Introduction
Aging is associated with a progressive reduction in
muscle mass and strength (1,2),
which is known as sarcopenia. Sarcopenia is recognized as an
important risk factor associated with disability and mortality
(3). Daily life is largely
affected by the loss of skeletal muscle mass, which subsequently
leads to skeletal muscle atrophy (4). Muscle atrophy is mainly caused by
musculoskeletal injury, denervation, ligament and joint
immobilization, joint inflammation, joint injuries, prolonged bed
rest, sepsis, aging, cancer and gluco-corticoid treatment (5–7).
In research, various model organisms of skeletal
muscle atrophy have been developed, via unloading (8,9),
immobilization (10), starvation
(11), denervation (12) and administration of
glucocorticoids (13). Among
them, high doses of dexamethasone (DEXA) stimulate muscle
proteolysis causing catabolic alterations in skeletal muscles
(14,15). The ubiquitin-proteasome and
lysosomal pathways are predominantly responsible for activation of
glucocorticoid-induced protein degradation (16). Proteins involved in these pathways
include atrogin-1, muscle-specific E3-ligases, muscle RING-finger
protein-1 (MuRF1), cathepsin L and lysosomal enzyme (17–19). Furthermore, upregulation of
myostatin is an important negative regulator of skeletal muscle
mass (20), which is associated
with glucocorticoid-induced catabolic muscle atrophy (21). Muscle structure and mass are
determined by the equilibrium between protein synthesis and
degradation, and various proteins are involved in disused muscle
atrophy (9). The mRNA expression
levels of these proteins can be readily detected using reverse
transcription polymerase chain reaction (RT-PCR), and
RT-quantitative (q) PCR has been used to determine the efficacy of
animal models of disused muscle atrophy (9,22).
In addition, apoptosis (23),
muscle fiber loss and destruction of the muscle antioxidant defense
system (24,25) are involved in
glucocorticoid-induced catabolic muscle atrophy (26). These findings suggest that
glucocorticoid-induced muscle atrophy is a valuable and efficient
animal model that may be used to identify agents that protect
against abnormal catabolic muscle atrophy (26–29).
Oxymetholone (17β-hydroxy-2-hydroxymethylidene-17
α-methyl-3-androstanone) is an orally active 17α-alkylated
anabolic-androgenic steroid (30). It has a fully saturated cyclic
hydrocarbon structure, which may limit the risk of hepato-toxicity
(31). Oxymetholone exhibits
higher anabolic activity and lower androgenic activity than
methyltestosterone, testosterone and testosterone propionate
(32). Oxymetholone has been
approved by the US Food and Drug Administration for the treatment
of anemia-associated problems that are caused by deficient red
blood cell production (33). To
date, oxymetholone has been used to treat various musculoskeletal
disorders and as a reference drug for the production of muscle
enhancers (26,33–35). However, it also exerts hepatotoxic
effects (36,37) and decreases anticoagulant
tolerance (38).
Numerous polysaccharides are able to activate
cellular components involved in host defense mechanisms (39). β-1,3/1,6-glucan is derived from
yeast cell walls and modulates numerous in vivo and in
vitro activities (40). It
has previously been associated with antitumor effects (41), radioprotective actions (42), increased host resistance to
bacterial, viral and parasitic infections (43), and adjuvant effects (44). Extracellular polysaccharides
purified from Aureobasidium pullulans SM-2001 (Polycan)
(EAP) contain 13% β-1,3/1,6-glucan (45,46) as a specific component, and have
exhibited favorable antiosteoporotic activities (46), anti-inflammatory activities
against xylene-induced acute (47) and formalin-induced chronic
(48) inflammation, potent
immunomodulatory activities in cyclophosphamide-induced
immunosuppressed mice (45),
nephroprotective effects (49),
ameliorating effects on ovalbumin-induced asthma (50), antiosteoarthritic effects
(51), and therapeutic effects
against experimental periodontitis and associated alveolar bone
losses (52), via powerful
immunomodulatory, antioxidant and anti-inflammatory mechanisms.
The present study aimed to investigate whether
administration of EAP prevented or improved glucocorticoid-induced
catabolic muscle atrophy and to examine its possible mechanism(s)
of action. EAP (100, 200 and 400 mg/kg) was administered orally,
once per day for 24 days; EAP treatment was initiated 2 weeks prior
to DEXA treatment in mice. The results from the EAP-treated mice
were then compared with those from mice treated with the
17α-alkylated anabolic-androgenic steroid, oxymetholone, at an oral
dose of 50 mg/kg (51,52).
Materials and methods
Test substances
Light brown EAP powder was supplied by Glucan
Corporation (Busan, South Korea) and was stored at 4°C. EAP
consisted of 13% β-1, 3/1,6-glucan and 40% β-glucans, as determined
using previously described analytical methods (45,46,53). Oxymetholone (50 mg tablet;
Celltrion, Incheon, South Korea) was used as a reference drug;
tablets were ground and were also stored at 4°C protected from
light. Ground 50 mg oxymetholone tablets were dissolved at a 15
mg/ml concentration (5 mg/ml oxymetholone) in deionized distilled
water. EAP was dissolved at 40 mg/ml in deionized distilled
water.
Animals and experimental design
A total of 60 adult male SPF/ICR mice (6 weeks old),
weighing 27–30 g were obtained from orient Bio, Inc. (Seongnam,
South Korea). After 10 days of acclimatization, the 48 mice that
were well acclimatized in the laboratory environment (8 mice per
group; a total of 6 groups) were used in the present study. The
mice were maintained in polycarbonate cages (n=4–5 mice/cage) in a
humidity (40–45%)- and temperature (20–25°C)-controlled room under
a 12-h light/dark cycle. Normal rodent pellets (cat. no. 38057;
Purina Feed, Seongnam, South Korea) and water were provided ad
libitum during acclimation.
Three doses of EAP (100, 200 and 400 mg/kg) were
administered orally in a volume of 10 ml/kg, once a day for 24
days; EAP treatment was initiated 2 weeks prior to DEXA treatment.
In addition, 50 mg/kg oxymetholone was administered orally, in a
similar manner to EAP. EAP was dissolved at 10, 20 or 40 mg/ml in
distilled water, and was administered orally in a volume of 10
ml/kg body weight using a zonde needle attached to a 1 ml syringe.
Ground 50 mg oxymetholone tablets were also dissolved in distilled
water at 15 mg/ml (5 mg/ml as oxymetholone) and administered orally
at 10 ml/kg, which was equivalent to 150 mg/kg (50 mg/kg as
oxymetholone). The dosage of oxymetholone was selected based on
previous efficacy tests in mice (26,33–35). Doses of 100, 200 and 400 mg/kg EAP
were selected based on previously reported in vivo efficacy
tests of EAP (45,46). In the present study, catabolic
muscle atrophy was initiated by subcutaneous treatment with 1 mg/kg
DEXA, once a day for 10 days, according to previously reported
methods (16,26). Water-soluble DEXA (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was dissolved in saline at 1.5
mg/ml (0.1 mg/ml DEXA) and was subcutaneously injected into the
cervical dorsal region in a volume of 10 ml/kg, equivalent to 15
mg/kg (1 mg/kg as DEXA itself). An equal volume of deionized
distilled water, instead of oxymetholone or EAP, was orally
administered in the DEXA control and intact vehicle groups, and an
equal volume of saline, instead of DEXA, was injected
subcutaneously into the intact vehicle control group. The present
study was conducted in accordance with international regulations of
the usage and welfare of laboratory animals, and was approved by
the Institutional Animal Care and use Committee, Daegu Haany
University (Gyeongsan, South Korea; approval no. DHU2016-051, May
27, 2016).
Body weight measurements
Body weight (g) was measured 1 day prior to, the day
of, and 1, 7, 14, 19, 23 and 24 days after treatment administration
using an electronic balance (Precisa Gravimetrics AG, Dietikon,
Switzerland). The gain in body weight during the 14 days of
pretreatment, the 10 days of DEXA treatment and the total 24-day
treatment periods was measured to decrease individual differences,
according to equation 1, where BW indicates body weight:
During 14 days of pretreatment= BW at 14 days after
initial administration - BW at first administration (Eq. 1a).
During 10 days of DEXA treatment = BW on the last
day of DEXA treatment - BW on the first day of DEXA
treatmenta (Eq. 1b). aA total of 2 weeks
after pretreatment.
During total 24 days of treatment = BW at sacrifice
- BW on the first day of pretreatment (Eq. 1c).
Calf and gastrocnemius muscle thickness
measurements
The thickness of the left hind calf was measured 1
day prior to, the day of, and 1, 7, 14, 19, 23 and 24 days after
treatment administration using electronic digital calipers
(Mitutoyo, Tokyo, Japan), similar to previous studies (26,35). Gastrocnemius muscle thickness in
the left hind limb was measured following muscle exposure after
sacrifice (all mice were sacrificed at the end of the 24-day
period; liver, kidney, pancreas, calf muscle mass and gastrocnemius
muscle tissues were collected following sacrifice), in order to
decrease variability from the surrounding tissues. Gastrocnemius
muscle thickness was measured according to the method used to
measure calf thickness; alterations in calf thickness (mm) during
14 days of pretreatment, 10 days of DEXA treatment and the total
24-day treatment period were measured to reduce individual
differences, according to equation 2, where CT indicates calf
thickness:
During 14 days of pretreatment = CT at 14 days after
initial administration - CT at first administration (Eq. 2a).
During 10 days of DEXA treatment = CT at the last
day of DEXA treatment - CT at the first day DEXA
treatmenta (Eq. 2b). aA total of 2 weeks
after pretreatment.
After 24 days of treatment = CT at sacrifice - CT on
the first day of pretreatment (Eq. 2c).
Calf muscle strength measurements
A total of 1 h after the last dose of oxymetholone,
vehicle or EAP was administered (10 days after the initial DEXA
treatment), the calf muscle strengths of individual mice were
measured as tensile strengths using a computerized testing machine
(SV-H1000, Japan Instrumentation System Co., Ltd., Tokyo, Japan) in
Newtons (N) according to established methods (26,35). Briefly, animals were restrained in
the machine using two separate 1-0 silk suture ties on the chest
and left ankle, and the peak tensile loads were documented as calf
muscle strengths during knee angle reach of 0° (10–20-mm
distance).
Gastrocnemius muscle weight
measurements
After gastrocnemius muscle thickness was measured
following sacrifice, the gastrocnemius muscles were separated
carefully from the tibia and fibula bones. The weights of
individual gastrocnemius muscles were measured in g (absolute
wet-weights) using an electronic balance, and to reduce the
differences from individual body weights, relative weights (% of
body weights) were calculated according to body weight at sacrifice
and absolute weight, following equation 3.
Relative muscle mass(% of body weight)=[absolute muscle massbody weight at sacrifice]×100
Serum biochemistry
To obtain sera for biochemical analysis, blood
samples were collected on the day of sacrifice using a separation
tube, and were then centrifuged at 600 × g for 10 min at ambient
temperature. Separated serum samples were stored at −150°C in an
ultra-deep freezer until further analysis. Serum creatine, creatine
kinase (CK) and lactate dehydrogenase (LDH) levels were measured
using an auto analyzer (Dri-Chem NX500i; FUJIFILM Medical Systems
U.S.A., Inc., Stamford, CT, USA).
Antioxidant defense systems
Following muscle mass measurements, gastrocnemius
muscles were separated and the malondialdehyde (MDA), glutathione
(GSH) and reactive oxygen species (ROS) contents, and superoxide
dismutase (SOD) and catalase (CAT) enzyme activities were assessed
in individual muscles. Separated gastrocnemius muscles were weighed
and homogenized in ice-cold 0.01 M Tris-HCl (pH 7.4), after which
they were centrifuged at 12,000 × g for 15 min at ambient
temperature, as described previously (54). Muscle tissue homogenates were
stored at −150°C in an ultra-deep freezer until analysis. The
degree of gastrocnemius muscle lipid peroxidation was measured by
assessing MDA values using the thiobarbituric acid test at 525 nm
using a UV/vis spectrometer (Optizen POP; Mecasys Co., Ltd.,
Daejeon, South Korea) (55). The
total protein contents were measured using the Lowry method
(56), whereas bovine serum
albumin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used as a standard. ROS level analyses were performed
using 2′,7′-dichlorofluorescein diacetate fluorescent dye as a
probe and fluorescence density was measured at 490/520 nm according
to the manufacturer’s protocol (Cellular Reactive oxygen Species
Detection assay kit; ab113851; Abcam, Cambridge, MA, USA); the
measured optical density values were corrected to the protein
contents of samples and were expressed as
RFu/μg−1 protein (57). In addition, prepared homogenates
were mixed with 0.1 ml 25% trichloroacetic acid (EMD Millipore,
Billerica, MA, USA) and were then centrifuged at 800 × g for 40 min
at 4°C. GSH contents were measured at 412 nm using 2-nitrobenzoic
acid (Sigma-Aldrich; Merck KGaA), and were expressed as
mg/g−1 tissue (58).
H2O2 decomposition in the presence of CAT was
estimated at 240 nm (59). CAT
activity was defined as the amount of enzyme required to decompose
1 nM H2O2 per min, at 25°C and pH 7.8, and
the results are expressed as U/mg−1 protein.
Furthermore, SOD activity was measured at 560 nm according to a
protocol previously described by Sun et al (60), and was expressed as
U/mg−1 protein. One unit of SOD enzymatic activity is
equal to the amount of enzyme that diminishes the initial
absorbance of nitroblue tetrazolium by 50% during 1 min.
RT-qPCR
Total RNA was extracted from gastrocnemius muscles
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to previous studies (9,26,35,61). The RNA concentration and quality
were determined using a CFX96™ Real-Time PCR Detection system using
iTaq™ SYBR-Green (both from Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The samples were treated with recombinant DNase I
(DNA-free DNA removal kit; Ambion, Austin, TX, USA) to remove
possible DNA contamination. RNA was reverse-transcribed using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manu facturer’s
protocol. The PCR cycling conditions were as follows: Initial
pre-denaturation of 95°C for 1 min, denaturation for 15 sec,
annealing of 55–65°C for 20 sec and extension of 72°C for 30 sec. A
total of 50 cycles were performed. 18S ribosomal RNA was used as an
internal control. PCR primer sequences are listed in Table I. For quantitative analysis, the
intact control muscle tissue was used as the control, and the
relative expression of Atrogin-1, MuRF 1, PI3K p85α, Akt1,
Adenosine A1R, TRPV4, Myostatin and SIRT1 was calculated using the
2−ΔΔCt method (62).
 | Table IOligonucleotides for quantitative
polymerase chain reaction used in the present study. |
Table I
Oligonucleotides for quantitative
polymerase chain reaction used in the present study.
| Target | Sequences
(5′-3′) | Size (bp) | Gene ID |
|---|
| Atrogin-1 | F:
CAGCTTCGTGAGCGACCTC | 244 | 67731 |
| R:
GGCAGTCGAGAAGTCCAGTC | | |
| MuRF 1 | F:
GACAGTCGCATTTCAAAGCA | 194 | 433766 |
| R:
GCCTAGCACTGACCTGGAAG | | |
| PI3K p85α | F:
GCCAGTGGTCATTTGTGTTG | 236 | 18708 |
| R:
ACACAACCAGGGAAGTCCAG | | |
| Akt1 | F:
ATGAACGACGTAGCCATTGTG | 116 | 11651 |
| R:
TTGTAGCCAATAAAGGTGCCAT | | |
| Adenosine A1R | F:
TGTTCCCAGGGCCTTTCAC | 155 | 11539 |
| R:
TAATGGACTGAGACTAGCTTGACTGGTA | | |
| TRPV4 | F:
CAGGACCTCTGGAAGAGTGC | 165 | 63873 |
| R:
AAGAGCTAGCCTGGACACCA | | |
| Myostatin | F:
CCTCCACTCCGGGAACTGA | 185 | 17700 |
| R:
AAGAGCCATCACTGCTGTCATC | | |
| SIRT1 | F:
TTCACATTGCATGTGTGTGG | 175 | 93759 |
| R:
TGAGGCCCAGTGCTCTAACT | | |
| 18S ribosomal
RNA | F:
AGCCTGAGAAACGGCTACC | 252 | 19791 |
| R:
TCCCAAGATCCAACTACGAG | | |
Histopathology
Samples from gastrocnemius muscles were separated
and fixed in 10% neutral buffered formalin, embedded in paraffin
wax, sectioned (3–4 μm), and stained with Sirius red for
collagen fibers or hematoxylin and eosin for general histopathology
(63,64). Histopathological profiles were
observed under a light microscope (Eclipse 80i; Nikon Corporation,
Tokyo, Japan). Mean muscle fiber diameters (μm/fiber) and
collagen fiber-occupied regions (%/mm2) in muscle
bundles were calculated using an automated image analyzer
(iSolution FL, version 9.1; Brooke Anco Corporation, Cicero, NY,
USA) in gastrocnemius muscle samples, according to previous studies
(9,15,21,26,35,63) with some modifications.
Immunohistochemistry
Following deparaffinization of gastrocnemius muscle
histological sections, citrate buffer antigen retrieval was
conducted as previously described (26,35,65). Briefly, a staining dish containing
10 mM citrate buffer (pH 6.0) was preheated at 95–100°C in a water
bath. Slides were immersed in the staining dish and incubated for
20 min prior to turning off the water bath. The staining dish was
placed at room temperature and the slides were allowed to cool for
20 min. Subsequently, sections were immunostained using the
avidin-biotin complex (ABC) method, to detect caspase-3, poly
(ADP-ribose) polymerase (PARP), nitrotyrosine, 4-hydroxynonenal
(4-HNE), inducible nitric oxide synthase (iNOS) and myostatin
expression (Table II) according
to previous studies (26,35). Briefly, endogenous peroxidase
activity was blocked by incubation in methanol and 0.3%
H2O2 for 30 min at ambient temperature, and
non-specific binding was blocked with normal horse serum blocking
solution (1:100; Vector Laboratories, Inc., Burlingame, CA, USA)
for 1 h at ambient temperature in a humidified chamber. Slides were
incubated with primary antibodies (Table II) overnight at 4°C in a
humidified chamber, and were then incubated with biotinylated
universal secondary antibody [1:50; Vectastain Elite ABC kit
(PK-6200); Vector Laboratories, Inc.] and ABC reagents (1:50;
Vectastain Elite ABC kit, Vector Laboratories, Inc.) for 1 h at
room temperature in a humidified chamber. Finally, sections were
treated with a peroxidase substrate kit (Vector Laboratories, Inc.)
for 3 min at room temperature. All of the sections were rinsed in
0.01 M PBS three times between steps. Cells or muscle fibers that
exhibited >20% immunoreactivity with each antibody were
considered positive, and the mean numbers of caspase-3, PARP,
nitrotyrosine, 4-HNE, iNOS and myostatin-immunoreactive fibers, as
dispersed in 1 mm2 of muscle bundles, were counted using
an image analysis process described by Kim et al (26,35) with some modifications. The
histopathologist was blinded to the group distribution when
performing the analysis.
 | Table IIPrimary antibodies and detection kits
used in the present study. |
Table II
Primary antibodies and detection kits
used in the present study.
| Antibodies or
detection kits | Cat. no. | Source | Dilution |
|---|
| Primary
antibodiesa | | | |
| Anti-cleaved
caspase-3 (Asp175) polyclonal antibody | 9661 | Cell Signaling
Technology Inc. (Danvers, MA, USA) | 1:400 |
| Anti-cleaved PARP
(Asp214) specific antibody | 9545 | Cell Signaling
Technology Inc. | 1:100 |
|
Anti-4-hydroxynonenal polyclonal
antibody | Ab46545 | Abcam (Cambridge,
UK) | 1:100 |
| Anti-nitrotyrosine
polyclonal antibody | 06-284 | EMD Millipore
(Billerica, CA, USA) | 1:200 |
| Anti-nitric oxide
synthase 2 (N-20) polyclonal antibody | sc-651 | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | 1:100 |
|
Anti-GDF8/Myoststin antibody | Ab71808 | Abcam | 1:50 |
| Detection kits | | | |
| Vectastain Elite
ABC kit | PK-6200 | Vector
Laboratories, Inc. (Burlingame, CA, USA) | 1:50 |
| Peroxidase
substrate kit | SK-4100 | Vector
Laboratories, Inc. | 1:50 |
Statistical analysis
All numerical values are expressed as the means ± SD
of 8 mice. Multiple comparison tests for different dose groups were
conducted. Variance homogeneity was examined using the Levene test
(66). If the Levene test
indicated no significant deviation from variance homogeneity, data
were analyzed by one-way analysis of variance followed by
least-significant differences multi-comparison test to determine
which pairs of group comparisons were significantly different. In
cases where significant deviations from variance homogeneity were
observed with the Levene test, the non-parametric Kruskal-Wallis
H-test was used. When a significant difference was observed with
the Kruskal-Wallis H test, the Mann-Whitney U test was conducted to
determine the specific pairs of group comparisons that were
significantly different. Statistical analyses were conducted using
SPSS 14K for Windows software (SPSS Inc., Chicago, IL, USA)
(67). Statistical significances
were set at P<0.01 and P<0.05. Percent changes between intact
vehicle and DEXA control groups were calculated to assess the
severities of catabolic muscle atrophy induced, and the percent
changes between the DEXA control and test material-treated mice
were calculated, in order to understand the efficacy of the test
substances according to the following equations 4 and 5:
Percentage change compared to the intact vehicle control group(%)=[Data of DEXA control−Data of intact vehicle controlData of intact vehicle control]×100
Percentage change compared to the DEXA control group(%)=[Data of test material treated mice−Data of DEXA controlData of DEXA control]×100
Results
Alterations in body weight
Significant decreases (P<0.01) in body weight
were demonstrated in the DEXA control mice compared with in the
intact control mice from 5 days after initial DEXA treatment to
sacrifice. Accordingly, body weight during the 10 days of DEXA
treatment, and after the total 24-day experimental period, was
significantly decreased (P<0.01) in the DEXA control mice
compared with in the intact vehicle control group. However, these
decreases in body weight were significantly inhibited (P<0.01)
by treatment with oxymetholone and all three doses of EAP (100, 200
and 400 mg/kg) from 5 days after initial DEXA treatment to
sacrifice. In addition, body weight after 10 days of DEXA
treatment, and after the total 24-day experimental period, was
significantly increased (P<0.01) in the oxymetholone- and
EAP-treated mice compared with in the DEXA control group. Anyway,
no test material treatment-related alterations in body weight were
detected compared with intact vehicle or DEXA control mice in this
experiment. Treatment with EAP (100, 200 and 400 mg/kg) exhibited
dose-dependent inhibitory effects on DEXA-induced decreases in body
weight, in particular 400 mg/kg EAP exhibited favorable inhibitory
activities on DEXA-induced decreases in body weight, which were
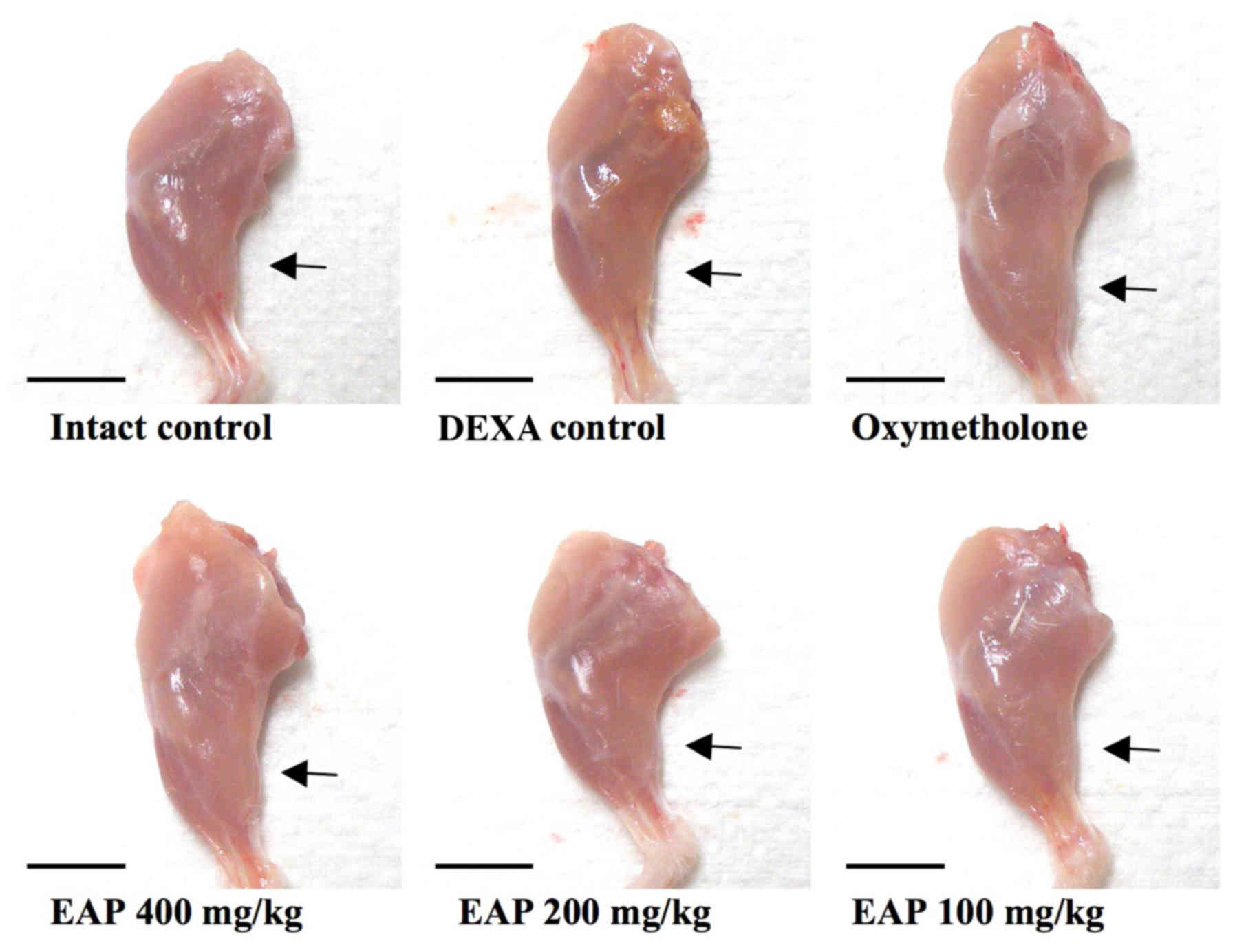
comparable with the effects of 50 mg/kg oxymetholone (Table III and Fig. 1).
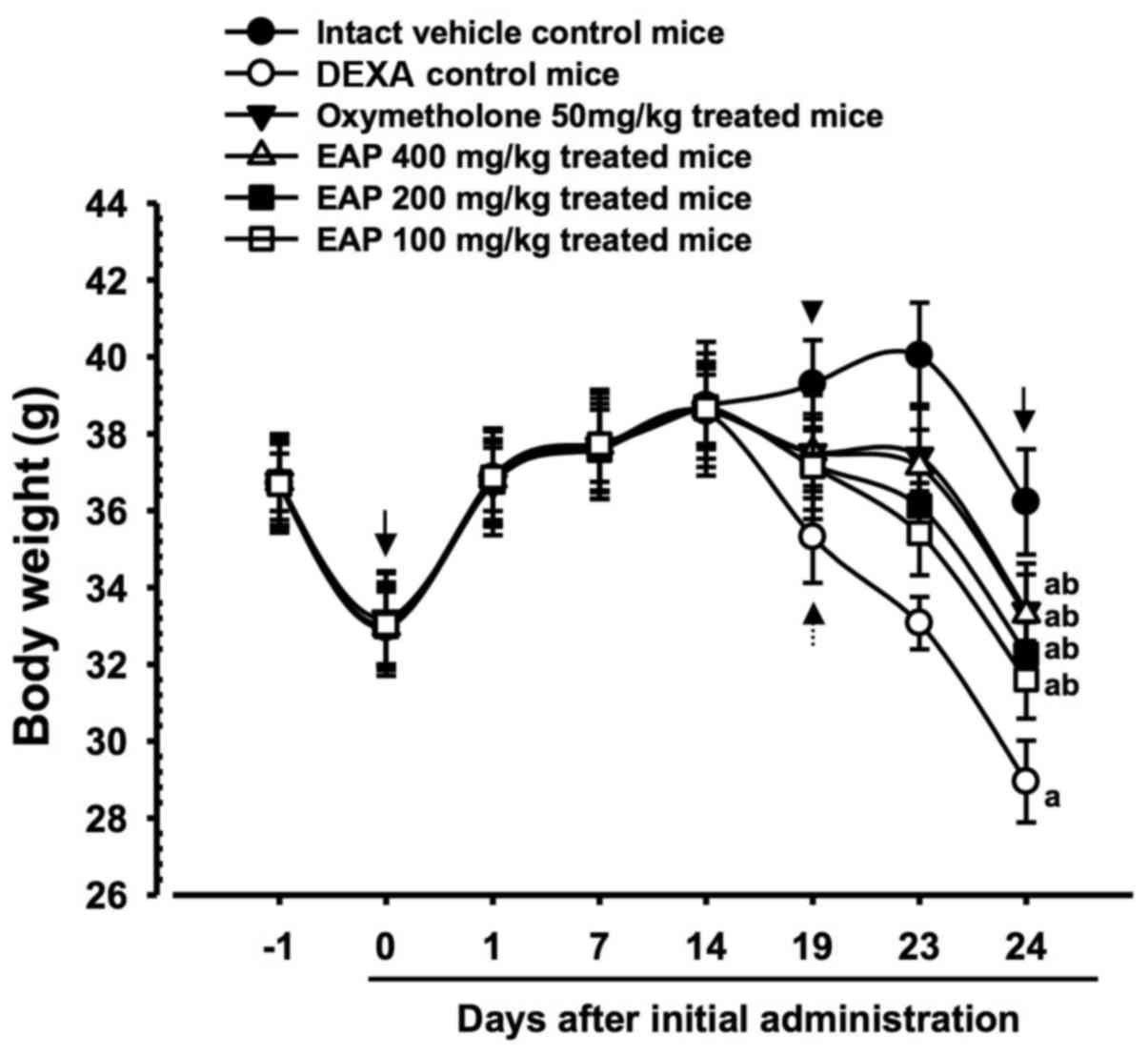 | Figure 1Body weight alterations in mice with
DEXA-induced muscle atrophy. Significant decreases in body weight
were detected in the DEXA control mice compared with in the intact
control mice from 5 days after initial DEXA treatment, 19 days
after initial administration (dotted arrow). However, these
decreases in body weight were significantly inhibited by treatment
with oxymetholone and all three doses of EAP (400, 200 and 100
mg/kg), from 5 days after initial DEXA treatment (arrowhead) to
sacrifice. EAP 400, 200 and 100 mg/kg exhibited clear
dose-dependent inhibitory effects on DEXA-induced decreases in body
weight, particularly EAP 400 mg/kg, which exerted comparable
effects to oxymetholone (50 mg/kg). No test material
treatment-associated body weight alterations were detected compared
with in the intact vehicle and DEXA control mice during the 14-day
pretreatment period. Data are presented as the mean ± standard
deviation of 8 mice. Day -1 and 24 indicates 1 day prior to initial
administration of test materials and the day of sacrifice,
respectively. Day 0 indicates initiation of test material
administration, at 2 weeks prior to initial DEXA treatment. All
animals were fasted overnight prior to initial administration of
test materials and sacrifice (arrows). aP<0.01
compared with the intact control group, as determined by LSD test.
bP<0.01 compared with the DEXA control group, as
determined by LSD test. DEXA, dexamethasone; EAP, extracellular
polysaccharides purified from Aureobasidium pullulans
SM-2001; LSD, least-significant difference. Results were
significant at 24 days |
 | Table IIIAlterations in body weight in mice
with DEXA-induced muscle atrophy. |
Table III
Alterations in body weight in mice
with DEXA-induced muscle atrophy.
| Group | Weight gain (g)
|
|---|
| 14 days of test
material pretreatment | 10 days of DEXA
treatment | Total 24 days of
treatment |
|---|
| Controls | | | |
| Intact | 5.74±0.63 | 1.30±0.34 | 3.23±0.96 |
| DEXA | 5.63±0.49 | −5.50±0.81a | −4.00±0.63b |
| Reference | | | |
| Oxymetholone | 5.43±0.56 | −1.19±0.47a,c | 0.20±0.66b,d |
| EAP-treated | | | |
| 400 mg/kg | 5.68±0.58 | −1.49±0.73a,c | 0.34±1.14b,d |
| 200 mg/kg | 5.71±0.41 | −2.58±0.52a,c | −0.73±0.55b,d |
| 100 mg/kg | 5.61±0.62 | −3.24±0.95a,c | −1.44±0.81b,d |
Effects on calf thickness
Significant decreases (P<0.01) in calf thickness
were demonstrated in the DEXA control mice compared with in the
intact control mice from 19 days after initial administration of
the test substances to the day of sacrifice. Accordingly, calf
thickness alterations after 10 days of DEXA treatment, and after
the total 24-day test substance administration period, were also
significantly decreased (P<0.01) in the DEXA control mice
compared with in the intact vehicle controls. However, 5 days after
the initial DEXA treatment, these decreases in calf thickness were
significantly inhibited (P<0.01) by treatment with the three
doses of EAP, and calf thickness during the 10 days of DEXA
treatment, and the total 24-day test substance administration
period, were also significantly increased (P<0.01) in these
groups compared with in the DEXA control group. Furthermore, 50
mg/kg oxymetholone-treated mice also exhibited significant
increases (P<0.01) in calf thickness from 5 days after the
initial DEXA treatment, and also exhibited significant increases
(P<0.01) in calf thickness during the 10 days of DEXA treatment
and the total 24-day test substance administration period. A dose
of 400 mg/kg EAP exhibited favorable inhibitory activities on
DEXA-induced decreases in calf thickness, which were comparable
with the effects of 50 mg/kg oxymetholone (Table IV and Fig. 2).
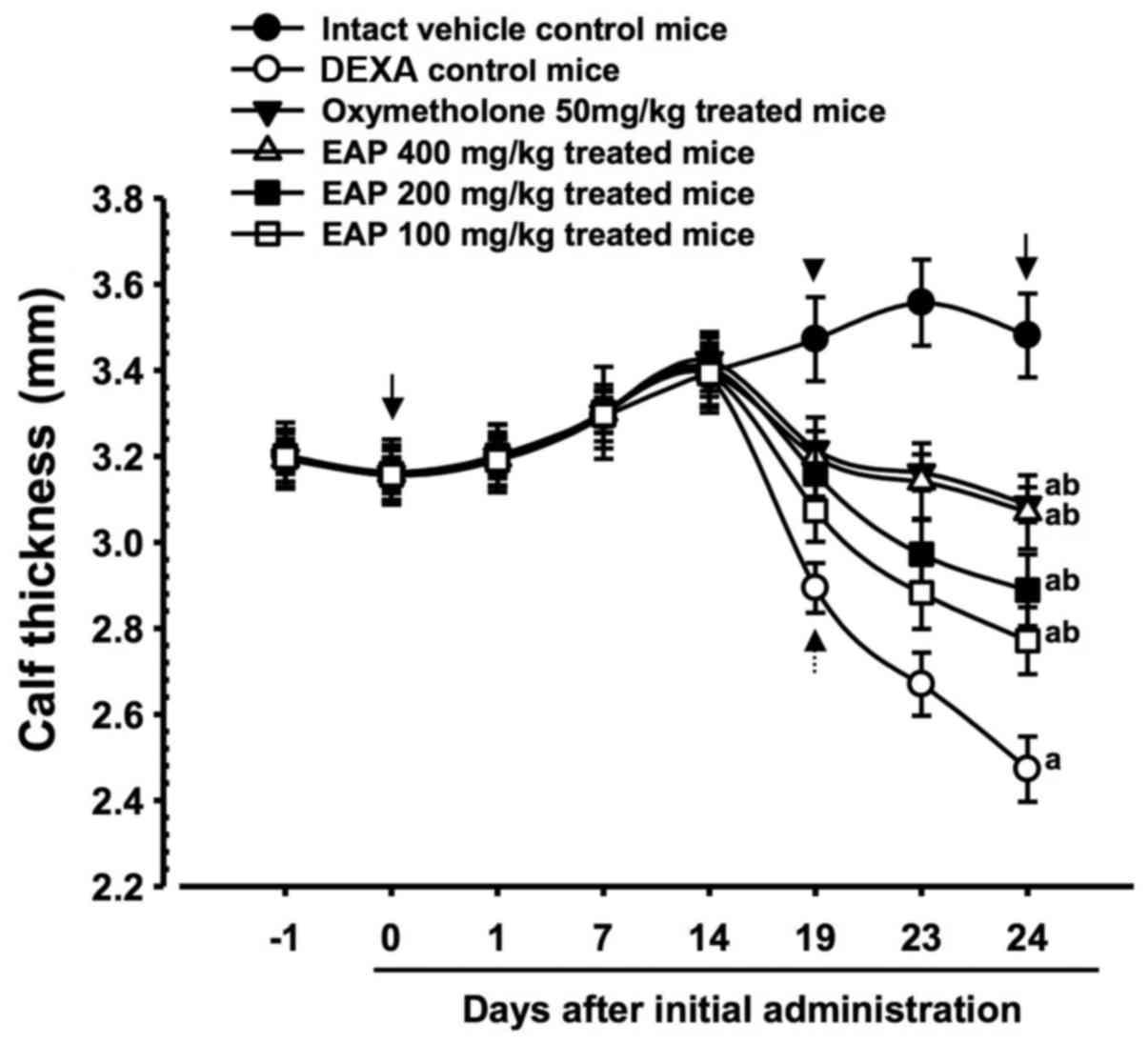 | Figure 2Calf thickness alterations in mice
with DEXA-induced muscle atrophy. Significant decreases in calf
thickness were revealed in the DEXA control mice compared with in
the intact control mice from 19 days after the initial test
substance administration to the day of sacrifice (dotted arrow).
However, these decreases in calf thicknes were significantly and
dose-dependently inhibited by treatment with all three doses of EAP
(400, 200 and 100 mg/kg) from 5 days after the intial DEXA
treatment (arrowhead). In addition, 50 mg/kg oxymetholone-treated
mice also exhibited significant increases in calf thickness from 5
days after the intial DEXA treatment compared with in the DEXA
control mice (arrowhead). EAP (400 mg/kg) exhibited favorable
inhibitory activities on DEXA-induced decreases in calf thickness,
as comparable to those of oxymetholone (50 mg/kg). Data are
presented as the mean ± standard deviation of 8 mice. Day -1 and 24
indicates 1 day prior to initial administration of test materials
and the day of sacrifice, respectively. Day 0 indicates initiation
of test material administration, at 2 weeks prior to initial DEXA
treatment. All animals were fasted overnight prior to initial
administration of test materials and sacrifice (arrows).
aP<0.01 compared with the intact control group, as
determined by LSD test. bP<0.01 compared with the
DEXA control group, as determined by LSD test. DEXA, dexamethasone;
EAP, extracellular polysaccharides purified from Aureobasidium
pullulans SM-2001; LSD, least-significant difference. Results
were significant at 24 days |
 | Table IVAlterations in calf thickness in mice
with DEXA-induced muscle atrophy. |
Table IV
Alterations in calf thickness in mice
with DEXA-induced muscle atrophy.
| Group | Calf thickness
alterations (mm)
|
|---|
| 14 days of test
material pretreatment | 10 days of DEXA
treatment | Total 24 days of
treatment |
|---|
| Controls | | | |
| Intact | 0.23±0.04 | 0.09±0.06 | 0.32±0.06 |
| DEXA | 0.23±0.04 | −0.91±0.04a | −0.68±0.05a |
| Reference | | | |
| Oxymetholone | 0.27±0.05 | −0.34±0.04a,b | −0.07±0.04a,b |
| EAP-treated | | | |
| 400 mg/kg | 0.24±0.05 | −0.33±0.04a,b | −0.09±0.04a,b |
| 200 mg/kg | 0.25±0.03 | −0.52±0.10a,b | −0.27±0.09a,b |
| 100 mg/kg | 0.24±0.03 | −0.62±0.04a,b | −0.39±0.05a,b |
Effects on gastrocnemius muscle thickness
after muscle exposure
Significant decreases (P<0.01) in gastrocnemius
muscle thickness following muscle exposure were observed in the
DEXA control mice compared with in the intact vehicle control mice.
However, significant increases (P<0.01) in gastrocnemius muscle
thickness were detected in the mice treated with oxymetholone and
all three doses of EAP compared with in the DEXA control group. EAP
(100, 200 and 400 mg/kg) exhibited dose-dependent inhibitory
effects on DEXA-induced decreases in gastrocnemius muscle
thickness. In particular, 400 mg/kg EAP exhibited favorable
inhibitory activities on gastrocnemius muscle thickness, which were
comparable with the effects of 50 mg/kg oxymetholone (Figs. 3 and 4).
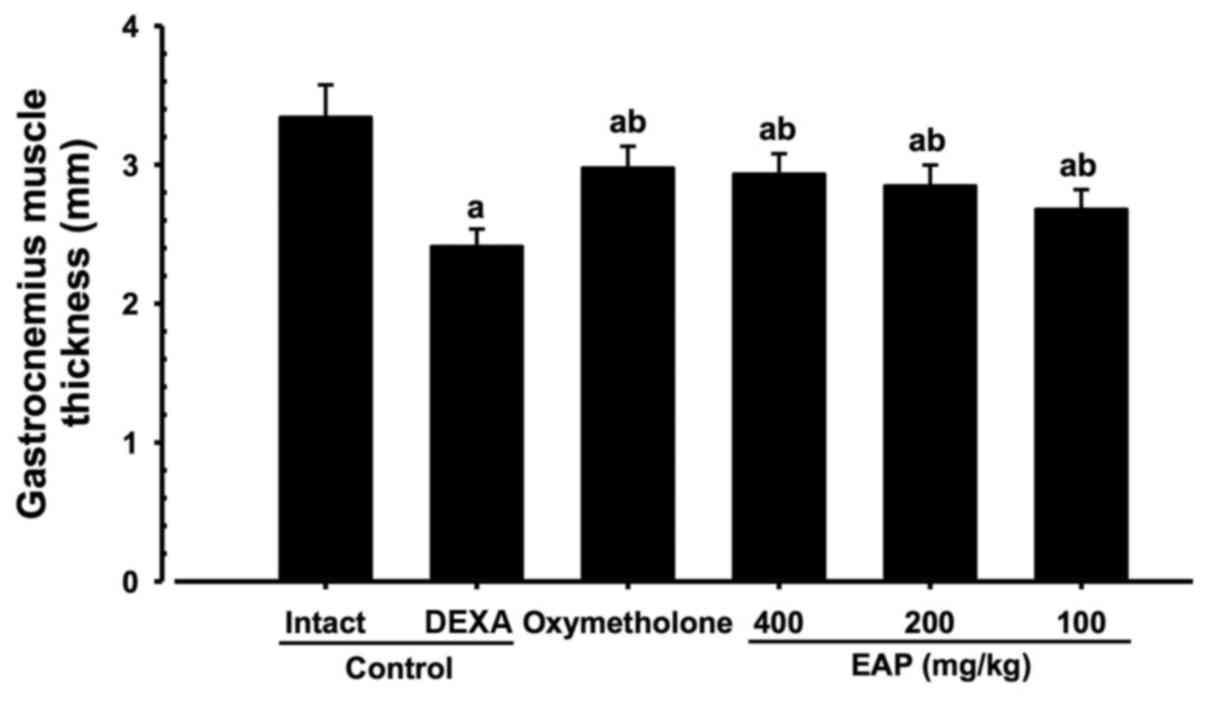 | Figure 4Alterations in gastrocnemius muscle
thickness following muscle exposure in mice with DEXA-induced
muscle atrophy. Significant decreases in gastrocnemius muscle
thickness following muscular exposure were detected in the DEXA
control mice compared with in the intact vehicle control mice.
However, significant increases in gastrocnemius muscle thickness
were observed in oxymetholone- and EAP-treated mice compared with
in the DEXA control group. EAP (400, 200 and 100 mg/kg) exhibited
marked dose-dependent inhibitory effects on DEXA-induced decreases
in gastroc-nemius muscle thickness; in particular, 400 mg/kg EAP
exhibited favorable inhibitory activities on decreases in
gastrocnemius muscle thickness, which were comparable with the
effects of oxymetholone (50 mg/kg). Data are presented as the mean
± standard deviation of 8 mice. Oxymetholone was orally
administered at 50 mg/kg, dissolved in deionized distilled water.
aP<0.01 compared with the intact control group, as
determined by LSD test. bP<0.01 compared with the
DEXA control group, as determined by LSD test. DEXA, dexamethasone;
EAP, extracellular polysaccharides purified from Aureobasidium
pullulans SM-2001; LSD, least-significant difference. |
Effects on gastrocnemius muscle mass
Significant decreases (P<0.01) in relative
weights and absolute wet weights of gastrocnemius muscle mass were
demonstrated in the DEXA control mice compared with in the intact
vehicle control mice. However, significant increases (P<0.01) in
gastrocnemius muscle weights were observed in the
oxymetholone-treated and 100, 200 and 400 mg/kg EAP-treated mice
compared with in the DEXA control group. EAP doses (100, 200, and
400 mg/kg) exhibited dose-dependent inhibitory effects on the
DEXA-induced decreases in gastrocnemius muscle weights; in
particular, 400 mg/kg EAP exhibited favorable inhibitory activities
on gastrocnemius muscle weight, which were comparable with the
effects of 50 mg/kg oxymetholone (Fig. 5).
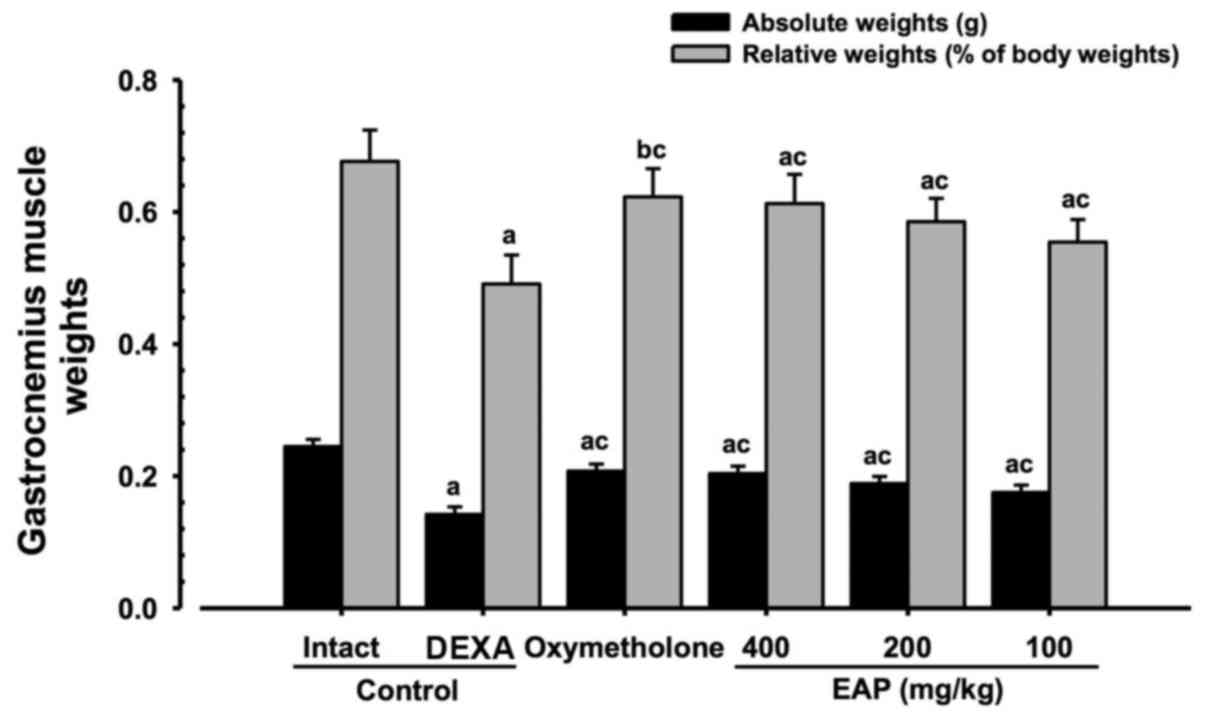 | Figure 5Alterations in gastrocnemius muscle
weight in mice with DEXA-induced muscle atrophy. Significant
decreases in absolute wet-weights and relative weights of
gastrocnemius muscle mass were revealed in the DEXA control mice
compared with in the intact vehicle control mice. However,
significant increases in gastrocnemius muscle mass weights were
observed in oxymetholone- and EAP-treated mice compared with in the
DEXA control group. EAP (400, 200 and 100 mg/kg) exhibited
dose-dependent inhibitory effects on DEXA-induced decreases in
gastrocnemius muscle weights; in particular, 400 mg/kg EAP
exhibited favorable inhibitory activities on decreases in
gastrocnemius muscle weights, which were comparable with the
effects of oxymetholone (50 mg/kg). Data are presented as the mean
± standard deviation of 8 mice. Oxymetholone was orally
administered at 50 mg/kg, dissolved in deionized distilled water.
aP<0.01 and bP<0.05 compared with the
intact control group, as determined by LSD test.
cP<0.01 compared with the DEXA control group, as
determined by LSD test. DEXA, dexamethasone; EAP, extracellular
polysaccharides purified from Aureobasidium pullulans
SM-2001; LSD, least-significant difference. |
Effects on calf muscle strength
Significant decreases (P<0.01) in the tensile
strength of calf muscles were demonstrated in the DEXA control mice
compared with in the intact vehicle control mice. However,
significant increases (P<0.01) in calf muscle strength were
observed in oxymetholone-treated and 200 and 400 mg/kg EAP-treated
mice compared with in the DEXA control group. In addition, 100
mg/kg EAP-treated mice exhibited non-significant increases in calf
muscle strength compared with in the DEXA control mice. EAP (100,
200 and 400 mg/kg) exhibited dose-dependent inhibitory effects on
DEXA-induced decreases in calf muscle strength; in particular, 400
mg/kg EAP exhibited favorable inhibitory activities on decreases in
calf muscle strength, which were comparable with the effects of 50
mg/kg oxymetholone (Fig. 6).
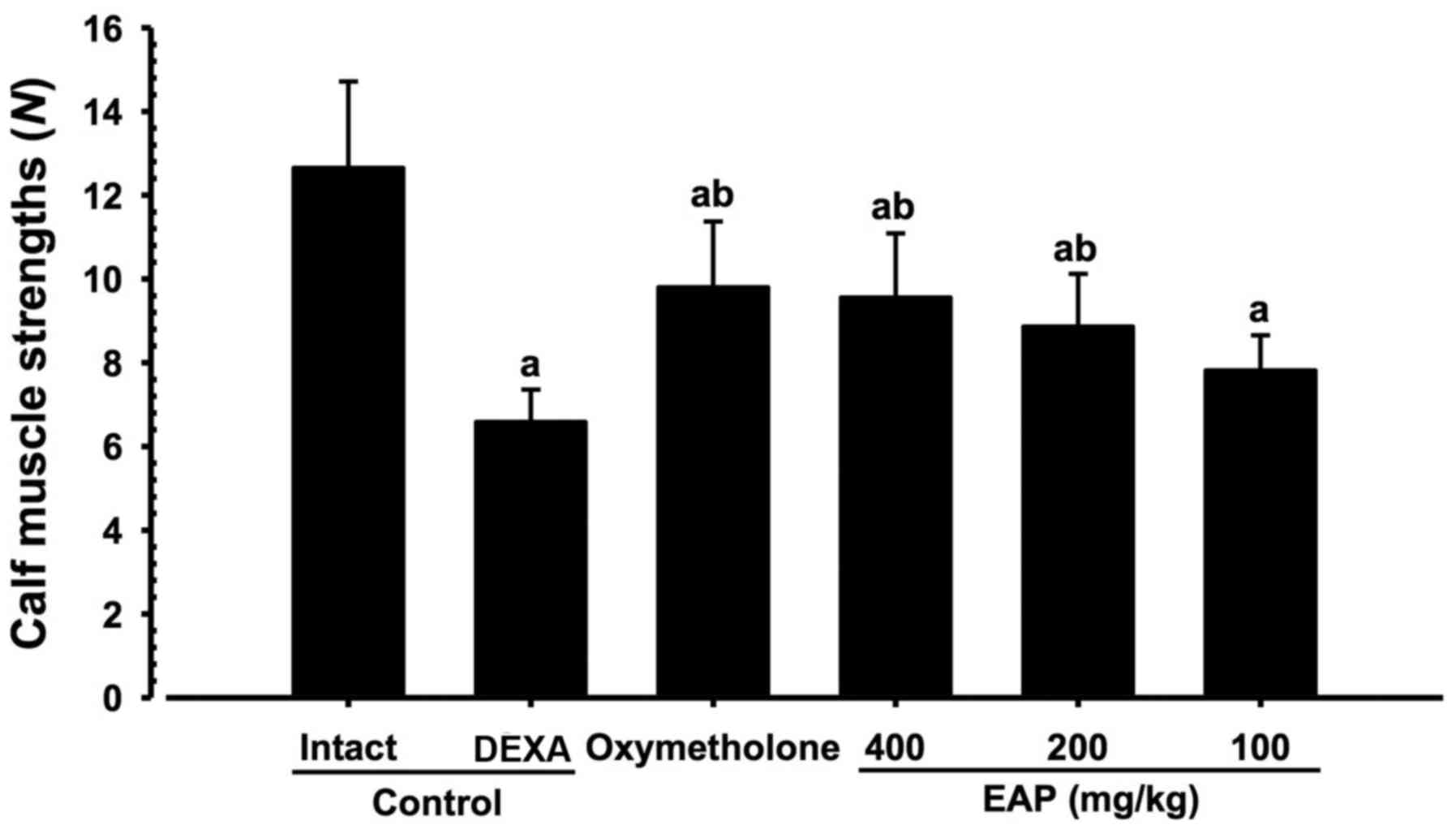 | Figure 6Alterations in calf muscle strength
in mice with DEXA-induced muscle atrophy. Significant decreases in
the tensile strength of calf muscles were revealed in the DEXA
control mice compared with in the intact vehicle control mice.
However, significant increases in calf muscle strength were
observed in the 50 mg/kg oxymetholone-treated and 400 and 200 mg/kg
EAP-treated mice compared with in the DEXA control group. In
addition, 100 mg/kg EAP-treated mice exhibited non-significant
increases in calf muscle strength compared with in the DEXA control
mice. EAP (400, 200 and 100 mg/kg) exhibited clear dose-dependent
inhibitory effects on DEXA-induced decreases in calf muscle
strength; in particular, 400 mg/kg EAP exhibited favorable
inhibitory activities on decreases in calf muscle strength, which
were comparable with the effects of oxymetholone (50 mg/kg). Data
are presented as the mean ± standard deviation of 8 mice.
Oxymetholone was orally administered at 50 mg/kg, dissolved in
deionized distilled water. aP<0.01 compared with the
intact control group, as determined by LSD test.
bP<0.01 compared with the DEXA control group, as
determined by LSD test. DEXA, dexamethasone; EAP, extracellular
polysaccharides purified from Aureobasidium pullulans
SM-2001; LSD, least-significant difference. |
Effects on serum biochemistry
Significant increases (P<0.01) in serum CK and
creatine levels, and decreases in serum LDH levels, were
demonstrated in the DEXA control mice compared with in the intact
vehicle control mice. However, significant decreases (P<0.05) in
serum CK and creatine levels were observed in oxymetholone- and
EAP-treated mice compared with in the DEXA control group, alongside
significant increases (P<0.05) in serum LDH levels. EAP (100,
200, and 400 mg/kg) exhibited dose-dependent inhibitory effects on
DEXA-induced increases in serum CK and creatine levels, and
decreases in serum LDH levels. In particular, 400 mg/kg EAP
exhibited favorable inhibitory activities on serum CK and creatine
level elevations, and decreases in serum LDH levels, which were
comparable with the effects of 50 mg/kg oxymetholone (Table V).
 | Table VAlterations in the serum biochemistry
of mice with DEXA-induced muscle atrophy. |
Table V
Alterations in the serum biochemistry
of mice with DEXA-induced muscle atrophy.
| Group | Serum levels
(units)
|
|---|
| Creatine
(mg/dl) | Creatine kinase
(IU/l) | LDH (IU/l) |
|---|
| Controls | | | |
| Intact | 0.33±0.06 | 83.63±19.40 | 647.25±131.86 |
| DEXA | 0.85±0.11a |
274.13±51.85b |
163.25±47.63a |
| Reference | | | |
| Oxymetholone | 0.45±0.05c,d |
146.50±18.15b,e |
304.13±71.06a,d |
| EAP-treated | | | |
| 400 mg/kg | 0.49±0.09a,d |
152.88±14.26b,e |
294.00±42.81a,d |
| 200 mg/kg | 0.57±0.10a,d |
176.63±15.40b,e |
264.25±53.73a,d |
| 100 mg/kg | 0.66±0.13a,d |
210.75±29.35b,f |
242.50±29.24a,g |
Effects on gastrocnemius muscle
antioxidant defense systems Alterations in muscle MDA levels
Significant increases (P<0.01) in MDA levels were
observed in the DEXA control group compared with in the intact
control group. However, the elevations in MDA levels were
significantly (P<0.01) and dose-dependently decreased following
treatment with EAP. Gastrocnemius muscle lipid peroxidation in
oxymetho-lone-treated mice was also significantly decreased
(P<0.01) compared with in the control mice. In particular, 400
mg/kg EAP exhibited favorable inhibitory activities on DEXA-induced
increases in muscle lipid peroxidation, which were comparable with
the effects of 50 mg/kg oxymetholone (Table VI).
 | Table VIAlterations in the gastrocnemius
muscle antioxidant defense system in mice with DEXA-induced muscle
atrophy. |
Table VI
Alterations in the gastrocnemius
muscle antioxidant defense system in mice with DEXA-induced muscle
atrophy.
| Group | Activity levels
(units)
|
|---|
| Malondialdehyde
(nM/mg protein) | Reactive oxygen
species (RFU/μg protein) | Glutathione (nM/mg
protein) | Superoxide
dismutase (nM/min/mg protein) | Catalase (U/mg
protein) |
|---|
| Controls | | | | | |
| Intact | 1.84±0.76 | 22.12±10.46 | 0.64±0.15 | 34.14±10.58 | 7.08±2.11 |
| DEXA | 8.32±1.11a | 67.40±12.82a | 0.16±0.07a | 11.19±1.97b | 1.84±0.24b |
| Reference | | | | | |
| Oxymetholone | 4.44±1.05a,c | 31.92±11.89c | 0.37±0.09a,c | 21.51±4.42b,d | 3.64±0.70b,d |
| EAP-treated | | | | | |
| 400 mg/kg | 4.48±1.20a,c | 31.89±10.67c | 0.38±0.10a,c | 20.81±4.57b,d | 3.56±0.83b,d |
| 200 mg/kg | 5.81±0.90a,c | 37.31±10.21c,e | 0.34±0.12a,c | 18.94±4.46b,d | 3.12±0.49b,d |
| 100 mg/kg | 6.42±0.76a,c | 45.19±12.22a,c | 0.29±0.08a,f | 17.52±2.30b,d | 2.70±0.52b,d |
Alterations in muscle ROS content
Significant increases (P<0.01) in muscle ROS
content were observed in the DEXA control group compared with in
the intact control group. However, elevated ROS levels were
significantly and dose-dependently decreased (P<0.01) following
treatment with EAP. In addition, gastrocnemius muscle ROS levels
were significantly (P<0.01) inhibited in 50 mg/kg
oxymetholone-treated mice compared with in the DEXA control mice.
In particular, 400 mg/kg EAP exhibited favorable inhibitory
activities on DEXA-induced muscle ROS elevations, which were
comparable with the effects of oxymetholone (Table VI).
Alterations in muscle GSH content
Significant decreases (P<0.01) in the levels of
the endogenous antioxidant, GSH, were detected in the DEXA control
group compared with in the intact control group. However, these
decreases in muscle GSH were significantly (P<0.05) inhibited
following 24 days of oral treatment with oxymetholone, and 100, 200
and 400 mg/kg EAP. EAP increased gastrocnemius muscle GSH content
in a dose-dependent manner compared with in the DEXA control mice.
In particular, 400 mg/kg EAP exhibited favorable inhibitory
activities on DEXA-induced decreases in muscle GSH content, which
were comparable with the effects of oxymetho-lone (Table VI).
Alterations in muscle SOD activity
Significant decreases (P<0.01) in the activity
levels of the endogenous antioxidant enzyme, SOD, were detected in
the DEXA control group compared with in the intact control group.
However, significant increases (P<0.01) in SOD activity were
observed in oxymetholone-treated, and 100, 200 and 400 mg/kg
EAP-treated mice compared with in the DEXA control mice. EAP
exerted dose-dependent increases on SOD activity in gastrocnemius
muscles compared with in the DEXA control mice. In particular, 400
mg/kg EAP exhibited favorable inhibitory activities on DEXA-induced
decreases in SOD activity levels, which were comparable with the
effects of 50 mg/kg oxymetholone (Table VI).
Alterations in muscle CAT activity
Significant decreases (P<0.01) in the activity
levels of the endogenous antioxidant enzyme, CAT, were detected in
the DEXA control group compared with in the intact control group.
However, these decreases in muscle CAT activity were significantly
and dose-dependently inhibited (P<0.01) following 24 days of
oral treatment with EAP. Gastrocnemius muscle CAT activity levels
in 50 mg/kg oxymetholone-treated mice were also significantly
increased (P<0.01) compared with in the DEXA control mice. In
particular, 400 mg/kg EAP exhibited favorable inhibitory activities
on DEXA-induced decreases in muscle CAT activity levels, which were
comparable with the effects of 50 mg/kg oxymetholone (Table VI).
Effects on gastrocnemius muscle mRNA
expression
Significant alterations (P<0.01) in the mRNA
expression levels of atrogin-1, MuRF1, PI3K, Akt1, A1R, TRPV4,
myostatin and SIRT1 were detected in the gastrocnemius muscles of
the DEXA control group compared with in the intact control group.
However, these alterations in muscle atrogin-1, MuRF1, PI3K, Akt1,
A1R, TRPV4, myostatin and SIRT1 expression were significantly
reversed (P<0.05), in a dose-dependent manner, by treatment with
EAP. In addition, the mRNA expression levels of atrogin-1, MuRF1,
PI3K, Akt1, A1R, TRPV4, myostatin and SIRT1 in gastrocnemius muscle
tissues, were significantly reversed in 50 mg/kg
oxymetholone-treated mice (P<0.01) compared with in the DEXA
control mice. In particular, 400 mg/kg EAP exhibited favorable
activities on DEXA-induced alterations in muscle atrogin-1, MuRF1,
PI3K, Akt1, A1R, TRPV4, myostatin and SIRT1 mRNA expression, which
were comparable with the effects of 50 mg/kg oxymetholone (Table VII).
 | Table VIIAlterations in gastrocnemius muscle
mRNA expression in mice with DEXA-induced muscle atrophy. |
Table VII
Alterations in gastrocnemius muscle
mRNA expression in mice with DEXA-induced muscle atrophy.
| Target | Groups
|
|---|
Controls
| Reference
| EAP-treated mice
(mg/kg)
|
|---|
| Intact | DEXA | Oxymetholone | 400 | 200 | 100 |
|---|
| Atrogin-1 | 0.99±0.07 | 4.90±0.67a | 2.20±0.44a,b | 2.29±0.44a,b | 3.24±0.66a,b | 3.81±0.51a,b |
| MuRF 1 | 1.08±0.22 | 6.15±0.97c | 2.96±0.54c,d | 3.05±0.61c,d | 3.54±0.83c,d | 4.03±0.58c,d |
| PI3K p85α | 1.03±0.13 | 0.62±0.09a | 1.13±0.39b | 1.11±0.33b | 0.92±0.06b,e | 0.84±0.13b,e |
| Akt1 | 1.01±0.06 | 0.52±0.07c | 0.85±0.10c,d | 0.85±0.09c,d | 0.78±0.12c,d | 0.70±0.10c,d |
| A1R | 1.03±0.14 | 0.49±0.12c | 0.86±0.06c,d | 0.86±0.11c,d | 0.72±0.08c,d | 0.67±0.12c,d |
| TRPV4 | 1.09±0.10 | 0.35±0.08c | 0.65±0.10c,d | 0.68±0.15c,d | 0.61±0.11c,d | 0.52±0.13c,d |
| Myostatin | 1.01±0.09 | 6.88±0.89a | 3.16±0.73a,b | 3.17±0.59a,b | 3.95±0.73a,b | 4.36±1.18a,b |
| SIRT1 | 1.01±0.18 | 10.49±2.97a | 3.66±1.13a,b | 3.49±1.00a,b | 4.72±1.61a,b | 5.28±1.15a,b |
Effects on gastrocnemius muscle
histopathology
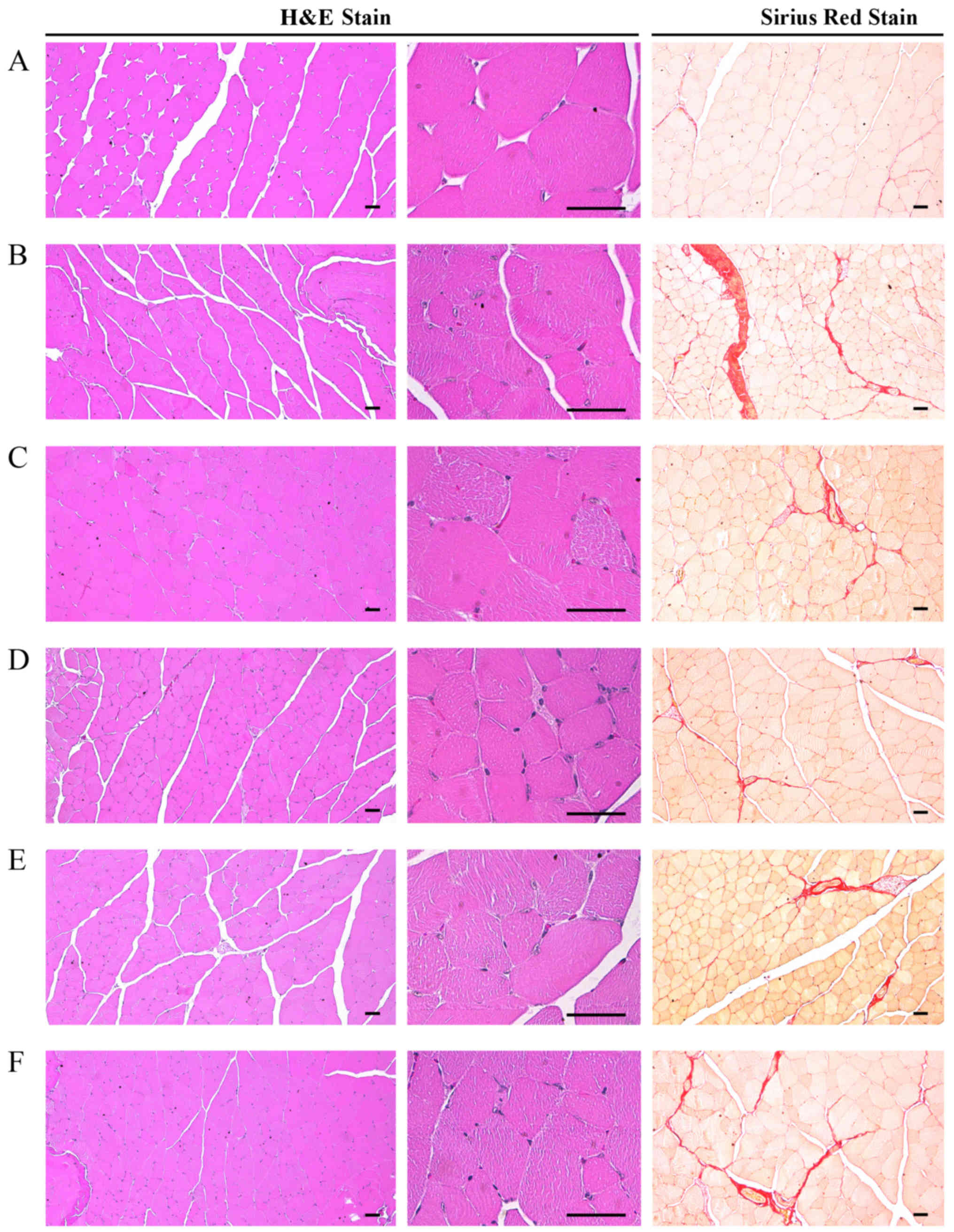
Marked alterations associated with catabolic muscle
atrophy, including focal fibrosis in muscle bundles,
microvacuolation and diminished muscle fibers, were induced by
treatment with DEXA in the control mice. Accordingly, significant
decreases (P<0.01) in mean muscle fiber diameters and increases
in collagen fiber-occupied region percentages in muscle bundles
were detected in the DEXA control mice compared with in the intact
control mice. However, these DEXA treatment-associated catabolic
alterations were significantly (P<0.05) and dose-dependently
decreased following treatment with EAP. The muscle
atrophy-associated alterations were also significantly inhibited
(P<0.01) in 50 mg/kg oxymetholone-treated mice compared with in
the DEXA control mice. In particular, 400 mg/kg EAP exhibited
favorable inhibitory activities on DEXA-induced decreases in mean
muscle fiber diameters and increases in collagen fiber-occupied
regions in muscle bundles, which were comparable with the effects
of oxymetholone (Table VIII
and Fig. 7).
 | Table VIIIAlterations in gastrocnemius muscle
histomorphometry in mice with DEXA-induced muscle atrophy. |
Table VIII
Alterations in gastrocnemius muscle
histomorphometry in mice with DEXA-induced muscle atrophy.
| Variable | Groups
|
|---|
Controls
| Reference
| EAP-treated mice
(mg/kg)
|
|---|
| Intact | DEXA | Oxymetholone | 400 | 200 | 100 |
|---|
| General
histomorphometry | | | | | | |
| Fiber diameter
(μm) | 51.36±10.37 | 23.35±5.26a | 38.03±6.72a,b | 37.39±7.30a,b | 34.77±6.65a,b | 31.87±4.30a,c |
| Collagen (%) | 4.09±1.71 | 31.96±4.71d | 16.72±3.30d,e | 15.93±4.62d,e | 19.36±3.02d,e | 23.57±5.64d,f |
|
Immunohistomorphometry
(fibers/mm2) | | | | | | |
| Caspase-3 | 2.13±2.53 | 41.13±10.45a | 20.88±5.03a,b | 19.00±2.83a,b | 23.63±5.26a,b | 28.00±6.55a,b |
| PARP | 4.88±2.59 | 75.00±12.94a | 32.13±10.25a,b | 31.25±10.94a,b | 38.63±14.34a,b | 48.50±12.04a,b |
| Nitrotyrosine | 5.63±2.67 | 67.75±12.37a | 34.63±13.09a,b | 31.38±14.51a,b | 38.25±15.18a,b | 46.00±13.31a,b |
| 4-HNE | 3.75±1.91 | 75.38±11.67a | 42.25±10.50a,b | 40.38±10.14a,b | 45.75±10.98a,b | 53.50±10.60a,b |
| iNOS | 7.63±2.77 | 50.25±11.85d | 20.38±4.63d,e | 16.25±3.54d,e | 27.63±7.85d,e | 32.75±8.76d,e |
| Myostatin | 1.13±0.83 | 51.88±11.15d | 21.75±4.83d,e | 20.38±5.40d,e | 32.75±4.98d,e | 36.38±7.63d,e |
Effects on gastrocnemius muscle
immunohistochemistry
Alterations in
caspase-3-immunolabelled muscle fibers
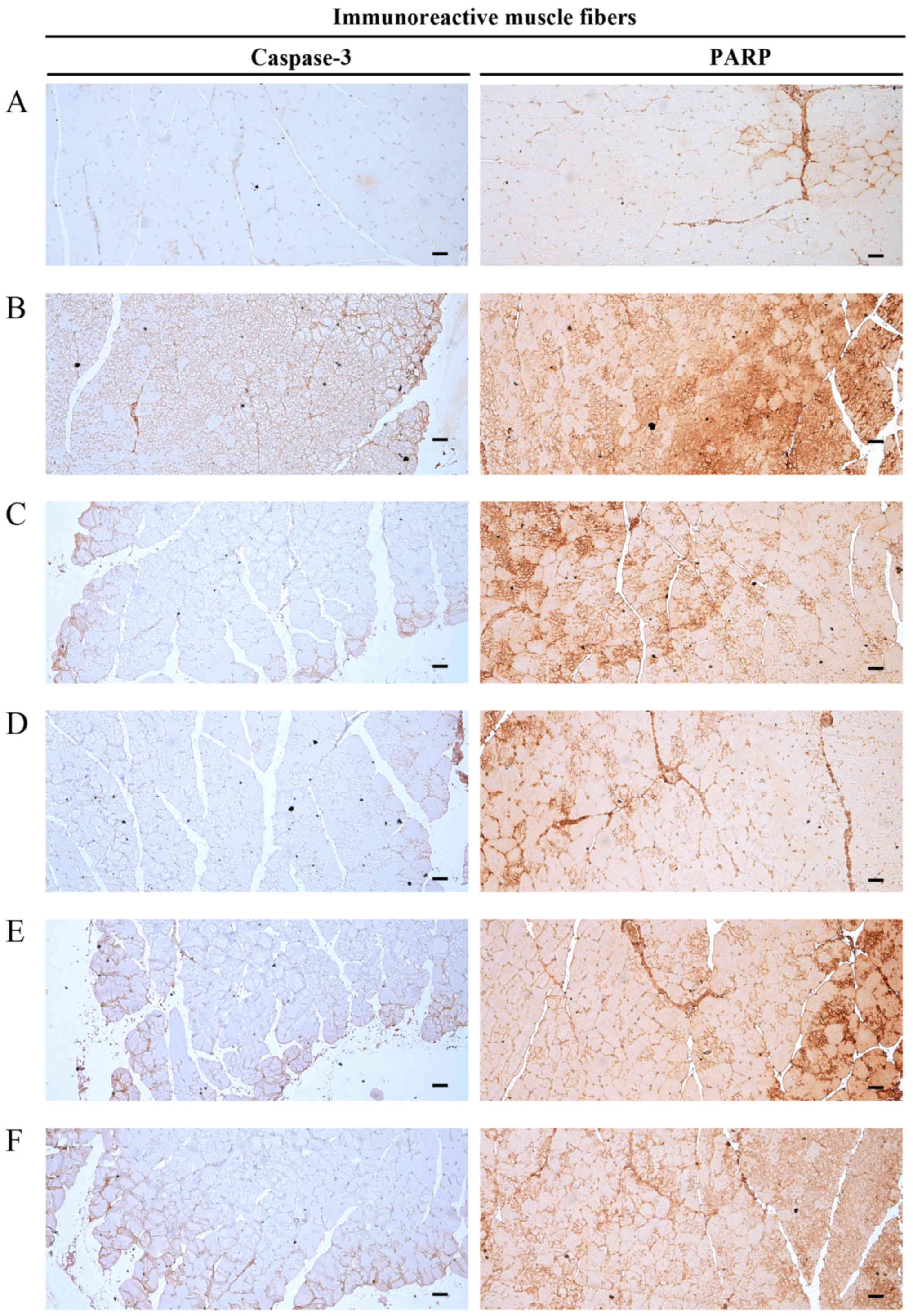
Significant increases (P<0.01) in caspase-3
(apoptotic marker) immunoreactivity in gastrocnemius muscle bundles
were observed in the DEXA control mice. EAP significantly and
dose-dependently reduced (P<0.01) these DEXA-induced increases
in caspase-3-immunoreactive muscle fibers. Oxymetholone also
significantly decreased (P<0.01) the number of
caspase-3-positive muscle fibers compared with in the DEXA control
mice. In particular, 400 mg/kg EAP exhibited favorable inhibitory
activities on DEXA-induced increases in caspase-3 immunoreactivity,
which were comparable with the effects of 50 mg/kg oxymetholone
(Table VIII and Fig. 8).
Alterations in PARP-immunolabelled
muscle fibers
Significant increases (P<0.01) in PARP (apoptotic
marker) immunoreactivity in gastrocnemius muscle bundles were
observed in the DEXA control mice. EAP significantly and
dose-dependently reduced (P<0.01) these DEXA-induced increases
in PARP-immunoreactive muscle fibers. Oxymetholone also
significantly decreased (P<0.01) the number of PARP-positive
muscle fibers compared with in the DEXA control mice. In
particular, 400 mg/kg EAP exhibited favorable inhibitory activities
on DEXA-induced increases in PARP immunoreactivity, which were
comparable with the effects of oxymetholone (Table VIII and Fig. 8).
Alterations in
nitrotyrosine-immunolabelled muscle fibers
Significant increases (P<0.01) in nitrotyrosine
(iNOS-associated oxidative stress marker) immunoreactivity in
gastrocnemius muscle bundles were observed in the DEXA control
mice. EAP significantly and dose-dependently reduced (P<0.01)
these DEXA-induced increases in the number of
nitrotyrosine-immunoreactive muscle fibers. Oxymetholone also
significantly decreased (P<0.01) the number of
nitrotyrosine-positive muscle fibers compared with in the DEXA
control mice. In particular, 400 mg/kg EAP exhibited favorable
inhibitory activities on DEXA-induced increases in nitrotyrosine
immunoreactivity, which were comparable with the effects of
oxymetholone (Table VIII and
Fig. 9).
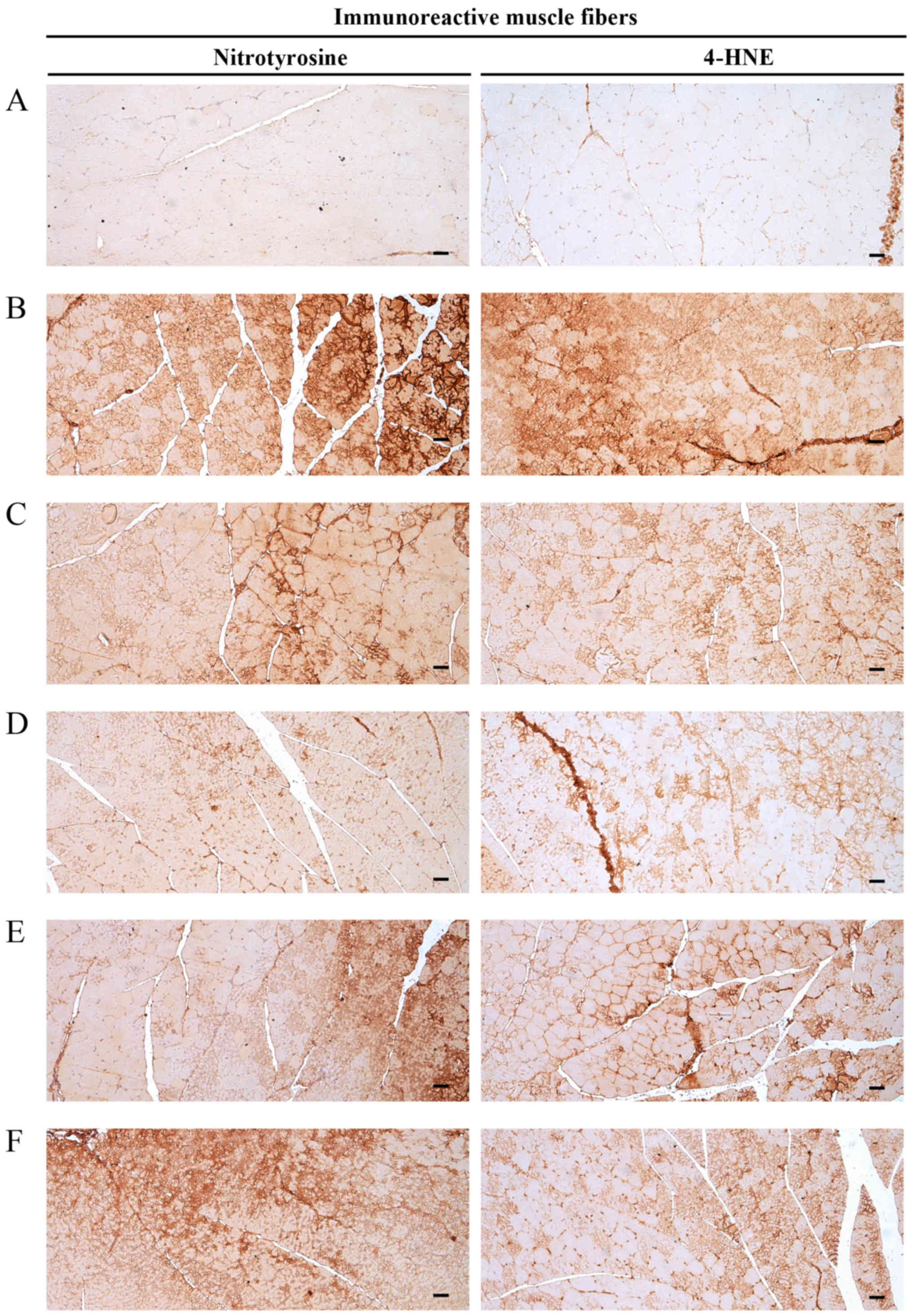 | Figure 9Representative gastrocnemius muscle
nitrotyrosine and 4-HNE immunoreactivity. Marked increases in the
immunoreactivity of the oxidative stress marker, nitrotyrosine, and
the lipid peroxidation marker, 4-HNE, were detected in the
gastrocnemius muscle bundles from DEXA control mice. However, EAP
dose-dependently and significantly reduced these DEXA-induced
increases in nitrotyrosine- and 4-HNE-immunoreactive fibers. In
addition, oxymetholone (50 mg/kg) significantly reduced the number
of nitrotyrosine- and 4-HNE-positive muscle fibers as compared with
in the DEXA control mice. In particular, 400 mg/kg EAP exhibited
favorable inhibitory activities on DEXA-induced increases in
nitrotyrosine- and 4-HNE-immunoreactive fibers, which were
comparable with the effects of oxymetholone (50 mg/kg). (A)
Deionized distilled water-administered and saline-treated mice
(intact vehicle control group). (B) Deionized distilled
water-administered and DEXA-treated control mice (DEXA control
group). (C) Oxymetholone (50 mg/kg)-administered and DEXA-treated
reference mice (oxymetholone group). (D) EAP (400
mg/kg)-administered and DEXA-treated experimental mice (EAP400
group). (E) EAP (200 mg/kg)-administered and DEXA-treated
experimental mice (EAP200 group). (F) EAP (100 mg/kg)-administered
and DEXA-treated experimental mice (EAP100 group). Scale bars=40
μm. 4-HNE, 4-hydroxynonenal; DEXA, dexamethasone; EAP,
extracellular polysaccharides purified from Aureobasidium
pullulans SM-2001. |
Alterations in 4-HNE-immunolabelled
muscle fibers
Significant increases (P<0.01) in 4-HNE (lipid
peroxidation marker) immunoreactivity in gastrocnemius muscle
bundles were observed in the DEXA control mice. EAP significantly
and dose-dependently reduced (P<0.01) these DEXA-induced
increases in muscle 4-HNE-immunoreactive fibers. Oxymetholone also
significantly decreased (P<0.01) the number of 4-HNE-positive
muscle fiber compared with in the DEXA control mice. In particular,
400 mg/kg EAP exhibited favorable inhibitory activities on
DEXA-induced increases in 4-HNE-immunoreactive fibers, which were
comparable with the effects of oxymetholone (Table VIII and Fig. 9).
Alterations in iNOS-immunolabelled
muscle fibers
Significant increases (P<0.01) in iNOS
(oxidative stress marker) immunoreactivity in gastrocnemius muscle
bundles were observed in the DEXA control mice. EAP significantly
and dose-dependently reduced (P<0.01) these DEXA-induced
increases in muscle iNOS-immunoreactive fibers. Oxymetholone also
significantly decreased (P<0.01) the number of iNOS-positive
muscle fibers compared with in the DEXA control mice. In
particular, 400 mg/kg EAP exhibited favorable inhibitory activities
on DEXA-induced increases in iNOS-immunoreactive fibers, which were
comparable with the effects of oxymetholone (Table VIII and Fig. 10).
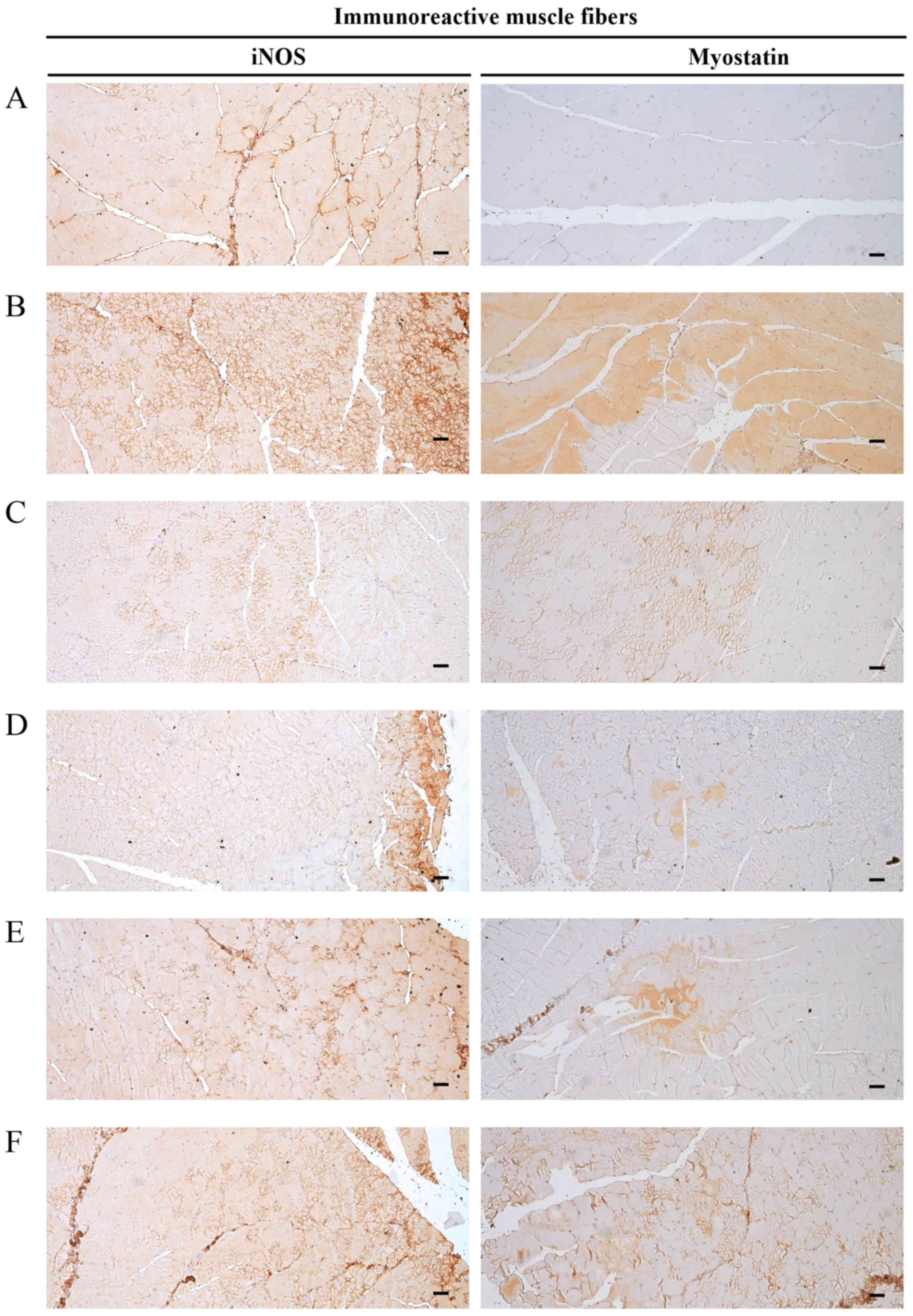 | Figure 10Representative gastrocnemius muscle
iNOS and myostatin immunoreactivity. Marked increases in the
immunoreactivity of the oxidative stress marker, iNOS, which is a
strong negative regulator of muscle growth, and myostatin, were
detected in the gastrocnemius muscle bundles from DEXA control
mice. However, EAP significantly and dose-dependently reduced these
DEXA–induced increases in iNOS- and myostatin-immunoreactive muscle
fibers. In addition, oxymetholone (50 mg/kg) significantly
decreased the number of iNOS- and myostatin-positive muscle fibers
compared with in the DEXA control mice. In particular, 400 mg/kg
EAP exhibited favorable inhibitory activities on DEXA-induced
increases in iNOS- and myostatin-immunoreactive fibers, which were
comparable with the effects of oxymetholone (50 mg/kg). (A)
Deionized distilled water-administered and saline-treated mice
(intact vehicle control group). (B) Deionized distilled
water-administered and DEXA-treated control mice (DEXA control
group). (C) Oxymetholone (50 mg/kg)-administered and DEXA-treated
reference mice (oxymetholone group). (D) EAP (400
mg/kg)-administered and DEXA-treated experimental mice (EAP400
group). (E) EAP (200 mg/kg)-administered and DEXA-treated
experimental mice (EAP200 group). (F) EAP (100 mg/kg)-administered
and DEXA-treated experimental mice (EAP100 group). Scale bars=40
μm. DEXA, dexamethasone; EAP, extracellular polysaccharides
purified from Aureobasidium pullulans SM-2001; iNOS,
inducible nitric oxide synthase. |
Alterations in
myostatin-immunolabelled muscle fibers
Significant increases (P<0.01) in myostatin
immunoreactivity in gastrocnemius muscle bundles were observed in
the DEXA control mice. EAP significantly and dose-dependently
reduced (P<0.05) these DEXA-induced increases in
myostatin-immunoreactive muscle fibers. Oxymetholone also
significantly decreased (P<0.01) the number of
myostatin-positive muscle fibers compared with in the DEXA control
mice. In particular, 400 mg/kg EAP exhibited favorable inhibitory
activities on DEXA-induced increases in myostatin-immunoreactive
fibers, which were comparable with the effects of oxymetholone
(Table VIII and Fig. 10).
Discussion
Atrophy begins with a decrease in muscle tension,
which is associated with reduced protein synthesis and increased
protein degradation (68). Four
types of proteolytic degradation are involved in muscle atrophy:
Calpain calcium-dependent signaling, lysosomal proteases
(cathepsins), the ubiquitin proteasome pathway and the caspase
signaling system (6,68–70). There is a common genetic program
involved in muscle proteolysis regardless of its etiology; however,
distinct signaling pathways are involved to modulate the system
(6,69,71). Oxidative stress is a well-known
and important inducer of muscle atrophy in response to disuse and
in catabolic muscle cachexia (71). In addition, apoptosis and loss of
muscle fibers are also involved in the early phase of muscle
atrophy, regardless of etiology (72,73).
Glucocorticoid-induced catabolic muscle atrophy is
characterized by a reduction and degradation in protein content,
organelles, cytoplasm, fiber diameter, resistance to fatigue and
muscle strength (16,21,23,28,74). Glucocorticoids are
immunosuppressants that are clinically used to suppress swelling
and acute inflammation. Millions of people take glucocorticoids as
chronic therapy to treat various diseases, including asthma,
rheumatoid arthritis, primary or secondary adrenal insufficiency,
and organ transplants (23).
Common side effects of glucocorticoids include nervousness,
insomnia, gastrointestinal upset, immunosuppression, arthralgia,
myopathy and edema (75).
Glucocorticoids have been in commercial use for >50 years
(76); however, their prolonged
use is associated with myopathy, particularly with prolonged high
doses. Long-term glucocorticoid therapy enhances the risk of muscle
weakness and myopathy by 50% (77,78). The characteristic features of
myopathy include weakness and muscle atrophy, oxidative stress,
mitochondrial dysfunction and insulin resistance. Histological
alterations associated with muscle atrophy include loss of myosin
filaments in sarcomeres, type II specific atrophy of muscle fibers,
preservation of Z-bands and thin filaments, and necrosis (74). Steroid-induced myopathy is not
only associated with the use of fluorinated steroids, including
triamcinolone, β-methasone and DEXA, but can also be caused by
non-fluorinated steroids, such as hydrocortisone and prednisolone
(79). In the present study, the
potential beneficial skeletal muscle-preserving effects of EAP were
examined in a mouse model of DEXA-induced catabolic muscle
atrophy.
All of the intact vehicle control mice exhibited
normal body weight gain throughout the experimental period,
including during the 10 days of acclimation (80,81). The DEXA-induced decreases in body
weight detected in the present study were considered to be related
to cachexia, due to the potent catabolic effects of DEXA (82,83). Conversely, the increased body
weight detected in mice treated with EAP may be associated with the
known immunomodulatory effects of EAP (45,84). Generally, good growth patterns are
associated with an enhanced immune system (85,86), which is induced by EAP
administration (84).
Oxymetholone is a 17α-alkylated anabolic-androgenic steroid
(33,34), which may inhibit the catabolic
cachexia-associated decreases in body weight induced by
glucocorticoid treatment (87–89).
Overuse of glucocorticoids can facilitate catabolic
muscle atrophy, which is characterized by decreased fiber diameter,
and reduced and degraded protein contents (16,21,23,26,28,74). In the present study, decreases in
calf thickness were detected 5 days after the initial DEXA
treatment in DEXA-treated mice, alongside decreased gastrocnemius
muscle thickness, and calf muscle strength and weight at sacrifice,
as a result of catabolic muscle atrophy. Conversely, oral
administration of 100, 200 and 400 mg/kg EAP, and 50 mg/kg
oxymetholone, inhibited DEXA-induced decreases in calf muscle
strength and thickness, and gastrocnemius muscle thickness and
weight, thus indicating that oxymetholone and EAP may reverse the
DEXA-induced atrophic alterations in calf muscles.
Creatine is a naturally occurring nitrogenous
organic acid that assists in the supply of energy to the entire
body, particularly muscle cells. Creatine is synthesized in the
kidney and liver; however, muscles do not possess the ability to
synthesize creatine. Creatine is stored in the muscle, according to
a concentration gradient, via a specific active transport mechanism
from the plasma (90). Skeletal
muscle is a large and relatively constant reservoir of creatine in
the body (91,92). Creatine is constantly metabolized
to its non-ionic cyclic derivative creatinine, over 1.7% of
creatine is metabolized per day via non-enzymatic hydrolytic
cyclization (93,94). Creatinine rapidly diffuses from
the muscle into the plasma and is transferred to the urine, with no
uptake into muscles (90,95). Therefore, plasma creatine levels
can be used as a serum biochemical indicator for skeletal muscle
damage, activity or muscle quantity (26,96,97). In the present study, marked
increases in serum creatine levels were verified alongside other
DEXA-associated catabolic muscle atrophic alterations; this finding
was similar to those of previous studies (16,26). Oral administration of 100, 200 and
400 mg/kg EAP significantly and dose-dependently limited the
DEXA-induced increases in serum creatine levels. Particularly, 400
mg/kg EAP exhibited favorable inhibitory effects on serum creatine
level elevations; these effects were comparable with those of 50
mg/kg oxymetholone, thus indicating that EAP exerts positive
muscle-preserving effects against glucocorticoid-induced
atrophy.
The medical significance of LDH is evident due to
its extensive presence in body tissues, including heart muscle and
blood cells. CK is an enzyme expressed by various tissues and cell
types, which is involved in the conversion of creatine and the
consumption of adenosine. Since LDH and CK are released during
tissue damage, they are considered markers of common disease and
injuries, particularly muscle damage. Plasma activities of CK and
LDH have been used commonly as markers of muscle tissue damage
(26,98,99). They are also markedly elevated in
animals with disused muscle atrophy (100). In a DEXA-induced animal model of
catabolic muscle atrophy, marked elevations in serum CK levels were
noted; however, serum LDH levels were generally decreased due to
reduced physiological activity and skeletal muscle fiber
concentration (24,26,101). Significantly elevated serum CK
levels, indicating decreases in serum LDH levels and muscle damage,
thus signifying reduced muscle activity, were demonstrated in the
DEXA control mice in the present study. However, significant and
dose-dependent decreases in serum CK and increases in serum LDH
levels were detected in 100, 200, and 400 mg/kg EAP-treated mice;
these effects were comparable with those of 50 mg/kg oxymetholone.
In particular, 400 mg/kg EAP exhibited favorable and potent
muscle-preserving effects.
Lipid peroxidation can harm surrounding tissues due
to the release of various toxic substances (102), and oxidative stress is a
significant inducer of muscle atrophy (71). Inhibition of increased lipid
peroxidation protects muscles against atrophic alterations
(57,103,104). Nitrotyrosine, which is a product
of tyrosine nitration that has been detected in numerous
pathological disorders, is known as a marker of iNOS-dependent
nitrate stress (105–107). In addition, it has been
demonstrated to damage antioxidant defense systems in muscle
tissues; this was associated with glucocorticoid-induced catabolic
muscle atrophic alterations (26,71,108). In the present study, EAP
dose-dependently protected the gastrocnemius muscle against
DEXA-triggered oxidative stress, reduced DEXA-induced increases in
lipid peroxidation and ROS formation, increased DEXA-induced
decreases in CAT and SOD activities and GSH contents, and reduced
DEXA-induced increases in nitrotyrosine and 4-HNE-immunolabelled
muscle fibers. Oxymetholone also exerted strong antioxidative
effects against DEXA-induced depletion of antioxidant defense
systems, consistent with other studies on anabolic steroids
(109,110) and previous results in
glucocorticoid-induced catabolic muscle atrophic mice (26).
Apoptosis and muscle fiber damage are associated
with the early phase of muscle atrophy regardless of etiology
(72,73), and caspase-3 and PARP serve key
roles in apoptosis (111,112).
Increases in the number of caspase-3 and PARP-immunoreactive muscle
fibers in muscle bundles indicate apoptosis and related damage
(26,113,114). Furthermore, treatment with
glucocorticoids has been reported to induce marked apoptosis in
muscles (23,26). Therefore, EAP-induced
dose-dependent inhibition of caspase-3 and PARP immunore-activity
in DEXA-treated gastrocnemius muscle bundles may provide direct
evidence that EAP can preserve muscle mass through inhibitory
effects against DEXA-induced muscle fiber apoptosis.
Muscle structure and mass are evaluated by the
equilibrium between protein synthesis and degradation (70). Protein degradation, which is
responsible for muscle wasting, is triggered by
ATP-ubiquitin-dependent proteolysis (9). A previous study reported that the
muscle-specific E3 ubiquitin ligases, including MuRF1 and
atrogin-1, are important for muscle atrophy (6). In addition, it has been revealed
that the expression levels of MuRF1 and atrogin-1 are increased in
atrophic skeletal muscles, and mice deficient in MuRF1 or atrogin-1
are resistant to muscle atrophy (5,115,116). In addition, marked increases in
the mRNA expression levels of MuRF1 and atrogin-1 have been
observed in glucocorticoid-induced catabolic atrophic muscles
(16,26,28). In the present study, marked
elevations in the mRNA expression levels of MuRF1 and atrogin-1 in
gastrocnemius muscles were detected in the DEXA control group
compared with in the intact vehicle control group; however, these
elevations were dose-dependently inhibited following treatment with
EAP, providing direct evidence to suggest that EAP exerts
muscle-protective effects apparently mediated through
downregulation of atrogin-1 and MuRF1. In particular, 400 mg/kg EAP
exhibited favorable inhibitory effects on muscle atrogin-1 and
MuRF1 mRNA expression; these effects were comparable with those of
oxymetholone.
Protein synthesis is activated by the insulin-like
growth factor 1 (IGF-1)/PI3K/Akt pathway (6,70).
PI3K, which is initiated by IGF or insulin, in turn activates the
serine/threonine kinase Akt (69). Marked downregulation of PI3K and
Akt1 mRNA expression were detected in DEXA-treated mice with
catabolic muscle atrophic alterations; this finding was consistent
with the results of a previous study (26). Conversely, EAP dose-dependently
upregulated the mRNA expression levels of Akt1 and PI3K compared
with in the DEXA control group, which indicated that EAP may resist
glucocorticoid-induced muscle atrophy and activate muscle protein
synthesis; these effects were comparable with those of
oxymetholone. Notably, 400 mg/kg EAP exhibited favorable
upregulating effects on Akt1 and PI3K mRNA expression, comparable
with those of oxymetholone.
Adenosine modulates numerous physiological
functions in various tissues, including skeletal muscle and the
cardiovascular system (117–119). Adenosine is considered to be
involved in the synergistic effects of contraction- and
insulin-stimulated glucose uptake in skeletal muscle, and in the
regulation of blood flow to skeletal muscle (120,121). Specific adenosine receptors are
associated with facilitation of the physiological effects of
adenosine (122). TRPV4 is a
member of the TRP channel superfamily (123,124), which serves an osmosensory or
mechanosensory role in numerous musculoskeletal tissues, and
prevents muscle atrophy and bone loss (124,125). Subcutaneous treatment with DEXA
significantly decreased the mRNA expression levels of TRPV4 and A1R
in gastrocnemius muscle, which may be associated with catabolic
muscle atrophy-related proteolysis; these findings were similar to
those of a previous study (26).
EAP dose-dependently upregulated A1R and TRPV4 mRNA expression
compared with in the DEXA control group, providing direct evidence
that 400 mg/kg EAP can increase muscle growth and resist
DEXA-induced catabolic muscle atrophy; these effects were
comparable with those of 50 mg/kg oxymetholone.
Myostatin is a secreted growth differentiation
factor that inhibits growth and muscle differentiation in
myogenesis. It is a powerful negative controller of muscle growth
(9,16). The sirtuin protein family
(SIRT1-7) possesses ADP ribosyltransferase activity and/or
NAD+-dependent deacetylase activity (126). SIRT1 controls numerous
biological processes, including differentiation, cell
proliferation, metabolism and apoptosis (127). In addition, it regulates
transcription of peroxisome proliferator-activated receptor-γ
co-activator 1α in skeletal muscle (128) and inhibits muscle regeneration,
which causes cachexia (129). In
catabolic muscle atrophy, the mRNA expression levels of SIRT1 and
myostatin have been detected alongside decreases in muscle mass
(16,25,26,130); similar findings were induced
with DEXA treatment in the present study. However, elevations in
the expression levels of SIRT1, a representative inhibitor of
muscle regeneration, and myostatin, a strong negative regulator of
muscle growth, were dose-dependently inhibited by treatment with
EAP. In addition, EAP dose-dependently inhibited increases in
myostatinimmunoreactive fibers, as determined by
immunohistochemical analysis, providing evidence of
muscle-shielding effects via downregulation of SIRT1 and myostatin.
Notably, 400 mg/kg EAP exhibited favorable inhibitory effects on
muscle myostatin and SIRT1 mRNA expression, and myostatin
immunoreactivity in muscle fibers; these effects were comparable
with those of 50 mg/kg oxymetholone.
Glucocorticoid-induced catabolic muscle atrophic
alterations have been reported to induce marked histopatho-logical
alterations, including microvacuolation, diminished muscle fiber
diameter, fibrosis and collagen deposition, as well protein
degradation (15,21,26); these alterations were observed in
the present study. However, in the present study, muscle
atrophy-associated alterations were reduced by treatment with
oxymetholone or EAP. These findings suggested that oxymetholone or
EAP may protect muscles against DEXA-induced catabolic atrophy. EAP
exhibited favorable inhibitory effects on histopathological muscle
fibrosis and atrophic alterations, which were compared with the
effects of oxymetholone.
In conclusion, EAP exerted favorable ameliorating
effects on DEXA-induced catabolic muscle atrophy via
anti-inflammatory and antioxidant effects, which were mediated by
modulation of the expression of genes associated with muscle
protein synthesis (Akt1, PI3K, A1R and TRPV4) and degradation
(atrogin-1, MuRF1, myostatin and SIRT1). Therefore, EAP may be
helpful in improving various muscle atrophy conditions with various
etiologies. Notably, 400 mg/kg EAP exhibited favorable
muscle-protective effects against DEXA-induced catabolic muscle
atrophy, which were comparable with the effects of 50 mg/kg
oxymetholone.
Acknowledgments
The present study was financially supported by the
Ministry of Trade, Industry, and Energy, Korea, under the ‘Regional
Specialized Industry Development Program’ (grant no. R0005069;
Development of functional food products for improving the
locomotive syndrome using black yeast β-glucan) supervised by the
Korea Institute for Advancement of Technology.
References
|
1
|
Brooks SV and Faulkner JA: Skeletal muscle
weakness in old age: Underlying mechanisms. Med Sci Sports Exerc.
26:432–439. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frontera WR, Hughes VA, Fielding RA,
Fiatarone MA, Evans WJ and Roubenoff R: Aging of skeletal muscle: A
12-yr longitudinal study. J Appl Physiol. 88:1321–1326. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metter EJ, Talbot LA, Schrager M and
Conwit R: Skeletal muscle strength as a predictor of all-cause
mortality in healthy men. J Gerontol A Biol Sci Med Sci.
57:B359–B365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glass DJ: Molecular mechanisms modulating
muscle mass. Trends Mol Med. 9:344–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glass DJ: Skeletal muscle hypertrophy and
atrophy signaling pathways. Int J Biochem Cell Biol. 37:1974–1984.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramírez C, Russo TL, Sandoval MC, Dentillo
AA, Couto MA, Durigan JL and Salvini TF: Joint inflammation alters
gene and protein expression and leads to atrophy in the tibialis
anterior muscle in rats. Am J Phys Med Rehabil. 90:930–939. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofer T, Marzetti E, Xu J, Seo AY, Gulec
S, Knutson MD, Leeuwenburgh C and Dupont-Versteegden EE: Increased
iron content and RNA oxidative damage in skeletal muscle with aging
and disuse atrophy. Exp Gerontol. 43:563–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onda A, Jiao Q, Nagano Y, Akimoto T,
Miyamoto T, Minamisawa S and Fukubayashi T: Acupuncture ameliorated
skeletal muscle atrophy induced by hindlimb suspension in mice.
Biochem Biophys Res Commun. 410:434–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Booth FW: Physiologic and biochemical
effects of immobilization on muscle. Clin orthop Relat Res.
219:15–20. 1987.
|
|
11
|
Thomas DR: Loss of skeletal muscle mass in
aging: Examining the relationship of starvation, sarcopenia and
cachexia. Clin Nutr. 26:389–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Léger B, Senese R, Al-Khodairy AW, Dériaz
O, Gobelet C, Giacobino JP and Russell AP: Atrogin-1, MuRF1, and
FoXo, as well as phosphorylated GSK-3beta and 4EBP1 are reduced in
skeletal muscle of chronic spinal cord-injured patients. Muscle
Nerve. 40:69–78. 2009. View Article : Google Scholar
|
|
13
|
Kawano F, Tanihata J, Sato S, Nomura S,
Shiraishi A, Tachiyashiki K and Imaizumi K: Effects of
dexamethasone on the expression of beta(1)-, beta (2)- and beta
(3)-adrenoceptor mRNAs in skeletal and left ventricle muscles in
rats. J Physiol Sci. 59:383–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dardevet D, Sornet C, Savary I, Debras E,
Patureau-Mirand P and Grizard J: Glucocorticoid effects on insulin-
and IGF-I-regulated muscle protein metabolism during aging. J
Endocrinol. 156:83–89. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanda F, Takatani K, Okuda S, Matsushita T
and Chihara K: Preventive effects of insulinlike growth factor-I on
steroid-induced muscle atrophy. Muscle Nerve. 22:213–217. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilson H, Schakman O, Combaret L, Lause P,
Grobet L, Attaix D, Ketelslegers JM and Thissen JP: Myostatin gene
deletion prevents glucocorticoid-induced muscle atrophy.
Endocrinology. 148:452–460. 2007. View Article : Google Scholar
|
|
17
|
Auclair D, Garrel DR, Chaouki Zerouala A
and Ferland LH: Activation of the ubiquitin pathway in rat skeletal
muscle by catabolic doses of glucocorticoids. Am J Physiol.
272:C1007–C1016. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hasselgren PO: Glucocorticoids and muscle
catabolism. Curr Opin Clin Nutr Metab Care. 2:201–205. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komamura K, Shirotani-Ikejima H, Tatsumi
R, Tsujita-Kuroda Y, Kitakaze M, Miyatake K, Sunagawa K and Miyata
T: Differential gene expression in the rat skeletal and heart
muscle in glucocorticoid-induced myopathy: Analysis by microarray.
Cardiovasc Drugs Ther. 17:303–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-β
superfamily member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin J, Du R, Yang YQ, Zhang HQ, Li Q, Liu
L, Guan H, Hou J and An XR: Dexamethasone-induced skeletal muscle
atrophy was associated with upregulation of myostatin promoter
activity. Res Vet Sci. 94:84–89. 2013. View Article : Google Scholar
|
|
22
|
Allen DL, Bandstra ER, Harrison BC, Thorng
S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG,
Leinwand LL, et al: Effects of spaceflight on murine skeletal
muscle gene expression. J Appl Physiol 1985. 106:582–595. 2009.
View Article : Google Scholar :
|
|
23
|
Dirks-Naylor AJ and Griffiths CL:
Glucocorticoid-induced apoptosis and cellular mechanisms of
myopathy. J Steroid Biochem Mol Biol. 117:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orzechowski A, Ostaszewski P, Wilczak J,
Jank M, Bałasińska B, Wareski P and Fuller J Jr: Rats with a
glucocorticoid-induced catabolic state show symptoms of oxidative
stress and spleen atrophy: The effects of age and recovery. J Vet
Med A Physiol Pathol Clin Med. 49:256–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alamdari N, Aversa Z, Castillero E, Gurav
A, Petkova V, Tizio S and Hasselgren PO: Resveratrol prevents
dexamethasone-induced expression of the muscle atrophy-related
ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through
a SIRT1-dependent mechanism. Biochem Biophys Res Commun.
417:528–533. 2012. View Article : Google Scholar :
|
|
26
|
Kim JW, Ku SK, Han MH, Kim KY, Kim SG, Kim
GY, Hwang HJ, Kim BW, Kim CM and Choi YH: The administration of
Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy
in mice. Int J Mol Med. 36:29–42. 2015a. View Article : Google Scholar
|
|
27
|
Benveniste O, Jacobson L, Farrugia ME,
Clover L and Vincent A: MuSK antibody positive myasthenia gravis
plasma modifies MURF-1 expression in C2C12 cultures and mouse
muscle in vivo. J Neuroimmunol. 170:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones A, Hwang DJ, Narayanan R, Miller DD
and Dalton JT: Effects of a novel selective androgen receptor
modulator on dexa-methasone-induced and hypogonadism-induced muscle
atrophy. Endocrinology. 151:3706–3719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Nakagawa K, Sarkar A, Maruyama J,
Iwasa H, Bao Y, Ishigami-Yuasa M, Ito S, Kagechika H, Hata S, et
al: Screening with a novel cell-based assay for TAZ activators
identifies a compound that enhances myogenesis in C2C12 cells and
facilitates muscle repair in a muscle injury model. Mol Cell Biol.
34:1607–1621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ringold HJ, Batres E, Halpern O and
Necoechea E: Steroids. CV. 12-Methyl and
2-hydroxymethylene-androstane derivatives. J Am Chem Soc.
81:427–432. 1959. View Article : Google Scholar
|
|
31
|
Lennon HD: Effects of various
17-alpha-alkyl substitutions and structural modifications of
steroids on sulfobromophthalein (BSP) retention in rabbits.
Steroids. 7:157–170. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dorfman RI and Kincl FA: Relative potency
of steroids in an anabolic-androgenic assay using the castrated
rat. Endocrinology. 72:259–266. 1963. View Article : Google Scholar
|
|
33
|
Pavlatos AM, Fultz O, Monberg MJ and
Vootkur A: Review of oxymetholone: A 17alpha-alkylated
anabolic-androgenic steroid. Clin Ther. 23:789–801; discussion 771.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Isaacs J, Loveland K, Mallu S, Adams S and
Wodicka R: The use of anabolic steroids as a strategy in reversing
denervation atrophy after delayed nerve repair. Hand (NY).
6:142–148. 2011. View Article : Google Scholar
|
|
35
|
Kim JW, Ku SK, Kim KY, Kim SG, Han MH, Kim
GY, Hwang HJ, Kim BW, Kim CM and Choi YH: Schisandrae Fructus
supplementation ameliorates sciatic neurectomy-induced muscle
atrophy in mice. Oxid Med Cell Longev. 2015:8724282015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Young GP, Bhathal PS, Sullivan JR, Wall
AJ, Fone DJ and Hurley TH: Fatal hepatic coma complicating
oxymetholone therapy in multiple myeloma. Aust N Z J Med. 7:47–51.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wood P and Yin JA: Oxymetholone
hepatotoxicity enhanced by concomitant use of cyclosporin A in a
bone marrow transplant patient. Clin Lab Haematol. 16:201–204.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walker ID, Davidson JF, Young P and Conkie
JA: Plasma fibrinolytic activity following oral anabolic steroid
therapy. Thromb Diath Haemorrh. 34:236–245. 1975.PubMed/NCBI
|
|
39
|
Tzianabos AO: Polysaccharide
immunomodulators as therapeutic agents: Structural aspects and
biologic function. Clin Microbiol Rev. 13:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Estrada A, Yun CH, Van Kessel A, Li B,
Hauta S and Laarveld B: Immunomodulatory activities of oat
beta-glucan in vitro and in vivo. Microbiol Immunol. 41:991–998.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lotzová E and Gutterman JU: Effect of
glucan on natural killer (NK) cells: Further comparison between NK
cell and bone marrow effector cell activities. J Immunol.
123:607–611. 1979.PubMed/NCBI
|
|
42
|
Hofer M and Pospísil M: Glucan as
stimulator of hematopoiesis in normal and gamma-irradiated mice. A
survey of the authors’ results. Int J Immunopharmacol. 19:607–609.
1997. View Article : Google Scholar
|
|
43
|
Lee JN, Lee DY, Ji IH, Kim GE, Kim HN,
Sohn J, Kim S and Kim CW: Purification of soluble beta-glucan with
immune-enhancing activity from the cell wall of yeast. Biosci
Biotechnol Biochem. 65:837–841. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Benach JL, Habicht GS, Holbrook TW and
Cook JA: Glucan as an adjuvant for a murine Babesia microti
immunization trial. Infect Immun. 35:947–951. 1982.PubMed/NCBI
|
|
45
|
Yoon HS, Kim JW, Cho HR, Moon SB, Shin HD,
Yang KJ, Lee HS, Kwon YS and Ku SK: Immunomodulatory effects of
Aureobasidium pullulans SM-2001 exopolymers on the
cyclo-phosphamide-treated mice. J Microbiol Biotechnol. 20:438–445.
2010.PubMed/NCBI
|
|
46
|
Jung MY, Kim JW, Kim KY, Choi SH and Ku
SK: Polycan, a β-glucan from Aureobasidium pullulans SM-2001,
mitigates ovariectomy-induced osteoporosis in rats. Exp Ther Med.
12:1251–1262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim HD, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS, Lee HS and Ku SK: Effects of β-glucan
from Aureobasidium pullulans on acute inflammation in mice. Arch
Pharm Res. 30:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HD, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS, Lee HS and Ku SK: Effect of Exopolymers
from Aureobasidum pullulans on formalin-induced chronic paw
inflammation in mice. J Microbiol Biotechnol. 16:1954–1960.
2006.
|
|
49
|
Ku SK, Lee YJ, Lee SD, Cho HR, Moon SB,
Kim KY, Kwon YS and Kim JW: Nephroprotective effect of Polycan on
acute renal failure induced by cisplatin in rats. ISRN Vet Sci.
2012:8621042012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ku SK, Kim JW, Cho HR, Kim KY, Min YH,
Park JH, Kim JS, Park JH, Seo BI and Roh SS: Effect of β-glucan
originated from Aureobasidium pullulans on asthma induced by
ovalbumin in mouse. Arch Pharm Res. 35:1073–1081. 2012a. View Article : Google Scholar
|
|
51
|
Kim JW, Cho HR and Ku SK: Efficacy test of
Polycan, a beta-glucan originated from Aureobasidium pullulans
SM-2001, on anterior cruciate ligament transection and partial
medial meniscectomy-induced-osteoarthritis rats. J Microbiol
Biotechnol. 22:274–282. 2012a. View Article : Google Scholar
|
|
52
|
Kim YS, Kang SJ, Kim JW, Cho HR, Moon SB,
Kim KY, Lee HS, Han CH, Ku SK and Lee YJ: Effects of Polycan, a
β-glucan, on experimental periodontitis and alveolar bone loss in
Sprague-Dawley rats. J Periodontal Res. 47:800–810. 2012b.
View Article : Google Scholar
|
|
53
|
Seo HP, Kim JM, Shin HD, Kim TK, Chang HJ,
Park BR and Lee JW: Production of β-1,3/1,6-glucan by Aureobasidium
pullulans SM-2001. Korean J Bitechnol Bioeng. 17:376–380. 2002.
|
|
54
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jamall IS and Smith JC: Effects of cadmium
on glutathione peroxidase, superoxide dismutase, and lipid
peroxidation in the rat heart: A possible mechanism of cadmium
cardiotoxicity. Toxicol Appl Pharmacol. 80:33–42. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
57
|
He HJ, Wang GY, Gao Y, Ling WH, Yu ZW and
Jin TR: Curcumin attenuates Nrf2 signaling defect, oxidative stress
in muscle and glucose intolerance in high fat diet-fed mice. World
J Diabetes. 3:94–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman’s reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aebi H: Catalase. Methods in Enzymatic
Analysis. Bergmeyer HU: Academic Press; New York, NY: pp. 673–686.
1974, View Article : Google Scholar
|
|
60
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
61
|
Yokoyama U, Minamisawa S, Quan H, Akaike
T, Suzuki S, Jin M, Jiao Q, Watanabe M, Otsu K, Iwasaki S, et al:
Prostaglandin E2-activated Epac promotes neointimal formation of
the rat ductus arteriosus by a process distinct from that of
cAMP-dependent protein kinase A. J Biol Chem. 283:28702–28709.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the the 2−ΔΔCt method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
63
|
Ogawa T, Nikawa T, Furochi H, Kosyoji M,
Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, et
al: Osteoactivin upregulates expression of MMP-3 and MMP-9 in
fibroblasts infiltrated into denervated skeletal muscle in mice. Am
J Physiol Cell Physiol. 289:C697–C707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tipoe GL, Leung TM, Liong EC, Lau TY, Fung
ML and Nanji AA: Epigallocatechin-3-gallate (EGCG) reduces liver
inflammation, oxidative stress and fibrosis in carbon tetrachloride
(CCl4)-induced liver injury in mice. Toxicology. 273:45–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shi SR, Chaiwun B, Young L, Cote RJ and
Taylor CR: Antigen retrieval technique utilizing citrate buffer or
urea solution for immunohistochemical demonstration of androgen
receptor in formalin-fixed paraffin sections. J Histochem Cytochem.
41:1599–1604. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Levene A: Pathological factors influencing
excision of tumours in the head and neck. Part I. Clin Otolaryngol
Allied Sci. 6:145–151. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ludbrook J: Update: Microcomputer
statistics packages. A personal view. Clin Exp Pharmacol Physiol.
24:294–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jackman RW and Kandarian SC: The molecular
basis of skeletal muscle atrophy. Am J Physiol Cell Physiol.
287:C834–C843. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sandri M: Signaling in muscle atrophy and
hypertrophy. Physiology (Bethesda). 23:160–170. 2008.
|
|
70
|
Glass DJ: Signaling pathways perturbing
muscle mass. Curr Opin Clin Nutr Metab Care. 13:225–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Powers SK, Kavazis AN and McClung JM:
Oxidative stress and disuse muscle atrophy. J Appl Physiol 1985.
102:2389–2397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Arakawa T, Katada A, Shigyo H, Kishibe K,
Adachi M, Nonaka S and Harabuchi Y: Electrical stimulation prevents
apoptosis in denervated skeletal muscle. NeuroRehabilitation.
27:147–154. 2010.PubMed/NCBI
|
|
73
|
Lim JY and Han TR: Effect of
electromyostimulation on apoptosis-related factors in denervation
and reinnervation of rat skeletal muscles. Muscle Nerve.
42:422–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mastagllia FL: Drug induced myopathies.
Pract Neurol. 6:4–13. 2006. View Article : Google Scholar
|
|
75
|
Seale JP and Compton MR: Side-effects of
corticosteroid agents. Med J Aust. 144:139–142. 1986.PubMed/NCBI
|
|
76
|
Zoorob RJ and Cender D: A different look
at corticosteroids. Am Fam Physician. 58:443–450. 1998.PubMed/NCBI
|
|
77
|
Bowyer SL, LaMothe MP and Hollister JR:
Steroid myopathy: Incidence and detection in a population with
asthma. J Allergy Clin Immunol. 76:234–242. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Covar RA, Leung DY, McCormick D, Steelman
J, Zeitler P and Spahn JD: Risk factors associated with
glucocorticoid-induced adverse effects in children with severe
asthma. J Allergy Clin Immunol. 106:651–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Owczarek J, Jasińska M and
Orszulak-Michalak D: Drug-induced myopathies. An overview of the
possible mechanisms. Pharmacol Rep. 57:23–34. 2005.PubMed/NCBI
|
|
80
|
Fox JG, Cohen BJ and Loew FM: Laboratory
animal medicine. Academic Press Inc.; Orlando, Fl: 1984
|
|
81
|
Tajima Y: Biological reference data book
on experimental animals. Soft Science Inc.; Tokyo: 1989
|
|
82
|
Gupta AK and Chow M: Prednicarbate
(Dermatop): Profile of a corticosteroid. J Cutan Med Surg.
8:244–247. 2004. View Article : Google Scholar
|
|
83
|
Cho YH, Chung IK, Cheon WH, Lee HS and Ku
SK: Effect of DHU001, a polyherbal formula on formalin-induced paw
chronic inflammation of mice. Toxicol Res. 27:95–102. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lee HS, Yang KJ, Shin HD, Park BR, Son CW,
Jang HJ, Park DC, Jung YM and Ku SK: Single oral dose toxicity
studies of Polycan, β-glucan originated from Aureobasidium in mice.
Toxicol Res. 21:361–365. 2005.
|
|
85
|
Duarte CG, dos Santos GL, Azzolini AE and
de Assis Pandochi AI: The effect of the antithyroid drug
propylthiouracil on the alternative pathway of complement in rats.
Int J Immunopharmacol. 22:25–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pinzone JJ, Fox ML, Sastry MK, Parenti DM
and Simon GL: Plasma leptin concentration increases early during
highly active antiretroviral therapy for acquired immunodeficiency
syndrome, independent of body weight. J Endocrinol Invest.
28:RC1–RC3. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hengge UR, Baumann M, Maleba R, Brockmeyer
NH and Goos M: Oxymetholone promotes weight gain in patients with
advanced human immunodeficiency virus (HIV-1) infection. Br J Nutr.
75:129–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hengge UR, Stocks K, Faulkner S, Wiehler
H, Lorenz C, Jentzen W, Hengge D and Ringham G: Oxymetholone for
the treatment of HIV-wasting: A double-blind, randomized,
placebo-controlled phase III trial in eugonadal men and women. HIV
Clin Trials. 4:150–163. 2003.PubMed/NCBI
|
|
89
|
Hengge UR, Stocks K, Wiehler H, Faulkner
S, Esser S, Lorenz C, Jentzen W, Hengge D, Goos M, Dudley RE, et
al: Double-blind, randomized, placebo-controlled phase III trial of
oxymetholone for the treatment of HIV wasting. AIDS. 17:699–710.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wyss M and Kaddurah-Daouk R: Creatine and
creatinine metabolism. Physiol Rev. 80:1107–1213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hunter A: The biological distribution of
creatine and creatinine. Creatine and Creatinine. Longmans; Green,
London: pp. 73–113. 1928
|
|
92
|
Balsom PD, Söderlund K and Ekblom B:
Creatine in humans with special reference to creatine
supplementation. Sports Med. 18:268–280. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fitch CD, Lucy DD, Bornhofen JH and
Dalrymple GV: Creatine metabolism in skeletal muscle. II. Creatine
kinetics in man. Neurology. 18:32–42. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bloch K and Schoenheimer R: Studies in
protein metabolism. XI. The metabolic relation of creatine and
creatinine studies with isotopic nitrogen. J Biol Chem.
131:111–119. 1939.
|
|
95
|
Heymsfield SB, Arteaga C, McManus C, Smith
J and Moffitt S: Measurement of muscle mass in humans: Validity of
the 24-hour urinary creatinine method. Am J Clin Nutr. 37:478–494.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sala A, Tarnopolsky M, Webber C, Norman G
and Barr R: Serum creatinine: A surrogate measurement of lean body
mass in children with acute lymphoblastic leukemia. Pediatr Blood
Cancer. 45:16–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stimpson SA, Turner SM, Clifton LG, Poole
JC, Mohammed HA, Shearer TW, Waitt GM, Hagerty LL, Remlinger KS,
Hellerstein MK, et al: Total-body creatine pool size and skeletal
muscle mass determination by creatine-(methyl-D3) dilution in rats.
J Appl Physiol 1985. 112:1940–1948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang Y, Huang JJ, Wang ZQ, Wang N and Wu
ZY: Value of muscle enzyme measurement in evaluating different
neuro-muscular diseases. Clin Chim Acta. 413:520–524. 2012.
View Article : Google Scholar
|
|
99
|
Choi M, Park H, Cho S and Lee M: Vitamin
D3 supplementation modulates inflammatory responses from the muscle
damage induced by high-intensity exercise in SD rats. Cytokine.
63:27–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cohen I, Bogin E, Chechick A and Rzetelny
V: Biochemical alterations secondary to disuse atrophy in the rat’s
serum and limb tissues. Arch Orthop Trauma Surg. 119:410–417. 1999.
View Article : Google Scholar
|
|
101
|
Pellegrino MA, D’Antona G, Bortolotto S,
Boschi F, Pastoris O, Bottinelli R, Polla B and Reggiani C:
Clenbuterol antagonizes glucocorticoid-induced atrophy and fibre
type transformation in mice. Exp Physiol. 89:89–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Comporti M: Lipid peroxidation and
cellular damage in toxic liver injury. Lab Invest. 53:599–623.
1985.PubMed/NCBI
|
|
103
|
Gore M, Fiebig R, Hollander J,
Leeuwenburgh C, Ohno H and Ji LL: Endurance training alters
antioxidant enzyme gene expression in rat skeletal muscle. Can J
Physiol Pharmacol. 76:1139–1145. 1998. View Article : Google Scholar
|
|
104
|
Süleyman H, Cadirci E, Albayrak A, Polat
B, Halici Z, Koc F, Hacimuftuoglu A and Bayir Y: Comparative study
on the gastroprotective potential of some antidepressants in
indomethacin-induced ulcer in rats. Chem Biol Interact.
180:318–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen JH, Tipoe GL, Liong EC, So HS, Leung
KM, Tom WM, Fung PC and Nanji AA: Green tea polyphenols prevent
toxin-induced hepatotoxicity in mice by down-regulating inducible
nitric oxide-derived prooxidants. Am J Clin Nutr. 80:742–751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mohiuddin I, Chai H, Lin PH, Lumsden AB,
Yao Q and Chen C: Nitrotyrosine and chlorotyrosine: Clinical
significance and biological functions in the vascular system. J
Surg Res. 133:143–149. 2006. View Article : Google Scholar
|
|
107
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Salvini TF, Durigan JL, Peviani SM and
Russo TL: Effects of electrical stimulation and stretching on the
adaptation of denervated skeletal muscle: Implications for physical
therapy. Rev Bras Fisioter. 16:175–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Delgado J, Saborido A and Megías A:
Prolonged treatment with the anabolic-androgenic steroid stanozolol
increases antioxidant defences in rat skeletal muscle. J Physiol
Biochem. 66:63–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yoo YE and Ko CP: Dihydrotestosterone
ameliorates degeneration in muscle, axons and motoneurons and
improves motor function in amyotrophic lateral sclerosis model
mice. PLoS One. 7:e372582012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar
|
|
112
|
Barrett KL, Willingham JM, Garvin AJ and
Willingham MC: Advances in cytochemical methods for detection of
apoptosis. J Histochem Cytochem. 49:821–832. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dam AD, Mitchell AS, Rush JW and
Quadrilatero J: Elevated skeletal muscle apoptotic signaling
following glutathione depletion. Apoptosis. 17:48–60. 2012.
View Article : Google Scholar
|
|
114
|
Yen YP, Tsai KS, Chen YW, Huang CF, Yang
RS and Liu SH: Arsenic induces apoptosis in myoblasts through a
reactive oxygen species-induced endoplasmic reticulum stress and
mitochondrial dysfunction pathway. Arch Toxicol. 86:923–933. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA.
98:14440–14445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cohen S, Brault JJ, Gygi SP, Glass DJ,
Valenzuela DM, Gartner C, Latres E and Goldberg AL: During muscle
atrophy, thick, but not thin, filament components are degraded by
MuRF1-dependent ubiquitylation. J Cell Biol. 185:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Berne RM, Knabb RM, Ely SW and Rubio R:
Adenosine in the local regulation of blood flow: A brief overview.
Fed Proc. 42:3136–3142. 1983.PubMed/NCBI
|
|
118
|
Segal SS and Kurjiaka DT: Coordination of
blood flow control in the resistance vasculature of skeletal
muscle. Med Sci Sports Exerc. 27:1158–1164. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hespel P and Richter EA: Role of adenosine
in regulation of carbohydrate metabolism in contracting muscle.
Skeletal Muscle Metabolism in Exercise and Diabetes. Richter EA,
Kiens B, Galbo H and Saltin B: Plenum Press; New York, NY: pp.
97–106. 1998, View Article : Google Scholar
|
|
120
|
Dobson JG Jr, Rubio R and Berne RM: Role
of adenine nucleotides, adenosine, and inorganic phosphate in the
regulation of skeletal muscle blood flow. Circ Res. 29:375–384.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Vergauwen L, Hespel P and Richter EA:
Adenosine receptors mediate synergistic stimulation of glucose
uptake and transport by insulin and by contractions in rat skeletal
muscle. J Clin Invest. 93:974–981. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lynge J and Hellsten Y: Distribution of
adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta
Physiol Scand. 169:283–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
124
|
Guilak F, Leddy HA and Liedtke W:
Transient receptor potential vanilloid 4: The sixth sense of the
musculoskeletal system? Ann N Y Acad Sci. 1192:404–409. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mizoguchi F, Mizuno A, Hayata T, Nakashima
K, Heller S, Ushida T, Sokabe M, Miyasaka N, Suzuki M, Ezura Y, et
al: Transient receptor potential vanilloid 4 deficiency suppresses
unloading-induced bone loss. J Cell Physiol. 216:47–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Haigis MC and Guarente LP: Mammalian
sirtuins - emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Amat R, Planavila A, Chen SL, Iglesias R,
Giralt M and Villarroya F: SIRT1 controls the transcription of the
peroxisome proliferator-activated receptor-gamma
Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through
the PGC-1alpha autoregulatory loop and interaction with MyoD. J
Biol Chem. 284:21872–21880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Toledo M, Busquets S, Ametller E,
López-Soriano FJ and Argilés JM: Sirtuin 1 in skeletal muscle of
cachectic tumour-bearing rats: A role in impaired regeneration? J
Cachexia Sarcopenia Muscle. 2:57–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Amat R, Solanes G, Giralt M and Villarroya
F: SIRT1 is involved in glucocorticoid-mediated control of
uncoupling protein-3 gene transcription. J Biol Chem.
282:34066–34076. 2007. View Article : Google Scholar : PubMed/NCBI
|