Introduction
Parkinson's disease (PD) is an age-related
neurodegenerative disorder that is characterised by hypokinesia
(1). An oxidative stress insult
is one of the principal causes that is associated with the profound
loss of dopamine (DA)-producing neurons in the substantia nigra
pars compacta (2,3). Even though the mechanisms underlying
neuronal damage remain unclear, apoptosis or oxidative stress, as
crucial contributors to the pathogenesis of PD, have been reported
(4–8). Oxidative stress-induced cell lesions
are usually induced by hydrogen peroxide
(H2O2), hydroxyl radicals and superoxide,
which are known as reactive oxygen species (ROS) and are generated
through metabolic processes in cells. The oxidative stress in
vitro model has been proposed by using
H2O2 as an inducer when it is added to the
cell culture medium (9).
The Bcl-2 family, as regulatory proteins, are vital
apoptosis-related factors in H2O2-induced
oxidative stress in vitro models. Among this family, Bcl-2
is an anti-apoptotic protein and forms a heterodimer with Bax, an
apoptotic activator, to control the fate of cells (10,11). The ratio of Bax to Bcl-2 protein
is a common indicator with which to determine whether a cell is
alive or is dead via apoptosis. In addition, the extracellular
signal-regulated kinase 1/2 (ERK1/2), c-Jun kinases (JNKs) and p38
kinases have been reported to participate in the regulation of
apoptosis. ERK1/2, p38 and JNK are activated when a cell is injured
by oxidative stress or a pro-inflammatory environment (12,13). Abnormal levels of phosphorylated
JNK, p38 and ERK1/2 in the brains of patients with Alzheimer's
disease (AD) are associated with oxidative stress (14). Consequently, the illumination of
the biochemical processes surrounding
H2O2-mediated neuronal apoptosis may aid in
the understanding of the pathogenesis of neurodegenerative diseases
and in discovering new drug targets for the treatment of
diseases.
α-synuclein, a protein highly expressed in the human
brain, is localised in the inner membrane of the mitochondria.
α-synuclein not only dose-dependently inhibits complex I activity
of the mitochondrial respiratory chain, but also aggregates to form
insoluble fibrils in PD characterised by Lewy bodies (15). Tyrosine hydroxylase (TH) catalyses
the synthesis of catecholamines in the rate-limiting step.
Alterations in TH activity may be involved in PD. TH may help to
produce H2O2 and other ROS in pathological
conditions. Nevertheless, TH is also a possible target for the
damaging alterations induced by ROS or may be a target for
radical-mediated injury (16). It
has been proposed that the abnormal expression of TH induced by
oxidative damage leads to a reduction in DA levels, which is
associated with the degeneration of dopaminergic neurons in PD
(17). Therefore, α-synuclein and
TH may be novel drug targets.
In recent years, natural substances extracted from
plants have attracted increasing attention due to their unique
biological activities, such as neuroprotective potential that can
protect cells from oxidative damage. A number of Chinese herbal
effects have been evaluated and have been shown to exert beneficial
effects in various models related to PD (18,19), suggesting that herbs, as drug
candidates, have a bright future in the treatment of PD.
Astragaloside IV (AS-IV), an ingredient extracted from Astra
galus membranaceus, is frequently used as a food additive and
in herbal medicine. AS-IV is included in some efficacious medicinal
prescriptions and as a supplement in various health foods (20). AS-IV has been shown to exert
anti-hypertensive (21), positive
inotropic (22),
anti-inflammatory (23) and
anti-infarction effects (24).
Despite the use of Astragalus membranaceus as a traditional
therapy for degenerative diseases in China, few scientific studies
investigating the antioxidant mechanism of AS-IV in neurons have
been reported to date, at least to the best of our knowledge.
Moreover, further research is required in order to fully examine
the effects of the antioxidant activity of AS-IV.
Thus, the aims of the present study were to evaluate
the neuroprotective effects of AS-IV in vitro using SH-SY5Y
cells exposed to H2O2 and to discover novel
targets of AS-IV. Our findings demonstrate that AS-IV protects the
cells from oxidative damage by downregulating the Bax/Bcl-2 ratio.
The effects of AS-IV were also mediated via the downregulation of
the expression of α-synuclein and the increase in TH expression via
the p38 signalling pathway. To the very best of our knowledge, this
is a fundamental new discovery of the mechanisms through which
AS-IV protects neuronal cells from damage.
Materials and methods
Chemicals and reagents
AS-IV (Fig. 1),
with a purity >98%, was obtained from the Nanjing Zelang Medical
Technology Co., Ltd. (Nanjing, China). Bovine serum albumin (BSA),
3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO), 2′,7′-dichlorofluorescein diacetate
(DCFH-DA), vitamin C (Vit C) and H2O2 were
all purchased from Sigma-Aldrich (St. Louis, MO, USA). The Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
obtained from KeyGen Biotech Co., Ltd. (Nanjing, China). Fetal
bovine serum (FBS) and Dulbecco's modified Eagle's medium/F12
(DMEM/F12) were both purchased from Gibco (Grand Island, NY, USA).
Anti-α-synuclein antibody (#ab138501) was purchased from Epitomics
(Burlingame, CA, USA). Anti-β-actin (#3700), anti-Bcl-2 (#15071)
and anti-Bax (#5023) monoclonal antibodies were all purchased from
Cell Signalling Technology, Inc. (Beverly, MA, USA). Anti-p38
mitogen-activated protein kinase (MAPK; sc-7972), anti-p-p38
(sc-17852-R), anti-p-JNK (sc-293136), anti-ERK1/2 (sc-514302),
anti-p-ERK1/2 (sc-16981-R) and anti-TH (sc-7847) mono clonal
antibodies were all purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). All of the other reagents were of analytical
grade.
Cell culture
The SH-SY5Y cell line (#CRL-2266, ATCC, Rockville,
MD, USA) was cultured in DMEM supplemented with penicillin (final
concentration, 100 U/ml), streptomycin (final concentration, 0.1
mg/ml) and 10% (v/v) FBS in a humidified atmosphere of 5%
CO2 and 95% air at 37°C. The SH-SY5Y cells were plated
in 6-well plates for assays and 96-well plates (for MTT assay) at a
density of approximately 1×104 cells/well. After 24 h,
the cells were treated with various concentrations of AS-IV (50-200
µmol/l containing 0.1% DMSO) for 24 h and were then exposed
to the same fresh medium containing 300 µmol/l
H2O2 for 4 h.
Determination of cell viability
By means of MTT assay, we first assessed the drug
treatment toxicity. The cells (8,000 cells/well) were plated in
96-well microplates and grown in DMEM for 24 h and exposed to
various concentrations of H2O2 (100, 200, 300
and 400 µmol/l).
The effects of various concentrations of AS-IV on
cell viability were analysed following treatment for 24 h. The
medium was then replaced by 200 µl of fresh medium plus 10
µl of the MTT solution (1 mg/ml). The microplates were
incubated at 37°C in 5% CO2 for 4 h. The precipitated
formazan was dissolved in 100 µl DMSO. The optical density
of the samples was measured at 492 nm. The untreated SH-SY5Y cells
were used as controls. For pre-treatment with vitamin C (Vit C),
the SH-SY5Y cells were treated with 200 mg/l Vit C (A8100;
Solarbio, Beijing, China) for 1 h and then exposed to
H2O2 for 4 h.
Cell morphological observation
Following exposure to 300 µmol/l
H2O2, the SH-SY5Y cells were observed under a
phase-contrast microscope (TE2000-U; Nikon, Tokyo, Japan).
Moreover, the SH-SY5Y cells were observed under a microscope
following exposure to H2O2 and DAPI
staining.
Detection of apoptotic cells by flow
cytometry
The cell apoptotic rate following exposure to
H2O2 was assayed by flow cytometry using the
Annexin V-FITC/propidium iodide (PI) double-labelling method. The
SH-SY5Y cells (1×105 cells/ml) were seeded in 60-mm
dishes and treated with AS-IV and H2O2 (300
µmol/l). The cells were trypsinised and collected by
centrifugation at 800 rpm for 5 min. An Annexin V-FITC apoptosis
detection kit was used to double-stain the cells according to the
manufacturer's instructions. The samples were analysed using a
FACSVantage SE flow cytometer (BD Biosciences, San Jose, CA,
USA).
Measurement of intracellular ROS
levels
The SH-SY5Y cells (4×104 cells/ml) were
seeded in 6-well plates for 48 h. The cells were treated with AS-IV
for 24 h prior to exposure to H2O2 (300
µmol/l) for 4 h. Following stimulation, the cells were
incubated with 10 µmol/l DCFH-DA, which was oxidised to the
highly fluorescent compound, DCF, at 37°C for 30 min and, the cells
were then washed 3 times with phosphate-buffered saline (PBS). The
fluorescence intensity, expressed as the intracellular ROS, was
measured with a FACSVantage SE flow cytometer.
Immunofluorescence staining
The immunofluorescence staining technique was
performed as follows: briefly, the cells, on coverslips, were fixed
with a 4% paraformaldehyde solution for 20 min and were
permeabilised with 0.5% Triton X-100 in PBS for 15 min, and the
background was blocked with 5% BSA in PBS for 1 h before staining
with primary and secondary antibodies. Primary antibodies to
α-synuclein (1:100; #ab138501; rabbit anti-α-synuclein monoclonal
antibody; Epitomics) and TH (1:100; sc-7847; goat anti-TH
monoclonal antibody; Santa Cruz Biotechnology, Inc.) were diluted
by 0.5% Triton X-100 and incubated with the cells for 90 min at
37°C. A secondary FITC-conjugated goat-anti-rabbit IgG (1:100;
sc-2012; Santa Cruz Biotechnology, Inc.) and a TRITC-conjugated
mouse-anti-goat IgG (sc-516243) were diluted in 0.5% Triton X-100
and were incubated with the cells for 60 min at room temperature.
The cells were then stained with DAPI for 3 min. The slides were
rinsed with PBS after each step. The slides were mounted with
glycerol and stored for detection.
Western blot analysis
The SH-SY5Y cells (2×106 cells/well) were
seeded and treated with 10 µmol/l SB203580 (S8307;
Sigma-Aldrich) for 1 h or various concentrations of AS-IV for 24 h
prior to exposure to H2O2 (300 µmol/l)
for 4 h. The treated cells were collected and were resuspended in a
lysis buffer (RIPA buffer with protease inhibitor cocktail) at 4°C
for 2 h. The lysate was centrifuged at 12,000 rpm for 15 min at
4°C, and the supernatant was then collected. The Bradford assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and a UV
spectrophotometer were used to equalize the protein loading. Equal
amounts of protein (40 µg) were subjected to 12% SDS-PAGE
and were transferred onto polyvinylidene difluoride membranes.
Blocking buffer [5% (v/v) non-fat dry-milk in TBS containing 0.1%
Tween-20 (TBST), pH 7.5] was used to treat the membranes for 1 h at
room temperature. The membranes were then incubated with primary
antibodies to Bcl-2 (1:1,000), Bax (1:1,000), α-synuclein
(1:1,000), TH (1:1,000), p38 (1:1,000), p-p38 (1:1,000), ERK1/2
(1:1,000), p-ERK1/2 (1:1,000), p-JNK (1:1,000) and β-actin
(1:1,000) overnight at 4°C and were then incubated with
HRP-conjugated rabbit or mouse IgG secondary antibodies (1:1,000)
for 1 h. The secondary antibodies HRP-conjugated goat-anti-rabbit
IgG (sc-2004), HRP-conjugated goat-anti-mouse IgG (sc-2005) and
HRP-conjugated mouse anti-goat IgG (sc-2354) were obtained from
Santa Cruz Biotechnology, Inc. The proteins were detected by an
enhanced chemiluminescence (ECL) solution (Amersham Biosciences,
Buckinghamshire, UK), and densitometric analysis was performed with
the use of a PDI ImageWare System (Bio-Rad Laboratories, Inc.).
Statistical analysis
The results were analysed using a one-way analysis
of variance (one-way ANOVA) followed by the Tukey's test to examine
the effects of the different drug concentrations. The data are
expressed as the means ± SEM. A Dunnett's test and an ANOVA
followed by a Bonferroni correction were performed to determine the
statistical significance. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Protective effects of AS-IV against
H2O2-induced SH-SY5Y cell damage
We initially examined the viability of the SH-SY5Y
cells, which was examined by MTT assay, after incubating the cells
for 2, 4 and 6 h with H2O2 in the absence or
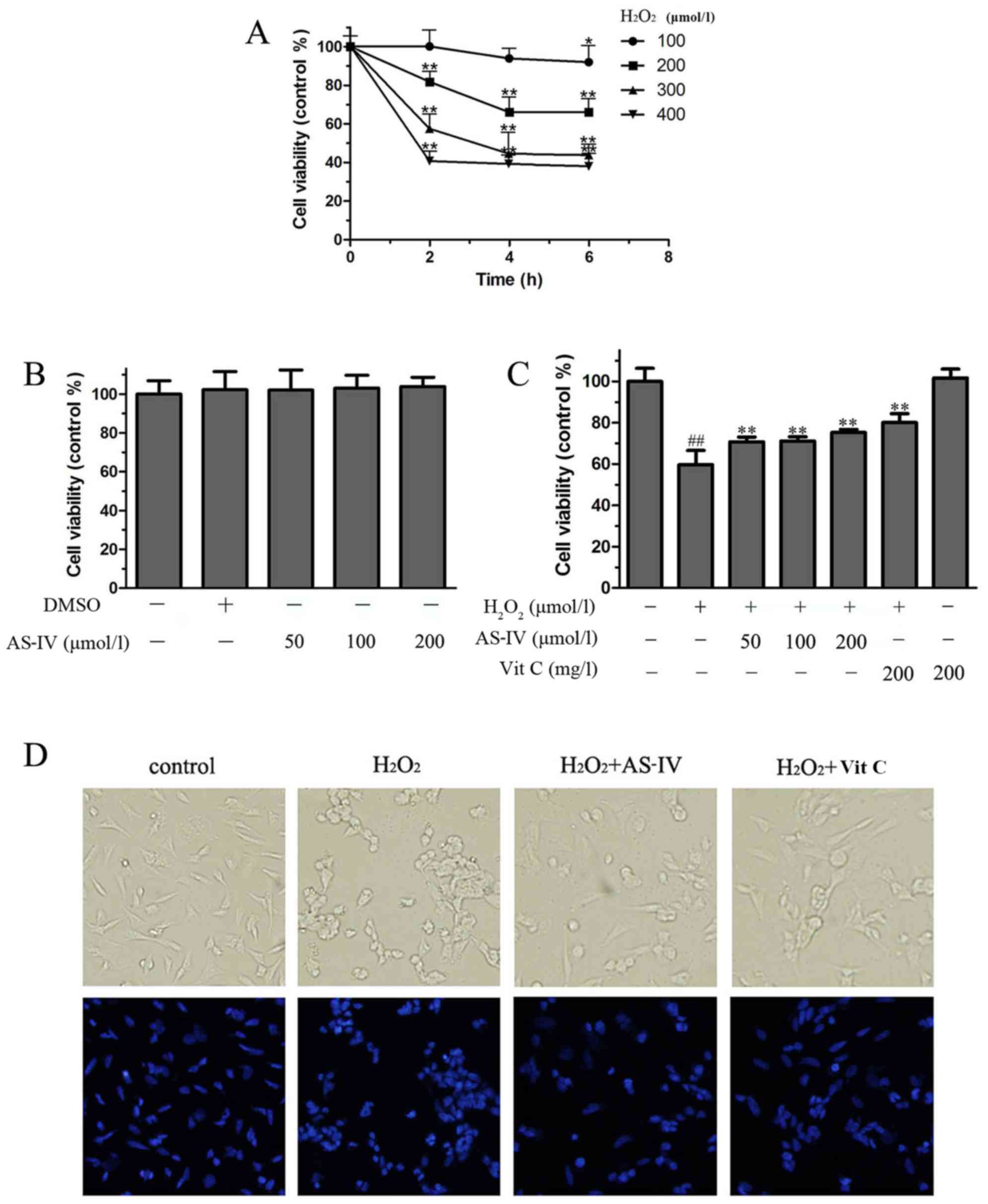
presence of AS-IV for 24 h. As shown in Fig. 2A, exposure to
H2O2 induced a gradual reduction in cell
viability in a time- and dose-dependent manner. The cell viability
was approximately 57% of the control value at 4 h following
H2O2 exposure (300 µmol/l). Therefore,
for all the subsequent experiments, a concentration of
H2O2 at 300 µmol/l was used for the
cell damage model.
As shown in Fig.
2B, cell viability was not affected by AS-IV at a concentration
of up to 200 µmol/l. After the SH-SY5Y cells were treated
with AS-IV (50–200 µmol/l) for 24 h and then exposed to
H2O2 (300 µmol/l) for 4 h, cell
viability was increased with the increasing concentrations of AS-IV
compared with the control group. As shown in Fig. 2C, cell viability following
exposure to H2O2 for only 4 h was 59% of the
control value. However, treatment with AS-IV (50, 100 and 200
µmol/l) for 24 h prior to exposure to
H2O2 increased cell viability to 70, 71 and
75%, respectively, which was similar to the activity of Vit C, a
drug that was used as a positive control. Evidently, AS-IV was
effective in protecting the SH-SY5Y cells against
H2O2-induced injury.
We also assessed the cell morphological changes
using a phase-contrast microscope. Exposure to 300 µmol/l
H2O2 for 4 h clearly induced the aggregation
and shrinkage of cell bodies and reduced the number of SH-SY5Y
cells. However, treatment with AS-IV (200 µmol/l) prior to
H2O2 exposure significantly prevented the
morphological manifestations of cell damage (Fig. 2D, upper panel). DAPI staining also
revealed that nuclear fragmentation and nuclear DNA condensation
occurred following exposure to 300 µmol/l
H2O2; however, pre-treatment with AS-IV
inhibited these apoptotic features, exerting effects similar to
those of Vit C at 200 mg/l (Fig.
2D, bottom panel). These results suggested that AS-IV exerted
an anti-apoptotic effect in SH-SY5Y cells exposed to
H2O2.
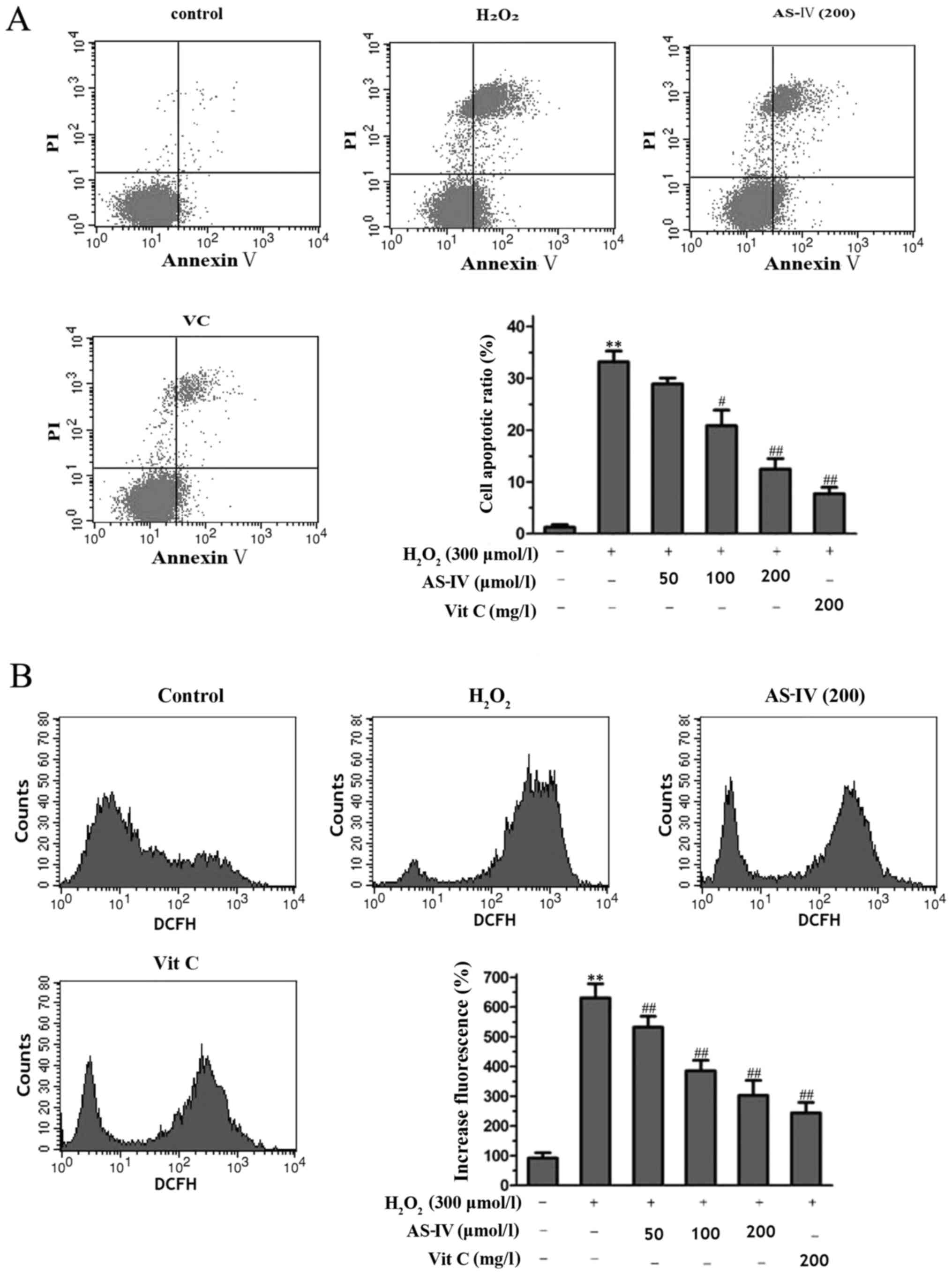
AS-IV inhibits the
H2O2-induced apoptosis of SH-SY5Y cells
The inhibitory effects of AS-IV on the apoptosis of
SH-SY5Y cells induced by H2O2 were assessed
by flow cytometry. As shown in Fig.
3A, the percentage of apoptotic cells increased to 33.2%
following exposure to 300 µmol/l H2O2
for 4 h. However, treatment with AS-IV (50, 100 and 200 mg/l) for
24 h prior to H2O2 exposure prevented
apoptosis in a concentration-dependent manner, and the rate of
apoptosis decreased to 28.9, 22.6 and 14.8%, respectively. AS-IV at
200 mg/l exerted similar effects to those of Vit C. Moreover,
incubation with AS-IV alone for 24 h had no effects on the cell
apoptotic ratio of the SH-SY5Y cells (data not shown).
AS-IV inhibits ROS production in SH-SY5Y
cells induced by H2O2
Intracellular ROS plays a crucial role in oxidative
stress-induced cell damage; thus, the effects of AS-IV on
H2O2-induced ROS production in SH-SY5Y cells
were assessed by flow cytometry. As shown in Fig. 3B, the cells with fluorescence
induced by 300 µmol/l H2O2 for 4 h
displayed a 6.5-fold increase greater than that of the control
group (P<0.05). By contrast, tre atment with AS-IV for 24 h
prior to exposure to H2O2 suppressed the
production of DCFH fluorescence (for ROS production) in a
concentration-dependent manner. The inhibitory effects of AS-IV at
200 µmol/l were significant (53.8% compared to the
H2O2-exposed group not treated with AS-IV
(P<0.05) and were similar to the effects of Vit C at 200
mg/l.
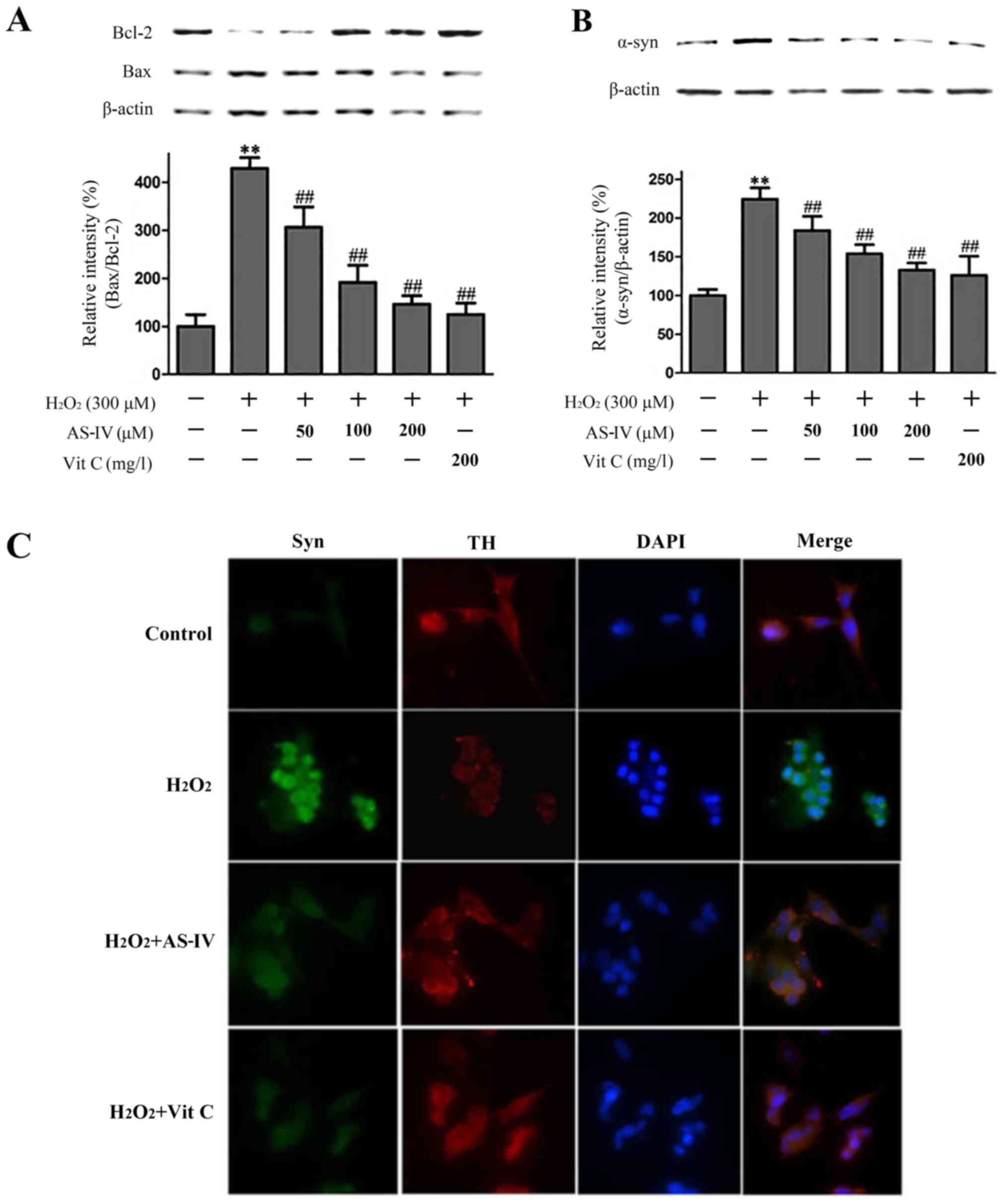
AS-IV decreases the
H2O2-induced increase in the Bax/Bcl-2 ratio
in SH-SY5Y cells
The levels of Bax and Bcl-2 were determined by
western blot analysis as Bcl-2 family members are involved in
apoptosis. As shown in Fig. 4A,
compared with the control, Bax protein expression was upregulated
in the H2O2-exposed group, while AS-IV
treatment downregulated Bax expression. The levels of Bcl-2
decreased in the H2O2 group and increased
following AS-IV pre-treatment. The Bax/Bcl-2 ratio wass increased
4.2-fold of the normal control group upon exposure to
H2O2, whereas this increase was attenuated in
the cells pre-treated with 50–200 µmol/l AS-IV.
AS-IV decreases the expression of
α-synuclein and increases the expression of TH in SH-SY5Y
cells
α-synuclein is a major component of Lewy bodies that
plays an important role in H2O2-induced
apoptosis in neurons (25). Thus,
to determine whether the observed neuroprotective effects of AS-IV
were associated with the expression of α-synuclein, we performed
western blot analysis using a monoclonal rabbit antibody against
α-synuclein. As shown in Fig. 4B,
when the cells were exposed to H2O2, the
expression of α-synuclein significantly increased 2.25-fold
compared with the control group. By contrast, pre-treatment with
AS-IV (50, 100 and 200 µmol/l) markedly decreased the
expression of α-synuclein by 22.2, 27.1 and 42.2%, respectively,
compared with the H2O2-exposed cells. To
further confirm this effect, we performed a double
immunofluorescence assay using a monoclonal rabbit antibody against
α-synuclein. As shown in Fig. 4C,
the results were consistent with those of western blot analysis.
These results suggest that AS-IV inhibited the overexpression of
α-synuclein induced by H2O2. Consequently, we
hypothesized that the neuroprotective effects of AS-IV against
oxidative stress-induced damage are mediated by the decrease in the
expression of α-synuclein.
Previous studies have shown that α-synuclein,
implicated in the pathogenesis of PD, is involved in the regulation
of DA metabolism, possibly by downregulating the expression of TH
(26). TH catalyses the
rate-limiting step in the biosynthesis of the catecholamines, DA,
norepinephrine and epinephrine (27). Therefore, TH expression was also
evaluated by double immunofluorescence assay. As shown in Fig. 4C, pre-treatment with AS-IV
prevented the H2O2-induced downregulation of
TH levels in the SH-SY5Y cells. The results indicated that AS-IV
treatment inhibited H2O2-induced α-synuclein
upregulation.
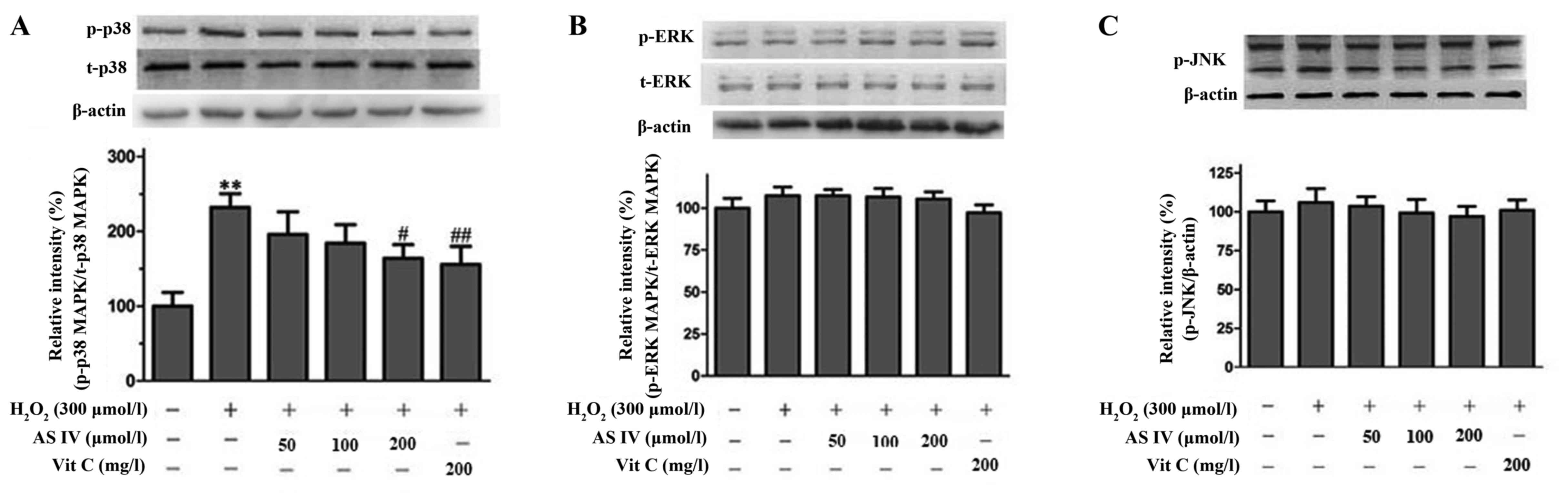
AS-IV attenuates the
H2O2-induced phosphorylation of p38 in
SH-SY5Y cells
To clarify the mechanisms underlying the
neuroprotective effects of AS-IV, we evaluated the expression of
p-p38, p-JNK and p-ERK to determine whether the protective effects
of AS-IV are mediated via the MAPK signalling pathway. Our results
revealed that the levels of phosphorylated p38 increased (Fig. 5A); however, no effect was observed
on the levels of phosphorylated ERKl/2 (Fig. 5B) and phosphorylated JNK (Fig. 5C) in the
H2O2-exposed cells. By contrast, AS-IV (200
µmol/l) pre-treatment markedly decreased the levels of
phosphorylated p38 compared with those in the untreated cells
exposed to H2O2 (Fig. 5A).
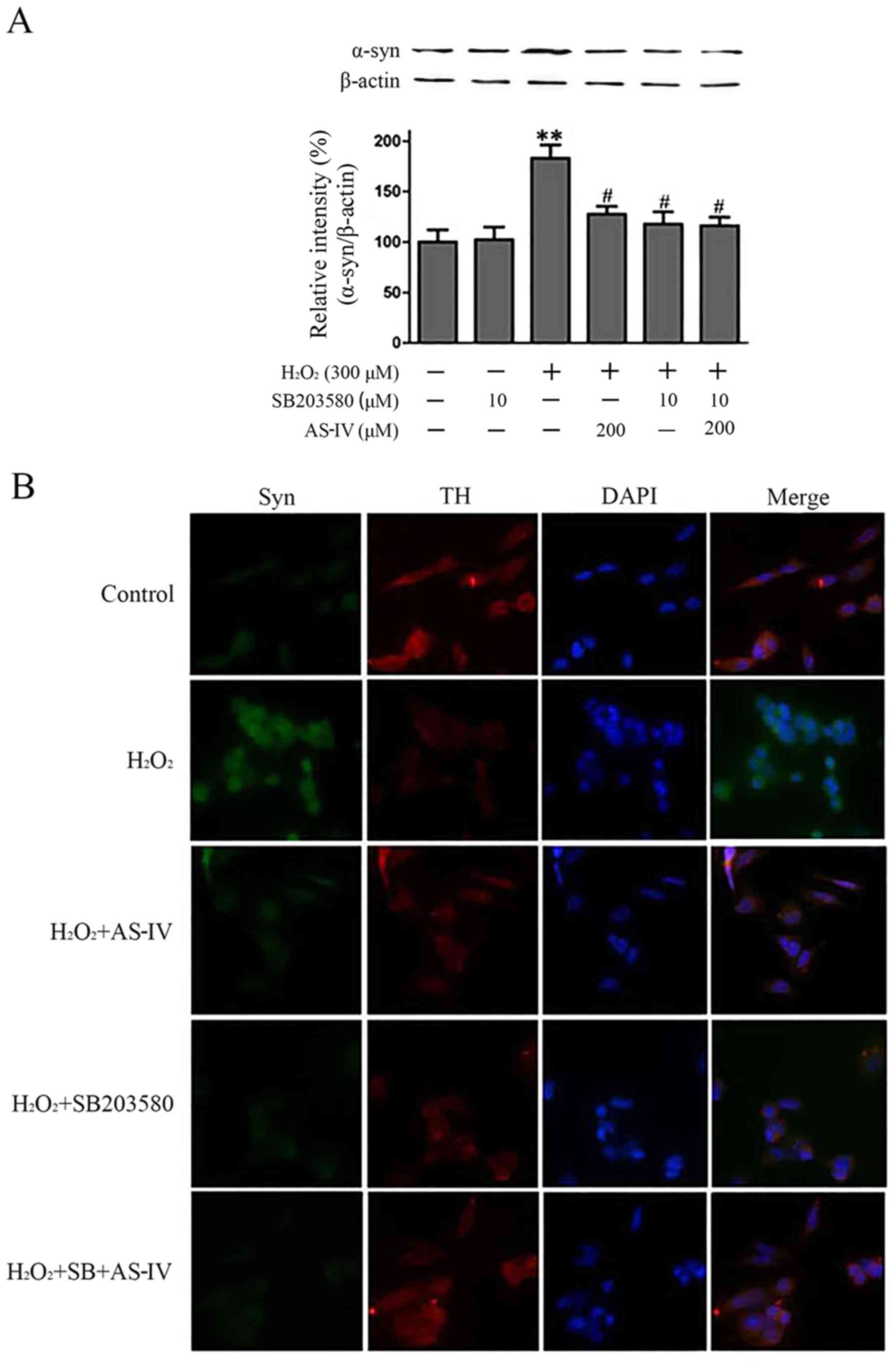
To elucidate whether H2O2
stimulates α-synuclein expression via the p38 MAPK signalling
pathway, a chemical inhibitor targeting p38 kinase (SB203580) was
used. The SH-SY5Y cells were pre-incubated with SB203580 (10
µmol/l) for 1 h and were then exposed to
H2O2 (300 µmol/l) for 4 h, and the
levels of α-synuclein were determined. As shown in Fig. 6A, AS-IV (200 µmol/l)
pre-treatment markedly decreased the expression of α-synuclein
0.73-fold compared with the untreated cells exposed to
H2O2.
Of note, when the H2O2-exposed
SH-SY5Y cells were incubated with SB203580 alone, the expression of
α-synuclein was attenuated compared with the
H2O2 group, and incubation of the cells with
SB203580, H2O2 and AS-IV (200 µmol/l)
together also decreased the expression of α-synuclein. We found
identical results in the double immunofluorescence analysis, as
shown in Fig. 6B. Thus, these
results indicated that AS-IV protected the SH-SY5Y cells from
oxidative stress-induced damage and that these effects were
mediated via the decrease in the expression of α-synuclein through
the p38 MAPK signalling pathway.
Discussion
PD is a neurodegenerative disorder resulting from
the gradual and progressive loss of dopaminergic neurons in the
substantia nigra. Accumulating evidence suggests that a
pathological mechanism for this is that oxidative stress is
implicated in different neurodegenerative diseases, such as PD and
AD (28–32). H2O2, as an
oxidative stress inducer, is widely used in vitro models
(4,33). The human neuroblastoma cell line,
SH-SY5Y, is extensively used as a cell model for researching
neuronal cell death induced by H2O2 (34,35). In this study,
H2O2-induced oxidative stress in SH-SY5Y
cells was used to examine H2O2-induced
neurotoxicity and the effects of AS-IV.
Although the exact mechanisms of oxidative stress
are not completely clear, the use of antioxidant agents as a method
of neuroprotection may be a potential treatment strategy for
neurode generative diseases (36). AS-IV, as a major active
constituent of Astragalus membranaceus, exerts multipotent
effects under pathophysiological conditions. As previously
demonstrated, AS-IV not only protected primary DA neurons from
6-OHDA-induced neurotoxicity and neurodegeneration, but also
promoted dopaminergic neurite outgrowth (37). In our study, we demonstrated that
AS-IV exerted protective effects against the
H2O2-induced loss of cell viability.
Similarly, the results of DAPI staining suggested that AS-IV
prevented H2O2-induced morphological changes
associated with apoptosis in the SH-SY5Y cells.
It is well known that ROS can injure the cardinal
cellular components, such as proteins, DNA and lipids, resulting in
subsequent cell death by necrosis or apoptosis. To provide further
evidence, we examined the effects of AS-IV on cells by flow
cytometry. When the SH-SY5Y cells were pre-treated with AS-IV for
24 h and were then cultured with 300 µmol/l
H2O2 for 4 h, the percentage of apoptotic
cells decreased and the production of ROS was also reduced compared
with the cells exposed to H2O2 alone.
Therefore, the protective effects of AS-IV may contribute to the
antioxidant activity. However, the balance and maintenance of the
intracellular redox state is largely dependent on the existance of
glutathione, particularly the ratio of glutathione (GSH) to
oxidized glutathione (GSSG). An increased GSSG-to-GSH ratio is an
important indicator of oxidative stress. Some studies have analysed
the status of GSH in SH-SY5Y cells induced by
H2O2 (38–40). In this study, we wished to
determine the mechanisms involved in the protective effects of
AS-IV against oxidative stress. We focused on α-synuclein, a very
important factor, and thus the levels and the status of GSH in the
SH-SY5Y cells were not analysed in our study. α-synuclein is a
14-kDa soluble, intrinsically unfolded protein that is expressed in
all neurons (41,42). The overexpression of α-synuclein
may be associated with the selective dege neration and toxicity of
dopaminergic neurons (43). TH,
as a key enzyme, plays a critical role in the differentiation and
survival of dopaminergic neurons. In this study, we found that
AS-IV inhibited the overexpression of α-synuclein induced by
H2O2 and increased the levels of TH. Our
results provide new evidence that AS-IV protects cells from
H2O2-induced injury and is associated with
the expression of α-synuclein and TH. Cell survival depends mostly
on the balance between the anti- and pro-apoptotic proteins of the
Bcl-2 family in the early phases of the apoptotic cascade. Any
changes in the balance between the anti- and pro-apoptotic proteins
will affect cell death. Bax (pro-apoptotic group) and Bcl-2
(anti-apoptotic group) play a vital role in the apoptotic pathway.
The ratio of Bax/Bcl-2 is a better predictor of apoptosis than the
absolute expression of either Bcl-2 or Bax alone (44). Our results revealed that the
expression of these Bcl-2 family members was influenced profoundly
by H2O2. Treatment with AS-IV upregulated the
expression of anti-apoptotic Bcl-2 and reduced the expression of
pro-apoptotic Bax. Thereby, AS-IV decreased the
H2O2-induced increase in the Bax/Bcl-2 ratio
in SH-SY5Y cells.
ROS play a key role as a second messenger in signal
transduction cascades and regulate signalling pathways. The
intracellular redox state may lead to the activation of MAPKs
(45).
H2O2, as an oxidative stress inducer, may
activate ERK and p38 proteins in cells, which may be closely
related to MAPK signalling (46,47). In our experiments, treatment with
AS-IV notably inhibited the H2O2-induced
increase in phosphorylated p38 levels. We further found that the
protective effects of AS-IV were associated with the expression of
α-synuclein and TH through the p38 MAPK signalling pathway using
chemical inhibitors targeting p38 kinase (SB203580).
In conclusion, the results of this study
demonstrated that AS-IV decreased
H2O2-induced cell damage, prevented cell
morphologic changes, and decreased ROS production and the apoptotic
rate. Furthermore, the neuroprotective effects of AS-IV against the
H2O2-induced apoptosis of SH-SY5Y cells were
associated with the downregulation of the Bax/Bcl-2 ratio,
decreased levels of α-synuclein and increased levels of TH via the
p38 MAPK signalling pathway. Our study may provide a novel
therapeutic strategy for PD, although further research into the
neuroprotective mechanisms of AS-IV is warranted.
Acknowledgments
We are grateful for the financial support from the
National Nature Science Foundation of China (no. 81370403),
Chongqing Foundation and Advanced Research Project (no.
CSTC2015jcyjBX0053), and Chongqing Medical University Scientific
Research Cultivating Fund (no. 201414).
References
|
1
|
Lesage S and Brice A: Parkinson's disease:
from monogenic forms to genetic susceptibility factors. Hum Mol
Genet. 18(R1): R48–R59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braak H, Rüb U, Gai WP and Del Tredici K:
Idiopathic Parkinson's disease: possible routes by which vulnerable
neuronal types may be subject to neuroinvasion by an unknown
pathogen. J Neural Transm Vienna. 110:517–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schapira AH: Mitochondrial dysfunction in
neurodegenerative diseases. Neurochem Res. 33:2502–2509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jenner P and Olanow CW: Oxidative stress
and the pathogenesis of Parkinson's disease. Neurology. 47(Suppl
3): S161–S170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owen AD, Schapira AH, Jenner P and Marsden
CD: Oxidative stress and Parkinson's disease. Ann N Y Acad Sci.
786:217–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orth M and Schapira AH: Mitochondrial
involvement in Parkinson's disease. Neurochem Int. 40:533–541.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown JM and Yamamoto BK: Effects of
amphetamines on mitochondrial function: role of free radicals and
oxidative stress. Pharmacol Ther. 99:45–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gandhi S and Wood NW: Molecular
pathogenesis of Parkinson's disease. Hum Mol Genet. 14:2749–2755.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoh T, Sakai N, Enokido Y, Uchiyama Y
and Hatanaka H: Free radical-independent protection by nerve growth
factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered
apoptosis. J Biochem. 120:540–546. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
12
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakano H, Nakajima A, Sakon-Komazawa S,
Piao JH, Xue X and Okumura K: Reactive oxygen species mediate
crosstalk between NF-kappaB and JNK. Cell Death Differ. 13:730–737.
2006. View Article : Google Scholar
|
|
14
|
Zhu X, Lee HG, Raina AK, Perry G and Smith
MA: The role of mitogen-activated protein kinase pathways in
Alzheimer's disease. Neurosignals. 11:270–281. 2002. View Article : Google Scholar
|
|
15
|
Liu G, Zhang C, Yin J, Li X, Cheng F, Li
Y, Yang H, Uéda K, Chan P and Yu S: α-Synuclein is differentially
expressed in mitochondria from different rat brain regions and
dose-dependently down-regulates complex I activity. Neurosci Lett.
454:187–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao F, Zhang L, Wessel J, Zhang K, Wen G,
Kennedy BP, Rana BK, Das M, Rodriguez-Flores JL, Smith DW, et al:
Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine
biosynthesis: discovery of common human genetic variants governing
transcription, autonomic activity, and blood pressure in vivo.
Circulation. 116:993–1006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Javoy-Agid F, Hirsch EC, Dumas S,
Duyckaerts C, Mallet J and Agid Y: Decreased tyrosine hydroxylase
messenger RNA in the surviving dopamine neurons of the substantia
nigra in Parkinson's disease: an in situ hybridization study.
Neuroscience. 38:245–253. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houghton PJ and Howes MJ: Natural products
and derivatives affecting neurotransmission relevant to Alzheimer's
and Parkinson's disease. Neurosignals. 14:6–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen LW, Wang YQ, Wei LC, Shi M and Chan
YS: Chinese herbs and herbal extracts for neuroprotection of
dopaminergic neurons and potential therapeutic treatment of
Parkinson's disease. CNS Neurol Disord Drug Targets. 6:273–281.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: a literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang WD, Zhang C, Liu RH, Li HL, Zhang
JT, Mao C, Moran S and Chen CL: Preclinical pharmacokinetics and
tissue distribution of a natural cardioprotective agent
astragaloside IV in rats and dogs. Life Sci. 79:808–815. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li ZP and Cao Q: Effects of astragaloside
IV on myocardial calcium transport and cardiac function in ischemic
rats. Acta Pharmacol Sin. 23:898–904. 2002.
|
|
23
|
Zhang WJ, Hufnagl P, Binder BR and Wojta
J: Antiinflammatory activity of astragaloside IV is mediated by
inhibition of NF-kappaB activation and adhesion molecule
expression. Thromb Haemost. 90:904–914. 2003.PubMed/NCBI
|
|
24
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spillantini MG, Schmidt ML, Lee VM,
Trojanowski JQ, Jakes R and Goedert M: Alpha-synuclein in Lewy
bodies. Nature. 388:839–840. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singleton AB, Farrer M, Johnson J,
Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra
A, Nussbaum R, et al: alpha-Synuclein locus triplication causes
Parkinson's disease. Science. 302:8412003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima A, Hayashi N, Kaneko YS, Mori K,
Sabban EL, Nagatsu T and Ota A: Role of N-terminus of tyrosine
hydroxylase in the biosynthesis of catecholamines. J Neural Transm
(Vienna). 116:1355–1362. 2009. View Article : Google Scholar
|
|
28
|
Fahn S and Cohen G: The oxidant stress
hypothesis in Parkinson's disease: evidence supporting it. Ann
Neurol. 32:804–812. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith MA, Perry G, Richey PL, Sayre LM,
Anderson VE, Beal MF and Kowall N: Oxidative damage in Alzheimer's.
Nature. 382:120–121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Markesbery WR: Oxidative stress hypothesis
in Alzheimer's disease. Free Radic Biol Med. 23:134–147. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emerit J, Edeas M and Bricaire F:
Neurodegenerative diseases and oxidative stress. Biomed
Pharmacother. 58:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Halliwell B: Oxidative stress and
neurodegeneration: where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu XJ, Zheng YJ, Cui YY, Zhu L, Lu Y and
Chen HZ: Propofol attenuates oxidative stress-induced PC12 cell
injury via p38 MAP kinase dependent pathway. Acta Pharmacol Sin.
28:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heo SR, Han AM, Kwon YK and Joung I: 62
protects SH-SY5Y neuroblastoma cells against
H2O2-induced injury through the PDK1/Akt
pathway. Neurosci Lett. 450:45–50. 2009. View Article : Google Scholar
|
|
35
|
Xiong Y, Ding H, Xu M and Gao J:
Protective effects of asiatic acid on rotenone- or
H2O2-induced injury in SH-SY5Y cells.
Neurochem Res. 34:746–754. 2009. View Article : Google Scholar
|
|
36
|
Yuan H, Zheng JC, Liu P, Zhang SF, Xu JY
and Bai LM: Pathogenesis of Parkinson's disease: oxidative stress,
environmental impact factors and inflammatory processes. Neurosci
Bull. 23:125–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan WS, Durairajan SS, Lu JH, Wang Y, Xie
LX, Kum WF, Koo I, Yung KK and Li M: Neuroprotective effects of
astragaloside IV in 6-hydroxydopamine-treated primary nigral cell
culture. Neurochem Int. 55:414–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tian X, Guo LP, Hu XL, Huang J, Fan YH,
Ren TS and Zhao QC: Protective effects of Arctium lappa L. roots
against hydrogen peroxide-induced cell injury and potential
mechanisms in SH-SY5Y cells. Cell Mol Neurobiol. 35:335–344. 2015.
View Article : Google Scholar
|
|
39
|
Jia Z, Zhu H, Misra HP and Li Y: Potent
induction of total cellular GSH and NQO1 as well as mitochondrial
GSH by 3H-1,2-dithiole-3-thione in SH-SY5Y neuroblastoma cells and
primary human neurons: protection against neurocytotoxicity
elicited by dopamine, 6-hydroxydopamine, 4-hydroxy-2-nonenal, or
hydrogen peroxide. Brain Res. 1197:159–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu XL, Niu YX, Zhang Q, Tian X, Gao LY,
Guo LP, Meng WH and Zhao QC: Neuroprotective effects of kukoamine B
against hydrogen peroxide-induced apoptosis and potential
mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol. 40:230–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Totterdell S, Hanger D and Meredith GE:
The ultrastructural distribution of alpha-synuclein-like protein in
normal mouse brain. Brain Res. 1004:61–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uversky VN: Alpha-synuclein misfolding and
neurodegenerative diseases. Curr Protein Pept Sci. 9:507–540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu S, Uéda K and Chan P: Alpha-synuclein
and dopamine metabolism. Mol Neurobiol. 31:243–254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eguchi M, Monden K and Miwa N: Role of
MAPK phosphorylation in cytoprotection by pro-vitamin C against
oxidative stress-induced injuries in cultured cardiomyoblasts and
perfused rat heart. J Cell Biochem. 90:219–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ruffels J, Griffin M and Dickenson JM:
Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human
SH-SY5Y neuroblastoma cells: role of ERK1/2 in
H2O2-induced cell death. Eur J Pharmacol.
483:163–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC,
Lee SY and Jang CG: Loganin protects against hydrogen
peroxide-induced apoptosis by inhibiting phosphorylation of JNK,
p38 and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58:533–541.
2011. View Article : Google Scholar : PubMed/NCBI
|