Introduction
Melanocytes, melanin-producing cells, originate from
embryonic neural crest cells (NCCs) (1). They are found in the epidermis, hair
and iris, but are also distributed in the nervous system, inner ear
and heart (2,3). Other types of cells able to produce
melanin are cells of the retinal pigmented epithelium, the
epithelia of the iris and the ciliary body in the eye, as well as
adipocytes and some neurons (4,5).
The most important inducer of melanogenesis is the
α-melanocyte-stimulating hormone (α-MSH), which exerts its effects
through melanocortin receptor 1(MC1R) (6). Melanin biosynthesis is catalyzed by
tyrosinase as the rate-limiting enzyme, together with
tyrosinase-related proteins 1 and 2 (7). The main transcriptional activator of
these melanogenic enzymes is the microphthalmia transcription
factor, melanogenesis-associated transcription factor (MITF)
(8), the activity of which may be
regulated by several signaling pathways. It is well known that the
phosphoinositide 3-kinase (PI3K)/Akt pathway negatively regulates
the expression of MITF (9), while
the activation of extracellular signal-regulated kinase (ERK)
phosphorylates MITF at Ser73, thus leading to its ubiquitination
and degradation (10).
The regulation of melanogenesis in cutaneous
melanocytes has been extensively investigated (11–13); however, little is known about the
role of α-MSH and MC1R in the growth of human uveal melanocytes and
melanin production. It has been reported that several cell lines of
iridal origin or choroidal melanocytes from human donor eyes
exhibit no detectable amount of MC1R and show no response to α-MSH
treatment, independently from iris color (14). These results suggest a
differential regulation of melanogenesis in the area of the eye and
may explain the difference in the in vivo functions between
cutaneous and uveal melanocytes, such as the tanning response
following exposure to solar radiation, that is evident in skin, but
not in melanocytes from the iris.
Although it may seem that melanin production is
hardly regulated in the adult eye, it has been demonstrated that
prostaglandin analogs used for the treatment of elevated
intraocular pressure in patients with primary open-angle glaucoma
may cause an increase in pigmentation in periocular skin and a
spotted iris hyperpigmentation (15).
It has been demonstrated that melanin synthesis can
be modulated by various effectors, including mangosteen
(Garcinia mangostana) leaf extract (16), baicalein (one of the major
flavonoids in Scutellaria baicalensis) (17), Wnts (a large family of secreted
glycoproteins that act as ligands to activate receptor-mediated
signaling pathways) (18),
extract of Angelicae Gigantis Radix (19) and lactoferricin B (a peptide of
bovine lactoferrin) (20).
Several molecules are known to be endowed with lightening activity
on cutaneous melanocytes (21),
whilst currently no molecules with a similar activity on ocular
melanocytes have been described, at least to the best of our
knowledge. Argan oil, for instance, is derived from the fruit of
Argania spinosa and is widely used in folk medicine and in
cosmetics for the treatment of eczema, psoriasis, skin
inflammation, to heal burns and wounds, to cure brittle
fingernails, and to prevent hair loss and dry hair. Recently, it
has been shown that argan oil exerts a depigmenting effect in
vitro on mouse cutaneous melanoma cells (22).
Therefore, in this study, we used argan oil to
examine melanin biosynthesis and its regulation in iris-derived
melanocytes, using melanin content and tyrosinase as markers of
melanogenesis, and MITF, ERK1/2 and Akt as markers of its
regulation.
Materials and methods
Chemicals and antibodies
PD98059 (P215), wortmannin (W1628), LY294002 (L9908)
and CHAPS (C5849) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Goat polyclonal anti-tyrosinase antibody (Tyrosinase C-19;
cat. no. sc-7833) was from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA), rabbit polyclonal anti-phosphorylated MITF at Ser73
antibody (cat. no. SAB4503940) was from Sigma-Aldrich, mouse
monoclonal anti-β-actin antibody (cat. no. MA1-91399) was from
Thermo Fisher Scientific (Waltham, MA, USA), and rabbit monoclonal
anti-MITF (cat. no. 12590), rabbit polyclonal anti-phosphorylated
(cat. no. 9101) and total ERK (cat. no. 9102), rabbit polyclonal
anti phosphorylated (cat. no. 9271) and total Akt (cat. no. 9272)
antibodies were all from Cell Signaling Technology, Inc. (Beverly,
MA, USA). Forskolin was from Sigma-Aldrich (cat. no. F6886). Argan
oil (composition shown in Table
I) was purchased from Natural Sourcing LLC (Oxford, CT, USA)
and used as a fine emulsion in growth medium.
 | Table IComposition of argan oil used in the
experiments. |
Table I
Composition of argan oil used in the
experiments.
| Compounds | Amount (%) |
|---|
| Oleic acid | 43–55 |
| Linoleic acid | 30–38 |
| Palmitic acid | 11–16 |
| Stearic acid | 3.5–7 |
| Linolenic acid | ≤0.5 |
| Arachidonic
acid | ≤0.5 |
| Gadoleic acid | ≤0.5 |
| Others | ≤0.5 |
|
Unsaponifiables | 0.5–1.5 |
Cell culture
The B16-F1 mouse cutaneous melanoma cells were a
kind gift from Dr Vera Cardile (Department of BIOMETEC, University
of Catania, Catania, Italy); the cells were routinely cultured as
monolayers in Falcon Petri dishes (Lincoln Park, NJ, USA) in a
humidified incubator at 37°C with DMEM and 10% FCS. The 92.1 human
uveal melanoma cell line was purchased from the Cell Factory-IST
(Genoa, Italy) and grown in monolayer cultures in regular RPMI-1640
medium supplemented with 10% heat-inactivated FBS, 100 IU/ml
penicillin, 100 µg/ml streptomycin and 2 mmol/l of
L-glutamine in a humidified atmosphere (5% CO2/95% air)
at 37°C.
Cell viability
Monolayer cultures of 92.1 cells were seeded in
96-well plates at 104 cells/well in 0.1 ml of complete
culture medium in the absence or in presence of argan oil. To
determine cell viability, the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrasodium bromide (MTT)
assay was used (Chemicon, Temecula, CA, USA). At the end of the
treatment time, the cells in each well were incubated at 37°C with
additional 0.01 ml MTT (5 mg/ml) for 4 h; subsequently, 0.1 ml
isopropanol in the presence of 20 µl of 5% (w/v) sodium
dodecyl sulfate (SDS) in water was added and, after 1 h, the
absorbance was measured at 570 nm in a plate reader (VarioSkan;
Thermo Fisher Scientific) (23,24).
Melanin content
Melanin content was determined as previously
described (25). Briefly, the
92.1 and B16-F1 cells were seeded in 6-well plates at a cell
density of 1×105 cells/well and 2×105
cells/well, respectively, and treated with either forskolin or
argan oil at various concentrations and for different periods of
time, as indicated in the figure legends. The MAP kinase inhibitor,
PD98059 (25 µM), or the PI3K/Akt inhibitors, wortmannin (50
nM) and LY294002 (20 µM), were incubated with the cells in
the absence or presence of forskolin (50 µM) for 48 h.
Following incubation, the medium was removed, and the cells were
mechanically detached and pelleted. Melanin in cell pellets was
dissolved in 1 M NaOH/10% DMSO by boiling for 1 h. Determination of
the melanin content was carried out by optical reading at 490 nm
and the relative melanin quantity was normalized to the protein
concentration of each sample, measured by BCA protein assay (BCA
protein assay kit; cat. no. sc-202389; Santa Cruz Biotechnology,
Inc.).
Western blot analysis
The cells were plated in 60 mm dishes at a cell
density of 2×105 cells/dish and treated with either
forskolin or argan oil at various concentrations and for different
periods of time, as indicated in the figure legends. In some
experiments, the MAP kinase inhibitor, PD98059 (25 µM), or
PI3K/Akt inhibitors, wortmannin (50 nM) and LY294002 (20
µM), were incubated with the cells in the absence or
presence of forskolin for 48 h. Following incubation, the cells
were lysed in the dish by CHAPS buffer cell lysis (5 mM
MgCl2, 1 mM EGTA, 100 mM NaCl, 10% Glicerol, 1% CHAPS,
25 mM Hepes, 1 mM sodium orthovanadate) and the protein
concentration was determined by BCA protein assay. Western blot
analysis was performed as previously described (26). The membranes were incubated with
primary antibodies (1:1,000, 4°C, overnight) and then incubated
with secondary antibodies rabbit anti-goat (sc-2768; Santa Cruz
Biotechnology, Inc.), donkey anti-rabbit (NA934V; Amersham), sheep
anti-mouse (NA931V; Amersham) (1:2,000, at room temperature for 1
h). The immunocomplexes were detected by enhanced chemiluminescence
reagent (ECL; GE Healthcare Life Sciences, Little Chalfont, UK).
All blots were standardized for equal loading by actin monoclonal
antibody staining.
Evaluation of tyrosinase and MITF gene
expression
The 92.1 uveal melanoma cells were plated in 60 mm
dishes at a density of 2×105 cells/dish and treated with
1/100 v/v argan oil in complete culture medium for 3 days with
daily changing of the emulsion. The cells were cultured for 2 more
days in the absence of argan oil to examine the reversibility of
the effect. Following incubation, RNA was extracted from the
monolayer cells using TRIzol reagent (Thermo Fisher Scientific)
according to the manufacturer's instruction and re-dissolved in 30
µl RNase-free water (27).
Reverse transcription-semi-quantitative PCR was conducted as
previously described. The RNA concentration and purity were
estimated by optical density at 260 and 280 nm (28). First-strand cDNA was reverse
transcribed in a 20 µl reaction volume with SuperScript III
(Thermo Fisher Scientific). The PCR reactions were performed using
Platinum PCR SuperScript (Thermo Fisher Scientific). Cycling
parameters were initial denaturing, 94°C for 2 min, denaturing,
94°C for 30 sec, annealing at specific temperature (55–56°C) for 40
sec, extension (72°C for 30 sec), and a final extension step at
72°C for 5 min. Specific primers for human MITF, tyrosinase and
β-actin genes were used in semi-quantitative RT-PCR. A 92 bp
product, using a forward primer (5′-AAGGCCAAGTGACACCAGCC-3′) and
reverse primer (5′-GAAACAGAGCAACGAGATGGG-3′), was amplified for
human tyrosinase. A 116 bp product, using a forward primer
(5′-AAGTGAACGTGTTCGAGAGG-3′) and reverse primer
(5′-GATGATGCGGCTGTGATGG-3′), was amplified for MITF. The primers
used for β-actin were forward, 5′-TAGACAAGATGGTGAAGG-3′ and
reverse, 5′-GCAGGGATGATGTTCTGG-3′ and their amplification product
was 650 bp.
Statistical analysis
The statistical significance between 2 groups was
analyzed using the Student's t-test. One-way analysis of variance
(ANOVA), followed by Tukey's post hoc test, was used to compare the
means for the multiple groups. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Tyrosinase expression and melanin content
in cutaneous and uveal melanoma cells: effects of specific
modulators
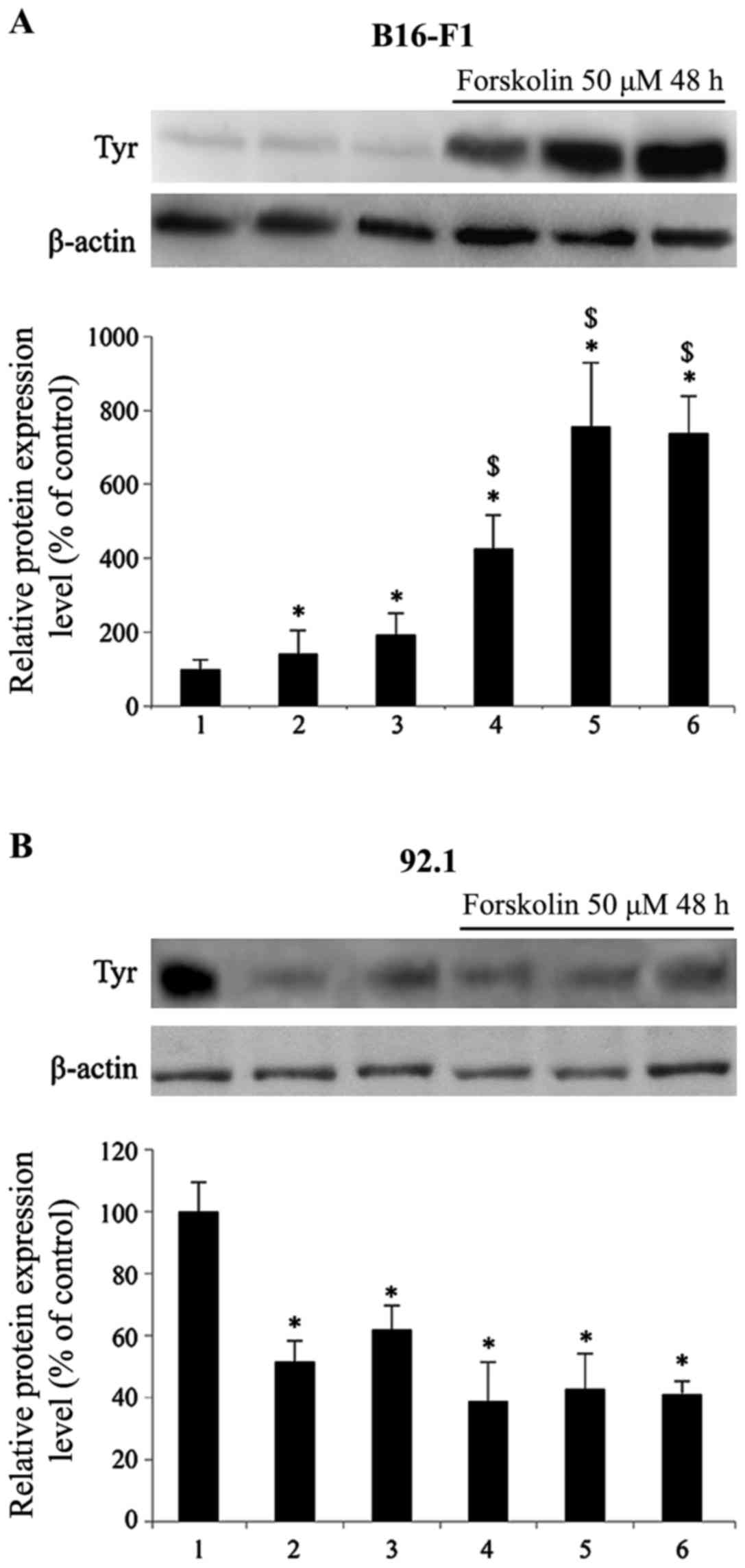
Fig. 1 illustrates
the response of B16-F1 murine cutaneous cells (Fig. 1A) and 92.1 human uveal melanoma
cells (Fig. 1B) following 48 h of
treatment with different compounds known to modulate melanogenesis
and tyrosinase expression, namely the adenyl cyclase and PKA
activator, forskolin (29), the
mitogen-activated protein (MAP) kinase inhibitor, PD98059 (30), and the PI3K/Akt inhibitors,
wortmannin and LY294002 (31),
are known inducers of melanogenesis and tyrosinase expression in
skin melanocytes. The results presented in Fig. 1A revealed that in the B16-F1 mouse
cutaneous melanoma cells, tyrosinase expression in the presence of
25 µM PD98059 and wortmannin (50 nM)/LY294002 (20 µM)
increased by 1.4- and 1.9- fold, respectively. The incubation of
the B16-F1 cells in the presence of 50 µM forskolin induced
a greater increase in tyrosinase expression by 4.3-fold in
comparison to the control cells. Incubation with forskolin in the
presence of PD98059 and wortmannin/LY294002 further increased
tyrosinase protein expression by 1.7-fold in comparison to the
cells treated with forskolin alone.
Surprisingly, the expression of tyrosinase in the
92.1 human uveal melanoma cells (Fig.
1B) significantly decreased following incubation with PD98059
and wortmannin/LY294002 by 48 and 38%, respectively, in comparison
to the control cells. Incubation with forskolin induced a greater
reduction in tyrosinase expression (by 61%) in comparison to the
control cells; this decrease was not further enhanced by the
simultaneous presence of PD98059 and wortmannin/LY294002,
indicating a differential regulation of enzyme expression in the
B16-F1 cells and 92.1 cells.
The results presented in Fig. 2 basically confirmed the
above-mentioned results, using melanin as a marker of
differentiation. As shown in Fig.
2A, incubation of the B16-F1 mouse cutaneous melanoma cells
with the adenyl cyclase activator, forskolin, increased melanin
production by >4-fold. The inhibition of ERK1/2 and PI3K/Akt
with the specific inhibitors increased the melanin content both in
the absence (by 1.5- and 2.6-fold in comparison to the control
cells) and in the presence of forskolin (by 1.6- and 1.8- fold in
comparison to the forskolin-treated cells). By contrast, in the
92.1 human uveal melanoma cells (Fig.
2B), the inhibition of ERK1/2 and PI3K/Akt led to a significant
decrease in the melanin content in the absence of forskolin (57 and
59%, respectively in comparison to the control cells). Treatment
with forskolin alone decreased the melanin content by 57% in
comparison to the control cells, and the inhibition of both ERK1/2
and PI3K/Akt in the forskolin-treated cells decreased the melanin
content by 47 and 55%, respectively.
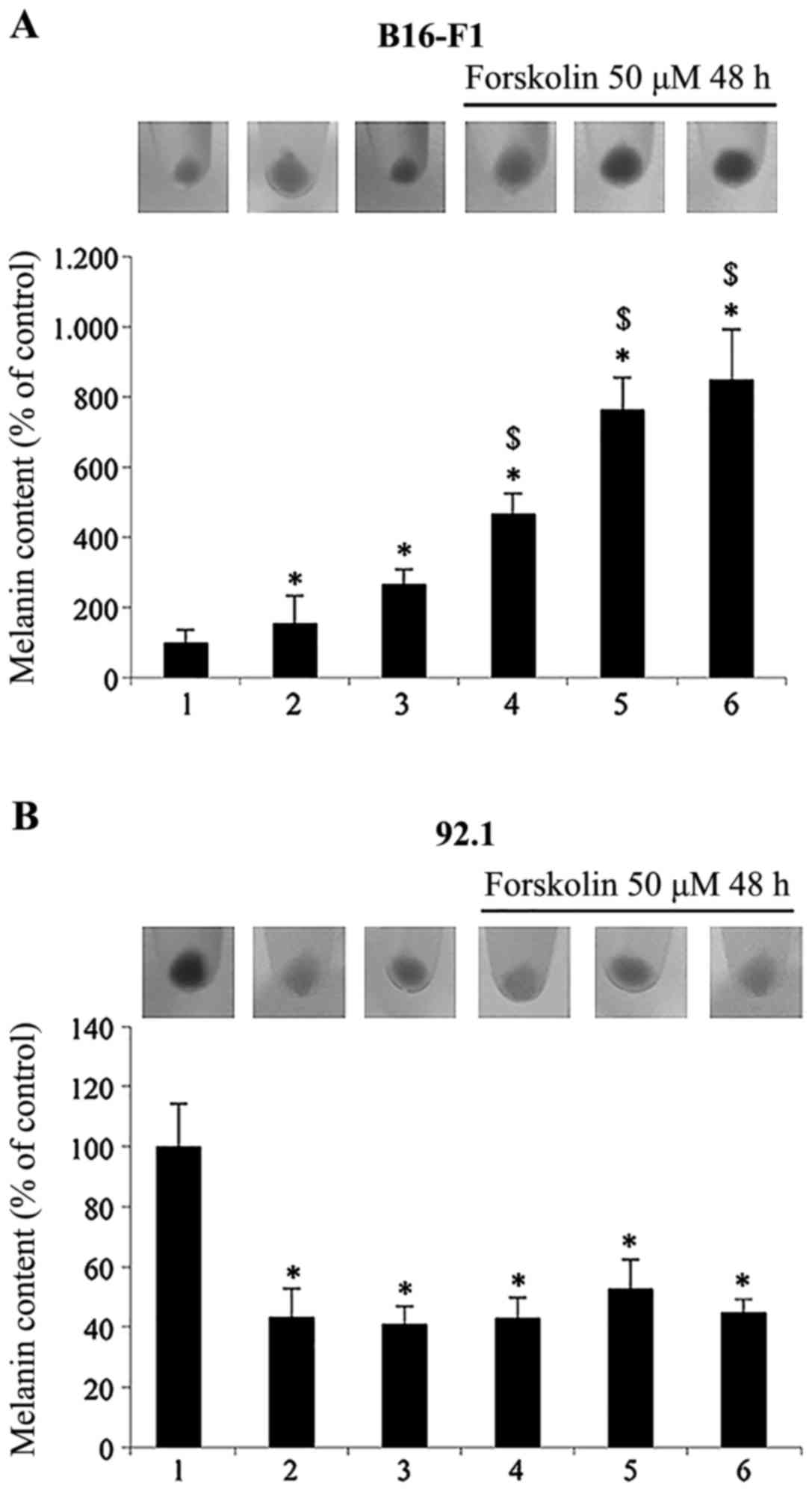 | Figure 2Determination of melanin content in
(A) B16-F1 mouse cutaneous melanoma cells and (B) 92.1 human uveal
melanoma cells. The cells were incubated with 25 µM of
PD98059 or wortmannin (50 nM) plus LY294002 (20 µM) in the
absence or presence of forskolin 50 µM for 48 h and melanin
content was measured. Values are the means ± SD from 3 independent
experiments (n=3) performed in triplicate. Statistically
significant differences by one-way ANOVA and Tukey's post hoc test
are indicated by symbols (*p<0.05 vs. control cells,
$p<0.05 vs. forskolin-treated cells). Results,
normalized with protein content of each well, are expressed as a
percentage of the control. The cell pellets, collected from the
cells incubated with the compounds for the indicated periods of
time, were photographed. The lanes and bars are labeled as follows:
1, control; 2, PD98059; 3, wortmannin plus LY294002; 4, forskolin;
5, forskolin plus PD98059; 6, forskolin plus wortmannin plus
LY294002. |
Taken together, these data clearly indicate the
differential regulation of tyrosinase expression by ERK1/2 and
PI3K/Akt in the two melanoma cell lines of different origin.
Effect of argan oil on 92.1 cell
viability
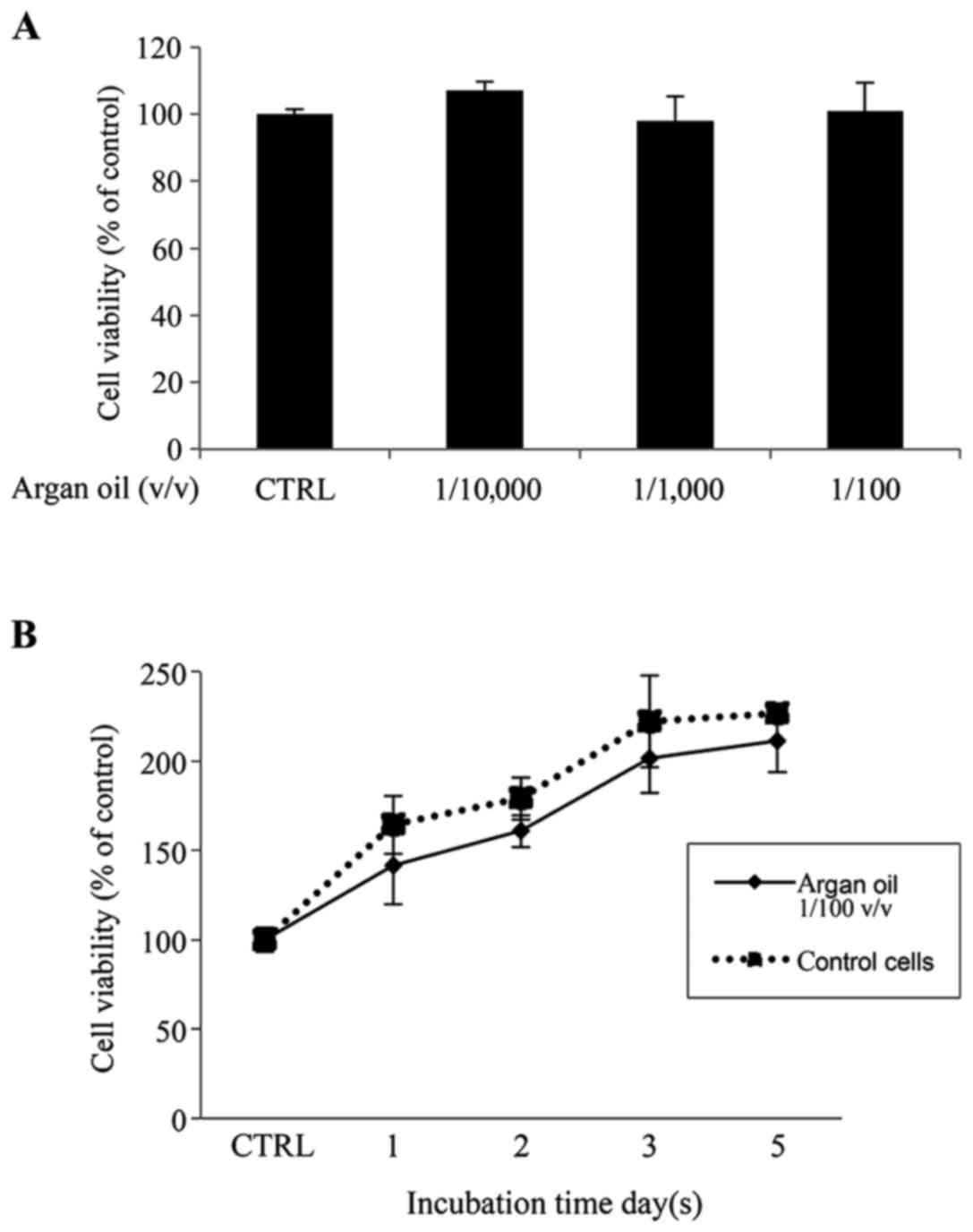
Cell viability following a 5-day exposure to argan
oil fine emulsion in growth medium at 1/100, 1/1,000 and 1/10,000
v/v dilution exerted no toxic effects (Fig. 3A). Therefore, the 1/100 v/v
dilution was selected for use in the subsquent experiments. A cell
growth curve at this dilution also exhibited no significant
difference in cell viability between the untreated cells and cells
treated for 1–5 days with argan oil (Fig. 3B).
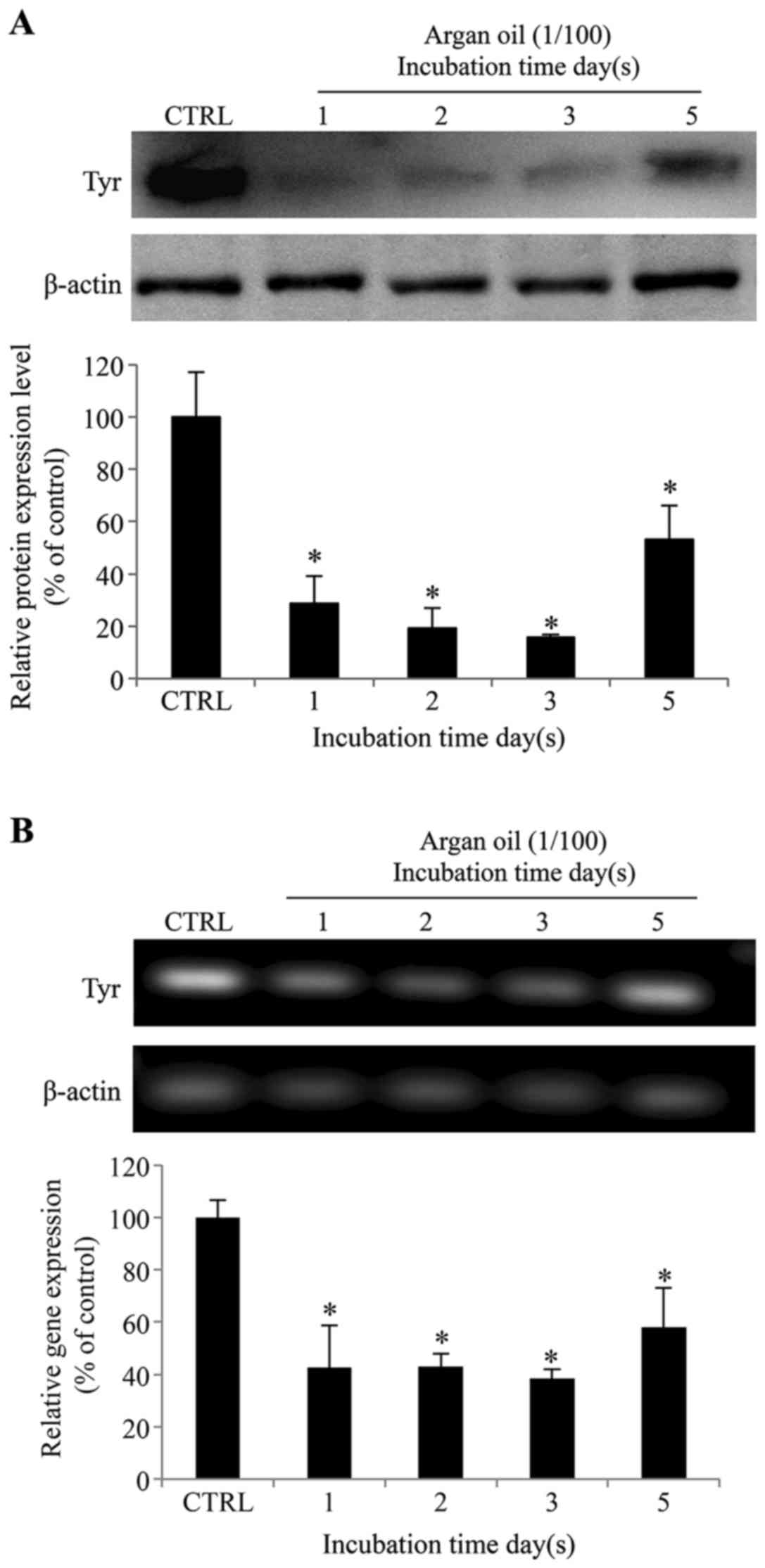
Argan oil elicits a time-dependent
decrease in tyrosinase protein and gene expression in 92.1
cells
To investigate the possible role of argan oil in
melanogenesis, we evaluated tyrosinase expression in the 92.1 cells
following incubation for 1, 2, 3 and 5 days with 1% argan oil. As
shown in Fig. 4A, argan oil
induced a decrease in tyrosinase protein expression progressing
until day 3, and then an increase after 5 days. In particular, a
decrease of 71, 81, and 84% in comparison to the the control cells
was observed following treatment of the cells with 1% argan oil for
1, 2 and 3 days, respectively. After 5 days, an increase in
tyrosinase expression (a recovery of 2.9-fold compared to day 3)
was observed in comparison to the shorter incubation periods, while
maintaining a 47% reduction, in comparison to the control cells.
Semi-quantitative RT-PCR (Fig.
4B) revealed a similar time-dependent variation in tyrosinase
gene expression, with a reduction of 61, 59, 63 and 48% after 1, 2,
3 and 5 days, respectively, in comparison to the control cells.
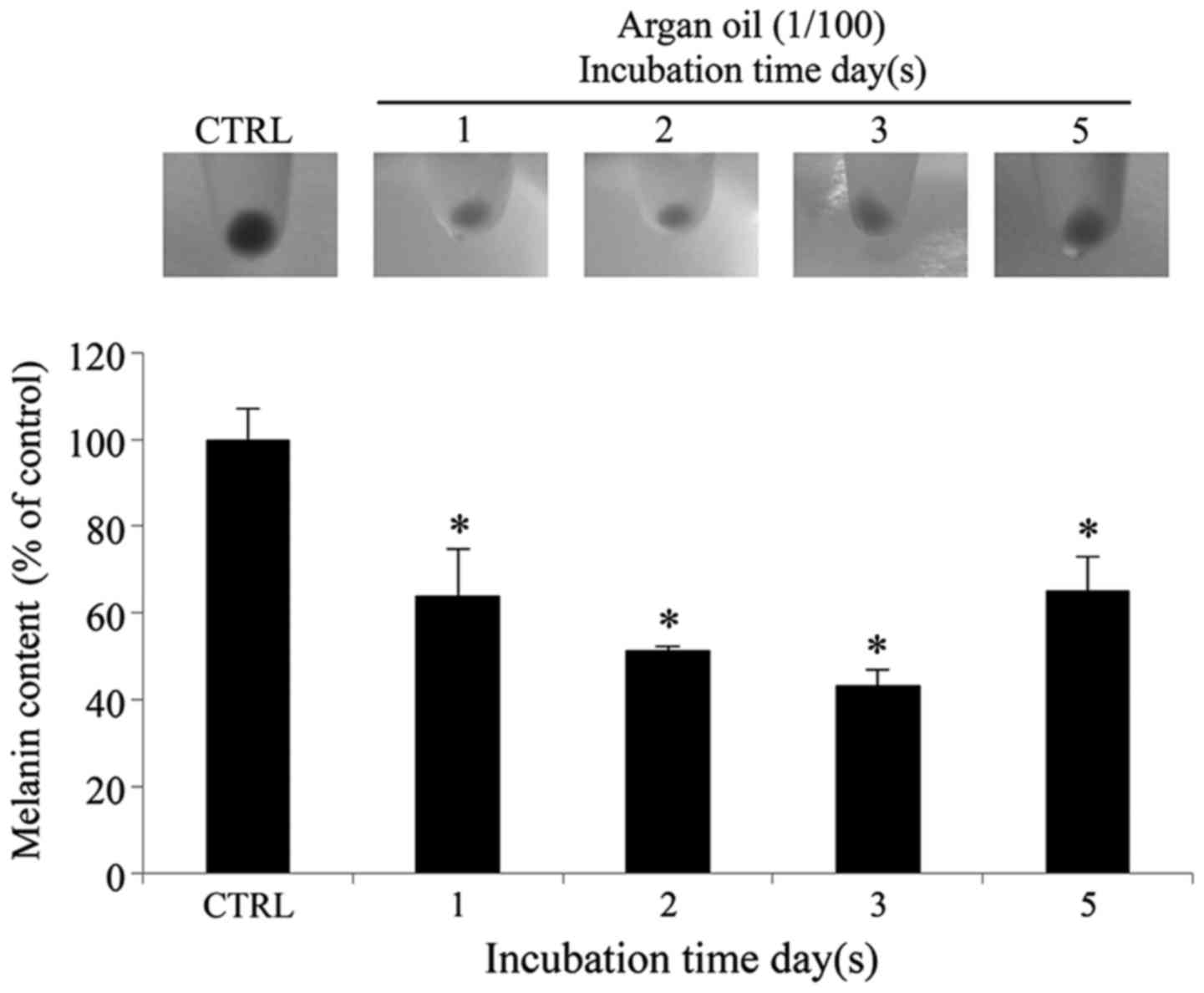
Argan oil treatment reduces the melanin
content in 92.1 cells
In agreement with the data already obtained,
treatment of the 92.1 cells with argan oil also indcued a
significant and progressive decrease in melanin content up until
day 3 of stimulation (36, 48 and 57% of reduction after 1, 2 and 3
days, respectively, in comparison to the control cells) with a
partial recovery after 5 days of treatment (35% of reduction at day
5 in comparison with the control cells) (Fig. 5). The different gray intensity of
the cell pellets, just before their lysis in hot NaOH for the
detection of melanin content, clearly illustrates the depigmenting
effect following treatment with argan oil (Fig. 5).
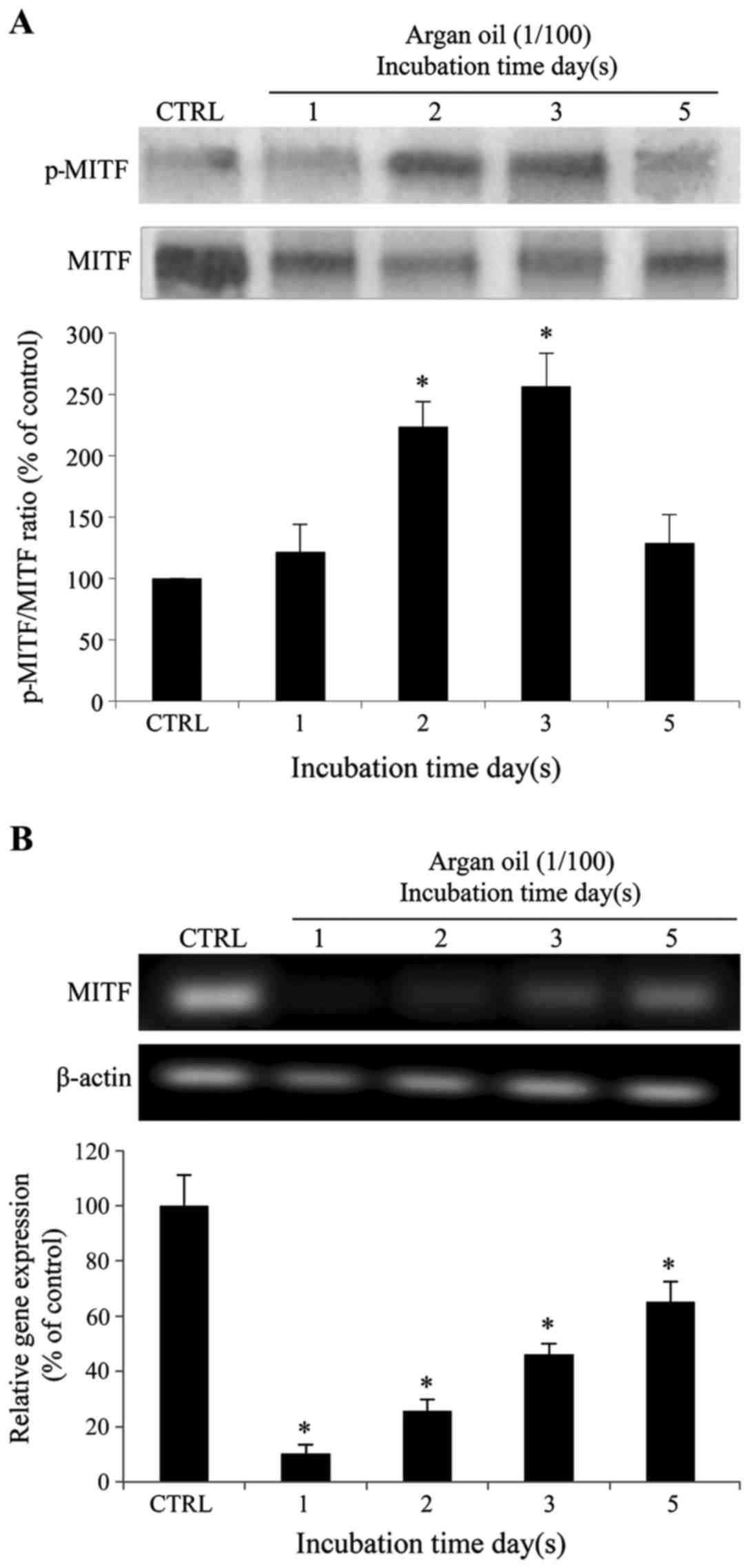
Argan oil promotes MITF degradation,
decreases MITF gene expression and impairs ERK1/2 and AKT
phosphorylation in 92.1 cells
Due to the observed effects of argan oil on melanin
biosynthesis and tyrosinase expression, we investigated the
expression of the transcription factor, MITF.
As shown in Fig.
6A, as already observed for tyrosinase expression and melanin
production, treatment with argan oil progressively increased the
phosphorylation and further proteasome the degradation (10) of MITF at Ser73 (20% at day 1, 120%
at day 2 and 160% at day 3) with a partial recovery following
treatment for 5 days (20% increase in comparison to the control
cells). Argan oil also decreased MITF gene expression (Fig. 6B). However, a different trend was
observed in this case; this involved a sharp reduction following
incubation with argan oil for 1 and 2 days, followed by a
progressive recovery following incubation for 3 and 5 days (90, 74,
54 and 35% of reduction after 1, 2, 3 and 5 days, respectively, in
comparison to the control cells).
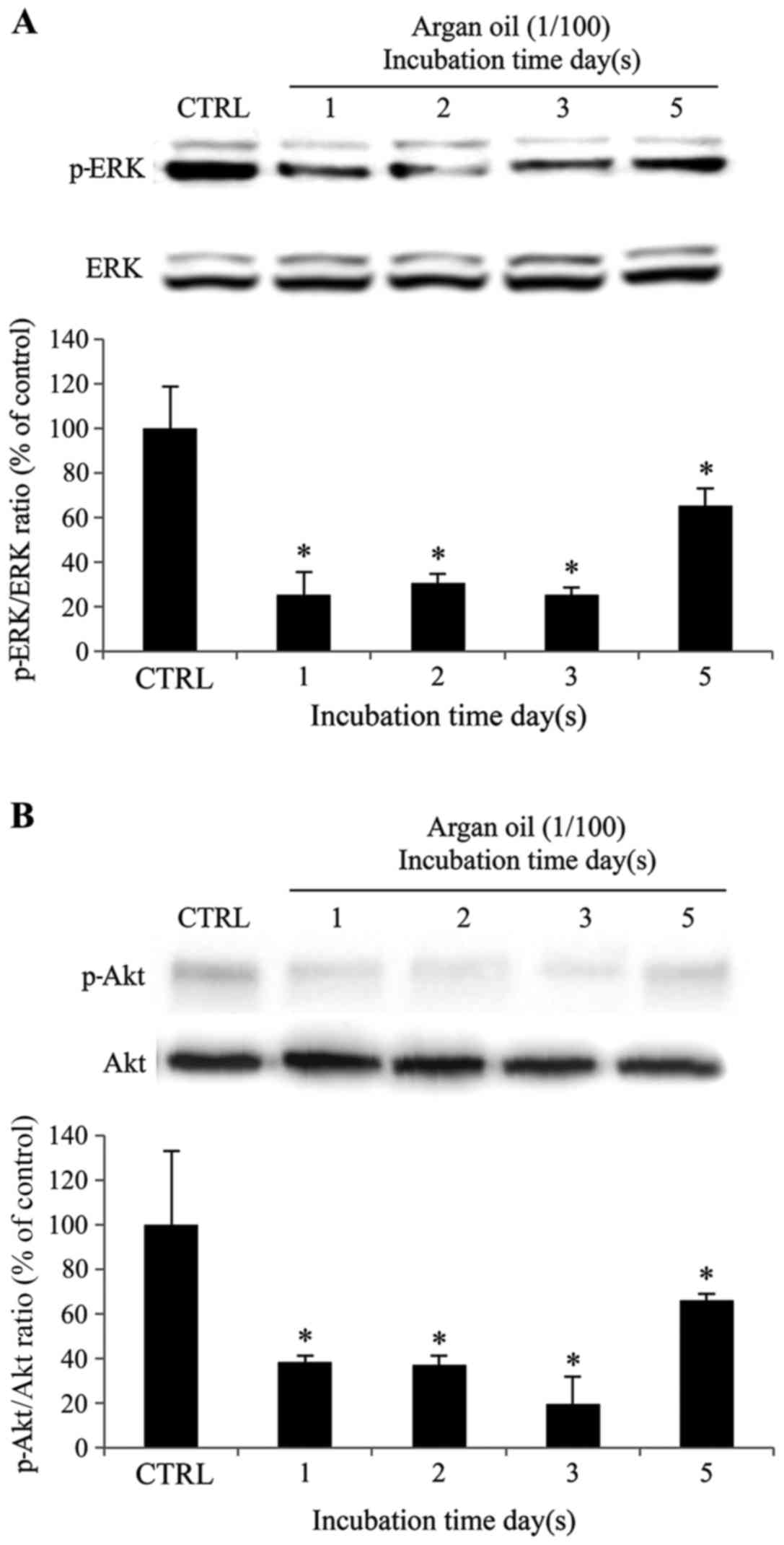
Given that specific inhibitors of ERK1/2 and
PI3K/Akt phosphorylation decreased tyrosinase expression and the
melanin content in 92.1 cells, we wished to determine whether argan
oil can influence ERK1/2 and PI3K/Akt activation and
expression.
As shown in Fig.
7A, p-ERK1/2 and ERK protein expression levels were examined.
As expected, 1% argan oil reduced ERK1/2 phosphorylation by 75, 70,
75 and 35% following incubation of the 92.1 cells for 1, 2, 3 and 5
days, respectively, compared to the control cells.
As shown in Fig.
7B, p-Akt and Akt protein expression levels were also examined.
Incubation of the 92.1 cells with 1% argan oil induced a decrease
in the levels of p-Akt by 62, 63, 80 and 33% following incubation
for 1, 2, 3 and 5 days, in comparison to the control cells.
Discussion
The human iris contains two types of pigmented
cells, epithelial cells and melanocytes. Iris pigmented epithelial
cells are located in a double layer at the posterior surface of the
iris, and do not produce melanin in vitro (32). Their melanin content does not vary
significantly between irises of different colors, and thus it is
believed that they play a minor role in iris color variations
(33–35). Uveal melanocytes are dispersed in
the iris stroma and do produce melanin in vitro (36); their melanin content varies in
different colored irises (both in vivo and in vitro),
and thus that they represent the main factor in determining iris
color (33–36). Ocular pigmentation in humans is
assumed to stabilize past infancy; however, a study on eye color
changes in a group of twin pairs, enrolled in the Louisville Twin
Study, revealed that the iris pigmentation was variable throughout
adolescence in 10–15% of subjects (37). The uneven darkening of iris color
is a well-known side-effect of glaucoma treatment with
prostaglandins or prostaglandin analogues, such as isopropyl
unoprostone or latanoprost (15,38). Thus, the changes in iris color
during a human lifetime may be more frequent than supposed both for
physiological and pathological events.
Uveal and cutaneous melanocytes have different
functions. Cutaneous melanocytes continuously synthesize and
transfer melanin to keratinocytes, in a juxtacrine-regulated
interaction in which keratinocytes respond secreting growth factors
for melanocytes (39). Similar to
cutaneous melanocytes, conjunctival melanocytes transfer melanin to
conjunctival epithelial cells (40). Finally, ocular albinism
depigmentation occurs in the eye, but not in the skin (41), indicating that melanogenesis in
uveal and cutaneous melanocytes involves different pathways,
starting from the lack of response of uveal melanocytes to MSH, the
melanocyte stimulating hormone, the main orchestrator of cutaneous
melanogenesis (14).
Considering the pivotal role of the ERK1/2 and
PI3K/Akt pathways in the regulation of cutaneous melanogenesis
(42), we set out to explore the
role of these two pathways in the regulation of ocular
melanogenesis by using molecules able to interfere with the
regulation of melanogenesis. We used B16-F1 mouse cutaneous
melanoma cells and 92.1 human uveal melanoma cells, both with a
detectable melanin content (43).
Our results revealed that ERK1/2 and PI3K-Akt played
an opposing role in the regulation of melanogenesis in the iris
melanoma cells in comparison to the cutaneous melanoma cells.
Surprisingly, specific inhibitors of both kinases significantly
decreased tyrosinase protein expression and melanin content in the
92.1 cells, but not in the B16-F1 cells, where significant
increases were observed. Even more surprisingly, in the presence of
forskolin, we did not observe an increase (such as in the B16-F1
cells), but rather a marked decrease in tyrosinase protein
expression and melanin content in the 92.1 cells.
Argan oil is already widely used for cosmetic and
medicinal purposes and one in vitro study showed its
depigmenting activity on B16-F1 cells (17). Moreover, another in vitro
study showed its ability to reduce ERK1/2 and Akt phosphorylation
in the HTC liver cell line (44).
For these reasons, we used it in our cellular model in order to
modulate ocular melanogenesis.
Argan oil exhibited a time-dependent depigmenting
activity, with a decrease in both tyrosinase gene and protein
expression, and in the melanin content, an increase in the
phosphorylation of MITF at Ser73, probably leading to its
ubiquitination and degradation, and a decrease in MITF gene
expression. Moreover, argan oil decreased ERK1/2 and Akt
phosphorylation and, taking into account our results, this may be
the mechanism through which argan oil exerts its depigmenting
effects on human uveal melanoma cells.
The understanding of the molecular mechanisms which
regulate uveal melanogenesis may have significance in the treatment
of several eye diseases. It has been observed that the development
of certain eye diseases may be related to exposure to ultraviolet
and visible light. In this regard, a population-based study
demonstrated that exposure to sunlight may be associated with
age-related macular degeneration, a greater incidence in blue
irises than in brown irises patients (45).
For these reasons, the study of ocular melanogenesis
mechanisms and the identification of molecules able to modulate it,
similarly to what has been done for cutaneous melanogenesis, may
have both cosmetic and medical implications. Further studies are
therefore warranted in order to fully determine the mechanisms of
ocular melanogenesis.
References
|
1
|
Plonka PM, Passeron T, Brenner M, Tobin
DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D,
Peters E, et al: What are melanocytes really doing all day long…?
Exp Dermatol. 18:799–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tachibana M: Sound needs sound melanocytes
to be heard. Pigment Cell Res. 12:344–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brito FC and Kos L: Timeline and
distribution of melanocyte precursors in the mouse heart. Pigment
Cell Melanoma Res. 21:464–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu DN, Simon JD and Sarna T: Role of
ocular melanin in ophthalmic physiology and pathology. Photochem
Photobiol. 84:639–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Randhawa M, Huff T, Valencia JC, Younossi
Z, Chandhoke V, Hearing VJ and Baranova A: Evidence for the ectopic
synthesis of melanin in human adipose tissue. FASEB J. 23:835–843.
2009. View Article : Google Scholar :
|
|
6
|
Suzuki I, Cone RD, Im S, Nordlund J and
Abdel-Malek ZA: Binding of melanotropic hormones to the
melanocortin receptor MC1R on human melanocytes stimulates
proliferation and melanogenesis. Endocrinology. 137:1627–1633.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olivares C and Solano F: New insights into
the active site structure and catalytic mechanism of tyrosinase and
its related proteins. Pigment Cell Melanoma Res. 22:750–760. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oka M, Kageyama A, Fukunaga M, Bito T,
Nagai H and Nishigori C: Phosphatidylinositol
3-kinase/Akt-dependent and -independent protection against
apoptosis in normal human melanocytes. J Invest Dermatol.
123:930–936. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hemesath TJ, Price ER, Takemoto C,
Badalian T and Fisher DE: MAP kinase links the transcription factor
Microphthalmia to c-Kit signalling in melanocytes. Nature.
391:298–301. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bae JS, Han M, Yao C and Chung JH:
Chaetocin inhibits IBMX-induced melanogenesis in B16F10 mouse
melanoma cells through activation of ERK. Chem Biol Interact.
245:66–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong HS, Gu GE, Jo AR, Bang JS, Yun HY,
Baek KJ, Kwon NS, Park KC and Kim DS: Baicalin-induced Akt
activation decreases melanogenesis through downregulation of
microphthalmia-associated transcription factor and tyrosinase. Eur
J Pharmacol. 761:19–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Hu DN, Zhao H, McCormick SA,
Nordlund JJ and Boissy RE: Uveal melanocytes do not respond to or
express receptors for α-melanocyte-stimulating hormone. Invest
Ophthalmol Vis Sci. 47:4507–4512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu DN, Stjernschantz J and McCormick SA:
Effect of prostaglandins A2, E1 F2
α and latanoprost on cultured human iridal melanocytes. Exp
Eye Res. 70:113–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamid MA, Sarmidi MR and Park CS:
Mangosteen leaf extract increases melanogenesis in B16F1 melanoma
cells by stimulating tyrosinase activity in vitro and by
up-regulating tyrosinase gene expression. Int J Mol Med.
29:209–217. 2012.
|
|
17
|
Li X, Guo L, Sun Y, Zhou J, Gu Y and Li Y:
Baicalein inhibits melanogenesis through activation of the ERK
signaling pathway. Int J Mol Med. 25:923–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo H, Yang K, Deng F, Xing Y, Li Y, Lian
X and Yang T: Wnt3a inhibits proliferation but promotes
melanogenesis of melan-a cells. Int J Mol Med. 30:636–642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lv N, Koo JH, Yoon HY, Yu J, Kim KA, Choi
IW, Kwon KB, Kwon KS, Kim HU, Park JW, et al: Effect of Angelica
gigas extract on melanogenesis in B16 melanoma cells. Int J Mol
Med. 20:763–767. 2007.PubMed/NCBI
|
|
20
|
Huang HC, Lin H and Huang MC: The
lactoferricin B-derived peptide, LfB17-34, induces melanogenesis in
B16F10 cells. Int J Mol Med. 39:595–602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Solano F, Briganti S, Picardo M and Ghanem
G: Hypopigmenting agents: An updated review on biological, chemical
and clinical aspects. Pigment Cell Res. 19:550–571. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Villareal MO, Kume S, Bourhim T, Bakhtaoui
FZ, Kashiwagi K, Han J, Gadhi C and Isoda H: Activation of MITF by
argan oil leads to the inhibition of the tyrosinase and dopachrome
tautomerase expressions in B16 murine melanoma cells. Evid Based
Complement Alternat Med. 2013:3401072013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anfuso CD, Motta C, Giurdanella G, Arena
V, Alberghina M and Lupo G: Endothelial PKCα-MAPK/ERK-phospholipase
A2 pathway activation as a response of glioma in a
triple culture model. A new role for pericytes? Biochimie.
99:77–87. 2014. View Article : Google Scholar
|
|
24
|
Anfuso CD, Giurdanella G, Motta C, Muriana
S, Lupo G, Ragusa N and Alberghina M: PKCα-MAPK/ERK-phospholipase
A2 signaling is required for human melanoma-enhanced
brain endothelial cell proliferation and motility. Microvasc Res.
78:338–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rusciano D, Lorenzoni P, Lin S and Burger
MM: Hepatocyte growth factor/scatter factor and hepatocytes are
potent downregulators of tyrosinase expression in B16 melanoma
cells. J Cell Biochem. 71:264–276. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lupo G, Anfuso CD, Ragusa N, Tirolo C,
Marchetti B, Gili E, La Rosa C and Vancheri C: Activation of
cytosolic phospholipase A2 and 15-lipoxygenase by
oxidized low-density lipoproteins in cultured human lung
fibroblasts. Biochim Biophys Acta. 1771:522–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giurdanella G, Anfuso CD, Olivieri M, Lupo
G, Caporarello N, Eandi CM, Drago F, Bucolo C and Salomone S:
Aflibercept, bevacizumab and ranibizumab prevent glucose-induced
damage in human retinal pericytes in vitro, through a
PLA2/COX-2/VEGF-A pathway. Biochem Pharmacol.
96:278–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scuderi MR, Anfuso CD, Lupo G, Motta C,
Romeo L, Guerra L, Cappellani A, Ragusa N, Cantarella G and
Alberghina M: Expression of Ca2+ -independent and
Ca2+ -dependent phospholipases A2 and
cyclooxygenases in human melanocytes and malignant melanoma cell
lines. Biochim Biophys Acta. 1781:635–642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheli Y, Luciani F, Khaled M, Beuret L,
Bille K, Gounon P, Ortonne JP, Bertolotto C and Ballotti R: αMSH
and cyclic AMP elevating agents control melanosome pH through a
protein kinase A-independent mechanism. J Biol Chem.
284:18699–18706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Englaro W, Bertolotto C, Buscà R, Brunet
A, Pagès G, Ortonne JP and Ballotti R: Inhibition of the
mitogen-activated protein kinase pathway triggers B16 melanoma cell
differentiation. J Biol Chem. 273:9966–9970. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hah YS, Cho HY, Lim TY, Park DH, Kim HM,
Yoon J, Kim JG, Kim CY and Yoon TJ: Induction of melanogenesis by
rapamycin in human MNT-1 melanoma cells. Ann Dermatol. 24:151–157.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu DN, Ritch R, McCormick SA and
Pelton-Henrion K: Isolation and cultivation of human iris pigment
epithelium. Invest Ophthalmol Vis Sci. 33:2443–2453.
1992.PubMed/NCBI
|
|
33
|
Eagle RC Jr: Iris pigmentation and
pigmented lesions: An ultrastructural study. Trans Am Ophthalmol
Soc. 86:581–687. 1988.PubMed/NCBI
|
|
34
|
Imesch PD, Bindley CD, Khademian Z, Ladd
B, Gangnon R, Albert DM and Wallow IH: Melanocytes and iris color.
Electron microscopic findings. Arch Ophthalmol. 114:443–447. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilkerson CL, Syed NA, Fisher MR, Robinson
NL, Wallow IH and Albert DM: Melanocytes and iris color. Light
microscopic findings. Arch Ophthalmol. 114:437–442. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu DN: Regulation of growth and
melanogenesis of uveal melanocytes. Pigment Cell Res. 13(Suppl 8):
81–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bito LZ, Matheny A, Cruickshanks KJ,
Nondahl DM and Carino OB: Eye color changes past early childhood.
The Louisville Twin Study. Arch Ophthalmol. 115:659–663. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wistrand PJ, Stjernschantz J and Olsson K:
The incidence and time-course of latanoprost-induced iridial
pigmentation as a function of eye color. Surv Ophthalmol. 41(Suppl
2): S129–S138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seiberg M: Keratinocyte-melanocyte
interactions during melanosome transfer. Pigment Cell Res.
14:236–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu DN, McCormick SA, Seedor JA, Ritterband
DC and Shah MK: Isolation, purification and cultivation of
conjunctival melanocytes. Exp Eye Res. 84:655–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bassi MT, Schiaffino MV, Renieri A, De
Nigris F, Galli L, Bruttini M, Gebbia M, Bergen AA, Lewis RA and
Ballabio A: Cloning of the gene for ocular albinism type 1 from the
distal short arm of the X chromosome. Nat Genet. 10:13–19. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim HJ, Kim IS, Dong Y, Lee IS, Kim JS,
Kim JS, Woo JT and Cha BY: Melanogenesis-inducing effect of
cirsimaritin through increases in microphthalmia-associated
transcription factor and tyrosinase expression. Int J Mol Sci.
16:8772–8788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onken MD, Li J and Cooper JA: Uveal
melanoma cells utilize a novel route for transendothelial
migration. PLoS One. 9:e1154722014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samane S, Noël J, Charrouf Z, Amarouch H
and Haddad PS: Insulin-sensitizing and anti-proliferative effects
of Argania spinosa seed extracts. Evid Based Complement Alternat
Med. 3:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitchell P, Smith W and Wang JJ: Iris
color, skin sun sensitivity, and age-related maculopathy. The Blue
Mountains Eye Study. Ophthalmology. 105:1359–1363. 1998. View Article : Google Scholar : PubMed/NCBI
|