Introduction
Ischemic stroke is a devastating neurological
disorder and is one of the leading causes of death worldwide. It
entails heavy financial burden for patients and their families, and
society. Treatment is limited to interventions such as
thrombolysis, anticoagulation and antiplatelet therapies.
Neuroprotective treatments have been reported to restore
neurological function (1). Stem
cell transplantation is a promising strategy to restore
neurological function after stroke. Dental pulp stem cells (DPSCs)
trigger tissue regeneration and differentiation into cells such as
osteoblasts, odontoblast-like cells, adipocytes, and neurons.
Gronthos and his colleagues first isolated DPSCs from human dental
pulp in 2000 (2). DPSCs represent
adult multipotent cells originating in the cranial neural crest.
They secrete neurotrophic factors. Previous studies suggest that
human DPSCs potentially differentiate into functional neural
progenitors or neurons in vitro, which integrate into rat
brains in vivo, playing a therapeutic role in brain and
spinal cord injury, and neurodegenerative diseases (3–7).
However, a major challenge relates to the limited
viability and neuronal differentiation in vivo.
Brain-derived neurotrophic factor (BDNF) is a member of the
neurotrophin growth factor family that includes nerve growth factor
(NGF), neurotrophin-3 (NT-3), and neurotrophins-4/5 (NT-4/5)
(8). BDNF acts via high-affinity
receptor tyrosine kinase B (TrkB), expressed in the central nervous
system (9). As reported
previously, BDNF exerts trophic effects on cortical, hippocampal
and cerebellar neurons in vitro. BDNF protects motor
neurons, hippocampal, and substantia nigra dopaminergic neurons
against brain injury. It plays a vital role in neuronal growth,
differentiation, and migration (10). Ischemic brain injury triggers BDNF
expression, as part of neuroprotective response (11). Intraventricular injection of BDNF
before focal cerebral ischemia (12), intracerebral infusion of BDNF
following stroke (13), and
transplantation of BDNF-treated mesenchymal or neural stem cells
alleviated tissue damage in the brain and improved behavioral
recovery (14–16).
However, studies investigating the fate of
transplanted DPSCs in the injured brain and their neuroprotective
function when combined with BDNF are limited. We propose that DPSCs
and BDNF are neuroprotective in experimental mouse models of
cerebral ischemia, and restore neurological function after stroke.
In this study, we determined the fate of transplanted DPSCs in the
rat brain. Additionally, we investigated whether intravenous
administration of rat-derived DPSCs enhanced neurological outcomes
after ischemic stroke. We demonstrated that BDNF combined with
DPSCs increased the viability of transplanted cells, facilitated
the differentiation and neuroprotective effects compared with DPSCs
or BDNF alone.
Materials and methods
Experimental design
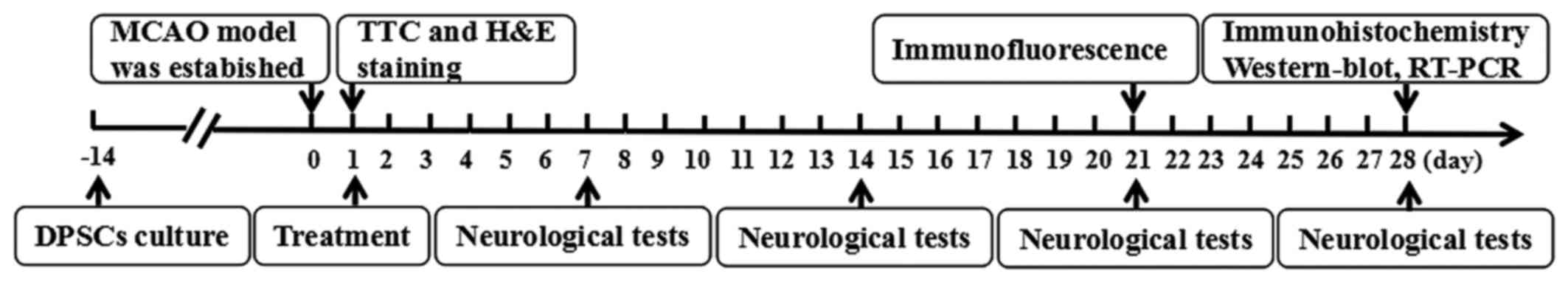
We randomized the rats into four groups immediately
after middle cerebral artery occlusion (MCAO) (Fig. 1). Rats were anesthetized via
intraperitoneal injection with 10% chloral hydrate (0.35 ml/100 g)
24 h after stroke. The control group was treated with 0.3 ml saline
as vehicle during the subacute phase. Animals in the BDNF group
were treated with 0.3 ml BDNF (50 µg/ml) injection via the
tail vein. Rats in the DPSCs group were injected with 0.3 ml DPSCs
suspension (1×107/ml) via the tail vein very slowly.
Rats in the DPSCs+BDNF group were injected with 0.3 ml DPSCs
(1×107/ml) and BDNF (50 µg/ml) via the tail
vein.
Isolation, identification, in vitro
fluorescent labeling
DPSCs were obtained from the incisors of 4-week-old
male Sprague-Dawley (SD) rats under sterile conditions as
previously described (17). In
brief, dental pulp was treated with 3 mg/ml collagenase type I at
37°C for 1 h with gentle shaking, followed by centrifugation (1,500
rpm for 5 min). The supernatant and enzymes were discarded, and the
residue was cultured in α-modified Eagle's medium (α-MEM)
containing penicillin (1%, 100 U/ml), streptomycin (1%, 100
µg/ml) and fetal bovine serum (FBS 10%; Gibco, Grand Island,
NY, USA) at 37°C and 5% CO2. The medium was replaced
every 3 days. Cells at 80% confluency were passaged. DPSCs were
identified by flow cytometry based on the surface markers on stem
cells. In brief, ~1×106 cells were incubated with 2% FBS
in phosphate-buffered saline (PBS) at 4°C for 30 min and 1
µl of rat monoclonal antibody specific for Sca-1, CD34, CD44
(18) and CD45 (19). Cells in the control experiment
were cultured in buffer solution without primary antibodies. DPSCs
were pre-labeled with green fluorescent dye PKH67 up to 90%. The
final density of the labeled cell suspension was ~1×107
cells/ml.
Induced differentiation
To test the pluripotency of the isolated DPSCs,
passage 3 cells were seeded in 6-well culture plates up to 20,000
cells/cm2. The proliferation medium was replaced by the
inductive medium. For mineralized nodules, the
differentiation-inducing medium was supplemented with ascorbic
acid, dexamethasone, and an excess of phosphate. Osteogenesis was
promoted by supplementing 50 g/ml ascorbic acid 2-phosphate, 10 nm
dexamethasone and 10 µM β-glycerolphosphate. Adipogenesis
was induced with 100 nm dexamethasone and 50 µg/ml
indometacin. The culture was maintained for 3 weeks, during which
the medium was changed every 2–3 days. Finally, the cells were
stained with Alizarin Red and Oil Red O, and visualized under light
microscopy.
Focal cerebral ischemia
The animal model of focal cerebral ischemia was
generated by transient occlusion of the right middle cerebral
artery (MCA) (20). Adult male SD
rats weighing 250±10 g each, were purchased from the Animal Center
of the Second Affiliated Hospital of Harbin Medical University. All
the procedures were approved by the Ethics Committee of the Harbin
Medical University, and were compliant with the NIH guidelines for
use and care of laboratory animals. In brief, rats were
anesthetized with 10% chloral hydrate (0.35 ml/100 g). A midline
cervical incision was used to dissect the right common carotid
artery (CCA), external carotid artery (ECA) and internal carotid
artery (ICA). A 4-0 monofilament nylon thread with a
silicone-coated tip (diameter 0.37 mm, length 3–4 mm) was extended
from the ECA into the ICA to mask the origin of the right MCA. The
nylon thread was secured and the incision was closed. The rat body
temperature was maintained at 37±1°C using an incandescent lamp.
Two hours later, the thread was withdrawn to perform reperfusion.
Sham-operated animals were subjected to similar procedures without
occlusion of the right MCA. MCAO was confirmed by suspending the
rats by the tail for contralateral forelimb flexion. Further,
circling behavior on the floor was detected. The weight of rats
after ischemia was recorded at 0, 1, 7, 14 and 28 days
post-MCAO.
2,3,5-Triphenyltetrazolium chloride (TTC)
staining
TTC staining was used to verify ischemic injury at
24 h post-MCAO. The brain wa rapidly removed on ice under
anesthesia. Fresh brains were frozen for 25 min at −20°C, sliced
into five consecutive coronal sections, incubated in 2% TTC
(Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 20 min in the dark.
Healthy zones appeared red and infarcted tissues were white in
color. Brain sections were photographed using a digital camera. The
infarct volume was determined as a percentage of dead cerebral
tissue.
Histological examination
The brain tissues from rats were embedded in
paraffin. Serial coronal sections were produced and mounted on
slides. The specimens were stained with hematoxylin and eosin
(H&E) according to standardized protocols, and visualized by
light microscopy.
Adhesive removal test and modified
neurological severity scores (mNSS)
Two small pieces of adhesive-backed paper dots,
measuring 113.1 mm2 in size, were used as bilateral
tactile stimuli to the distal-radial region of the wrist in each
forelimb. Rats were pre-trained for a week before MCA occlusion.
They were monitored on days 1, 7, 14, 21 and 28 after stroke. The
mean duration of the three trials for the removal of adhesive
papers was recorded (21). The
mNSS encompass a series of motor, sensory, balance and reflex tests
graded on a scale of 0–18 (normal 0; maximal deficit score 18).
Scores reflect the degree of inability to perform a specific test:
higher scores suggest severe neurological deficit (22).
Immunofluorescence
The rats were anesthetized with 10% chloral hydrate
0.35 ml/100 g and their chests were split to expose the hearts. The
animals were rapidly perfused with 400 ml saline, followed by 300
ml 4% paraformaldehyde (pH 7.4) through the left ventricle. The
brain tissues of the rats were post-fixed in 4% paraformaldehyde
(pH 7.4) at 4°C for 24 h. Brains were dehydrated in a graded series
of sucrose at 20 and 30% daily. Frozen sections of the brain were
blocked with normal goat serum for 20 min at room temperature, and
incubated with anti-nestin (1:100), anti-doublecortin (DCX)
(1:100), and anti-neuronal specific filament (NF-H) (1:200)
overnight at 4°C. They were washed with PBS three times (5 min each
time) followed by 1 h reaction with rhodamine-conjugated goat
anti-rabbit and anti-mouse IgGs. Brain sections were
counter-stained with DAPI for 10 min at 37°C. The survival and
spatial distribution of PKH67-labeled DPSCs were observed using a
laser scanning confocal microscope. Five randomly-selected fields
were used to count the average number of positive cells in the
regions. The antibodies are listed in Table I.
 | Table IThe antibodies. |
Table I
The antibodies.
| Antibody | Conjugation | Manufacturer | Dilution
(FACS) | Dilution (IF) | Dilution (IHC) | Dilution (WB) |
|---|
| Sca-1 | FITC | Biolegend | 1:100 | | | |
| CD34 | APC | Biolegend | 1:100 | | | |
| CD44 | PE | Biolegend | 1:100 | | | |
| CD45 | Percp | Biolegend | 1:100 | | | |
| Nestin | | Abcam | | 1:100 | 1:200 | 1:1,000 |
| DCX | | Abcam | | 1:100 | 1:200 | 1:500 |
| NF-H | | Abcam | | 1:200 | 1:200 | 1:1,000 |
| Goat
anti-rabbit | Rhodamine | Zhongshan | | 1:100 | | |
| Goat
anti-mouse | Rhodamine | Zhongshan | | 1:100 | | |
| Goat
anti-rabbit | HRP | Zhongshan | | | 1:400 | |
| Goat
anti-mouse | HRP | Zhongshan | | | 1:400 | |
| Goat
anti-rabbit | HRP | Zhongshan | | | | 1:5,000 |
| Goat
anti-mouse | HRP | Zhongshan | | | | 1:5,000 |
Immunohistochemistry
Paraffin sections were washed with xylene and
ascending alcohol, to air-dry and hydration, and incubated in 1%
H2O2 for 10 min. Antigen retrieval was
performed by heating the sections at 95°C for 10 min. Paraffin
sections were incubated with anti-nestin (1:200), anti-DCX (1:200),
anti-NF-H (1:200) at 4°C overnight and biotinylated secondary
antibodies for 20 min at room temperature. The sections were
incubated with avidin-biotin complex (ABC) (1:400) for another 20
min, followed by washing with PBS three times, 5 min each time. The
sections were counterstained with hematoxylin, followed by
dehydration, and analyzed under light microscope. The antibodies
are listed in Table I.
Western blot analysis
Animals were euthanized 4 weeks after MCAO using an
overdose of anesthesia. Infarcted cerebral tissues were lysed with
RIPA buffer containing protease/phosphatase inhibitors and
phenylmethanesulfonyl fluoride (PMSF) on ice. After centrifugation
at 15,000 rpm for 15 min, protein concentration was measured using
a bicinchoninic acid (BCA) protein assay kit. Protein samples (30
µg each) were electrophoresed and transferred to
polyvinylidene difluoride (PVDF) membrane, which was blocked with
5% nonfat dry milk in PBS containing 0.2% Tween-20 1.5 h at room
temperature. After gentle shaking, and washing four times (10 min
each) followed by incubation with primary antibodies: anti-nestin
(1:1,000), anti-DCX (1:500), anti-NF-H (1:1,000) and anti-β-actin
overnight at 4°C, the membrane was incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:5,000) at room
temperature for 1.5 h. An ECL advance western blotting detection
kit was used to detect chemiluminescence in the dark. The
antibodies are listed in Table
I.
RT-PCR
Total RNA was extracted from the right cerebral
cortex with TRIzol (Invitrogen, Carlsbad, CA, USA). The cDNA
synthesis was performed using the reverse transcription system with
oligo(dT)20 according to the manufacturer's
instructions. A control reaction without the reverse transcriptase
was also carried out. The amplifications were conducted on a
real-time PCR system (Bio-Rad, Hercules, CA, USA) using
Accupower® 2X SYBR-Green I qPCR Master Mix (Bioneer,
Daejeon, Korea) under the following conditions: pre-denaturation at
95°C for 15 min, followed by 40 cycles of 95°C for 10 sec, 53°C for
30 sec, and cooling at 4°C for 5 min. The melting temperatures (Tm)
of the amplification products immediately after the last reaction
cycle were used to determine the specificity. The 2−ΔΔCT
method was used to compare the gene expression of neural markers in
each group. All the PCRs were performed in triplicate and validated
by the presence of a single peak in the melting curve. The primers
are shown in Table II.
 | Table IIPrimers used in quantitative
RT-PCR. |
Table II
Primers used in quantitative
RT-PCR.
| Primers | Sequences | Product size | Manufacturer |
| Nestin | Forward:
5′-CTGGGCAAGTGGAACGTAGA-3′ | | |
| Reverse:
5′-CCTCCCACCGCTGTTGAT-3′ | 150 bp | Invitrogen |
| DCX | Forward:
5′-CCTCAGGGAGTGCGCTACAT-3′ | | |
| Reverse:
5′-CGACCAGTTGGGATTGACATT-3′ | 150 bp | Invitrogen |
| NF-H | Forward:
5′-GATGGCCCTGGATATTGAGA-3′ | | |
| Reverse:
5′-TTCGCTTTTGACTTTTATGTGAG-3′ | 148 bp | Invitrogen |
| β-actin | Forward:
5′-TGTCACCAACTGGGACGATA-3′ | | |
| Reverse:
5′-GGGGTGTTGAAGGTCTCAAA-3′ | 165 bp | Sangon Biotech |
Statistical analysis
All data are expressed as mean ± standard error of
the mean (SEM). Means were compared using one-way ANOVA with
Bonferroni's multiple comparison tests. P<0.05 was considered
statistically significant. GraphPad Prism version 5 was used for
all the statistical analyses.
Results
Assessment of stroke
The transient MCAO is an established animal model of
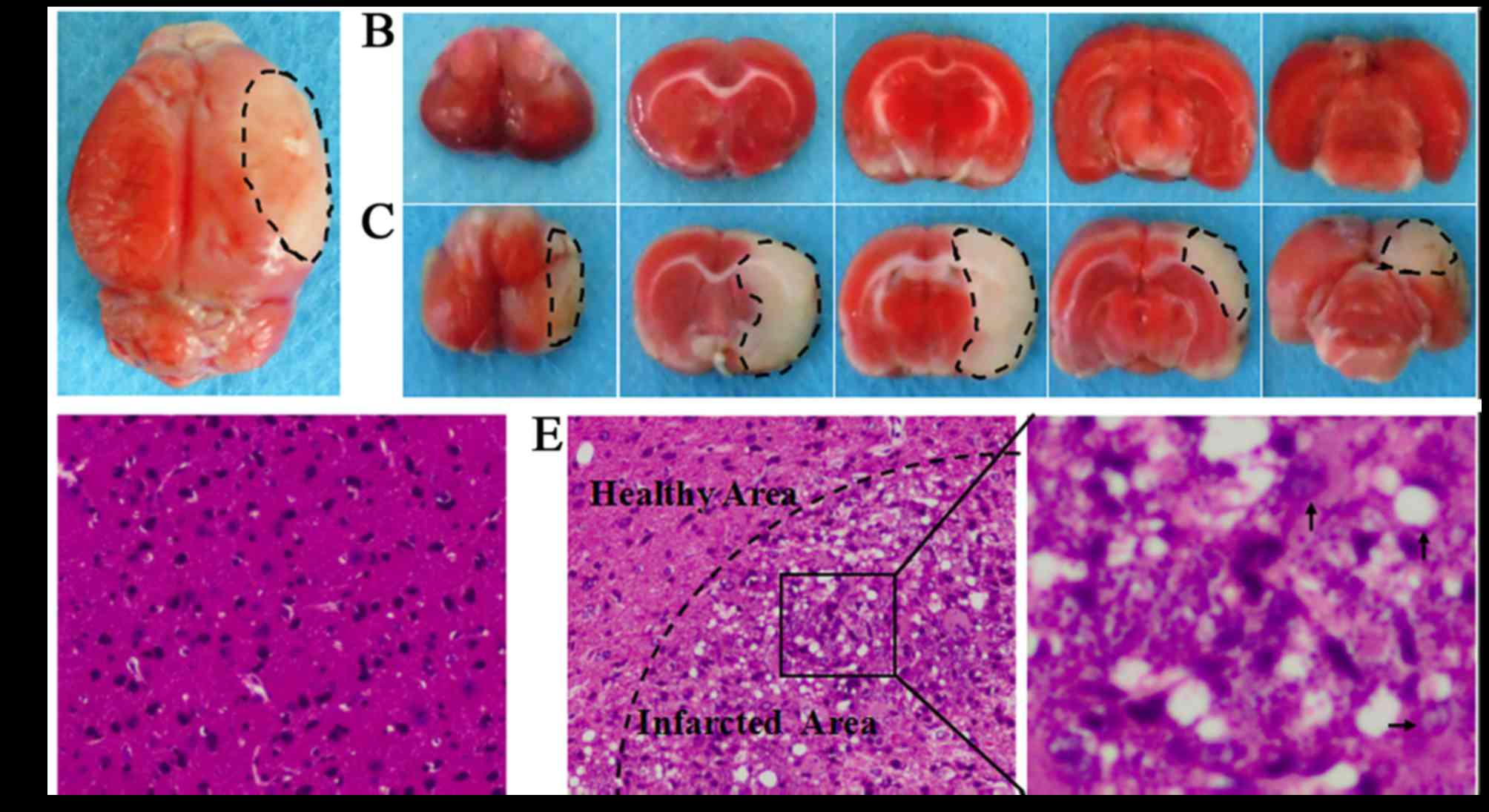
stroke. In the present study, the infarct zone ratio for each brain
was calculated as follows: (left hemisphere area-right uninfarcted
area)/(left hemisphere area*2), TTC staining showed that
the ischemic areas accounted for an average 32.1±1.8% of the total
volume at 24 h (Fig. 2).
MCAO-induced injury mainly involves frontal, parietal, temporal,
occipital and striatum regions of the right cerebral hemisphere. No
visible lesions occurred in the sham-operated animals. H&E
staining revealed normal brain tissues in the sham-operated group,
with uniform neuronal distribution. Neurons were round or oval,
with pink cytoplasm, abundant and with clear nucleus. No
inflammatory cells were detected. In the MCAO group, infarcted
brain tissues were found, with a limited number of disordered
neurons containing pale cytoplasm, eosinophils and abundance of
vacuoles. In addition, a large infiltration of necrotic neurons and
inflammatory cells was observed. Widespread ischemic damage in the
brain resulted in significant neurological deficit. The 18-point
mNSS and the results of the adhesive removal tests increased
significantly.
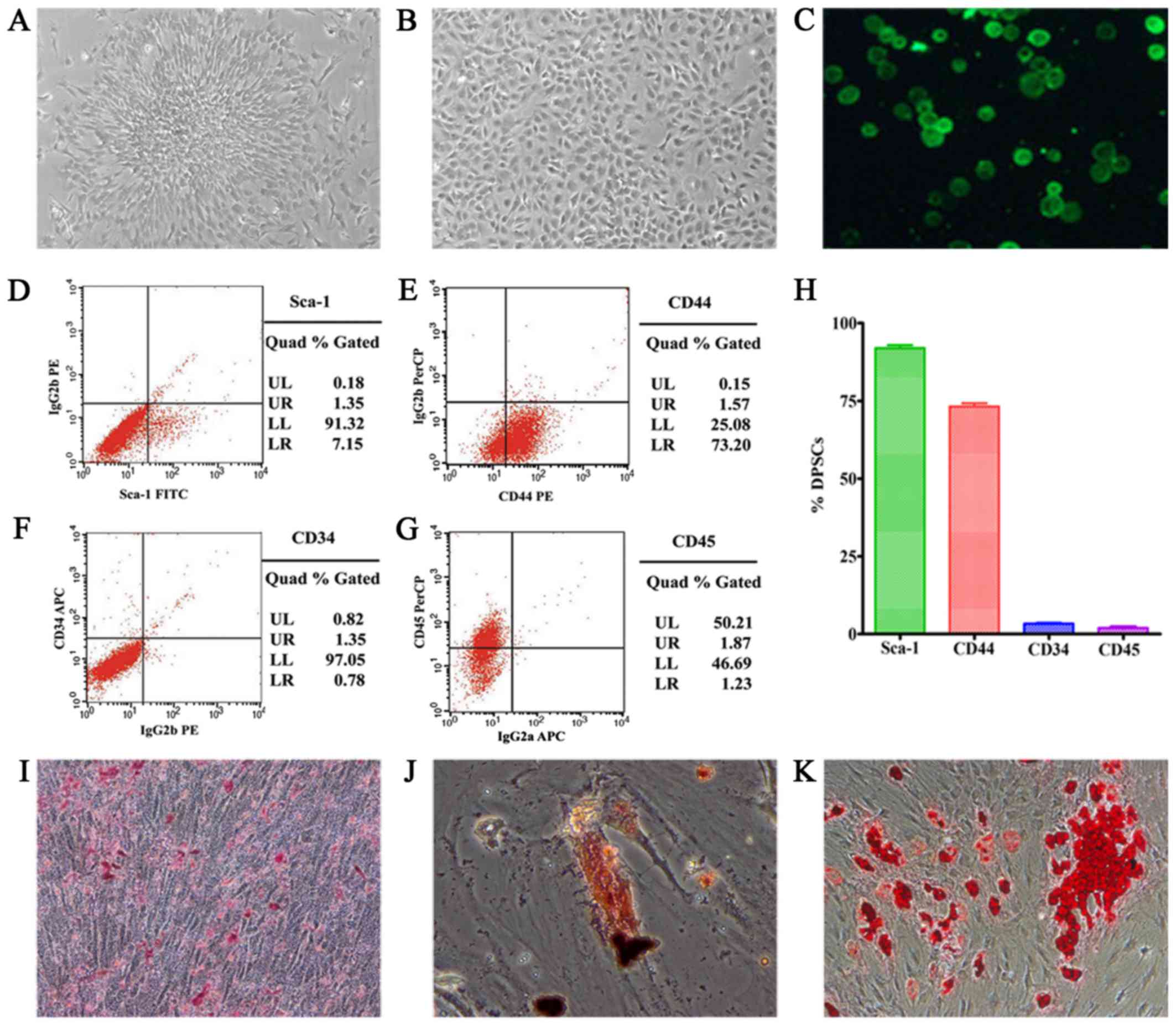
Characteristics of DPSCs
Cultured DPSCs exhibited plastic adherence, colony
formation, fibroblast-like morphology, self-renewing capacity,
expression of surface markers and multilineage differentiation
(Fig. 3). Fluorescence-activated
cell sorting (FACS) analyses were used to evaluate the expression
of various surface markers on the isolated cells. In conclusion,
91.32% of the passage-3 cells expressed stem cell marker Sca-1; and
73.20% expressed CD44. In contrast, few cells expressed
hematopoietic stem cell markers, 1.35% expressed CD34; 1.23%
expressed CD45. In addition, randomly distributed mineralized
nodules were detected in the culture dish. Osteogenesis and
adipogenesis were characterized by the presence of calcium deposits
and fat droplets. In conclusion, the cells derived from SD rat
dental pulp appeared to be largely DPSCs.
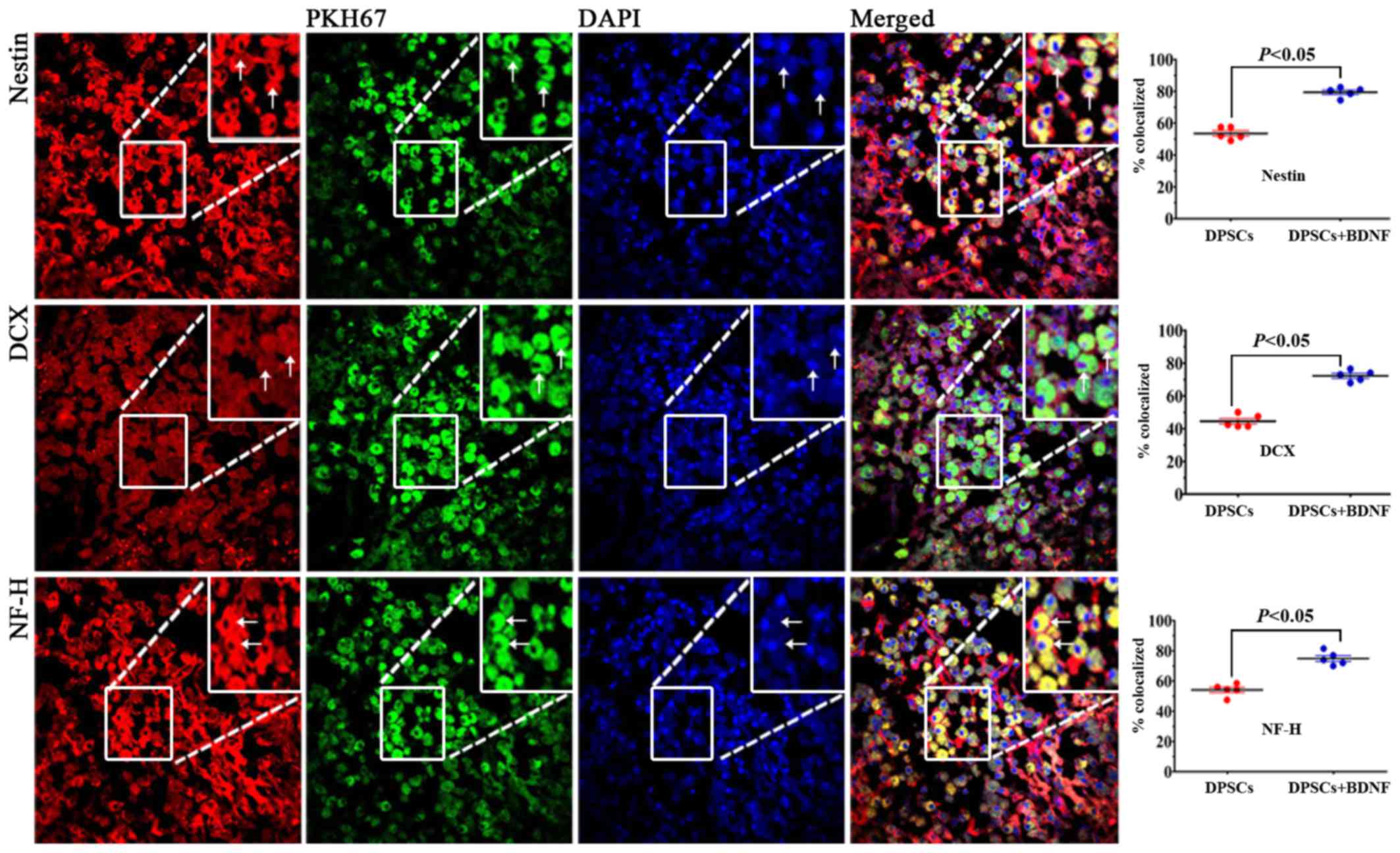
Migration and distribution of
transplanted DPSCs
In the present study, we observed PKH67 fluorescent
signal 3 weeks after DPSCs transplantation (Fig. 4). PKH67-labeled cells were
abundant in the DPSC group around the periphery of the infarcted
area, but not in the contralateral nonischemic brain (data not
shown) under laser scanning confocal microscope. Several
PKH67-labeled cells were found in the DPSCs+BDNF treatment group
than in the DPSC group, as BDNF prolonged the viability of
transplanted DPSCs. No PKH67-labeled cells were found in the brains
of control rats. These results suggest that DPSCs penetrated
through the blood-brain barrier (BBB) (23), proliferated, and migrated toward
the ischemic areas of stroke. Homing ability is the characteristic
of stem cells. Grafted cells can migrate and localize in injured
areas of the brain tissues. PKH67 represents an ideal cell-labeling
agent to track transplanted cells in vivo.
Expression of neural-specific
markers
PKH67-labeled cells emitted green fluorescence at
488 nm. Rhodamine-conjugated antibody emitted red fluorescence at
594 nm, and DAPI displayed blue fluorescence at 405 nm. We found
that most of the PKH67-labeled DPSCs accumulated in the peripheral
ischemic regions of MCAO models (Fig.
4). They displayed neuronal morphology and were identified with
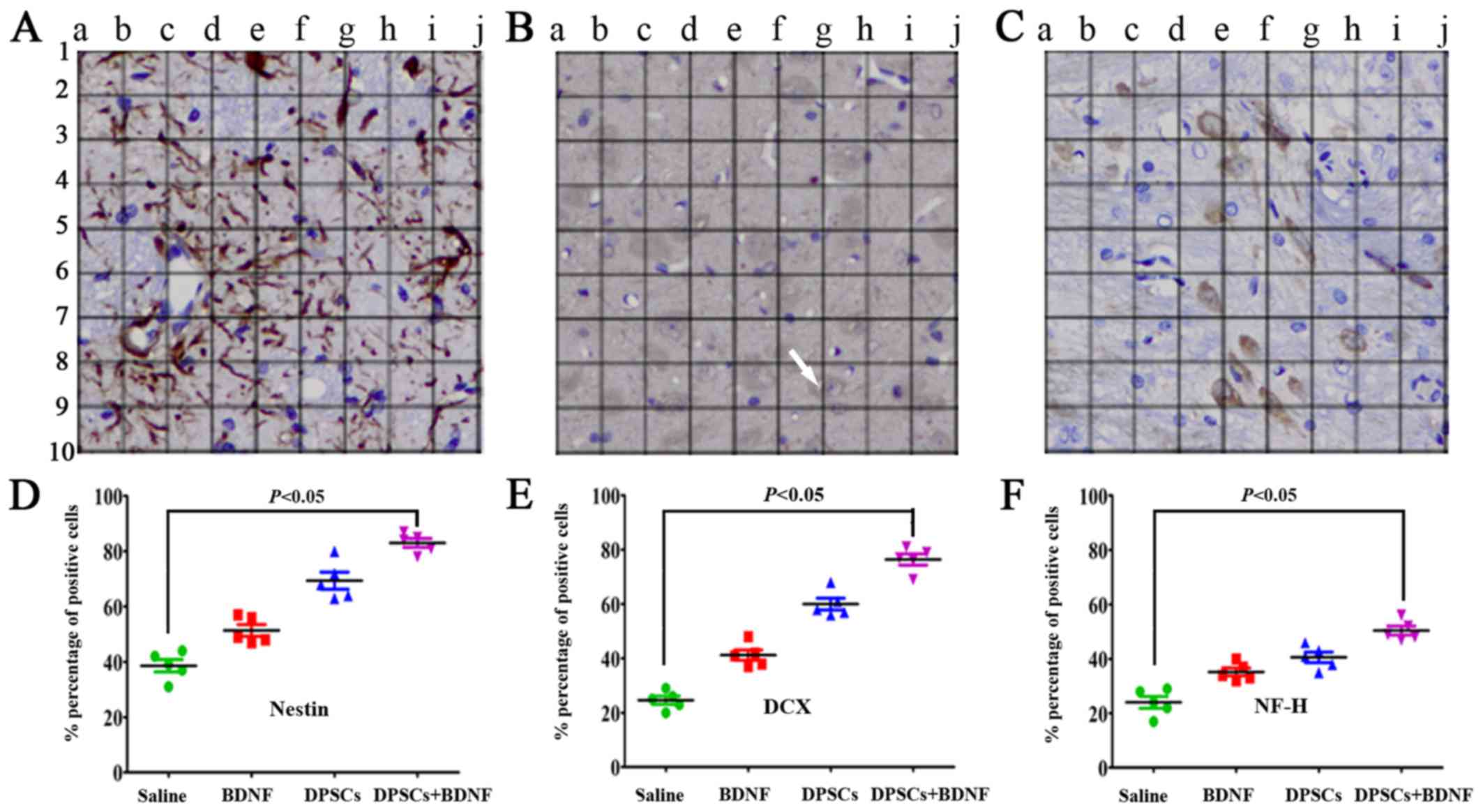
a panel of neural-specific markers including nestin (24), DCX (25), NF-H and DAPI. The arrow in
Fig. 5B shows that DCX was
localized mainly in the cytoplasma. We systematically analyzed and
compared the expression of markers in each group.
Immunofluorescence revealed increased number of positive cells in
DPSCs+BDNF group compared with DPSCs group. The percentage of
positive cells was significantly higher in the different treatment
groups than in the saline control group (P<0.05). The number of
positive cells in the DPSCs+BDNF group was significantly higher
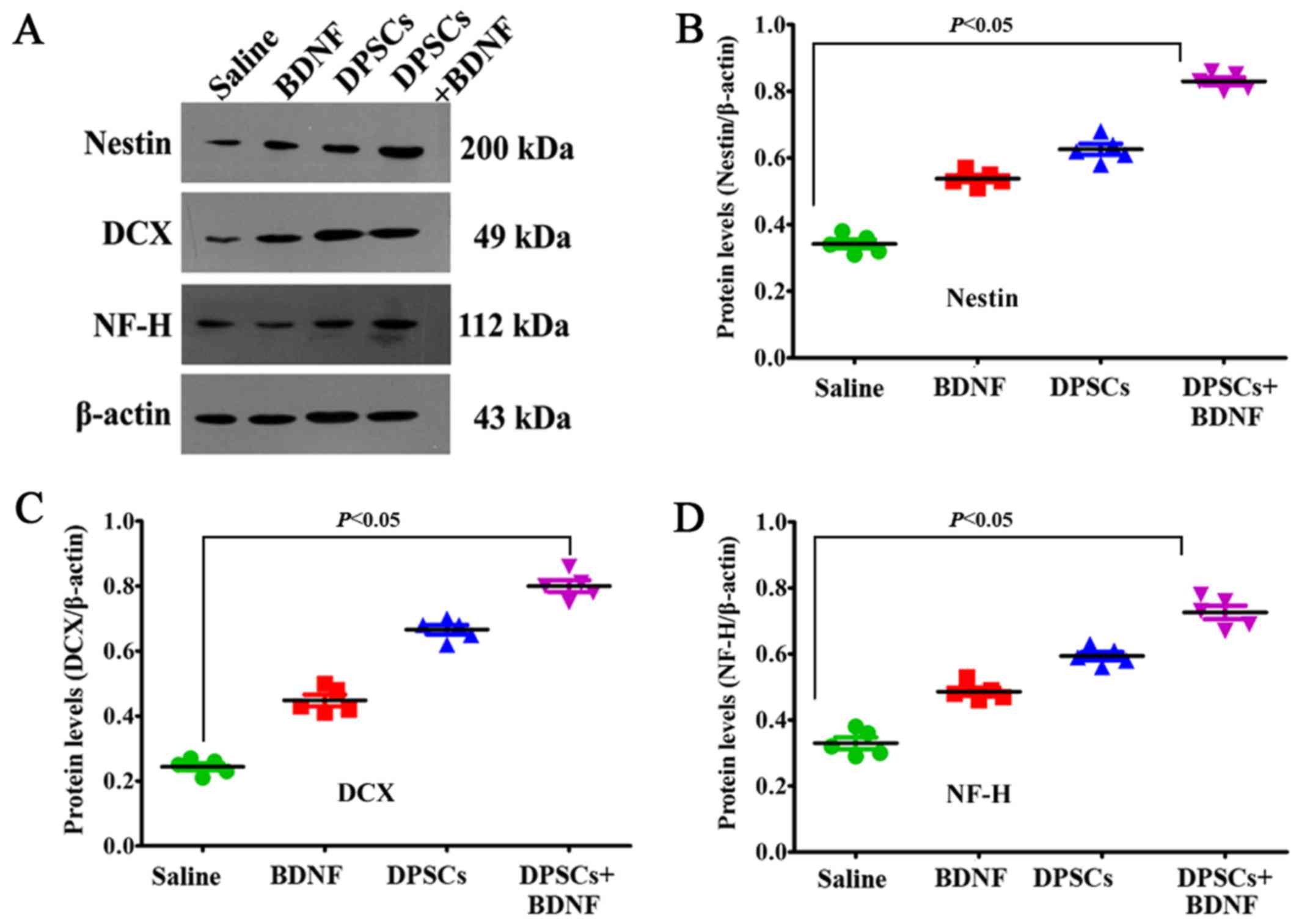
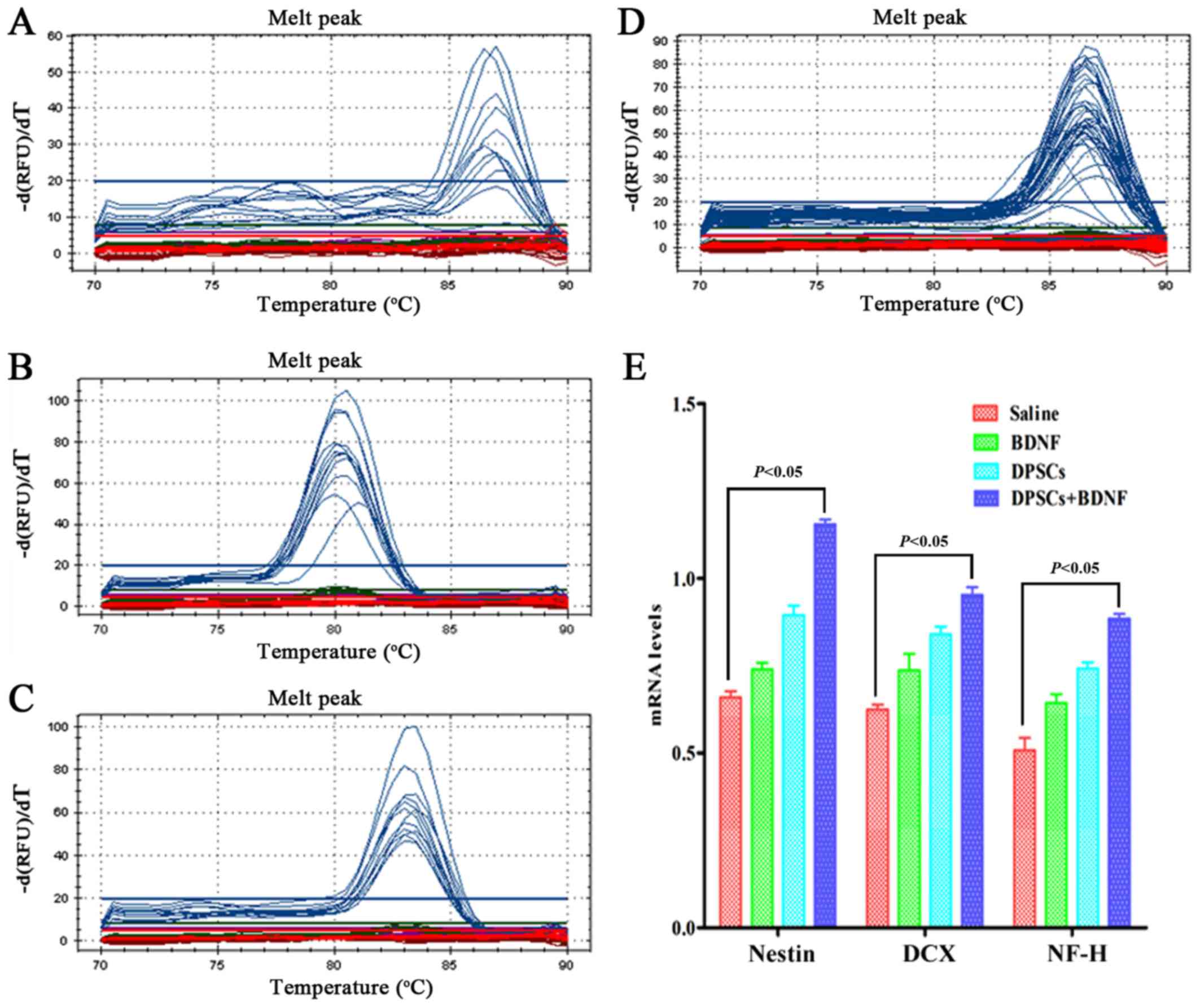
than in the BDNF and the DPSCs groups (P<0.05) (Fig. 5). Using western blot analysis and
RT-PCR analysis, we further confirmed an increase in
neuron-specific markers in the DPSCs+BDNF group compared with the
other groups. Protein and gene markers in DPSCs or BDNF group were
also higher than in the saline control group (P<0.05) (Figs. 6 and 7). Data suggest that BDNF promoted DPSC
differentiation into neuron-like cells and triggered
neurogenesis.
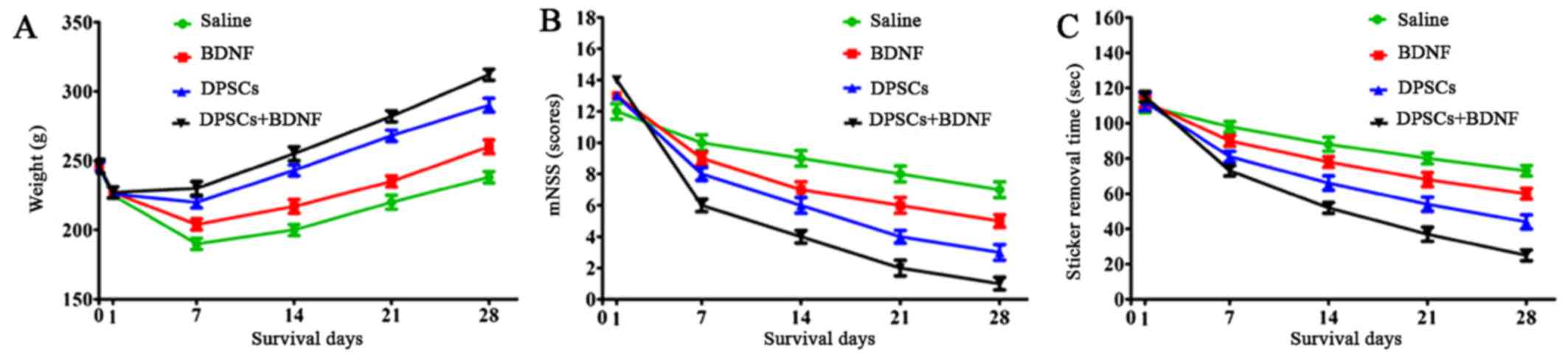
Weight comparison
The body weight of MCAO models decreased
significantly 24 h after surgery and recovered slowly eventually.
The weight was higher in the experimental groups than in the saline
control group. The recovery in the DPSCs+BDNF group was
significantly higher than in the BDNF and DPSCs groups (P<0.05).
The groups differed significantly from each other suggesting that
BDNF and DPSCs prevented neuronal ischemia (Fig. 8).
Treatment enhances sensorimotor
functional recovery
The stroke models were functionally assessed using
the adhesive-removal test and the mNSS on days 1, 7, 14, 21 and 28
after MCAO. All the animals showed functional impairment on day 1
postoperatively, followed by gradual recovery. Compared with the
saline group, animals treated with BDNF or DPSCs exhibited improved
neurological performance in the adhesive removal task and in mNSS
at 1 week post-treatment (P<0.05). The combination treatment
group showed greater functional recovery compared with other groups
(P<0.05). The greatest improvement was observed in the
DPSCs+BDNF-treated rats on day 28 post-MCAO (P<0.01). These data
suggest that functional deficits following ischemia due to MCAO
were successfully treated by intravenous transplantation of DPSCs
or BDNF injection (Fig. 7).
Further, DPSCs and BDNF showed synergistic neuroprotection against
stroke.
Discussion
Induced pluripotent stem cells (26), embryonic stem cells (27), neural stem cells (28), bone marrow-derived mesenchymal
stem cells (29) and DPSCs
(30) exhibit neuroprotective
effects in ischemic stroke animal models. DPSCs are easily
harvested, cultured, amplified and cryo-preserved. They originate
in the cranial neural crest and exhibit higher differentiation
compared with other stem cells. Allogeneic stem cell
transplantation may be used in clinical trials without the risk of
immune rejection and ethical concerns. DPSCs represent ideal stem
cells for treatment of neurological diseases. In the present study,
we showed that: i) cells can be labeled with the green fluorescent
dye PKH67 and tracked in vivo without cytotoxicity; ii)
intravenously transplanted DPSCs retain their proliferative
capacity in the peripheral ischemic regions of MCAO models; iii)
PKH67-labeled DPSCs were co-localized with neuronal markers and
DAPI; iv) DPSC transplantation combined with BDNF injection
enhanced the expression of neural differentiation markers including
nestin, DCX, and NF-H, suggesting that BDNF promotes DPSC survival
and differentiation into neuron-like cells; v) combination
treatment with DPSCs and BDNF restores neurological function better
than BDNF or DPSCs alone. MCAO is traditionally used to mimic
ischemic stroke. TTC and H&E staining revealed cerebral
infarction in rat brains. Rats subjected to MCAO showed obvious
weight loss and behavioral deficits postoperatively unlike the
sham-operated rats. Our data indicated successful creation of focal
cerebral ischemia models. We studied rat DPSCs displaying stem cell
morphology, phenotype and multilineage differentiation potentials.
Isolated DPSCs expressed mesenchymal stem cell markers Sca-1 and
CD44, and rarely expressed hematopoietic or endothelial cell
markers CD34 and CD45. Calcium deposition and fat droplets were
found using Alizarin Red and Oil Red O staining, which revealed
DPSC differentiation into bone and adipose cells. The green
fluorescent dye PKH67 was used to label cell membrane and track
transplanted stem cells in vivo. The high labeling
efficiency suggested absence of cytotoxicity. Cells colocalized
with green fluorescent, neural-specific markers and DAPI were
observed in the peripheral ischemic regions under laser scanning
confocal microscope, indicating DPSC survival in focal cerebral
ischemia. According to previous experimental research and basic
theory, stem cells have homing characteristic, grafted cells
migrate and localize in injured areas in vivo. In the past,
our group demonstrated similar results that transplanted bone
marrow cells relocated to and resided mostly around the infarct
penumbra in a mouse model of ischemic cerebral stroke (31). Many researchers have proved that
stroke-induced stromal cell-derived factor-1 (SDF-1) and its
receptor CXC chemokine receptor 4 (CXCR4) play a critical role in
attracting transplanted cells into injured areas. Stroke-induced
stromal cell derived factor-1 (SDF-1) may play a critical role in
attracting transplanted cells to ischemic areas. Intravenously
transplanted stem cells reach the site of injured area by
interaction with SDF-1 and its CXC chemokine receptor 4 (CXCR4)
(32–35). Intravenous delivery of stem cells
is safe and effective without surgical trauma or clinical
complications (31,36). No adverse events were apparent
after cellular transplantation or BDNF injection. DCX is expressed
by neuronal precursor cells and is a biological marker for immature
neurons. It is the 'gold standard' for evaluation of neurogenesis
(25). Nestin, a marker of neural
stem cells, is an intermediate filament protein synthesized during
the early stages of neuronal development (24). NF-H is a mature neuronal marker.
Engrafted DPSCs expressed neural-specific markers including nestin,
DCX and NF-H, indicating successful differentiation into
neuron-like cells. Functionally differentiated cells integrate into
neural networks and replace the damaged or lost neural tissues.
Additional evidence is needed to corroborate the findings.
Animals in the BDNF, DPSCs and DPSCs+BDNF groups,
showed better neurological performance than those in the saline
group. Functional recovery occurs as a result of direct cell
replacement. In addition, DPSCs secrete a series of neurotrophic
factors, including glial-derived neurotrophic factor (GDNF), BDNF
and NGF, which attenuate apoptosis and control neuronal survival,
growth, and differentiation (37). Further, DPSCs modulate tissue
microenvironment and alleviate inflammation. Central nervous system
inflammation contributes to the pathogenesis of stroke and
neurological deficits. Studies suggest that BDNF not only decreases
local pro-inflammatory cytokines, but also increases the levels of
anti-inflammatory mediators (38). Further, BDNF promotes angiogenesis
and neurogenesis in MCAO rats. A test grid of frames was used to
conduct semi-quantitative analysis of positive cells expressing
nestin, DCX and NF-H (39). We
found that the expression of nestin, DCX and NF-H was higher in the
DPSCs+BDNF group than in the DPSCs. Expression of markers in the
DPSCs or BDNF group was also higher than in the control group. BDNF
injection may improve the viability and differentiation of
transplanted DPSCs resulting in enhanced neuroprotection. The
results suggest that combining BDNF and DPSCs had a synergistic
protective effect against stroke, and represents an effective
strategy to restore neurological function. The study provides
experimental evidence supporting clinical investigation of cell and
gene therapy in neurological diseases.
However, the study limitations relate to the short
follow-up of the surviving DPSCs after transplantation in
vivo. Further investigations are needed to elucidate the
molecular mechanisms underlying the role of combined BDNF and DPSCs
administration in cerebral ischemia.
In conclusion, notwithstanding the lack of
established mechanisms underlying the role of stem cell therapies,
the present study for the first time demonstrated that intravenous
transplantation of rat-derived DPSCs promotes effective functional
recovery in animal models of stroke without any adverse effect.
BDNF administration may promote DPSC survival and differentiation
into functional neurons. DPSC transplantation together with BDNF
injection is a promising clinical strategy for the management of
cerebral ischemia.
Acknowledgments
The authors thank the Laboratory Center of the
Second Affiliated Hospital of Harbin Medical University for
experimental and technical assistance.
Notes
[1]
Funding
This study was co-supported by the China
Postdoctoral Science Foundation (no. 2012M520771) and the
Heilongjiang Postdoctoral Fund (no. LBH-Z12152).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
JF designed the experiment and provided guidance. XZ
and YZ implemented the experiment and were major contributors in
writing the manuscript. XZ and YZ contributed equally. HL
participated in the design of the experiment and performed the
fluorescence-activated cell sorting (FACS) analyses. RW analyzed
the immunofluorescence and immunohistochemistry. DY and BL
performed the PCR and western blot analysis. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
The experimental design and procedures were
conducted according to institutional guides for animal experiments
approved by the Institutional Animal Care and Use Committees of
Harbin Medical University.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Grossman AW and Broderick JP: Advances and
challenges in treatment and prevention of ischemic stroke. Ann
Neurol. 74:363–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nosrat IV, Widenfalk J, Olson L and Nosrat
CA: Dental pulp cells produce neurotrophic factors, interact with
trigeminal neurons in vitro, and rescue motoneurons after spinal
cord injury. Dev Biol. 238:120–132. 2001. View Article : Google Scholar
|
|
4
|
Király M, Kádár K, Horváthy DB, Nardai P,
Rácz GZ, Lacza Z, Varga G and Gerber G: Integration of neuronally
predifferentiated human dental pulp stem cells into rat brain in
vivo. Neurochem Int. 59:371–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY
and He XH: Intraventricular injection of human dental pulp stem
cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS
One. 8:e667482013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nosrat IV, Smith CA, Mullally P, Olson L
and Nosrat CA: Dental pulp cells provide neurotrophic support for
dopaminergic neurons and differentiate into neurons in vitro;
implications for tissue engineering and repair in the nervous
system. Eur J Neurosci. 19:2388–2398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apel C, Forlenza OV, de Paula VJ, Talib
LL, Denecke B, Eduardo CP and Gattaz WF: The neuroprotective effect
of dental pulp cells in models of Alzheimer's and Parkinson's
disease. J Neural Transm (Vienna). 116:71–78. 2009. View Article : Google Scholar
|
|
8
|
Lee HJ, Lim IJ, Lee MC and Kim SU: Human
neural stem cells genetically modified to overexpress brain-derived
neurotrophic factor promote functional recovery and neuroprotection
in a mouse stroke model. J Neurosci Res. 88:3282–3294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Q, Li B, Feng H, Xiao Z, Chen B, Zhao
Y, Huang J and Dai J: The promotion of cerebral ischemia recovery
in rats by laminin-binding BDNF. Biomaterials. 32:5077–5085. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cotman CW and Berchtold NC: Exercise: A
behavioral intervention to enhance brain health and plasticity.
Trends Neurosci. 25:295–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kokaia Z, Andsberg G, Yan Q and Lindvall
O: Rapid alterations of BDNF protein levels in the rat brain after
focal ischemia: Evidence for increased synthesis and anterograde
axonal transport. Exp Neurol. 154:289–301. 1998. View Article : Google Scholar
|
|
12
|
Schäbitz WR, Schwab S, Spranger M and
Hacke W: Intraventricular brain-derived neurotrophic factor reduces
infarct size after focal cerebral ischemia in rats. J Cereb Blood
Flow Metab. 17:500–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita K, Wiessner C, Lindholm D,
Thoenen H and Hossmann KA: Post-occlusion treatment with BDNF
reduces infarct size in a model of permanent occlusion of the
middle cerebral artery in rat. Metab Brain Dis. 12:271–280. 1997.
View Article : Google Scholar
|
|
14
|
Kurozumi K, Nakamura K, Tamiya T, Kawano
Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, et al:
BDNF gene-modified mesenchymal stem cells promote functional
recovery and reduce infarct size in the rat middle cerebral artery
occlusion model. Mol Ther. 9:189–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nomura T, Honmou O, Harada K, Houkin K,
Hamada H and Kocsis JD: I.V. infusion of brain-derived neurotrophic
factor gene-modified human mesenchymal stem cells protects against
injury in a cerebral ischemia model in adult rat. Neuroscience.
136:161–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang DJ, Lee N, Choi C, Jeon I, Oh SH,
Shin DA, Hwang TS, Lee HJ, Kim SU, Moon H, et al: Therapeutic
effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF)
in a rodent model of middle cerebral artery occlusion. Cell
Transplant. 22:1441–1452. 2013. View Article : Google Scholar
|
|
17
|
Ellis KM, O'Carroll DC, Lewis MD, Rychkov
GY and Koblar SA: Neurogenic potential of dental pulp stem cells
isolated from murine incisors. Stem Cell Res Ther. 5:302014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang XF, Yuan SJ and Yang C: Effects of
total flavonoids from Drynaria fortunei on the proliferation and
osteogenic differentiation of rat dental pulp stem cells. Mol Med
Rep. 6:547–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hata M, Omi M, Kobayashi Y, Nakamura N,
Tosaki T, Miyabe M, Kojima N, Kubo K, Ozawa S, Maeda H, et al:
Transplantation of cultured dental pulp stem cells into the
skeletal muscles ameliorated diabetic polyneuropathy: Therapeutic
plausibility of freshly isolated and cryopreserved dental pulp stem
cells. Stem Cell Res Ther. 6:1622015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reglodi D, Tamás A and Lengvári I:
Examination of sensorimotor performance following middle cerebral
artery occlusion in rats. Brain Res Bull. 59:459–466. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Winderlich JN, Kremer KL and Koblar SA:
Adult human dental pulp stem cells promote blood-brain barrier
permeability through vascular endothelial growth factor-α
expression. J Cereb Blood Flow Metab. 36:1087–1097. 2016.
View Article : Google Scholar
|
|
24
|
Michalczyk K and Ziman M: Nestin structure
and predicted function in cellular cytoskeletal organisation.
Histol Histopathol. 20:665–671. 2005.PubMed/NCBI
|
|
25
|
Couillard-Despres S, Winner B, Schaubeck
S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG
and Aigner L: Doublecortin expression levels in adult brain reflect
neurogenesis. Eur J Neurosci. 21:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SJ, Chang CM, Tsai SK, Chang YL, Chou
SJ, Huang SS, Tai LK, Chen YC, Ku HH, Li HY, et al: Functional
improvement of focal cerebral ischemia injury by subdural
transplantation of induced pluripotent stem cells with fibrin glue.
Stem Cells Dev. 19:1757–1767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tae-Hoon L and Yoon-Seok L:
Transplantation of mouse embryonic stem cell after middle cerebral
artery occlusion. Acta Cir Bras. 27:333–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang L, Wong S, Snyder EY, Hamblin MH and
Lee JP: Human neural stem cells rapidly ameliorate symptomatic
inflammation in early-stage ischemic-reperfusion cerebral injury.
Stem Cell Res Ther. 5:1292014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitkari B, Kerkelä E, Nystedt J, Korhonen
M, Mikkonen V, Huhtala T and Jolkkonen J: Intra-arterial infusion
of human bone marrow-derived mesenchymal stem cells results in
transient localization in the brain after cerebral ischemia in
rats. Exp Neurol. 239:158–162. 2013. View Article : Google Scholar
|
|
30
|
Yamagata M, Yamamoto A, Kako E, Kaneko N,
Matsubara K, Sakai K, Sawamoto K and Ueda M: Human dental
pulp-derived stem cells protect against hypoxic-ischemic brain
injury in neonatal mice. Stroke. 44:551–4. 2013. View Article : Google Scholar
|
|
31
|
Zhang XM, Du F, Yang D, Yu CJ, Huang XN,
Liu W and Fu J: Transplanted bone marrow stem cells relocate to
infarct penumbra and co-express endogenous proliferative and
immature neuronal markers in a mouse model of ischemic cerebral
stroke. BMC Neurosci. 11:1382010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenkranz K, Kumbruch S, Lebermann K,
Marschner K, Jensen A, Dermietzel R and Meier C: The chemokine
SDF-1/CXCL12 contributes to the 'homing' of umbilical cord blood
cells to a hypoxic-ischemic lesion in the rat brain. J Neurosci
Res. 88:1223–1233. 2010.
|
|
33
|
Miller JT, Bartley JH, Wimborne HJ, Walker
AL, Hess DC, Hill WD and Carroll JE: The neuroblast and angioblast
chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated
by reactive astrocytes in brain following neonatal hypoxic-ischemic
injury. BMC Neurosci. 6:632005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hill WD, Hess DC, Martin-Studdard A,
Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE and
Conway SJ: SDF-1 (CXCL12) is upregulated in the ischemic penumbra
following stroke: Association with bone marrow cell homing to
injury. J Neuropathol Exp Neurol. 63:84–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M
and Yu Q: SDF-1/CXCR4 axis induces human dental pulp stem cell
migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci
Rep. 7:401612017. View Article : Google Scholar
|
|
36
|
Zhang XM, Du F, Yang D, Wang R, Yu CJ,
Huang XN, Hu HY, Liu W and Fu J: Granulocyte colony-stimulating
factor increases the therapeutic efficacy of bone marrow
mononuclear cell transplantation in cerebral ischemia in mice. BMC
Neurosci. 12:612011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kvinnsland IH, Luukko K, Fristad I,
Kettunen P, Jackson DL, Fjeld K, von Bartheld CS and Byers MR:
Glial cell line-derived neurotrophic factor (GDNF) from adult rat
tooth serves a distinct population of large-sized trigeminal
neurons. Eur J Neurosci. 19:2089–2098. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G
and Liu X: Effects of brain-derived neurotrophic factor on local
inflammation in experimental stroke of rat. Mediators Inflamm.
2010:3724232010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melvin NR and Sutherland RJ: Quantitative
caveats of standard immunohistochemical procedures: Implications
for optical disector-based designs. J Histochem Cytochem.
58:577–584. 2010. View Article : Google Scholar :
|