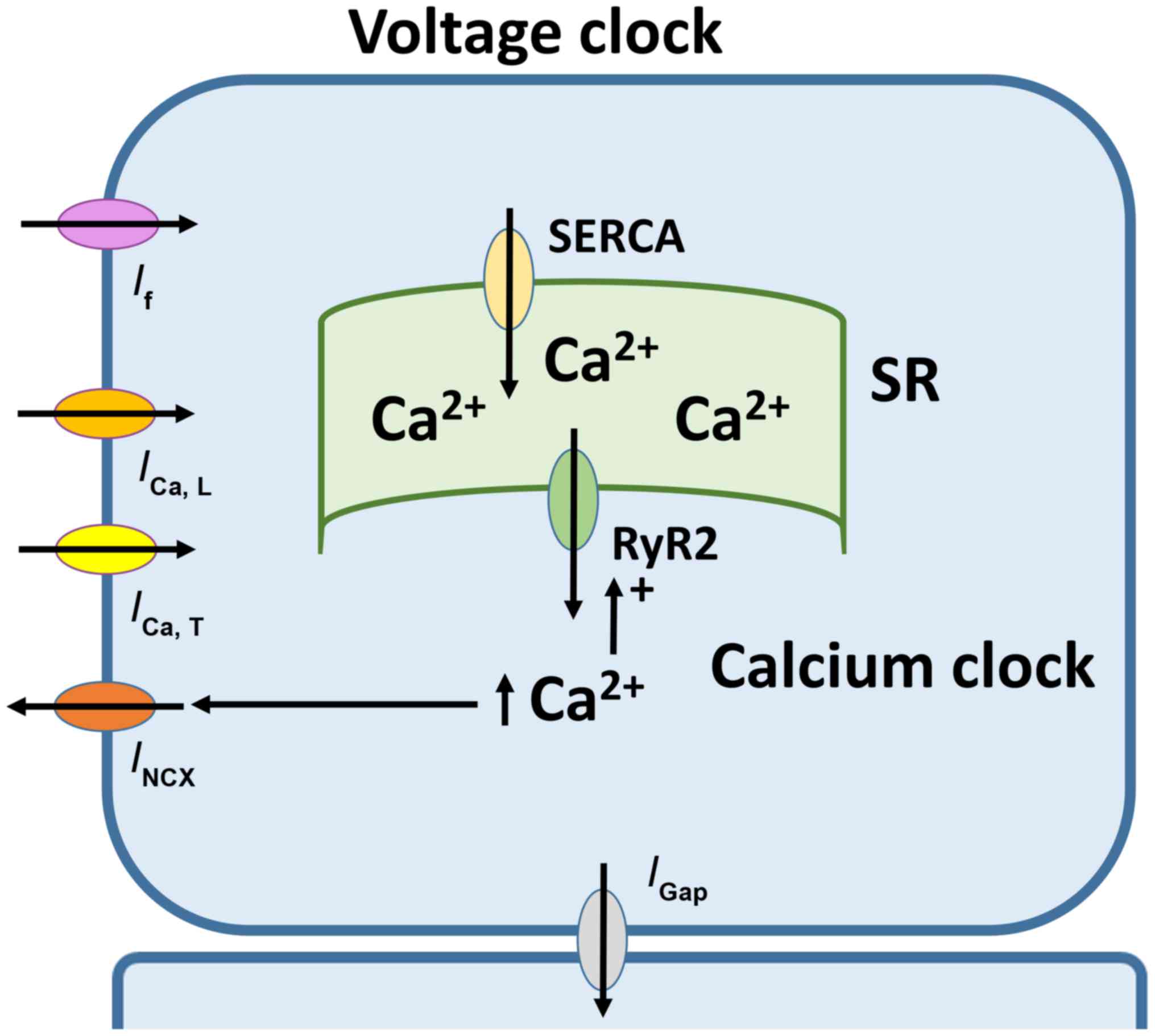
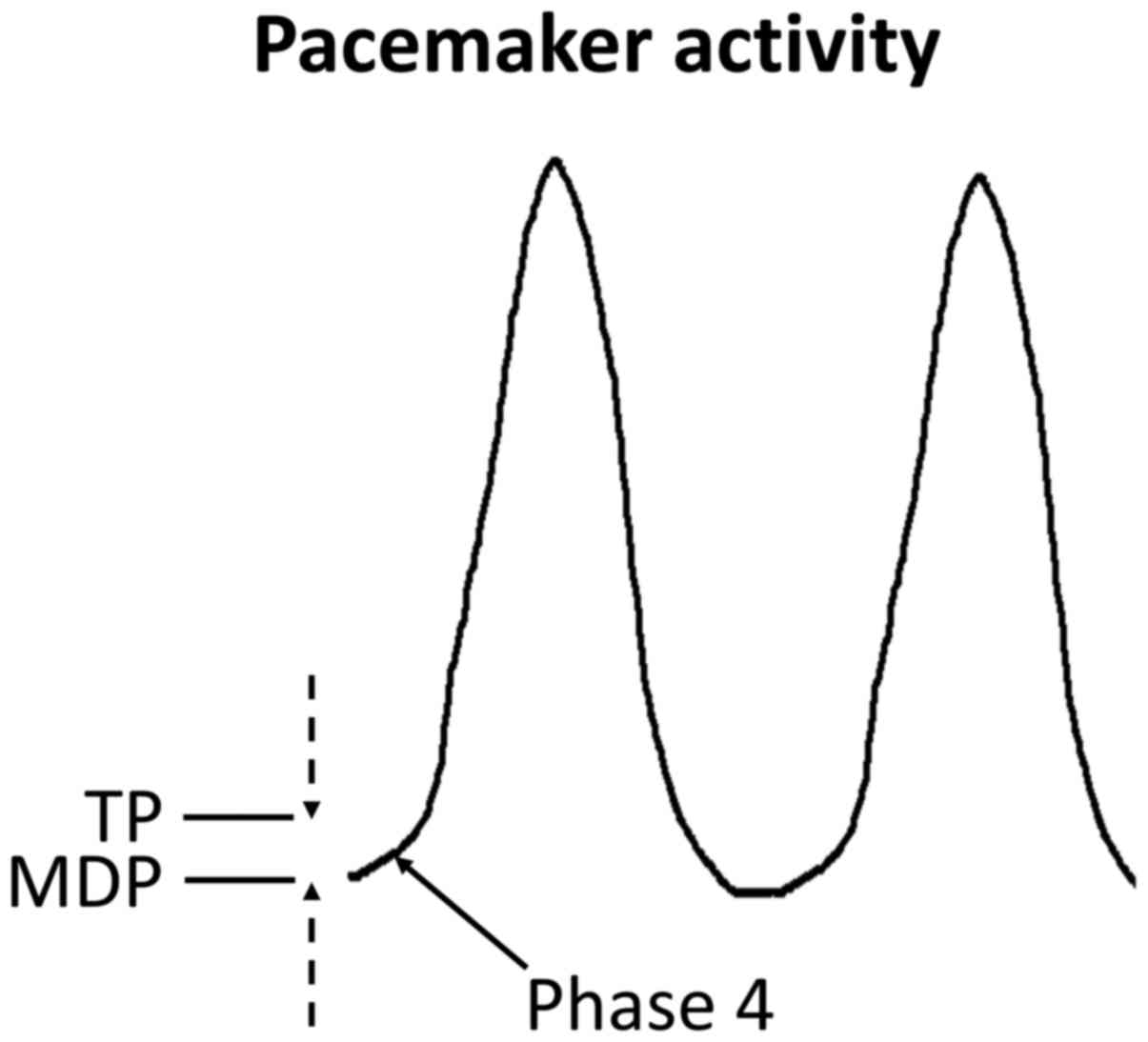
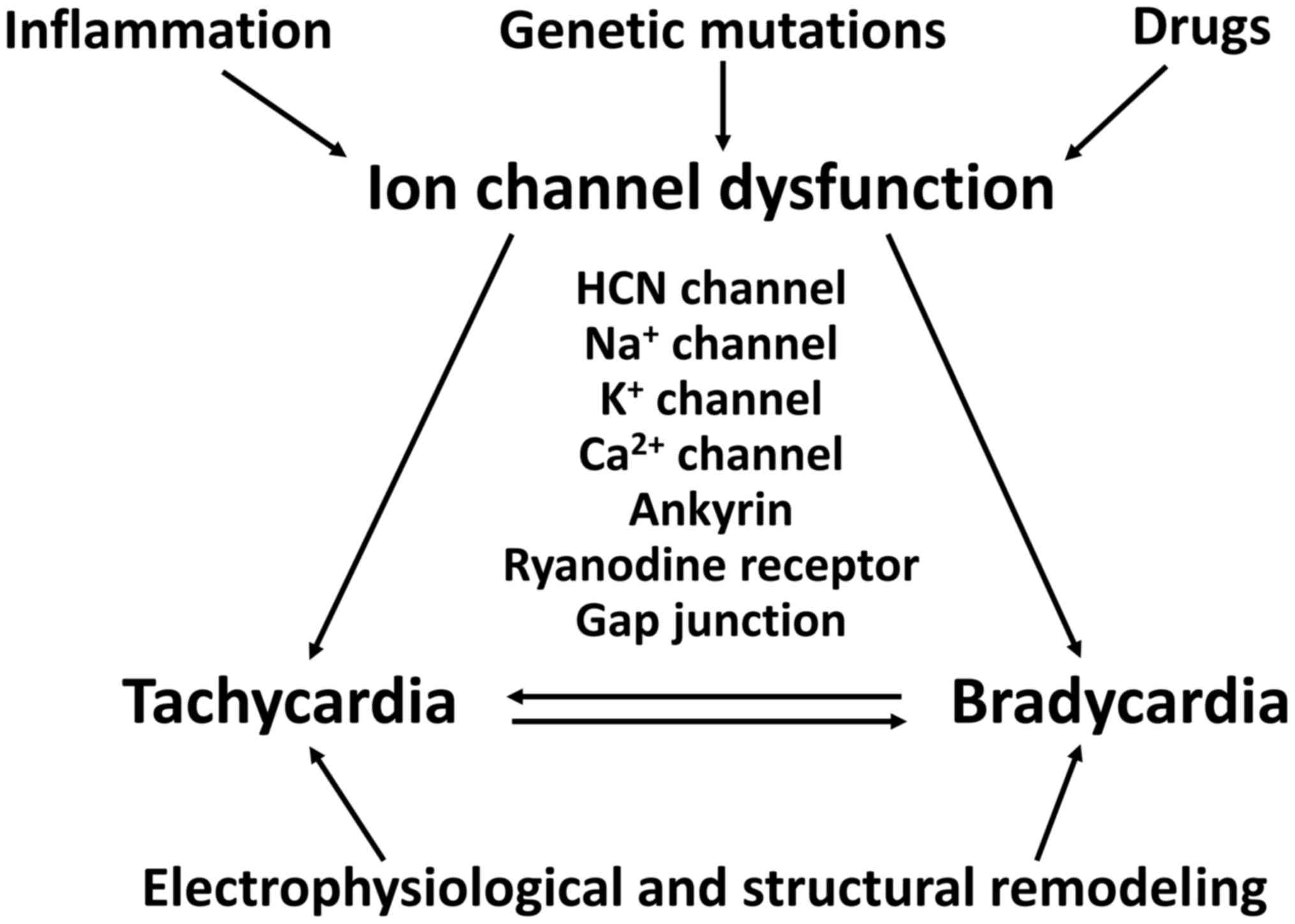
|
1
|
Ferrer MI: The sick sinus syndrome in
atrial disease. JAMA. 206:645–646. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaplan BM, Langendorf R, Lev M and Pick A:
Tachycardia-bradycardia syndrome (so-called 'sick sinus syndrome').
Pathology, mechanisms and treatment. Am J Cardiol. 31:497–508.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubenstein JJ, Schulman CL, Yurchak PM and
DeSanctis RW: Clinical spectrum of the sick sinus syndrome.
Circulation. 46:5–13. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomes JA, Kang PS, Matheson M, Gough WB Jr
and El-Sherif N: Coexistence of sick sinus rhythm and atrial
flutter-fibrillation. Circulation. 63:80–86. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bayés de Luna AJ: Bloqueo a nivel
auricular. Rev Esp Cardiol. 32:5–10. 1979.
|
|
6
|
Bayes de Luna A, Fort de Ribot R, Trilla
E, Julia J, Garcia J, Sadurni J, Riba J and Sagues F:
Electrocardiographic and vector-cardiographic study of interatrial
conduction disturbances with left atrial retrograde activation. J
Electrocardiol. 18:1–13. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bayés de Luna A, Cladellas M, Oter R,
Torner P, Guindo J, Martí V, Rivera I and Iturralde P: Interatrial
conduction block and retrograde activation of the left atrium and
paroxysmal supraventricular tachyarrhythmia. Eur Heart J.
9:1112–1118. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayés de Luna A, Oter MC and Guindo J:
Interatrial conduction block with retrograde activation of the left
atrium and paroxysmal supraventricular tachyarrhythmias: Influence
of preventive anti-arrhythmic treatment. Int J Cardiol. 22:147–150.
1989. View Article : Google Scholar
|
|
9
|
Bayés de Luna A, Guindo J, Viñolas X,
Martinez-Rubio A, Oter R and Bayés-Genís A: Third-degree
inter-atrial block and supraventricular tachyarrhythmias. Europace.
1:43–46. 1999. View Article : Google Scholar
|
|
10
|
Bayés de Luna A, Platonov P, Cosio FG,
Cygankiewicz I, Pastore C, Baranowski R, Bayés-Genis A, Guindo J,
Viñolas X, Garcia-Niebla J, et al: Interatrial blocks. A separate
entity from left atrial enlargement: A consensus report. J
Electrocardiol. 45:445–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conde D, Seoane L, Gysel M, Mitrione S,
Bayés de Luna A and Baranchuk A: Bayés' syndrome:The association
between interatrial block and supraventricular arrhythmias. Expert
Rev Cardiovasc Ther. 13:541–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baranchuk A and Bayés de Luna A: The
P-wave morphology: What does it tell us. Herzschrittmacherther
Elektrophysiol. 26:192–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baranchuk A, de Luna AB and Breithardt G:
To the Editor - The role of advanced interatrial block pattern as a
predictor of atrial fibrillation. Heart Rhythm. 13:e872016.
View Article : Google Scholar
|
|
14
|
Tse G: Both transmural dispersion of
repolarization and transmural dispersion of refractoriness are poor
predictors of arrhythmogenicity: A role for the index of Cardiac
Electrophysiological Balance (QT/QRS). J Geriatr Cardiol. In
press.
|
|
15
|
Zhao J, Liu T and Li G: Relationship
between two arrhythmias: Sinus node dysfunction and atrial
fibrillation. Arch Med Res. 45:351–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choy L, Yeo JM, Tse V, Chan SP and Tse G:
Cardiac disease and arrhythmogenesis: Mechanistic insights from
mouse models. Int J Cardiol Heart Vasc. 12:1–10. 2016.PubMed/NCBI
|
|
17
|
Tse G and Yan BP: Electrophysiological
mechanisms of long and short QT syndromes: Insights from mouse
models. IJC Heart & Vasculature. In press.
|
|
18
|
Tse G, Lai ET, Lee AP, Yan BP and Wong SH:
Electrophysiological mechanisms of gastrointestinal
arrhythmogenesis: Lessons from the heart. Front Physiol.
7:2302016.PubMed/NCBI
|
|
19
|
Tse G, Wong ST, Tse V, Lee YT, Lin HY and
Yeo JM: Cardiac dynamics: alternans and arrhythmogenesis. J
Arrhythm. In press.
|
|
20
|
Tse G: Novel conduction-repolarization
indices for the stratification of arrhythmic risk. J Geriatr
Cardiol. 13:811–812. 2016.PubMed/NCBI
|
|
21
|
Tse G: (Tpeak-Tend)/QRS and
(Tpeak-Tend)/(QT x QRS): Novel markers for predicting arrhythmic
risk in the Brugada syndrome. Europace. In press.
|
|
22
|
Tse G and Yan BP: Novel arrhythmic risk
markers incorporating QRS dispersion: QRSd × (Tpeak - Tend)/QRS and
QRSd × (Tpeak - Tend)/(QT × QRS). Ann Noninvasive Electrocardiol.
Aug 18–2016.Epub ahead of print. View Article : Google Scholar
|
|
23
|
Wong J, Tan T, Chan C, Laxton V, Chan Y,
Liu T, Wong J and Tse G: The role of connexins in wound healing and
repair: novel therapeutic approaches. Front Physiol. In press.
|
|
24
|
Tse G and Yan BP: Traditional and novel
electrocardiographic conduction and repolarization markers of
sudden cardiac death. Europace. Oct 4–2016.Epub ahead of print.
View Article : Google Scholar
|
|
25
|
Tse G, Wong ST, Tse V and Yeo JM:
Variability in local action potential durations, dispersion of
repolarization and wavelength restitution in aged wild type and
Scn5a/- mouse hearts modelling human Brugada syndrome. J Geriatr
Cardiol. In press.
|
|
26
|
Chen Z, Sun B, Tse G, Jiang J and Xu W:
Reversibility of both sinus node dysfunction and reduced HCN4 mRNA
expression level in an atrial tachycardia pacing model of
tachycardia-bradycardia syndrome in rabbit hearts. Int J Clin Exp
Pathol. 9:8526–8531. 2016.
|
|
27
|
Yeh YH, Burstein B, Qi XY, Sakabe M,
Chartier D, Comtois P, Wang Z, Kuo CT and Nattel S: Funny current
downregulation and sinus node dysfunction associated with atrial
tachyarrhythmia: A molecular basis for tachycardia-bradycardia
syndrome. Circulation. 119:1576–1585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monfredi O and Boyett MR: Sick sinus
syndrome and atrial fibrillation in older persons - A view from the
sinoatrial nodal myocyte. J Mol Cell Cardiol. 83:88–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lakatta EG, Vinogradova T, Lyashkov A,
Sirenko S, Zhu W, Ruknudin A and Maltsev VA: The integration of
spontaneous intracellular Ca2+ cycling and surface membrane ion
channel activation entrains normal automaticity in cells of the
heart's pacemaker. Ann N Y Acad Sci. 1080:178–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baruscotti M, Bucchi A and Difrancesco D:
Physiology and pharmacology of the cardiac pacemaker ('funny')
current. Pharmacol Ther. 107:59–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DiFrancesco D: Pacemaker mechanisms in
cardiac tissue. Annu Rev Physiol. 55:455–472. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ludwig A, Zong X, Jeglitsch M, Hofmann F
and Biel M: A family of hyperpolarization-activated mammalian
cation channels. Nature. 393:587–591. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi W, Wymore R, Yu H, Wu J, Wymore RT,
Pan Z, Robinson RB, Dixon JE, McKinnon D and Cohen IS: Distribution
and prevalence of hyperpolarization-activated cation channel (HCN)
mRNA expression in cardiac tissues. Circ Res. 85:e1–e6. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moroni A, Gorza L, Beltrame M, Gravante B,
Vaccari T, Bianchi ME, Altomare C, Longhi R, Heurteaux C, Vitadello
M, et al: Hyperpolarization-activated cyclic nucleotide-gated
channel 1 is a molecular determinant of the cardiac pacemaker
current I(f). J Biol Chem. 276:29233–29241. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yaniv Y, Lakatta EG and Maltsev VA: From
two competing oscillators to one coupled-clock pacemaker cell
system. Front Physiol. 6:282015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dobrzynski H, Boyett MR and Anderson RH:
New insights into pacemaker activity: Promoting understanding of
sick sinus syndrome. Circulation. 115:1921–1932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boyett MR, Honjo H and Kodama I: The
sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc
Res. 47:658–687. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gellens ME, George ALJ Jr, Chen LQ,
Chahine M, Horn R, Barchi RL and Kallen RG: Primary structure and
functional expression of the human cardiac tetrodotoxin-insensitive
voltage-dependent sodium channel. Proc Natl Acad Sci USA.
89:554–558. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stühmer W, Conti F, Suzuki H, Wang XD,
Noda M, Yahagi N, Kubo H and Numa S: Structural parts involved in
activation and inactivation of the sodium channel. Nature.
339:597–603. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kontis KJ, Rounaghi A and Goldin AL:
Sodium channel activation gating is affected by substitutions of
voltage sensor positive charges in all four domains. J Gen Physiol.
110:391–401. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horn R, Patlak J and Stevens CF: Sodium
channels need not open before they inactivate. Nature. 291:426–427.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
West JW, Patton DE, Scheuer T, Wang Y,
Goldin AL and Catterall WA: A cluster of hydrophobic amino acid
residues required for fast Na(+)-channel inactivation. Proc Natl
Acad Sci USA. 89:10910–10914. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kellenberger S, Scheuer T and Catterall
WA: Movement of the Na+ channel inactivation gate during
inactivation. J Biol Chem. 271:30971–30979. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kellenberger S, West JW, Catterall WA and
Scheuer T: Molecular analysis of potential hinge residues in the
inactivation gate of brain type IIA Na+ channels. J Gen Physiol.
109:607–617. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kellenberger S, West JW, Scheuer T and
Catterall WA: Molecular analysis of the putative inactivation
particle in the inactivation gate of brain type IIA Na+ channels. J
Gen Physiol. 109:589–605. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smith MR and Goldin AL: Interaction
between the sodium channel inactivation linker and domain III
S4-S5. Biophys J. 73:1885–1895. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shryock JC, Song Y, Rajamani S,
Antzelevitch C and Belardinelli L: The arrhythmogenic consequences
of increasing late INa in the cardiomyocyte. Cardiovasc Res.
99:600–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balser JR, Nuss HB, Chiamvimonvat N,
Pérez-García MT, Marban E and Tomaselli GF: External pore residue
mediates slow inactivation in mu 1 rat skeletal muscle sodium
channels. J Physiol. 494:431–442. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vilin YY, Makita N, George AL Jr and Ruben
PC: Structural determinants of slow inactivation in human cardiac
and skeletal muscle sodium channels. Biophys J. 77:1384–1393. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
John RM and Kumar S: Sinus Node and Atrial
Arrhythmias. Circulation. 133:1892–1900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koval M, Isakson BE and Gourdie RG:
Connexins, pannexins and innexins: Protein cousins with overlapping
functions. FEBS Lett. 588:11852014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Veeraraghavan R, Gourdie RG and Poelzing
S: Mechanisms of cardiac conduction: A history of revisions. Am J
Physiol Heart Circ Physiol. 306:H619–H627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Veeraraghavan R, Poelzing S and Gourdie
RG: Intercellular electrical communication in the heart: A new,
active role for the intercalated disk. Cell Commun Adhes.
21:161–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Davis LM, Kanter HL, Beyer EC and Saffitz
JE: Distinct gap junction protein phenotypes in cardiac tissues
with disparate conduction properties. J Am Coll Cardiol.
24:1124–1132. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gourdie RG, Green CR, Severs NJ, Anderson
RH and Thompson RP: Evidence for a distinct gap-junctional
phenotype in ventricular conduction tissues of the developing and
mature avian heart. Circ Res. 72:278–289. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gourdie RG, Severs NJ, Green CR, Rothery
S, Germroth P and Thompson RP: The spatial distribution and
relative abundance of gap-junctional connexin40 and connexin43
correlate to functional properties of components of the cardiac
atrioventricular conduction system. J Cell Sci. 105:985–991.
1993.PubMed/NCBI
|
|
57
|
Beyer EC, Paul DL and Goodenough DA:
Connexin43: A protein from rat heart homologous to a gap junction
protein from liver. J Cell Biol. 105:2621–2629. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Davis LM, Rodefeld ME, Green K, Beyer EC
and Saffitz JE: Gap junction protein phenotypes of the human heart
and conduction system. J Cardiovasc Electrophysiol. 6:813–822.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Saffitz JE, Green KG and Schuessler RB:
Structural determinants of slow conduction in the canine sinus
node. J Cardiovasc Electrophysiol. 8:738–744. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wilders R, Verheijck EE, Kumar R, Goolsby
WN, van Ginneken AC, Joyner RW and Jongsma HJ: Model clamp and its
application to synchronization of rabbit sinoatrial node cells. Am
J Physiol. 271:H2168–H2182. 1996.PubMed/NCBI
|
|
61
|
Bukauskas FF and Verselis VK: Gap junction
channel gating. Biochim Biophys Acta. 1662:42–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Musil LS and Goodenough DA: Biochemical
analysis of connexin43 intracellular transport, phosphorylation,
and assembly into gap junctional plaques. J Cell Biol.
115:1357–1374. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sáez JC, Nairn AC, Czernik AJ, Fishman GI,
Spray DC and Hertzberg EL: Phosphorylation of connexin43 and the
regulation of neonatal rat cardiac myocyte gap junctions. J Mol
Cell Cardiol. 29:2131–2145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kwak BR, Hermans MM, De Jonge HR, Lohmann
SM, Jongsma HJ and Chanson M: Differential regulation of distinct
types of gap junction channels by similar phosphorylating
conditions. Mol Biol Cell. 6:1707–1719. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
De Mello WC: Effect of intracellular
injection of calcium and strontium on cell communication in heart.
J Physiol. 250:231–245. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dahl G and Isenberg G: Decoupling of heart
muscle cells: Correlation with increased cytoplasmic calcium
activity and with changes of nexus ultrastructure. J Membr Biol.
53:63–75. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Burt JM: Block of intercellular
communication: Interaction of intracellular H+ and Ca2+. Am J
Physiol. 253:C607–C612. 1987.PubMed/NCBI
|
|
68
|
Maurer P and Weingart R: Cell pairs
isolated from adult guinea pig and rat hearts: Effects of [Ca2+]i
on nexal membrane resistance. Pflugers Arch. 409:394–402. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hermans MM, Kortekaas P, Jongsma HJ and
Rook MB: pH sensitivity of the cardiac gap junction proteins,
connexin 45 and 43. Pflugers Arch. 431:138–140. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Morley GE, Taffet SM and Delmar M:
Intramolecular interactions mediate pH regulation of connexin43
channels. Biophys J. 70:1294–1302. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Meyer R, Malewicz B, Baumann WJ and
Johnson RG: Increased gap junction assembly between cultured cells
upon cholesterol supplementation. J Cell Sci. 96:231–238.
1990.PubMed/NCBI
|
|
72
|
Meyer RA, Lampe PD, Malewicz B, Baumann WJ
and Johnson RG: Enhanced gap junction formation with LDL and
apolipoprotein B. Exp Cell Res. 196:72–81. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Massey KD, Minnich BN and Burt JM:
Arachidonic acid and lipoxygenase metabolites uncouple neonatal rat
cardiac myocyte pairs. Am J Physiol. 263:C494–C501. 1992.PubMed/NCBI
|
|
74
|
Schubert AL, Schubert W, Spray DC and
Lisanti MP: Connexin family members target to lipid raft domains
and interact with caveolin-1. Biochemistry. 41:5754–5764. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yabek SM and Jarmakani JM: Sinus node
dysfunction in children, adolescents, and young adults. Pediatrics.
61:593–598. 1978.PubMed/NCBI
|
|
76
|
Schulze-Bahr E, Neu A, Friederich P, Kaupp
UB, Breithardt G, Pongs O and Isbrandt D: Pacemaker channel
dysfunction in a patient with sinus node disease. J Clin Invest.
111:1537–1545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Duhme N, Schweizer PA, Thomas D, Becker R,
Schröter J, Barends TR, Schlichting I, Draguhn A, Bruehl C, Katus
HA, et al: Altered HCN4 channel C-linker interaction is associated
with familial tachycardia-bradycardia syndrome and atrial
fibrillation. Eur Heart J. 34:2768–2775. 2013. View Article : Google Scholar
|
|
78
|
DiFrancesco D: HCN4, Sinus Bradycardia and
Atrial Fibrillation. Arrhythm Electrophysiol Rev. 4:9–13. 2015.
View Article : Google Scholar
|
|
79
|
Milano A, Vermeer AM, Lodder EM, Barc J,
Verkerk AO, Postma AV, van der Bilt IA, Baars MJ, van Haelst PL,
Caliskan K, et al: HCN4 mutations in multiple families with
bradycardia and left ventricular noncompaction cardiomyopathy. J Am
Coll Cardiol. 64:745–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Schweizer PA, Schröter J, Greiner S, Haas
J, Yampolsky P, Mereles D, Buss SJ, Seyler C, Bruehl C, Draguhn A,
et al: The symptom complex of familial sinus node dysfunction and
myocardial noncompaction is associated with mutations in the HCN4
channel. J Am Coll Cardiol. 64:757–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou J, Ding WG, Makiyama T, Miyamoto A,
Matsumoto Y, Kimura H, Tarutani Y, Zhao J, Wu J, Zang WJ, et al: A
novel HCN4 mutation, G1097W, is associated with atrioventricular
block. Circ J. 78:938–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ueda K, Nakamura K, Hayashi T, Inagaki N,
Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y,
Yasunami M, et al: Functional characterization of a
trafficking-defective HCN4 mutation, D553N, associated with cardiac
arrhythmia. J Biol Chem. 279:27194–27198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Baruscotti M, Bucchi A, Viscomi C,
Mandelli G, Consalez G, Gnecchi-Rusconi T, Montano N, Casali KR,
Micheloni S, Barbuti A, et al: Deep bradycardia and heart block
caused by inducible cardiac-specific knockout of the pacemaker
channel gene Hcn4. Proc Natl Acad Sci USA. 108:1705–1710. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mesirca P, Alig J, Torrente AG, Müller JC,
Marger L, Rollin A, Marquilly C, Vincent A, Dubel S, Bidaud I, et
al: Cardiac arrhythmia induced by genetic silencing of 'funny' (f)
channels is rescued by GIRK4 inactivation. Nat Commun. 5:4664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Makiyama T, Akao M, Shizuta S, Doi T,
Nishiyama K, Oka Y, Ohno S, Nishio Y, Tsuji K, Itoh H, et al: A
novel SCN5A gain-of-function mutation M1875T associated with
familial atrial fibrillation. J Am Coll Cardiol. 52:1326–1334.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bezzina C, Veldkamp MW, van Den Berg MP,
Postma AV, Rook MB, Viersma JW, van Langen IM, Tan-Sindhunata G,
Bink-Boelkens MT, van Der Hout AH, et al: A single Na(+) channel
mutation causing both long-QT and Brugada syndromes. Circ Res.
85:1206–1213. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bezzina CR, Barc J, Mizusawa Y, Remme CA,
Gourraud JB, Simonet F, Verkerk AO, Schwartz PJ, Crotti L, Dagradi
F, et al: Common variants at SCN5A–SCN10A and HEY2 are associated
with Brugada syndrome, a rare disease with high risk of sudden
cardiac death. Nat Genet. 45:1044–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bezzina CR and Remme CA: Dilated
cardiomyopathy due to sodium channel dysfunction: What is the
connection. Circ Arrhythm Electrophysiol. 1:80–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bezzina CR, Rook MB, Groenewegen WA,
Herfst LJ, van der Wal AC, Lam J, Jongsma HJ, Wilde AA and Mannens
MM: Compound heterozygosity for mutations (W156X and R225W) in
SCN5A associated with severe cardiac conduction disturbances and
degenerative changes in the conduction system. Circ Res.
92:159–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Remme CA, Wilde AA and Bezzina CR: Cardiac
sodium channel overlap syndromes: Different faces of SCN5A
mutations. Trends Cardiovasc Med. 18:78–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tan HL, Bink-Boelkens MT, Bezzina CR,
Viswanathan PC, Beaufort-Krol GC, van Tintelen PJ, van den Berg MP,
Wilde AA and Balser JR: A sodium-channel mutation causes isolated
cardiac conduction disease. Nature. 409:1043–1047. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chang CC, Acharfi S, Wu MH, Chiang FT,
Wang JK, Sung TC and Chahine M: A novel SCN5A mutation manifests as
a malignant form of long QT syndrome with perinatal onset of
tachycardia/bradycardia. Cardiovasc Res. 64:268–278. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Letsas KP, Korantzopoulos P, Efremidis M,
Weber R, Lioni L, Bakosis G, Vassilikos VP, Deftereos S, Sideris A
and Arentz T: Sinus node disease in subjects with type 1 ECG
pattern of Brugada syndrome. J Cardiol. 61:227–231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Girmatsion Z, Biliczki P, Bonauer A,
Wimmer-Greinecker G, Scherer M, Moritz A, Bukowska A, Goette A,
Nattel S, Hohnloser SH, et al: Changes in microRNA-1 expression and
IK1 up-regulation in human atrial fibrillation. Heart Rhythm.
6:1802–1809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bennett V and Healy J: Organizing the
fluid membrane bilayer: Diseases linked to spectrin and ankyrin.
Trends Mol Med. 14:28–36. 2008. View Article : Google Scholar
|
|
96
|
Le Scouarnec S, Bhasin N, Vieyres C, Hund
TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, et
al: Dysfunction in ankyrin-B-dependent ion channel and transporter
targeting causes human sinus node disease. Proc Natl Acad Sci USA.
105:15617–15622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mohler PJ, Splawski I, Napolitano C,
Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT and Bennett
V: A cardiac arrhythmia syndrome caused by loss of ankyrin-B
function. Proc Natl Acad Sci USA. 101:9137–9142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mohler PJ, Schott JJ, Gramolini AO, Dilly
KW, Guatimosim S, duBell WH, Song LS, Haurogné K, Kyndt F, Ali ME,
et al: Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia
and sudden cardiac death. Nature. 421:634–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mohler PJ, Le Scouarnec S, Denjoy I, et
al: Defining the cellular phenotype of 'ankyrin-B syndrome'
variants: Human ANK2 variants associated with clinical phenotypes
display a spectrum of activities in cardiomyocytes. Circulation.
115:432–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mangoni ME, Couette B, Bourinet E, Platzer
J, Reimer D, Striessnig J and Nargeot J: Functional role of L-type
Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad
Sci USA. 100:5543–5548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Trebak M, Zhang W, Ruhle B, Henkel MM,
González-Cobos JC, Motiani RK, Stolwijk JA, Newton RL and Zhang X:
What role for store-operated Ca2+ entry in muscle.
Microcirculation. 20:330–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ju YK, Lee BH, Trajanovska S, Hao G, Allen
DG, Lei M and Cannell MB: The involvement of TRPC3 channels in
sinoatrial arrhythmias. Front Physiol. 6:862015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Swaminathan PD, Purohit A, Soni S, Voigt
N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, et al:
Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J
Clin Invest. 121:3277–3288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Erickson JR, Joiner ML, Guan X, Kutschke
W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE,
Aykin-Burns N, et al: A dynamic pathway for calcium-independent
activation of CaMKII by methionine oxidation. Cell. 133:462–474.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Luu M, Stevenson WG, Stevenson LW, Baron K
and Walden J: Diverse mechanisms of unexpected cardiac arrest in
advanced heart failure. Circulation. 80:1675–1680. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Stevenson WG, Stevenson LW, Middlekauff HR
and Saxon LA: Sudden death prevention in patients with advanced
ventricular dysfunction. Circulation. 88:2953–2961. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Faggioni M, van der Werf C and Knollmann
BC: Sinus node dysfunction in catecholaminergic polymorphic
ventricular tachycardia: Risk factor and potential therapeutic
target. Trends Cardiovasc Med. 24:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sumitomo N, Sakurada H, Taniguchi K, et
al: Association of atrial arrhythmia and sinus node dysfunction in
patients with catecholaminergic polymorphic ventricular
tachycardia. Circ J. 71:1606–1609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Faggioni M, Savio-Galimberti E,
Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D and Knollmann
BC: Suppression of spontaneous ca elevations prevents atrial
fibrillation in calsequestrin 2-null hearts. Circ Arrhythm
Electrophysiol. 7:313–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Glukhov AV, Kalyanasundaram A, Lou Q, Hage
LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M,
Györke S, et al: Calsequestrin 2 deletion causes sinoatrial node
dysfunction and atrial arrhythmias associated with altered
sarcoplasmic reticulum calcium cycling and degenerative fibrosis
within the mouse atrial pacemaker complex1. Eur Heart J.
36:686–697. 2015. View Article : Google Scholar
|
|
111
|
Jongsma HJ: Diversity of gap junctional
proteins: Does it play a role in cardiac excitation. J Cardiovasc
Electrophysiol. 11:228–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Eckardt D, Theis M, Degen J, Ott T, van
Rijen HV, Kirchhoff S, Kim JS, de Bakker JM and Willecke K:
Functional role of connexin43 gap junction channels in adult mouse
heart assessed by inducible gene deletion. J Mol Cell Cardiol.
36:101–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bagwe S, Berenfeld O, Vaidya D, Morley GE
and Jalife J: Altered right atrial excitation and propagation in
connexin40 knockout mice. Circulation. 112:2245–2253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Verheule S, van Batenburg CA, Coenjaerts
FE, Kirchhoff S, Willecke K and Jongsma HJ: Cardiac conduction
abnormalities in mice lacking the gap junction protein connexin40.
J Cardiovasc Electrophysiol. 10:1380–1389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
VanderBrink BA, Sellitto C, Saba S, Link
MS, Zhu W, Homoud MK, Estes NA III, Paul DL and Wang PJ:
Connexin40-deficient mice exhibit atrioventricular nodal and
infra-Hisian conduction abnormalities. J Cardiovasc Electrophysiol.
11:1270–1276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thery C, Gosselin B, Lekieffre J and
Warembourg H: Pathology of sinoatrial node. Correlations with
electrocardiographic findings in 111 patients. Am Heart J.
93:735–740. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ellinor PT, Lunetta KL, Albert CM, Glazer
L, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP,
Lubitz SA, et al: Meta-analysis identifies six new susceptibility
loci for atrial fibrillation. Nat Genet. 44:670–675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Funaya H, Kitakaze M, Node K, Minamino T,
Komamura K and Hori M: Plasma adenosine levels increase in patients
with chronic heart failure. Circulation. 95:1363–1365. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lou Q, Hansen BJ, Fedorenko O, Csepe TA,
Kalyanasundaram A, Li N, Hage LT, Glukhov AV, Billman GE, Weiss R,
et al: Upregulation of adenosine A1 receptors facilitates
sinoatrial node dysfunction in chronic canine heart failure by
exacerbating nodal conduction abnormalities revealed by novel
dual-sided intramural optical mapping. Circulation. 130:315–324.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li G, Liu E, Liu T, Wang J, Dai J, Xu G,
Korantzopoulos P and Yang W: Atrial electrical remodeling in a
canine model of sinus node dysfunction. Int J Cardiol. 146:32–36.
2011. View Article : Google Scholar
|
|
121
|
Herrmann S, Fabritz L, Layh B, Kirchhof P
and Ludwig A: Insights into sick sinus syndrome from an inducible
mouse model. Cardiovasc Res. 90:38–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tse G and Yeo JM: Conduction abnormalities
and ventricular arrhythmogenesis: The roles of sodium channels and
gap junctions. Int J Cardiol Heart Vasc. 9:75–82. 2015.
|
|
123
|
Pezhouman A, Cao H, Lee HH, Belardinelli
L, Weiss JN and Karagueuzian HS: Abstract 16247: Oxidative Stress
Initiates Atrial Fibrillation in Fibrotic Hearts by Early
Afterdepolarization-Mediated Triggered Activity. The Key Role of
Late INa. Circulation. 130:A162472014.
|
|
124
|
Morita N, Mandel WJ, Kobayashi Y and
Karagueuzian HS: Cardiac fibrosis as a determinant of ventricular
tachyarrhythmias. J Arrhythm. 30:389–394. 2014. View Article : Google Scholar
|
|
125
|
Tse G, Tse V and Yeo JM: Ventricular
anti-arrhythmic effects of heptanol in hypokalaemic,
Langendorff-perfused mouse hearts. Biomed Rep. 4:313–324.
2016.PubMed/NCBI
|
|
126
|
Tse G, Tse V, Yeo JM and Sun B: Atrial
anti-arrhythmic effects of heptanol in Langendorff-perfused mouse
hearts. PLoS One. 11:e01488582016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tse G, Wong ST, Tse V and Yeo JM:
Restitution analysis of alternans using dynamic pacing and its
comparison with S1S2 restitution in heptanol-treated, hypokalaemic
Langendorff-perfused mouse hearts. Biomed Rep. 4:673–680.
2016.PubMed/NCBI
|
|
128
|
Tse G, Sun B, Wong ST, Tse V and Yeo JM:
Ventricular anti-arrhythmic effects of hypercalcaemia treatment in
hyperkalaemic, Langendorff-perfused mouse hearts. Biomed Rep.
5:301–310. 2016.PubMed/NCBI
|
|
129
|
Tse G, Yeo JM, Tse V, Kwan J and Sun B:
Gap junction inhibition by heptanol increases ventricular
arrhythmogenicity by reducing conduction velocity without affecting
repolarization properties or myocardial refractoriness in
Langendorff-perfused mouse hearts. Mol Med Rep. 14:4069–4074.
2016.PubMed/NCBI
|
|
130
|
Tse G, Lai ET, Tse V and Yeo JM: Molecular
and electrophysiological mechanisms underlying cardiac
arrhythmogenesis in diabetes mellitus. J Diabetes Res.
2016:28487592016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tse G, Yeo JM, Chan YW, Lai ET and Yan BP:
What is the arrhythmic substrate in viral myocarditis? Insights
from clinical and animal studies. Front Physiol. 7:3082016.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tse G, Yan BP, Chan YW, Tian XY and Huang
Y: Reactive oxygen species, endoplasmic reticulum stress and
mitochondrial dysfunction: The link with cardiac arrhythmogenesis.
Front Physiol. 7:3132016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tse G, Lai ET, Yeo JM and Yan BP:
Electrophysiological mechanisms of Bayés syndrome: Insights from
clinical and mouse studies. Front Physiol. 7:1882016.
|
|
134
|
Li RA: Gene- and cell-based bio-artificial
pacemaker: What basic and translational lessons have we learned.
Gene Ther. 19:588–595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xue T, Cho HC, Akar FG, Tsang SY, Jones
SP, Marbán E, Tomaselli GF and Li RA: Functional integration of
electrically active cardiac derivatives from genetically engineered
human embryonic stem cells with quiescent recipient ventricular
cardiomyocytes: Insights into the development of cell-based
pacemakers. Circulation. 111:11–20. 2005. View Article : Google Scholar
|
|
136
|
Nattel S: Inward rectifier-funny current
balance and spontaneous automaticity: Cautionary notes for biologic
pacemaker development. Heart Rhythm. 5:1318–1319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Miake J, Marbán E and Nuss HB: Biological
pacemaker created by gene transfer. Nature. 419:132–133. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Azene EM, Xue T, Marbán E, Tomaselli GF
and Li RA: Non-equilibrium behavior of HCN channels: Insights into
the role of HCN channels in native and engineered pacemakers.
Cardiovasc Res. 67:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Qu J, Barbuti A, Protas L, Santoro B,
Cohen IS and Robinson RB: HCN2 overexpression in newborn and adult
ventricular myocytes: Distinct effects on gating and excitability.
Circ Res. 89:E8–E14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xue T, Siu CW, Lieu DK, Lau CP, Tse HF and
Li RA: Mechanistic role of I(f) revealed by induction of
ventricular automaticity by somatic gene transfer of
gating-engineered pacemaker (HCN) channels. Circulation.
115:1839–1850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kass-Eisler A, Falck-Pedersen E, Alvira M,
Rivera J, Buttrick PM, Wittenberg BA, Cipriani L and Leinwand LA:
Quantitative determination of adenovirus-mediated gene delivery to
rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci USA.
90:11498–11502. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Mühlhauser J, Jones M, Yamada I, Cirielli
C, Lemarchand P, Gloe TR, Bewig B, Signoretti S, Crystal RG and
Capogrossi MC: Safety and efficacy of in vivo gene transfer into
the porcine heart with replication-deficient, recombinant
adenovirus vectors. Gene Ther. 3:145–153. 1996.PubMed/NCBI
|
|
143
|
Chan YC, Siu CW, Lau YM, Lau CP, Li RA and
Tse HF: Synergistic effects of inward rectifier (I) and pacemaker
(I) currents on the induction of bioengineered cardiac
automaticity. J Cardiovasc Electrophysiol. 20:1048–1054. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lieu DK, Chan YC, Lau CP, Tse HF, Siu CW
and Li RA: Overexpression of HCN-encoded pacemaker current silences
bioartificial pacemakers. Heart Rhythm. 5:1310–1317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Saito Y, Nakamura K, Yoshida M, Sugiyama
H, Ohe T, Kurokawa J, Furukawa T, Takano M, Nagase S, Morita H, et
al: Enhancement of Spontaneous Activity by HCN4 Overexpression in
Mouse Embryonic Stem Cell-Derived Cardiomyocytes - A Possible
Biological Pacemaker. PLoS One. 10:e01381932015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kong CW, Akar FG and Li RA: Translational
potential of human embryonic and induced pluripotent stem cells for
myocardial repair: Insights from experimental models. Thromb
Haemost. 104:30–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Weng Z, Kong CW, Ren L, Karakikes I, Geng
L, He J, Chow MZ, Mok CF, Keung W, Chow H, et al: A simple,
cost-effective but highly efficient system for deriving ventricular
cardiomyocytes from human pluripotent stem cells. Stem Cells Dev.
23:1704–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Plotnikov AN, Shlapakova I, Szabolcs MJ,
Danilo P Jr, Lorell BH, Potapova IA, Lu Z, Rosen AB, Mathias RT,
Brink PR, et al: Xenografted adult human mesenchymal stem cells
provide a platform for sustained biological pacemaker function in
canine heart. Circulation. 116:706–713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Plotnikov AN, Sosunov EA, Qu J, Shlapakova
IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson
RB, et al: Biological pacemaker implanted in canine left bundle
branch provides ventricular escape rhythms that have
physiologically acceptable rates. Circulation. 109:506–512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Cho HC, Kashiwakura Y and Marbán E:
Creation of a biological pacemaker by cell fusion. Circ Res.
100:1112–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kehat I, Khimovich L, Caspi O, Gepstein A,
Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J and Gepstein
L: Electromechanical integration of cardiomyocytes derived from
human embryonic stem cells. Nat Biotechnol. 22:1282–1289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Verkerk AO and Wilders R:
Hyperpolarization-activated current, If, in mathematical models of
rabbit sinoatrial node pacemaker cells. BioMed Res Int.
2013:8724542013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Tse G: Mechanisms of cardiac arrhythmias.
J Arrhythm. 32:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|