Introduction
Corneal diseases exhibit a high prevalence and are
prone to cause blindness. Normal corneal endothelial cells (CECs)
are crucial for maintaining corneal transparency and clear vision.
The CEC layer primarily transports nutrients in the aqueous humor
to the cornea through ion channels and ion pumps. These cells also
transport water molecules from the matrix to the aqueous humor to
offset passive matrix swelling caused by water absorption and to
maintain the relatively dehydrated state of the matrix, thus
further maintaining the transparency of the cornea (1). Human corneal endothelial cells
(HCECs) undergo contact inhibition due to cell junctions and the
arrest of cell proliferation at the the G1 phase, thus ensuring
their monolayer features and maintaining a normal corneal
endothelial cell density (ECD) (2). After birth, HCECs cannot regenerate,
and the loss of endothelial cells caused by injury, inflammation
and eye surgery is compensated through an increase in the adjacent
cell volume, a reduction in the cell density (3), or a transition into fibroblast-like
cells (4), which have poor repair
functions in the acute and chronic injury of CECs (5,6).
In the normal physiological state, with increased age, the ECD
gradually decreases at a rate of 0.3–0.6% annually, and the CEC
volume also gradually increases (7). Once the corneal ECD is lower than
the critical density for maintaining the physiological functions of
endothelial cells (400–700/mm2), the cornea will undergo
irreversible pathological changes, and the barrier and ionic
transporter expression of CECs will be decompensated to produce
corneal edema and the reduction of corneal transparency, thus
inducing visual impairment. Therefore, the restoration of the
normal amount, morphology and ionic transporter expression of CECs
is of significance in the drug and surgical treatment of corneal
endothelial diseases.
Penetrating keratoplasty and endothelial
keratoplasty are currently the only methods for effectively
treating corneal endothelial decompensation diseases. However,
these surgeries have high requirements for donor corneal
endothelial quality and cell number. The lack of donor corneal
material suitable for corneal transplantation remains the largest
limiting factor in current corneal transplantation surgery. In
addition, issues such as post-operative immune rejection,
endothelial dysfunction and secondary glaucoma are also common
reasons for surgical failure (8).
For patients at the early stage of the diseases (when corneal
limbal stem cells and corneal endothelial progenitor cells still
maintain their normal functions) and for patients who cannot
receive corneal transplantation surgery in time, drug treatment is
very important (5,9). The in vitro disruption of the
junctions between CECs and the application of reagents, such as
growth factors in the culture environment can stimulate CEC
replication (10). However, CECs
obtained from culture, whether primary cells or passaged cells,
often undergo fibrotic transitions with respect to cell morphology
(11–14). It has been reported that
endothelial-to-mesenchymal transition (EndMT) and
epithelial-to-mesenchymal transition (EMT) are major factors
limiting the production of the CEC layer graft in tissue
engineering, which can not only result in the dissociation of cell
junctions, the lack of apical-basal polarity, changes in cell
morphology and an increase in cell motility, but can also lead to
the re-organization of the Rho guanosine triphosphatase
family-dependent actin cytoskeleton, promoting the production of
extracellular matrix proteins and changing gene expression
(15,16). Therefore, in cultured CECs, EndMT
and EMT, are the two most important factors which can cause CECs to
lose their normal cell morphology and can induce cell fibrosis
(17).
Thiazovivin (2,4-disubstituted thiazole, TZV) is a
novel Rho associated coiled-coil containing protein kinase (ROCK)
inhibitor. Currently, in vitro studies on ROCK inhibitors
primarily focus on the effect of selective inhibitors, such as
Y-27632 and Y-39983 on the proliferation of cynomolgus monkey and
rabbit CECs and cell scratch tests (18). Studies on TZV are restricted to
embryonic stem cells, adult endometrial stromal cells and neurons
(19–21). In this study, we cultured primary
HCECs and then passaged them. In addition, the effects of TZV on
EndMT/EMT, cell morphology, junction proteins and ionic transporter
expression in HCECs in vitro were investigated, and the
underlying mechanisms were also examined.
Materials and methods
Materials
Human corneal tissue material
All procedures in this study conformed to the
Declaration of Helsinki developed by the World Medical Association
(WMA) and the ethics principles of the International Ethical
Guidelines for biomedical research involving human subjects
developed by the Council for International Organizations of Medical
Sciences (CIOMS). This study was approved by the Ethics Committee
of Zhongshan Ophthalmic Center, Sun Yat-sen University. Written
informed consent was obtained from all donors prior to obtaining
the samples. The human corneal tissues used in this study were
corneal limbi left from corneal transplantation surgery and were
all from the Eye Bank of Guangdong Province. After receiving the
donor corneal materials, the Eye Bank performed screening for
health, past disease history and genetic disease history of the
corneal donors to ensure that the corneal tissues were in excellent
condition and suitable to be used as the graft material for corneal
transplantation surgery.
Experimental materials
FNC coating mix was purchased from Athena
Environmental Sciences, Inc. (Baltimore, MD, USA); Opti-MEM-I (1X)
reduced serum medium, pen strep (penicillin streptomycin), 0.25%
trypsin-EDTA (1X), PBS (10X), bovine serum albumin (BSA),
Australian fetal bovine serum and goat serum were all purchased
from Gibco (Mountain View, CA, USA). Petri dishes (35 mm) with
lids, culture plates, chamber culture plates, DAPI, Super ECL
chemiluminescent solution, and collagenase IV were all purchased
from Sigma (St. Louis, MO, USA). N-cadherin (D4R1N) XP®
rabbit monoclonal antibody (mAb) (#13116), E-cadherin (24E10)
(24E10) rabbit mAb (Alexa Fluor® 488 conjugate, #3199),
Na+/K+-ATPase α1 (D4Y7E) rabbit mAb (#23565),
neuron-specific enolase (NSE) antibody, anti-rabbit IgG (H+L),
F(ab′) fragment (Alexa Fluor® 488 conjugate; #4412),
anti-rabbit IgG, HRP-linked antibody (#7074) and GAPDH (14C10)
rabbit mAb (#2118) were all purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Purified mouse anti-human
Zonula occludens-1 (ZO-1) antibody (#610967) was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Anti-α smooth muscle actin
antibody (α-SMA; ab5694) and goat anti-mouse IgG H&L (Alexa
Fluor® 594; ab150120) were purchased from Abcam
(Cambridge, UK). Anti-ROCK-1/2 antibody (#07-1458) was purchased
from Merck Millipore (Billerica, MA, USA). Epidermal growth factor
(EGF; E9644), calcium chloride (C5670), chondroitin sulfate A
(C9819) and L-ascorbic acid (A4403) were all purchased from Sigma.
TZV and DMSO were purchased from Selleck Chemicals (Houston, TX,
USA). Y-27632 (CalBiochem, San Diego, CA, USA), qPCR reagent
SYBR® Premix Ex Taq™, RNA extraction reagent RNAiso
Plus, reverse transcriptase reagent PrimeScript™ RT Master Mix, and
the CCK-8 reagent kit were purchased from Takara Bio (Dalian,
China).
Methods
Cell culture
The corneal materials used in this study were from
corneal limbi left from corneal transplantation surgery (n=6). The
ECD range of the corneal graft materials was
1,500–2,000/mm2. The age of the corneal donors was 22–48
years. Descemet's membrane and the endothelial layer were isolated
under a dissection microscope and immersed in 4% type IV
collagenase for 15–20 min. After complete culture medium was added
and centrifuged 3 times (1,500 rpm/min, 5 min), Descemet's membrane
and CECs were inoculated together onto FNC-coated 35-mm Petri
dishes with lids, 4-,8- and 6-well chamber culture plates and
placed in a 37°C, 5% CO2 incubator. After 15–20 days,
when the primary HCECs effectively covered the bottom of the Petri
dish and the confluence reached approximately 80%, passage and
other subsequent experiments were performed. The complete culture
medium contained Opti-MEM-I, 8% FBS, 1‰ Pen/Strep (Gibco), 5 ng/ml
EGF (Sigma), 20 µg/ml ascorbic acid, 200 mg/l calcium
chloride (Sigma) and 0.08% chondroitin sulfate A.
Immunofluorescence staining
The samples were fixed in 4% paraformaldehyde for 30
min and blocked in 1% BSA or goat serum for 1 h. The samples were
washed with 1X PBS 3 times for 5 min each on a rotary destaining
shaker at 50 rpm. The primary antibodies (NSE antibody, N-cadherin
antibody, E-cadherin antibody, ZO-1 antibody, α-SMA antibody,
Na+/K+-ATPase antibody and ROCK antibody)
were diluted in primary antibody dilution solution according to the
ratio in the instruction manual. The samples were incubated with
primary antibodies at 4°C for 12 h. The samples were washed with 1X
PBS 3 times for 5 min each on a rotary destaining shaker at 50 rpm.
Based on the sources of the primary antibodies, corresponding
secondary antibodies were selected. Secondary antibodies were
diluted using the secondary antibody dilution solution (0.01 M PBS
containing 1% BSA and 0.3% Triton X-100, pH 7.4) according to the
instruction manual. The samples were incubated with secondary
antibodies at 4°C for 4 h. The samples were washed with 1X PBS 3
times for 5 min each on a rotary destaining shaker at 50 rpm. The
samples were then incubated in 0.5 µg/ml DAPI at room
temperature for 10 min, washed with 1X PBS, and finally mounted in
an anti-fluorescence quenching agent. The results were observed
using an automatic upright fluorescence microscope (Imager Z1;
Zeiss, Jena, Germany).
Determination of proliferative
ability
The cell suspension was inoculated onto 96-well
plates (100 µl/well). Culture plates were placed in an
incubator for pre-culture (37°C, 5% CO2). Approximately
10 µl of CCK-8 solution was added to each well. The culture
plates were placed in an incubator for 1–4 h. The absorbance at 450
nm was measured using a microplate reader (Synergy H1, Hybrid
Reader; BioTek, Winooski, VT, USA).
Western blot analysis
Proteins were extracted, and protein concentrations
were determined using a BCA reagent kit. All samples were diluted
to the same concentration using RIPA buffer; 4X SDS sample loading
buffer was added, and the samples were boiled for 5 min, mixed
thoroughly and centrifuged. The samples were loaded onto a 10%
sodium dodecyl sulfate polyacrylamide gel for electrophoresis.
After electrophoresis was completed, the proteins were transferred
onto a PVDF membrane and blocked in 5% non-fat milk at room
temperature for 1 h on a shaker with stable shaking. The diluted
primary antibody (ROCK) was added; the dilution was 1:1,000. The
diluted rabbit-anti GADPH antibody was added as an internal control
at a 1:2,000 dilution. The membrane was incubated overnight at 4°C
on a shaker. After washing in 1X TBST for 10 min 3 times, the
membrane was placed in the corresponding secondary antibodies
diluted in 5% non-fat milk (secondary antibody concentration
1:5,000) and incubated at room temperature for 1 h. Following
incubation with secondary antibodies, the membrane was washed in 1X
TBST for 10 min 3 times. Solution A and B in super ECL were mixed
at equal volumes; after mixing thoroughly, the solution was added
onto the membrane. The membrane was exposed and fixed, and the
X-ray film was scanned using a scanner (5200 automatic
chemiluminescence image analysis system; Tanon, Shanghai,
China).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the cultured cells was first
extracted using TriPure Isolation reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). The RNA concentration and purity
were detected using UV spectroscopy (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The RNA (1 µg) was reverse
transcribed into cDNA using the Transcriptor First Strand cDNA
Synthesis kit (Roche Applied Science, Penzberg, Germany). PCR
amplification was performed using a Light Cycler instrument and the
Light Cycler Fast Start DNA Master SYBR-Green 1 kit (Roche Applied
Science) according to the manufacturer's instructions. Finally,
gene expression was quantified relatively by ABI Prism 7700
Sequence Detection system. The primers for Snail were
forward, CCACACTGGCGAGAAG and reverse, AGAAGGTCCGAGCACAC; the
primers for GADPH were forward, GCACCGTCAAGGCTGAGAAC and reverse,
TGGTGAAGACGCCAGTGGA. GAPDH was used as an internal control, and the
relative expression level of Snail was calculated using the
2−ΔΔCt method.
Cell treatment
Primary and passaged HCECs were cultured, and the
cells were identified by observing the cell morphology under a
light microscope and through NSE immunofluorescence staining
experiments. When the confluence of primary cells was close to
70–80%, 0.25% trypsin-EDTA (1X) was added for digestion. Following
centrifugation, the cells were sub-cultured (1–3 generations).
Primary and passaged HCECs were cultured onto 8-well chamber
culture plates. When the cell confluence reached approximately 80%,
various concentrations of TZV (2, 4 and 6 µM), 0.6‰ DMSO, or
10 µM Y-27632 were added. After 24 and 48 h, the cell
morphology was observed (10 µM Y-27632 was detected after 48
h), and the expression of E-cadherin, N-cadherin, α-SMA, ZO-1, and
Na+/K+-ATPase were evaluated by
immunofluorescence staining experiments. The expression of ROCK was
detected by western blot analysis, and the mRNA expression of
Snail was detected by RT-PCR.
Statistical analysis
All data are presented as the means ± SD and
analyzed using SPSS 13.0 software. Comparisons between groups were
processed by one-way analysis of variance. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
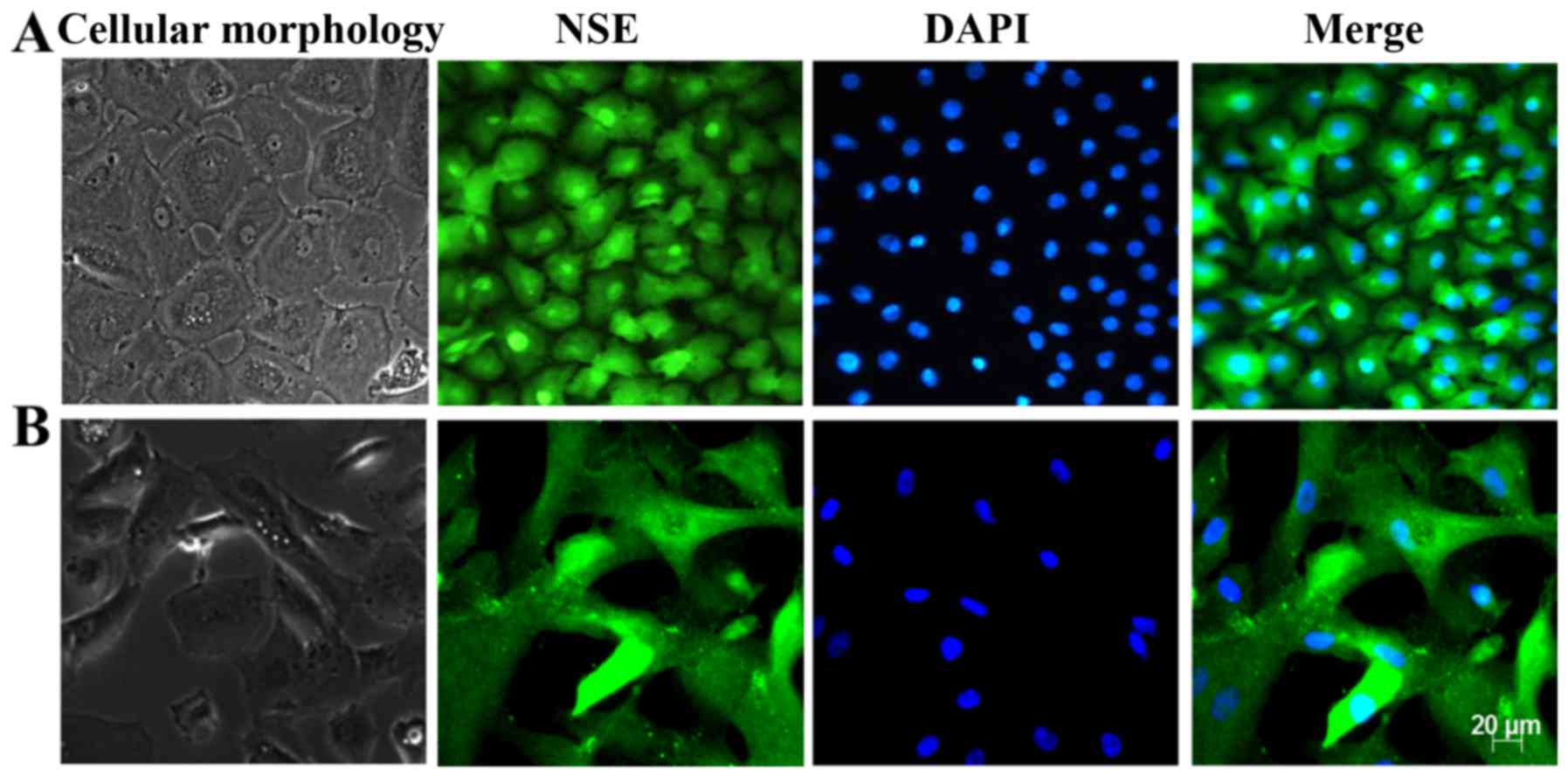
Identification of HCECs
Primary and subcultured CECs were identified by
observing the cell morphology using a light microscope and
immunofluorescence staining of the CEC marker NSE. The results are
presented in Fig. 1. Under a
light microscope, the cells exhibited a regular hexagonal or
polygonal shape, the size was homogeneous and the arrangement was
dense. In addition, the cells were homogeneously stained with the
NSE antibody, and the hexagonal morphology of the cells was clear.
These results revealed that these cells displayed the
characteristics of HCECs.
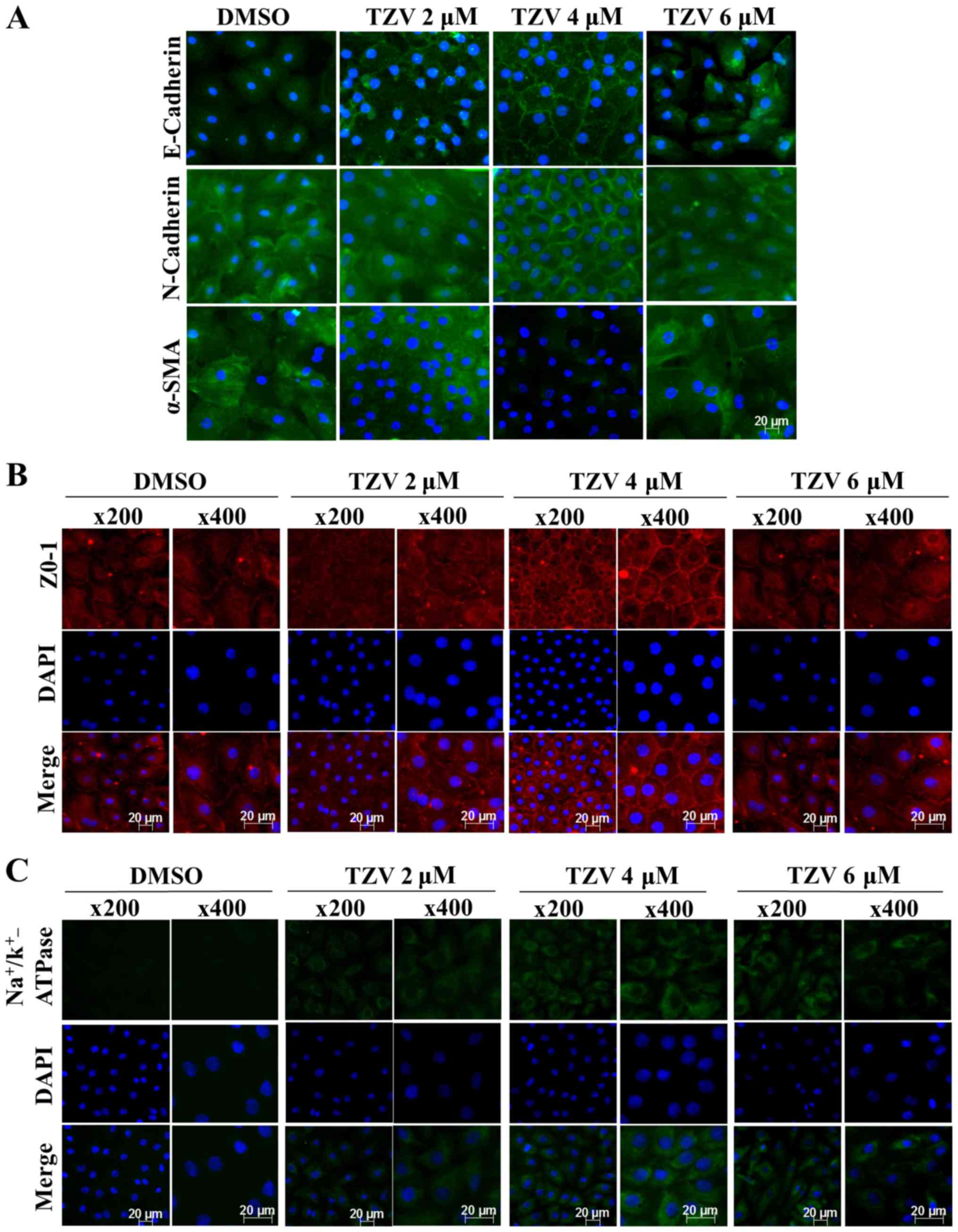
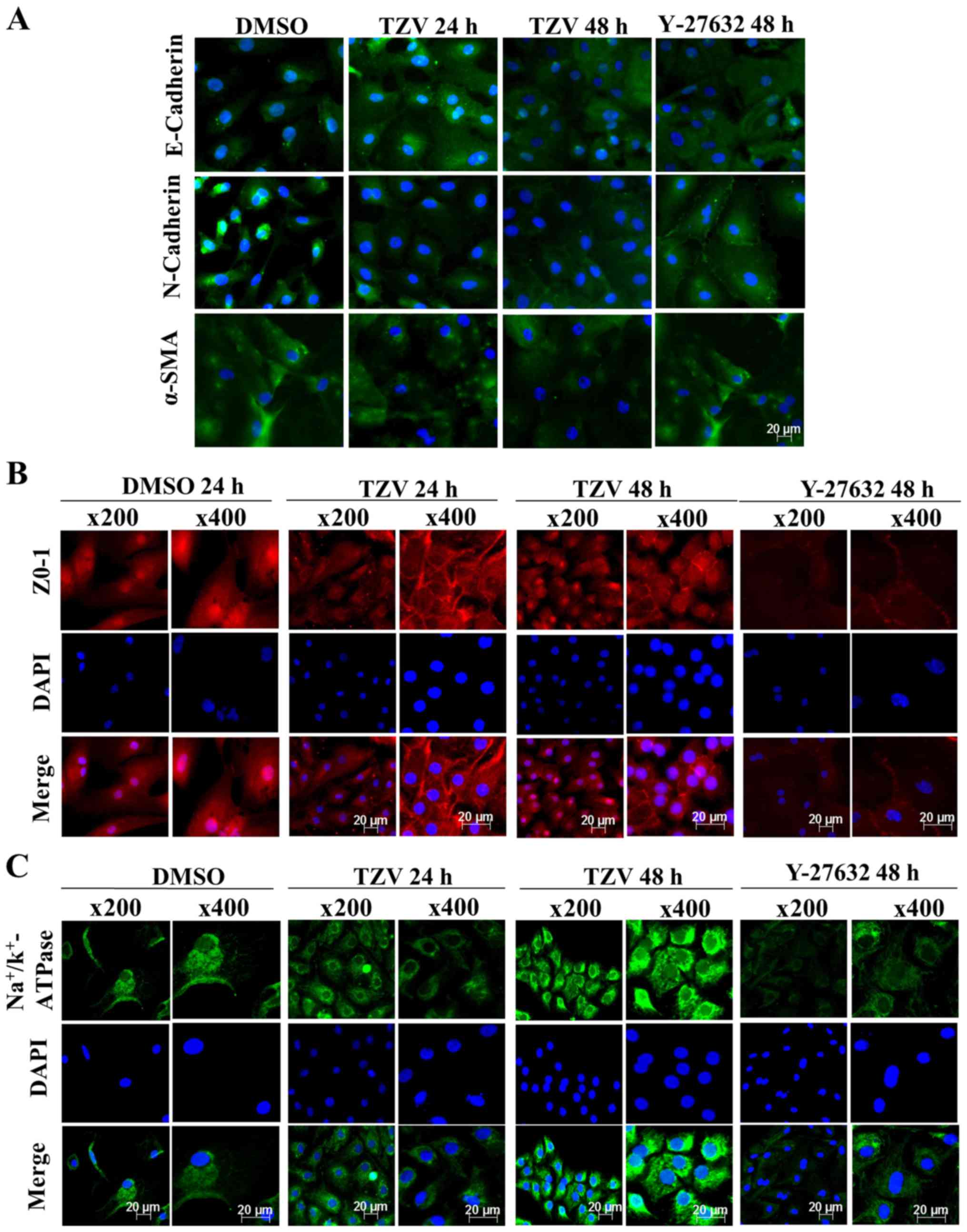
Screening of the optimal drug
concentration of TZV
Fig 2 presents the
effect of TZV on primary HCEC adherens junction proteins
(E-cadherin and N-cadherin), the EndMT marker, α-SMA, a tight
junction protein (ZO-1) and an ionic transporter expression protein
(Na+/K+-ATPase). The immunofluorescence
results of E-cadherin (Fig. 2A)
demonstrated that the cell density of the HCECs without the
addition of TZV (0.6‰ DMSO control) was relatively lower than that
following the addition of TZV. Furthermore, the fluorescence of
E-cadherin was weaker, the cell morphology exhibited round or
polygonal shapes, and there were no tight junctions between most
cells. However, following the addition of TZV, the fluorescence
expression increased. When the drug concentration was 2 µM,
the cell number increased compared with that in the DMSO control
group; however, the changes in morphology were not obvious. When
the drug concentration increased to 6 µM, the cell volume
increased, and some cells exhibited a long spindle shape and a
fibroblast-like phenotype. Following the addition of 4 µM
TZV for 24 h, the fluorescence expression of E-cadherin was
stronger, and the cells exhibited a tightly connected hexagonal or
pentagonal morphology. N-cadherin was highly expressed in the HCECs
and localized to the cell boundary, which could stabilize the
junction between cells to maintain the normal hexagonally shaped
HCEC cell structure. Thus, it appears that among the two adherens
junction protein in HCECs, N-cadherin plays a more important role
than E-cadherin (6). When 4
µM TZV and HCECs were co-cultured for 24 h, compared with
the DMSO group and other drug concentration groups, the
fluorescence expression of N-cadherin and α-SMA was weaker, the
HCEC volume was smaller, the cell junction was dense and clear, and
the cell morphology exhibited hexagonal or pentagonal shapes. When
the drug concentration was 2 µM, compared with the DMSO
group, the changes in cell morphology and cell number were not
obvious, and cell junctions were not observed. When the TZV
concentration increased to 6 µM, the cell volume
significantly increased; most cells exhibited a long spindle shape
and showed a tendency toward fibrosis; furthermore, the N-cadherin
and α-SMA expression increased, paralleling the trend of E-cadherin
expression. After 24 h of 4 µM TZV treatment,
immunofluorescence analysis of ZO-1 expression revealed that the
cell density increased, the cell morphology was hexagonal or
pentagonal shape, the ZO-1 expression level was higher, and the
cell junctions were denser and clearer than those in the other
control groups (Fig. 2B). When
the drug concentration continuously increased, the cells exhibited
spindle-shape changes. In addition, Fig. 2C presents the expression of
Na+/K+-ATPase in the HCECs following
treatment with various concentrations of TZV. As shown in Fig. 2C, following treatment with
gradient concentrations of TZV at 2, 4 and 6 µM in the HCECs
for 24 h, the fluorescence expression of
Na+/K+-ATPase significantly increased when
TZV was at 4 µM. The above-mentioned results indicated that
4 µM TZV had the optimal effects on cell morphology, cell
junction, cell density and pump junction in primary HCECs.
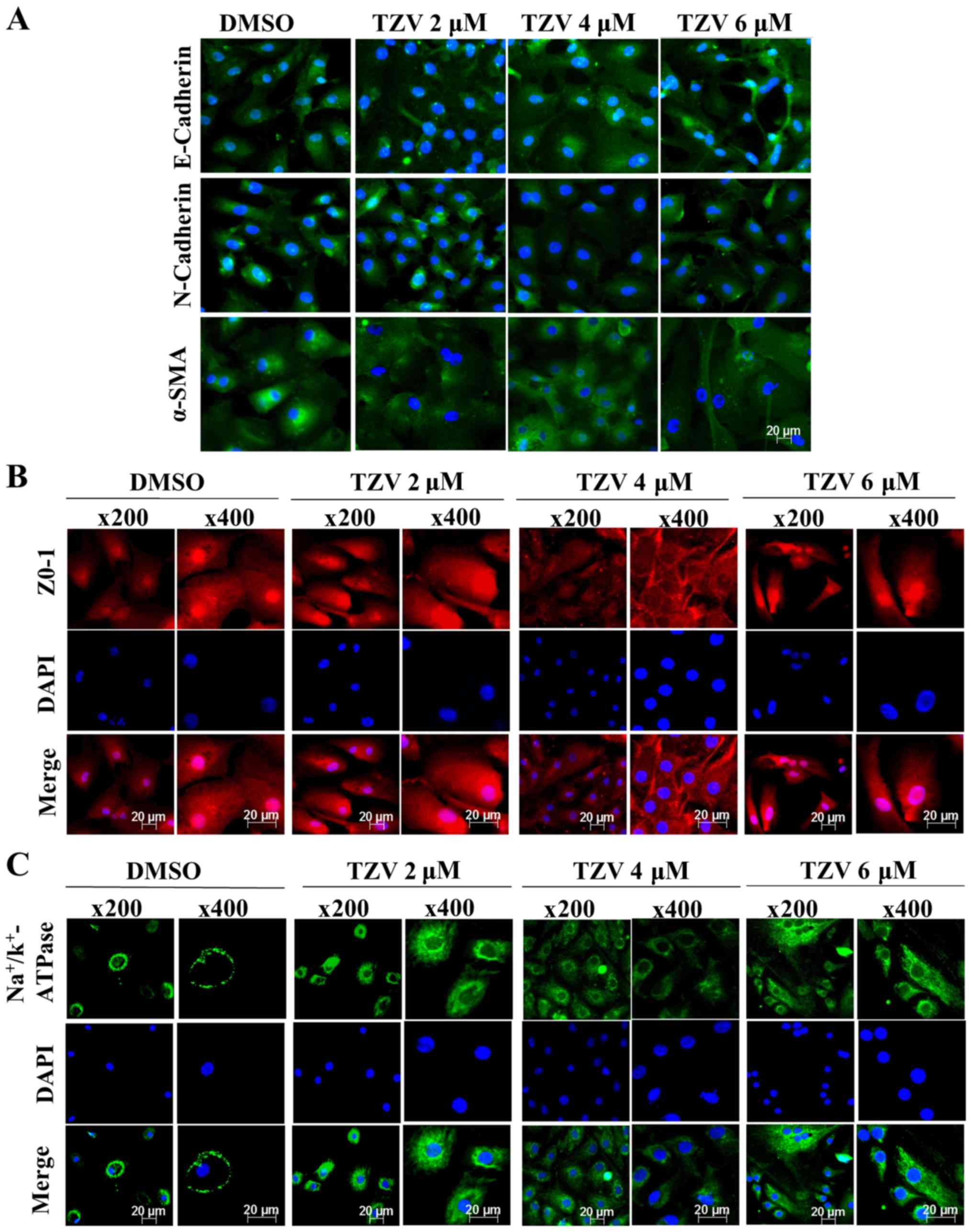
Fig. 3 presents
the immunofluorescence results of E-cadherin, N-cadherin, α-SMA,
ZO-1, and Na+/K+-ATPase in passaged HCECs.
Compared with the primary HCECs, most of the passaged HCECs were
spindle-shaped or polygonal-shaped, with no or few tight junctions
between the cells. However, when 4 µM TZV and passaged HCECs
were co-cultured for 24 h, the cell volume decreased, the
morphology was close to a round or polygonal shape, the junctions
between the cells increased, and the cell ionic transporter
expression was at the optimal status compared with the findings in
the other control groups.
Therefore, these results revealed that compared with
the other concentrations of TZV (2 and 6 µM), with
co-culture of 4 µM TZV and HCECs, the state of HCECs was
maintained at the optimum.
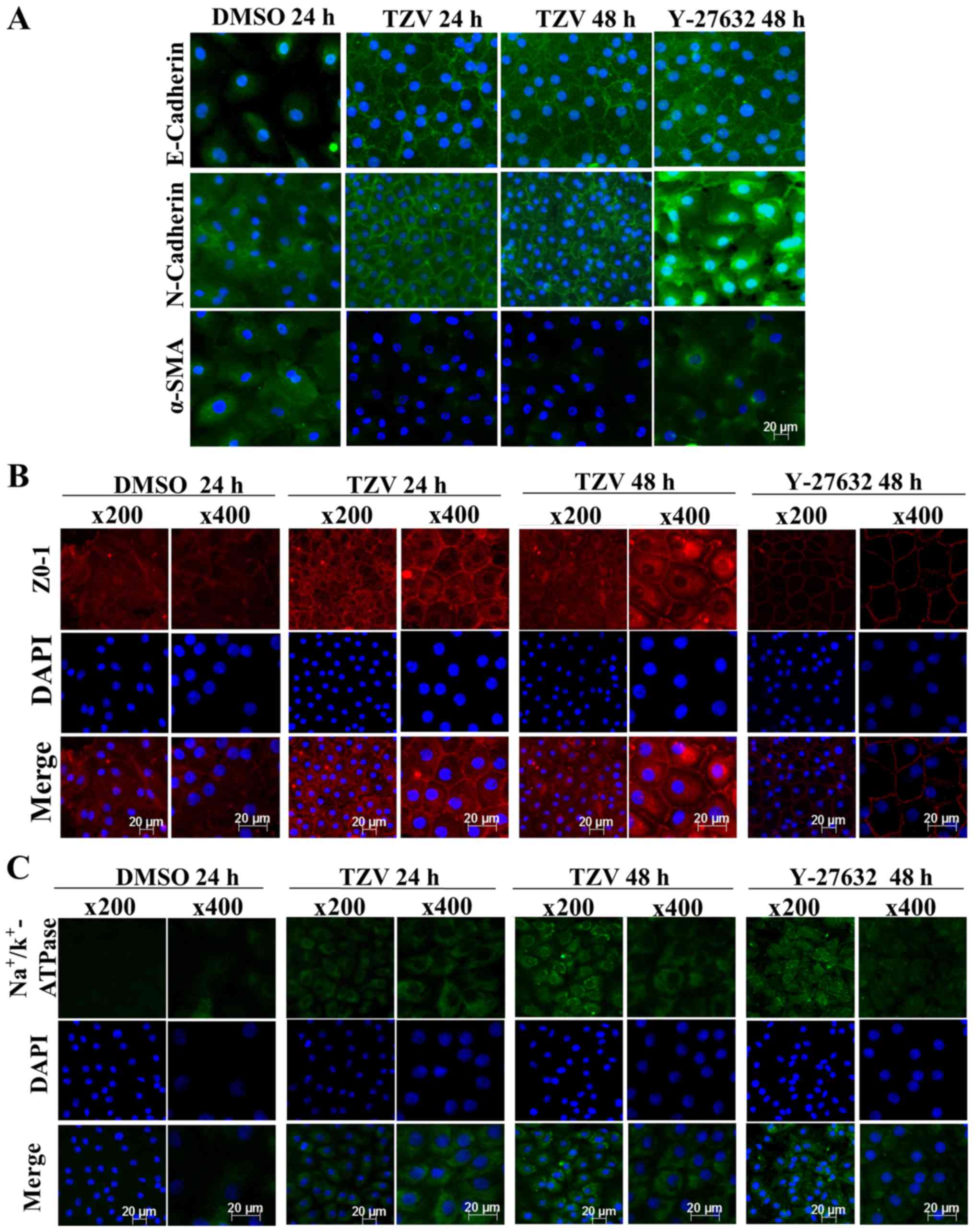
Screening of the optimal action time of
TZV
Fig. 4 presents
the immunofluorescence results of adherens junction proteins
(E-cadherin and N-cadherin), the EndMT marker, α-SMA, a ZO-1 and an
ionic transporter expression protein
(Na+/K+-ATPase) after the HCECs were treated
with the optimal drug concentration of TZV (4 µM) for
different periods of time (24 and 48 h). Following treatment with 4
µM TZV for 24 h, the HCECs were tightly and regularly
arranged, the cell junctions were clear, and the cell morphology
exhibited an even hexagonal arrangement. With the extension of
time, following treatment with TZV for 48 h, the morphology of the
HCECs improved further. Fig. 5
presents the effects of 4 µM TZV at different periods of
time (24 and 48 h) on the expression of E-cadherin, N-cadherin,
α-SMA, ZO-1 and Na+/K+-ATPase in the passaged
HCECs. Similarly, following treatmetn with TZV for 48 h, the HCECs
reached the optimal state. Therefore, the above-mentioned results
indicate that for junction proteins and ionic transporter
expression, the optimal effective action time of TZV on primary and
passaged HCECs was 48 h.
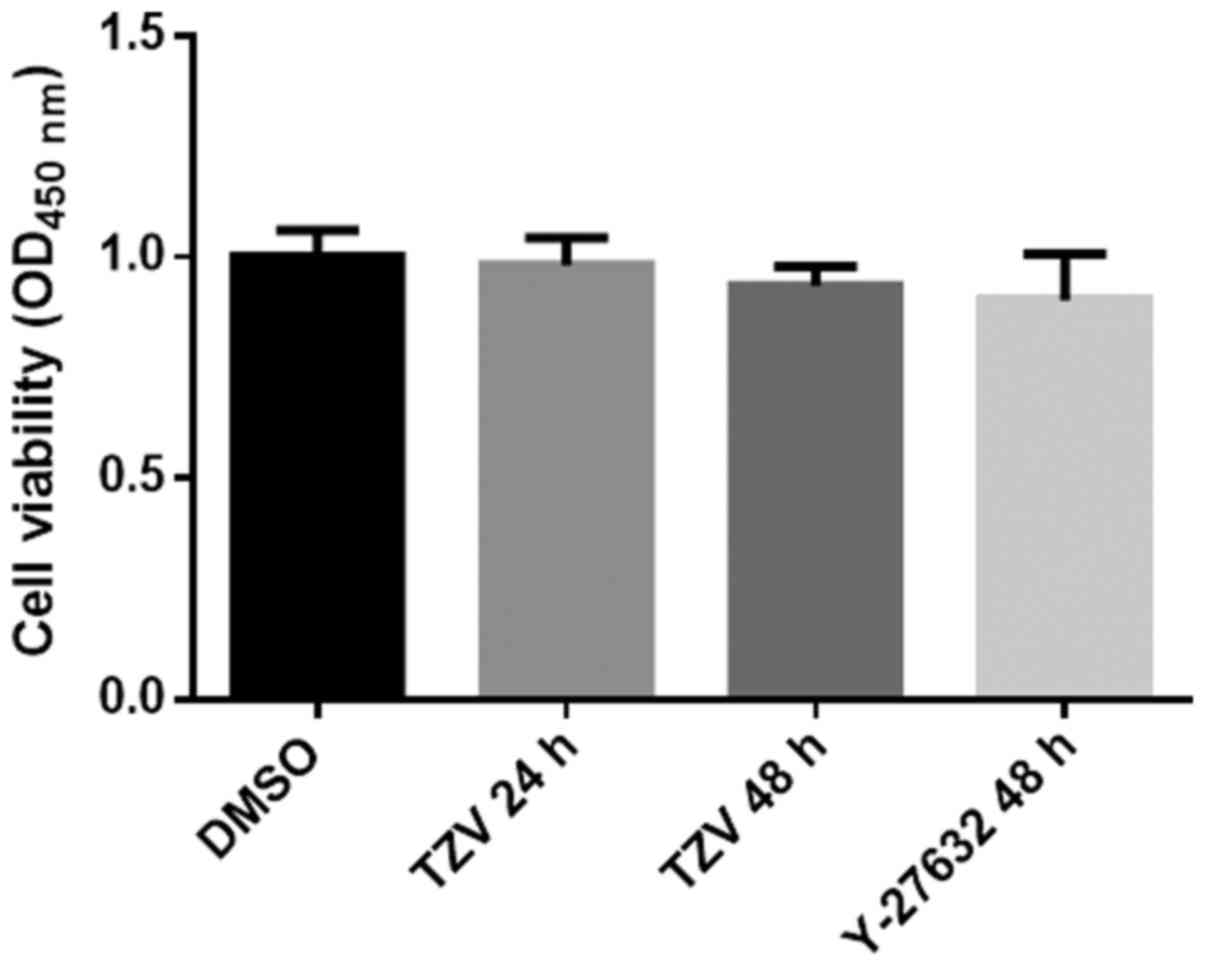
Effects of TZV on HCEC proliferation
Fig. 6 presents
the effects of TZV on the proliferation of HCECs. As shown in
Fig. 6, we found that compared
with the DMSO group and the positive control (Y-27632 group), there
was no significant difference in the TZV-treated group. The results
from CCK-8 assay revealed that TZV had no effect on HCEC
proliferation.
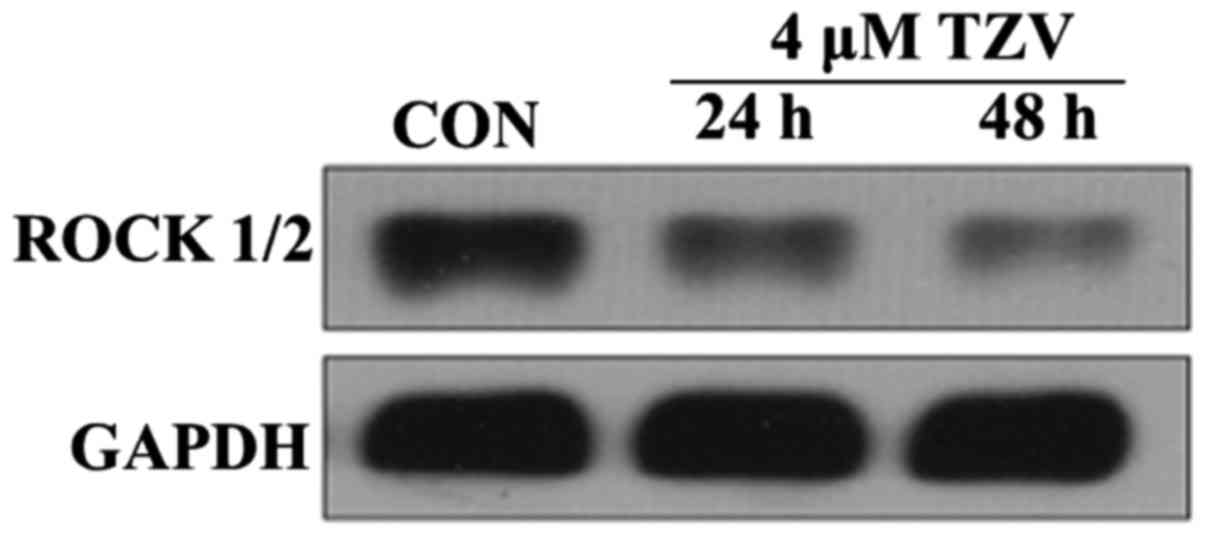
Effects of TZV on ROCK
ROCK belongs to the serine/threonine protein kinase
family. ROCK is primarily distributed in the cytoplasm and
functions to adjust the motility of cytoskeleton components. The
high expression and over-activation of ROCK can cause disease
development (22). In this study,
the effects of TZV on ROCK expression were observed using western
blot analysis. Fig. 7 presents
the protein expression of ROCK1/2 in the HCECs cultured with 4
µm TZV for different periods of time. The results revealed
that after the HCECs were treated with TZV for 24 and 48 h, the
expression of ROCK1/2 significantly decreased. In addition, we
found that TZV treatment for 48 h led to a much lower expression of
ROCK1/2, compared with TZV treatment for 24 h. These results
indicate that TZV indeed effectively inhibited ROCK protein
expression in HCECs.
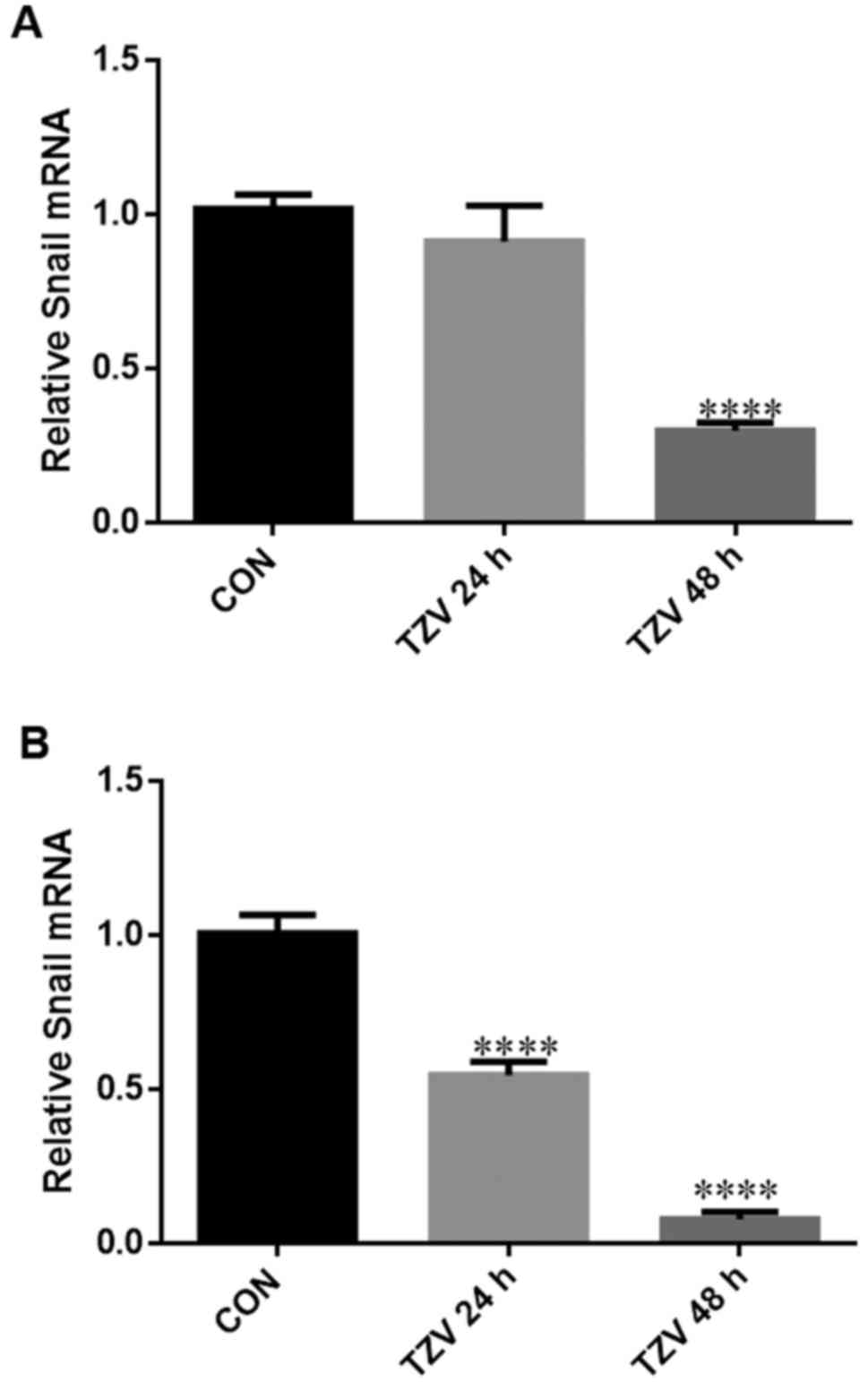
TZV inhibits EndMT/EMT in HCECs
Fig. 8 presents
the effects of TZV on the mRNA expression level of the EndMT/EMT
regulatory gene, Snail. The expression of the EMT-inducing
factor, Snail, can cause the disruption of cell junctions,
which is an important marker for transition into a mesenchymal
cell-like status (23,24). After the primary and passaged
HCECs were treated with 4 µM TZV for 24 and 48 h, the
expression level of Snail was detected by RT-PCR. Compared
with the DMSO group, 4 µM TZV significantly inhibited
Snail expression after primary culture of the HCECs for 48 h
(P<0.001), and no significant difference was observed after the
HCECs were cultured with TZV for 24 h (Fig. 8A). As shown in Fig. 8B, we found that the passaged HCECs
cultured with 4 µM TZV for 24 and 48 h exhibited significant
differences compared with the control.
Discussion
The morphological integrity and healthy function of
HCECs are clinical indicators of corneal function. To date, at
least to the best of our knowledge, there are no reports on the
effects of the ROCK inhibitor, TZV, on the cell morphology and
ionic transporter expression in HCECs. ROCK can regulate the
synthesis, degradation, motility and contraction of cytoskeleton
proteins and participates in the basic life cycle of cells, thus
playing important roles in cell junctions, morphology and motility
(25,26). Specifically, ROCK can regulate
actin stress fiber assembly and cell contraction via the
phosphorylation of its downstream molecules, myosin light chain,
myosin light chain phosphatase, ezrin-radixin-moesin proteins and
LIM kinase (27), and can form
local cell junctions via the phosphorylation of
Na+/H+ exchanger 1 (NHE1) (28). Furthermore, in addition to
regulating cytoskeleton remodeling and cell migration, ROCK
signaling pathways are also closely associated with gene
transcription, the promotion of cell cycle progression of G1 cells,
and cell apoptosis (29,30). Currently, ROCK inhibitors have
been extensively applied in a variety of pathological studies,
including cancer, neurodegeneration, kidney failure, asthma,
glaucoma, osteoporosis, erectile dysfunction, and insulin
resistance (31). For example,
the ROCK inhibitor fasudil is on the Japanese market for the
treatment of cerebral ischemic diseases. Currently, fasudil has
been applied in more than 124,000 cases (32). In the field of ophthalmology, some
reports suggest that the selective ROCK inhibitor Y-27632 can
promote cell scratch repair in animal (cynomolgus monkey) CECs
(33–35). The ROCK inhibitor Y-39983 can be
used to treat glaucoma and has entered clinical trials (36).
In this study, to the best of our knowledge, for the
first time, we examined the effects of in vitro co-culture
of gradient concentrations of the ROCK inhibitor TZV with primary
and passaged HCECs to observe changes in cell density, cell
morphology, cell junctions, and ionic transporter expression of
HCECs and identified the optimal drug concentration and time course
of action of TZV in in vitro cultured HCECs. After either
primary HCECs or passaged HCECs were co-cultured with 4 µM
TZV for 48 h, corneal EndMT/EMT was inhibited, as evidenced by the
significant upregulation of the expression of the ZO-1 and adhesion
protein E-cadherin and the obvious downregulation of N-cadherin
expression. This phenomenon of simultaneous upregulation of
E-cadherin expression and downregulation of N-cadherin expression
is known as the cadherin switch (12,37); additionally, the mRNA expression
levels of the EndMT/EMT-inducing gene snail are
downregulated. Furthermore, cells in EndMT/EMT can particularly
express α-SMA, which is often used as an EndMT marker in CECs
(16). In this study, the
expression levels of the EndMT marker protein α-SMA (38) was downregulated in response to the
action of TZV at the above mentioned drug concentration and time
course of action; in addition, the cell density increased, the cell
volume decreased, tight junctions between the cells were enhanced
and became clear, the cell morphology became hexagonal or
pentagonal, and the expression of cellular ionic transporter
expression protein Na+/K+-ATPase
increased.
In conclusion, the novel ROCK inhibitor, TZV (4
µM), was effective in improving the morphology, cell
junctions, and ionic transporter expression of HCECs via inhibiting
EndMT/EMT but had no effect on HCEC proliferation. In the above
described experiments, we determined a method with which to block
EndMT in vitro while enhancing the expansion of CECs. More
importantly, we observed that the number of primary HCECs acquired
from the treatment of donor corneal rings left from human corneal
transplantation with type IV collagenase expanded 10-fold. In
addition, these primary cells exhibited significant sensitivity to
TZV. Therefore, we can use these cells to engineer a functional
corneal endothelium. Moreover, for the drug application of TZV, we
are inclined to perform direct anterior chamber injection of the
drugs at the optimal concentration to improve the quality of
endothelial cells in the early stage of decompensation. These
experiments require further study at the organismal level.
Acknowledgments
All authors are grateful for the financial support
from the National Natural Science Foundation of China (no.
81371065), and acknowledge the support given by the Zhongshan
Ophthalmic Center, Sun Yat-sen University for using the country's
key laboratory and thank the Eye Bank of Guangdong Province for
providing the corneal limbi left from corneal transplantation
surgery.
References
|
1
|
Mishima S: Clinical investigations on the
corneal endothelium-XXXVIII Edward Jackson Memorial Lecture. Am J
Ophthalmol. 93:1–29. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wulle KG: Electron microscopy of the fetal
development of the corneal endothelium and Descemet's membrane of
the human eye. Invest Ophthalmol. 11:897–904. 1972.PubMed/NCBI
|
|
3
|
Laing RA, Sanstrom MM, Berrospi AR and
Leibowitz HM: Changes in the corneal endothelium as a function of
age. Exp Eye Res. 22:587–594. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh JS, Haroldson TA and Patel SP:
Characteristics of the low density corneal endothelial monolayer.
Exp Eye Res. 115:239–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Z, Campolmi N, Gain P, Ha Thi BM,
Dumollard JM, Duband S, Peoc'h M, Piselli S, Garraud O and Thuret
G: Revisited microanatomy of the corneal endothelial periphery: New
evidence for continuous centripetal migration of endothelial cells
in humans. Stem Cells. 30:2523–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuda M, Sawa M, Edelhauser HF, Bartels
SP, Neufeld AH and Kenyon KR: Cellular migration and morphology in
corneal endothelial wound repair. Invest Ophthalmol Vis Sci.
26:443–449. 1985.PubMed/NCBI
|
|
7
|
Edelhauser HF: The resiliency of the
corneal endothelium to refractive and intraocular surgery. Cornea.
19:263–273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan DTH, Dart JKG, Holland EJ and
Kinoshita S: Corneal transplantation. Lancet. 379:1749–1761. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirata-Tominaga K, Nakamura T, Okumura N,
Kawasaki S, Kay EP, Barrandon Y, Koizumi N and Kinoshita S: Corneal
endothelial cell fate is maintained by LGR5 through the regulation
of hedgehog and Wnt pathway. Stem Cells. 31:1396–1407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peh GS, Toh KP, Wu FY, Tan DT and Mehta
JS: Cultivation of human corneal endothelial cells isolated from
paired donor corneas. PLoS One. 6:e283102011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okumura N, Kay EP, Nakahara M, Hamuro J,
Kinoshita S and Koizumi N: Inhibition of TGF-β signaling enables
human corneal endothelial cell expansion in vitro for use in
regenerative medicine. PLoS One. 8:e580002013. View Article : Google Scholar
|
|
12
|
Engelmann K and Friedl P: Optimization of
culture conditions for human corneal endothelial cells. In Vitro
Cell Dev Biol. 25:1065–1072. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu C and Joyce NC: Proliferative response
of corneal endothelial cells from young and older donors. Invest
Ophthalmol Vis Sci. 45:1743–1751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engelmann K, Bednarz J and Valtink M:
Prospects for endothelial transplantation. Exp Eye Res. 78:573–578.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McNiven MA: Breaking away: Matrix
remodeling from the leading edge. Trends Cell Biol. 23:16–21. 2013.
View Article : Google Scholar
|
|
16
|
Roy O, Leclerc VB, Bourget JM, Thériault M
and Proulx S: Understanding the process of corneal endothelial
morphological change in vitro. Invest Ophthalmol Vis Sci.
56:1228–1237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JG, Ko MK and Kay EP: Endothelial
mesenchymal transformation mediated by IL-1β-induced FGF-2 in
corneal endothelial cells. Exp Eye Res. 95:35–39. 2012. View Article : Google Scholar
|
|
18
|
Pipparelli A, Arsenijevic Y, Thuret G,
Gain P, Nicolas M and Majo F: ROCK inhibitor enhances adhesion and
wound healing of human corneal endothelial cells. PLoS One.
8:e620952013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu L, Gao Q, Ni L, Wang M and Shen F:
Fasudil inhibits epithelial-myofibroblast transdifferentiation of
human renal tubular epithelial HK-2 cells induced by high glucose.
Chem Pharm Bull. 61:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park S, Kim D, Jung YG and Roh S:
Thiazovivin, a Rho kinase inhibitor, improves stemness maintenance
of embryo-derived stem-like cells under chemically defined culture
conditions in cattle. Anim Reprod Sci. 161:47–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang H, Liu Z, Ma Y, Zhong C, Yin Q, Zhou
C, Shi L, Cai Y, Zhao H, Wang H, et al: Generation of haploid
embryonic stem cells from Macaca fascicularis monkey parthenotes.
Cell Res. 23:1187–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka T, Nishimura D, Wu RC, Amano M, Iso
T, Kedes L, Nishida H, Kaibuchi K and Hamamori Y: Nuclear Rho
kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem.
281:15320–15329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang RY, Guilford P and Thiery JP: Early
events in cell adhesion and polarity during epithelial-mesenchymal
transition. J Cell Sci. 125:4417–4422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaibuchi K, Kuroda S and Amano M:
Regulation of the cytoskeleton and cell adhesion by the Rho family
GTPases in mammalian cells. Annu Rev Biochem. 68:459–486. 1999.
View Article : Google Scholar
|
|
26
|
Somlyo AP and Somlyo AV: Signal
transduction by G-proteins, rho-kinase and protein phosphatase to
smooth muscle and non-muscle myosin II. J Physiol. 522:177–185.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cytoskeleton. Mol Cell
Biol. 16:5313–5327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tominaga T and Barber DL: Na-H exchange
acts downstream of RhoA to regulate integrin-induced cell adhesion
and spreading. Mol Biol Cell. 9:2287–2303. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac, and Cdc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Valdez JM, Zhang B, Wei L, Chang
J and Xin L: ROCK inhibitor Y-27632 suppresses dissociation-induced
apoptosis of murine prostate stem/progenitor cells and increases
their cloning efficiency. PLoS One. 6:e182712011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao JK, Seto M and Noma K: Rho kinase
(ROCK) inhibitors. J Cardiovasc Pharmacol. 50:17–24. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okumura N, Ueno M, Koizumi N, Sakamoto Y,
Hirata K, Hamuro J and Kinoshita S: Enhancement on primate corneal
endothelial cell survival in vitro by a ROCK inhibitor. Invest
Ophthalmol Vis Sci. 50:3680–3687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okumura N, Koizumi N, Ueno M, Sakamoto Y,
Takahashi H, Hirata K, Torii R, Hamuro J and Kinoshita S:
Enhancement of corneal endothelium wound healing by Rho-associated
kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 95:1006–1009.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okumura N, Koizumi N, Kay EP, Ueno M,
Sakamoto Y, Nakamura S, Hamuro J and Kinoshita S: The ROCK
inhibitor eye drop accelerates corneal endothelium wound healing.
Invest Ophthalmol Vis Sci. 54:2493–2502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tokushige H, Inatani M, Nemoto S, Sakaki
H, Katayama K, Uehata M and Tanihara H: Effects of topical
administration of y-39983, a selective rho-associated protein
kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest
Ophthalmol Vis Sci. 48:3216–3222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piera-Velazquez S and Jimenez SA:
Molecular mechanisms of endothelial to mesenchymal cell transition
(EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis
Tissue Repair. 5(Suppl 1): S72012.PubMed/NCBI
|