Introduction
Pulmonary arterial hypertension (PAH) is a complex
and life-threatening disease, the characteristics of which are the
continuous and evolutional rise in pulmonary vascular pressure,
remodeling of the arteries, right ventricular failure and
eventually, death (1). Several
factors are involved in the development of the disease, including
vasoconstriction, thrombosis and remodeling which have been widely
recognized. Apart from these, inflammatory mechanisms also play an
important role in both experimental and human pulmonary
hypertension (PH) (2–4). The actions of immunity and
inflammation affect multiple types of PH, such as idiopathic PAH
and diseases associated with PAH, accompanied by the evaluation of
circulating antibodies and pro-inflammatory cytokines, such as
interleukin-1 (IL)-1 and -6 (3,4).
The transcription factor, nuclear factor-κB (NF-κB),
which is made up of a homodimer or heterodimer of different
members, includes p65, p50, p52, cRel and RelB (5–7).
NF-κB is widely expressed to mediate various biological processes
as a transcription factor, which activates the genes involved in
inflammation, immune responses, apoptosis and cell growth (5,8–10).
The most common dimers of NF-κB are the p65 and p50 dimers. IκB,
the inhibitory unit of NF-κB, combines with NF-κB through its
C-terminal specific predominate ankyrin repeat sequence, and
inhibits the transfer of NF-κB to the nucleus by covering the
nuclear localization sequence (NLS). In resting cells, the
complexes of NF-κB and IκB predominate in an inactive form in the
cytoplasm. When the cells are stimulated by extracellular signals,
IκB kinase (IKK) complex activation predominates IκB
phosphorylation, and exposes NF-κB. 'Free' NF-κB then quickly
shifts to the nucleus, combined with the predominance of a specific
κB sequence, and the induction of related gene transcription. IKK
is made-up of IKKα and β (8–10).
p65 is an important factor in the progress of inflammation and
immunity. Nuclear p65 can cause a large amount of gene
transcription in reaction to inflammatory stimuli as an active form
(5,11).
Nuclear factor of activated T cells-1 (NFAT-1) has
been reported to play a significant role in the physiopathology of
pulmonary arterial smooth muscle cells (PASMCs) as an integrator of
calcium signaling (12).
Moreover, the transcription factor, NFAT-1, participates in chronic
hypoxia induced pulmonary arterial remodeling by upregulating
α-smooth muscle actin (α-SMA) in rats (12,13). Activated NFAT participates in the
process of the hypoxia-induced proliferation of human pulmonary
artery smooth muscle cells (PASMCs) (14). There is also evidence to indicate
that NFAT factors can interact with other nuclear transcription
factors and promote the transcription of numerous inflammatory
cytokines, such as activator protein 1 (AP-1), GATA binding protein
4 (GATA-4), myocyte enhancer factor-2 (MEF-2), NF-κB p65, tumor
necrosis factor (TNF) and ILs (4,15).
The biosynthesis of serotonin (5-hydroxytryptamine,
5-HT) is mediated by tryptophan hydroxylase (TPH). One
non-reversible inhibitor of TPH-1, 4-chloro-DL-phenylalanine
(PCPA), has been shown to reduce monocrotaline (MCT)-induced
inflammation and remodeling in rat lungs (16). Nevertheless, the synthesis amongst
these transcriptional effector signaling pathways and their role in
inflammation and remodeling have not been discussed in PAH to date,
at least to the best of our knowledge. It is not known whether the
inhibitory effects of PCPA on PAH are associated with the NFAT and
NF-κB pathways. Therefore, in this study, we aimed to explore the
mechanisms underlying the protective effects of PCPA against
MCT-induced PAH, focusing on NFAT and NF-κB in particular.
Materials and methods
Establishment of the animal models
All experiments involving rats were approved by the
animal care and experimental protocols which complied with the
Institutional Animal Care and Use Committee of China Medical
University, Shenyang, China. All experiments abided by the
guidelines of China Medical University and were approved by the
local authority. Sixty-eight male Sprague-Dawley (SD) rats
(weightin, 180±10 g) were obtained from the Animal Resource Centre,
China Medical University (certificate no. Liaoning 034). They were
separated into the following groups: i) the control group, which
received the vehicle (1:4 mixture of dehydrated ethanol-normal
saline) + physiological saline (0.9%); ii) the MCT group, which
received MCT + physiological saline; iii) the MCT + P1 group, which
received MCT + PCPA at 50 mg/kg once a day; and iv) the MCT + P2
group, which received MCT + PCPA at 100 mg/kg once a day.
The MCT and 2 PCPA treatment groups of rats were
administered either a single dose of MCT (Sigma-Aldrich, St. Louis,
MO, USA) at 60 mg/kg body weight, which was dissolved in the
vehicle (1:4 mixture of dehydrated ethanol-normal saline) by
intraperitoneal (i.p.) injections to induce PAH, or the same volume
of the vehicle, as previously described (2). The PCPA-effected groups of rats
received PCPA (i.p.), which dissolved in physiological saline
(0.9%) continuously for 21 days once a day. Over the same period,
the rats in the control and MCT group were administered an equal
volume of the vehicle (0.9% physiological saline, i.p.). All the
experimental animals were fed with sufficient food and water, and
were kept under a natural environment (day/night round at 50–70%
dampness and 18–22°C temperature). The parameters measured by us in
the complete experiment were carried out in a blinded manner.
Hemodynamic measurements
On the 22nd day, all rats were narcotized with 3%
sodium pentobarbital (40 mg/kg). The data of pulmonary arterial
pressure (PAP) and systemic arterial pressure (SAP) were kept as
records under the same factors, as previously described (17,18). To measure PAP, a PV-1 catheter was
inserted into the right jugular vein via the right atrium and
ventricle, and was finally introduced into the pulmonary artery
(16,18). A polyethylene catheter (PE-50) was
introduced into the right carotid artery for the measurement of SAP
(16,18). Hemodynamic indexes were surveyed
by a pressure pickup and a polygraph system (RM6000; Nihon Kohden,
Tokyo, Japan) for recording.
Pulmonary arterial morphometry
We performed histopathological observations as
previously described (16). We
used 4% paraform and sterile physiological saline to exsanguinate
the rats. We then detached the right inferior lobe of the lungs and
fixed them with 4% paraform. After embedding in paraffin, the lungs
were sectioned to produce 5-µm-thick sections which were
stained with hematoxylin and eosin (H&E; ZLI-9615; ZSGB-BIO,
Beijing, China). Twelve pulmonary arteries per rat were
investigated using a Metamorph (Universal Imaging Corp., West
Chester, PA, USA)/DP10/BX51 (Olympus, Tokyo, Japan) system in 3
rats/group (16). Percentage
medial muscle thickness was measured to reflect pulmonary
remodeling, which was calculated as follows: pulmonary wall
thickness (%) = (external diameter - internal diameter)/external
diameter ×100%, as previously described (16).
Collagen and elastic fiber dyeing
The paraffin-embedded sections were stained with
Orcein stain, Van Gieson stain or Victoria-ponceau's double stain
(all from Shanghai Jing Ke Chemical Technology Co., Ltd., Shanghai,
China) to localize elastin and collagen in lungs and pulmonary
arteries.
Immunohistochemistry
We used the ultrasensitive SP and diaminobenzidine
(DAB) staining kits (both from Maixin-Bio, Fuzhou, China) to stain
the paraffin-embedded lung tissues and arterial sections. Primary
rabbit anti-NFAT-1 (BA2799; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) polyclonal antibody was diluted 1:200, rabbit
polyclonal anti-NF-κB p65 (sc-372; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was diluted 1:300. We incubated the samples
with 0.01 M phosphate-buffered saline (PBS) in place of the primary
antibody as a negative control. We then used a BX51 microscope
(Olympus) to analyze the digital images. In eaach group, we
observed 15 pulmonary arteries from 3 rats, the external diameter
being 60–80 µm. We used average optical density to calculate
the NFAT-1 and p65 protein levels.
Preparation of total protein
To obtain total protein, the inferior lobe of the
left lungs and pulmonary arteries were homogenized using a polytron
homogenizer (Kinematica, Lucerne, Switzerland). We then utilized
the Heraeus Sepatech to separate the homogenate at 4°C and 15,000 ×
g for 20 min. The supernatant was collected and storing it at −70°C
for further analysis. The total proteins were determined by the
Bradford method.
Preparation of nuclear and cytosolic
protein
Nuclear and cytosolic proteins were prepared as
previously described (19). The
fresh samples were homogenized and the homogenate was then
collected using a nuclear protein extraction kit (Beyotime
Institute of Biotechnology, Haimen, China). The extract methods of
nuclear fractions and cytosolic fractions were according to the
manufacturer's instructions. We stored the aliquots at −70°C until
analysis. Using BCA (Beyotime Institute of Biotechnology) method
determined the nuclear and cytosolic protein concentration.
Western blot analysis
The same amount of protein was segregated through
SDS-PAGE and electro-transferred onto polyvinylidene difluoride
(PVDF) membranes, as previously described (17). The PVDF membranes were incubated
with 5% non-fat dry milk, 1X TBS and 0.05% Tween-20 for 2 h at room
temperature. The primary antibodies, rabbit polyclonal anti-NFAT-1
antibody (1:400, BA2799; Wuhan Boster Biological Technology, Ltd.),
rabbit polyclonal anti-extracellular signal-regulated kinase
(ERK)1/2 antibody (1:500, sc-292838), mouse monoclonal
anti-p-ERK1/2 (1:500, sc-81492), rabbit polyclonal anti-IKKα
antibody (1:500, sc-7218), rabbit polyclonal anti-p-IKKα antibody
(1:600, sc-101706) and rabbit polyclonal anti-NF-κB p65 antibody
(1:400, sc-372) (all from Santa Cruz Biotechnology, Inc.), goat
polyclonal anti-intercellular adhesion molecule-1 (ICAM-1) antibody
(1:200, zs-1511; Bioworld Technology, Inc., St. Louis Park, MN,
USA), goat polyclonal anti-IL-6 antibody (1:300, sc-1265), mouse
monoclonal anti-α-tubulin antibody (1:2,000, sc8035) and mouse
polyclonal anti-β-actin antibody (1:2,000, sc-47778) (all from
Santa Cruz Biotechnology, Inc.) were incubated with the membranes
at 4°C overnight. The following day, the PVDF membranes were
incubated with the corresponding horseradish peroxidase
(HRP)-conjugated secondary antibodies at room temperature for 2 h.
Subsequently, using the super ECL plus (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) were observed the immunoreactive bands.
Densitometry was used to quantify the relative protein expression
using Quantity One software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
The data are presented as the means ± standard
deviation (SD). SPSS version 16.0 sofware (SPSS, Inc., Chicago, IL,
USA) was used to carry out all the statistic analyses. One-way
analysis of variance (ANOVA) with Fisher's least significant
difference (LSD) or Dunnett's T3 test were used for statistical
comparative analysis. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Influence of PCPA on MCT-induced
haemodynamics and morphological changes in rats
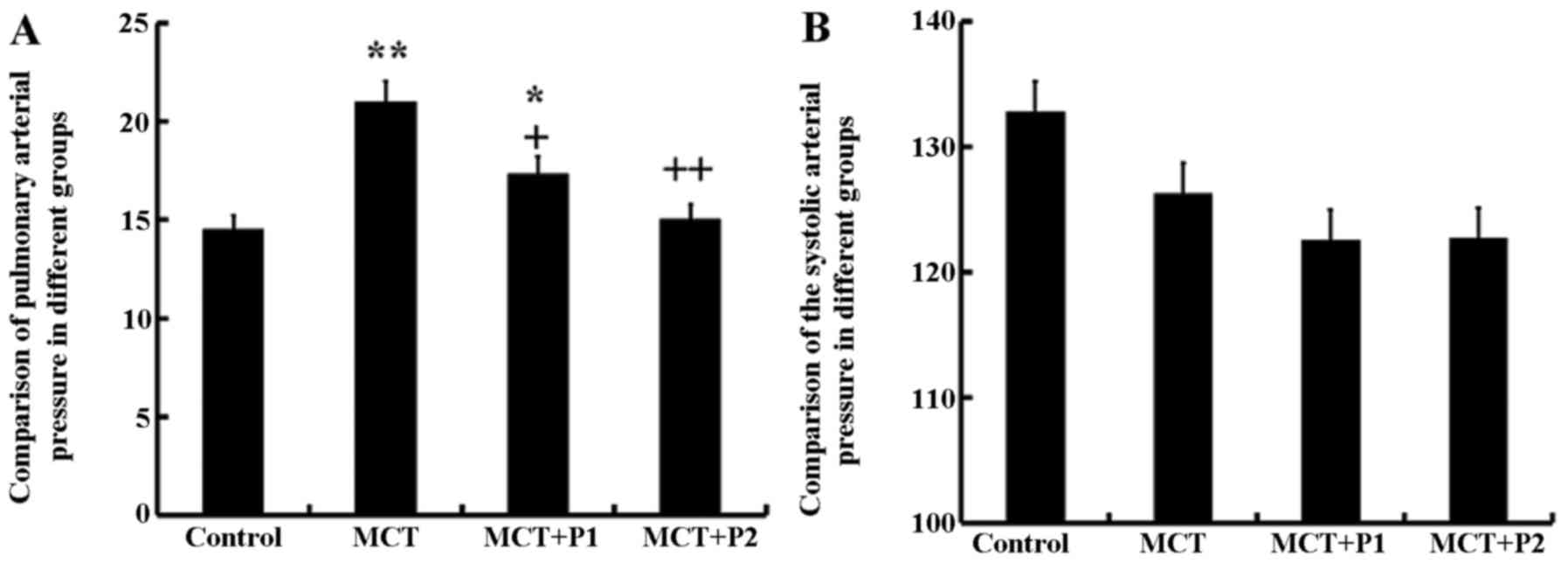
The rats in all groups were fed for 21 days. In the
MCT group, the mean PAP was significantly increased (21.06±3.4
mmHg, P<0.01 vs. control group). PCPA significantly inhibited
the mean PAP; in the MCT + P1 group PAP decreased to 17.4±2.5 mmHg
(P<0.05) and in the MCT + P2 group, it decreased to 15.1±3.1
mmHg (P<0.01) compared with the MCT group. However, the values
of SAP in the 4 groups exhibited no significant differences
(Fig. 1).
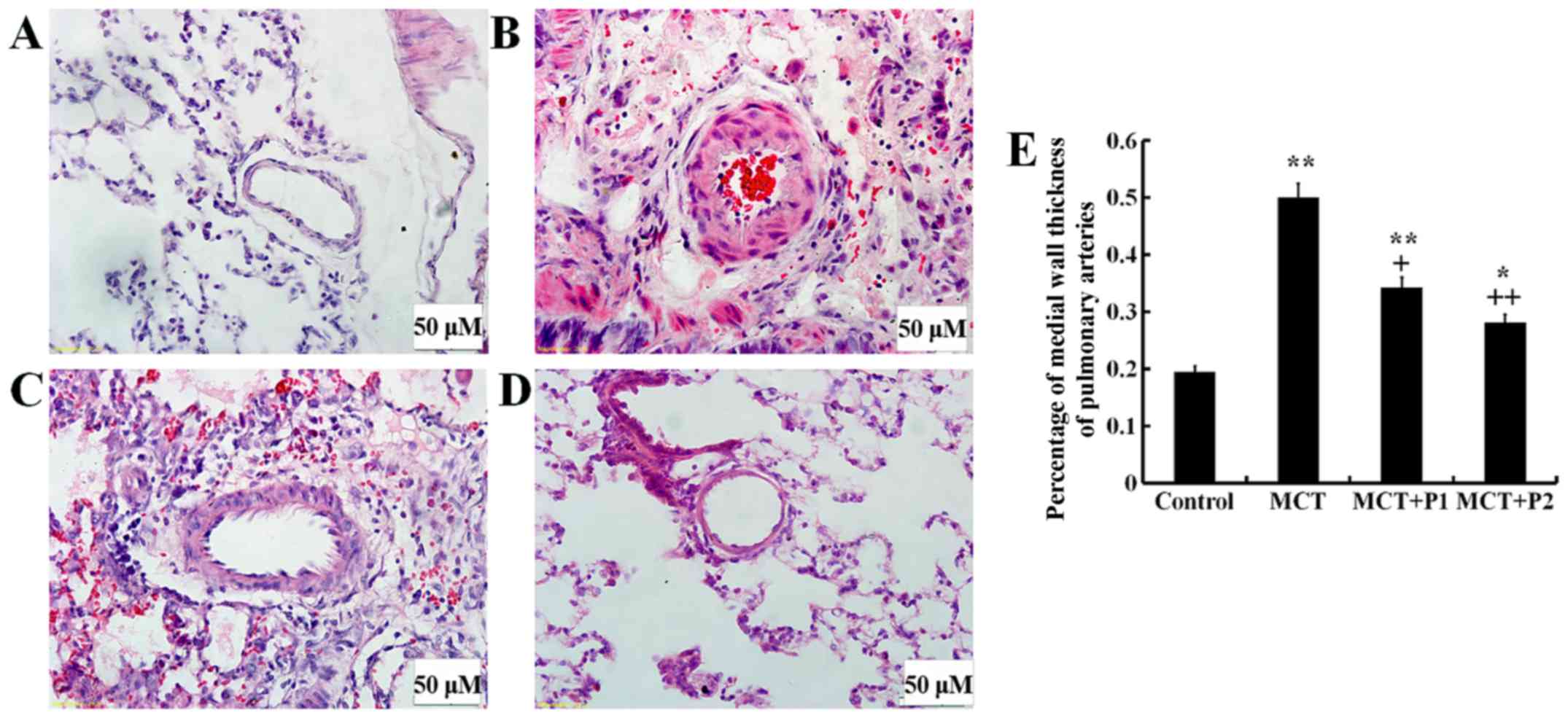
The muscularization of lung tissue was examined
under a light microscope. In the MCT group, the thickness of the
arterial wall was significantly increased (49.7±9.2%, P<0.01 vs.
control 19.1±7.7%). PCPA inhibited this thickness in a
dose-dependent manner in the MCT + P1 group (34.3±8.2%, P<0.01
vs. MCT) and MCT + P2 group (28.1±10.7%, P<0.01 vs. MCT)
(Fig. 2).
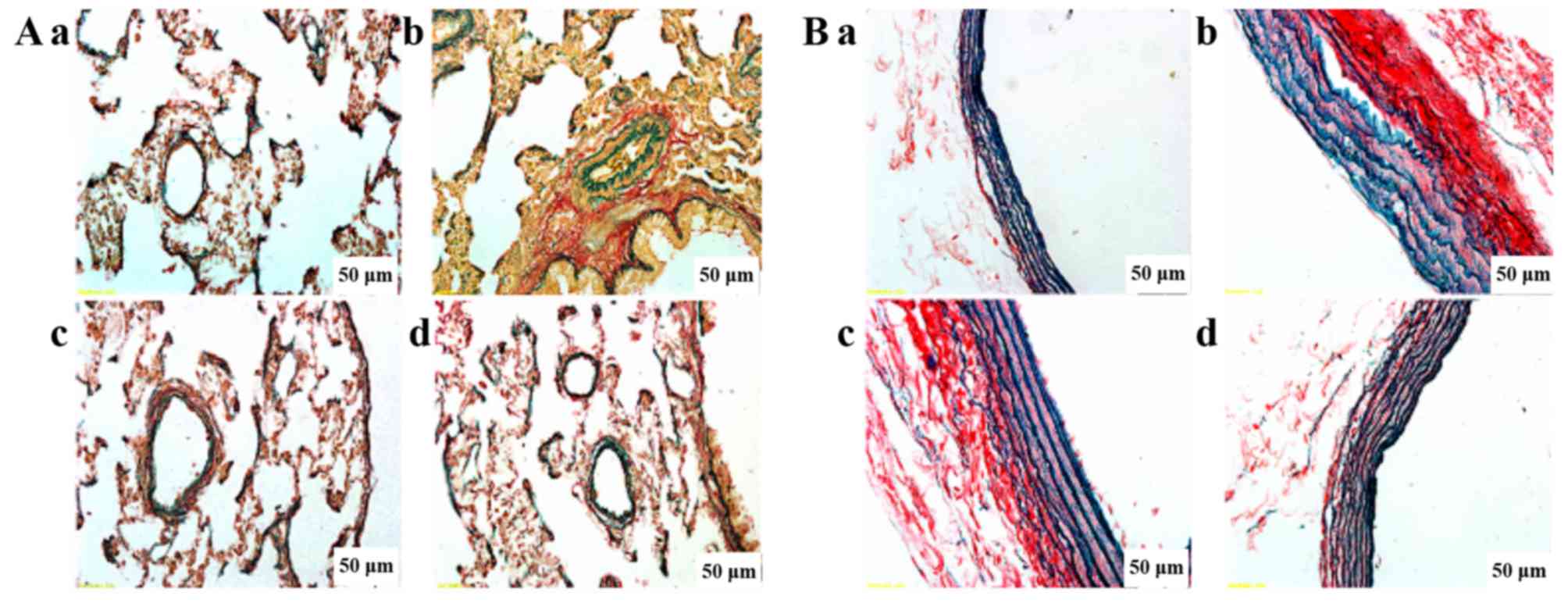
Evaluation of elastin and collagen
Fig. 3 shows
double staining in elastin and collagen in the lungs. We observed
that the collagen was conspicuously diffused and increased in the
MCT group, and the elastic fibers were also disrupted and increased
in the MCT group. PCPA inhibited collagen deposition and decreased
the structural destruction of the lungs in a dose-dependent manner.
In particular, the high dose of PCPA markedly inhibited elastin and
collagen hyperplasia and maintained the integrity of the arterial
structure simultaneously.
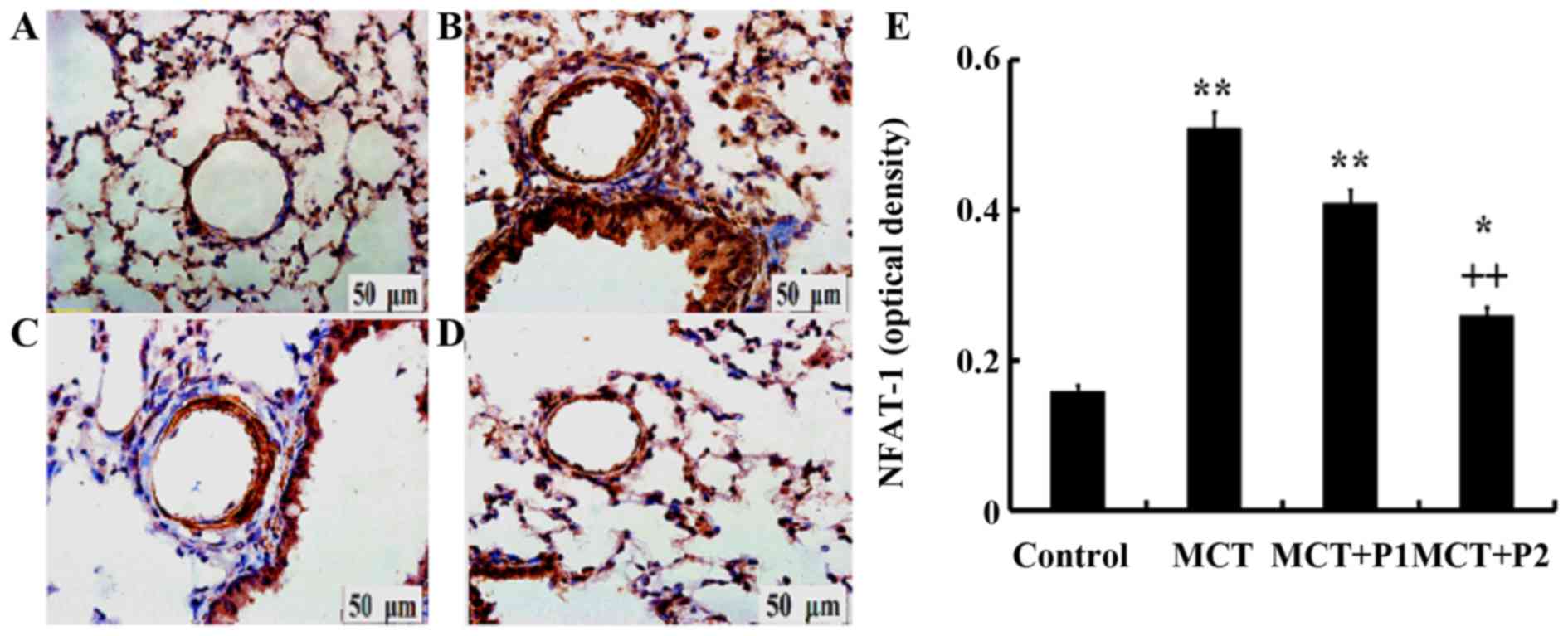
Influence of PCPA on NFAT-1
expression
Immunohistochemistry revealed a stronger expression
of NFAT-1 in the group with MCT-induced PAH using anti-NFAT-1
antibodies compared with the controls. PCPA (50 mg/kg/day) had
little influence on NFAT-1 protein in rats. However, treatment with
PCPA at 100 mg/kg/day led to marked decrease in the expression of
NFAT-1 compared with the MCT group, in which NFAT-1 expression was
increased (Fig. 4).
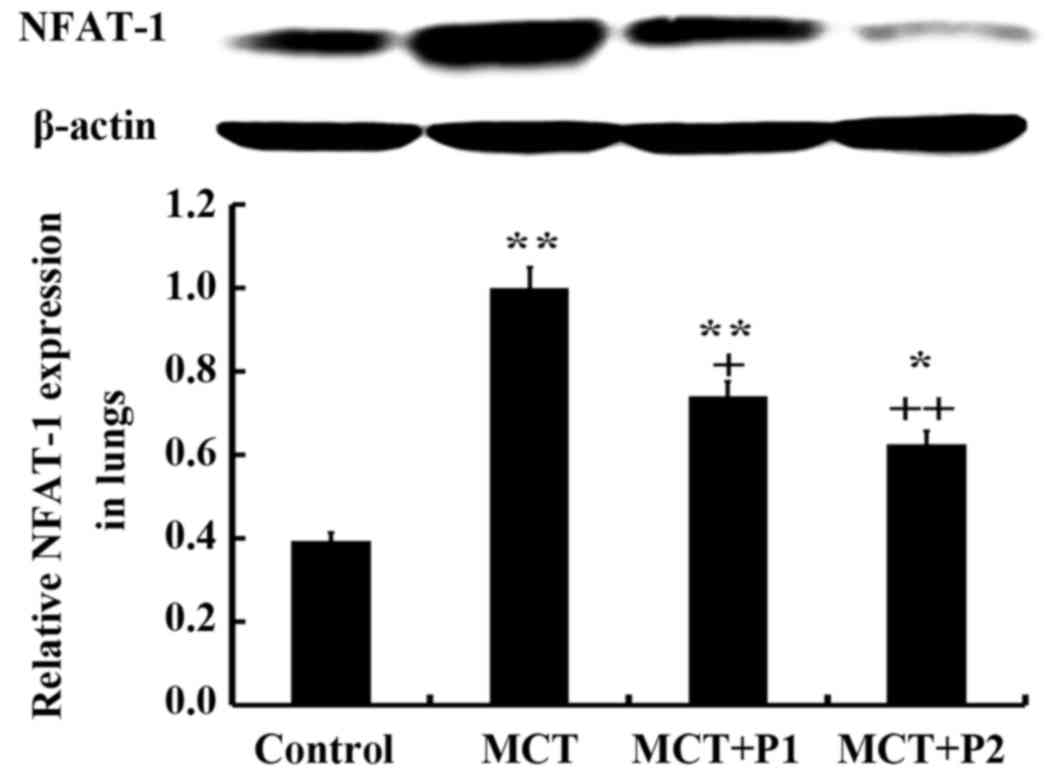
The results of western blot analysis revealed that
the rats in the MCT group had significantly elevated protein
expression levels of NFAT-1 compared with the control group rats
(1.03±0.01 vs. 0.59±0.04, respectively, P<0.01). PCPA (50 mg/kg)
suppressed NFAT-1 protein expression (0.78±0.04, P<0.05 vs.
MCT). However, PCPA at 100 mg/kg decreased NFAT-1 protein
expression, which was increased by MCT more significantly
(0.67±0.05, P<0.01 vs. MCT) (Fig.
5).
Evaluation of NF-κB p65
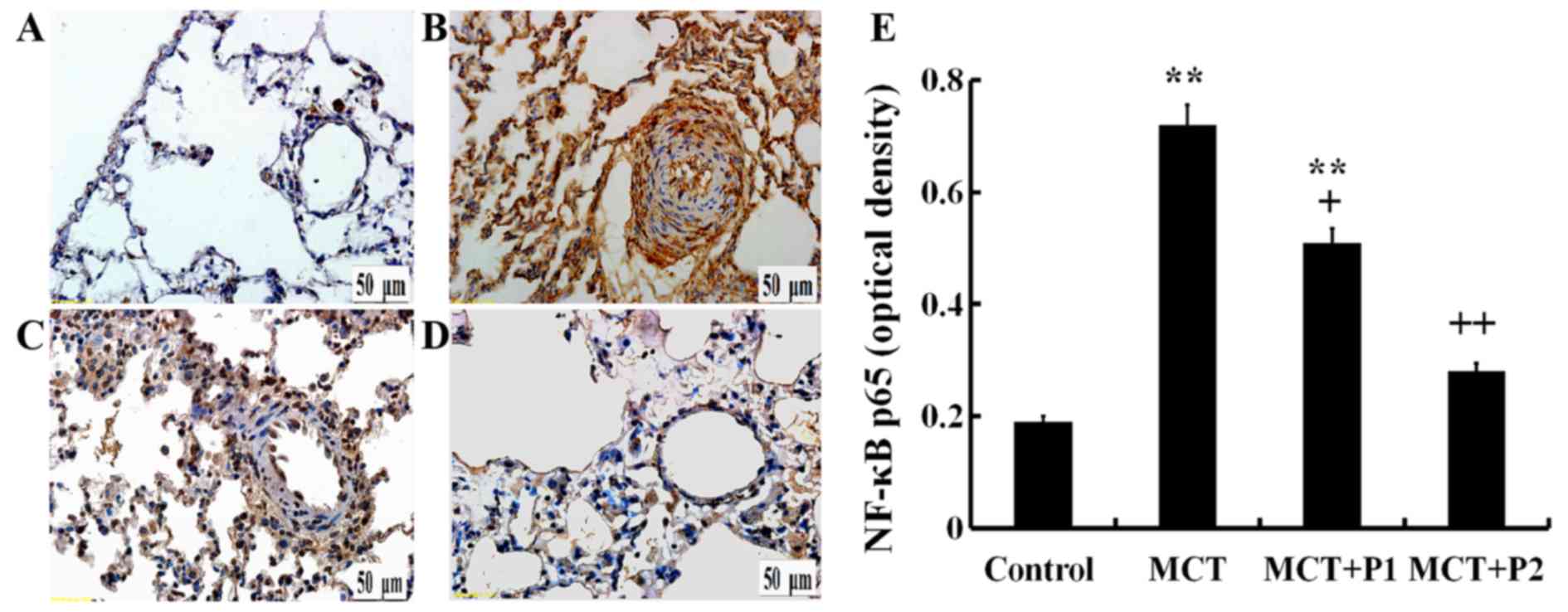
Immunohistochemistry revealed a stronger p65
expression in the rat lungs of the MCT group using anti-p65
antibodies compared with the control group. Treatment with PCPA at
50 mg/kg/day led to a slight, yet significant decrease in p65
protein expression. The results revealed that treatment with PCPA
at 100 mg/kg/day even more significantly reduced the increase in
p65 expression induced by MCT (Fig.
6).
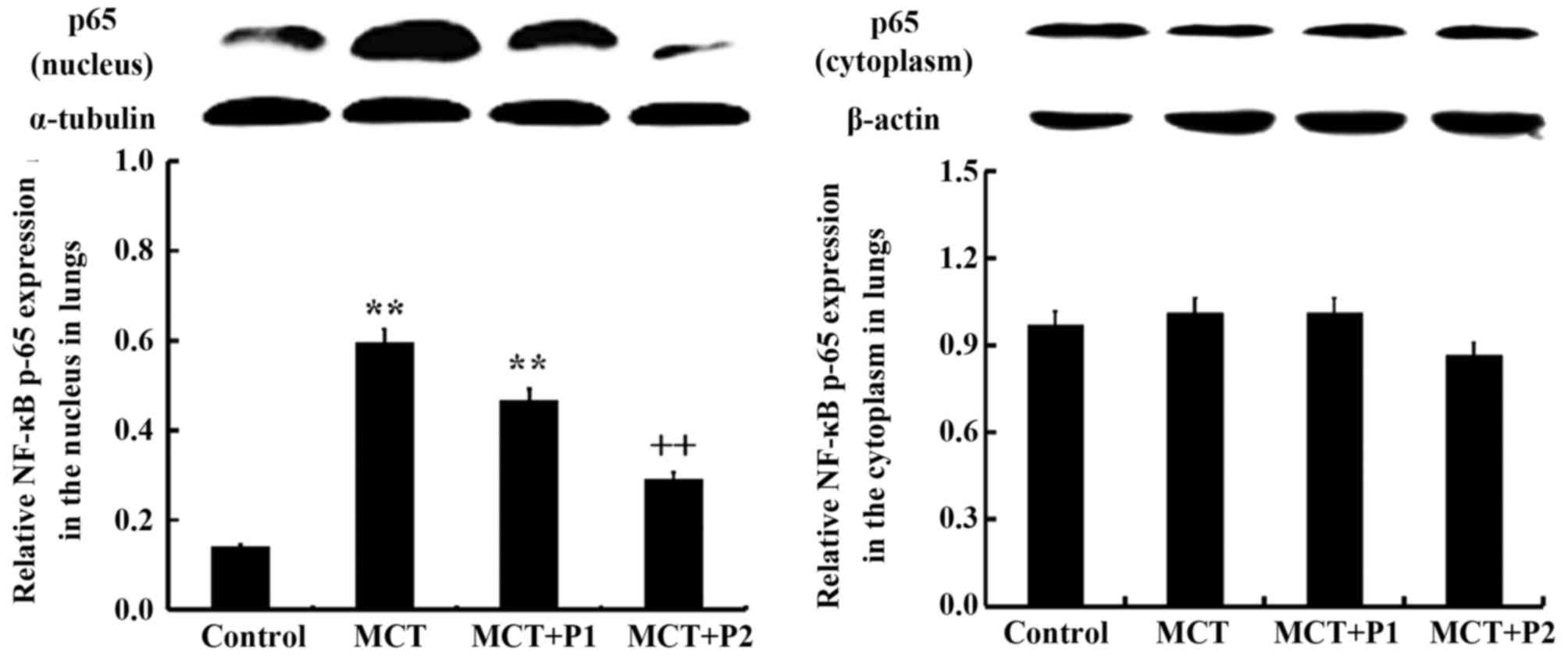
Western blot analysis revealed that the expression
of p65 protein was markedly increased in the nuclear protein
isolated from the lungs of the rats in the MCT group compared with
those of the control group (P<0.01 vs. control). PCPA at 50
mg/kg suppressed MCT-induced nuclear p65 expression, but without
statistical significance (P>0.05 vs. MCT). However, PCPA at 100
mg/kg markedly attenuated the expression of nuclear p65 induced by
MCT (P<0.01 vs. MCT) (Fig. 7).
No significant difference was observed in the cytoplasmic
expression of p65 among the 4 groups.
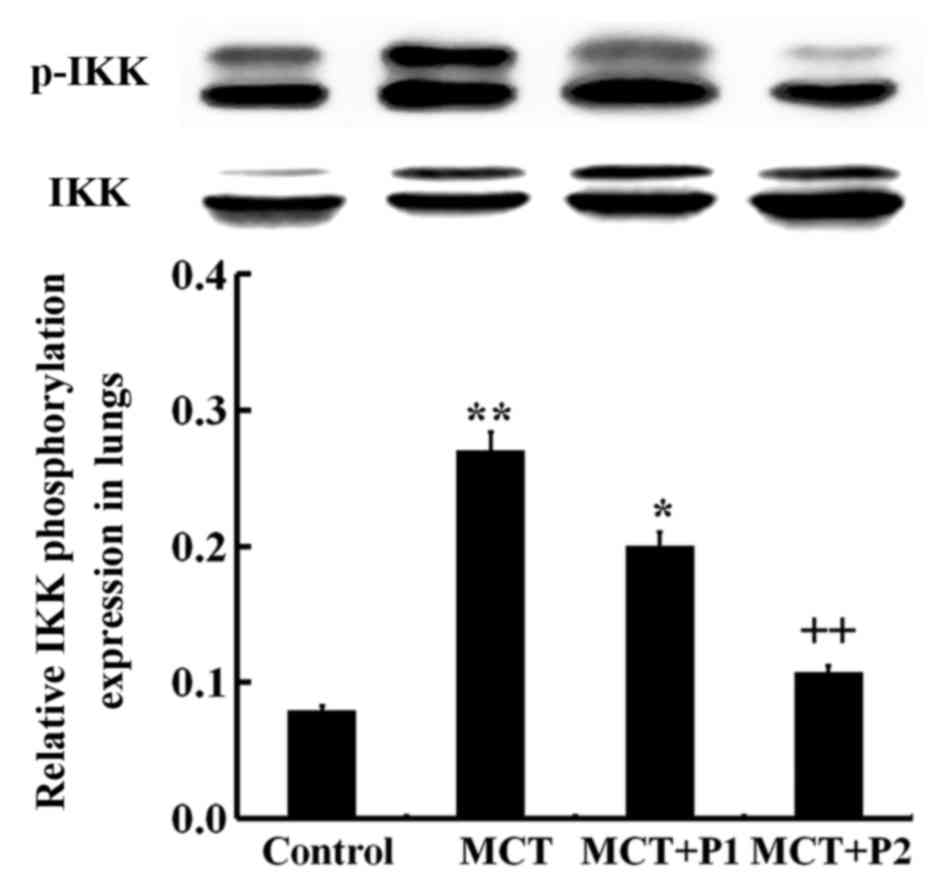
Effect of PCPA on ERK, p-ERK, IKK and
p-IKK expression
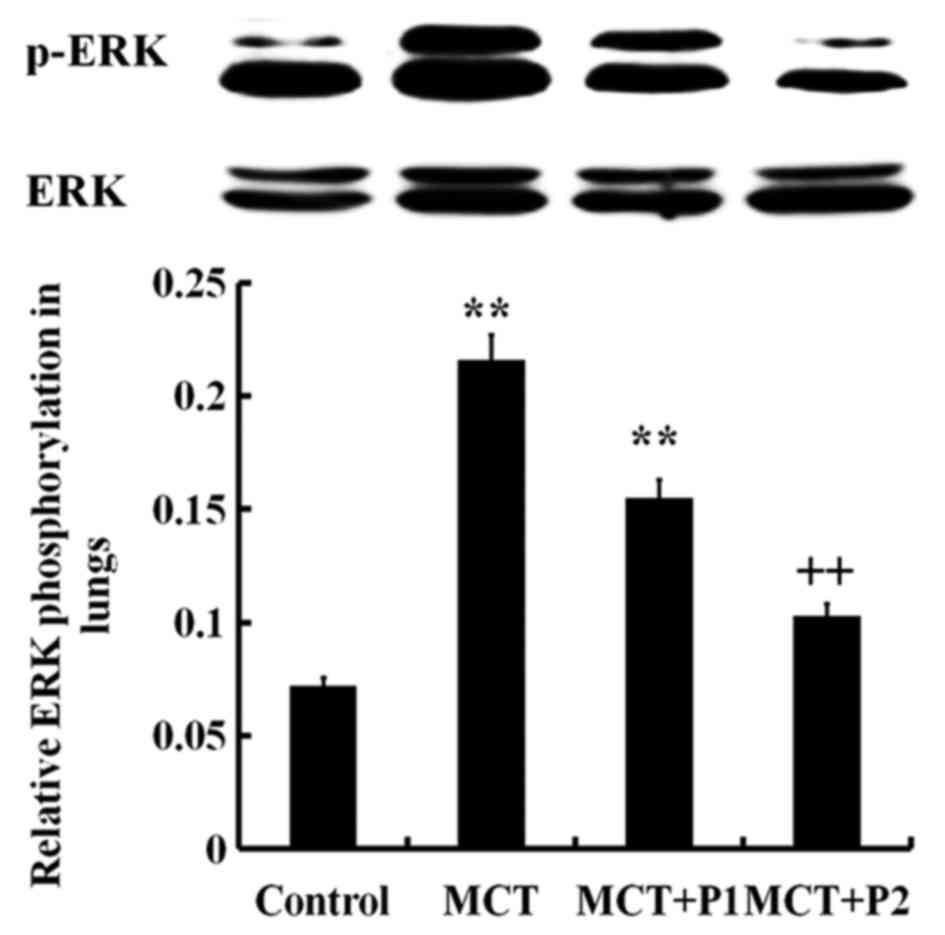
Western blot analysis revealed an increased level of
phosphorylated ERK in the lungs of the MCT group compared with the
control group (P<0.01; Fig.
8). PCPA decreased the ERK phosphorylation level induced by
MCT, particularly in the MCT + P2 group (P<0.01 vs. MCT)
(Fig. 8). The IKK phosphorylation
level in the MCT group was sign ificantly increased (P<0.01 vs.
control). Treatment with PCPA at 100 mg/kg/day notably decreased
IKK phosphorylation in the lungs (P<0.01 vs. MCT) (Fig. 9). These results indicated that
PCPA inhibited the MCT-induced activation of ERK and IKK in the
lungs.
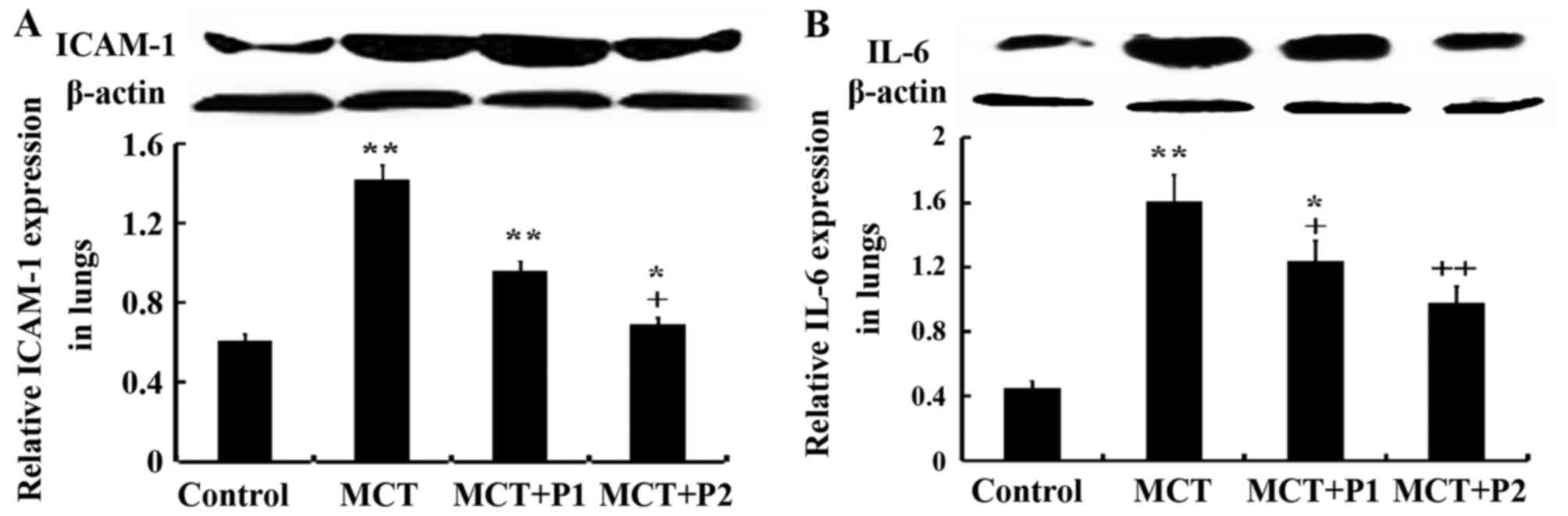
Influence of PCPA on ICAM-1 and IL-6
expression
Fig. 10 shows
that ICAM-1 and IL-6 expression was markedly increased by MCT
compared to the control. The expression of ICAM-1 was elevated from
0.45±0.02 in the control group to 1.61±0.26 in the MCT group
(P<0.01). PCPA at 50 mg/kg/day decreased ICAM-1 expression to
1.24±0.12 (P>0.05) compared with the MCT group, and PCPA at 100
mg/kg/day reduced ICAM-1 expression to 0.98±0.15 (P<0.05).
Similarly, the expression of IL-6 was markedly increased by MCT,
from 0.61±0.04 in the control group to 1.42±0.21 (P<0.01) in the
MCT group. PCPA at 50 mg/kg/day decreased IL-6 expression to
0.96±0.05 (P<0.05) compared with the MCT group, and PCPA at 100
mg/kg/day reduce IL-6 expression to 0.69±0.15 (P<0.01) (Fig. 10). Thus, PCPA, particularly at a
high dose reduced the expression of IL-6 and ICAM-1.
Discussion
In this study, we demonstrated that the expression
levels of NFAT-1, nuclear NF-κB p65, phosphorylated ERK, IKK, IL-6
and ICAM-1 were markedly elevated by MCT in the lungs. PCPA
markedly reduced the expression of NFAT-1 and proteins related to
the NF-κB signaling pathway, and thus inhibited lung inflammation
and remodeling. This is a demonstration of the inter-dependence
between NFAT and NF-κB signaling in mediating lung tissue
inflammation, which has an effect on MCT-induced PAH; the 5-HT
signaling pathways may be involved in this process.
The occurrence of PAH is a complex
pathophysiological process, and its pathogenesis is not yet
completely clear. There is evidence to indicate that the synthesis
of 5-HT may influence the pathological process of PAH. Our
laboratory previously has proved that PCPA inhibited 5-HT, TPH-1,
serotonin transporter (SERT), serotonin receptor and associated
serotonin signaling pathways (16). The PCPA exerted protective effects
against PCPA by attenuating MCT-induced lung inflammation and
remodeling, and this was related to the reduction of SERT, TPH-1,
matrix metalloproteinases (MMPs), tissue inhibitors of
metalloproteinases (TIMPs) and some inflammatory factors (16). Evidence indicates that PCPA
inhibits inflammation in lungs by suppressing TPH in allergic
airway inflammation models (20).
Fluoxetine, is an inhibitor of SERT, and its
inhibitory effects on pulmonary vascular remodeling in MCT-induced
PAH are mediated through the downregulation of the ERK, Akt and
RhoA/ROCK signaling pathways (17). In addition, fluoxetine suppresses
lung tissue inflammation (17,18). Ketanserin, a 5-HT2A receptor
antagonist, had been shown to modestly suppress inflammation and
eosinophil infiltration in allergic airway inflammation models
(21). These findings demonstrate
that 5-HT is involved in the processes of remodeling and lung
inflammation in PAH. The phenomenon can be decreased by inhibiting
the 5-HT upstream and downstream signaling pathways.
NFAT, as a transcription factor, promotes the
transcription of a number of inflammatory cytokines, such as ILs
and TNF, and can activate T and B cells (22). Bonnet et al (23) demonstrated that in PAH, including
scleroderma-associated PAH and idiopathic PAH (IPAH), NFATc2 is
upregulate and activated in some circulating inflammatory cells. A
recent study found that following treatment with 5-HT, calcineurin
and NFAT pathway activation occurred in PASMCs, which was related
to dosage (24). In addition,
following treatment with sidenafil, an inhibitor of PDE5, the
serotonin-induced activation of calcineurin/NFATc2 signaling
pathway was suppressed, which suggested that one of the major
targets of sildenafil was the calcineurin/NFAT cascade in the
pulmonary system (24). In the
present study, the expression of NFAT-1 was markedly increased in
the MCT group, which was inhibited by PCPA in a dose-dependent
manner. Consistent with previous results, inference with PCPA may
regulate NFAT-1 by inhibiting serotonin.
NF-κB is involved in inflammation, immunoreactions
and various other biological processes. Several factors can
activate NF-κB, including inflammatory cytokines, growth factors or
chemokines, such as transforming growth factor-β (TGF-β), serotonin
and connective tissue growth factor (CTGF). A NF-κB homodimer or a
heterodimer is formed by two types of subunits, a class of subunit
p65 and another type of subunit p50 and p52. p65, a particularly
curcial subunit, can mediate the processes of inflammation and
tumor formation (5). NF-κB can be
activated by the multi-subunit IKK composed of two catalytic
subunits, IKKα and β, which responds to various cellular stimuli,
including bacterial or viral antigens, cytokines, growth factors
and mitogens (25). In
unstimulated cells, NF-κB is usually found in the cytoplasm and
binds to inhibitory proteins IκBs (25,26). Upon activation, IκB is
phosphorylated by IKK, and thus IκBα is degraded and NF-κB is
released and then translocates to the nucleus (27,28). Rapidly, IκBα can be synthesized by
the NF-κB-mediated expression of its gene (29,30). In addition, as a pivotal mediator
in signal transduction, NF-κB is involved in the effecter phase of
inflammation when responding to multiple inflammatory cytokines,
e.g., IL-1 and TNF (31). In
addition to promoting the activation of a wide range of cytokines,
activated NF-κB also promotes the expression of ICAM-1, -2 and
other adhesion molecules and nitric oxide syntheses (NOS) by
activating endothelial cells (32–34).
In the present study, we found that the expression
levels of nuclear p65, IKK phosphorylation, ICAM-1 and IL-6 were
significantly increased by MCT in the lungs. PCPA markedly
suppressed the NF-κB p65 nuclear translocation, inhibited IKK
phosphorylation and inflammatory cytokine production. The
above-mentioned results indicated that PCPA suppressed 5-HT,
mediating the NF-κB signaling pathway. These inflammatory mediators
or cytokines can stimulate the systemic inflammatory response
syndrome, increasing the damage to the lungs.
There is synergy between NF-κB and NFAT, which can
facilitate transcriptional activation of each other in
cardiomyocytes (26). Liu et
al (26) demonstrated that a
complex was formed through the direct interaction of NFAT with p65,
promoting NF-κB nuclear translocation induced by IKKβ and NFAT
nuclear localization enhanced by p65-RHD. In accordance with the
results from other studies, our study showed that NFAT-1 promoted
IKK phosphorylation and dissociation through the IKK-NF-κB
signaling pathway, and p65 nuclear translocation. Activated NF-κB
can promote the production of large amounts of IL-6 and other
cellular factor, and can then activate endothelial cells to produce
ICAM-1.
During the progression of PAH, factors which can
regulate the stability of p65 are of importance. It has been
demonstrated that ERK plays a crucial role in transferring
inflammatory information from the extracellular environment to the
cytoplasm or nucleus, which can modulate inflammatory responses
(35,36). The ERK signaling pathway can
regulate PASMCs exposed to 5-HT during mitosis (37). The ERK and NFAT-3 signal pathways
and hyperphosphate-induced response have a marked effect on the
development of cardiac hypertrophy (38). In our previous study, MCT-induced
vascular remodeling in lungs, which was caused by the crosstalk
among SERT, RhoA/ROCK and ERK signaling pathway, was suppressed by
fluoxetine (17). Targeting NF-κB
can secondarily suppress the NFAT signaling pathway, and can thus
be considered as a original therapeutic method in putting into use
cardiac hypertrophy (26). Our
present results demonstrate that PCPA suppressed the expression and
activation of the phosphorylation ERK in a dose-dependent manner,
indicating that PCPA attenuates inflammation and remodeling in PAH
by mediating the NFAT-1 and IKK-NF-κB signaling pathways.
In conclusion, our study demonstrates that PCPA
exerts a protection effect on MCT-induced inflammation and
remodeling in lungs, which is related to the NFAT and NF-κB
signaling pathways. The detailed mechanisms however, require
further investigation.
Acknowledgments
This study was supported by the Science Research
Project of the Education Department of Liaoning Province
(LK201640), the National Natural Science Foundation of China (nos.
81273511 and 81503058) and the Natural Science Foundation of
Liaoning Province (no. 2014021065).
References
|
1
|
Lai YC, Potoka KC, Champion HC, Mora AL
and Gladwin MT: Pulmonary arterial hypertension: the clinical
syndrome. Circ Res. 115:115–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaumais MC, Ranchoux B, Montani D,
Dorfmüller P, Tu L, Lecerf F, Raymond N, Guignabert C, Price L,
Simonneau G, et al: N-acetylcysteine improves established
monocrotaline-induced pulmonary hypertension in rats. Respir Res.
15:652014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabinovitch M, Guignabert C, Humbert M and
Nicolls MR: Inflammation and immunity in the pathogenesis of
pulmonary arterial hypertension. Circ Res. 115:165–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Chami H and Hassoun PM: Immune and
inflammatory mechanisms in pulmonary arterial hypertension. Prog
Cardiovasc Dis. 55:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Sha M, Wang Q, Ma Y, Geng X, Gao Y,
Feng L and Shen Y and Shen Y: Small ubiquitin-related modifier 2/3
interacts with 65 and stabilizes it in the cytoplasm in
HBV-associated hepatocellular carcinoma. BMC Cancer. 15:6752015.
View Article : Google Scholar
|
|
6
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
8
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H and Sun SC: NF-κB in inflammation
and renal diseases. Cell Biosci. 5:632015. View Article : Google Scholar
|
|
11
|
Gilmore TD and Wolenski FS: NF-κB: where
did it come from and why? Immunol Rev. 246:14–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parpaite T, Cardouat G, Mauroux M,
Gillibert-Duplantier J, Robillard P, Quignard JF, Marthan R,
Savineau JP and Ducret T: Effect of hypoxia on TRPV1 and TRPV4
channels in rat pulmonary arterial smooth muscle cells. Pflugers
Arch. 468:111–130. 2016. View Article : Google Scholar
|
|
13
|
de Frutos S, Spangler R, Alò D and Bosc
LV: NFATc3 mediates chronic hypoxia-induced pulmonary arterial
remodeling with alpha-actin up-regulation. J Biol Chem.
282:15081–15089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Li JF, Zhao L, Liu J, Wan J, Wang
YX, Wang J and Wang C: Inhibition of SOC/Ca2+/NFAT
pathway is involved in the anti-proliferative effect of sildenafil
on pulmonary artery smooth muscle cells. Respir Res. 10:1232009.
View Article : Google Scholar
|
|
15
|
Hogan PG, Chen L, Nardone J and Rao A:
Transcriptional regulation by calcium, calcineurin, and NFAT. Genes
Dev. 17:2205–2232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai Y, Wang HM, Liu M, Wang Y, Lian GC,
Zhang XH, Kang J and Wang HL: 4-Chloro-DL-phenylalanine protects
against monocrotaline induced pulmonary vascular remodeling and
lung inflammation. Int J Mol Med. 33:373–382. 2014.
|
|
17
|
Wang HM, Wang Y, Liu M, Bai Y, Zhang XH,
Sun YX and Wang HL: Fluoxetine inhibits monocrotaline-induced
pulmonary arterial remodeling involved in inhibition of RhoA-Rho
kinase and Akt signalling pathways in rats. Can J Physiol
Pharmacol. 90:1506–1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XQ, Wang HM, Yang CG, Zhang XH, Han DD
and Wang HL: Fluoxetine inhibited extracellular matrix of pulmonary
artery and inflammation of lungs in monocrotaline-treated rats.
Acta Pharmacol Sin. 32:217–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Abate A, George AG, Weng YH and
Dennery PA: Maturational differences in lung NF-kappaB activation
and their role in tolerance to hyperoxia. J Clin Invest.
114:669–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dürk T, Duerschmied D, Müller T, Grimm M,
Reuter S, Vieira RP, Ayata K, Cicko S, Sorichter S, Walther DJ, et
al: Production of serotonin by tryptophan hydroxylase 1 and release
via platelets contribute to allergic airway inflammation. Am J
Respir Crit Care Med. 187:476–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Bie JJ, Henricks PA, Cruikshank WW,
Hofman G, Jonker EH, Nijkamp FP and Van Oosterhout AJ: Modulation
of airway hyperresponsiveness and eosinophilia by selective
histamine and 5-HT receptor antagonists in a mouse model of
allergic asthma. Br J Pharmacol. 124:857–864. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Macian F: NFAT proteins: key regulators of
T-cell development and function. Nat Rev Immunol. 5:472–484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonnet S, Rochefort G, Sutendra G, Archer
SL, Haromy A, Webster L, Hashimoto K, Bonnet SN and Michelakis ED:
The nuclear factor of activated T cells in pulmonary arterial
hypertension can be therapeutically targeted. Proc Natl Acad Sci
USA. 104:11418–11423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li M, Liu Y, Sun X, Li Z, Liu Y, Fang P,
He P, Shi H, Xie M, Wang X, et al: Sildenafil inhibits
calcineurin/NFATc2-mediated cyclin A expression in pulmonary artery
smooth muscle cells. Life Sci. 89:644–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang HJ, Hong SH, Kang KH, Park C and Choi
YH: Anti-inflammatory effects of Hwang-Heuk-San, a traditional
Korean herbal formulation, on lipopolysaccharide-stimulated murine
macrophages. BMC Complement Altern Med. 15:4472015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Q, Chen Y, Auger-Messier M and
Molkentin JD: Interaction between NFκB and NFAT coordinates cardiac
hypertrophy and pathological remodeling. Circ Res. 110:1077–1086.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
28
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun SC, Ganchi PA, Ballard DW and Greene
WC: NF-kappa B controls expression of inhibitor I kappa B alpha:
evidence for an inducible autoregulatory pathway. Science.
259:1912–1915. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng B, Ling J, Lee AJ, Wang Z, Chang Z,
Jin W, Kang Y, Zhang R, Shim D, Wang H, et al: Defective feedback
regulation of NF-kappaB underlies Sjogren's syndrome in mice with
mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl
Acad Sci USA. 107:15193–15198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tak PP and Firestein GS: NF-kappaB: a key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamura R, Chen Y, Shinozaki M, Arao K,
Wang L, Tang W, Hirano S, Ogura H, Mitsui T, Taketani S, et al:
Eudesmane-type sesquiterpene lactones inhibit multiple steps in the
NF-κB signaling pathway induced by inflammatory cytokines. Bioorg
Med Chem Lett. 22:207–211. 2012. View Article : Google Scholar
|
|
33
|
Foulds S, Galustian C, Mansfield AO and
Schachter M: Transcription factor NF kappa B expression and
postsurgical organ dysfunction. Ann Surg. 233:70–78. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KN, Ko SC, Ye BR, Kim MS, Kim J, Ko
EY, Cho SH, Kim D, Heo SJ and Jung WK:
5-Bromo-2-hydroxy-4-methyl-benzaldehyde inhibited LPS-induced
production of pro-inflammatory mediators through the inactivation
of ERK, p38 and NF-κB pathways in RAW 264.7 macrophages. Chem Biol
Interact. 258:108–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu G, Feng L, Song P, Xu F, Li A, Wang Y,
Shen Y, Wu X, Luo Q, Wu X, et al: Isomeranzin suppresses
inflammation by inhibiting M1 macrophage polarization through the
NF-κB and ERK pathway. Int Immunopharmacol. 38:175–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Suzuki YJ, Day RM and Fanburg BL:
Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth
muscle cell mitogenesis caused by serotonin. Circ Res. 95:579–586.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu YL, Huang CC, Chang CC, Chou CY, Lin
SY, Wang IK, Hsieh DJ, Jong GP, Huang CY and Wang CM:
Hyper-phosphate-induced myocardial hypertrophy through the
GATA-4/NFAT-3 signaling pathway is attenuated by ERK inhibitor
treatment. Cardiorenal Med. 5:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|