Introduction
Osteonecrosis of the femoral head (ONFH) is a
refractory disease affecting young individuals, which is
characterised by hip pain and dysfunction or even lameness
(1,2). While several risk factors are known,
glucocorticoid (GC) medication is thought to be the principle one,
causing 10–30% of non-traumatic ONFH (3). GC has been widely used in the
treatment of rheumatic and auto-immune diseases, and for
chemotherapy and acute medical conditions, including traumatic
spinal cord injury (4–7). Hence, ONFH is a potential
complication for numerous patients receiving GC treatment. The
pathogenesis of ONFH includes ischaemia, necrosis, repair and
deformity in the femoral head (8–10).
All of these changes occur sequentially, resulting in collapse of
the femoral head. GC may cause ONFH through several mechanisms.
First, a GC overdose often exerts adverse effects on the vascular
system, including hypertension, atherosclerosis and coagulation
abnormalities, leading to diminished blood supply in the femoral
head (11,12). Secondary to GC administration,
patients present with a decreased pool of mesenchymal stem cells
(MSCs) in the proximal femur (13) and a reduced activity of MSCs
(14). MSCs have a vital role in
the osteonecrosis repair process. When osteonecrosis appears,
fibrous tissue carrying undifferentiated mesenchymal cells invades
the necrotic area and then initiates new bone formation; however,
this is slower than the spread of fibrous tissue, therefore leading
to incomplete reconstruction of the necrotic area (15–18). GC has been reported to inhibit the
proliferation and osteogenic differentiation of MSCs. The
inhibitive role of GC may be associated with the induction of
apoptosis and downregulation of runt-elated transcription factor 2
(Runx2)/core-binding factor α1 (Cbfa1), which is considered to be
important in osteogenesis, particularly for the development of
osteoblasts (19,20). Furthermore, MSCs extracted from
steroid-induced ONFH patients have a compromised osteogenic
ability, an elevated adipogenic ability and a lower proliferation
rate (21–25). Therefore, the enhancement of
proliferation and osteogenesis in bone marrow-derived MSCs (BMSCs)
in patients receiving GC by other drugs may improve the repair
process or even prevent ONFH.
Valproic acid (VPA) has been approved by the Food
and Drug and Administration of the US to treat epilepsy for >20
years (26). It has been reported
that VPA promotes the osteoblast differentiation and maturation
processes through the regulation of cell histone acetylation
(27,28). Histone acetylation is a reversible
epigenetic process delicately modulated by histone
acetyltransferases (HATs) and histone deacetylases (HDACs). HATs
are responsible for histone hyperacetylation, resulting in
relaxation of the chromatin structure, and allow the binding of DNA
sequences with transcription factors, thereby activating
transcription and downstream expression. Conversely, HDACs
deacetylate histones and silence transcription, thereby
counteracting the effects of HATs (29). This epigenetic regulation of gene
expression has a dominant role in cell stemness, determination,
commitment and differentiation (30–33). HDACs are mainly classified into 4
groups: Class I HDACs (1, 2, 3 and 8), class IIa HDACs (4, 5, 7 and
9), class IIb HDACs (6 and 10), class III HDACs (sirtuin-1 to -7)
and a class IV HDAC (HDAC11) (34). VPA, a potent class I and II HDAC
inhibitor (HDACi), has been reported to promote osteogenic
differentiation in a number of cell types, including osteoblasts,
BMSCs adipose-derived stem cells and dental pulp stem cells in
vitro (27,35–38). In vivo, VPA promotes bone
healing at its clinically applied concentration (39). GC treatment may have a systemic
influence on the vascular system and BMSC pool (40,41). Thus, an increasing number of
studies focus on systemic administration of drugs to prevent or
treat ONFH (42–44).
To the best of our knowledge, the effect of VPA on
BMSC proliferation and osteogenic differentiation upon GC treatment
has remained elusive, as well as whether systemic administration of
VPA may improve the osteogenic differentiation of BMSCs and thus
prevent the occurrence of ONFH after GC administration. The results
of the present study indicated that VPA reduces the inhibitive
effect of GC on proliferation, apoptosis and osteogenic
differentiation of BMSCs in vitro and prevents the
occurrence of ONFH in rats.
Materials and methods
Cell culture
The present study was performed according to the
principles of the Helsinki Declaration, and written informed
consent was obtained from each patient. The experimental procedures
were approved by the Ethical Review Board of Shanghai Jiaotong
University Affiliated Sixth People's Hospital (Shanghai, China).
BMSCs were isolated from human bone marrow harvested from patients
suffering femoral neck fracture during hip arthroplasty surgery.
The BMSCs were purified and cultured according to a previously
published protocol by our group (45). BMSCs of passages 3–6 were used in
all experiments. Patient consent was provided before any procedures
were performed. BMSCs from proximal femurs of rats were extracted
and cultured using the same methods.
Cell proliferation and apoptosis
assay
First, the effect of VPA, dexamethasone (DEX) and a
combination of the two drugs on BMSC proliferation and apoptosis
was assessed. BMSCs were seeded in 96-well plates (3-wells per
group) with the same number of cells and divided into 5 groups: i)
Control; ii) VPA 0.5 mM; iii) VPA 1 mM; iv) VPA 2 mM; and v) VPA 5
mM. DEX at 10−5 M was selected due to its significant
inhibitive effect on cell proliferation and osteogenesis reported
in previous studies (46,47). In addition, the following DEX
groups with or without VPA treatment were established: i) DEX; ii)
DEX+VPA 0.5 mM; iii) DEX+VPA 1 mM; iv) DEX+VPA 2 mM; and v) DEX+VPA
5 mM.
The Cell Counting Kit-8 (CCK-8) assay was used
according to the manufacturer's instructions at 24, 72, 120 and 168
h after BMSC adherence and the above treatments. At these
time-points, 10 µl CCK-8 reagent was added into 100
µl of culture medium in each well, followed by incubation
for another 3 h. The absorbance value was measured using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 450 nm.
To evaluate the effect on apoptosis, BMSCs cultured
with 5 mM VPA, DEX and DEX plus 5 mM VPA for 5 days were subjected
to Annexin V-fluorescein isothiocyanate and propidium iodine double
staining (Dojindo, Kumamoto, Japan), and the apoptotic rate was
measured by flow cytometry according to the manufacturer's
protocols.
Osteogenic induction assay
BMSC differentiation was initiated 48 h after the
cells were plated, with the basic medium in each group supplemented
with 10−2 M β-sodium glycerophosphate, 50 µg/ml
L-ascorbic acid and 10−7 M DEX. The medium was changed
every 3 days. First, the osteogenesis-associated mRNA levels of
BMSCs treated with VPA alone for 7 and 14 days were measured via a
polymerase chain reaction (PCR) assay. Next, BMSCs were
osteo-induced with VPA at gradient concentrations for 7 and 21 days
in a mineralisation assay. Subsequently, the effect of DEX in the
presence or absence of VPA on BMSC osteogenesis and mineralisation
was tested. For PCR and western blot assays, BMSCs were cultured
for 7 days. For alizarin red S staining, BMSCs in each group were
cultured for 21 days. Alkaline phosphatase (ALP) activity was
measured after 14 days of osteoinduction.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from osteogenic-induced
BMSCs in each group with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The RT reaction was performed
using EasyScript one-step gDNA Removal and cDNA Synthesis Supermix
(TransGen Biotech, Beijing, China) from 1 µg of total RNA
according to the manufacturer's protocols. qPCR of type I collagen
(COL I, ALP, osteocalcin (OCN) and Runx2 were performed with the
TransStart Tip Green qPCR SuperMix (TransGen Biotech). The relative
amount of mRNA was normalised to β-actin (48). The forward and reverse primers of
each complementary (c)DNA were designed as follows: β-actin,
5′-GTCATCCATGGCGAACTGGT-3′ and 5′-CGTCATCCATGGCGAACTGG-3′; Runx2,
5′-CCGAGACCAACCGAGTCATTTA-3′ and 5′-AAGAGGCTGTTTGACGCCAT-3′; ALP,
5′-CAAGGATGCTGGGAAGTCCG-3′ and 5′-CTCTGGGCGCATCTCATTGT-3′; OCN,
5′-CCCCCTCTAGCCTAGGACC-3′ and 5′-ACCAGGTAATGCCAGTTTGC-3′; COL I,
5′-CAGCCGCTTCACCTACAGC-3′ and 5′-TTTTGTATTCAATCACTGTCTTGCC-3′. The
PCR system was as follows: cDNA, 1 µl; double-distilled
water, 3.4 µl; Tip Green qPCR SuperMix, 5 µl; passive
reference dye (50X), 0.2 µl; forward primer (10
µmol/l), 0.2 µl; reverse primer (10 µmol/l),
0.2 µl. The total volume of the system was 10 µl. The
reaction conditions were 95°C for 30 sec initial denaturation,
followed by 40 cycles of 95°C for 5 sec and finally 60°C for 30
sec. Furthermore, a 65–95°C solubility curve was constructed.
Western blot analysis
Proteins were extracted with a cell lysis buffer
(radioimmunoprecipitation assay lysis and extraction Buffer, Thermo
Fisher Scientific, Inc.) supplemented with proteinase inhibitor
(#78430; Thermo Fisher Scientific, Inc.), and the total protein
concentration was detected with a bicinchoninic acid assay.
Following denaturation at 95°C for 5 min, 30-µg aliquots of
protein were subjected to 10% SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (Merck KGaA, Darmstadt, Germany).
After being blocked with 5% dried skimmed milk, the membranes were
labelled with a primary antibody to COL I (ab138492), Runx2
(ab23981), ALP (ab186422) and β-actin (ab115777) (Abcam, Cambridge,
UK) at a concentration of 1:1,000 at 4°C overnight and then
immersed in the secondary antibody working solution containing
anti-rabbit immunoglobulin G (#14708; Cell Signaling Technology,
Inc., Danvers, MA, USA) (1:1,000) at 37°C for 1 h. After
chemiluminescence staining (Pierce™ ECL, #32106; Thermo Fisher
Scientific, Inc.) with a commercial assay, the target bands were
detected with a gel image-processing system. The protein levels
were normalised against β-actin. Histone extraction was performed
according to the protocol of a previous study (35), and the membranes were incubated
with primary antibodies to HDAC1 (AH379; Beyotime Institute of
Biotechnology, Shanghai, China), HDAC2 (AH382; Beyotime Institute
of Biotechnology), acetylated histone H3 (ac-H3) (#06-599; EMD
Millipore, Billerica, MA, USA), ac-H4 (#06-598; EMD Millipore) and
histone H4 (ab31830; Abcam), which was used as the loading control,
at a concentration of 1:500 at 4°C overnight. Proteins were
detected after incubation with goat anti-rabbit IgG secondary
antibody (1:1,000; #14708; Cell Signaling Technology, Inc.) at 37°C
for 1 h and visualized using chemiluminescence in the same way
according to manufacturer's protocols mentioned above.
ALP activity assay
The osteogenic differentiation of human (h)BMSCs was
evaluated by the ALP activity assay. After culture for 7 days, the
cell layers were gently washed with cold PBS and lysed in 200
µl of 0.2% Triton X-100 for 30 min. The lysates were
centrifuged at 20,000 × g for 15 min at 4°C and sonicated. Next, 30
µl of the supernatant was mixed with 150 µl of the
working solution (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's protocol. The
formation of p-nitrophenol from p-nitrophenylphosphate, the
substrate of ALP, was evaluated by measuring the absorbance at 405
nm with a microplate reader (Bio-Rad 680; Bio-Rad Laboratories,
Inc.). The ALP activity was calculated by determining the ratio of
the absorbance of the experimental samples to that of the standard
and was expressed as mM of p-nitrophenol produced per minute per mg
of protein.
Alizarin red S staining
After osteogenic induction for 21 days, cells in
48-well plates (3-wells in each group) were fixed with 4%
paraformaldehyde for 20 min, then rinsed twice with PBS (pH 7.4)
and stained with 40 mM alizarin red working solution for 10 min at
room temperature. After being rinsed twice with PBS again, these
cells were visualised under a light microscope. Subsequently, 100
mmol/l cetylpyridinium chloride was added to each well and
semi-quantitative analyses were performed by measurement of optical
density values at 560 nm.
Animal model and grouping
All animal experiments in the present study were
approved by the Animal Care and Use committee of Shanghai Sixth
People's Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China). A total of 36 Sprague Dawley (SD) rats (weight,
250–300 g, 7 weeks old) purchased from Shanghai Animal Experimental
Centre, (Shanghai, China) were divided into 3 groups. Six rats in
the control group received no treatment; 15 rats in the
methylprednisolone (MP) group were intramuscularly injected with MP
20 mg/kg/day (49,50) for 3 continuous days per week over
a period of 3 weeks (total MP, 180 mg/kg) to induce ONFH, and 15
rats in the MP+VPA group were intraperitoneally injected with 300
mg/kg VPA (51,52) once a day for 3 weeks in a row
until MP injection was ceased. MP rather than DEX was selected, as
it is more commonly used in the clinic.
Serum ALP activity
Blood samples of 1 ml were harvested randomly from 3
SD rats in each group on the last day of weeks 1, 2 and 3 after
injection. Serum separation was performed by centrifugation at 300
× g for 15 min at 4°C. The serum was then mixed with the working
solution for the ALP activity assay (#A059-2; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) and processed according
to the manufacturer's protocol. The optical density values were
measured at 405 nm with a microplate reader (Bio-Rad 680; Bio-Rad
Laboratories, Inc.).
Micro-computed tomography (CT)
scanning
To evaluate bone morphologic changes in the rats,
the right femoral head of each rat was scanned with a micro-CT
scanner (Skyscan 1176; Bruker MicroCT, Kontich, Belgium) at a
resolution of 9 microns. Two-dimensional (2D) images were analysed
using CTAn software (v.1.13; Bruker MicroCT). Primarily, the
trabecular bone parameters of whole subchondral bone in the femoral
head were measured. Specifically, the bone mineral density (BMD),
bone volume per tissue volume (BV/TV) and trabecular thickness
(Tb.Th) were quantified.
Angiography
After cardiac perfusion with heparinised saline,
Microfil (MV-112; Flow Tech, Inc., Carver, MA, USA) was injected
into the abdominal aorta until a constant outflow of the compound
was observed from the abdominal vein. All the surgical steps were
under anaesthesia after intraperitoneal injection of 4% chloral
hydrate (360 mg/kg). Subsequently, the rats were stored at 4°C
overnight to ensure the polymerisation of the contrast agent.
Femoral heads were fixed with 10% formalin and decalcified with a
10% EDTA solution. Finally, the samples were scanned via micro-CT
as described above.
Histological and immunohistochemical
(IHC) analyses
After decalcification and paraffin embedding,
femoral heads were sectioned at a thickness of 5 µm in the
coronal plane. Certain sections were stained with haematoxylin and
eosin (H&E) at room temperature for 5 min to evaluate the
trabecular structure, while others were deparaffinised, subjected
to antigen retrieval and incubated with anti-OCN (1:50; ab13420;
Abcam), anti-Runx2 (1:200; ab23981; Abcam) and anti-vascular
endothelial growth factor (VEGF; 1:50; BA0407; Boshide, Wuhan,
China) primary antibodies at 4°C overnight and then incubated with
the Super Vison Polymer anti-rabbit lgG-HRP kit at 37°C for 30 min
according to the manufacturer's protocols (SV0002; Boshide).
Staining was visualized with 3,3-diaminobenzidine and samples were
counterstained with haematoxylin for 1 min at room temperature.
Photomicrographs were acquired using a Leica DM 4000 (Leica
Microsystems, Wetzlar, Germany).
Statistical analysis
SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used to
analyse the values in each group. Values are expressed as the mean
± standard deviation. Comparisons of data among the groups were
performed using one-way analysis of variance with
Student-Newman-Keuls post hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
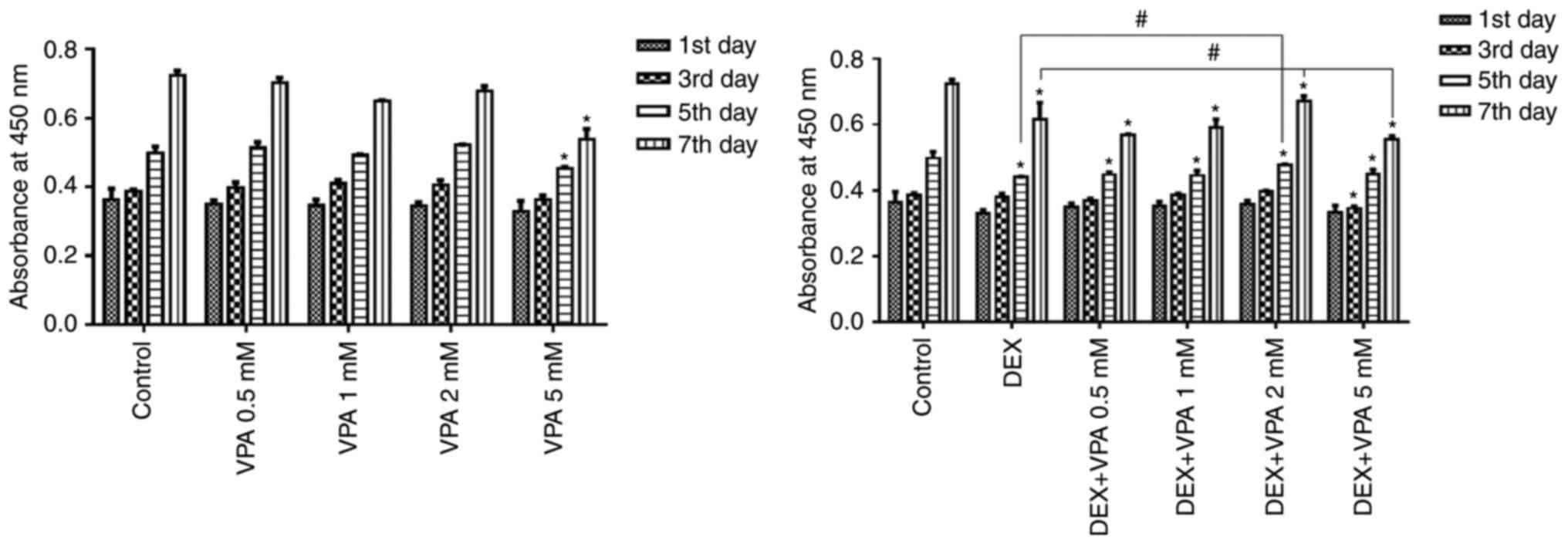
VPA at a high concentration inhibits BMSC
proliferation, while VPA at lower concentrations inhibits
DEX-induced decreases in BMSC proliferation
The CCK-8 assay indicated that the amount of BMSCs
in all groups gradually increased in a time-dependent manner, but
proliferation was significantly repressed after incubation with 5
mM VPA and with 10−5 M DEX in the presence or absence of
VPA. The BMSC proliferation rate was not significantly influenced
by 0.5–2 mM VPA, but these concentrations of VPA attenuated the
inhibitory effect on BMSC proliferation exerted by DEX in a
dose-dependent manner, except for the 5-mM concentration of VPA,
which decreased the proliferation compared with that in the groups
treated with DEX alone (Fig.
1).
VPA decreases the apoptotic rate of BMSCs
induced by DEX
The apoptosis assay indicated that DEX promoted cell
apoptosis. The apoptotic cells in the DEX group was >40%, which
was significantly higher than that in the control group (24.83%).
Although 5 mM VPA inhibited BMSC proliferation, it exerted no
significant effect on cell apoptosis. However, when 5 mM VPA was
added to the DEX culture, the amount of apoptotic BMSCs was
significantly reduced (Fig.
2).
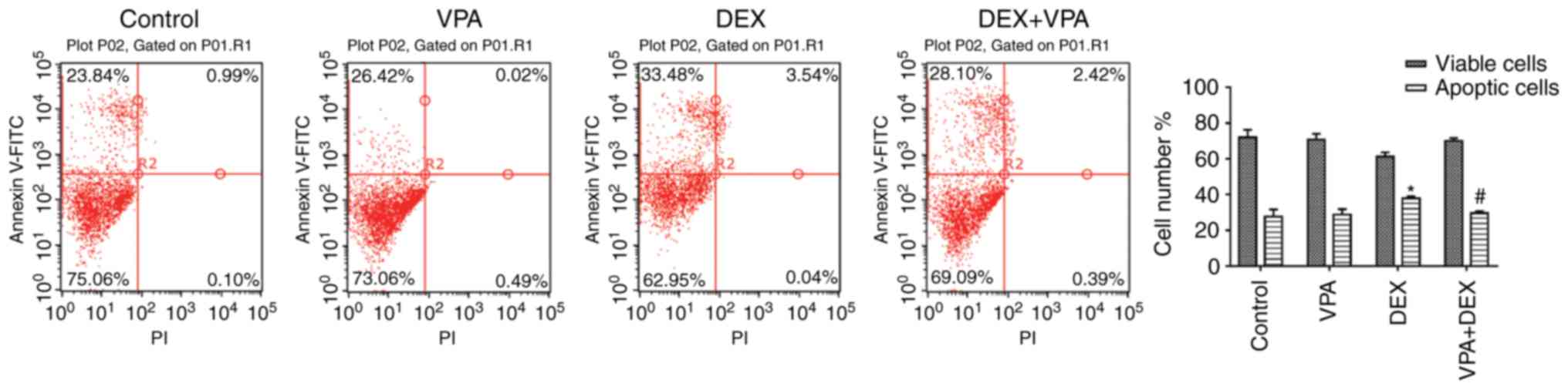 | Figure 2Effects of VPA, DEX and a combination
of DEX and VPA on the apoptosis of BMSCs. After treatment with 5 mM
VPA, DEX and DEX+5 mM VPA, the apoptotic rate of BMSCs was detected
by flow cytometry after Annexin V-FITC and PI staining, and the
apoptotic rates were calculated. *P<0.05, vs. the
control, #P<0.05 vs. DEX group. FITC, fluorescein
isothiocyanate; PI, propidium iodide; DEX group. BMSCs, bone marrow
mesenchymal stem cells; DEX, dexamethasone; VPA, valproic acid. |
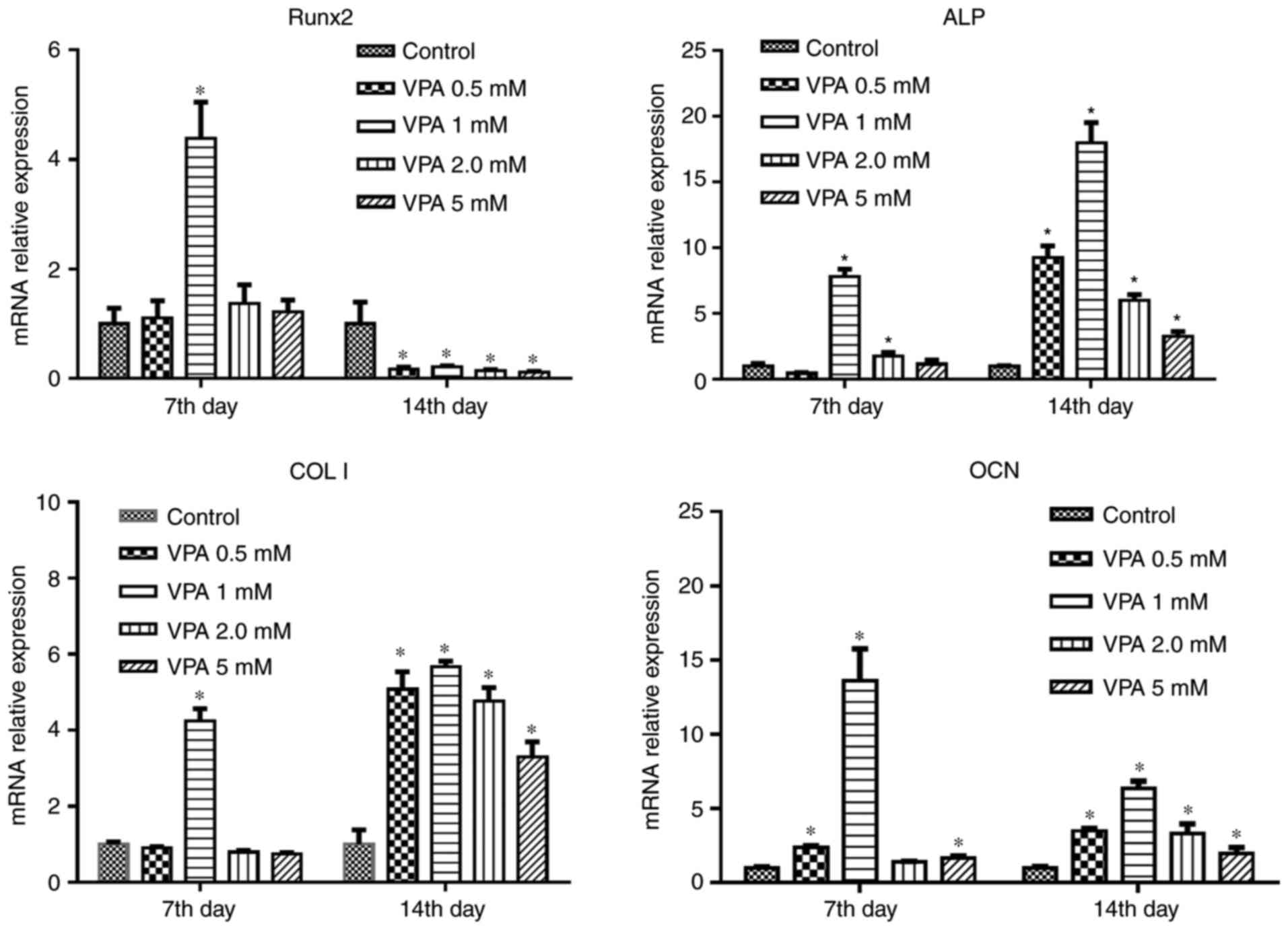
VPA increases osteogenic differentiation
and improves the osteogenic capacity of BMSCs compromised by DEX
treatment by inhibition of HDAC activity
First, BMSCs were treated with different
concentrations of VPA, and RT-qPCR analysis revealed that 1 mM VPA
significantly increased the mRNA levels of Runx2, ALP, COL I and
OCN after 7 days of culture. After osteogenic induction for 14
days, VPA at all concentrations promoted the expression of these
osteogenesis-associated genes to varying degrees, while decreasing
that of Runx2. The most efficacious concentration of VPA at 7 and
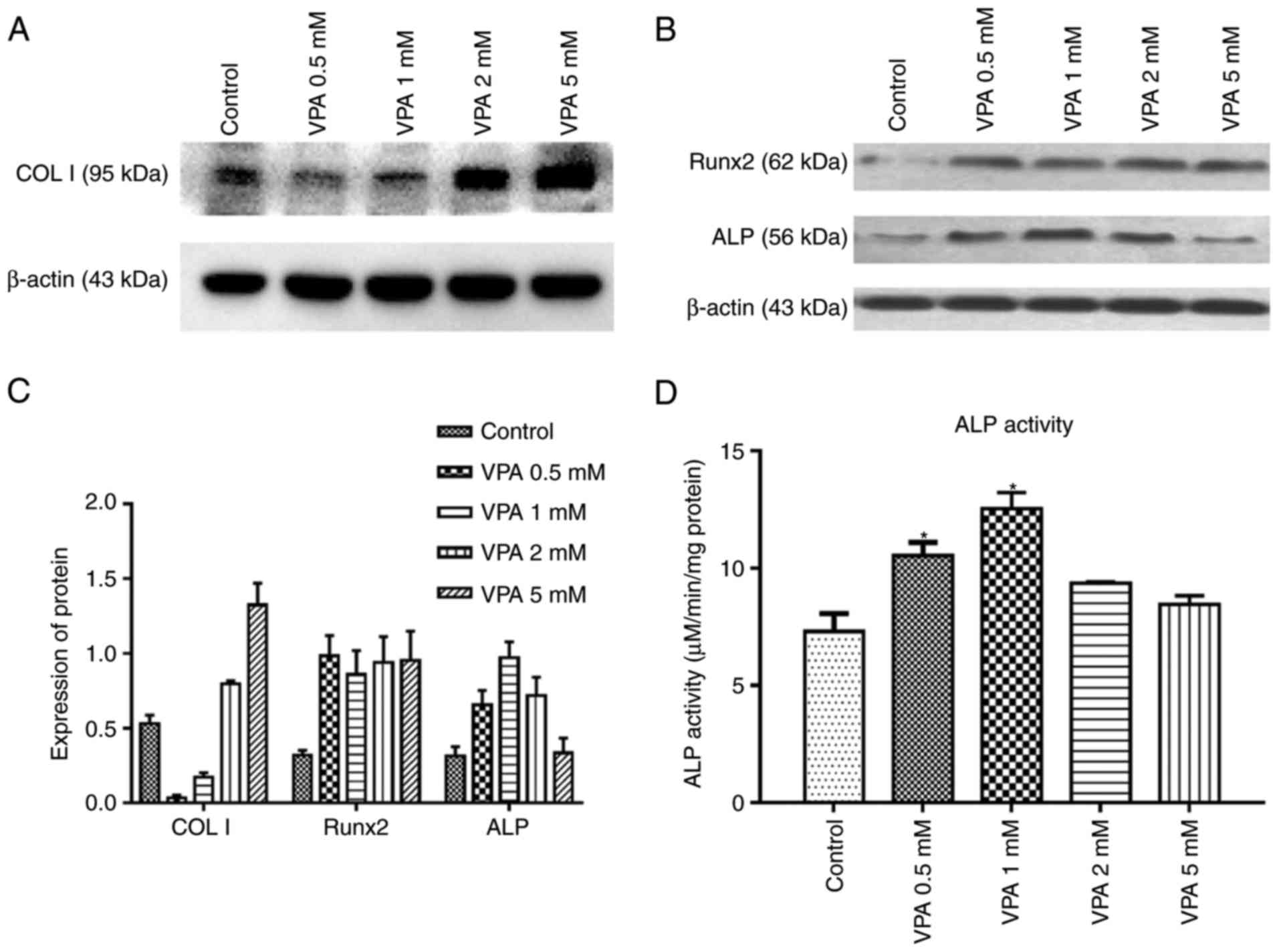
14 days was 1 mM (Fig. 3). Next,
the protein expression of Runx2, COL I and ALP, as well as ALP
activity, was investigated in the same treatment groups. The
results indicated that VPA elevated ALP activity and the protein
expression of ALP, COL I and Runx2 (Fig. 4A–C). With VPA treatment at 1 mM, a
similar trend to that in ALP activity was observed regarding ALP
expression (Fig. 4D). Given that
5 mM VPA caused a downward trend in proliferation, only the lower
concentrations (0.5–2 mM) were added into osteogenic medium
containing 10−5 M DEX, in which BMSCs were cultured for
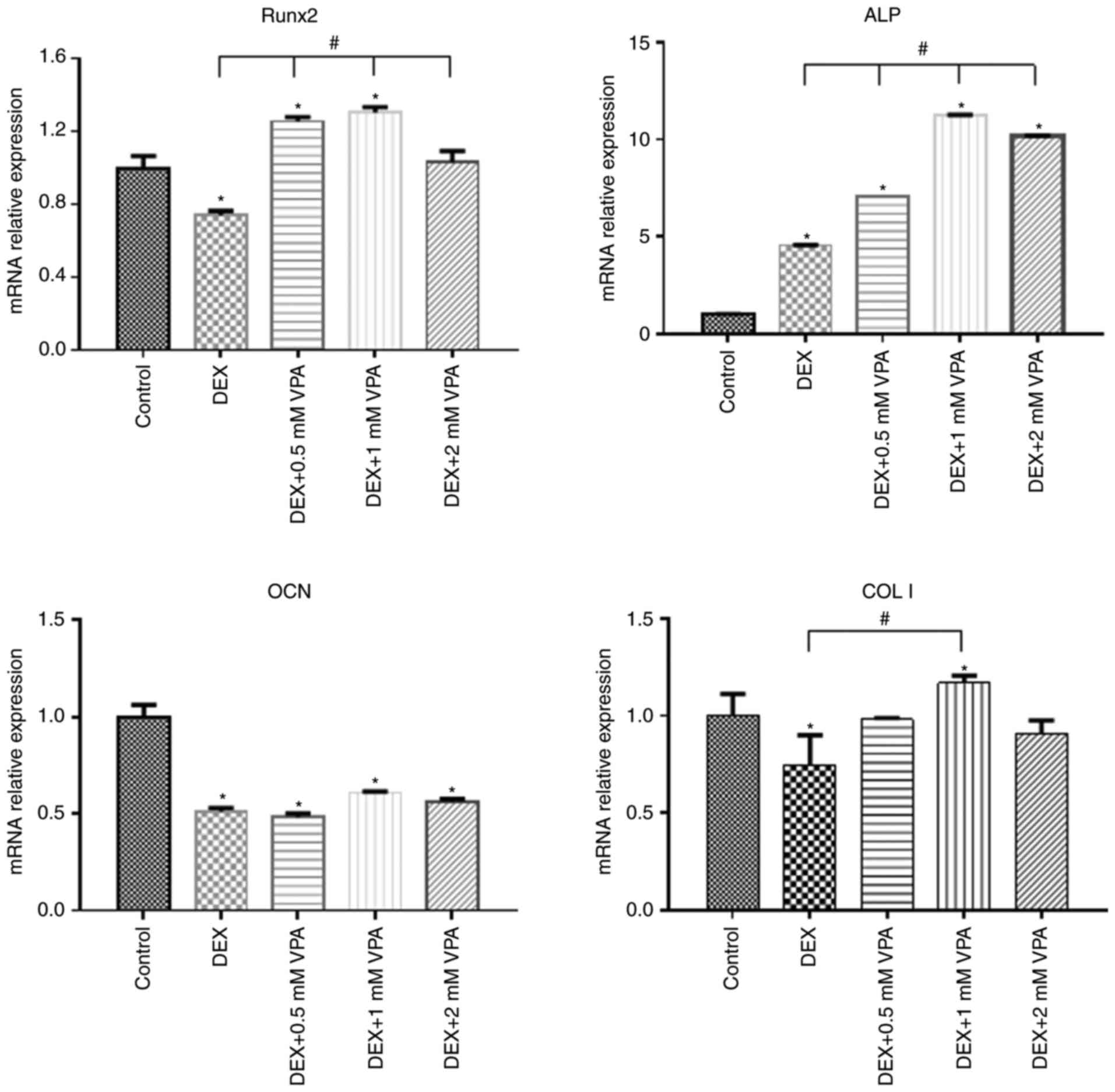
7 days. The results indicated that DEX decreased the protein levels
of Runx2, COL I and ALP in comparison with those in the control
group, while the addition of 0.5 and 1 mM VPA enhanced the levels
of all of these proteins to varying degrees. Specifically, 0.5 and
1 mM VPA recovered Runx2 expression to the same level as that in
the control group, and rescued ALP expression. Furthermore,
supplementation with 0.5 mM VPA gave rise to significantly higher
expression levels of COL I than those in the control and DEX
groups. However, the addition of 2 mM VPA repressed the expression
levels of Runx2, COL I and ALP (Fig.
5A). Considering that VPA is an HDACi, the protein expression
of HDAC1 and HDAC2 as class I HDACs as well as the levels of ac-H3
and ac-H4 were measured. The results indicated that the two HDACs
were lowered in all experimental groups, including the DEX and
DEX+VPA groups, while ac-H3 and ac-H4 were lowered in the DEX group
and upregulated with the addition of 0.5 and 1 mM, but not 2 mM VPA
(Fig. 5B and C). Similarly, the
mRNA levels of Runx2 and COL I were significantly lowered by DEX
and elevated by the addition of VPA. Although DEX increased ALP,
even higher ALP levels were observed after the addition of VPA.
Likewise, 1 mM VPA exerted the greatest effect on COL I, Runx2 and
ALP gene expression. However, VPA did not rescue the lowered OCN
expression under DEX treatment (Fig.
6).
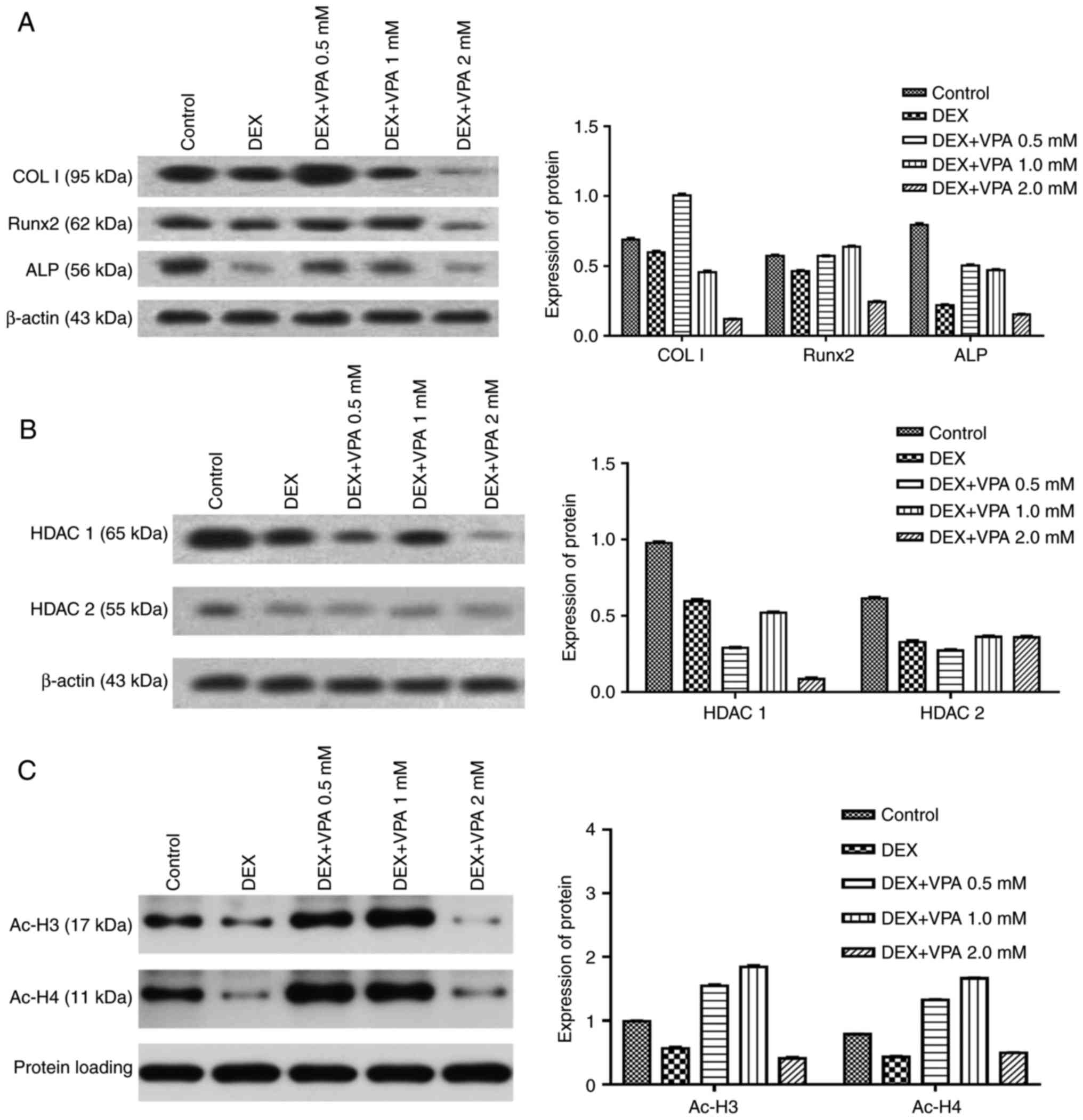 | Figure 5Expression of proteins associated
with osteogenesis in BMSCs treated with various concentrations of
VPA in combination with DEX. (A) Protein expression of Runx2, ColI
and ALP in BMSCs. (B and C) The protein expression of HDAC1 and -2,
ac-H3 and ac-H4. DEX, dexamethasone; BMSCs, bone marrow mesenchymal
stem cells; VPA, valproic acid; Runx2, runt-related transcription
factor 2; ALP, alkaline phosphatase; OCN, osteocalcin; ColI, type I
collagen; HDAC, histone deacetylase; ac-H3, acetylated histone
H3. |
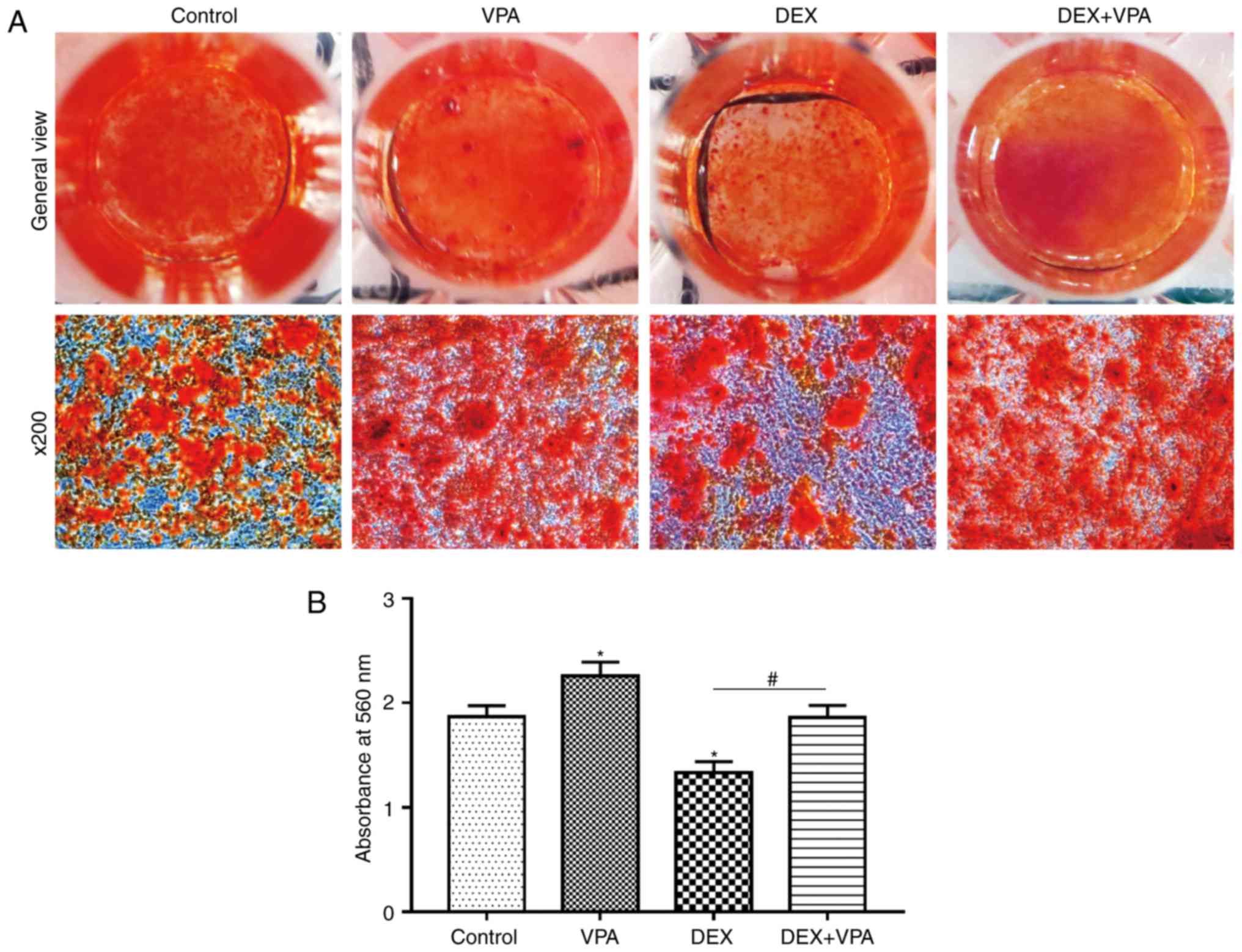
VPA promotes bone mineralisation and
improves mineralisation compromised by DEX treatment
Alizarin red S staining was used to assess calcium
deposition, a characteristic of late-stage osteogenic
differentiation of BMSCs. Fewer calcium deposits in the DEX group
compared with those in the control group were observed, while more
deposits were visible after VPA treatment. In addition,
supplementation of VPA in addition to DEX significantly improved
the mineralisation of BMSCs in comparison with DEX treatment alone
(Fig. 7A). The staining eluate
was then harvested with 1 ml cetylpyridinium chloride and the
absorbance values were measured at 560 nm. The quantified results
indicated the same trend as that of the microscopic views presented
(Fig. 7B).
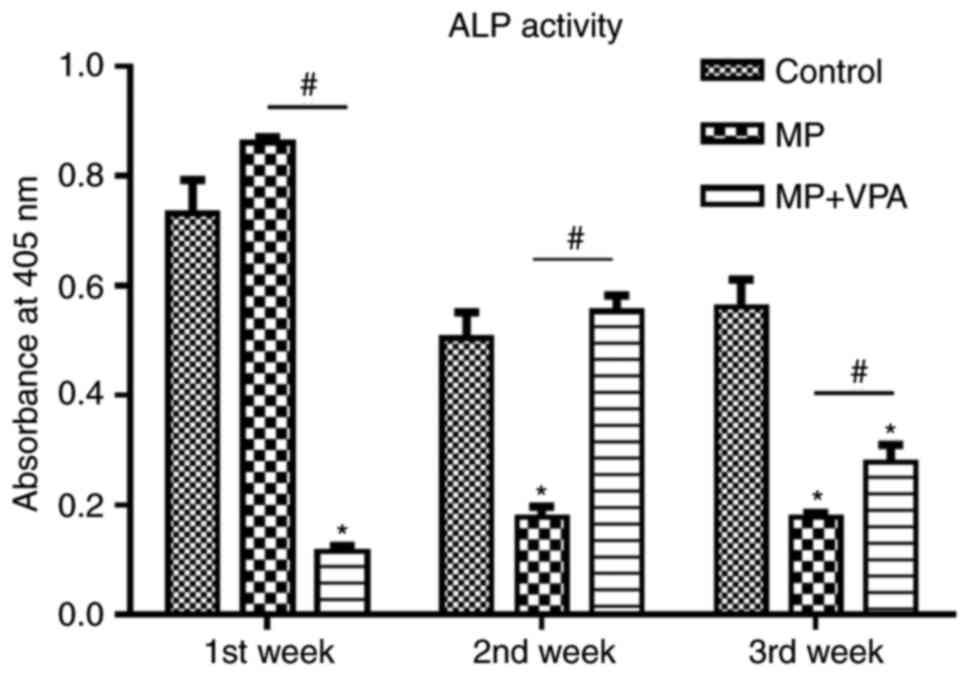
VPA increases serum ALP in rats treated
with MP
Within the 3 weeks of the animal experiment, serum
ALP activity in the MP group was highest in the first week. At 1
week, it was also significantly higher than that in the MP+VPA
group. However, ALP activity in the MP group waned in the following
2 weeks and became lower than that in the control. Of note, with
the addition of VPA, ALP activity was rescued to the level of the
control in the second week, while this effect was attenuated but
still significant in the third week (Fig. 8).
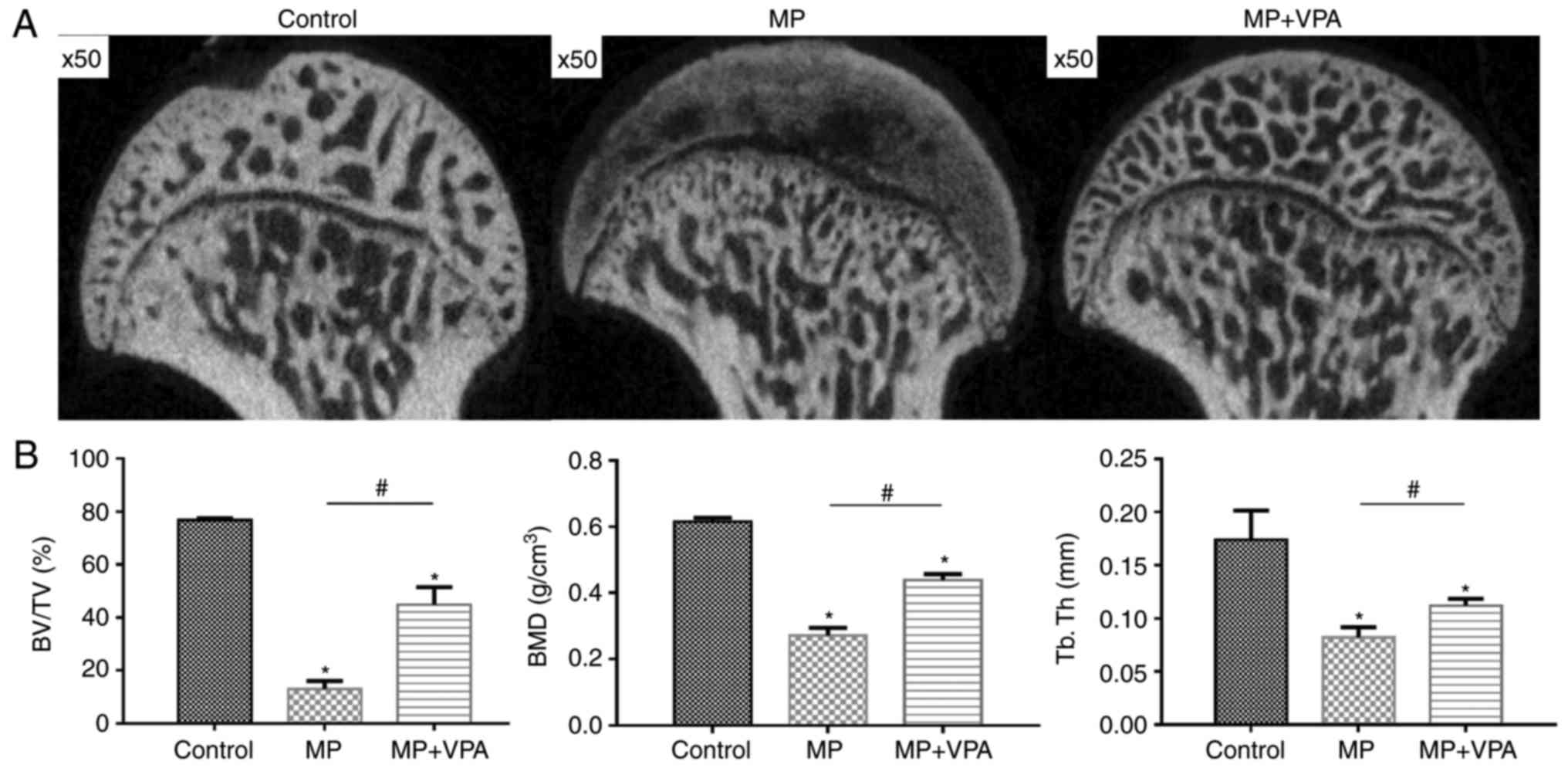
VPA improves subchondral bone formation
under MP treatment
The trabecular changes in the subchondral area of
the femoral heads were detected by micro-CT scan at 6 weeks after
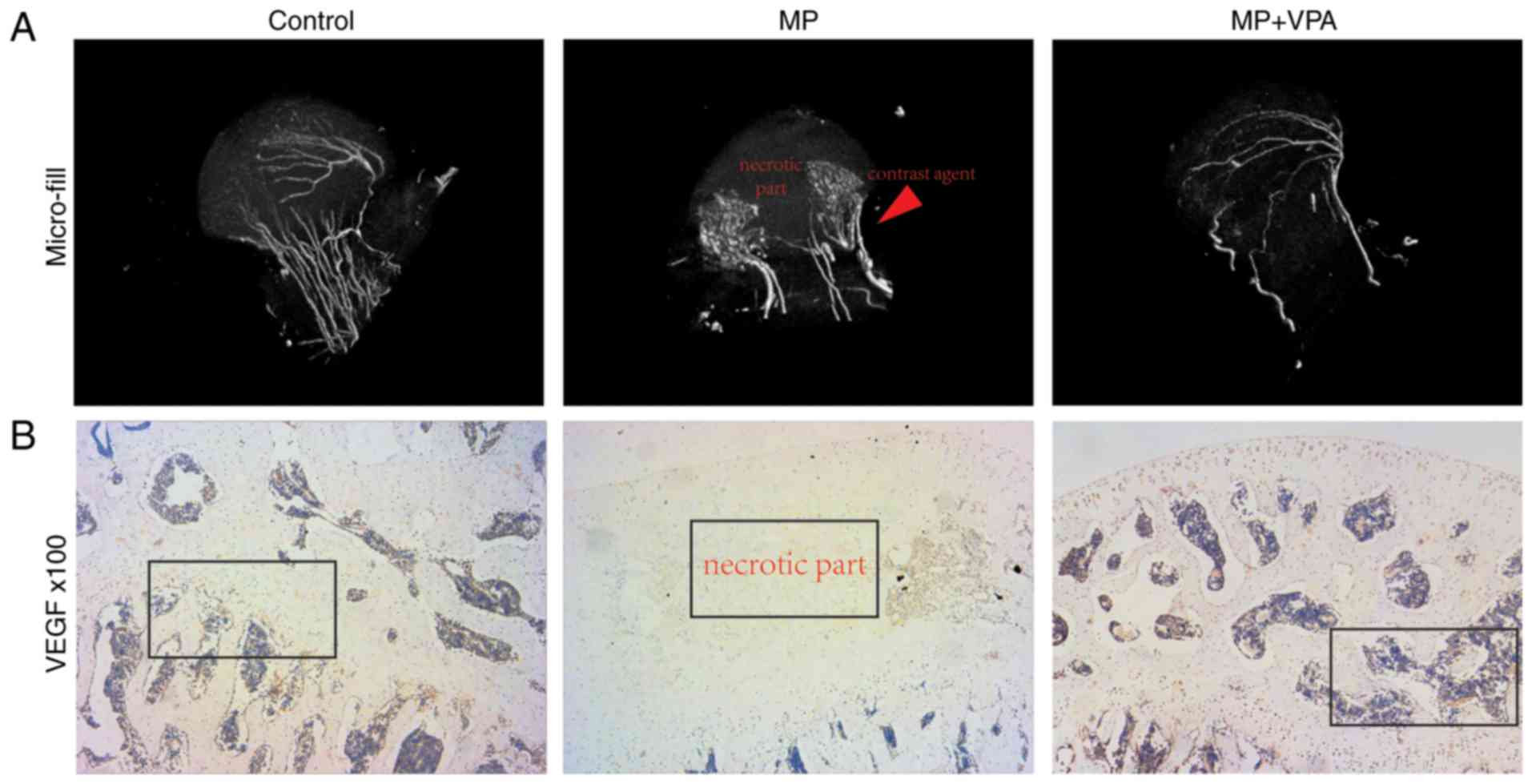
the first injection of MP. A total of 11 rats in the MP group
exhibited visible osteonecrosis of the femoral head in the micro-CT
images, while only 2 rats in the MP+VPA group had obvious
osteonecrosis (Fig. 9A). The BMD
of the rats in the MP group was 0.27±0.01 g/cm3, which
was significantly lower than that in the control group (0.61±0.01
g/cm3), while supplementation with VPA significantly
increased the BMD of the area to 0.43±0.01 g/cm3. In
addition, a similar effect was identified regarding the BV/TV and
Tb.Th among these 3 groups of rats (Fig. 9B).
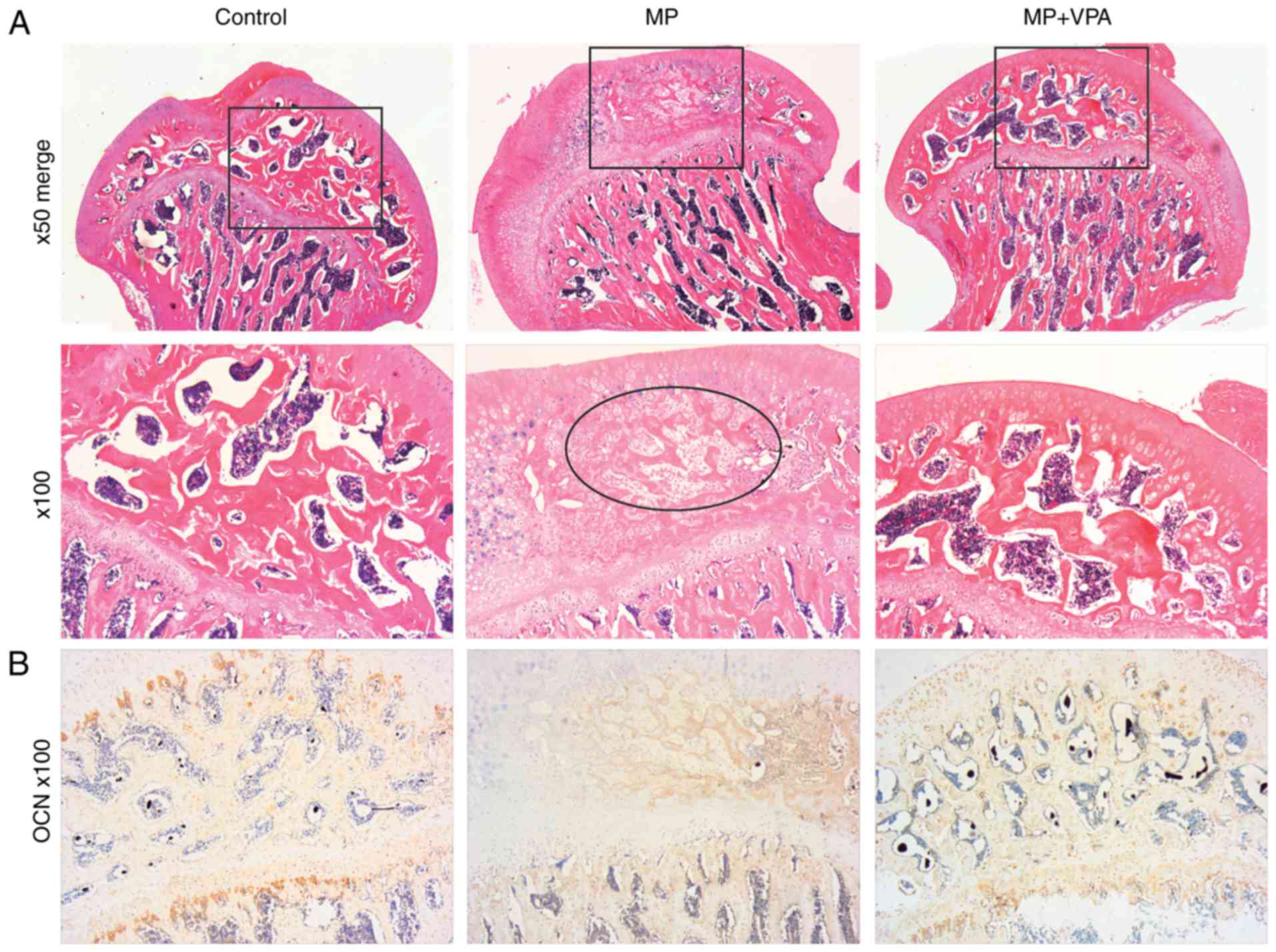
Similar to the micro-CT results, H&E staining of
subchondral bone tissue revealed typical signs of osteonecrosis.
Specifically, at low magnification, the MP group exhibited
amorphous substance in the inter-trabecular spaces with few normal
haematopoietic and fat cells on H&E staining. The trabeculae
decreased in volume and became thinner in disarray and pale in
staining in comparison with the control. At a magnification of
×100, diffuse karyolytic and karyorrhectic nuclei of osteocytes
were identified, and the marrow of amorphous matter was lacking
normal cell nuclei, where there were mainly karyorrhectic and
pyknotic speckles, revealing coagulation necrosis of the fatty
hematopoietic tissue of the marrow. Furthermore, the ingrowth of
fibrous tissue, a characteristic property of the repair process,
was observed in this stage. However, with supplementation of VPA,
no obvious necrotic area was present (Fig. 10A). The present study further
assessed OCN expression as a marker of mature bone by IHC staining.
The results indicated almost no positive area in the necrotic part
of the subchondral bone after MP treatment, while the control and
MP+VPA groups featured widespread OCN-expressing cells in the same
area (Fig. 10B).
VPA preserves the blood supply to the
femoral head
To detect the blood supply to the femoral head in
the present study, micro-angiography of the femoral head was
performed by perfusing the vessels with Microfil and observing them
by micro-CT. The reconstructed 3D micro-CT images exhibited
disrupted blood supply in the femoral head in the MP group, with
vascularisation blocked around the femoral neck. However, the main
vessel distributing to the femoral head in rats treated with MP+VPA
was preserved (Fig. 11A).
Correlation of micro-CT images and H&E staining revealed
structural trabeculae in the vascularised area of the femoral
heads. By contrast, the devascularised area displayed a lack of
trabeculae and was referred to as the necrotic area. The
vascularisation of the subchondral area of the femoral head was
further detected by IHC analysis of VEGF. Nearly no staining for
VEGF was observed in the subchondral bone area in the MP group,
while a stained area was observed in the marrow cavity around the
trabeculae in the MP+VPA and control groups (Fig. 11B).
VPA promotes the osteogenic capacity of
BMSCs in rats
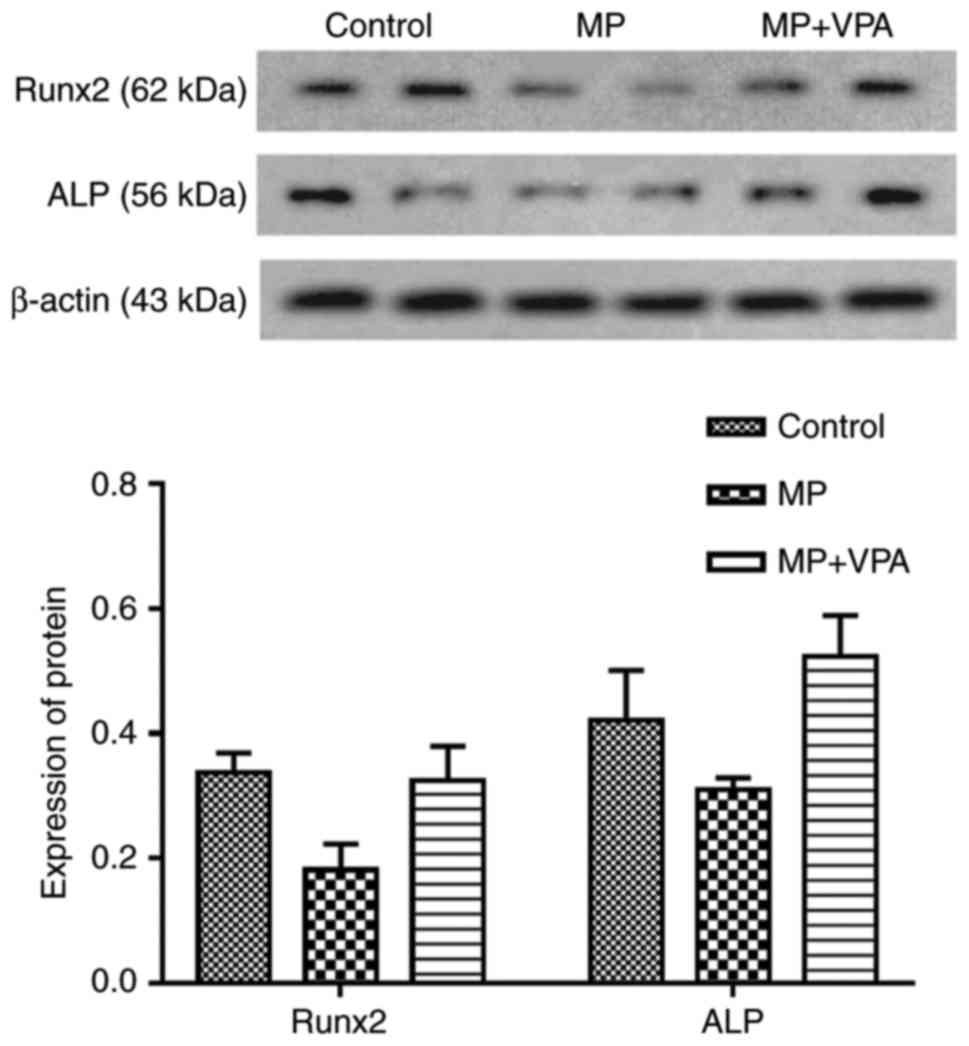
The BMSCs were subjected to osteogenic induction for
3 days after being extracted from the proximal femurs of rats from
each group and cultured for 3–5 passages. Western blot analysis was
performed to measure the expression of Runx2 directly influenced by
VPA as reported in previous studies (53), as well as ALP, its downstream
protein. The results indicated that Runx2 and ALP expression were
lowered in the MP group and improved with the addition of VPA
(Fig. 12).
Discussion
ONFH is a common side effect of exogenous GC usage.
One of the major underlying mechanisms is the inhibitory role of
GCs in the osteogenesis of BMSCs, which influences the repair
process of osteonecrosis. High doses of GCs may affect numerous
processes involved in the differentiation of BMSCs. GCs may
downregulate the expression of Runx2/Cbfa1, the key factor
associated with osteogenic differentiation and maturation of
osteoblasts (54). Several
studies have indicated that GC antagonises Runx2 and subsequently
compromises the expression of ALP, osteopontin and OCN in MSCs,
resulting in inhibition of bone remodelling and fracture healing
(19,55,56). The repair process is initiated
when osteonecrosis takes place and is gradually outpaced by the
proliferation of fibrous tissue, leading to incomplete
reconstruction of the necrotic area devastated by the ceased blood
supply. The ceased blood supply due to injury of vessels
distributing to the femoral head is also attributed to failure of
tissue reconstruction in the necrotic area. Consequently, the
approach to prevent ONFH is more promising than to treat it,
avoiding the loss of vessels that contributes to necrosis, which
was applied in the present study.
VPA is an effective and widely used antiepileptic
drug, which has been reported to influence osteogenesis as an HDACi
(26). HDACis have been
demonstrated to enhance osteoblast differentiation in vitro
(34,53,57) and new bone formation in
vivo (37,39). To the best of our knowledge, the
present study was the first to indicate that VPA attenuates the
inhibitive effect of GCs on the osteogenic differentiation of
BMSCs, and that systemic injection of VPA prevents GC-induced ONFH
in rats.
First, the effect of VPA alone on BMSC viability and
apoptosis was observed. The results indicated that concentrations
of VPA as high as 5 mM inhibited BMSC proliferation after 7 days of
incubation, while VPA concentrations of 0.5–2 mM did not
significantly influence BMSC proliferation. Hatakeyama et al
(58) reported that (0.625–6.25
mM) VPA inhibited the proliferation of mouse embryonic stem cells.
Lee et al (35,38) also reported that the proliferation
of adipose-derived stem cells decreased in a dose-dependent manner
after VPA treatment at 0.4–10 mM, but the extent of inhibition was
not as great in umbilical cord blood-derived stem cells, displaying
a significant decrease only with 10 mM VPA. The decrease in cell
proliferation in the presence of high concentrations of VPA may be
associated with cell apoptosis or cell cycle arrest. Lee et
al (35,38) reported that the G2/M phase
population of the cells was increased by VPA in a dose-dependent
manner, while no increase in the sub-G1 phase was observed,
demonstrating that the decrease in proliferation by VPA was not due
to promoting apoptosis. In line with this, the present results also
indicated that VPA concentrations as high as 5 mM inhibited the
proliferation of BMSCs, but did not significantly affect cell
apoptosis. Accordingly, VPA may cause cell cycle arrest rather than
cell apoptosis. Furthermore, cycle arrest is a prerequisite for
cell differentiation, and thus, it is possible that VPA influences
the osteogenic differentiation of BMSCs (58,59). However, in the present study,
monotreatment with DEX significantly inhibited BMSC proliferation,
while this effect was attenuated by co-treatment with 0.5–2 mM VPA.
In a cell apoptosis assay, the percentage of apoptotic cells
increased under DEX treatment, but improved after addition of 5 mM
VPA. DEX has been reported to promote apoptosis of several types of
stem cell (60–63). However, VPA may be able to hamper
this negative effect of DEX by inducing cell cycle arrest, which
requires further study. Taken together, the results of the present
study suggested that low concentrations of VPA (0.5–2 mM) do not
significantly influence BMSC proliferation and attenuate the
negative effect on proliferation exerted by DEX. The underlying
mechanisms may include a reduction of DEX-induced cell apoptosis by
VPA.
Next, the effect of VPA on osteogenic
differentiation of BMSCs was assessed. The results indicated that
VPA alone promoted ALP, COL I, Runx2 and OCN mRNA expression.
Specifically, BMSCs treated with 1 mM VPA for 7 days exhibited the
highest osteogeneis-associated gene expression, while all
concentrations of VPA significantly elevated these genes after 14
days of incubation, with the exception of Runx2. Runx2 expression
was gradually reduced along with osteoblast maturation, and mature
osteoblasts do not contain significant amounts of Runx2 protein
(64). Hence, it is possible that
VPA accelerated the osteogenic differentiation process of BMSCs,
leading to a reduction of Runx2, but an elevation of COL I, ALP and
OCN, all of which are expressed in mature osteoblasts. Similar
results were obtained for the protein expression of ALP, COL I and
Runx2, as well as for ALP activity. VPA at a concentration of 1 mM
was selected due to its efficacy in the mineralisation assay,
resulting in the promotion of calcium nodule deposition. All of the
present results indicated that VPA promotes osteogenic
differentiation of BMSCs.
DEX has been reported to inhibit osteogenic
differentiation and bone formation in vitro and in
vivo (19,55). The present study indicated that
DEX inhibited COL I, Runx2 and ALP protein expression after
osteogenic induction of BMSCs for 7 days. This inhibition was
abrogated or attenuated by co-treatment with 0.5 and 1 mM of VPA
for Runx2 or ALP, respectively. In addition, 0.5 mM VPA reversed
the inhibitory effect of DEX on COL I, resulting in expression
levels above those in the control group. Similar results were
obtained for the mineralization of hBMSCs, manifesting in a
protective effect of VPA on calcium deposition on BMSCs in the
presence of DEX. Likewise, the gene expression of Runx2 and COL I
was downregulated by DEX and reversed by the addition of VPA, and
ALP expression exhibited the highest level with the addition of 1
mM of VPA. However, the lowered gene expression of OCN was not
improved with the addition of VPA. Rimando et al (65) used high concentrations of DEX
(10−5 M) for long-term treatment (7 days) of BMSCs and
identified glucocorticoid receptor (GR)-HDAC6 complex as the
repressor of the promoter region of OCN, leading to the
downregulation of OCN mRNA. However, VPA inhibits class I HDACs
more efficiently than class II HDACs, e.g., HDAC5 and -6 (27). It is possible that VPA at
concentrations of 0.5–2 mM is not sufficient to inhibit HDAC6 to
improve OCN mRNA expression. Consistent with the results of
previous studies (66–71), the present study also indicated
that VPA and DEX significantly inhibit HDAC1 and -2 expressions in
BMSCs. Previous studies have demonstrated that GR elements are
located in the promoter of the HDAC2 gene and that DEX treatment
for 7 days reduced GR expression (66,67). Accordingly, HDAC2 expression may
be lowered by DEX. DEX also affects histone acetylation. DEX acts
by binding to GR, which, upon activation, translocates to the
nucleus and either increases or decreases gene expression (68). DEX has been reported to inhibit
Runx2 expression by recruiting HDAC1 to the Runx2 promoter, which
then mediates the deacetylation of histone H4 and downregulates
Runx2 expression (69). In
addition, the complex of DEX and GR recruits HDAC2 to the complex
of nuclear factor-κB and activator protein 1 and reduces the
associated inflammatory cytokine expression (70,71). The results of the present study
also indicate that the levels of ac-H3 and ac-H4 were lower with
DEX treatment alone than with the addition of VPA. Although DEX
lowered the expression of HDAC1 and -2, as VPA did, the present
results indicated that DEX did not have the same inhibitory effect
on HDAC activity as VPA did. This may be the reason for DEX
inhibiting osteogenic differentiation, and the addition of VPA may
improve or reverse this inhibitory effect. As stated above, DEX
inhibits osteogenic differentiation by binding to the promoter of
Runx2 with GR, recruiting HDACs and bringing transcription to an
end. Consequently, VPA has a beneficial effect on osteogenic
differentiation inhibited by DEX, which may be explained by the
inhibition of HDACs and rendering of histone acetylation, giving
rise to initiation of Runx2 expression.
The present study hypothesized that VPA has a
beneficial effect against GC-induced ONFH. In a previous study, Xu
et al (35) reported that
pre-treatment of mouse adipose-derived stem cells with VPA for 2
days produced an increased amount of bone formation after injection
into bone defect areas. Rashid et al (39) demonstrated that intraperitoneal
injection of VPA for 1 week accelerated the healing of maxillary
bone cavities. To the best of our knowledge, no previous in
vivo experiment has been performed to assess the preventive
effect of VPA against GC-induced ONFH. GC-induced ONFH was
successfully achieved in rats by injection of 20 mg/kg MP on 3
consecutive days over 3 weeks. Previously, animal models of ONFH
have been established using a combination of 20 mg/kg MP and 2
mg/kg lipopolysaccharide (72–74), as LPS causes vasculitis, which
occurs in most autoimmune diseases (75). However, not all patients receiving
GC treatment are diagnosed with autoimmune diseases, e.g. those
with spinal cord injury. Accordingly, it is better to establish an
animal model of ONFH by induction with MP only to study GC-induced
ONFH. The GC-induced rat model of ONFH has been successfully
established in previous studies (49,50). In the present study, this ONFH rat
model exhibited characteristics of classically-defined necrosis on
histology with karyolytic and karyorrhectic osteocytes; it also
featured necrotic marrow filled with acellular substance or
fibroblasts invasion, and sinusoidal vessels devoid of red blood
cells, demonstrating that devascularisation and necrosis had
occurred in the femoral head. Of the rats that had received MP
injection, a randomly selected 50% were simultaneously administered
300 mg/kg VPA once a day for 3 weeks with no intermittence, as
administration of 250–300 mg/kg VPA has been reported to cause HDAC
inhibition and improve functional recovery in stroke (76), nerve injury (77), retinal ischaemic injury (78) or survival of haemorrhagic shock
(79). However, it remains
elusive whether 300 mg/kg of VPA is the optimal dose, e.g. whether
larger doses would increase the efficacy or whether smaller doses
would suffice, particularly in long-term treatment periods of ≥3
weeks. Therefore, the dose-dependent effect on the prevention of
GC-induced ONFH will be assessed in a future study.
In the present study, blood samples were collected
from the experimental rats for measurement of serum ALP activity,
which reflects the process of bone formation in general. The
results indicated that MP+VPA attenuated the suppression of ALP
activity caused by MP during the second and third weeks. VPA
treatment enhanced the serum ALP levels, which may explain for how
VPA attenuates the inhibitory effect of MP on ALP activity
(80). However, serum ALP is an
indicator of bone formation rather than osteonecrosis progression
(81–83). The bone density of the femoral
head was then measured, and histological and angiographic analyses
were performed. Overall, 11 out of 15 rats that had received MP
treatment presented with ONFH, but it only occurred in 2 out of 15
rats subjected to MP+VPA treatment. The results of the micro-CT
scanning indicated that VPA yielded a greater preservation of
trabeculae and bone volume in animals treated with MP (MP+VPA
group). On the contrary, no normal-shaped trabeculae were observed
in the subchondral area, which was only filled with a silt-like
substance in single treatment of MP. On histological analysis, the
femoral heads exhibited no apparent necrotic area in the group
subjected to VPA treatment, with evenly distributed expression of
OCN along the trabeculae, indicating that VPA treatment preserves a
relatively large amount of mature bone under MP challenge. The
major blood supply into the femoral head was also preserved in the
VPA group, and as in the control group, an area of VEGF-expressing
tissue was observed in the marrow cavity, indicating that VPA may
be able to protect vessels as well. A further study on the effect
of VPA against MP- or DEX-induced vessel deficits will be performed
in the future. Finally, BMSCs were collected from rats in each
group and western blot analysis indicated that the protein
expression of Runx2 and ALP was higher in the MP+VPA group compared
with that in the MP group, indicating that the osteogenic capacity
may have been improved by co-treatment with VPA, as indicated in
the in vitro experiments. It may also be possible to prevent
ONFH by improvement of the osteogenic capacity of BMSCs.
In summary, the present study reported that VPA
enhanced the osteogenic differentiation of BMSCs, and attenuated
the negative effect of GCs on BMSC proliferation, apoptosis and
osteogenic differentiation. To the best of our knowledge, the
present study was also the first to apply VPA as a preventive agent
against GC-induced ONFH. The present results demonstrate that VPA
is a promising drug for preventing ONFH, possibly via the
enhancement of the osteogenic differentiation of BMSCs.
Acknowledgments
Not applicable.
Notes
[1]
Funding
Financial support from the National Natural Science
Foundation of China (grant no. 81371959), Ministry of Major Disease
Joint Program of Shanghai Health System (grant no.
2014ZYJB0301).
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
DZ was responsible for designing the concept,
acquisition of data, analysis and interpretation and manuscript
writing. CZ and YF contributed to the design, analysis and
interpretation. YC analysed and interpreted the data and animal
experiments. JY, ST and SG were involved in the experimental method
optimisation. ZW contributed to the Micro-CT scanning and analyses.
Each author read and approved the final version of the
manuscript.
[4] Ethical
approval and consent to participate
The present study was performed according to the
principles of the Helsinki Declaration, and written consent was
obtained from each patient. The experimental procedures were
approved by the Ethical Review Board of Shanghai Jiaotong
University Affiliated Sixth People's Hospital (Shanghai, China).
Patient consent was provided before any procedures were
performed.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mont MA, Jones LC and Hungerford DS:
Nontraumatic osteonecrosis of the femoral head: Ten years later. J
Bone Joint Surg Am. 88:1117–1132. 2006.PubMed/NCBI
|
|
3
|
Tan G, Kang PD and Pei FX: Glucocorticoids
affect the metabolism of bone marrow stromal cells and lead to
osteonecrosis of the femoral head: A review. Chin Med J.
125:134–139. 2012.PubMed/NCBI
|
|
4
|
Tait AS, Butts CL and Sternberg EM: The
role of glucocorticoids and progestins in inflammatory, autoimmune,
and infectious disease. J Leukoc Biol. 84:924–931. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kirwan JR: The effect of glucocorticoids
on joint destruction in rheumatoid arthritis. The arthritis and
rheumatism council Low-dose glucocorticoid study group. N Engl J
Med. 333:142–146. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salmon SE, Crowley JJ, Grogan TM, Finley
P, Pugh RP and Barlogie B: Combination chemotherapy,
glucocorticoids, and interferon alfa in the treatment of multiple
myeloma: A Southwest oncology group study. J Clin Oncol.
12:2405–2414. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall ED and Braughler JM: Glucocorticoid
mechanisms in acute spinal cord injury: A review and therapeutic
rationale. Surg Neurol. 18:320–327. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mont MA, Zywiel MG, Marker DR, McGrath MS
and Delanois RE: The natural history of untreated asymptomatic
osteonecrosis of the femoral head: A systematic literature review.
J Bone Joint Surg Am. 92:2165–2170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boss JH: Experimental models of
osteonecrosis of the femoral head. J Orthop Sci. 9:533–534. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavernia CJ, Sierra RJ and Grieco FR:
Osteonecrosis of the femoral head. J Am Acad Orthop Surg.
7:250–261. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerachian MA, Cournoyer D, Harvey EJ, Chow
TY, Bégin LR, Nahal A and Séguin C: New insights into the
pathogenesis of glucocorticoid-induced avascular necrosis:
Microarray analysis of gene expression in a rat model. Arthritis
Res Ther. 12:R1242010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cenni E, Fotia C, Rustemi E, Yuasa K,
Caltavuturo G, Giunti A and Baldini N: Idiopathic and secondary
osteonecrosis of the femoral head show different thrombophilic
changes and normal or higher levels of platelet growth factors.
Acta Orthop. 82:42–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernigou P, Beaujean F and Lambotte JC:
Decrease in the mesenchymal stem-cell pool in the proximal femur in
corticosteroid-induced osteonecrosis. J Bone Joint Surg Br.
81:349–355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernigou P and Beaujean F: Abnormalities
in the bone marrow of the iliac crest in patients who have
osteonecrosis secondary to corticosteroid therapy or alcohol abuse.
J Bone Joint Surg Am. 79:1047–1053. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia Y, Chu TW and Zhou Y: Repair process
in experimentally induced avascular necrosis of femoral head in
rabbits. Chongqing Med. 2006.
|
|
16
|
Takaoka K, Yoshioka T, Hosoya T, Ono K and
Takase T: The repair process in experimentally induced avascular
necrosis of the femoral head in dogs. Arch Orthop Trauma Surg.
99:109–115. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malizos KN, Quarles LD, Seaber AV, Rizk WS
and Urbaniak JR: An experimental canine model of osteonecrosis:
Characterization of the repair process. J Orthop Res. 11:350–357.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shapiro F, Connolly S, Zurakowski D,
Menezes N, Olear E, Jimenez M, Flynn E and Jaramillo D: Femoral
head deformation and repair following induction of ischemic
necrosis. J Bone Joint Surg Am. 91:2903–2914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koromila T, Baniwal SK, Song YS, Martin A,
Xiong J and Frenkel B: Glucocorticoids antagonize RUNX2 during
osteoblast differentiation in cultures of ST2 pluripotent
mesenchymal cells. J Cell Biochem. 115:27–33. 2014. View Article : Google Scholar
|
|
20
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004. View Article : Google Scholar
|
|
21
|
Lee JS, Lee JS, Roh HL, Kim CH, Jung JS
and Suh KT: Alterations in the differentiation ability of
mesenchymal stem cells in patients with nontraumatic osteonecrosis
of the femoral head: Comparative analysis according to the risk
factor. J Orthop Res. 24:604–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Houdek MT, Wyles CC, Packard BD, Terzic A,
Behfar A and Sierra RJ: Decreased osteogenic activity of
mesenchymal stem cells in patients with corticosteroid-induced
osteonecrosis of the femoral head. J Arthroplasty. 31:893–898.
2016. View Article : Google Scholar
|
|
23
|
Wang BL, Sun W, Shi ZC, Lou JN, Zhang NF,
Shi SH, Guo WS, Cheng LM, Ye LY, Zhang WJ and Li ZR: Decreased
proliferation of mesenchymal stem cells in corticosteroid-induced
osteonecrosis of femoral head. Orthopedics. 31:4442008.
|
|
24
|
Zhou DA, Zheng HX, Wang CW, Shi D and Li
JJ: Influence of glucocorticoids on the osteogenic differentiation
of rat bone marrow-derived mesenchymal stem cells. BMC
Musculoskeletal Disord. 15:2392014. View Article : Google Scholar
|
|
25
|
Zhang W, Yang N and Shi XM: Regulation of
mesenchymal stem cell osteogenic differentiation by
glucocorticoid-induced leucine zipper (GILZ). J Biol Chem.
283:4723–4729. 2008. View Article : Google Scholar
|
|
26
|
Waterhouse E: Intravenous valproate for
pediatric status epilepticus. Epilepsy Curr. 3:208–209. 2003.
View Article : Google Scholar
|
|
27
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho HH, Park HT, Kim YJ, Bae YC, Suh KT
and Jung JS: Induction of osteogenic differentiation of human
mesenchymal stem cells by histone deacetylase inhibitors. J Cell
Biochem. 96:533–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Ruijter AJ, van Gennip AH, Caron HN,
Kemp S and van Kuilenburg AB: Histone deacetylases (HDACs):
Characterization of the classical HDAC family. Biochem J.
370:737–749. 2003. View Article : Google Scholar
|
|
30
|
Delcuve GP, Rastegar M and Davie JR:
Epigenetic control. J Cell Physiol. 219:243–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng QF, Wang HM, Wang ZF, Liu JY, Zhang
Q, Zhang L, Lu YH, You H and Jin GH: Reprogramming of histone
methylation controls the differentiation of monocytes into
macrophages. FEBS J. 284:1309–1323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin H, Zhao A, Zhang C and Fu X:
Epigenetic control of reprogramming and transdifferentiation by
histone modifications. Stem Cell Rev. 12:708–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sepulveda H, Aguilar R, Prieto CP, Bustos
F, Aedo S, Lattus J, van Zundert B, Palma V and Montecino M:
Epigenetic signatures at the RUNX2-P1 and Sp7 gene promoters
control osteogenic lineage commitment of umbilical Cord-derived
mesenchymal stem cells. J Cell Physiol. 232:2519–2527. 2017.
View Article : Google Scholar
|
|
34
|
Shahbazian MD and Grunstein M: Functions
of site-specific histone acetylation and deacetylation. Annu Rev
Biochem. 76:75–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hatakeyama Y, Hatakeyama J, Takahashi A,
Oka K, Tsuruga E, Inai T and Sawa Y: The effect of valproic Acid on
mesenchymal pluripotent cell proliferation and differentiation in
extracellular matrices. Drug Target Insights. 5:1–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paino F, La Noce M, Tirino V, Naddeo P,
Desiderio V, Pirozzi G, De Rosa A, Laino L, Altucci L and Papaccio
G: Histone deacetylase inhibition with valproic acid downregulates
osteocalcin gene expression in human dental pulp stem cells and
osteoblasts: Evidence for HDAC2 involvement. Stem Cells.
32:279–289. 2014. View Article : Google Scholar :
|
|
37
|
Xu Y, Hammerick KE, James AW, Carre AL,
Leucht P, Giaccia AJ and Longaker MT: Inhibition of histone
deacetylase activity in reduced oxygen environment enhances the
osteogenesis of mouse adipose-derived stromal cells. Tissue Eng
Part A. 15:3697–3707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee S, Park JR, Seo MS, Roh KH, Park SB,
Hwang JW, Sun B, Seo K, Lee YS, Kang SK, et al: Histone deacetylase
inhibitors decrease proliferation potential and multilineage
differentiation capability of human mesenchymal stem cells. Cell
Prolif. 42:711–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rashid MM, Akiba Y, Akiba N, Kaku M and
Uoshima K: Effect of valproic acid on bone healing in rat.
IADR/AADR/CADR General Session and Exhibition. 2013.
|
|
40
|
Grose AW, Gardner MJ, Sussmann PS, Helfet
DL and Lorich DG: The surgical anatomy of the blood supply to the
femoral head: Description of the anastomosis between the medial
femoral circumflex and inferior gluteal arteries at the hip. J Bone
Joint Surg Br. 90:1298–1303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zlotorowicz M and Czubak J: Vascular
anatomy and blood supply to the femoral head. Osteonecrosis. 19–25.
2014.
|
|
42
|
Li GY, Feng Y, Cheng TS, Yin JM and Zhang
CQ: Edaravone, a novel free radical scavenger, prevents
steroid-induced osteonecrosis in rabbits. Rheumatology. 52:438–447.
2013. View Article : Google Scholar
|
|
43
|
Chen CH and Wang GJ: Alendronate in the
prevention of collapse of the femoral head in nontraumatic
osteonecrosis. Osteonecrosis. 7:265–271. 2014.
|
|
44
|
Nishida K, Yamamoto T, Motomura G,
Jingushi S and Iwamoto Y: Pitavastatin may reduce risk of
steroid-induced osteonecrosis in rabbits: A preliminary
histological study. Clin Orthop Relat Res. 466:1054–1058. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou D, Qi C, Chen YX, Zhu YJ, Sun TW,
Chen F and Zhang CQ: Comparative study of porous
hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone
regeneration in calvarial defects. Int J Nanomedicine.
12:2673–2687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo S, Yang Y, Chen J, Zhong Z, Huang H,
Zhang J and Cui L: Tanshinol stimulates bone formation and
attenuates dexamethasone-induced inhibition of osteogenesis in
larval zebrafish. J Orthopaedic Translation. 4:35–45. 2015.
View Article : Google Scholar
|
|
47
|
Fujita T, Fukuyama R, Enomoto H and Komori
T: Dexamethasone inhibits insulin-induced chondrogenesis of ATDC5
cells by preventing PI3K-Akt signaling and DNA binding of Runx2. J
Cell Biochem. 93:374–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
49
|
Zhang YL, Yin JH, Ding H, Zhang W, Zhang
CQ and Gao YS: Vitamin K2 prevents
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Int J Biol Sci. 12:347–358. 2016. View Article : Google Scholar :
|
|
50
|
Guo SC, Tao SC, Yin WJ, Qi X, Sheng JG and
Zhang CQ: Exosomes from human synovial-derived mesenchymal stem
cells prevent glucocorticoid-induced osteonecrosis of the femoral
head in the rat. Int J Biol Sci. 12:1262–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Siéssere S, Semprini M, Lopes RA, Sala MA
and Mattos MC: Morphological and morphometric alterations induced
by valproic acid on rat fetuses' Meckel's cartilage, lingual
musculature, and submandibular gland. Int J Morphol. 22:133–137.
2004. View Article : Google Scholar
|
|
52
|
Welbat JU, Chaisawang P,
Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Sripanidkulchai B
and Wigmore P: Kaempferia parviflora extract ameliorates the
cognitive impairments and the reduction in cell proliferation
induced by valproic acid treatment in rats. Ann Anat. 206:7–13.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cho HH, Park HT, Kim YJ, Bae YC, Suh KT
and Jung JS: Induction of osteogenic differentiation of human
mesenchymal stem cells by histone deacetylase inhibitors. J Cell
Biochem. 96:533–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS,
Park SW, Kim SY and Shin CS: Activation of peroxisome
proliferator-activated receptor-gamma inhibits the Runx2-mediated
transcription of osteocalcin in osteoblasts. J Biol Chem.
278:23270–23277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Morimoto E, Li M, Khalid AB, Krum SA,
Chimge NO and Frenkel B: Glucocorticoids Hijack Runx2 to stimulate
Wif1 for suppression of osteoblast growth and differentiation. J
Cell Physiol. 232:145–153. 2017. View Article : Google Scholar :
|
|
56
|
Shi XM, Chutkan N, Hamrick MW and Isales
CM: Mechanism of glucocorticoid-induced osteoporosis: An update.
Intech. 2012. View
Article : Google Scholar
|
|
57
|
Marquez-Curtis LA, Qiu Y, Xu A and
Janowska-Wieczorek A: Migration, proliferation, and differentiation
of cord blood mesenchymal stromal cells treated with histone
deacetylase inhibitor valproic acid. Stem Cells Int.
2014:6104952014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tomé M, López-Romero P, Albo C, Sepúlveda
JC, Fernández-Gutiérrez B, Dopazo A, Bernad A and González MA:
miR-335 orchestrates cell proliferation, migration and
differentiation in human mesenchymal stem cells. Cell Death Differ.
18:985–995. 2011. View Article : Google Scholar :
|
|
59
|
Ciciarello M, Zini R, Rossi L, Salvestrini
V, Ferrari D, Manfredini R and Lemoli RM: Extracellular purines
promote the differentiation of human bone marrow-derived
mesenchymal stem cells to the osteogenic and adipogenic lineages.
Stem Cells Dev. 22:1097–1111. 2013. View Article : Google Scholar :
|
|
60
|
Ding H, Wang T, Xu D, Cha B, Liu J and Li
Y: Dexamethasone-induced apoptosis of osteocytic and osteoblastic
cells is mediated by TAK1 activation. Biochem Biophys Res Commun.
460:157–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim SM, Kim YG, Park JW, Lee JM and Suh
JY: The effects of dexamethasone on the apoptosis and osteogenic
differentiation of human periodontal ligament cells. J Periodontal
Implant Sci. 43:168–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pang J, Huang H, Zhang Q, et al: The
effect of dexamethasone on the proliferation and apoptosis of human
bone marrow mesenchymal stem cells in vitro. Modern Med J China.
2008.
|
|
63
|
Gao B, Huang Q, Jie Q, Zhang HY, Wang L,
Guo YS, Sun Z, Wei BY, Han YH, Liu J, et al: Ginsenoside-Rb2
inhibits dexamethasone-induced apoptosis through promotion of
GPR120 induction in bone marrow-derived mesenchymal stem cells.
Stem Cells Dev. 24:781–790. 2015. View Article : Google Scholar
|
|
64
|
Komori T: Regulation of osteoblast
differentiation by Runx2. Adv Exp Med Biol. 658:43–49. 2010.
View Article : Google Scholar
|
|
65
|
Rimando MG, Wu HH, Liu YA, Lee CW, Kuo SW,
Lo YP, Tseng KF, Liu YS and Lee OK: Glucocorticoid receptor and
Histone deacetylase 6 mediate the differential effect of
dexamethasone during osteogenesis of mesenchymal stromal cells
(MSCs). Sci Rep. 6:373712016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pujols L, Mullol J, Pérez M, Roca-Ferrer
J, Juan M, Xaubet A, Cidlowski JA and Picado C: Expression of the
human glucocorticoid receptor alpha and beta isoforms in human
respiratory epithelial cells and their regulation by dexamethasone.
Am J Respir Cell Mol Biol. 24:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li LB, Leung DY, Martin RJ and Goleva E:
Inhibition of histone deacetylase 2 expression by elevated
glucocorticoid receptor beta in steroid-resistant asthma. Am J
Respir Crit Care Med. 182:877–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Adcock IM: Glucocorticoid-regulated
transcription factors. Pulm Pharmacol Ther. 14:211–219. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang YY, Li X, Qian SW, Guo L, Huang HY,
He Q, Liu Y, Ma CG and Tang QQ: Down-regulation of type I Runx2
mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol
Endocrinol. 26:798–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Adcock IM, Cosio B, Tsaprouni L, Barnes PJ
and Ito K: Redox regulation of histone deacetylases and
glucocorticoid-mediated inhibition of the inflammatory response.
Antioxid Redox Signal. 7:144–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ito K, Yamamura S, Essilfie-Quaye S, Cosio
B, Ito M, Barnes PJ and Adcock IM: Histone deacetylase 2-mediated
deacetylation of the glucocorticoid receptor enables NF-kappaB
suppression. J Exp Med. 203:7–13. 2006. View Article : Google Scholar
|
|
72
|
Okazaki S, Nagoya S, Matsumoto H, Mizuo K,
Sasaki M, Watanabe S, Yamashita T and Inoue H: Development of
non-traumatic osteonecrosis of the femoral head requires toll-like
receptor 7 and 9 stimulations and is boosted by repression on
nuclear factor kappa B in rats. Lab Invest. 95:92–99. 2015.
View Article : Google Scholar
|
|
73
|
Chen S, Li J, Peng H, Zhou J and Fang H:
Administration of erythropoietin exerts protective effects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Int J Mol Med. 33:840–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Han N, Yan ZQ, Guo CA, Shen F, Liu J, Shi
YX and Zhang ZY: Effect of rifampicin on the risk of
steroid-induced osteonecrosis of the femoral head. Orthop Surg.
2:124–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tseng MT, Hsieh SC, Shun CT, Lee KL, Pan
CL, Lin WM, Lin YH, Yu CL and Hsieh ST: Skin denervation and
cutaneous vasculitis in systemic lupus erythematosus. Brain.
129:977–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Suda S, Katsura K, Kanamaru T, Saito M and
Katayama Y: Valproic acid attenuates ischemia-reperfusion injury in
the rat brain through inhibition of oxidative stress and
inflammation. Eur J Pharmacol. 707:26–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rao T, Wu F, Xing D, Peng Z, Ren D, Feng
W, Chen Y, Zhao Z, Wang H, Wang J, et al: Effects of valproic Acid
on axonal regeneration and recovery of motor function after
peripheral nerve injury in the rat. Arch Bone Jt Surg. 2:17–24.
2014.PubMed/NCBI
|
|
78
|
Zhang Z, Qin X, Tong N, Zhao X, Gong Y,
Shi Y and Wu X: Valproic acid-mediated neuroprotection in retinal
ischemia injury via histone deacetylase inhibition and
transcriptional activation. Exp Eye Res. 94:98–108. 2012.
View Article : Google Scholar
|
|
79
|
Hwabejire JO, Lu J, Liu B, Li Y, Halaweish
I and Alam HB: Valproic acid for the treatment of hemorrhagic
shock: A dose-optimization study. J Surg Res. 186:363–370. 2014.
View Article : Google Scholar
|
|
80
|
Krishnamoorthy G, Karande S, Ahire N,
Mathew L and Kulkarni M: Bone metabolism alteration on
antiepileptic drug therapy. Indian J Pediatr. 76:377–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lee HS, Lee CS, Jang JS, Lee JD and Um SM:
Changes of serum alkaline phosphatase and osteocalcin during
fracture healing. J Korean Orthopaedic Association. 37:411–415.
2002. View Article : Google Scholar
|
|
82
|
Komnenou A, Karayannopoulou M,
Polizopoulou ZS, Constantinidis TC and Dessiris A: Correlation of
serum alkaline phosphatase activity with the healing process of
long bone fractures in dogs. Vet Clin Pathol. 34:35–38. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Floerkemeier T, Hirsch S, Budde S, Radtke
K, Thorey F, Windhagen H and von Lewinski G: Bone turnover markers
failed to predict the occurrence of osteonecrosis of the femoral
head: A preliminary study. J Clin Lab Anal. 26:55–60. 2012.
View Article : Google Scholar : PubMed/NCBI
|