Introduction
Colorectal cancer (CRC), as the third most common
cancer affecting the gastrointestinal tract, is a common type of
cancer worldwide and is associated with a high mortality rate
(1–3). The majority of cases of mortality
from CRC occur in patients with metastases from the primary cancer
(4). In patients with CRC, ~10%
have lung metastasis (5,6); the lungs are the second most common
site of metastasis in CRC, following the liver (7). Currently, the diagnosis of CRC in
patients with pulmonary metastases depends on radiology and biopsy
(8). However, radiology-guided
biopsy has its limitation of sensitivity (9). Therefore, to improve the survival of
patients with CRC, it is necessary to explore novel biomarkers and
the molecular mechanism of metastatic progression in CRC that may
be useful for earlier diagnosis and treatment of patients with lung
metastases.
It has been reported that non-coding RNA, including
transfer RNA (tRNA), microRNA (miRNA), long non-coding RNA and
circular RNA (circRNA), are involved in biological and pathological
processes (10–12). In contrast to tRNA, miRNA and long
non-coding RNA, circRNA are less well characterized. circRNA were
first identified in RNA viruses as early as the 1970s (13); however, the majority of these were
misconstrued as 'splicing rubbish' (14). Since a study that firstly ascribed
an actual function to one of these circRNA molecules (15), the potential of circRNA has
elevated the scientific community's awareness of these molecules
(16). With the development of
RNA deep sequencing technology and bioinformatics, study has
revealed that large numbers of circRNA are endogenous, abundant,
conserved and stable in mammalian cells (15,17–22), and regulate gene expression at the
transcriptional or post-transcriptional level by interacting with
miRNA or other molecules (23).
Given that circRNA interact with miRNA to regulate their target
genes, circRNA may be involved in diseases correlated with miRNA
(24). Recently, circRNA have
been reported to mediate cancer progression (12), and the global abundance of circRNA
was demonstrated to be lower in CRC than in normal tissue (25). However, to date, little is known
about the genome-wide expression and function analysis of circRNA
in CRC tissues of patients with lung metastasis.
Therefore, the present study examined differentially
expressed circRNA in tissues from patients with CRC with and
without lung metastasis so as to identify novel diagnostic and
prognostic markers in patients with CRC with lung metastasis.
Materials and methods
Study approval
All samples were collected from patients between
January, 2013 and December, 2015 in the Department of
Gastrointestinal and Hernia Surgery, the First Affiliated Hospital
of Kunming Medical University (Kunming, China). The project was
reviewed and approved by the Ethics Committee of the First
Affiliated Hospital of Kunming Medical University. All patients
included in the present study provided written informed consent
prior to surgery.
Samples
Primary CRC samples were obtained from 3 male
patients (age, 66.33±7.37 years) with CRC and lung metastasis (Exp
A) and 3 male patients (age, 64.67±11.93 years) with CRC without
metastasis (Ctrl A), respectively. All patients were histologically
confirmed to have CRC based on colonoscopy (26) and did not receive any other forms
of therapy at the time of enrollment. At the time of surgery, all
tissue samples were immediately frozen in liquid nitrogen and
stored at −80°C for further use.
RNA extraction and quality control
According to the manufacturer's protocol, total RNA
was extracted from each sample using a homogenizer and TRIzol
regent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Subsequently, the quantification and quality of purified RNA
were assessed using a NanoDrop ND-1000 (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.). The integrity of RNA was assessed
by electrophoresis on a denaturing 1.5% agarose gel.
Microarray analysis
Sample labeling and human circRNA array
hybridization were performed according to the manufacturer's
protocol (Arraystar, Inc., Rockville, MD, USA). Briefly, total RNA
were digested with RNase R (Epicentre; Illumina, Inc., San Diego,
CA, USA) to remove linear RNA and enrich circRNA. Subsequently, the
enriched circRNA were amplified and transcribed into fluorescent
cRNA utilizing a random priming method (Arraystar Super RNA
Labeling kit; Arraystar, Inc.). The labeled cRNA were purified
using an RNeasy mini kit (Qiagen GmbH, Hilden, Germany). The
concentration and specific activity of the labeled cRNA (pmol
Cy3/µg cRNA) were measured using a NanoDrop ND-1000. A total
of 1 µg of each labeled cRNA was fragmented by adding 5
µl 10X blocking agent and 1 µl 25X fragmentation
buffer (Arraystar Super RNA Labeling kit; Arraystar, Inc.).
Subsequently, the mixture was heated at 60°C for 30 min, and
finally 25 µl 2X hybridization buffer was added to dilute
the labeled cRNA. Following this, 50 µl hybridization
solution was dispensed into the gasket slide and assembled to the
circRNA expression microarray slide. The slides were incubated for
17 h at 65°C in an Agilent Hybridization Oven (Agilent
Technologies, Inc., Santa Clara, CA, USA). The hybridized arrays
were washed using Agilent wash buffer 1 and wash buffer 2, fixed
and scanned using the Agilent Scanner G2505C (both from Agilent
Technologies, Inc.).
Data analysis
Scanned images were imported into Agilent Feature
Extraction software (version 11.0.1.1; Agilent Technologies, Inc.)
for raw data extraction. When comparing two groups of profile
differences (such as CRC with lung metastasis vs. without lung
metastasis), the fold change (i.e. the ratio of the group averages)
between the groups for each circRNA is computed. The statistical
significance of the difference may be conveniently estimated by a
t-test. circRNA with fold changes ≥2 and P-values of <0.05 were
selected as being significantly differentially expressed.
Gene ontology (GO) analysis
GO analysis (geneontology.org) may be used to construct meaningful
annotation of gene products in a wide variety of organisms. GO
contains three domains, including biological process (BP), cellular
components (CC) and molecular function (MF). The -log10
(P-value) indicates the enrichment score representing the
significance of GO term enrichment among genes producing
differentially expressed circRNA. Fold enrichment represents the
proportion of the changes in genes as proportions in the GO
database in this function (such as, the greater the proportion, the
more reliability, which is seen as the more significant changes in
BP, CC or MF).
Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analysis
KEGG (genome.jp/kegg/) was utilized to harvest pathway
clusters covering our knowledge of the molecular interaction and
reaction networks in genes producing differentially expressed
circRNA.
Annotation for circRNA/miRNA
interactions
In the present study, circRNA/miRNA interactions
were predicted with Arraystar's home-made miRNA target prediction
software (Arraystar, Inc.) based on TargetScan (targetscan.org/vert_71/) and miRanda (microrna.org/microrna/home.do). The
differentially expressed circRNA within all the comparisons were
annotated in detail with the circRNA/miRNA interaction
information.
Results
Overview of differentially expressed
circRNA in the tissues of patients with CRC and lung
metastasis
High-throughput microarray is an efficient approach
for studying the biological function of RNA. As demonstrated in
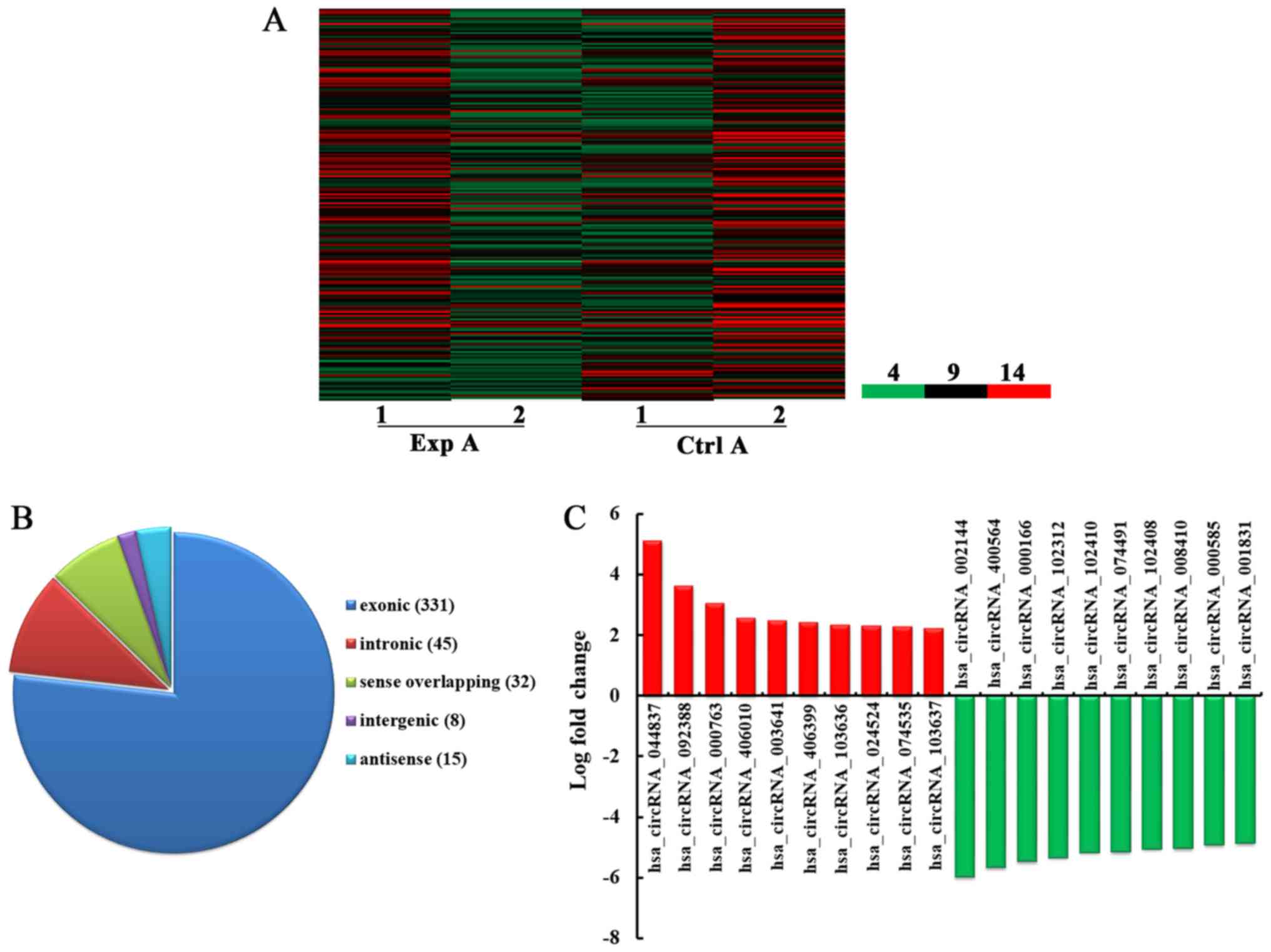
Fig. 1A, a total of 431 circRNA
were detected to be differentially expressed with fold change ≥2.0,
P<0.05 and false discovery rate <0.05 in the Exp A group
compared with the Ctrl A group. Among them, 192 and 239 circRNA
were upregulated and downregulated, respectively. Among the
dysregulated circRNA, in the light of their relation with their
coding genes, the circRNA were classified into five categories: 331
were exonic, 45 were intronic, 32 were sense overlapping, eight
were intergenic and 15 were antisense (Fig. 1B). According to the log fold
change, the top 10 upregulated and downregulated circRNA were
selected (Fig. 1C).
BP analysis of the genes producing
upregulated circRNA
According to the number of linear counterparts of
differentially overexpressed circRNA involved in BPs, the top 10
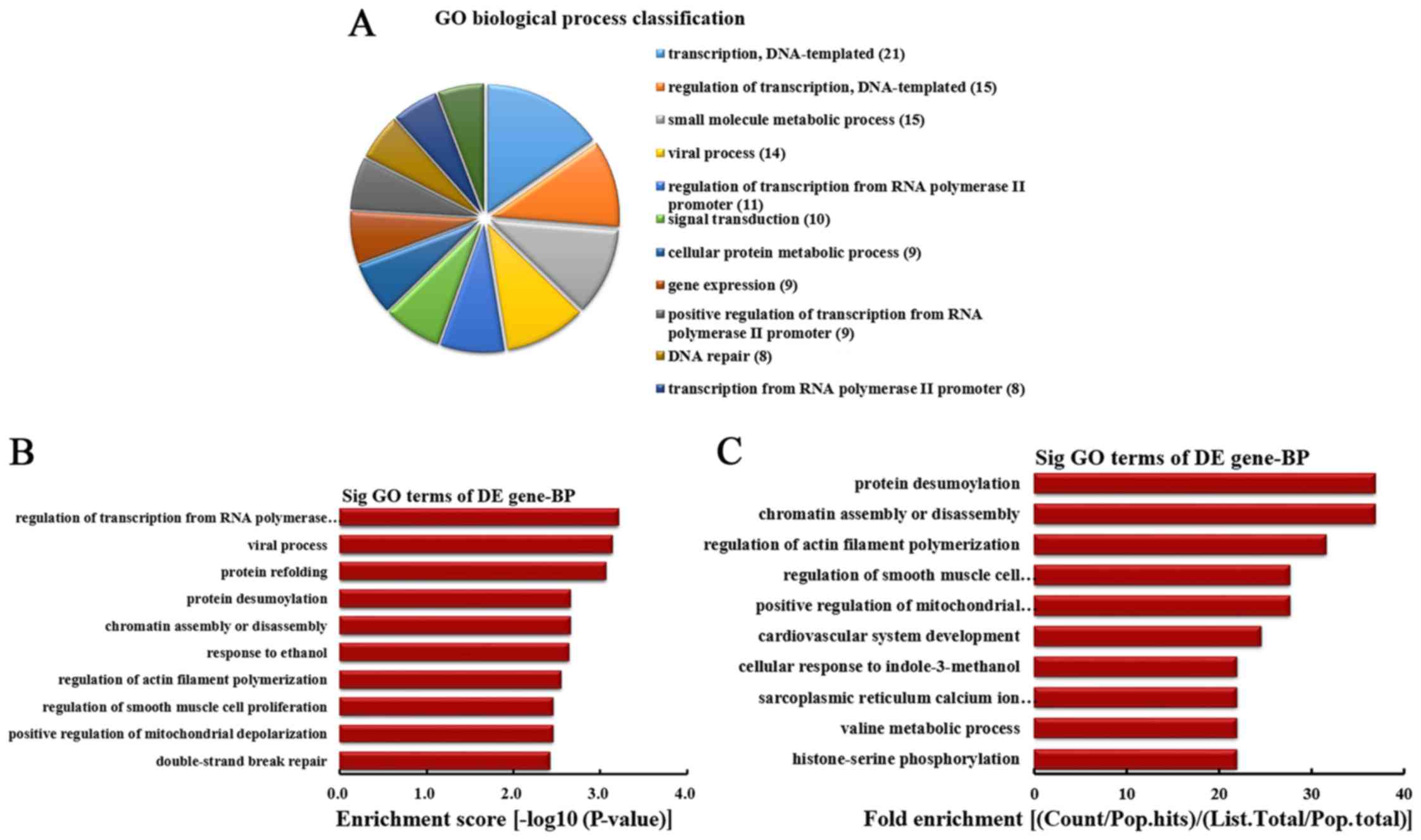
BPs were classified (Fig. 2A).
Among them, 21 linear counterparts of differentially overexpressed
circRNA were involved in transcription, DNA-templated, 15 were
involved in regulation of transcription, DNA-templated, 15 were
involved in small molecule metabolic process, 14 were involved in
viral process, 11 were involved in the regulation of transcription
from RNA polymerase II promoter, 10 were involved in signal
transduction, 9 were involved in cellular protein metabolic
process, 9 were involved in gene expression, 9 were involved in
positive regulation of transcription from RNA polymerase II
promoter and 8 were involved in DNA repair. According to the
enrichment score, the top 10 BPs were selected (Fig. 2B). These included regulation of
transcription from RNA polymerase II promoter, viral process,
protein refolding, protein desumoylation, chromatin assembly or
disassembly, response to ethanol, regulation of actin filament
polymerization, regulation of smooth muscle cell proliferation,
positive regulation of mitochondrial depolarization and
double-strand break repair. Additionally, according to the fold
enrichment, the top 10 BPs were selected (Fig. 2C). These included protein
desumoylation, chromatin assembly or disassembly, regulation of
actin filament polymerization, regulation of smooth muscle cell
proliferation, positive regulation of mitochondrial depolarization,
cardiovascular system development, cellular response to
indole-3-methanol, sarcoplasmic reticulum calcium ion transport,
valine metabolic process and histone-serine phosphorylation.
BP analysis of the genes producing
downregulated circRNA
According to the number of linear counterparts of
down-regulated circRNA involved in BPs, the top 10 BPs were
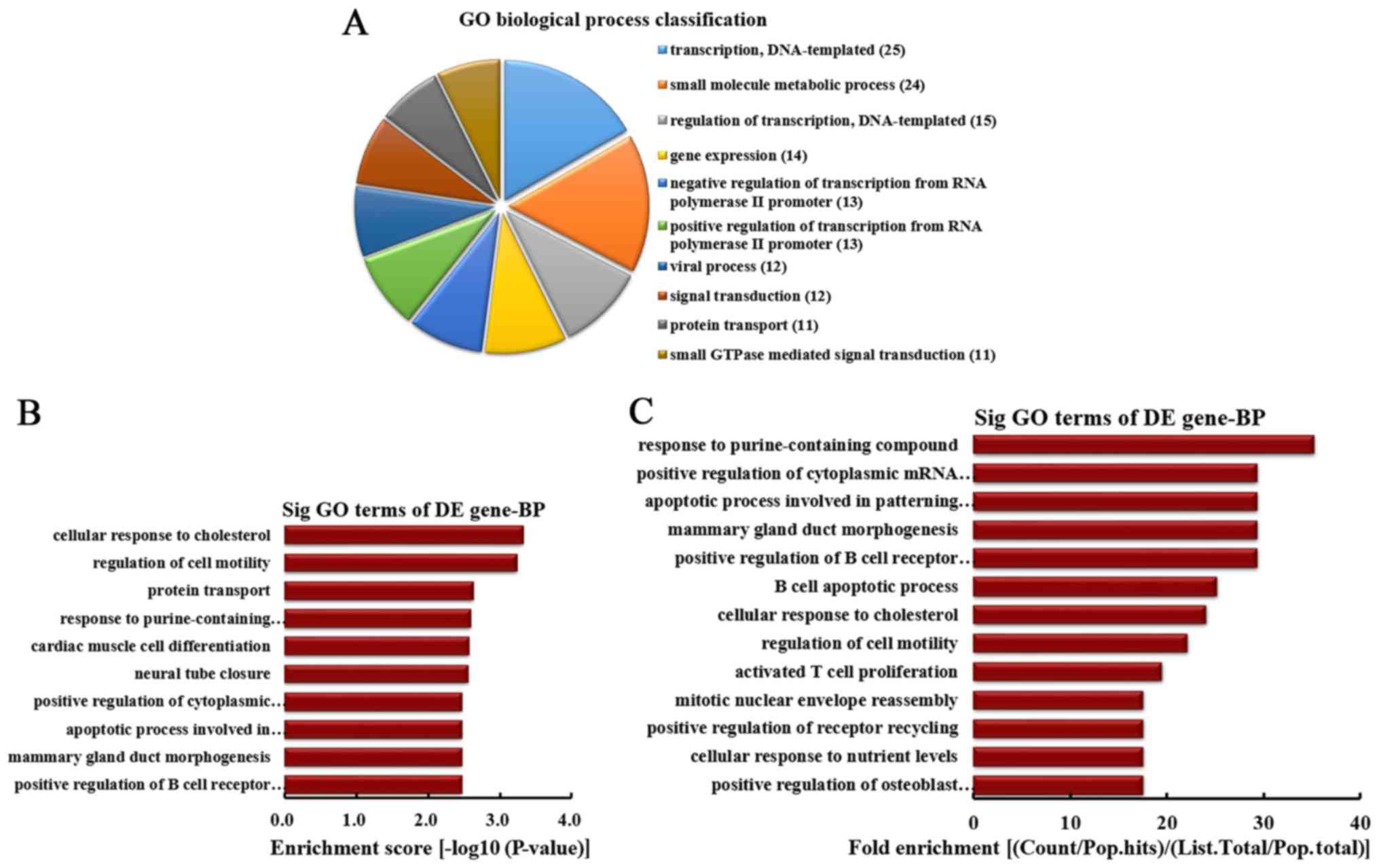
classified (Fig. 3A). Among them,
25 linear counterparts of differentially downregulated circRNA were
involved in transcription, DNA-templated, 24 were involved in small
molecule metabolic process, 15 were involved in regulation of
transcription, DNA-templated, 14 were involved in gene expression,
13 were involved in negative regulation of transcription from RNA
polymerase II promoter, 13 were involved in positive regulation of
transcription from RNA polymerase II promoter, 12 were involved in
viral process, 12 were involved in signal transduction, 11 were
involved in protein transport and 11 were involved in small
GTPase-mediated signal transduction. According to the enrichment
score, the top 10 BPs were selected (Fig. 3B). These included cellular
response to cholesterol, regulation of cell motility, protein
transport, response to purine-containing compound, cardiac muscle
cell differentiation, neural tube closure, positive regulation of
cytoplasmic mRNA processing body assembly, apoptotic process
involved in patterning of blood vessels, mammary gland duct
morphogenesis and positive regulation of B cell receptor signaling
pathway. Furthermore, according to the fold enrichment, the top 13
BPs were selected (Fig. 3C).
These included response to purine-containing compound, positive
regulation of cytoplasmic mRNA processing body assembly, apoptotic
process involved in patterning of blood vessels, mammary gland duct
morphogenesis, positive regulation of B cell receptor signaling
pathway, B cell apoptotic process, cellular response to
cholesterol, regulation of cell motility, activated T cell
proliferation, mitotic nuclear envelope reassembly, positive
regulation of receptor recycling, cellular response to nutrient
levels and positive regulation of osteoblast proliferation.
CC analysis of the genes producing
upregulated circRNA
According to the number of linear counterparts of
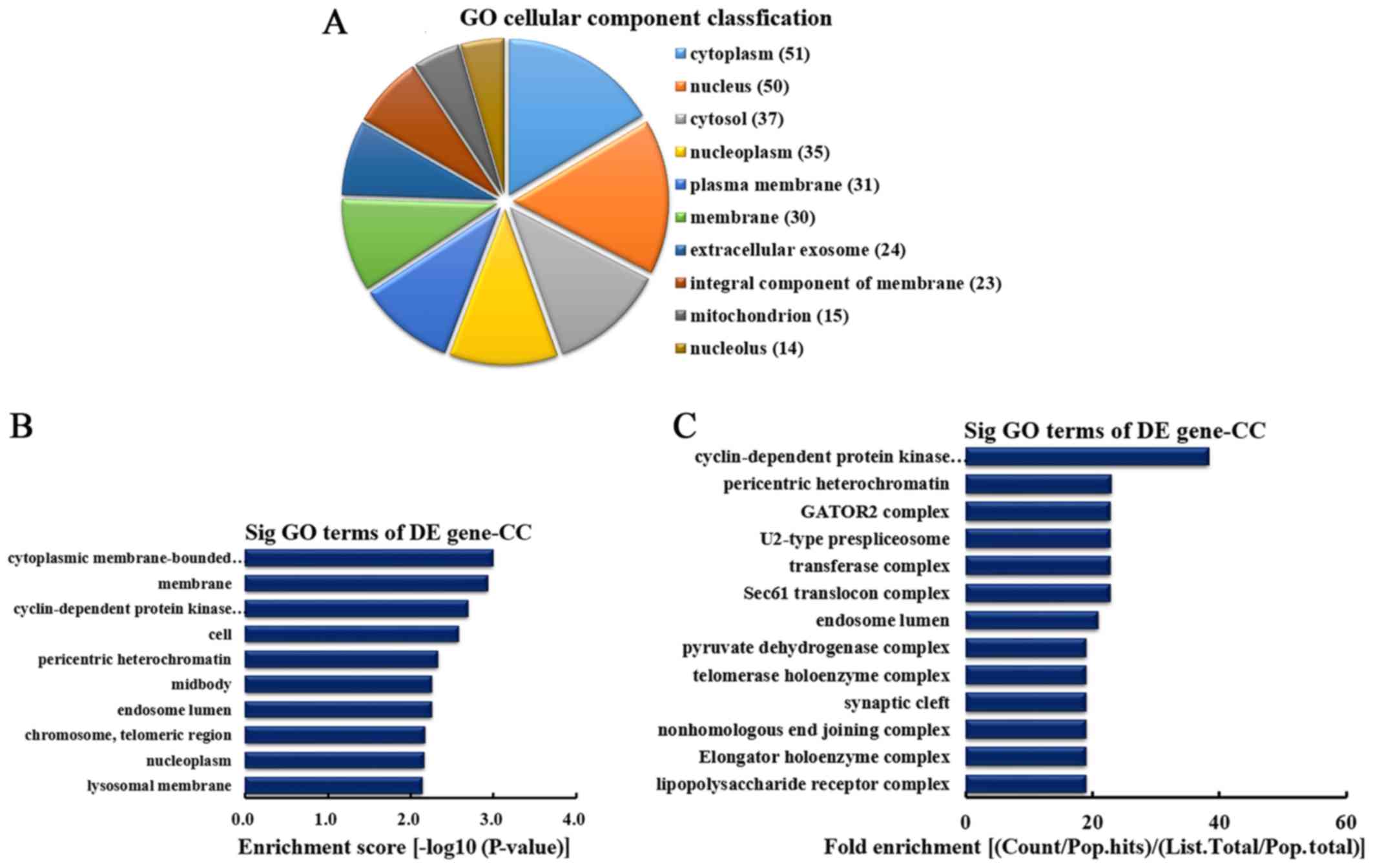
differentially overexpressed circRNA identified in CC, the top 10
CC categories were classified (Fig.
4A). Among them, 51 linear counterparts of differentially
overexpressed circRNA were involved in the cytoplasm, 50 were
involved in the nucleus, 37 were involved in the cytosol, 35 were
involved in the nucleoplasm, 31 were involved in plasma membrane,
30 were involved in the membrane, 24 were involved in the
extracellular exosome, 23 were involved in the integral component
of the membrane, 15 were involved in the mitochondrion and 14 were
involved in the nucleolus. According to enrichment score, the top
10 CCs were selected (Fig. 4B).
These included cytoplasmic membrane-bounded vesicle, membrane,
cyclin-dependent protein kinase activating kinase holoenzyme
complex, cell, pericentric heterochromatin, midbody, endosome
lumen, chromosome (telomeric region), nucleoplasm and lysosomal
membrane. Furthermore, according to the fold enrichment, the top 13
CCs were selected (Fig. 4C).
These included cyclin-dependent protein kinase activating kinase
holoenzyme complex, pericentric heterochromatin, GATOR2 complex,
U2-type prespliceosome, transferase complex, Sec61 translocon
complex, endosome lumen, pyruvate dehydrogenase complex, telomerase
holoenzyme complex, synaptic cleft, nonhomologous end joining
complex, elongator holoenzyme complex and lipopolysaccharide
receptor complex.
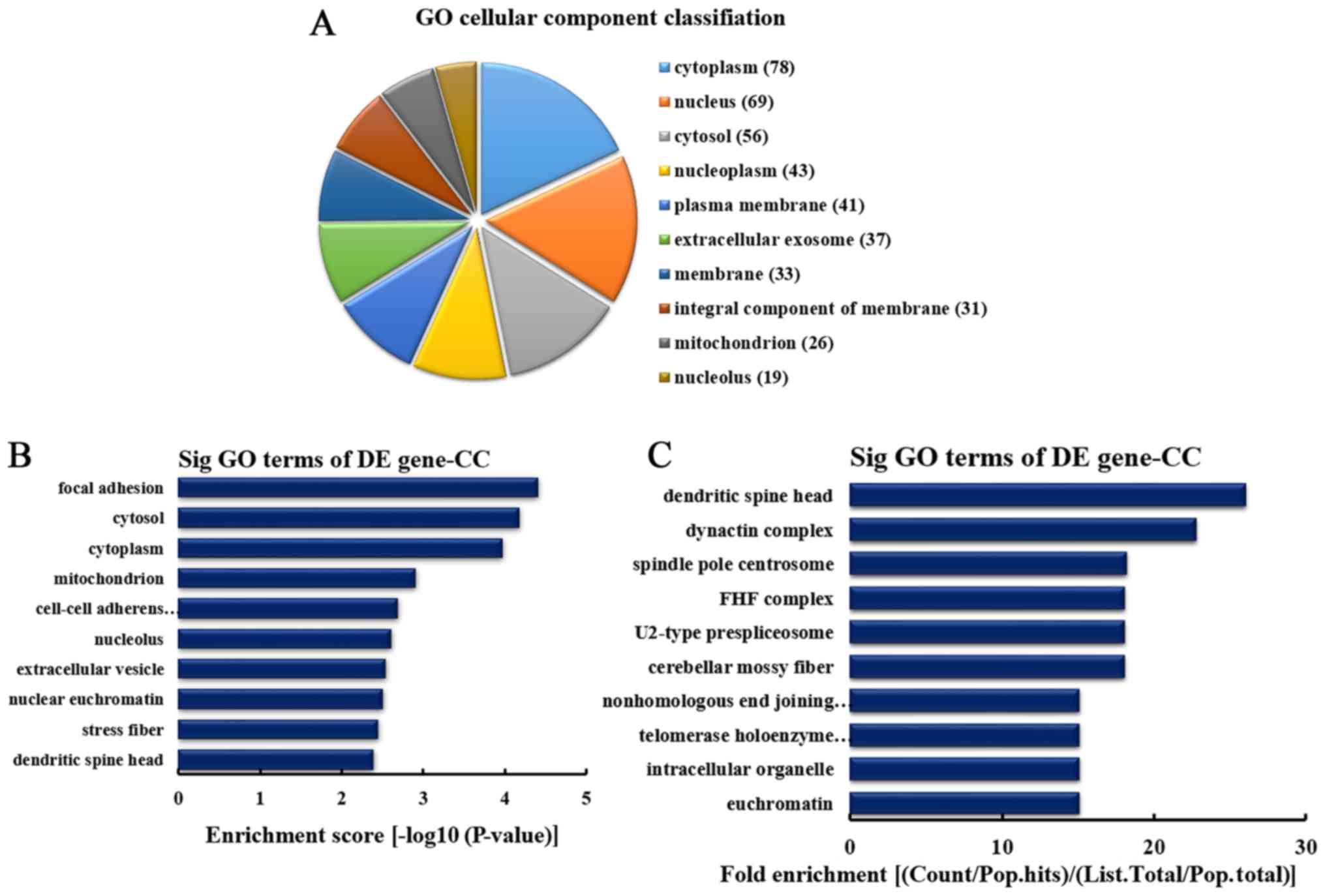
CC analysis of the genes producing
downregulated circRNA
According to the number of linear counterparts of
differentially downregulated circRNA identified in CCs, the top 10
CCs were classified (Fig. 5A).
Among them, 78 linear counterparts of differentially downregulated
circRNA were involved in the cytoplasm, 69 were involved in the
nucleus, 56 were involved in the cytosol, 43 were involved in the
nucleoplasm, 41 were involved in the plasma membrane, 37 were
involved in the extracellular exosome, 33 were involved in the
membrane, 31 were involved in the integral component of the
membrane, 26 were involved in mitochondrion and 19 were involved in
the nucleolus. According to the enrichment score, the top 10 CC
categories were selected (Fig.
5B). These included focal adhesion, cytosol, cytoplasm,
mitochondrion, cell-cell adherens junction, nucleolus,
extracellular vesicle, nuclear euchromatin, stress fiber and
dendritic spine head. Additionally, according to the fold
enrichment, the top 10 CC categories were selected (Fig. 5C). These included dendritic spine
head, dynactin complex, spindle pole centrosome, Fuse Toes complex,
U2-type prespliceosome, cerebellar mossy fiber, nonhomologous end
joining complex, telomerase holoenzyme complex, intracellular
organelle and euchromatin.
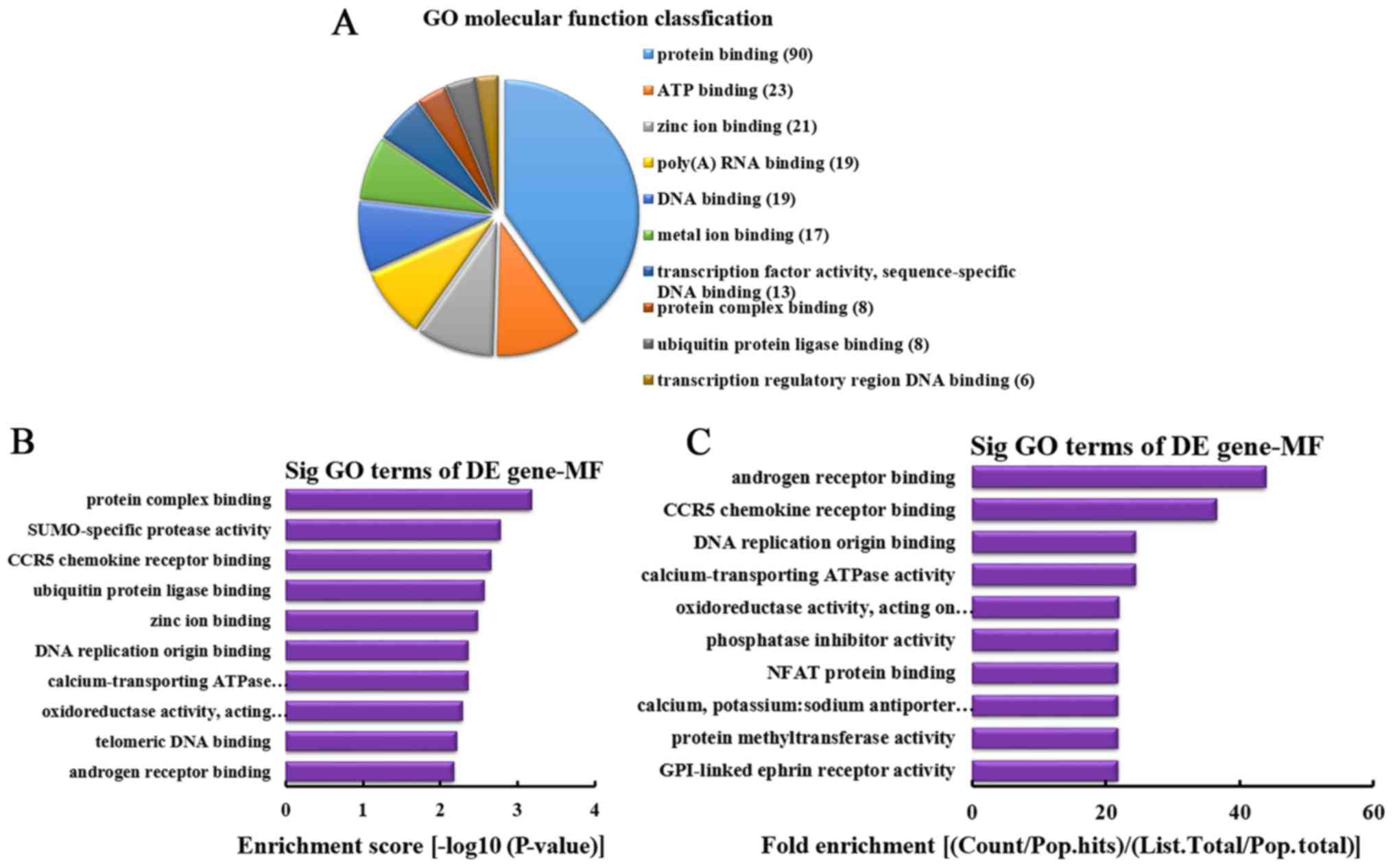
MF analysis of the genes producing
upregulated circRNA
According to the number of linear counterparts of
differentially overexpressed circRNA identified in MF, the top 10
MFs were classified (Fig. 6A).
Among them, 90 linear counterparts of differentially overexpressed
circRNA were involved in protein binding, 23 were involved in ATP
binding, 21 were involved in zinc ion binding, 19 were involved in
poly(A) RNA binding, 19 were involved in DNA binding, 17 were
involved in metal ion binding, 13 were involved in transcription
factor activity, sequence-specific DNA binding, 8 were involved in
protein complex binding, 8 were involved in ubiquitin protein
ligase binding and 6 were involved in transcription regulatory
region DNA binding. According to the enrichment score, the
following top 10 MFs were selected (Fig. 6B): Protein complex binding,
SUMO-specific protease activity, C-C chemokine receptor type 5
(CCR5) chemokine receptor binding, ubiquitin protein ligase
binding, zinc ion binding, DNA replication origin binding,
calcium-transporting ATPase activity, oxidoreductase activity
[acting on paired donors, with incorporation or reduction of
molecular oxygen, NAD(P)H as one donor, and incorporation of one
atom of oxygen], telomeric DNA binding and androgen receptor
binding. Additionally, according to the fold enrichment, the
following top 10 MFs were identified (Fig. 6C): Androgen receptor binding, CCR5
chemokine receptor binding, DNA replication origin binding,
calcium-transporting ATPase activity, oxidoreductase activity
[acting on paired donors, with incorporation or reduction of
molecular oxygen, NAD(P)H as one donor, and incorporation of one
atom of oxygen], phosphatase inhibitor activity, nuclear factor
(NF) of activated T cells protein binding, calcium, potassium:
sodium antiporter activity, protein methyltransferase activity and
glycosylphosphatidylinositol-linked ephrin receptor activity.
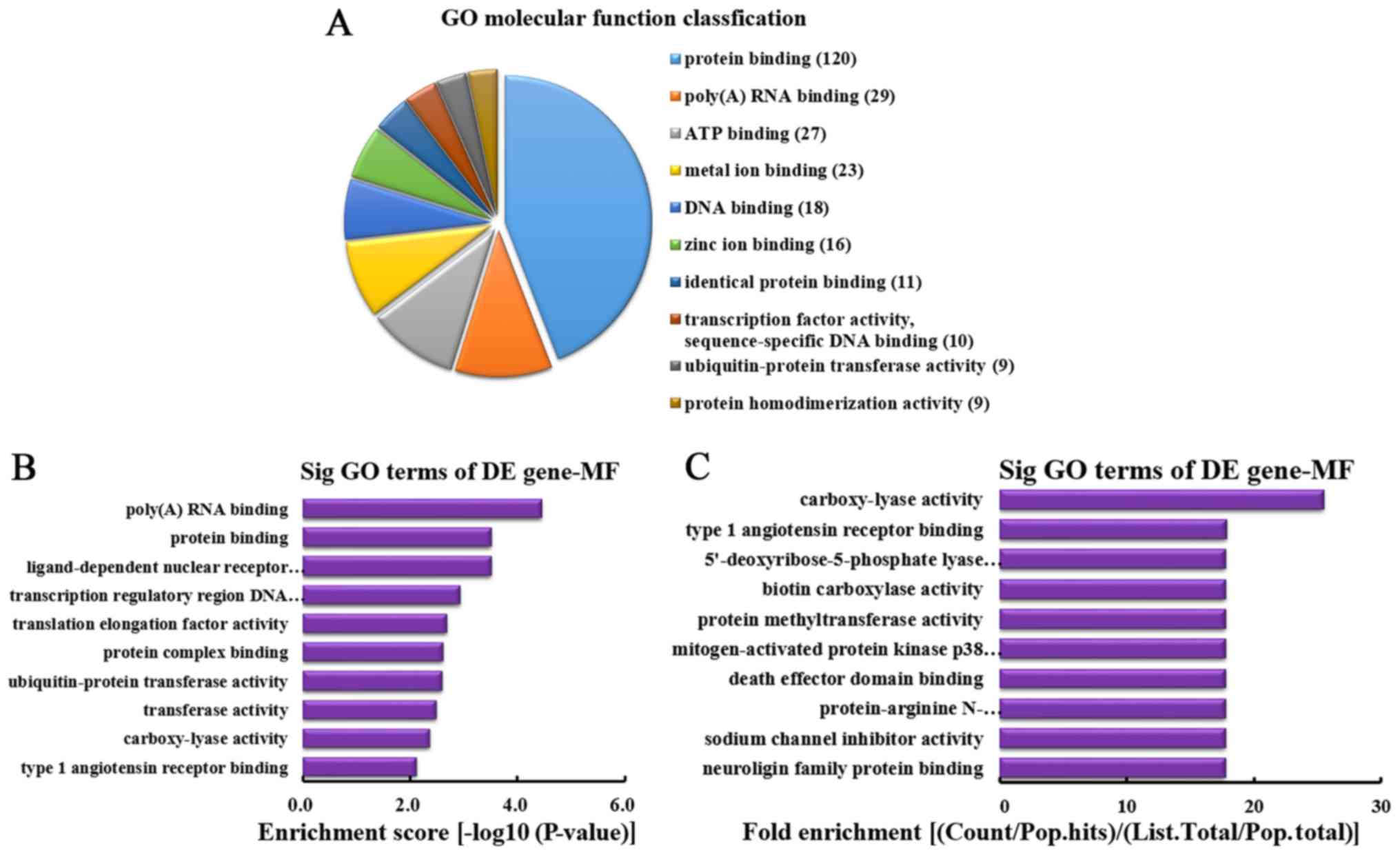
MF analysis of the genes producing
downregulated circRNA
According to the number of linear counterparts of
differentially downregulated circRNA identified in MF, the top 10
MFs were classified (Fig. 7A).
Among them, 120 linear counterparts of differentially downregulated
circRNA were involved in protein binding, 29 were involved in
poly(A) RNA binding, 27 were involved in ATP binding, 23 were
involved in metal ion binding, 18 were involved in DNA binding, 16
were involved in zinc ion binding, 11 were involved in identical
protein binding, 10 were involved in transcription factor activity,
sequence-specific DNA binding, 9 were involved in ubiquitin-protein
transferase activity and 9 were involved in protein
homodimerization activity. According to the enrichment score, the
following top 10 MF categories were selected (Fig. 7B): Poly(A) RNA binding, protein
binding, ligand-dependent nuclear receptor transcription
coactivator activity, transcription regulatory region DNA binding,
translation elongation factor activity, protein complex binding,
ubiquitin-protein transferase activity, transferase activity,
carboxy-lyase activity and type 1 angiotensin receptor binding.
According to the fold enrichment, the following top 10 MFs were
selected (Fig. 7C): Carboxy-lyase
activity, type 1 angiotensin receptor binding,
5′-deoxyribose-5-phosphate lyase activity, biotin carboxylase
activity, protein methyltransferase activity, mitogen-activated
protein kinase p38 binding, death effector domain binding,
protein-arginine N-methyltransferase activity, sodium channel
inhibitor activity and neuroligin family protein binding.
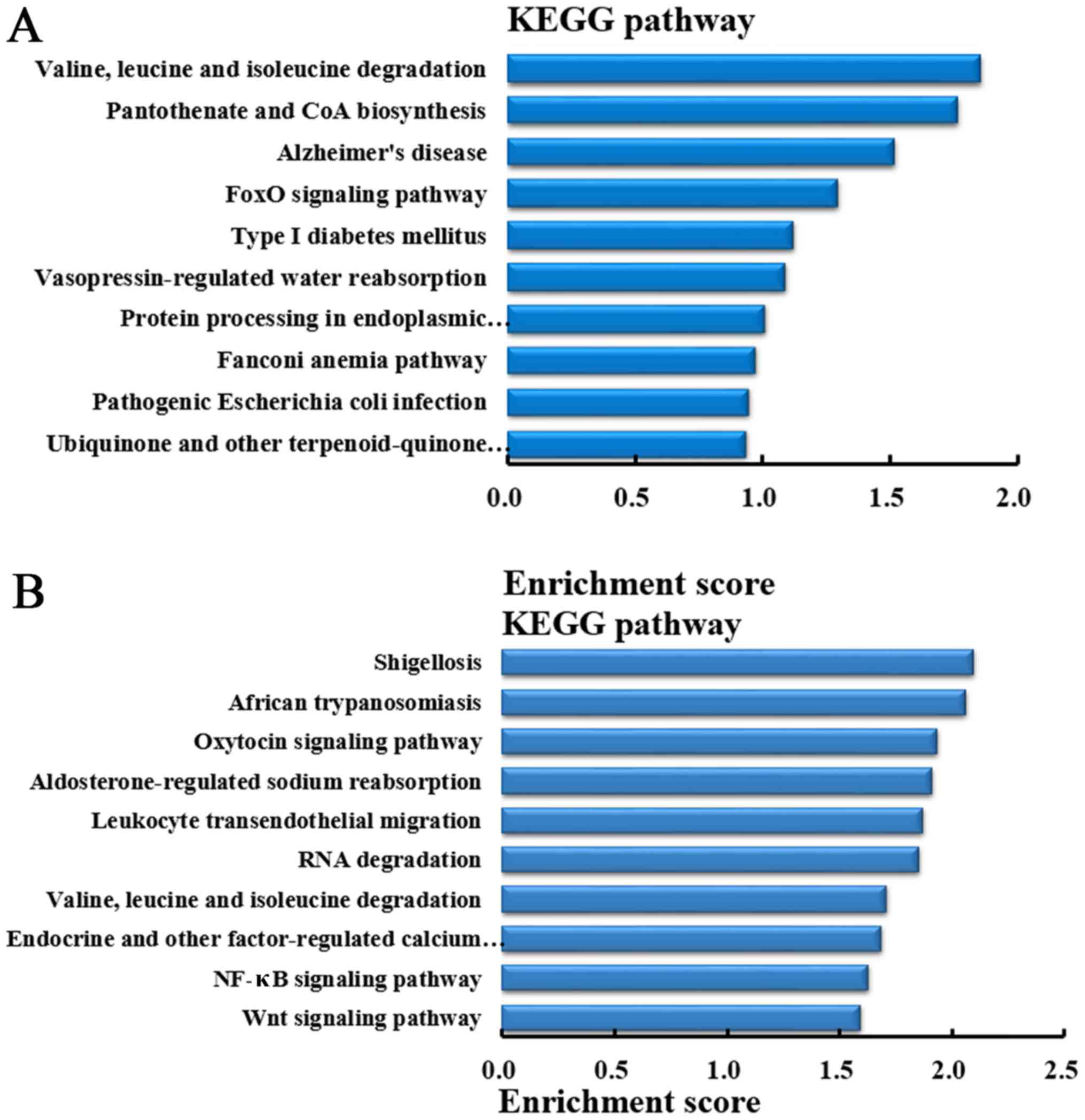
Pathway analysis of the genes producing
differentially expressed circRNA
In order to understand the pathways that
differentially expressed circRNA involved in lung metastasis in
CRC, KEGG pathway analysis was performed to analyze the genes
producing differentially expressed circRNA. According to the
enrichment score, the top 10 pathways in which the genes producing
upregulated circRNA were involved in are demonstrated in Fig. 8A. While, based on the enrichment
score, the top 10 pathways in which the genes producing
downregulated circRNA were involved are demonstrated in Fig. 8B.
Candidate circRNA as potential miRNA
sponges
To investigate the circRNA/microRNA interactions,
the binding sites of circRNA were predicted. As demonstrated in
Table I, it was identified that
hsa_circRNA_105055 (upregulated), hsa_circRNA_086376
(downregulated) and hsa_circRNA_102761 (downregulated) could bind
with miR-7 regulating target genes PRKCB, EPHA3, BRCA1 and ABCC1.
Additionally, hsa_circRNA_102348 and hsa_circRNA_102562 could bind
with miR-410 regulating target genes PDHX, ATM and ABCC1.
 | Table IPotential and common miRNA sponging
by circRNA. |
Table I
Potential and common miRNA sponging
by circRNA.
| circRNA | Common sponging
miRNA | miRNA target
genes |
|---|
|
hsa_circRNA_105055 | miR-7 | PRKCB |
|
hsa_circRNA_086376 | | EPHA3 |
|
hsa_circRNA_102761 | | BRCA1
ABCC1 |
|
hsa_circRNA_102348 | miR-410 | PDHX |
|
hsa_circRNA_102562 | | ATM
ABCC1 |
Discussion
CRC is one of the most frequent malignant tumors in
developed countries (27,28). Of the patients with CRC, ~50%
eventually develop distant metastases, such as pulmonary
metastasis, resulting in poor outcomes even when these patients
have resectable primary tumors (29). Therefore, it is important to
investigate the molecular mechanism involved in CRC with pulmonary
metastasis. In the present study, it was identified that abnormal
circRNA were expressed in CRC tissues from patients with pulmonary
metastasis compared with CRC tissues without pulmonary metastasis.
GO, KEGG pathway and circRNA/miRNA interaction analyses were
conducted to determine the potential function and regulatory
mechanisms of circRNA in CRC tissues from patients with pulmonary
metastasis.
First, through high-throughput microarray, it was
identified that 431 circRNA were differentially expressed in CRC
tissues from patients with pulmonary metastasis compared with CRC
tissues without pulmonary metastasis. Among them, 192 and 239
circRNA were upregulated and down-regulated, respectively. Among
the abnormally expressed circRNA, in the light of their relation
with their coding genes, the circRNA were classified into five
categories: 331 were exonic, 45 were intronic, 32 were sense
overlapping, 8 were intergenic and 15 were antisense. Previous
study indicated that 39 circRNA were significantly differentially
expressed between normal colon mucosa and CRC samples, 11 of them
were upregulated in cancer and 28 were downregulated (25). Circ_001569 has been demonstrated
to be upregulated, while Cir-ITCH has been indicated to be
typically downregulated in CRC in comparison with peritumoral
tissue (30,31). The expression of circRNA were
altered in primary and metastatic sites of epithelial ovarian
cancer (32). Furthermore, study
has indicated that 469 circRNA were markedly differentially
expressed between bladder carcinoma and normal tissues with a fold
change ≥2.0 and P<0.05, among which 285 circRNA were upregulated
and 184 were downregulated (33).
To the best of our knowledge, the present study is the first to
report the differentially expressed circRNA in CRC tissues from
patients with pulmonary metastasis.
Then, GO analysis of the genes producing
differentially expressed circRNA was performed to investigate the
potential enriched GO of differentially expressed circRNA. It was
demonstrated that the genes producing upregulated circRNA were
involved in DNA repair. It has been reported that DNA repair
commonly appeared in CRC (34–36), and the carriers of allele A in DNA
repair genes XRCC1 were connected with a higher risk of
disseminated CRC (37). The
present study also revealed that the genes producing downregulated
circRNA were enriched in signal transduction. Previous study has
indicated that various signal transduction pathways were important
in genetic programming and growth control of metastatic CRC and CRC
(38–41). Therefore, these studies combined
with the present study demonstrated that metastatic CRC may result
from the interaction effects of multiple circRNA involved in
regulation of multi-systems.
Additionally, KEGG pathway analysis was performed to
analyze the genes producing differentially expressed circRNA. It
was demonstrated that the genes producing downregulated circRNA
were involved in the NF-κB and Wnt signaling pathway in the CRC
tissues from patients with lung metastasis compared with the CRC
tissues without metastasis. Previous study indicated that
Akt/protein kinase B (PKB) was a major antiapoptotic pathway that
was frequently hyperactivated in the majority of cancer types
(42), and the antiapoptotic
NF-κB signaling pathway was one of the downstream targets of
activated Akt/PBK and controlled the expression of genes and
cellular processes involved in cell proliferation, oncogenesis,
angiogenesis, and apoptosis (43,44). Furthermore, a previous study also
indicated a critical role for aberrant Wnt signaling in CRC that
was involved in diverse cellular processes, such as cell migration
(45).
In addition, bioinformatics methods were performed
in the present study to predict circRNA/microRNA interactions. The
results demonstrated that hsa_circRNA_105055 (upregulated),
hsa_circRNA_086376 (downregulated) and hsa_circRNA_102761
(downregulated) may bind with miR-7 regulating target genes PRKCB,
EPHA3, BRCA1 and ABCC1. Additionally, hsa_circRNA_102348 and
hsa_circRNA_102562 may bind with miR-410 regulating target genes
PDHX, ATM and ABCC1. Previous study indicated that miR-7 could
inhibit CRC cell proliferation and induce apoptosis by targeting
XRCC2 (46). PRKCB and EPHA3 were
differentially expressed in CRC tissues (47–49). It has also been reported that
BRCA1 and ERCC1 mRNA were associated with lymph node metastasis in
Chinese patients with CRC (50).
BRCA1 gene overexpression was correlated with radiation response in
CRC (51), and BRCA1 mutation may
be associated with early onset of CRC (52). Study has also indicated that the
expression of ABCC1 was altered in association with colon
carcinogenesis (53). For
miR-410, previous study revealed that miR-410 could regulate
apoptosis by targeting Bak1 in human CRC cells (54). PDHX may be targeted by miR-26a to
regulate glucose metabolism of CRC, which inhibited the conversion
of pyruvate to acetyl coenzyme A in the citric acid cycle (55). ATM may be negatively regulated by
miR-203 to induce oxaliplatin resistance in CRC (56). To the best our knowledge, the
present study is the first to report that hsa_circRNA_105055,
hsa_circRNA_086376 and hsa_circRNA_102761 may bind with miR-7
regulating target genes PRKCB, EPHA3, BRCA1 and ABCC1, and
hsa_circRNA_102348 and hsa_ circRNA_102562 may bind with miR-410
regulating target genes PDHX, ATM and ABCC1. However, these binding
interactions need to be verified through functional analysis in
future study.
In conclusion, the present study demonstrated that
431 circRNA were differentially expressed in CRC tissues from
patients with pulmonary metastasis compared with tissues without
metastasis. By utilizing GO, KEGG pathway and circRNA/miRNA
interaction analyses, the present study revealed that
hsa_circRNA_105055 (upregulated), hsa_circRNA_086376
(downregulated) or hsa_circRNA_102761 (downregulated) may regulate
the pulmonary metastasis of CRC through binding with miR-7 to
regulate PRKCB (the target gene of miR-7) that is involved in the
NF-κB or Wnt signaling pathway. However, this requires further
study. The present findings may provide a novel perspective on
circRNA and lay a foundation for future research of potential roles
of circRNA in CRC with pulmonary metastasis.
Acknowledgments
The present study was supported by the Project of
Department of Health in Yunnan Province (grant no. 2014NS124), the
China Guanghua Foundation, the General Joint Project of Yunnan
Provincial Science and Technology Department and Kunming Medical
University (grant no. 2015FB024) and the Major Project of Yunnan
Provincial Bureau of Education (grant no. ZD2015010).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah SA, Haddad R, Al-Sukhni W, Kim RD,
Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S and Wei AC:
Surgical resection of hepatic and pulmonary metastases from
colorectal carcinoma. J Am Coll Surg. 202:468–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rotolo N, De Monte L, Imperatori A and
Dominioni L: Pulmonary resections of single metastases from
colorectal cancer. Surg Oncol. 16(Suppl 1): S141–S144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goya T, Miyazawa N, Kondo H, Tsuchiya R,
Naruke T and Suemasu K: Surgical resection of pulmonary metastases
from colorectal cancer. 10-year follow-up. Cancer. 64:1418–1421.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galandiuk S, Wieand HS, Moertel CG, Cha
SS, Fitzgibbons RJ Jr, Pemberton JH and Wolff BG: Patterns of
recurrence after curative resection of carcinoma of the colon and
rectum. Surg Gynecol Obstet. 174:27–32. 1992.PubMed/NCBI
|
|
8
|
McAuliffe JC, Qadan M and D'Angelica MI:
Hepatic resection, hepatic arterial infusion pump therapy, and
genetic biomarkers in the management of hepatic metastases from
colorectal cancer. J Gastrointest Oncol. 6:699–708. 2015.PubMed/NCBI
|
|
9
|
Chen D, Sun Q, Cheng X, Zhang L, Song W,
Zhou D, Lin J and Wang W: Genome-wide analysis of long noncoding
RNA (lncRNA) expression in colorectal cancer tissues from patients
with liver metastasis. Cancer Med. 5:1629–1639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattick JS, Taft RJ and Faulkner GJ: A
global view of genomic information - moving beyond the gene and the
master regulator. Trends Genet. 26:21–28. 2010. View Article : Google Scholar
|
|
11
|
Malouf GG, Zhang J, Yuan Y, Compérat E,
Rouprêt M, Cussenot O, Chen Y, Thompson EJ, Tannir NM, Weinstein
JN, et al: Characterization of long non-coding RNA transcriptome in
clear-cell renal cell carcinoma by next-generation deep sequencing.
Mol Oncol. 9:32–43. 2015. View Article : Google Scholar
|
|
12
|
Li J, Yang J, Zhou P, Le Y, Zhou C, Wang
S, Xu D, Lin HK and Gong Z: Circular RNAs in cancer: Novel insights
into origins, properties, functions and implications. Am J Cancer
Res. 5:472–480. 2015.PubMed/NCBI
|
|
13
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perkel JM: Assume nothing: The tale of
circular RNA. Biotechniques. 55:55–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
20
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bachmayr-Heyda A, Reiner AT, Auer K,
Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW,
Zeillinger R and Pils D: Correlation of circular RNA abundance with
proliferation - exemplified with colorectal and ovarian cancer,
idiopathic lung fibrosis, and normal human tissues. Sci Rep.
5:80572015. View Article : Google Scholar
|
|
26
|
Thorson AG, Christensen MA and Davis SJ:
The role of colonoscopy in the assessment of patients with
colorectal cancer. Dis Colon Rectum. 29:306–311. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jemal A, Thun MJ, Ries LA, Howe HL, Weir
HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, et al: Annual
report to the nation on the status of cancer, 1975–2005, featuring
trends in lung cancer, tobacco use, and tobacco control. J Natl
Cancer Inst. 100:1672–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yiu HY, Whittemore AS and Shibata A:
Increasing colorectal cancer incidence rates in Japan. Int J
Cancer. 109:777–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin PC, Lin JK, Lin CC, Wang HS, Yang SH,
Jiang JK, Lan YT, Lin TC, Li AF, Chen WS, et al: Carbohydrate
antigen 19-9 is a valuable prognostic factor in colorectal cancer
patients with normal levels of carcinoembryonic antigen and may
help predict lung metastasis. Int J Colorectal Dis. 27:1333–1338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ, et al: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang G, Zhu H, Shi Y, Wu W, Cai H and
Chen X: cir-ITCH plays an inhibitory role in colorectal cancer by
regulating the Wnt/β-catenin pathway. PLoS One. 10:e01312252015.
View Article : Google Scholar
|
|
32
|
Ahmed I, Karedath T, Andrews SS, Al-Azwani
IK, Mohamoud YA, Querleu D, Rafii A and Malek JA: Altered
expression pattern of circular RNAs in primary and metastatic sites
of epithelial ovarian carcinoma. Oncotarget. 7:36366–36381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang M, Zhong Z, Lv M, Shu J, Tian Q and
Chen J: Comprehensive analysis of differentially expressed profiles
of lncRNAs and circRNAs with associated co-expression and ceRNA
networks in bladder carcinoma. Oncotarget. 7:47186–47200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karam RA, Al Jiffry BO, Al Saeed M, Abd El
Rahman TM, Hatem M and Amer MG: DNA repair genes polymorphisms and
risk of colorectal cancer in Saudi patients. Arab J Gastroenterol.
17:117–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Freitas IN, de Campos FG, Alves VA,
Cavalcante JM, Carraro D, Coudry Rde A, Diniz MA, Nahas SC and
Ribeiro U Jr: Proficiency of DNA repair genes and microsatellite
instability in operated colorectal cancer patients with clinical
suspicion of lynch syndrome. J Gastrointest Oncol. 6:628–637.
2015.PubMed/NCBI
|
|
36
|
Scarbrough PM, Weber RP, Iversen ES,
Brhane Y, Amos CI, Kraft P, Hung RJ, Sellers TA, Witte JS, Pharoah
P, et al: A cross-cancer genetic association analysis of the DNA
repair and DNA damage signaling pathways for lung, ovary, prostate,
breast, and colorectal cancer. Cancer Epidemiol Biomarkers Prev.
25:193–200. 2016. View Article : Google Scholar
|
|
37
|
Dimberg J, Skarstedt M, Slind Olsen R,
Andersson RE and Matussek A: Gene polymorphism in DNA repair genes
XRCC1 and XRCC6 and association with colorectal cancer in Swedish
patients. APMIS. 124:736–740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Győrffy B, Stelniec-Klotz I, Sigler C,
Kasack K, Redmer T, Qian Y and Schäfer R: Effects of RAL signal
transduction in KRAS- and BRAF-mutated cells and prognostic
potential of the RAL signature in colorectal cancer. Oncotarget.
6:13334–13346. 2015. View Article : Google Scholar
|
|
39
|
Lu X, Li C, Wang YK, Jiang K and Gai XD:
Sorbitol induces apoptosis of human colorectal cancer cells via p38
MAPK signal transduction. Oncol Lett. 7:1992–1996. 2014.PubMed/NCBI
|
|
40
|
Ji H, Greening DW, Barnes TW, Lim JW,
Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, et al:
Proteome profiling of exosomes derived from human primary and
metastatic colorectal cancer cells reveal differential expression
of key metastatic factors and signal transduction components.
Proteomics. 13:1672–1686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nowakowska-Zajdel E, Mazurek U, Stachowicz
M, Niedworok E, Fatyga E and Muc-Wierzgoń M: Cellular signal
transduction pathways by leptin in colorectal cancer tissue:
Preliminary results. ISRN Endocrinol. 2011:5753972011. View Article : Google Scholar
|
|
42
|
Marte BM and Downward J: PKB/Akt:
Connecting phosphoinositide 3-kinase to cell survival and beyond.
Trends Biochem Sci. 22:355–358. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mayo MW and Baldwin AS: The transcription
factor NF-kappaB: Control of oncogenesis and cancer therapy
resistance. Biochim Biophys Acta. 1470:M55–M62. 2000.PubMed/NCBI
|
|
44
|
Hidalgo M and Rowinsky EK: The
rapamycin-sensitive signal transduction pathway as a target for
cancer therapy. Oncogene. 19:6680–6686. 2000. View Article : Google Scholar
|
|
45
|
Qi J, Yu Y, Akilli Öztürk Ö, Holland JD,
Besser D, Fritzmann J, Wulf-Goldenberg A, Eckert K, Fichtner I and
Birchmeier W: New Wnt/β-catenin target genes promote experimental
metastasis and migration of colorectal cancer cells through
different signals. Gut. 65:1690–1701. 2016. View Article : Google Scholar
|
|
46
|
Xu K, Chen Z, Qin C and Song X: miR-7
inhibits colorectal cancer cell proliferation and induces apoptosis
by targeting XRCC2. Onco Targets Ther. 7:325–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y and Zheng T: Screening of hub genes
and pathways in colorectal cancer with microarray technology.
Pathol Oncol Res. 20:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slaby O, Sachlova M, Brezkova V, Hezova R,
Kovarikova A, Bischofová S, Sevcikova S, Bienertova-Vasku J, Vasku
A, Svoboda M, et al: Identification of microRNAs regulated by
isothiocyanates and association of polymorphisms inside their
target sites with risk of sporadic colorectal cancer. Nutr Cancer.
65:247–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huhn S, Bevier M, Pardini B, Naccarati A,
Vodickova L, Novotny J, Vodicka P, Hemminki K and Försti A:
Colorectal cancer risk and patients' survival: Influence of
polymorphisms in genes somatically mutated in colorectal tumors.
Cancer Causes Control. 25:759–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuanming L, Lineng Z, Baorong S, Junjie P
and Sanjun C: BRCA1 and ERCC1 mRNA levels are associated with lymph
node metastasis in Chinese patients with colorectal cancer. BMC
Cancer. 13:1032013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang MY, Wang JY, Chang HJ, Kuo CW, Tok
TS and Lin SR: CDC25A, VAV1, TP73, BRCA1 and ZAP70 gene
overexpression correlates with radiation response in colorectal
cancer. Oncol Rep. 25:1297–1306. 2011.PubMed/NCBI
|
|
52
|
Sopik V, Phelan C, Cybulski C and Narod
SA: BRCA1 and BRCA2 mutations and the risk for colorectal cancer.
Clin Genet. 87:411–418. 2015. View Article : Google Scholar
|
|
53
|
Kobayashi M, Funayama R, Ohnuma S, Unno M
and Nakayama K: Wnt-β-catenin signaling regulates ABCC3 (MRP3)
transporter expression in colorectal cancer. Cancer Sci.
107:1776–1784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu C, Zhang A, Cheng L and Gao Y: miR 410
regulates apoptosis by targeting Bak1 in human colorectal cancer
cells. Mol Med Rep. 14:467–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z,
Yuan Q, Zhao X, Xu N and Liang S: MicroRNA-26a regulates glucose
metabolism by direct targeting PDHX in colorectal cancer cells. BMC
Cancer. 14:4432014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R,
Hu X, Ye X, Lu J, Fan F, Xia L, et al: miR-203 induces oxaliplatin
resistance in colorectal cancer cells by negatively regulating ATM
kinase. Mol Oncol. 8:83–92. 2014. View Article : Google Scholar
|