Introduction
The frequency of Y/autosome translocations in the
general population is ~1 in 2,000 (1,2).
Like any other chromosome, the Y chromosome can be translocated
onto an autosome or an X chromosome, either in a balanced or
unbalanced way. In the most common form of Y/autosome
translocation, the heterochromatic portion of Yq is translocated
onto the short arm of an acrocentric chromosome (2). These translocations have been
observed in phenotypically normal individuals and have also been
reported in multiple families, indicating that fertility is usually
not affected (2,3). In contrast, the rare trans-location
involved in the Y chromosome is that the euchromatic part of this
chromosome is translocated onto non-acrocentric autosomal regions,
which is frequently associated with azoospermia, a variety of male
gamete deformities and idiopathic sterility (4–6). A
few cases of reciprocal translocations involved in the long arm of
the Y chromosome and the short arm of an autosome have been
reported (7,8). Almost all of these reported studies
focused on the description of trans-location breakpoints (6,9),
but provide little insight into the underlying relationship between
the Y-autosome translocation and meiotic abnormalities (10).
Here, we present the meiotic behavior of
spermatocytes in a male 46,X,t(Y;1) carrier with non-obstructive
azoospermia. To shed light on possible effects of this reciprocal
translocation on human male fertility, we studied synapsis and
recombination between homologous chromosomes, meiotic prophase I
progression and expression of genes around the breakpoint of
chromosome 1 in the testis of our subject by using fluorescence
immunocytogenetic approaches.
Materials and methods
Patient and karyotyping
A 29-year-old male was presented to the Center for
Reproductive Medicine, Department of Obstetrics and Gynecology,
Anhui Provincial Hospital Affiliated to Anhui Medical University,
Hefei, China. Semen analysis was conducted according to World
Health Organization (WHO Laboratory Manual for the Examination of
Human Semen and Semen-Cervical Interaction, 2010) and revealed that
the patient was suffering from azoospermia. After obtaining written
informed consent from the patient and fertile men, testicular
tissues were sampled. Five fertile men, having at least one healthy
child, were recruited as normal controls for this study and similar
experiments were performed on them as mentioned for the patient.
All the procedures of this study were approved by the Institutional
Review Board and Ethics Committee of the University of Science and
Technology of China.
Spermatocyte spreading and
immunofluorostaining
Testicular tissues were processed as we described
previously (11,12). Rabbit anti-SYCP3 (15093Ab; Abcam,
Cambridge, UK), human anti-CREST (HCT-0100; ImmunoVision,
Springdale, AR, USA), mouse anti-MLH1 (551092; BD Pharmingen
Biosciences, San Diego, CA, USA), mouse anti-Brca1 (sc-6954; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), mouse anti-γ-H2AX
(05–636; Millipore, Billerica, MA, USA) and rabbit anti-Rad51
(sc-8349; Santa Cruz Biotechnology, Inc.) were used as primary
antibodies. The primary antibodies were detected using the
following secondary antibodies: Alexa 555 donkey anti-rabbit, Alexa
488 goat anti-mouse, Alexa 488 donkey anti-mouse (all from
Molecular Probes, Carlsbad, CA, USA) and
1-amino-4-methylcoumarin-3-acetic acid (AMCA) donkey anti-human
(Cat. no. 709-155-098; Jackson ImmunoResearch, West Grove, PA,
USA).
For the immune-detection of H1T2 and pH3, PFA-fixed
testicular sections were incubated overnight at 4°C with primary
antibodies: anti-H1T2 (Abcam; ab184838, 1:500) and anti-pH3 (Ser10)
(sc-8656-R, 1:500; Santa Cruz Biotechnology, Inc.). After rinsing
thoroughly with TBS, the primary antibodies were detected by an
Alexa 555 donkey anti-rabbit (1:250; Molecular Probes) secondary
antibody for 1 h at 37°C and sections were counterstained using
DAPI.
An epifluorescence microscope Olympus BX61 (Olympus
Inc., Tokyo, Japan) and Image Pro-Plus version 5.1 software (Media
Cybernetics Inc., Bethesda, MD, USA) were used for imaging and cell
evaluation.
Real-time PCR
A number of genes around the breakpoint on
chromosome 1 and Y were selected along with genes on other
chromosomes that were not involved in the translocation (as
control) to compare their expression in the 46,X,t(Y;1) carrier and
control. RNA isolation, RT-PCR, and real-time PCR were performed as
previously described (13). All
PCR primers used are listed in Table
I. For real-time PCR analyses, Ct values of samples were
normalized to the corresponding Ct values of glyceraldehyde
3-phosphate dehydrogenase (GAPDH). Quantification of the
fold-change in gene expression was determined by the comparative Ct
method.
 | Table IList of genes, their location on the
chromosomes and the sequence of oligonucleotides used to compare
the RT-PCR Ct values between the 46,X,t(Y;1) carrier and
control. |
Table I
List of genes, their location on the
chromosomes and the sequence of oligonucleotides used to compare
the RT-PCR Ct values between the 46,X,t(Y;1) carrier and
control.
| Name | Gene location | Direction | Sequence
(5′→3′) |
|---|
| USP1 | 1p31.3 | Forward |
GTCCCACCGTCGAGGATTAAC |
| Reverse |
CCAATTAGATGGGCGGGAGC |
| LEPR | 1p31 | Forward |
TCCTTCAGTGGGGCTATTGG |
| Reverse |
GGTGGCATGCAAGACAACTTAAA |
| INSL5 | 1p31.3 | Forward |
GCAAGCTGAGACAGGAAACTC |
| Reverse |
GCCATCAGTGCAACACAAAGT |
| MSH4 | 1p31 | Forward |
GGAGGGGTCGCTCAGAAAC |
| Reverse |
AGAGACCACCTGGACCGAAG |
| NFIA | 1p31.3-p31.2 | Forward |
CGACTTGGAAATGTGAACGCA |
| Reverse |
CATCCTGGGTGAGACAGAGC |
| TEX11 | Xq13.1 | Forward |
ACTTGGCTGTGCAATGTGAC |
| Reverse |
TGCACGACTCAGGAACATGG |
| CDK2 | 12q13 | Forward |
CTGGACACGCTGCTGGAT |
| Reverse |
GGTTTAAGGTCTCGGTGGAGG |
| DAZ | Yq11.223 | Forward |
GTTCTACCTCCGAGGGTTCG |
| Reverse |
TTTGCAGCAGACATGGTGGT |
| TSPY1 | Yp11.2 | Forward |
TATCGCCGCAGACACCAC |
| Reverse |
AACAACTGGGAGTCCCCTGA |
| SRY | Yp11.3 | Forward |
GTGGTCTCGCGATCAGAGG |
| Reverse |
CCTTCCGACGAGGTCGATAC |
| RPS4Y1 | Yp11.3 | Forward |
TTTGATGTGGTGCATGTGAAGG |
| Reverse |
TGCTGCTACTGCAATTTAGCC |
| SLC25A6 | Xp22.32 and
Yp11.3 | Forward |
CATTGCGATCCAACCATCGG |
| Reverse |
GGGCTAGCGCTGAAGTACAA |
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). A Chi-square test was
applied to compare RAD51 foci and meiotic progression between the
patient and controls. MLH1 foci were compared between the patient
and controls using the Mann-Whitney U test. The Student's t-test
was used to compare the expression of studied genes between patient
and control.
Results
Semen analysis revealed that the patient was
suffering from azoospermia. Karyotyping on G-banded metaphases from
peripheral blood lymphocytes revealed a karyotype of
46,X,t(Y;1)(p11.3;p31) in all the 100 studied cells of the patient
(Fig. 1A), which was also
confirmed by immunostaining of pachytene spermatocyte spreads
(Fig. 1B–D).
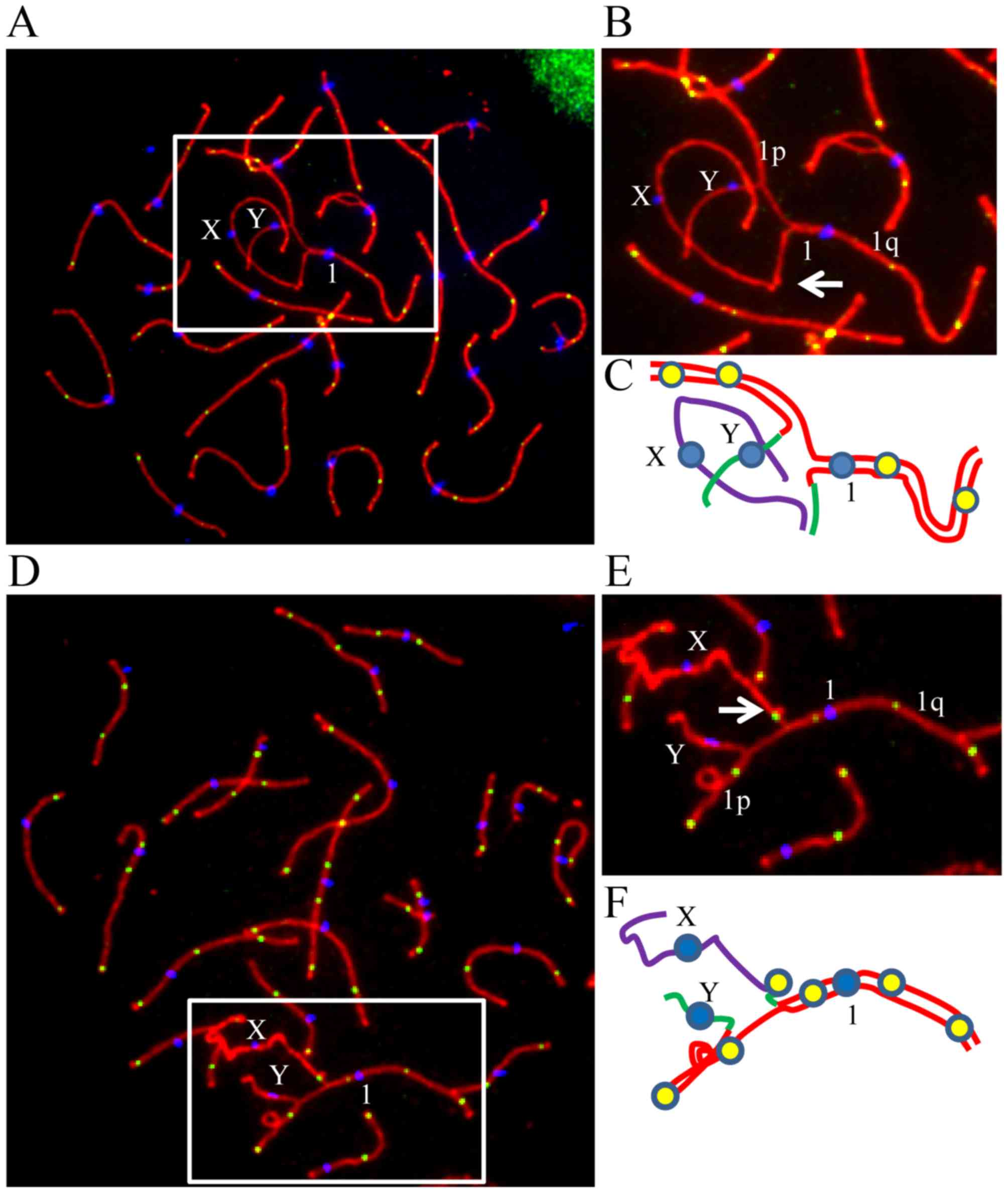
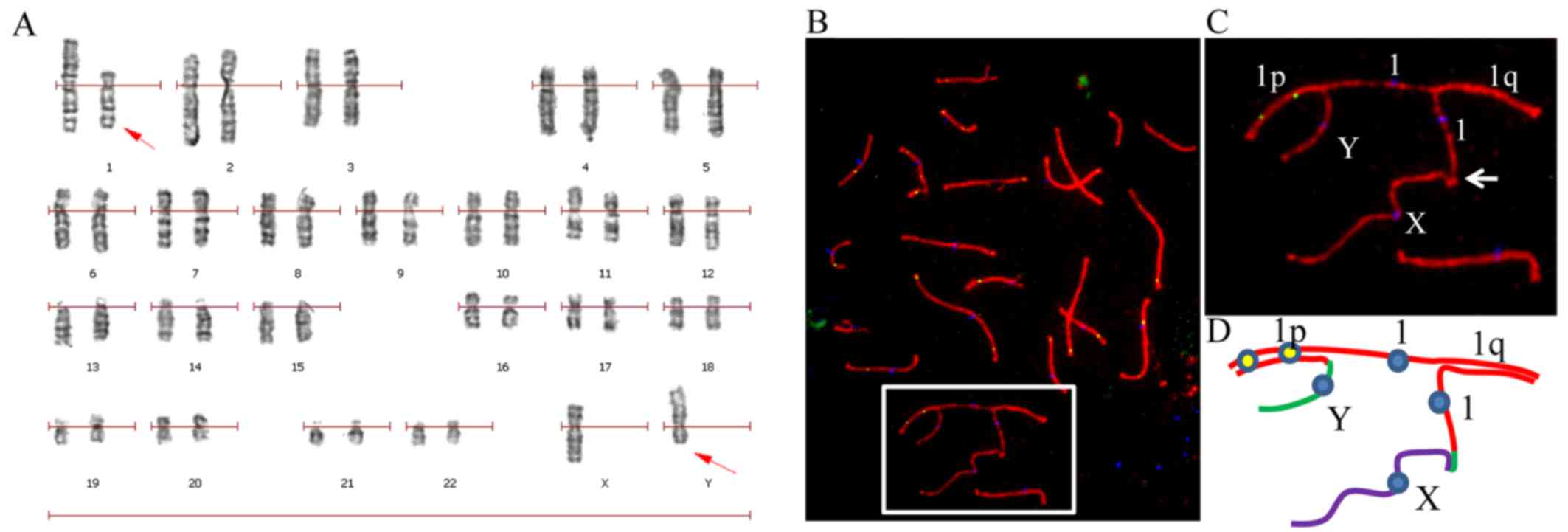 | Figure 146,X,t(Y;1) translocation in the
patient was identified by karyotyping and confirmed by
immunostaining of pachytene spermatocyte spreads. (A) Karyotyping
of G-banded peripheral blood lymphocytes identified a 46,X,t(Y;1)
karyotype. (B) A representative pachytene spermatocyte spread
immunostained for CREST (blue), SYCP3 (red) and MLH1 (green) from
the 46,X,t(Y;1) carrier confirms the chromosomal translocation. (C)
Enlarged area from B, showing the translocation. (D) A schematic
configuration of chromosomes involved in the translocation from the
cell shown in B. Chromosomes X, Y and 1 are presented in purple,
green and red lines, respectively. |
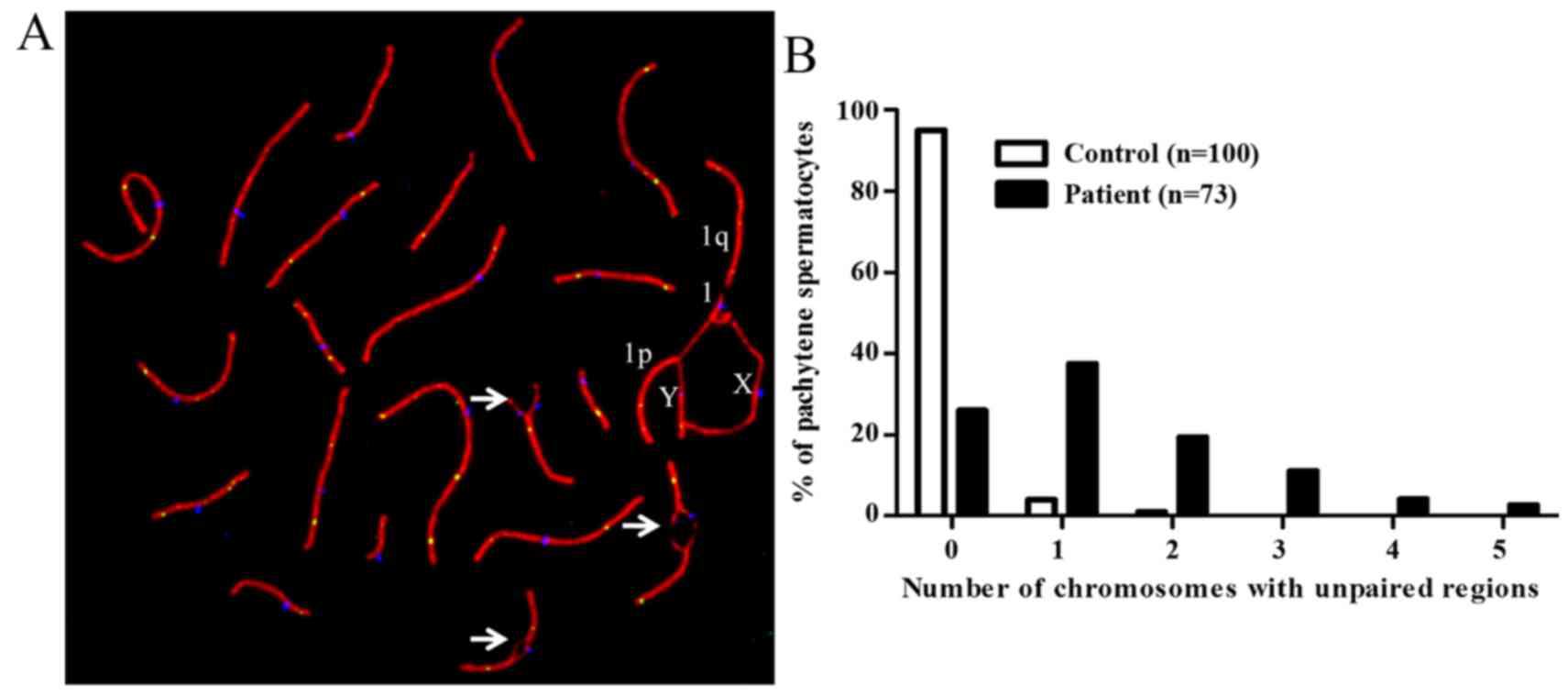
Abnormal homologous chromosome pairing in
pachytene spermatocytes of the 46,X,t(Y;1) carrier
Analysis of immunostained pachytene spermatocyte
spreads (n=73) revealed reciprocal translocation on p arms of Y and
1 chromosome. der(Y) was found paired with 1 and X while
der(1) was associated with the X
chromosome (Fig. 1B–D). It was
observed that only 26% of the studied spermatocytes had normal
chromosomes pairing while 74% spermatocytes contained either 1
(37%), 2 (19%), 3 (11%), 4 (4%) or 5 (3%) bivalents that showed
unpaired regions (Fig. 2A and
B).
Reduced recombination between the
homologous region of sex chromosomes in the 46,X,t(Y;1)
carrier
In all the 73 studied spermatocyte spreads from the
patient, the association and recombination between the homologous
regions of sex chromosomes were determined and compared with the
control. X chromosome was found to be associated with translocated
part of Y chromosome in 93.2% of the cells (Fig. 3A–D), while in 6.8% of the
spermatocytes, they were not found to be associated with each
other. In the patient, recombination between the X and translocated
part of Y on derived chromosome 1 (represented by MLH1 focus) was
observed to be present in the homologous region in 2.7% of the
cells (Fig. 3E), which was
significantly lower (P<0.001) than the recombination in
homologous regions on X and Y chromosomes in the control
individuals (Table II),
indicating that the recombination in the homologous region of XY
chromosomes was impaired in the patient.
 | Table IIMean MLH1 foci per cell in the
controls and the 46,X,t(Y;1) carrier. |
Table II
Mean MLH1 foci per cell in the
controls and the 46,X,t(Y;1) carrier.
| Donor | No. of cells
analyzed (n) | Mean no. of MLH1
foci on autosomes (except chromosome 1) | SD | Range of MLH1
foci |
|---|
| Control |
| C1 | 83 | 46.4 | 6.8 | 30–59 |
| C2 | 93 | 43.8 | 4.8 | 32–57 |
| C3 | 100 | 44.0 | 5.6 | 31–55 |
| C4 | 75 | 44.2 | 7.9 | 25–63 |
| C5 | 92 | 44.3 | 4.6 | 33–57 |
| Mean ± SD | | 45.2±6.3 | | |
| t(Y;1) | 73 | 40.2a | 12.8 | 11–64 |
Reduced meiotic recombination between
autosomes and homologous region of the chromosomes involved in the
translocation in the 46,X,t(Y;1) carrier
In order to determine the effect of chromosomal
translocation on recombination during meiosis, the MLH1 foci, the
meiotic recombination markers, were counted in pachytene
spermatocytes of the patient and the results are presented in
Tables II and III. A total of 73 spermatocyte spreads
were analyzed to calculate the recombination frequencies between
autosomes of the 46,X,t(Y;1) translocation carrier. Interestingly,
the mean number of MLH1 foci per cell on chromosomes that were not
involved in the translocation was also significantly lower
(P<0.001) in the patient (40.01) than in the controls (45.22)
(Table II), indicating an
inter-chromosomal effect (ICE). When the crossover frequencies were
compared for homologous region of the chromosomes involved in
translocation, a significant decrease in the number of crossovers
was observed on p arm of chromosome Y (P<0.001), p arm of
chromosome 1 (P<0.001) and on q arm (P<0.001) of chromosome 1
as compared to the controls (Table
III).
 | Table IIIComparison of crossovers on the
chromosomes involved in the translocation between the controls and
the 46,X,t(Y;1) carrier. |
Table III
Comparison of crossovers on the
chromosomes involved in the translocation between the controls and
the 46,X,t(Y;1) carrier.
| Chromosome arm | No. of MLH1 foci
|
|---|
Controls
| t(Y;1)
|
|---|
| Mean | Range | Mean | Range |
|---|
| 1p | 1.607 | 0–3 | 1.082a | 0–3 |
| 1q | 1.885 | 0–3 | 1.274a | 0–3 |
| Yp | 0.849 | 0–1 | 0.027a | 0–1 |
| Yq | 0 | 0 | 0 | 0 |
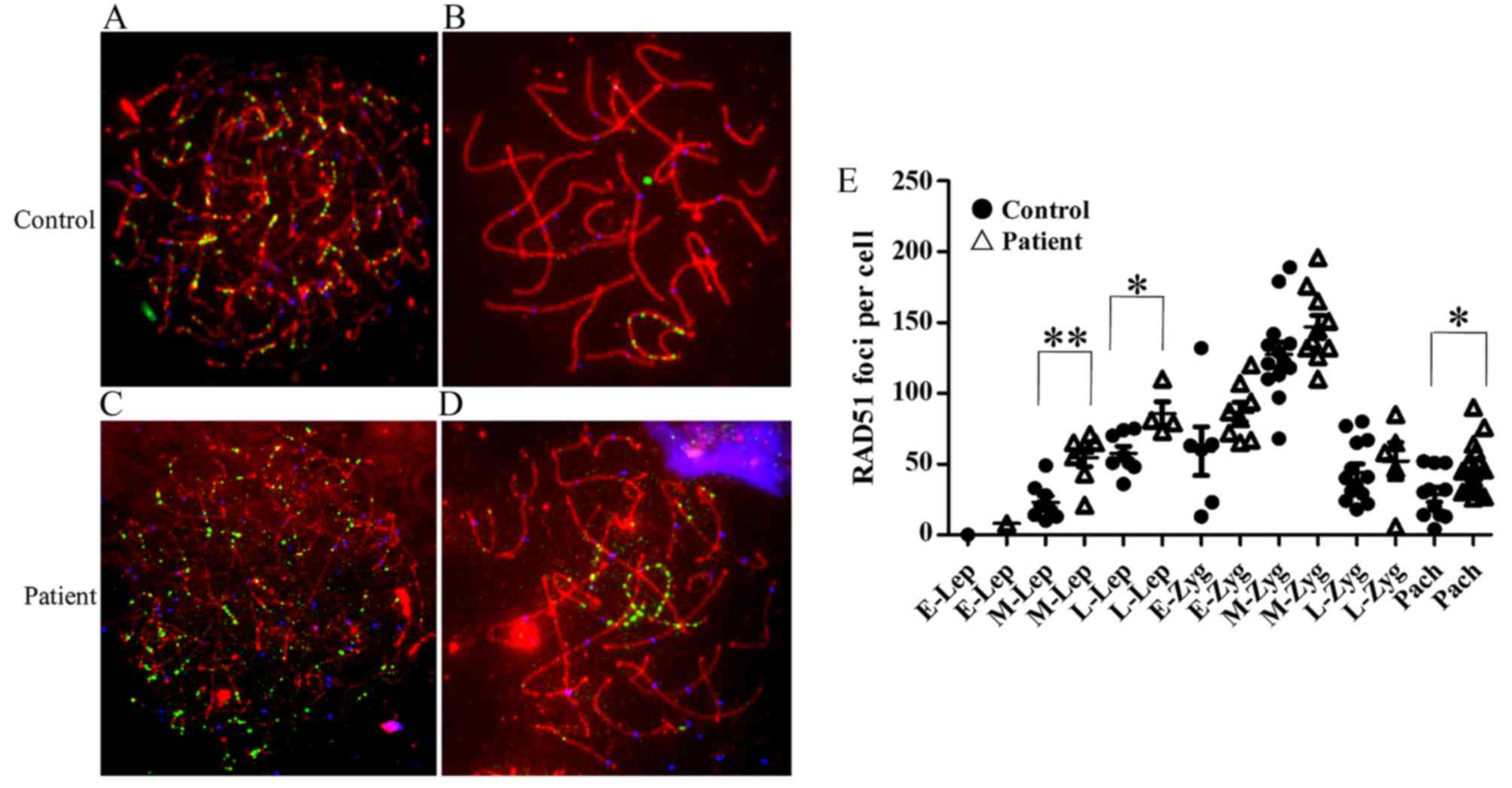
Delayed DNA double-strand break repair
during recombination in spermatocytes of the 46,X,t(Y;1)
carrier
RAD51 forms cytologically detectable complexes
involved in DNA double-strand breaks (DSBs) at sites of ongoing
recombination (14,15). We studied the kinetics of
formation and disappearance of RAD51 foci on meiotic chromosomes of
the 46,X,t(Y;1) carrier and control (Fig. 4A–D). The following criteria were
used to classify the sub-stages of prophase I. During leptonema,
the chromatin begins to condense, and proteinaceous axial elements
begin to form between sister chromatids. As axial elements of
homologous chromosomes align and come into contact during zygonema,
a central element and transverse filaments form between homologues,
completing the structure called the synaptonemal complex (SC).
Homologous autosomes are fully synapsed throughout pachynema, the
period during which reciprocal recombination (crossing over)
occurs. During diplonema, the central element of the SC
disassembles and homologous chromosomes begin to repel one another,
but remain held together at chiasmata (crossing-over sites).
Prophase concludes with diakinesis, during which further chromatin
condensation occurs (16–19).
The mean number of RAD51 foci/cell was significantly
higher at middle leptotene (P=0.001) and late leptotene (P=0.01) as
well at the pachytene (P=0.02) stage in the 46,X,t(Y;1) carrier
(Fig. 4E).
It has been documented that the chromosome or
chromosomal regions that have not experienced synapsis undergo
inactivation and are decorated by γ-H2AX and BRCA1 signals in
spermatocytes (20,21). Upon immunostaining of pachytene
spermatocytes, we also observed signals for γ-H2AX (Fig. 5A and C) and BRCA1 (Fig. 5B and D) on unsynapsed regions of
autosomes and sex chromosomes of the 46,X,t(Y;1) carrier,
indicating silencing of these chromosomal regions.
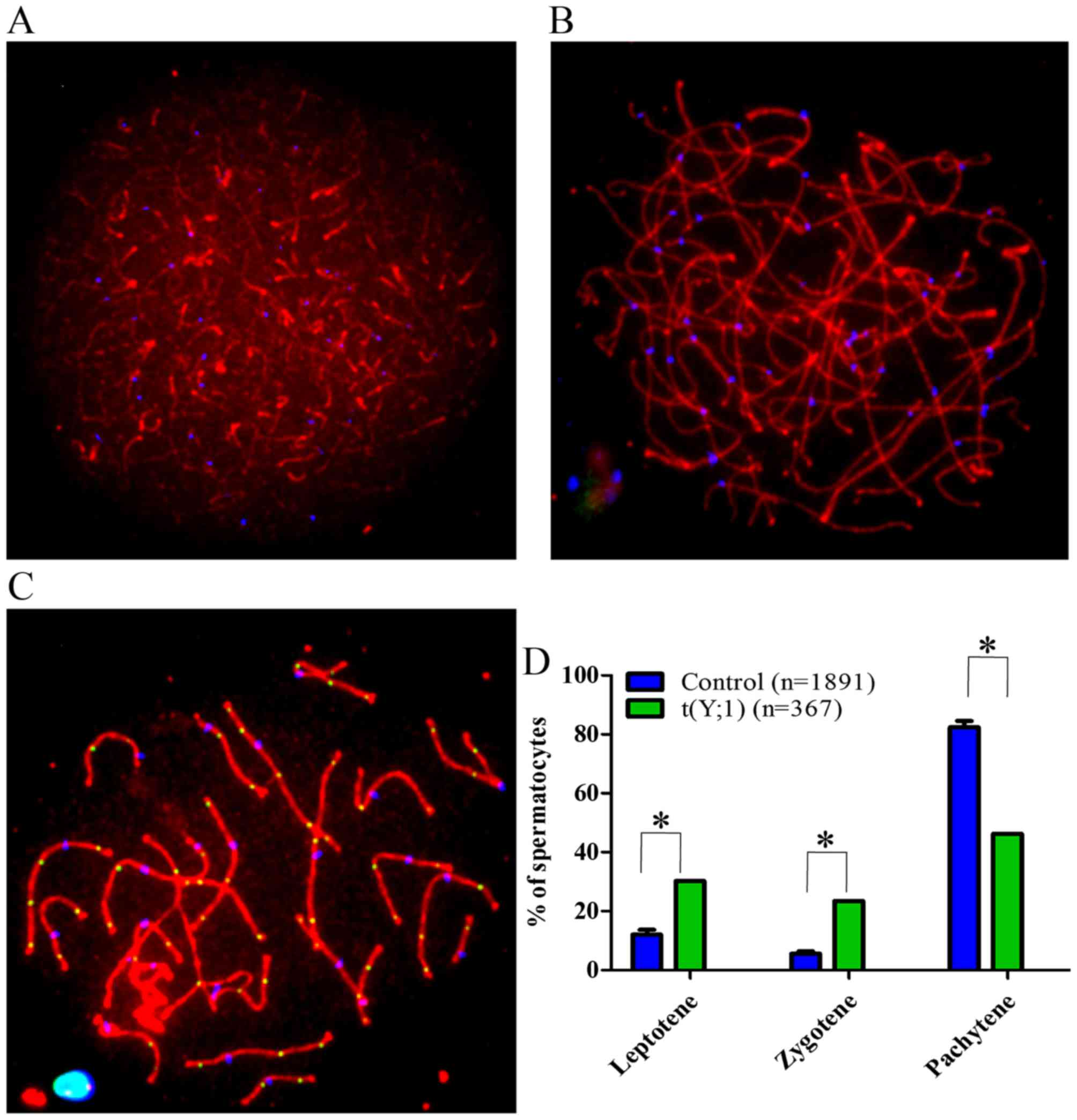
Disturbed meiotic prophase progression in
spermatocytes of the 46,X,t(Y;1) carrier
To determine whether t(Y;1) trans-location affects
the meiotic prophase progression, a total of 369 randomly selected
spermatocytes in different sub-stages of meiotic prophase I were
studied in the patient and the results were compared with the
controls (Fig. 6A–C). An increase
in leptotene (P<0.001) and zygotene (P<0.001) while a
decrease in the pachytene spermatocytes (P<0.001) were observed
in the 46,X,t(Y;1) carrier when compared with the controls
(Fig. 6D).
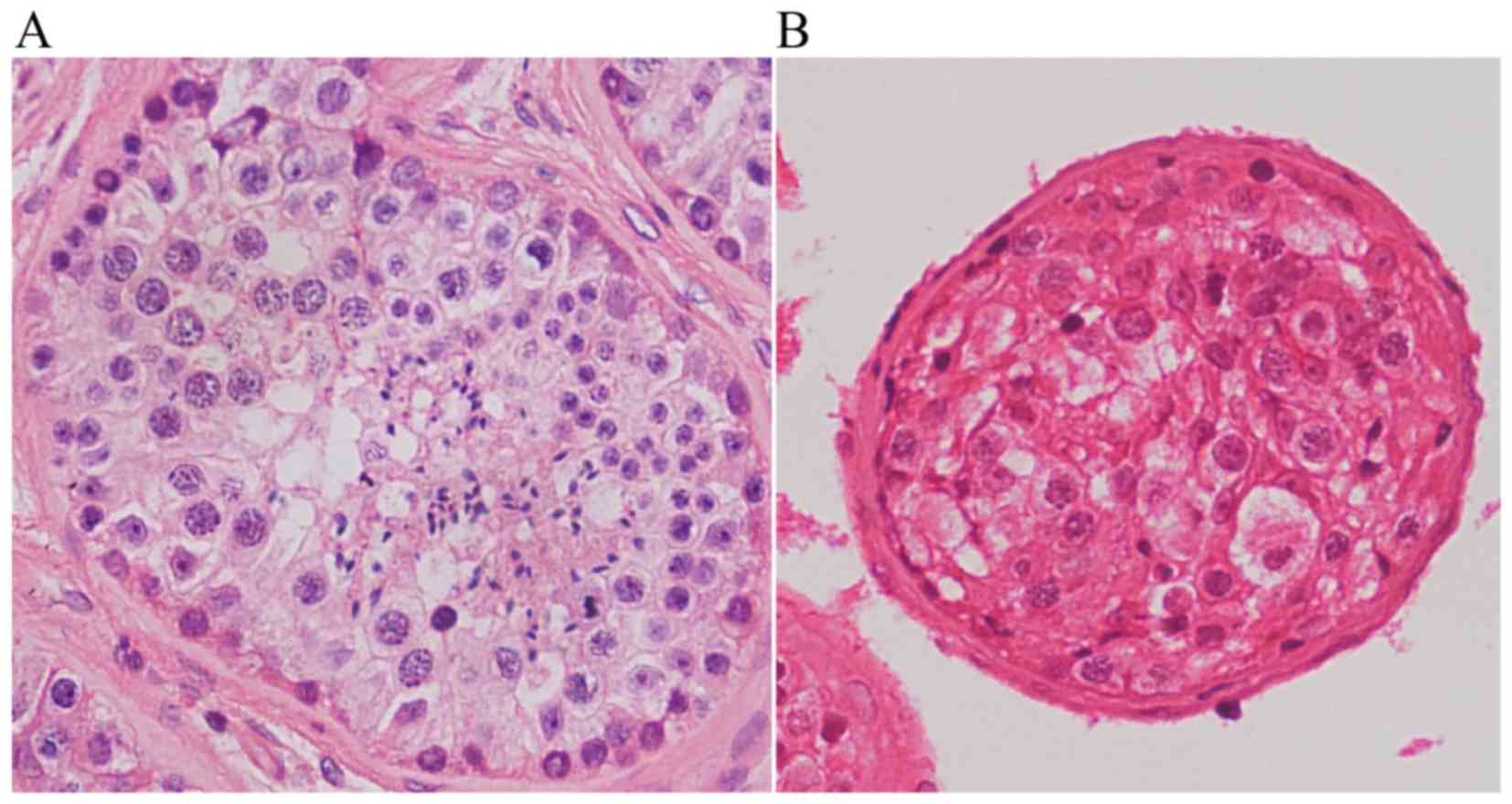
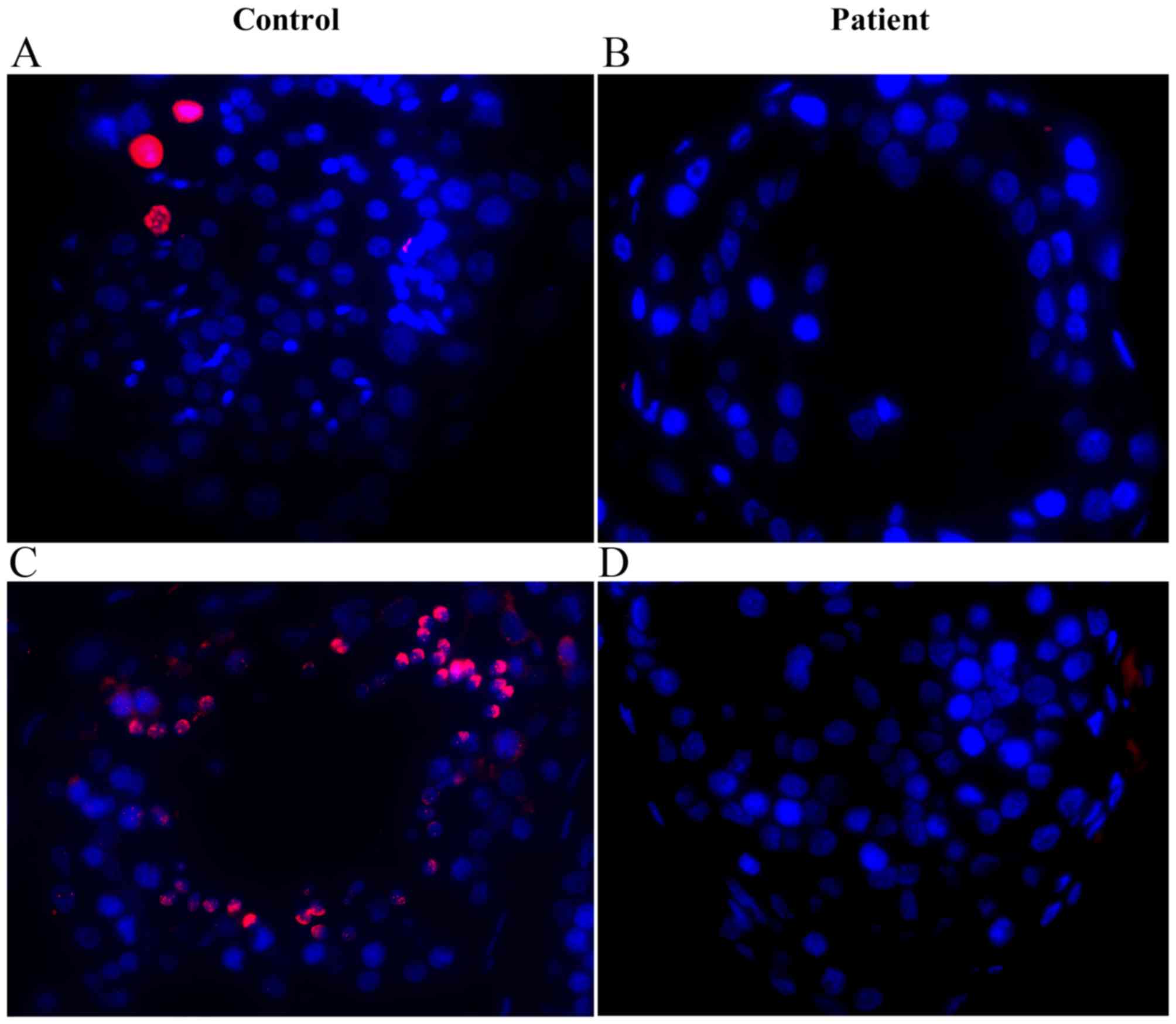
Disturbed spermatogenesis in testicular
sections of the 46,X,t(Y;1) carrier
Histological examination of the hematoxylin and
eosin (H&E)-stained testicular sections revealed normal
spermatogenesis in the testicular sections of a control man
(Fig. 7A), while reduced germ
cells with no spermatids or sperm were observed in the patient
(Fig. 7B). These observations
were consistent with the findings in testicular sections
immunostained for pH3 (phosphorylated histone H3), a specific
marker of chromosomes in cells at diplotene and/or metaphase stage,
and H1T2, a marker for spermatids (Fig. 8). Numerous pH3- and H1T2-positive
cells were observed in the control testis (Fig. 8A and C), however, no such cells
were observed in the translocation carrier's testis (Fig. 8B and D), indicating
spermatogenesis did not progress beyond the pachytene stage in the
patient.
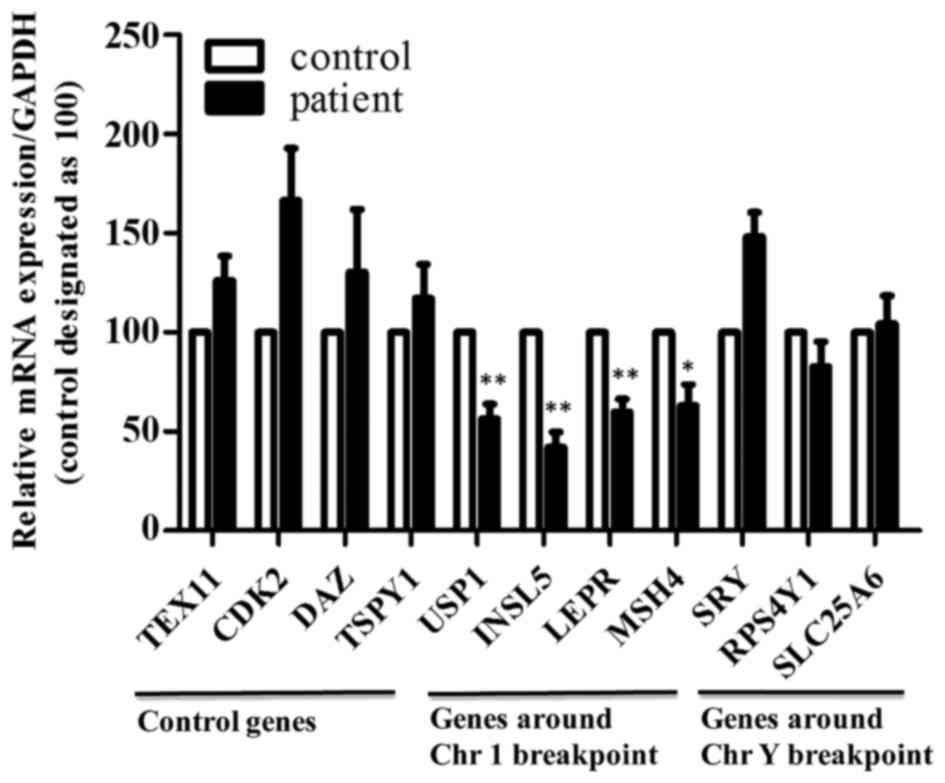
Downregulation of genes present around
the breakpoint on chromosome 1 in the 46,X,t(Y;1) carrier
CT for various studied genes critical for
meiosis/spermatogenesis and located around the breakpoint of
chromosome 1 and Y, and on the chromosomes that are not involved in
the translocation were compared between the patient and control, to
determine the effect of chromosomal translocation on gene
expression in the testes, if any. Our results revealed decreased
expression of ubiq-uitin specific peptidase 1 (USP1), insulin like
factor 5 (INSL5), leptin receptor (LEPR) and MutS homolog 4 (MSH4)
present around the breakpoint of chromosome 1, and no change for
those on Y chromosome and other chromosomes in the testis of the
46,X,t(Y;1) carrier as compared to the control (Fig. 9). These results indicate that
downregulation of these genes may have affected the spermatogenesis
in this patient.
Discussion
Balanced reciprocal translocations are the
structural abnormalities involving mostly two autosomes while a few
involve a gonosome (X or Y chromosome) and an autosome (22). These rearrangements are usually
associated with infertility and/or a higher risk of chromosomal
imbalances among offspring (23).
The relationship between Y-autosome translocation and male
sterility has been reported but the mechanism of the sterilizing
effect remains elusive (1,10,23).
This is the first report of immnunofluorescence meiotic analysis on
a reciprocal translocation t(Y;1)(p11.3;p31) carrier with an
azoospermic phenotype.
Homologous chromosome pairing, synapsis and
recombination are critical to meiosis, and failure in either of
these processes has been associated with meiotic arrest and
infertility (24). Meiotic
studies on infertile male carriers of reciprocal translocations
have shown that the regions surrounding the breakpoints often fail
to completely pair and synapse (24,25). Furthermore, these asynapsed
regions have been found to be associated with the sex chromosomes
(11,26). In the present study, we also
observed abnormal chromosomal pairing in most of the studied
spermatocytes in our patient (Fig.
2B). As expected, the recombination between homologous regions
of the chromosomes involved in the translocation was significantly
decreased in our patient (Fig. 3
and Table III). Interestingly,
the mean number of MLH1 foci per cell on chromosomes that were not
involved in the translocation were also significantly lower
(P<0.001) in the patient (40.01) than in the controls (45.22)
(Table II), highlighting an
ICE.
RAD51 forms cytologically detectable complexes
involved in DSBs at sites of ongoing recombination (14,15). In our trans-location carrier, we
observed increased RAD51 foci during the pachytene as compared to
controls (Fig. 4), which may
indicate an inefficient DSB repair mechanism in the patient
(Fig. 5).
During male meiosis, the X and Y chromosomes are
transcriptionally silenced, forming a condensed sex body. Meiotic
sex chromosome inactivation (MSCI) is characterized by the
localization of phosphorylated histone H2AX on the sex chromosomes,
which is thought to be critical for chromatin condensation and
transcriptional inactivation (27). BRCA1 is also critical for MSCI,
recruiting ataxia telangiectasia (ATR and RAD3 related) to
phosphorylate H2AX (28). Recent
studies, however, have suggested that the phenomenon of meiotic
inactivation is not limited to the sex chromosomes; unsynapsed
autosomal chromosomes have been shown to undergo a similar
transcriptional silencing in germ cells of mice (21,29). Our results complement the above
studies as upon immunostaining of pachytene spermatocytes, we also
observed signals for γ-H2AX (Fig.
5C) and BRCA1 (Fig. 5D) on
unsynapsed regions of autosomes and sex chromosomes in
spermatocytes of the 46,X,t(Y;1) carrier.
The local consequences of asynapsis may include
persistence of DNA DSBs (30),
silencing of genes in the unsynapsed chromosomal segment (21) and intrusion of the rearranged
autosomes in the sex body (31).
The ultimate physiological consequence of asynapsis in the male is
subfertility or sterility due to partial or complete meiotic arrest
at the first meiotic prophase (32), even though in many instances, as
in multiple heterozygotes for Robertsonian translocation, pachytene
block can be leaky or missing and spermatogenesis may fail at
meta-phase I of meiosis (33–35). Here, we studied spermatocytes in
different sub-stages of meiotic prophase I from our patient and
observed an increased proportion of leptotene (P<0.001) and
zygotene (P<0.001) and decreased proportion of pachytene
spermatocytes (P<0.001) as compared to the controls, indicating
that the meiotic progression in our translocation carrier was
disturbed (Fig. 6). After
immunostaining of the spermatocytes with anti-pH3, a specific
marker for chromatin of diplotene and metaphase cells (36,37), we observed the absence of
pH3-positive cells in the testis of the 46,X,t(Y;1) carrier, which
indicate that meiosis failed to progress beyond the pachytene stage
in our patient (Fig. 8A and B).
These findings are also consistent with the studies that
translocation can activate the pachytene checkpoint that is
responsible for the delay/arrest of pachytene progression when
either the process required for crossover is defective or when
there are defects in the structure of the meiotic chromosome axis
(38). Obviously, the chromosomal
rearrangements in our patient may have also activated the pachytene
checkpoint in spermatocytes as the meiosis did not progress beyond
the pachytene stage (Figs. 7 and
8).
It has been reported that balanced chromosomal
rearrangements are associated with aberrant gene expression, which
have many underlying causes, e.g. direct disruption of genes or
their regulatory elements by chromosomal rearrangement at
breakpoints, or silencing of genes due to failure in synapsis
between homologous chromosomes around breakpoint (39,40). To test these, we detected the
expression of a series of genes that are required for
spermatogenesis and located around the breakpoints. USP1 is located
at 1p31.3 and responsible for deubiquitination of FANCD2, an
important member of the Fanconi anemia (FA) pathway that regulates
the repair of DNA crosslinks (41). Targeted deletion of mouse Usp1
results in male infertility by interfering with homologous
recombination repair (42). INSL5
is a member of the insulin superfamily and is located at 1p31.3. It
has been reported that Insl5−/− male mice display
impaired fertility due to marked reduction in sperm motility
(43). LEPR belongs to the
cytokine receptor class I superfamily located at 1p31 (44). De Luca et al reported that
homozygous LEPRB mutant mice are sterile (45). MSH4, another important gene
present in the breakpoint region on chromosome 1 (1p31), is a
member of the mammalian mismatch repair gene family and is
responsible for post replicative DNA mismatch repair as well as the
control of meiotic recombination (46,47). Disruption of the MSH4 gene in mice
results in male and female sterility due to meiotic synapsis and
recombination failure (48).
Significant decreases in the expression of all these genes were
observed in the testis of our 46,X,t(Y;1) patient as compared to
the control (Fig. 9), which
indicates that failure in synapsis of the homologous region around
the breakpoint of chromosome 1 is responsible for the decreased
expression of these genes. These findings are complementary to our
results as abnormal pairing, synapsis, recombination and delayed
DSB repair were observed, and confirmed by the presence of γ-H2AX
(Fig. 5C) and BRCA1 (Fig. 5D) signals on unsynapsed regions of
chromosome 1 and sex chromosomes in spermatocytes of the
46,X,t(Y;1) carrier.
We thus propose that in our patient, the
translocation results in the failure in pairing and synapsis of
homologous regions around breakpoints and consequently cause
inefficient DSB repair, and silencing of the genes in this region.
Some of the silenced genes, such as MSH4, are required for meiotic
synapsis, recombination and spermatogenesis; their decreased
expression will further exacerbate meiotic and spermatogenic
aberration in translocation carriers, which is also consistent with
our finding that our patient showed ICE in recombination as MSH4
was downregulated.
In conclusion, we report a potential mechanism that
caused azoospermia in our 46,X,t(Y;1) carrier. We observed that the
studied chromosomal translocation disturbed the process of meiotic
pairing, synapsis and recombination, which resulted in the
silencing of genes located in the unsynapsed chromosomal regions.
As some of the silenced genes are required for meiosis and
spermatogenesis, their reduced expression could further exacerbate
meiotic and spermatogenesis failure, and ultimately cause meiotic
arrest and azoospermia in translocation carriers.
Acknowledgments
We gratefully acknowledge the patient and
individuals for sample donation during this study. This study was
supported by the National Basic Research Program (nos. 2013CB947900
and 2014CB943101) of China (973), by grants from National Natural
Science Foundation of China (nos. 31371519, 31301227 and
313111245), by the Project of Cultivate Scientific Research
Foundation of the Fourth Affiliated Hospital, Anhui Medical
University (no. F1407D) and the Knowledge Innovation Program of the
Chinese Academy of Sciences (no. KSCX2-EW-R-07).
References
|
1
|
Alves C, Carvalho F, Cremades N, Sousa M
and Barros A: Unique (Y;13) translocation in a male with
oligozoospermia: Cytogenetic and molecular studies. Eur J Hum
Genet. 10:467–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu LY: Phenotype/karyotype correlations
of Y chromosome aneuploidy with emphasis on structural aberrations
in postnatally diagnosed cases. Am J Med Genet. 53:108–140. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alitalo T, Tiihonen J, Hakola P and de la
Chapelle A: Molecular characterization of a Y;15 translocation
segregating in a family. Hum Genet. 79:29–35. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brisset S, Izard V, Misrahi M, Aboura A,
Madoux S, Ferlicot S, Schoevaert D, Soufir JC, Frydman R and
Tachdjian G: Cytogenetic, molecular and testicular tissue studies
in an infertile 45, X male carrying an unbalanced (Y;22)
translocation: Case report. Hum Reprod. 20:2168–2172. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gunel M, Cavkaytar S, Ceylaner G and
Batioglu S: Azoospermia and cryptorchidism in a male with a de novo
reciprocal t(Y;16) translocation. Genet Couns. 19:277–280.
2008.PubMed/NCBI
|
|
6
|
Jiang YT, Zhang HG, Wang RX, Yu Y, Zhang
ZH and Liu RZ: Novel Y chromosome breakpoint in an infertile male
with a de novo translocation t(Y;16): A case report. J Assist
Reprod Genet. 29:1427–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conte RA, Kleyman SM, Klein V, Bialer MG
and Verma RS: Characterization of a de novo t(Y;9) (q11.2;q22) by
FISH technique. Ann Genet. 39:10–15. 1996.PubMed/NCBI
|
|
8
|
Vásquez-Velásquez AI, Arnaud-López L,
Figuera LE, Padilla-Gutiérrez JR, Rivas F and Rivera H: Ambiguous
genitalia by p9 p deletion inherent to a dic(Y;9)(q12;p24). J Appl
Genet. 46:415–418. 2005.
|
|
9
|
Röpke A, Stratis Y, Dossow-Scheele D,
Wieacker P, Kliesch S and Tüttelmann F: Mosaicism for an unbalanced
Y;21 translocation in an infertile man: A case report. J Assist
Reprod Genet. 30:1553–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun F, Oliver-Bonet M, Turek PJ, Ko E and
Martin RH: Meiotic studies in an azoospermic human translocation
(Y;1) carrier. Mol Hum Reprod. 11:361–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang H, Wang L, Cui Y, Xu Z, Guo T, Cheng
D, Xu P, Yu W and Shi Q: Meiotic chromosome behavior in a human
male t(8;15) carrier. J Genet Genomics. 41:177–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Z, Yang Q, Ye N, Wang L, Li J, Yu D,
Cooke HJ and Shi Q: Complex relationship between meiotic
recombination frequency and autosomal synaptonemal complex length
per cell in normal human males. Am J Med Genet A. 158A:581–587.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma
T, Zheng W, Sun R, Shen W, Sha J, et al: Proliferating cell nuclear
antigen (PCNA) regulates primordial follicle assembly by promoting
apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One.
6:e160462011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashley T, Plug AW, Xu J, Solari AJ, Reddy
G, Golub EI and Ward DC: Dynamic changes in Rad51 distribution on
chromatin during meiosis in male and female vertebrates.
Chromosoma. 104:19–28. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moens PB, Chen DJ, Shen Z, Kolas N,
Tarsounas M, Heng HH and Spyropoulos B: Rad51 immunocytology in rat
and mouse spermatocytes and oocytes. Chromosoma. 106:207–215. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonsalves J, Sun F, Schlegel PN, Turek PJ,
Hopps CV, Greene C, Martin RH and Pera RA: Defective recombination
in infertile men. Hum Mol Genet. 13:2875–2883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vidal F, Navarro J, Templado C, Marina S
and Egozcue J: Development and behavior of synaptonemal complexes
in human spermatocytes by light and electron microscopy. Hum Genet.
68:142–147. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
von Wettstein D, Rasmussen SW and Holm PB:
The synaptonemal complex in genetic segregation. Annu Rev Genet.
18:331–413. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walpita D, Plug AW, Neff NF, German J and
Ashley T: Bloom's syndrome protein, BLM, colocalizes with
replication protein A in meiotic prophase nuclei of mammalian
spermatocytes. Proc Natl Acad Sci. 96:5622–5627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahadevaiah SK, Turner JM, Baudat F,
Rogakou EP, de Boer P, Blanco-Rodríguez J, Jasin M, Keeney S,
Bonner WM and Burgoyne PS: Recombinational DNA double-strand breaks
in mice precede synapsis. Nat Genet. 27:271–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turner JM, Aprelikova O, Xu X, Wang R, Kim
S, Chandramouli GV, Barrett JC, Burgoyne PS and Deng CX: BRCA1,
histone H2AX phosphorylation, and male meiotic sex chromosome
inactivation. Curr Biol. 14:2135–2142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perrin A, Douet-Guilbert N, Le Bris MJ,
Keromnes G, Langlois ML, Barrière P, Amice J, Amice V, De
Braekeleer M and Morel F: Segregation of chromosomes in sperm of a
t(X;18) (q11;p11.1) carrier inherited from his mother: Case report.
Hum Reprod. 23:227–230. 2008. View Article : Google Scholar
|
|
23
|
Pinho MJ, Neves R, Costa P, Ferrás C,
Sousa M, Alves C, Almeida C, Fernandes S, Silva J, Ferrás L, et al:
Unique t(Y;1) (q12;q12) reciprocal translocation with loss of the
hetero-chromatic region of chromosome 1 in a male with azoospermia
due to meiotic arrest: A case report. Hum Reprod. 20:689–696. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferguson KA, Chow V and Ma S: Silencing of
unpaired meiotic chromosomes and altered recombination patterns in
an azoospermic carrier of a t(8;13) reciprocal translocation. Hum
Reprod. 23:988–995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oliver-Bonet M, Ko E and Martin RH: Male
infertility in reciprocal translocation carriers: The sex body
affair. Cytogenet Genome Res. 111:343–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leng M, Li G, Zhong L, Hou H, Yu D and Shi
Q: Abnormal synapses and recombination in an azoospermic male
carrier of a reciprocal translocation t(1;21). Fertil Steril.
91:1293.e1217–1222. 2009. View Article : Google Scholar
|
|
27
|
Turner JM: Meiotic sex chromosome
inactivation. Development. 134:1823–1831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turner JM, Mahadevaiah SK,
Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX and Burgoyne
PS: Silencing of unsynapsed meiotic chromosomes in the mouse. Nat
Genet. 37:41–47. 2005.
|
|
29
|
Baarends WM, Wassenaar E, van der Laan R,
Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers JH, de Boer P and
Grootegoed JA: Silencing of unpaired chromatin and histone H2A
ubiquitination in mammalian meiosis. Mol Cell Biol. 25:1041–1053.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schoenmakers S, Wassenaar E, van Cappellen
WA, Derijck AA, de Boer P, Laven JS, Grootegoed JA and Baarends WM:
Increased frequency of asynapsis and associated meiotic silencing
of heterologous chromatin in the presence of irradiation-induced
extra DNA double strand breaks. Dev Biol. 317:270–281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Forejt J: Hybrid sterility in the mouse.
Trends Genet. 12:412–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Homolka D, Jansa P and Forejt J:
Genetically enhanced asynapsis of autosomal chromatin promotes
transcriptional dysregulation and meiotic failure. Chromosoma.
121:91–104. 2012. View Article : Google Scholar :
|
|
33
|
Burgoyne PS, Mahadevaiah SK and Turner JM:
The consequences of asynapsis for mammalian meiosis. Nat Rev Genet.
10:207–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahadevaiah SK, Bourc'his D, de Rooij DG,
Bestor TH, Turner JM and Burgoyne PS: Extensive meiotic asynapsis
in mice antagonises meiotic silencing of unsynapsed chromatin and
consequently disrupts meiotic sex chromosome inactivation. J Cell
Biol. 182:263–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Manterola M, Page J, Vasco C, Berríos S,
Parra MT, Viera A, Rufas JS, Zuccotti M, Garagna S and
Fernández-Donoso R: A high incidence of meiotic silencing of
unsynapsed chromatin is not associated with substantial pachytene
loss in heterozygous male mice carrying multiple simple
robertsonian translocations. PLoS Genet. 5:e10006252009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mark M, Jacobs H, Oulad-Abdelghani M,
Dennefeld C, Féret B, Vernet N, Codreanu CA, Chambon P and
Ghyselinck NB: STRA8-deficient spermatocytes initiate, but fail to
complete, meiosis and undergo premature chromosome condensation. J
Cell Sci. 121:3233–3242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zimmermann C, Romero Y, Warnefors M,
Bilican A, Borel C, Smith LB, Kotaja N, Kaessmann H and Nef S: Germ
cell-specific targeting of DICER or DGCR8 reveals a novel role for
endo-siRNAs in the progression of mammalian spermatogenesis and
male fertility. PLoS One. 9:e1070232014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Subramanian VV and Hochwagen A: The
meiotic checkpoint network: Step-by-step through meiotic prophase.
Cold Spring Harb Perspect Biol. 6:a0166752014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harewood L, Schütz F, Boyle S, Perry P,
Delorenzi M, Bickmore WA and Reymond A: The effect of
translocation-induced nuclear reorganization on gene expression.
Genome Res. 20:554–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kleinjan DA and van Heyningen V:
Long-range control of gene expression: Emerging mechanisms and
disruption in disease. Am J Hum Genet. 76:8–32. 2005. View Article : Google Scholar :
|
|
41
|
Wang W: Emergence of a DNA-damage response
network consisting of Fanconi anaemia and BRCA proteins. Nat Rev
Genet. 8:735–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim JM, Parmar K, Huang M, Weinstock DM,
Ruit CA, Kutok JL and D'Andrea AD: Inactivation of murine Usp1
results in genomic instability and a Fanconi anemia phenotype. Dev
Cell. 16:314–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burnicka-Turek O, Mohamed BA, Shirneshan
K, Thanasupawat T, Hombach-Klonisch S, Klonisch T and Adham IM:
INSL5-deficient mice display an alteration in glucose homeostasis
and an impaired fertility. Endocrinology. 153:4655–4665. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tartaglia LA: The leptin receptor. J Biol
Chem. 272:6093–6096. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Luca C, Kowalski TJ, Zhang Y, Elmquist
JK, Lee C, Kilimann MW, Ludwig T, Liu SM and Chua SC Jr: Complete
rescue of obesity, diabetes, and infertility in db/db mice by
neuron-specific LEPR-B transgenes. J Clin Invest. 115:3484–3493.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paquis-Flucklinger V, Santucci-Darmanin S,
Paul R, Saunières A, Turc-Carel C and Desnuelle C: Cloning and
expression analysis of a meiosis-specific MutS homolog: The human
MSH4 gene. Genomics. 44:188–194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Winand NJ, Panzer JA and Kolodner RD:
Cloning and characterization of the human and Caenorhabditis
elegans homologs of the Saccharomyces cerevisiae MSH5 gene.
Genomics. 53:69–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kneitz B, Cohen PE, Avdievich E, Zhu L,
Kane MF, Hou H Jr, Kolodner RD, Kucherlapati R, Pollard JW and
Edelmann W: MutS homolog 4 localization to meiotic chromosomes is
required for chromosome pairing during meiosis in male and female
mice. Genes Dev. 14:1085–1097. 2000.PubMed/NCBI
|