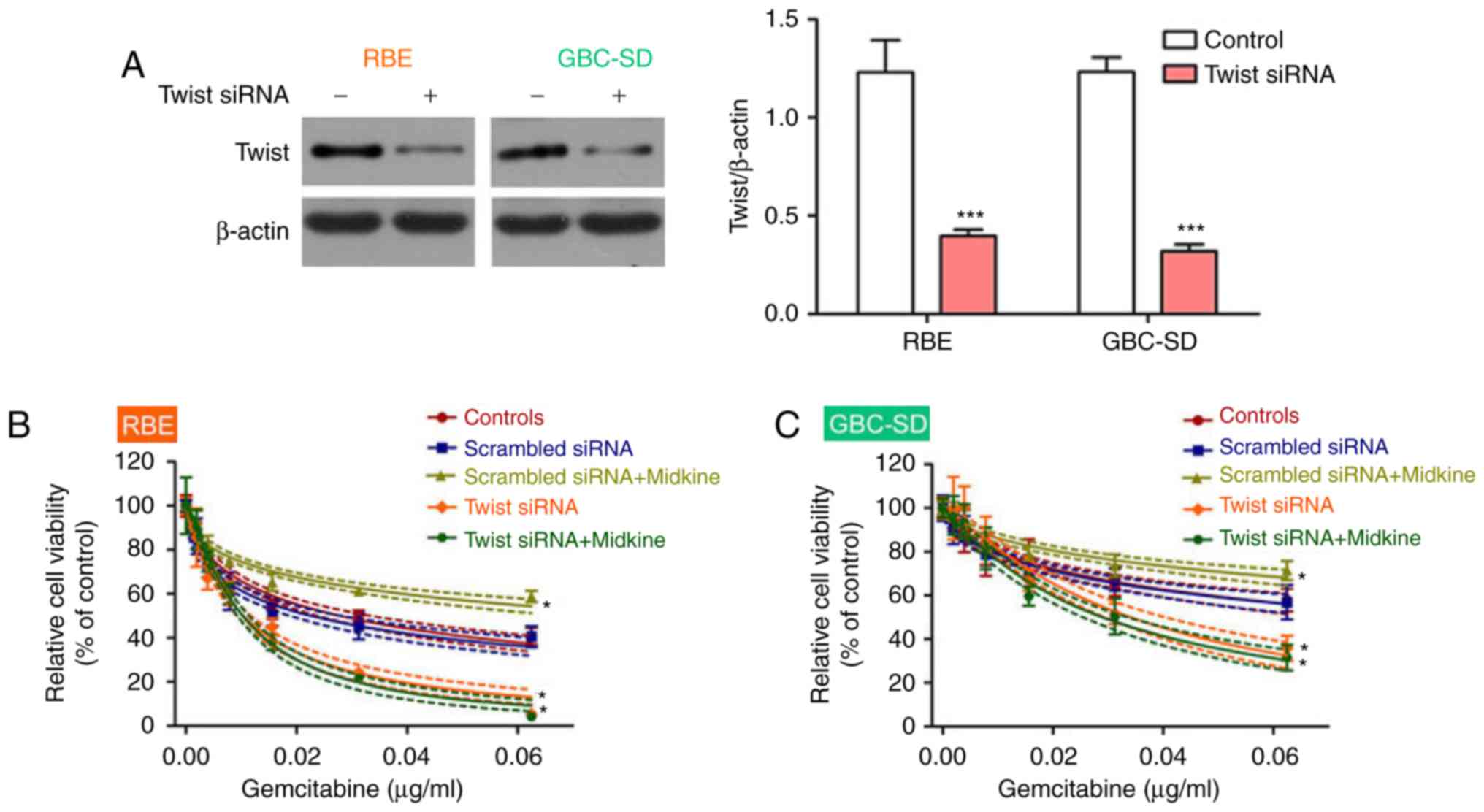
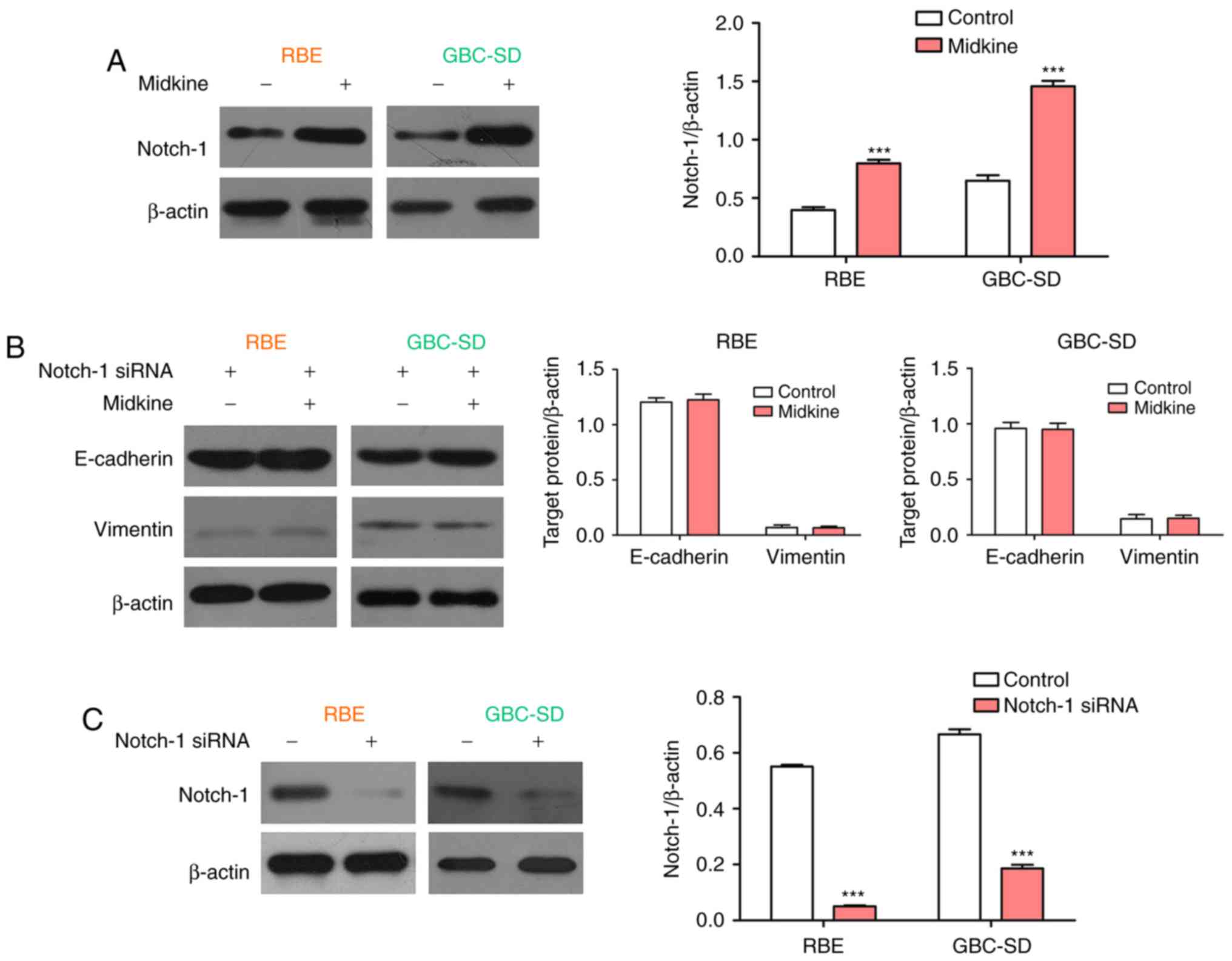
|
1
|
Hennedige TP, Neo WT and Venkatesh SK:
Imaging of malignancies of the biliary tract-an update. Cancer
Imaging. 14:142014.
|
|
2
|
Randi G, Malvezzi M, Levi F, Ferlay J,
Negri E, Franceschi S and La Vecchia C: Epidemiology of biliary
tract cancers: An update. Ann Oncol. 20:146–159. 2009. View Article : Google Scholar
|
|
3
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: A
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hezel AF and Zhu AX: Systemic therapy for
biliary tract cancers. Oncologist. 13:415–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goyal L, Chong DQ, Duda DG and Zhu AX:
Chemotherapy and antiangiogenics in biliary tract cancer. Lancet
Oncol. 16:882–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghosn M, Kourie HR, El Rassy E, Chebib R,
El Karak F, Hanna C and Nasr D: Optimum chemotherapy for the
management of advanced biliary tract cancer. World J Gastroenterol.
21:4121–4125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park K, Kim KP, Park S and Chang HM:
Comparison of gemcitabine plus cisplatin versus capecitabine plus
cisplatin as first-line chemotherapy for advanced biliary tract
cancer. Asia Pac J Clin Oncol. 13:13–20. 2017. View Article : Google Scholar
|
|
9
|
Stein A, Arnold D, Bridgewater J,
Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J,
Schrum S, et al: Adjuvant chemotherapy with gemcitabine and
cisplatin compared to observation after curative intent resection
of cholangiocarcinoma and muscle invasive gallbladder carcinoma
(ACTICCA-1 trial)-a randomized, multidisciplinary, multinational
phase III trial. BMC Cancer. 15:5642015. View Article : Google Scholar
|
|
10
|
Lamarca A, Benafif S, Ross P, Bridgewater
J and Valle JW: Cisplatin and gemcitabine in patients with advanced
biliary tract cancer (ABC) and persistent jaundice despite optimal
stenting: Effective intervention in patients with luminal disease.
Eur J Cancer. 51:1694–1703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yonemoto N, Furuse J, Okusaka T, Yamao K,
Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H and
Sato T: A multi-center retrospective analysis of survival benefits
of chemotherapy for unresectable biliary tract cancer. Jpn J Clin
Oncol. 37:843–851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Böhlen P and Kovesdi I: HBNF and MK
members of a novel gene family of heparin-binding proteins with
potential roles in embryogenesis and brain function. Prog Growth
Factor Res. 3:143–157. 1991. View Article : Google Scholar
|
|
13
|
Vu Van D, Heberling U, Wirth MP and
Fuessel S: Validation of the diagnostic utility of urinary midkine
for the detection of bladder cancer. Oncol Lett. 12:3143–3152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edfeldt K, Daskalakis K, Bäcklin C, Norlén
O, Tiensuu Janson E, Westin G, Hellman P and Stålberg P: DcR3, TFF3
and midkine are novel serum biomarkers in small intestinal
neuroendocrine tumors. Neuroendocrinology. 105:170–181. 2017.
View Article : Google Scholar
|
|
15
|
Krzystek-Korpacka M, Gorska S, Diakowska
D, Kapturkiewicz B, Podkowik M, Gamian A and Bednarz-Misa I:
Midkine is up-regulated in both cancerous and inflamed bowel,
reflecting lymph node metastasis in colorectal cancer and clinical
activity of ulcerative colitis. Cytokine. 89:68–75. 2017.
View Article : Google Scholar
|
|
16
|
Vongsuvanh R, van der Poorten D, Iseli T,
Strasser SI, McCaughan GW and George J: Midkine increases
diagnostic yield in AFP negative and NASH-related hepatocellular
carcinoma. PLoS One. 11:e01558002016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamashita T, Shimada H, Tanaka S, Araki K,
Tomifuji M, Mizokami D, Tanaka N, Kamide D, Miyagawa Y, Suzuki H,
et al: Serum midkine as a biomarker for malignancy, prognosis, and
chemosensitivity in head and neck squamous cell carcinoma. Cancer
Med. 5:415–425. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao J, Li WY and Gao SG: The advances of
Midkine with peripheral invasion in pancreatic cancer. Am J Cancer
Res. 5:2912–2917. 2015.PubMed/NCBI
|
|
19
|
Mirkin BL, Clark S, Zheng X, Chu F, White
BD, Greene M and Rebbaa A: Identification of midkine as a mediator
for intercellular transfer of drug resistance. Oncogene.
24:4965–4974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lorente M, Torres S, Salazar M, Carracedo
A, Hernández-Tiedra S, Rodríguez-Fornés F, García-Taboada E,
Meléndez B, Mollejo M, Campos-Martín Y, et al: Stimulation of ALK
by the growth factor midkine renders glioma cells resistant to
autophagy-mediated cell death. Autophagy. 7:1071–1073. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu YY, Mao XY, Song YX, Zhao F, Wang ZN,
Zhang WX, Xu HM and Jin F: Midkine confers Adriamycin resistance in
human gastric cancer cells. Tumor Biol. 33:1543–1548. 2012.
View Article : Google Scholar
|
|
22
|
Hu R, Yan Y, Li Q, Lin Y, Jin W, Li H, Lu
Y and Pang T: Increased drug efflux along with midkine gene high
expression in childhood B-lineage acute lymphoblastic leukemia
cells. Int J Hematol. 92:105–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vaquero J, Guedj N, Clapéron A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017. View Article : Google Scholar
|
|
24
|
Sung WJ, Kim H and Park KK: The biological
role of epithelial-mesenchymal transition in lung cancer (Review).
Oncol Rep. 36:1199–1206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nomura A, Majumder K, Giri B, Dauer P,
Dudeja V, Roy S, Banerjee S and Saluja AK: Inhibition of NF-kappa B
pathway leads to deregulation of epithelial-mesenchymal transition
and neural invasion in pancreatic cancer. Lab Invest. 96:1268–1278.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HS, Lee KS, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Yang HD, Park M, Park WS, et al: MicroRNA-31
functions as a tumor suppressor by regulating cell cycle and
epithelial-mesenchymal transition regulatory proteins in liver
cancer. Oncotarget. 6:8089–8102. 2015.PubMed/NCBI
|
|
27
|
Zhang X, Liu X, Luo J, Xiao W, Ye X, Chen
M, Li Y and Zhang GJ: Notch3 inhibits epithelial-mesenchymal
transition by activating Kibra-mediated Hippo/YAP signaling in
breast cancer epithelial cells. Oncogenesis. 5:e2692016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu G, Shao G, Pan Q, Sun L, Zheng D and Li
M: MicroRNA-9 regulates non-small cell lung cancer cell invasion
and migration by targeting eukaryotic translation initiation factor
5A2. Am J Transl Res. 9:478–488. 2017.PubMed/NCBI
|
|
29
|
Zhao G, Nie Y, Lv M, He L, Wang T and Hou
Y: ERβ-mediated estradiol enhances epithelial mesenchymal
transition of lung adenocarcinoma through increasing transcription
of midkine. Mol Endocrinol. 26:1304–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Güngör C, Zander H, Effenberger KE,
Vashist YK, Kalinina T, Izbicki JR, Yekebas E and Bockhorn M: Notch
signaling activated by replication stress-induced expression of
midkine drives epithelial-mesenchymal transition and
chemoresistance in pancreatic cancer. Cancer Res. 71:5009–5019.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Hoque MO, Wu F, Trink B,
Sidransky D and Ratovitski EA: Midkine induces
epithelial-mesenchymal transition through Notch2/Jak2-Stat3
signaling in human keratinocytes. Cell Cycle. 7:1613–1622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Yan
ML, Huang L, Tian YF, Chen YL and Wang YD: Hypoxia induces
TWIST-activated epithelial-mesenchymal transition and proliferation
of pancreatic cancer cells in vitro and in nude mice. Cancer Lett.
383:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Zhang X, Zhang Y, Zhu D, Zhang L,
Li Y, Zhu Y, Li D and Zhou J: HIF-2α promotes
epithelial-mesenchymal transition through regulating Twist2 binding
to the promoter of E-cadherin in pancreatic cancer. J Exp Clin
Cancer Res. 35:262016. View Article : Google Scholar
|
|
34
|
Lee JY and Kong G: Roles and epigenetic
regulation of epithelial-mesenchymal transition and its
transcription factors in cancer initiation and progression. Cell
Mol Life Sci. 73:4643–4660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Espinoza I, Pochampally R, Xing F, Watabe
K and Miele L: Notch signaling: Targeting cancer stem cells and
epithelial-to-mesenchymal transition. Onco Targets Ther.
6:1249–1259. 2013.PubMed/NCBI
|
|
36
|
Ma J, Xia J, Miele L, Sarkar FH and Wang
Z: Notch signaling pathway in pancreatic cancer progression.
Pancreat Disord Ther. 3(pii): 10001142013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Yin Y, Hu Y, Feng Y, Bian Z, Yao S,
Li M, You Q and Huang Z: miR-139 5p sensitizes colorectal cancer
cells to 5-fluorouracil by targeting NOTCH-1. Pathol Res Pract.
212:643–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mirone G, Perna S, Shukla A and Marfe G:
Involvement of Notch-1 in resistance to regorafenib in colon cancer
cells. J Cell Physiol. 231:1097–1105. 2016. View Article : Google Scholar
|
|
39
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Osipo C, Patel P, Rizzo P, Clementz AG,
Hao L, Golde TE and Miele L: ErbB-2 inhibition activates Notch-1
and sensitizes breast cancer cells to a gamma-secretase inhibitor.
Oncogene. 27:5019–5032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oyasiji T, Zhang J, Kuvshinoff B, Iyer R
and Hochwald SN: Molecular targets in biliary carcinogenesis and
implications for therapy. Oncologist. 20:742–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang HC, Kim IJ, Park JH, Shin Y, Ku JL,
Jung MS, Yoo BC, Kim HK and Park JG: Identification of genes with
differential expression in acquired drug-resistant gastric cancer
cells using high-density oligonucleotide microarrays. Clin Cancer
Res. 10:272–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qi M, Ikematsu S, Ichihara-Tanaka K,
Sakuma S, Muramatsu T and Kadomatsu K: Midkine rescues Wilms' tumor
cells from cisplatin-induced apoptosis: Regulation of Bcl-2
expression by Midkine. J Biochem. 127:269–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21(pii): E9652016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brabletz S, Bajdak K, Meidhof S, Burk U,
Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J
and Brabletz T: The ZEB1/miR-200 feedback loop controls Notch
signalling in cancer cells. EMBO J. 30:770–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Espinoza I and Miele L: Deadly crosstalk:
Notch signaling at the intersection of EMT and cancer stem cells.
Cancer Lett. 341:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu YY, Zheng MH, Zhang R, Liang YM and Han
H: Notch signaling pathway and cancer metastasis. Adv Exp Med Biol.
727:186–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vinson KE, George DC, Fender AW, Bertrand
FE and Sigounas G: The Notch pathway in colorectal cancer. Int J
Cancer. 138:1835–1842. 2016. View Article : Google Scholar
|
|
49
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Emerging role of Notch in stem cells and cancer. Cancer Lett.
279:8–12. 2009. View Article : Google Scholar :
|
|
50
|
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad
A, Banerjee S, Azmi AS, Miele L and Sarkar FH: Notch-1 induces
epithelial-mesenchymal transition consistent with cancer stem cell
phenotype in pancreatic cancer cells. Cancer Lett. 307:26–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fender AW, Nutter JM, Fitzgerald TL,
Bertrand FE and Sigounas G: Notch-1 promotes stemness and
epithelial to mesenchymal transition in colorectal cancer. J Cell
Biochem. 116:2517–2527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hao L, Rizzo P, Osipo C, Pannuti A, Wyatt
D, Cheung LW, Sonenshein G, Osborne BA and Miele L: Notch-1
activates estrogen receptor-alpha-dependent transcription via IKK
alpha in breast cancer cells. Oncogene. 29:201–213. 2010.
View Article : Google Scholar
|