Introduction
Deep venous thrombosis (DVT) is a common traumatic
and postoperative complication in patients undergoing orthopedic
surgery. Several studies have revealed that DVT is closely
associated with the inflammatory reaction. The inflammatory factors
can damage the venous wall, causing thrombosis (1). This process involves a vicious
cycle, which promotes the mutual effects between the inflammatory
response and DVT (2).
Interleukin (IL)-18 is a type of proinflammatory
cytokine belonging to the interleukin family, and is detectable in
the majority of mammalian cells, including vascular endothelial
cells. IL-18 has a multi-directional biological functional
activity, and is extensively involved in the regulatory processes
of inflammatory and immune reactions. Few studies exist on the
correlation between IL-18 and DVT, however, studies on the
correlation of IL-18 with inflammatory reactions in the body, cell
function regulation and cardiovascular system diseases have
suggested that it may be one of the important factors involved in
causing thrombosis. It has been revealed (3) that IL-18 can activate nuclear
factor-κB (NF-κB) and mediate human cerebral microvascular
endothelial cell (HCMEC) damage, inducing the dysfunction and
apoptosis of cells. NF-κB is associated with the expression of
tissue factor (TF), and the transcriptional regulatory effects of
NF-κB and TF are key in the pathogenesis of DVT (4), with NF-κB inhibitor used in the
prevention and treatment of DVT. In summary, IL-18 and NF-κB are
closely associated with the functional changes of endothelial
cells, and there exists a type of intrinsic association between
them, promoting the incidence of DVT.
Therefore, the present study investigated the effect
of the expression of IL-18 and related markers on DVT to examine
the correlation between IL-18 and DVT.
Materials and methods
Ethical statement
SPF female Sprague-Dawley (SD) rats aged 8-10 weeks
and weighing 180±20 g were raised in the SPF Animal Experiment
Building, Experimental Animal Center, Kunming Medical University
(Kunming, China). Rats were housed at room temperature, 50-60%
humidity, <14 mg/m3 under a 12 h light/dark cycle.
Food and water was provided ad libitum. All animal
experiments were performed following approval from the Animal
Experiment and Ethics Committee of Kunming Medical University.
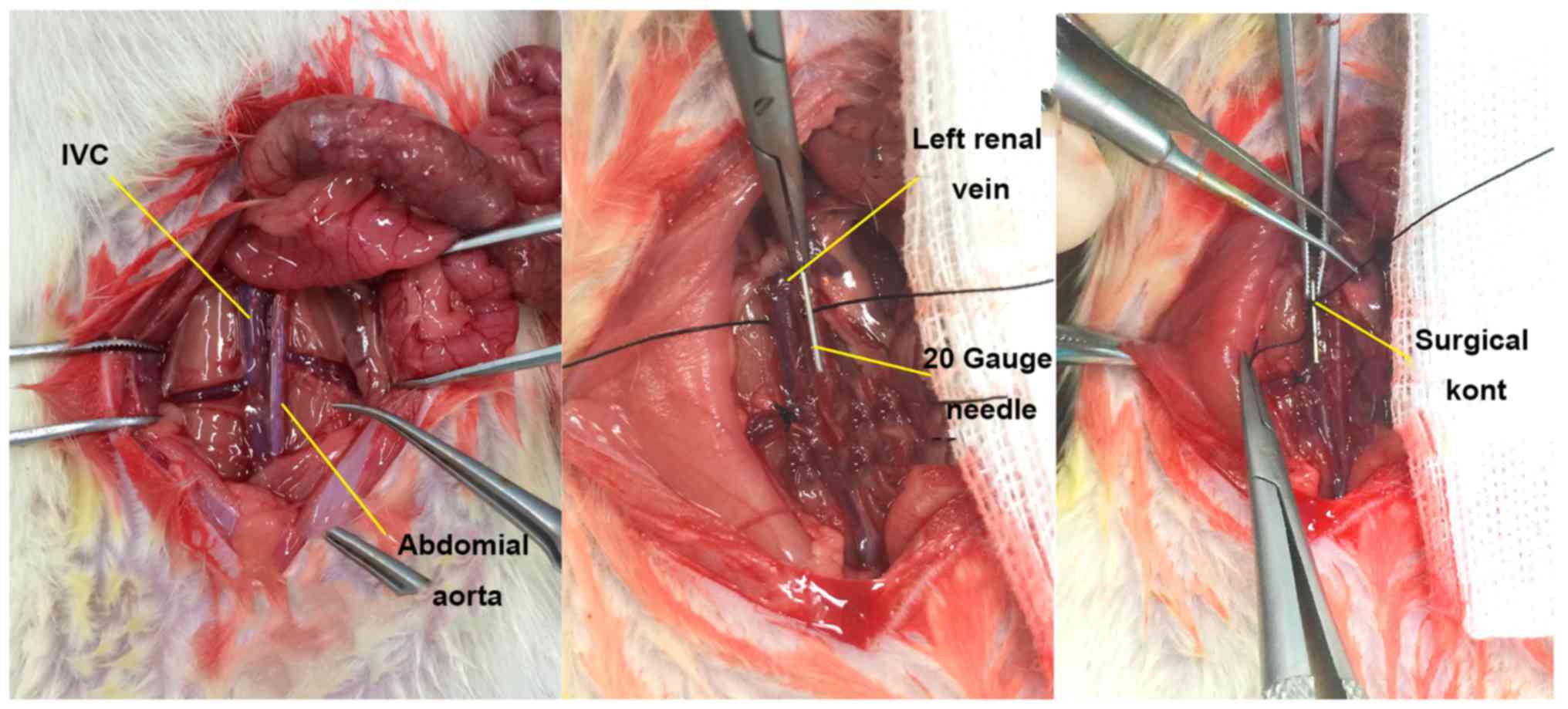
Establishment of the rat inferior vena
cava (IVC) thrombosis model
The SD rats were randomized into three groups. In
the DVT model group (n=40), the IVC thrombosis model was
established in rats using the flow restriction method (5) (Fig.
1). In the Sham group (n=40), the IVC was exposed and then
sutured, without any invasive treatment. In the control group
(n=40), no invasive procedure was performed on the rats. At 2, 8,
24 and 72 h, 10 rats were randomly selected from each group for
observation of IVC thrombosis at the preset time-points.
Subsequently, ~5-8 ml blood was collected using sodium citrate
anticoagulant vacutainer (cat. no. 363095; BD Biosciences, Franklin
Lakes, NJ, USA), and was centrifuged at 760 × g for 10 min at 4°C,
followed by isolation of plasma and preservation at −80°C. The
expression levels of IL-18 and NF-κB in the plasma samples of each
group were detected using the ELISA method according to the ELISA
kit manufacturer's protocol (IL-18: cat. no. CSB-E04610r; NF-κB:
cat. no. CSB-E13148r; Cusabio Biotech Co., Ltd., Wuhan, China).
Construction of overexpression and
suppression vectors of the rat IL-18 gene
Construction of the overexpression vector was
performed as follows: The complete CDS sequence of the rat IL-18
gene was obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The primer
sequences were designed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and Primer
5.0 (Premier Biosoft International, Palo Alto, CA, USA) software:
IL-18 (585 bp), forward, 5′-CGGAATTCATGGCTGCCATGTCAGAA-3′ and
reverse, 5′-CGGGATCCCTAACTTTGATGTAAGTT-3′.
The details of PCR amplification are as follows, mixed: 30.5 liters
double distilled water, 2 liters upstream primers, 2 liters
downstream primers, 2 liters cDNA, 4 liters dNTP, 4 liters
MgCl2, 5 liters 10X EXTaq Buffer (Mg2+ free),
0.5 liter EXTaq; total 50 liters. The thermocycling procedure
comprised: 94°C for 3 min; 94°C for 30 sec; 52°C for 30 sec; 72°C
for 1 min; 94°C for 30 sec, 30 cycles; 72°C for 10 min and hold at
4°C; annealing temperature 56°C.
The gene fragment was amplified by polymerase chain
reaction (PCR). The target gene fragment and plasmid vector were
constructed according to the manufacturer's protocol of the
pCDHcDNA cloning and expression lentivectors,
pCDH-MCS-T2A-copGFP-MSCV (cat. no. CD523A-1; System Biosciences,
Inc., Mountain View, CA, USA).
Construction of the suppression vector first
involved amplification of the rat IL-18 gene retrovirus oligo
fragments: Two 22-base candidate small interfering (si)RNA target
sequences were designed using Oligoengine 2.0 online software
(http://bioinfo.clontech.com/rnaidesigner/frontpage.jsp):
5′-TGCTGTTGACAGTGAGCGCAGGTCTCCACTGCTGCCCTTGTAGTGAAGCCACAGATGTACAAGGGCAGCAGTGGAGACCTGTGCCTACTGCCTCGGA-3′
and
5′-TGCTGTTGACAGTGAGCGCCCAGCTGGTGTCCTAGCAGATTAGTGAAGCCACAGATGTAATCTGCTAGGACACCAGCTGGGTGCCTACTGCCTCGGA-3′.
For PCR, the oligo used microRNA (miR)30, forward (XhoI),
5′-CAGAAGGCTCGAGAAGGTATATTGCTGTTGACAGTGAGCG-3′ and reverse
(EcoRI), 5′-CTAAAGTAGCCCCTTGAATTCCGAGGCAGTAGGCA-3′. These
oligo fragments were amplified, and the target fragment and plasmid
vector were constructed using the expression arrest
microRNA-adapted retroviral vector (LMP microRNA-adapted vector),
according to the manufacturer's protocol (cat. no. EAV4071; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Acquisition of plasmid virus with IL-18
overexpression and inhibition, and efficiency determination
In the present study, 293T cells (The Cell Bank of
Type Culture Collection of Chinese Academy of Sciences, Shanghai,
China) were used as the host cells for packaging viruses, and rat
fibroblasts were used as the infected cells. For packaging and
infection of the viral vectors, the pCDH-GFP lentivirus (12 g) was
packaged with the pCL-ECO plasmid (8 g) and was transfected into
the overexpression plasmid IL-18-pCDH-GFP using HEPES and the
Calcium Phosphate Cell Transfection kit (Beyotime Institute of
Biotechnology, Jiangsu, China) according to the manufacturer's
protocols Normal cells and cells transfected with null vectors were
used as controls. The MSCV-LMP retrovirus (12.5 g) had two
packaging plasmids: pAPAX (7.5 g) and pMD2.G (5 g). This was
transfected into IL-18-LMP short hairpin (sh)RNAmir1 and plasmid 2,
whereas transfection with null vectors were used as controls.
The target plasmid and packaging plasmid were added
to 1.5 ml EP tubes in sequence and centrifuged at 300 × g for 10
sec at room temperature. Subsequently, 60 µl 2 M calcium
chloride was added to each tube with 420 µl sterile
distilled water, followed by vortex mixing and centrifugation
(solution A). Subsequently, new 1.5 ml EP tubes were selected and
500 µl HEPES buffer was added to each (solution B). On a
vortex, the solution (A) was slowly dripped into solution B (~25-28
drops), and then stood at room temperature for 30 min. This mixture
was then slowly dripped into the 293T cell culture flask and stood
for 6-8 h, followed by replacement of the medium and further
culture. The transfection time was from 2 h following the
application of the plasmid mixture. At 48 h, medium containing the
virus was collected and filtered using a filtration membrane
(0.45-µm). Subsequently, 7 ml fresh medium was poured into a
culture dish, from which the rat fibroblasts medium prepared on the
2nd day of transfection was removed and added to medium containing
the virus, with 2 µl of Polybrene added to promote
infection. Subsequently, virus secreted for the second time was
collected and was used to infect the target cells again, to which
polybrene was added. The infection efficiency was observed when the
total infection time was 48 h.
Determination of IL-18 overexpression and
inhibition efficiency
RNA was extracted from the infected cells, and was
synthesized into cDNA according to the RevertAid First Strand cDNA
synthesis kit (MBI Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) protocol. Subsequently, the relative
expression of IL-18 was determined using reverse
transcription-quantitative (RT-q)PCR analysis according to the
manufacturer's protocol of Maxima® SYBR-Green/ROX qPCR
Master mix (2X) (MBI Fermentas; Thermo Fisher Scientific, Inc.).
The ABI PRISM® 7300HT system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for detection of the relative
expression of IL-18 via for RT-qPCR analysis. The ther-mocycling
conditions constituted: Initial denaturation at 95°C for 10 min (1
cycle), followed by denaturation at 95°C for 15 sec, and annealing
and extension at 60°C for 60 sec (40 cycles). The primer sequences
were obtained using the 2−ΔΔCq method (6) (ABI DataAssist™ v3.0 software (Thermo
Fisher Scientific, Inc.) and were as follows: Rat IL-18 (82 bp),
forward, 5′-TCTGTAGCTCCATGCTTTCCG-3′ and reverse,
5′-GATCCTGGAGGTTGCAGAAGA-3′; Rat GAPDH (146 bp), forward,
5′-ACGGCAAGTTCAACGGCACAG-3′ and reverse,
5′-GACGCCAGTAGACTCCACGACA-3′.
Acquisition of virus
At 48 and 72 h, the virus stock solution containing
the IL-18-pCDH-GFP overexpression plasmid and IL-18-LMP shRNAmir1
inhibition plasmid was collected, centrifuged with an
ultrafiltration tube at 14,000 × g for 30 min at low-temperature,
followed by centrifugation with a high-speed refrigerated
centrifuge at 85,000 × g for 2 h. The precipitation was retained,
and 50-100 µl PBS was added into each tube, and then placed
on a horizontal shaker overnight at 4°C. The virus concentrate was
then collected and preserved at −80°C.
Treatment and modeling of rats with IL-18
gene overexpression and inhibition
The SD rats were randomly assigned into four groups:
In the overexpression group (n=10), rats were administered with an
intravenous tail injection of IL-18-pCDH-GFP overexpression virus,
(300 µl/rat). In the inhibition group (n=10), rats were
administered with IL-18 LMP shRNAmir1 inhibition virus (300
µl/rat). Rats in the control group (n=5) were injected with
sterile saline (300 µl/rat). Rats in the normal group (n=5)
received no treatment. After 24 h, the IVC thrombus model was
prepared using the flow restriction method (5) in the overexpression, inhibition and
control groups. At 24 h post-model establishment, the rats were
anatomized, and the IVC and embolus below the ligation suture were
collected for measurement of the weight and length. In the normal
group, the IVC (length of ~1.0-1.5 cm) was collected under the left
renal vein inlet.
The total protein was extracted from the vein wall
using radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) and centrifugation at 14,000 × g for 5 min at 4°C.
Total protein was quantified via a bicinchoninc acid (BCA) protein
assay; 30 µg proteins were separated via 10% SDS-PAGE, which
were then transferred to nitrocellulose membranes. For blocking, 5%
milk was applied for 2.5 h at 20-25°C. The expression levels of
IL-18 (1:1,000; cat. no. sc-7954; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and NF-κB (1:1,000; cat. no. p65-10745-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA) were analyzed using
western blot analysis. The membrane was incubated with the
aforementioned primary antibodies overnight at 4°C. Subsequently,
the membranes were incubated with goat anti-rabbit IgG horseradish
peroxidase (HRP; H+L) secondary antibody (1:10,000; cat. no. 31460;
Thermo Fisher Scientific, Inc.) for 2 h at 4°C. The protein was
developed using ECL Plus according to the manufacturer's protocol
of the ECL kit (Beyotime Institute of Biotechnology). Images of the
staining were captured using a GEL imaging system (GelDoc XR
System; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the
band results were semi-quantitatively analyzed using Quantity One
4.6 software (Bio-Rad Laboratories, Inc.) to obtain the
corresponding optical density values. The ratio was attained using
the following formula: Ratio=target/β-actin.
RNA extracted from the vein wall and synthesized
into cDNA as aforementioned via RT, and the target gene expression
was analyzed using qPCR analysis. A reaction master mix was
prepared by adding the following components for each 25 µl
reaction to a tube at room temperature: 12.5 µl
Maxima® SYBR-Green/ROX qPCR Master Mix (2X), 0.3
µl PCR forward primer (10 µM), 0.3 µl PCR
reverse primer (10 µM), 500 ng template DNA and water,
nuclease-free to 25 µl. The primer sequences were analyzed
using the 2−ΔΔCq method (6): IL-18 (240 bp), forward, 5′-AAT CAG
ACC ACT TTG GCA GAC-3′ and reverse, 5′-TTT TAC AGG AGA GGG TAG ACA
TCC-3′; NF-κB (297 bp), forward, 5′-TCC AAC ACA GGC ATC ACC CA-3′
and reverse, 5′-CTT CAC ATC TCC CGT AAC CGC-3′; GAPDH (146 bp),
forward, 5′-ACG GCA AGT TCA ACG GCA CAG-3′ and reverse, 5′-GAC GCC
AGT AGA CTC CAC GAC A-3′. The thermocycling conditions constituted:
Initial denaturation at 95°C for 10 min (1 cycle); denaturation at
95°C for 15 sec; annealing at 60°C for 30 sec and extension at 72°C
for 30 sec (40 cycles).
Effect of IL-18 activated NF-κB on human
umbilical vein endothelial cells (HUVECs)
Fresh normal HUVECs (cat. no. 1-0025; CHI
Scientific, Inc., Jiangsu, China) were collected and cultured at
37°C, in a 5% CO2 saturated humidity incubator for 2-3
h. The original culture medium was discarded, and the cells were
digested using 0.25% EDTA trypsin at 37°C for 2 min for
contraction. The cells were the inoculated in 2-4 culture flasks
for preparing stationary culture. The medium was initially
displaced at 72 h, and was subsequently displaced every 1-2 days
according to the cell growth and experimental arrangement.
Measurements associated with the
IL-18-activated NF-κB signaling pathway
The HUVECs were divided into three groups according
to different pretreatment methods: Group A (control), comprised
cells routinely cultured for 24 h. Group B (IL-18) comprised cells
pretreated with IL-18 (50 ng/ml; recombinant human IL-18, cat. no.
CYT-269; Prospec-Tany Technogene Ltd., Rehovot, Israel) for 24 h.
Group C (IL-18+QNZ) comprised cells were pretreated with IL-18 50
ng/ml for 12 h, followed by addition of 50 µM/ml NF-κB
activation inhibitor QNZ (EVP4593, cat. no. s4902; Selleck
Chemicals, Houston, TX, USA) and further culture until 24 h.
Subsequently, nucleoprotein and plasma protein were isolated and
extracted from the HUVECs according to the manufacturer's protocol
of the Nuclear and Cytoplasmic Protein Extraction kit (cat. no.
P0028; Beyotime Institute of Biotechnology). The expression levels
of NF-κB-p65 (p65 polyclonal antibody, cat. no. 10745-1-AP) and
inhibitor of NF-B (IκBα polyclonal antibody, cat. no 10268-1-AP)
(both from ProteinTech Group, Inc.) in the cytoplasm and nucleus
were examined, respectively, using western blot and
immunofluorescence staining methods. The results were observed
under a fluorescence microscope (Nikon Eclipse 90i; Nikon
Corporation, Tokyo, Japan)
Effect of IL-18-activated NF-κB on cell
state and function
For the detection of apoptosis, pretreated cells
were subjected to mitochondrial membrane potential detection using
a mitochondrial membrane potential assay kit with JC-1 according to
the manufacturer's protocol (cat. no. C2006; Beyotime Institute of
Biotechnology), in which a fluorescence microscope (Nikon Eclipse
90i) was used for observation. The cells in each group were then
analyzed using Annexin V-FITC/PI flow cytometry (CyFlow®
Space; SysmexPartec GmbH, Görlitz, Germany) according to the
manufacturer's protocol of the Annexin V-FITC Apoptosis Detection
kit (cat. no. C1062; Beyotime Institute of Biotechnology).
Detection of endothelial cell-related
markers
The endothelial cells were divided into three groups
and were pretreated as described previously. Total protein was
isolated from 5-10×105 treated cells using SDS Lysis
Buffer (Beyotime Institute of Biotechnology), and incubated on ice
for 30 min. Cells were centrifuged at 14,000 × g for 5 min at 4°C.
Total protein was quantified by a BCA protein assay; 30 µg
proteins were separated via 10% SDS-PAGE and then transferred onto
nitrocellulose membranes, which were blocked with 5% milk for 2.5 h
at 20-25°C. The membranes were incubated with primary antibodies
(1:1,000) against vWF (C-12, cat. no. sc-365712), P-selectin
(CTB201, cat. no. sc-8419) and tissue plasminogen activator (t-PA;
C-16, cat. no. sc-5239) (all from Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Subsequently, the membranes were then incubated
with goat anti-rabbit IgG HRP (H+L) secondary antibodies (1:10,000)
for 2 h at 4°C.
For RT-qPCR analysis, total RNA was extracted from
the HUVECs of each group and synthesized into cDNA. A reaction
master mix was prepared by adding the following components for each
25 µl reaction to a tube at room temperature: 12.5 µl
Maxima® SYBR-Green/ROX qPCR Master Mix (2X), 0.3
µl PCR forward primer (10 µM), 0.3 µl PCR
reverse primer (10 µM), 500 ng template DNA and water,
nuclease-free to 25 µl. The themocycling conditions
constituted: Initial denaturation at 95°C for 10 min (1 cycle);
denaturation at 95°C for 15 sec; annealing/extension for 60°C for
60 sec (40 cycles).
The primers included were as follows: Human vWF,
forward 5′-TCCTCCTACTCTGCCCCCC-3′ and reverse,
5′-TCCATCCGCTGAATCACCTC-3′; human P-selectin, forward
5′-CCGTGCGTAATTACTCCCCC-3′ and reverse,
5′-AGGCTTTCTCGGCTTCATCTG-3′; human t-PA, forward,
5′-CCCAGATCGAGACTCAAAGCC-3′ and revesre,
5′-TGACCCATTCCCAAAGTAGCAG-3′; human β-actin, forward,
5′-ACGGCAAGTTCAACGGCACAG-3′ and reverse,
5′-GACGCCAGTAGACTCCACGACA-3′. The 2−ΔΔCq method
(6) was used for experimental
analysis.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). Measurement data are
expressed as the mean ± standard deviation. Intergroup comparisons
were performed using one-way analysis of variance (F test) and
multiple comparisons were performed using the LSD method. A 95%
confidence interval was adopted, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Rat modeling
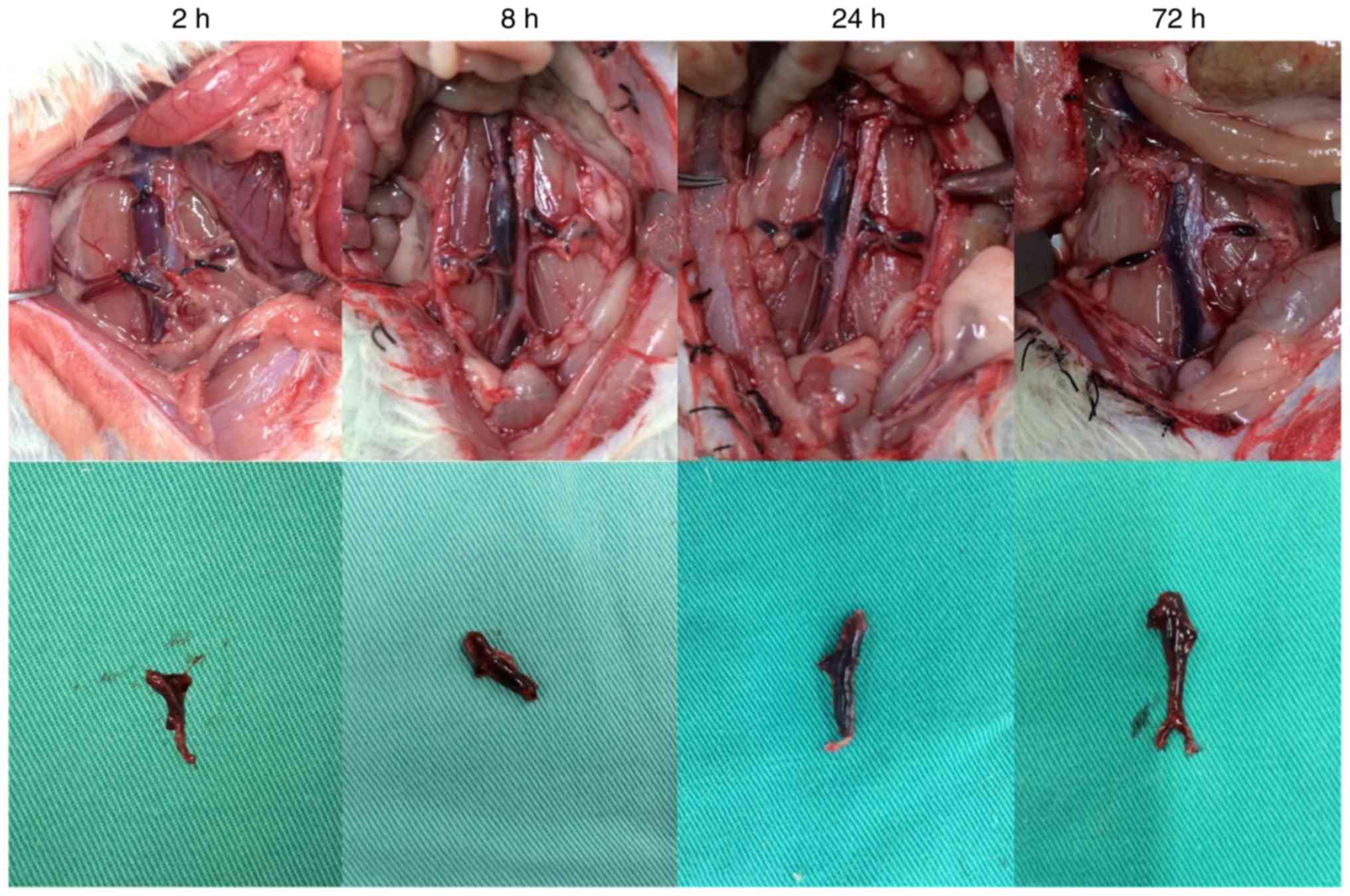
In the DVT model group, thrombosis was clearly
visible following model establishment (Fig. 2). During the experiment, the
average diameter of the rat IVC was 2.898±0.279 mm. Considering the
diameter of a 20-G needle as 0.91 mm, the average residual
cross-sectional area of the IVC lumen following modeling using the
flow restriction method was 9.87±1.67%. A favorable thrombosis rate
was obtained if the residual cross-sectional area of the lumen was
controlled at ~10% in the IVC thrombosis model using the flow
restriction method. This was consistent with the results reported
by Brill et al (5).
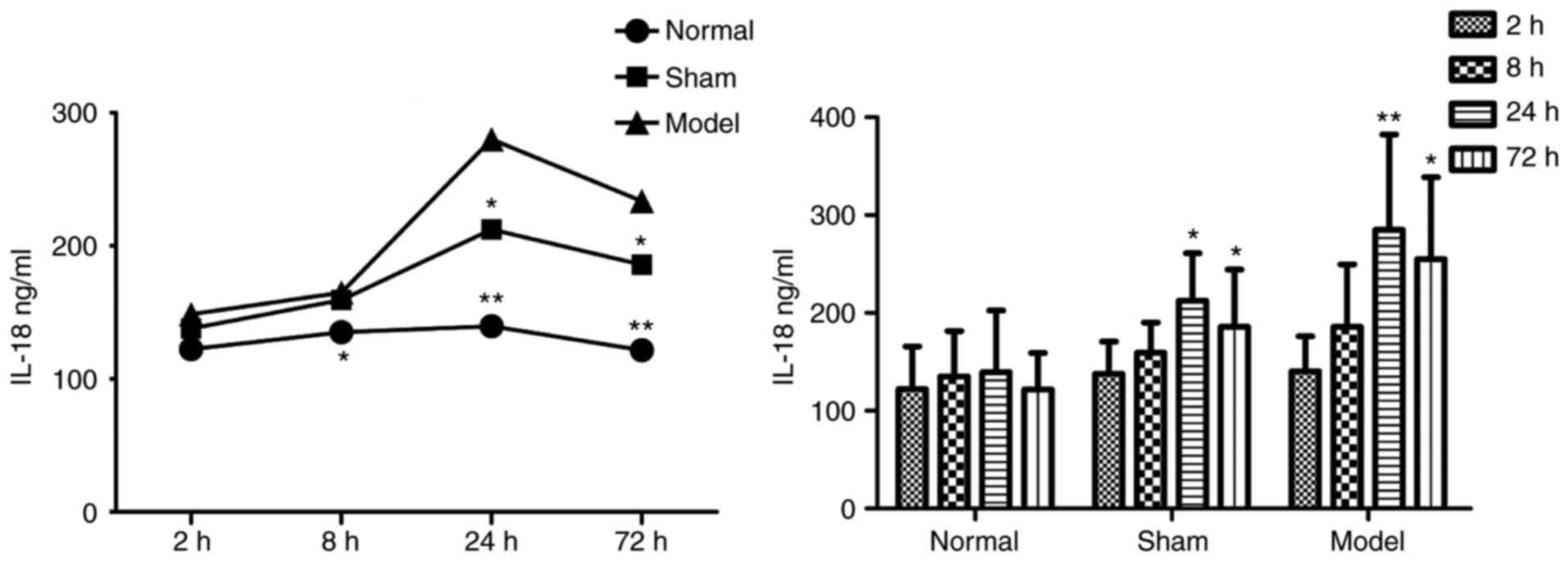
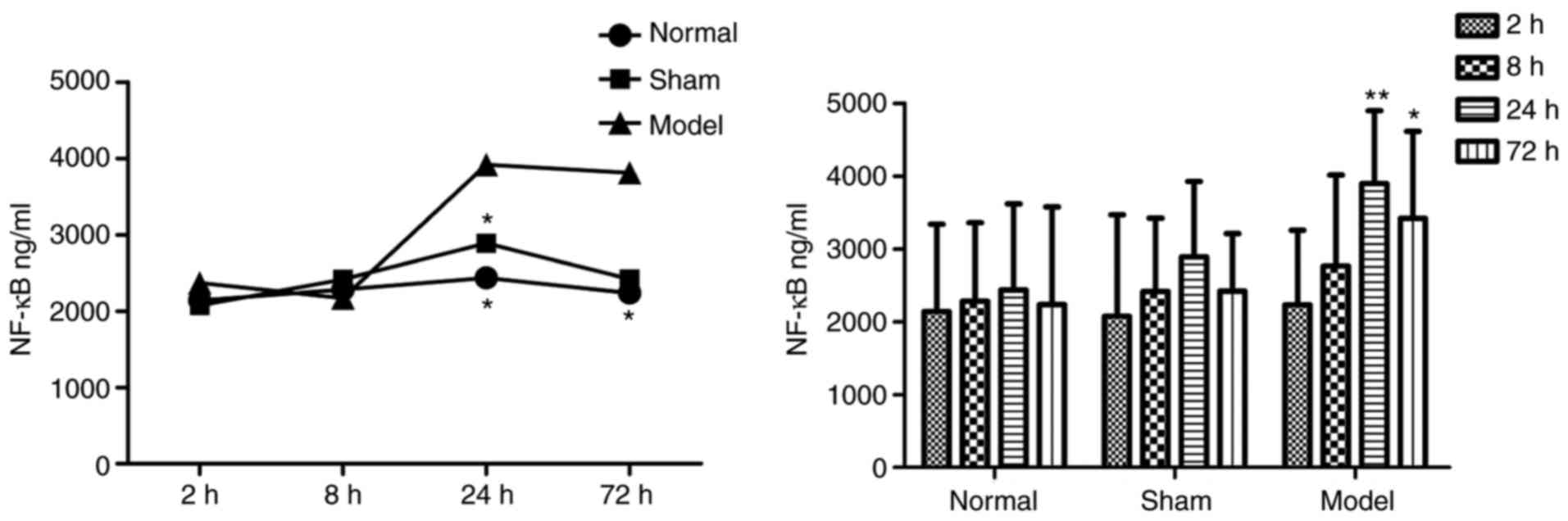
Blood ELISA results
In the plasma of the DVT model group, the protein
concentrations of IL-18 (Table I
and Fig. 3) and NF-κB (Table II and Fig. 4) were increased at all
time-points, and were significantly higher than those in the other
two groups.
 | Table IProtein concentrations of IL-18. |
Table I
Protein concentrations of IL-18.
| Time-point (h) | Concentration
(ng/ml)
| n |
|---|
| Normal | Sham | Model |
|---|
| 2 | 122.283±43.703 | 137.986±32.865 | 140.607±35.471 | 30 |
| 8 | 135.133±46.451 | 159.335±30.756 |
185.760±64.050a | 30 |
| 24 | 139.516±62.830 |
212.496±48.515a |
285.153±97.398b | 30 |
| 72 | 121.682±37.450 |
185.883±58.455a |
255.160±83.531b | 29 |
| Total | 129.653±47.288 | 173.925±51.021 | 215.683±91.342 | 119 |
 | Table IIProtein concentrations of NF-κB. |
Table II
Protein concentrations of NF-κB.
| Time-point (h) | Concentration
(ng/ml)
| n |
|---|
| Normal | Sham | Model |
|---|
| 2 |
2,140.567±1,197.236 |
2,072.758±1,396.933 |
2,230.484±1,025.352 | 30 |
| 8 |
2,282.417±1,078.633 |
2,412.840±1,010.100 |
2,762.880±1,250.555 | 30 |
| 24 |
2,436.232±1,181.866 |
2,889.602±1,038.773 |
3,897.117±998.730a | 30 |
| 72 |
2,235.919±1,343.126 |
2,417.126±794.822 |
3,418.390±1,196.602a | 29 |
| Total |
2,273.784±1,161.730 |
2,448.081±1,080.393 |
3,068.470±1,255.448 | 119 |
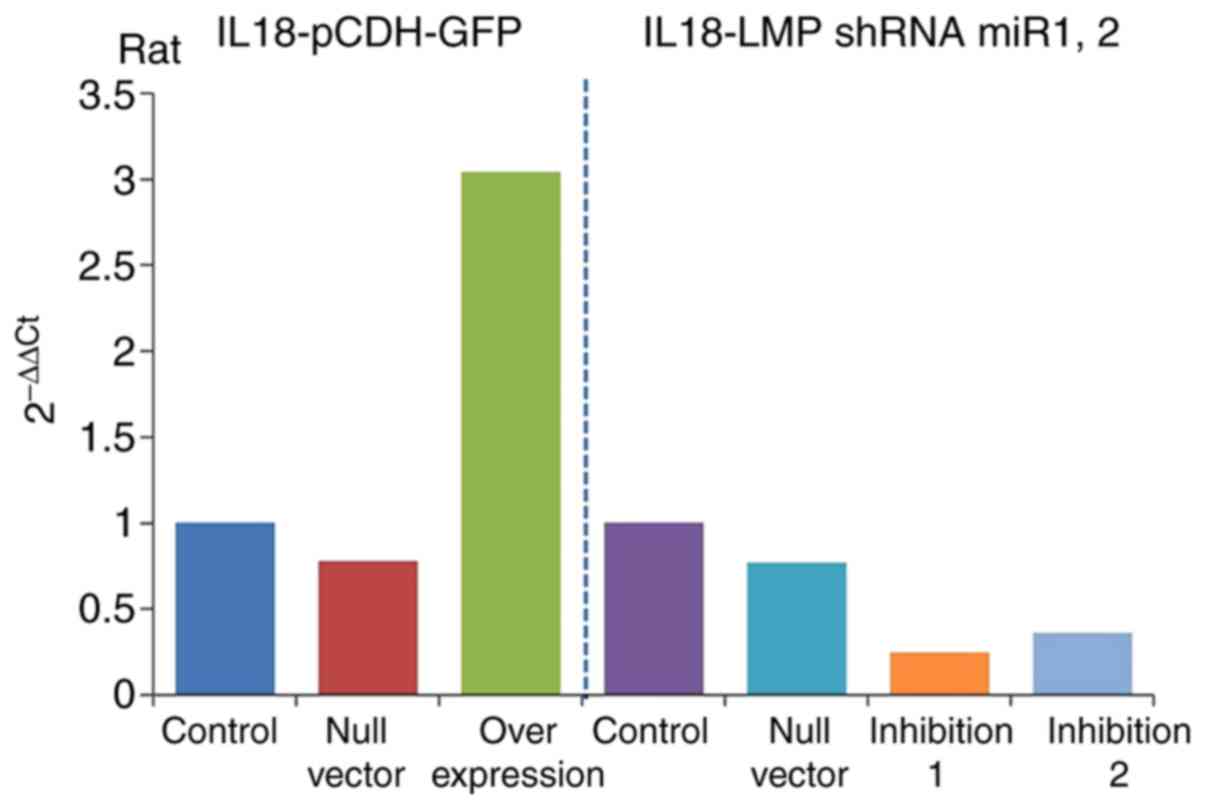
Detection of IL-18 gene overexpression
and inhibition efficiencies
The results of RT-qPCR analysis (Table III) revealed the expression of
IL-18 the gene in the overexpression and inhibition groups and
showed significant differences compared with that in the control
group. The gene expression of IL-18 was significantly increased in
the overexpression group (IL-18-pCDH-GFP) and significantly
decreased in the inhibition group. It was downregulated more in the
inhibition 1 group (IL-18-LMP shRNAmir1), as shown in Fig. 5. This indicated ideal efficiencies
of the plasmid virus with overexpression and inhibition of
IL-18.
 | Table IIIExpression of interleukin-18. |
Table III
Expression of interleukin-18.
| Group | 2−ΔΔCq
(rat) |
|---|
| Control | 1 |
| Null vector | 0.78 |
| Overexpression | 3.04 |
| Control | 1 |
| Null vector | 0.77 |
| Inhibition 1 | 0.25 |
| Inhibition 2 | 0.36 |
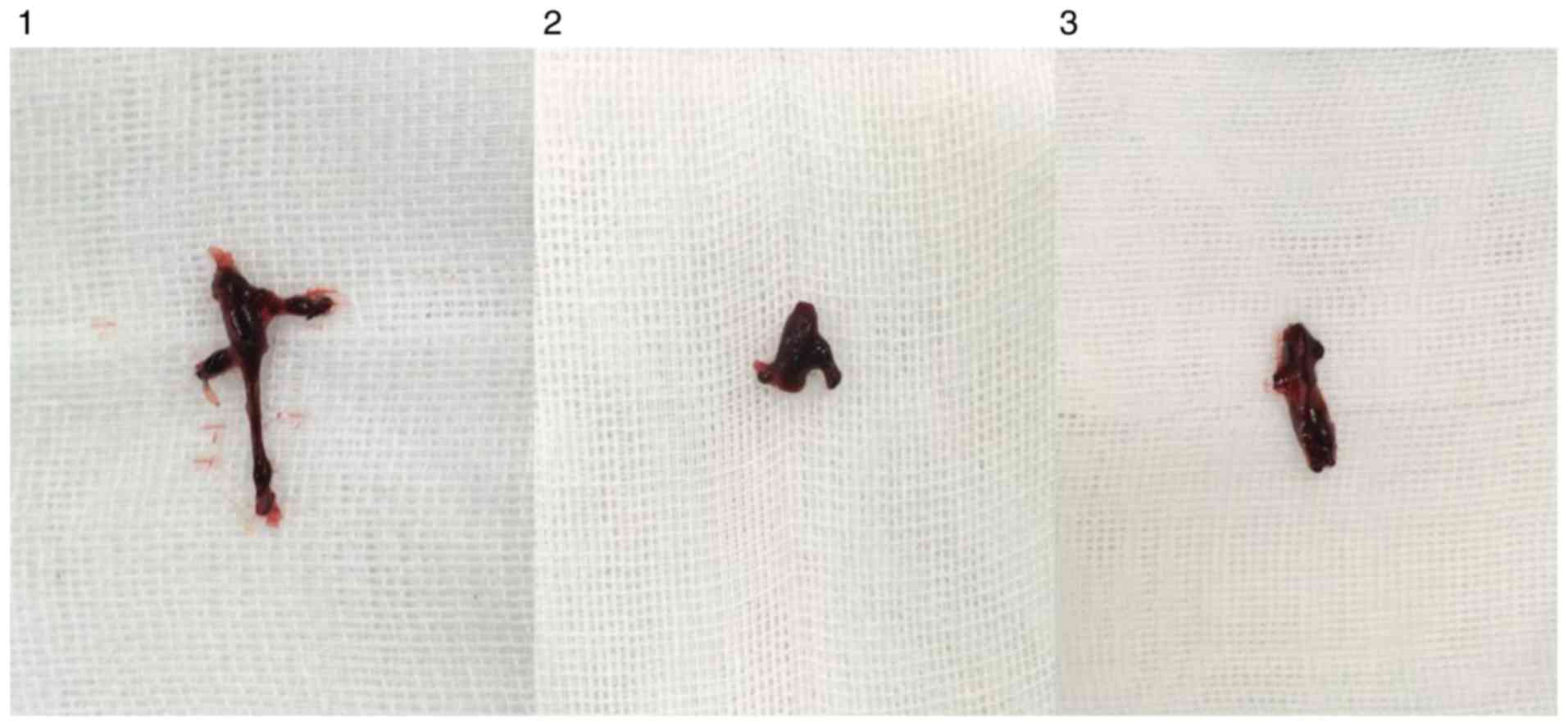
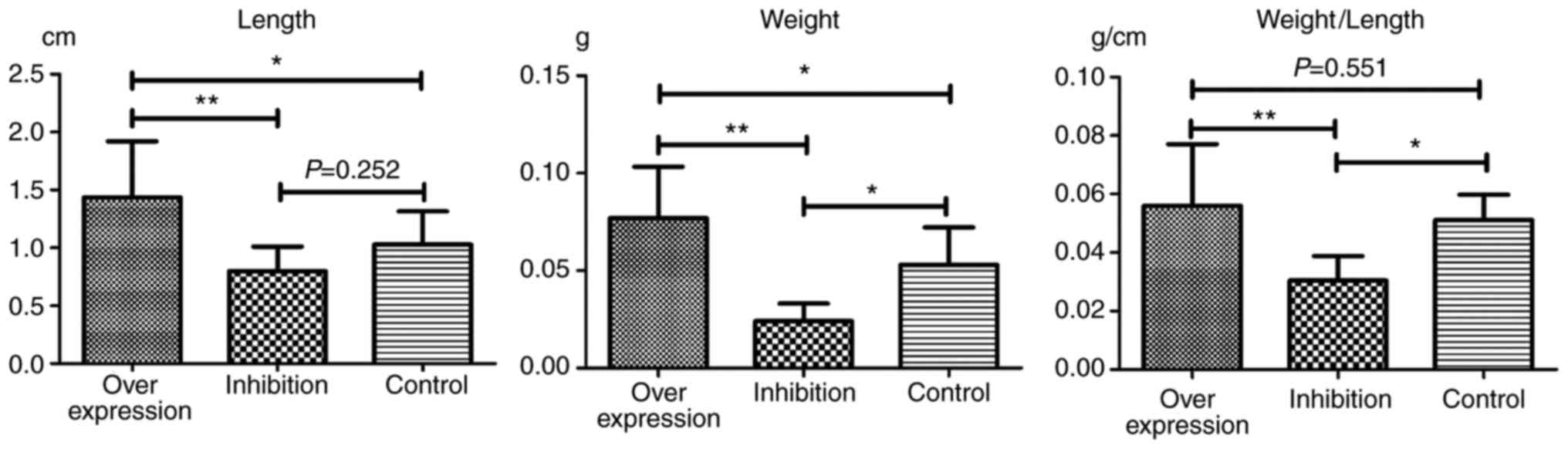
Observation of thrombosis in rats
In each model group, varying degrees of IVC
thromboses were visible at 24 h post-model establishment (Fig. 6). Compared with the control group:
i) Thrombus length differed significantly in the overexpression and
inhibition groups (F=7.965, P=0.002). This was significantly higher
in the overexpression group compared with the inhibition group
(P=0.001). ii) Thrombus weight differed significantly in the
overexpression and inhibition groups (F=18.178, P<0.001). This
was significantly higher in the overexpression group compared with
the normal (P=0.036) and inhibition groups (P<0.001), and was
significantly lower in the inhibition group compared with the
normal group (P=0.013). iii) Weight-length ratio, which indirectly
reflects the integrity of the thrombus (7,8)
did not differ significantly between the overexpression and the
control groups (P=0.551). However, thrombosis in the inhibition
group was significantly weaker, compared with that in the
overexpression (P=0.001) and control groups (P=0.019) (Table IV and Fig. 7).
 | Table IVLengths and weights of thrombi. |
Table IV
Lengths and weights of thrombi.
| Group | Length (cm) | Weight (g) | Weight/length
(g/cm) |
|---|
| Overexpression | 1.45±0.48a |
0.0769±0.0264a | 0.0561±0.0210 |
| Inhibition | 0.79±0.21 |
0.0241±0.0895a |
0.0304±0.0084a |
| Control | 1.03±0.29 | 0.0529±0.0193 | 0.0511±0.0086 |
| Total | 1.10±0.45 | 0.0510±0.0306 | 0.0448±0.0188 |
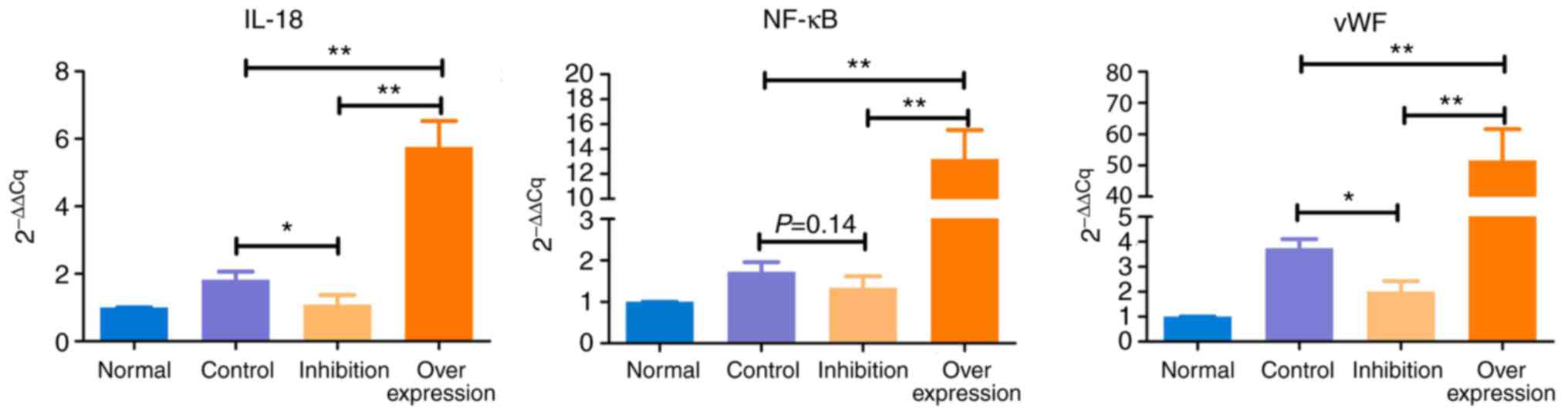
Results of RT-qPCR anaylsis
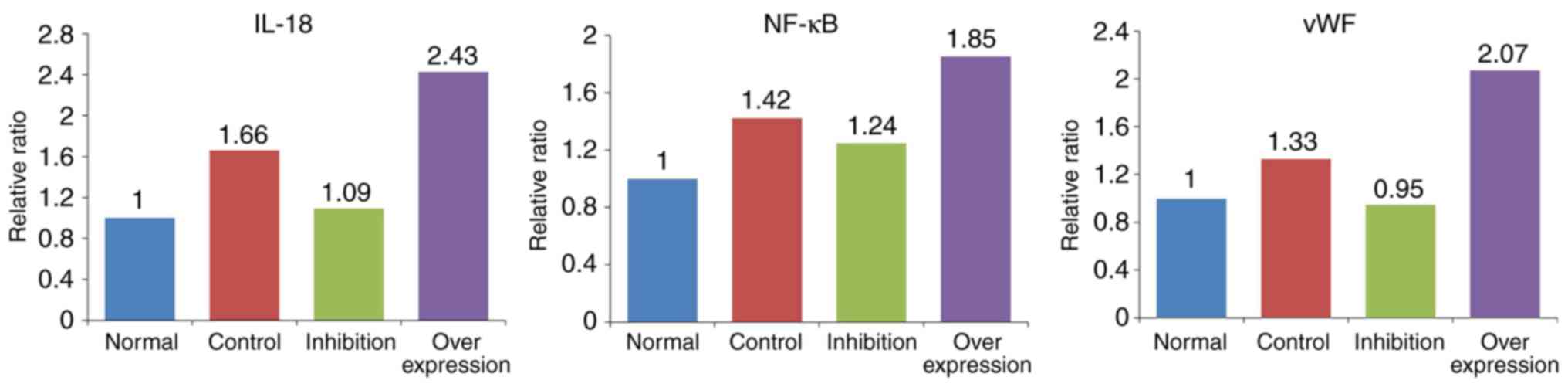
The expression levels of IL-18, NF-κB and vWF showed
similar trends in the various model groups (Table V and Fig. 8): i) Expression levels of IL-18,
NF-κB and vWF in the control group were significantly higher,
compared with those in the normal group (P<0.05). ii) Compared
with the normal group, expression levels of IL-18 and NF-κB were
marginally increased (P>0.05), and the expression of vWF was
significantly increased (P<0.05) in the inhibition group. iii)
Expression levels of IL-18, NF-κB and vWF in the overexpression
group were significantly higher, compared with those in the normal
rats (P<0.005).
 | Table VResults of reverse
transcription-quantitative polymerase chain reaction analysis. |
Table V
Results of reverse
transcription-quantitative polymerase chain reaction analysis.
| Group | 2−ΔΔCq
|
|---|
| n | IL-18 | NF-κB | vWF |
|---|
| Normal | 5 | 1.000 | 1.000 | 1.000 |
| Control | 5 | 1.824a | 1.726a | 3.739b |
| Inhibition | 10 | 1.089 | 1.345 | 2.008a |
| Overexpression | 10 | 5.757b | 13.204b | 51.565b |
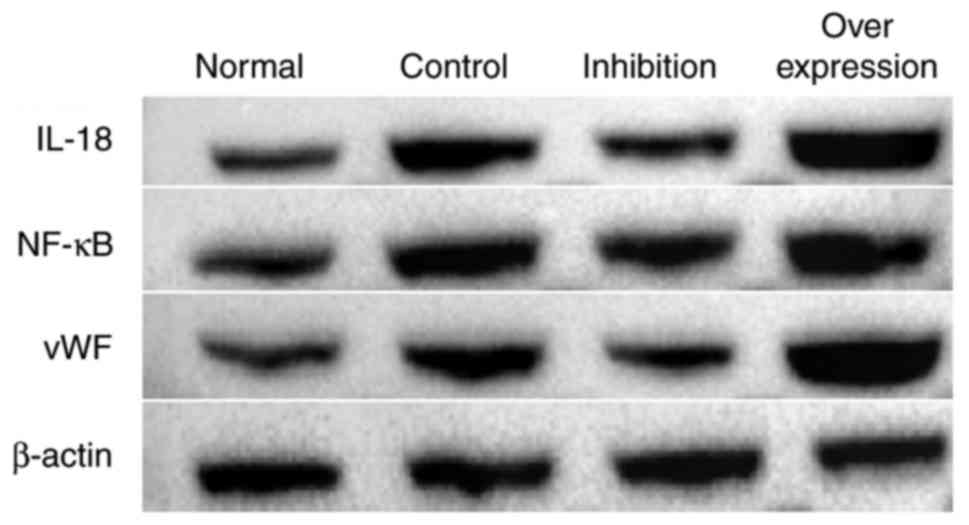
Results of western blot analysis
In the intravenous tissue, the expression levels of
IL-18, NF-κB and vWF in the normal group were significantly lower
compared with those in rats with DVT. In the rats infected with the
virus and with IL-18 overexpression and inhibition, the expression
of IL-18 in the intravenous tissue was significantly decreased in
the inhibition group, and increased in the overexpression group.
The expression levels of NF-κB and vWF in each group presented with
similar tendencies as with the expression of IL-18 (Figs. 9 and 10, Table
VI).
 | Table VIRelative protein expression levels in
different groups (ratio). |
Table VI
Relative protein expression levels in
different groups (ratio).
| Group | IL-18 | NF-κB | vWF |
|---|
| Normal | 0.686 | 0.693 | 0.767 |
| Control | 1.141 | 0.986 | 1.021 |
| Inhibition | 0.751 | 0.866 | 0.725 |
| Overexpression | 1.667 | 1.285 | 1.589 |
Effect of the IL-18-activated NF-κB
signaling pathway on HUVECs
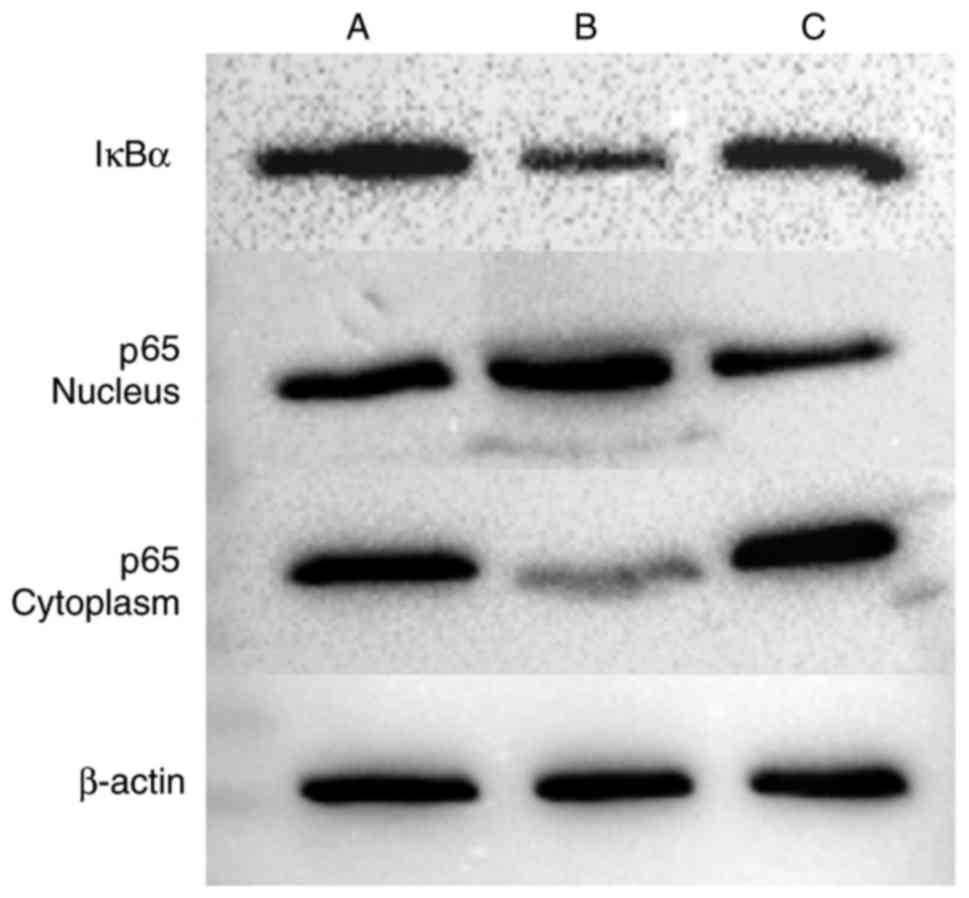
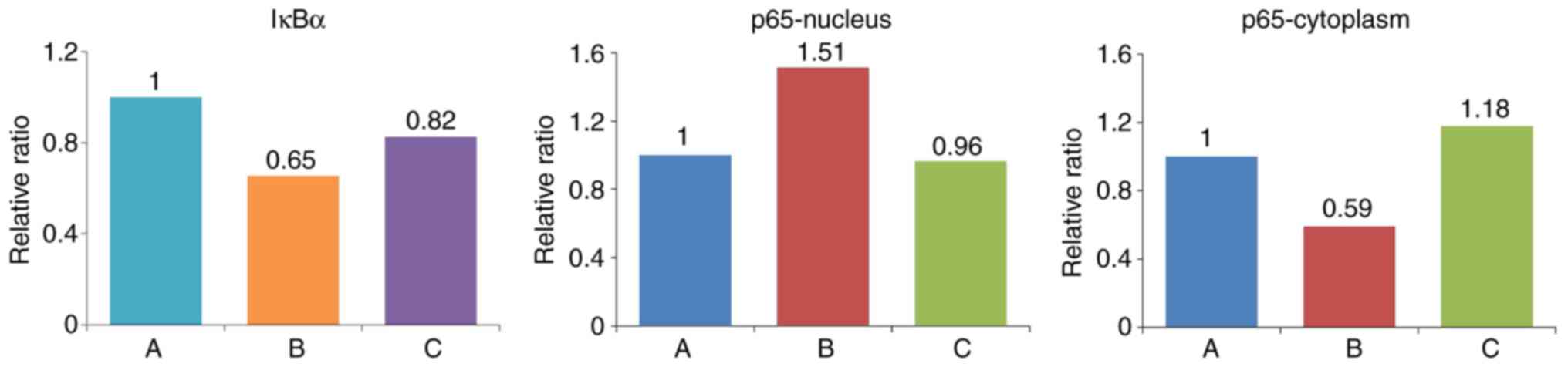
Western blot analysis was performed to detect the
expression of IκBα in cells, and the expression of p65 in the
cytoplasm and nucleus (Figs. 11
and 12, Table VII). The expression of IκBα was
significantly reduced following the application of IL-18, and was
increased further following the application of QNZ. The expression
of p65 in the nucleus was upregulated in the IL-18 group, compared
with that in the control group, and was similar to the normal group
following the application of QNZ. The expression of p65 in the
cytoplasm was significantly decreased in the IL-18 group, and was
increased following administration of QNZ.
 | Table VIIRelative expressions of IκBα and p65
(ratio). |
Table VII
Relative expressions of IκBα and p65
(ratio).
| Group | IκBα | p65-nucleus | p65-cytoplasm |
|---|
| A (Control) | 0.743997 | 0.777252 | 0.978306 |
| B (IL-18) | 0.486842 | 1.177360 | 0.578926 |
| C (IL-18+QNZ) | 0.614516 | 0.749936 | 1.153425 |
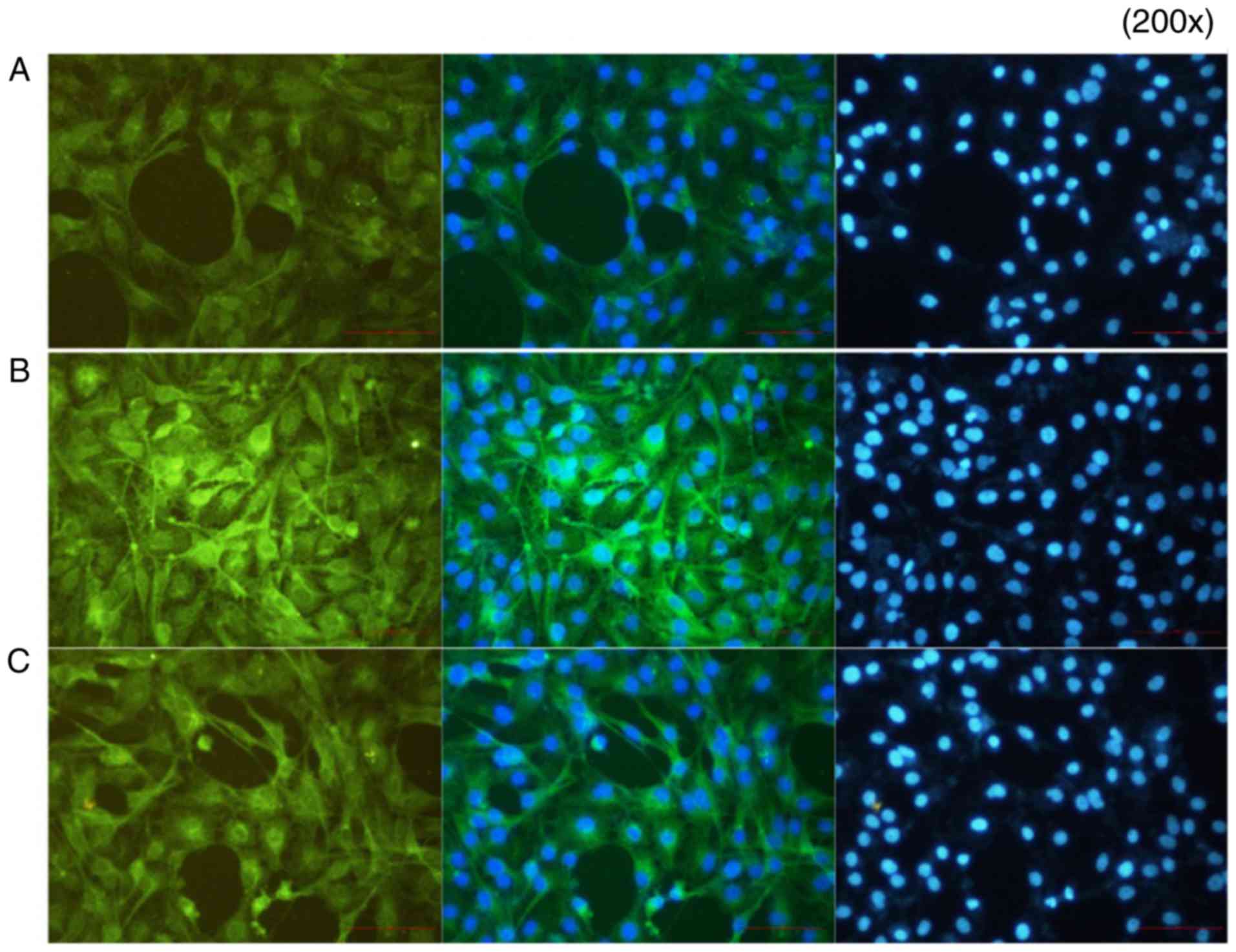
Immunofluorescence results
In group A (control), p-p65 was expressed at a low
level. In group B, the p65-p50 dimer had migrated to the nucleus
following the activation of NF-κB by IL-18, and the fluorescence
signal appeared shiny and bright green in color. In group C,
following application of the QNZ inhibitor, the green fluorescence
intensity was reduced, and the activation of NF-κB was suppressed
(Fig. 13).
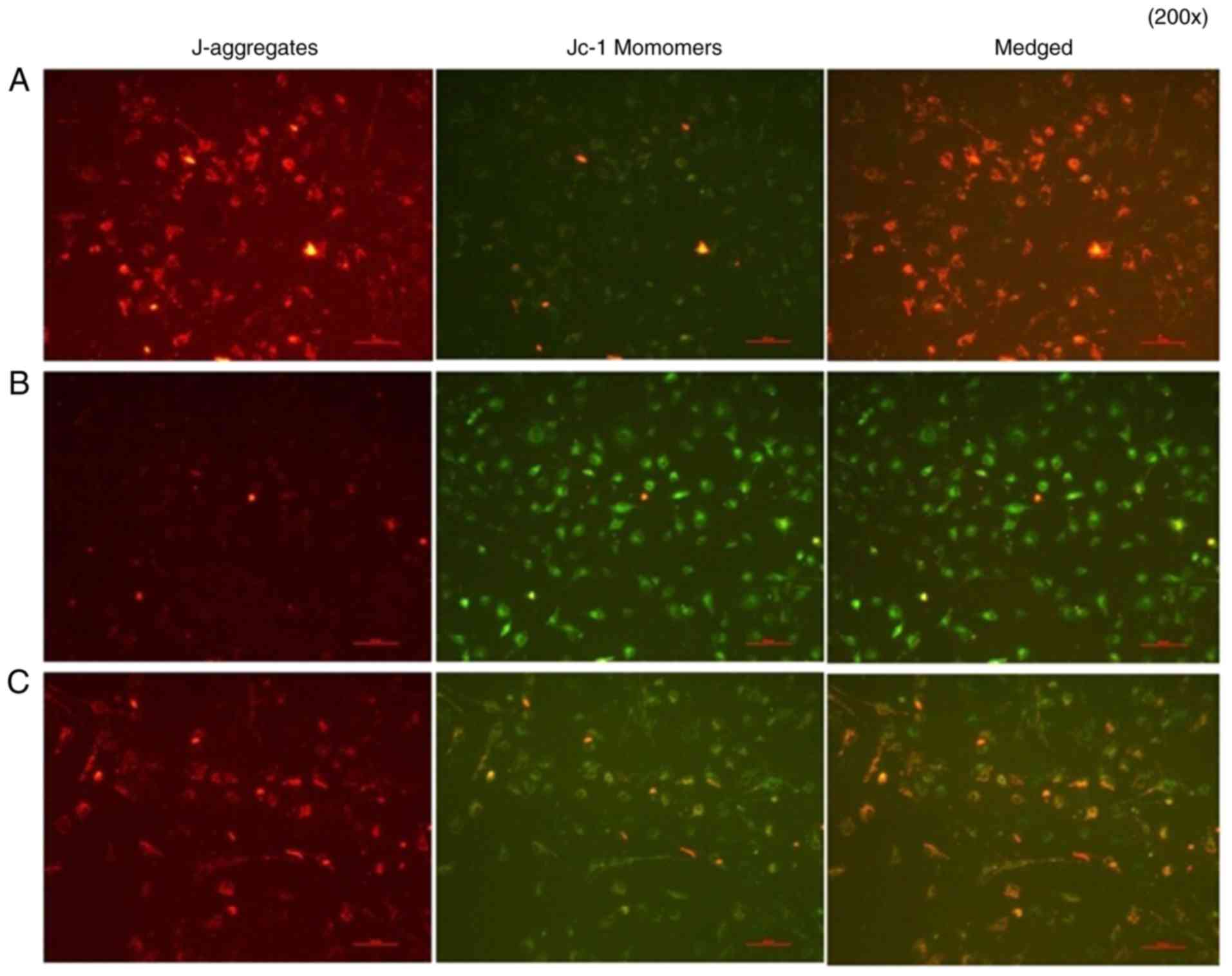
Analysis of cell apoptosis
A mitochondrial membrane potential assay was
performed. In group A group, normal cells were in good condition,
J-aggregates showed significant red fluorescence, whereas JC-1
monomers showed weak green fluorescence. In group B group, IL-18
showed significantly weakened red fluorescence, whereas bright
green fluorescence appeared, decreasing the mitochondrial membrane
potential. In group C, the application of QNZ suppressed IL-18 from
activating NF-κB, the number of cells with normal mitochondrial
membrane potential was significantly increased (red fluorescence)
and the apoptotic cells were reduced (green fluorescence) (Fig. 14).
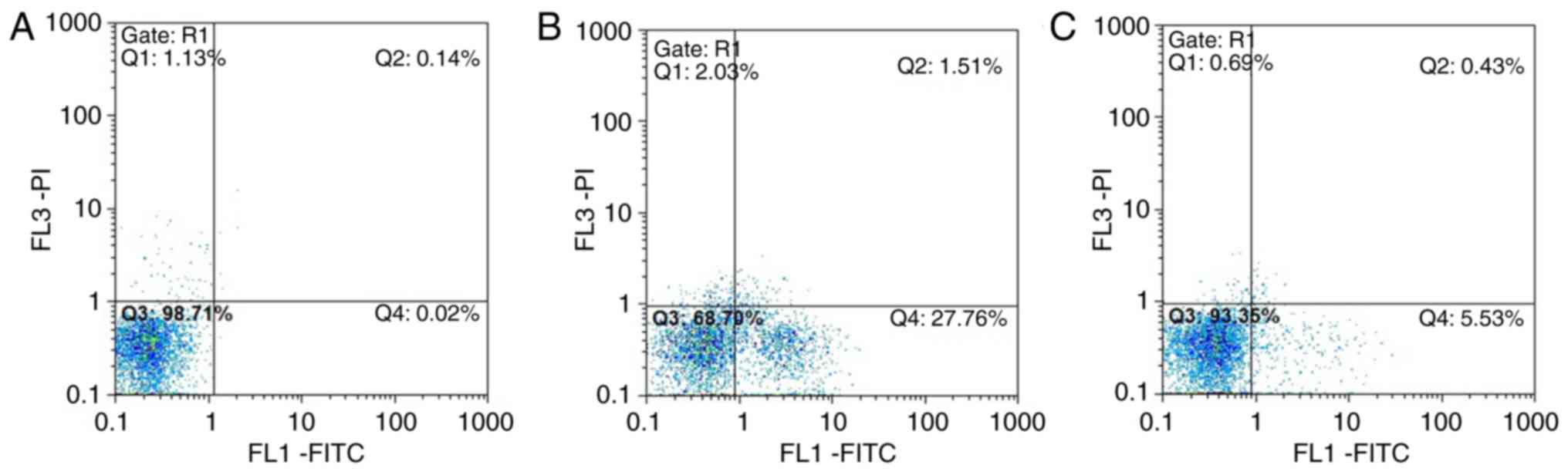
Annexin V-FITC/PI flow cytometry
results
In group A, normal viable cells accounted for
98.71%, and the viable apoptotic cells, early apoptotic and
necrotic cells accounted for 0.02 and 0.14%, respectively. In group
B, normal viable cells accounted for only 68.70%, and the early
apoptotic cells accounted for 27.76%, which was significantly
higher compared with that in group A group. The number of late
apoptotic and necrotic cells was increased compared with that in
group A. In group C, normal viable cells accounted for 93.35%, and
the number of early apoptotic cells was significantly lower
compared with that in the group B, whereas the number of late
apoptotic and necrotic cells accounted for 0.43%, which was close
to the number in group A (Fig.
15A–C).
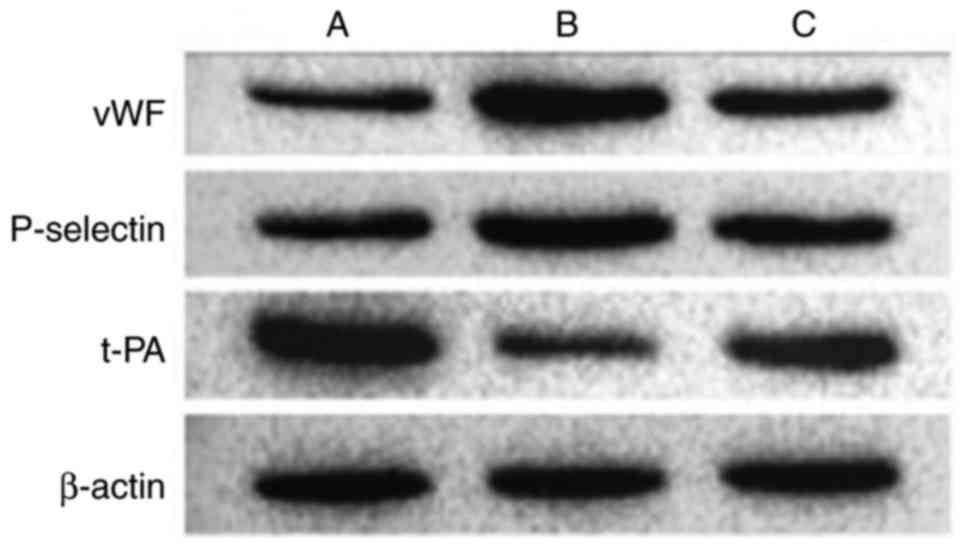
Results of western blot analysis of
endothelial cell-related markers
Various markers were affected following the
activation of NF-κB by IL-18. Compared with the control group, the
expression levels of vWF and P-selectin were significantly
upregulated in the other groups. t-PA was expressed at a high level
in normal cells, but was significantly inhibited in group B. The
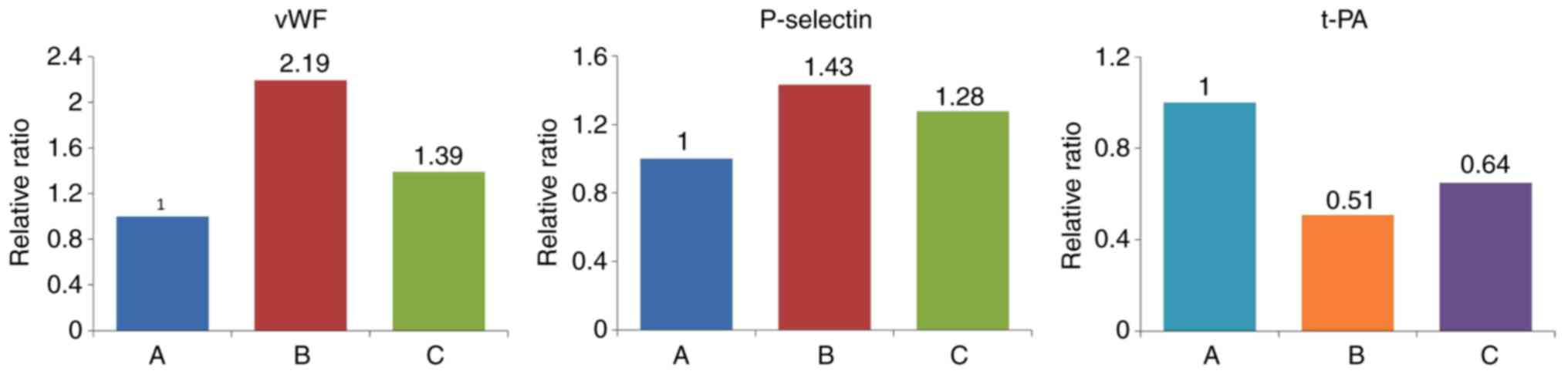
effect of IL-18 was significantly attenuated in group C (Table VIII, Figs. 16 and 17).
 | Table VIIIRelative expression levels of vWF,
P-selectin and t-PA (ratio). |
Table VIII
Relative expression levels of vWF,
P-selectin and t-PA (ratio).
| Group | vWF | P-selectin | t-PA |
|---|
| A (Control) | 0.593 | 1.029 | 1.281 |
| B (IL-18) | 1.301 | 1.476 | 0.650 |
| C (IL-18+QNZ) | 0.825 | 1.315 | 0.832 |
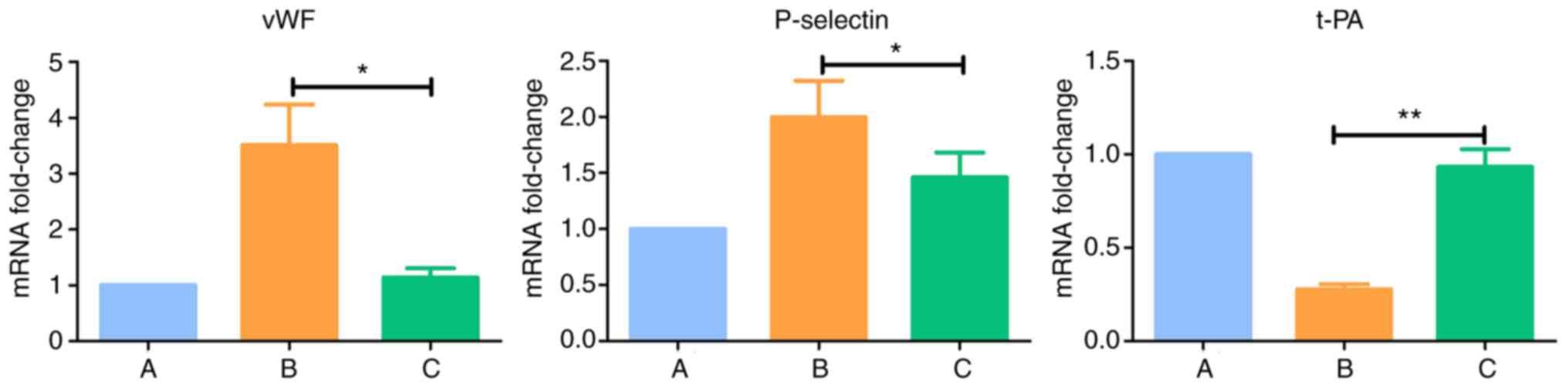
Results of RT-qPCR analysis of
endothelial cell-related markers
Compared with normal cells in the group A, the
expression levels of vWF and P-selectin were upregulated
(P<0.05) and the expression of t-PA was downregulated
(P<0.005) in group B. There were no significant differences the
expression levels of vWF, P-selectin and t-PA between group C and
group A (P>0.05) (Table IX
and Fig. 18).
 | Table IXGene expression levels determined
using reverse transcription-quantitative polymerase chain reaction
analysis. |
Table IX
Gene expression levels determined
using reverse transcription-quantitative polymerase chain reaction
analysis.
| Group | 2−ΔΔCq
|
|---|
| vWF | P-selectin | t-PA |
|---|
| A (Control) | 1.000 | 1.000 | 1.000 |
| B (IL-18) | 3.513a | 1.997a | 0.276b |
| C (IL-18+QNZ) | 1.138 | 1.461 | 0.932 |
Discussion
IL-18 is expressed in a variety of cells in the
body, and is mainly involved in the regulation of inflammatory and
immune reactions. It is also important in the release and
interaction of proinflammatory cytokines. Endothelial cells act as
key targets of immune and inflammatory mediators in the circulating
system. Morel et al (9)
confirmed that IL-18 promoted the expression levels of E-selectin,
vascular cell adhesion protein-1 and other adhesion molecules in
dermal microvascular endothelial cells. These have important
effects on inflammatory-related diseases, including rheumatoid
arthritis, and may be involved through activating NF-κB or
phosphatidylinosde 3-kinase cell signaling pathways. A study by
Chandrasekar et al (3) on
cardiovascular diseases revealed that IL-18 activated the
NF-κB-mediated signal transduction pathway, which in turn activated
cardiac microvascular endothelial cell apoptotic signaling, and led
to the growth and functional abnormalities of endothelial cells.
This suggested that IL-18 was involved through NF-κB signal
transduction pathway activation, rather than relying on IL-1β,
tumor necrosis factor-α and interferon-γ to induce the death of
HCMECs. In the present study, it was found that IL-18, NF-κB and
vWF were expressed at high levels in the rat model, and that
upregulation of the expression of IL-18 using viral vectors
increased the severity of thrombophilia and thrombosis, leading to
increased expression levels of NF-κB and vWF. Baker et al
(10) found that the sustained
activation of NF-κB remained harmful, and triggered a series of
chronic inflammatory processes by promoting the expression of
inflammatory factors, subsequently resulting in cell damage and
induction of diseases. Therefore, the present study hypothesized
that IL-18 in venous thrombosis can activate NF-κB to induce
abnormalities in cell growth and functional status, and is
important in the formation of DVT.
In the present study, recombinant human IL-18 was
used added to the HUVECs cultured in vitro, and induced
significant changes in the expression levels of NF-κB-p65. In the
cell resting state, NF-κB is located in the cytoplasm in a
non-active form. In the activated state, the p65-p50 dimer and IκBα
are isolated and transferred to the nucleus, where they are
involved in activating intracellular signaling pathways and the
transcription of target gene. The isolated IκBα ultimately
undergoes proteolysis. When the conditions change, the activator
may produce new IκBα and then move into the nucleus. It then
separates NF-κB from DNA and reconstitutes into the p65-p50-IκBα
complex to move back into the cytoplasm (11,12). The expression of p65 in the
nucleus of the HUVECs was significantly upregulated following
treatment with IL-18, whereas expression in the cytoplasm was
significantly decreased compared with that in normal cells. This
further confirmed that IL-18 effectively activated NF-κB in the
dissociation of p65-p50-IκBα, and that the p65-p50 dimer was
rapidly transferred into the nucleus to induce its corresponding
effect. The expression of p65 in the nucleus was downregulated by
using the NF-κB activator, inhibitor QNZ, and its relative
expression in the cytoplasm was close to that in the normal cells.
This revealed that the activation of NF-κB by IL-18 produced large
quantities of phosphorylated p65-p50 dimers and then migrated into
the nucleus, which was significantly suppressed following the
application of QNZ. Several studies involving cell and animal
models (13-15) have demonstrated that QNZ
effectively suppresses the activation of NF-κB-mediated
intracellular signal transduction pathway and target gene
transcription.
Thrombotic disease is a common complication
occurring during orthopedic surgery and trauma. Arterial thrombosis
and embolization occur rarely. More commonly, DVT and pulmonary
embolism are important factors, which may affect the perioperative
treatment and increase mortality rates. In the 1850s, Virchow
described three factors of venous thrombosis, which included blood
stagnation, hypercoagulable state and vein wall damage, and these
remain publicly recognized. With the progression of thrombotic
disease-related studies, it is increasingly considered that
endothelial cell injury, the adhesion, aggregation and release of
platelets, and activation of soluble coagulation factors are three
independent elements of equal significance (16). Endothelial cells are important in
the pathogenesis of thrombotic diseases as they regulate
physiological anticoagulation and pathological thrombosis.
In the present study, a large number of HUVECs
showed decreased abnormal status of mitochondrial membrane
potential following the application of IL-18. Annexin V-FITC/PI
flow cytometry revealed that a large number of cells were early
apoptotic, and the numbers of late apoptotic and necrotic cells
were also increased compared with those in the normally-cultured
cells. Previous studies (17,18) have confirmed that the abnormal
expression of inflammatory factors cause changes in endothelial
cell apoptosis, leading to vascular wall injury. This ultimately
promotes the formation of local blood clots and further stimulates
inflammatory cell infiltration, thereby resulting in abnormal
progression of thrombosis and atherosclerotic plaques. Apoptosis
remains the main process for maintaining a steady state of cell
population under normal conditions. However, the rapid or abnormal
apoptosis of cells lead to changes in the normal cell function and
can lead to diseases. In the present study, following application
of the NF-κB activation inhibitor QNZ, the number of normal viable
cells, viable apoptotic cells, non-viable apoptotic and necrotic
cells were similar to those in the normal group. This further
confirmed that IL-18 induced abnormal growth of HUVECs and
increased the number of viable apoptotic cells, which was
significantly improved by inhibiting the activation of NF-κB.
In the present study, the endothelial cell
dysfunction markers, vWF, P-selectin and t-PA, were altered
following treatment of the HUVECs with IL-18. vWF is a type of
macromolecular glycoprotein, which is synthesized primarily by
vascular endothelial cells. The synthesized and secreted vWF is
increased when endothelial cells are damaged or stimulated, which
promotes the adhesion of platelets and binds with GPIb and
subcutaneous collagen. It is also involved in the formation of
emboli and platelets at the injured site in endothelial cells
(19). It also can produce
complexes by binding with plasma VIII factor to prevent its
degradation and protect its activity. Studies have found (20,21) that the expression of vWF is
upregulated in patients at high risk of atherosclerosis, which is
also considered a marker of endothelial injury. The results of the
present study also confirmed that the upregulation of IL-18
activated NF-κB-induced HUVEC dysfunction and increased the
expression of vWF. This in turn altered the coagulation property in
the venous lumen, leading to thrombophilia. Vascular endothelial
cells can synthesize P-selectin, which is stored in Weibel-Palade
bodies. When endothelial cells are dysfunctional or stimulated, the
Weibel-Palade bodies fuse with the cell membrane. P-selectin is a
membrane glycoprotein, which is present on the cell surface and
acts as a receptor for neutrophils and monocytes, mediating the
interactions between endothelial cells, platelets and leukocytes.
Therefore, P-selectin is often used as a marker of endothelial
dysfunction. The increased expression of P-selectin can lead to the
occurrence of thrombophilia, inducing the activation of
procoagulants and formation of fibrin (22,23). Studies (7,24)
have revealed that P-selectin has a significant effect on
thrombosis, where the upregulation of soluble P-selectin in the
blood can be used as a clinical risk factor in venous thrombosis.
Treatment of the HUVECs with IL-18 significantly increased the
expression of P-selectin, unlike vWF, and its abnormal expression
was effectively suppressed following inhibition of the activation
of NF-κB. Physiologically, thrombosis requires prompt removal to
restore the patency of the vein. Therefore, there is a complex
fibrinolytic system in the body, including plasminogen, plasmin
inhibitor, plasminogen activator and plasminogen activator
inhibitor. Endothelial cells can synthe-size t-PA and
urokinase-type plasminogen activator (u-PA). Of these, t-PA has a
higher affinity to fibrin. Following fibrin formation, t-PA and
plasminogen in the emboli bind to the fibrin. The t-PA can activate
the plasminogen present on the fibrin surface, having a
fibrinolytic effect locally (25). Recombinant t-PA synthesized by
genetic engineering has been used as an antithrombotic drug
(24). In the present study, it
was found that IL-18 led HUVECs to produce t-PA, which
significantly improved following the application of inhibitors of
NF-κB activation. These results demonstrated that IL-18 activated
NF-κB causing the dysfunction of venous endothe-lial cells, which
may in turn act as one of the most important pathways for
demonstrating the mechanism of association between IL-18 and DVT
formation.
In conclusion, IL-18, in addition to NF-κB and vWF,
are closely associated with DVT, and regulation of the expression
of IL-18 can alter NF-κB, vWF and other related markers. A high
expression of IL-18 can activate NF-κB, causing damage, apoptosis
and other changes of the venous endothelial cells. This in turn
leads to cell dysfunction, further affecting the endothelial cells
and causing abnormal changes in thrombotic disease-related cell
function markers, vWF, P-selectin and t-PA. The mechanism
underlying DVT is complicated. IL-18 was shown to be involved in
the regulation of inflammatory and immune reactions in vivo,
and regulated the expression of NF-κB and vWF, but also affected
endothelial function. This may be one of the important mechanisms
involved in DVT. The association between IL-18 and related markers
and DVT requires further detailed investigation, including the
examination of platelets, erythrocytes, leukocytes, fibrinolytic
abnormalities and anti-procoagulant abnormalities.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China Science Fund Project (grant nos.
30960389 and 8176010097), the Guizhou Province Science and
Technology Program Joint Fund Project [grant no. Qian Ke He LH Zi
(2016) no. 7228] and the Guizhou Medical University Doctoral Start
Fund Project [grant no. Yuan Bo He J Zi (2015) 019].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and XZ made substantial contributions to the
conception of the present study. GL drafted and wrote the
manuscript preparation and performed the data analyses. RZ
generated overexpression and suppression vectors employed in the
present study. RL and CY made substantial contributions to data
analysis and critically revised the manuscript for important
intellectual content.
Ethics approval and consent to
participate
All animal experiments were performed following
approval from the Animal Experiment and Ethics Committee of Kunming
Medical University (Kunming, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Downing LJ, Strieter RM, Kadell AM, Wilke
CA, Austin JC, Hare BD, Burdick MD, Greenfield LJ and Wakefield TW:
IL-10 regulates thrombus-induced vein wall inflammation and
thrombosis. J Immunol. 161:1471–1476. 1998.
|
|
2
|
Strukova S: Blood coagulation-dependent
inflammation. Coagulation-dependent inflammation and
inflammation-dependent thrombosis. Front Biosci. 11:59–80. 2006.
View Article : Google Scholar
|
|
3
|
Chandrasekar B, Vemula K, Surabhi RM,
Li-Weber M, Owen-Schaub LB, Jensen LE and Mummidi S: Activation of
intrinsic and extrinsic proapoptotic signaling pathways in
interleukin-18-mediated human cardiac endothelial cell death. J
Biol Chem. 279:20221–20233. 2004. View Article : Google Scholar
|
|
4
|
Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG,
Chen M, Liu JG, Pei XH, Wang LJ, Lin ZX, et al: NF-kappaB
transcription factor p50 critically regulates tissue factor in deep
vein thrombosis. J Biol Chem. 284:4473–4483. 2009. View Article : Google Scholar
|
|
5
|
Brill A, Fuchs TA, Chauhan AK, Yang JJ, De
Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S and
Wagner DD: Von Willebrand factor-mediated platelet adhesion is
critical for deep vein thrombosis in mouse models. Blood.
117:1400–1407. 2011. View Article : Google Scholar
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
7
|
Myers DD, Hawley AE, Farris DM, Wrobleski
SK, Thanaporn P, Schaub RG, Wagner DD, Kumar A and Wakefield TW:
P-selectin and leukocyte microparticles are associated with venous
thrombogenesis. J Vasc Surg. 38:1075–1089. 2003. View Article : Google Scholar
|
|
8
|
Henke PK, Varga A, De S, Deatrick CB,
Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL,
Upchurch GR Jr and Wakefield TW: Deep vein thrombosis resolution is
modulated by monocyte CXCR2-mediated activity in a mouse model.
Arterioscler Thromb Vasc Biol. 24:1130–1137. 2004. View Article : Google Scholar
|
|
9
|
Morel JC, Park CC, Woods JM and Koch AE: A
novel role for interleukin-18 in adhesion molecule induction
through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent
signal transduction pathways. J Biol Chem. 276:37069–37075. 2001.
View Article : Google Scholar
|
|
10
|
Baker RG, Hayden MS and Ghosh S:
NF-kappaB, inflammation, and metabolic disease. Cell Metab.
13:11–22. 2011. View Article : Google Scholar
|
|
11
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View
Article : Google Scholar
|
|
12
|
Sen R: The origins of NF-κB. Nat Immunol.
12:686–688. 2011. View
Article : Google Scholar
|
|
13
|
Tobe M, Isobe Y, Tomizawa H, Nagasaki T,
Takahashi H and Hayashi H: A novel structural class of potent
inhibitors of NF-kappa B activation: Structure-activity
relationships and biological effects of 6-aminoquinazoline
derivatives. Bioorg Med Chem. 11:3869–3878. 2003. View Article : Google Scholar
|
|
14
|
Armstrong SJ, Wiberg M, Terenghi G and
Kingham PJ: Laminin activates NF-kappaB in Schwann cells to enhance
neurite outgrowth. Neurosci Lett. 439:42–46. 2008. View Article : Google Scholar
|
|
15
|
Yang RH, Strong JA and Zhang JM: NF-kappaB
mediated enhancement of potassium currents by the chemokine
CXCL1/growth related oncogene in small diameter rat sensory
neurons. Mol Pain. 5:262009. View Article : Google Scholar
|
|
16
|
Blann AD and Lip GY: Virchow's triad
revisited: The importance of soluble coagulation factors, the
endothelium, and platelets. Thromb Res. 101:321–327. 2001.
View Article : Google Scholar
|
|
17
|
Pan J, Zhang SQ and Chen B: Interferon-γ
induces the apoptosis of vascular endothelial cell in vitro. J
Bengbu Med Coll. 30:388–389. 2005.
|
|
18
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar
|
|
19
|
Blann AD: Plasma von Willebrand factor,
thrombosis, and the endothelium: The first 30 years. Thromb
Haemost. 95:49–55. 2006.
|
|
20
|
Jansson JH, Nilsson TK and Johnson O: Von
Willebrand factor in plasma: A novel risk factor for recurrent
myocardial infarction and death. Br Heart J. 66:351–355. 1991.
View Article : Google Scholar
|
|
21
|
Cortellaro M, Boschetti C, Cofrancesco E,
Zanussi C, Catalano M, de Gaetano G, Gabrielli L, Lombardi B,
Specchia G and Tavazzi L: The PLAT Study: Hemostatic function in
relation to atherothrombotic ischemic events in vascular disease
patients. Principal results. PLAT Study Group. Progetto Lombardo
Atero-Trombosi (PLAT) Study Group. Arterioscler Thromb.
12:1063–1070. 1992. View Article : Google Scholar
|
|
22
|
Cambien B and Wagner DD: A new role in
hemostasis for the adhesion receptor P-selectin. Trends Mol Med.
10:179–186. 2004. View Article : Google Scholar
|
|
23
|
Furie B and Furie BC: Role of platelet
P-selectin and micropar-ticle PSGL-1 in thrombus formation. Trends
Mol Med. 10:171–178. 2004. View Article : Google Scholar
|
|
24
|
Rosenbaum T, Rammos S, Kniemeyer HW and
Gobel U: Extended deep vein and inferior vena cava thrombosis in a
15-year-old boy: Successful lysis with recombinant tissue-type
plasminogen activator 2 weeks after onset of symptoms. Eur J
Pediatr. 152:978–980. 1993. View Article : Google Scholar
|
|
25
|
Kruithof EK and Dunoyer-Geindre S: Human
tissue-type plas-minogen activator. Thromb Haemost. 112:243–254.
2014. View Article : Google Scholar
|