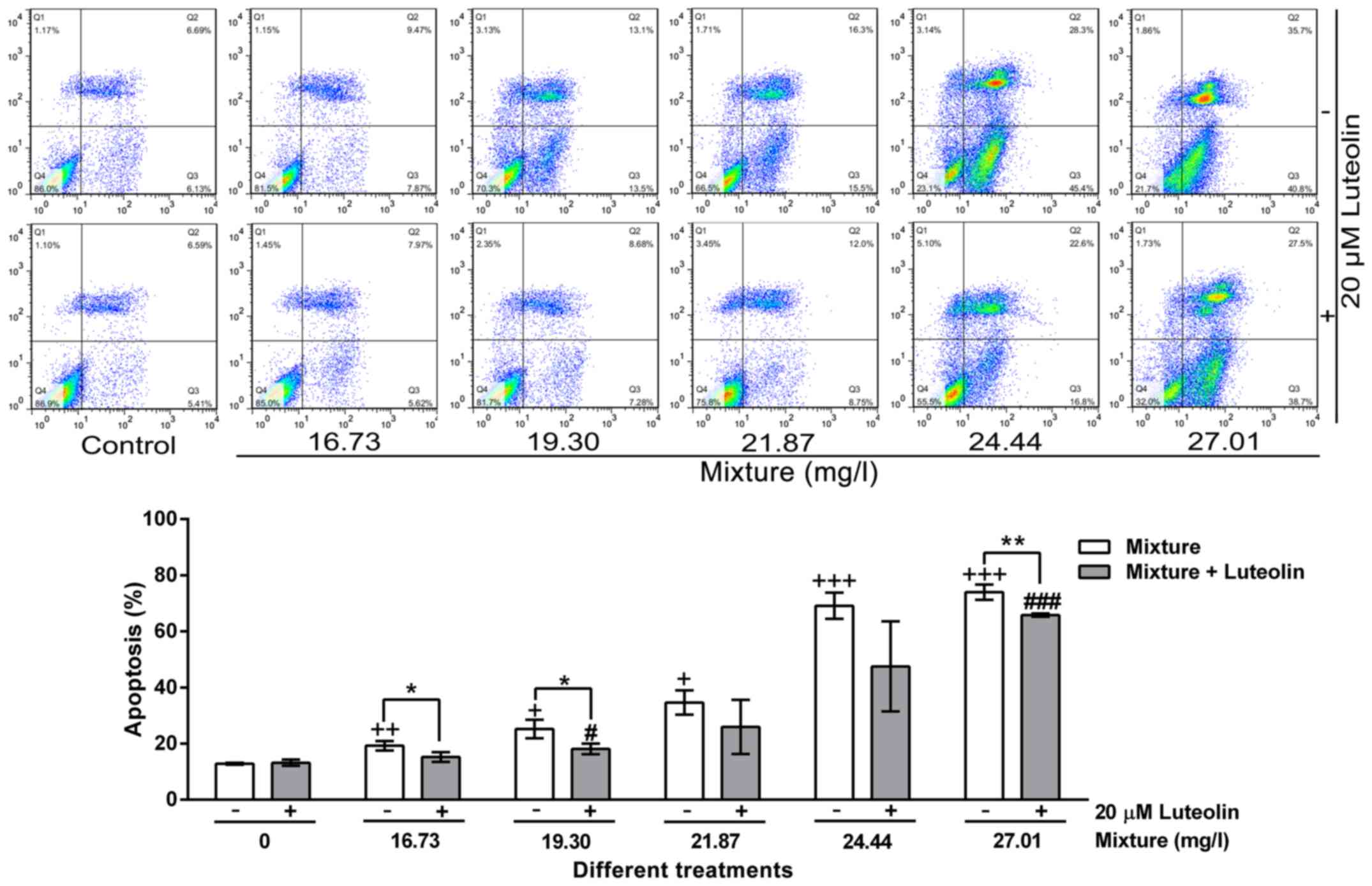
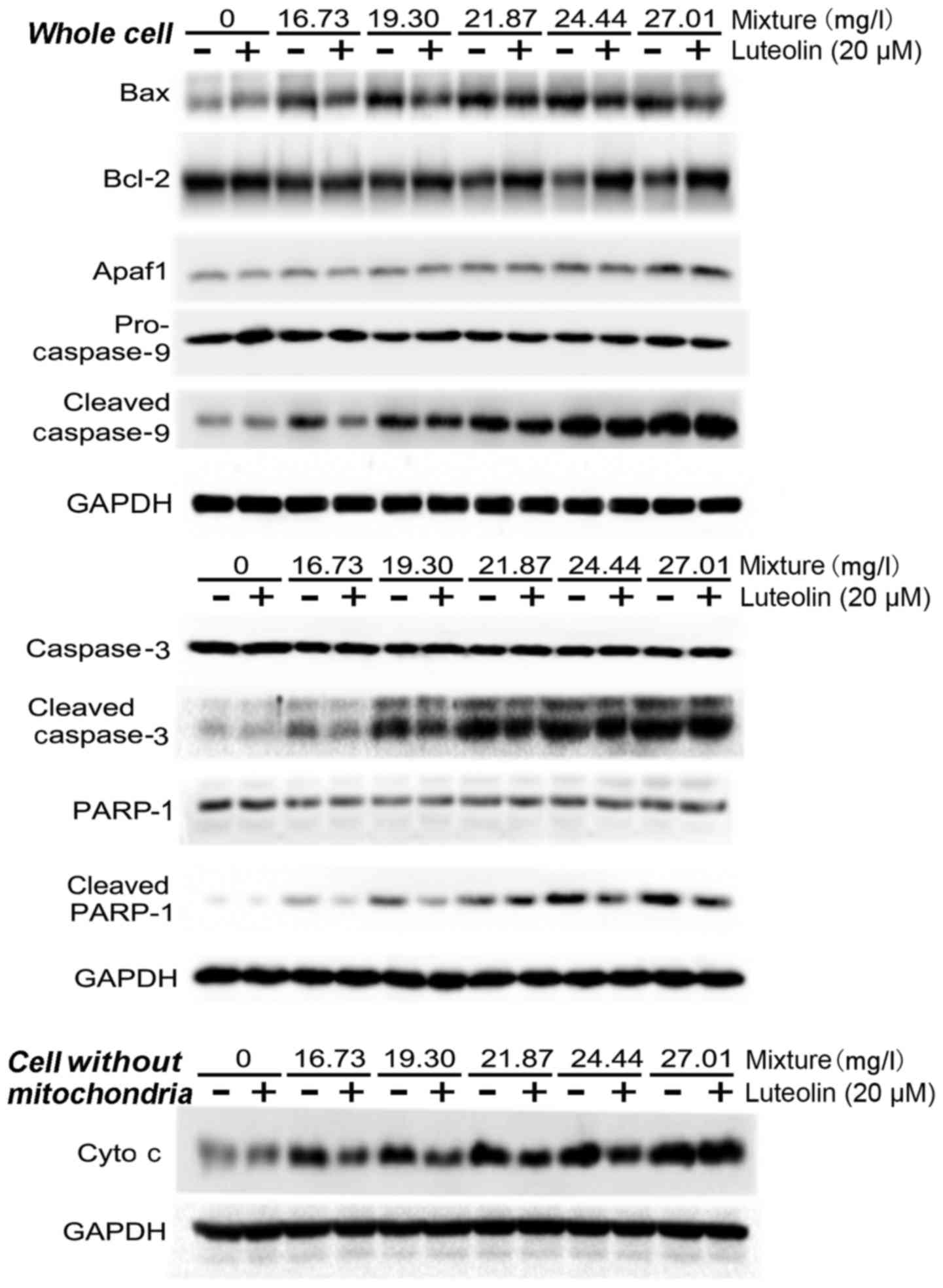
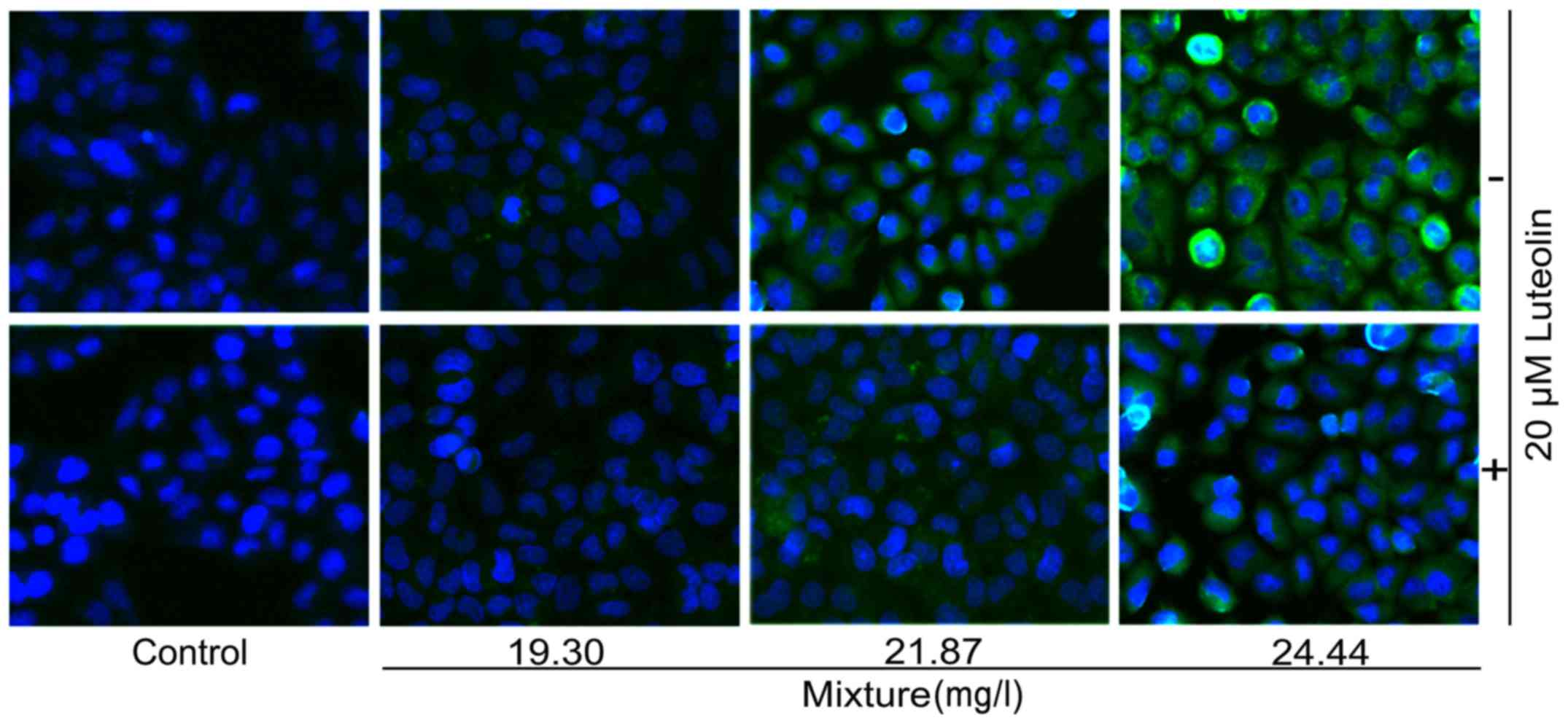
|
1
|
Liu Z, Zhang Q, Han T, Ding Y, Sun J, Wang
F and Zhu C: Heavy metal pollution in a soil-rice system in the
Yangtze River Region of China. Int J Environ Res Public Health.
13:632015. View Article : Google Scholar
|
|
2
|
Niu Y, Niu Y, Pang Y and Yu H: Assessment
of heavy metal pollution in sediments of inflow rivers to Lake
Taihu, China. Bull Environ Contam Toxicol. 95:618–623. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan Y and Li H: Investigating heavy metal
pollution in mining brownfield and its policy implications: a case
study of the Bayan Obo Rare Earth Mine, Inner Mongolia, China.
Environ Manage. 57:879–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu R, Zhang W, Hu G, Lin C and Yang Q:
Heavy metal pollution and Pb isotopic tracing in the intertidal
surface sediments of Quanzhou Bay, southeast coast of China. Mar
Pollut Bull. 105:416–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Liao Q, Shao S, Zhang N, Shen Q
and Liu C: Heavy metal pollution, fractionation, and potential
ecological risks in sediments from Lake Chaohu (Eastern China) and
the surrounding rivers. Int J Environ Res Public Health.
12:14115–14131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong S, Geng H, Zhang F, Liu Z, Wang T
and Song B: Risk assessment and prediction of heavy metal pollution
in groundwater and river sediment: a case study of a typical
agricultural irrigation area in Northeast China. Int J Anal Chem.
2015:9215392015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uluturhan E and Kucuksezgin F: Heavy metal
contaminants in Red Pandora (Pagellus erythrinus) tissues from the
Eastern Aegean Sea, Turkey. Water Res. 41:1185–1192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao J, Bowman L, Magaye R, Leonard SS,
Castranova V and Ding M: Apoptosis induced by tungsten
carbide-cobalt nanoparticles in JB6 cells involves ROS generation
through both extrinsic and intrinsic apoptosis pathways. Int J
Oncol. 42:1349–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhijie Gao LW, Zheng H and Yao X: Analysis
on concentration of heavy metals lead, mercury, cadmium, chromium
in seafood in Ningbo in 2012. Zhongguo Shipin Weisheng Zazhi.
26:76–78. 2014.
|
|
10
|
Lin X, Gu Y, Zhou Q, Mao G, Zou B and Zhao
J: Combined toxicity of heavy metal mixtures in liver cells. J Appl
Toxicol. 36:1163–1172. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verma R, Xu X, Jaiswal MK, Olsen C, Mears
D, Caretti G and Galdzicki Z: In vitro profiling of epigenetic
modifications underlying heavy metal toxicity of tungsten-alloy and
its components. Toxicol Appl Pharmacol. 253:178–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou J, Jin L, Wu N, Tan Y, Song Y, Gao M,
Liu K, Zhang X and He J: DNA damage and oxidative stress in human B
lymphoblastoid cells after combined exposure to hexavalent chromium
and nickel compounds. Food Chem Toxicol. 55:533–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stephenson AP, Schneider JA, Nelson BC,
Atha DH, Jain A, Soliman KF, Aschner M, Mazzio E and Renee Reams R:
Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells:
attenuation of thymine base lesions by glutathione and
N-acetylcysteine. Toxicol Lett. 218:299–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lankoff A, Banasik A, Duma A, Ochniak E,
Lisowska H, Kuszewski T, Góźdź S and Wojcik A: A comet assay study
reveals that aluminium induces DNA damage and inhibits the repair
of radiation-induced lesions in human peripheral blood lymphocytes.
Toxicol Lett. 161:27–36. 2006. View Article : Google Scholar
|
|
15
|
Bal W and Kasprzak KS: Induction of
oxidative DNA damage by carcinogenic metals. Toxicol Lett.
127:55–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JK, Kang KA, Ryu YS, Piao MJ, Han X,
Oh MC, Boo SJ, Jeong SU, Jeong YJ, Chae S, et al: Induction of
endoplasmic reticulum stress via reactive oxygen species mediated
by luteolin in melanoma cells. Anticancer Res. 36:2281–2289.
2016.PubMed/NCBI
|
|
17
|
Kanai K, Nagata S, Hatta T, Sugiura Y,
Sato K, Yamashita Y, Kimura Y and Itoh N: Therapeutic
anti-inflammatory effects of luteolin on endotoxin-induced uveitis
in Lewis rats. J Vet Med Sc. 78:1381–1384. 2016. View Article : Google Scholar
|
|
18
|
Fan W, Qian S, Qian P and Li X: Antiviral
activity of luteolin against Japanese encephalitis virus. Virus
Res. 220:112–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majumdar D, Jung KH, Zhang H, Nannapaneni
S, Wang X, Amin AR, Chen Z, Chen ZG and Shin DM: Luteolin
nanoparticle in chemoprevention: in vitro and in vivo anticancer
activity. Cancer Prev Res (Phila). 7:65–73. 2014. View Article : Google Scholar
|
|
20
|
Kure A, Nakagawa K, Kondo M, Kato S,
Kimura F, Watanabe A, Shoji N, Hatanaka S, Tsushida T and Miyazawa
T: Metabolic fate of luteolin in rats: its relationship to
anti-inflammatory effect. J Agric Food Chem. 64:4246–4254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Francisco V, Figueirinha A, Costa G,
Liberal J, Ferreira I, Lopes MC, García-Rodríguez C, Cruz MT and
Batista MT: The flavone luteolin inhibits liver X receptor
activation. J Nat Prod. 79:1423–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou F, Qu L, Lv K, Chen H, Liu J, Liu X,
Li Y and Sun X: Luteolin protects against reactive oxygen
species-mediated cell death induced by zinc toxicity via the
PI3K-Akt-NF-κB-ERK-dependent pathway. J Neurosci Res. 89:1859–1868.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Meng F, Zhang L, Liu A, Qin H, Lan
X, Li L and Du G: Luteolin isolated from the medicinal plant
Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in
β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y
cells. Molecules. 16:2084–2096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi EM: Luteolin protects osteoblastic
MC3T3-E1 cells from antimycin A-induced cytotoxicity through the
improved mitochondrial function and activation of PI3K/Akt/CREB.
Toxicol In Vitro. 25:1671–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hossain S, Bhowmick S, Jahan S, Rozario L,
Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK and Shahjalal
H: Maternal lead exposure decreases the levels of brain development
and cognition-related proteins with concomitant upsurges of
oxidative stress, inflammatory response and apoptosis in the
offspring rats. Neurotoxicology. 56:150–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karimi R, Vacchi-Suzzi C and Meliker JR:
Mercury exposure and a shift toward oxidative stress in avid
seafood consumers. Environ Res. 146:100–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bagchi D, Bagchi M and Stohs SJ: Chromium
(VI)-induced oxidative stress, apoptotic cell death and modulation
of p53 tumor suppressor gene. Mol Cell Biochem. 222:149–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iakimova ET, Woltering EJ, Kapchina-Toteva
VM, Harren FJ and Cristescu SM: Cadmium toxicity in cultured tomato
cells - role of ethylene, proteases and oxidative stress in cell
death signaling. Cell Biol Int. 32:1521–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roth JA and Eichhorn M: Down-regulation of
LRRK2 in control and DAT transfected HEK cells increases
manganese-induced oxidative stress and cell toxicity.
Neurotoxicology. 37:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmid M, Zimmermann S, Krug HF and Sures
B: Influence of platinum, palladium and rhodium as compared with
cadmium, nickel and chromium on cell viability and oxidative stress
in human bronchial epithelial cells. Environ Int. 33:385–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin XL, Gu YL, Zhou Q, Mao GC, Ma DJ, Zhao
JS and Zou BB: Study: Joint toxicity of heavy metal mixtures
related to proportions of fish consumption. J Ningbo Univ (NSEE).
29:22–27. 2016.In Chinese.
|
|
32
|
Guillouzo A, Corlu A, Aninat C, Glaise D,
Morel F and Guguen-Guillouzo C: The human hepatoma HepaRG cells: a
highly differentiated model for studies of liver metabolism and
toxicity of xenobiotics. Chem Biol Interact. 168:66–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu Y, Wang Y, Zhou Q, Bowman L, Mao G, Zou
B, Xu J, Liu Y, Liu K, Zhao J, et al: Inhibition of nickel
nanoparticles-induced toxicity by epigallocatechin-3-gallate in JB6
cells may be through down-regulation of the MAPK signaling
pathways. PLoS One. 11:e01509542016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asif M, Shafaei A, Jafari SF, Mohamed SK,
Ezzat MO, Abdul Majid AS, Oon CE, Petersen SH, Kono K and Abdul
Majid AM: Isoledene from Mesua ferrea oleo-gum resin induces
apoptosis in HCT 116 cells through ROS-mediated modulation of
multiple proteins in the apoptotic pathways: a mechanistic study.
Toxicol Lett. 257:84–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosseini A, Sharifi AM, Abdollahi M,
Najafi R, Baeeri M, Rayegan S, Cheshmehnour J, Hassani S, Bayrami Z
and Safa M: Cerium and yttrium oxide nanoparticles against
lead-induced oxidative stress and apoptosis in rat hippocampus.
Biol Trace Elem Res. 164:80–89. 2015. View Article : Google Scholar
|
|
37
|
Verma K, Mehta SK and Shekhawat GS: Nitric
oxide (NO) counteracts cadmium induced cytotoxic processes mediated
by reactive oxygen species (ROS) in Brassica juncea: cross-talk
between ROS, NO and antioxidant responses. Biometals. 26:255–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dutta RK, Nenavathu BP, Gangishetty MK and
Reddy AV: Studies on antibacterial activity of ZnO nanoparticles by
ROS induced lipid peroxidation. Colloids Surf B Biointerfaces.
94:143–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mathy-Hartert M, Hogge L, Sanchez C,
Deby-Dupont G, Crielaard JM and Henrotin Y: Interleukin-1beta and
interleukin-6 disturb the antioxidant enzyme system in bovine
chondrocytes: a possible explanation for oxidative stress
generation. Osteoarthritis Cartilage. 16:756–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akkerman JW, Rijksen G, Gorter G and Staal
GE: Platelet functions and energy metabolism in a patient with
hexokinase deficiency. Blood. 63:147–153. 1984.PubMed/NCBI
|
|
41
|
Chinopoulos C, Tretter L and Adam-Vizi V:
Depolarization of in situ mitochondria due to hydrogen
peroxide-induced oxidative stress in nerve terminals: inhibition of
alpha-ketoglutarate dehydrogenase. J Neurochem. 73:220–228. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tretter L, Chinopoulos C and Adam-Vizi V:
Enhanced depolarization-evoked calcium signal and reduced
[ATP]/[ADP] ratio are unrelated events induced by oxidative stress
in synaptosomes. J Neurochem. 69:2529–2537. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bennett M, Macdonald K, Chan SW, Luzio JP,
Simari R and Weissberg P: Cell surface trafficking of Fas: a rapid
mechanism of p53-mediated apoptosis. Science. 282:290–293. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Egger L, Madden DT, Rhême C, Rao RV and
Bredesen DE: Endoplasmic reticulum stress-induced cell death
mediated by the proteasome. Cell Death Differ. 14:1172–1180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outer mitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Meichner K, Fogle JE, English L and Suter
SE: Expression of apoptosis-regulating proteins Bcl-2 and Bax in
lymph node aspirates from dogs with lymphoma. J Vet Intern Med.
30:819–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
et al: Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|