Introduction
Infantile hemangioma (IH), which is a benign
vascular neoplasm, is the most common tumor affecting infants,
resulting from the abnormal proliferation of endothelial cells and
pericytes (1). A previous study
demonstrated that IH often exhibits diverse and dramatic clinical
behaviors (1). The majority of
IHs are self-limiting, while some require specific treatment
(2). Of note, the majority of
lesions exhibit no potential for complications or aggressiveness
(1). If left untreated, they are
characterized by a rapid growth phase during the first year of
life, which is then followed by slow involution spontaneously
(3,4). Conversely, approximately 10% of IHs
exhibit rapid growth, and can have exhibit destructive, disfiguring
and even vision- or life-threatening consequences, such as
telangiectasias, fibro-fatty tissue, scars, excessive atrophic skin
and pigment changes (1,5).
Currently, the non-selective β-adrenergic-blocker,
propranolol, is the preferred treatment for problematic
proliferating IHs, which has been identified as a safe and
effective treatment option for achieving a significant reduction in
the size of IHs and lead to a considerable shortening of the
natural course of IHs without any observed adverse effects
(6–8). Additionally, propranolol has been
shown to exert marked and rapid effects, particularly on IHs
involving dyspnea, palpebral occlusion, hemodynamic compromise, or
ulcerations (6). However, the
mechanisms underlying the effects of propranolol have not yet been
fully elucidated. The mechanisms of action of propranolol may not
only involve a single mechanism, but rather to a combination of
events.
MicroRNAs (miRNAs or miRs) are functional non-coding
RNAs approximately 18–25 nucleotides in length, which can
post-transcriptionally regulate mRNA gene expression by acting on
the 3′-untranslated region (3′-UTR) of mRNAs (9). miRNAs participate in essential
biological processes, including development, proliferation,
cellular differentiation, stress responses, metabolism and
apoptosis, as well as tumor initiation and progression (10,11). Moreover, miRNAs play important
roles in both tumor suppression and oncogene regulation and exhibit
complex patterns of tissue- and disease-specific expression, having
the ability to regulate many targets that are crucial to the
carcinogenic process (11).
It has been previously demonstrated that propranolol
induces the downregulation of miR-1 in a rat model of myocardial
infarction (12), and to also the
induce the downregulation of miR-382 in rat hearts (9). However, whether propranolol can
regulate miR-1 and miR-382 expression in IH remains unknown. This
sparked our interest in the role of these two miRNAs in
propranolol-treated IHs. In the present study, we found that
miR-382 was upregulated in XPTS-1 cells constructed in our
laboratory when compared to the normal control, while miR-1
expression was not significantly altered. Furthermore, miR-382 was
found to promote the progression of IHs and was further identified
to be a target miRNA of propranolol in XPTS-1 cells, which was
downregulated by propranolol treatment. Moreover, the migratory and
proliferative ability of the XPTS-1 cells was inhibited by
propranolol, and propranolol also promoted XTPS-1 cell apoptosis.
The restoration of miR-382 expression promoted XTPS-1 cell
migration and proliferation by targeting the phosphatase and tensin
homolog (PTEN)-mediated AKT/mammalian target of rapamycin (mTOR)
pathway. Taken together, our findings indicate that the
downregulation of miR-382 by propranolol inhibits the progression
of IHs via the PTEN-mediated AKT/mTOR pathway.
Materials and methods
Cell culture
A hemangioma-derived endothelial cell line isolated
from proliferating IH tissues was previously established in our
laboratory and named XPTS-1 (13). The XPTS-1 cells were cultured in
RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA),
10 ng/ml epidermal growth factor, 100 U/ml penicillin and 100
µg/ml streptomycin (Sigma-Aldrich) in a humidified
atmosphere chamber containing 5% CO2 at 37°C. Human
umbilical vein endothelial cells (HUVECs) were purchased from the
Cell Resource Center, Shanghai Institute of Biochemistry and Cell
Biology at the Chinese Academy of Sciences (Shanghai, China) and
used as a control.
Treatment of XPTS-1 cells with
propranolol
XPTS-1 cells (2.5×105 cells/well) were
seeded in 6-well plates in RPMI-1640 medium with 10% FBS, 10 ng/ml
epidermal growth factor, 100 U/ml penicillin and 100 µg/ml
streptomycin. In order to examine the effects of propranolol on
XPTS-1 cells, various final concentrations of propranolol (0, 30,
60, 90 and 120 µM) (Sigma-Aldrich) were added to the medium.
Following treatment for 48 h, the cells were collected and used to
analyze the expression of miR-382 by reverse
transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA and miRNA were extracted from the XPTS-1
cell line and the HUVECs using TRIzol reagent and a PureLink miRNA
Isolation kit (Invitrogen). Following quantification using a
biophotometer (Eppendorf BioPhotometer Plus; Eppendorf, Hamburg,
Germany), the extracted total RNA was reverse transcribed using a
High-Capacity cDNA Archive kit and a TaqMan miRNA reverse
transcription kit (both from Applied Biosystems, Foster City, CA,
USA) according to the manufacturers' instructions. The reverse
transcription products were mixed with TaqMan universal PCR Master
Mix II, and quantitative PCR (qPCR) was performed on an Applied
Biosystems Prism 7500 Fast Sequence Detection system (Applied
Biosystems). The primers for mRNA were as follows: miR-1 (assay ID:
002222; Applied Biosystems), miR-382 (miScript miR-382 primer assay
kit; Qiagen, Inc., Valencia, CA, USA), PTEN (forward, 5′-GGA CGA
ACT GGT GTA ATG AT-3′ and reverse, 5′-TCT ACT GTT GTG AAG TAC
AGC-3′). RNU48 or β-actin were used as endogenous controls. All
reactions were carried out in triplicate. The mRNA and miRNA
expression levels were determined using the 2−∆Ct
method.
Cell transfection
To overexpress miRNA-382, after cloning the mature
miR-382 or its negative control miRNA (miR-control) into the
pENTR™/H1/TO vector (Invitrogen), the XPTS-1 cells treated with or
without 120 µM propranolol were transfected. Cells without
transfection were used as control group. Following transfection for
24 h, for miR-382 or mRNA PTEN analysis, total RNA was extracted
using the methods described above. Total proteins were separated
for western blot analysis experiments.
Transwell cell migration assay
Migration assays were performed using Transwell
inserts (8.0 µm pore size). For the migration assay, the
XPTS-1 cells were treated with various final concentrations of
propranolol (0, 30, 60, 90 or 120 µM). Subsequently,
approximately 2×104 cells/well in RPMI-1640 medium
containing 10% FBS, 10 ng/ml epidermal growth factor, 100 U/ml
penicillin, 100 µg/ml streptomycin and 10 µg/ml
mitomycin C were added to the upper wells. Here, mitomycin C was
used to suppress cell proliferation. The same medium without cells
was added to the lower wells. Cells that had migrated through the
filter after 24, 36 or 48 h were stained with 0.1% crystal violet
(Sigma-Aldrich) and counted using a phase contrast microscope
(Eclipse TE2000-U; Nikon, Tokyo, Japan), as previously described
(14). The cell migration rate at
48 h was obtained according to the following formula: rate = the
number of migrated cells/the number of total cells. The same
experiments were carried out on miR-382- or miR-control-transfected
XPTS-1 cells, which were treated with or without 120 µM
propranolol for 48 h.
Bromodeoxyuridine (BrdU) assay
The BrdU Cell Proliferation Assay kit (Cat. no 2750;
Millipore Corp., Bedford, MA, USA) was used to detect cell
proliferation ability according to the manufacturer's instructions.
The XPTS-1 cells treated with 0 or 120 µM propranolol were
synchronized and plated in 96-wells (3×103 cells/well)
in RPMI-1640 medium and 10 µl BrdU solution was added
followed by incubation for 24, 36 and 48 h. Subsequently, 100
µl/well of the fixing solution were added followed by
incubation for 15 min. This was followed by the addition of 100
µl/well of prediluted mouse monoclonal BrdU detection
antibody solution (Part no. 2750c) and further incubation for 1 h.
Subsequently, 100 µl/well of prepared HRP-conjugated goat
anti-mouse IgG secondary antibody (Part no. 2750e) was added
followed by incubation for 30 min. Finally, 100 µl of TMB
substrate were added before being incubated for 30 min. The amount
of BrdU incorporated into the cells was determined at 450 nm using
a microplate reader (Benchmark 550; Bio-Rad, Hercules, CA, USA).
The same experiments were carried out on miR-382- or
miR-control-transfected XPTS-1 cells, which were treated with or
without 120 µM propranolol for 48 h.
Apoptosis assay
Cell apoptosis was assessed by Annexin V-propidium
iodide (PI). Following treatment with propranolol (0 or 120
µM) for 48 h, floating as well as adherent cells were
harvested by trypsinization (trypsin from Sigma-Aldrich) and washed
with PBS. The levels of Annexin V and PI expression in the XPTS-1
cells were detected using the Annexin V-isothiocyanate (FITC)
Apoptosis Detection kit (Invitrogen) according to the
manufacturer's instructions. The results were analyzed using a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) within 1 h. The same experiments were carried out on miR-382-
or miR-control-transfected XPTS-1 cells, which were treated with or
without 120 µM propranolol for 48 h.
Western blot analysis
The cultured XPTS-1 cells were lysed with RIPA
buffer added with a cocktail of protease inhibitors (Roche
Diagnostics, Indianapolis, IN, USA) for protein analysis. The
protein concentration was determined using a BCA kit
(Sigma-Aldrich). Target proteins were analyzed with immunoblotting
following transfer onto PVDF membranes (Sigma-Aldrich) on an
SDS-PAGE gel. All the primary or secondary antibodies were
purchased from Abcam (Cambridge, MA, USA). Primary antibodies and
anti-β-actin antibodies were added at a dilution of 1:4,000
overnight at 4°C. HRP-conjugated secondary antibodies (ab6721) were
used at 1:10,000 at room temperature for 1 h. The reactive bands
were detected with enhanced chemiluminescence (ECL, GE Healthcare,
Buckinghamshire, UK) according to the manufacturer's instructions
and the relative levels of each protein to β-actin were analyzed.
Quantitative analysis of the bands was determined using Gel-Pro
Analyzer 4.0 software (Media Cybernetics, Bethesda, MD, USA). The
primary antibodies used were as follows: PTEN (ab32199); p-AKT
(p-AKT1, phospho S473, ab81283); AKT (AKT1, ab32505); p-mTOR
(phospho S2448, ab109268); mTOR (ab32028); p-p70S6K (phospho T421 +
S424, ab32525); p70S6K (ab32359).
Statistical analysis
Data were obtained from 3 repeated assays and are
expressed as the means ± standard deviation (SD). Differences
between groups were considered statistically significant with
values of P<0.01 or P<0.05 and were analyzed using the
Student's t-test. The western blot analyses experiments were
performed several times, and the results were similar in all
experiments. Ultimately, the images of the blots with the best
quality were selected for use.
Results
Expression of miR-382 in XPTS-1
cells
To validate the role of miR-1 and miR-382 in
propranolol-treated IHs, we first detected the level of miR-1 and
miR-382 in XPTS-1 cells constructed in our laboratory by RT-qPCR.
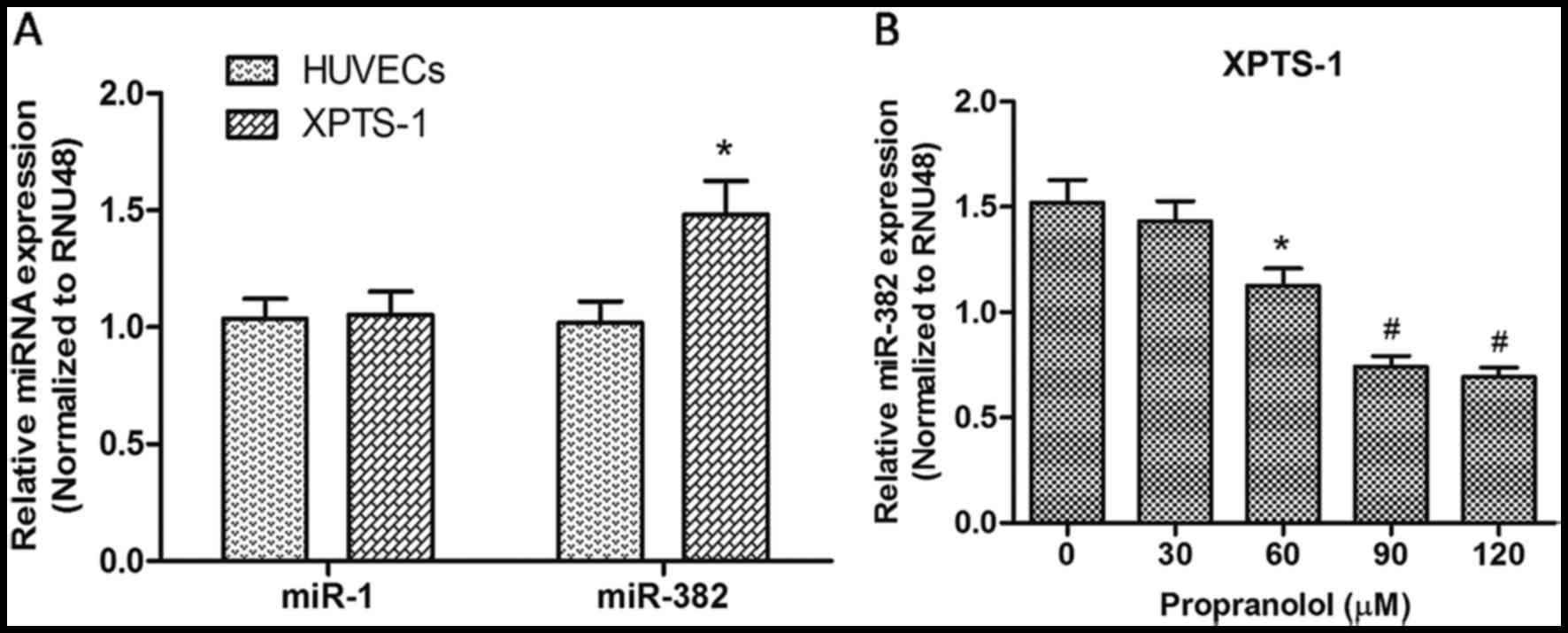
HUVECs were used as a control. As shown in Fig. 1A, the expression of miR-382 in the
XPTS-1 cells was significantly upregulated when compared with that
in HUVECs (P<0.05); however, no significant differences were
observed in miR-1 expression was between the two cell lines
(P>0.05). These results suggest that miR-382 is a relevant miRNA
in the progression of IH. Following treatment with propranolol at
various concentrations for 48 h, the level of miR-382 was
re-detected by RT-qPCR. As shown in Fig. 1B, the expression of miR-382 was
downregulated gradually with the increasing concentrations of
propranolol, which indicated that propranolol decreased the
expression of miR-382 in XPTS-1 cells.
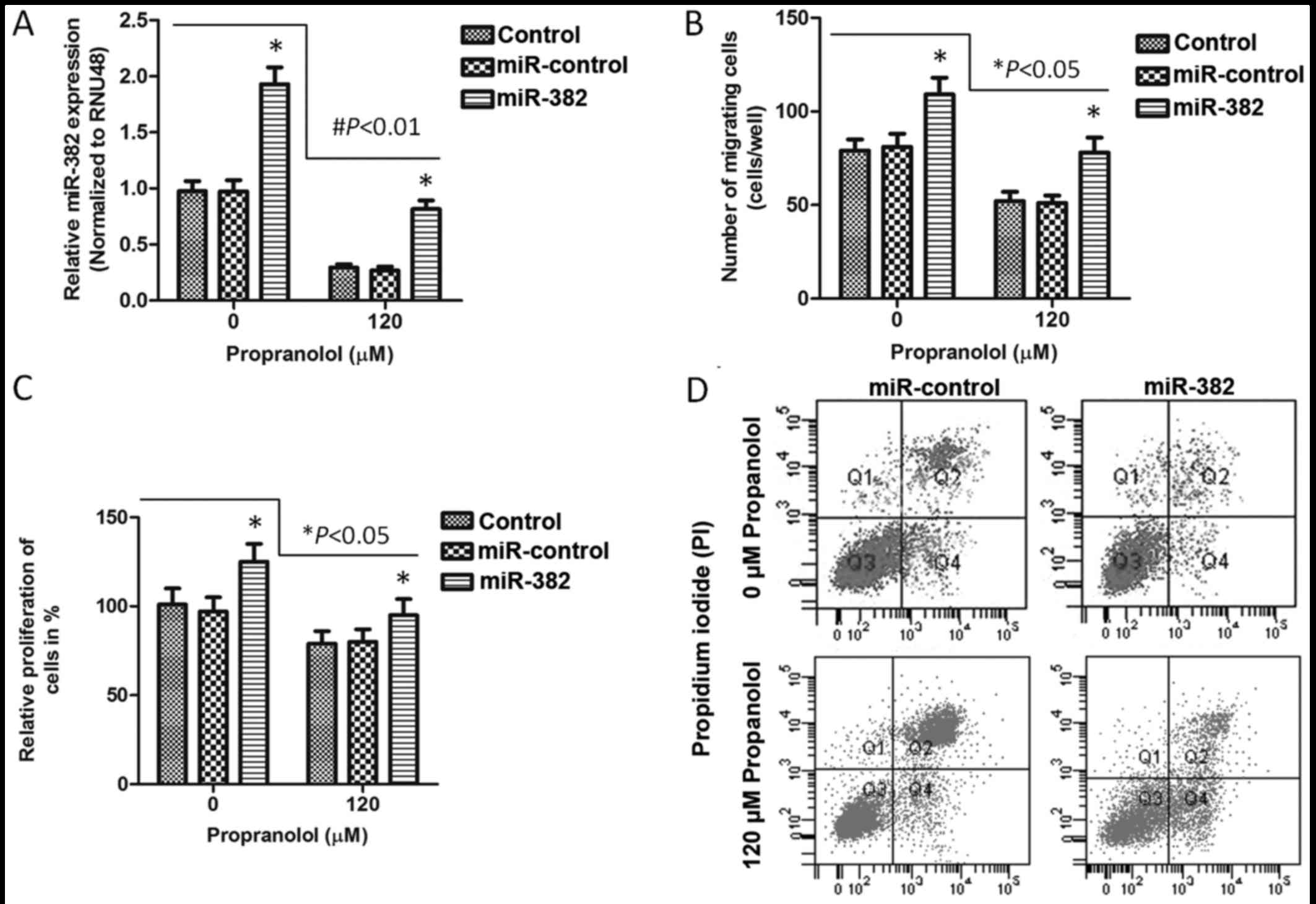
Propranolol inhibits XPTS-1 cell
migration and proliferation, and promotes cell apoptosis
The cell migratory ability of the
propranolol-treated XPTS-1 cells was evaluated by Transwell
migration assay. As shown by our results, the number of migrated
cells was significantly reduced with the increasing concentrations
of propranolol (60 µM, P<0.05; 90 and 120 µM,
P<0.01) for each time point (Fig.
2A). In addition, the cell migration rate at 48 h was
calculated and shown in Fig. 2B,
which further verified the inhibitory effects of propranolol on
XPTS-1 cell migration. On the basis of this data, cell
proliferation and apoptosis were detected by BrdU incorporation
assay and flow cytometry, respectively in the XPTS-1 cells treated
with 120 µM propranolol. As shown in Fig. 2C, the cell proliferative ability
was markedly decreased by propranolol as time progressed (24, 36
and 48 h; P<0.05), while cell apoptosis was significantly
increased following treatment with propranolol (Fig. 2D and E). These results suggested
that propranolol inhibited XPTS-1 cell migration and proliferation
in a time- and concentration-dependent manner, and promoted XPTS-1
cell apoptosis. However, whether these effects are associated with
the downregulation miR-382 remained unclear.
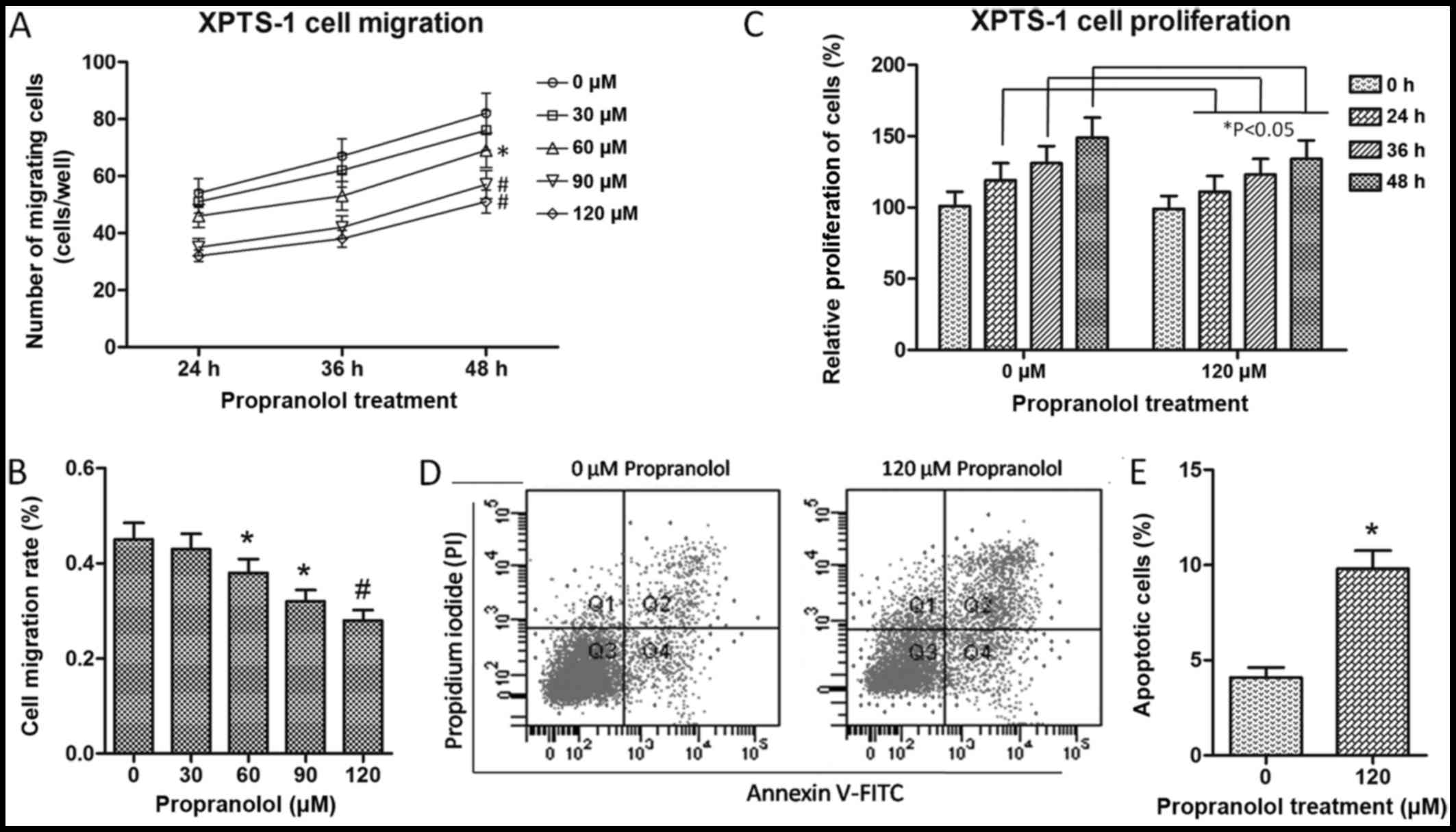 | Figure 2XPTS-1 cell migration, proliferation
and apoptosis. (A) Migration of XPTS-1 cells treated with
propranolol. (B) XPTS-1 cell migration rate. Cell migration rate
was calculated at 48 h following propranolol treatment.
#P<0.01 and *P<0.05 when compared with
0 µM propranolol, as shown by the Student's t-test. (C)
Proliferation of XPTS-1 cells treated with propranolol.
*P<0.05 when compared with the corresponding time
point, as shown by the Student's t-test. Cell migration and
proliferation were examined by Transwell migration assay and
bromodeoxyuridine (BrdU) incorporation assay, respectively. Each
assay was repeated in 3 independent experiments. Data are presented
as the means ± SD. (D) The Annexin V and propidium iodide
(PI)-positive populations of XPTS-1 cells were analyzed by flow
cytometry. XPTS-1 cells were pre-treated with 0 and 120 µM
propranolol for 48 h, respectively. The 4 quandrants in the flow
cytometry plots indicate the following: Q1, necrotic cells; Q2,
late apoptotic cells; Q3, live cells; Q4, early apoptotic cells.
(E) The quantification of apoptotic XPTS-1 cells.
*P<0.05 when compared with 0 µM propranolol,
as shown by the Student's t-test. |
Overexpression of miR-382 promotes XPTS-1
cell migration and proliferation, and inhibits cell apoptosis
In order to investigate the association between the
downregulation of miR-382 and decreased cell migration and
proliferation, and increased cell apoptosis in XPTS-1 cells treated
with propranolol, we transfected the XPTS-1 cells with an miR-382
overexpression vector, and examined the transfection efficiency by
RT-qPCR (Fig. 3A). The XPTS-1
cells were treated with or without 120 µM propranolol for 48
h. Our results revealed that the overexpression of miR-382 promoted
XPTS-1 cell migration (P<0.05; Fig. 3B) and proliferation (P<0.05;
Fig. 3C), whereas it inhibited
cell apoptosis (Fig. 3D), when
compared with the control (untransfected cells) or miR-control
group. In this study, miR-382 was found to contribute the
progression of IHs. The effects of propranolol on XPTS-1 cell
migration, proliferation and apoptosis were also examined in
miR-382 overexpressing XPTS-1 cells. Combined with the results in
Figs. 1 and 2, we concluded that propranolol
inhibited XPTS-1 cell migration and proliferation, and promoted
cell apoptosis by reducing the expression of miR-382.
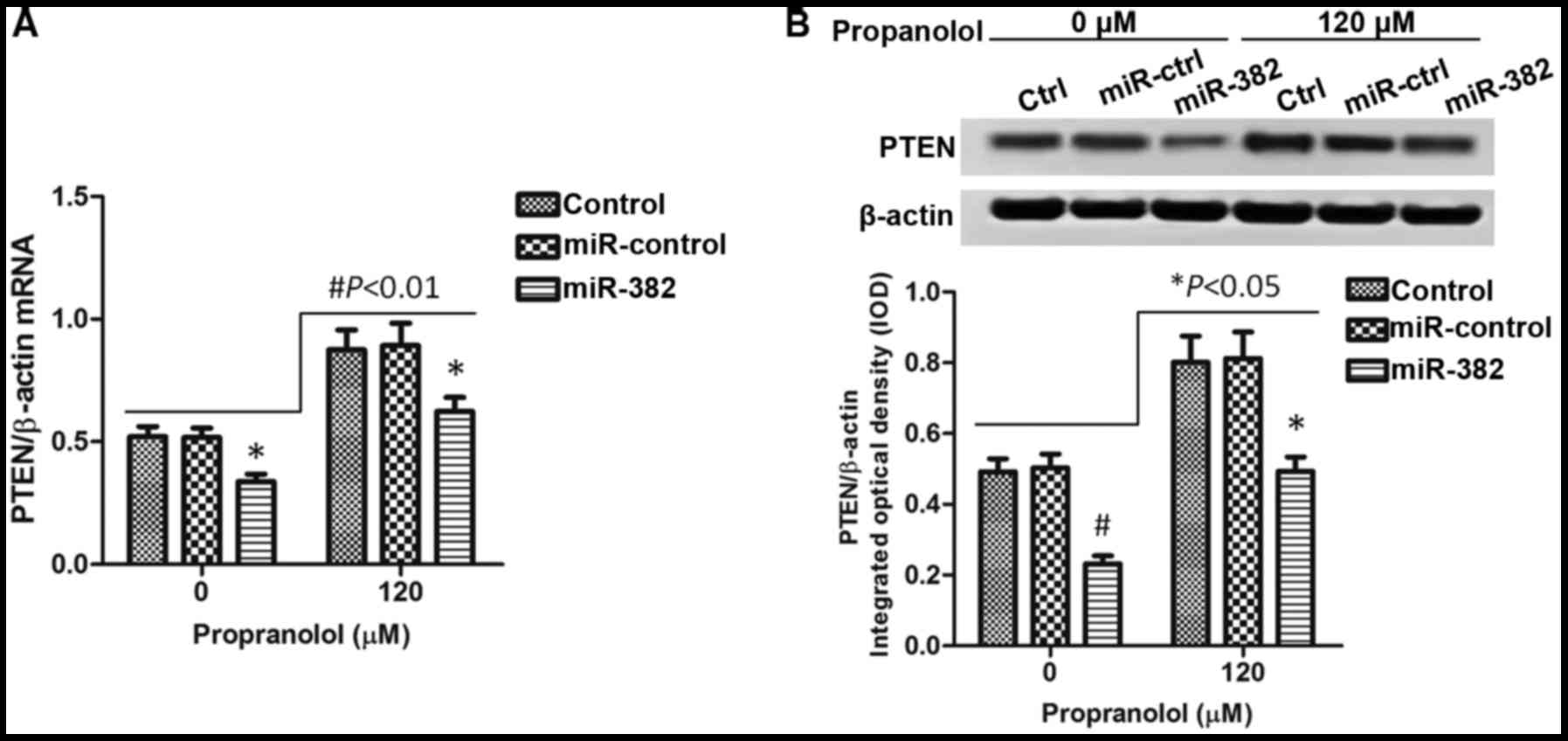
Overexpressiong of miR-382 inhibits PTEN
expression
A previous study demonstrated that miR-328
expression induced by hypoxia promotes angiogenesis and acts as an
angiogenic oncogene by suppressing PTEN (15). Based on this result, we
hypothesized that the inhibitory effects of propranolol on the
progression of IHs which involve the downregulation of miR-382 are
also associated with PTEN. To prove our hypothesis, we detected the
expression of PTEN in XPTS-1 cells treated with or without
propranolol. As shown in Fig. 4A,
the overexpression of miR-382 significantly downregulated PTEN
expression when compared with the control (untransfected) or
miR-control group (P<0.05). In the XPTS-1 cells treated with
propranolol, which was shown to inhibit miR-382 expression, we
observed that the PTEN expression level increased (P<0.01),
which is accordance with our expectations. Hence, PTEN plays a role
in the inhibitory effects of propranolol on the progression of IHs
associated with the decreased expression of miR-382. When examining
protein expression, similar results were observed Fig. 4B.
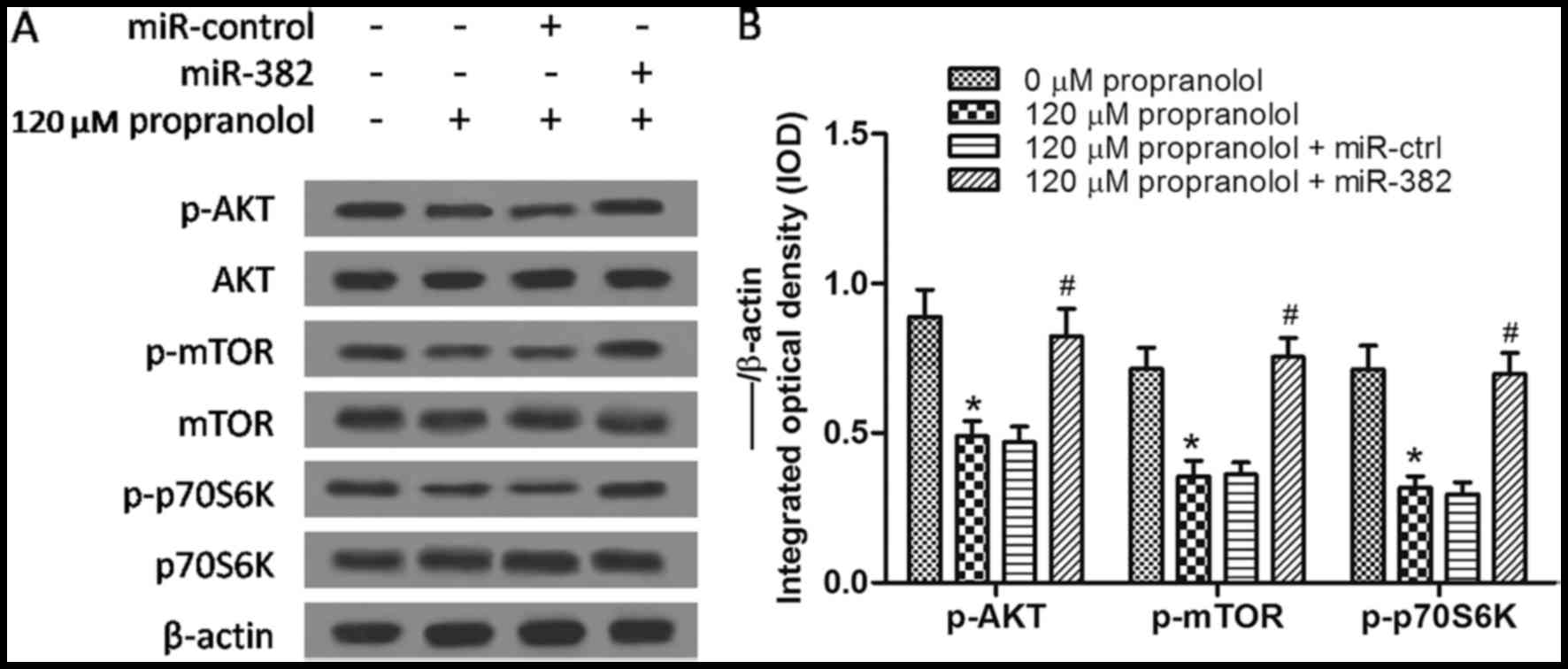
Propranolol inhibits the AKT/mTOR
signaling pathway by increasing PTEN expression
It is well known that PTEN negatively regulates the
phosphoinositide 3-kinase (PI3K) signaling pathway, which is
involved in intracellular signaling for cell growth, proliferation
and survival (16,17). As PTEN is an inhibitor of the
PI3K/AKT/mTOR signaling pathway and propranolol increases PETN
expression by decreasing miR-382, we hypothesized that the
PI3K/AKT/mTOR signaling pathway may be inhibited in
propranolol-treated XPTS-1 cells. As shown by our results of
western blot analysis, the expression of p-AKT, p-mTOR and p-p70S6K
was downregulated by propranolol (P<0.05) when compared with the
untreated group, and these effects were partly reversed by the
overexpression of miR-382 (Fig. 5A
and B). All of the above-mentioned results indicated that the
downregulation of miR-382 by propranolol inhibited the progression
of IHs via the PTEN-mediated AKT/mTOR signaling pathway.
Discussion
IH is the most common tumor affecting infants, which
is a benign vascular neoplasm (1). The majority of IHs have an
uncomplicated course and do not require treatment, while some
patients with IH experience a series of complications and require
special treatment (18). Since
2008, the efficacy of propranolol in the treatment of complicated
IHs has been discovered, and propranolol has become the primary
treatment of choice (19,20). Accumulating evidence has proven
that miRNAs participate in a variety of biological and pathological
processes. Altered miRNA expression has been implicated in
oncogenesis (21,22). In addition, propranolol has been
found to regulate miRNA expression in rat hearts (9). All of the above-meniotned findings
sparked our interest in the miRNA-related mechanisms concerning the
effect of propranolol in IHs.
In a previous study, we established the
hemangioma-derived endothelial cell line from proliferating IH
tissues and named these cells XPTS-1 (13). We first determined that miR-382
was upregulated in the XPTS-1 cells and was downregulated following
treatment with propranolol, which indicated that miR-382 is a novel
IH-relevant miRNA regulated by propranolol. We then discovered that
propranolol inhibited the migration and proliferation of XPTS-1
cells, and promote cell apoptosis. The inhibitory effect of
propranolol on tumor progression has also been reported in breast
cancer (23), intracerebral and
spinal hemangiomas (24), and
tufted angioma (25). In this
study, following transfection of the XPTS-1 cells with miR-382
overexpression vector, the inhibitory effecs of propranolol on IH
progression were reversed to a certain extent. This indicates that
propranolol exerts its inhibitory effects on IH progression by
targeting miR-382. We then proved that the PTEN-mediated AKT/mTOR
signaling pathway plays a role in this process. In the XPTS-1
cells, propranolol reduced miR-382 expression, and attenuated the
inhibitory effects of miR-382 on PTEN expression, further promoting
the suppressive effects of PTEN on the AKT/mTOR signaling pathway,
and inhibiting the migration and proliferation of these cells.
The PI3K/AKT/mTOR signaling pathway plays a key role
in cellular growth and survival, which has been implicated in the
pathogenesis of a variety of tumors (26–28). Hence, the inhibition of the
PI3K/AKT/mTOR pathway is of therapeutic interest. Although the
PI3K/AKT/mTOR signaling pathway has been predicted to be implicated
in IHs (1), the exact role of
this pathway has not yet been fully described. In the present
study, we indentified that the PTEN-mediated AKT/mTOR signaling
pathway is downstream of miR-382, which is regulated by
propranolol.
In conclusion, the findings of our study indicated
that down-regulation of miR-382 by propranolol inhibited the
progression of IHs via the PTEN-mediated AKT/mTOR signaling
pathway. Our study provides new insight and mechanisms to explain
the functions of propranolol in IHs. In the future, we aim to
conduct further research to sufficiently justify this mechanism at
the organism level in IHs.
Abbreviations:
|
IHs
|
infantile hemangiomas
|
|
miR-382
|
microRNA 382
|
|
miR-1
|
microRNA 1
|
|
PTEN
|
phosphatase and tensin homolog
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
FITC
|
isothiocyanate
|
|
FBS
|
fetal bovine serum
|
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81172589).
References
|
1
|
Ji Y, Chen S, Li K, Li L, Xu C and Xiang
B: Signaling pathways in the development of infantile hemangioma. J
Hematol Oncol. 7:132014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yilmaz L, Dangoisse C and Semaille P:
Infantile hemangioma and propranolol: a therapeutic 'revolution'.
Literature review. Rev Med Brux. 3:4479–4484. 2012.
|
|
3
|
Mulliken JB, Fishman SJ and Burrows PE:
Vascular anomalies. Curr Probl Surg. 37:517–584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drolet BA, Esterly NB and Frieden IJ:
Hemangiomas in children. N Engl J Med. 341:173–181. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margileth AM and Museles M: Cutaneous
hemangiomas in children. Diagnosis and conservative management.
JAMA. 194:523–526. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji Y, Chen S, Xu C, Li L and Xiang B: The
use of propranolol in the treatment of infantile haemangiomas: an
update on potential mechanisms of action. Br J Dermatol. 172:24–32.
2015. View Article : Google Scholar
|
|
7
|
England RW, Hardy KL, Kitajewski AM, Wong
A, Kitajewski JK, Shawber CJ and Wu JK: Propranolol promotes
accelerated and dysregulated adipogenesis in hemangioma stem cells.
Ann Plast Surg. 73(Suppl 1): S119–S124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laranjo S, Costa G, Paramés F, Freitas I,
Martins JD, Trigo C and Pinto FF: The role of propranolol in the
treatment of infantile hemangioma. Rev Port Cardiol. 33:289–295.
2014.PubMed/NCBI
|
|
9
|
Hou Y, Sun Y, Shan H, Li X, Zhang M, Zhou
X, Xing S, Sun H, Chu W, Qiao G and Lu Y: β-adrenoceptor regulates
miRNA expression in rat heart. Med Sci Monit. 18:BR309–BR314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaedcke J, Grade M, Camps J, Søkilde R,
Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi
BM, Møller S, et al: The rectal cancer microRNAome - microRNA
expression in rectal cancer and matched normal mucosa. Clin Cancer
Res. 18:4919–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slattery ML, Wolff E, Hoffman MD, Pellatt
DF, Milash B and Wolff RK: MicroRNAs and colon and rectal cancer:
differential expression by tumor location and subtype. Genes
Chromosomes Cancer. 50:196–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Zhang Y, Shan H, Pan Z, Li X, Li B,
Xu C, Zhang B, Zhang F, Dong D, et al: MicroRNA-1 downregulation by
propranolol in a rat model of myocardial infarction: a new
mechanism for ischaemic cardioprotection. Cardiovasc Res.
84:434–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li P, Xiao XE, Xu Q and Guo ZT:
Establishment of human infancy hemangioma-derived endothelial cell
line XPTS-1 and animal model of human infancy hemangioma. Zhonghua
Kou Qiang Yi Xue Za Zhi. 46:129–133. 2011.In Chinese. PubMed/NCBI
|
|
14
|
Han L, Liang XH, Chen LX, Bao SM and Yan
ZQ: SIRT1 is highly expressed in brain metastasis tissues of
non-small cell lung cancer (NSCLC) and in positive regulation of
NSCLC cell migration. Int J Clin Exp Pathol. 6:2357–2365.
2013.PubMed/NCBI
|
|
15
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Snaddon J, Parkinson EK, Craft JA,
Bartholomew C and Fulton R: Detection of functional PTEN lipid
phosphatase protein and enzyme activity in squamous cell carcinomas
of the head and neck, despite loss of heterozygosity at this locus.
Br J Cancer. 84:1630–1634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blanco-Aparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haggstrom AN, Drolet BA, Baselga E,
Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
Newell B, et al: Prospective study of infantile hemangiomas:
clinical characteristics predicting complications and treatment.
Pediatrics. 118:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drolet BA, Frommelt PC, Chamlin SL,
Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE,
Liberman L, et al: Initiation and use of propranolol for infantile
hemangioma: report of a consensus conference. Pediatrics.
131:128–140. 2013. View Article : Google Scholar :
|
|
21
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
22
|
Li W, Xie L, He X, Li J, Tu K, Wei L, Wu
J, Guo Y, Ma X, Zhang P, et al: Diagnostic and prognostic
implications of microRNAs in human hepatocellular carcinoma. Int J
Cancer. 123:1616–1622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdin AA, Soliman NA and Saied EM: Effect
of propranolol on IL-10, visfatin, Hsp70, iNOS, TLR2, and survivin
in amelioration of tumor progression and survival in Solid Ehrlich
Carcinoma-bearing mice. Pharmacol Rep. 66:1114–1121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miquel J, Bruneau B and Dupuy A:
Successful treatment of multifocal intracerebral and spinal
hemangiomas with propranolol. J Am Acad Dermatol. 70:e83–e84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto Y, Kounami S, Okuhira H, Nakamura
Y and Furukawa F: Successful treatment of tufted angioma with
propranolol. J Dermatol. 41:1120–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamming DW, Ye L, Sabatini DM and Baur JA:
Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin
Invest. 123:980–989. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benjamin D, Colombi M, Moroni C and Hall
MN: Rapamycin passes the torch: a new generation of mTOR
inhibitors. Nat Rev Drug Discov. 10:868–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|