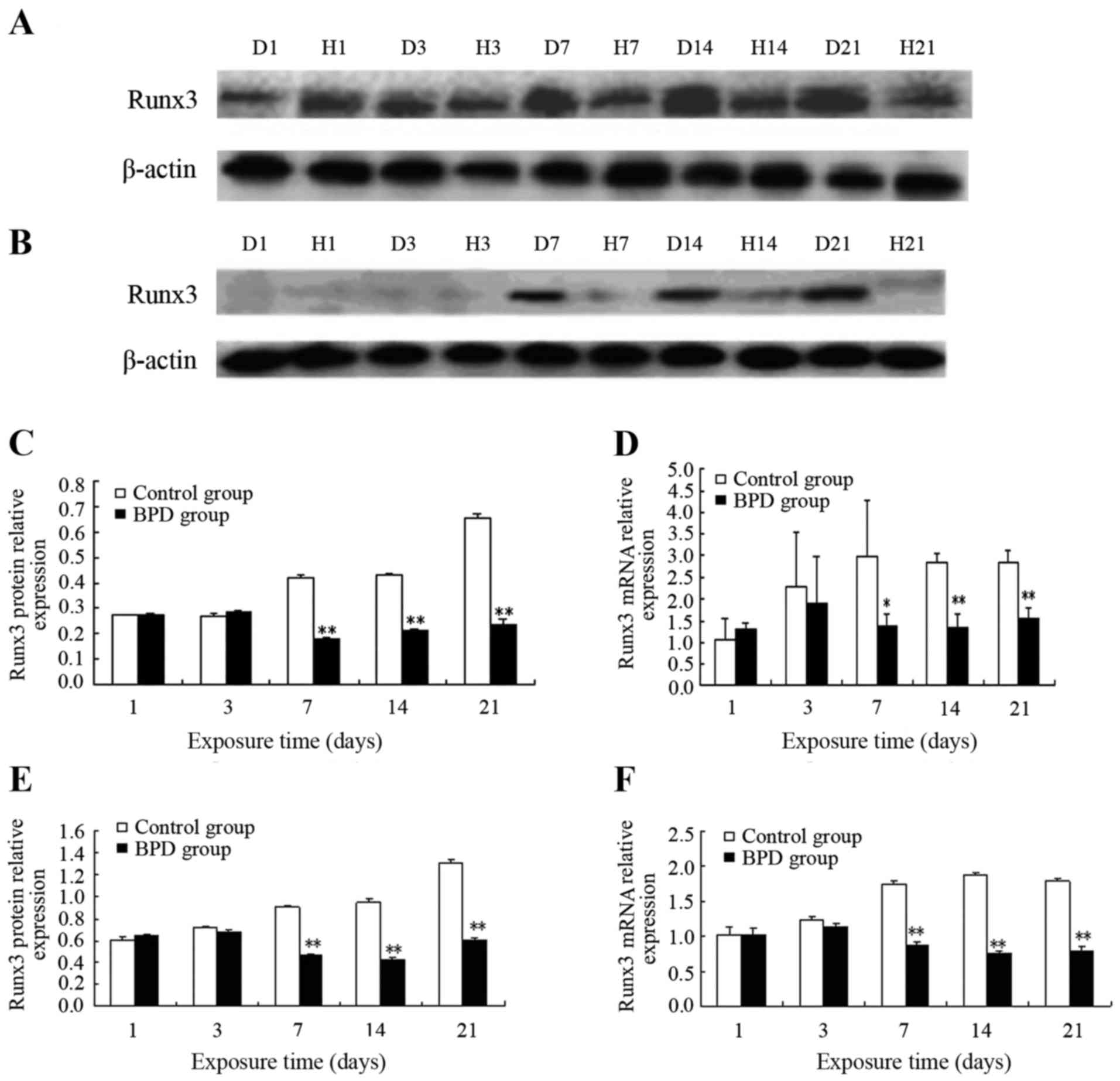
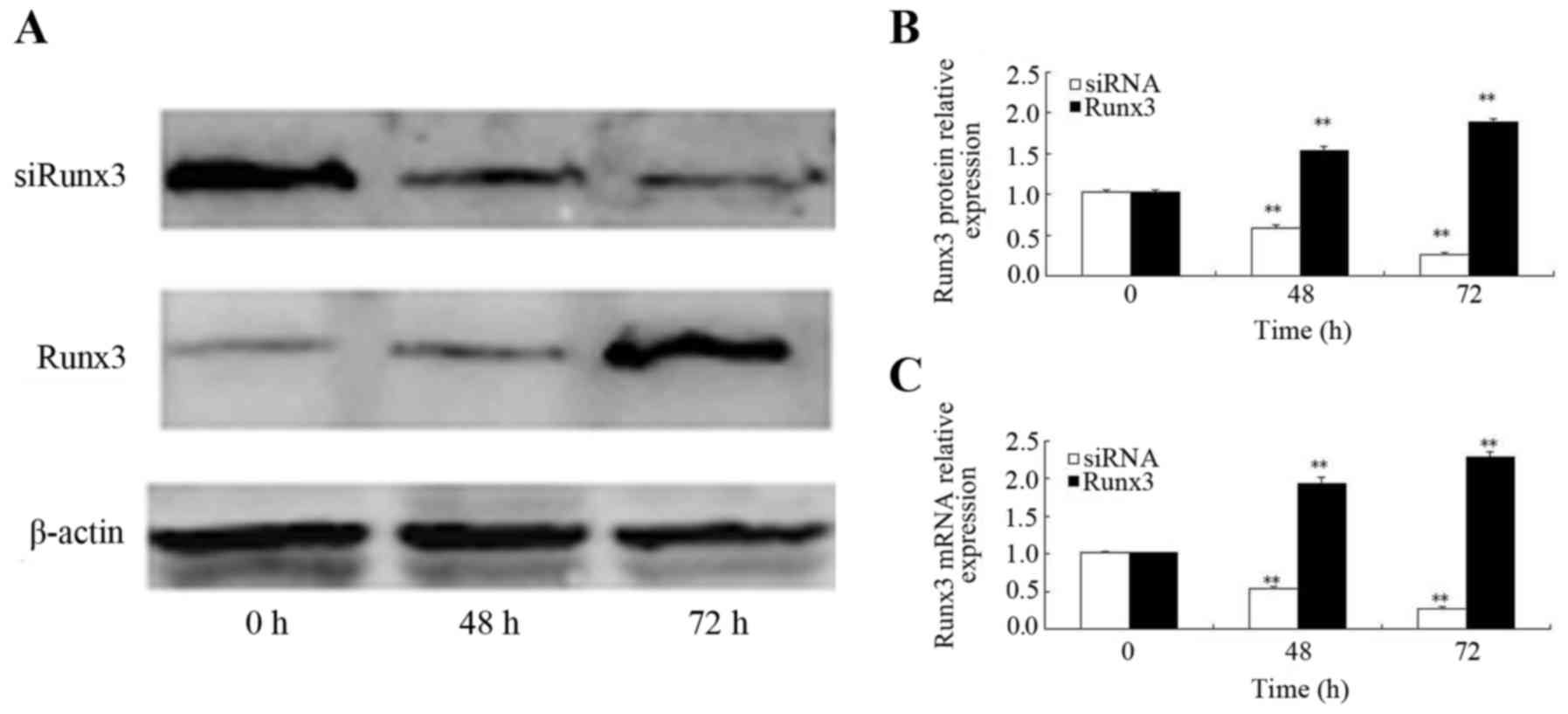
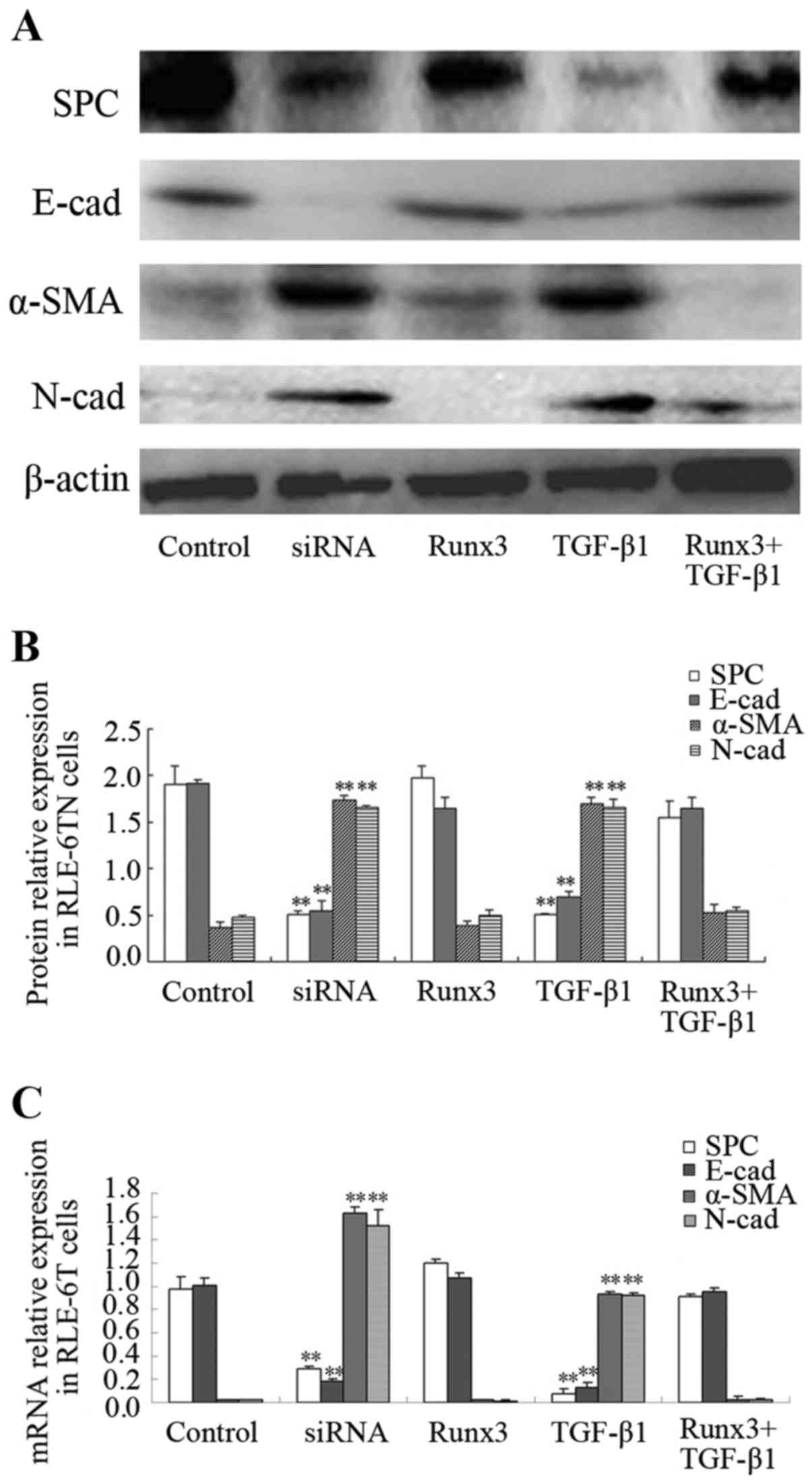
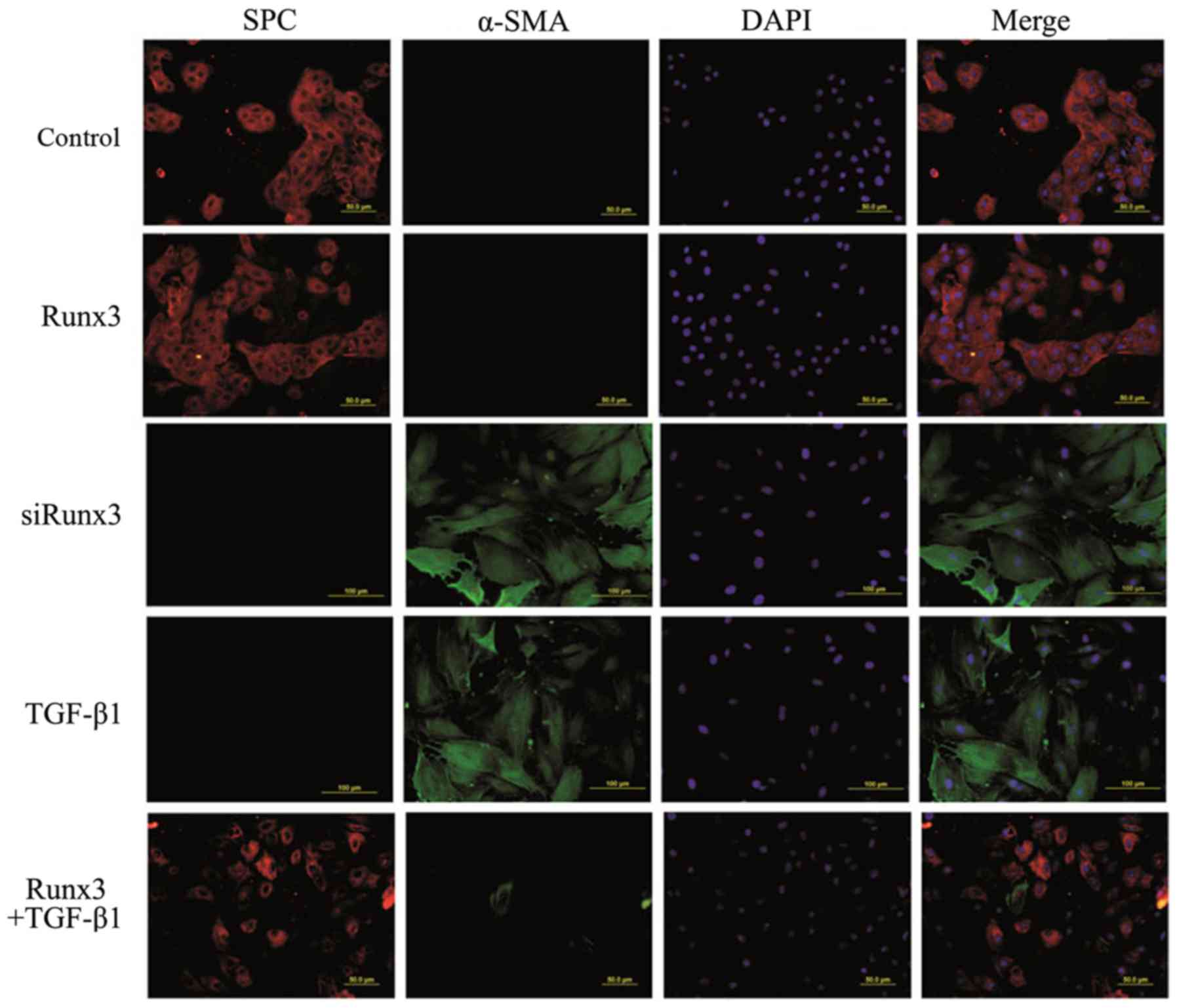
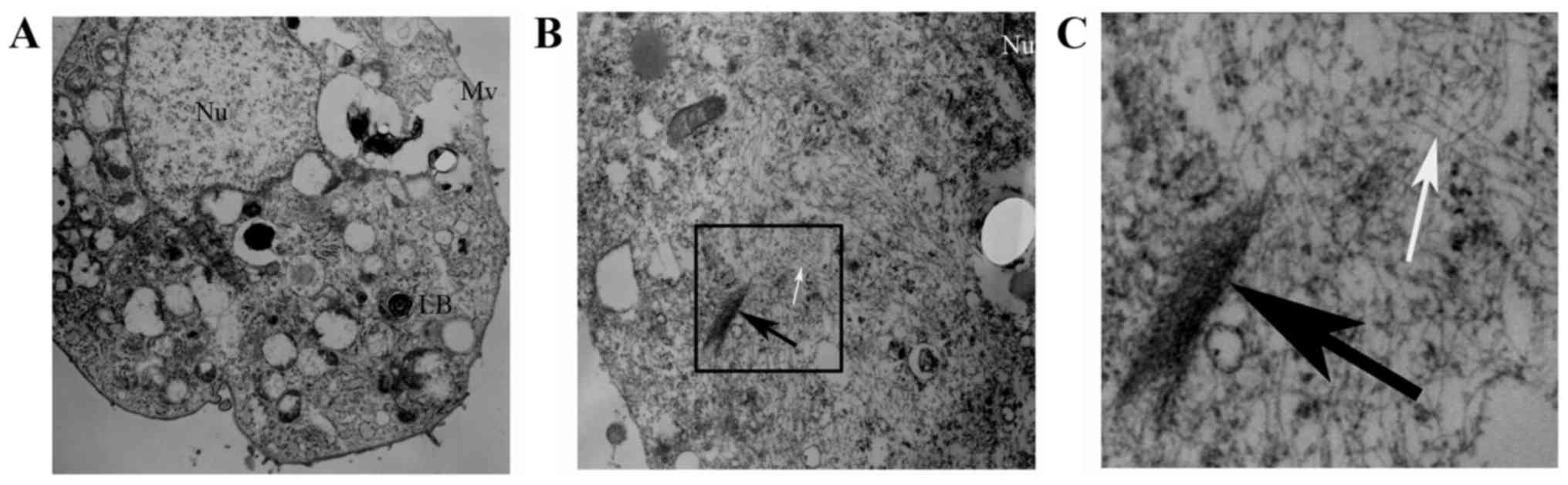
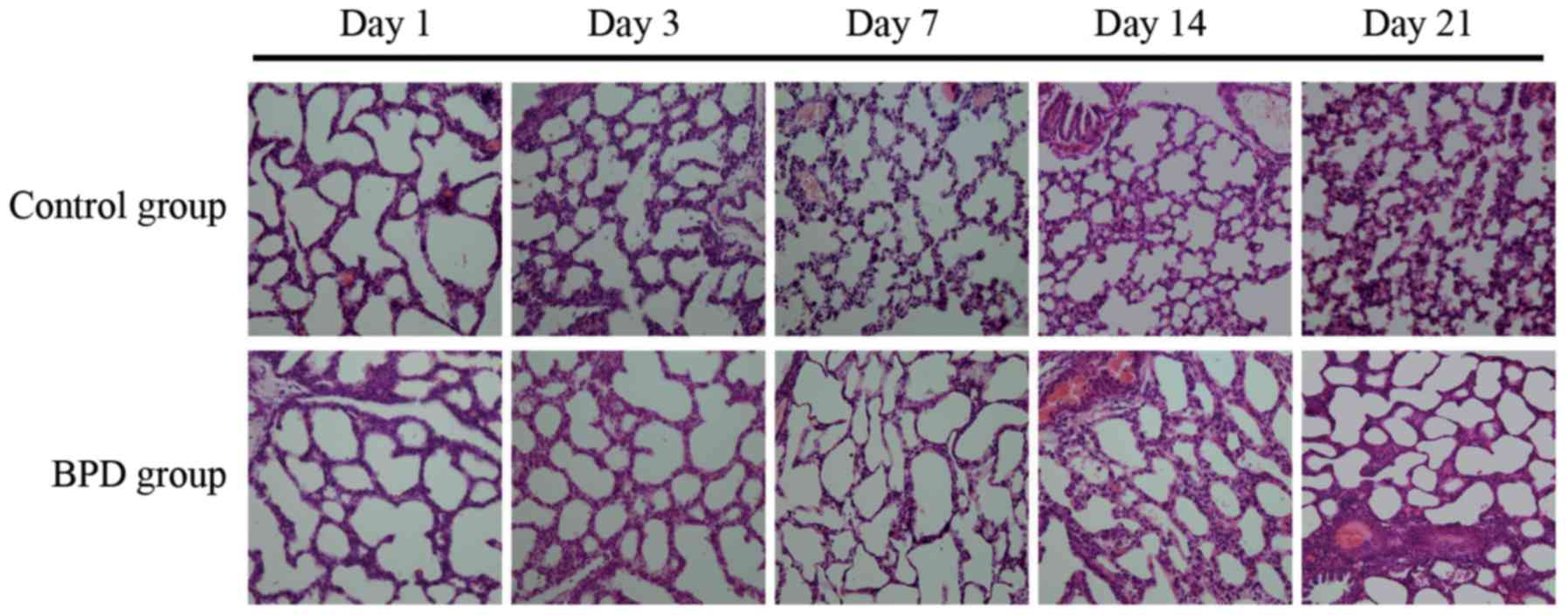
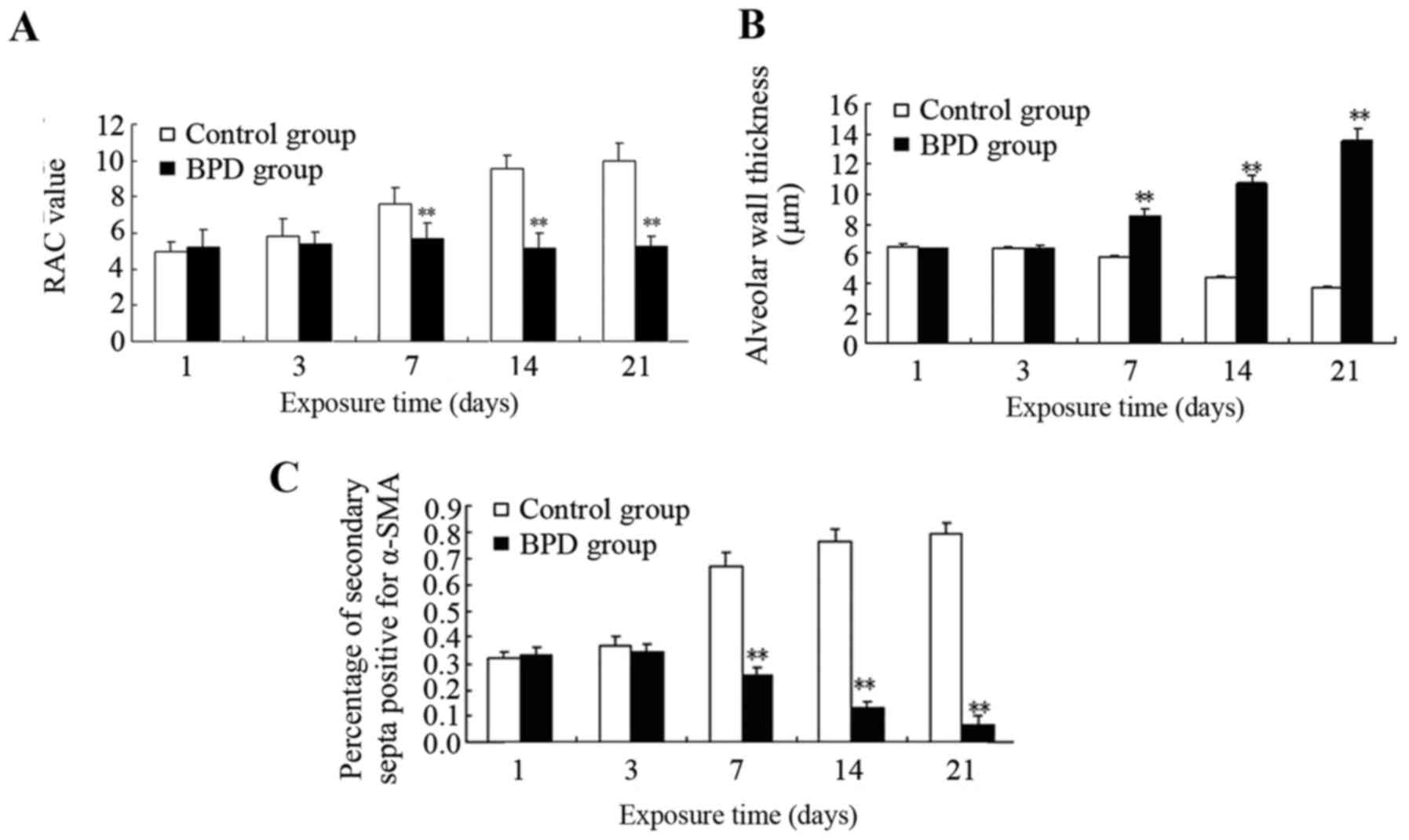
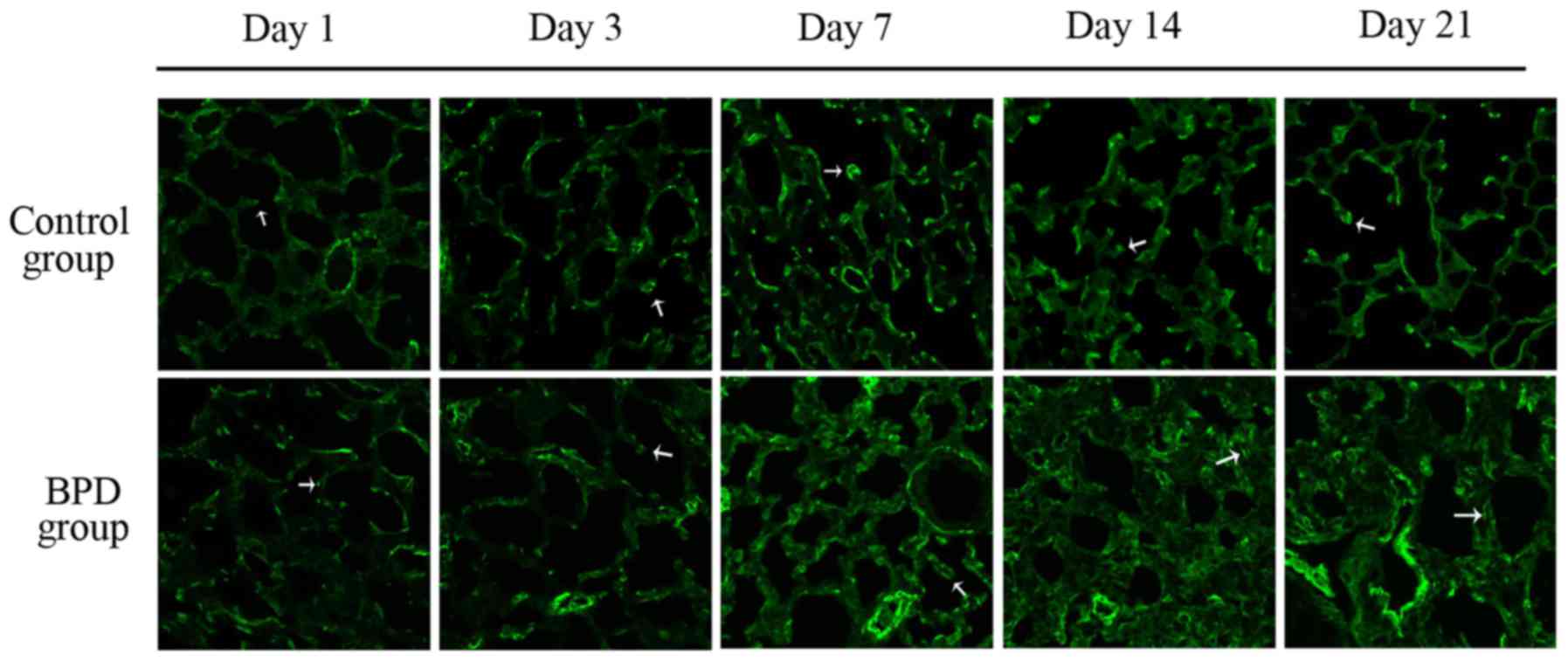
|
1
|
Fanaroff AA, Stoll BJ, Wright LL, Carlo
WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB,
Laptook AR, et al: Trends in neonatal morbidity and mortality for
very low birth-weight infants. Am J Obstet Gynecol. 196:147.e1–e8.
2007. View Article : Google Scholar
|
|
2
|
Walsh MC, Szefler S, Davis J, Allen M, Van
Marter L, Abman S, Blackmon L and Jobe A: Summary proceedings from
the bronchopulmonary dysplasia group. Pediatrics. 117(Suppl 1):
S52–S56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Husain AN, Siddiqui NH and Stocker JT:
Pathology of arrested acinar development in postsurfactant
bronchopulmonary dysplasia. Hum Pathol. 29:710–717. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Huang C, Reddy Chintagari N,
Bhaskaran M, Weng T, Guo Y, Xiao X and Liu L: miR-375 regulates rat
alveolar epithelial cell trans-differentiation by inhibiting
Wnt/β-catenin pathway. Nucleic Acids Res. 41:3833–3844. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bitterman PB, Polunovsky VA and Ingbar DH:
Repair after acute lung injury. Chest. 105(Suppl 3): 118S–121S.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Fu J, Xue X, Yao L, Qiao L, Hou A,
Jin L and Xing Y: Epithelial-mesenchymal transitions in
bronchopulmonary dysplasia of newborn rats. Pediatr Pulmonol.
49:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue K, Shiga T and Ito Y: Runx
transcription factors in neuronal development. Neural Dev.
3:202008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito Y: RUNX genes in development and
cancer: regulation of viral gene expression and the discovery of
RUNX family genes. Adv Cancer Res. 99:33–76. 2008. View Article : Google Scholar
|
|
9
|
Lee JM, Kwon HJ, Lai WF and Jung HS:
Requirement of Runx3 in pulmonary vasculogenesis. Cell Tissue Res.
356:445–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Chen L, Zhang X, Xu X, Xing H,
Zhang Y, Li W, Yu H, Zeng J and Jia J: RUNX3 regulates vimentin
expression via miR-30a during epithelial-mesenchymal transition in
gastric cancer cells. J Cell Mol Med. 18:610–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei PJ, Bai J, Liu H, Li C, Wu YP, Yu ZQ
and Zheng JN: RUNX3 expression is lost in glioma and its
restoration causes drastic suppression of tumor invasion and
migration. J Cancer Res Clin Oncol. 137:1823–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee YS, Lee JW, Jang JW, Chi XZ, Kim JH,
Li YH, Kim MK, Kim DM, Choi BS, Kim EG, et al: Runx3 inactivation
is a crucial early event in the development of lung adenocarcinoma.
Cancer Cell. 24:603–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishina S, Shiraha H, Nakanishi Y, Tanaka
S, Matsubara M, Takaoka N, Uemura M, Horiguchi S, Kataoka J,
Iwamuro M, et al: Restored expression of the tumor suppressor gene
RUNX3 reduces cancer stem cells in hepatocellular carcinoma by
suppressing Jagged1-Notch signaling. Oncol Rep. 26:523–531.
2011.PubMed/NCBI
|
|
14
|
Levanon D, Brenner O, Negreanu V, Bettoun
D, Woolf E, Eilam R, Lotem J, Gat U, Otto F, Speck N, et al:
Spatial and temporal expression pattern of Runx3 (Aml2) and Runx1
(Aml1) indicates non-redundant functions during mouse
embryogenesis. Mech Dev. 109:413–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KS, Lee YS, Lee JM, Ito K, Cinghu S,
Kim JH, Jang JW, Li YH, Goh YM, Chi XZ, et al: Runx3 is required
for the differentiation of lung epithelial cells and suppression of
lung cancer. Oncogene. 29:3349–3361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JM, Shin JO, Cho KW, Hosoya A, Cho SW,
Lee YS, Ryoo HM, Bae SC and Jung HS: Runx3 is a crucial regulator
of alveolar differentiation and lung tumorigenesis in mice.
Differentiation. 81:261–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You K, Xu X, Fu J, Xu S, Yue X, Yu Z and
Xue X: Hyperoxia disrupts pulmonary epithelial barrier in newborn
rats via the deterioration of occludin and ZO-1. Respir Res.
13:362012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Willis BC, Liebler JM, Luby-Phelps K,
Nicholson AG, Crandall ED, du Bois RM and Borok Z: Induction of
epithelial-mesenchymal transition in alveolar epithelial cells by
transforming growth factor-beta1: potential role in idiopathic
pulmonary fibrosis. Am J Pathol. 166:1321–1332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cooney TP and Thurlbeck WM: The radial
alveolar count method of Emery and Mithal: a reappraisal 1 -
postnatal lung growth. Thorax. 37:572–579. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Emanuel RL, Torday JS, Mu Q, Asokananthan
N, Sikorski KA and Sunday ME: Bombesin-like peptides and receptors
in normal fetal baboon lung: roles in lung growth and maturation.
Am J Physiol. 277:L1003–L1017. 1999.PubMed/NCBI
|
|
21
|
Cullen A, Van Marter LJ, Allred EN, Moore
M, Parad RB and Sunday ME: Urine bombesin-like peptide elevation
precedes clinical evidence of bronchopulmonary dysplasia. Am J
Respir Crit Care Med. 165:1093–1097. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito Y: Oncogenic potential of the RUNX
gene family: 'overview'. Oncogene. 23:4198–4208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Speck NA and Gilliland DG: Core-binding
factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2:502–513.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mundlos S, Otto F, Mundlos C, Mulliken JB,
Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et
al: Mutations involving the transcription factor CBFA1 cause
cleidocranial dysplasia. Cell. 89:773–779. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas DM, Johnson SA, Sims NA, Trivett
MK, Slavin JL, Rubin BP, Waring P, McArthur GA, Walkley CR,
Holloway AJ, et al: Terminal osteoblast differentiation, mediated
by runx2 and p27KIP1, is disrupted in osteosarcoma. J
Cell Biol. 167:925–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bae SC and Choi JK: Tumor suppressor
activity of RUNX3. Oncogene. 23:4336–4340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato N, Tamura G, Fukase M, Shibuya H and
Motoyama T: Hypermethylation of the RUNX3 gene promotor in
testicular yolk sac tumor of infants. Am J Pathol. 163:387–391.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bone KR, Gruper Y, Goldenberg D, Levanon D
and Groner Y: Translation regulation of Runx3. Blood Cells Mol Dis.
45:112–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka S, Shiraha H, Nakanishi Y, Nishina
S, Matsubara M, Horiguchi S, Takaoka N, Iwamuro M, Kataoka J,
Kuwaki K, et al: Runt-related transcription factor 3 reverses
epithelial-mesenchymal transition in hepatocellular carcinoma. Int
J Cancer. 131:2537–2546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemieux E, Bergeron S, Durand V, Asselin
C, Saucier C and Rivard N: Constitutively active MEK1 is sufficient
to induce epithelial-to-mesenchymal transition in intestinal
epithelial cells and to promote tumor invasion and metastasis. Int
J Cancer. 125:1575–1586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou B, Buckley ST, Patel V, Liu Y, Luo J,
Krishnaveni MS, Ivan M, DeMaio L, Kim KJ, Ehrhardt C, et al:
Troglitazone attenuates TGF-β1-induced EMT in alveolar epithelial
cells via a PPARγ-independent mechanism. PLoS One. 7:e388272012.
View Article : Google Scholar
|
|
34
|
Kojima T, Takano K, Yamamoto T, Murata M,
Son S, Imamura M, Yamaguchi H, Osanai M, Chiba H, Himi T, et al:
Transforming growth factor-beta induces epithelial to mesenchymal
transition by down-regulation of claudin-1 expression and the fence
function in adult rat hepatocytes. Liver Int. 28:534–545. 2008.
View Article : Google Scholar
|
|
35
|
Nakanishi H, Sugiura T, Streisand JB,
Lonning SM and Roberts JD Jr: TGF-beta-neutralizing antibodies
improve pulmonary alveologenesis and vasculogenesis in the injured
newborn lung. Am J Physiol Lung Cell Mol Physiol. 293:L151–L161.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vicencio AG, Lee CG, Cho SJ, Eickelberg O,
Chuu Y, Haddad GG and Elias JA: Conditional overexpression of
bioactive transforming growth factor-beta1 in neonatal mouse lung:
a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol
Biol. 31:650–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan JM, Ng YY, Hill PA, Nikolic-Paterson
DJ, Mu W, Atkins RC and Lan HY: Transforming growth factor-beta
regulates tubular epithelial-myofibroblast transdifferentiation in
vitro. Kidney Int. 56:1455–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ng YY, Huang TP, Yang WC, Chen ZP, Yang
AH, Mu W, Nikolic-Paterson DJ, Atkins RC and Lan HY: Tubular
epithelial-myofibroblast transdifferentiation in progressive
tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int.
54:864–876. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Joss-Moore L, Carroll T, Yang Y, Fitzhugh
M, Metcalfe D, Oman J, Hale M, Dong L, Wang ZM, Yu X, et al:
Intrauterine growth restriction transiently delays alveolar
formation and disrupts retinoic acid receptor expression in the
lung of female rat pups. Pediatr Res. 73:612–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramaniam M, Bausch C, Twomey A,
Andreeva S, Yoder BA, Chang L, Crapo JD, Pierce RA, Cuttitta F and
Sunday ME: Bombesin-like peptides modulate alveolarization and
angiogenesis in bronchopulmonary dysplasia. Am J Respir Crit Care
Med. 176:902–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang Y, Tong D, Lou G, Zhang Y and Geng
J: Expression of RUNX3 gene, methylation status and
clinicopathological significance in breast cancer and breast cancer
cell lines. Pathobiology. 75:244–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee TI, Jenner RG, Boyer LA, Guenther MG,
Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K,
et al: Control of developmental regulators by Polycomb in human
embryonic stem cells. Cell. 125:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai KW, Koh KX, Loh M, Tada K, Subramaniam
MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B, Ito Y, et
al: Singapore Gastric Cancer Consortium: MicroRNA-130b regulates
the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer.
46:1456–1463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ito K, Liu Q, Salto-Tellez M, Yano T, Tada
K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, et al: RUNX3, a
novel tumor suppressor, is frequently inactivated in gastric cancer
by protein mislocalization. Cancer Res. 65:7743–7750. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soong R, Shah N, Peh BK, Chong PY, Ng SS,
Zeps N, Joseph D, Salto-Tellez M, Iacopetta B and Ito Y: The
expression of RUNX3 in colorectal cancer is associated with disease
stage and patient outcome. Br J Cancer. 100:676–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yanada M, Yaoi T, Shimada J, Sakakura C,
Nishimura M, Ito K, Terauchi K, Nishiyama K, Itoh K and Fushiki S:
Frequent hemizygous deletion at 1p36 and hypermethylation
downregulate RUNX3 expression in human lung cancer cell lines.
Oncol Rep. 14:817–822. 2005.PubMed/NCBI
|