Introduction
Epilepsy is one of the most prevalent chronic
neurological disorders, which affects ~65,000,000 individuals
worldwide and is a global burden in terms of seizure-related
disability, comorbidities and mortality rates (1). Clinically, temporal lobe epilepsy
(TLE) is the most common form of drug-resistant and intractable
epilepsy, and is characterized by recurrent spontaneous seizures
(SRS) due to neuronal hyperactivity in the brain (2). Abnormal hippocampal neurogenesis is
a prominent feature of TLE, which may contribute to the hippocampal
network plasticity associated with epilepsy (3). The newborn neuron can be labeled by
doublecortin (DCX), a marker of transit-amplifying cells and
immature newborn neurons, which begin to have neuronal potential
(4). These newborn neurons are
continuously generated in the subgranular zone of the hippocampal
dentate gyrus, migrate into the granule layer and terminally
differentiate, mainly into new granule cell neurons (5). The newly generated ectopic granule
cells have immature synapses and exhibit spontaneous discharge,
which may be an important pathophysiological basis for chronic
spontaneous seizures (6).
However, the molecular mechanisms that regulate hippocampal
neurogenesis during the spontaneous seizure period remain to be
fully elucidated.
Ephrins, ligands for Eph receptors, are
membrane-bound proteins which are the largest member of the
receptor tyrosine kinase family and can be divided into A and B
subclasses, binding either A-class or B-class ligands; ephrin
ligands and receptors are important in the regulation of
morphologic processes, including axon guidance, angiogenesis, cell
migration and positioning, in the central nervous system (7). Increasing roles of the transmembrane
ephrin-B3 ligand have been revealed in the central nervous system.
In the hippocampal dentate gyrus, ephrin-B3/Eph-B1 is involved in
several functions, including neuronal progenitor sorting,
stochastic cell migration and guidance of neuronal growth cones
during the early developing brain (8). Ephrin-B3 can also reduce cell death
by inhibiting the functions of EphA4 receptors during adult
neurogenesis in the subventricular zone of the brain (9). Based on these reports, the present
study hypothesized that ephrin-B3 may affect hippocampal
neurogenesis during the spontaneous seizure period following
pilocarpine-induced epilepsy.
Ephrin-B3 can function by the regulation of other
signaling pathways via cell-cell interactions, including the
regulation of glutamate receptor signaling by inducing the tyrosine
phosphorylation of NR2 subunits in glutamatergic CA1 synapses in
the hippocampus (10). Of note,
ephrin-B3 is involved in the activation of the reelin signaling
pathway, which is essential in controlling neuronal migration in
the neocortex, hippocampus and cerebellum of reelin-deficient
mutant reeler mice (11,12). Reelin is an extracellular protein,
which affects several stages of neuronal migration and layering in
the developing brain; reelin protein acts mainly by binding to
low-density lipoprotein receptors and apolipoprotein E receptor 2,
and then initiates the tyrosine phosphorylation of intracytoplasmic
docking disabled protein 1 (Dab1) (13). The reelin pathway control the
shapes, sizes and types of dendritic spines, and synaptic
homeostasis; its dysregulation may occur in neurological and
psychiatric disorders, including epilepsy, targeting hippocampal
circuits (14). Although the
functions of ephrin-B3 and the reelin pathway during physiological
or pathological neurodevelopment are clear, the function of
ephrin-B3 in modulation of the reelin pathway in epilepsy remains
to be fully elucidated.
The aim of the present study was to investigate the
role of ephrin-B3 in hippocampal neurogenesis and the reelin
pathway in a pilocarpine-induced rat model, and to examine the
potential mechanism underlying the effect of ephrin-B3 on cell
proliferation and the reelin pathway in pilocarpine-induced SE
rats.
Materials and methods
Pilocarpine-induced SE
The animals were provided by the Shanghai Laboratory
Animal Center (Shanghai, China) and housed at the Experimental
Animal Center of Central South University (Changsha, China) at a
temperature of 23±1°C with a regular 12-h light/dark cycle and free
access to food and water. All animal experiments were performed in
accordance with the official recommendations of the Institutional
Animal Care and Use Committee of the Institute of Laboratory Animal
Science of Central South University (no. 201403142).
A total of 70 healthy, young male Sprague-Dawley
rats (aged 6–8 weeks, 200–250 g) were treated with pilocarpine (25
mg/kg, i.p., Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) to
induce epilepsy. The rats were administered with lithium chloride
(127 mg/kg, i.p., Sigma-Aldrich; EMD Millipore) 18–22 h prior to
pilocarpine injection. According to Racine's classification, SE was
defined as continuous seizures lasting at least 30 min, and rats
classified as Racine stage IV–V that fulfilled the SE criterion
were used in the present study (15). Following pilocarpine injection,
the rats spent ~30 min in the SE phase in Racine stage IV or V. The
matched controls (n=35) were injected i.p. with the same volume of
normal saline instead of lithium chloride and pilocarpine, and were
used as corresponding controls. All rats were administered with
chloral hydrate injection (3 ml/kg, i.p.; Tonghua Pharmaceutical,
Tonghua, China) to terminate behavioral seizures or limit
behavioral seizures.
Ephrin-B3 stimulation experiment
On day 7 following the onset of SE, the epileptic
and control rats were anesthetized with 10% chloral hydrate (3
ml/kg, i.p.) and were then fixed on a stereotaxic apparatus (RWD
Life Science, Shenzhen, China). The skin was shaved and sterilized
with iodine complex. The scalp was dissected in the middle line and
retracted bilaterally to expose the sagittal and coronal sutures.
Based on the Rat Brain Stereotaxic Atlas (16), bilateral hippocampal regions were
selected as sites for implantation. Two vertical bone holes were
drilled symmetrically 1.6 mm adjacent to the sagittal suture and
3.6 mm posterior to the anterior fontanel, with the anterior
fontanel as the center. A micro-osmotic pump (ALZET®;
DURECT Corporation, Cupertino, CA, USA) connected to a cannula
(ALZET® brain infusion kit) was inserted 2.8 mm under
the dura, and an infusion of Eph B3-Fc suspension (recombinant
mouse EphB3-Fc chimera; 50 µg/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) or Fc (50 µg/ml; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) were
injected into the brain for 7 days. The cannula was cemented onto
the skull with dental acrylic. Following removal of the pumps from
the skull, the wound was washed repeatedly with sterile saline and
was sutured closed. All rats survived, and remained active
following surgery.
Behavioral and electroencephalography
(EEG) recordings
Spontaneous behavioral seizures were observed in all
animals, including controls, following the induction of SE and were
usually detected for 60 days between 9 and 11 a.m., and between 3
and 5 p.m. each day using video monitoring. The recordings of the
spontaneous seizures consisted of the latency period of SRS,
seizure duration, frequency and intensity.
For the EEG recording, the rats were anesthetized
using a mixture of 3% isoflurane in 30% oxygen and 70% nitrous
oxide, and maintained with 1.5% isoflurane, and the animal was then
fixed into the stereotaxic apparatus (RWD Life Science, Shenzhen,
China). Electrodes were placed at the following positions on the
skull using an electro-microdriver (RWD Life Science): 2.5 mm
anterior to the bregma, 2.5 mm bilateral to the midline and 2.5 mm
posterior to the lambda on the midline). The electrodes were then
fixed with dental cement. Following recovery for 3 days, EEG
monitoring was performed to evaluate the frequency and duration of
EEG seizures. An EEG seizure was identified according to the
definition of a period of consistent and repetitive changes in
amplitude and frequency of electrical activity for at least 10 sec,
which was different from interictal activity (17). Data were collected and analyzed
using an acquisition system (Physical Signal Recorder RM6240;
Chengdu Instrument Factory, Chengdu, China).
Histological examination
The rats were deeply anesthetized and then
transcardially perfused with a successive administration of 0.9%
sterile saline and 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4;
4°C). Following perfusion, the brains were removed from the skull
and postfixed in 4% PFA for 24 h at 4°C. Subsequently, the brains
were rinsed in PBS and cryoprotected in 30% sucrose solution at 4°C
for 72 h. In particular, the coronal slices involving the middle
region of the dorsal hippocampus were collected from between −3.6
and −4.0 mm to the bregma and sectioned into 25 µm-thick
sections for staining using a freezing microtome (Leica CM 1850;
Leica Microsystems GmbH, Wetzlar, Germany).
Immunohistochemical labeling was used to evaluate
the protein distribution and expression. Based on standard
protocols, the sections were rinsed in PBS, and then incubated in
0.1% hydrogen peroxide for 30 min to minimize endogenous peroxidase
activity. The sections were then washed three times in PBS, placed
in citrate buffer (pH 6.0) which had been heated to 90°C, and then
maintained for 20 min at room temperature to retrieve antigen.
Following three washes with PBS, the sections were blocked with 10%
normal goat serum (Sigma-Aldrich, Merck KGaA; Darmstadt, Germany)
and avidin (200 µl/ml; Avidin/Biotin blocking kit; Vector
Laboratories, Burlingame, CA, USA) in PBS for 2 h at room
temperature. Subsequently, the sections were incubated with primary
rabbit ephrin-B3 (1:50, sc-271328; Santa Cruz Biotechnology, Inc.,
Dallas, Texas, USA); mouse reelin (1:50, sc-25346; Santa Cruz
Biotechnology, Inc.); and rabbit p-Dab1 (1:50, Tyr232; Affinity
Biologicals, Inc., Ancaster, ON, Canada) antibodies diluted in a
solution containing 10% serum and avidin in PBS at 4°C for 48 h.
Following rinsing in PBS, the sections were incubated in
biotinylated anti-mouse or anti-rabbit or anti-goat secondary
antiserum (1:200, 5260-0045, 5260-0038 and 5260-0035; KPL, Inc.,
Gaithersburg, MA, USA) for 1 h at room temperature. Following
washing in PBS, the sections were incubated with the ABC reagent
kit (VECTASTAIN®, Vector Laboratories) for 2 h,
visualized using DAB (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China), and counterstained with hematoxylin. Images of the
sections were captured using a Leica light microscope (Leica DM5000
B; Leica Microsystems GmbH) and analyzed using the ImagePro Plus
6.0 software package (Media Cybernetics, Inc., Rockville, MD, USA)
by two investigators, in a blinded-manner, to calculate the mean
optical density of immunoreactive products in each microscopic view
(magnification, ×40).
Immunofluorescent labeling was performed based on
standard protocols as described previously (2). The sections were incubated with
primary antibodies, including goat doublecortin protein (DCX,
1:125; sc-8066; Santa Cruz Biotechnology, Inc.) and rabbit neuronal
nuclei (NeuN, 1:1,000; ab177487; Abcam, Cambridge, MA, USA) at 4°C
overnight and visualized with appropriate Alexa Fluor-conjugated
secondary antibodies (1:1,000; A32723 and A32732; Invitrogen,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room
temperature for 6 h. All sections were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; Wuhan Boster Biological
Technology, Ltd.) and images were captured using a laser scanning
confocal microscope (LSM 510; Leica Microsystems GmbH). For
quantification analysis, five microscopic fields were selected to
observe the distribution of newborn neurons and calculate the cell
counts in each view (magnification, ×40) using the ImagePro Plus
6.0 software package (Media Cybernetics, Inc.) by two investigators
in a blinded manner. The positive cell counts were normalized to
the total number of cells in the regions analyzed in the
hippocampus.
SYBR Green-based reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The rats were sacrificed 7, 14 and 60 days following
the termination of SE, and the hippocampus was rapidly dissected
and transferred to liquid nitrogen. All the hippocampal samples
were stored at −80°C until processing. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
and reverse transcribed into cDNA using a RevertAid™ First-Strand
complementary DNA (cDNA) synthesis kit (Thermo Fisher Scientific,
Inc.). The qPCR sample contained the following: 1 µl cDNA, 1
µl sense primer, 1 µl antisense primer, 9.5 µl
RNase-free water and 12.5 µl Maxima®
SYBR-Green/ROX qPCR Master mix (Thermo Fisher Scientific, Inc.) to
obtain a 25 µl reaction volume. The sequences of PCR primers
and PCR product lengths were as follows: Ephrin-B3 (NM_001406),
forward 5′-ACTCAGCCTGGAGCCTGTCTAC-3′ and reverse
5′-CGATCTGAGGGTAAAGCACGTA-3′ (212 bp); and β-actin (NM_007993.3),
forward 5′-AATAAGTGGTTACAGGAAG-3′ and reverse
5′-GTATTAAGGCGGAAGATT-3′ (164 bp). The reactions were performed
using the following cycling conditions: Enzyme activation at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 15
sec, annealing at 60°C for 1 min and extension at 72°C for 20 sec.
The relative expression levels were calculated as ratios normalized
against those of β-actin and assessed using the ABI StepOnePlus™
real-time PCR system (Thermo Fisher Scientific, Inc.). The qPCR
data were analyzed using the 2−∆∆Cq method as described
previously (18).
Western blot analysis
The stored hippocampal samples from the rats
sacrificed 7, 14 and 60 days following the termination of SE were
homogenized in 400 µl RIPA lysis buffer (Beyotime Institute
of Biotechnology, Shanghai, China) supplemented with the protease
inhibitor PMSF (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) and the phosphatase inhibitor PhosSTOP (Roche
Diagnostics, Basel, Switzerland). The lysates were centrifuged at
14,000 × g for 20 min at 4°C. Bicinchoninic acid assays were used
to determine protein concentrations, as described previously
(19). Equal quantities of
protein (50 µg) extracts were separated by electrophoresis
on 12% polyacrylamide gels at 120 V. The proteins were then
transferred onto polyvinylidence difluoride membranes in 120 mM
glycine, 125 mM Tris, 0.1% sodium dodecyl sulfate and 20% methanol
at 150 mA for 1.5 h. The membranes were blocked in 5% non-fat dry
milk in Tris-buffered saline containing 0.1%Tween-20 (TBST) at 4°C.
The membranes were then incubated with the following primary
antibodies: Anti-ephrin-B3 (1:1,000; sc-271328; Santa Cruz
Biotechnology, Inc.); anti-p-Dab1 antibodies (1:1,000; Tyr232;
Affinity Biologicals); anti-Dab1 (1:2,000; sc-271136; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Secondary antibodies
(1:2,000; 5220-0283, 5220-0460 and 5450-0010; KPL, Inc.) conjugated
to horseradish peroxidase were used at a dilution of 1:1,000 in
blocking solution at room temperature for 2 h. The membranes were
then washed three times with TBST and labeling was visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). The specific bands visible on images were scanned using the
Bio-Rad Gel Doc 2000 imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and measured with Image Lab 3.0 image analysis
software (Bio-Rad Laboratories, Inc.). The relative intensities of
ephrin-B3 were normalized to the internal reference protein β-actin
(1:1,000; ABP50151; Beyotime Institute of Biotechnology) and the
relative intensity of p-Dab1 was normalized to total Dab1.
Statistical analysis
All results are presented as the mean ± standard
deviation. Statistical analyses were performed using GraphPad Prism
6.0 (GraphPad Software, Inc., La Jolla, CA, USA). All continuous
variables were tested to confirm that they fit a normal
distribution or homoscedasticity according to the results of
Levene's test prior to further analysis. Comparisons among groups
were analyzed using two-way analysis of variance (ANOVA), followed
by multiple Tukey's post hoc test (α=0.05) to assess the difference
between any two groups. EEG data between groups was compared using
one-way ANOVA, followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Dynamic expression pattern of ephrin-B3
in rats during the development of SE
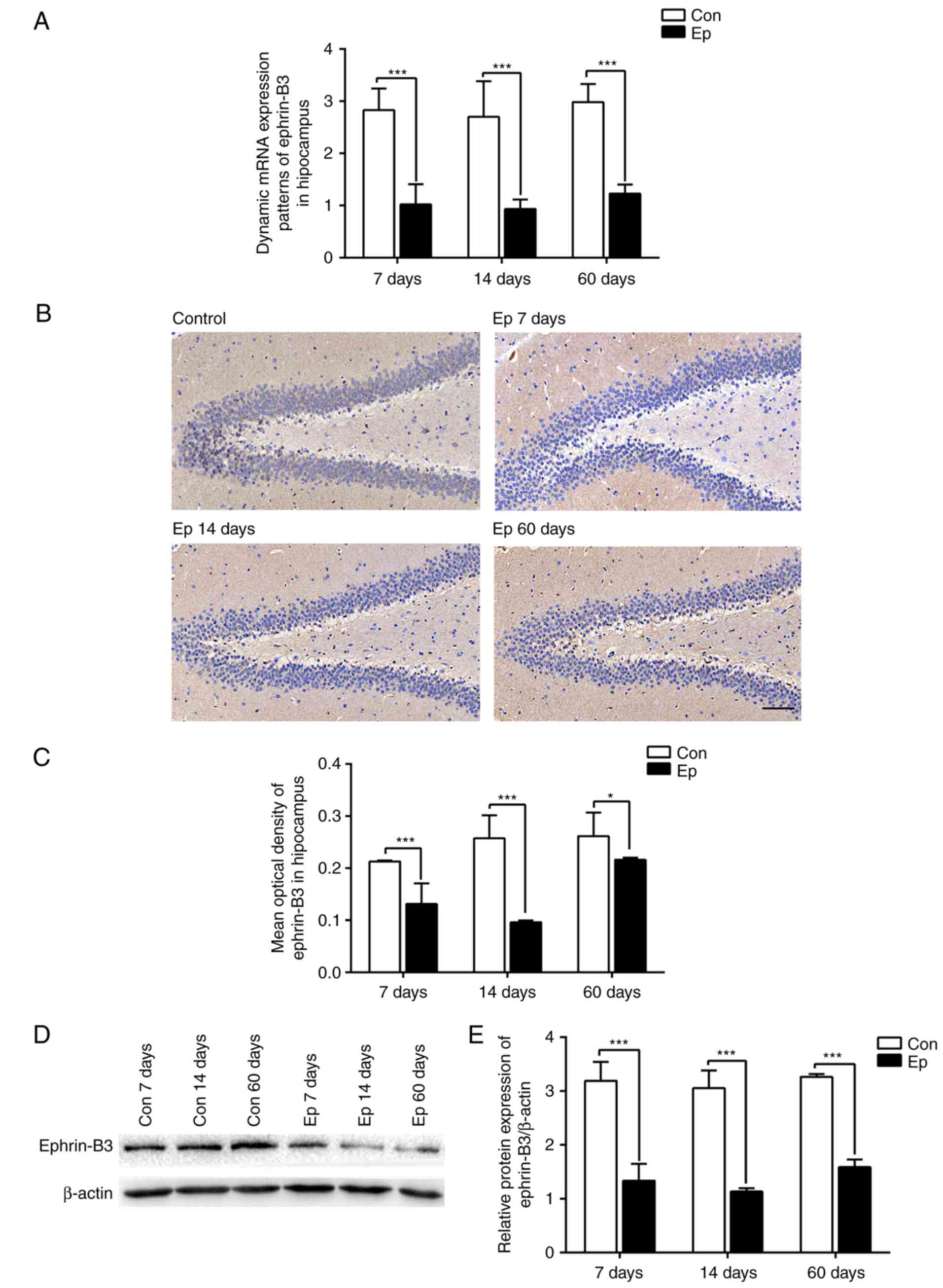
The results of the RT-qPCR analysis showed a
significant downregulation in the gene expression of ephrin-B3 in
the hippocampal tissues of epileptic rats (Ep group) during the
three phases of SE development (P<0.05; Fig. 1A). Following pilocarpine-induced
SE, the expression of ephrin-B3 was lower in the 7 days group
compared with that in the 60 days group, and the lowest expression
was observed in the 14 days group (P<0.05).
Subsequently, immunohistochemistry was used to
clarify the distribution of ephrin-B3 in the rat hippocampus;
representative images are shown in Fig. 1B. The results of the
immunohistochemistry revealed that eprhin-B3 was prominent in
granule cells in the dentate gyrus, subgranular zone and hilus in
the hippocampus, was sparse in the inner and outer molecular
layers, and was mainly expressed in the cell membrane.
Additionally, the expression of ephrin-B3 in the hippocampus was
decreased in the 14 days group, compared with that in the 7 days
group, but was increased in the 60 days group (Fig. 1C). These results were confirmed by
detecting the protein expression of ephrin-B3 by western blot
analysis, which showed similar dynamic changes to gene expression
(Fig. 1D and E).
Hippocampal neurogenesis in rats during
the development of SE
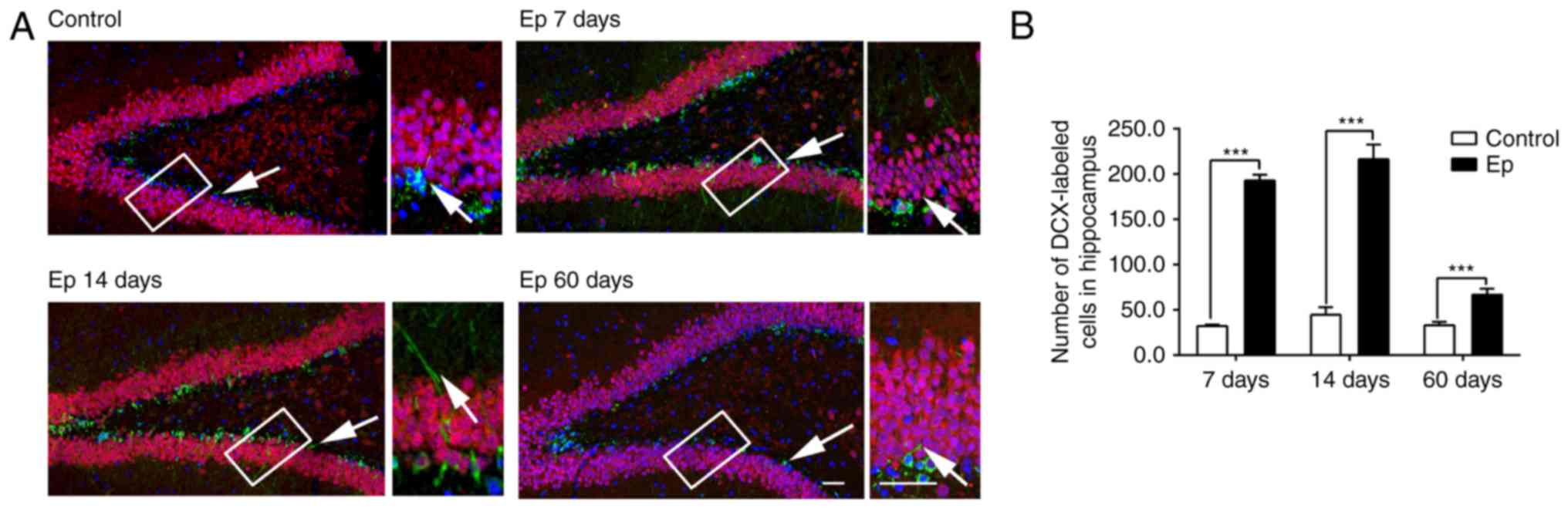
To examine hippocampal neurogenesis following
pilocarpine-induced SE, DCX and NeuN were used for double-labeling
to measure the changes in newborn neurons in the hippocampus
post-SE, as they can recognize late mitotic neuronal precursors and
newly-generated postmitotic neurons. DCX is a marker of
transit-amplifying cells and immature neurons, which begin to have
neuronal potential, whereas NeuN labels postmitotic neurons, which
have been found to be mature granule cells. The DCX/NeuN-positive
cells showed the features of newly generated neurons in the rats;
representative images are shown in Fig. 2A. The number of newborn neurons
was significantly increased in the rats following
pilocarpine-induced SE (P<0.05; Fig. 2B). Consistent with the increased
number of newborn neurons, the DCX-positive cells in the Ep rats
clustered along the subgranular zone and revealed premature
branching of the dendritic outgrowth within the granule cell layer,
random and nonradial orientation of the outgrowth, and numerous
abnormal neuronal processes when compared with the control group.
The images indicated the abnormal positioning of DCX-positive cells
in the hippocampus and abnormal morphology of dendritic outgrowth
following pilocarpine induction.
Reelin pathway expression in rats during
the development of SE
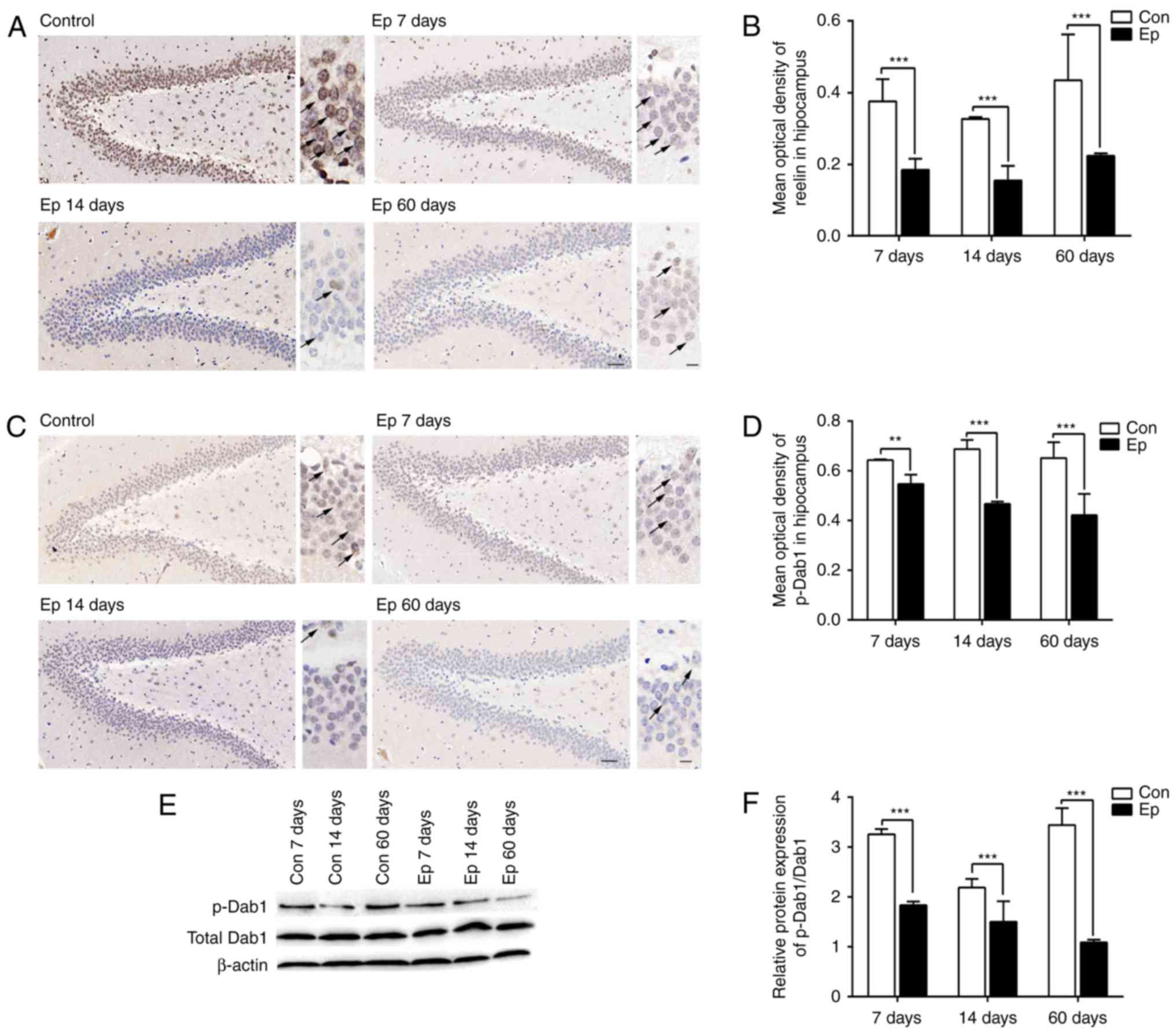
As shown in Fig.
3A, the immunohistochemistry results revealed a significant
downregulation in the expression of reelin in the rat hippocampus
during the three phases of SE, with a lower expression at 7 days,
compared with that at 60 days, and the lowest expression observed
at 14 days (P<0.05; Fig. 3B).
The immunohistochemistry also revealed the distribution of reelin,
which was mainly expressed in the interneurons in the dentate
gyrus, hilus and subgranular zone, and labeled as numerous punctate
structures on the cell membrane. The phosphorylation of Dab1 is
necessary for the activation of reelin signaling. The
immunohistochemistry revealed that, compared with the control rats,
the Ep rats showed significantly decreased expression of p-Dab1 on
days 7, 14 and 60 post-SE induction (P<0.05; Fig. 3C and D). The immunohistochemistry
also revealed that p-Dab1 was labeled in the dentate granule cell
body in the hilus and dentate gyrus, the inner molecular layer and
stratum radiatum of the hippocampus. In addition, the expression
levels of p-Dab1 and total Dab1 were assessed using western blot
analysis following pilocarpine-induced SE (Fig. 3E and F). The expression of p-Dab1
was downregulated, whereas that of total Dab1 remained
unchanged.
Effects of ephrin-B3 stimulator on
hippocampal neurogenesis
To further examine whether ephrin-B3 can affect
hippocampal neurogenesis in the hippocampus in Ep rats, a
stimulator of ephrin-B3 with EphB3-Fc/Fc-control was injected into
the Ep rats 7 days following pilocarpine-induced SE (Fig. 4A). The expression level of
ephrin-B3 was confirmed to be upregulated following injection with
EphB3-Fc, whereas no changes were found in the Fc-control groups
(Fig. 4B). As shown in Fig. 4C and D, following EpB3-Fc
injection, the number of newborn neurons was significantly
decreased, compared with that following Fc-control treatment
(P<0.05). DCX-labeled cells in the Ep rats post-EphB3-Fc
treatment were scattered in the subgranular zone, with short
dendrites that extended through <1/3 of the granular cell layer,
compared with those in the Fc-control group. No significant
difference was observed in the control groups between the EphB3-Fc
and Fc-control treatments. These results suggested that EphB3-Fc
reduced the number of newborn neurons and abnormal dendritic
outgrowth following pilocarpine-induced SE.
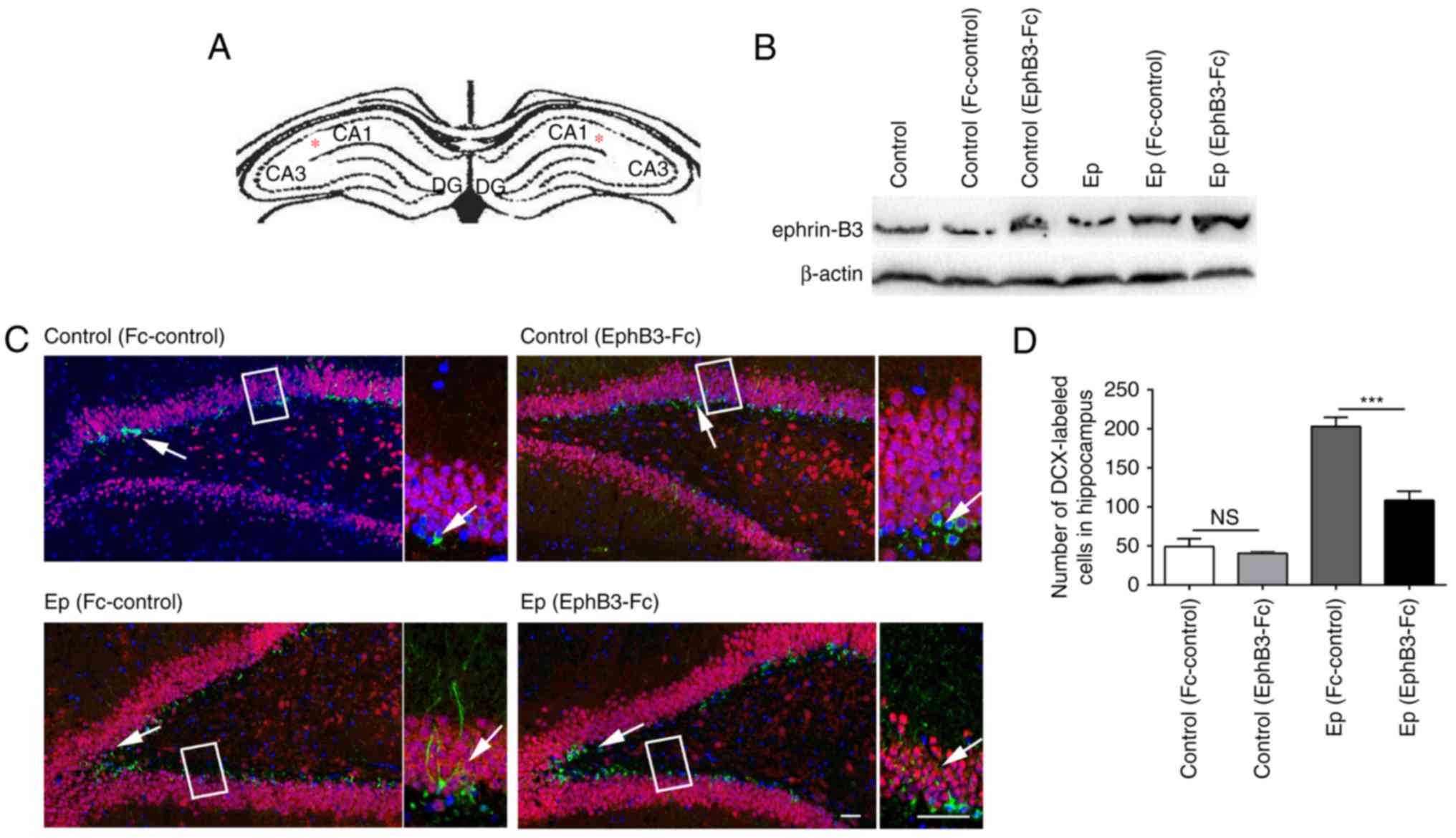 | Figure 4Effect of EphB3-Fc on hippocampal
neurogenesis post-SE. (A) Schematic of a typical coronal
hippocampal section, illustrating the region of EphB3-Fc injection.
Asterisks represent the injection sites. (B) Expression of
ephrin-B3 increased following EphB3-Fc infusion, detected by
western blot analysis. (C) Representative immunofluorescent results
of hippocampal neurogenesis using DCX (green), NeuN (red) and DAPI
(blue) antibodies to identify progenitor cells (green) and mature
neurons (red). The arrows indicate the DCX/DAPI co-labeled newborn
neurons. High-magnification bright-field images are shown in the
boxed section. (D) Bar graph results of the immunofluorescent
results. Following EphB3-Fc treatment, Ep rats showed fewer
DCX-labeled cells, compared with the Fc-control rats, whereas no
significant difference was found between EphB3-Fc and Fc-control
treatment in the control rats. Data are presented as the mean ±
standard deviation. Scale bar=50 µm.
***P<0.0001, ns, (P>0.05) by two-way analysis of
variance with Tukey's post hoc test (n=5 slices/per group). Ep,
epilepsy; DCX, doublecortin; DAPI, 4′,6-diamidino-2-phenylindole;
ns, not significant. |
Effects of ephrin-B3 stimulator on the
reelin pathway
To further examine whether ephrin-B3 can affect the
protein expression levels of reelin and p-Dab1 in the hippocampus
of Ep rats, the present study detected the expression levels of
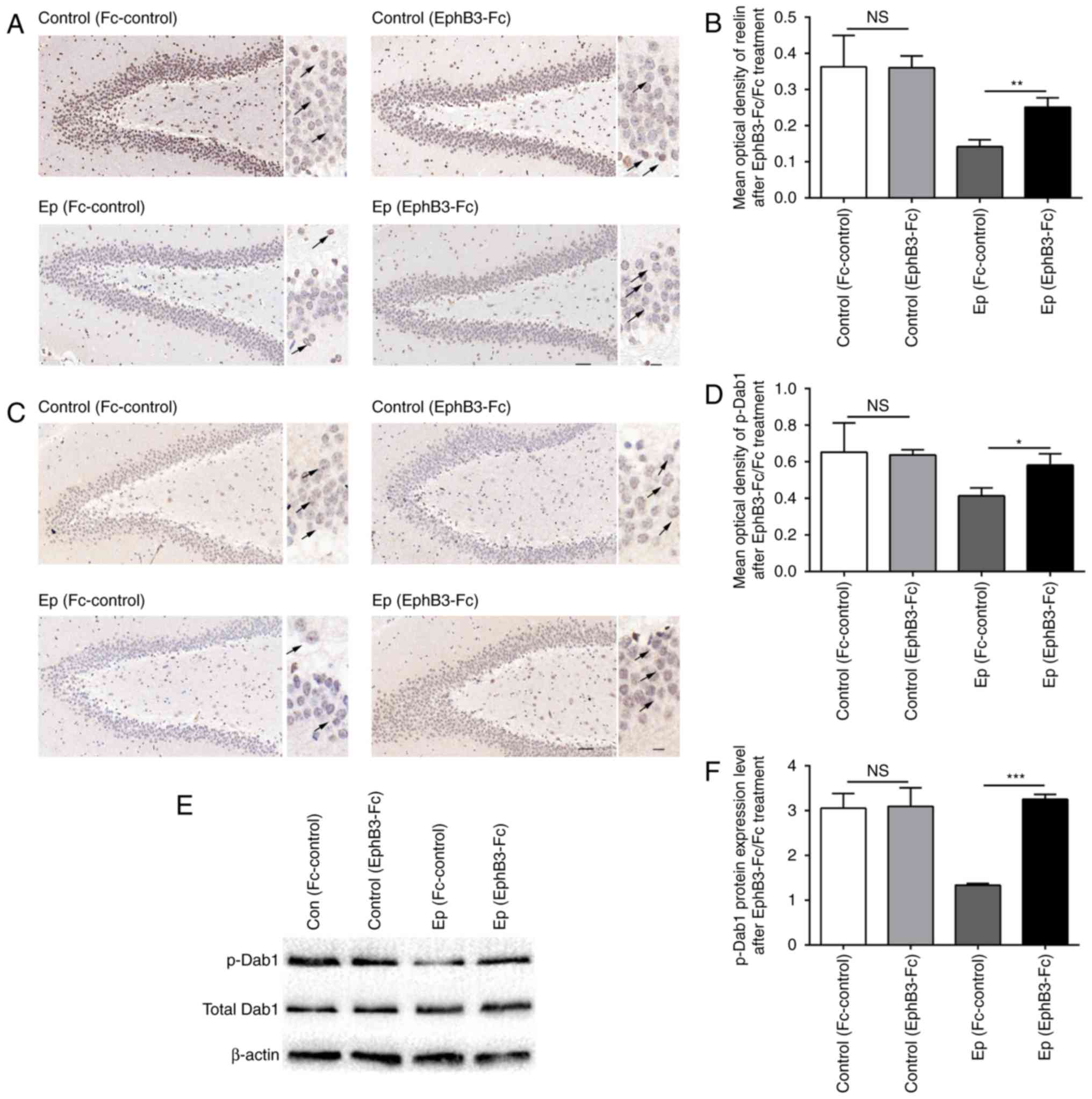
reelin and p-Dab1 following EphB3-Fc injection (Fig. 5). The immunohistochemistry results
showed that the injection of ephrin-B3 stimulator markedly
increased the expression levels of reelin in the hippocampus of Ep
rats post-SE (P<0.05), whereas no significant increase in the
protein expression of reelin was observed in the rats injected with
Fc-control (Fig. 5A). In
addition, as shown in Fig. 5B and
C, the expression of p-Dab1 detected by immunohistochemistry
was increased following EphB3-Fc treatment (P<0.05), whereas
Fc-control treatment had no effect on the level of p-Dab1 post-SE.
The expression of p-Dab1 was also confirmed using western blot
analysis (Fig. 5D and E), which
also showed upregulation in the hippocampus of Ep rats following
EphB3-Fc treatment. By contrast, the hippocampal level of total
Dab1 was not altered following EphB3-Fc or Fc-control treatment. No
significant difference was found in the expression of reelin or
p-Dab1 in the control rats between the EphB3-Fc and Fc-control
treatment groups.
Characteristics of behaviors and EEG
following ephrin-B3 stimulation treatment in the rat model
All the rats were monitored to record their
behaviors and EEG. The control animals without injections of
pilocarpine did not exhibit seizure activities. Following several
minutes of pilocarpine injection, the rats exhibited facial muscle,
forelimb and tail clonus, and ataxic lurching, head bobbing and wet
dog shakes. In the latent stage of SE (14 days post-SE), no
behavioral changes or seizures were observed in the Ep rats.
Spontaneous recurrent seizures occurred at 60 days post-SE in the
Ep rats (chronic stage of SE). However, following EphB3-Fc
treatment, it was found that the Ep rats exhibited reduced average
latency periods to the onset of seizure, seizure thresholds and
seizure frequencies, compared with the Fc-control-treated rats
(P<0.05); whereas Fc-control treatment had no effect on
seizures. No significant difference was found in seizure activities
of the control rats following EphB3-Fc or Fc-control treatment
(data not shown).
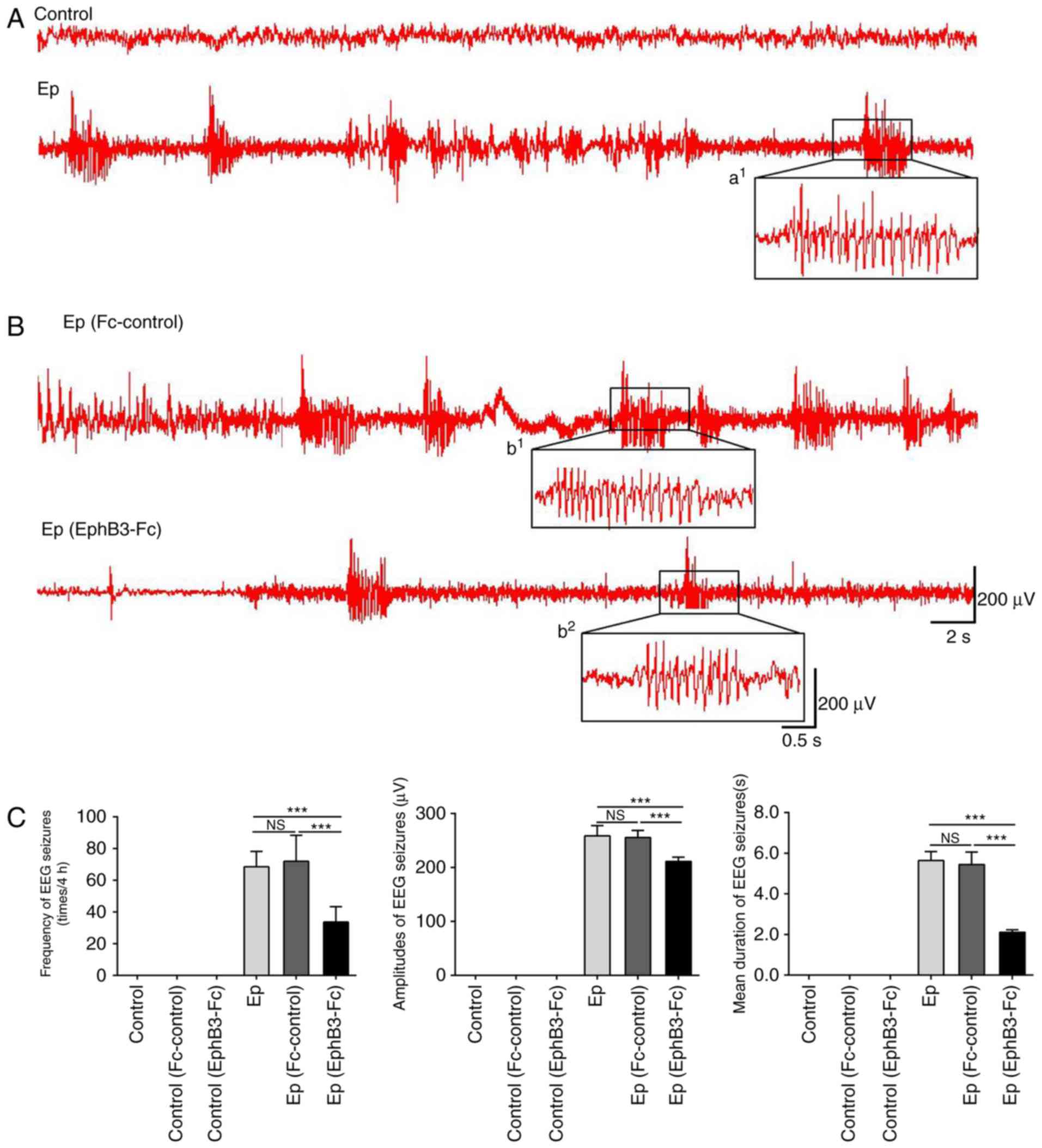
EEG recordings that showed high frequency and
amplitude, poly-spike paroxysmal electrical activities, and
persisted for at least 10 sec were considered to be EEG seizures
(Fig. 6A–C). No EEG changes or
seizures were observed in the control rats. The pilocarpine-induced
rats exhibited EEG seizures characterized by a recruiting rhythmic
activity followed by spiking activity of progressively increased
frequency and voltage. Treatment with EphB3-Fc significantly
suppressed the frequency, duration and the firing amplitudes of
spontaneous seizures in the Ep rats. However, no significant
differences were found in frequency, duration or epileptiform
activity in the control rats following EphB3-Fc or Fc-control
treatment.
Discussion
The present study is the first, to the best of our
knowledge, to demonstrate the role of ephrin-B3 in neurogenesis and
the reelin pathway during the development of SE in epileptic rats.
By integrating the data, it was found that the expression of
ephrin-B3 was significantly downregulated in epileptic rats; the
expression of ephrin-B3 was lower in the acute phase, compared with
that in the chronic phase, and was lowest in the latent phase. The
reelin pathway showed similar dynamic changes to the expression of
ephrin-B3. The pilocarpine-induced rats exhibited increased
hippocampal neurogenesis during the spontaneous period of SE. The
effects of ephrin-B3 on hippocampal neurogenesis and reelin pathway
expression levels were examined, and it was found that, following
the administration of exogenous ephrin-B3, the epileptic rats
showed subsequent increases in hippocampal reelin pathway
expression and reduced neurogenesis, accompanied by the suppression
of spontaneous seizures. These results suggested the underlying
functions of ephrin-B3 on the modulation of the reelin pathway, and
the suppression of neurogenesis and seizure activity in
epilepsy.
Increasing investigations have started to
investigate the role of Eph receptor and its ligand ephrins in the
central nervous system (20).
Ephrin-B3, the ligand of EphB receptor is critical in the
regulation of cell signaling, neuronal migration and cell
proliferation, dendritic spine maturity, formation of excitatory
synapses, and synaptic plasticity in the central nervous system
(21,22). In a previous animal study, mice
lacking ephrin-B3 or EphB1 receptor exhibited disrupted hippocampal
neurogenesis, including polarity, cell positioning and
proliferation (8). In the present
study, it was demonstrated that ephrin-B3 was significantly
decreased post-SE in epileptic rats, which corroborated with the
previous finding that the involvement of ephrin-B3 is an important
regulator of neuronal cell positioning in the central nervous
system (12). The present study
also found that the expression of ephrin-B3 was marginally
increased in epileptic rats in the chronic phase. A possible
explanation for this increase is that ephrin-B3 binding to its
cognate receptor can induce a small depression of excitatory
synaptic transmission in cultured hippocampal neurons (23). The increase of ephrin-B3 may serve
as a compensatory mechanism to maintain homeostatic balance in the
chronic stage of SE.
Hippocampal neurogenesis in several animal models of
epilepsy can result in alteration of the excitability of neurons,
which contributes to spontaneous recurrent seizures (24,25). Seizures can activate progenitor
cells in the subgranular zone of the dentate gyrus in the
hippocampus, and these newborn neurons integrate into the
hippocampal circuitry and contribute to hippocampal network
plasticity, thereby affecting epilepsy (25,26). Consistent with the findings in the
present study, Song et al also found that newborn neurons
exhibited abnormal dendritic development, including longer apical
and basal dendrites, and were accompanied by long-term seizure
activity in an intraventricular kainic acid model of epilepsy
(27). Of note, the hippocampal
neurogenesis and neuronal migration stimulated by SE were prominent
at least within 28 days, which coincided with the finding in the
latent phase in the present study (24). By comparing the number and
features of newborn neurons following EphB3-Fc injection, the
epileptic rats showed a reduction in proliferation, altered
migration and relatively normal branching of newborn neurons. These
results further support the hypothesis that ephrin-B3 is involved
in regulating the proliferation and migration of neural progenitors
during the development of SE.
Furthermore, the present study examined the role of
ephrin-B3 in the regulation of reelin pathway during the
development of SE. In association with other observations in human
temporal lobe epilepsy specimens (28), the present study showed the
downregulation of reelin pathway expression in epileptic rats,
which indicated that this pathway may also be involved in neuronal
migration post-SE. Several studies have suggested that reelin is
also associated with epilepsy; for example, reelin deficiency has
an effect on granule cell dispersion in mesial temporal lobe
epilepsy (29), and heterozygous
reelin mutation is associated with a clinical phenotype of temporal
lobe epilepsy in humans (30).
Baek et al also found that the misexpression of reelin can
lead to a migration defect in neurons of focal malformations in
cortical development in the human and mouse brain (31). In addition, a previous study
demonstrated that Dab1-deficient mice exhibit interictal
epileptiform abnormalities and a reduced latency to
pilocarpine-induced SE (32),
which is similar to the findings in the present study. However,
following the administration of exogenous ephrin-B3 with EphB3-Fc
in the present study, subsequent upregulation in the expression of
reelin and downstream p-Dab1 were observed in the hippocampus. This
finding is consistent with a report by Senturk et al, which
showed the ephrin-B3 can activate the reelin pathway in
reelin-knockout mice (12).
However, the findings in the present study are the first, to the
best of our knowledge, to demonstrate the dynamic changes in the
reelin pathway and the role of ephrin-B3 in modulating the reelin
pathway during the development of SE.
There were limitations to the present study. First,
only one time point was examined, rather than longitudinal
behavioral and molecular changes following injection. This is due
to our findings that ephrin-B3 and reelin decreased from 7 days and
were expressed at its lowest level in 14 days. Secondly, the
present study only showed the expression of the reelin pathway
following exogenous ephrin-B3 injection, with no intervening of the
reelin pathway; future investigations aim to include interventional
methods to further investigate the downstream proteins and confirm
the effect of ephrin-B3 on the reelin pathway. Finally,
preclustered Eph-B3-Fc molecules increase ephrin-B3 protein by its
bidirectional signaling (33),
therefore, it is possible to cluster ephrin-B1 or B2 (7). However, ephrin-B3 is the primarily
activated protein by EphB3-Fc in cortical and hippocampal neurons
according to previous studies (12). Therefore, may be useful to examine
the function of ephrin-B1 or B2 and attempt other genetic methods,
including ephrin-B3 transgenic mice, in the future.
In conclusion, the observations in the present study
provide evidence that ephrin-B3 is key in the amelioration of
neurogenesis and suppression of neuronal excitation in epilepsy.
Furthermore, ephrin-B3 was found to be involved in modulation of
the reelin signaling pathway during the development of epilepsy.
These findings support the hypothesis that the administration of
ephrin-B3 may assist in preventing recurrent seizures, at least in
an animal model. Further investigations are required to validate
whether, and to what extent, the results obtained in the present
study can be extrapolated to patients.
Acknowledgments
The authors would like to thank Miss Qiuxiang Li at
the Department of Neurology, Xiangya Hospital, Central South
University (Changsha, China) for tissue preparation and also Dr
Zhaohui Luo and Dr Zhiguo Wu from the Department of Neurology,
Xiangya Hospital, Central South University (Changsha, China) for
their outstanding technical assistance with experimental
apparatus.
Notes
[1]
Funding
This study was supported by grants from National
Natural Science Foundation of China (grant nos. 81771407, 81100967
and 81371435).
[2] Availability
of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
BX and LF designed the research. TTL performed the
majority of the experiments. YL and YS contributed to the study
design and analyzed the data. TTL, BX and LF wrote the article. All
authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
All experiments performed in the present study
involving animals were performed with the ethical approval of the
Institutional Animal Care and Use Committee of the Institute of
Laboratory Animal Science of Central South University (no.
201403142).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Moshe SL, Perucca E, Ryvlin P and Tomson
T: Epilepsy: New advances. Lancet. 385:884–898. 2015. View Article : Google Scholar
|
|
2
|
Liu TT, Feng L, Liu HF, Shu Y and Xiao B:
Altered axon initial segment in hippocampal newborn neurons,
associated with recurrence of temporal lobe epilepsy in rats. Mol
Med Rep. 16:3169–3178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Peng Z, Xiao B and Houser CR:
Activation of ERK by spontaneous seizures in neural progenitors of
the dentate gyrus in a mouse model of epilepsy. Exp Neurol.
224:133–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Bohlen und Halbach O:
Immunohistological markers for proliferative events, gliogenesis,
and neurogenesis within the adult hippocampus. Cell Tissue Res.
345:1–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parent JM and Kron MM: Neurogenesis and
Epilepsy. 2012.
|
|
6
|
Ishii K, Kubo K, Endo T, Yoshida K, Benner
S, Ito Y, Aizawa H, Aramaki M, Yamanaka A, Tanaka K, et al:
Neuronal Heterotopias affect the activities of distant brain areas
and lead to behavioral deficits. J Neurosci. 35:12432–12445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klein R and Kania A: Ephrin signalling in
the developing nervous system. Curr Opin Neurobiol. 27C:16–24.
2014. View Article : Google Scholar
|
|
8
|
Chumley MJ, Catchpole T, Silvany RE,
Kernie SG and Henkemeyer M: EphB receptors regulate stem/progenitor
cell proliferation, migration, and polarity during hippocampal
neurogenesis. J Neurosci. 27:13481–13490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furne C, Ricard J, Cabrera JR, Pays L,
Bethea JR, Mehlen P and Liebl DJ: EphrinB3 is an anti-apoptotic
ligand that inhibits the dependence receptor functions of EphA4
receptors during adult neurogenesis. Biochim Biophys Acta.
1793:231–238. 2009. View Article : Google Scholar :
|
|
10
|
Antion MD, Christie LA, Bond AM, Dalva MB
and Contractor A: Ephrin-B3 regulates glutamate receptor signaling
at hippocampal synapses. Mol Cell Neurosci. 45:378–388. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forster E: Reelin, neuronal polarity and
process orientation of cortical neurons. Neuroscience. 269:102–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senturk A, Pfennig S, Weiss A, Burk K and
Acker-Palmer A: Ephrin Bs are essential components of the Reelin
pathway to regulate neuronal migration. Nature. 472:356–360. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leeb C, Eresheim C and Nimpf J: Clusterin
is a ligand for apolipoprotein E receptor 2 (ApoER2) and very low
density lipoprotein receptor (VLDLR) and signals via the
Reelin-signaling pathway. J Biol Chem. 289:4161–4172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosch C, Masachs N, Exposito-Alonso D,
Martínez A, Teixeira CM, Fernaud I, Pujadas L, Ulloa F, Comella JX,
DeFelipe J, et al: Reelin regulates the maturation of dendritic
spines, synaptogenesis and glial ensheathment of newborn granule
cells. Cereb Cortex. 26:4282–4298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khazipov R, Zaynutdinova D, Ogievetsky E,
Valeeva G, Mitrukhina O, Manent JB and Represa A: Atlas of the
postnatal rat brain in stereotaxic coordinates. Front Neuroanat.
9:1612015. View Article : Google Scholar
|
|
17
|
Kanamori K: Faster flux of
neurotransmitter glutamate during seizure-Evidence from
13C-enrichment of extracellular glutamate in kainate rat model.
PLoS One. 12:e01748452017. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
19
|
Wu Q, Li Y, Shu Y, Feng L, Zhou L, Yue ZW,
Luo ZH, Wu ZG and Xiao B: NDEL1 was decreased in the CA3 region but
increased in the hippocampal blood vessel network during the
spontaneous seizure period after pilocarpine-induced status
epilepticus. Neuroscience. 268:276–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lisabeth EM, Falivelli G and Pasquale EB:
Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol.
5:2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hruska M and Dalva MB: Ephrin regulation
of synapse formation, function and plasticity. Mol Cell Neurosci.
50:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ricard J, Salinas J, Garcia L and Liebl
DJ: EphrinB3 regulates cell proliferation and survival in adult
neurogenesis. Mol Cell Neurosci. 31:713–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piccinin S, Cinque C, Calo L, Molinaro G,
Battaglia G, Maggi L, Nicoletti F, Melchiorri D, Eusebi F, Massey
PV, et al: Interaction between Ephrins and mGlu5 metabotropic
glutamate receptors in the induction of long-term synaptic
depression in the hippocampus. J Neurosci. 30:2835–2843. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu Y, Xiao B, Wu Q, Liu T, Du Y, Tang H,
Chen S, Feng L, Long L and Li Y: The Ephrin-A5/EphA4 interaction
modulates neurogenesis and angiogenesis by the p-Akt and p-ERK
pathways in a mouse model of TLE. Mol Neurobiol. 53:561–576. 2016.
View Article : Google Scholar
|
|
25
|
Kron MM, Zhang H and Parent JM: The
developmental stage of dentate granule cells dictates their
contribution to seizure-induced plasticity. J Neurosci.
30:2051–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parent JM, Yu TW, Leibowitz RT, Geschwind
DH, Sloviter RS and Lowenstein DH: Dentate granule cell
neurogenesis is increased by seizures and contributes to aberrant
network reorganization in the adult rat hippocampus. J Neurosci.
17:3727–3738. 1997.PubMed/NCBI
|
|
27
|
Song C, Xu W, Zhang X, Wang S, Zhu G, Xiao
T, Zhao M and Zhao C: CXCR4 antagonist AMD3100 suppresses the
long-term abnormal structural changes of newborn neurons in the
intraventricular kainic acid model of epilepsy. Mol Neurobiol.
53:1518–1532. 2016. View Article : Google Scholar
|
|
28
|
Kobow K, Jeske I, Hildebrandt M, Hauke J,
Hahnen E, Buslei R, Buchfelder M, Weigel D, Stefan H, Kasper B, et
al: Increased reelin promoter methylation is associated with
granule cell dispersion in human temporal lobe epilepsy. J
Neuropathol Exp Neurol. 68:356–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haas CA and Frotscher M: Reelin deficiency
causes granule cell dispersion in epilepsy. Exp Brain Res.
200:141–149. 2010. View Article : Google Scholar
|
|
30
|
Michelucci R, Pulitano P, Di Bonaventura
C, Binelli S, Luisi C, Pasini E, Striano S, Striano P, Coppola G,
La Neve A, et al: The clinical phenotype of autosomal dominant
lateral temporal lobe epilepsy related to reelin mutations.
Epilepsy Behav. 68:103–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baek ST, Copeland B, Yun EJ, Kwon SK,
Guemez-Gamboa A, Schaffer AE, Kim S, Kang HC, Song S, Mathern GW,
et al: An AKT3-FOXG1-reelin network underlies defective migration
in human focal malformations of cortical development. Nat Med.
21:1445–1454. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Korn MJ, Mandle QJ and Parent JM:
Conditional disabled-1 deletion in mice alters hippocampal
neurogenesis and reduces seizure threshold. Front Neurosci.
10:632016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dobrzanski P, Hunter K, Jones-Bolin S,
Chang H, Robinson C, Pritchard S, Zhao H and Ruggeri B:
Antiangiogenic and anti-tumor efficacy of EphA2 receptor
antagonist. Cancer Res. 64:910–919. 2004. View Article : Google Scholar : PubMed/NCBI
|