Diabetes mellitus is one of the most prevalent
metabolic diseases, affecting 347 million individuals worldwide
(1). This heterogeneous disease
ultimately arises from either the failure of pancreatic β cells to
produce insulin, and/or the development of insulin resistance in
peripheral tissues (2). Type 1
diabetes (T1D; juvenile-onset, approximately 10% of all patients
with diabetes) is often caused by an autoimmune attack on
pancreatic β cells, resulting in the loss of insulin secretion. T1D
represents the insulin-dependent form of diabetes, requiring daily
insulin therapy. Type 2 diabetes (T2D; adult-onset, 90% of all
patients with diabetes) is caused by insulin resistance, associated
with relative hyperinsulinemia. T2D is usually a
non-insulin-dependent form of diabetes. Nevertheless, it requires
active and often complex therapeutic interventions. Obesity and
associated inflammation are common risk factors for T2D (3,4).
Aberrant lipid metabolism and signalling are tightly interconnected
with the pathogenesis of obesity, inflammation and diabetes. In
this review, we highlight the mechanisms through which the key
signalling sphingolipid molecule, sphingosine-1-phosphate (S1P),
and S1P-producing enzyme sphingosine kinase (SphK) have been shown
to affect diabetes-related pathologies.
There are two major isoforms of SphK (SphK1 and
SphK2), each having diverse and compensatory biological functions
(Figs. 1 and 2) (5).
Both SphKs can phosphorylate sphingosine (Sph) to form S1P, thus
activating a variety of extracellular and intracellular signalling
mechanisms. Controlled by SphKs, the conversion of pro-apoptotic
Sph into the pro-survival molecule, S1P, maintains the equilibrium
amid opposing cellular functionsm, such as cell growth,
proliferation, secretion and migration on the one side; and
apoptosis, senescence, autophagy and growth arrest on the other
(6,7). This balance, known as the
'sphingolipid rheostat', has been suggested to be critical to cell
fate (8,9). For example, tipping the balance in
favour of Sph accumulation may cause insulin resistance, whereas an
increased S1P level has been shown to promote insulin action
(10–12).
The highly bioactive lipid, S1P, is involved in
maintaining metabolic stability; however, it can also mediate the
development of serious pathological conditions (13–15). S1P binds specifically to five
(S1P1–5) transmembrane G protein-coupled receptors
(GPCRs) (16) and activates
cellular responses via S1P receptor-mediated mechanisms and/or by
targeting a complex network of intracellular messengers. The
biological actions of S1P are cell type- and receptor
subtype-specific as reviewed previously (17). In this review, we summarise the
recent evidence implicating SphKs/S1P signalling in
diabetes-associated intracellular abnormalities and metabolic
aberrations.
The SphK isoforms (Sphk1 and SphK2) are structurally
related, with five highly conserved domains (C1 to C5), although
they differ in size, intracellular localization and function. SphK
isoforms are encoded by two different genes, SPHK1
(chromosome 17, cytoband q25.1) and SPHK2 (chromosome 19,
cytoband q13.33). SphK1 is a 48 kDa protein first purified from rat
kidney cells (18). There are
three splice isoforms of SphK1 (1a, b and c); all are cytosolic
proteins differing slightly in subcellular distribution. SphK2 is
larger in size (69 kDa) and has sequence homology to SphK1. There
are two recently discovered splice isoforms of SphK2 (a and b)
(18). SphK2 contains an extended
N-terminal region with a proline-rich polypeptide insertion and
several other unique sites within the N-terminal sequence. The
N-terminal region of SphK2 includes a nuclear export sequence
(NES), important for shuttling the enzyme between the nucleus and
cytoplasm. The SphK2 sulphite-binding site facilitates the membrane
localization of SphK2 (19),
while the caspase-1 cleavage site regulates SphK2 maturation and
secretion from cells during the induction of apoptosis (21).
Furthermore, SphK isoforms differ in developmental
expression, tissue distribution and subcellular localization
(5,6). SphK1 predominates in the lungs and
spleen (7,8,11),
whereas SphK2 is more common in the heart, brain and liver
(9,10,12,13). Notably, SphK1 and SphK2 have been
shown to regulate different intracellular processes. For instance,
SphK1 promotes cell survival and proliferation, whereas SphK2 is
involved in the induction of apoptosis and cell growth arrest
(22). The divergent roles of
SphK isoforms in diabetes-related pathologies will be discussed in
greater detail below.
The different steady-state localization of the SphK
isoforms corresponds to the specific intracellular functioning of
the enzymes. SphK1/2 are redistributed to distinct intracellular
sites in an agonist-dependent manner. SphK1/2 substrates (Sph and
dihydrosphingosine) and product (S1P) are lipids and therefore, the
subcellular membrane localization of SphK in close proximity to
substrates is necessary for the enzyme to fulfill its housekeeping
and signalling functions.
SphK localization to specific intracellular
compartments is critical to the functional consequences of
signalling, such as the stimulation of cancer cell growth (23). Under basal conditions, SphK1
predominates in the cytosol where it maintains low levels of
intracellular S1P required for normal cell metabolism (24). It has been documented that the
translocation of SphK1 to the plasma membrane is required for its
oncogenic effect (23). However,
the targeting of SphK1 to a specialized subcellular compartment
enables its regulation of different functions. For example, the
translocation of SphK1 to the endoplasmic reticulum (EndRet)
promotes cell apoptosis (25),
whereas the translocation of SphK1 to the nuclear envelope promotes
G1/S transition during cell division (22).
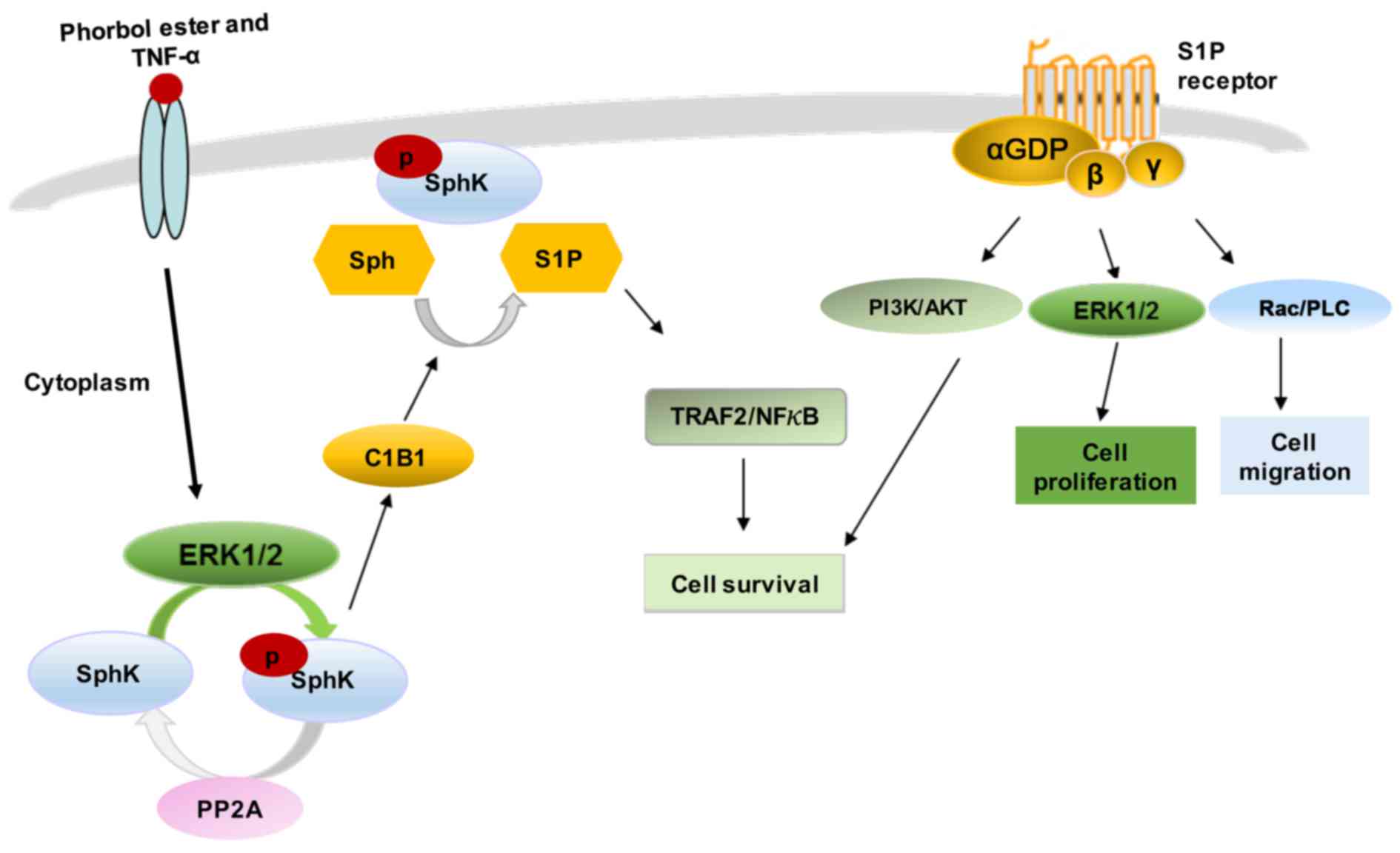
Various stimuli, such as growth factors and
cytokines can activate SphK1 by phosphorylation of the enzyme at
Ser-225, mediated by mitogen-activated protein kinase (MAPK) ERK1/2
(20,21,26,27). This phosphorylation promotes SphK1
to undergo conformational changes accompanied by a rapid increase
in the catalytic activity of the enzyme and its subcellular
translocation from the cytosol to plasma membrane (26). The continuous retention of SphK1
at the plasma membrane requires binding to phosphatidylserine or
phosphatidic acid (20,21,27). In addition to phosphorylation,
SphK1 membrane translocation can be also induced by protein-protein
interactions (28). SphK1
contains a calmodulin-binding site that binds calcium and
integrin-binding protein 1 (CIB1) in a calcium-dependent manner.
CIB1 functions as a calcium-myristoyl switch, providing a novel
mechanism for SphK1 translocation to the plasma membrane (28).
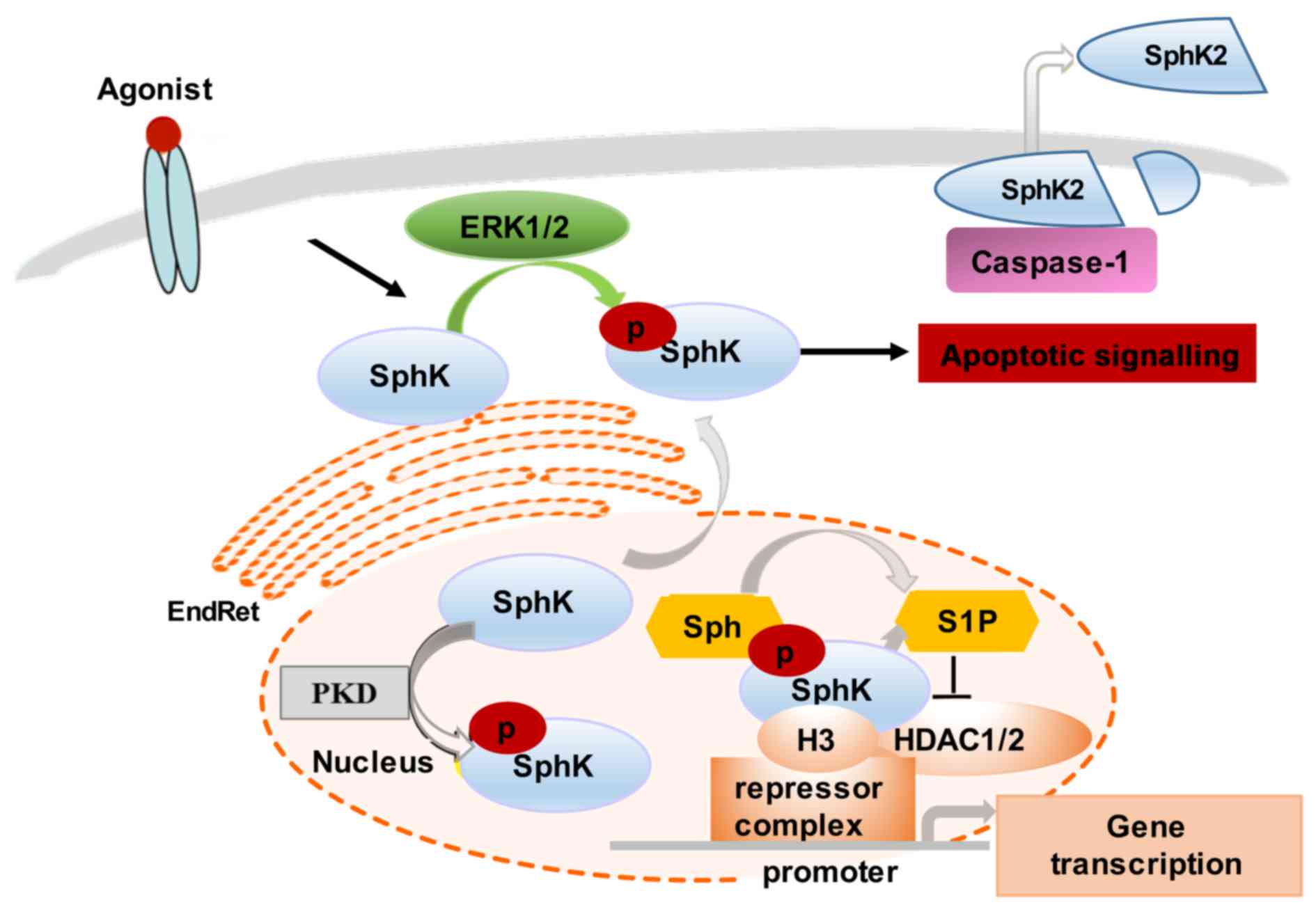
In comparison to SphK1, SphK2 function-associated
localization is less well understood. However, it has been shown
that SphK2 can be found both in the nucleus and the cytoplasm,
shuttling between these two compartments (28). Similar to SphK1, SphK2 cellular
levels and distribution are cell type-specific, agonist-dependent
and modifiable by cell culture conditions. For example, SphK2
translocates to the EndRet following serum starvation, which
coincides with the induction of apoptosis (29–31). Notably, SphK2 can be released from
apoptotic cells by caspase-1-mediated cleavage at its amino
terminus (30) (Fig. 2).
Several stimuli, such as epidermal growth factor
(EGF) and phorbol ester (PMA) (protein kinase C stimulant) activate
SphK2 through MAPK ERK1-mediated phosphorylation at Ser-351 and/or
Thr-578 (32). The EGF-induced
phosphorylation and activation of SphK2 has been linked to breast
cancer cell migration and to the increased invasive capacity of
tumor cells (32). The nuclear
localization of SphK2 is required for the epigenetic regulation of
specific target genes. For instance, SphK2 produces nuclear S1P
that binds and, thus, inhibits histone deacetylase 1 and 2
(HDAC1/2) activity, preventing the deacetylation of histone 3.
Nuclear SphK2 may also regulate cyclin-dependent kinase inhibitor
p21 and transcription regulator c-fos activity (15,29). The overexpression of SphK2 has
been shown to stimulate the PMA-induced expression of c-fos
mRNA, and, thus, indirectly influence a large group of genes
controlled by c-fos (15,29). The SphK2 regulation of
HDAC1-dependent deacetylation of histone H3 also results in
repression of p21 gene transcription, thus interfering with
cell cycle progression and cellular senescence. The nuclear
signalling of SphK is regulated by protein kinase D (PKD)-induced
phosphorylation, which promotes its export from the nucleus
(30).
The multifunctional signalling lipid, S1P, mediates
the effects of numerous biological stimuli, including cytokines,
growth factors and hormones (8,33).
S1P regulates diverse signaling via five transmembrane GPCRs,
S1P1–5, originally known as endothelial differentiation
gene (EDG) receptors (34). S1P
may bind its receptors in a paracrine or autocrine manner, followed
by differential coupling to specific G proteins (34).
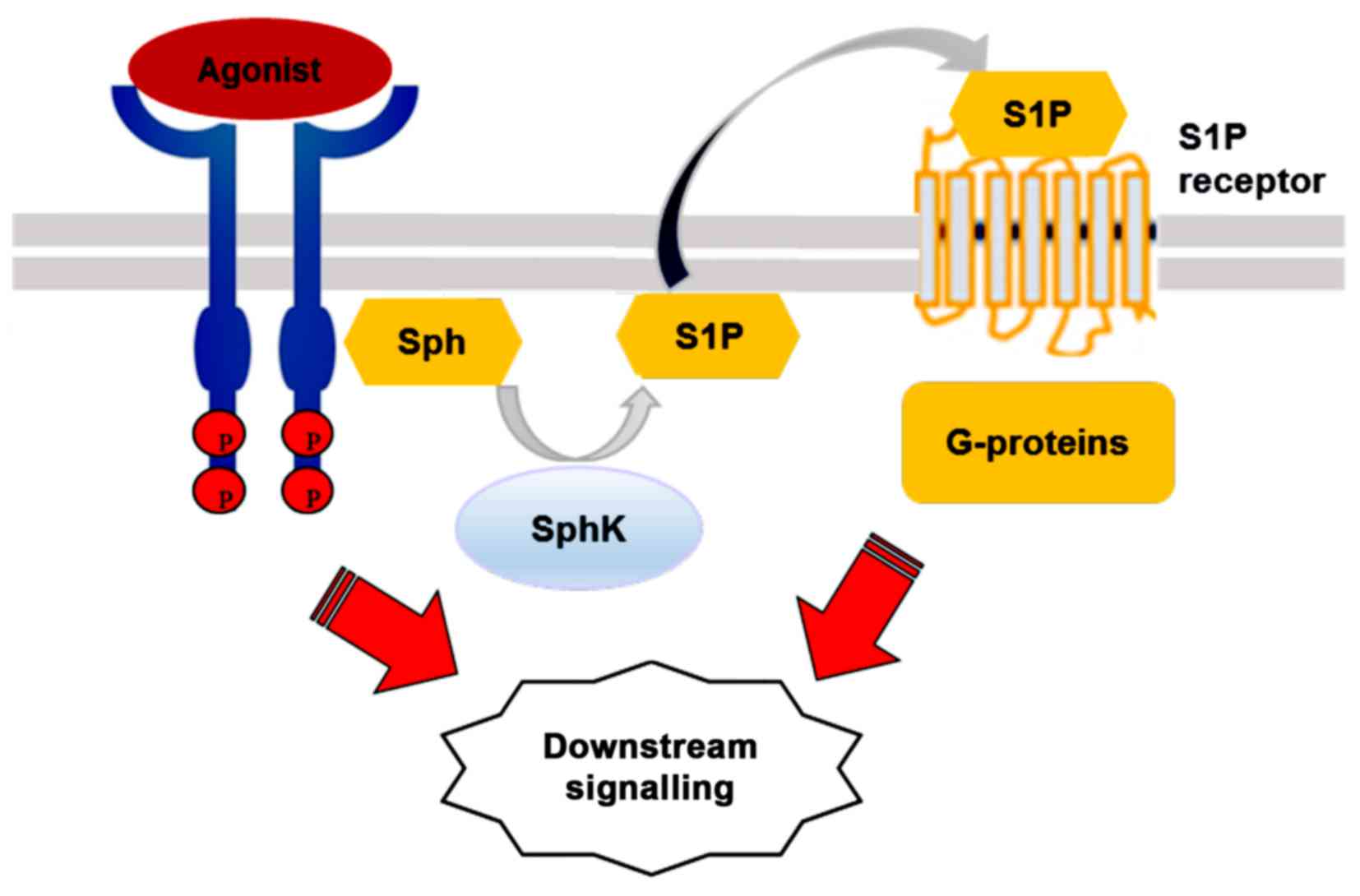
According to the 'inside-out' signalling model,
activated SphK1 is translocated to the plasma membrane where it
generates S1P. The lipid is then released locally and, in an
autocrine manner, binds to one or more S1P receptor subtypes on the
same cell, or, in a paracrine manner, activates the receptors on
neighbouring cells (33).
Activated SphK1 may also be secreted from cells to produce S1P from
extracellular Sph (35).
Amongst its intracellular targets, S1P has been
shown to interact with several cytoplasmic and nuclear proteins
(33), including HDAC1/2, which
is involved in regulating histone acetylation and the epigenetic
regulation of specific target genes, such as p21 and c-fos
(36). S1P can also act as a
co-factor, stimulating E3 ligase activity in TNF
receptor-associated factor 2 (TRAF2) and controlling the survival
response (37). Moreover, S1P
interacts with inner mitochondrial membrane protein prohibitin 2
(PHB2) to regulate cytochrome c oxidase assembly and
mitochondrial respiration (38).
Finally, S1P has been shown to bind the transcription factor
peroxisome proliferator-activated receptor γ (PPARγ) (39), human telomerase reverse
transcriptase (hTERT) (40) and
beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1)
(41). The essential cellular
effects of S1P are summarised in Fig.
3 and were recently reviewed in full detail elsewhere (42).
Reduced sensitivity to insulin of the hormone target
tissues, such as skeletal muscle, liver and adipose deposits is
defined as insulin resistance. Hepatic insulin resistance has been
confirmed as the major risk factor for T2D onset. Previous studies
have shown that SphK activation improves hepatic insulin signalling
in obesity and diabetes (43–45,47). Notably, in a previous study, low
total SphK activity was detected in the livers of mice fed a
high-fat diet (HFD). The mice also had elevated liver
triacylglycerol (TAG) and diacylglycerol (DAG) levels, and
demonstrated glucose intolerance. SphK1 overexpression in the liver
reduced hepatic TAG synthesis and the total TAG content in the
HFD-fed mice (46). However,
these SphK1-overexpressing mice exhibited no changes in glucose
metabolism, including the states of gluconeogenesis, glycogen
synthesis and glucose tolerance (46), suggesting it has minimal influence
on carbohydrate metabolism.
It was recently found that SphK2 is the major SphK
isoform in the liver. The overexpression of the SphK2 gene
has been shown to elevate hepatic S1P expression and improve
glucose/lipid metabolism in KK/Ay diabetic mice. The
adenoviral-mediated expression of SphK2 activated the Akt pathway,
a key signalling mechanism in the insulin-induced regulation of
glucose metabolism, thus, confirming an important role of SphK2 in
regulating hepatic insulin signalling (47).
Insulin resistance is also associated with the
pathological transformation of proteins and lipid biosynthesis in
the EndRet. The perturbation of specific EndRet functions, the
so-called EndRet stress, can be caused by excess nutrient intake
(one of the major causes of obesity) that further induces the
activation of multiple pathological chain-reaction mechanisms,
including the unfolded protein response (UPR) and aberrant lipid
biosynthesis (48). Notably,
SphK/S1P-signalling activation ameliorates hepatic insulin
resistance induced by EndRet stress. Accordingly, SphK2 activation
improves insulin signalling and its metabolic actions under
conditions of EndRet stress in HFD-fed mice. Activated SphK2 also
reduces hepatic lipid accumulation, thus improving the effects of
insulin in these mice in vivo, an effect confirmed in
vitro in primary hepatocytes (47).
Decreased insulin secretion and T2D can be triggered
by pancreatic β-cell death that is often caused by excessive levels
of circulating lipids (lipotoxicity) in obese or overweight
patients. Increased sphingolipid metabolites that are observed
during lipotoxicity also induce β-cell dysfunction, leading to
apoptosis (49). For example,
increased intracellular ceramide promotes an apoptotic cascade and
initiates β-cell death in diabetic fatty rodent models of T2D in
vivo and human β-cells in vitro (50–52).
Contrary to the deleterious effect of ceramide,
SphK/S1P have been shown to promote insulin release, to stimulate
the development of intra-islet vasculature, improve glucose sensing
and prevent inflammation-linked attacks of the immune system
(58). The relative intracellular
balance of sphingolipid species, such as ceramide and S1P
critically determines the direction of β-cell fate; deciding
between activating apoptosis or proliferation, or stimulating
insulin secretion, and/or islet-cell inflammatory responses
(49).
There is abundant evidence demonstrating the
pro-survival role of S1P in pancreatic β-cells. S1P has been shown
to improve β-cell function in the HIT-T15 cell line and isolated
mouse islets, through phospholipase C (PLC) activation (59). S1P has also been shown to protect
pancreatic islet cells from IL-1β-induced apoptosis (60). The exposure of INS-1 cells and
isolated pancreatic islets to IL-1β and TNF-α has been shown to
activate SphK2 as a self-protective mechanism, reducing β-cell
inflammatory damage (61).
Furthermore, SphK1 activation promotes β-cell survival in diabetic
obese mice in vivo (10).
The roles of SphK/S1P in β-cell survival processes have also been
addressed in several cell lines and animal models following
lipotoxicity-induced β-cell damage. It was previously demonstrated
that the inhibition of SphK/S1P signalling, activated in INS-1
β-cells by palmitate treatment, potentiated β-cell apoptosis;
however, SphK1 overexpression significantly mitigated β-cell
apoptosis under lipotoxic conditions (62). In another study, the assessment of
SphK1 (−/−) and wild-type HFD-fed mice demonstrated that HFD-fed
SphK1(−/−) mice developed evident diabetes, accompanied by reduced
β-cell mass and a 3-fold decrease in insulin secretion (10). Furthermore, the oral
administration of FTY720 (a S1P receptor agonist) to diabetic
(db/db) mice facilitated β-cell mass preservation and normalised
fasting blood glucose (48,63). In addition to its pro-survival
effect, SphK activation has been shown to promote
glucose-stimulated insulin secretion (GSIS) in MIN6 cells and mouse
pancreatic islets (64).
SphK activation and S1P formation have recently been
linked to endogenic adiponectin signalling during the induction of
β-cell survival. The pro-survival effect of adiponectin has been
shown to be modulated by increased S1P formation, employing the
AMP-activated protein kinase (AMPK)-dependent pathway in obese mice
(44,65). Supporting these findings, S1P2
receptor inhibition has been shown to attenuate streptozotocin
(STZ)-induced β-cell apoptosis in T1D models (66). Collectively, current studies
indicate divergent signalling mechanisms and positive involvement
of the different SphK isoforms and S1P receptor subtypes in
protecting pancreatic β cells from apoptosis and malfunction.
Skeletal muscles consume energy and provide a sink
for insulin-stimulated glucose disposal and glycogen formation,
thus contributing to the regulation of whole body metabolism.
Skeletal muscle insulin resistance is often considered to be the
initiating event for T2D, evident prior to β-cell failure and overt
hyperglycaemia. Previous studies have implicated SphK/S1P
signalling in skeletal muscle insulin resistance, followed by
decreased whole-body insulin sensitivity (67,68). Notably, SphK1 overexpression
promotes basal and insulin-stimulated glucose uptake in C2C12 cells
(67) and a remarkable reduction
in blood glucose in diabetic mice (12,67). In support of this, pharmacological
SphK inhibition reduces insulin-stimulated glucose disposal
(69).
The adiponectin receptor is another alternative
mechanism potentially explaining the protective effects of SphK.
The overexpression of the adiponectin receptor AdipR1 improves
local insulin sensitivity in rat skeletal muscle, at the same time
reducing the concentration of both S1P and ceramide (73). Overall, experimental findings
suggest a fundamental role of SphK signalling as a tool for
ceramide utilisation and the modulation of skeletal muscle insulin
resistance. However, the contribution of S1P to skeletal muscle
insulin resistance requires further clarification.
Intriguingly, despite intensive research in the
field, the role of SphK/S1P signalling in regulating insulin
sensitivity remains controversial, as alternative studies
demonstrate the pro-inflammatory effects of the SphK/S1P pathway.
However, it is well established that obesity and
adipocyte-triggered inflammation give rise to insulin resistance in
peripheral tissues, and that SphK1 signalling mediates
lipolysis-associated inflammation in adipocytes. Excessive
lipolysis can induce inflammation via the increased production of
inflammatory cytokines, such as IL-6 and/or the acute activation of
β3-adrenergic receptors. The pharmacological inhibition of SphK1
activity blocks ADRB3-induced IL-6 production in adipocytes, both
in vitro and in vivo (74). Furthermore, the selective
inhibition of SphK1 protects adipocytes from lipopolysaccharide
(LPS)-induced inflammation in Zucker diabetic fatty rats (75). SphK1 deficiency upregulates the
gene expression of the anti-inflammatory molecules (IL-10 and
adiponectin) and improves overall insulin sensitivity in the
adipose and muscle tissues of SphK1 knockout mice in vivo
(68). Further detailed
investigations of the involvement of SphK in inflammatory processes
are required. However, taken together, the accumulating evidence
indicates that the inhibition of the SphK/S1P axis is a potential
therapeutic target for the treatment of insulin resistance.
SphK/S1P signalling has been linked to several
diabetic microvascular complications, such as neuropathy (76–78), retinopathy (79,80) and nephropathy (81).
Aberrant sphingolipid metabolism and/or generation
of specific sphingolipid metabolites are thought to aggravate
diabetic complications, including the pathogenesis of DN. DN is
characterised by a series of pathological events, such as early
glomerular proliferation and hypertrophy, accumulation of
extracellular matrix (ECM) components and renal fibrosis that may
progress to end-stage renal disease. The incidence of DN accounts
for 30% of diabetic patients diagnosed with glomerular sclerosis
and/or tubulointerstitial (renal) fibrosis (82). S1P stimulates the survival,
proliferation and migration of renal mesangial cells (83,84), and induces the upregulation of the
pro-fibrotic growth factors, collagen and fibronectin synthesis
(81,85). Phosphorylated Smads, secreted
phospholipase A2 and matrix metalloproteinase-9 mediate the effect
of S1P in renal cells (81,85).
SphK/S1P signalling has previously been linked to
glomerular proliferation. However, S1P can promote not only renal
mesangial cell proliferation, but also renal inflammation and
fibrosis (86). The stimulatory
effect of S1P on renal mesangial cell proliferation was first
demonstrated in Swiss 3T3 fibroblasts (87). In a previous study, activated SphK
and 10-fold upregulated S1P levels stimulated the proliferation of
glomerular mesangial cells in rats with STZ-induced diabetes in
vivo (88). Further studies
have confirmed an association of SphK1 activation and S1P
production with renal hypertrophy and increased levels of
fibronectin (81,89). SphK1/S1P promotes glomerular
mesangial cell proliferation via increased fibronectin production,
but also through the activation of transforming growth factor-β1
(TGF-β1) and AP-1 signalling (90).
Expressed in glomerular mesangial cells, S1P2 and
S1P3 receptors mediate renal fibrosis, glomerular cell
proliferation and pathological angiogenesis (91,92). Lymphocyte migration to the site of
inflammation is mediated through binding to the S1P1 receptor
(93), indicating the involvement
of this receptor subtype in potentially damaging immune reactions
in kidneys and other organs (94–96). However, an inflammation-associated
role of the SphK/S1P pathway remains controversial, as other
authors have demonstrated that S1P can reduce inflammatory signals
in cultured renal mesangial cells by downregulating prostaglandin
E2 synthesis and the expression of pro-inflammatory mediators, such
as cytokine-triggered secretory phospholipase A2 and inducible
nitric oxide (NO) synthase (97).
The relevance of SphK/S1P signalling to DN
progression was investigated in SphK-deficient mice in vivo
(35). One study on
SphK2-deficient mice showed reduced plasma creatinine
concentrations, suggesting that SphK2 protected cells from renal
ischaemia (98), and another
study detected the worsening of nephropathy conditions in
SphK1-deficient mice (86). The
loss of SphK1 activity has been shown to aggravate cytogenesis in a
mouse model of polycystic kidney disease and renal injury (35). Similarly, the lentiviral-mediated
overexpression of human SphK1 in mice subjected to
ischaemia-perfusion injury demonstrated less tubular necrosis and
reduced inflammation (98).
Overall, the current evidence suggests the involvement of both SphK
isoforms, probably to a different degree and affecting distinct
targets, in regulating microvascular complications, such as DN.
Further studies are warranted in order to clarify which SphK
isoform is involved in inflammation-associated signalling and
whether S1P receptors should be targeted for nephropathy drug
design.
However, several recent investigations have
indicated a positive effect of S1P receptor signalling in
maintaining vascular health. The transactivation of S1P1/3
receptors stimulates eNOS, increasing NO production and
vasodilation (116,117). The increased expression of
S1P1/3 receptors improves recovery following cardiac microvascular
dysfunction associated with diabetes (118). The S1P1 receptor also mediates
estrogen-induced activation of Akt/eNOS signalling in endothelial
cells (119). Estrogen
replacement therapy in post-menopausal women prevents
diabetes-associated cardiovascular complications (120), thus indicating that S1P
receptors should be further explored as potential drug targets for
the treatment of diabetes-associated vascular pathologies.
The complex interactions among members of the
sphingolipid signalling pathway, insulin signalling and diabetic
pathologies have been extensively investigated. Yet the role of
SphK/S1P signalling in the development of diabetes mellitus remains
unclear. The divergence of SphK/S1P signalling seems to be
dependent on cell type, the expression pattern of S1P receptor
subtypes and the relative expression of the specific SphK isoforms.
In diabetes mellitus, SphK activation has been known to promote
β-cell survival and insulin secretion, prevent vascular pathologies
related to diabetes (39,116,119), ameliorate peripheral insulin
resistance and obesity in diabetic patients and animal models
(121,122).A low S1P content has been linked
to increased incidence of coronary artery disease (97,122–124) and diabetes mellitus (97,125–127).
In addition, emerging evidence has also linked
SphK/S1P signalling to the development of diabetes-related vascular
complications, such as neuropathy (76–78), retinopathy (79,80,128,129) and nephropathy (81). However, the roles of the different
S1P receptor subtypes and two SphK isoforms in the pathogenesis of
diabetes remain to be confirmed in vivo and in future
clinical trials. SphK therefore represents a potential therapeutic
target for diabetes mellitus. The FTY720 (fingolimoid), an S1P
receptor agonist has demonstrated promising therapeutic effects,
including the stimulation of lipolysis, decreased accumulation of
skeletal muscle ceramide, improved systemic glucose homeostasis,
and β-cell survival in diabetic mouse models (63,118,130). Combination therapy with FTY720
plus insulin glargine has been shown to be a promising therapeutic
strategy for the treatment of diabetes mellitus (130). A second generation of S1P3
modulators (siponimod, ponesimod, KRP-203, ONO-4641, RPC1063,
CS-0777 and GSK2018682) targets different S1P receptor subtypes
(131). These substances promise
to elucidate the mechanisms underlying the divergent outcomes of
S1P signalling, and consequently, the potential efficacy of
targeting the SphK/S1P pathway for the treatment of diabetes and
associated pathologies.
|
1
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA,
et al: Global Burden of Metabolic Risk Factors of Chronic Diseases
Collaborating Group (Blood Glucose): National, regional, and global
trends in fasting plasma glucose and diabetes prevalence since
1980: Systematic analysis of health examination surveys and
epidemiological studies with 370 country-years and 2·7 million
participants. Lancet. 378:31–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Expert Committee on the Diagnosis and
Classification of Diabetes Mellitus: Report of the expert committee
on the diagnosis and classification of diabetes mellitus. Diabetes
Care. 26(Suppl 1): S5–S20. 2003. View Article : Google Scholar
|
|
3
|
Shao S, Yang Y, Yuan G, Zhang M and Yu X:
Signaling molecules involved in lipid-induced pancreatic beta-cell
dysfunction. DNA Cell Biol. 32:41–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newsholme P, Keane D, Welters HJ and
Morgan NG: Life and death decisions of the pancreatic beta-cell:
The role of fatty acids. Clin Sci (Lond). 112:27–42. 2007.
View Article : Google Scholar
|
|
5
|
Alemany R, van Koppen CJ, Danneberg K, Ter
Braak M and Meyer zu Heringdorf D: Regulation and functional roles
of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol.
374:413–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Brocklyn JR, Lee MJ, Menzeleev R,
Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada
S, Liu CH, et al: Dual actions of sphingosine-1-phosphate:
Extracellular through the Gi-coupled receptor Edg-1 and
intracellular to regulate proliferation and survival. J Cell Biol.
142:229–240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olivera A and Spiegel S:
Sphingosine-1-phosphate as second messenger in cell proliferation
induced by PDGF and FCS mitogens. Nature. 365:557–560. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuvillier O, Pirianov G, Kleuser B, Vanek
PG, Coso OA, Gutkind S and Spiegel S: Suppression of
ceramide-mediated programmed cell death by sphingosine-1-phosphate.
Nature. 381:800–803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pyne S, Chapman J, Steele L and Pyne NJ:
Sphingomyelin-derived lipids differentially regulate the
extracellular signal-regulated kinase 2 (ERK-2) and c-Jun
N-terminal kinase (JNK) signal cascades in airway smooth muscle.
Eur J Biochem. 237:819–826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi Y, Chen J, Lay A, Don A, Vadas M and
Xia P: Loss of sphingosine kinase 1 predisposes to the onset of
diabetes via promoting pancreatic β-cell death in diet-induced
obese mice. FASEB J. 27:4294–4304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holland WL, Brozinick JT, Wang LP, Hawkins
ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, et
al: Inhibition of ceramide synthesis ameliorates glucocorticoid-,
saturated-fat-, and obesity-induced insulin resistance. Cell Metab.
5:167–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruce CR, Risis S, Babb JR, Yang C,
Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K,
Takuwa Y, et al: Overexpression of sphingosine kinase 1 prevents
ceramide accumulation and ameliorates muscle insulin resistance in
high-fat diet-fed mice. Diabetes. 61:3148–3155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spiegel S and Milstien S: The outs and the
ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol.
11:403–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pyne S and Pyne NJ: Translational aspects
of sphingosine 1-phosphate biology. Trends Mol Med. 17:463–472.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maceyka M and Spiegel S: Sphingolipid
metabolites in inflammatory disease. Nature. 510:58–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mendelson K, Evans T and Hla T:
Sphingosine 1-phosphate signalling. Development. 141:5–9. 2014.
View Article : Google Scholar :
|
|
17
|
Hla T and Dannenberg AJ: Sphingolipid
signaling in metabolic disorders. Cell Metab. 16:420–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pitson SM: Regulation of sphingosine
kinase and sphingolipid signaling. Trends Biochem Sci. 36:97–107.
2011. View Article : Google Scholar
|
|
19
|
Don AS and Rosen H: A lipid binding domain
in sphingosine kinase 2. Biochem Biophys Res Commun. 380:87–92.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barr RK, Lynn HE, Moretti PA, Khew-Goodall
Y and Pitson SM: Deactivation of sphingosine kinase 1 by protein
phosphatase 2A. J Biol Chem. 283:34994–35002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sutherland CM, Moretti PA, Hewitt NM,
Bagley CJ, Vadas MA and Pitson SM: The calmodulin-binding site of
sphingosine kinase and its role in agonist-dependent translocation
of sphingosine kinase 1 to the plasma membrane. J Biol Chem.
281:11693–11701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hait NC, Allegood J, Maceyka M, Strub GM,
Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S
and Spiegel S: Regulation of histone acetylation in the nucleus by
sphingosine-1-phosphate. Science. 325:1254–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wattenberg BW, Pitson SM and Raben DM: The
sphingosine and diacylglycerol kinase superfamily of signaling
kinases: Localization as a key to signaling function. J Lipid Res.
47:1128–1139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leclercq TM and Pitson SM: Cellular
signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB
Life. 58:467–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giussani P, Maceyka M, Le Stunff H, Mikami
A, Lépine S, Wang E, Kelly S, Merrill AH Jr, Milstien S and Spiegel
S: Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic
reticulum-to-golgi trafficking of ceramide. Mol Cell Biol.
26:5055–5069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pitson SM, Moretti PA, Zebol JR, Lynn HE,
Xia P, Vadas MA and Wattenberg BW: Activation of sphingosine kinase
1 by ERK1/2-mediated phosphorylation. EMBO J. 22:5491–5500. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stahelin RV, Hwang JH, Kim JH, Park ZY,
Johnson KR, Obeid LM and Cho W: The mechanism of membrane targeting
of human sphingosine kinase 1. J Biol Chem. 280:43030–43038. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siow D and Wattenberg B: The
compartmentalization and trans-location of the sphingosine kinases:
Mechanisms and functions in cell signaling and sphingolipid
metabolism. Crit Rev Biochem Mol Biol. 46:365–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maceyka M, Sankala H, Hait NC, Le Stunff
H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr, et
al: SphK1 and SphK2, sphingosine kinase isoenzymes with opposing
functions in sphingolipid metabolism. J Biol Chem. 280:37118–37129.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding G, Sonoda H, Yu H, Kajimoto T,
Goparaju SK, Jahangeer S, Okada T and Nakamura S: Protein kinase
D-mediated phosphorylation and nuclear export of sphingosine kinase
2. J Biol Chem. 282:27493–27502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Igarashi N, Okada T, Hayashi S, Fujita T,
Jahangeer S and Nakamura S: Sphingosine kinase 2 is a nuclear
protein and inhibits DNA synthesis. J Biol Chem. 278:46832–46839.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hait NC, Bellamy A, Milstien S, Kordula T
and Spiegel S: Sphingosine kinase type 2 activation by ERK-mediated
phosphorylation. J Biol Chem. 282:12058–12065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvarez SE, Milstien S and Spiegel S:
Autocrine and paracrine roles of sphingosine-1-phosphate. Trends
Endocrinol Metab. 18:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strub GM, Maceyka M, Hait NC, Milstien S
and Spiegel S: Extracellular and intracellular actions of
sphingosine-1-phosphate. Adv Exp Med Biol. 688:141–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Venkataraman K, Thangada S, Michaud J, Oo
ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL and Hla T:
Extracellular export of sphingosine kinase-1a contributes to the
vascular S1P gradient. Biochem J. 397:461–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maceyka M, Harikumar KB, Milstien S and
Spiegel S: Sphingosine-1-phosphate signaling and its role in
disease. Trends Cell Biol. 22:50–60. 2012. View Article : Google Scholar :
|
|
37
|
Xia P, Wang L, Moretti PA, Albanese N,
Chai F, Pitson SM, D'Andrea RJ, Gamble JR and Vadas MA: Sphingosine
kinase interacts with TRAF2 and dissects tumor necrosis
factor-alpha signaling. J Biol Chem. 277:7996–8003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Artal-Sanz M and Tavernarakis N:
Prohibitin and mitochondrial biology. Trends Endocrinol Metab.
20:394–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parham KA, Zebol JR, Tooley KL, Sun WY,
Moldenhauer LM, Cockshell MP, Gliddon BL, Moretti PA, Tigyi G,
Pitson SM and Bonder CS: Sphingosine 1-phosphate is a ligand for
peroxisome proliferator-activated receptor-γ that regulates
neoangiogenesis. FASEB J. 29:3638–3653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panneer Selvam S, De Palma RM, Oaks JJ,
Oleinik N, Peterson YK, Stahelin RV, Skordalakes E, Ponnusamy S,
Garrett-Mayer E, Smith CD and Ogretmen B: Binding of the
sphingolipid S1P to hTERT stabilizes telomerase at the nuclear
periphery by allosterically mimicking protein phosphorylation. Sci
Signal. 8:ra582015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takasugi N, Sasaki T, Suzuki K, Osawa S,
Isshiki H, Hori Y, Shimada N, Higo T, Yokoshima S, Fukuyama T, et
al: BACE1 activity is modulated by cell-associated
sphingosine-1-phosphate. J Neurosci. 31:6850–6857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pyne S, Adams DR and Pyne NJ: Sphingosine
1-phosphate and sphingosine kinases in health and disease: Recent
advances. Prog Lipid Res. 62:93–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fox TE, Bewley MC, Unrath KA, Pedersen MM,
Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, Flanagan
JM, et al: Circulating sphingolipid biomarkers in models of type 1
diabetes. J Lipid Res. 52:509–517. 2011. View Article : Google Scholar :
|
|
44
|
Tao C, Sifuentes A and Holland WL:
Regulation of glucose and lipid homeostasis by adiponectin: Effects
on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res
Clin Endocrinol Metab. 28:43–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Osawa Y, Seki E, Kodama Y, Suetsugu A,
Miura K, Adachi M, Ito H, Shiratori Y, Banno Y, Olefsky JM, et al:
Acid sphingomyelinase regulates glucose and lipid metabolism in
hepatocytes through AKT activation and AMP-activated protein kinase
suppression. FASEB J. 25:1133–1144. 2011. View Article : Google Scholar :
|
|
46
|
Kowalski GM, Kloehn J, Burch ML,
Selathurai A, Hamley S, Bayol SA, Lamon S, Watt MJ, Lee-Young RS,
McConville MJ and Bruce CR: Overexpression of sphingosine kinase 1
in liver reduces triglyceride content in mice fed a low but not
high-fat diet. Biochim Biophys Acta. 1851:210–219. 2015. View Article : Google Scholar
|
|
47
|
Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee
J, Lee HY, Soo Choi C, Kim BK and Park TS: Activation of
sphingosine kinase 2 by endoplasmic reticulum stress ameliorates
hepatic steatosis and insulin resistance in mice. Hepatology.
62:135–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boden G: Endoplasmic reticulum stress:
Another link between obesity and insulin resistance/inflammation?
Diabetes. 58:518–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boslem E, Meikle PJ and Biden TJ: Roles of
ceramide and sphingolipids in pancreatic β-cell function and
dysfunction. Islets. 4:177–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu Q, Shan X, Miao H, Lu Y, Xu J, You N,
Liu C, Liao DF and Jin J: Acute activation of acid ceramidase
affects cytokine-induced cytotoxicity in rat islet beta-cells. FEBS
Lett. 583:2136–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Maedler K, Oberholzer J, Bucher P, Spinas
GA and Donath MY: Monounsaturated fatty acids prevent the
deleterious effects of palmitate and high glucose on human
pancreatic beta-cell turnover and function. Diabetes. 52:726–733.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Veluthakal R, Palanivel R, Zhao Y,
McDonald P, Gruber S and Kowluru A: Ceramide induces mitochondrial
abnormalities in insulin-secreting INS-1 cells: Potential
mechanisms underlying ceramide-mediated metabolic dysfunction of
the beta cell. Apoptosis. 10:841–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lupi R, Dotta F, Marselli L, Del Guerra S,
Masini M, Santangelo C, Patané G, Boggi U, Piro S, Anello M, et al:
Prolonged exposure to free fatty acids has cytostatic and
pro-apoptotic effects on human pancreatic islets: Evidence that
beta-cell death is caspase mediated, partially dependent on
ceramide pathway, and Bcl-2 regulated. Diabetes. 51:1437–1442.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Boslem E, MacIntosh G, Preston AM, Bartley
C, Busch AK, Fuller M, Laybutt DR, Meikle PJ and Biden TJ: A
lipidomic screen of palmitate-treated MIN6 β-cells links
sphingolipid metabolites with endoplasmic reticulum (ER) stress and
impaired protein trafficking. Biochem J. 435:267–276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Véret J, Coant N, Berdyshev EV, Skobeleva
A, Therville N, Bailbé D, Gorshkova I, Natarajan V, Portha B and Le
Stunff H: Ceramide synthase 4 and de novo production of ceramides
with specific N-acyl chain lengths are involved in
glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem J.
438:177–189. 2011. View Article : Google Scholar
|
|
56
|
Kelpe CL, Moore PC, Parazzoli SD,
Wicksteed B, Rhodes CJ and Poitout V: Palmitate inhibition of
insulin gene expression is mediated at the transcriptional level
via ceramide synthesis. J Biol Chem. 278:30015–30021. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo J, Qian Y, Xi X, Hu X, Zhu J and Han
X: Blockage of ceramide metabolism exacerbates palmitate inhibition
of pro-insulin gene expression in pancreatic beta-cells. Mol Cell
Biochem. 338:283–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jessup CF, Bonder CS, Pitson SM and Coates
PT: The sphingolipid rheostat: A potential target for improving
pancreatic islet survival and function. Endocr Metab Immune Disord
Drug Targets. 11:262–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shimizu H, Okajima F, Kimura T, Ohtani K,
Tsuchiya T, Takahashi H, Kuwabara A, Tomura H, Sato K and Mori M:
Sphingosine 1-phosphate stimulates insulin secretion in HIT-T 15
cells and mouse islets. Endocr J. 47:261–269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rütti S, Ehses JA, Sibler RA, Prazak R,
Rohrer L, Georgopoulos S, Meier DT, Niclauss N, Berney T, Donath
MY, et al: Low- and high-density lipoproteins modulate function,
apoptosis, and proliferation of primary human and murine pancreatic
beta-cells. Endocrinology. 150:4521–4530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mastrandrea LD, Sessanna SM and Laychock
SG: Sphingosine kinase activity and sphingosine-1 phosphate
production in rat pancreatic islets and INS-1 cells: Response to
cytokines. Diabetes. 54:1429–1436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Véret J, Coant N, Gorshkova IA, Giussani
P, Fradet M, Riccitelli E, Skobeleva A, Goya J, Kassis N, Natarajan
V, et al: Role of palmitate-induced sphingoid base-1-phosphate
biosynthesis in INS-1 β-cell survival. Biochim Biophys Acta.
1831:251–262. 2013. View Article : Google Scholar
|
|
63
|
Zhao Z, Choi J, Zhao C and Ma ZA: FTY720
normalizes hyperglycemia by stimulating β-cell in vivo
re-generation in db/db mice through regulation of cyclin D3 and p57
(KIP2). J Biol Chem. 287:5562–5573. 2012. View Article : Google Scholar
|
|
64
|
Cantrell Stanford J, Morris AJ, Sunkara M,
Popa GJ, Larson KL and Özcan S: Sphingosine 1-phosphate (S1P)
regulates glucose-stimulated insulin secretion in pancreatic beta
cells. J Biol Chem. 287:13457–13464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Holland WL, Miller RA, Wang ZV, Sun K,
Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et
al: Receptor-mediated activation of ceramidase activity initiates
the pleiotropic actions of adiponectin. Nat Med. 17:55–63. 2011.
View Article : Google Scholar
|
|
66
|
Imasawa T, Koike K, Ishii I, Chun J and
Yatomi Y: Blockade of sphingosine 1-phosphate receptor 2 signaling
attenuates streptozotocin-induced apoptosis of pancreatic
beta-cells. Biochem Biophys Res Commun. 392:207–211. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma MM, Chen JL, Wang GG, Wang H, Lu Y, Li
JF, Yi J, Yuan YJ, Zhang QW, et al: Sphingosine kinase 1
participates in insulin signalling and regulates glucose metabolism
and homeostasis in KK/Ay diabetic mice. Diabetologia. 50:891–900.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang J, Badeanlou L, Bielawski J, Ciaraldi
TP and Samad F: Sphingosine kinase 1 regulates adipose
proinflammatory responses and insulin resistance. Am J Physiol
Endocrinol Metab. 306:E756–E768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mikłosz A, Łukaszuk B, Baranowski M,
Górski J and Chabowski A: Effects of inhibition of serine
palmitoyltransferase (SPT) and sphingosine kinase 1 (SphK1) on
palmitate induced insulin resistance in L6 myotubes. PLoS One.
8:e855472013. View Article : Google Scholar
|
|
70
|
Rapizzi E, Taddei ML, Fiaschi T, Donati C,
Bruni P and Chiarugi P: Sphingosine 1-phosphate increases glucose
uptake through trans-activation of insulin receptor. Cell Mol Life
Sci. 66:3207–3218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rapizzi E, Donati C, Cencetti F, Nincheri
P and Bruni P: Sphingosine 1-phosphate differentially regulates
proliferation of C2C12 reserve cells and myoblasts. Mol Cell
Biochem. 314:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Takuwa N, Ohkura S, Takashima S, Ohtani K,
Okamoto Y, Tanaka T, Hirano K, Usui S, Wang F, Du W, et al:
S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic
mice involves reactive oxygen species. Cardiovasc Res. 85:484–493.
2010. View Article : Google Scholar :
|
|
73
|
Patel SA, Hoehn KL, Lawrence RT, Sawbridge
L, Talbot NA, Tomsig JL, Turner N, Cooney GJ, Whitehead JP, Kraegen
EW and Cleasby ME: Overexpression of the adiponectin receptor
AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity.
Endocrinology. 153:5231–5246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang W, Mottillo EP, Zhao J, Gartung A,
VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL, et al:
Adipocyte lipolysis-stimulated interleukin-6 production requires
sphingosine kinase 1 activity. J Biol Chem. 289:32178–32185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tous M, Ferrer-Lorente R and Badimon L:
Selective inhibition of sphingosine kinase-1 protects adipose
tissue against LPS-induced inflammatory response in Zucker diabetic
fatty rats. Am J Physiol Endocrinol Metab. 307:E437–E446. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Janes K, Little JW, Li C, Bryant L, Chen
C, Chen Z, Kamocki K, Doyle T, Snider A, Esposito E, et al: The
development and maintenance of paclitaxel-induced neuropathic pain
require activation of the sphingosine 1-phosphate receptor subtype
1. J Biol Chem. 289:21082–21097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guan H, Song L, Cai J, Huang Y, Wu J, Yuan
J, Li J and Li M: Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim
pathway and contributes to apoptosis resistance in glioma cells.
PLoS One. 6:e199462011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Abuhusain HJ, Matin A, Qiao Q, Shen H,
Kain N, Day BW, Stringer BW, Daniels B, Laaksonen MA, Teo C, et al:
A metabolic shift favoring sphingosine 1-phosphate at the expense
of ceramide controls glioblastoma angiogenesis. J Biol Chem.
288:37355–37364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xie B, Shen J, Dong A, Rashid A, Stoller G
and Campochiaro PA: Blockade of sphingosine-1-phosphate reduces
macrophage influx and retinal and choroidal neovascularization. J
Cell Physiol. 218:192–198. 2009. View Article : Google Scholar
|
|
80
|
Maines LW, French KJ, Wolpert EB,
Antonetti DA and Smith CD: Pharmacologic manipulation of
sphingosine kinase in retinal endothelial cells: Implications for
angiogenic ocular diseases. Invest Ophthalmol Vis Sci.
47:5022–5031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lan T, Liu W, Xie X, Xu S, Huang K, Peng
J, Shen X, Liu P, Wang L, Xia P and Huang H: Sphingosine kinase-1
pathway mediates high glucose-induced fibronectin expression in
glomerular mesangial cells. Mol Endocrinol. 25:2094–2105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Katsuma S, Hada Y, Ueda T, Shiojima S,
Hirasawa A, Tanoue A, Takagaki K, Ohgi T, Yano J and Tsujimoto G:
Signalling mechanisms in sphingosine 1-phosphate-promoted mesangial
cell proliferation. Genes Cells. 7:1217–1230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Klawitter S, Hofmann LP, Pfeilschifter J
and Huwiler A: Extracellular nucleotides induce migration of renal
mesangial cells by upregulating sphingosine kinase-1 expression and
activity. Br J Pharmacol. 150:271–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xin C, Ren S, Kleuser B, Shabahang S,
Eberhardt W, Radeke H, Schäfer-Korting M, Pfeilschifter J and
Huwiler A: Sphingosine 1-phosphate cross-activates the Smad
signaling cascade and mimics transforming growth
factor-beta-induced cell responses. J Biol Chem. 279:35255–35262.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yaghobian D, Don AS, Yaghobian S, Chen X,
Pollock CA and Saad S: Increased sphingosine 1-phosphate mediates
inflammation and fibrosis in tubular injury in diabetic
nephropathy. Clin Exp Pharmacol Physiol. 43:56–66. 2016. View Article : Google Scholar
|
|
87
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Geoffroy K, Troncy L, Wiernsperger N,
Lagarde M and El Bawab S: Glomerular proliferation during early
stages of diabetic nephropathy is associated with local increase of
sphingosine-1-phosphate levels. FEBS Lett. 579:1249–1254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lan T, Shen X, Liu P, Liu W, Xu S, Xie X,
Jiang Q, Li W and Huang H: Berberine ameliorates renal injury in
diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P
signaling pathway. Arch Biochem Biophys. 502:112–120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Huang K, Huang J, Chen C, Hao J, Wang S,
Huang J, Liu P and Huang H: AP-1 regulates sphingosine kinase 1
expression in a positive feedback manner in glomerular mesangial
cells exposed to high glucose. Cell Signal. 26:629–638. 2014.
View Article : Google Scholar
|
|
91
|
Liu W, Lan T, Xie X, Huang K, Peng J,
Huang J, Shen X, Liu P and Huang H: S1P2 receptor mediates
sphingosine-1-phosphate-induced fibronectin expression via MAPK
signaling pathway in mesangial cells under high glucose condition.
Exp Cell Res. 318:936–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Imasawa T, Kitamura H, Ohkawa R, Satoh Y,
Miyashita A and Yatomi Y: Unbalanced expression of sphingosine
1-phosphate receptors in diabetic nephropathy. Exp Toxicol Pathol.
62:53–60. 2010. View Article : Google Scholar
|
|
93
|
Xia P, Wang L, Gamble JR and Vadas MA:
Activation of sphingosine kinase by tumor necrosis factor-alpha
inhibits apoptosis in human endothelial cells. J Biol Chem.
274:34499–34505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Vessey DA, Kelley M, Li L, Huang Y, Zhou
HZ, Zhu BQ and Karliner JS: Role of sphingosine kinase activity in
protection of heart against ischemia reperfusion injury. Med Sci
Monit. 12:BR318–BR324. 2006.PubMed/NCBI
|
|
95
|
Jin ZQ and Karliner JS: Low dose N,
N-dimethylsphingosine is cardioprotective and activates cytosolic
sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc
Res. 71:725–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jin ZQ, Goetzl EJ and Karliner JS:
Sphingosine kinase activation mediates ischemic preconditioning in
murine heart. Circulation. 110:1980–1989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Besler C, Heinrich K, Rohrer L, Doerries
C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N,
et al: Mechanisms underlying adverse effects of HDL on
eNOS-activating pathways in patients with coronary artery disease.
J Clin Invest. 121:2693–2708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Park SW, Kim M, Kim JY, Brown KM, Haase
VH, D'Agati VD and Lee HT: Proximal tubule sphingosine kinase-1 has
a critical role in A1 adenosine receptor-mediated renal protection
from ischemia. Kidney Int. 82:878–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Paneni F, Beckman JA, Creager MA and
Cosentino F: Diabetes and vascular disease: Pathophysiology,
clinical consequences, and medical therapy: Part I. Eur Heart J.
34:2436–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Grundy SM, Benjamin IJ, Burke GL, Chait A,
Eckel RH, Howard BV, Mitch W, Smith SC Jr and Sowers JR: Diabetes
and cardiovascular disease: A statement for healthcare
professionals from the American Heart Association. Circulation.
100:1134–1146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Schnell O, Cappuccio F, Genovese S, Standl
E, Valensi P and Ceriello A: Type 1 diabetes and cardiovascular
disease. Cardiovasc Diabetol. 12:1562013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fioretto P, Dodson PM, Ziegler D and
Rosenson RS: Residual microvascular risk in diabetes: Unmet needs
and future directions. Nat Rev Endocrinol. 6:19–25. 2010.
View Article : Google Scholar
|
|
103
|
Rosenberg DE, Jabbour SA and Goldstein BJ:
Insulin resistance, diabetes and cardiovascular risk: Approaches to
treatment. Diabetes Obes Metab. 7:642–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Keul P, Sattler K and Levkau B: HDL and
its sphingosine-1-phosphate content in cardioprotection. Heart Fail
Rev. 12:301–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Karliner JS: Sphingosine kinase regulation
and cardioprotection. Cardiovasc Res. 82:184–192. 2009. View Article : Google Scholar :
|
|
107
|
Karliner JS: Sphingosine kinase and
sphingosine 1-phosphate in the heart: A decade of progress. Biochim
Biophys Acta. 1831:203–212. 2013. View Article : Google Scholar
|
|
108
|
Whetzel AM, Bolick DT and Hedrick CC:
Sphingosine-1-phosphate inhibits high glucose-mediated ERK1/2
action in endothelium through induction of MAP kinase
phosphatase-3. Am J Physiol Cell Physiol. 296:C339–C345. 2009.
View Article : Google Scholar :
|
|
109
|
Whetzel AM, Bolick DT, Srinivasan S,
Macdonald TL, Morris MA, Ley K and Hedrick CC: Sphingosine-1
phosphate prevents monocyte/endothelial interactions in type 1
diabetic NOD mice through activation of the S1P1 receptor. Circ
Res. 99:731–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Khafaji HA and Suwaidi JM: Atypical
presentation of acute and chronic coronary artery disease in
diabetics. World J Cardiol. 6:802–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jin ZQ, Karliner JS and Vessey DA:
Ischaemic postconditioning protects isolated mouse hearts against
ischaemia/reperfusion injury via sphingosine kinase isoform-1
activation. Cardiovasc Res. 79:134–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Vessey DA, Kelley M, Li L and Huang Y:
Sphingosine protects aging hearts from ischemia/reperfusion injury:
Superiority to sphingosine 1-phosphate and ischemic pre- and
post-conditioning. Oxid Med Cell Longev. 2:146–151. 2009.
View Article : Google Scholar :
|
|
113
|
Bonder CS, Sun WY, Matthews T, Cassano C,
Li X, Ramshaw HS, Pitson SM, Lopez AF, Coates PT, Proia RL, et al:
Sphingosine kinase regulates the rate of endothelial progenitor
cell differentiation. Blood. 113:2108–2117. 2009. View Article : Google Scholar :
|
|
114
|
Yu H, Yuan L, Xu M, Zhang Z and Duan H:
Sphingosine kinase 1 improves cutaneous wound healing in diabetic
rats. Injury. 45:1054–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Furuya H, Wada M, Shimizu Y, Yamada PM,
Hannun YA, Obeid LM and Kawamori T: Effect of sphingosine kinase 1
inhibition on blood pressure. FASEB J. 27:656–664. 2013. View Article : Google Scholar :
|
|
116
|
Igarashi J and Michel T: Sphingosine
1-phosphate and isoform-specific activation of phosphoinositide
3-kinase beta. Evidence for divergence and convergence of
receptor-regulated endothelial nitric-oxide synthase signaling
pathways. J Biol Chem. 276:36281–36288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
De Palma C, Meacci E, Perrotta C, Bruni P
and Clementi E: Endothelial nitric oxide synthase activation by
tumor necrosis factor alpha through neutral sphingomyelinase 2,
sphingosine kinase 1, and sphingosine 1 phosphate receptors: A
novel pathway relevant to the pathophysiology of endothelium.
Arterioscler Thromb Vasc Biol. 26:99–105. 2006. View Article : Google Scholar
|
|
118
|
Yin Z, Fan L, Wei L, Gao H, Zhang R, Tao
L, Cao F and Wang H: FTY720 protects cardiac microvessels of
diabetes: A critical role of S1P1/3 in diabetic heart disease. PLoS
One. 7:e429002012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sukocheva O, Wadham C, Gamble J and Xia P:
Sphingosine-1-phosphate receptor 1 transmits estrogens' effects in
endothelial cells. Steroids. 104:237–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Margolis KL, Bonds DE, Rodabough RJ,
Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J and
Howard BV; Women's Health Initiative Investigators: Effect of
oestrogen plus progestin on the incidence of diabetes in
postmenopausal women: Results from the Women's Health Initiative
Hormone Trial. Diabetologia. 47:1175–1187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Russo SB, Ross JS and Cowart LA:
Sphingolipids in obesity, type 2 diabetes, and metabolic disease.
Handb Exp Pharmacol. 216:373–401. 2013. View Article : Google Scholar
|
|
122
|
Kontush A and Chapman MJ: Functionally
defective high-density lipoprotein: A new therapeutic target at the
crossroads of dyslipidemia, inflammation, and atherosclerosis.
Pharmacol Rev. 58:342–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Barter PJ, Puranik R and Rye KA: New
insights into the role of HDL as an anti-inflammatory agent in the
prevention of cardiovascular disease. Curr Cardiol Rep. 9:493–498.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
deGoma EM, deGoma RL and Rader DJ: Beyond
high-density lipoprotein cholesterol levels evaluating high-density
lipoprotein function as influenced by novel therapeutic approaches.
J Am Coll Cardiol. 51:2199–2211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Levkau B: HDL-S1P: Cardiovascular
functions, disease-associated alterations, and therapeutic
applications. Front Pharmacol. 6:2432015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tong X, Peng H, Liu D, Ji L, Niu C, Ren J,
Pan B, Hu J, Zheng L and Huang Y: High-density lipoprotein of
patients with type 2 diabetes mellitus upregulates cyclooxgenase-2
expression and prostacyclin I-2 release in endothelial cells:
Relationship with HDL-associated sphingosine-1-phosphate.
Cardiovasc Diabetol. 12:272013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tong X, Lv P, Mathew AV, Liu D, Niu C,
Wang Y, Ji L, Li J, Fu Z, Pan B, et al: The compensatory enrichment
of sphingosine-1-phosphate harbored on glycated high-density
lipoprotein restores endothelial protective function in type 2
diabetes mellitus. Cardiovasc Diabetol. 13:822014. View Article : Google Scholar
|
|
128
|
Zhu D, Sreekumar PG, Hinton DR and Kannan
R: Expression and regulation of enzymes in the ceramide metabolic
pathway in human retinal pigment epithelial cells and their
relevance to retinal degeneration. Vision Res. 50:643–651. 2010.
View Article : Google Scholar :
|
|
129
|
Mizugishi K, Yamashita T, Olivera A,
Miller GF, Spiegel S and Proia RL: Essential role for sphingosine
kinases in neural and vascular development. Mol Cell Biol.
25:11113–11121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tsuji T, Inoue M, Yoshida Y, Fujita T,
Kaino Y and Kohno T: Therapeutic approach for type 1 diabetes
mellitus using the novel immunomodulator FTY720 (fingolimod) in
combination with once-daily injection of insulin glargine in
non-obese diabetic mice. J Diabetes Investig. 3:132–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gonzalez-Cabrera PJ, Brown S, Studer SM
and Rosen H: S1P signaling: new therapies and opportunities.
F1000Prime Rep. 6:1092014.
|