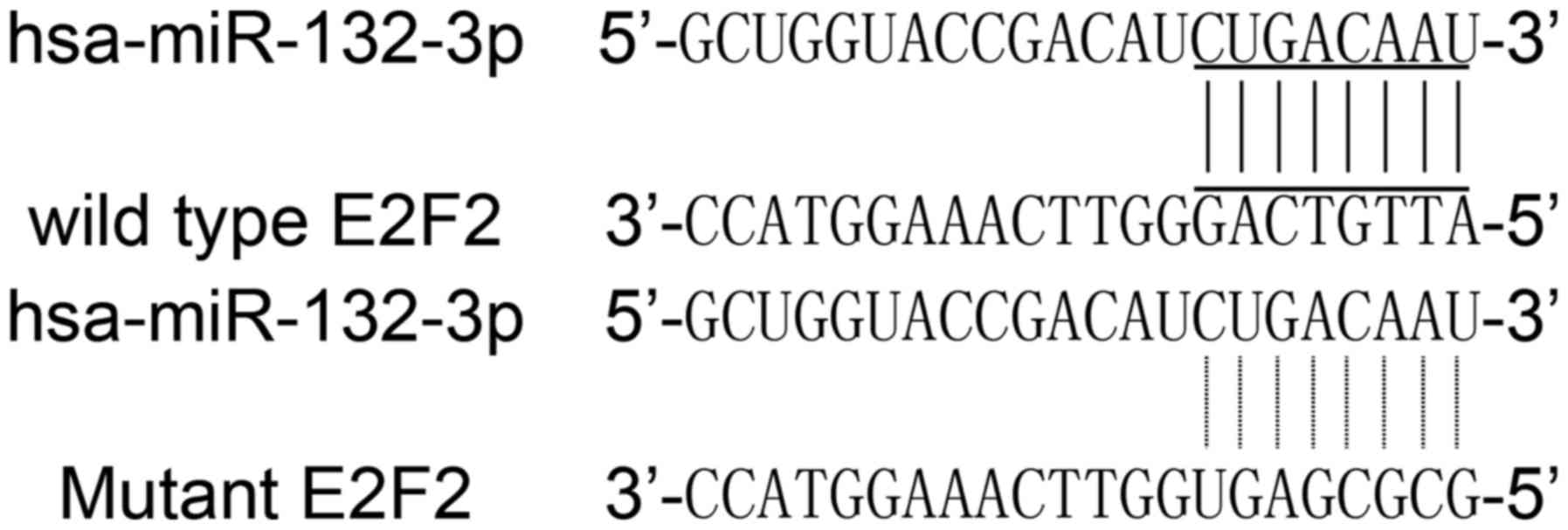Introduction
In the western world, prostate cancer is one of the
most common cancers following skin cancer, with estimated one in
nine men to have prostate cancer when they are at the age of 75,
and there are 20,000 cases being newly diagnosed in Australia each
year, as published by the Prostate Cancer Foundation of Australia
(information available at http://www.prostate.org.au, 2010). The well-proven
risk factors for prostate cancer include race, age, and family
history of prostate cancer (1).
Moreover, considerable data show that genetic basis is a risk
factor for prostate cancer (2,3).
Previously, a microarray-based, genome-wide,
comparative genomic hybridization analysis was performed to
investigate the profiles of chromosomal changes in primary HCC and
repeated amplification of the E2F5 gene harbored by the 8q21.2
locus in HCC was discovered (4).
Representing the E2F transcription factor family, E2F5 binds to the
promoters of the target genes associated with cell cycle control
and subsequently has regulatory role in the expression of the
target genes (5). Present
downstream of the cascades of the growth factor signaling, the E2F
family serves a crucial role in cell proliferation and growth by
regulation of the genes associated with cell cycle progression
(6). Hence, the members of the
E2F family are likely to be implicated in oncogenesis. There are
two subclassess of the members of the E2F family, including
repressor (E2F4-E2F8) and activator (E2F1-E2F3) (5). It has been reported that
overexpressed E2F activators trigger uncontrolled proliferated
cells in a variety of human cancers including gastrointestinal,
lung, ovarian and breast cancers (7–10).
A significant quantity of studies support the possibility that some
E2F repressors may act as oncogenes in tumorigenesis though the E2F
repressors are considered to serve as tumor inhibitors (5).
Our understanding of the correlation between gene
messenger RNAs (mRNAs) and human disease has changed and the
discovery of miRNAs at the turn of the 21st century marked that
cell biology had made a step into the new era, and since then has
extended to the sequences in the residual 90% of eukaryotic genomes
that produce non-coding RNAs. The microRNAs (miRNAs) play as
meta-controllers of gene expression and are pivotal for the
cellular alterations required for development (11). miRNA is an interesting and
potential target to improve specificity of diagnosis because the
expression of miRNA reveals the origin of the tumor and it has been
associated with initiation and progression of prostate cancer
(12–15). Currently, although there is no
differential miRNA signature that enables to distinct healthy from
disease patients, surprising findings have been derived for PCa. In
a study of Volinia et al, the expression profile of 228
miRNAs was analyzed in 7 normal tissues and 56 prostate tumor
tissues (16). Ambs et al
conducted a study in a cohort of 76 micro-dissected tissues
consisted of 16 controls and 60 tumor specimens and confirmed the
upregulation of miR-93, miR-196a, miR-25, miR-92, miR-32, miR-26a,
miR-181a, and let-7i (17). There
was significantly upregulated expression of miR-101, miR-195, and
miR-30c in patients who had extra-prostatic extension of cancer
cells, indicating a potential role in prediction the progression of
prostate cancer (18).
The data of miRNA microarray assay showed that
miR-132 is dysregulated in prostate cancer compared with the
control (19), indicating a role
of miR-132 in the tumorigenesis of prostate cancer. Prediction
algorithm was used to predict the target of miR-132, and based on
the physiopathological functions of the virtual target genes
obtained, we identified E2F5 in the follow-up study focusing on the
reported involvement of E2F5 in the pathogenesis of prostate cancer
(20). In this study, we
validated E2F5 as a target of miR-132 and verified the involvement
of miR-132 and E2F5 in the development of prostate cancer.
Materials and methods
Patients samples
Thirty-two prostate cancer patients who underwent
surgery at the Tianqiao Hospital in Jinan of Shandong (Jinan,
China) were recruited for this study between December 2013 and
September 2014. The tumor sections as well as adjacent
non-cancerous tissue were dissected. After resection, the specimens
were immediately frozen in liquid nitrogen and then stored at
−80°C. Participants or their first-degree relatives signed the
informed consents before start of the experiment after careful
explaination of all the potential risk factors. The Ethics
Committee of The Tianqiao Hospital in Jinan of Shandong (Jinan,
China) approved this study.
RNA isolation and real-time PCR
For analysis of the expression of E2F5 mRNA and
miR-132 from tissue samples and LNCaP cells. TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) was used to
extract the total RNA from LNCaP cells and tissue samples. And then
oligo(dT) primer and SuperScript III reverse transcriptase
(Invitrogen Life Technologies) were used to synthesize the cDNA.
Mx3000P qPCR (Stratagene, La Jolla, CA, USA) was used to perform
the quantitative reverse transcriptase polymerase chain reaction
(qRT-PCR) with a mixture including 1X SYBR-Green Tbr
polymerase (Finnzymes, Espoo, Finland), primers, 0.5X ROX. The
thermal cycling was carried out one cycle for 10 min at 95°C,
followed by 45 cycles of 10 sec at 95°C, 55°C for 30 sec and 72°C
for 30 sec. The 2−ΔΔCt method was used to analyze the
relative quantification. The internal controls, GAPDH and
RUN44, were used to control the expression of targets.
Cell culture and transfection
The LNCaP cells were prepared in RMPI-1640 medium
including 10% fetal bovine serum (FBS) (both from Gibco, Carlsbad,
CA, USA) at 37°C in a humidified atmosphere of 5%
CO2/95% air. On transfection day, cells were cultured to
80%, Lipofectamine 2000 (Invitrogen Life Technologies) was used for
luciferase assay, transfection assay, apoptosis assay and
proliferation assay in accordance with the manufacturer's
instructions.
Cell proliferation assay
The cell proliferation reagent WST-1 (Roche
Diagnostics, Indianapolis, IN, USA) was used to determine the
growth of LNCaP cells based on the manufacturer's instructions. The
optical density of the LNCaP cells was determined according to the
absorption at 450 nm at different time-points using a microplate
spectrophotometer (Tecan Group, Ltd., Männedorf, Switzerland). All
experiments were repeated three times.
Luciferase assay
We search a public database (TargetScan, www.targetscan.org) for the target gene of miR-132 and
E2F5 was found to contain the putative miR-132 binding site.
PsiCHECK-2 reporter vector (Promega, Madison, WI, USA) was used to
generate psiCHECK-2-E2F5-3′UTR by amplifying and inserting E2F5
3′UTR into the SpeI and HindIII sites of the empty
vector and mutagenesis was performed for the same site, and
introduced to the control vector (both from Ambion, Cambridgeshire,
UK) at the same time. Lipofectamine 2000 (Invitrogen Life
Technologies) was used in all the transfection assays in accordance
with the manufacturer's instructions. Each test was repeated at
least three times. Renilla luciferase activity was used as
the internal control. Dual-Luciferase Reporter assay system
(Promega, Massachusetts, MA, USA) was used to measure the
activities of firefly and Renilla luciferase 48 h
post-transfection. Three independent experiments were
performed.
Western blot analysis
In order to detect the expression of E2F5 mRNA and
miR-132, we collected the transfected cells, lysis buffer
(BioSharp, Hefei, China) with 0.1% Triton X-100, 10 mmol/l sodium
pyrophosphate, 2 mmol/l EDTA, 50 mmol/l NaF and 150 mmol/l NaCl, to
lyse the LNCaP cells. The cellular lysates were centrifuged at
15,000 rpm for 15 min. Approximately 35 µg of protein was loaded
using boiling water for 5 min with loading buffer.
SDS-polyacrylamide gel (SDS-PAGE) (10%) was used to separate the
target protein, and then the protein was transfer-blotted onto
polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA,
USA). In order to avoid unspecific binding, the PVDF membranes were
treated in the dark with 5% non-fat dried milk in TBST (BioSharp)
(150 mmol/l NaCl, 0.1% Tween-20 and 20 mmol/l Tris-HCl, pH 7.5).
Then washed the membranes twice using TBST (BioSharp), next,
treated with anti-β-actin antibodies (1:1,000; Sigma-Aldrich, St.
Louis, MO, USA) and anti-E2F5 (1:1,000; Abcam, Cambridge, UK) for
12 h at 4°C in accordance with the manufacturer's instructions. The
membranes were washed twice again using TBST (BioSharp), diluted
HRP-conjugated anti-rabbit IgG (1:3,000; Abcam) to incubate the
PVDF membrane at room temperature for 1 h. The enhanced
chemiluminescence system (Amersham Pharmacia Biotech, Braunschweig,
Germany) was applied to detect the blots. The internal control was
the expression of β-actin. Three independent tests were
performed.
Analysis of apoptosis
The Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (C1062; Beyotime Institute of
Biotechnology, Beijing, China) with Annexin V-FITC was used to
stain the LNCaP cells according to the manufacturer's instructions.
The flow cytometry (Cell Lab Quanta SC; Beckman Coulter Inc.,
Miami, FL, USA) with 585/42 nm PI and 530/30 nm FITC emission
filters was used to detect the apoptosis of LNCaP cells. All tests
were performed in triplicate.
Statistical analysis
SPSS statistical software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. The Mann-Whitney U test was
used to determine the statistical significance for continuous
variables and the χ2 test or Fisher's exact test for
categorical variables. P-values <0.05 were considered
statistically significant. The data are shown as the mean ±
standard deviation (SD).
Results
miR-132 is upregulated in cancerous
tissue of prostate cancer patients
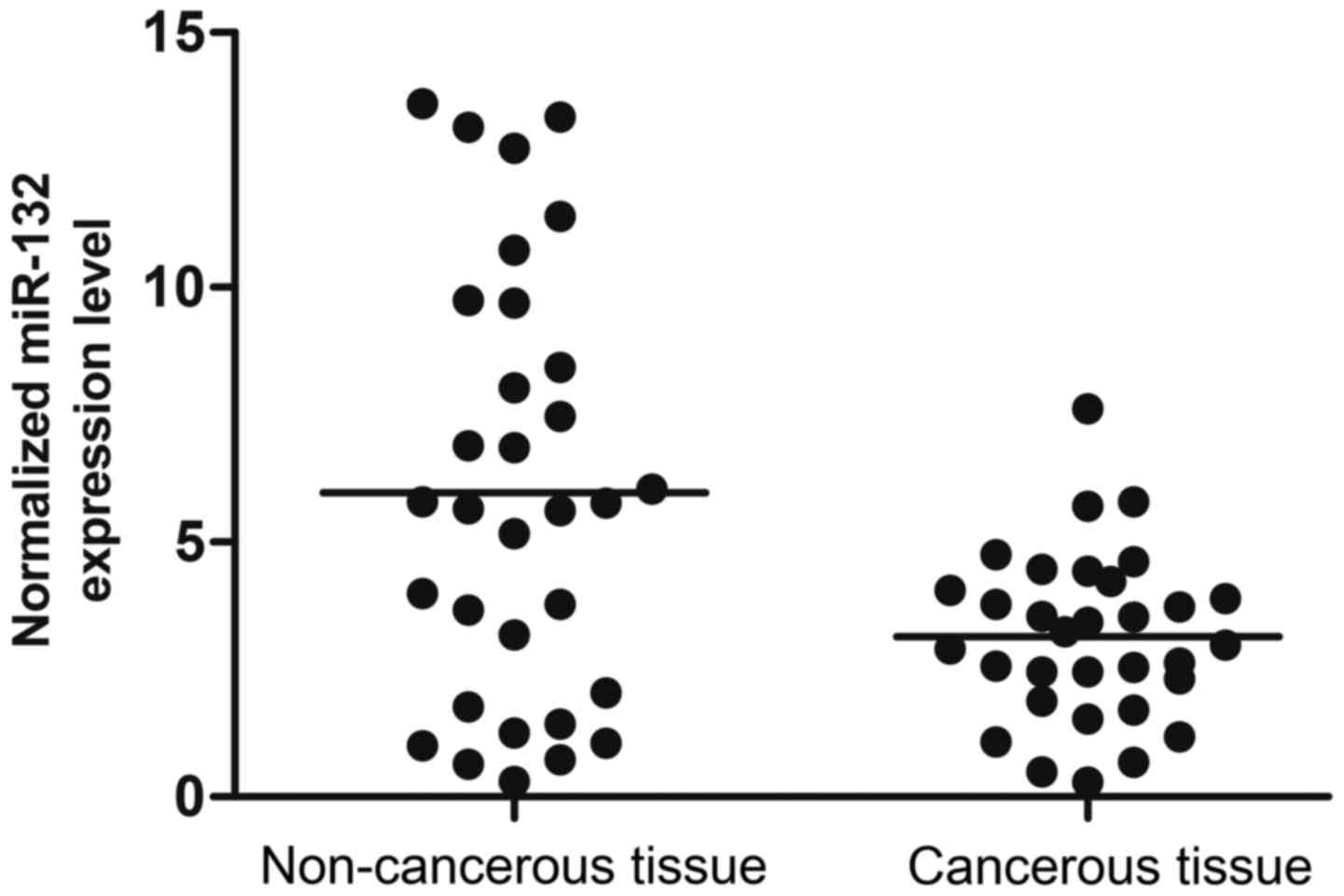
We collected tumor tissue and adjacent non-cancerous
tissue of prostate cancer patients (n=32). Using real-time PCR, we
found that the expression level of miR-132 was higher in cancerous
tissue compared with the control (Fig. 1). The results indicated that
miR-132 is negatively related to the tumorigenesis of prostate
cancer.
E2F5 was a direct target of miR-132
We first searched candidate target genes of miR-132
using publicly available databases, and consequently identified
E2F5 as the candidate target gene with the potential binding site
in the 3′UTR (Fig. 2). Thus, E2F5
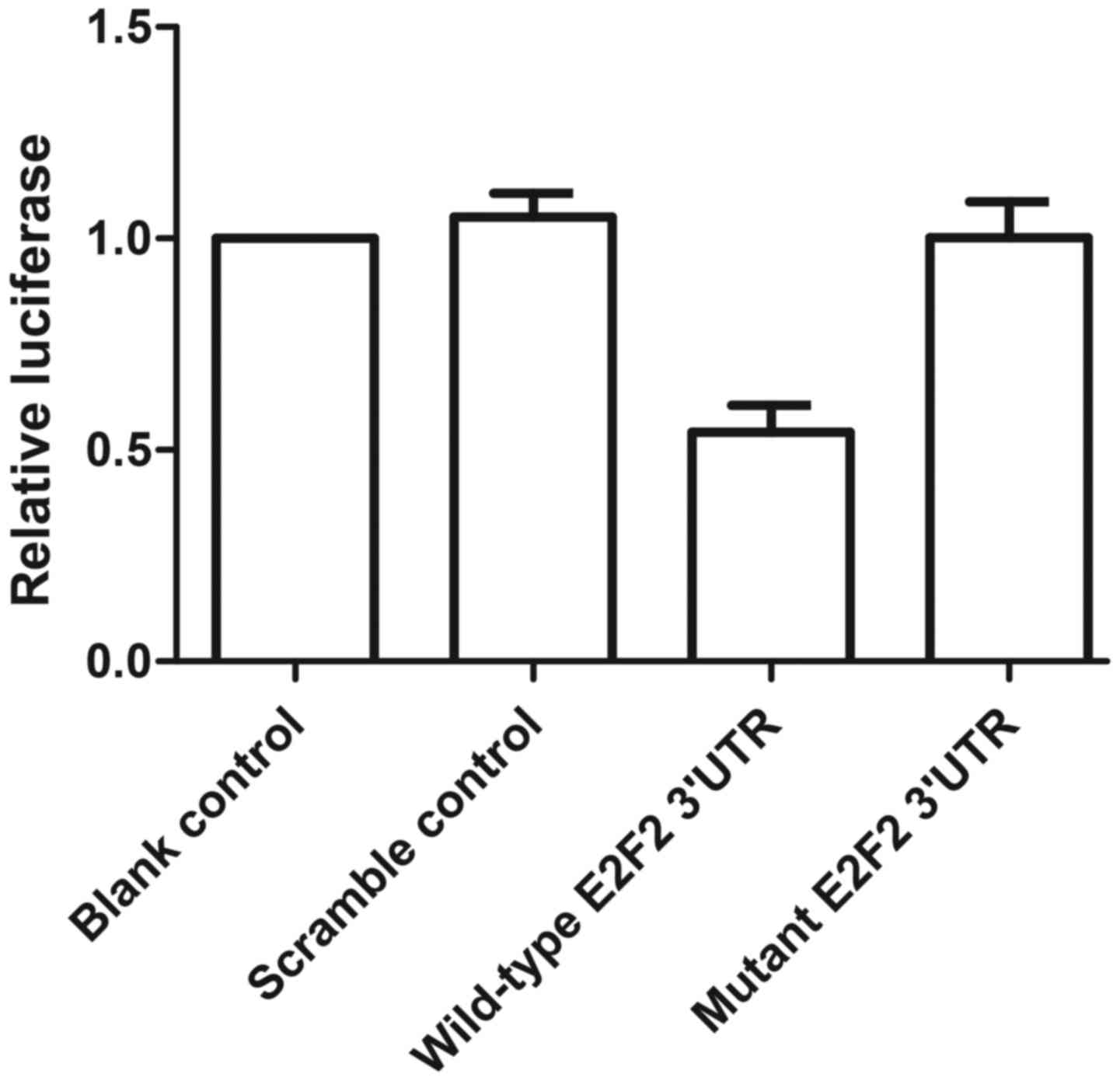
was selected for further study. We conducted luciferase activity
reporter assay in prostate cancer cells, and found that the
fluorescence from the cells co-transfected with miR-132 and
wild-type E2F5 3′UTR decreased significantly (Fig. 2), while cells co-transfected with
miR-132 and mutant E2F5 3′UTR were comparable compared with
scramble control (Fig. 3). The
results confirmed that E2F5 was an immediate target of miR-132 in
the cells.
Interaction between miR-132 and E2F5
To further clarify the role of miR-132 and E2F5 in
tumorigenesis of prostate cancer, we collected 32 pairs of tissue
samples (cancerous tissue, n=32; non-cancerous tissue, n=32). We
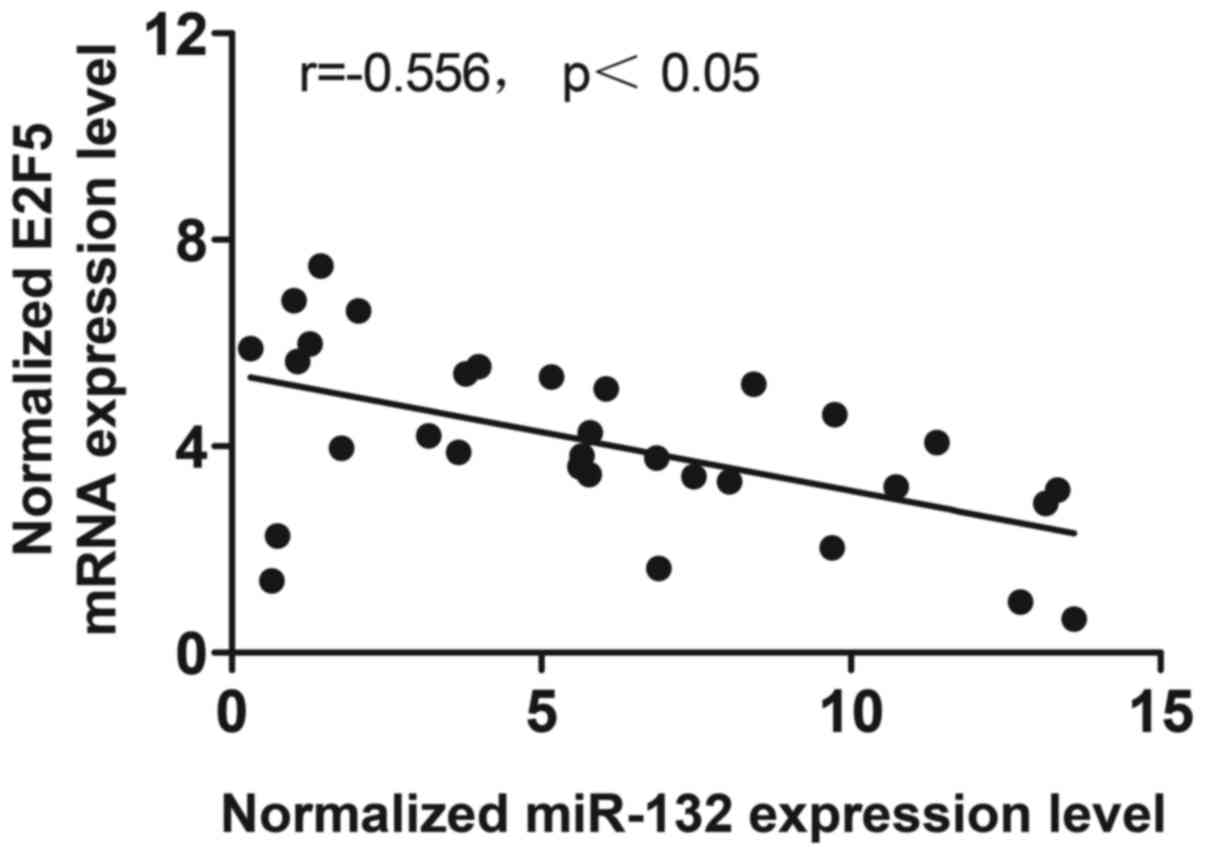
first analyzed the correlation between the expression level of
miR-132 and E2F5 mRNA among the tissues (n=32), which showed
negative regulatory relationship (Fig. 4; r=−0.556, p<0.05). We then
performed real-time PCR, and found the expression of E2F5 mRNA
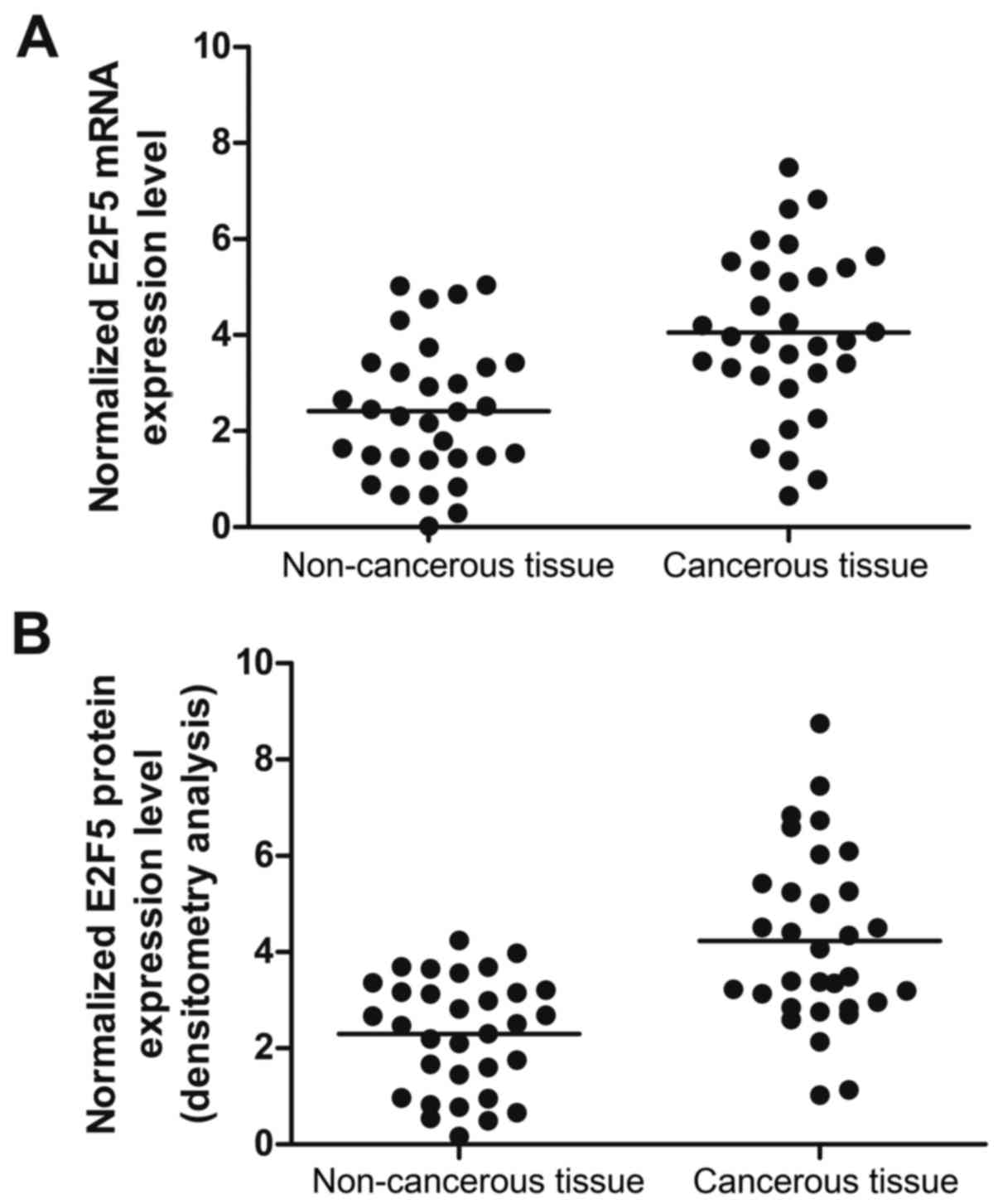
(Fig. 5A) was increased in
cancerous tissue compared with the control; the expression of E2F5
protein (Fig. 5B) was determined
by densitometry analysis and it increased in cancerous tissue
compared with chemotherapy sensitivity. These results suggested the
presence of a negative regulator relationship between miR-132 and
E2F5. To further verify the negative regulatory relationship
between miR-132 and E2F5, we transfected the cells with scramble
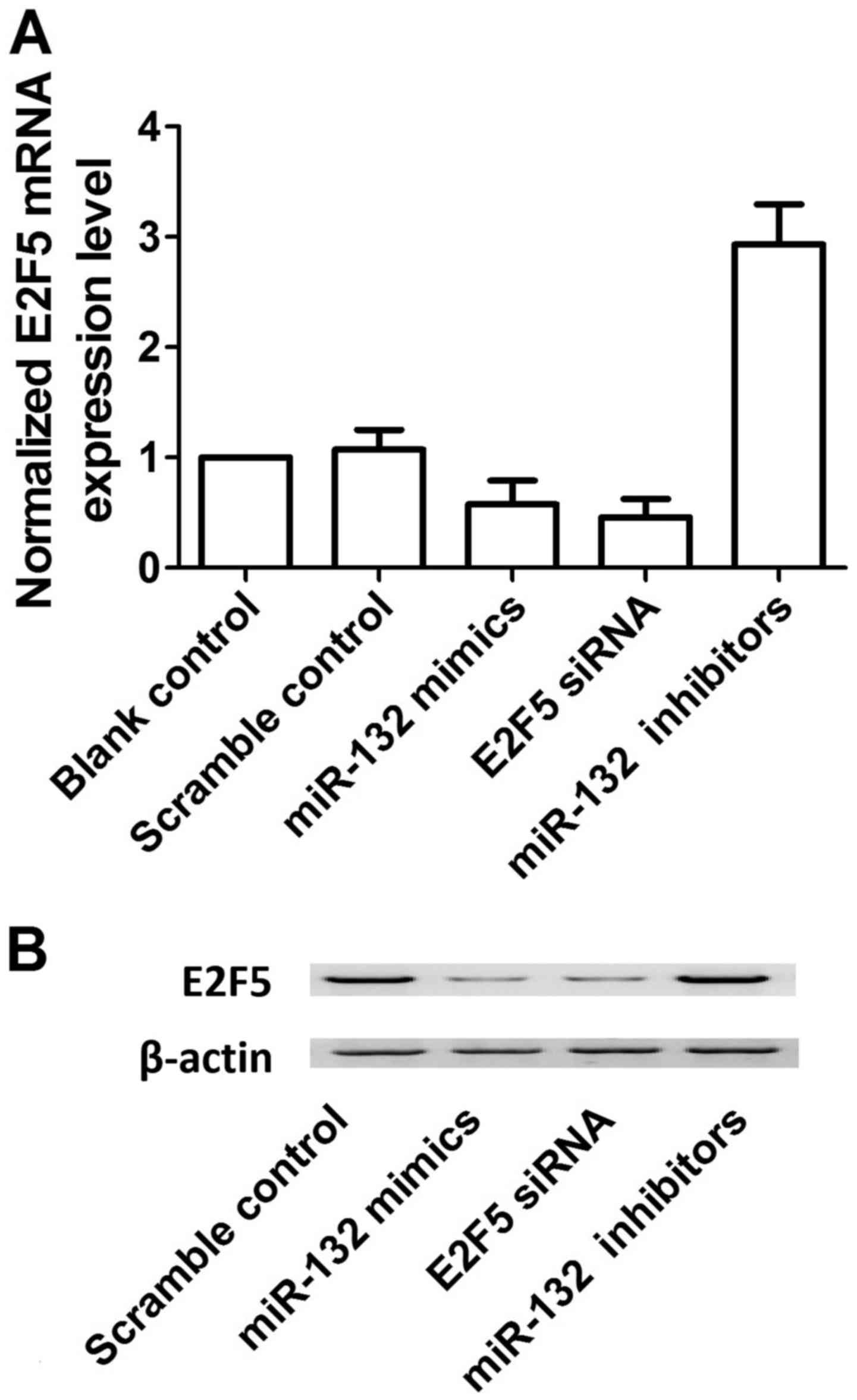
control, miR-132 mimics, E2F5 siRNA and miR-132 inhibitors. As
shown in Fig. 6, overexpression
of miR-132 decreased the protein (upper panel) and mRNA (lower
panel) expression level of E2F5 compared with the scramble control,
while down-regulation of miR-132 increased the protein (upper
panel) and mRNA (lower panel) expression level of E2F5, verifying
the negative regulatory relationship between miR-132 and E2F5.
miR-132 and E2F5 interfere with the
viability of prostate cancer cells
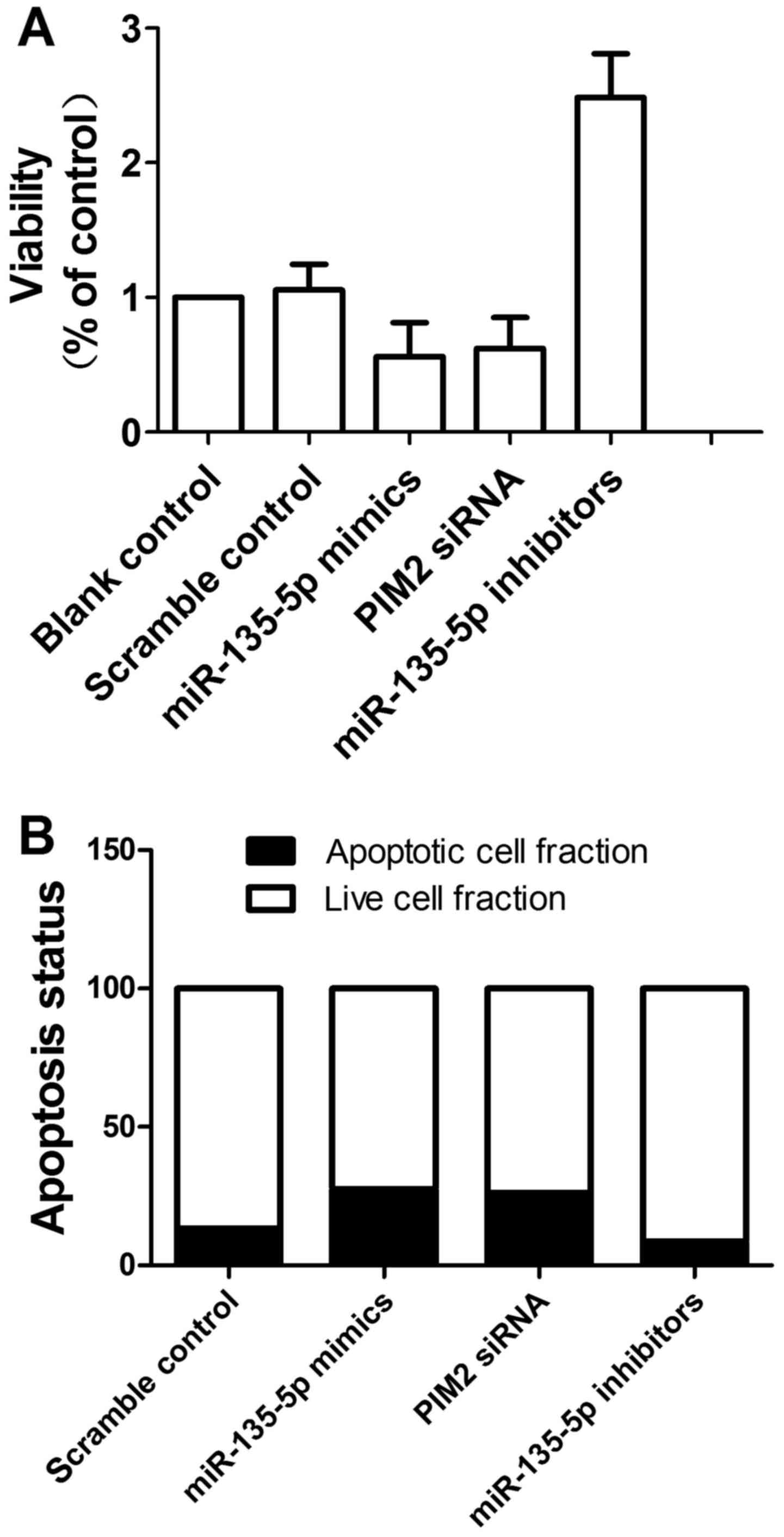
We also investigated the relative viability of cells
when transfected with scramble control, miR-132 mimics, E2F5 siRNA
and miR-132 inhibitors. As shown in Fig. 7A, downregulation of miR-132
attenuated viability of cells when compared with the scramble
controls, while upregulation of miR-132 and downregulation of E2F5
enhanced viability, indicating miR-132 positively interfered with
the viability of cells, while E2F5 negatively interfered with the
viability of cells.
miR-132 and E2F5 interfere with apoptosis
in cells
We then investigated the relative apoptosis of cells
when transfected with scramble control, miR-132 mimics, E2F5 siRNA
and miR-132 inhibitors. When transfected with miR-132 mimics and
E2F5 siRNA, the number of survival cells were more and the number
of apoptotic cells were less than the scramble controls, while
cells transfected with miR-132 inhibitors showed comparably less
survival cells and more apoptotic cells. The results indicated
miR-132 inhibited apoptosis and E2F5 accelerated apoptosis.
Discussion
miR-132 is present in the intron of a non-coding
gene on chromosome 17 in humans and originates from the miR-212/132
cluster (21). miR-132 is
associated with angiotensin II (Ang II)-mediated dysfunction of the
vascular smooth muscle, as demonstrated previously (22). In the tumorigenesis, the
downregulated level of miR-132 prevents proliferation, migration,
metastasis and invasion in breast cancer via affecting HN1
(23). Moreover, colorectal
cancer metastasis and invasion are inhibited by miR-132 by
affecting ZEB2 (24).
Nevertheless, the mechanism by which miR-132 function as a
regulator in tumor metastasis remains to be further investigated.
In this study, we collected tumor tissue and adjacent non-cancerous
tissue of prostate cancer patients (n=32). Using real-time PCR, we
found that the expression level of miR-132 was higher in cancerous
tissue compared with the control (Fig. 1). In addition, we also performed
real-time PCR, and found the expression of E2F5 mRNA (Fig. 5A) increased in cancerous tissue
compared with the control; the expression of E2F5 protein (Fig. 5B) was determined by densitometry
analysis and it increased in cancerous tissue compared with
chemotherapy sensitive, indicating that the presence of a negative
regulatory relationship between miR-132 and E2F5.
A conserved putative miR-132 target site in E2F5's
3′UTR was discovered by searching the online miRNA database.
Subsequently, we conducted luciferase activity reporter assay in
prostate cancer cells, and found that the fluorescence from the
cells cotransfected with miR-132 and wild-type E2F5 3′UTR decreased
significantly (Fig. 2), while
cells cotransfected with miR-132 and mutant E2F5 3′UTR were
comparable compared with scramble control (Fig. 3). We next analyzed the correlation
between the expression level of miR-132 and E2F5 mRNA among the
tissues (n=32), which showed negative regulatory relationship
(Fig. 4; r=−0.556,
p<0.05).
Representing the E2F transcription factor family,
E2F5 binds to the promoters of the target genes associated with
cell cycle control and subsequently has regulatory role in the
expression of the target genes (5). Present downstream of the cascades of
the growth factor signaling, the E2F family serves a crucial role
in cell proliferation and growth by regulation of the genes
associated with cell cycle progression (6). Hence, the members of the E2F family
are likely to be implicated in oncogenesis. There are two
subclassess of the members of the E2F family, including repressor
(E2F4-E2F8) and activator (E2F1-E2F3). It has been reported that
overexpressed E2F activators trigger uncontrolled proliferation of
cells in a variety of human cancers including gastrointestinal,
lung, ovarian and breast cancers (7–10).
A significant quantity of studies support the possibility that some
E2F repressors may act as oncogenes in tumorigenesis though the E2F
repressors are considered to serve as tumor inhibitors and although
the E2F repressors are expected to behave as tumor suppressors
(5). In this study, we
investigated the relative viability of cells when transfected with
scramble control, miR-132 mimics, E2F5 siRNA and miR-132
inhibitors. As shown in Fig. 7A,
down-regulation of miR-132 attenuated viability of cells when
compared with the scramble controls, while upregulation of miR-132
and downregulation of E2F5 enhanced viability, indicating miR-132
positively interfered with the viability of cells, while E2F5
negatively interfered with the viability of cells.
We then investigated the relative apoptosis of cells
when transfected with scramble control, miR-132 mimics, E2F5 siRNA
and miR-132 inhibitors. When transfected with miR-132 mimics and
E2F5 siRNA, the number of surviving cells are more and the number
of apoptotic cells are less than the scramble controls, while cells
transfected with miR-132 inhibitors showed comparably less
surviving cells and more apoptotic cells. The results indicated
miR-132 inhibited apoptosis and E2F5 accelerated apoptosis.
In conclusion, these findings indicate that E2F5 is
a potential new target of miR-132 which is critical regulator in
the development of prostate cancer, and reveals that E2F5 is a
direct target gene of miR-132 although it is previously
uncharacterized.
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Crawford ED: Epidemiology of prostate
cancer. Urology. 62(Suppl 1): 3–12. 2003. View Article : Google Scholar
|
|
2
|
Coughlin SS and Hall IJ: A review of
genetic polymorphisms and prostate cancer risk. Ann Epidemiol.
12:182–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witte JS: Prostate cancer genomics:
towards a new under-standing. Nat Rev Genet. 10:77–82. 2009.
View Article : Google Scholar
|
|
4
|
Kim TM, Yim SH, Shin SH, Xu HD, Jung YC,
Park CK, Choi JY, Park WS, Kwon MS, Fiegler H, et al: Clinical
implication of recurrent copy number alterations in hepatocellular
carcinoma and putative oncogenes in recurrent gains on 1q. Int J
Cancer. 123:2808–2815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren B, Cam H, Takahashi Y, Volkert T,
Terragni J, Young RA and Dynlacht BD: E2F integrates cell cycle
progression with DNA repair, replication, and G(2)/M checkpoints.
Genes Dev. 16:245–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim
YD and Kim HY: E2F1 expression is related with the poor survival of
lymph node-positive breast cancer patients treated with
fluorouracil, doxorubicin and cyclophosphamide. Breast Cancer Res
Treat. 82:11–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reimer D, Sadr S, Wiedemair A, Stadlmann
S, Concin N, Hofstetter G, Müller-Holzner E, Marth C and Zeimet AG:
Clinical relevance of E2F family members in ovarian cancer - an
evaluation in a training set of 77 patients. Clin Cancer Res.
13:144–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eymin B, Gazzeri S, Brambilla C and
Brambilla E: Distinct pattern of E2F1 expression in human lung
tumours: E2F1 is upregulated in small cell lung carcinoma.
Oncogene. 20:1678–1687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee J, Park CK, Park JO, Lim T, Park YS,
Lim HY, Lee I, Sohn TS, Noh JH, Heo JS, et al: Impact of E2F-1
expression on clinical outcome of gastric adenocarcinoma patients
with adjuvant chemoradiation therapy. Clin Cancer Res. 14:82–88.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saugstad JA: MicroRNAs as effectors of
brain function with roles in ischemia and injury, neuroprotection,
and neurodegeneration. J Cereb Blood Flow Metab. 30:1564–1576.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coppola V, De Maria R and Bonci D:
MicroRNAs and prostate cancer. Endocr Relat Cancer. 17:F1–F17.
2010. View Article : Google Scholar
|
|
14
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar
|
|
15
|
Casanova-Salas I, Rubio-Briones J,
Fernández-Serra A and López-Guerrero JA: miRNAs as biomarkers in
prostate cancer. Clin Transl Oncol. 14:803–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theodore SC, Davis M, Zhao F, Wang H, Chen
D, Rhim J, Dean-Colomb W, Turner T, Ji W, Zeng G, et al: MicroRNA
profiling of novel African American and Caucasian prostate cancer
cell lines reveals a reciprocal regulatory relationship of miR-152
and DNA methyltranferase 1. Oncotarget. 5:3512–3525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Wu XY, Ling XH, Lin ZY, Fu X, Deng
YH, He HC and Zhong W: Analysis of genetic aberrations on
chromosomal region 8q21-24 identifies E2F5 as an oncogene with copy
number gain in prostate cancer. Med Oncol. 30:4652013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agarwal C, Dhanalakshmi S, Singh RP and
Agarwal R: Inositol hexaphosphate inhibits growth and induces G1
arrest and apoptotic death of androgen-dependent human prostate
carcinoma LNCaP cells. Neoplasia. 6:646–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lau P, Bossers K, Janky R, Salta E,
Frigerio CS, Barbash S, Rothman R, Sierksma AS, Thathiah A,
Greenberg D, et al: Alteration of the microRNA network during the
progression of Alzheimer's disease. EMBO Mol Med. 5:1613–1634.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin W, Reddy MA, Chen Z, Putta S, Lanting
L, Kato M, Park JT, Chandra M, Wang C, Tangirala RK, et al: Small
RNA sequencing reveals microRNAs that modulate angiotensin II
effects in vascular smooth muscle cells. J Biol Chem.
287:15672–15683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang ZG, Chen WX, Wu YH, Liang HF and
Zhang BX: miR-132 prohibits proliferation, invasion, migration, and
metastasis in breast cancer by targeting HN1. Biochem Biophys Res
Commun. 454:109–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng YB, Luo HP, Shi Q, Hao ZN, Ding Y,
Wang QS, Li SB, Xiao GC and Tong SL: miR-132 inhibits colorectal
cancer invasion and metastasis via directly targeting ZEB2. World J
Gastroenterol. 20:6515–6522. 2014. View Article : Google Scholar : PubMed/NCBI
|