Introduction
Cryptococcosis, a potentially fatal infectious
fungal infection, primarily caused by either Cryptococcus
neoformans (C. neoformans) or Cryptococcus gattii
(C. gattii), has gradually increased in incidence worldwide
over the past 20 years, particularly in China (1,2).
Infection proceeds via inhalation and subsequent dissemination to
the central nervous system to cause meningoencephalitis. Worldwide,
the most common risk for cryptococcosis caused by C.
neoformans is immunodeficiency, such as AIDS, and infections
caused by C. gattii are more often reported in
immunocompetent patients (3).
Epidemiological studies from far east Asian countries, particularly
from China, have indicated that C. neoformans infects mostly
HIV-uninfected patients for whom a predisposing underlying factor
may or may not be apparent (4,5).
A previous study strongly suggested that
anti-granulocyte-macrophage colony-stimulating factor
autoantibodies are a risk factor for central nervous system
infection by C. gattii cryptococcosis in otherwise
immunocompetent individuals (6).
Thus, this raises the question of whether the so-called healthy
hosts of cryptococcosis caused by C. neoformans are, in
fact, accompanied by other immunocompromising conditions that have
not been identified.
The innate immune system, including cellular
components, monocytes, macrophages and many other effectors, is the
first line of defense against pathogens, and broadly protects
against invading microorganisms. Monocytes are blood-borne cells
that differentiate into macrophages within tissues, which are
infiltrated following an inflammatory signal (7). Once within tissues, macrophages can
further develop distinct functional phenotypes, which is determined
by the cytokine milieu induced by pathogens (8,9).
Macrophages are important components with versatile
functions prominently involved in host defense and immunity against
foreign microorganisms, including bacteria, viruses, fungi and
parasites (10). Macrophages
possess a broad array of cell-surface receptors, intracellular
mediators and essential secretory molecules for the recognition,
engulfment and destruction of invading pathogens, and for the
regulation of other types of immune cells (11). In light of the important role of
monocytes in the immune response, additional investigations of the
immune mechanism in monocytes exposed to C. neoformans would
be of interest.
Toll-like receptors (TLRs), are the first identified
and the most well characterized pattern-recognition receptors
(PRRs) that recognize components derived from a wide range of
pathogens known as pathogen-associated molecular patterns (PAMPs).
TLRs subsequently initiate an anti-infection innate immune response
and help initiate and shape adaptive immune responses (12,13). Among the TLRs, TLR2 can recognize
microbes, including components from Gram-positive bacteria in the
presence of TLR1 or TLR6 (14).
The importance of TLR2 in host defense against C. neoformans
has been widely studied in animal experiments (15,16).
The activation of TLRs initiates a transmembrane
signaling cascade and triggers intracellular signaling molecules,
including interleukin-1 receptor-associated kinase (IRAK)1, IRAK4
and TNF receptor associated factor 6 (TRAF6), and then initiates a
series of immune responses (17).
Although the inflammatory response is valuable for dealing with
pathogens, if the inflammatory response is unregulated, it can take
a toll on the body and lead to serious disease. Thus, negative
regulators of the response are critically important.
MicroRNAs (miRs or miRNAs) are a class of highly
conserved small non-coding RNAs, 19–24 nucleotides in length, which
post-transcriptionally regulate gene expression by targeting the 3′
untranslated region (3′UTR) of target mRNAs (18). miRNAs prevent protein synthesis by
degrading mRNAs and inhibiting their translation (19). Accumulating evidence has indicatd
that miRNAs play a novel role in the regulation of the immune
system, including the development and differentiation of immune
cells, antibody production and innate immune regulation (20,21).
The abnormal expression of miRNAs is also involved
in various diseases, ranging from the development and
differentiation of cells to tumors (22), and inflammatory and autoimmune
disorders (23,24). Recent evidence has indicated that
miRNAs, such as miR-9, miR-21, miR-125a, miR-132, miR-146a/b and
miR-155 are inducible by PAMPs, such as lipopolysaccharides (LPS).
These miRNAs are also engaged in regulating innate immune responses
(25–27). miR-146a is one of the most
prominent miRNAs induced by TLR signals through nuclear factor-κB
(NF-κB) activation and then feeds back to suppress TLR-triggered
NF-κB activation (28).
It has been well established that the cytokine
profile of the host markedly affects the outcome of cryptococcal
disease, and the negative regulators of miRNAs are critically
important for immunomodulation. However, the role of miRNAs and the
molecular basis of the inflammatory response induced by C.
neoformans in monocytes remain unknown. Thus, the aim of this
study was to investigate the effects of the unique regulatory
pattern of miRNAs in C. neoformans-exposed monocytic
cells.
We analyzed the differential miRNA expression
profiles in C. neoformans-exposed THP-1 cells, and predicted
their target genes and function. Our data indicate that many
miRNAs, including miR-146a, are altered in primary human
macrophages exposed to C. neoformans. We demonstrate that
C. neoformans components induce NF-κB activation through a
TLR signaling pathway, resulting in the upregulation of the
miR-146a, which, upon processing, downregulates the mRNA and
protein levels of IRAK1 and TRAF6, reducing the activity of the
NF-κB pathway. The interaction of miR-146a with NF-κB in C.
neoformans-exposed THP-1 cells is thought to be crucial to the
inflammatory response, determining Cryptococcosis progression and
correlating with disease outcome in humans.
Materials and methods
Cells and bacterial culture
The human macrophage cell line, THP-1 (Shanghai Cell
Bank, Chinese Academy of Sciences, Shanghai, China), was cultured
in RPMI-1640 (HyClone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Carlsbad, CA, USA), penicillin (100
U/ml), and streptomycin (100 mg/ml) (all from Beyotime Institute of
Biotechnology, Shanghai, China), in a humidified incubator
containing 5% CO2 at 37°C. C. neoformans (WN148;
Center for the Preservation of Medical Mycology, Shanghai, China)
was cultured in YPD broth (1% yeast extract, 2% peptone and 2%
dextrose) for 3–5 days at 30°C, then harvested, washed with sterile
saline, and inactivated by heating at 56°C for 60 min. The
efficiency of heat-killing was assessed by culture in Sabouraud
glucose broth (Shanghai Hao Yang Biotechnology Co., Ltd., Shanghai,
China). The heat-killed C. neoformans number was counted and
adjusted to the desired concentration. The THP-1 cells were
incubated with the heat-killed C. neoformans WN148 for 0, 3,
6, 9 and 12 h. For all experiments, the cells were exposed to WN148
at an MOI of 1:5.
NF-κB inhibitor (PDTC) pre-treatment
The appropriate amount of THP-1 cells was planted
according to the experiment. When the THP-1 cells were grown to 80%
confluency, the fresh anti-culture medium was replaced. The cells
were pre-treated with NF-κB inhibitor (PDTC, S1809; Beyotime
Institute of Biotechnology) and the final concentration was 10
µM. After 12 h of pre-treatment, the cells of the
experimental group were treated with C. neoformans (MOI =
5). After 3 and 6 h, the cells were collected.
In vitro exposure model
The THP-1 cells were seeded at 5×106
cells/flask and grown to 70% confluency at 37°C in 5%
CO2. THP-1 monolayers (containing approximately
107 cells) were incubated with 5×107 C.
neoformans (MOI of 5) for 6 h as the induction model. The
exposed cells were lysed with 0.1% TRIzol (Invitrogen, Carlsbad,
CA, USA), and CFU counts of the cell lysates were determined by
RT-qPCR (7900 real-time PCR system; Applied Biosystems, Foster
City, CA, USA).
Small RNA library construction and
Illumina sequencing
miRNA expression profiling of C.
neoformans-exposed THP-1 cells for 0 and 6 h (this is a
representative experiment of 4). miRNAs were isolated by separating
total RNAs on denaturing polyacrylamide gel electrophoresis and
cutting a portion of the gel corresponding to the size 18–30
nucleotides. Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer's instructions, and the
RNA concentration and purity were determined using the Agilent
Technologies 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA,
USA). Small RNA library construction and sequencing, analysis of
sequencing data, stem-loop RT-qPCR for miRNAs and bioinformatics
analysis were carried out by the Amplicon Gene Biotechnology
Companies (Shanghai, China).
THP-1 cell transfection
miR-146a functional analyses were performed using
synthetic miR-146a mimic, miR-146a inhibitor, relative controls, or
FAM-negative control miRNA obtained from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China) and reconstituted in nuclease-free water at
a concentration of 20 mM. Prior to transfection, the cells were
transferred to fresh culture medium at a concentration of
1×106 cells/ml. The following day, the THP-1 cells
adjusted to 1×106 cells/well were transfected with
miR-146a mimic (20 nM) or inhibitor (40 nM) using Lipofectamine
2000 (Invitrogen), according to the manufacturer's instructions.
Following transfection, the cells were allowed to recover for 6 h
at 37°C and fresh RPMI-1640 medium was changed thereafter, and the
cells were then exposed to C. neoformans for 3 h.
Supernatants from cell cultures were collected and assayed for
cytokine secretion, and cell pellets were used for RNA isolation
and RT-qPCR.
RT-qPCR
Total RNA of related factors in treated and
untreated THP-1 cells was prepared using TRIzol reagent
(Invitrogen) according to the manufacturer's instructions. miR-146a
expression was measured using a TaqMan MicroRNA reverse
transcription kit (Takara, Dalian, China) and normalized to an
internal actin control, as previously described (29). For the other genes, IRAKI, TRAF6
and actin, reverse transcription was carried out using 1 mg of RNA
to produce cDNA. The primer pairs used are listed in Table I. RT-qPCR for miRNAs was performed
using a standard SYBR Green PCR kit (Takara) in an 7900 real-time
PCR (Applied Biosystems) according to the instructions from the
respective manufacturers. Triplicate samples were analyzed in
triplicate wells in each experiment. The 2−ΔΔCq method
was used to quantify the relative levels of gene expression.
 | Table ISequences of primers used for
RT-qPCR |
Table I
Sequences of primers used for
RT-qPCR
| Gene | Category | Primer sequences
(5′→3′) |
|---|
| Actin | Sense |
ACAATGTGGCCGAGGCTTT |
| Actin | Antisense |
GCACGAAGGCTCATCATTCA |
| IRAK1 | Sense |
GGACACGGACACCTTCAGC |
| IRAK1 | Antisense |
CAGCCTCCTCTTCCACCAG |
| TRAF6 | Sense |
TTGATGGCATTACGAGAAGCAG |
| TRAF6 | Antisense |
GCAAACAACCTTCATTTGGACAT |
| mi R-146a | Sense |
GCTACAAAAGCTGGGAA |
| mi R-146a | Antisense |
CTGATGCGTGAAGTGCTG |
| mi R-146a | Reverse |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCCA |
Enzyme-linked immunosorbent assay (ELISA)
for the determination of cytokine levels
Supernatants were collected from cell cultures at
different time points following exposure to various stimuli.
Secreted cytokines, such as tumor necrosis factor-α (TNF-α), and
interleukin-1β (IL-1β) in the supernatants were measured using
ELISA kits according to the manufacturer's instructions
(eBioscience, San Diego, CA, USA). The absorbance at 405 nm was
measured using a microplate reader (Bio-Rad iMark microplate
reader; Bio-Rad, Hercules, CA, USA). The absorbance at 405 was
converted to protein concentrations (pg/ml) using standard curves
of recombinant human or mouse cytokines.
Western blot analysis
The protein expression levels of IRAKI, TRAF6 and
NF-κB p65 (p65) in treated and untreated THP-1 cells were detected
by western blot analysis. Briefly, the THP-1 cells were lysed with
RIPA buffer (Beyotime Institute of Biotechnology). Supernatants
were collected following centrifugation at 13,000 rpm for 20 min at
4°C, and the protein concentration was determined using the BCA
Protein assay kit (Beyotime Institute of Biotechnology). Total
protein from the THP-1 cells was resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred onto Immobilon-P polyvinylidene difluoride (PVDF;
Millipore, Billerica, MA, USA) membranes. After blocking the
membranes with 5% skim milk for 60 min, they were then probed with
primary rabbit anti-IRAK1 (ab67841), anti-TRAF6 (ab13853; both from
Abcam, Cambridge, UK), and anti-p65 (sc-109) antibodies at a
concentration of 1:200 (Santa Cruz Biotechnology, Inc., Delaware,
CA, USA). After washing the membranes, they were probed with the
corresponding secondary antibodies [goat anti-rabbit secondary
antibody (G1210-2); PB001; Shanghai Immune Biotech Co., Ltd.,
Shanghai, China] before developing them using an ECL western blot
detection. The protein band intensities were measured using online
ImageJ software provided by the Transformation Center of Changzheng
Hospital. Background intensity was subtracted from each sample and
then normalized to the actin loading control (sc-47778; Santa Cruz
Biotechnology, Inc.).
Statistical analysis
The majority of the experiments were performed
independently at least 3 times and yielded similar results. Data
are presented in figures as the means ± SD using the SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). For multiple group
comparisons, one-way ANOVA (p<0.05) was performed, followed by a
two-sided, unpaired Student's t-test. An unpaired two-tailed
Student's t-test was used to compare 2 independent groups.
Differences at a level of p<0.05 were considered statistically
significant.
Results
Identification of miRNAs with an altered
expression in monocytes during C. neoformans infection
To identify the miRNAs in human monocytes whose
expression was altered during C. neoformans infection, we
performed all miRNA expression profiling in THP-1 cells
post-infection with the standardized strain, C. neoformans,
using the Illumina sequencing miRNA expression assay. The miRNAs in
the THP-1 cells at 6 h following C. neoformans infection
were compared with miRNAs from the control cells exposed for 0 h.
The time point of 6 h post-infection was selected to reflect the
phases of acclimatization to the extracellular environment and
induction of the members of the innate immune system. This assay
revealed that the expression of miRNAs was significantly altered
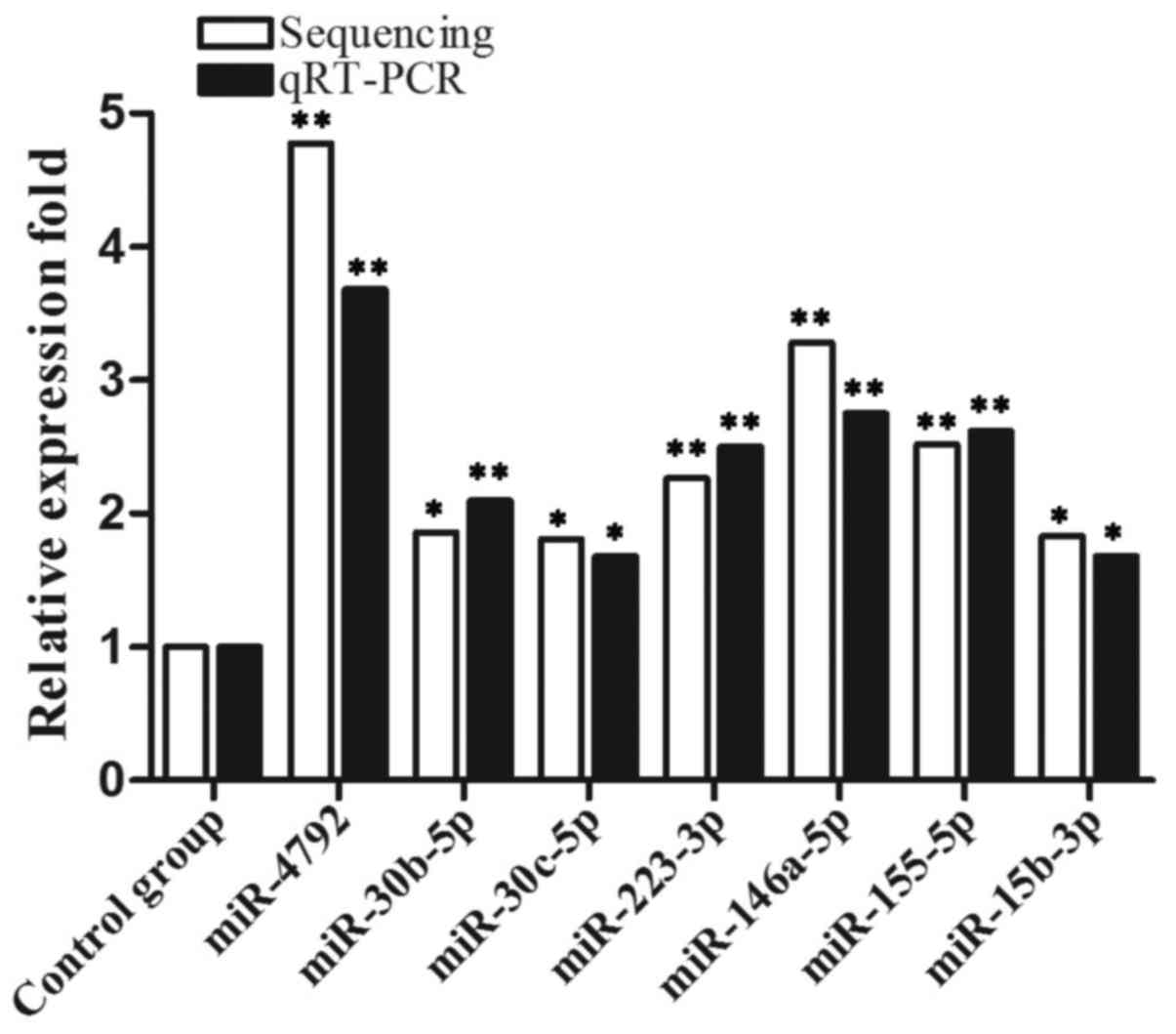
during C. neoformans infection. Among the altered miRNAs, 7
miRNAs, miR-4792, miR-30b-5p (miR-30b), miR-30c-5p, miR-223-3p,
miR-15b-3p, miR-146a-5p (miR-146a) and miR-155-5p were
significantly upregulated compared to the control group. To
validate the miRNA expression profiling results, we used RT-qPCR to
assay the expression of several upregulated miRNAs (Fig. 1). When compared with the
expression profiles established by Illumina sequencing, the RT-qPCR
validation results demonstrated great congruence between the
expression patterns determined using these two techniques for these
miRNAs.
C. neoformans induces the expression of
miR-146a and inflammatory cytokines in an NF-κB-dependent
manner
The aforementioned findings together with related
literature reports on the effect of miR-146a on the immune response
(28), prompted us to select
miR-146a for further investigation in our study. The THP-1 cells
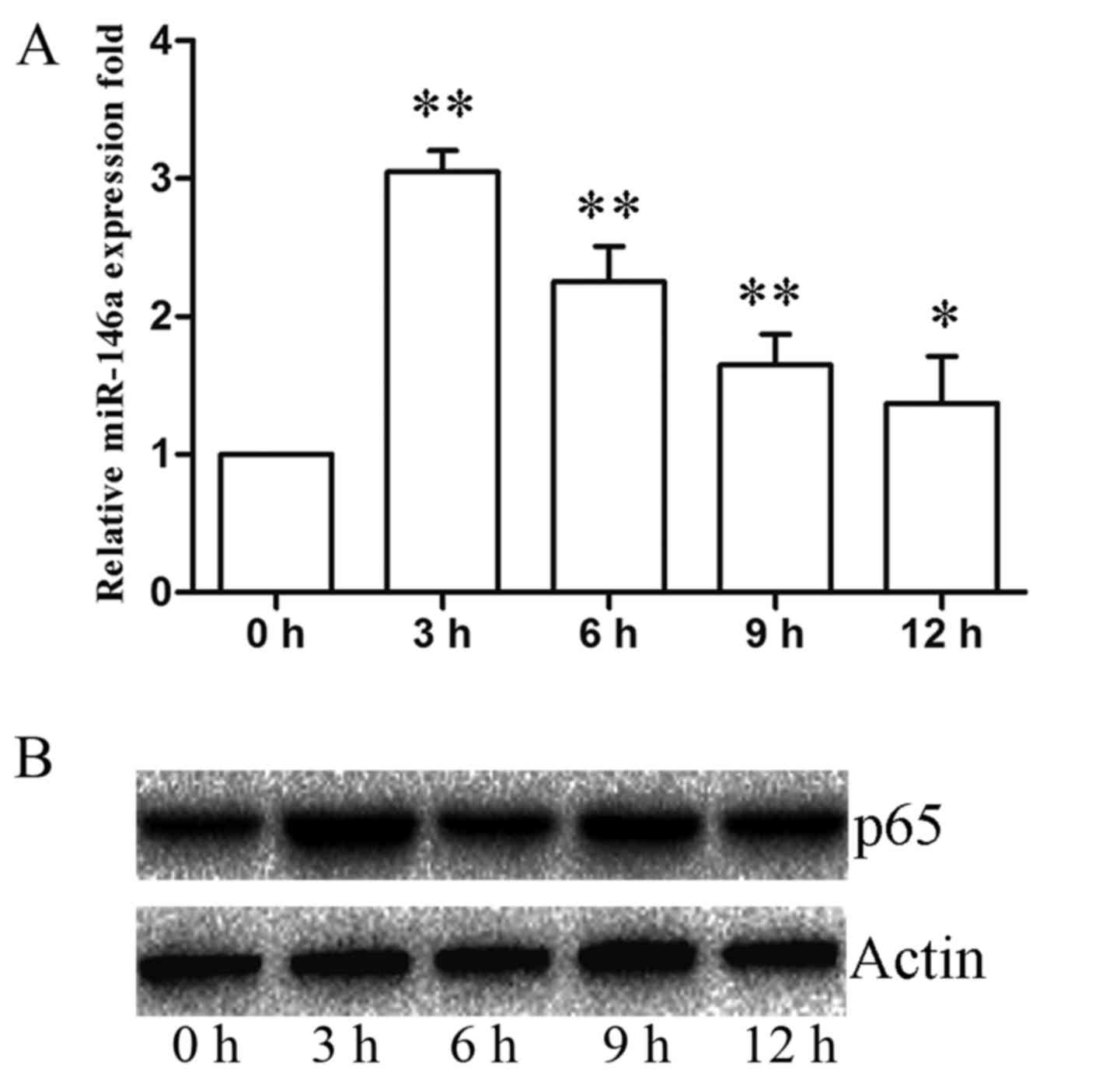
were stimulated with C. neoformans (MOI of 5) for 0, 3, 6, 9
and 12 h. The expression of miR-146a immediately increased in the
early phase of incubation and reached peak levels at 3 h, then
gradually decreased from 3 to 12 h (Fig. 2A). We first examined the levels of
miR-146a during C. neoformans infection in the human
macrophage cell line cells.
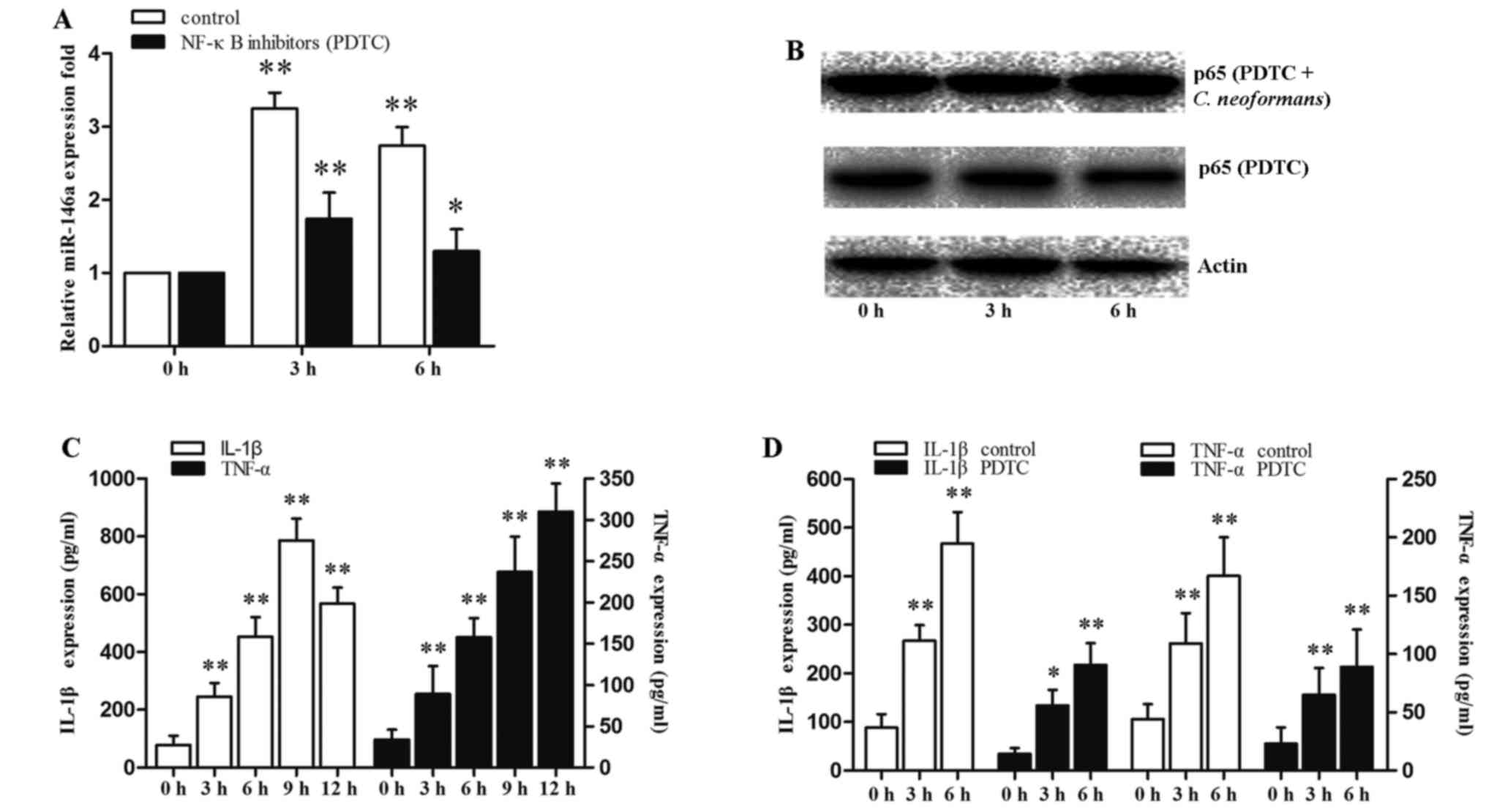
A previous study demonstrated that LPS stimulation
led to NF-κB activation and an increase in miR-146a expression in
THP-1 cells (30). To identify
the NF-κB pathway involved in the regulation of miR-146a expression
following C. neoformans infection, the THP-1 cells were
exposed to C. neoformans and PDTC (NF-κB inhibitor) were
used. The activation of NF-κB was determined by the presence of the
p65 subunit of NF-κB in the nuclear compartment of the cells where
it exerts its transcriptional activity.
The expression of miR-146a was rapidly induced by
3.5-fold at 3 h, and the induction of miR-146a then decreased with
an increase of p65 expression, upon C. neoformans infection
(Fig. 2). Although the THP-1
cells were pre-treated with the NF-κB inhibitor, PDTC, for 12 h,
which cannot completely inhibit the p65 protein level (Fig. 3B, PDTC group). The C.
neoformans-induced activation of NF-κB in the THP-1 cells was
partially blocked by PDTC treatment at 3 h. However, the expression
of NF-κB at 6 h was increased with the induction of C.
neoformans (Fig. 3B, PDTC +
C. neoformans group). Treatment with PDTC suppressed the
C. neoformans-induced upregulation of miR-146a (Fig. 3A). The secretion of IL-1p and
TNF-α into the extracellular medium was quantified by ELISA. The
expression levels of IL-1β and TNF-α increased progressively in the
THP-1 cells following incubation. PDTC treatment suppressed the
expression levels of IL-1β and TNF-α (Fig. 3C and D). The results revealed that
the miR-146a level was significantly increased by C.
neoformans infection in the early phase, and was associated
with the expression of inflammatory cytokines. These data indicated
that C. neoformans infection increases miR-146a expression
via a NF-κB-dependent mechanism.
NF-κB is negatively regulated by miR-146a
in the miR-146a- NF-κB axis in THP-1 cells infected with C.
neoformans
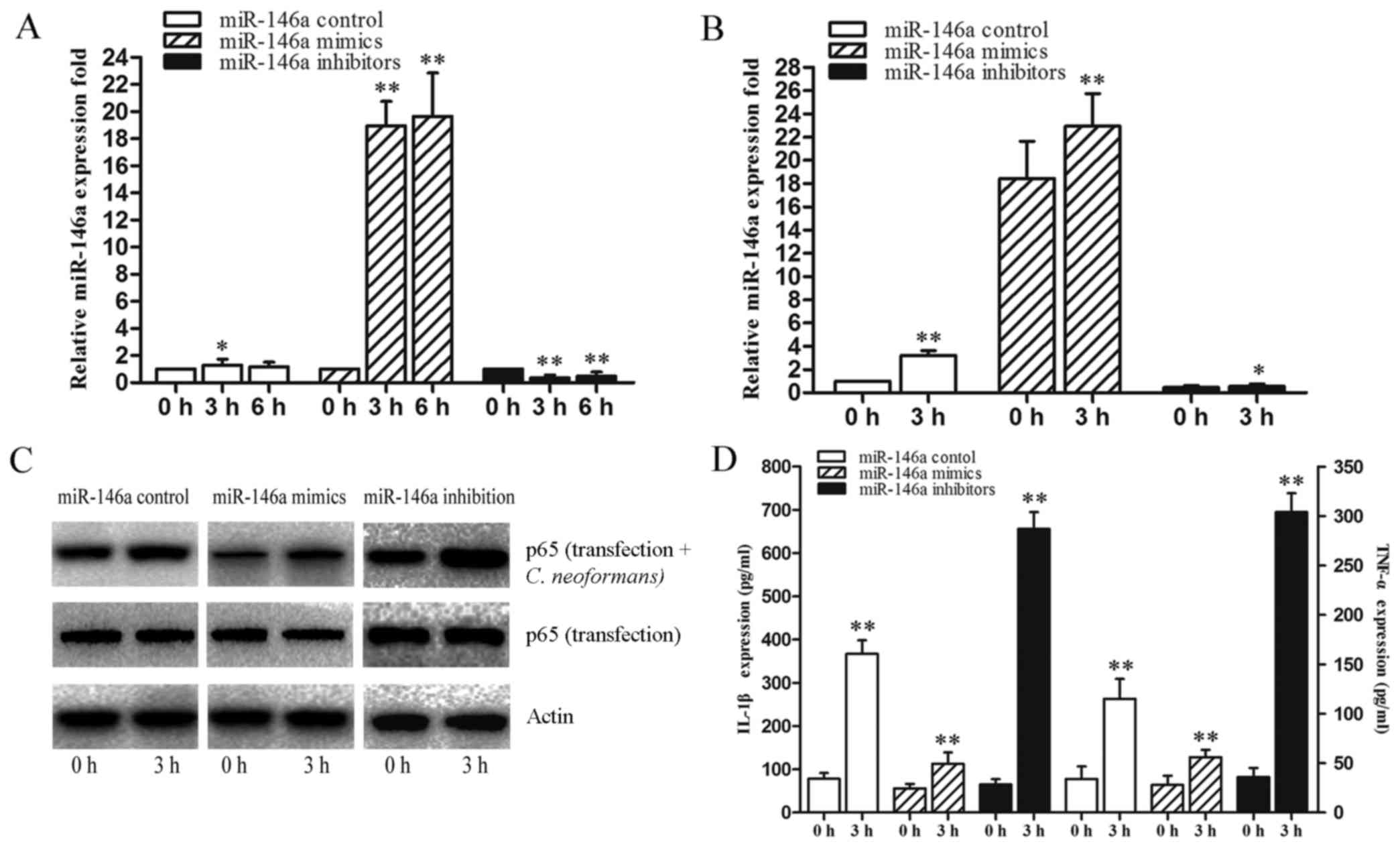
To provide additional evidence of the mechanisms of
action of miR-146a as regards the regulation of the miR-146a-NF-κB
axis, we examined the effects of miR-146a on NF-κB expression in
THP-1 cells transfected with miR-146a mimics and miR-146a
inhibitors. The efficiency of transfection is shown in Fig. 4A and B. When the THP-1 cells were
transfected with miR-146a mimics and inhibitors they were allowed
to recover for 6 h and subsequently infected by C.
neoformans for 3 h. Transfection with miR-146a mimics
significantly attenuated the expression of NF-κB, and transfection
with miR-146a inhibitors elevated the expression of NF-κB compared
to the miR-146a control (Fig. 4C,
transfection group). The expression of NF-κB infected by C.
neoformans was increased compared to the transfection group
(Fig. 4C, transfection + C.
neoformans group), which suggests that miR-146a significantly
regulates the activation of NF-κB induced by C. neoformans.
The changes in the activation of NF-κB affected the release of
IL-1β and TNF-α (Fig. 4D). The
results revealed that NF-κB activation was regulated by miR-146a
via transfection with miR-146a mimics and inhibitors. These data
indicated that miR-146a exerts negative regulatory effects on the
NF-κB pathway.
IRAKI and TRAF6 are regulated by miR-146a
in the miR-146a- NF-κB axis in THP-1 cells infected with C.
neoformans
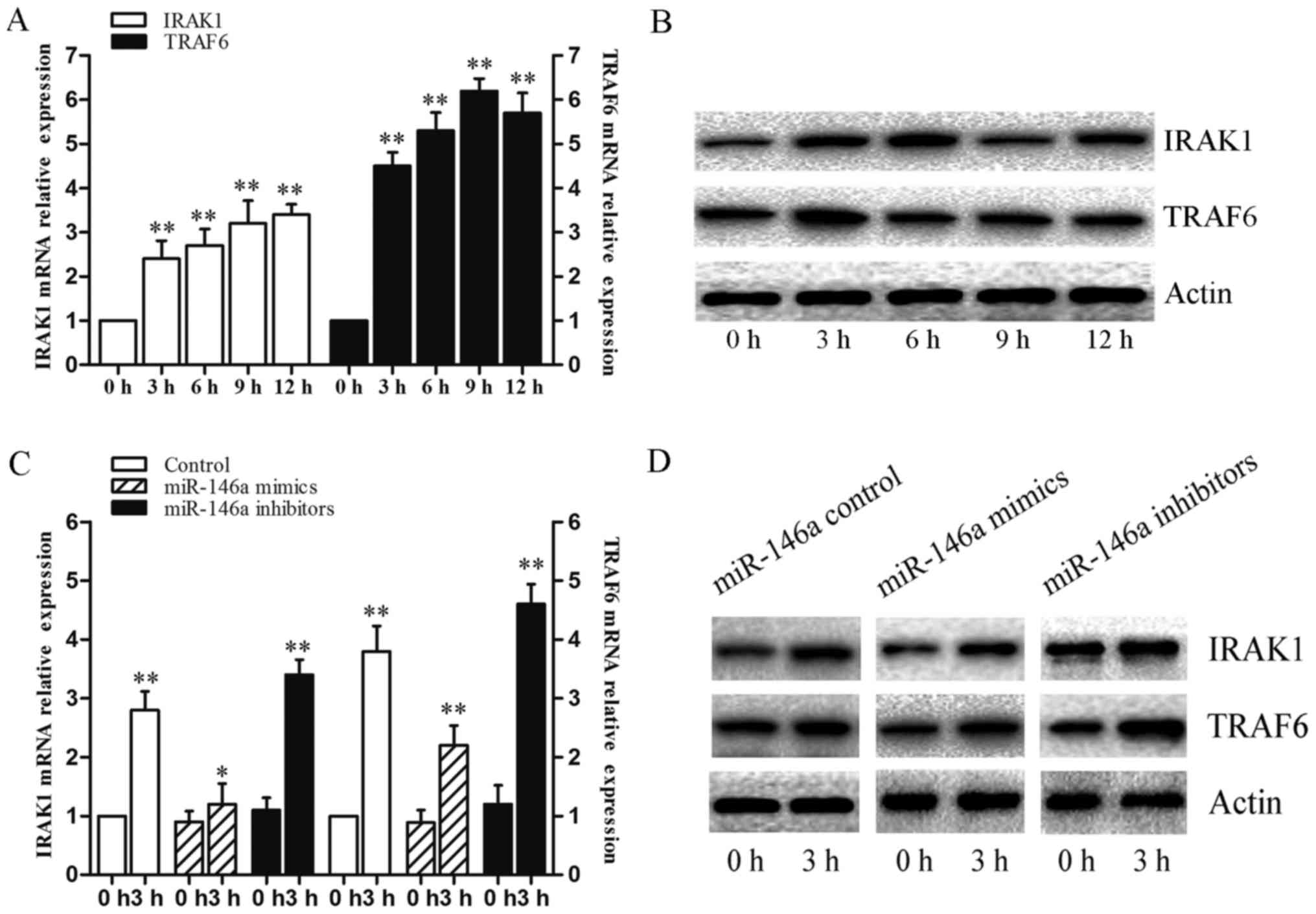
To further assess the function of miR-146a, it is
important to determine the host mRNAs that are regulated by
miR-146a. We selected two key signaling proteins, IRAK1 and TRAF6,
whose 3′UTRs were previously conhrmed to be complementary to
miR-146a (28), as miR-146a
primarily regulates these adaptor molecules via translational
repression (31). To identify the
function of miR-146a that results in the suppression of these
target genes, we measured the mRNA and protein levels of IRAK1 and
TRAF6 in THP-1 cells infected with C. neoformans for 0, 3,
6, 9 and 12 h. The mRNA and protein levels of IRAK1 and TRAF6 were
significantly affected in these groups (Fig. 5A and B). To identify the mechanism
that results in the suppression of these target genes, we measured
the mRNA and protein levels of IRAK1 and TRAF6 in THP-1 cells
transfected with miR-146a mimics or inhibitors. The results
revealed that transfection with miR-146a mimics signihcantly
decreased the mRNA levels of IRAK1 and TRAF6. Furthermore, the
overexpression of miR-146a resulted in the downregulation of the
protein levels of IRAK1 and TRAF6 (Fig. 5C and D). These data suggested that
IRAK1 and TRAF6 are the main target of miR-146a on the NF-κB
pathway during C. neoformans infection in THP-1 cells.
Discussion
Macrophages play important and indispensable roles
in the anti-infection immune response. Studies have shown that
macrophages are widely involved in the immune response to fungal
infection (32,33). Insufficient macrophage activation
may lead to the incomplete elimination of invading pathogens, but
superabundant macrophage activation may result in tissue damage,
inflammatory diseases, and even autoimmune disorders. Therefore,
the inflammatory processes of macrophages in infection need to be
accurately regulated. Over the past decade, research has focused on
macrophage regulation by miRNAs and epigenetics-associated
molecules (34,35).
As novel regulators of gene expression at the
post-transcriptional level, miRNAs have emerged to play important
roles in many biological processes ranging from cellular
development and differentiation to tumorigenesis (22). miRNAs have also been shown to be
involved in innate immunity (36). During the activation of an innate
immune response, the expression of some miRNAs, including miR-146a,
miR-132, miR-155 and miR-125a, rapidly changes (28,37). Recent publications have indicated
that miR-146a may play a key role in the innate immune response and
also participates in the pathogenesis of immune diseases, such as
infection, lupus and cancer (38–41).
In the present study, we observed that the
expression of 7 miRNAs, miR-4792, miR-30b-5p (miR-30b), miR-30c-5p,
miR-223-3p, miR-15b-3p, miR-146a-5p (miR-146a) and miR-155-5p was
significantly upregulated compared to the control group (Fig. 1). We demonstrated that miR-146a
expression was significantly induced by C. neoformans
infection in THP-1 cells. Of note, in the control human monocytes,
the basal expression levels of miR-146a were rapidly increased and
reached a peak at 3 h, then gradually decreased from 3 to 12 h
(Fig. 2A). This observation is in
conflict with the expression pattern of miR-146a in THP-1 cells
infected by other pathogens or bioactive components shown in
another study which reported that miR-146a expression was gradually
increased (42). The difference
in miR-146a expression in THP-1 cells may be partially due to the
differences in bacterial load or strains.
Activated signaling molecules induce the nuclear
translocation of NF-κB and activator protein (AP)-1, resulting in
the production of inflammatory cytokines, such as TNF-α, IL-1β and
IL-6 (43,44). In a recent study, miR-146a
expression induced by the activation of TLR signaling was
NF-κB-dependent in human and mouse macrophages (45). The induction of miR-146a is known
to be NF-κB-dependent and thus is a pivotal component of a negative
feedback loop invoked by TLR signaling. The C. neoformans
-induced expression of the miRNA, miR-146a, has been shown to be
NF-κB-dependent, and the inhibition of NF-κB by PDTC, an NF-κB
inhibitor, decreased miR-146a expression (34). We found that C. neoformans
infection induced the upregulation of miR-146a in macrophages, and
this enhancement occurred in an NF-κB-dependent manner (Fig. 2 and 3A and B). Furthermore, the negative
regulatory effects of miR-146a on NF-κB activity in THP-1 cells
infected with C. neoformans may be a secondary effect of the
induction of inflammation (Fig. 3C
and D).
We transfected macrophages with miR-146a mimic or
inhibitor in the present study and found that the overexpression of
miR-146a attenuated the activation of NF-κB. Moreover, miR-146a
negatively regulated NF-κB expression in immunity against C.
neoformans induction (Fig. 4B and
D).
It is well known that miRNAs function by binding to
the 3′UTR of target mRNAs to induce degradation or the suppression
of translation. Thus far, many proteins have been identified as
targets of miR-146a, including TRAF6, IRAK1, IRAK2, STAT1, TLR4 and
Notch1 (41,46,47). Among these miR-146a targets, TRAF6
and IRAK1 have been demonstrated to be important molecules in the
signaling pathway (48). More
importantly, IRAK1 and TRAF6 are known to be part of the common
signaling pathway derived from TLR-2, -4 and -5, and the IL-1|3
receptor, leading to speculation that increased miR-146a expression
may act as a negative feedback pathway. Previously, Li et al
(49) and Boone et al
(50) observed LPS tolerance in
monocytes caused by the impairment of IRAK1 and TRAF6 kinase
activity, respectively. As discussed previously, miR-146a targets
and suppresses IRAK1 and TRAF6 (51).
In this study, we also found that the overexpression
of miR-146a in THP-1 cells resulted in the downregulation of the
mRNA and protein levels of IRAK1 and TRAF6, which, in contrast,
subsequently decreased NF-κB activity (Fig. 5). This suggests that miR-146a may
exert a negative regulatory effect on NF-κB signaling via the
negative regulation of IRAK1 and TRAF6, which play a critical role
in immunity against C. neoformans.
In conclusion, we found that miRNAs were integrally
involved in the inflammatory reaction in monocytes infected by
C. neoformans. miR-146a was upregulated by the NF-κB
pathway, whereas miR-146a negatively regulated NF-κB activation by
targeting IRAK1 and TRAF6, and then inhibiting the activation of
NF-κB and the release of inflammatory cytokines in monocytes, which
helps to fine-tune the immune response. Taken together, our results
suggest that miR-146a may represent a future therapeutic target for
regulating the inflammatory response of the host innate immune
response to C. neoformans infection.
Acknowledgments
This study was supported by the National Basic
Research Program of China (973 program, no. 2013CB531603), the
Development Fund for Department of Dermatology and the Institute of
Shanghai Medical Mycology of Changzheng Hospital, the Second
Military Medical University.
References
|
1
|
Chayakulkeeree M and Perfect JR:
Cryptococcosis. Infect Dis Clin North Am. 20:507–544. v–vi. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu SX, Guo NR, Li XF, Liao WQ, Chen M,
Zhang QQ, Li CY, Li RY, Bulmer GS, Li DM, et al: Human pathogenic
fungi in China - emerging trends from ongoing national survey for
1986, 1996, and 2006. Mycopathologia. 171:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hajjeh RA, Conn LA, Stephens DS, Baughman
W, Hamill R, Graviss E, Pappas PG, Thomas C, Reingold A, Rothrock
G, et al Cryptococcal Active Surveillance Group: Cryptococcosis:
Population-based multistate active surveillance and risk factors in
human immunodeficiency virus-infected persons. J Infect Dis.
179:449–454. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SST, Nimmo G, Speed B, Currie B,
Ellis D, Marriott D, Pfeiffer T, Parr D and Byth K: Epidemiology
and host- and varietydependent characteristics of infection due to
Cryptococcus neoformans in Australia and New Zealand. Australasian
Cryptococcal Study Group Clin Infect Dis. 31:92000.
|
|
5
|
Mihara T, Izumikawa K, Kakeya H,
Ngamskulrungroj P, Umeyama T, Takazono T, Tashiro M, Nakamura S,
Imamura Y, Miyazaki T, et al: Multilocus sequence typing of
Cryptococcus neoformans in non-HIV associated cryptococcosis in
Nagasaki, Japan. Med Mycol. 51:252–260. 2013. View Article : Google Scholar
|
|
6
|
Saijo T, Chen J, Chen SC, Rosen LB, Yi J,
Sorrell TC, Bennett JE, Holland SM, Browne SK and Kwon-Chung KJ:
Anti-granulocyte-macrophage colony-stimulating factor
autoantibodies are a risk factor for central nervous system
infection by Cryptococcus gattii in otherwise immunocompetent
patients. MBio. 5:e00912–e00914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez FO, Helming L and Gordon S:
Alternative activation of macrophages: An immunologic functional
perspective. Annu Rev Immunol. 27:451–483. 2009. View Article : Google Scholar
|
|
10
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor PR, Martinez-Pomares L, Stacey M,
Lin HH, Brown GD and Gordon S: Macrophage receptors and immune
recognition. Annu Rev Immunol. 23:901–944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiménez-Dalmaroni MJ, Radcliffe CM, Harvey
DJ, Wormald MR, Verdino P, Ainge GD, Larsen DS, Painter GF,
Ulevitch R, Beutler B, et al: Soluble human TLR2 ectodomain binds
diacylglycerol from microbial lipopeptides and glycolipids. Innate
Immun. 21:175–193. 2015. View Article : Google Scholar :
|
|
15
|
Nakamura K, Miyagi K, Koguchi Y, Kinjo Y,
Uezu K, Kinjo T, Akamine M, Fujita J, Kawamura I, Mitsuyama M, et
al: Limited contribution of Toll-like receptor 2 and 4 to the host
response to a fungal infectious pathogen, Cryptococcus neoformans.
FEMS Immunol Med Microbiol. 47:148–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yauch LE, Mansour MK, Shoham S, Rottman JB
and Levitz SM: Involvement of CD14, toll-like receptors 2 and 4,
and MyD88 in the host response to the fungal pathogen Cryptococcus
neoformans in vivo. Infect Immun. 72:5373–5382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
20
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang C and Wei W: The miRNA expression
profile of the uveal melanoma. Sci China Life Sci. 54:351–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baltimore D, Boldin MP, O'Connell RM, Rao
DS and Taganov KD: MicroRNAs: New regulators of immune cell
development and function. Nat Immunol. 9:839–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lodish HF, Zhou B, Liu G and Chen CZ:
Micromanagement of the immune system by microRNAs. Nat Rev Immunol.
8:120–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Case SR, Martin RJ, Jiang D, Minor MN and
Chu HW: MicroRNA-21 inhibits toll-like receptor 2 agonist-induced
lung inflammation in mice. Exp Lung Res. 37:500–508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monk CE, Hutvagner G and Arthur JS:
Regulation of miRNA transcription in macrophages in response to
Candida albicans. PLoS One. 5:e136692010. View Article : Google Scholar
|
|
27
|
Gantier MP: New perspectives in MicroRNA
regulation of innate immunity. J Interferon Cytokine Res.
30:283–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nahid MA, Pauley KM, Satoh M and Chan EK:
miR-146a is critical for endotoxin-induced tolerance: IMPLICATION
IN INNATE IMMUNITY. J Biol Chem. 284:34590–34599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hardison SE and Brown GD: C-type lectin
receptors orchestrate antifungal immunity. Nat Immunol. 13:817–822.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feriotti C, Loures FV, Frank de Araujo E,
da Costa TA and Calich VL: Mannosyl-recognizing receptors induce an
M1-like phenotype in macrophages of susceptible mice but an M2-like
phenotype in mice resistant to a fungal infection. PLoS One.
8:e548452013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
Type I IFN production in macrophages by targeting TRAF6, IRAK1, and
IRAK2. J Immunol. 183:2150–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang P, Hou J, Lin L, Wang C, Liu X, Li D,
Ma F, Wang Z and Cao X: Inducible microRNA-155 feedback promotes
type I IFN signaling in antiviral innate immunity by targeting
suppressor of cytokine signaling 1. J Immunol. 185:6226–6233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tili E, Michaille JJ, Cimino A, Costinean
S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, et al:
Modulation of miR-155 and miR-125b levels following
lipopolysaccharide/TNF-alpha stimulation and their possible roles
in regulating the response to endotoxin shock. J Immunol.
179:5082–5089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jazdzewski K, Liyanarachchi S, Swierniak
M, Pachucki J, Ringel MD, Jarzab B and de la Chapelle A:
Polymorphic mature microRNAs from passenger strand of pre-miR-146a
contribute to thyroid cancer. Proc Natl Acad Sci USA.
106:1502–1505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pauley KM, Satoh M, Chan AL, Bubb MR,
Reeves WH and Chan EK: Upregulated miR-146a expression in
peripheral blood mononuclear cells from rheumatoid arthritis
patients. Arthritis Res Ther. 10:R1012008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamasaki K, Nakasa T, Miyaki S, Ishikawa
M, Deie M, Adachi N, Yasunaga Y, Asahara H and Ochi M: Expression
of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum.
60:1035–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y,
Huang X, Zhou H, de Vries N, Tak PP, et al: MicroRNA-146A
contributes to abnormal activation of the type I interferon pathway
in human lupus by targeting the key signaling proteins. Arthritis
Rheum. 60:1065–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu S, He L, Li Y, Wang T, Feng L, Jiang L,
Zhang P and Huang X: miR-146a facilitates replication of dengue
virus by dampening interferon induction by targeting TRAF6. J
Infect. 67:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seki E and Brenner DA: Toll-like receptors
and adaptor molecules in liver disease: Update. Hepatology.
48:322–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao JL, Rao DS, Boldin MP, Taganov KD,
O'Connell RM and Baltimore D: NF-kappaB dysregulation in
microRNA-146a-deficient mice drives the development of myeloid
malignancies. Proc Natl Acad Sci USA. 108:9184–9189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu
J, Sun Z and Shen WF: MiR-146a inhibits oxidized low-density
lipoprotein-induced lipid accumulation and inflammatory response
via targeting toll-like receptor 4. FEBS Lett. 585:854–860. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mei J, Bachoo R and Zhang CL:
MicroRNA-146a inhibits glioma development by targeting Notch1. Mol
Cell Biol. 31:3584–3592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takeda K: Evolution and integration of
innate immune recognition systems: The Toll-like receptors. J
Endotoxin Res. 11:51–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li L, Cousart S, Hu J and McCall CE:
Characterization of interleukin-1 receptor-associated kinase in
normal and endotoxin-tolerant cells. J Biol Chem. 275:23340–23345.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boone DL, Turer EE, Lee EG, Ahmad RC,
Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et
al: The ubiquitin-modifying enzyme A20 is required for termination
of Toll-like receptor responses. Nat Immunol. 5:1052–1060. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nahid MA, Satoh M and Chan EK: Mechanistic
role of microRNA-146a in endotoxin-induced differential
cross-regulation of TLR signaling. J Immunol. 186:1723–1734. 2011.
View Article : Google Scholar
|