Introduction
D-pinitol, the 3-O-methyl form of D-chiro-inositol,
which is abundant in Pinaceae and Leguminosae plants is a natural
inositol derivative functioning as an active principle in soy foods
and legumes (1,2). D-pinitol has been shown to possess
multifunctional properties, including antidiabetic, antilipidemic
and anticancer effects (2–5).
As one of the potential mechanisms for its anti-diabetic effect, it
has been reported that D-pinitol increases insulin-mediated glucose
uptake in the liver by activating the phosphatidylinositol-3-kinase
(PI3K)/Akt signalling pathway in a type 2 diabetic experimental rat
model (2). It has been also
demonstrated that D-pinitol improves insulin sensitivity by
stimulating the translocation of glucose transporter type 4 (GLUT4)
to the plasma membrane in the skeletal muscle of diabetic mice
(6). Consistent with the results
in the diabetic animal model, D-pinitol supplementation improves
glycemic control in healthy control and diabetic patients, although
there are conflicting results (7,8).
In addition to its attenuating effect on insulin resistance,
D-pinitol appears to ameliorate hyperlipidemia, inflammation and
oxidative stress in the liver, kidney and pancreas in diabetic
animals. Sivakumar et al (9) demonstrated that D-pinitol protects
the kidney by attenuating hyperglycemia-induced proinflammatory
cytokines and oxidative stress, resulting in histological and
ultra-structural improvement in streptozotocin-induced diabetic
rats. This indicates that the anti-oxidative effect of D-pinitol
may be a pivotal factor in its renoprotective effect against
diabetes.
The introduction of cyclosporine A (CsA)
revolutionized solid organ transplantation, reducing rejection
rates and improving early graft survival (10). Although there is a trend in
introducing novel immunosuppressive agents, CsA remains the
backbone of current immunosuppression for kidney transplantation.
In addition, accumulating evidence has shown that CsA is effective
in a significant number of patients with steroid-resistant
nephrotic syndrome (10), and it
has also been used to treat frequently relapsing nephrotic syndrome
(11). Despite its significant
contributions to transplantation and treatment of proteinuric renal
diseases, patients treated with CsA can suffer from a range of side
effects, including nephrotoxicity (12). The acute effects of CsA present
mainly as vasoconstriction of the afferent arteriole, which can
occur even following administration of the first dose, and CsA can
also cause renal morphological changes known as chronic CsA
nephropathy (13). Although the
exact mechanism of CsA-induced renal injury remains to be fully
elucidated, evidence has demonstrated that CsA-induced oxidative
stress is critical in causing structural and functional impairment
of the kidney (14–16), This suggests that the attenuation
of oxidative stress has a protective effect in kidneys exposed to
CsA. Our previous study demonstrated that oleanolic acid, a natural
pentacyclic triterpenoid, had a renoprotective effect by decreasing
oxidative stress generated by CsA (17).
The present study aimed to identify whether
D-pinitol has a protective effect against CsA-induced nephropathy
and to analyze the underlying mechanism with a focus on the
anti-oxidative effect of D-pinitol.
Materials and methods
Experimental design
The present study was approved by The Institutional
Animal Care and Use Committee of The Catholic University of Korea
Yeouido St. Mary's Hospital (Seoul, Korea; approval no.
YEO20131602FA). Five-week-old male ICR mice (DooYeol Biotech.,
Seoul, Korea) with initial weights of 15–20 g were housed at room
temperature (22±1°C) under an alternating 12 h-light and 12 h-dark
cycle. The animals were provided with free access to a low-salt
diet (0.01% sodium; Research Diets, Inc., New Brunswick, NJ, USA)
and water ad libitum. The mice were allowed to acclimatize
for 1 week prior to experiments. The mice were assigned into four
groups: i) Vehicle-treated control group (control, n=8), in which
mice were administered with a daily subcutaneous injection of
vehicle (1 ml/kg olive oil; Merck Millipore, Darmstadt, Germany);
ii) vehicle and D-pinitol-treated group (pinitol, n=8), in which
mice were administered with a daily subcutaneous injection of olive
oil (1 ml/kg) and D-pinitol (50 mg/kg; Merck Millipore) daily; iii)
CsA only group (CsA, n=8), in which mice were administered with a
daily subcutaneous injection of CsA (30 mg/kg; Chong Kun Dang
Pharmaceutical Corp., Seoul, Korea); iv) CsA and D-pinitol-treated
group (CsA+pinitol, n=8), in which mice were administered with a
daily subcutaneous injection of CsA (30 mg/kg) and D-pinitol (50
mg/kg) orally for 4 weeks. The doses of CsA and D-pinitol were
selected based on a previous study (6).
Assessment of basic parameters of renal
function
Twenty-four-hour urine collection was performed
using metabolic cages (Nalgene, Rochester, NY, USA) prior to
sacrifice. Proteinuria and urine creatinine levels were measured
using ELISA kits (Exocell, Inc., Philadelphia, PA, USA).
Measurement of serum creatinine concentration and urine osmolality
was performed at Samkwang Medical Laboratories (Seoul, Korea) using
enzymatic colorimetric methods (Modular DPP system; Roche
Diagnostics GmbH, Hamburg, Germany). Creatinine clearance was
calculated using a standard formula: Urine creatinine (mg/dl) ×
urine volume (ml/24 h)/serum creatinine (mg/dl) ×1440 (min/24 h).
Whole-blood CsA concentration was determined by liquid
chromatography-tandem mass spectrometry using an API3200 LC-MS
system (MDS Sciex, Foster City, CA, USA) equipped with an
electrospray ionization interface to generate negative ions
[M+NH4]+.
Histopathology
On the day of sacrifice, the kidneys were retrieved,
washed with heparinized saline, fixed in a
periodate-lysine-paraformaldehyde solution and embedded in wax.
Following dewaxing, sections with a thickness of 4-µm were
processed and stained with Masson's trichrome. Tubulointerstitial
fibrosis was defined as a matrix-rich expansion of the interstitium
with tubular dilatation, atrophy, cast formation, and sloughing of
tubular epithelial cells or thickening of the tubular basement
membrane. At least 20 fields per section were assessed by counting
the percentage of injured area per field of cortex at 200×
magnification using a color-image analyzer (TDI Scope Eye Version
3.5 for Windows; Olympus, Tokyo, Japan).
Immunohistochemistry
For immunohistochemistry, the 4-µm-thick
sections were deparaffinized, hydrated in ethanol and treated with
an antigen unmasking solution containing 10 mM sodium citrate
buffer (pH 6.0) followed by washing with PBS. The sections were
incubated with 3% H2O2 in methanol to block
endogenous peroxidase activity. Non-specific binding was blocked
with 10% normal goat serum in PBS. The sections were incubated with
anti-α-smooth muscle actin (α-SMA; dilution, 1:1,000; cat. no.
ab32575) or anti-collagen IV (dilution, 1:1,000; cat. no. ab6586)
(both from Abcam, Cambridge, UK) antibodies overnight in a
humidified chamber at 4°C. Antibodies were then localized through
incubation with a peroxidase-conjugated horse anti-rabbit IgG
(dilution, ready to use) for 1 h at room temperature and DAB
substrate solution using the Vector Immpress kit (cat. no. MP-7401;
Vector Laboratories, Inc., Burlingame, CA, USA). The sections were
dehydrated in ethanol, cleared in xylene and mounted without
counterstaining. The sections were examined in a blinded-manner
using light microscopy (Olympus BX-50; Olympus). For quantification
of the proportional area of staining, ~20 views (magnification,
×400) were randomly located in the renal cortex and
cortico-medullary junction of each slide. Images were captured and
analyzed to determine density × positive area/glomerular total area
using ImageJ software 1.49 (National Institutes of Health,
Bethesda, MD, USA).
Immunoblot analysis
Immunoblot analysis was performed to analyze the
effect of D-pinitol on the expression of several important proteins
in the pathogenesis of UUO-induced renal fibrosis. Whole cell
lysates and nuclear fractions of the renal cortical tissues were
extracted using Pro-Prep protein extraction solution (Intron
Biotechnology, Inc., Seongnam, Korea) and the NE-PER nuclear kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
respectively, according the manufacturer's protocol (18). Protein concentration of whole cell
lysates and nuclear fractions were determined using BCA assay kit
(Thermo Fisher Scientific, Inc.). Equal amount (20 µg) of
each sample was then separated by 12% SDS-PAGE, and transferred
onto nitrocellulose membranes. Subsequent to blocking in 3% bovine
serum albumin in TBS-T solution for 1 h at room temperature, the
membranes were incubated with primary antibodies overnight at 4°C.
Following incubation with secondary antibodies conjugated with
horseradish peroxidase for 1 h, at room temperature, the protein
was visualized using an enhanced chemiluminescence detection method
(GE Healthcare Life Sciences, Chalfont, UK). Primary antibodies
against nuclear erythroid factor 2-related factor 2 (Nrf2; 1:1,000;
cat. no. sc722), Kelch-like ech-associated protein 1 (Keap1;
1:1,000; cat. no. sc33569), NAD(P)H:quinone oxidoreductase 1 (NQO1;
1:1.000; cat. no. sc16464) and acetylated Forkhead box O1 (FoxO1;
1:1,000; cat. no. sc49437) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), and antibodies against Sirt1
(1:1,000; cat. no. 9475), Akt (1:1,000; cat. no. 9272), phospho-Akt
(1:1,000; cat. no. 9271) and lamin B1 (1:1,000; cat. no. 125860)
were from Cell Signaling Technology, Inc (Danvers, MA, USA).
Antibodies against superoxide dismutase SOD1 (SOD1; 1:10,000; cat.
no. ADI-SOD-100; Enzo Life Sciences, Inc., Farmingdale, NY, USA),
SOD2 (dilution, 1:10,000; cat. no. ab16956), catalase (dilution,
1:2,000; cat. no. ab31630), FoxO1 (dilution, 1:1,000; cat. no.
ab39670), phospho-FoxO1A S329 (dilution, 1:1,000; cat. no. ab58519)
(all from Abcam), heme oxygenase-1 (HO-1; dilution, 1:1,000; cat.
no. PA5-27338; BD Biosciences, Franklin Lakes, NJ, USA), and
β-actin (dilution, 1:10,000; cat. no. 061M4808; Merck Millipore)
were also commercially obtained.
Terminal
deoxynucleotidyltransferase-mediated biotin nick end-labeling
(TUNEL) assay
Apoptosis was assessed using TUNEL assay. Apoptotic
cells in the formalin-fixed and paraffin-embedded tissue were
detected using the ApopTag In Situ Apoptosis Detection kit
(EMD Millipore, Billerica, MA, USA) according to the manufacturer's
protocol. TUNEL-positive cells were evaluated under a light
microscope (Olympus BX-50; Olympus) at 400× magnification.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences between groups were examined for statistical
significance using one-way analysis of variance with Bonferroni
correction using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was independently performed at least
three times.
Results
Effect of D-pinitol on renal functional
changes
Following 28 days of treatment, the parameters of
renal functions were measured as summarized in Table I. The 24-h urine volume was
significantly increased in the CsA group and the CsA+pinitol group.
The CsA-treated mice had significantly lower urinary osmolality.
The serum creatinine level was significantly increased and
creatinine clearance was decreased in the CsA group. However,
D-pinitol treatment enhanced creatinine clearance by reducing the
CsA-induced elevation of serum creatinine, and increased urine
osmolality (Table I).
 | Table IPhysical and biochemical
characteristics of the four groups at the end of the 4-week
period. |
Table I
Physical and biochemical
characteristics of the four groups at the end of the 4-week
period.
| Characteristic | Control (n=8) | Pinitol (n=8) | CsA (n=8) | CsA+pinitol
(n=8) |
|---|
| Body weight
(g) | 33.68±1.73 | 32.94±0.90 | 29.81±1.19 | 28.88±2.45 |
| Urine volume
(ml) | 2.16±2.39 | 2.03±1.88 | 5.25±1.44a | 6.26±3.20b |
| Serum Cr
(mg/dl) | 0.11±0.02 | 0.13±0.02 | 0.27±0.11c | 0.17±0.16d |
| Urine osmolality
(mOsm/kg) | 1324.90±534.50 | 1280.00±230.20 |
494.00±208.80e | 705.80±280.00 |
| Cr clearance
(ml/min/100 g BW) | 1.29±0.15 | 1.38±0.09 | 0.52±0.16f | 1.03±0.19 |
| Urine protein/Cr
ratio (mg/mg) | 23.30±4.48 | 17.80±11.88 | 8.40±5.70g | 3.80±2.00 |
| CsA concentration
(ng/ml) | N/A | N/A | 896.20±328.40 | 882.20±302.90 |
Effect of D-pinitol on renal
morphological changes
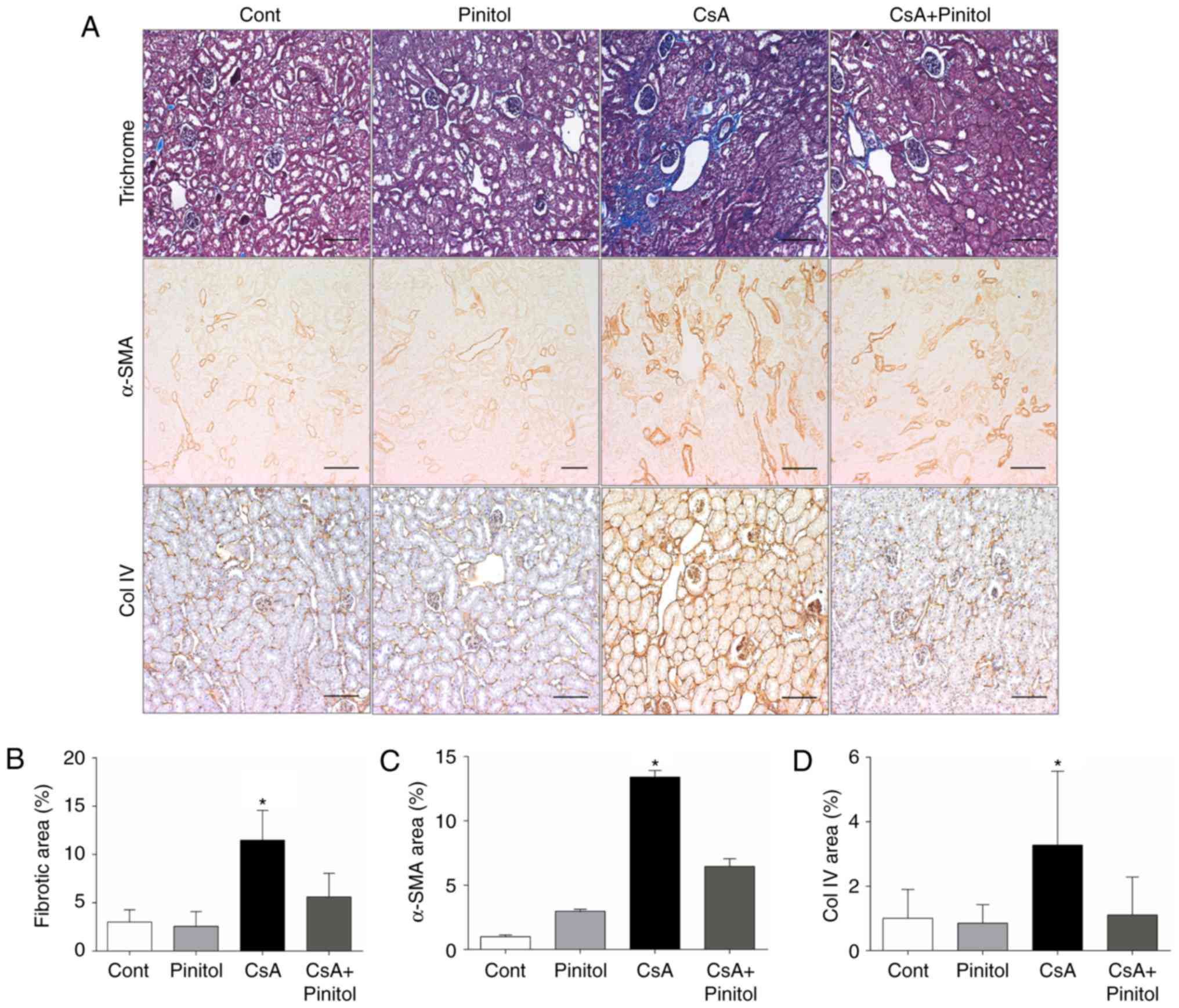
To evaluate whether D-pinitol affected renal
morphological changes, staining with Masson-trichrome, α-SMA, and
collagen IV was performed. The tubulointerstitial fibrosis,
analyzed using Masson-trichrome staining, was increased in the
kidney tissues of the CsA-treated mice, compared with that in the
control group. By contrast, D-pinitol treatment significantly
attenuated renal fibrosis in the CsA-treated mice (Fig. 1A and B). CsA treatment also led to
the significant upregulation of α-SMA, a marker of myofibroblasts,
and type IV collagen in the kidneys of the mice. By contrast,
D-pinitol mitigated the CsA-induced expression of α-SMA and type IV
collagen (Fig. 1A, C and D).
Effect of D-pinitol on renal oxidative
status
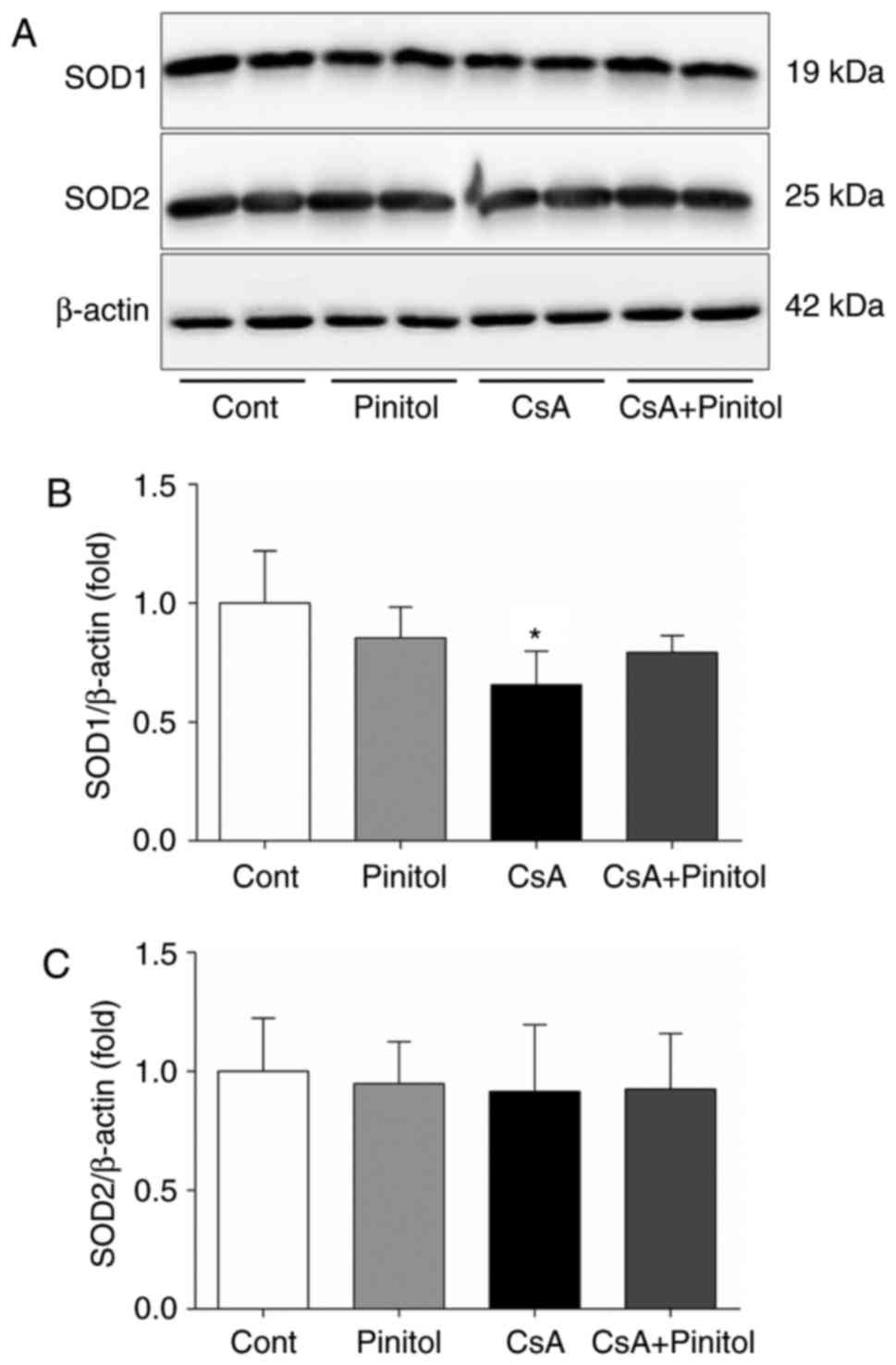
Following 28 days of CsA administration, there was a
significant decrease in renal SOD1 (Fig. 2A and B). D-pinitol treatment
significantly ameliorated the CsA-induced reduction in SOD1. By
contrast, no significant difference was found in the expression of
SOD2 among the groups (Fig. 2A and
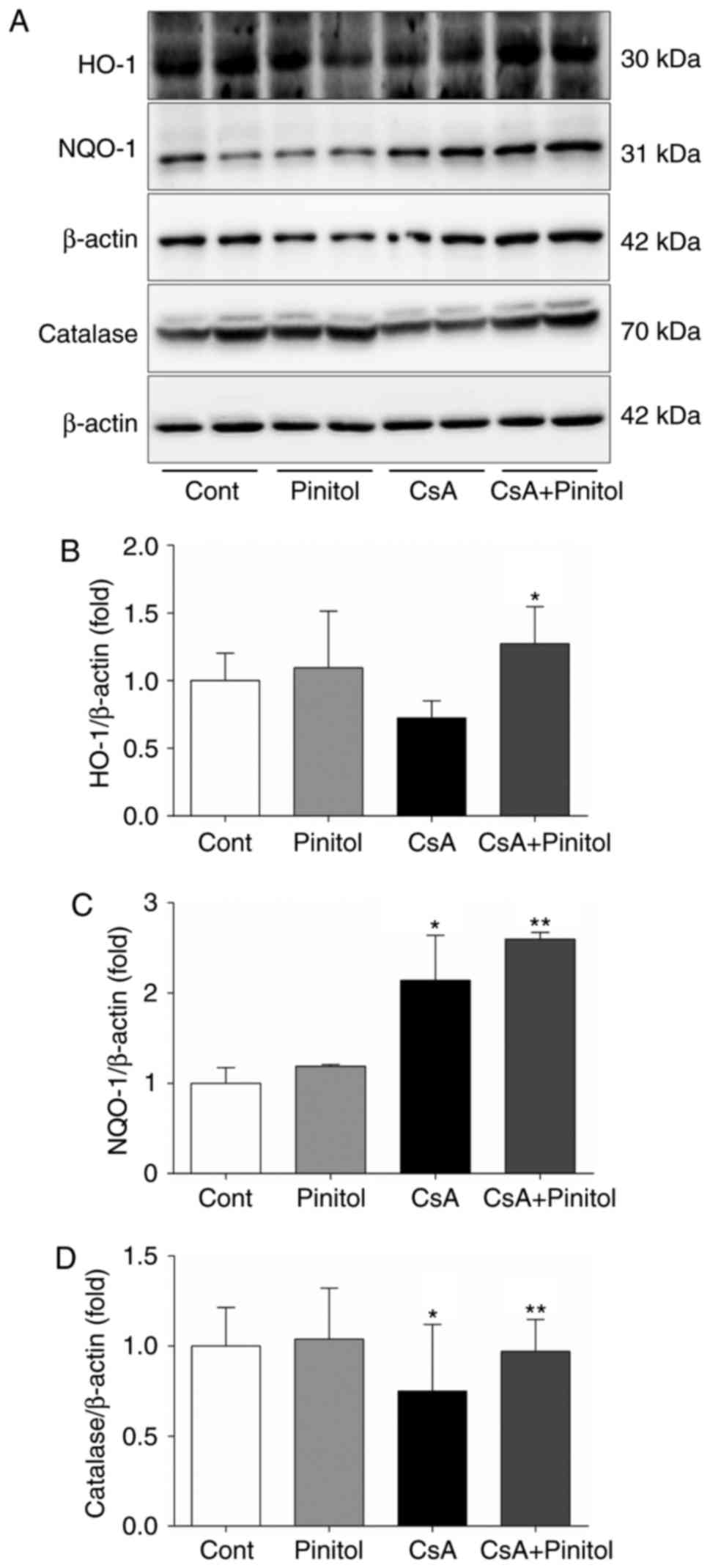
C). The renal levels of HO-1, NQO-1 and catalase were also
determined. It was found that D-pinitol treatment of the
CsA-treated mice restored the decreased expression of HO-1 in the
kidney (Fig. 3A and B). There was
increased expression of NQO1 in the CsA-treated mice, compared with
that in the control, which was more marked following D-pinitol
treatment (Fig. 3A and C).
D-pinitol treatment restored the decreased expression of catalase
in the CsA-treated mice (Fig. 3A and
D). Taken together, the intra-renal levels of cytosolic
antioxidant enzymes were increased by D-pinitol treatment,
suggesting that the increased activity of cytosolic antioxidant
enzymes may be attributed to the renoprotective effects of
D-pinitol against CsA-induced nephropathy.
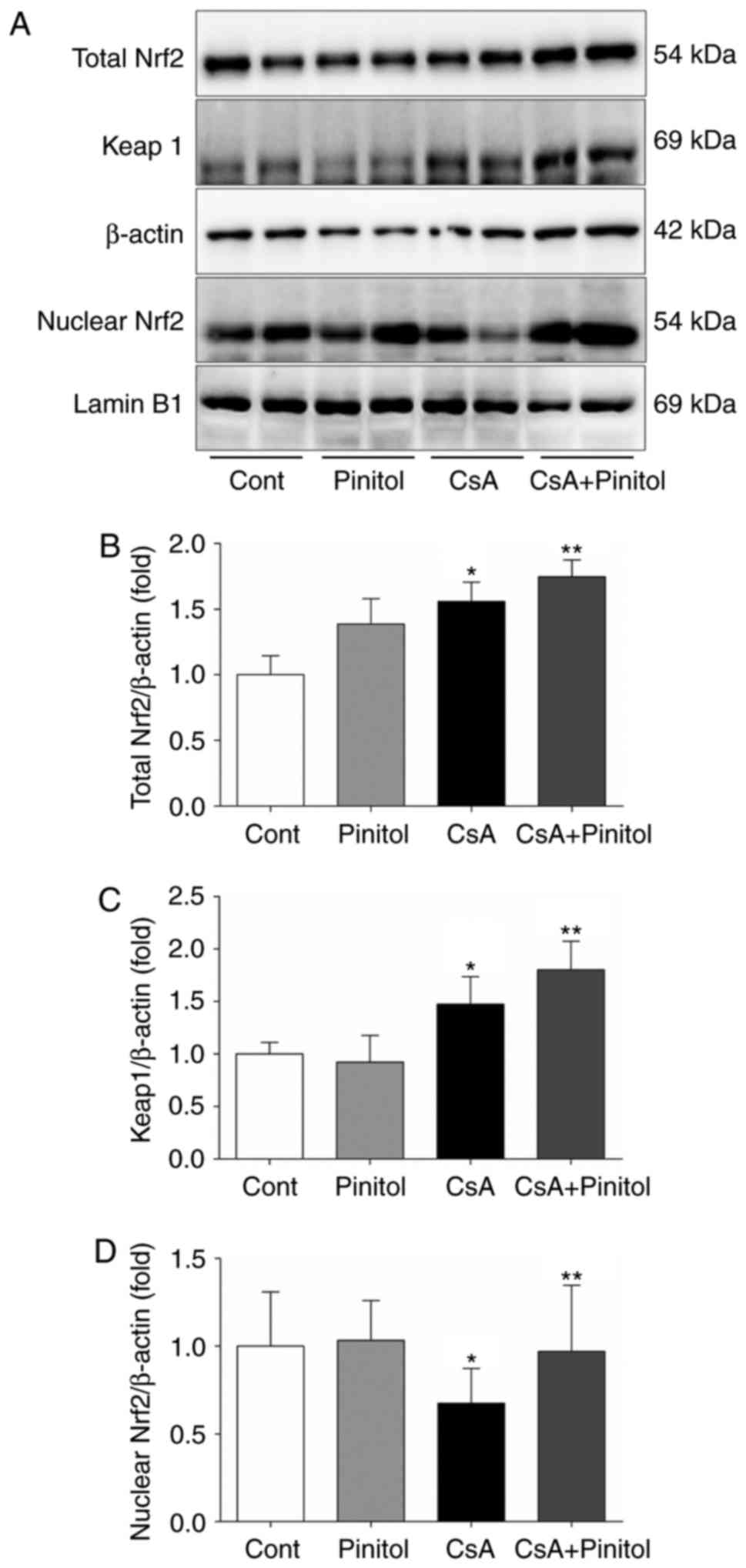
Effect of D-pinitol on the Nrf2
signalling pathway
It has been reported that HO-1, catalase and SOD1
are major transcriptional target genes of Nrf2 (19). Accordingly, it was necessary to
determine whether D-pinitol treatment affected the Nrf2/Keap1
signalling pathway. The results of the western blot analysis showed
that CsA markedly decreased the nuclear expression of Nrf2, whereas
the expression levels of Keap1 and total Nrf2 were marginally
increased in the CsA mice (Fig.
4A–C). It was noted that D-pinitol treatment restored the
nuclear expression of Nrf2 in the kidneys of CsA-treated mice
(Fig. 4A and D).
Effect of D-pinitol on the renal
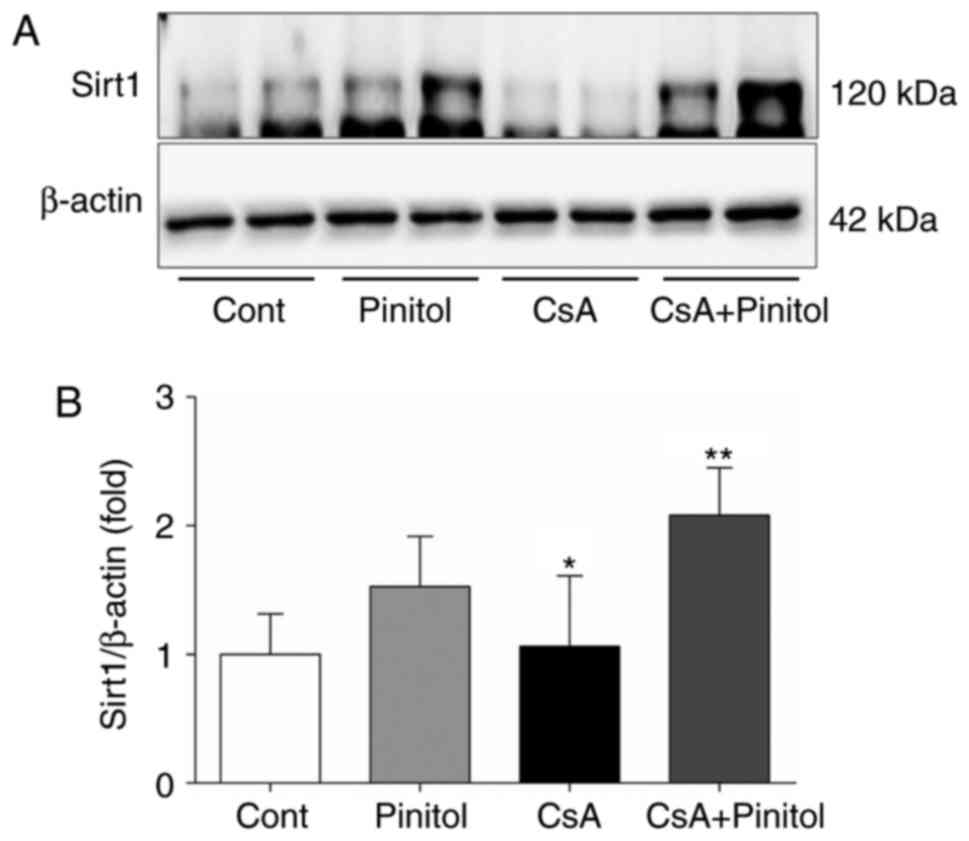
expression of Sirt1, Akt and FoxO1
Sirt1 and its downstream pathway are crucial in
several biological pathways, including apoptosis and oxidative
stress responses in chronic kidney disease (20). Therefore, the effect of D-pinitol
treatment on Sirt1 and its downstream pathway was determined. The
results of the western blot analysis revealed that the intrarenal
expression level of Sirt1 was increased in the CsA+pinitol group,
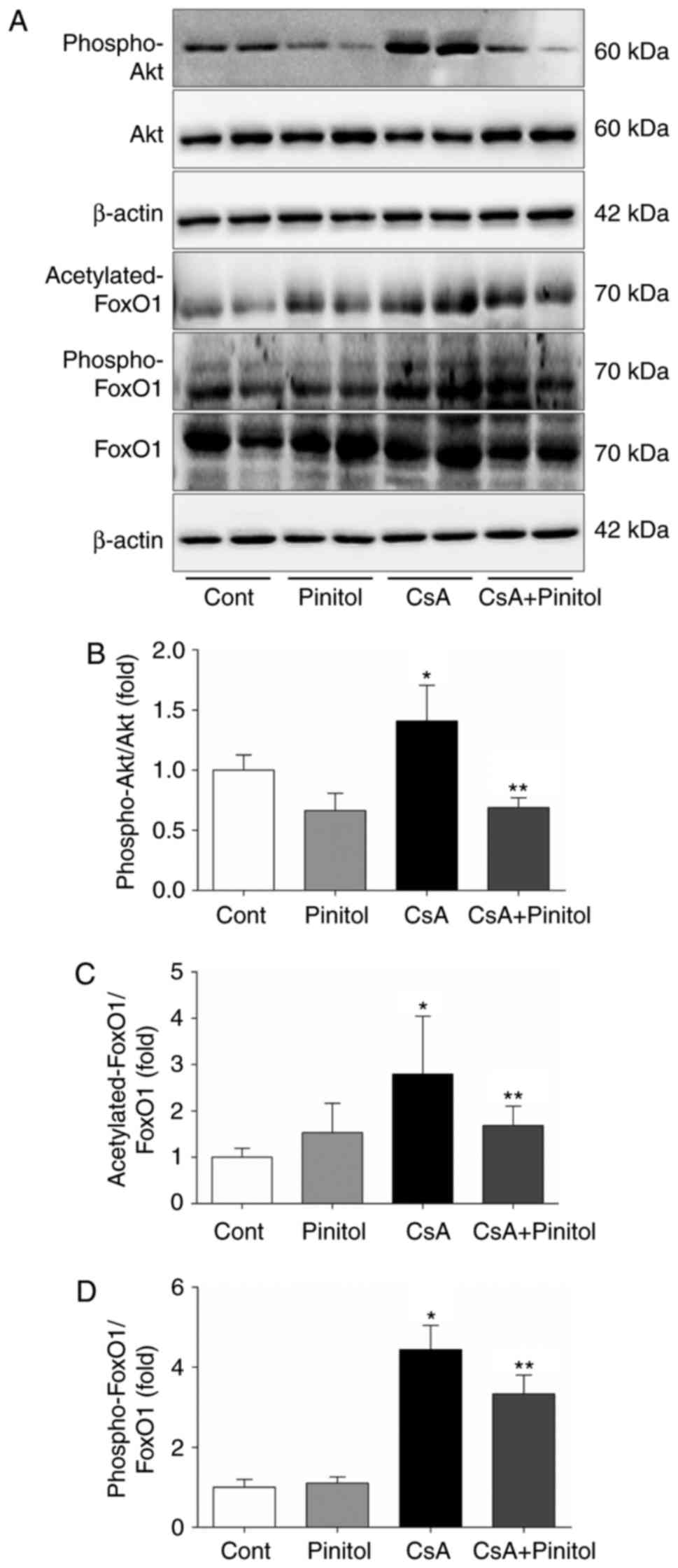
compared with that in the other groups (Fig. 5A and B). By contrast, the ratios
of phospho-Akt/Akt, acetylated FoxO1/FoxO1 and phospho-FoxO1/FoxO1
were all increased in the kidneys of the CsA-treated mice (Fig. 6). These ratios were significantly
decreased following treatment with D-pinitol.
Effect of D-pinitol on renal
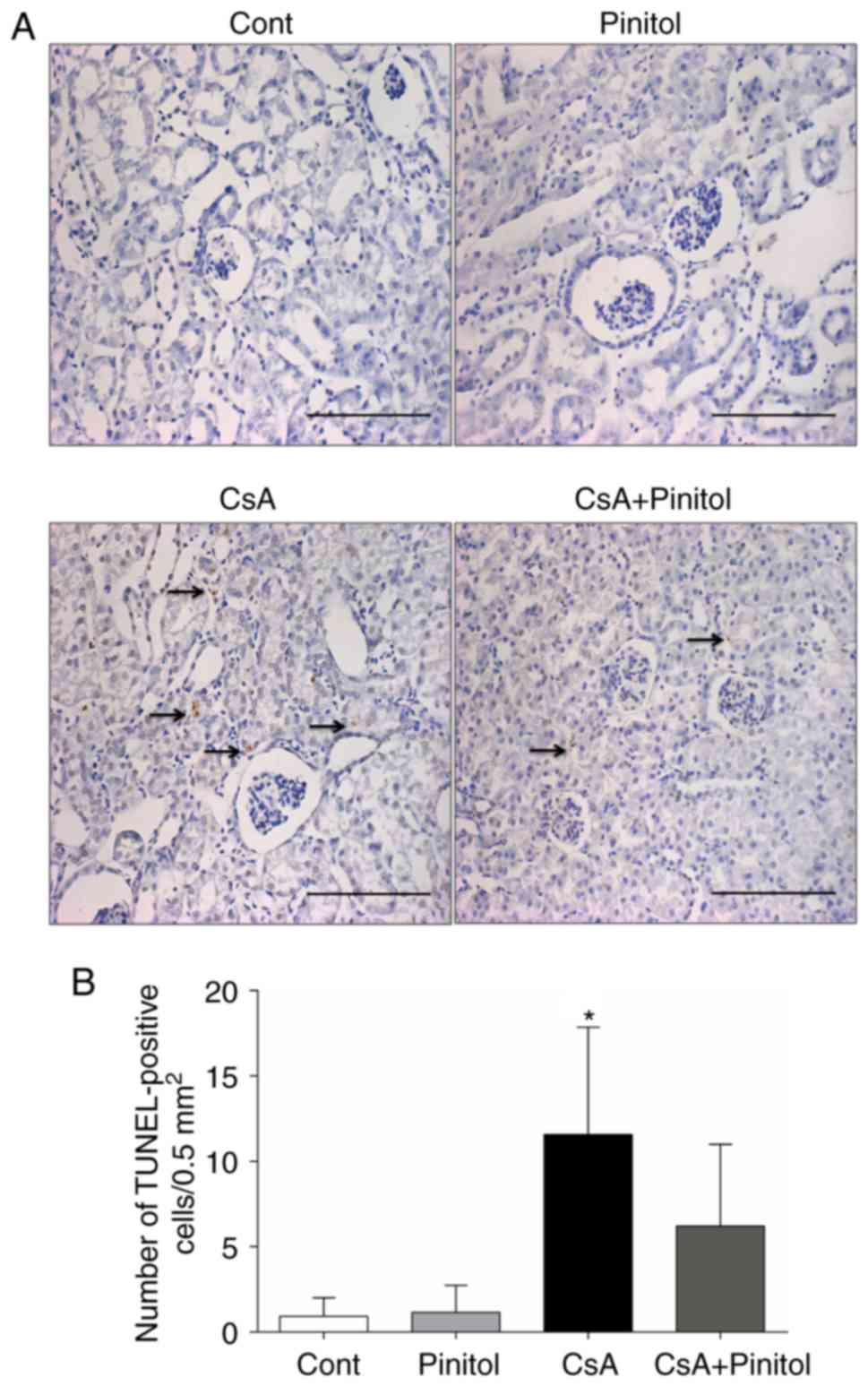
apoptosis
To evaluate whether D-pinitol treatment affected
intrarenal apoptosis, TUNEL assay was performed. It was found that
the number of TUNEL-positive cells in the renal interstitium of the
CsA-treated mice was significantly increased, compared with that in
the control group. This was reversed by D-pinitol treatment
(Fig. 7), indicating that
D-pinitol treatment attenuated apoptosis in CsA nephropathy.
Discussion
In the present study, it was demonstrated that
D-pinitol treatment recovered renal function in a mouse model of
chronic CsA nephropathy. This renoprotective effect of D-pinitol
was associated with improvement in renal fibrosis, apoptotic cell
injury and oxidative stress through restoration of the decreased
Nrf2 and Sirt1 pathway. It was shown that Nrf2 and its downstream
genes, including SOD1, HO-1, NQO1 and catalase, were all increased
by D-pinitol. Additionally, D-pinitol treatment reversed the
CsA-induced decline in the expression of Sirt1, increased
phosphorylation of Akt and FoxO1, and acetylation of FoxO1.
Although these are apparently different signalling pathways,
D-pinitol administration appeared to ameliorate oxidative stress
damage with subsequent reduction in fibrosis and apoptosis in
chronic CsA-induced nephropathy.
Nrf2 is critical in cellular defense mechanism in
response to increased oxidative stress (17). Upon oxidative insult, Nrf2 is
dissociated from its cytoplasmic repressor Keap1, and then
translocated to the nucleus, where it binds to antioxidant response
element (ARE) to stimulate the transcription of its target genes,
including HO-1, SOD1 and NQO1 (17,21). Diverse mechanisms appear to be
involved in the activation of Nrf2, including the release of Nrf2
from Keap1, downregulated expression of Keap1, disruption of the
Keap1-Cullin 3 complex, and the nuclear translocation of Nrf2
(21). Evidence supports that
nuclear Nrf2 content is markedly declined with increased reactive
oxygen species (ROS) production in chronic kidney disease (15,22–24). Our previous studies also revealed
a deterioration of the Nrf2 pathway with impairment of downstream
antioxidant defense mechanism in several experimental kidney
disease models, including chronic CsA nephropathy (17,20,25,26). In contrast to our previous study
(17), the total renal expression
of Nrf2 was increased by CsA treatment alone in the present study
(Fig. 4A and B). Considering
previous reports, which have shown various CsA concentrations in
the blood (23,27,28), this discrepancy may be due to
differences in blood concentrations of CsA; the blood concentration
of CsA was ~1,800 ng/ml in our previous study (17) but was <900 ng/ml, almost half
of the previous value, in the present study (Table I). It was observed that CsA
provoked oxidative stress and the expression level of Nrf2 was
induced by low level of ROS, but not by a high level of ROS
(29). Therefore, although the
mechanism involved in the discrepant effect of ROS on the
expression of Nrf2 is in accordance with the concentration of ROS,
it may be that lower blood concentrations of CsA increase the
expression of Nrf2 via ROS. CsA also showed a discrepant effect on
the expression of Nrf2. In the present study, the Nrf2 increased by
CsA failed to stimulate the transcription of downstream antioxidant
enzymes, which was incomplete and inadequate to protect the kidney
from the nephrotoxicity caused by CsA itself (Figs. 1Figure 2Figure 3–4). It was hypothesized that this
non-protective effect of Nrf2 on CsA-induced nephrotoxicity may be
due to failure of the nuclear translocation of Nrf2 in CsA-treated
mice (Fig. 4D). Based on these
findings, CsA appeared to sequentially stimulate the expression of
Nrf2, inhibit the nuclear translocation of Nrf2, and finally lose
its effect on increasing the expression of Nrf2, as CsA
concentration in the blood increased. However, D-pinitol treatment
of the CsA-treated mice induced an increase in the nuclear
expression of Nrf2 even with increased expression of Keap1,
indicating that D-pinitol is a facilitator of the nuclear
translocation of cytoplasmic Nrf2 released from Keap1. Taken
together, D-pinitol appeared to attenuate CsA-induced renal injury
through restoring the activity of Nrf2 and acting as a free radical
scavenger (9,30,31). It may protect the kidney against
high levels of ROS by promoting the nuclear translocation of Nrf2
and scavenging low levels of ROS.
Another intracellular mechanism underpinning the
effect of D-pinitol may be associated with the expression of Sirt1
coordinately with the Akt and FoxO1 pathway in the CsA nephropathy
model. Sirtuin, an NAD-dependent deacetylase, has a renoprotective
effect, and diverse target molecules through direct deacetylation
or epigenetic gene modulation have been confirmed as effectors of
its renoprotective function (32). Among its isoforms, Sirt1 has
various substrates involved in energy metabolism with a crucial
role in several biological pathways, including apoptosis, longevity
and oxidative stress responses in chronic kidney diseases (33). The present study suggested a novel
role of D-pinitol in restoring the altered expression of Sirt1 in a
chronic CsA nephropathy model. As FoxO signalling can be
selectively activated by Sirt1, the effect of D-pinitol on the
expression of FoxO was investigated in the present study. The FoxO
subfamily of Forkhead box transcription factors can activate an
overlapping set of genes, which regulate cell cycle, apoptosis and
metabolism, thereby coordinating cellular responses to various
nutrient status and oxidative stress (34). Whereas nuclear Sirt1 can
deacetylate and reactivate the transcriptional activity of FoxO1,
the inactivated form of FoxO1, which is acetylated by dissociation
from Sirt1, can be translocated back to the cytoplasm (35). The present study showed that the
expression of Sirt1 was decreased in the chronic CsA nephropathy
model, whereas the ratio of acetylated FoxO1/FoxO1 was increased.
Therefore, it may be that the suppression of Sirt1 by CsA caused
increased acetylation of FoxO1, which then moved back to the
cytoplasm with its ensuing inactivation. However, D-pinitol
treatment reversed the changes of Sirt1 and FoxO1 to improve renal
function and morphological derangement.
The activity of FoxO1 can be modulated by the
PI3K/Akt pathway (34). The
exposure of cells to oxidative stress can result in activation of
the PI3K/Akt pathway and any fluctuation of Akt phosphorylation can
be involved in the pathogenesis of several complex diseases
(34). Akt allows the
translocation of FoxO from the nucleus to the cytoplasm (34). Emerging evidence has shown that,
upon oxidative stress, AKT is activated and subsequently
phosphorylates FoxO1 proteins, which are then translocated to the
cytoplasm (34–36). Modifications of FoxO1, including
phosphorylation and acetylation, may assist in driving the
expression of genes involved in combating oxidative stress
(2,36). It has been demonstrated that
acetylated FoxO1 can become more sensitive to Akt-dependent
phosphorylation, suggesting that acetylation and phosphorylation
can cooperatively regulate the function of FoxO1 (37). To fine tune the activity of FoxO1
with complexity, this provides an additional dimension to the
regulatory mechanism of FoxO1 associated with its dual
post-translational modifications. The distinct modification of
subsequent downstream FoxO1 by Sirt1 and Akt may be involved in the
renoprotective effect of D-pinitol in the CsA nephropathy model
(38), although the mechanism at
a molecular level requires further evaluation.
Although the results in the present study
demonstrated a novel mechanism involved in the protective effect of
D-pinitol against CsA-induced nephropathy, a number of points
require addressing. First, unlike humans, mice are resistant to
CsA-induced renal injury due to CsA-induced nephrotoxicity being
species-specific. Therefore, salt depletion with higher-dose CsA is
required to induce morphologic nephrotoxicity, compared with that
in clinical practice (14).
Secondly, the present study was unable to elucidate the molecular
causality or the association between Nrf2 and Sirt1 in CsA-induced
renal injury. Previous studies have suggested a plausible
explanation. Kulkarni et al (39) showed that the fasting-induced
activation of Sirt1 increased the expression of Nrf2 and
accumulation of Nrf2 to the ARE region. It also activated ARE in
tissues and cells of the liver, acting upstream of the Nrf2-ARE
anti-oxidative pathway. Similarly, Huang et al (40) showed that the depletion of Sirt1
inhibited Nrf2-ARE pathway activation. It was concluded that Sirt1
regulation was a crucial promoter of the Nrf2-ARE pathway. However,
how Sirt1 regulates the activity of Nrf2 or subsequent gene
expression remains to be elucidated. Due to the response of Sirt1
to oxidative stress, the effect of Sirt1 on the Nrf2/ARE pathway
may be determined and requires further investigation to elucidate
key missing links. In conclusion, the present study showed that
D-pinitol attenuated the CsA-induced renal fibrotic processes by
mitigating oxidative stress through the Nrf2 and
Sirt1-PI3K/Akt/FoxO1 pathways. This suggested that D-pinitol offers
potential as a therapeutic agent for the progression of chronic
tubulointerstitial fibrosis.
Acknowledgments
The authors would like to thank Dr Jong Hee Chung
(Department of Statistics, The Graduate School of Ewha Womans
University, Seoul, Republic of Korea) for her statistical advice.
This study was supported by a grant (grant no.
NRF-2015R1C1A1A02037258) of the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Science, ICT and Future Planning, Republic of
Korea.
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Lin TH, Tan TW, Tsai TH, Chen CC, Hsieh
TF, Lee SS, Liu HH, Chen WC and Tang CH: D-pinitol inhibits
prostate cancer metastasis through inhibition of alphaVbeta3
integrin by modulating FAK, c-Src and NF-κB pathways. Int J Mol
Sci. 14:9790–9802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao Y, Zhang M, Wu T, Xu M, Cai H and
Zhang Z: Effects of D-pinitol on insulin resistance through the
PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J
Agric Food Chem. 63:6019–6026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi MS, Lee MK, Jung UJ, Kim HJ, Do GM,
Park YB and Jeon SM: Metabolic response of soy pinitol on
lipid-lowering, antioxidant and hepatoprotective action in hamsters
fed-high fat and high cholesterol diet. Mol Nutr Food Res.
53:751–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sethi G, Ahn KS, Sung B and Aggarwal BB:
Pinitol targets nuclear factor-kappaB activation pathway leading to
inhibition of gene products associated with proliferation,
apoptosis, invasion, and angiogenesis. Mol Cancer Ther.
7:1604–1614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rengarajan T, Nandakumar N, Rajendran P,
Ganesh MK, Balasubramanian MP and Nishigaki I: D-pinitol mitigates
tumor growth by modulating interleukins and hormones and induces
apoptosis in rat breast carcinogenesis through inhibition of
NF-kappaB. J Physiol Biochem. 71:191–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dang NT, Mukai R, Yoshida K and Ashida H:
D-pinitol and myo-inositol stimulate translocation of glucose
transporter 4 in skeletal muscle of C57BL/6 mice. Biosci Biotechnol
Biochem. 74:1062–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernández-Mijares A, Bañuls C, Peris JE,
Monzó N, Jover A, Bellod L, Victor VM and Rocha M: A single acute
dose of pinitol from a naturally-occurring food ingredient
decreases hyperglycaemia and circulating insulin levels in healthy
subjects. Food Chem. 141:1267–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stull AJ, Wood KV, Thyfault JP and
Campbell WW: Effects of acute pinitol supplementation on plasma
pinitol concentration, whole body glucose tolerance, and activation
of the skeletal muscle insulin receptor in older humans. Horm Metab
Res. 41:381–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sivakumar S, Palsamy P and Subramanian SP:
Impact of D-pinitol on the attenuation of proinflammatory
cytokines, hyperglycemia-mediated oxidative stress and protection
of kidney tissue ultrastructure in streptozotocin-induced diabetic
rats. Chem Biol Interact. 188:237–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nankivell BJ, P'Ng CH, O'Connell PJ and
Chapman JR: Calcineurin inhibitor nephrotoxicity through the lens
of longitudinal histology: Comparison of cyclosporine and
tacrolimus eras. Transplantation. 100:1723–1731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Büscher AK, Beck BB, Melk A, Hoefele J,
Kranz B, Bamborschke D, Baig S, Lange-Sperandio B, Jungraithmayr T,
Weber LT, et al: Rapid response to cyclosporin A and favorable
renal outcome in nongenetic versus genetic steroid-resistant
nephrotic syndrome. Clin J Am Soc Nephrol. 11:245–253. 2016.
View Article : Google Scholar
|
|
12
|
Gooch JL, King C, Francis CE, Garcia PS
and Bai Y: Cyclosporine A alters expression of renal microRNAs: New
insights into calcineurin inhibitor nephrotoxicity. PLoS One.
12:e01752422017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hošková L, Málek I, Kopkan L and Kautzner
J: Pathophysiological mechanisms of calcineurin inhibitor-induced
nephrotoxicity and arterial hypertension. Physiol Res. 66:167–180.
2017.
|
|
14
|
Lim SW, Doh KC, Jin L, Jin J, Piao SG, Heo
SB, Chung BH and Yang CW: Ginseng treatment attenuates autophagic
cell death in chronic cyclosporine nephropathy. Nephrology
(Carlton). 19:490–499. 2014. View Article : Google Scholar
|
|
15
|
Ruiz S, Pergola PE, Zager RA and Vaziri
ND: Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang CW, Ahn HJ, Kim WY, Shin MJ, Kim SK,
Park JH, Kim YO, Kim YS, Kim J and Bang BK: Influence of the
renin-angiotensin system on epidermal growth factor expression in
normal and cyclosporine-treated rat kidney. Kidney Int. 60:847–857.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong YA, Lim JH, Kim MY, Kim EN, Koh ES,
Shin SJ, Choi BS, Park CW, Chang YS and Chung S: Delayed treatment
with oleanolic acid attenuates tubulointerstitial fibrosis in
chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J
Transl Med. 12:502014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reisman SA, Aleksunes LM and Klaassen CD:
Oleanolic acid activates Nrf2 and protects from acetaminophen
hepatotoxicity via Nrf2-dependent and Nrf2-independent processes.
Biochem Pharmacol. 77:1273–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung S, Kim S, Kim M, Koh ES, Yoon HE,
Kim HS, Park CW, Chang YS and Shin SJ: T-type calcium channel
blocker attenuates unilateral ureteral obstruction-induced renal
interstitial fibrosis by activating the Nrf2 antioxidant pathway.
Am J Transl Res. 8:4574–4585. 2016.PubMed/NCBI
|
|
20
|
Kitada M and Koya D: Role of sirtuins in
kidney disease. Curr Opin Nephrol Hypertens. 23:75–79. 2014.
View Article : Google Scholar
|
|
21
|
Huang K, Huang J, Xie X, Wang S, Chen C,
Shen X, Liu P and Huang H: Sirt1 resists advanced glycation end
products-induced expressions of fibronectin and TGF-β1 by
activating the Nrf2/ARE pathway in glomerular mesangial cells. Free
Radic Biol Med. 65:528–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parra Cid T, Conejo Garcia JR, Carballo
Alvarez F and de Arriba G: Antioxidant nutrients protect against
cyclosporine A nephrotoxicity. Toxicology. 189:99–111. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon HE, Ghee JY, Piao S, Song JH, Han DH,
Kim S, Ohashi N, Kobori H, Kuro-o M and Yang CW: Angiotensin II
blockade upregulates the expression of klotho, the anti-ageing
gene, in an experimental model of chronic cyclosporine nephropathy.
Nephrol Dial Transplant. 26:800–813. 2011. View Article : Google Scholar :
|
|
24
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–F671.
2010. View Article : Google Scholar
|
|
25
|
Chung S, Yoon HE, Kim SJ, Kim SJ, Koh ES,
Hong YA, Park CW, Chang YS and Shin SJ: Oleanolic acid attenuates
renal fibrosis in mice with unilateral ureteral obstruction via
facilitating nuclear translocation of Nrf2. Nutr Metab (Lond).
11:22014. View Article : Google Scholar
|
|
26
|
Kim S, Kim SJ, Yoon HE, Chung S, Choi BS,
Park CW and Shin SJ: Fimasartan, a novel angiotensin-receptor
blocker, protects against renal inflammation and fibrosis in mice
with unilateral ureteral obstruction: The possible role of nrf2.
Int J Med Sci. 12:891–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piao SG, Kang SH, Lim SW, Chung BH, Doh
KC, Heo SB, Jin L, Li C and Yang CW: Influence of N-acetylcysteine
on klotho expression and its signaling pathway in experimental
model of chronic cyclosporine nephropathy in mice. Transplantation.
96:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doh KC, Lim SW, Piao SG, Jin L, Heo SB,
Zheng YF, Bae SK, Hwang GH, Min KI, Chung BH and Yang CW: Ginseng
treatment attenuates chronic cyclosporine nephropathy via reducing
oxidative stress in an experimental mouse model. Am J Nephrol.
37:421–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gęgotek A and Skrzydlewska E: The role of
transcription factor Nrf2 in skin cells metabolism. Arch Dermatol
Res. 307:385–396. 2015. View Article : Google Scholar
|
|
30
|
Sivakumar S, Palsamy P and Subramanian SP:
Attenuation of oxidative stress and alteration of hepatic tissue
ultrastructure by D-pinitol in streptozotocin-induced diabetic
rats. Free Radic Res. 44:668–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MJ, Yoo KH, Kim JH, Seo YT, Ha BW, Kho
JH, Shin YG and Chung CH: Effect of pinitol on glucose metabolism
and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res
Clin Pract. 77(Suppl 1): S247–S251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carafa V, Rotili D, Forgione M, Cuomo F,
Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A and
Altucci L: Sirtuin functions and modulation: From chemistry to the
clinic. Clin Epigenetics. 8:612016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wakino S, Hasegawa K and Itoh H: Sirtuin
and metabolic kidney disease. Kidney Int. 88:691–698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Medema RH and Jäättelä M: Cytosolic FoxO1:
Alive and killing. Nat Cell Biol. 12:642–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Y, Yang J, Liao W, Liu X, Zhang H,
Wang S, Wang D, Feng J, Yu L and Zhu WG: Cytosolic FoxO1 is
essential for the induction of autophagy and tumour suppressor
activity. Nat Cell Biol. 12:665–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szydłowski M, Jabłońska E and Juszczyński
P: FOXO1 transcription factor: A critical effector of the PI3K-AKT
axis in B-cell development. Int Rev Immunol. 33:146–157. 2014.
View Article : Google Scholar
|
|
37
|
Matsuzaki H, Daitoku H, Hatta M, Aoyama H,
Yoshimochi K and Fukamizu A: Acetylation of Foxo1 alters its
DNA-binding ability and sensitivity to phosphorylation. Proc Natl
Acad Sci USA. 102:11278–11283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim DH, Park CH, Park D, Choi YJ, Park MH,
Chung KW, Kim SR, Lee JS and Chung HY: Ginsenoside Rc modulates
Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res.
37:813–820. 2014. View Article : Google Scholar
|
|
39
|
Kulkarni SR, Donepudi AC, Xu J, Wei W,
Cheng QC, Driscoll MV, Johnson DA, Johnson JA, Li X and Slitt AL:
Fasting induces nuclear factor E2-related factor 2 and ATP-binding
cassette transporters via protein kinase A and sirtuin-1 in mouse
and human. Antioxid Redox Signal. 20:15–30. 2014. View Article : Google Scholar :
|
|
40
|
Huang K, Chen C, Hao J, Huang J, Wang S,
Liu P and Huang H: Polydatin promotes Nrf2-ARE anti-oxidative
pathway through activating Sirt1 to resist AGEs-induced
upregulation of fibronetin and transforming growth factor-β1 in rat
glomerular messangial cells. Mol Cell Endocrinol. 399:178–189.
2015. View Article : Google Scholar
|