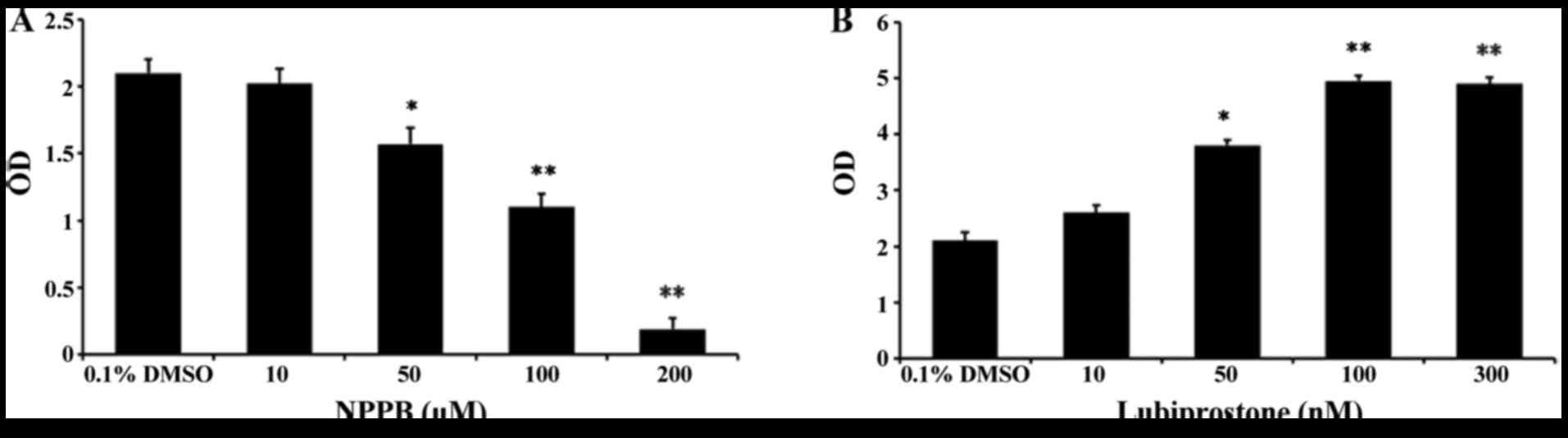
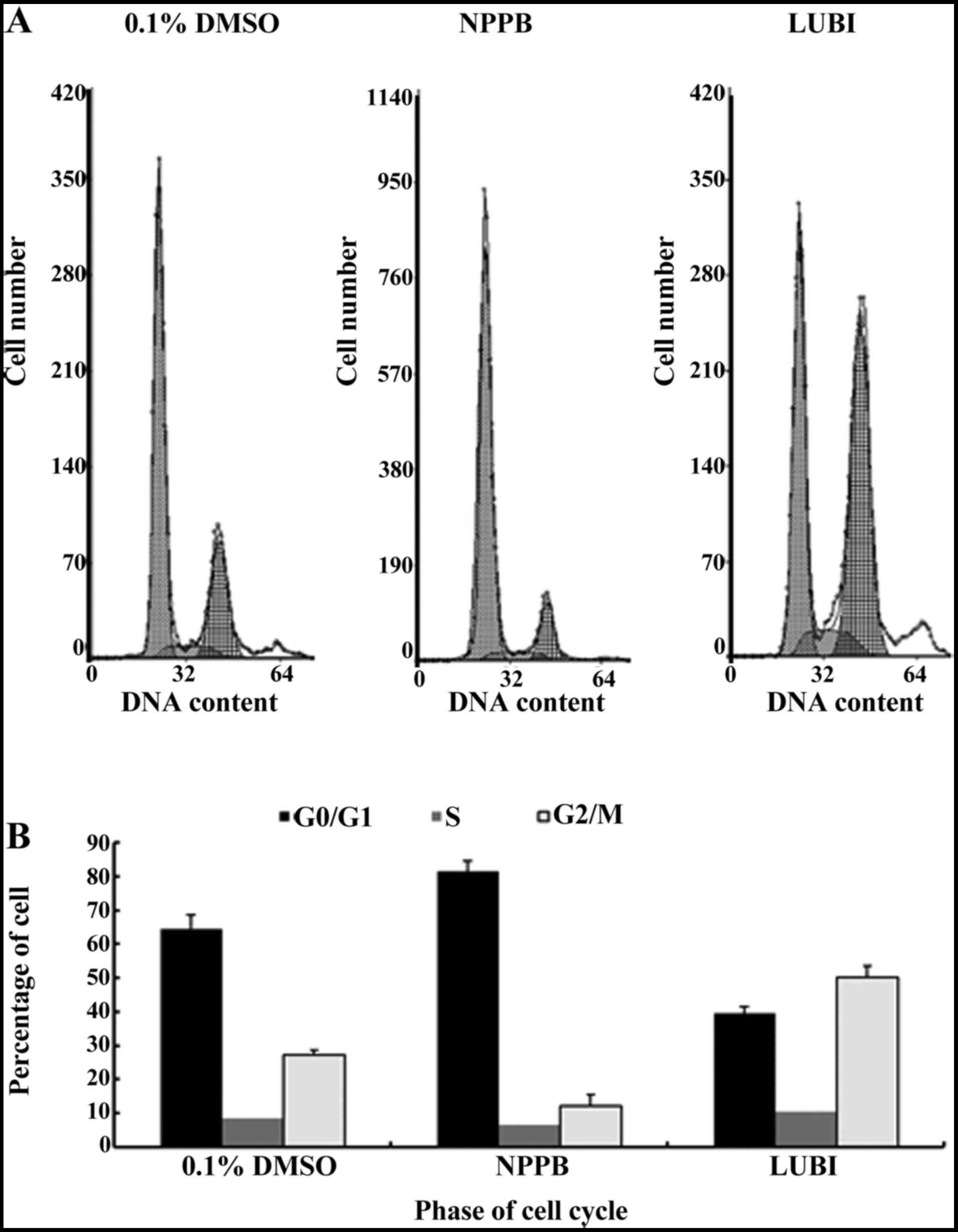
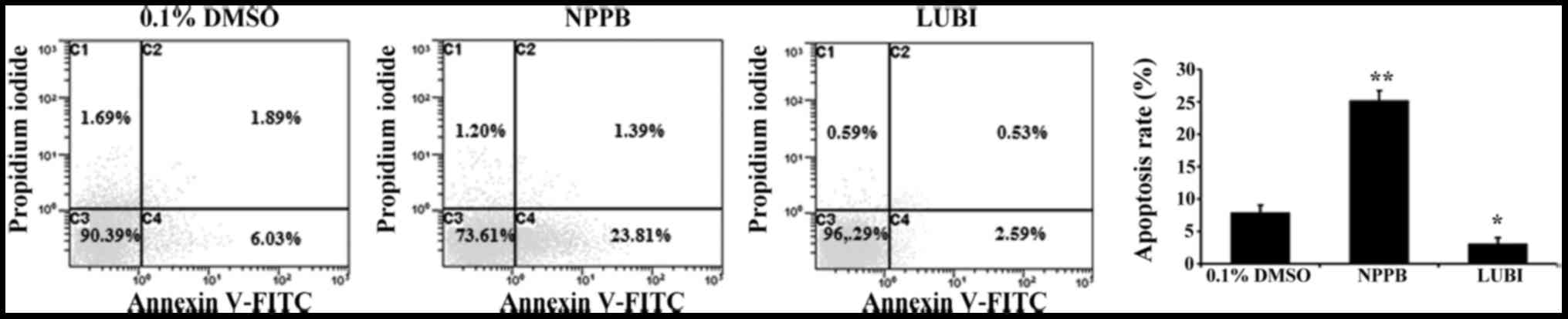
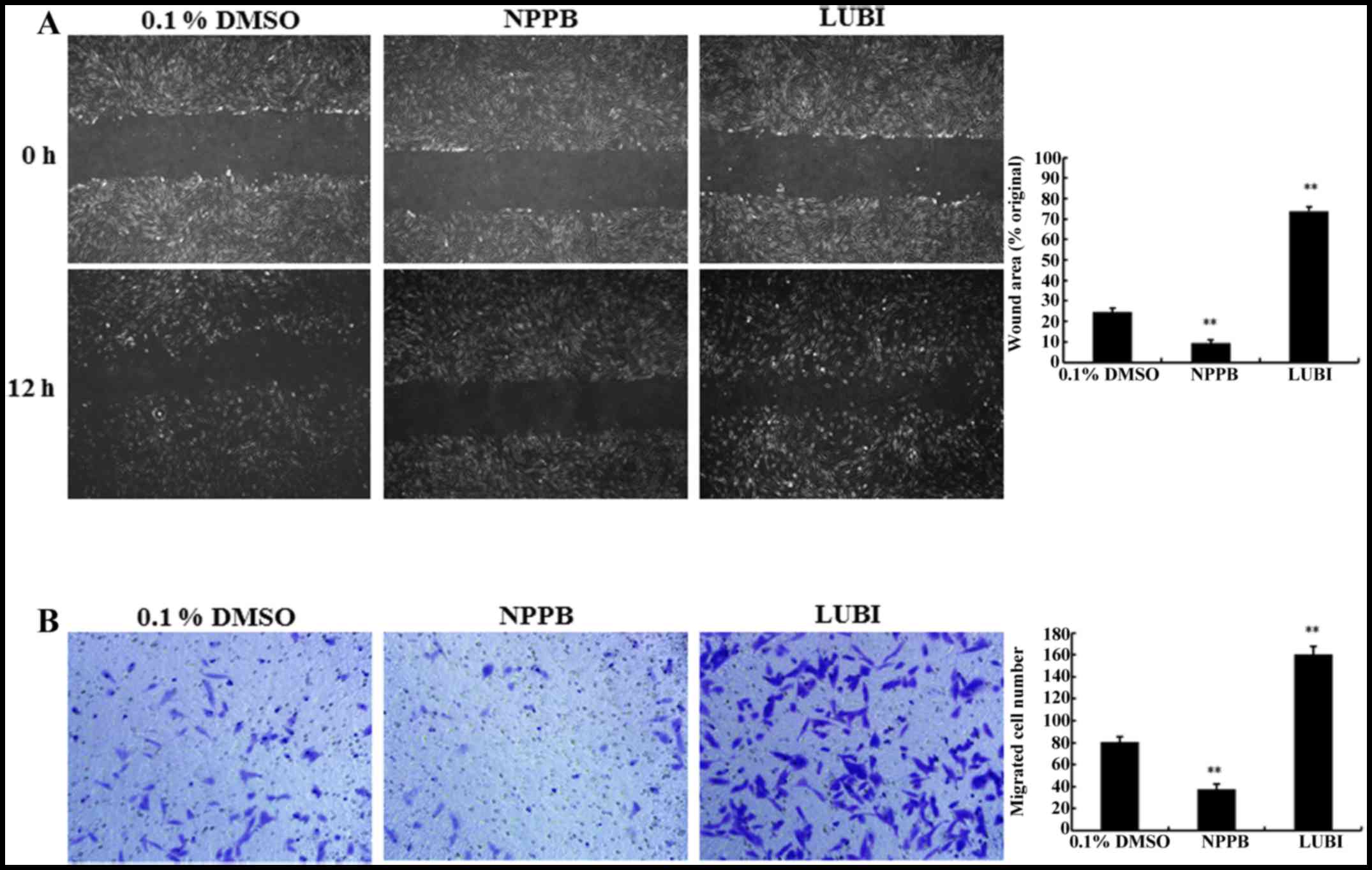
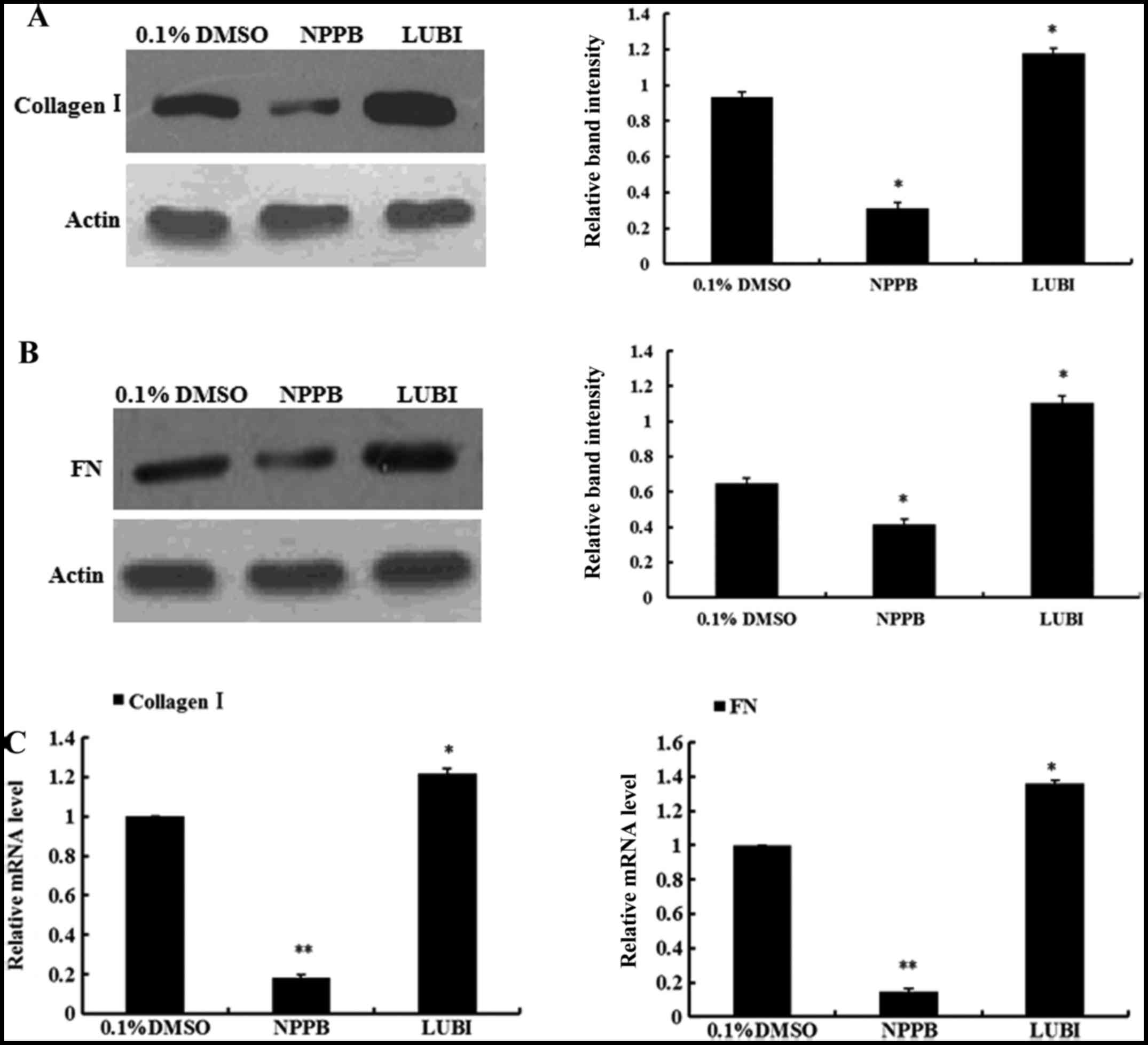
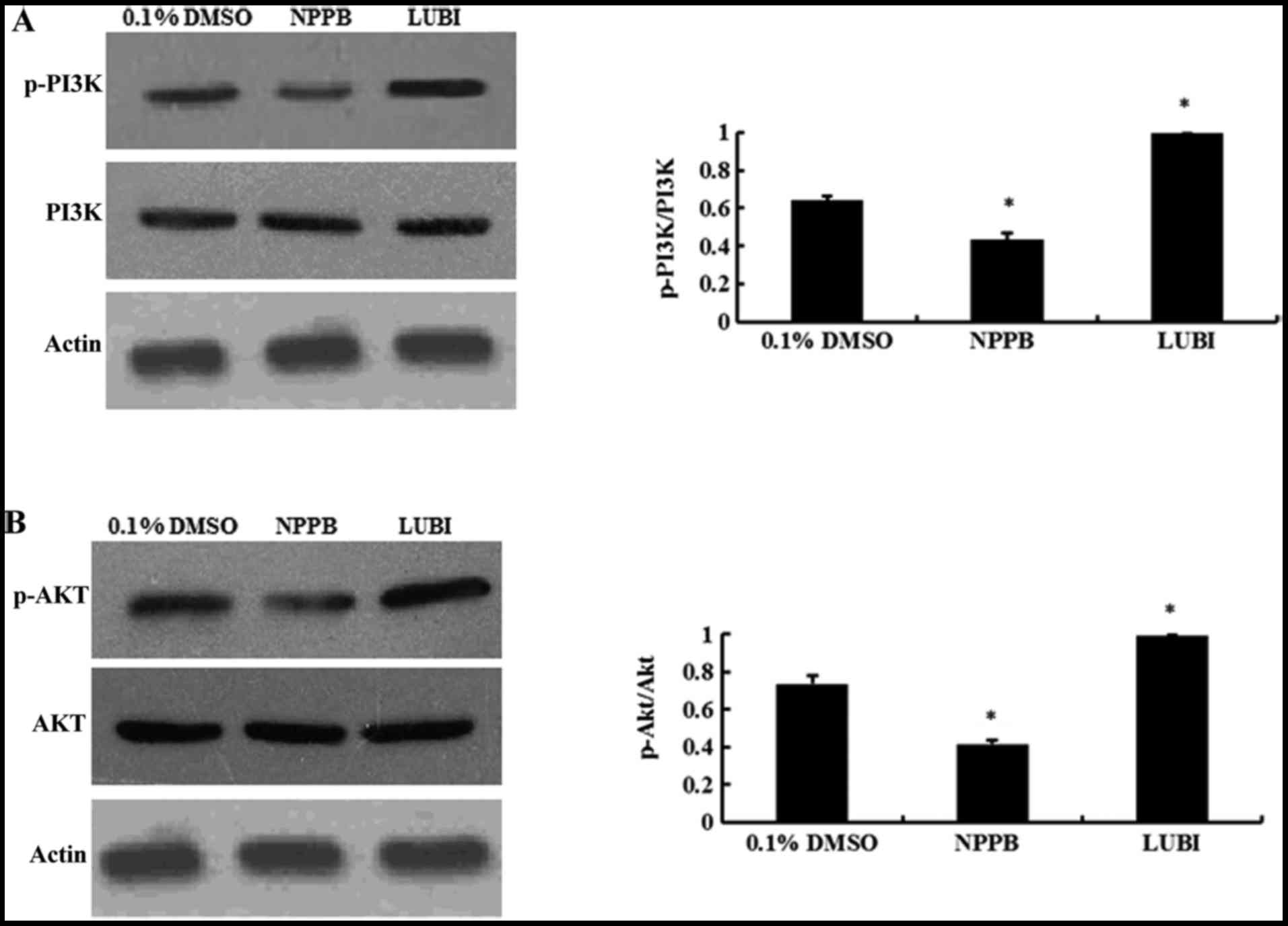
|
1
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foster PJ and Johnson GJ: Glaucoma in
China: how big is the problem. Br J Ophthalmol. 85:1277–1282. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khaw PT, Chang L, Wong TT, Mead A, Daniels
JT and Cordeiro MF: Modulation of wound healing after glaucoma
surgery. Curr Opin Ophthalmol. 12:143–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DA: Antifibrosis agents and glaucoma
surgery. Invest Ophthalmol Vis Sci. 35:3789–3791. 1994.PubMed/NCBI
|
|
5
|
Mielke C, Dawda VK and Anand N:
Intraoperative 5-fluorouracil application during primary
trabeculectomy in Nigeria: a comparative study. Eye (Lond).
17:829–834. 2003. View Article : Google Scholar
|
|
6
|
Wong TT, Khaw PT, Aung T, Foster PJ, Htoon
HM, Oen FT, Gazzard G, Husain R, Devereux JG, Minassian D, et al:
The singapore 5-fluorouracil trabeculectomy study: effects on
intraocular pressure control and disease progression at 3 years.
Ophthalmology. 116:175–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin DH, Ren J, Juzych MS, Hughes BA, Kim
C, Song MS, Yang KJ and Glover KB: Primary glaucoma triple
procedure in patients with primary open-angle glaucoma: the effect
of mitomycin C in patients with and without prognostic factors for
filtration failure. Am J Ophthalmol. 125:346–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perkins TW, Gangnon R, Ladd W, Kaufman PL
and Heatley GA: Trabeculectomy with mitomycin C: intermediate-term
results. J Glaucoma. 7:230–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang L, Crowston JG, Cordeiro MF, Akbar
AN and Khaw PT: The role of the immune system in conjunctival wound
healing after glaucoma surgery. Surv Ophthalmol. 45:49–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atreides SP, Skuta GL and Reynolds AC:
Wound healing modulation in glaucoma filtering surgery. Int
Ophthalmol Clin. 44:61–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Desjardins DC, Parrish RK II, Folberg R,
Nevarez J, Heuer DK and Gressel MG: Wound healing after filtering
surgery in owl monkeys. Arch Ophthalmol. 104:1835–1839. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song Y, Zhan L, Yu M, Huang C, Meng X, Ma
T, Zhang L and Li J: TRPV4 channel inhibits TGF-β1-induced
proliferation of hepatic stellate cells. PLoS One. 9:e1011792014.
View Article : Google Scholar
|
|
13
|
Roach KM, Duffy SM, Coward W,
Feghali-Bostwick C, Wulff H and Bradding P: The K+
channel KCa3.1 as a novel target for idiopathic
pulmonary fibrosis. PLoS One. 8:e852442013. View Article : Google Scholar
|
|
14
|
Roach KM, Feghali-Bostwick C, Wulff H,
Amrani Y and Bradding P: Human lung myofibroblast TGFβ1-dependent
Smad2/3 signalling is Ca(2+)-dependent and regulated by
KCa3.1 K(+) channels. Fibrogenesis Tissue Repair.
8:52015. View Article : Google Scholar
|
|
15
|
Sun H, Harris WT, Kortyka S, Kotha K,
Ostmann AJ, Rezayat A, Sridharan A, Sanders Y, Naren AP and Clancy
JP: Tgf-beta down-regulation of distinct chloride channels in
cystic fibrosis-affected epithelia. PLoS One. 9:e1068422014.
View Article : Google Scholar
|
|
16
|
Nilius B and Droogmans G: Amazing chloride
channels: an overview. Acta Physiol Scand. 177:119–147. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng YJ, Furukawa T, Tajimi K and Inagaki
N: Cl− channel blockers inhibit transition of quiescent
(G0) fibroblasts into the cell cycle. J Cell Physiol.
194:376–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng YJ, Furukawa T, Ogura T, Tajimi K
and Inagaki N: M phase-specific expression and
phosphorylation-dependent ubiquitination of the ClC-2 channel. J
Biol Chem. 277:32268–32273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng G, Shao Z, Chaudhari B and Agrawal
DK: Involvement of chloride channels in TGF-beta1-induced apoptosis
of human bronchial epithelial cells. Am J Physiol Lung Cell Mol
Physiol. 293:L1339–L1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu L, Yang H, Zuo W, Yang L, Zhang H, Ye
W, Mao J, Chen L and Wang L: Differential expression and roles of
volume-activated chloride channels in control of growth of normal
and cancerous nasopharyngeal epithelial cells. Biochem Pharmacol.
83:324–334. 2012. View Article : Google Scholar
|
|
21
|
Okada Y, Shimizu T, Maeno E, Tanabe S,
Wang X and Takahashi N: Volume-sensitive chloride channels involved
in apoptotic volume decrease and cell death. J Membr Biol.
209:21–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao J, Chen L, Xu B and Wang L, Li H, Guo
J, Li W, Nie S, Jacob TJ and Wang L: Suppression of ClC-3 channel
expression reduces migration of nasopharyngeal carcinoma cells.
Biochem Pharmacol. 75:1706–1716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao J, Yuan J, Wang L, Zhang H, Jin X, Zhu
J, Li H, Xu B and Chen L: Tamoxifen inhibits migration of estrogen
receptor-negative hepatocellular carcinoma cells by blocking the
swelling-activated chloride current. J Cell Physiol. 228:991–1001.
2013. View Article : Google Scholar
|
|
24
|
Guan YY, Wang GL and Zhou JG: The ClC-3
Cl− channel in cell volume regulation, proliferation and
apoptosis in vascular smooth muscle cells. Trends Pharmacol Sci.
27:290–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sang H, Li T, Li H and Liu J: Gab1
regulates proliferation and migration through the PI3K/Akt
signaling pathway in intrahepatic cholangiocarcinoma. Tumour Biol.
36:8367–8377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou
Y, Hu S, Tian F, Wang J and Chen Y: Effects of exendin-4 on bone
marrow mesenchymal stem cell proliferation, migration and apoptosis
in vitro. Sci Rep. 5:128982015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng R, Xiong Y, Zhu F, Ma Z, Liao W, He
Y, He J, Li W, Yang J, Lu Q, et al: Fenofibrate attenuated
glucose-induced mesangial cells proliferation and extracellular
matrix synthesis via PI3K/AKT and ERK1/2. PLoS One. 8:e768362013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hitchings RA and Grierson I: Clinico
pathological correlation in eyes with failed fistulizing surgery.
Trans Ophthalmol Soc U K. 103:84–88. 1983.PubMed/NCBI
|
|
29
|
Addicks EM, Quigley HA, Green WR and Robin
AL: Histologic characteristics of filtering blebs in glaucomatous
eyes. Arch Ophthalmol. 101:795–798. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Wang L, Zhu L, Nie S, Zhang J,
Zhong P, Cai B, Luo H and Jacob TJ: Cell cycle-dependent expression
of volume-activated chloride currents in nasopharyngeal carcinoma
cells. Am J Physiol Cell Physiol. 283:C1313–C1323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao R, Lau CP, Tse HF and Li GR:
Regulation of cell proliferation by intermediate-conductance
Ca2+-activated potassium and volume-sensitive chloride
channels in mouse mesenchymal stem cells. Am J Physiol Cell
Physiol. 295:C1409–C1416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen LX, Zhu LY, Jacob TJ and Wang LW:
Roles of volume-activated Cl− currents and regulatory
volume decrease in the cell cycle and proliferation in
nasopharyngeal carcinoma cells. Cell Prolif. 40:253–267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Souktani R, Ghaleh B, Tissier R,
d'Anglemont de Tassigny A, Aouam K, Bedossa P, Charlemagne D,
Samuel J, Henry P and Berdeaux A: Inhibitors of swelling-activated
chloride channels increase infarct size and apoptosis in rabbit
myocardium. Fundam Clin Pharmacol. 17:555–561. 2003. View Article : Google Scholar
|
|
34
|
Small DL, Tauskela J and Xia Z: Role for
chloride but not potassium channels in apoptosis in primary rat
cortical cultures. Neurosci Lett. 334:95–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maeno E, Ishizaki Y, Kanaseki T, Hazama A
and Okada Y: Normotonic cell shrinkage because of disordered volume
regulation is an early prerequisite to apoptosis. Proc Natl Acad
Sci USA. 97:9487–9492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Li H, Yang L, Deng Z, Luo H, Ye
D, Bai Z, Zhu L, Ye W, Wang L, et al: The ClC-3 chloride channel
associated with microtubules is a target of paclitaxel in its
induced-apoptosis. Sci Rep. 3:26152013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J: The IL-23/IL-17 axis may be
important in obesity-associated cancer by way of the activation of
multiple signal pathways. Int J Obes. 34:1227–1229. 2010.
View Article : Google Scholar
|
|
38
|
Martelli AM, Evangelisti C, Chiarini F,
Grimaldi C, Cappellini A, Ognibene A and McCubrey JA: The emerging
role of the phosphatidylinositol 3-kinase/Akt/mammalian target of
rapamycin signaling network in normal myelopoiesis and
leukemogenesis. Biochim Biophys Acta. 1803:991–1002. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weichhart T and Säemann MD: The
PI3K/Akt/mToR pathway in innate immune cells: emerging therapeutic
applications. Ann Rheum Dis. 67(Suppl 3): iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao T, Qi Y, Li Y and Xu K: PI3 kinase
regulation of neural regeneration and muscle hypertrophy after
spinal cord injury. Mol Biol Rep. 39:3541–3547. 2012. View Article : Google Scholar
|
|
41
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Y, Li W, Liu H, Peng Y, Yang Q, Xiao
L, Liu Y and Liu F: Inhibition effect of small interfering RNA of
connective tissue growth factor on the expression of extracellular
matrix molecules in cultured human renal proximal tubular cells.
Ren Fail. 36:278–284. 2014. View Article : Google Scholar
|
|
44
|
Qin D, Zhang GM, Xu X and Wang LY: The
PI3K/Akt signaling pathway mediates the high glucose-induced
expression of extracellular matrix molecules in human retinal
pigment epithelial cells. J Diabetes Res 2015. 920280:2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan F, Guo R, Cheng W, Chai L, Wang W, Cao
C and Li S: High glucose inhibits ClC-2 chloride channels and
attenuates cell migration of rat keratinocytes. Drug Des Devel
Ther. 9:4779–4791. 2015.PubMed/NCBI
|
|
46
|
Heo KS, Ryoo SW, Kim L, Nam M, Baek ST,
Lee H, Lee AR, Park SK, Park Y, Myung CS, et al: Cl− channel is
essential for LDL-induced cell proliferation via the activation of
Erk1/2 and PI3K/Akt and the upregulation of Egr-1 in human aortic
smooth muscle cells. Mol Cells. 26:468–473. 2008.PubMed/NCBI
|
|
47
|
Xia Li L, Wang Y, Cao Z, Da X, Guo Z, Qian
G, Liu J, Fan X, Sun YL, et al: Suppression of the PI3K-Akt pathway
is involved in the decreased adhesion and migration of bone
marrow-derived mesenchymal stem cells from non-obese diabetic mice.
Cell Biol Int. 35:961–966. 2011. View Article : Google Scholar : PubMed/NCBI
|