Introduction
Alzheimer's disease (AD) is a progressive and
neurodegenerative disorder with dementia resulting from the
combination of both genetic and environmental risk factors
(1). Several studies have linked
psychosocial stress and stress hormone levels, such as
glucocorticoids (GCs), with AD generation and development (2,3).
Clinical studies have shown that plasma cortisol levels are
significantly increased in AD and mild cognitive impairment
patients, and dysfunction of the hypothalamus-pituitary-adrenal
(HPA) axis is associated with a higher risk of AD (4). Furthermore, it has been reported
that the rate of dementia progression is correlated with the plasma
levels of GCs in AD patients (3).
GCs are known to alter neuronal plasticity, impair learning and
memory, produce atrophy in several areas of the brain, reduce
hippocampal dendritic complexity (5,6),
and promote hippocampal cell death (7). Yet, the precise mechanisms by which
they contribute to AD remain to be fully elucidated (8).
Currently, there are no effective drugs for
preventing neuronal damage induced by chronic GC exposure. Ginseng
has been safely used to improve health conditions, and delay
senescence for more than 2,000 years in China. Ginsenosides are the
major active components in ginseng. It has been reported that
ginsenosides have neuroprotective effects in animals and humans
(9,10). Ginsenoside Rg1 (Rg1) is a
steroidal saponin which is abundantly found in ginseng. Recent
studies have revealed that Rg1 (2.5–10 mg/kg) prevents spatial
learning and memory impairment, and may be a useful agent for
neurodegenerative disorders such as AD (11). However, the protective effect of
Rg1 on neuronal inflammation damage induced by chronic GC exposure
and the underlying mechanisms have not yet been fully elucidated.
Moreover, mifepristone (RU486) is a synthetic glucocorticosteroid
receptor (GR) antagonist (12).
RU486 has been used as a tool to study the mechanisms of steroid
receptor function (13). Recent
studies have reported that RU486 may act as a neuroprotective agent
against excitotoxicity and traumatic brain injury (14,15). In the present study, RU486 was
used as a positive control.
Dexamethasone (DEX) is a type of synthetic
glucocorticoid. DEX (5 mg/kg) is often used to mimic GC-induced
neurodegenerative diseases (16,17). Our most recent study showed that
chronic DEX (5 mg/kg) exposure for 28 days significantly induced
neurodegeneration and increased neuroinflammation via NLRP-1
inflammasome activation (18). A
previous study by us also demonstrated that Rg1 protects against
learning and memory impairments and ROS oxidative damage in chronic
restraint stress-exposed mice (19). ROS oxidative stress is a method by
which to activate NLRP-1 inflammasomes. In the present study we
hypothesized that Rg1 inhibits the activation of NLRP-1
inflammasomes and attenuates neuronal damage induced by chronic DEX
exposure. Our results indicated that Rg1 has protective effects
against neuronal inflammation damage induced by chronic DEX (5
mg/kg, 28 days) exposure.
Materials and methods
Animals and treatment
All experiments were carried out according to
protocols approved by the Commitee on the Care and Use of
Laboratory Animals at Anhui Medical University. Adult male ICR mice
(25–30 g, Grade II) were obtained from the Center for Laboratory
Animals of Anhui. The mice were housed in groups of four to six
with unrestricted access to food and water and a 12-h light/dark
cycle.
Animals were randomly divided into six groups (10
mice in each group): control group, DEX (5 mg/kg) exposure group,
DEX (5 mg/kg) + RU486 (5 mg/kg) treatment group and DEX (5 mg/kg) +
Rg1 (1, 2, 4 mg/kg) treatment groups. DEX (Sigma, St. Louis, MO,
USA) was dissolved in alcohol at a concentration of 500 mg/ml to be
used as a stock solution and diluted in normal saline (NS) at a
concentration of 0.5 mg/ml before use. RU486 (Beijing Zizhu
Pharmaceutical Co., Ltd., Beijing, China) was prepared by
dissolving RU486 in distilled water at a concentration of 0.5
mg/ml. The Rg1 (content of Rg1 >98%, obtained from the Institute
of Pharmaceutical Research of Xiehe Medical University of China)
was dissolved in distilled water at concentrations of 0.1, 0.2 and
0.4 mg/ml. In the DEX, RU486 and Rg1 treatment groups, the mice
were injected subcutaneously (s.c.) with DEX (5 mg/kg/day) for 28
days. In the control group, the mice were injected (s.c.) with NS
with an equal volume of alcohol. The RU486 (5 mg/kg) group was
treated with RU486 solution (0.5 mg/ml) intragastrically (i.g., 0.1
ml/10 g) for 28 days. The Rg1 (1, 2, 4 mg/kg) groups were treated
with Rg1 (0.1, 0.2 and 0.4 mg/ml) intragastrically (i.g., 0.1 ml/10
g) for 28 days. The control group and DEX exposure received
equivalent volumes of distilled water for 28 days.
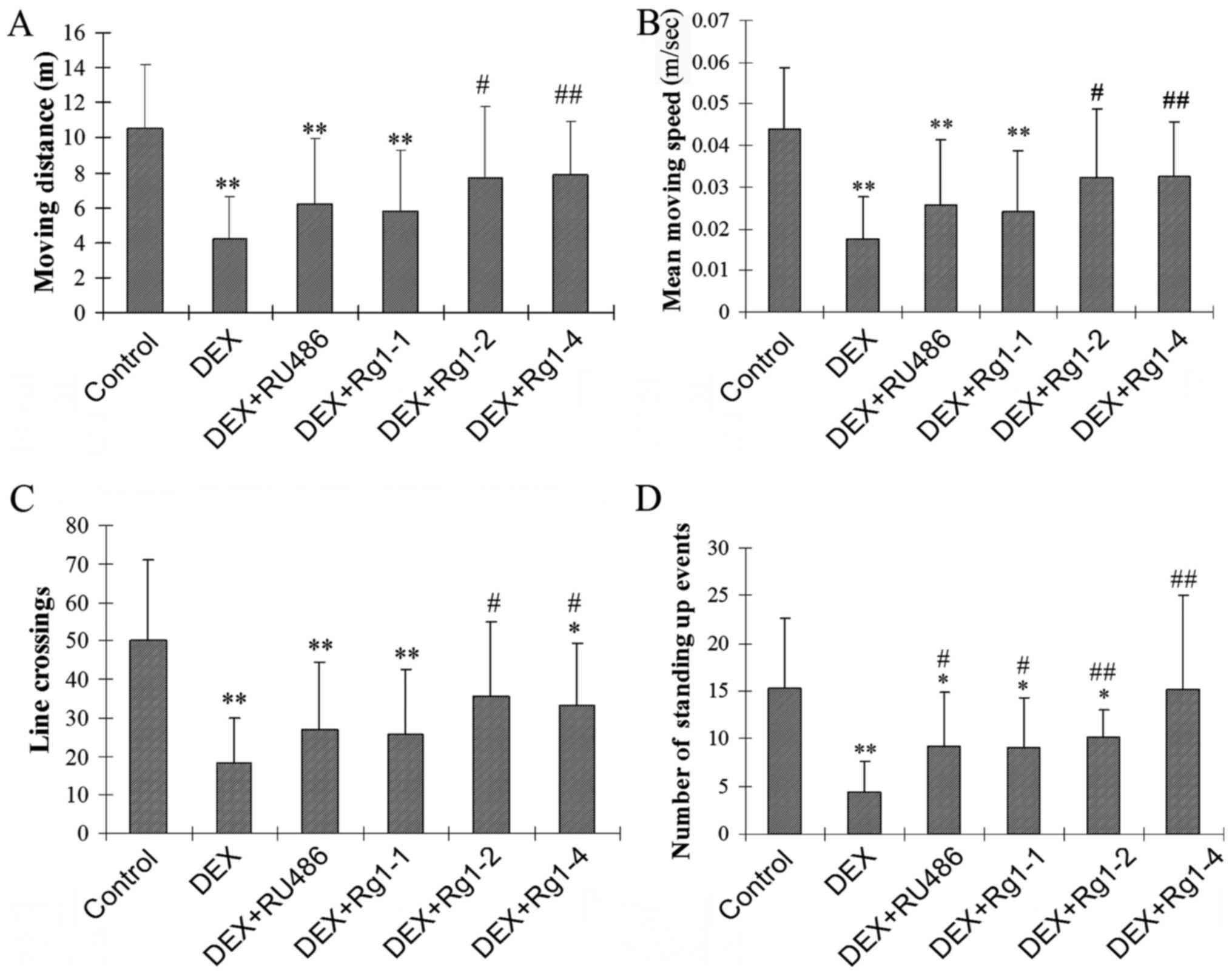
Open field test
The open field test was performed to study the
effects of Rg1 on DEX exposure-induced motor activity and
exploratory behavior impairments. It is often used to detect the
locomotor behavior, anxiety and exploration of animals (20). The open field test apparatus
(ShangHai Biowill Co., Ltd., Shanghai, China) consisted of a
computer-tracking cage (60×60×50 cm) which was separated as 9
squares (1 central and 8 peripheral) by two vertical lines and
transverse lines as previously described (21). The mouse was placed in the cage
for 2 min in each testing period. The ANY-maze Behavioral Tracking
Software (Stoelting Co., Wood Dale, IL, USA) was used to record the
path of its motor activity and exploration for 3 min. The number of
line crossings, the moving distance (m), the mean moving speed
(m/sec) and the number of standing up events (indicates exploratory
behavior) were calculated by the software to represent the motor
and exploration behaviors (22).
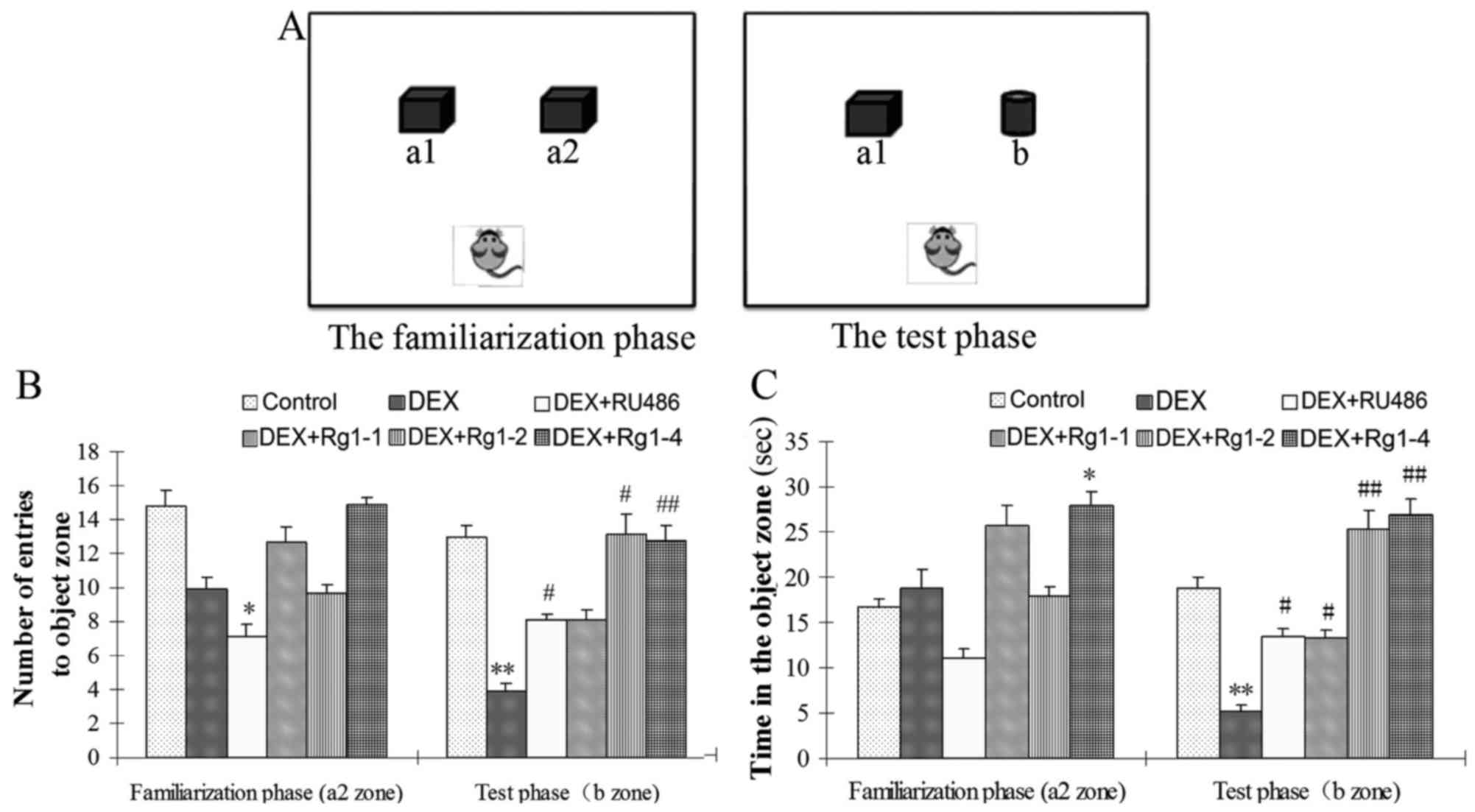
Novel object recognition (NOR) test
The NOR test is usually used to evaluate recognition
memory (23). The open field
apparatus and the ANY-maze Behavioral Tracking system are used to
detect the NOR task. The NOR test consists of three phases: the
habituation phase, the familiarization phase, and the test phase.
In the habituation phase, the mice are placed in an empty arena to
adapt to the environment. The next day, the mice are placed in the
familiar arena with two identical objects (a1, a2) (Fig. 2A). Twenty-four hours after
familiarization, the mice are placed in the open field with (a1)
the familiar object and (b) a novel object to examine long-term
recognition memory. The number of entries to a2 zone and the time
exploring the a2 zone in the familiarization phase, the number of
entries to b zone, and the time exploring the b zone in the test
phase were recorded to evaluate the effects of Rg1 on long-term
recognition memory impairments.
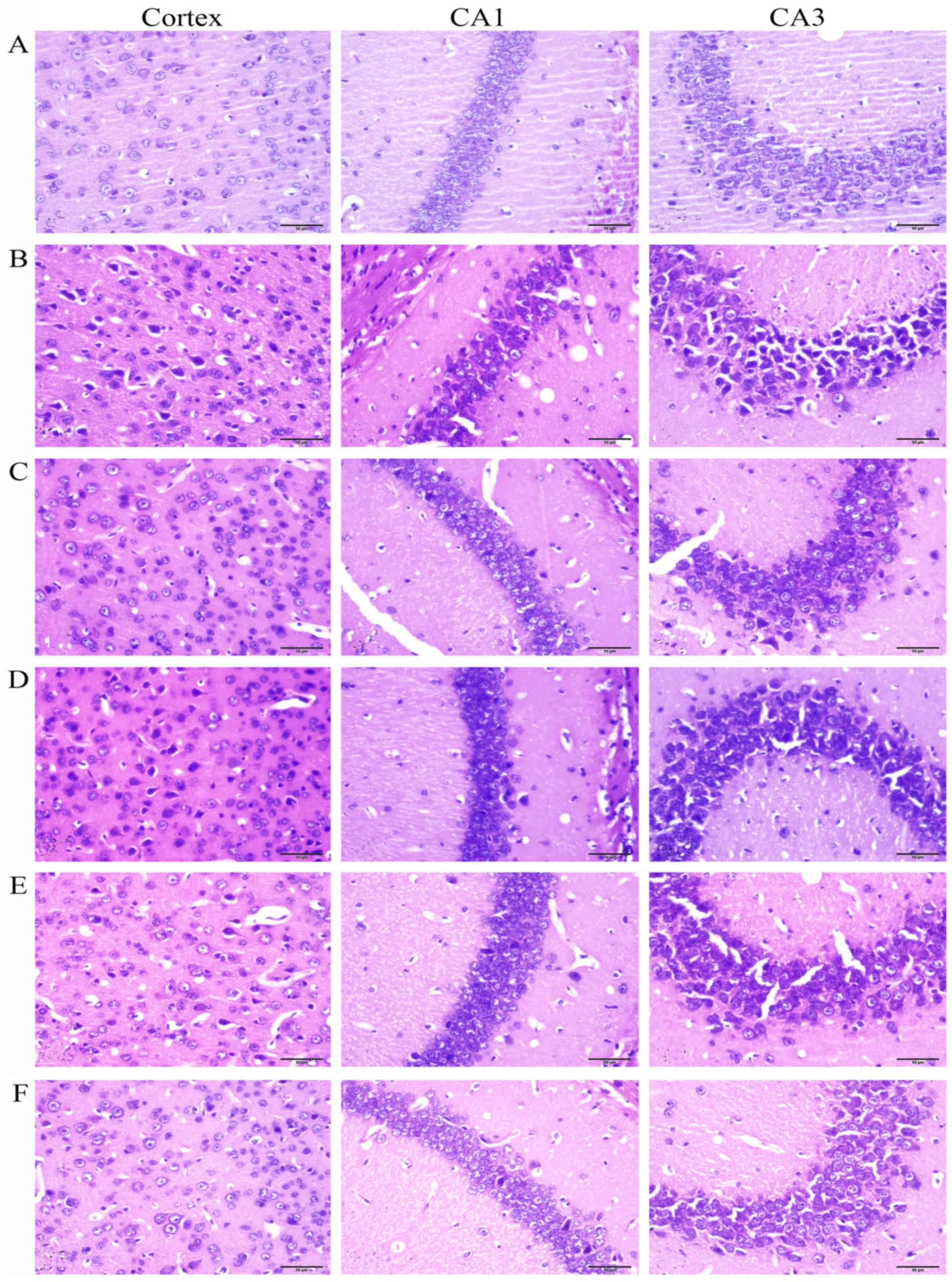
Histological examination
The mice were anesthetized by injection of 3.5%
chloral hydrate (i.p., 0.1 ml/10 g body weight). The brains (n=5)
were carefully removed and fixed in 4% paraformaldehyde. The brain
tissues were embedded in paraffin and sliced into 5-µm
sections using a section cutter (Leica, Mannheim, Germany). The
sections were stained with hematoxylin and eosin (H&E) and the
neuronal morphology in the frontal cortex and hippocampus CA1 and
CA3 was observed by a microscope (Olympus IX71; Olympus, Tokyo,
Japan).
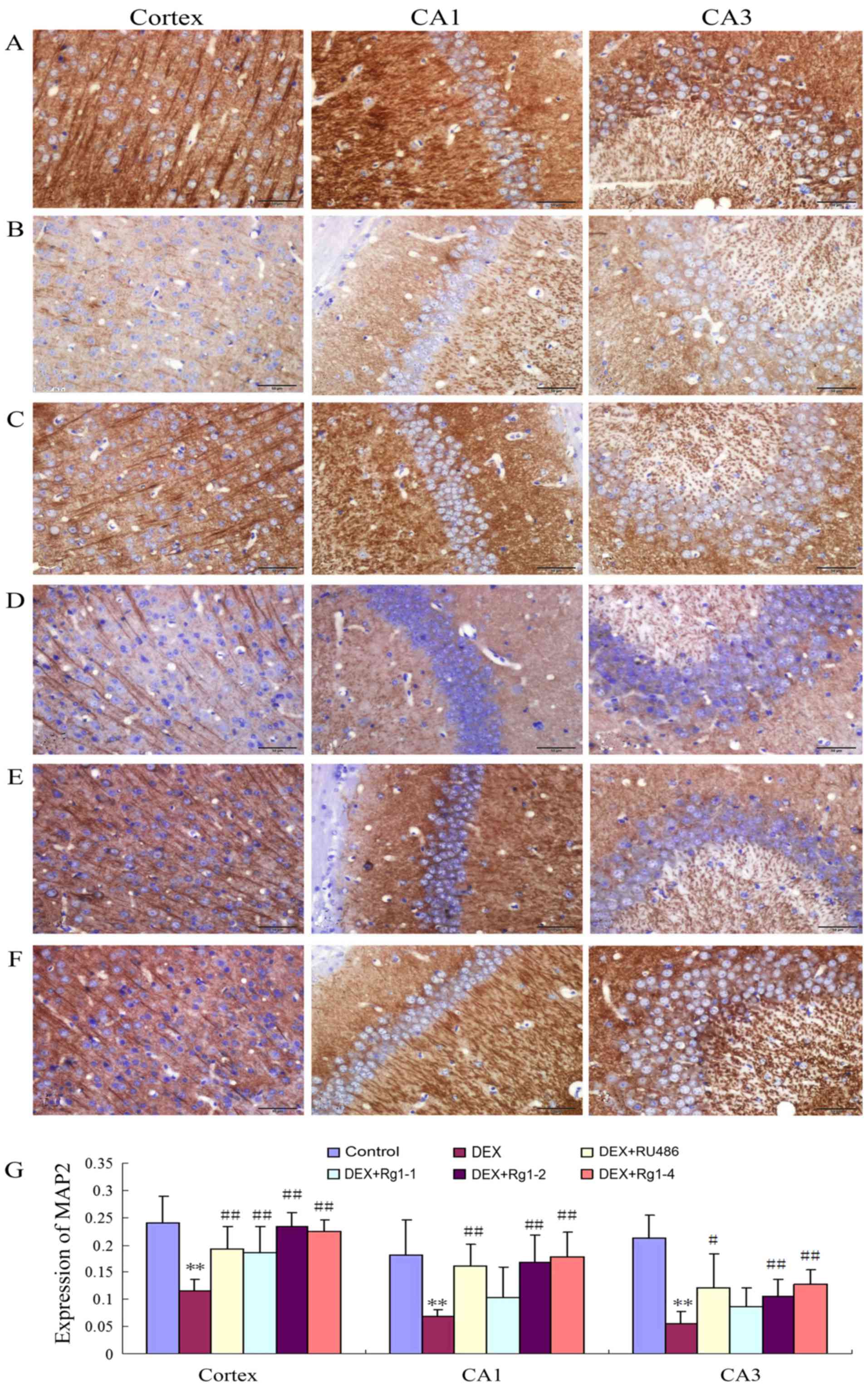
Immunohistochemistry
The immunohistochemical analysis was performed as
previously described (18). The
brain sections were deparaffinized and incubated with 3% hydrogen
peroxide and then blocked with non-immune goat serum. The sections
were washed with PBS and incubated in the mouse anti-MAP2 antibody
(1:200; ab11268; Abcam, Cambridge, UK) overnight at 4°C. The next
day, the sections were washed 3 times with PBS, and incubated in
the secondary antibody for 1 h. Then the sections were washed and
visualized by using an ABC kit (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). The sections were mounted
and examined by a light microscope (Olympus IX71; Olympus). The
positive neurons were stained brown. Image-Pro Plus 6.0 analysis
system was used to analyze the mean optical density in the frontal
cortex, hippocampus CA1 and CA3 by an observer blinded to the
experimental protocol.
Immunoblot analysis
The immunoblot method was performed according to our
previous description (18). The
total protein in the frontal cortex and hippocampus was extracted.
Equal amounts (40 µg) of protein were transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% skim milk in TBS-T for 1 h at room temperature. The
membranes were washed with TBS-T 3 times, and incubated with
primary antibodies for NLRP-1, ASC, caspase-1, caspase-5, IL-1β,
IL-18, glucocorticoid receptor (GR) and β-actin (1:1,000) overnight
at 4°C. The antibodies for ASC (BS2215), caspase-1 (BS1730),
caspase-5 (BS7048), IL-1β (BS3506) and GR (BS6617) were provided by
Bioworld Technology, Inc. (St. Louis Park, MN, USA). The NLRP-1
antibody (ab16091) was provided by Abcam. The IL-18 antibody
(sc-6179) was obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). The dilution of all primary antibodies was at 1:500. On
the next day, the membranes were washed 3 times with TBS-T and
incubated with HRP-conjugated secondary antibody (1:5,000) for 1 h.
After the membranes were extensively washed, the proteins were
detected using the ECL kit (Amersham Biosciences, Little Chalfont,
UK). The protein bands were visualized using Chemi Q4800 Mini
Imaging System (Bioshine, Shanghai, China), and Image J Software
[National Institutes of Health (NIH), Bethesda, MD, USA] was used
for densitometry and quantification of the protein bands. The
immunoreactive band intensities were normalized to its
corresponding band of β-actin.
Statistical analysis
All data are reported as mean ± SD. Statistical
differences were performed using SPSS 17.0. The data were analyzed
by one-way ANOVA followed by Bonferroni's post hoc test for
between-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Rg1 on motor and exploratory
behavior impairments in mice induced by chronic DEX exposure
In the present study, the open field test was used
to examine motor and exploratory behavior. The results showed that
DEX exposure significantly decreased the motor activity and
exploratory behavior in male mice. DEX exposure for 28 days
significantly decreased the total moving distance (m), the mean
moving speed (m/sec), the number of line crossings, and the number
of standing up movements (Fig.
1A–D, P<0.01). Rg1 (2 and 4 mg/kg) treatment significantly
increased the total m, the mean m/sec, the number of line
crossings, and the number of standing up events in the male mice
which were decreased by chronic DEX exposure (Fig. 1A–D, P<0.05 or P<0.01).
Effects of Rg1 on chronic DEX-induced NOR
in mice
The NOR test results showed that, in the
familiarization phase, DEX exposure for 28 days had no significant
effects on the number of entries into the a2 zone and the exploring
time in the a2 zone, which is the novel object zone (b zone) in the
test phase. While in the test phase, DEX exposure significantly
reduced the number of entries into the new object zone (b zone) and
the exploring time in the b zone. Compared with the DEX exposure
group, RU486 and Rg1 (2 and 4 mg/kg) treatment increased the number
of entries into the b zone and the exploring time in the b zone in
the final test phase (Fig. 2B and
C, P<0.05 or P<0.01).
Effects of Rg1 on chronic DEX-induced
neuronal damage in the hippocampus and frontal cortex
The H&E staining showed that no significant
neuronal abnormalities were observed in the frontal cortex and
hippocampus CA1 and CA3 in the control group (Fig. 3A). While in the DEX treatment
group (Fig. 3B), the neurons
showed significant nuclear condensation and acidophilia
degeneration. Compared with the DEX treatment group, RU486 and Rg1
(2 and 4 mg/kg) treatment significantly alleviated the neuronal
damage (Fig. 3C, E and F).
Effects of Rg1 on MAP2 expression in the
frontal cortex and hippocampus in mice
The results showed that MAP2 expression was abundant
in the frontal cortex and hippocampus in the control group
(Fig. 4A and G). In the DEX
treatment group, the MAP2 expression was significantly decreased in
the frontal cortex and hippocampus (Fig. 4B and G, P<0.01). Compared with
the DEX treatment group, RU486 and Rg1 (2 and 4 mg/kg)
significantly increased the expression of MAP2 in the frontal
cortex and hippocampus CA1 and CA3 (Fig. 4C and E–G, P<0.05 or
P<0.01).
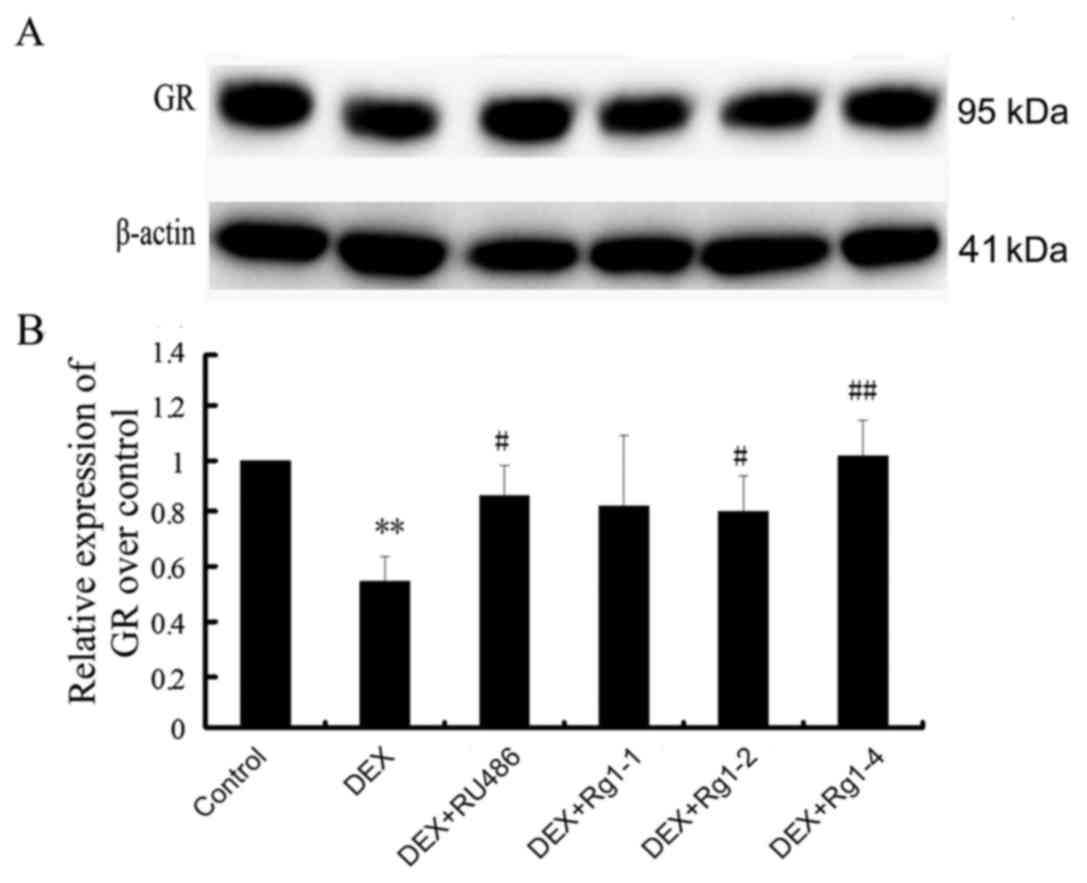
Effects of Rg1 on GR expression in the
frontal cortex and hippocampus
We further detected GR expression in the frontal
cortex and hippocampus brain tissue by immunoblot analysis. The
results revealed that GR expression was abundant in the hippocampus
and cortex in the control groups. In the DEX treatment group, GR
expression was significantly decreased (Fig. 5, P<0.01). Compared with the DEX
treatment group, RU486 and Rg1 (2 and 4 mg/kg) treatment
significantly increased the expression of GR (Fig. 5, P<0.05 or P<0.01).
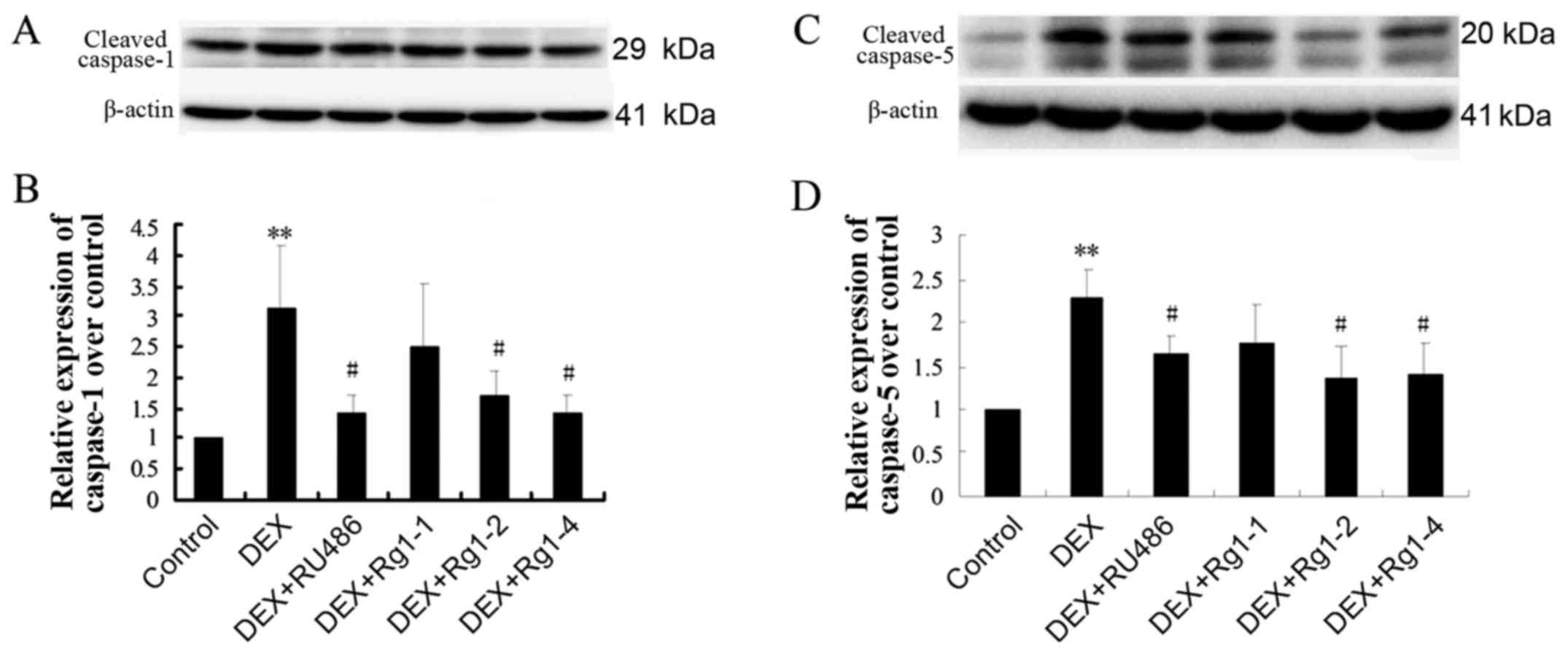
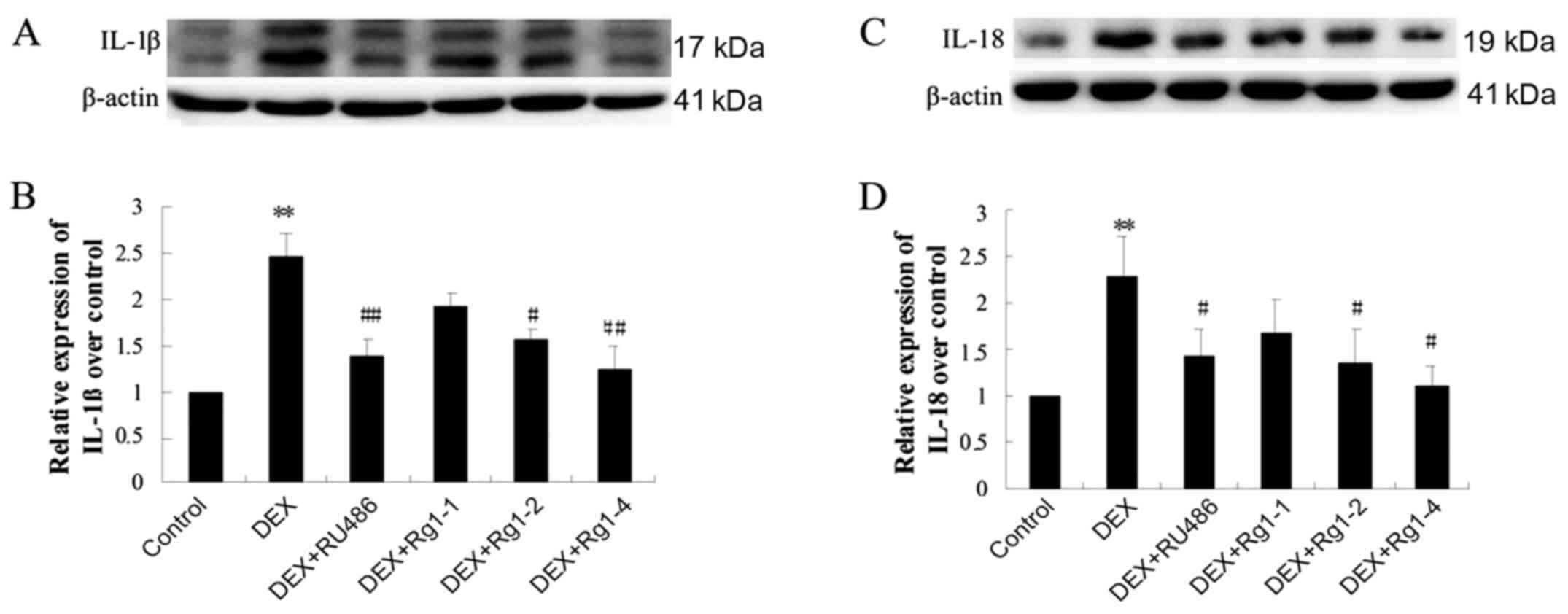
Effects of Rg1 on expression levels of
NLRP-1, ASC, caspase-1, caspase-5, IL-1β and IL-18 in the frontal
cortex and hippocampus
To confirm whether Rg1 can regulate NLRP1
inflammasome activation, which is involved in chronic DEX-induced
hippocampal neuron damage, we further detected the expression of
NLRP-1, ASC, caspase-1, caspase-5, IL-1β and IL-18 in the frontal
cortex and hippocampus brain tissue by immunoblotting. The results
showed that DEX exposure for 28 days significantly increased the
expression of NLRP-1 and ASC (Fig.
6, P<0.05 and P<0.01), caspase-1 and caspase-5 (Fig. 7, P<0.05 and P<0.01) and
IL-1β and IL-18 (Fig. 8,
P<0.01). Compared with the DEX treatment group, RU486 and Rg1 (2
and 4 mg/kg) treatment decreased the expression of NLRP-1 and ASC
(Fig. 6, P<0.05), caspase-1
and caspase-5 (Fig. 7, P<0.05
and P<0.01) and IL-1β and IL-18 (Fig. 8, P<0.05 or P<0.01). These
data indicate that Rg1 decreased the activation of NLRP1
inflammasomes activated by chronic DEX exposure.
Discussion
A growing number of reports suggest that neuronal
inflammation is a contributing factor to neurodegenerative
diseases, such as AD (24–26).
It has been reported that inflammasomes play an important role in
many neurodegenerative diseases such as Parkinson's disease (PD)
and AD (27). The present study
was designed to investigate the effects of the ginseng extract
ginsenoside Rg1 on neuroinflammation damage in the frontal cortex
and hippocampus in mice following chronic DEX exposure. In the
present study, the results showed that Rg1 (2 and 4 mg/kg) and
RU486 (GR antagonist) treatment increased spontaneous motor
activity and exploratory behavior in an open field test, and
increased the number of entries into the new object zone in the NOR
test. Rg1 (2 and 4 mg/kg) and RU486 significantly protected against
neuronal damage induced by chronic DEX exposure. Rg1 (2 and 4
mg/kg) and RU486 were able to decrease the expression of NLRP-1
inflammasomes and attenuate the neuronal damage in the frontal
cortex and hippocampus in mice. Additional, Rg1 and RU486 treatment
increased GR expression and decreased IL-1β and IL-18 expression in
brain tissue. The results suggest that Rg1 may inhibit chronic DEX
exposure-induced neuronal inflammation, which may prevent the
generation and progression of AD.
Growing evidence has linked high levels of GCs with
neurodegenerative diseases, such as vulnerability to depression, AD
and PD (28,29). Numerous studies have shown that
chronic high concentrations of GCs have adverse effects on neurons,
and even cause hippocampal neuronal death (3,5,6).
DEX is a type of synthetic glucocorticoid. It has been reported
that chronic exposure to DEX (5 mg/kg) can induce learning and
memory impairment and mimic GC-induced neurodegenerative diseases
(16,17,30). In our previous studies, we found
that both chronic DEX (5 mg/kg) exposure and restrain stress
induced cognition impairments and neuronal damage in the
hippocampus and cortex in mice (19,31). Our most recent study revealed that
DEX (5 mg/kg) exposure for 28 days significantly increased
expression of NLRP-1 and accelerated neurodegeneration in the
hippocampus and frontal cortex in mice (18). However, currently, there are no
effective drugs for preventing chronic GC exposure-mediated
neuronal damage. It was found that ginsenoside Rg1 attenuated the
inflammation, neuronal apoptosis and alleviated the cognitive
impairment in an AD model (32,33). Our previous study also showed that
Rg1 could protect against learning and memory impairments and ROS
oxidative damage in chronic restrain stress-exposed mice (19). Yet, it is still unclear whether
Rg1 could alleviate chronic GC-induced cognitive impairment and
neuronal inflammation damage.
Behavioral tests play an important role in
evaluating the ability of recognition in animal models of cognitive
dysfunction (34). The open field
test is generally used to evaluate locomotor activity and
spontaneous exploration in a novel environment (35). In the present study, we observed
that chronic DEX-exposed animals showed impaired motor and
exploration activities such as reduced number of lines crossed and
stand up events in the open field test. Rg1 (2 and 4 mg/kg)
significantly increased the number of lines crossed and stand up
movements of the mice. The NOR test is also widely used to
investigate memory alterations. As Ennaceur (36) reported, animals were placed to an
arena with a familiar and a novel object. He found that the animals
approached frequently and spent more time exploring the novel
object than the familiar one. In the present study, we found that
DEX exposure for 28 days significantly decreased the entering
number and the exploring time in the novel object zone. Compared
with the DEX treatment group, RU486 and Rg1 (2 and 4 mg/kg)
treatment increased the entering number and the exploring time in
the novel object zone. These results suggest that Rg1 may alleviate
the cognitive dysfunction induced by chronic GC exposure.
As a cytoskeletal protein, MAP2 is a marker of
structural integrity in neurons (37). It has been observed that there is
a significant reduction in MAP2 in the cortex and hippocampus in
elder rats (38). The present
study showed that DEX (5 mg/kg) exposure for 28 days significantly
induced neuronal damage; the neurons showed significant acidophilia
degeneration and nuclear condensation in the cortex and hippocampus
CA1, CA3, and the expression of MAP2 was also significantly reduced
in the frontal cortex and hippocampus. Treatment with Rg1 (2 and 4
mg/kg) and RU486 significantly alleviated the histological changes,
increased the expression of MAP2, and attenuated the neuronal
injury in the frontal cortex and hippocampus CA1 and CA3.
Neuroinflammation has emerged as an important cause
of the cognitive decline during aging and AD (25,26,39). GCs are commonly used as
anti-inflammatory and immunosuppressive agents in the clinic.
However, research has showed that GCs also have a proinflammatory
action (40,41). The GR, as a member of the nuclear
receptor superfamily, plays a key role in regulated the actions of
GCs (42). GR contributes to the
regulation of proinflammatory molecules at the level of gene
transcription (43). Sun et
al (13) reported that LPS
treatment slightly downregulated GR expression. Rg1 treatment
significantly increased the GR expression compared to the LPS
group. The GR antagonist RU486 inhibited the neuroprotective
effects of Rg1, indicating that the anti-inflammatory effects of
Rg1 are dependent on GR. Du et al (44) reported that Rg1, a novel GR
agonist of plant origin, possesses GC and estrogen-like activities
and can effectively inhibit acute and chronic inflammation, but it
does not cause an adverse reaction as noted with DEX. In the
present study, we found that DEX (5 mg/kg) exposure for 28 days
significantly downregulated GR expression and increased IL-1β and
IL-18 expression in the hippocampus and frontal cortex in mice. Rg1
and RU486 increased GR expression and reduced IL-1β and IL-18
expression in the hippocampus and frontal cortex in mice. The
results suggest that both Rg1 and RU486 can increase GR expression
and are involved in anti-inflammatory effects in chronic
DEX-exposed mice. However, the mechanisms through which Rg1
inhibits the downregulation of GR expression induced by DEX are
still unclear and warrant further study.
The inflammasome-associated pathway plays an
important role in the pathogenesis of neurodegenerative diseases.
The NLRP1 inflammasome is a multi-protein complex which consists of
NLRP1, the adaptor ASC and caspase-1 (45). NLRP1 inflammasomes mediate
activation of caspase-1 which promotes cleavage of mature
proinflammatory cytokines from pro-IL-1β and pro-IL-18 into IL-1β
and IL-18 (46). The adaptor ASC
is a critical component of inflammasomes by linking NLRPs to
caspase-1 activation (47).
Therefore, activation of inflammasomes provides a molecular
platform for caspase-1 activation which promotes IL-1β and IL-18
release (48,49). Caspase-5 is also a proinflammatory
cysteine protease. Caspase-5 together with caspase-1 are components
of the NLRP1 inflammasome complex and enhance activation of
caspase-1 (50). The present
study showed that DEX exposure for 28 days significantly increased
the expression levels of NLRP-1, caspase-1, caspase-5, ASC, IL-1β
and IL-18 in the hippocampus and frontal cortex brain tissue. Rg1
and RU486 significantly decreased the expression levels of NLRP-1,
caspase-1, caspase-5, ASC, IL-1β and IL-18 in the hippocampus and
frontal cortex brain tissue. These data suggest that Rg1 may
suppress neuroinflammation and inhibit chronic DEX exposure-induced
inflammation injury in the hippocampus and frontal cortex.
In summary, the present study suggests that chronic
GC exposure induces neurodegeneration and NLRP-1 inflammasome
activation in the hippocampus and frontal cortex. Rg1 protects
against the neuroinflammation and neuronal damage induced by
chronic DEX exposure. Additionally, the inhibition of NLRP-1
inflammasomes was involved in the action mechanisms of Rg1 in this
experimental model. However, this study only offered an
experimental basis for Rg1 in the treatment of chronic DEX
exposure, and other related molecular mechanisms of Rg1 in regards
to DEX exposure warrant further investigation.
Acknowledgments
The present study was financially supported by
grants from the National Nature Science Foundation of China
(81371329 and 81671384) and the Natural Science Foundation of Anhui
Province Education Department (KJ2015A298, KJ2016A357). We thank
Bao Li and Li Gui (Synthetic Laboratory of Basic Medicine College,
Anhui Medical University) for their excellent technical
assistance.
References
|
1
|
Joshi YB, Chu J and Praticò D: Stress
hormone leads to memory deficits and altered tau phosphorylation in
a model of Alzh–eimer's disease. J Alzheimers Dis. 31:167–176.
2012.
|
|
2
|
Moceri VM, Kukull WA, Emanuel I, van Belle
G and Larson EB: Early-life risk factors and the development of
Alzheimer's disease. Neurology. 54:415–420. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csernansky JG, Dong H, Fagan AM, Wang L,
Xiong C, Holtzman DM and Morris JC: Plasma cortisol and progression
of dementia in subjects with Alzheimer-type dementia. Am J
Psychiatry. 163:2164–2169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aznar S and Knudsen GM: Depression and
Alzheimer's disease: Is stress the initiating factor in a common
neuropathological cascade? J Alzheimers Dis. 23:177–193. 2011.
|
|
5
|
Kleen JK, Sitomer MT, Killeen PR and
Conrad CD: Chronic stress impairs spatial memory and motivation for
reward without disrupting motor ability and motivation to explore.
Behav Neurosci. 120:842–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conrad CD, McLaughlin KJ, Harman JS, Foltz
C, Wieczorek L, Lightner E and Wright RL: Chronic glucocorticoids
increase hippocampal vulnerability to neurotoxicity under
conditions that produce CA3 dendritic retraction but fail to impair
spatial recognition memory. J Neurosci. 27:8278–8285. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacPherson A, Dinkel K and Sapolsky R:
Glucocorticoids worsen excitotoxin-induced expression of
pro-inflammatory cytokines in hippocampal cultures. Exp Neurol.
194:376–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EJ, Pellman B and Kim JJ: Stress
effects on the hippocampus: A critical review. Learn Mem.
22:411–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rausch WD, Liu S, Gille G and Radad K:
Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars).
66:369–375. 2006.
|
|
10
|
Xie CL, Wang WW, Xue XD, Zhang SF, Gan J
and Liu ZG: A systematic review and meta-analysis of
Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep.
5:77902015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Wang J, Xing Y, Gong L, Li H, Wu
Z, Li Y, Wang J, Wang Y, Dong L, et al: Effects of ginsenoside Rg1
or 17β-estradiol on a cognitively impaired, ovariectomized rat
model of Alzheimer's disease. Neuroscience. 220:191–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baulieu EE: Contragestion and other
clinical applications of RU 486, an antiprogesterone at the
receptor. Science. 245:1351–1357. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun XC, Ren XF, Chen L, Gao XQ, Xie JX and
Chen WF: Glucocorticoid receptor is involved in the neuroprotective
effect of ginsenoside Rg1 against inflammation-induced dopaminergic
neuronal degeneration in substantia nigra. J Steroid Biochem Mol
Biol. 155(Pt A): 94–103. 2016. View Article : Google Scholar
|
|
14
|
Behl C, Lezoualc'h F, Trapp T, Widmann M,
Skutella T and Holsboer F: Glucocorticoids enhance oxidative
stress-induced cell death in hippocampal neurons in vitro.
Endocrinology. 138:101–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCullers DL, Sullivan PG, Scheff SW and
Herman JP: Mifepristone protects CA1 hippocampal neurons following
traumatic brain injury in rat. Neuroscience. 109:219–230. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green KN, Billings LM, Roozendaal B,
McGaugh JL and LaFerla FM: Glucocorticoids increase amyloid-beta
and tau pathology in a mouse model of Alzheimer's disease. J
Neurosci. 26:9047–9056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maloney SE, Noguchi KK, Wozniak DF, Fowler
SC and Farber NB: Long-term effects of multiple glucocorticoid
exposures in neonatal mice. Behav Sci (Basel). 1:4–30. 2011.
View Article : Google Scholar
|
|
18
|
Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang
Y and Li W and Li W: Chronic glucocorticoids exposure enhances
neurodegeneration in the frontal cortex and hippocampus via NLRP-1
inflammasome activation in male mice. Brain Behav Immun. 52:58–70.
2016. View Article : Google Scholar
|
|
19
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koros E, Piasecki J, Kostowski W and
Bienkowski P: Saccharin drinking rather than open field behaviour
predicts initial ethanol acceptance in Wistar rats. Alcohol
Alcohol. 33:131–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Senna PN, Ilha J, Baptista PP, do
Nascimento PS, Leite MC, Paim MF, Gonçalves CA, Achaval M and
Xavier LL: Effects of physical exercise on spatial memory and
astroglial alterations in the hippocampus of diabetic rats. Metab
Brain Dis. 26:269–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frye CA, Paris JJ and Rhodes ME: Engaging
in paced mating, but neither exploratory, anti-anxiety, nor social
behavior, increases 5alpha-reduced progestin concentrations in
midbrain, hippocampus, striatum, and cortex. Reproduction.
133:663–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antunes M and Biala G: The novel object
recognition memory: Neurobiology, test procedure, and its
modifications. Cogn Process. 13:93–110. 2012. View Article : Google Scholar :
|
|
24
|
Zotova E, Bharamb—e V, Cheaveau M, Morgan
W, Holmes C, Harris S, Neal JW, Love S, Nicoll JA and Boche D:
Inflammatory components in human Alzheimer–'s disease and after
active amyloid-β42 immunization. Brain. 136:2677–2696. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calsolaro V and Edison P:
Neuroinflammation in Alzheimer's disease: Current evidence and
future directions. Alzheimers Dement. 12:719–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morales I, Guzmán-Martínez L,
Cerda-Troncoso C, Farías GA and Maccioni RB: Neuroinflammation in
the pathogenesis of Alzheimer's disease. A rational framework for
the search of novel therapeutic approaches. Front Cell Neurosci.
8:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jha S, Srivastava SY, Brickey WJ, Iocca H,
Toews A, Morrison JP, Chen VS, Gris D, Matsushima GK and Ting JP:
The inflammasome sensor, NLRP3, regulates CNS inflammation and
demyelination via caspase-1 and interleukin-18. J Neurosci.
30:15811–15820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sotiropoulos I, Catania C, Pinto LG, Silva
R, Pollerberg GE, Takashima A, Sousa N and Almeida OF: Stress acts
cumulatively to precipitate Alzheimer's disease-like tau pathology
and cognitive deficits. J Neurosci. 31:7840–7847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen KC, Blalock EM, Curran-Rauhut MA,
Kadish I, Blalock SJ, Brewer L, Porter NM and Landfield PW:
Glucocorticoid-dependent hippocampal transcriptome in male rats:
Pathway-specific alterations with aging. Endocrinology.
154:2807–2820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danilczuk Z, Ossowska G, Lupina T, Cieślik
K and Zebrowska-Łupina I: Effect of NMDA receptor antagonists on
behavioral impairment induced by chronic treatment with
dexamethasone. Pharmacol Rep. 57:47–54. 2005.PubMed/NCBI
|
|
31
|
Li WZ, Li WP, Yao YY, Zhang W, Yin YY, Wu
GC and Gong HL: Glucocorticoids increase impairments in learning
and memory due to elevated amyloid precursor protein expression and
neuronal apoptosis in 12-month old mice. Eur J Pharmacol.
628:108–115. 2010. View Article : Google Scholar
|
|
32
|
Kril JJ, Patel S, Harding AJ and Halliday
GM: Neuron loss from the hippocampus of Alzheimer's disease exceeds
extracellular neurofibrillary tangle formation. Acta Neuropathol.
103:370–376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Li X, Zhang L, Liu L, Jing G and
Cai H: Ginsenoside Rg1 suppressed inflammation and neuron apoptosis
by activating PPARγ/HO-1 in hippocampus in rat model of cerebral
ischemia-reperfusion injury. Int J Clin Exp Pathol. 8:2484–2494.
2015.
|
|
34
|
Baxter MG: 'I've seen it all before':
Explaining age-related impairments in object recognition.
Theoretical comment on Burke et al 2010. Behav Neurosci.
124:706–709. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wi S, Yu JH, Kim M and Cho SR: In vivo
expression of reprogramming factors increases hippocampal
neurogenesis and synaptic plasticity in chronic hypoxic-ischemic
brain injury. Neural Plast. 2016:25808372016.Epub ahead of print.
View Article : Google Scholar :
|
|
36
|
Ennaceur A: One-trial object recognition
in rats and mice: Methodological and theoretical issues. Behav
Brain Res. 215:244–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Stefano G, Casoli T, Fattoretti P,
Gracciotti N, Solazzi M and Bertoni-Freddari C: Distribution of
map2 in hippocampus and cerebellum of young and old rats by
quantitative immunohistochemistry. J Histochem Cytochem.
49:1065–1066. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chauhan N and Siegel G: Age-dependent
organotypic expression of microtubule-associated proteins (MAP1,
MAP2, and MAP5) in rat brain. Neurochem Res. 22:713–719. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sartori AC, Vance DE, Slater LZ and Crowe
M: The impact of inflammation on cognitive function in older
adults: Implications for healthcare practice and research. J
Neurosci Nurs. 44:206–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frank MG, Miguel ZD, Watkins LR and Maier
SF: Prior exposure to glucocorticoids sensitizes the
neuroinflammatory and peripheral inflammatory responses to E. coli
lipopolysaccharide. Brain Behav Immun. 24:19–30. 2010. View Article : Google Scholar
|
|
41
|
Hermoso MA, Matsuguchi T, Smoak K and
Cidlowski JA: Glucocorticoids and tumor necrosis factor alpha
cooperatively regulate toll-like receptor 2 gene expression. Mol
Cell Biol. 24:4743–4756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carter BS, Meng F and Thompson RC:
Glucocorticoid treatment of astrocytes results in temporally
dynamic transcriptome regulation and astrocyte-enriched mRNA
changes in vitro. Physiol Genomics. 44:1188–1200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chrousos GP: Stress and disorders of the
stress system. Nat Rev Endocrinol. 5:374–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du J, Cheng B, Zhu X and Ling C:
Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant
origin, maintains glucocorticoid efficacy with reduced side
effects. J Immunol. 187:942–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martinon F and Tschopp J: NLRs join TLRs
as innate sensors of pathogens. Trends Immunol. 26:447–454. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mariathasan S, Newton K, Monack DM, Vucic
D, French DM, Lee WP, Roose-Girma M, Erickson S and Dixit VM:
Differential activation of the inflammasome by caspase-1 adaptors
ASC and Ipaf. Nature. 430:213–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stutz A, Golenbock DT and Latz E:
Inflammasomes: Too big to miss. J Clin Invest. 119:3502–3511. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fernandes-Alnemri T, Wu J, Yu JW, Datta P,
Miller B, Jankowski W, Rosenberg S, Zhang J and Alnemri ES: The
pyroptosome: A supramolecular assembly of ASC dimers mediating
inflammatory cell death via caspase-1 activation. Cell Death
Differ. 14:1590–1604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Salminen A, Ojala J, Suuronen T,
Kaarniranta K and Kauppinen A: Amyloid-beta oligomers set fire to
inflammasomes and induce Alzheimer's pathology. J Cell Mol Med.
12(6A): 2255–2262. 2008. View Article : Google Scholar : PubMed/NCBI
|