Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignancies, and it is the second cause of
cancer-related mortality worldwide (1). Despite significant improvements in
diagnosis and treatment techniques, the early recurrence and
intrahepatic and distant organ metastasis rates following curative
hepatectomy in patients with HCC remains generally high. Thus,
recurrence and metastasis are a major obstacle which influence the
survival time of patients with HCC (2). Therefore, in order to improve the
survival rate of patients with HCC, it would be beneficial to
identify novel biomarkers for diagnostic and prognostic
targets.
Mitotic chromosome condensation is an essential
cellular characteristic of all proliferative cells and is
responsible for restructuring chromatin into rod-shaped mitotic
chromosomes and ensuring the segregation of sister chromatids
during cell division (3,4). The condensin complexes were detected
and purified from Xenopus egg extracts for the first time
(5), which was considered to be a
key factor in understanding the mitotic chromosome condensation to
achieve mitosis-specific chromosome compaction and exact chromosome
segregation (6). Existing
research indicates that two types of condensin complexes are found
in vertebrates, condensin I and II complexes, which both contain
non-structural maintenance of chromosomes (non-SMC) regulatory
subunits (6). The non-SMC
subunits have been proposed to control the activity of
ATP-dependent DNA supercoiling and chromosome segregation, and the
depletion of any one of the subunits in condensin I or II can cause
defective mitotic chromosome condensation (7–9).
Non-SMC condensin I complex subunit G (NCAPG), a
mitosis-related chromosome condensation protein, is one of the
non-SMC subunits that exists in the condensin I complex (10). As the counterpart of the
Xenopus chromosome-associated polypeptide G (XCAP-G) gene,
it was first purified from HeLa cell nuclear extracts (5). NCAPG is a polypeptide consisting of
1,015 amino acids with a relative molecular mass of 114.1 kDa
(5), and is encoded by the
NY-MEL-3 gene located on human chromosome band 4p15.32 (11). Research has indicated that NCAPG
is cell cycle-related (11,12) and can influence the proliferation
of HCC cells (13); however, the
biological functions of NCAPG in HCC remain unknown. In the present
study, we aimed to investigate the association between
clinicopathological parameters and the NCAPG protein expression
level in patients with HCC, and the effects of NCAPG on the cell
cycle, apoptosis, invasion and migration.
Materials and methods
Patients and tissue samples
A total of 88 HCC tissue specimens and paracancerous
tissue specimens were provided by patients who underwent surgeries
at the Second Affiliated Hospital of Nanchang University, Nanchang,
China from 2012 to 2013. Based on the World Health Organization
standard, the histological diagnosis and tumor differentiation
grade of all the specimens were evaluated by the Department of
Pathology of the Second Affiliated Hospital of Nanchang University.
The patient's age, gender, tumor size, alpha-fetoprotein (AFP)
levels and other clinicopathological factors were obtained from
surgical and pathological records. Prior to specimen collection, no
patients had received any treatments, including radiotherapy and
chemotherapy. Fresh liver cancer tissue specimens and paracancerous
tissue specimens were immediately placed in liquid nitrogen, and
then stored at −80°C. In this study, ethics approval was provided
by the Medical Ethics Committee of the Second Affiliated Hospital
to Nanchang University, and written informed consent was obtained
from all patients prior to obtaining the samples. This study was
performed in accordance with the ethical standards of the
Declaration of Helsinki.
Cell lines and culture
The normal control cell line, L02 (Cat. no.
BNCC100012), and 5 human HCC cell lines [SMMC-7721 (Cat. no.
BNCC100526), HepG2 (Cat. no. BNCC338070), MHCC-97H (Cat. no.
BNCC337738), Hu7 (Cat. no. BNCC100280) and MHCC-LM3 (Cat. no.
BNCC338460; all from BeNa Culture Collection, Beijing China)] were
selected to conduct the assays, and the L02 cell line was selected
as the normal control. These cell lines were cultured in
high-glucose DMEM containing 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C and in a
humidified atmosphere of 5% CO2.
Immunohistochemistry
A two-step immunohistochemical method (PV-9000;
ZSGB-BIO Co., Ltd., Beijing, China) was adopted to perform the
immunostaining. After being fixed in 10% neutral formalin solution,
we embedded the HCC tissues in paraffin blocks and cut the tissue
into 4-mm-thick paraffin sections for immunostaining. After being
deparaffinized and hydrated, all the sections were added to 3%
H2O2 for 15 min to eliminate endogenous
peroxidase and then repaired in a microwave oven at 130°C for 10
min. Subsequently, the tissue sections were incubated with NCAPG
mouse monoclonal antibody (1:200) (Abcam, Cambridge, UK) at 4°C
overnight. Phosphate-buffered saline (PBS) was used to wash the
sections 3 times at 5 min intervals. The secondary antibody
biotinylated goat anti-mouse serum IgG (Cat. no. SPN-9002; PV-9000;
ZSGB-BIO Co., Ltd.) was added at 37°C for 30 min, after using PBS
to wash for 5 min 3 times; the diaminobenzidine and hematoxylin
dyes were employed, and the sections were sealed with neutral
resins. Two independent pathologists evaluated all of the sections'
immunoreactivity blindly and randomly. The positive staining of
NCAPG was located in the nucleus, adn thus the nuclear staining of
the tumor cells was identified as positive immunostaining. The
staining intensity and the percentage of tumor cells with positive
staining was used to evaluate the results; the staining intensity
was graded as follows: 0, no staining; 1+, mild staining; 2+,
moderate staining; or 3+, strong staining. The percentage of
staining was scored as follows: 0, no staining; 1, <10%
staining; 2, 10–40% staining; or 3, >40% staining. The overall
staining index was then computed by multiplying these two scores to
reach a value from 0 to 9 for each immunostained section and
summarized and designated as follows: 0–1, negative NCAPG
expression; or 2–9, positive expression.
Cell transfection and siRNA
treatment
NCAPG siRNA and negative control siRNA were
purchased from GenePharma Co. (Shanghai GenePharma Co., Ltd.,
Shanghai, China). The MHCC-LM3 and Hu7 cells were assigned to the
blank group, control groups and NCAPG siRNA groups. Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to
transiently transfect the cells with siRNA. Three different siRNA
sequences were transfected into the cells in the NCAPG siRNA group,
and the sequences were as follows: NCAPG_s1,
GGAGUUCAUUCAUUACCUUTTAAGGUAAUGAAUGAACUCCTT; NCAPG_s2,
GCUGAAACAUUGCAGAAAUTTAUUUCUGCAAUGUUUCAGCTT; NCAPG_s3,
GGACUAAUCAGGAAUGCUUTTAAGCAUUCCUGAUUAGUCCTT. The cells were seeded
in 6-well plates and transfected with transfection mixture for 48
h, and the transfected cells were then harvested and used in
further assays.
Cell proliferation assay
Cell proliferation was detected using the Cell
Counting kit-8 (CCK-8) assay. The HCC cells were seeded into
96-well plates at a density of 1×104 cells/well and
incubated for 24 h at 37°C in 5% CO2. These wells were
divided into 3 groups (NC, NCAPG siRNA-s1 and NCAPG siRNA-s2
groups) and each group occupied 5 replicate wells, and each
treatment group was transfected with siRNA-NCAPG. After 0, 24, 48,
72 and 96 h of cultivation, 10 μl CCK-8 reagent (CCK-8;
Dojindo Laboratories, Kumamoto, Japan) were added to each well
followed by cultured for 3 h at 37°C in 5% CO2. The
absorbance at 450 nm was measured using a microplate reader
(Bio-Rad, Berkeley, CA, USA).
Measurement of cell apoptosis by flow
cytometry
Cell apoptosis was examined by FACS analysis using
the Annexin V-FITC/PI apoptosis detection kit from BD Biosciences
(San Jose, CA, USA). Briefly, the cells subjected to the different
treatments were harvested and washed twice in cold PBS, then gently
re-suspended in 100 μl binding buffer and added to a plastic
12×75 mm test tube, followed by the addition of 5 μl of
Annexin V-FITC and 5 μl of propidium iodide solution in the
dark at 37°C. Following incubation for 15 min, 400 μl of 1X
binding buffer were added to the cell suspension. The cells were
examined by a BD FACScan flow cytometer (BD Biosciences) after 1
h.
Cell cycle analysis by flow
cytometry
Cell cycle analysis was detected by FACS analysis.
Briefly, the cells (1×106) were collected at 48 h by
trypsinization after the various treatments, washed twice with cold
PBS, resuspended and fixed in 1 ml of cold 70% ethanol overnight at
4°C. After washing once in cold PBS, the cells were re-suspended in
500 μl of PI/RNase staining solution (Tianjin Sungene
Biotech Co., Ltd., Tianjin, China) in the dark at room temperature
for 30 min. Subsequently, the cells were analyzed by BD FACScan
flow cytometer (BD Biosciences) and CellQuest software.
Cell invasion and migration assays
Cell invasion was evaluated using Transwell inserts
with 8 μm pores (Corning, Inc., Corning, NY, USA). Briefly,
48 h after transfection, the NCAPG siRNA-treated cells and control
groups cells were re-suspended in DMEM medium without serum and
growth factor, and then 200 μl cell suspension was seeded in
the upper Transwell chambers. Subsequently, 500 μl medium
containing 20% FBS was added to the bottom of the chambers. The
24-well culture plate was incubated at 37°C in a humidified
atmosphere containing 5% CO2 for 48 h. Cells remaining
on the upper surface of the Transwell chambers were mechanically
removed using a cotton swab, and then the underside of the
Transwell chambers was fixed, washed and stained by hexamethyl
pararosaniline. Five random fields on the lower surface of each
Transwell chamber were counted. The migration assay procedures
differed from the invasion assay, in that they were performed in
the Transwell chambers that were not pre-coated with Matrix
gel.
Western blot analysis
Western blot analysis was performed to detect the
total protein expression of the treated cells 48 h after
transfection. The cells were lysed in radioimmunoprecipitation
assay (RIPA) buffer with 1% phenylmethanesulfonyl fluoride to
extract the total protein. Protein concentrations were
electrophoresed on an 8% sodium dodecyl sulfate-polyacrylamide gel
and transferred onto polyvinylidene fluoride membranes, and the
membranes were then incubated with anti-NCAPG (Cat. no. ab56382),
anti-Bax (Cat. no. ab32503), anti-Bcl-2 (Cat. no. ab32124), cleaved
caspase-3 (Cat. no. ab13847), anti-CDK2 (Cat. no. ab32147), cyclin
A1 (Cat. no. ab53699), anti-HOXB9 (Cat. no. ab208920), anti-β-actin
(Cat. no. ab8226), anti-N-cadherin (Cat. no. ab18203),
anti-E-cadherin (Cat. no. ab1416) antibodies (all from Abcam) at
4°C overnight. Tris-HCl buffer solution + Tween-20 was used to wash
the membranes 3 times per 10 min. Subsequently, they were incubated
with horseradish peroxidase-conjugated secondary antibody (Cat.
nos. ab6789 and ab6721; 1:10,000; Abcam) for 1 h at room
temperature, and signals were detected by enhanced chemiluminescent
substrates (Millipore, Billerica, MA, USA).
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. Wilcoxon's paired
test was used to compare the expression of NCAPG in the HCC tissue
specimens and paracancerous tissue specimens. Chi-square tests were
used to examine possible correlations between NCAPG expression and
clinicopathological characteristics. Overall survival was assessed
using Kaplan-Meier curves, and the difference in overall survival
was stratified by NCAPG expression and evaluated using the log-rank
test. The Cox proportional hazards regression model was used to
assess the hazard ratio and to identify factors that independently
predicted overall survival. The in vitro data are expressed
as the means ± standard error and analyzed using one-way analysis
of variance using factorial design to compare the growth curves of
the different siRNA treatment groups. The P-value was based on the
two-sided statistical analysis, and value of P<0.05 was
considered to indicate a statistically significant difference.
Results
NCAPG protein is highly expressed in HCC
tissues compared with adjacent non-tumor tissues
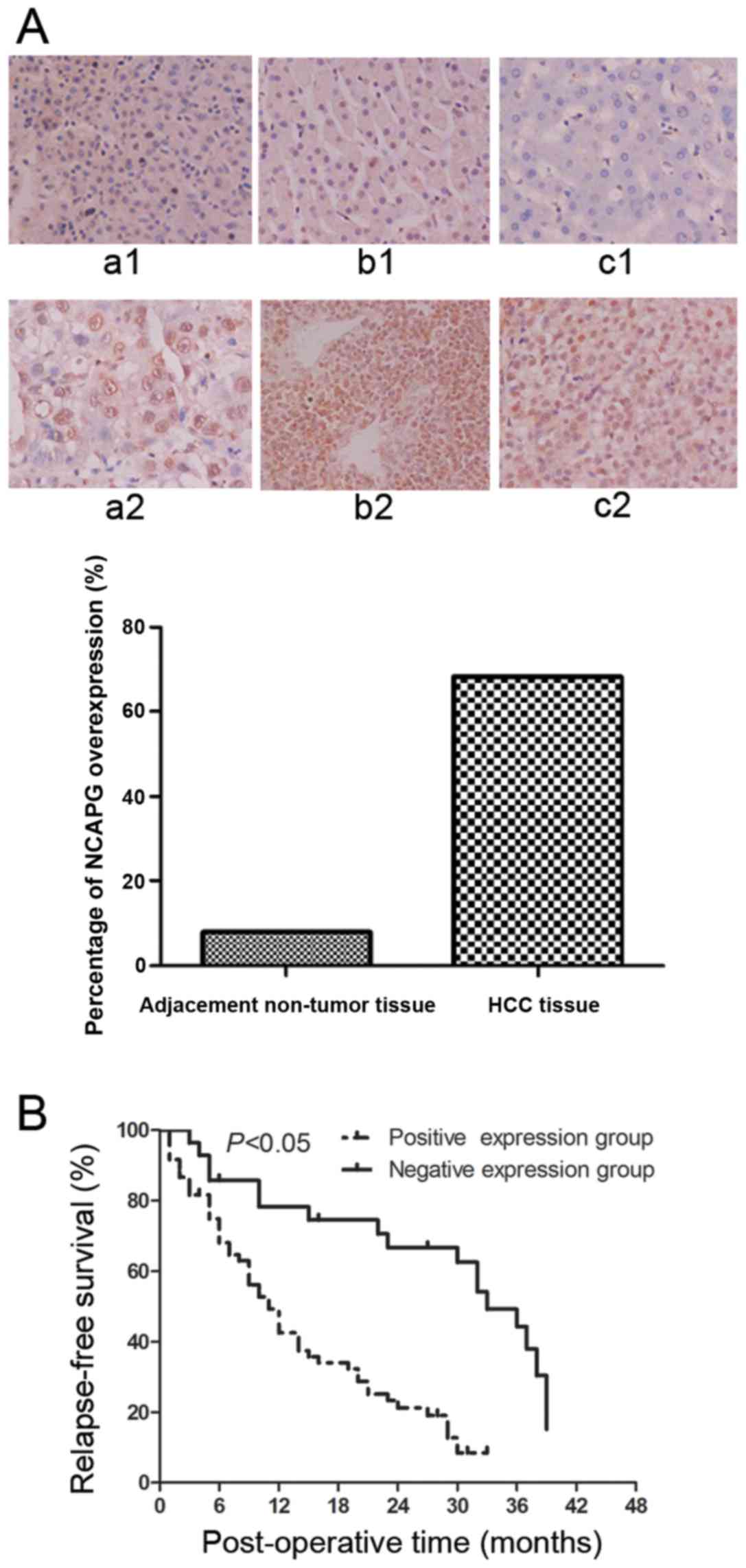
We performed immunohistochemistry to investigate
NCAPG protein expression levels in a total of 88 HCC tissues and
adjacent non-tumor tissues and found that NCAPG was expressed in
the cell nuclei. However, the protein expression of NCAPG was at a
low level in 7/88 (7.95%) of the adjacent non-tumor tissues
(Fig. 1A, panels a1–c1), whereas
the protein expression of NCAPG was at a high level in 60/88
(68.2%) of the HCC tissues (Fig.
1A, panels a2–c2). Therefore, NCAPG protein was overexpressed
in the HCC tissues compared to the paired normal hepatocellular
tissues (P<0.001).
Association between NCAPG protein
expression and clinicopathological characteristics in patients with
HCC
The data on the association of NCAPG overexpression
with the clinicopathological characteristics of the patients with
HCC are shown in Table I. The
protein expression of NCAPG in the tumor tissues was strongly
associated with the recurrence (P=0.031), the time of recurrence
(P=0.006), metastasis (P=0.020), differentiation (P=0.021) and TNM
stage (P=0.036). Kaplan-Meier curves were plotted to stratify NCAPG
expression for the overall survival of the patients with HCC. Our
data indicated that NCAPG overexpression was associated with a poor
overall survival of these patients (Fig. 1B, P<0.05).
 | Table IAssociatoin between NCAPG protein
expression and clinicopathological characeristics of patients with
HCC. |
Table I
Associatoin between NCAPG protein
expression and clinicopathological characeristics of patients with
HCC.
| Characteristics | No. of patients | NCAPG-positive n
(%) | NCAPG-negative n
(%) | χ2 | P-value |
|---|
| Sex |
| Male | 75 | 51 (68.0) | 24 (32.0) | 0.008 | 1.000 |
| Female | 13 | 9 (69.2) | 4 (30.8) | | |
| Age (years) |
| <60 | 77 | 53 (68.8) | 24 (31.2) | 0.120 | 0.738 |
| ≥60 | 11 | 7 (63.6) | 4 (36.4) | | |
| Hepatitis B |
| Positive | 80 | 54 (67.5) | 26 (32.5) | 0.189 | 1.000 |
| Negative | 8 | 6 (75.0) | 2 (25.0) | | |
| AFP
(μg/l) |
| <400 | 58 | 38 (65.5) | 20 (34.5) | 0.557 | 0.630 |
| ≥400 | 30 | 22 (73.3) | 8 (26.7) | | |
| Cirrhosis |
| Yes | 59 | 37 (62.7) | 22 (37.3) | 2.469 | 0.147 |
| No | 29 | 23 (79.3) | 6 (20.7) | | |
| Tumor size
(cm) |
| ≤3.0 | 14 | 9 (64.3) | 5 (35.7) | 0.116 | 0.760 |
| >3.0 | 74 | 51 (68.9) | 23 (31.1) | | |
| No. of tumors |
| 1 | 67 | 43 (64.2) | 24 (35.8) | 2.073 | 0.186 |
| >1 | 21 | 17 (81.0) | 4 (19.0) | | |
| Tumor capsule |
| Yes | 38 | 24 (63.2) | 14 (36.8) | 0.778 | 0.489 |
| No | 50 | 36 (72.0) | 14 (28.0) | | |
|
Differentiation |
| Well | 22 | 10 (45.5) | 12 (54.5) | 7.748 | 0.021 |
| Moderate | 43 | 31 (72.1) | 12 (27.9) | | |
| Poor | 23 | 19 (82.6) | 4 (17.4) | | |
| TNM stage |
| I+II | 65 | 40 (61.5) | 25 (38.5) | 5.059 | 0.036 |
| III+IV | 23 | 20 (87.0) | 3 (13.0) | | |
| BCLC stage |
| A | 59 | 36 (61.0) | 23 (39.0) | 4.346 | 0.114 |
| B | 14 | 12 (85.7) | 2 (14.3) | | |
| C | 15 | 12 (80.0) | 3 (20.0) | | |
| Metastasisa |
| Yes | 39 | 32 (82.1) | 7 (17.9) | 6.211 | 0.020 |
| No | 49 | 28 (57.1) | 21 (42.9) | | |
| Recurrence |
| Yes | 67 | 50 (74.6) | 17 (25.4) | 5.376 | 0.031 |
| No | 21 | 10 (47.6) | 11 (52.4) | | |
| Recurrence time
(months) |
| <6 | 36 | 30 (83.3) | 6 (16.7) | 10.319 | 0.006 |
| ≥6≤ to <12 | 8 | 7 (87.5) | 1 (12.5) | | |
| ≥12 | 44 | 23 (52.3) | 21 (47.7) | | |
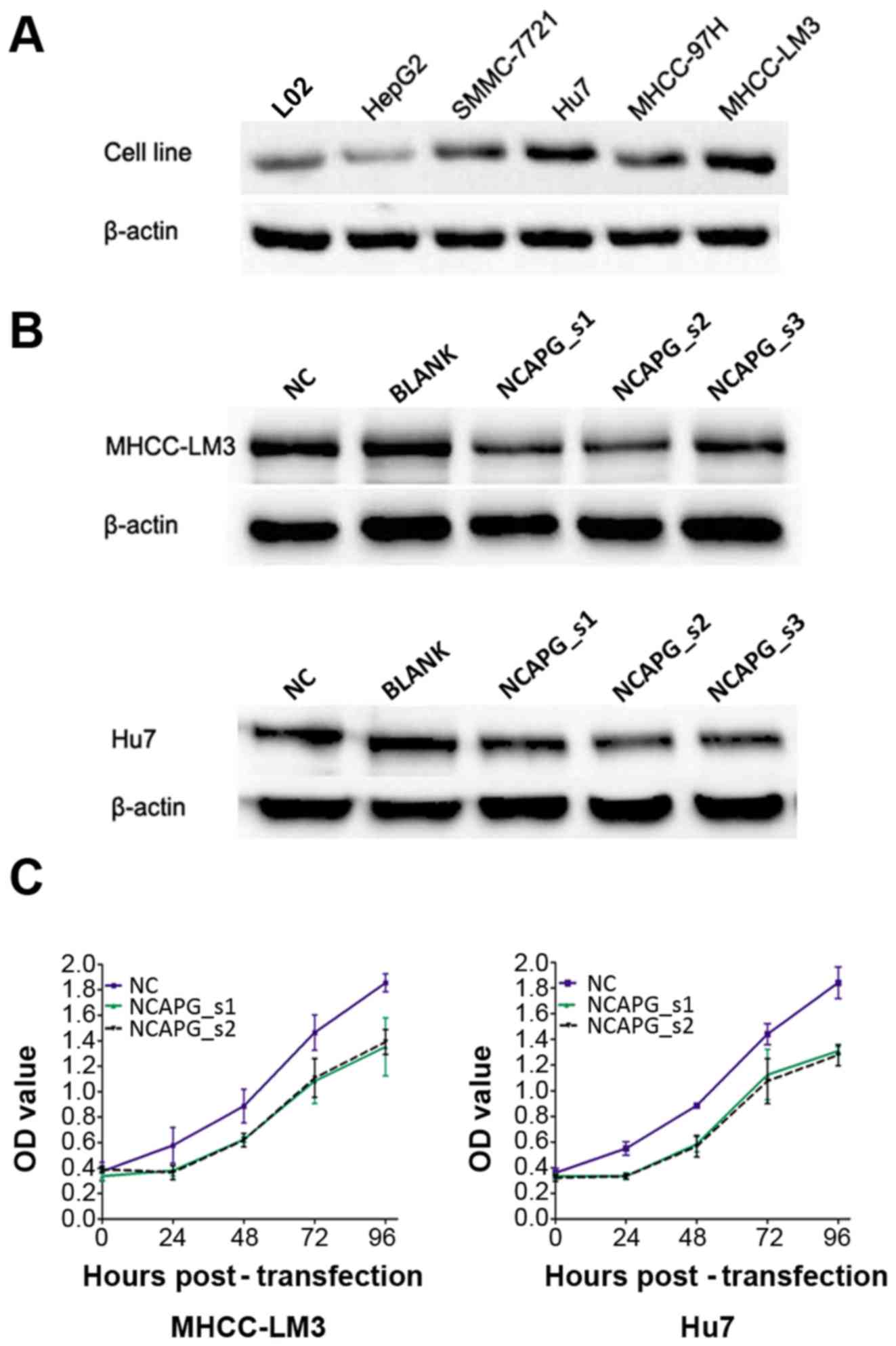
Detection of NCAPG protein in different
cell lines
The results of western blot analysis revealed that
the MHCC-LM3 and Hu7 cell lines had a higher protein expression of
NCAPG among these cell lines, which were selected for subsequent
functional assays (Fig. 2A).
siRNA-mediated knockdown of NCAPG
inhibits NCAPG protein levels and the proliferation of 2 HCC cell
lines
NCAPG siRNA was used to transfect the 2 HCC cell
lines. After 48 h of transfection, the protein expression level of
NCAPG was downregulated, as shown by western blot analysis. It was
shown that the NCAPG_s1, NCAPG_s2 siRNA sequences were the most
effective (Fig. 2B). In order to
better understand the effects of NCAPG knockdown on cell viability,
CCK-8 assay was employed. The results revealed that the silencing
of NCAPG expression induced a marked reduction in the viability of
the 2 cell lines compared with the control groups (Fig. 2C, P<0.05). These results
demonstrate that NCAPG suppression inhibits the proliferative
ability of the HCC cells.
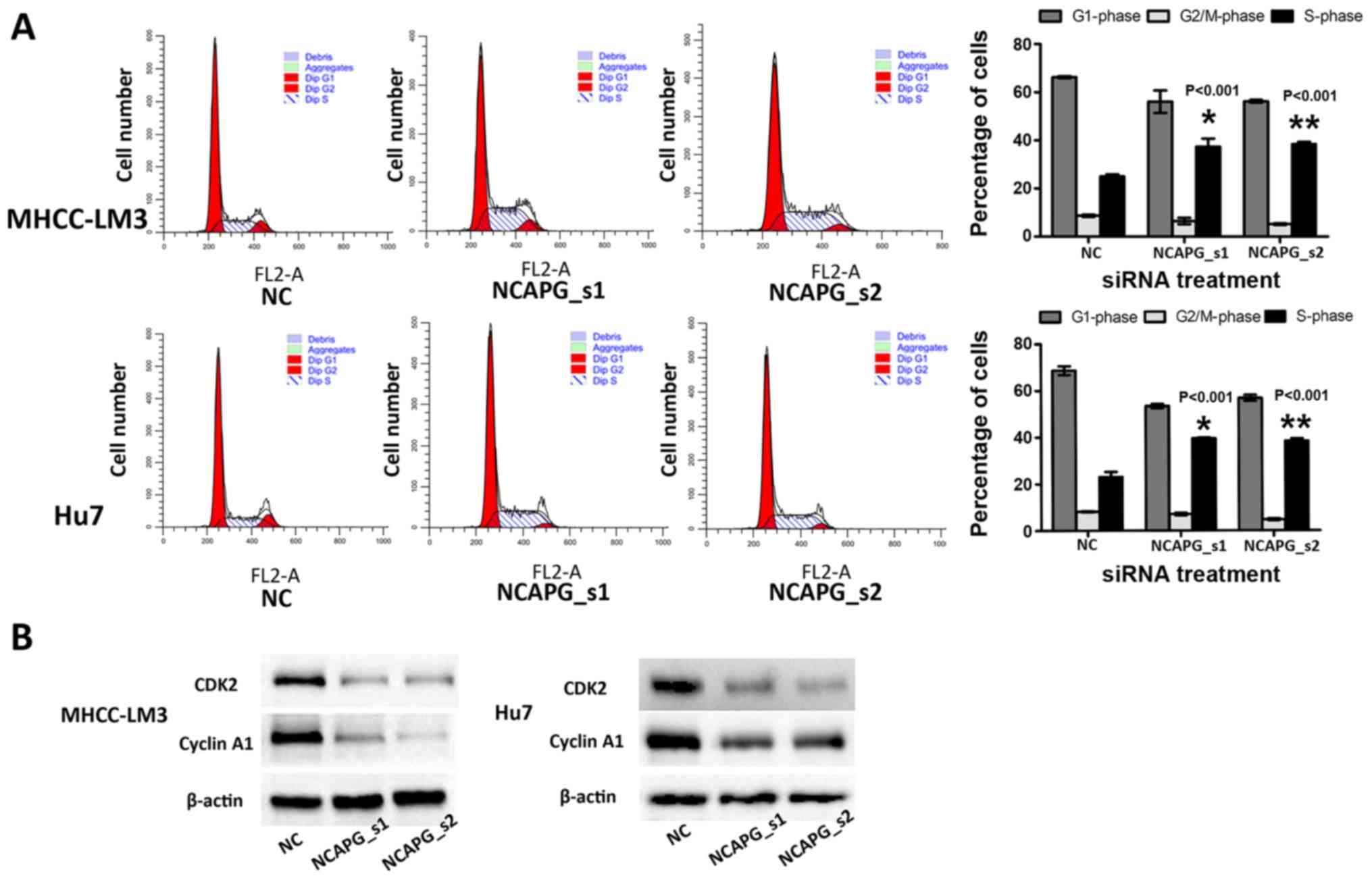
Depletion of NCAPG affects the cell cycle
distribution in the 2 HCC cell lines
Flow cytometry was employed to examine the effects
of NCAPG siRNA on the cell cycle of HCC cells. The percentage of
cells in the G1 phase and G2 phase was lower in the NCAPG
siRNA-transfected cells than in the negative siRNA control groups
(Fig. 3A, P<0.001).
Additionally, the percentage of cells in the S phase was higher in
the NCAPG siRNA-transfected cells compared with the negative siRNA
control cells (Fig. 3A,
P<0.001). Additionally, the levels of cyclin A1 and CDK2 were
reduced in the NCAPG siRNA-transfected cells (Fig. 3B).
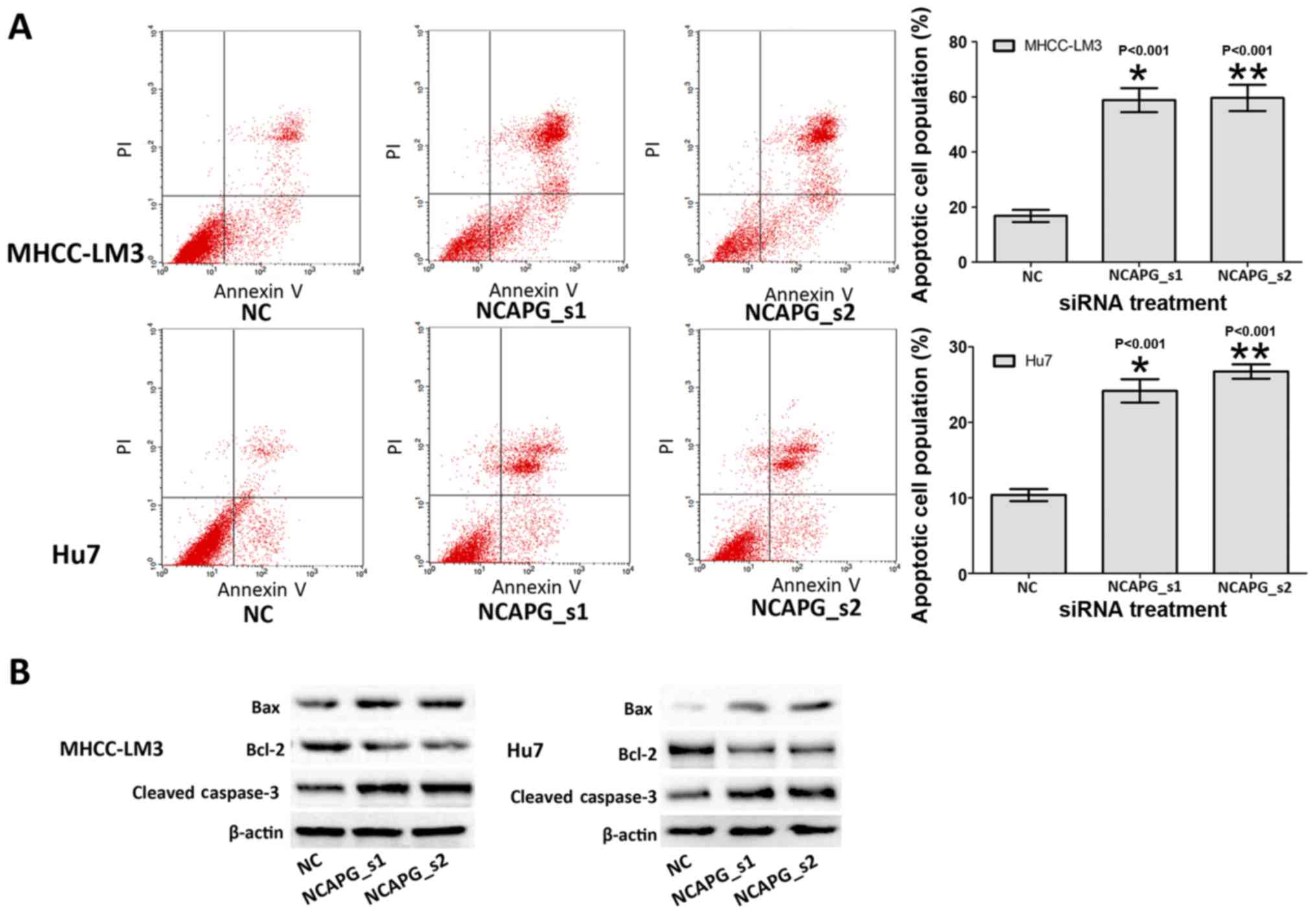
Depletion of NCAPG induces the apoptosis
of the 2 HCC cell lines
To determine whether NCAPG affects the apoptosis of
HCC cells, we investigated the apoptotic rate of the treated HCC
cells by flow cytometry. The results revealed that the apoptotic
rates of the cells in the NCAPG siRNA-transfected group were
markedly higher than those in the negative siRNA control groups
(Fig. 4A, P<0.001). This
suggested that the silencing of NCAPG induced the apoptosis of HCC
cells. Subsequently, the expression level of cell
apoptosis-associated proteins indicated abnormal alternations in
the NCAPG siRNA-transfected groups, including the increased
expression of Bax and caspase-3, and the decreased expression of
Bcl-2 (Fig. 4B). These results
indicated that the alterations in cell apoptosis may be related to
apoptosis-associated proteins by the down-regulation of NCAPG.
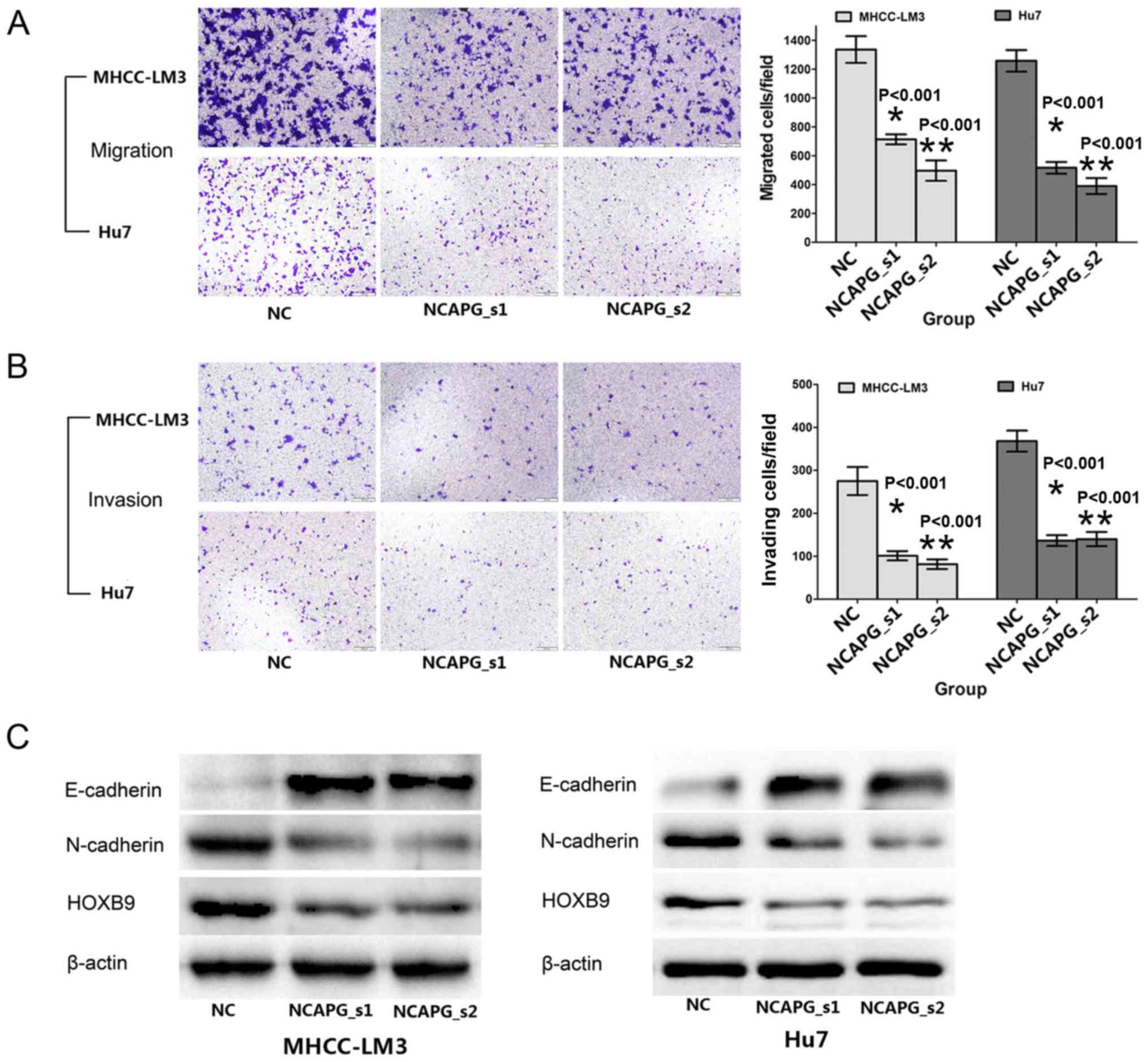
NCAPG silencing inhibits the migration
and invasion of the 2 HCC cell lines
The effects of NCAPG knockdown on the migratory and
invasive activity of human HCC cells were reflected by Transwell
assay. Compared with the negative siRNA control groups, the cells
transfected with NCAPG siRNA exhibited weaker migratory and
invasive abilities (Fig. 5A and
B, P<0.001). To further explore the influence of NCAPG, we
analyzed metastasis-related proteins, such as E- and N-cadherin and
HOXB9, by western blot analysis. The results revealed that the
expression of E-cadherin was upregulated, while that of N-cadherin
and HOXB9 was downregulated upon transfection with NCAPG siRNA
(Fig. 5C). These results indicate
that NCAPG plays a critical role in HCC cell migration and
invasion.
Discussion
NCAPG, a component of the condensin complex, is
highly associated with the condensation of mitotic chromosomes and
the proper segregation of sister chromatid in the division of the
nucleus. Currently, several studies on NCAPG have suggested that
NCAPG plays the role of a tumor promoter in the development of HCC.
Jäger et al (11)
investigated the expression of NCAPG in normal and tumor cells and
tissue by RT-PCR and northern blot analysis. Their findings showed
that NCAPG had the highest expression in the testis, a multifarious
expression in tumor cells and a low expression in the thymus, and
there was no detectable signal in other normal tissues. As we know,
testes and tumor cells have a stronger ability of division and
proliferation than other normal tissues. This indicates that a
higher expression of NCAPG may promote the ability of division and
proliferation of tumor cells. Furthermore, our our data indicated
that the depletion of NCAPG led to HCC cell cycle arrest at the S
phase and induced apoptosis. Satow et al (13) found that the NCAPG protein levels
in HCC were higher compared with the adjacent non-tumorous liver
tissue and that using siRNA-NCAPG to knockdown NCAPG protein
expression significantly inhibited the proliferation of HCC cells
and the growth rate of HCC xenografts inoculated into nude mice.
Our immunohistochemistry statistical results and cell proliferation
assays also confirmed this.
At specific phases of the cell cycle, the sequential
activation of CDKs can regulate the progression of the mammalian
cell division cycle. CDK activities are dependent on the
association with cyclins and co-factors, whose levels oscillate
throughout the cell cycle (14).
Additionally, the activity of CDK2-cyclin A complexes are the key
factors for DNA replication. Previous research has indicated that
CDK2-cyclin A complexes can promote DNA replication in vitro
(15). Therefore, decreases in
CDK2 and cyclin A protein levels can inhibit DNA replication in
vivo (16,17) and result in S-phase arrest
(14,18,19). In this study, the expression of
cell-cycle-associated proteins, including cyclin A1 and CDK2
proteins, was detected by western blot analysis to elucidate the
molecular mechanisms of cell cycle distribution changes. Our
results revealed that the silencing of NCAPG, compared to the
control groups, resulted in a decrease in the percentage of cells
in the G1 and G2 phase, an increase in the number of cells in the S
phase by flow cytometry and a decrease in cyclin A1 and CDK2
protein expression, which indicates that NCAPG may be a cell
proliferation promoter in HCC cells. In addition, we demonstrated,
by western blot analysis, that apoptosis was induced by
transfection with NCAPG siRNA, and there was a corresponding change
in the levels of cell apoptosis-associated proteins; NCAPG
silencing upregulated Bax and cleaved caspase-3 and downregulated
Bcl-2 expression. This result indicates that NCAPG acts as an
anti-apoptotic factor in the cell apoptotic process.
Recurrence and metastasis are the main causes of a
poor prognosis of patients with HCC. Therefore, reducing the
migratory and invasive abilities of HCC cells can effectively
reduce reoccurrence and metastasis and prolong the survival time.
HOXB9 has been shown to promote the invasion and metastasis of
malignant tumors in previous studies (20–24). Hayashida et al (21) showed that HOXB9 can promote
epithelial-mesenchymal transition (EMT) in breast cancer. In the
process of EMT, epithelial cells lose polarity and obtain more
migratory ability. Yuan et al (22) confirmed that the depletion of
HOXB9 significantly inhibited the invasion and metastasis of HCC
cells. Conversely, the invasive and metastatic ability was promoted
by increasing the expression of HOXB9. Our western blot analysis
results revealed that the expression of HOXB9 was downregulated by
transfection with NCAPG siRNA in the HCC cells. Additionally, the
expression of E-cadherin was upregulated and the expression of
N-cadherin was downregulated by transfection with NCAPG siRNA in
the HCC cells. Our Transwell assay results demonstrated that the
invasive and metastatic ability of the HCC cell lines was
suppressed in vitro. These results indicate that NCAPG may
act as a promoter of invasion and metastasis in HCC by regulating
the expression of HOXB9, N- and E-cadherin.
In conclusion, our results suggest that NCAPG
expression is associated with the clinicopathological
characteristics, cell cycle, apoptosis and cell migration and
invasion in human HCC, as an oncogenic protein playing an important
role in the occurrence and development of primary liver cancer.
Therefore, NCAPG may be a potential target for the diagnosis and
therapy of patients with HCC.
Acknowledgments
The present study was supported in part by a grant
from the Second Affiliated Hospital of Nanchang University (no.
2016YNQN12004) and the Natural Science Foundation of Jiangxi
Province, China (no. 20151BAB205101).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giordano S and Columbano A: Met as a
therapeutic target in HCC: Facts and hopes. J Hepatol. 60:442–452.
2014. View Article : Google Scholar
|
|
3
|
Hirano T, Kobayashi R and Hirano M:
Condensins, chromosome condensation protein complexes containing
XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren
protein. Cell. 89:511–521. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerlich D, Hirota T, Koch B, Peters JM,
Ellenberg J and Condensin I: Condensin I stabilizes chromosomes
mechanically through a dynamic interaction in live cells. Curr
Biol. 16:333–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimura K, Cuvier O and Hirano T:
Chromosome condensation by a human condensin complex in Xenopus egg
extracts. J Biol Chem. 276:5417–5420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herzog S, Nagarkar Jaiswal S, Urban E,
Riemer A, Fischer S and Heidmann SK: Functional dissection of the
Drosophila melanogaster condensin subunit Cap-G reveals its
exclusive association with condensin I. PLoS Genet. 9:e10034632013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seipold S, Priller FC, Goldsmith P, Harris
WA, Baier H and Abdelilah-Seyfried S: Non-SMC condensin I complex
proteins control chromosome segregation and survival of
proliferating cells in the zebrafish neural retina. BMC Dev Biol.
9:402009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirano T: At the heart of the chromosome:
SMC proteins in action. Nat Rev Mol Cell Biol. 7:311–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirano T: Condensins: Organizing and
segregating the genome. Curr Biol. 15:R265–R275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eberlein A, Takasuga A, Setoguchi K, Pfuhl
R, Flisikowski K, Fries R, Klopp N, Fürbass R, Weikard R and Kühn
C: Dissection of genetic factors modulating fetal growth in cattle
indicates a substantial role of the non-SMC condensin I complex,
subunit G (NCAPG) gene. Genetics. 183:951–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jäger D, Stockert E, Jäger E, Güre AO,
Scanlan MJ, Knuth A, Old LJ and Chen YT: Serological cloning of a
melanocyte rab guanosine 5′-triphosphate-binding protein and a
chromosome condensation protein from a melanoma complementary DNA
library. Cancer Res. 60:3584–3591. 2000.
|
|
12
|
Lam WW, Peterson EA, Yeung M and Lavoie
BD: Condensin is required for chromosome arm cohesion during
mitosis. Genes Dev. 20:2973–2984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satow R, Shitashige M, Kanai Y, Takeshita
F, Ojima H, Jigami T, Honda K, Kosuge T, Ochiya T, Hirohashi S, et
al: Combined functional genome survey of therapeutic targets for
hepatocellular carcinoma. Clin Cancer Res. 16:2518–2528. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wheeler LW, Lents NH and Baldassare JJ:
Cyclin A-CDK activity during G1 phase impairs MCM chromatin loading
and inhibits DNA synthesis in mammalian cells. Cell Cycle.
7:2179–2188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fotedar A, Cannella D, Fitzgerald P,
Rousselle T, Gupta S, Dorée M and Fotedar R: Role for cyclin
A-dependent kinase in DNA replication in human S phase cell
extracts. J Biol Chem. 271:31627–31637. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogryzko VV, Wong P and Howard BH: WAF1
retards S-phase progression primarily by inhibition of
cyclin-dependent kinases. Mol Cell Biol. 17:4877–4882. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishimi Y, Komamura-Kohno Y, You Z, Omori A
and Kitagawa M: Inhibition of Mcm4,6,7 helicase activity by
phosphorylation with cyclin A/Cdk2. J Biol Chem. 275:16235–16241.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song X, Li L, Shi Q, Lehmler HJ, Fu J, Su
C, Xia X, Song E and Song Y: Polychlorinated biphenyl quinone
metabolite promotes p53-dependent DNA damage checkpoint activation,
S Phase cycle arrest and extrinsic apoptosis in human liver
hepatocellular carcinoma HepG2 cells. Chem Res Toxicol.
28:2160–2169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sever-Chroneos Z, Angus SP, Fribourg AF,
Wan H, Todorov I, Knudsen KE and Knudsen ES: Retinoblastoma tumor
suppressor protein signals through inhibition of cyclin-dependent
kinase 2 activity to disrupt PCNA function in S phase. Mol Cell
Biol. 21:4032–4045. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayashida T, Takahashi F, Chiba N,
Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco M,
Wijendran V, Shioda T, et al: HOXB9, a gene overexpressed in breast
cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad
Sci USA. 107:1100–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan R, Wang K, Hu J, Yan C, Li M, Yu X,
Liu X, Lei J, Guo W, Wu L, et al: Ubiquitin-like protein FAT10
promotes the invasion and metastasis of hepatocellular carcinoma by
modifying β-catenin degradation. Cancer Res. 74:5287–5300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiba N, Comaills V, Shiotani B, Takahashi
F, Shimada T, Tajima K, Winokur D, Hayashida T, Willers H, Brachtel
E, et al: Homeobox B9 induces epithelial-to-mesenchymal
transition-associated radioresistance by accelerating DNA damage
responses. Proc Natl Acad Sci USA. 109:2760–2765. 2012. View Article : Google Scholar :
|
|
24
|
Sha L, Dong L, Lv L, Bai L and Ji X: HOXB9
promotes epithelial-to-mesenchymal transition via transforming
growth factor-β1 pathway in hepatocellular carcinoma cells. Clin
Exp Med. 15:55–64. 2015. View Article : Google Scholar
|