Introduction
Cholangiocarcinoma (CC) is the second most frequent
primary hepatic tumor entity following hepatocellular carcinoma and
accounts for 3% of all gastrointestinal tumors (1–3).
This aggressive tumor has a poor 5-year-survival rate of 5-10%. CC
can be classified into the extrahepatic subtype, which comprises
75% of all cases, and the intrahepatic subtype comprising the
remaining 25% (4). Due to the
late and unspecific appearance of clinical symptoms, mostly
presenting through biliary obstruction, CC is often diagnosed in
advanced tumor stages, and ~80% of patients are unsuitable for
surgical R0-resection, which currently represents the only curative
treatment modality (5–7). Unfortunately, palliative treatment
options, including R1/R2-resection, chemotherapy or radiotherapy,
show relatively poor results in prolonging the survival rates of
the patients. Often, they even lower the patients' quality of life
(QoL) due to severe adverse effects (1,7,8).
The current standard in palliative management of patients with
advanced CC is biliary decompression and relief of obstruction by
biliary drainage and stent insertion (9). However, compared with a median
survival rate of 3 months without any intervention, for patients
treated with biliary drainage, the median survival rate is only
4-10 months (7). In the context
of the poor prognosis for patients with non-resectable CC, there is
an urgent requirement to develop novel and favorable palliative
treatment strategies with high patient tolerability and improved
outcome. Photodynamic therapy (PDT) is a novel and non-to-minimally
invasive treatment option for several premalignant and malignant
diseases, including various forms of gastrointestinal cancer. PDT
involves the application and tumor-specific accumulation of a
photosensitizing drug, and its subsequent activation with light of
a specific wavelength. In the presence of molecular oxygen, the
activation of the PS leads to the generation of reactive oxygen
species (ROS), which damage the neoplastic tissue and cause
apoptotic and/or necrotic cell death (10). As a tumor-specific ablative method
with few adverse effects and no formation of resistance on
repetition, unlike that following chemotherapy, PDT offers novel
perspectives for the palliative treatment of advanced CC.
Randomized controlled trials have demonstrated that PDT alone or in
combination with stenting significantly improved the survival rates
and QoL of patients with non-resectable CC (6,8,11-15). Ortner et al (6) showed that PDT combined with stenting
increased the median survival rate of patients with non-resectable
CC from 3.3 months (biliary stent treatment alone) to 16.4 months
(combined treatment of stenting and PDT). Therefore, PDT may
represent a promising strategy for improved palliative treatment of
advanced and non-resectable CC (16).
The efficacy of PDT is particularly dependent on the
quality of the PS used (17),
therefore, there is a constant need for further development of
photosensitizers with improved efficacy. Phthalocyanines (Pcs)
belong to second generation of photosensitizers and are
structurally related to porphyrins, which have been well known as
PS for several years (18–20).
In particular, metallophthalocyanines bearing a zinc, silicon,
aluminum or indium as a central metal atom offer favorable
properties for an ideal PS (21).
In addition to being photostable, Pc-compounds show a high efficacy
in producing ROS upon illumination (20). The high absorption peak of Pcs
between 600 and 800 nm allows deep tissue penetration of up to 6-8
mm when photoactivating Pcs with red to deep red light (22,23). Pcs have been shown to
preferentially accumulate in malignant tissue and show high
retention in tumor cells (24,25). In addition, Pcs do not cause
cytotoxic effects in a non-illuminated state (26). In the present study, the antitumor
effects and suitability of two novel phthalocyanines:
Tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine (ZnPc)
and dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-tr
ioxaundecan-1-sulfonyl) silicon phthalocyanine (Pc32) were
investigated as PS for PDT of CC.
Materials and methods
Compounds
ZnPc and Pc32 were prepared by minor modification to
the procedures described previously (27). The compounds were dissolved in
DMSO of spectroscopic grade (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The concentrations of stock solutions were calculated by
measuring their optical density at 694 nm with a UV/Vis
spectrometer (Ultraspec 2100; GE Healthcare Life Sciences,
Chalfont, UK) and based on the Lambert-Beer relationship with
ε694 nm = 2.04×105 M−1
cm−1 (ZnPC) or by measuring the compounds weight with a
microbalance (Sartorius AG, Göttingen, Germany) and based on the
molar solution concentration equation: Molarity (mol/l) = mass
(g)/volume (l) x[1/molecular weight (g/mol)] (Pc32). ZnPc and Pc32
were donated by the Department of Chemistry, Gebze Institute of
Technology (Gebze, Turkey). The stock solutions were prepared at
concentration of 15-25 mM. Working solutions (0.2-10 μM)
were made up in medium so that the DMSO concentration never
exceeded 0.25%.
Cell culture
The poorly differentiated EGI-1 CC cell line
(Leibnitz Institute DSMZ, Braunschweig, Germany; no. ACC385)
(28), and the partly papillary
and partly tubular TFK-1 CC cell line (Leibnitz Institute DSMZ, no.
ACC344) (29) were derived from
patients with extrahepatic bile tract carcinoma, which had not been
exposed to chemotherapy or radiotherapy. The two cell lines were
cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 100 ml/l fetal calf
serum (FCS; Biochrom AG, Berlin, Germany), 100 U/ml penicillin, 100
μg/ml streptomycin and 2 mM L-glutamine (both from Biochrom
AG, Berlin, Germany). The cells were maintained under 37°C in a
humidified atmosphere containing 5% CO2. The culture
medium was replaced every second day, and once a week the cells
were passaged using 1% trypsin/EDTA.
Light source and irradiation
Irradiation of the cancer cells was performed with a
broad-band light source equipped with a 100-W halogen lamp (EFR 12
V/100 W GZ -6.35 lamp Omnilux; Thomann GmbH, Burgebrach, Germany)
with a spectral output of 400-800 nm. The optimal conditions for
PDT treatment were reached at a cell density of 70-80%
monolayer-confluence in the respective plastic dishes used for the
examinations. To prevent infrared irradiation, a heat-reflecting
filter cutting off wavelengths of ≥700 nm was embedded in the
optical path. The illuminated area (5.5×4.5 cm) had an average
power density of 80 W/m2. The light energy dose was
measured with a P-9710 radiometer controlled by a silicon photocell
(Optometer P 9710 Gigahertz-Optik (Munich, Germany). The total
light energy dose was calculated by integrating the energy signal
over the entire period of irradiation (25).
PDT treatment
For PDT treatment, the cells were seeded and
cultivated in an experiment-dependent number and subsequently
incubated for 10-24 h with ZnPc (0.2-10 μM) or Pc32 (1-10
μM) in the dark at 37°C in a humidified atmosphere
containing 5% CO2. For the measurement of growth
inhibition 1.5×103 EGI-1 cells, and 1×103
TFK-1 cells per 100-μl well were seeded in 96-well
microtiter plates and cultivated for 48 h prior to incubation with
the PS. For the determination of cytotoxicity, the cells were
seeded at a density of 2.5×104 cells/100 μl well
in a 96-well microtiter plate. Following the incubation period, the
PS-containing medium was replaced with PBS, and the cells were
irradiated with 10 J/cm2. The temperature during
irradiation was measured with a digital thermometer placed inside
of the irradiation system and did not exceed 37°C. Following
irradiation, the PBS was replaced with PS-free medium and the cells
were incubated at 37°C with 5% CO2 in a humidified
atmosphere for another 24-96 h. 'Dark controls' were treated in the
same manner, with a PS-incubation period of 24 h, but without
subsequent photoactivation by PDT. Dark controls were included to
determine the cytotoxic effects of the PS in the absence of light
for 24-72 h (25).
Measurement of growth inhibition
The PDT-induced changes in the number of CC cells
were determined by crystal violet staining of cellular DNA, as
described previously (30). In
brief, the cells were fixed with 1% glutaraldehyde and stained with
0.1% crystal violet. The unbound dye was removed by washing with
water. The DNA-bound crystal violet was solubilized with 0.2%
Triton X-100 in PBS. Light extinction, which increases linearly
with cell number, was analyzed at 570 nm using an ELISA reader.
Intracellular uptake and
accumulation
The cells were cultured on glass cover slips and
incubated with increasing concentrations of ZnPc (0.2-5 μM)
and Pc32 (1-10 μM). Following incubation for 24 h, the cells
were washed with PBS and fixed for at least 30 min at −20°C in
methanol. The intracellular distribution of ZnPc was analyzed by
measuring its fluorescence with a confocal laser microscope (Leica
DMI 6000; Leica Microsystems GmbH, Wetzlar, Germany) with an
excitation HeNe laser (700 nm) and detection of PMT at 400-800 nm,
performed with a ×63 glycerin immersion objective. Digital images
were processed using Leica LAS AF Lite software (version 3.2.0;
Leica Microsystems GmbH).
Determination of cytotoxicity
The PDT-induced cytotoxicity was determined by
measuring the release of lactate dehydrogenase (LDH) from the
cytosol of the damaged cells into the supernatant (Cytotoxicity
Detection KitPLUS LDH; cat. no. 04744926001; Roche Diagnostics
GmbH, Mannheim, Germany) (31).
Following PDT treatment, cells incubated with 1% Triton X-100
served as a control for maximum LDH release. Following PDT, the
supernatant was transferred to fresh plates and mixed with catalyst
and dye solution for 30 min, resulting in the formation of formazan
dye proportional to LDH enzyme activity. The absorbance was
measured at 490/630 nm using an ELISA reader (Dynex Technologies,
Denkendorf, Germany) and cytotoxicity was determined by calculating
the percentage of LDH release in the treated sample, compared with
the LDH release of the untreated cells, the value of which was 0%,
and the maximum LDH release of the Triton X-100 lysed cells, the
value of which represented 100% (32). All experiments were performed in
triplicate.
Measurement of apoptosis-specific
caspase-3 activity
Changes in caspase-3 activity were measured from the
cleavage of the fluorogenic substrate AC-DEVD-AMC (EMD Millipore,
Billerica, MA, USA), as described previously (32). At 8 and 24 h post-PDT, the cells
were harvested and lysed with lysis buffer. Subsequently, the
lysates were incubated for 1 h at 37°C with a substrate solution
containing 20 μg/ml AC-DEVD-AMC, 20 mM HEPES, 10% glycerol
and 2 mM DTT at pH 7.5. Substrate cleavage was measured
fluorometrically using a VersaFluor fluorometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA; filter sets: ex 360/40 nm,
em 460/10 nm). Campthotecin was used as a positive control.
Western blot analysis
Western blot analysis was performed as described
previously (33). Briefly,
whole-cell extracts were prepared by lysing the cells in RIPA
buffer. Protein concentration of the lysates was determined by
using bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.) and ≤20 μg protein were subjected to gel
electrophoresis with 10 or 12% gels and then transferred onto a
PVDF membrane by electroblotting for 1.5 h. Following blocking in
5% skim milk powder solution (Merck Millipore) for at least 1 h,
the blots were incubated with primary antibodies at 4°C overnight.
The antibodies were directed against B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax 1:1,000; sc-493), extracllular
signal-regulated kinase (ERK)1/2 (1:1,000; sc-94), GAPDH (1:1,000;
sc-25778), Cyclin D1 (1:1,000; sc-8396) and Cyclin B1 (1:1,000;
sc-752) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA),
and Bcl-2 (1:1,000; B3170) and β-actin (1:2,000; A5441) from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following
incubation with horseradish peroxidase-coupled anti-IgG antibodies
(1:10,000; NA931V and NA934V; GE Healthcare Life Sciences) at room
temperature for at least 1 h, the blots were developed using
enhanced chemiluminescent detection (ECL Clarity Max; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and processed with
Chemiluminescence Imager Celvin® S 420 (Biostep,
Burkhardsdorf, Germany). Relative changes in the expression of Bax,
Cyclin D1, Cyclin B1 and ERK1/2 of the PDT-treated, vs. untreated
control cells were measured densitometrically using ImageJ software
(version 2.0.0-rc-43/1.50e; National Institutes of Health,
Bethesda, MD, USA). Protein expression was normalized to
housekeeping gene expression of β-actin or GAPDH. The protein level
of untreated and housekeeping gene-normalized cells was set as 1.
Values >1 indicated upregulation and values <1 indicated
downregulation of a respective protein expression.
Measurement of ROS
The cells were incubated with ZnPc and Pc32 for 24 h
and then illuminated with 10 J/cm2. ROS formation was
determined 2 and 7 h post-PDT for ZnPc and 2 h post-PDT for Pc32
using CellROX Green and CellROX Orange (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Controls underwent
PDT without previous ZnPc loading. The cells were analyzed using a
fluorescence microscope (Axioskop 40, Zeiss; ×40 objective, 1.30
NA; Carl Zeiss AG, Oberkochen, Germany) equipped with a digital
camera (DX4-285FW, Kappa Optronics (Gleichen, Germany). CellROX
Green depicts ROS formation in the nucleus and mitochondria (ex/em
approximately 470/525 nm) by producing bright green fluorescence,
whereas orange fluorescence (CellROX Orange) depicts ROS in the
cytoplasm of the cells (ex/em approximately 546/575 nm).
Cell cycle analysis by flow cytometry
(FACS)
At 24 h post-PDT-treatment (10 J/cm2),
the cells were washed with PBS and fixed in ice-cold ethanol at
−20°C. Following incubation for at least 30 min, the ethanol was
removed by centrifugation (273.4 × g at 4°C for 4 min) and the cell
pellets were washed twice in PBS. The cell pellets were resuspended
in PBS containing 40 μg RNase-A and incubated for 30 min at
37°C. Subsequently, the cells were pelleted (241.3 × g at 4°C)
again and resuspended in PBS containing 50 μg/ml propidium
iodide. Then cells were analyzed using the FACSCanto II analyzer
(BD Biosciences, Franklin Lakes, NJ, USA) and FCS Express 6
software (De Novo Software, Los Angeles, CA, USA). Corresponding to
their phase-specific DNA-content, the cells were divided into G1,
S, G2/M phases and, when present, the sub-G1 phase which was
interpreted as apoptotic.
Determination of antiangiogenic effects
using a chorioallantoic membrane (CAM) assay
The CAM assay was performed as described in a
previous study (34). In brief,
fertilized chicken eggs (Lohmann Tierzucht, Cuxhaven, Germany) were
bred in an incubator at 37°C in constant humidity for 3 days. After
3 days, a square window was cut into the shell of each egg to
assure a living embryo and allow detachment of the developing CAM
from the shell. The window was sealed and the eggs were incubated
for additional 7 days. On day 10, the tapes were removed and two
small silicone rings were placed onto the CAM, and either 20
μl of vehicle (negative control), ZnPc (10 μM) or
Pc32 (10 μM) were topically applied. The eggs were then
sealed and incubated in the dark at 37°C in a humidified incubator.
After 24 h, the treated areas were illuminated with 10
J/cm2. Changes in the microvasculature of the CAM were
examined 24 h post-PDT and in vivo images were captured
using a stereomicroscope equipped with a digital camera (Di-Li,
Kaiserslautern, Germany).
Statistical analysis
Unless stated otherwise, data are presented as the
mean ± standard deviation. Significance between values was analyzed
with GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla,
CA, USA) using an unpaired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Photosensitizer uptake and intracellular
accumulation
The intracellular uptake and accumulation of ZnPc
and Pc32 were investigated using confocal laser scanning
microscopy. Following incubation for 24 h with non-photoactivated
ZnPc, the EGI-1 and TFK-1 cells showed a dose-dependent uptake and
predominantly cytoplasmic accumulation (Fig. 1A). By contrast, the uptake of Pc32
was comparably lower; even at 10 μM, only a marginal to
moderate cytoplasmic accumulation was observed (Fig. 1B).
 | Figure 1Uptake and loading times of ZnPc and
Pc32 in cholangiocarcinoma cells. (A) Dose-dependent uptake and
cytoplasmic accumulation of ZnPc in EGI-1 and TFK-1 cells at 24 h,
as determined by confocal scanning microscopy (ex/em 700/400-800).
(B) Pc32 uptake at the highest concentration of 10 μM
following incubation for 24 h. (C) Efficacy of ZnPc-PDT (10
J/cm2) correlated with the preloading time of the
photosensitizer. The longer the loading time, the more pronounced
the antiproliferative effect of ZnPc-PDT was in (C) EGI-1 and (D)
TFK-1 cells. Results are representative of at least three
independent experiments. Magnification, ×63. *P<0.05,
10 h incubation, vs. 24 h incubation. ZnPc,
tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine; Pc32,
dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy. |
Growth inhibition of photoactivated and
non-photoactivated ZnPc and Pc32
The optimal loading time prior to the PDT was
investigated in TFK-1 and EGI-1 cells following incubation with
increasing concentrations of ZnPc (0.2-5 μM) for 10, 16 and
24 h, and analyzed using crystal violet staining. The 'dark toxic'
effects of non-photoactivated ZnPc (2.5-10 μM) and the
antiproliferative effect of non- and photoactivated Pc32 (1-10
μM) were investigated following prior to incubation for 24 h
and subsequently analyzed using crystal violet staining.
Preloading of ZnPc for 10 h was sufficient to elicit
pronounced PDT-induced growth inhibitory effects. However, a
further increase in the preloading time of up to 24 h increased the
antiproliferative effectiveness of PDT in the two cell lines
(Fig. 1C and D). Based on these
findings, the subsequent investigations were performed following a
preloading period of 24 h of the respective PS. Incubation with
non-photoactivated ZnPc or Pc32 (dark toxicity) for 24 h did not
affect the proliferation of either CC cell lines at any time point.
Even the high 10 μM concentration of ZnPc or Pc32 did not
induce any appreciable antiproliferative effects, showing that
neither phthalocyanines were toxic in the absence of light
(Fig. 2A and B).
 | Figure 2Growth inhibitory effects of ZnPc-PDT
in cholangiocarcinoma cells. ZnPc-PDT (10 J/cm2) dose-
and time-dependently decreased the number of (A) EGI-1 and (B)
TFK-1 cells of ≥90%. Non-photoactivated ZnPc did not exhibit any
appreciable 'dark toxicity' in either cell line. ZnPc-PDT induced a
marked decrease in the expression of mitogen-activated protein
kinase-pathway related protein ERK1/2 in (C) EGI-1 and (D) TFK-1
cells. Results are representative of at least three independent
crystal violet experiments and two western blot experiments.
*P<0.05 non-photoactivated, vs. photoactivated ZnPc
(72 h-post PDT). ZnPc, tetra-triethyleneoxysulfonyl substituted
zinc phthalocyanine; Pc32, dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy; ERK1/2,
extracellular signal-regulated kinase 1/2. |
The growth inhibitory effects of PDT with ZnPc
(0.2-10 μM) and Pc32 (1-10 μM) were determined using
crystal violet staining. In the two cell lines, light activated
ZnPc-PDT led to pronounced time- and dose-dependent growth
inhibition. The IC50 values of ZnPc-PDT of EGI-1 and
TFK-1 were 0.5±0.17 and 1.7±0.13 μM, respectively, as
determined 24 h post-PDT. Compared with the TFK-1 cells, the
response of the EGI-1 cells was more pronounced. However, in both
cell lines, an overall decrease of ≥90% was observed, and there
were no signs of re-proliferation of cells that evaded immediate
PDT-induced death at moderate ZnPc-doses (1-2.5 μM)
(Fig. 2A and B). The effect of
ZnPc-PDT on the expression of ERK1/2, which is involved in cell
growth, differentiation and proliferation, was also evaluated by
western blot analysis. ZnPc-PDT led to a decrease in the expression
of ERK1/2 in the EGI-1 and TFK-1 cells in a dose-dependent manner
(Fig. 2C and D).
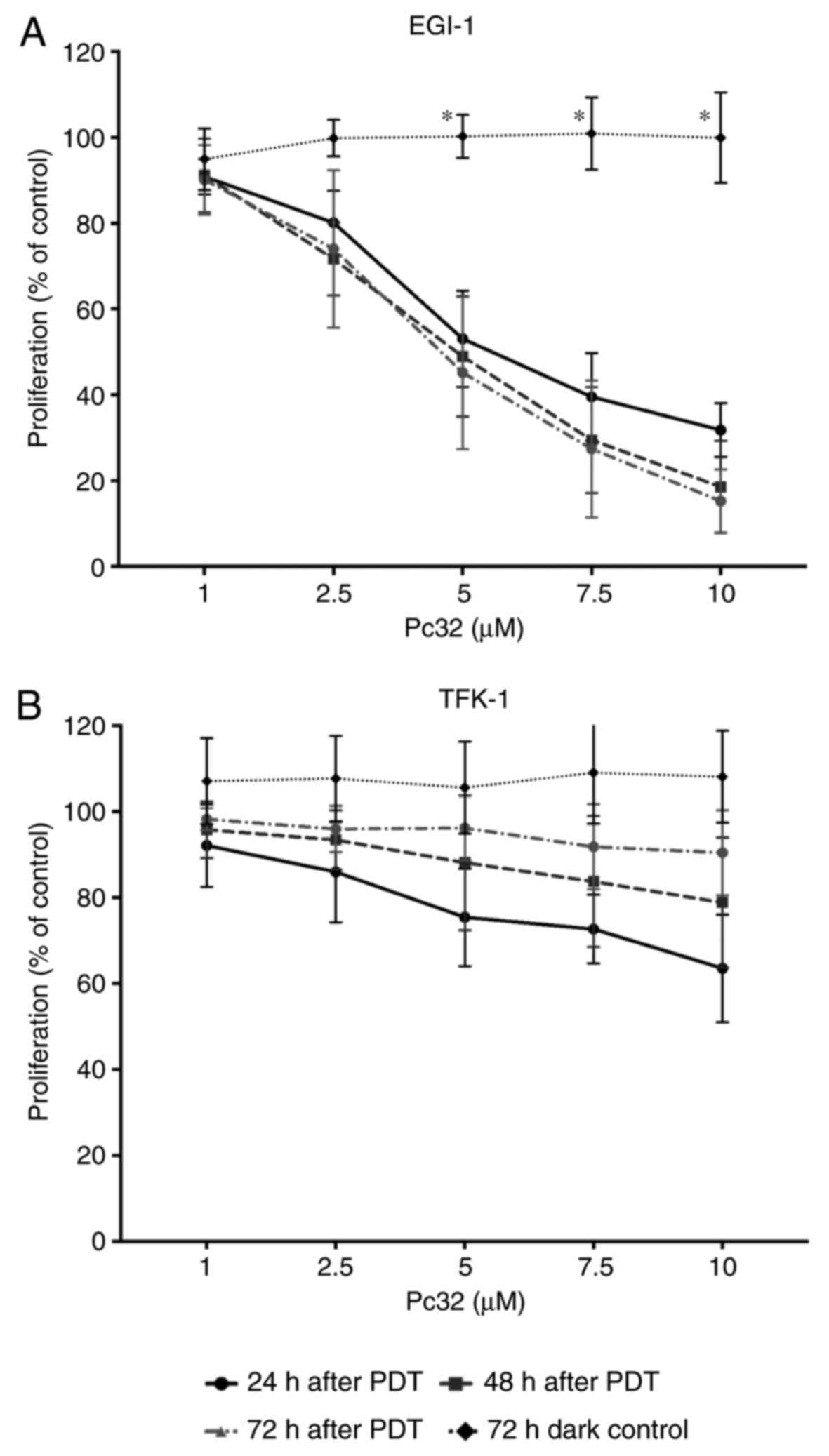
Pc32-PDT (1-10 μM) induced a sustained time-
and dose-dependent decrease in the proliferation of EGI-1 cells
(Fig. 3A), but did not induce
pronounced antiproliferative effects in the TFK-1 cells. Pc32-PDT
was not able to stop the re-proliferation of TFK-1 cells that
evaded immediate PDT-induced cell death (Fig. 3B). As described for ZnPc above,
incubation with non-photoactivated Pc32 (dark toxicity) did not
affect the proliferation of either CC cell lines at any time point.
Even the high 10 μM concentration of Pc32 did not induce any
appreciable antiproliferative effects, showing that the Pc32
phthalocyanine was not cytotoxic in the absence of light (Fig. 3).
Induction of ROS formation
ZnPc- and Pc32-PDT-induced ROS generation was
analyzed using fluorogenic dyes, which are sensitive for
cytoplasmic (orange) or mitochondrial/nuclear (green) ROS and
subsequent fluorescence microscopy. Compared with the control
(Fig. 4A), at 2 h post-ZnPc-PDT,
a marked dose-dependent increase in ROS formation was observed in
the cytoplasm and the mitochondria/nucleus of the EGI-1 cells
(Fig. 4B). Even at low
ZnPc-concentrations of 0.5 μM, marked generation of ROS was
observed in the cytoplasm and mitochondria/nuclei (data not shown).
However, despite the dose-dependent increase in overall ROS
formation, there was no preferred localization of ROS in either the
nucleus/mitochondria or the cytoplasm. By contrast, 2 h following
Pc32-PDT, a comparably lower increase of ROS formation was observed
in the EGI-1 cells (Fig. 4C).
This finding was in accordance with the reduced uptake and limited
antiproliferative effects of Pc32 shown in the prior experiments.
The same pattern of results was observed for the TFK-1 cells
following exposure to ZnPc-PDT, compared with the control (Fig. 4D and E), even at a low
concentration of 1.7 μM (data not shown), and following
exposure to Pc32-PDT (Fig.
4F).
 | Figure 4ZnPc- and Pc32-PDT induced formation
of ROS in cholangiocarcinoma cells. In EGI-1 cells, compared with
the (A) control, at (B) 2 h post-ZnPc-PDT (5 μM/10
J/cm2), a marked induction of ROS generation was
observed in the cytoplasm (orange) and the nucleus/mitochondria
(green). (C) At 2 h post-Pc32-PDT (5 μM/10
J/cm2), minimal induction of ROS formation occurred. In
TFK-1 cells, compared with the (D) control, at (E) 2 h
post-ZnPc-PDT (5 μM/10 J/cm2), a marked induction
of ROS generation was observed in the cytoplasm (orange) and the
nucleus/mitochondria (green). (F) At 2 h post-Pc32-PDT (5
μM/10 J/cm2), minimal induction of ROS formation
occurred. Results are representative of four independent
experiments. Magnification, ×40. ZnPc, tetra-triethyleneoxysulfonyl
substituted zinc phthalocyanine; Pc32, dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy; ROS, reactive
oxygen species. |
Evaluation of immediate cytotoxicity
following exposure to PDT with ZnPc and Pc32
The immediate cytotoxic effects of photoactivated
ZnPc in TFK-1 and EGI-1 cells were determined by measuring the LDH
release. The leakage of intracellular LDH into the culture medium
is an indicator of ROS-induced cytotoxicity due to a damage of the
integrity of the membrane of cells or cell organelles, including
mitochondria, golgi vesicles and nuclei. In the two cell lines,
ZnPc-PDT (0.5-5 μM) resulted in a significant time- and
concentration-dependent increase of immediate cytotoxicity
following 1-6 h (Fig. 5). The
EGI-1 cells showed a maximum LDH release of 34.8% at 6 h post-PDT
(Fig. 5A), whereas the effect was
less pronounced in the TFK-1 cells. However, in the TFK-1 cells, an
increase in LDH release of ~17.8% became apparent (Fig. 5B), showing the cytotoxic potential
of ZnPc for PDT of CC. Compared with these findings, Pc32-PDT
(0.5-5 μM) led to only weak immediate cytotoxic effects in
the two cell lines. The EGI-cells showed a maximum LDH release of
9.5% at 6 h post-Pc32-PDT (Fig.
5C), whereas a maximum LDH release of 8.5% was measured in the
TFK-1 cells (Fig. 5D).
 | Figure 5Immediate cytotoxicity of ZnPc- and
Pc32-PDT in cholangiocarcinoma cells. ZnPc-PDT (0.5-5 μM/10
J/cm2) induced a more marked time- and dose-dependent
release of cytotoxicity-indicating LDH into the supernatant of (A)
EGI-1 and (B) TFK-1 cells, compared with Pc32-PDT (0.5-5
μM/10 J/cm2) in the (C) EGI-1 and (D) TFK-1
cells. Results are representative of three independent experiments.
*P<0.05 cytotoxicity 1 vs. 6 h post-PDT or 0.5 vs. 5
μM 6 h post-PDT. ZnPc, tetra-triethyleneoxysulfonyl
substituted zinc phthalocyanine; Pc32, dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy; LDH, lactate
dehydrogenase. |
Apoptotic effects of ZnPc-PDT in CC
cells
In addition to the induction of necrotic cell death
via immediate cytotoxicity, PDT can also lead to apoptosis by
ROS-mediated disruption of mitochondrial integrity. Due to the
limited growth inhibitory and cytotoxic effects of Pc32-PDT, the
possible apoptotic effects were predominantly examined for
ZnPc-PDT. The apoptosis-specific activation of caspase-3 was
determined at 8 and 24 h post-PDT. In addition, the changes in the
proportions of cells in the apoptosis-indicating sub-G1 phase of
the cell cycle were elucidated by FACS-analysis. ZnPc-PDT (0.2-5
μM) led to a time- and dose-dependent increase of caspase-3
activity in the EGI-1 and TFK-1 cells of up to 26- and 6-fold,
compared with the control cells, which were illuminated but not
loaded with ZnPc (Fig. 6A and B).
In particular, the induction of capsase-3 activity in the EGI-1
cells occurred rapidly, reaching the highest value at 8 h post-PDT
with 5 μM ZnPc (Fig.
6A).
 | Figure 6Induction of apoptosis in
cholangiocarcinoma cells by ZnPc-PDT. The effector-caspase-3 was
measured 8 and 24 h post PDT with ZnPc (0.2-5 μM) in (A)
EGI-1 and (B) TFK-1 cells. (C) A dose-dependent increase in the
apoptotic subG1-peak was determined by measuring changes in the
hypoploide DNA content following ZnPc-PDT treatment in EGI-1 and
TFK-1 cells. Western blot analysis of expression of pro-apoptotic
Bax and anti-apoptotic Bcl-2 at 12 and 24 h in (D) EGI-1 and (E)
TFK-1 cells. Densitometric analysis with ImageJ revealed the
relative increase or decrease in the protein expression of pro- and
antiapoptotic proteins Bax and Bcl-2 following ZnPc-PDT treatment,
normalized to β-actin. Results are representative of at least three
independent experiments. *P<0.05 caspase-3 activity 8
or 24 h post-PDT, vs. control. ZnPc, tetra-triethyleneoxysulfonyl
substituted zinc phthalocyanine; Pc32, dihydroxy-2,9(10),16(17), 23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
Due to the process of partial DNA-fragmentation
(karrhyorexis) and its loss during the formation of apoptotic
bodies (35), the two cell lines
showed a concentration-dependent increase in the proportion of
cells containing sub-G1 phase-specific hypoploide DNA content 24 h
post-ZnPc-PDT (Fig. 6C). These
effects occurred in both cell lines, but were less pronounced in
the TFK-1 cells. Compared with the untreated controls, ZnPc-PDT (5
μM, 10 J/cm2) led to a 5-fold increase in the
apoptotic sub-G1 peak, but there was an increase of >18-fold in
the EGI-1 cells.
The activity of caspase-3 and changes in sub-G1-
phase-specific DNA content can be driven by either
intrinsic-mitochondrial or extrinsic apoptotic pathways. To
elucidate the induction of apoptosis via one way or the other, the
expression of two proteins associated with the mitochon-
dria-driven apoptotic pathway, namely Bax and Bcl-2, were evaluated
by western blot analysis. ZnPc-PDT induced a time- and
dose-dependent decrease in the protein expression of anti-apoptotic
Bcl-2 and a simultaneous increase in the protein expression of
pro-apoptotic Bax in the EGI-1 cells at 12 and 24 h post-PDT
(Fig. 6D). Compared with the
EGI-1 cells, the TFK-1 cells showed a less marked decrease in the
expression of Bcl-2, and a less pronounced increase in the
expression of Bax, and these effects occurred later (Fig. 6E).
Changes of cell cycle distribution
The effect of ZnPc-PDT on the cell cycle
distribution of the EGI-1 and TFK-1 cells was measured by flow
cytometry. Additionally, the expression levels of two cell cycle
phase-specific proteins Cyclin D1 and Cyclin B1 were evaluated by
western blot analysis. At 24 h post-ZnPc-PDT, the EGI-1 cells
showed a dose-dependent increase in the number of cells in the
G0/G1-phase, with a corresponding decrease in
the number of cells in the G2/M-phase (Fig. 7A and B). At 6 and 24 h post-PDT
treatment, a corresponding decrease in the expression of cell cycle
promoter Cyclin D1, acting at the G1- to S-phase
transition, was observed (Fig.
7C). By contrast, the TFK-1 cells responded with a decrease of
cells in the G0/G1-phase and a corresponding
increase of cells in the G2/M-phase (Fig. 7D and E). These changes were
accompanied by a decrease in the expression of cell cycle promoter
Cyclin B1, which is required for the transition of cells from the
G2- to M-phase (Fig.
7F).
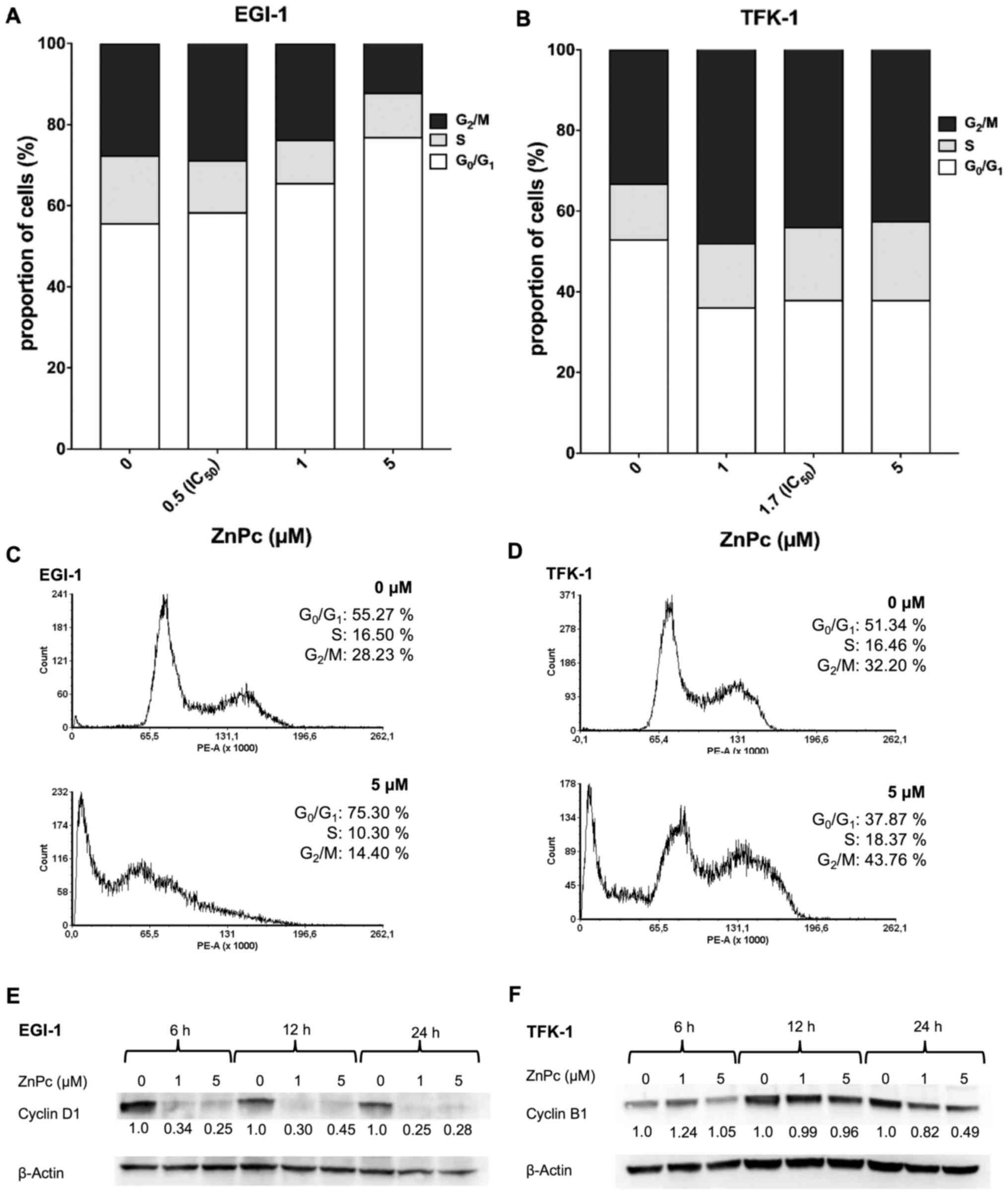 | Figure 7Effects of ZnPc-PDT on the cell cycle
of cholangiocarcinoma cells. FACS analysis (A) proportions from the
(C) output revealed a ZnPc-PDT-induced dose-dependent increase of
EGI-1 cells in the G0/G1-phase, with a
corresponding decrease of cells in the G2/M-phase, at 24
h post-PDT. (E) Accordingly, the expression of Cyclin D1 decreased
in a time- and dose-dependent manner. In TFK-1 cells, the FACS (B)
proportions from the (D) output showed that ZnPc-PDT led to an
increase of cells in the G2/M-phase and decrease in the
G0/G1-phase. (F) A pronounced decrease of
Cyclin B1, in line with the observed cell cycle arrest, was
observed in TFK-1 cells at 24 h. Results are representative of a
minimum of three independent experiments. ZnPc,
tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine; Pc32,
dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy. |
Angiogenesis inhibition
The influence of ZnPc and Pc32-PDT on the formation
of new blood vessels was evaluated using an angiogenesis assay with
the CAM of fertilized chicken eggs (CAM assay). An area of 5 mm in
diameter of 10-day old CAMs was incubated with either 10 μM
of ZnPc or Pc32 for 24 h and then illuminated (10
J/cm2). Prior to the PDT treatment, the CAM consisted of
a regular vascular network with an intact capillary bed. In the
control-treated CAMs, no changes in the development of the vascular
network were observed. There was a constant increase in microvessel
formation, as shown in the progression of new branches at the
distal end of small vessels (Fig.
8A). Pc32-PDT did not alter the development or distribution of
new blood vessels (Fig. 8B). By
contrast, ZnPC-PDT induced the degeneration of the existing
vascular network, leading to nonperfused regions (Fig. 8C). In addition, changes in the
branching of the small supplying vessels became apparent,
suggesting avascular and antiangiogenic properties of ZnPc-PDT.
 | Figure 8Blood vessel formation of the
developing chicken chorioallantoic membrane (CAM) after ZnPc- or
Pc32-PDT. Images show chicken CAMs in prior to and 24 h following
PDT with (A) PBS (control), (B) Pc32 (10 μM) or (C) ZnPc (10
μM) as photosensitizers. In the control CAMs and in the
Pc32-treated CAM, the vascular network was homogenous in
arrangement in an antiparallel manner; after 24 h, new blood
vessels had formed, as shown by branching of the small blood
vessels. Only ZnPc-PDT induced a degeneration of the vascular
network, shown as non-perfused regions and changes in branching of
the small supplying vessels (arrows). Results are representative of
three eggs per condition. Magnification, ×12. CAM, chorioallantoic
membrane; ZnPc, tetra-triethyleneoxysulfonyl substituted zinc
phthalocyanine; Pc32, dihydroxy-2,9(10),16(17),23(24)-tetrakis(4,7,10-trioxaundecan-1-sulfonyl)
silicon phthalocyanine; PDT, photodynamic therapy. |
Discussion
Palliative treatment options for CC are
disappointing. Patients suffering from advanced CC have a poor
prognosis in terms of survival rate and experience a low QoL.
Therefore, there is an urgent requirement for novel effective
treatment approaches, including PDT. In a previous study ZnPc was
proposed as a promising photosensitizer for PDT of gastrointestinal
cancer entities, including esophageal and neuroendocrine
gastrointestinal carcinoma (25,36). The present study focused on the
efficacy and the underlying mechanisms of PDT treatment of CC cells
with the two novel metallophthalocyanines, ZnPc and Pc32.
A main requirement for a PS is the absence of
cytotoxic effects without photoactivation, or 'dark toxicity', to
avoid unwanted side effects and the destruction of healthy tissue
(37), whereas the
photoactivation of the PS requires a persistent antineoplastic
effect on the PDT-treated tumor cells/tissue. In the present study
it was shown that neither incubation with non-photoactivated ZnPc,
nor with non-photoactivated Pc32 (Fig. 2) led to undesired 'dark toxic'
effects on the CC cells.
The photoactivated ZnPc-PDT led to a marked and
sustained time- and dose-dependent antiproliferative effect in the
two CC cell lines. Correspondingly, a decrease in the expression of
the mitogen-activated protein kinase (MAPK)-related protein,
ERK1/2, was observed, indicating the downregulation of
proliferation-promoting processes, cellular differentiation and
survival at a molecular level in the two cell lines (Fig. 2C and D) (38). As other second generation
photosensitizers, including the clinically relevant
5-aminolevulinic acid, fail to induce permanent antiproliferative
effects in gastrointestinal cancer (39), the findings in the present study
showed the potential and advantageous suitability of ZnPc as a
novel PS for PDT of CC.
To determine whether there is a difference between
silicon phthalocyanine and a zinc phthalocyanine on the growth
reduction of CC, photoactivated Pc32 was used for comparison. Pc32
led to a time- and dose-dependent antiproliferative effect only in
the less differentiated EGI-1 cells, but did not show comparable
effects in the more differentiated TFK-1 cells. This finding is of
importance, as other silicon-phthalocyanines have already been
shown to induce potent antiproliferative effects in several
different tumor entities, including gastrointestinal colon
carcinoma cells (40), but have
failed in CC cells. This underlines the importance of individually
evaluating the potential of a PS in a specific tumor entity and
shows that the findings in one tumor entity cannot easily be
transferred to another.
Individual treatment-protocols, including
light-dose, pre-incubation time and dose of PS, have an important
influence on the clinical outcome, and differ from one cancer type
to another (41–43). The time interval between the
application of a PS and its subsequent photoactivation is essential
for the subcellular uptake kinetics and distribution in a cancer
cell. Fabris et al showed that incubation for 2 h was
sufficient for a subcellular accumulation of a zinc phthalocyanine
derivative in the golgi apparatus, whereas a more mitochondrial
localization of this PS occurred only after 24 h (44). The present study evaluated the
effect of different incubation times (10-24 h) on the effectiveness
of the ZnPc-PDT in CC cells. PS loading for 24 h led to a more
marked concentration-dependent antiproliferative effect of the
subsequent PDT treatment, compared with shorter loading times of
10-16 h (Fig. 1C and D). After 10
h of PS loading, the PDT treatment induced significant
antiproliferative effects only at high concentrations (>2.5
μM) of ZnPc. At concentrations <2.5 μM the effects
were weak and the PDT-surviving CC cells tended to re-proliferate,
which did not occur when the loading time was prolonged to 24 h. In
a clinical setting, it can be beneficial to adapt the individual
PDT-protocol for different CC entities, and identify a reasonable
compromise between well-tolerated concentrations of PS with
preferably low incubation times, but maintaining a sustained and
pronounced antiproliferative effect of the PDT-treatment.
The efficacy of PDT on the target cells/tissue is
determined by the photophysical properties of the PS, its uptake
and the intracellular localization of accumulation (45,46). Investigations of other ZnPc and
Pc32 derivatives have shown an accumulation of the PS in
mitochondria, lysosomes and the golgi apparatus in various
carcinoma cells (44,47–50). In the loading experiments
performed in the present study, a predominantly cytoplasmic
distribution of ZnPc was observed in the CC cells. However, marked
generation of ROS was observed in the nucleus and mitochondria
following PDT, suggesting there was also an appreciable
accumulation of the photosensitizers in the nucleus, mitochondria
or other organelles. These results are in line with our previous
findings concerning the intracellular distribution and
ROS-formation of ZnPc in gastrointestinal cancer cells, including
esophageal cancer, indicating the phototoxic potency of
photoactivated ZnPc and its preferred subcellular accumulation
(23). The intracellular
accumulation and formation of ROS were less pronounced with Pc32
following illumination than with ZnPc.
PDT-induced cell death is mediated via ROS
formation, which can cause either necrosis and/or apoptosis
(44). By measuring LDH release,
a marked immediate cytotoxic effect of ZnPc-PDT was found in the
two CC cell lines. Challa and Chan previously showed that
increasing concentrations of extracellular LDH can indicate the
primary necrosis of dying cells due to disturbance of cellular
integrity. However, LDH release may also indicate the secondary
necrosis of cells that have undergone apoptosis but are not taken
up by phagocytosis under in vitro conditions (35,51). By contrast, Pc32-PDT did not lead
to comparable cytotoxic effects in two cell lines. This observation
supports the superiority of ZnPc in cellular uptake, ROS-formation
and antiproliferative efficacy.
The inflammation-free elimination of cancer cells
via apoptosis is a preferred therapeutic aim. Therefore, the
present study also examined the apoptotic effects of ZnPc-PDT in
the CC cells. It was shown that ZnPc-PDT induced
mitochondrial-driven apoptosis with corresponding changes in the
expression of regulatory proteins, Bax and Bcl-2. The expression of
pro-apoptotic Bax increased, whereas the expression of
anti-apoptotic Bcl-2 decreased, leading to a time- and
dose-dependent increase in caspase-3 activity (Fig. 6). These data are in line with
findings of Lam et al, who demonstrated the
mitochondria-driven apoptosis of phthalocyanine-based PDT in
epidermoid carcinoma cells (52)
and Tampa et al, who elucidated that increasing levels of
pro-apoptotic proteins and decreasing levels of anti-apoptotic
proteins were the main apoptotic pathway in oral keratinocytes
following zinc-sulphonated phthalocyanine-PDT (53).
The induction of cell cycle arrest at a specific
cell cycle checkpoint by chemotherapeutic treatment is another
mechanism to inhibit cancer cell proliferation in a
non-inflammatory manner (54).
Cell cycle arrest in the G1- and G2-phase can
be induced by genotoxic compounds and ionizing radiation, which
cause double stranded DNA breaks. In addition, S-phase arrest can
be triggered by anti-metabolites, including 5-fluorouracil and
hydroxyurea, whereas alkylating agents or
topoisomerase-II-inhibitors can induce cell cycle arrest in the
G2-phase (55). In the
present study, the concentration-dependent arrest of EGI-1 cells in
the G0/G1-phase was observed 24 h following
ZnPc-PDT, which was accompanied by a corresponding decrease of
cells in the G2/M-phase and a dose-dependent decrease in
the expression of Cyclin D1 (56–58). In the more differentiated TFK-1 CC
cells ZnPc-PDT also induced arrest in the
G0/G1-phase, however, in contrast to the
EGI-1 cells, the number of cells in the G2/M-phase
increased with an accompanied corresponding decrease in the
expression of the G2/M-phase-promoter Cyclin B1
(59). The data obtained on the
cell cycle behavior of CC cells within various differentiation
states offer valuable information for effective combination
therapies, in which ZnPc-PDT is combined with chemotherapeutics to
act at specific cell cycle phases in the future.
In addition to the described distinction in the
induction of cell cycle arrest, the present study showed further
differences between the two cell lines. PDT with either PS had a
more marked and earlier antiproliferative effect on EGI-1 cells,
compared with that on TFK-1 cells (Figs. 2 and 3). Correspondingly, higher immediate
cytotoxicity (Fig. 5) and an
earlier and more marked induction of apoptosis were observed in the
EGI-1 cells (Fig. 6). The reasons
for these variances between the two cell lines may lie in the
individual differentiation of the two cancer cell lines and/or a
dissimilar expression of regulatory proteins. The poorly
differentiated EGI-1 cells (28,60) presumably overexpress p53, which is
well known as an essential cellular protein, reacting to cell
stress and DNA damage, and is involved in the regulation of cell
cycle progression and apoptosis (61,62). By contrast, the partly papillary
and partly tubular growing TFK-1 cells (29) lack marked expression of p53
(62). This indicates differences
in the underlying mechanisms of PDT-induced cytotoxicity, and
growth- and proliferation-inhibition in the context of cell
line-specific genetic aberrations. It also supports the clinical
need to perform PDT with an individual and PS-adjusted protocol,
depending on the genetic characteristics, p53-status and
differentiation of the individual type of CC.
At a certain size, the further growth of a solid
tumor, including in CC, requires the formation of new blood vessels
for the supply of oxygen and nutrients. Therefore, the development
of novel anticancer compounds with antiangiogenic potency
represents an attractive approach for future treatment strategies
(63). Previous data show a
correlation between microvessel density and reduced 5-year survival
rates, higher recurrence rates and increased nodal spread in
intrahepatic CC (64). Although
ZnPc-PDT markedly inhibited neoangiogenesis and caused a
degeneration of the vascular complex, as examined in the CAM assay
of fertilized chicken eggs, no antiangiogenic and/or avascular
effects were observed following Pc32-PDT.
Taken together, the results of the present study
showed the suitability of the novel ZnPc for PDT of CC and its
superiority compared with Pc32. Further investigations are required
to examine the specific underlying mechanisms, particularly in the
context of individual cancerous genetic alterations. Additionally,
future investigations are required to examine the in vivo
potency of the novel ZnPc-PDT in a monotherapeutic approach and in
combination with clinically relevant chemotherapeutics.
Acknowledgments
Not applicable.
References
|
1
|
Aljiffry M, Walsh MJ and Molinari M:
Advances in diagnosis, treatment and palliation of
cholangiocarcinoma: 1990–2009. World J Gastroenterol. 15:4240–4262.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergquist A and Von Seth E: Epidemiology
of cholangiocarcinoma. Best Pract Res Clin Gastroenterol.
29:221–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baradari V, Höpfner M, Huether A, Schuppan
D and Scherübl H: Histone deacetylase inhibitor MS-275 alone or
combined with bortezomib or sorafenib exhibits strong
antiproliferative action in human cholangiocarcinoma cells. World J
Gastroenterol. 13:4458–4466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doherty B, Nambudiri VE and Palmer WC:
Update on the diagnosis and treatment of cholangiocarcinoma. Curr
Gastroenterol Rep. 19:22017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarnagin WR, Fong Y, Dematteo RP, Gonen M,
Burke EC, Bodniewicz BSJ, Youssef BAM, Klimstra D and Blumgart LH:
Staging, resectability, and outcome in 225 patients with hilar
cholangiocarcinoma. Ann Surg. 234:507–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ortner ME, Caca K, Berr F, Liebetruth J,
Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J and
Lochs H: Successful photodynamic therapy for nonresectable.
cholangiocarcinoma: A randomized prospective study.
Gastroenterology. 5085:1355–1363. 2003. View Article : Google Scholar
|
|
7
|
Moole H, Tathireddy H, Dharmapuri S, Moole
V, Boddireddy R, Yedama P, Dharmapuri S, Uppu A, Bondalapati N and
Duvvuri A: Success of photodynamic therapy in palliating patients
with nonresectable cholangiocarcinoma: A systematic review and
meta-analysis. World J Gastroenterol. 23:1278–1288. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ortner M: Photodynamic therapy in
cholangiocarcinoma: An overview. Photodiagnosis Photodyn Ther.
1:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abu-hamda EM and Baron TH: Endoscopic
management of cholangiocarcinoma. Semin Liver Dis. 24:165–175.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witzigmann H, Berr F, Ringel U, Caca K,
Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J,
Hauss J and Wiedmann M: Surgical and palliative management and
outcome in 184 patients with hilar cholangiocarcinoma. Ann Surg.
244:230–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoepf T, Jakobs R, Arnold J, Apel D and
Riemann JF: Palliation of nonresectable bile duct cancer: Improved
survival after photodynamic therapy. Am J Gastroenterol.
100:2426–2430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad GA, Wang KK, Baron TH, Buttar NS,
Wongkeesong LM, Roberts LR, LeRoy AJ, Lutzke LS and Borkenhagen LS:
Factors associated with increased survival after photodynamic
therapy for cholangiocarcinoma. Clin Gastroenterol Hepatol.
5:743–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee TY, Cheon YK, Shim CS and Cho YD:
Photodynamic therapy prolongs metal stent patency in patients with
unresectable hilar cholangiocarcinoma. World J Gastroenterol.
18:5589–5594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCaughan JS Jr, Mertens BF, Cho C,
Barabash RD and Payton HW: Photodynamic therapy to treat tumors of
the extrahepatic biliary ducts. A case report Arch Surg.
126:111–113. 1991.
|
|
16
|
Allison RR, Zervos E and Sibata CH:
Cholangiocarcinoma: An emerging indication for photodynamic
therapy. Photodiagnosis Photodyn Ther. 6:84–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allison RR and Sibata CH: Oncologic
photodynamic therapy photosensitizers: A clinical review.
Photodiagnosis Photodyn Ther. 7:61–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonnett R: Photosensitizers of the
porphyrin and phthalocyanine series for photodynamic therapy. Chem
Soc Rev. 24:1995. View Article : Google Scholar
|
|
19
|
Josefsen LB and Boyle RW: Photodynamic
therapy: Novel third-generation photosensitizers one step closer?
Br J Pharmacol. 154:1–3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Z, Shao J, Yang T, Wang J and Jia L:
Pharmaceutical development, composition and quantitative analysis
of phthalocyanine as the photosensitizer for cancer photodynamic
therapy. J Pharm Biomed Anal. 87:98–104. 2014. View Article : Google Scholar
|
|
21
|
Neagu M, Constantin C, Tampa M, Matei C,
Lupu A, Manole E, Ion RM, Fenga C and Tsatsakis AM: Toxicological
and efficacy assessment of post-transition metal (Indium)
phthalocyanine for photodynamic therapy in neuroblastoma.
Oncotarget. 7:69718–69732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ochsner M: Light scattering of human skin:
A comparison between Zinc(II)-phthalocyanine and photofrin II. J
Photochem Photobiol B. 32:3–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yslas EI, Prucca C, Romanini S, Durantini
EN, Bertuzzi M and Rivarola V: Biodistribution and phototherapeutic
properties of Zinc(II) 2,9,16,23-tetrakis (methoxy) phthalocyanine
s. Photodiagnosis Photodyn Ther. 6:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuzyniak W, Ermilov EA, Atilla D, Gürek
AG, Nitzsche B, Derkow K, Hoffmann B, Steinemann G, Ahsen V and
Höpfner M: Tetra-triethyleneoxysulfonyl substituted zinc
phthalocyanine for photodynamic cancer therapy. Photodiagnosis
Photodyn Ther. 13:148–157. 2016. View Article : Google Scholar
|
|
26
|
Liu W, Chen N, Jin H, Huang J, Wei J, Bao
J, Li C, Liu Y, Li X and Wang A: Intravenous repeated-dose toxicity
study of ZnPcS2P2-based-photodynamic therapy in beagle dogs. Regul
Toxicol Pharmacol. 47:221–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Atilla D, Saydan N, Durmus M, Gürek AG,
Khan T, Rück A, Walt H, Nyokong T and Ahsen V: Synthesis and
photodynamic potential of tetra- and octa-triethyleneoxysulfonyl
substituted zinc phthalocyanines. J Photochem Photobiol A Chem.
186:298–307. 2007. View Article : Google Scholar
|
|
28
|
Scherdin G, Garbrecht M and Klouche M: In
vitro interaction of á-difluoromethylornithine (DFMO) and human
recombinant interferon-a (rIFN-a) on human cancer cell lines.
Immunobiology. 175:1–143. 1987.
|
|
29
|
Saijyo S, Kudo T, Suzuki M, Katayose Y,
Shinoda M, Muto T, Fukuhara K, Suzuki T and Matsuno S:
Establishment of a new extrahepatic bile duct carcinoma cell line,
TFK-1. Tohoku J Exp Med. 177:61–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nitzsche B, Gloesenkamp C, Schrader M,
Ocker M, Preissner R, Lein M, Zakrzewicz A, Hoffmann B and Höpfner
M: Novel compounds with antiangiogenic and antiproliferative
potency for growth control of testicular germ cell tumours. Br J
Cancer. 103:18–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai Y, Xia Q, Su Q, Luo R, Sun Y, Shi Y
and Jiang W: MTOR inhibitor RAD001 (everolimus) induces apoptotic,
not autophagic cell death, in human nasopharyngeal carcinoma cells.
Int J Mol Med. 31:904–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gloesenkamp C, Nitzsche B, Lim AR, Normant
E, Vosburgh E, Schrader M, Ocker M, Scherübl H and Höpfner M: Heat
shock protein 90 is a promising target for effective growth
inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol.
40:1659–1667. 2012.PubMed/NCBI
|
|
33
|
Höpfner M, Baradari V, Huether A, Schöfl C
and Scherübl H: The insulin-like growth factor receptor 1 is a
promising target for novel treatment approaches in neuroendocrine
gastrointestinal tumours. Endocr Relat Cancer. 13:135–149. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodrigues JR, Charris J, Camacho J,
Barazarte A, Gamboa N, Nitzsche B, Höpfner M, Lein M, Jung K and
Abramjuk C:
N'-Formyl-2-(5-nitrothiophen-2-yl)benzothiazole-6-carbohydrazide as
a potential anti-tumour agent for prostate cancer in experimental
studies. J Pharm Pharmacol. 65:411–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krysko DV, Vanden Berghe T, D'Herde K and
Vandenabeele P: Apoptosis and necrosis: Detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuzyniak W, Schmidt J, Glac W, Berkholz J,
Steinemann G, Hoffmann B, Ermilov EA, Gürek AG, Ahsen V, Nitzsche B
and Höpfner M: Novel zinc phthalocyanine as a promising
photosensitizer for photodynamic treatment of esophageal cancer.
Int J Oncol. 50:953–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mfouo-tynga I and Abrahamse H: Cell death
pathways and phthalocyanine as an efficient agent for photodynamic
cancer therapy. Int J Mol Sci. 16:10228–10241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roskoski R Jr: ERK1/2 MAP kinases:
Structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Höpfner M, Maaser K, Theiss A, Lenz M,
Sutter AP, Kashtan H, von Lampe B, Riecken EO, Zeitz M and Scherübl
H: Hypericin activated by an incoherent light source has
photodynamic effects on esophageal cancer cells. Int J Colorectal
Dis. 18:239–247. 2003.PubMed/NCBI
|
|
40
|
Whitacre CM, Feyes DK, Satoh T, Grossmann
J, Mulvihill JW, Mukhtar H and Oleinick NL: Photodynamic therapy
with the phthalocyanine photosensitizer pc 4 of SW480 human colon
cancer xenografts in athymic mice 1. Clin Cancer Res. 6:2021–2027.
2000.PubMed/NCBI
|
|
41
|
Nanashima A, Isomoto H, Abo T, Nonaka T,
Morisaki T, Arai J, Takagi K, Ohnita K, Shoji H, Urabe S, et al:
How to access photodynamic therapy for bile duct carcinoma. Ann
Transl Med. 2:232014.PubMed/NCBI
|
|
42
|
Oniszczuk A, Wojtunik-Kulesza KA,
Oniszczuk T and Kasprzak K: The potential of photodynamic therapy
(PDT)-experimental investigations and clinical use. Biomed
Pharmacother. 83:912–929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Straten D, Mashayekhi V, de Bruijn H,
Oliveira S and Robinson D: Oncologic photodynamic therapy: Basic
principles, current clinical status and future directions. Cancers
(Basel). 9:E192017. View Article : Google Scholar
|
|
44
|
Fabris C, Valduga G, Miotto G, Borsetto L,
Jori G, Garbisa S and Reddi E: Photosensitization with Zinc (II)
phthalocyanine as a switch in the decision between apoptosis and
necrosis. Cancer Res. 61:7495–7500. 2001.PubMed/NCBI
|
|
45
|
Morgan J, Potter WR and Oseroff AR:
Comparison of photodynamic targets in a carcinoma cell line and its
mitochondrial DNA-deficient derivative. Photochem Photobiol.
71:747–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oleinick NL, Morris RL and Belichenko I:
The role of apoptosis in response to photodynamic therapy: What,
where, why, and how. Photochem Photobiol Sci. 1:1–21. 2002.
View Article : Google Scholar
|
|
47
|
Rodriguez ME, Zhang P, Azizuddin K, Delos
Santos GB, Chiu SM, Xue LY, Berlin JC, Peng X, Wu H, Lam M, et al:
Structural factors and mechanisms underlying the improved
photodynamic cell killing with Silicon phthalocyanine
photosensitizers directed to lysosomes v/s mitochondria. Photochem
Photobiol. 85:1189–1200. 2012. View Article : Google Scholar
|
|
48
|
Tynga IM, Houreld NN and Abrahamse H: The
primary subcellular localization of Zinc phthalocyanine and its
cellular impact on viability, proliferation and structure of breast
cancer cells (MCF-7). J Photochem Photobiol B. 120:171–176. 2013.
View Article : Google Scholar
|
|
49
|
Shao J, Dai Y, Zhao W, Xie J, Xue J, Ye J
and Jia L: Intracellular distribution and mechanisms of actions of
photosensitizer Zinc (II)-phthalocyanine solubilized in Cremophor
EL against human hepatocellular carcinoma HepG2 cells q. Cancer
Lett. 330:49–56. 2013. View Article : Google Scholar
|
|
50
|
Biology C, Marino J, García MC, Furmento
VA, Blank VC, Awruch J and Roguin LP: Lysosomal and mitochondrial
permeabilization mediates zinc (II) cationic phthalocyanine
phototoxicity. Int J Biochem Cell Biol. 45:2553–2562. 2013.
View Article : Google Scholar
|
|
51
|
Challa S and Chan FK: Going up in flames:
Necrotic cell injury and inflammatory diseases. Cell Mol Life Sci.
67:3241–3253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lam M, Oleinick NL and Nieminen A:
Photodynamic therapy-induced apoptosis in epidermoid carcinoma
cells. J Biol Chem. 276:47379–47386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tampa M, Matei C, Popescu S, Georgescu S
and Neagu M: Zinc trisulphonated phthalocyanine used in
photodynamic therapy of dysplastic oral keratinocytes. Rev Chim.
64:639–645. 2013.
|
|
54
|
Dickson MA and Schwartz GK: Development of
cell-cycle inhibitors for cancer therapy. Drug Dev Contemp Oncol.
16:36–43. 2009.
|
|
55
|
Gabrielli B, Brooks K and Pavey S:
Defective cell cycle checkpoints as targets for anti-cancer
therapies. Front Pharmacol. 3:92012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S transition. Mol
Cell Biol. 15:3463–3469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: The roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar :
|
|
58
|
Rezaei PF, Fouladdel S, Ghaffari SM, Amin
G and Azizi E: Induction of G1 cell cycle arrest and cyclin D1
down-regulation in response to pericarp extract of Baneh in human
breast cancer T47D cells. DARU. 20:1012012. View Article : Google Scholar
|
|
59
|
Su C, Lin J, Chen G, Lin W and Chung J:
Down-regulation of Cdc25c, CDK1 and cyclin B1 and up-regulation of
wee1 by curcumin promotes human colon cancer colo 205 cell entry
into G2/M-phase of cell cycle. Cancer Gen Proteomics. 3:55–62.
2006.
|
|
60
|
Huether A, Höpfner M, Baradari V, Schuppan
D and Scherübl H: Sorafenib alone or as combination therapy for
growth control of cholangiocarcinoma. Biochem Pharmacol.
73:1308–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Levine AJ: P53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Caca K, Feisthammel J, Klee K, Tannapfel
A, Witzigmann H, Wittekind C, Mössner J and Berr F: Inactivation of
the INK4a/ARF locus and p53 in sporadic extrahepatic bile duct
cancers and bile tract cancer cell lines. Int J Cancer. 97:481–488.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Thelen A, Scholz A, Weichert W, Wiedenmann
B, Neuhaus P, Gessner R, Benckert C and Jonas S: Tumor-associated
angiogenesis and lymphangiogenesis correlate with progression of
intrahepatic cholangiocarcinoma. Am J Gastroenterol. 105:1123–1132.
2010. View Article : Google Scholar
|