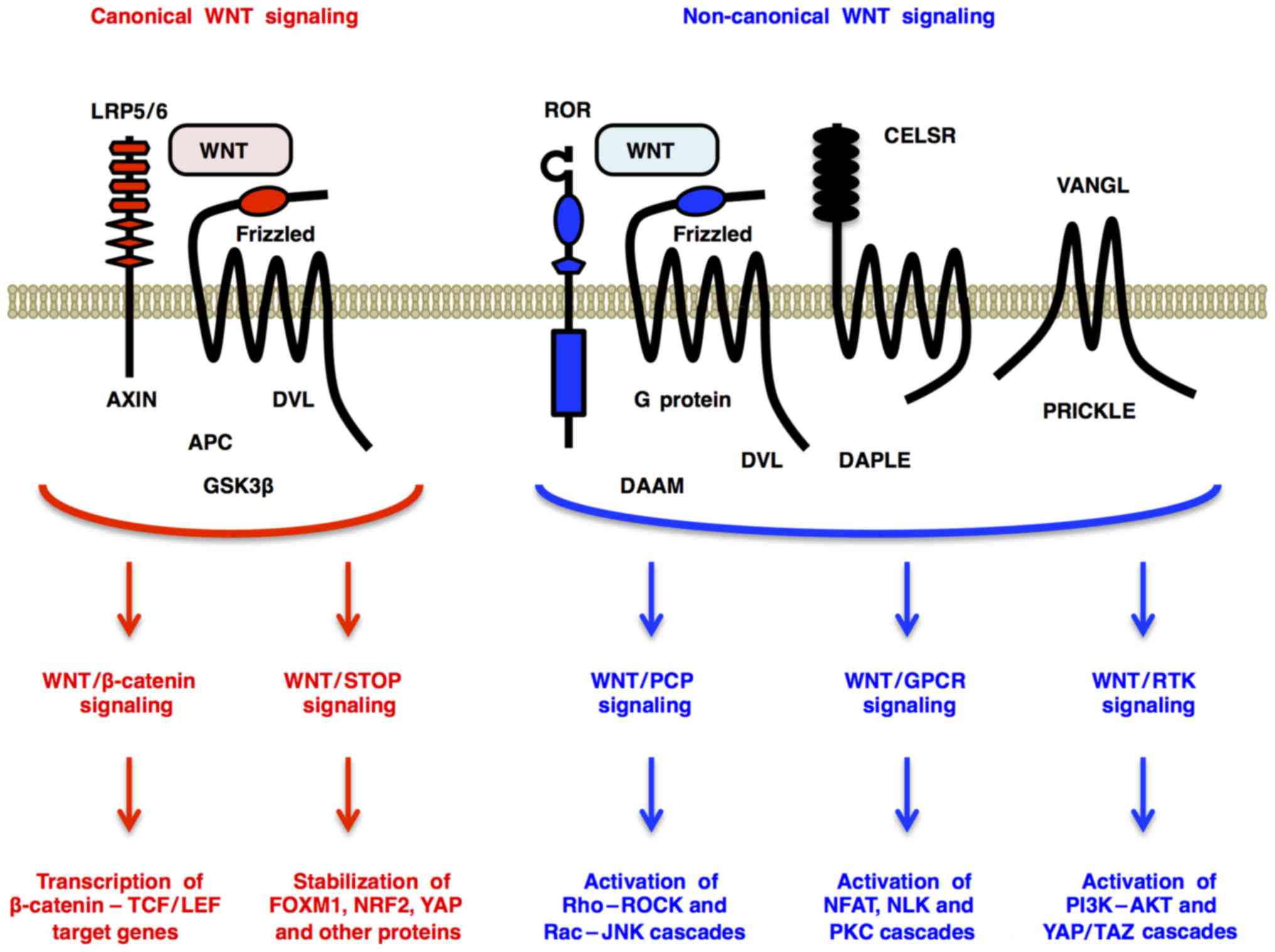
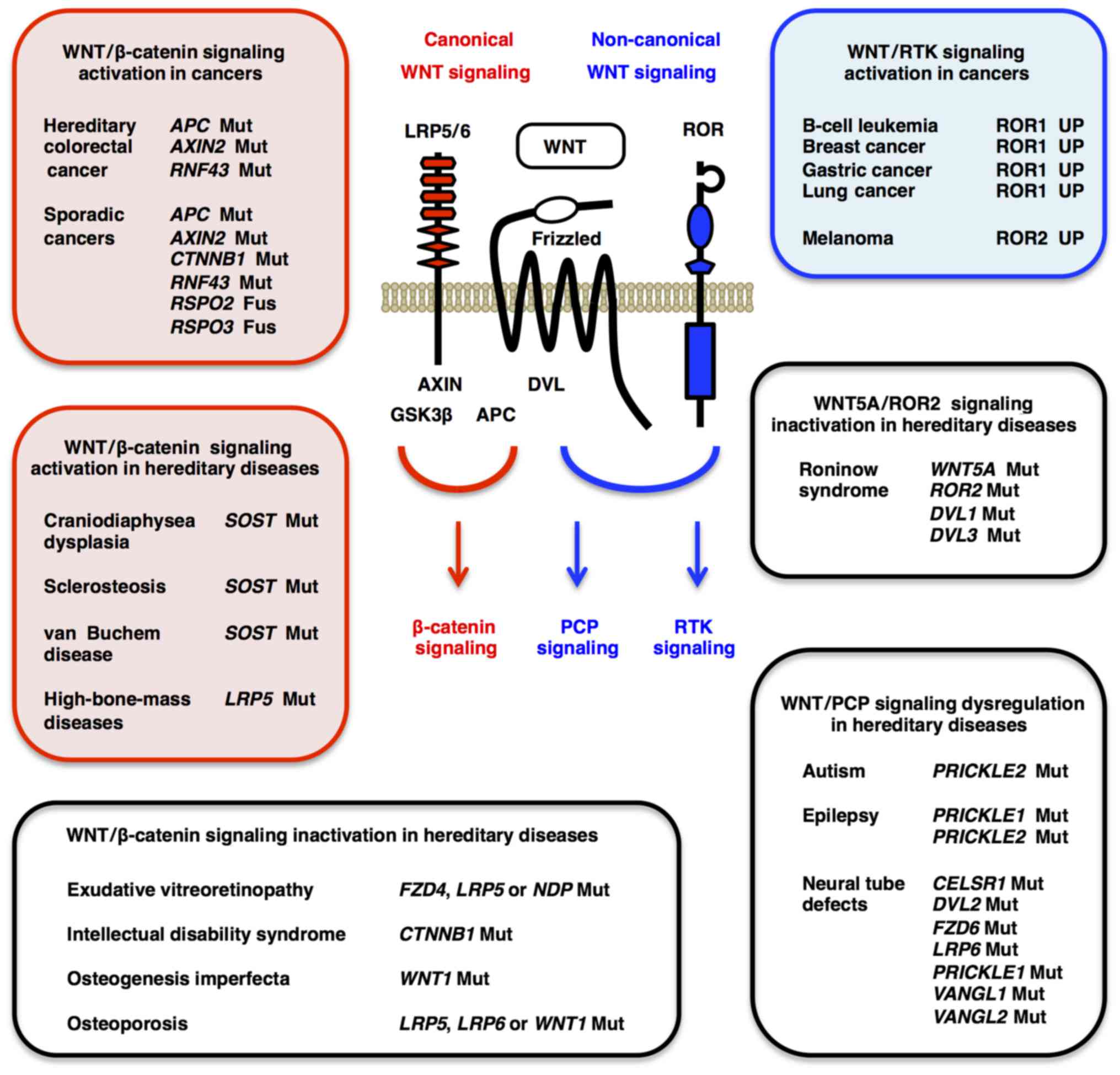
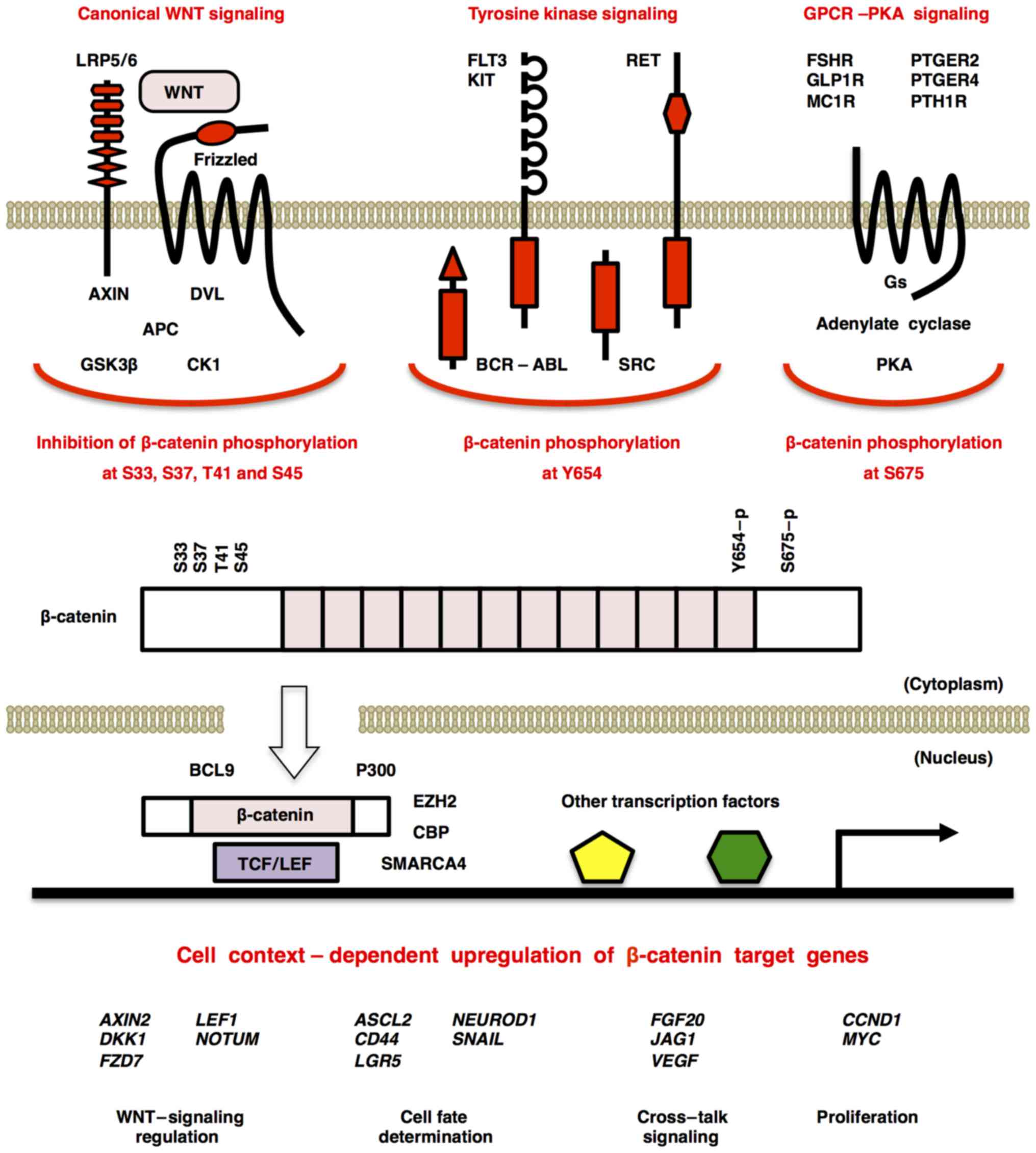
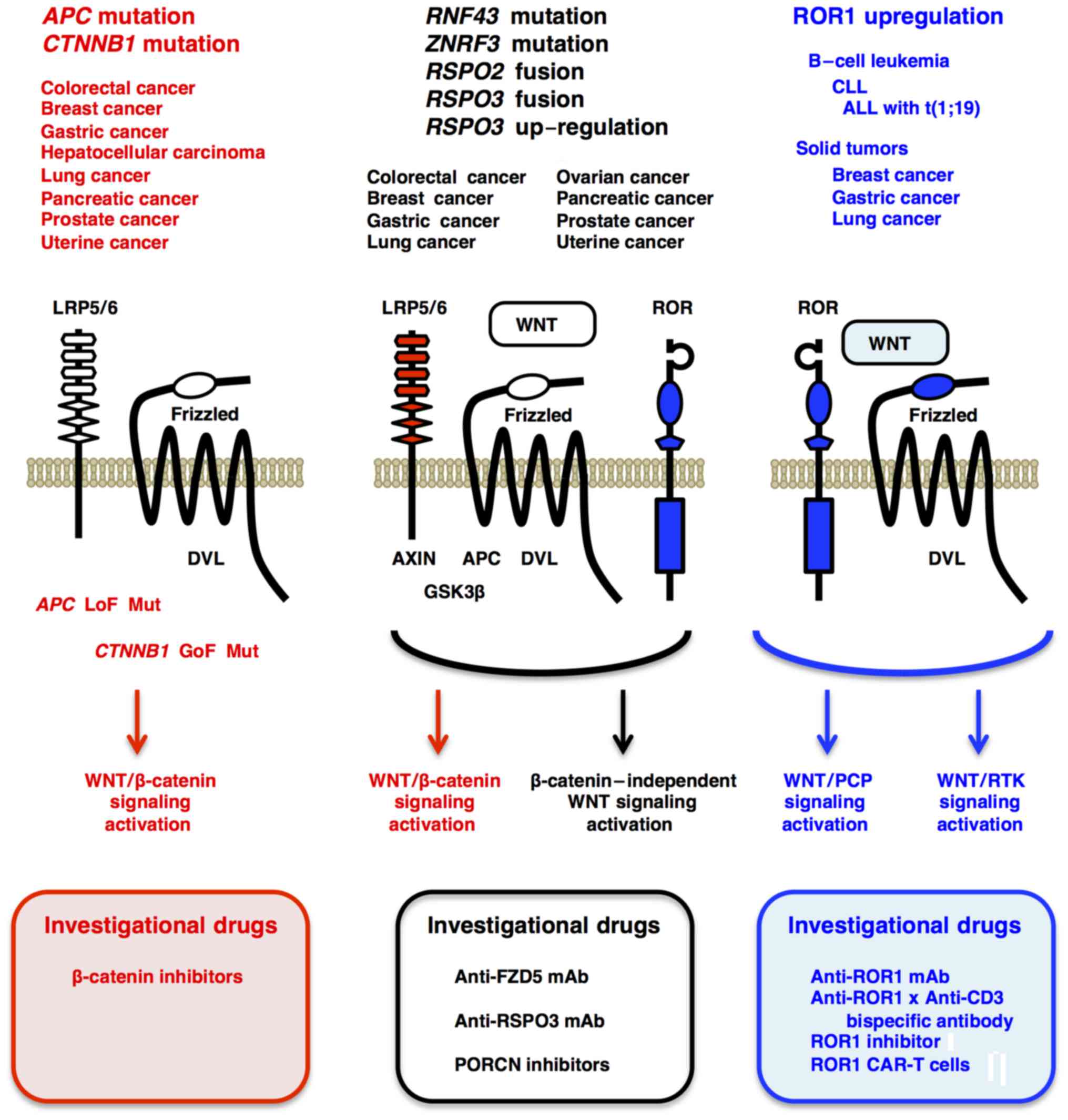
|
1
|
Katoh M: WNT and FGF gene clusters
(Review). Int J Oncol. 21:1269–1273. 2002.PubMed/NCBI
|
|
2
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niehrs C: The complex world of WNT
receptor signalling. Nat Rev Mol Cell Biol. 13:767–779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang K, Wang X, Zhang H, Wang Z, Nan G, Li
Y, Zhang F, Mohammed MK, Haydon RC, Luu HH, et al: The evolving
roles of canonical WNT signaling in stem cells and tumorigenesis:
Implications in targeted cancer therapies. Lab Invest. 96:116–136.
2016. View Article : Google Scholar :
|
|
5
|
Acebron SP and Niehrs C:
β-catenin-independent roles of Wnt/LRP6 signaling. Trends Cell
Biol. 26:956–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rada P, Rojo AI, Offergeld A, Feng GJ,
Velasco-Martín JP, González-Sancho JM, Valverde ÁM, Dale T,
Regadera J and Cuadrado A: WNT-3A regulates an Axin1/NRF2 complex
that regulates antioxidant metabolism in hepatocytes. Antioxid
Redox Signal. 22:555–571. 2015. View Article : Google Scholar :
|
|
7
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
8
|
Zhang S, Chen L, Cui B, Chuang HY, Yu J,
Wang-Rodriguez J, Tang L, Chen G, Basak GW and Kipps TJ: ROR1 is
expressed in human breast cancer and associated with enhanced
tumor-cell growth. PLoS One. 7:e311272012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuo W and Kang Y: Lnc-ing ROR1-HER3 and
Hippo signalling in metastasis. Nat Cell Biol. 19:81–83. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamb R, Bonuccelli G, Ozsvári B,
Peiris-Pagès M, Fiorillo M, Smith DL, Bevilacqua G, Mazzanti CM,
McDonnell LA, Naccarato AG, et al: Mitochondrial mass, a new
metabolic biomarker for stem-like cancer cells: Understanding
WNT/FGF-driven anabolic signaling. Oncotarget. 6:30453–30471. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: A little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katoh M and Nakagama H: FGF receptors:
Cancer biology and therapeutics. Med Res Rev. 34:280–300. 2014.
View Article : Google Scholar
|
|
17
|
Yu M, Ting DT, Stott SL, Wittner BS,
Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, et
al: RNA sequencing of pancreatic circulating tumour cells
implicates WNT signalling in metastasis. Nature. 487:510–513. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bozdag S, Li A, Riddick G, Kotliarov Y,
Baysan M, Iwamoto FM, Cam MC, Kotliarova S and Fine HA:
Age-specific signatures of glioblastoma at the genomic, genetic,
and epigenetic levels. PLoS One. 8:e629822013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ,
Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et
al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt
signaling in antiandrogen resistance. Science. 349:1351–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu Z, Churchman M, Roberts K, Li Y, Liu Y,
Harvey RC, McCastlain K, Reshmi SC, Payne-Turner D, Iacobucci I, et
al: Genomic analyses identify recurrent MEF2D fusions in acute
lymphoblastic leukaemia. Nat Commun. 7:133312016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pettinato G, Ramanathan R, Fisher RA,
Mangino MJ, Zhang N and Wen X: Scalable differentiation of human
iPSCs in a multicellular spheroid-based 3D culture into
hepatocyte-like cells through direct Wnt/β-catenin pathway
inhibition. Sci Rep. 6:328882016. View Article : Google Scholar
|
|
22
|
Motono M, Ioroi Y, Ogura T and Takahashi
J: WNT-C59, a small-molecule WNT inhibitor, efficiently induces
anterior cortex that includes cortical motor neurons from human
pluripotent stem cells. Stem Cells Transl Med. 5:552–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuno K, Mae SI, Okada C, Nakamura M,
Watanabe A, Toyoda T, Uchida E and Osafune K: Redefining definitive
endoderm subtypes by robust induction of human induced pluripotent
stem cells. Differentiation. 92:281–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohamed TM, Stone NR, Berry EC, Radzinsky
E, Huang Y, Pratt K, Ang YS, Yu P, Wang H, Tang S, et al: Chemical
enhancement of in vitro and in vivo direct cardiac reprogramming.
Circulation. 135:978–995. 2017. View Article : Google Scholar
|
|
25
|
Tao L, Zhang J, Meraner P, Tovaglieri A,
Wu X, Gerhard R, Zhang X, Stallcup WB, Miao J, He X, et al:
Frizzled proteins are colonic epithelial receptors for C. difficile
toxin B. Nature. 538:350–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collery RF, Volberding PJ, Bostrom JR,
Link BA and Besharse JC: Loss of zebrafish mfrp causes
nanophthalmia, hyperopia, and accumulation of subretinal
macrophages. Invest Ophthalmol Vis Sci. 57:6805–6814. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lammi L, Arte S, Somer M, Jarvinen H,
Lahermo P, Thesleff I, Pirinen S and Nieminen P: Mutations in AXIN2
cause familial tooth agenesis and predispose to colorectal cancer.
Am J Hum Genet. 74:1043–1050. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gala MK, Mizukami Y, Le LP, Moriichi K,
Austin T, Yamamoto M, Lauwers GY, Bardeesy N and Chung DC: Germline
mutations in oncogene-induced senescence pathways are associated
with multiple sessile serrated adenomas. Gastroenterology.
146:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muzny DM, Bainbridge MN, Chang K, Dinh HH,
Drummond JA, Fowler G, Kovar CL, Lewis LR, Morgan MB, Newsham IF,
et al Cancer Genome Atlas Network: Comprehensive molecular
characterization of human colon and rectal cancer. Nature.
487:330–337. 2012. View Article : Google Scholar
|
|
31
|
Giannakis M, Mu XJ, Shukla SA, Qian ZR,
Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et
al: Genomic correlates of immune-cell infiltrates in colorectal
carcinoma. Cell Rep. 15:857–865. 2016. View Article : Google Scholar :
|
|
32
|
Seshagiri S, Stawiski EW, Durinck S,
Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman
V, Jaiswal BS, et al: Recurrent R-spondin fusions in colon cancer.
Nature. 488:660–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ciriello G, Gatza ML, Beck AH, Wilkerson
MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et
al TCGA Research Network: Comprehensive molecular portraits of
invasive lobular breast cancer. Cell. 163:506–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al Cancer Genome Atlas Research Network: Comprehensive
molecular characterization of gastric adenocarcinoma. Nature.
513:202–209. 2014. View Article : Google Scholar :
|
|
35
|
Guichard C, Amaddeo G, Imbeaud S, Ladeiro
Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M,
Degos F, et al: Integrated analysis of somatic mutations and focal
copy-number changes identifies key genes and pathways in
hepatocellular carcinoma. Nat Genet. 44:694–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Campbell JD, Alexandrov A, Kim J, Wala J,
Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et
al Cancer Genome Atlas Research Network: Distinct patterns of
somatic genome alterations in lung adenocarcinomas and squamous
cell carcinomas. Nat Genet. 48:607–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bailey P, Chang DK, Nones K, Johns AL,
Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC,
et al Australian Pancreatic Cancer Genome Initiative: Genomic
analyses identify molecular subtypes of pancreatic cancer. Nature.
531:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC,
Attard G, et al: Integrative clinical genomics of advanced prostate
cancer. Cell. 161:1215–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al
Cancer Genome Atlas Research Network: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barker N: Adult intestinal stem cells:
Critical drivers of epithelial homeostasis and regeneration. Nat
Rev Mol Cell Biol. 15:19–33. 2014. View Article : Google Scholar
|
|
41
|
Flanagan DJ, Phesse TJ, Barker N, Schwab
RH, Amin N, Malaterre J, Stange DE, Nowell CJ, Currie SA, Saw JT,
et al: Frizzled7 functions as a Wnt receptor in intestinal
epithelial Lgr5(+) stem cells. Stem Cell Reports. 4:759–767. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang X and Cong F: Novel regulation of
Wnt signaling at the proximal membrane level. Trends Biochem Sci.
41:773–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Katoh M: Mutation spectra of histone
methyltransferases with canonical SET domains and EZH2-targeted
therapy. Epigenomics. 8:285–305. 2016. View Article : Google Scholar
|
|
45
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kajiguchi T, Katsumi A, Tanizaki R, Kiyoi
H and Naoe T: Y654 of β-catenin is essential for FLT3/ITD-related
tyrosine phosphorylation and nuclear localization of β-catenin. Eur
J Haematol. 88:314–320. 2012. View Article : Google Scholar
|
|
47
|
Jin B, Ding K and Pan J: Ponatinib induces
apoptosis in imatinib-resistant human mast cells by
dephosphorylating mutant D816V KIT and silencing β-catenin
signaling. Mol Cancer Ther. 13:1217–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fernández-Sánchez ME, Barbier S, Whitehead
J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon
A, Brullé L, et al: Mechanical induction of the tumorigenic
β-catenin pathway by tumour growth pressure. Nature. 523:92–95.
2015. View Article : Google Scholar
|
|
49
|
van Veelen W, Le NH, Helvensteijn W,
Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink
F, van der Valk MA, et al: β-catenin tyrosine 654 phosphorylation
increases Wnt signalling and intestinal tumorigenesis. Gut.
60:1204–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuechler A, Willemsen MH, Albrecht B,
Bacino CA, Bartholomew DW, van Bokhoven H, van den Boogaard MJ,
Bramswig N, Büttner C, Cremer K, et al: De novo mutations in
β-catenin (CTNNB1) appear to be a frequent cause of intellectual
disability: Expanding the mutational and clinical spectrum. Hum
Genet. 134:97–109. 2015. View Article : Google Scholar
|
|
51
|
Dixon MW, Stem MS, Schuette JL, Keegan CE
and Besirli CG: CTNNB1 mutation associated with familial exudative
vitreoretinopathy (FEVR) phenotype. Ophthalmic Genet. 37:468–470.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lui JH, Hansen DV and Kriegstein AR:
Development and evolution of the human neocortex. Cell. 146:18–36.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Inestrosa NC and Arenas E: Emerging roles
of Wnts in the adult nervous system. Nat Rev Neurosci. 11:77–86.
2010. View Article : Google Scholar
|
|
54
|
Bayod S, Felice P, Andrés P, Rosa P,
Camins A, Pallàs M and Canudas AM: Downregulation of canonical Wnt
signaling in hippocampus of SAMP8 mice. Neurobiol Aging.
36:720–729. 2015. View Article : Google Scholar
|
|
55
|
Wakabayashi T, Hidaka R, Fujimaki S,
Asashima M and Kuwabara T: Diabetes impairs Wnt3 protein-induced
neurogenesis in olfactory bulbs via glutamate transporter 1
inhibition. J Biol Chem. 291:15196–15211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Marzo A, Galli S, Lopes D, McLeod F,
Podpolny M, Segovia-Roldan M, Ciani L, Purro S, Cacucci F, Gibb A,
et al: Reversal of synapse degeneration by restoring Wnt signaling
in the adult hippocampus. Curr Biol. 26:2551–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen
Y, Yang TH, Kim HM, Drake D, Liu XS, et al: REST and stress
resistance in ageing and Alzheimer's disease. Nature. 507:448–454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dias C, Feng J, Sun H, Shao NY,
Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R, LaBonté B,
Ribeiro E, et al: β-catenin mediates stress resilience through
Dicer1/microRNA regulation. Nature. 516:51–55. 2014.PubMed/NCBI
|
|
59
|
Madison JM, Zhou F, Nigam A, Hussain A,
Barker DD, Nehme R, van der Ven K, Hsu J, Wolf P, Fleishman M, et
al: Characterization of bipolar disorder patient-specific induced
pluripotent stem cells from a family reveals neurodevelopmental and
mRNA expression abnormalities. Mol Psychiatry. 20:703–717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Karantalis V and Hare JM: Use of
mesenchymal stem cells for therapy of cardiac disease. Circ Res.
116:1413–1430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen Y and Alman BA: Wnt pathway, an
essential role in bone regeneration. J Cell Biochem. 106:353–362.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang R, Oyajobi BO, Harris SE, Chen D,
Tsao C, Deng HW and Zhao M: Wnt/β-catenin signaling activates bone
morphogenetic protein 2 expression in osteoblasts. Bone.
52:145–156. 2013. View Article : Google Scholar
|
|
64
|
Ke HZ, Richards WG, Li X and Ominsky MS:
Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases.
Endocr Rev. 33:747–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Boudin E, Fijalkowski I, Piters E and Van
Hul W: The role of extracellular modulators of canonical Wnt
signaling in bone metabolism and diseases. Semin Arthritis Rheum.
43:220–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Silva BC and Bilezikian JP: Parathyroid
hormone: Anabolic and catabolic actions on the skeleton. Curr Opin
Pharmacol. 22:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maeda K, Kobayashi Y, Udagawa N, Uehara S,
Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et
al: Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim SJ, Bieganski T, Sohn YB, Kozlowski K,
Semënov M, Okamoto N, Kim CH, Ko AR, Ahn GH, Choi YL, et al:
Identification of signal peptide domain SOST mutations in autosomal
dominant craniodiaphyseal dysplasia. Hum Genet. 129:497–502. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brunkow ME, Gardner JC, Van Ness J, Paeper
BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et
al: Bone dysplasia sclerosteosis results from loss of the SOST gene
product, a novel cystine knot-containing protein. Am J Hum Genet.
68:577–589. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
70
|
Balemans W, Patel N, Ebeling M, Van Hul E,
Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ,
et al: Identification of a 52 kb deletion downstream of the SOST
gene in patients with van Buchem disease. J Med Genet. 39:91–97.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Van Wesenbeeck L, Cleiren E, Gram J, Beals
RK, Bénichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y,
et al: Six novel missense mutations in the LDL receptor-related
protein 5 (LRP5) gene in different conditions with an increased
bone density. Am J Hum Genet. 72:763–771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Canalis E: Wnt signalling in osteoporosis:
Mechanisms and novel therapeutic approaches. Nat Rev Endocrinol.
9:575–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Laine CM, Joeng KS, Campeau PM, Kiviranta
R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ,
et al: WNT1 mutations in early-onset osteoporosis and osteogenesis
imperfecta. N Engl J Med. 368:1809–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gong Y, Slee RB, Fukai N, Rawadi G,
Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et
al Osteoporosis-Pseudoglioma Syndrome Collaborative Group: LDL
receptor-related protein 5 (LRP5) affects bone accrual and eye
development. Cell. 107:513–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mani A, Radhakrishnan J, Wang H, Mani A,
Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, et
al: LRP6 mutation in a family with early coronary disease and
metabolic risk factors. Science. 315:1278–1282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Simsek Kiper PO, Saito H, Gori F, Unger S,
Hesse E, Yamana K, Kiviranta R, Solban N, Liu J, Brommage R, et al:
Cortical-bone fragility: Insights from sFRP4 deficiency in Pyle's
disease. N Engl J Med. 374:2553–2562. 2016. View Article : Google Scholar
|
|
77
|
Estrada K, Styrkarsdottir U, Evangelou E,
Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP,
et al: Genome-wide meta-analysis identifies 56 bone mineral density
loci and reveals 14 loci associated with risk of fracture. Nat
Genet. 44:491–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Katoh M: Therapeutics targeting
angiogenesis: Genetics and epigenetics, extracellular miRNAs and
signaling networks (Review). Int J Mol Med. 32:763–767.
2013.PubMed/NCBI
|
|
80
|
Hayakawa Y, Ariyama H, Stancikova J,
Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H,
Westphalen CB, et al: Mist1 expressing gastric stem cells maintain
the normal and neoplastic gastric epithelium and are supported by a
perivascular stem cell niche. Cancer Cell. 28:800–814. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rafii S, Butler JM and Ding BS: Angiocrine
functions of organ-specific endothelial cells. Nature. 529:316–325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Katoh M: Therapeutics targeting FGF
signaling network in human diseases. Trends Pharmacol Sci.
37:1081–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou W, Wang G and Guo S: Regulation of
angiogenesis via Notch signaling in breast cancer and cancer stem
cells. Biochim Biophys Acta. 1836:304–320. 2013.PubMed/NCBI
|
|
86
|
Zhang P, Yan X, Chen Y, Yang Z and Han H:
Notch signaling in blood vessels: From morphogenesis to
homeostasis. Sci China Life Sci. 57:774–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rostama B, Peterson SM, Vary CP and Liaw
L: Notch signal integration in the vasculature during remodeling.
Vascul Pharmacol. 63:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hilbert T and Klaschik S: The
angiopoietin/TIE receptor system: Focusing its role for
ischemia-reperfusion injury. Cytokine Growth Factor Rev.
26:281–291. 2015. View Article : Google Scholar
|
|
89
|
Zhang X, Gaspard JP and Chung DC:
Regulation of vascular endothelial growth factor by the Wnt and
K-ras pathways in colonic neoplasia. Cancer Res. 61:6050–6054.
2001.PubMed/NCBI
|
|
90
|
Korn C, Scholz B, Hu J, Srivastava K,
Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG and
Augustin I: Endothelial cell-derived non-canonical Wnt ligands
control vascular pruning in angiogenesis. Development.
141:1757–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gilmour DF: Familial exudative
vitreoretinopathy and related retinopathies. Eye (Lond). 29:1–14.
2015. View Article : Google Scholar
|
|
92
|
Kirikoshi H, Sagara N, Koike J, Tanaka K,
Sekihara H, Hirai M and Katoh M: Molecular cloning and
characterization of human Frizzled-4 on chromosome 11q14-q21.
Biochem Biophys Res Commun. 264:955–961. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang K, Harada Y, Wei X, Shukla D,
Rajendran A, Tawansy K, Bedell M, Lim S, Shaw PX, He X, et al: An
essential role of the cysteine-rich domain of FZD4 in Norrin/Wnt
signaling and familial exudative vitreoretinopathy. J Biol Chem.
286:10210–10215. 2011. View Article : Google Scholar :
|
|
94
|
Musada GR, Syed H, Jalali S, Chakrabarti S
and Kaur I: Mutation spectrum of the FZD-4, TSPAN12 and ZNF408
genes in Indian FEVR patients. BMC Ophthalmol. 16:902016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tang M, Ding X, Li J, Hu A, Yuan M, Yang
Y, Zhan Z, Li Z and Lu L: Novel mutations in FZD4 and
phenotype-genotype correlation in Chinese patients with familial
exudative vitreoretinopathy. Mol Vis. 22:917–932. 2016.PubMed/NCBI
|
|
96
|
Fei P, Zhang Q, Huang L, Xu Y, Zhu X, Tai
Z, Gong B, Ma S, Yao Q, Li J, et al: Identification of two novel
LRP5 mutations in families with familial exudative
vitreoretinopathy. Mol Vis. 20:395–409. 2014.PubMed/NCBI
|
|
97
|
Ye X, Wang Y and Nathans J: The
Norrin/Frizzled4 signaling pathway in retinal vascular development
and disease. Trends Mol Med. 16:417–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Stenman JM, Rajagopal J, Carroll TJ,
Ishibashi M, McMahon J and McMahon AP: Canonical Wnt signaling
regulates organ-specific assembly and differentiation of CNS
vasculature. Science. 322:1247–1250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang Z, Liu CH, Sun Y, Gong Y, Favazza TL,
Morss PC, Saba NJ, Fredrick TW, He X, Akula JD, et al:
Pharmacologic activation of Wnt signaling by lithium normalizes
retinal vasculature in a murine model of familial exudative
vitreoretinopathy. Am J Pathol. 186:2588–2600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Birdsey GM, Shah AV, Dufton N, Reynolds
LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana
E, et al: The endothelial transcription factor ERG promotes
vascular stability and growth through Wnt/β-catenin signaling. Dev
Cell. 32:82–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Arthofer E, Hot B, Petersen J, Strakova K,
Jäger S, Grundmann M, Kostenis E, Gutkind JS and Schulte G: WNT
stimulation dissociates a Frizzled 4 inactive-state complex with
Gα12/13. Mol Pharmacol. 90:447–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Adler PN: The frizzled/stan pathway and
planar cell polarity in the Drosophila wing. Curr Top Dev Biol.
101:1–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang Y and Mlodzik M: Wnt-Frizzled/planar
cell polarity signaling: Cellular orientation by facing the wind
(Wnt). Annu Rev Cell Dev Biol. 31:623–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gao B, Song H, Bishop K, Elliot G, Garrett
L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al: Wnt
signaling gradients establish planar cell polarity by inducing
Vangl2 phosphorylation through Ror2. Dev Cell. 20:163–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nishimura T, Honda H and Takeichi M:
Planar cell polarity links axes of spatial dynamics in neural-tube
closure. Cell. 149:1084–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pan X, Sittaramane V, Gurung S and
Chandrasekhar A: Structural and temporal requirements of Wnt/PCP
protein Vangl2 function for convergence and extension movements and
facial branchiomotor neuron migration in zebrafish. Mech Dev.
131:1–14. 2014. View Article : Google Scholar :
|
|
107
|
Gödde NJ, Pearson HB, Smith LK and Humbert
PO: Dissecting the role of polarity regulators in cancer through
the use of mouse models. Exp Cell Res. 328:249–257. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cantrell VA and Jessen JR: The planar cell
polarity protein Van Gogh-Like 2 regulates tumor cell migration and
matrix metalloproteinase-dependent invasion. Cancer Lett.
287:54–61. 2010. View Article : Google Scholar
|
|
109
|
O'Connell MP, Fiori JL, Xu M, Carter AD,
Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier
M, et al: The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A
signaling in metastatic melanoma. Oncogene. 29:34–44. 2010.
View Article : Google Scholar :
|
|
110
|
Wang W, Runkle KB, Terkowski SM, Ekaireb
RI and Witze ES: Protein depalmitoylation is induced by Wnt5a and
promotes polarized cell behavior. J Biol Chem. 290:15707–15716.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Webster MR, Kugel CH III and Weeraratna
AT: The Wnts of change: How Wnts regulate phenotype switching in
melanoma. Biochim Biophys Acta. 1856:244–251. 2015.PubMed/NCBI
|
|
112
|
Katoh M: Function and cancer genomics of
FAT family genes (Review). Int J Oncol. 41:1913–1918.
2012.PubMed/NCBI
|
|
113
|
Matis M and Axelrod JD: Regulation of PCP
by the Fat signaling pathway. Genes Dev. 27:2207–2220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Katoh M and Katoh M: Identification and
characterization of human PRICKLE1 and PRICKLE2 genes as well as
mouse Prickle1 and Prickle2 genes homologous to Drosophila tissue
polarity gene prickle. Int J Mol Med. 11:249–256. 2003.PubMed/NCBI
|
|
115
|
De Marco P, Merello E, Piatelli G, Cama A,
Kibar Z and Capra V: Planar cell polarity gene mutations contribute
to the etiology of human neural tube defects in our population.
Birth Defects Res A Clin Mol Teratol. 100:633–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Allache R, Lachance S, Guyot MC, De Marco
P, Merello E, Justice MJ, Capra V and Kibar Z: Novel mutations in
Lrp6 orthologs in mouse and human neural tube defects affect a
highly dosage-sensitive Wnt non-canonical planar cell polarity
pathway. Hum Mol Genet. 23:1687–1699. 2014. View Article : Google Scholar :
|
|
117
|
Bassuk AG, Wallace RH, Buhr A, Buller AR,
Afawi Z, Shimojo M, Miyata S, Chen S, Gonzalez-Alegre P, Griesbach
HL, et al: A homozygous mutation in human PRICKLE1 causes an
autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome.
Am J Hum Genet. 83:572–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tao H, Manak JR, Sowers L, Mei X, Kiyonari
H, Abe T, Dahdaleh NS, Yang T, Wu S, Chen S, et al: Mutations in
prickle orthologs cause seizures in flies, mice, and humans. Am J
Hum Genet. 88:138–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sowers LP, Loo L, Wu Y, Campbell E, Ulrich
JD, Wu S, Paemka L, Wassink T, Meyer K, Bing X, et al: Disruption
of the non-canonical Wnt gene PRICKLE2 leads to autism-like
behaviors with evidence for hippocampal synaptic dysfunction. Mol
Psychiatry. 18:1077–1089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
White JJ, Mazzeu JF, Hoischen A, Bayram Y,
Withers M, Gezdirici A, Kimonis V, Steehouwer M, Jhangiani SN,
Muzny DM, et al Baylor-Hopkins Center for Mendelian Genomics: DVL3
alleles resulting in a -1 frameshift of the last exon mediate
autosomal-dominant Robinow syndrome. Am J Hum Genet. 98:553–561.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Copp AJ and Greene ND: Genetics and
development of neural tube defects. J Pathol. 220:217–230.
2010.
|
|
122
|
Muñoz-Soriano V, Belacortu Y and Paricio
N: Planar cell polarity signaling in collective cell movements
during morphogenesis and disease. Curr Genomics. 13:609–622. 2012.
View Article : Google Scholar :
|
|
123
|
Wu G, Huang X, Hua Y and Mu D: Roles of
planar cell polarity pathways in the development of neutral tube
defects. J Biomed Sci. 18:662011. View Article : Google Scholar :
|
|
124
|
de la Hoz AB, Maortua H, García-Rives A,
Martínez-González MJ, Ezquerra M and Tejada MI: 3p14 de novo
interstitial microdeletion in a patient with intellectual
disability and autistic features with language impairment: A
comparison with similar cases. Case Rep Genet.
2015:8763482015.PubMed/NCBI
|
|
125
|
Mazzeu JF, Pardono E, Vianna-Morgante AM,
Richieri-Costa A, Ae Kim C, Brunoni D, Martelli L, de Andrade CE,
Colin G and Otto PA: Clinical characterization of autosomal
dominant and recessive variants of Robinow syndrome. Am J Med Genet
A. 143:320–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Person AD, Beiraghi S, Sieben CM,
Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ
Jr, van Bokhoven H, Hoogeboom JM, et al: WNT5A mutations in
patients with autosomal dominant Robinow syndrome. Dev Dyn.
239:327–337. 2010.
|
|
127
|
Afzal AR, Rajab A, Fenske CD, Oldridge M,
Elanko N, Ternes-Pereira E, Tüysüz B, Murday VA, Patton MA, Wilkie
AO, et al: Recessive Robinow syndrome, allelic to dominant
brachydactyly type B, is caused by mutation of ROR2. Nat Genet.
25:419–422. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
128
|
Oldridge M, Fortuna AM, Maringa M,
Propping P, Mansour S, Pollitt C, DeChiara TM, Kimble RB,
Valenzuela DM, Yancopoulos GD, et al: Dominant mutations in ROR2,
encoding an orphan receptor tyrosine kinase, cause brachydactyly
type B. Nat Genet. 24:275–278. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
129
|
Green JL, Kuntz SG and Sternberg PW: Ror
receptor tyrosine kinases: Orphans no more. Trends Cell Biol.
18:536–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Minami Y, Oishi I, Endo M and Nishita M:
Ror-family receptor tyrosine kinases in noncanonical Wnt signaling:
Their implications in developmental morphogenesis and human
diseases. Dev Dyn. 239:1–15. 2010.
|
|
131
|
Petrova IM, Malessy MJ, Verhaagen J,
Fradkin LG and Noordermeer JN: Wnt signaling through the Ror
receptor in the nervous system. Mol Neurobiol. 49:303–315. 2014.
View Article : Google Scholar
|
|
132
|
Debebe Z and Rathmell WK: Ror2 as a
therapeutic target in cancer. Pharmacol Ther. 150:143–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bunn KJ, Lai A, Al-Ani A, Farella M, Craw
S and Robertson SP: An osteosclerotic form of Robinow syndrome. Am
J Med Genet A. 164A:2638–2642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Liu C, Lin C, Gao C, May-Simera H, Swaroop
A and Li T: Null and hypomorph Prickle1 alleles in mice phenocopy
human Robinow syndrome and disrupt signaling downstream of Wnt5a.
Biol Open. 3:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ford CE, Qian Ma SS, Quadir A and Ward RL:
The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in
human carcinogenesis. Int J Cancer. 133:779–787. 2013. View Article : Google Scholar
|
|
136
|
Asad M, Wong MK, Tan TZ, Choolani M, Low
J, Mori S, Virshup D, Thiery JP and Huang RY: FZD7 drives in vitro
aggressiveness in Stem-A subtype of ovarian cancer via regulation
of non-canonical Wnt/PCP pathway. Cell Death Dis. 5:e13462014.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Qin L, Yin YT, Zheng FJ, Peng LX, Yang CF,
Bao YN, Liang YY, Li XJ, Xiang YQ, Sun R, et al: WNT5A promotes
stemness characteristics in nasopharyngeal carcinoma cells leading
to metastasis and tumorigenesis. Oncotarget. 6:10239–10252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Thiele S, Rachner TD, Rauner M and
Hofbauer LC: WNT5A and its receptors in the bone-cancer dialogue. J
Bone Miner Res. 31:1488–1496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Kumawat K and Gosens R: WNT-5A: Signaling
and functions in health and disease. Cell Mol Life Sci. 73:567–587.
2016. View Article : Google Scholar :
|
|
140
|
Wei H, Wang N, Zhang Y, Wang S, Pang X and
Zhang S: Wnt-11 overexpression promoting the invasion of cervical
cancer cells. Tumour Biol. 37:11789–11798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Arabzadeh S, Hossein G, Salehi-Dulabi Z
and Zarnani AH: WNT5A-ROR2 is induced by inflammatory mediators and
is involved in the migration of human ovarian cancer cell line
SKOV-3. Cell Mol Biol Lett. 21:92016. View Article : Google Scholar
|
|
142
|
Jiang W, Crossman DK, Mitchell EH, Sohn P,
Crowley MR and Serra R: WNT5A inhibits metastasis and alters
splicing of Cd44 in breast cancer cells. PLoS One. 8:e583292013.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Easter SL, Mitchell EH, Baxley SE, Desmond
R, Frost AR and Serra R: Wnt5a suppresses tumor formation and
redirects tumor phenotype in MMTV-Wnt1 tumors. PLoS One.
9:e1132472014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang MT, Holderfield M, Galeas J,
Delrosario R, To MD, Balmain A and McCormick F: K-Ras promotes
tumorigenicity through suppression of non-canonical Wnt signaling.
Cell. 163:1237–1251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Fukuda T, Chen L, Endo T, Tang L, Lu D,
Castro JE, Widhopf GF II, Rassenti LZ, Cantwell MJ, Prussak CE, et
al: Antisera induced by infusions of autologous Ad-CD154-leukemia B
cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a.
Proc Natl Acad Sci USA. 105:3047–3052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bicocca VT, Chang BH, Masouleh BK, Muschen
M, Loriaux MM, Druker BJ and Tyner JW: Crosstalk between ROR1 and
the Pre-B cell receptor promotes survival of t(1;19) acute
lymphoblastic leukemia. Cancer Cell. 22:656–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yu J, Chen L, Cui B, Widhopf GF II, Shen
Z, Wu R, Zhang L, Zhang S, Briggs SP and Kipps TJ: Wnt5a induces
ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and
proliferation. J Clin Invest. 126:585–598. 2016. View Article : Google Scholar :
|
|
148
|
Gentile A, Lazzari L, Benvenuti S,
Trusolino L and Comoglio PM: The ROR1 pseudokinase diversifies
signaling outputs in MET-addicted cancer cells. Int J Cancer.
135:2305–2316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hojjat-Farsangi M, Moshfegh A,
Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A and Mellstedt
H: The receptor tyrosine kinase ROR1 - an oncofetal antigen for
targeted cancer therapy. Semin Cancer Biol. 29:21–31. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Li C, Wang S, Xing Z, Lin A, Liang K, Song
J, Hu Q, Yao J, Chen Z, Park PK, et al: A ROR1-HER3-lncRNA
signalling axis modulates the Hippo-YAP pathway to regulate bone
metastasis. Nat Cell Biol. 19:106–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Widhopf GF II, Cui B, Ghia EM, Chen L,
Messer K, Shen Z, Briggs SP, Croce CM and Kipps TJ: ROR1 can
interact with TCL1 and enhance leukemogenesis in Eμ-TCL1 transgenic
mice. Proc Natl Acad Sci USA. 111:793–798. 2014. View Article : Google Scholar
|
|
152
|
Yamaguchi T, Lu C, Ida L, Yanagisawa K,
Usukura J, Cheng J, Hotta N, Shimada Y, Isomura H, Suzuki M, et al:
ROR1 sustains caveolae and survival signalling as a scaffold of
cavin-1 and caveolin-1. Nat Commun. 7:100602016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
O'Connell MP, Marchbank K, Webster MR,
Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q,
et al: Hypoxia induces phenotypic plasticity and therapy resistance
in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer
Discov. 3:1378–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lai SS, Xue B, Yang Y, Zhao L, Chu CS, Hao
JY and Wen CJ: Ror2-Src signaling in metastasis of mouse melanoma
cells is inhibited by NRAGE. Cancer Genet. 205:552–562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Niemann S, Zhao C, Pascu F, Stahl U,
Aulepp U, Niswander L, Weber JL and Müller U: Homozygous WNT3
mutation causes tetra-amelia in a large consanguineous family. Am J
Hum Genet. 74:558–563. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
156
|
Woods CG, Stricker S, Seemann P, Stern R,
Cox J, Sherridan E, Roberts E, Springell K, Scott S, Karbani G, et
al: Mutations in WNT7A cause a range of limb malformations,
including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel
phocomelia syndrome. Am J Hum Genet. 79:402–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Biason-Lauber A, Konrad D, Navratil F and
Schoenle EJ: A WNT4 mutation associated with Müllerian-duct
regression and virilization in a 46, XX woman. N Engl J Med.
351:792–798. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Mandel H, Shemer R, Borochowitz ZU,
Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D,
Gershoni-Baruch R, Choder M, et al: SERKAL syndrome: An
autosomal-recessive disorder caused by a loss-of-function mutation
in WNT4. Am J Hum Genet. 82:39–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kirikoshi H, Sekihara H and Katoh M:
WNT10A and WNT6, clustered in human chromosome 2q35 region with
head-to-tail manner, are strongly coexpressed in SW480 cells.
Biochem Biophys Res Commun. 283:798–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Adaimy L, Chouery E, Megarbane H, Mroueh
S, Delague V, Nicolas E, Belguith H, de Mazancourt P and Megarbane
A: Mutation in WNT10A is associated with an autosomal recessive
ectodermal dysplasia: The odonto-onycho-dermal dysplasia. Am J Hum
Genet. 81:821–828. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
161
|
van den Boogaard MJ, Créton M, Bronkhorst
Y, van der Hout A, Hennekam E, Lindhout D, Cune M and Ploos van
Amstel HK: Mutations in WNT10A are present in more than half of
isolated hypodontia cases. J Med Genet. 49:327–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yu P, Yang W, Han D, Wang X, Guo S, Li J,
Li F, Zhang X, Wong SW, Bai B, et al: Mutations in WNT10B are
identified in individuals with oligodontia. Am J Hum Genet.
99:195–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Massink MP, Créton MA, Spanevello F,
Fennis WM, Cune MS, Savelberg SM, Nijman IJ, Maurice MM, van den
Boogaard MJ and van Haaften G: H and van Haaften G.
Loss-of-function mutations in the WNT co-receptor LRP6 cause
autosomal-dominant oligodontia. Am J Hum Genet. 97:621–626. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Poulsen A, Ho SY, Wang W, Alam J, Jeyaraj
DA, Ang SH, Tan ES, Lin GR, Cheong VW, Ke Z, et al: Pharmacophore
model for Wnt/Porcupine inhibitors and its use in drug design. J
Chem Inf Model. 55:1435–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Grzeschik KH, Bornholdt D, Oeffner F,
König A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff
U, Höfling K, et al: Deficiency of PORCN, a regulator of Wnt
signaling, is associated with focal dermal hypoplasia. Nat Genet.
39:833–835. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
166
|
Liu C, Widen SA, Williamson KA, Ratnapriya
R, Gerth-Kahlert C, Rainger J, Alur RP, Strachan E, Manjunath SH,
Balakrishnan A, et al UK10K Consortium: A secreted
WNT-ligand-binding domain of FZD5 generated by a frameshift
mutation causes autosomal dominant coloboma. Hum Mol Genet.
25:1382–1391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Fröjmark AS, Schuster J, Sobol M,
Entesarian M, Kilander MB, Gabrikova D, Nawaz S, Baig SM, Schulte
G, Klar J, et al: Mutations in Frizzled 6 cause isolated
autosomal-recessive nail dysplasia. Am J Hum Genet. 88:852–860.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Parma P, Radi O, Vidal V, Chaboissier MC,
Dellambra E, Valentini S, Guerra L, Schedl A and Camerino G:
R-spondin1 is essential in sex determination, skin differentiation
and malignancy. Nat Genet. 38:1304–1309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Brüchle NO, Frank J, Frank V, Senderek J,
Akar A, Koc E, Rigopoulos D, van Steensel M, Zerres K and Bergmann
C: RSPO4 is the major gene in autosomal-recessive anonychia and
mutations cluster in the furin-like cysteine-rich domains of the
Wnt signaling ligand R-spondin 4. J Invest Dermatol. 128:791–796.
2008. View Article : Google Scholar
|
|
170
|
Ekici AB, Hilfinger D, Jatzwauk M, Thiel
CT, Wenzel D, Lorenz I, Boltshauser E, Goecke TW, Staatz G,
Morris-Rosendahl DJ, et al: Disturbed Wnt signalling due to a
mutation in CCDC88C causes an autosomal recessive non-syndromic
hydrocephalus with medial diverticulum. Mol Syndromol. 1:99–112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez
I, Pavlova Y, Marivin A, Barbazán J, Murray F, Nitsche U, Janssen
KP, et al: Daple is a novel non-receptor GEF required for trimeric
G protein activation in Wnt signaling. eLife. 4:e070912015.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Voronkov A and Krauss S: Wnt/β-catenin
signaling and small molecule inhibitors. Curr Pharm Des.
19:634–664. 2013. View Article : Google Scholar
|
|
173
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Tai D, Wells K, Arcaroli J, Vanderbilt C,
Aisner DL, Messersmith WA and Lieu CH: Targeting the WNT signaling
pathway in cancer therapeutics. Oncologist. 20:1189–1198. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Pelay-Gimeno M, Glas A, Koch O and
Grossmann TN: Structure-based design of inhibitors of
protein-protein interactions: Mimicking peptide binding epitopes.
Angew Chem Int Ed Engl. 54:8896–8927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Kakugawa S, Langton PF, Zebisch M, Howell
SA, Chang TH, Liu Y, Feizi T, Bineva G, O'Reilly N, Snijders AP, et
al: Notum deacylates Wnt proteins to suppress signalling activity.
Nature. 519:187–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Madan B, Ke Z, Harmston N, Ho SY, Frois
AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, et al:
Wnt addiction of genetically defined cancers reversed by PORCN
inhibition. Oncogene. 35:2197–2207. 2016. View Article : Google Scholar
|
|
178
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang
T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al: Targeting
Wnt-driven cancer through the inhibition of Porcupine by LGK974.
Proc Natl Acad Sci USA. 110:20224–20229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Proffitt KD, Madan B, Ke Z, Pendharkar V,
Ding L, Lee MA, Hannoush RN and Virshup DM: Pharmacological
inhibition of the Wnt acyltransferase PORCN prevents growth of
WNT-driven mammary cancer. Cancer Res. 73:502–507. 2013. View Article : Google Scholar
|
|
181
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Cheng Y, Phoon YP, Jin X, Chong SY, Ip JC,
Wong BW and Lung ML: Wnt-C59 arrests stemness and suppresses growth
of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway
in the tumor microenvironment. Oncotarget. 6:14428–14439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Madan B, Ke Z, Lei ZD, Oliver FA, Oshima
M, Lee MA, Rozen S and Virshup DM: NOTUM is a potential
pharmacodynamic biomarker of Wnt pathway inhibition. Oncotarget.
7:12386–12392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Blyszczuk P, Müller-Edenborn B, Valenta T,
Osto E, Stellato M, Behnke S, Glatz K, Basler K, Lüscher TF,
Distler O, et al: Transforming growth factor-β-dependent Wnt
secretion controls myofibroblast formation and myocardial fibrosis
progression in experimental autoimmune myocarditis. Eur Heart J.
38:1413–1425. 2017.
|
|
185
|
Madan B, Patel MB, Zhang J, Bunte RM,
Rudemiller NP, Griffiths R, Virshup DM and Crowley SD: Experimental
inhibition of porcupine-mediated Wnt O-acylation attenuates kidney
fibrosis. Kidney Int. 89:1062–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Diamond JR, Eckhardt SG, Bendell JC,
Munster P, Morris VK, Kopetz S, Cattaruzza F, Kapoun AM, Dupont J
and Faoro L: A Phase 1a/b study of OMP-131R10, an anti-RSPO3
antibody, in advanced solid tumors and previously treated
metastatic colorectal cancer (CRC). Presented at: TAT 2016
Conference; Washington DC. 21–23 March, 2016;
|
|
187
|
Katoh M, Hirai M, Sugimura T and Terada M:
Cloning, expression and chromosomal localization of Wnt-13, a novel
member of the Wnt gene family. Oncogene. 13:873–876.
1996.PubMed/NCBI
|
|
188
|
Katoh M, Kirikoshi H, Terasaki H and
Shiokawa K: WNT2B2 mRNA, upregulated in primary gastric cancer, is
a positive regulator of the WNT- β-catenin-TCF signaling pathway.
Biochem Biophys Res Commun. 289:1093–1098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Jiang H, Li F, He C, Wang X, Li Q and Gao
H: Expression of Gli1 and Wnt2B correlates with progression and
clinical outcome of pancreatic cancer. Int J Clin Exp Pathol.
7:4531–4538. 2014.PubMed/NCBI
|
|
190
|
Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y
and Qiu Y: MicroRNA-324-3p regulates nasopharyngeal carcinoma
radioresistance by directly targeting WNT2B. Eur J Cancer.
49:2596–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Li SJ, Yang XN and Qian HY: Antitumor
effects of WNT2B silencing in GLUT1 overexpressing cisplatin
resistant head and neck squamous cell carcinoma. Am J Cancer Res.
5:300–308. 2014.
|
|
192
|
Kobayashi M, Huang CL, Sonobe M, Kikuchi R
and Date H: Ad-shWnt2b vector therapy demonstrates antitumor
activity in orthotopic intrapleural models as monitored with the in
vitro imaging system (IVIS). Anticancer Res. 36:5887–5893. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Tokuhara M, Hirai M, Atomi Y, Terada M and
Katoh M: Molecular cloning of human Frizzled-6. Biochem Biophys Res
Commun. 243:622–627. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Cantilena S, Pastorino F, Pezzolo A,
Chayka O, Pistoia V, Ponzoni M and Sala A: Frizzled receptor 6
marks rare, highly tumourigenic stem-like cells in mouse and human
neuroblastomas. Oncotarget. 2:976–983. 2011. View Article : Google Scholar
|
|
195
|
Kim BK, Yoo HI, Kim I, Park J and Kim Yoon
S: FZD6 expression is negatively regulated by miR-199a-5p in human
colorectal cancer. BMB Rep. 48:360–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Corda G, Sala G, Lattanzio R, Iezzi M,
Sallese M, Fragassi G, Lamolinara A, Mirza H, Barcaroli D, Ermler
S, et al: Functional and prognostic significance of the genomic
amplification of frizzled 6 (FZD6) in breast cancer. J Pathol.
241:350–361. 2017. View Article : Google Scholar :
|
|
197
|
Saitoh T, Hirai M and Katoh M: Molecular
cloning and characterization of human Frizzled-5 gene on chromosome
2q33.3-q34 region. Int J Oncol. 19:105–110. 2001.PubMed/NCBI
|
|
198
|
Steinhart Z, Pavlovic Z, Chandrashekhar M,
Hart T, Wang X, Zhang X, Robitaille M, Brown KR, Jaksani S,
Overmeer R, et al: Genome-wide CRISPR screens reveal a Wnt-FZD5
signaling circuit as a druggable vulnerability of RNF43-mutant
pancreatic tumors. Nat Med. 23:60–68. 2017. View Article : Google Scholar
|
|
199
|
Sagara N, Toda G, Hirai M, Terada M and
Katoh M: Molecular cloning, differential expression, and
chromosomal localization of human frizzled-1, frizzled-2, and
frizzled-7. Biochem Biophys Res Commun. 252:117–122. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Simmons GE Jr, Pandey S,
Nedeljkovic-Kurepa A, Saxena M, Wang A and Pruitt K: Frizzled 7
expression is positively regulated by SIRT1 and β-catenin in breast
cancer cells. PLoS One. 9:e988612014. View Article : Google Scholar
|
|
201
|
Vincan E, Flanagan DJ, Pouliot N, Brabletz
T and Spaderna S: Variable FZD7 expression in colorectal cancers
indicates regulation by the tumour microenvironment. Dev Dyn.
239:311–317. 2010.
|
|
202
|
Qiu X, Jiao J, Li Y and Tian T:
Overexpression of FZD7 promotes glioma cell proliferation by
upregulating TAZ. Oncotarget. 7:85987–85999. 2016.PubMed/NCBI
|
|
203
|
Song J, Gao L, Yang G, Tang S, Xie H, Wang
Y, Wang J, Zhang Y, Jin J, Gou Y, et al: MiR-199a regulates cell
proliferation and survival by targeting FZD7. PLoS One.
9:e1100742014. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Koike J, Takagi A, Miwa T, Hirai M, Terada
M and Katoh M: Molecular cloning of Frizzled-10, a novel member of
the Frizzled gene family. Biochem Biophys Res Commun. 262:39–43.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y,
Su F, Liu Q, Yao H and Song E: BRMS1L suppresses breast cancer
metastasis by inducing epigenetic silence of FZD10. Nat Commun.
5:54062014. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Terasaki H, Saitoh T, Shiokawa K and Katoh
M: Frizzled-10, upregulated in primary colorectal cancer, is a
positive regulator of the WNT-β-catenin-TCF signaling pathway. Int
J Mol Med. 9:107–112. 2002.PubMed/NCBI
|
|
207
|
Hanaoka H, Katagiri T, Fukukawa C,
Yoshioka H, Yamamoto S, Iida Y, Higuchi T, Oriuchi N, Paudyal B,
Paudyal P, et al: Radioimmunotherapy of solid tumors targeting a
cell-surface protein, FZD10: Therapeutic efficacy largely depends
on radio-sensitivity. Ann Nucl Med. 23:479–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Gurney A, Axelrod F, Bond CJ, Cain J,
Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et
al: Wnt pathway inhibition via the targeting of Frizzled receptors
results in decreased growth and tumorigenicity of human tumors.
Proc Natl Acad Sci USA. 109:11717–11722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Nielsen TO, Poulin NM and Ladanyi M:
Synovial sarcoma: Recent discoveries as a roadmap to new avenues
for therapy. Cancer Discov. 5:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Le PN, McDermott JD and Jimeno A:
Targeting the Wnt pathway in human cancers: Therapeutic targeting
with a focus on OMP-54F28. Pharmacol Ther. 146:1–11. 2015.
View Article : Google Scholar
|
|
211
|
Ferreira Tojais N, Peghaire C, Franzl N,
Larrieu-Lahargue F, Jaspard B, Reynaud A, Moreau C, Couffinhal T,
Duplàa C and Dufourcq P: Frizzled7 controls vascular permeability
through the Wnt-canonical pathway and crosstalk with endothelial
cell junction complexes. Cardiovasc Res. 103:291–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Phesse T, Flanagan D and Vincan E:
Frizzled7: A promising Achilles' heel for targeting the Wnt
receptor complex to treat cancer. Cancers (Basel). 8. pp. 502016,
View Article : Google Scholar
|
|
213
|
Katoh M: FGFR inhibitors: Effects on
cancer cells, tumor microenvironment and whole-body homeostasis
(Review). Int J Mol Med. 38:3–15. 2016.PubMed/NCBI
|
|
214
|
Shabani M and Hojjat-Farsangi M: Targeting
receptor tyrosine kinases using monoclonal antibodies: The most
specific tools for targeted-based cancer therapy. Curr Drug
Targets. 17:1687–1703. 2016. View Article : Google Scholar
|
|
215
|
Gentile A, Lazzari L, Benvenuti S,
Trusolino L and Comoglio PM: Ror1 is a pseudokinase that is crucial
for Met-driven tumorigenesis. Cancer Res. 71:3132–3141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Mellstedt H, Hojjat-Farsangi M,
Daneshmanesh AH, Moshfegh A, Byström S, Norin M, Olin T, Schultz J,
Vågberg J and Österborg A: First generation of a small chemical
molecule ROR1 RTK tyrosine kinase inhibitor. Ann Oncol. 27(Suppl
6): 15332016. View Article : Google Scholar
|
|
217
|
Khan AS, Hojjat-Farsangi M, Daneshmanesh
AH, Hansson L, Kokhaei P, Österborg A, Mellstedt H and Moshfegh A:
Dishevelled proteins are significantly upregulated in chronic
lymphocytic leukaemia. Tumour Biol. 37:11947–11957. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Barat B, Chichili G, Ciccarone V, Tamura
J, Gorlatov S, Spliedt M, Chen F, Koenig S, Moore P, Bonvini E, et
al: Development of a humanized ROR1 × CD3 bispecific DART molecule
for the treatment of solid and liquid tumors. (Abstract).
Proceedings of the 107th Annual Meeting of the American Association
for Cancer Research; Apr 16–20, 2016; New Orleans, LA
AACR: Cancer Res. 76(Suppl 14): Abstract
1489. 2016.
|
|
219
|
Blankenship JW, Misher L, Mitchell D,
Zhang N, Tan P, Hoyos GH, Ravikumar P, Bader R, McMahan CJ, Miller
RE, et al: Anti-ROR1 × anti-CD3 ADAPTIR™ molecule, ES425, redirects
T-cell cytotoxicity and inhibits tumor growth in preclinical models
of triple-negative breast cancer. (Abstract). Proceedings of the
107th Annual Meeting of the American Association for Cancer
Research; Apr 16–20, 2016; New Orleans, LA. Philadelphia
AACR: Cancer Res. 76(Suppl 14): Abstract
4995. 2016.
|
|
220
|
Berger C, Sommermeyer D, Hudecek M, Berger
M, Balakrishnan A, Paszkiewicz PJ, Kosasih PL, Rader C and Riddell
SR: Safety of targeting ROR1 in primates with chimeric antigen
receptor-modified T cells. Cancer Immunol Res. 3:206–216. 2015.
View Article : Google Scholar :
|
|
221
|
Tian E, Zhan F, Walker R, Rasmussen E, Ma
Y, Barlogie B and Shaughnessy JD Jr: The role of the Wnt-signaling
antagonist DKK1 in the development of osteolytic lesions in
multiple myeloma. N Engl J Med. 349:2483–2494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Voorzanger-Rousselot N, Goehrig D, Journe
F, Doriath V, Body JJ, Clézardin P and Garnero P: Increased
Dickkopf-1 expression in breast cancer bone metastases. Br J
Cancer. 97:964–970. 2007.PubMed/NCBI
|
|
223
|
McClung MR, Grauer A, Boonen S, Bolognese
MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta
JR, Wasserman SM, et al: Romosozumab in postmenopausal women with
low bone mineral density. N Engl J Med. 370:412–420. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Recker RR, Benson CT, Matsumoto T,
Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H,
et al: A randomized, double-blind phase 2 clinical trial of
blosozumab, a sclerostin antibody, in postmenopausal women with low
bone mineral density. J Bone Miner Res. 30:216–224. 2015.
View Article : Google Scholar
|
|
225
|
Roschger A, Roschger P, Keplingter P,
Klaushofer K, Abdullah S, Kneissel M and Rauch F: Effect of
sclerostin antibody treatment in a mouse model of severe
osteogenesis imperfecta. Bone. 66:182–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Fulciniti M, Tassone P, Hideshima T,
Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT,
Chauhan D, et al: Anti-DKK1 mAb (BHQ880) as a potential therapeutic
agent for multiple myeloma. Blood. 114:371–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Bendell JC, Murphy JE, Mahalingam D,
Halmos B, Sirard CA, Landau SB and Ryan DP: A Phase 1 study of
DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (pac)
in patients with DKK1 relapsed or refractory esophageal cancer (EC)
or gastro-esophageal junction tumors (GEJ). J Clin Oncol. 34(Suppl
4S): Abstract 111. 2016. View Article : Google Scholar
|
|
228
|
Betts AM, Clark TH, Yang J, Treadway JL,
Li M, Giovanelli MA, Abdiche Y, Stone DM and Paralkar VM: The
application of target information and preclinical
pharmacokinetic/pharmacodynamic modeling in predicting clinical
doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp
Ther. 333:2–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Florio M, Gunasekaran K, Stolina M, Li X,
Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li C, Sun B, et al:
A bispecific antibody targeting sclerostin and DKK-1 promotes bone
mass accrual and fracture repair. Nat Commun. 7:115052016.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Liu C and Yu X: ADP-ribosyltransferases
and poly ADP-ribosylation. Curr Protein Pept Sci. 16:491–501. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Katoh M and Katoh M: Identification and
characterization of human TIPARP gene within the CCNL amplicon at
human chromosome 3q25.31. Int J Oncol. 23:541–547. 2003.PubMed/NCBI
|
|
232
|
Roper SJ, Chrysanthou S, Senner CE,
Sienerth A, Gnan S, Murray A, Masutani M, Latos P and Hemberger M:
ADP-ribosyltransferases Parp1 and Parp7 safeguard pluripotency of
ES cells. Nucleic Acids Res. 42:8914–8927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Haince JF, Rouleau M, Hendzel MJ, Masson
JY and Poirier GG: Targeting poly(ADP-ribosyl)ation: A promising
approach in cancer therapy. Trends Mol Med. 11:456–463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Gunderson CC and Moore KN: Olaparib: An
oral PARP-1 and PARP-2 inhibitor with promising activity in ovarian
cancer. Future Oncol. 11:747–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Nathubhai A, Haikarainen T, Koivunen J,
Murthy S, Koumanov F, Lloyd MD, Holman GD, Pihlajaniemi T, Tosh D,
Lehtiö L, et al: Highly potent and isoform selective dual site
binding tankyrase/Wnt signaling inhibitors that increase cellular
glucose uptake and have antiproliferative activity. J Med Chem.
60:814–820. 2017. View Article : Google Scholar
|
|
236
|
Riffell JL, Lord CJ and Ashworth A:
Tankyrase-targeted therapeutics: Expanding opportunities in the
PARP family. Nat Rev Drug Discov. 11:923–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Wang W, Li N, Li X, Tran MK, Han X and
Chen J: Tankyrase inhibitors target YAP by stabilizing angiomotin
family proteins. Cell Rep. 13:524–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Kulak O, Chen H, Holohan B, Wu X, He H,
Borek D, Otwinowski Z, Yamaguchi K, Garofalo LA, Ma Z, et al:
Disruption of Wnt/β-catenin signaling and telomeric shortening are
inextricable consequences of tankyrase inhibition in human cells.
Mol Cell Biol. 35:2425–2435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Scarborough HA, Helfrich BA, Casás-Selves
M, Schuller AG, Grosskurth SE, Kim J, Tan AC, Chan DC, Zhang Z,
Zaberezhnyy V, et al: AZ1366: An inhibitor of tankyrase and the
canonical Wnt pathway that limits the persistence of non-small cell
lung cancer cells following EGFR inhibition. Clin Cancer Res.
23:1531–1541. 2017. View Article : Google Scholar
|
|
240
|
Lau T, Chan E, Callow M, Waaler J, Boggs
J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, et
al: A novel tankyrase small-molecule inhibitor suppresses APC
mutation-driven colorectal tumor growth. Cancer Res. 73:3132–3144.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Shultz MD, Cheung AK, Kirby CA, Firestone
B, Fan J, Chen CH, Chen Z, Chin DN, Dipietro L, Fazal A, et al:
Identification of NVP-TNKS656: The use of structure-efficiency
relationships to generate a highly potent, selective, and orally
active tankyrase inhibitor. J Med Chem. 56:6495–6511. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Arqués O, Chicote I, Puig I, Tenbaum SP,
Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J,
Silberschmidt D, et al: Tankyrase inhibition blocks Wnt/β-catenin
pathway and reverts resistance to PI3K and AKT inhibitors in the
treatment of colorectal cancer. Clin Cancer Res. 22:644–656. 2016.
View Article : Google Scholar
|
|
243
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Casás-Selves M, Kim J, Zhang Z, Helfrich
BA, Gao D, Porter CC, Scarborough HA, Bunn PA Jr, Chan DC, Tan AC,
et al: Tankyrase and the canonical Wnt pathway protect lung cancer
cells from EGFR inhibition. Cancer Res. 72:4154–4164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Feng W, Teng R, Zhao Y, Gao J and Chu H:
Epigenetic modulation of Wnt signaling contributes to neuropathic
pain in rats. Mol Med Rep. 12:4727–4733. 2015.PubMed/NCBI
|
|
246
|
Meijer L, Skaltsounis AL, Magiatis P,
Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA,
Brivanlou A, Dajani R, et al: GSK-3-selective inhibitors derived
from Tyrian purple indirubins. Chem Biol. 10:1255–1266. 2003.
View Article : Google Scholar
|
|
247
|
Wu Y, Ai Z, Yao K, Cao L, Du J, Shi X, Guo
Z and Zhang Y: CHIR99021 promotes self-renewal of mouse embryonic
stem cells by modulation of protein-encoding gene and long
intergenic non-coding RNA expression. Exp Cell Res. 319:2684–2699.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Atkinson JM, Rank KB, Zeng Y, Capen A,
Yadav V, Manro JR, Engler TA and Chedid M: Activating the
Wnt/β-catenin pathway for the treatment of melanoma: Application of
LY2090314, a novel selective inhibitor of glycogen synthase
kinase-3. PLoS One. 10:e01250282015. View Article : Google Scholar
|
|
249
|
Ding S, Wu TY, Brinker A, Peters EC, Hur
W, Gray NS and Schultz PG: Synthetic small molecules that control
stem cell fate. Proc Natl Acad Sci USA. 100:7632–7637. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Lalit PA, Salick MR, Nelson DO, Squirrell
JM, Shafer CM, Patel NG, Saeed I, Schmuck EG, Markandeya YS, Wong
R, et al: Lineage reprogramming of fibroblasts into proliferative
induced cardiac progenitor cells by defined factors. Cell Stem
Cell. 18:354–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Narcisi R, Arikan OH, Lehmann J, Ten Berge
D and van Osch GJ: Differential effects of small molecule WNT
agonists on the multilineage differentiation capacity of human
mesenchymal stem cells. Tissue Eng Part A. 22:1264–1273. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Fiskus W, Sharma S, Saha S, Shah B,
Devaraj SG, Sun B, Horrigan S, Leveque C, Zu Y, Iyer S, et al:
Pre-clinical efficacy of combined therapy with novel β-catenin
antagonist BC2059 and histone deacetylase inhibitor against AML
cells. Leukemia. 29:1267–1278. 2015. View Article : Google Scholar
|
|
253
|
Trautmann M, Sievers E, Aretz S, Kindler
D, Michels S, Friedrichs N, Renner M, Kirfel J, Steiner S, Huss S,
et al: SS18-SSX fusion protein-induced Wnt/β-catenin signaling is a
therapeutic target in synovial sarcoma. Oncogene. 33:5006–5016.
2014. View Article : Google Scholar
|
|
254
|
Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ,
Lee ES, Park JH, Yun CH, Chung JU, Lee KJ, et al: Wnt/β-catenin
small-molecule inhibitor CWP232228 preferentially inhibits the
growth of breast cancer stem-like cells. Cancer Res. 75:1691–1702.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Emami KH, Nguyen C, Ma H, Kim DH, Jeong
KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al: A small
molecule inhibitor of β-catenin/CREB-binding protein transcription
[corrected]. Proc Natl Acad Sci USA. 101:12682–12687. 2004.
View Article : Google Scholar
|
|
256
|
Fang L, Zhu Q, Neuenschwander M, Specker
E, Wulf-Goldenberg A, Weis WI, von Kries JP and Birchmeier W: A
small-molecule antagonist of the β-catenin/TCF4 interaction blocks
the self-renewal of cancer stem cells and suppresses tumorigenesis.
Cancer Res. 76:891–901. 2016. View Article : Google Scholar
|
|
257
|
Hwang SY, Deng X, Byun S, Lee C, Lee SJ,
Suh H, Zhang J, Kang Q, Zhang T, Westover KD, et al: Direct
targeting of β-catenin by a small molecule stimulates proteasomal
degradation and suppresses oncogenic Wnt/β-catenin signaling. Cell
Rep. 16:28–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui
H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, et al: Targeting
the β-catenin/TCF transcriptional complex in the treatment of
multiple myeloma. Proc Natl Acad Sci USA. 104:7516–7521. 2007.
View Article : Google Scholar
|
|
259
|
Lenz HJ and Kahn M: Safely targeting
cancer stem cells via selective catenin coactivator antagonism.
Cancer Sci. 105:1087–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao
JJ, Mani M, Lemieux M, Carrasco DE, Ryan J, Horst D, et al:
Targeted disruption of the BCL9/β-catenin complex inhibits
oncogenic Wnt signaling. Sci Transl Med. 4:148ra1172012. View Article : Google Scholar
|
|
261
|
Henderson WR Jr, Chi EY, Ye X, Nguyen C,
Tien YT, Zhou B, Borok Z, Knight DA and Kahn M: Inhibition of
Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses
pulmonary fibrosis. Proc Natl Acad Sci USA. 107:14309–14314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie
J, Hou FF, Kahn M and Liu Y: Multiple genes of the
renin-angiotensin system are novel targets of Wnt/β-catenin
signaling. J Am Soc Nephrol. 26:107–120. 2015. View Article : Google Scholar
|
|
263
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Son B, Lee S, Youn H, Kim E, Kim W and
Youn B: The role of tumor microenvironment in therapeutic
resistance. Oncotarget. 8:3933–3945. 2017.
|
|
265
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Malladi S, Macalinao DG, Jin X, He L,
Basnet H, Zou Y, de Stanchina E and Massagué J: Metastatic latency
and immune evasion through autocrine Inhibition of WNT. Cell.
165:45–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Xu WD, Wang J, Yuan TL, Li YH, Yang H, Liu
Y, Zhao Y and Herrmann M: Interactions between canonical Wnt
signaling pathway and MAPK pathway regulate differentiation,
maturation and function of dendritic cells. Cell Immunol.
310:170–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Naskar D, Maiti G, Chakraborty A, Roy A,
Chattopadhyay D and Sen M: Wnt5a-Rac1-NF-κB homeostatic circuitry
sustains innate immune functions in macrophages. J Immunol.
192:4386–4397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
D'Amico L, Mahajan S, Capietto AH, Yang Z,
Zamani A, Ricci B, Bumpass DB, Meyer M, Su X, Wang-Gillam A, et al:
Dickkopf-related protein 1 (Dkk1) regulates the accumulation and
function of myeloid derived suppressor cells in cancer. J Exp Med.
213:827–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Staal FJ and Arens R: Wnt signaling as
master regulator of T-lymphocyte responses: Implications for
transplant therapy. Transplantation. 100:2584–2592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Swafford D and Manicassamy S: Wnt
signaling in dendritic cells: Its role in regulation of immunity
and tolerance. Discov Med. 19:303–310. 2015.PubMed/NCBI
|
|
272
|
Holtzhausen A, Zhao F, Evans KS, Tsutsui
M, Orabona C, Tyler DS and Hanks BA: Melanoma-derived Wnt5a
promotes local dendritic-cell expression of IDO and
immunotolerance: Opportunities for pharmacologic enhancement of
immunotherapy. Cancer Immunol Res. 3:1082–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Hanks BA: Immune evasion pathways and the
design of dendritic cell-based cancer vaccines. Discov Med.
21:135–142. 2016.PubMed/NCBI
|
|
274
|
Kaur A, Webster MR and Weeraratna AT: In
the Wnt-er of life: Wnt signalling in melanoma and ageing. Br J
Cancer. 115:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Law NC, Weck J, Kyriss B, Nilson JH and
Hunzicker-Dunn M: Lhcgr expression in granulosa cells: Roles for
PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol.
27:1295–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Liu Z and Habener JF: Glucagon-like
peptide-1 activation of TCF7L2-dependent Wnt signaling enhances
pancreatic beta cell proliferation. J Biol Chem. 283:8723–8735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Bellei B, Pitisci A, Catricalà C, Larue L
and Picardo M: Wnt/β-catenin signaling is stimulated by
α-melanocyte-stimulating hormone in melanoma and melanocyte cells:
Implication in cell differentiation. Pigment Cell Melanoma Res.
24:309–325. 2011. View Article : Google Scholar
|
|
278
|
Furuyashiki T and Narumiya S: Stress
responses: The contribution of prostaglandin E(2) and its
receptors. Nat Rev Endocrinol. 7:163–175. 2011. View Article : Google Scholar
|
|
279
|
Brudvik KW, Paulsen JE, Aandahl EM, Roald
B and Taskén K: Protein kinase A antagonist inhibits β-catenin
nuclear translocation, c-Myc and COX-2 expression and tumor
promotion in Apc(Min/+) mice. Mol Cancer. 10:1492011. View Article : Google Scholar
|
|
280
|
Jansen SR, Holman R, Hedemann I, Frankes
E, Elzinga CR, Timens W, Gosens R, de Bont ES and Schmidt M:
Prostaglandin E2 promotes MYCN non-amplified
neuroblastoma cell survival via β-catenin stabilization. J Cell Mol
Med. 19:210–226. 2015. View Article : Google Scholar
|
|
281
|
Estus TL, Choudhary S and Pilbeam CC:
Prostaglandin-mediated inhibition of PTH-stimulated β-catenin
signaling in osteoblasts by bone marrow macrophages. Bone.
85:123–130. 2016. View Article : Google Scholar : PubMed/NCBI
|