Introduction
Diabetes mellitus (DM) comprises a group of
metabolic diseases characterized by high blood sugar levels, and
may cause a variety of complications if left untreated (1). Diabetic retinopathy (DR) is among
the most serious complications of DM, as it is the leading cause of
visual impairment and blindness among patients with diabetes
(2). DR has been found to be
associated with retinal microvascular damage induced by high
glucose levels (3). This
condition affects the structure of the retina, leading to metabolic
and functional disturbances. Retinal endothelial cells (RECs)
maintain the stability of the blood-retinal barrier, and remove
toxins and inflammatory factors; thus, they serve a key role in the
protection of visual function (4). Despite several studies on RECs
conducted in China and other countries (5,6),
the molecular mechanisms underlying REC damage and apoptosis remain
unclear.
Small GTPase proteins are monomeric G protein
molecules that serve key roles in regulating different cellular
functions (7,8). Ras homolog family member A (RhoA) is
a small GTPase protein that belongs to the Rho family (9). RhoA activates and combines with
Rho-associated protein kinase 1 (ROCK1), and is involved in the
signaling transduction pathways of various cellular functions. A
recent study demonstrated that the RhoA/ROCK1 signaling pathway
modulated hyperglycemia-induced microvascular endothelial
dysfunction, suggesting a potential target for the treatment of DR
(10).
microRNAs (miRNAs or miRs) are non-coding RNAs with
regulatory functions, which generally regulate protein translation
by inhibiting the expression of downstream target proteins
(11). In this regard, miRNAs are
involved in a variety of physiological processes, including
developmental timing, cell proliferation, apoptosis, hematopoiesis
and neural patterning (12).
miR-200b is a specific miRNA that regulates vascular endothelial
growth factor-mediated alterations in DR (13). Furthermore, it was previously
demonstrated that miR-133a mediated gene expression and
cardiomyocyte hypertrophy in diabetes (14). Bioinformatics methods have
identified RhoA as a direct target of miR-133b. Considering these
previous findings, the present study aimed to investigate the
effects of miR-133b on RhoA/ROCK1 signaling pathways in
high-glucose-induced human RECs (hRECs), in order to determine the
role of miR-133b in DR.
Materials and methods
Cell culture and treatment
hRECs were purchased from ScienCell (Research
Laboratories, Inc., Carlsbad, CA, USA). The cells were cultured in
human endothelial medium with 5% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% endothelial cell growth
supplement (ScienCell Research Laboratories, Inc.) at 37°C with 5%
CO2. To establish the high-glucose-induced DR model,
hRECs were cultured in high-glucose medium to a final concentration
of 25 mM. Normal human endothelial medium (5.5 mM glucose) was used
in the control group.
Cell transfection
hRECs were plated in 6-well plates (1×106
cells/well) on the day prior to transfection. miR-133b inhibitors,
miR-133b mimics and their controls were purchased from Sangon
Biotech Co., Ltd. (Shanghai, China). The cells were transfected
with the mimics or inhibitors using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hRECs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration of RNA was then measured with a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Next, total RNA
was reverse-transcribed into cDNA using a RevertAid™ First Strand
cDNA Synthesis kit (K1622; Fermentas; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. Subsequently, qPCR was
performed using a SYBR Green qPCR SuperMix (Invitrogen; Thermo
Fisher Scientific, Inc.). Thermocycling conditions comprised 40
cycles of: Denaturation at 95°C for 10 sec, annealing at 60°C for
10 sec, and extension at 72°C for 15 sec. The sequences of the
primers used were as follows: RhoA forward,
5′-GGAAAGCAGGTAGAGTTGGCT-3′ and reverse,
5′-GGCTGTCGATGGAAAAACACAT-3′; ROCK1 forward,
5′-AAGTGAGGTTAGGGCGAAATG-3′ and reverse,
5′-AAGGTAGTTGATTGCCAACGAA-3′; LIM domain kinase 1 (LIMK) forward,
5′-CGAGCACTCACACACCGTC-3′ and reverse, 5′-GATGGGCGTGCCATTGATTT-3′;
myosin light chain (MLC) forward, 5′-TGG GGG ATC GGT TTA CAG
ATG-3′, and reverse 5′-TTTCAGGATGCGTGTGAACTC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. GAPDH was used as the endogenous
control. The expression levels of RhoA, ROCK1, LIMK and MLC were
analyzed using the 2−ΔΔCq method (15).
Western blotting
Cells in different groups were lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and centrifuged at 12,000 × g at
4°C for 10 min. The concentration of proteins in the supernatant
was determined with a BCA Protein Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. A total of
40 µg protein was then separated by SDS-PAGE (10% gel) and
transferred to poly-vinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). Subsequent to blocking with 5% skimmed milk at
room temperature for 1 h, the membranes were incubated at 4°C
overnight with the following primary antibodies: Anti-RhoA (sc-418;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-ROCK1 (sc-17794; 1:500; Santa Cruz Biotechnology, Inc.),
anti-LIMK (ab119084; 1:2,000; Abcam, Cambridge, MA, USA), anti-MLC
(ab137063; 1:10,000; Abcam), anti-phosphorylated (p)-MLC (ab2480;
1:5,000; Abcam) and anti-GAPDH (sc-293335; 1:1,000; Santa Cruz
Biotechnology, Inc.). The membranes were then incubated with
horseradish peroxidase-conjugated secondary antibody (cat. no.
A0216; 1:1,000; Beyotime Institute of Biotechnology, Haimen, China)
at room temperature for 1 h. GAPDH was used as the reference
control. The expression of target proteins was detected with
SignalFire™ ECL Reagent (Cell Signaling Technology, Inc., Danvers,
MA, USA) and quantitatively analyzed with ImageJ software (version
1.43; National Institutes of Health, Bethesda, MD, USA).
Immunocytochemical assay
A total of 2×104 cells were incubated
into 90 mm culture dishes. After 5 days, cells on coverslips were
harvested and fixed with PBS containing 4% paraformaldehyde for 15
min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked
with blocking buffer (Abcam) for 30 min at room temperature. The
cells were then incubated at 4°C overnight with the following
primary antibodies: Anti-RhoA (sc-418; 1:500; Santa Cruz
Biotechnology, Inc.), anti-ROCK1 (sc-17794; 1:500; Santa Cruz
Biotechnology, Inc.), anti-LIMK (ab119084; 1:100; Abcam), anti-MLC
(ab137063; 1:500; Abcam), anti-p-MLC (ab2480; 1:500; Abcam) and
anti-GAPDH (sc-293335; 1:500; Santa Cruz Biotechnology, Inc.).
Subsequently, the cells were incubated with streptavidin antibodies
(cat. no. 21851; 1:1,000; Thermo Fisher Scientific, Inc.) at room
temperature for 30 min, followed by staining of the nuclei with
DAPI (Thermo Fisher Scientific, Inc.). Images were acquired using a
Nikon Eclipse E600 (Nikon Corporation, Tokyo, Japan).
MTT assay
Cell proliferation was evaluated using an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Briefly, cells
were seeded at a density of 5×103 cells per well in
96-well plates and incubated with 20 µl MTT solution (5
mg/ml) at 37°C for 4 h. The culture medium was then aspirated via
micropipetting, and 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) was added. The optical density at 570
nm was read on a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Annexin V-allophycocyanin
(APC)/7-aminoactinomycin D (7-AAD) staining assay
Cell apoptosis was assessed using an Annexin
V-APC/7-AAD Apoptosis Detection kit (KeyGen Biotech Co., Ltd.,
Nanjing, China) according to the manufacturer's protocol. Briefly,
following transfection, the cells were collected and resuspended in
500 µl binding buffer, 5 µl Annexin V-APC and 5
µl 7-AAD, and then incubated at room temperature for 15 min
in the dark. Subsequent to staining, the cells were analyzed by
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Dual-luciferase reporter assay
The possible miR-133b binding sites in the RhoA gene
3′-untranslated region (UTR) were predicted by bioinformatics
analysis using the TargetScan version 7.1 online tool (http://www.targetscan.org/vert_71/). To confirm
that RhoA is a direct target of miR-133b, its wild-type 3′-UTR
sequence (3′-UTR-WT) and a mutant 3′-UTR sequence (3′-UTR-MT) were
cloned into a luciferase reporter vector. Subsequently, the cells
were transfected with the luciferase reporter vector and miR-133b
or control mimics using Lipofectamine® 2000 according to
the manufacturer's protocol. Luciferase activity was then measured
using a Dual-Luciferase Reporter Gene Assay kit (Beyotime Institute
of Biotechnology) at 48 h after transfection.
Statistical analysis
The results are presented as the mean ± standard
error of the mean. Statistical analysis was performed using SPSS
software, version 20.0 (IBM Corp., Armonk, NY, USA). Statistical
analyses were performed using analysis of variance, followed by
Dunnett's multiple comparisons test. Statistically significant
differences were defined at a P-value of <0.05.
Results
High glucose attenuates apoptosis in
hRECs
To investigate whether high glucose regulated the
apoptosis of hRECs, the cells were cultured in normal- or
high-glucose medium for 1, 2 and 3 days. An Annexin V-APC/7-AAD
staining assay was then performed to assess cell apoptosis. As
shown in Fig. 1, the apoptotic
rate of hRECs significantly decreased in a time-dependent manner
compared with that in the normal glucose group. These data
indicated that the apoptotic rate of hRECs was significantly
attenuated by the high glucose concentration.
High glucose upregulates RhoA, ROCK1,
LIMK and p-MLC expression levels in hRECs
To investigate whether high glucose stimulates the
RhoA/ROCK1 pathway in hRECs, the cells were cultured in normal- or
high-glucose medium for 1, 2 and 3 days. RT-qPCR, western blotting
and immunocytochemical assays were then performed to measure the
expression levels of RhoA, ROCK1, LIMK, MLC and p-MLC. As shown in
Fig. 2A–D, the results of RT-qPCR
revealed that high glucose markedly upregulated the mRNA expression
levels of RhoA, ROCK1 and LIMK in a time-dependent manner compared
with the normal group. However, there was no significant difference
detected in the expression of MLC mRNA. Furthermore, the expression
of miR-133b was lower in hRECs treated with high glucose (Fig. 2E). As shown in Fig. 3, the results of western blotting
and immunocytochemistry revealed that high glucose significantly
induced the protein expression levels of RhoA, ROCK1, LIMK and
p-MLC in a time-dependent manner compared with the normal group.
However, there was no significant difference in the expression of
the MLC protein. These data indicated that high glucose upregulated
the mRNA and protein levels of RhoA, ROCK1 and LIMK, as well as
p-MLC protein, in hRECs.
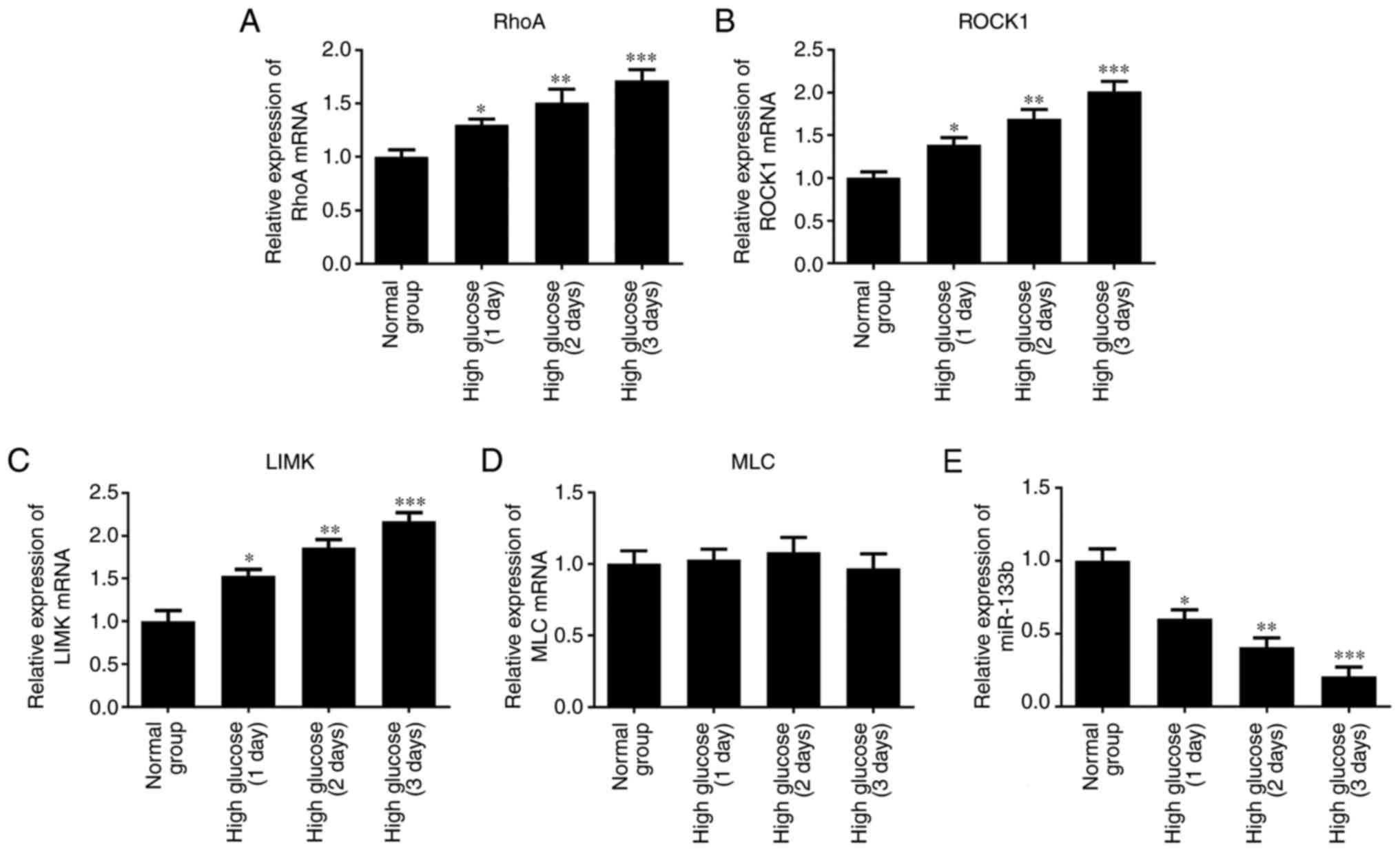 | Figure 2High glucose upregulates RhoA, ROCK1
and LIMK mRNA expression. hRECs were cultured in normal- or
high-glucose medium for 1, 2 and 3 days. The relative expression
levels of RhoA (A), ROCK1 (B), LIMK (C) and MLC (D) mRNA, and the
relative expression of miR-133b (E) were measured by reverse
transcription-quantitative polymerase chain reaction. n=3.
*P<0.05, **P<0.01 and
***P<0.001 vs. normal group. hRECs, human retinal
endothelial cells; miR, microRNA; RhoA, ras homolog family member
A; ROCK1, Rho-associated protein kinase 1; LIMK, LIM domain kinase
1; MLC, myosin light chain. |
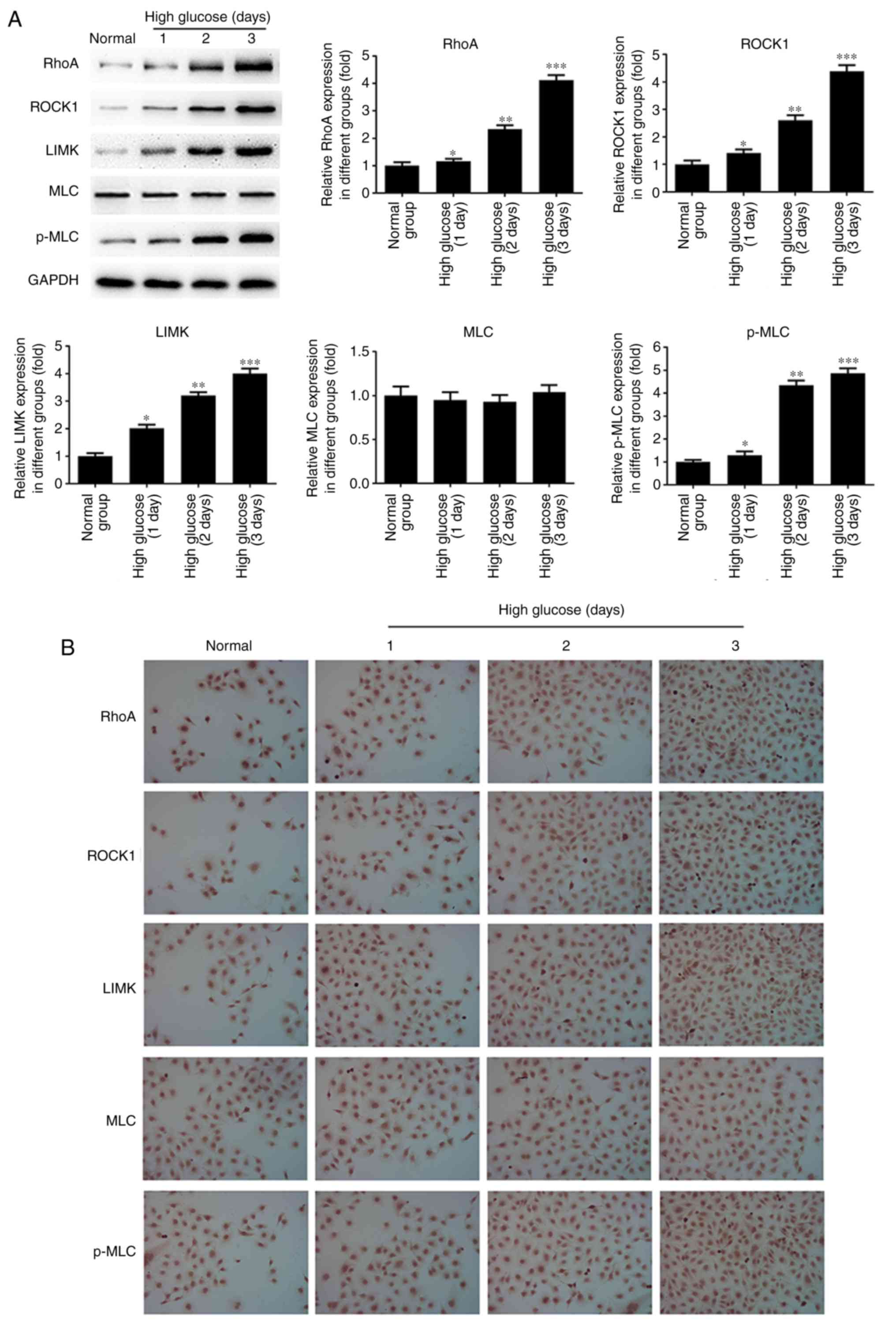 | Figure 3High glucose upregulates RhoA, ROCK1
and LIMK protein expression. hRECs were cultured in normal- or
high-glucose medium for 1, 2 and 3 days. (A) Western blot bands and
quantitative analysis of RhoA, ROCK1, LIMK, MLC and p-MLC protein
expression. (B) Immunocytochemical analysis was performed to assess
the expression of RhoA, ROCK1, LIMK, MLC and p-MLC proteins. n=3.
*P<0.05, **P<0.01 and
***P<0.001 vs. normal group. hRECs, human retinal
endothelial cells; miR, microRNA; RhoA, ras homolog family member
A; ROCK1, Rho-associated protein kinase 1; LIMK, LIM domain kinase
1; MLC, myosin light chain; p-MLC, phosphorylated-MLC. |
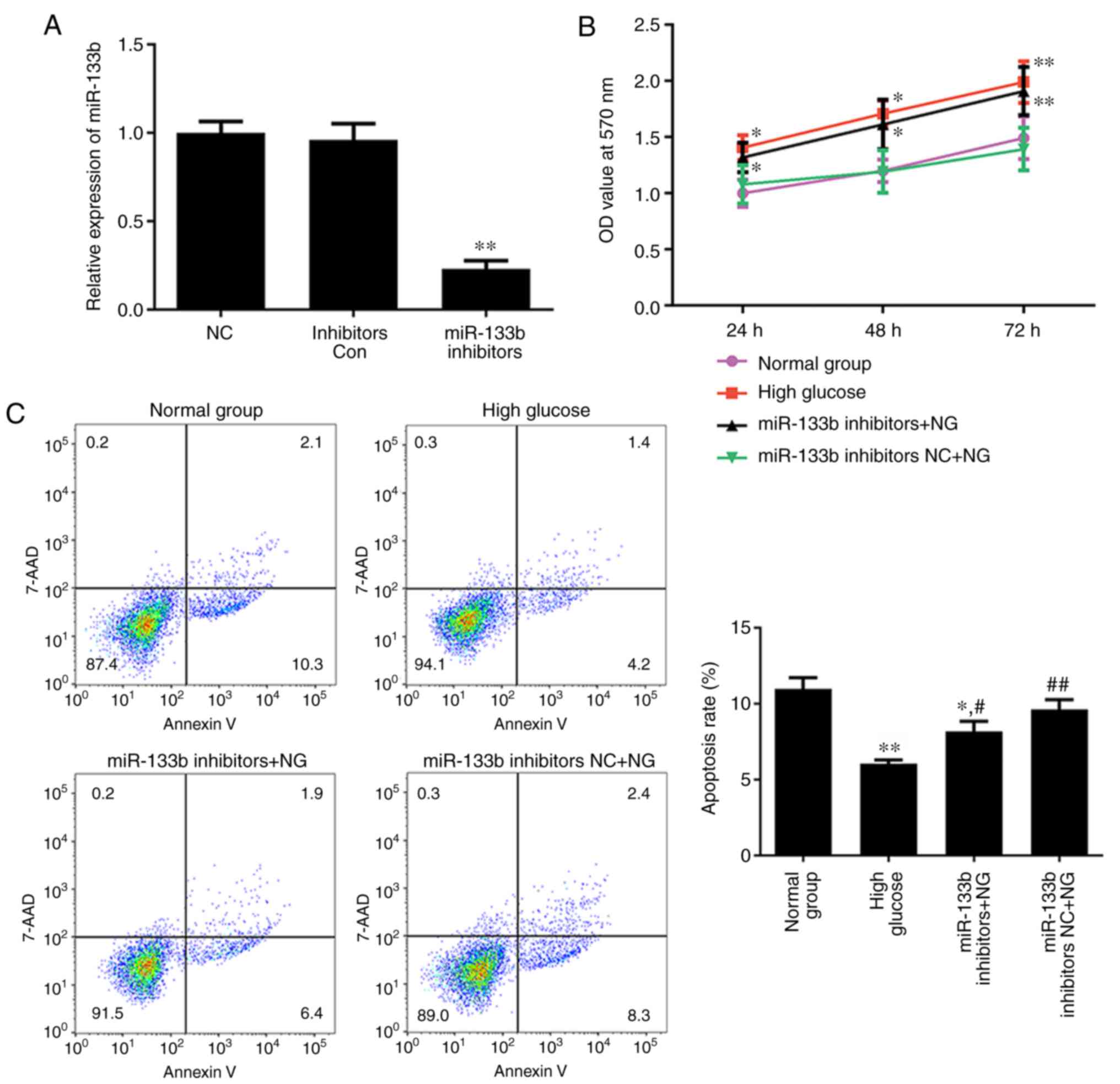
Inhibition of miR-133b promotes
proliferation and represses apoptosis in hRECs
To investigate whether miR-133b is involved in the
proliferation and apoptosis of hRECs, the normal-glucose-treated
cells were transfected with miR-133b inhibitors to reduce miR-133b
expression (Fig. 4A). MTT and
Annexin V-APC/7-AAD staining assays were then performed to assess
cell proliferation and apoptosis, respectively. As shown in
Fig. 4B, high glucose or miR-133b
inhibitors significantly promoted the proliferation of hRECs,
whereas the apoptotic rate of hRECs was significantly decreased in
cells treated with high glucose or transfected with miR-133b
inhibitors as compared with the normal group (Fig. 4C). These data indicated that
miR-133b inhibitors promoted the proliferation and repress the
apoptosis of hRECs.
Inhibition of miR-133b increases RhoA,
ROCK1, LIMK and p-MLC expression levels in hRECs
To determine whether miR-133b inhibitors stimulate
the RhoA/ROCK1 pathway in hRECs, the normal-glucose-treated cells
were transfected with miR-133b inhibitors or inhibitor control.
RT-qPCR, western blotting and immunocytochemistry were then
performed to measure the expression levels of RhoA, ROCK1, LIMK,
MLC and p-MLC. As shown in Fig.
5, the results of RT-qPCR revealed that high glucose or
miR-133b inhibitors significantly increased the expression levels
of RhoA, ROCK1 and LIMK mRNA in hRECs compared with the normal
group. However, there was no significant difference in the
expression of MLC mRNA. As shown in Fig. 6, the results of western blotting
and immunocytochemistry revealed that high glucose or miR-133b
inhibitors markedly induced the expression of RhoA, ROCK1, LIMK and
p-MLC proteins in hRECs compared with the normal group. However,
there was no significant difference in the expression of the MLC
protein. These data indicated that miR-133b expression inhibition
increased the RhoA, ROCK1 and LIMK expression levels at the mRNA
and protein levels, as well as the p-MLC protein level, in
hRECs.
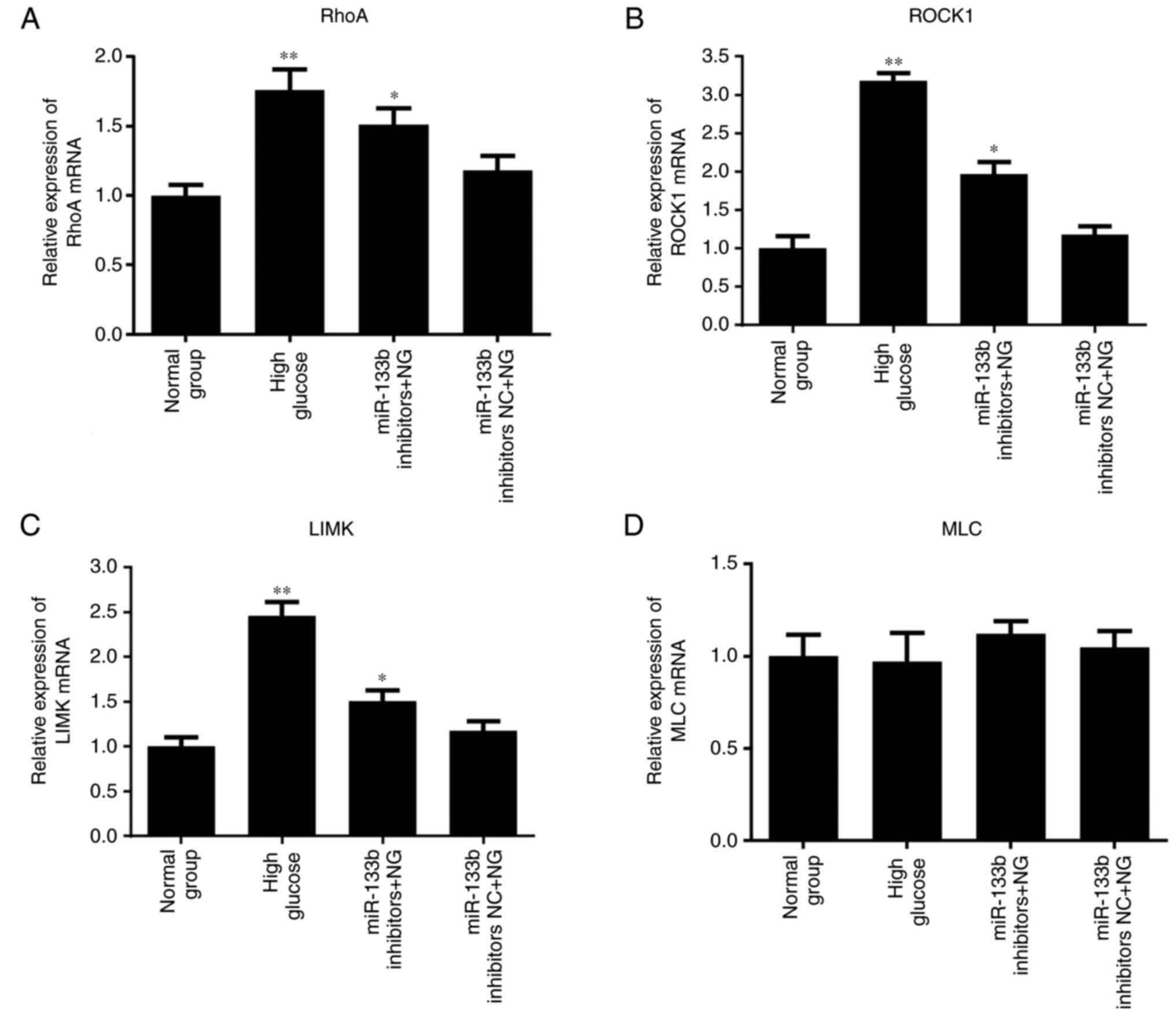 | Figure 5miR-133b inhibitors upregulate RhoA,
ROCK1 and LIMK mRNA expression levels. Relative mRNA expression
levels of RhoA (A), ROCK1 (B), LIMK (C) and MLC (D), measured by
reverse transcription-quantitative polymerase chain reaction. n=3.
*P<0.05 and **P<0.01 vs. normal group;
#P<0.05 and ##P<0.01 vs. high glucose.
hRECs, human retinal endothelial cells; miR, microRNA; RhoA, ras
homolog family member A; ROCK1, Rho-associated protein kinase 1;
LIMK, LIM domain kinase 1; MLC, myosin light chain; NC, negative
control; NG, normal glucose. |
 | Figure 6miR-133b inhibitors upregulate RhoA,
ROCK1 and LIMK protein expression levels. (A) Western blot bands
and quantitative analysis of RhoA, ROCK1, LIMK, MLC and p-MLC
protein levels. (B) Immunocytochemistry was performed to assess the
expression of RhoA, ROCK1, LIMK, MLC and p-MLC proteins. n=3.
*P<0.05 and **P<0.01 vs. normal group;
#P<0.05 and ##P<0.01 vs. high glucose.
hRECs, human retinal endothelial cells; miR, microRNA; RhoA, ras
homolog family member A; ROCK1, Rho-associated protein kinase 1;
LIMK, LIM domain kinase 1; MLC, myosin light chain; p-MLC,
phosphorylated-MLC; NC, negative control; NG, normal glucose. |
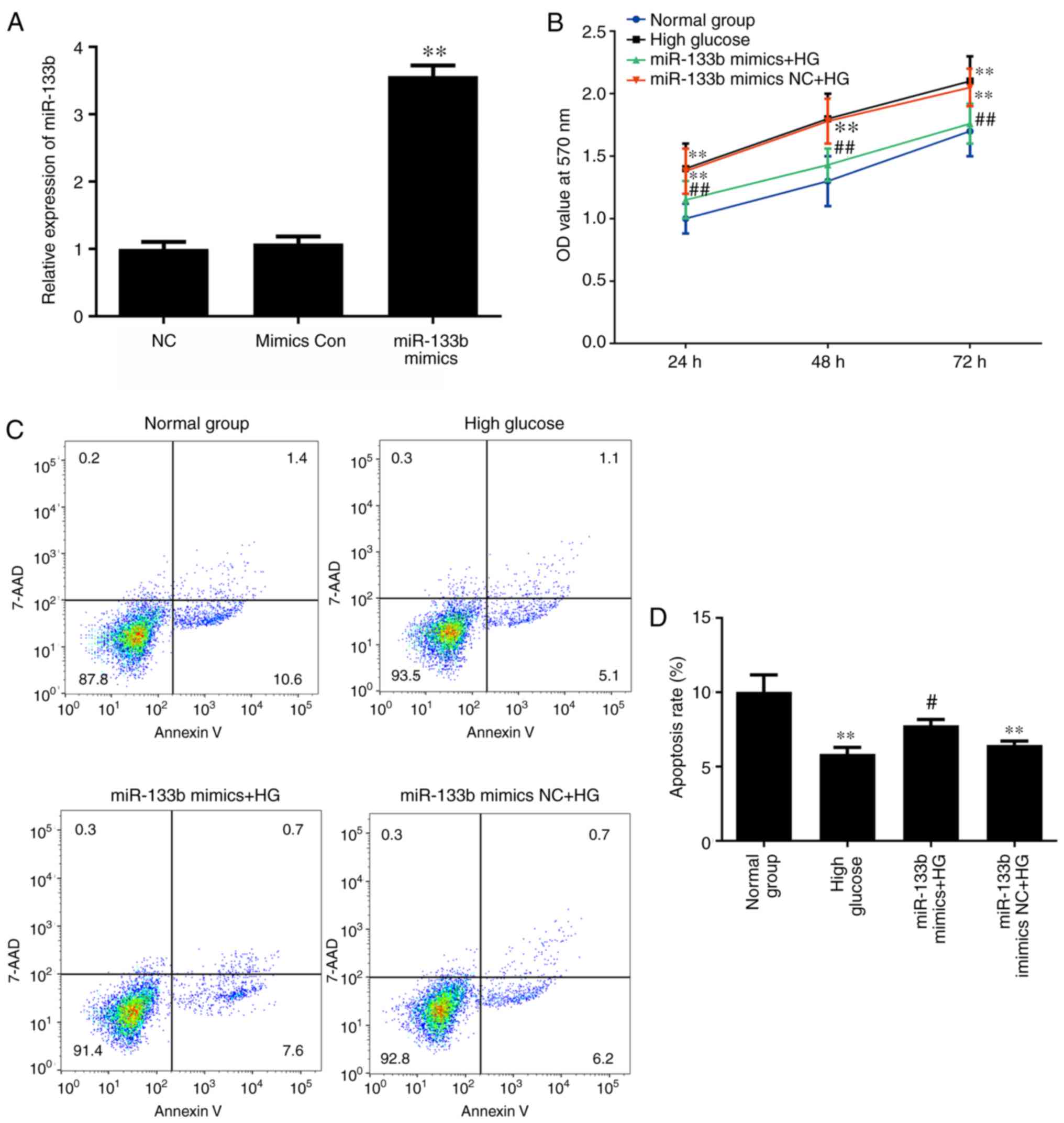
Overexpression of miR-133b inhibits
proliferation and increases apoptosis in high-glucose-induced
hRECs
To determine whether miR-133b overexpression is
involved in the proliferation and apoptosis of high-glucose-induced
hRECs, the cells were transfected with miR-133b mimics to increase
the levels of miR-133b expression (Fig. 7A). MTT and Annexin V-APC/7-AAD
staining assays were then performed to assess the cell
proliferation and apoptosis, respectively. As shown in Fig. 7B, miR-133b mimics significantly
inhibited the proliferation of high-glucose-induced hRECs when
compared with that in cells treated with high glucose alone. In
addition, the apoptotic rate of high-glucose-induced hRECs
significantly increased following transfection compared with the
high-glucose group (Fig. 7C).
These data indicated that miR-133b overexpression inhibited the
proliferation and increased the apoptosis of high-glucose-induced
hRECs.
Overexpression of miR-133b decreases
RhoA, ROCK1, LIMK and p-MLC expression levels in
high-glucose-induced hRECs
To further investigate whether miR-133b mimics
repress the RhoA/ROCK1 pathway in high-glucose-induced hRECs, the
cells were transfected with miR-133b mimics or mimic control.
RT-qPCR, western blotting and immunocytochemistry were then
performed to measure the expression levels of RhoA, ROCK1, LIMK,
MLC and p-MLC. As shown in Fig.
8, the results of RT-qPCR demonstrated that miR-133b mimics
significantly decreased the mRNA expression levels of RhoA, ROCK1
and LIMK in high-glucose-induced hRECs compared with those in the
high-glucose group. However, there was no significant difference in
the expression of MLC mRNA. As shown in Fig. 9, the results of western blotting
and immunocytochemistry revealed that miR-133b mimics markedly
suppressed the protein expression levels of RhoA, ROCK1, LIMK and
p-MLC in high-glucose-induced hRECs compared with those in the
high-glucose group. However, there was no significant difference
observed in the expression of the MLC protein. These data indicated
that miR-133b over-expression decreased the mRNA and protein levels
of RhoA, ROCK1 and LIMK, as well as p-MLC protein expression, in
high-glucose-induced hRECs.
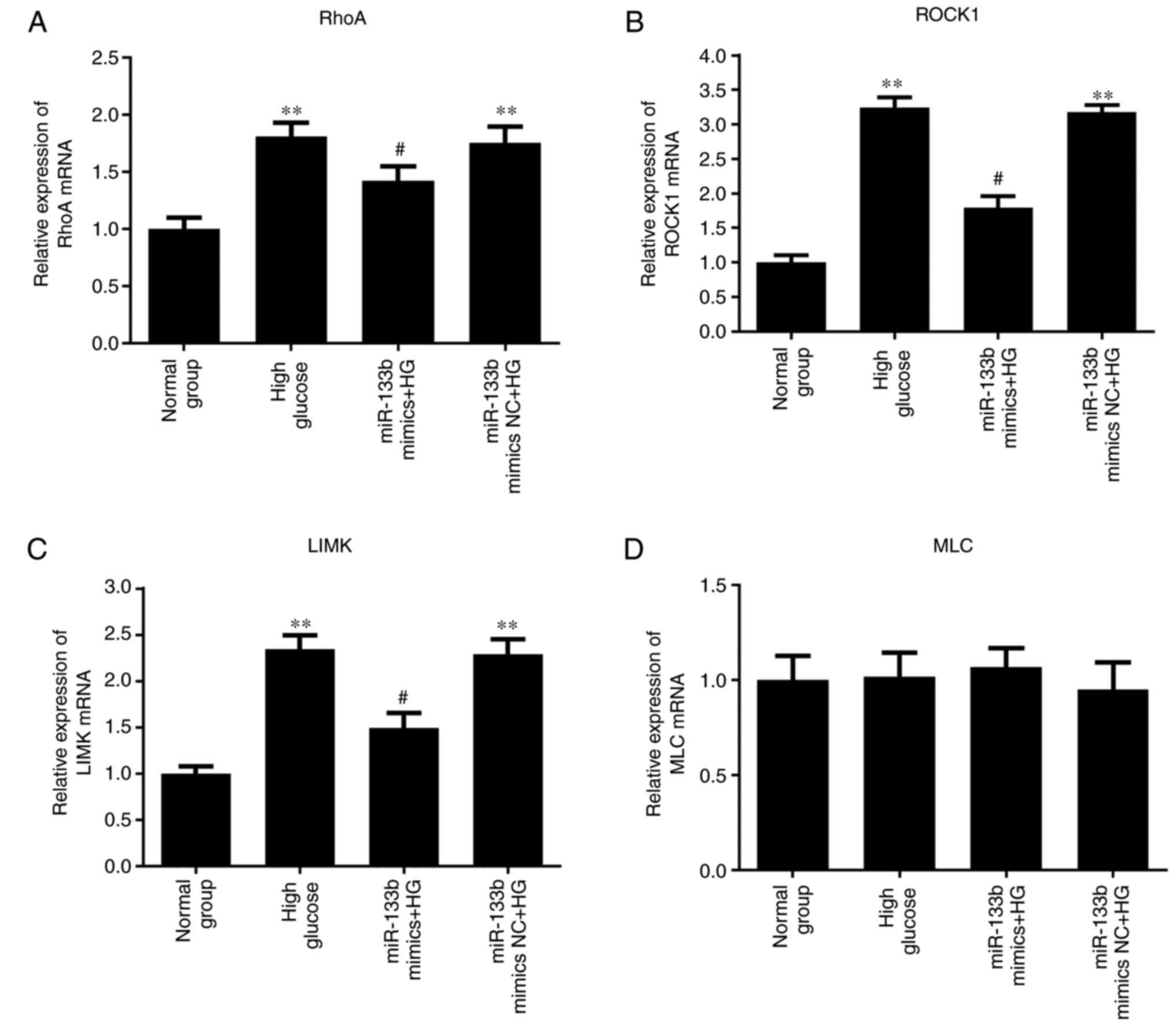 | Figure 8miR-133b overexpression downregulates
RhoA, ROCK1 and LIMK mRNA expression levels. The relative mRNA
expression levels of RhoA (A), ROCK1 (B), LIMK (C) and MLC (D) were
measured by reverse transcription-quantitative polymerase chain
reaction. n=3. *P<0.05 and **P<0.01 vs.
normal group. #P<0.05 and ##P<0.01 vs.
high glucose group. hRECs, human retinal endothelial cells; miR,
microRNA; RhoA, ras homolog family member A; ROCK1, Rho-associated
protein kinase 1; LIMK, LIM domain kinase 1; MLC, myosin light
chain; NC, negative control; HG, high glucose. |
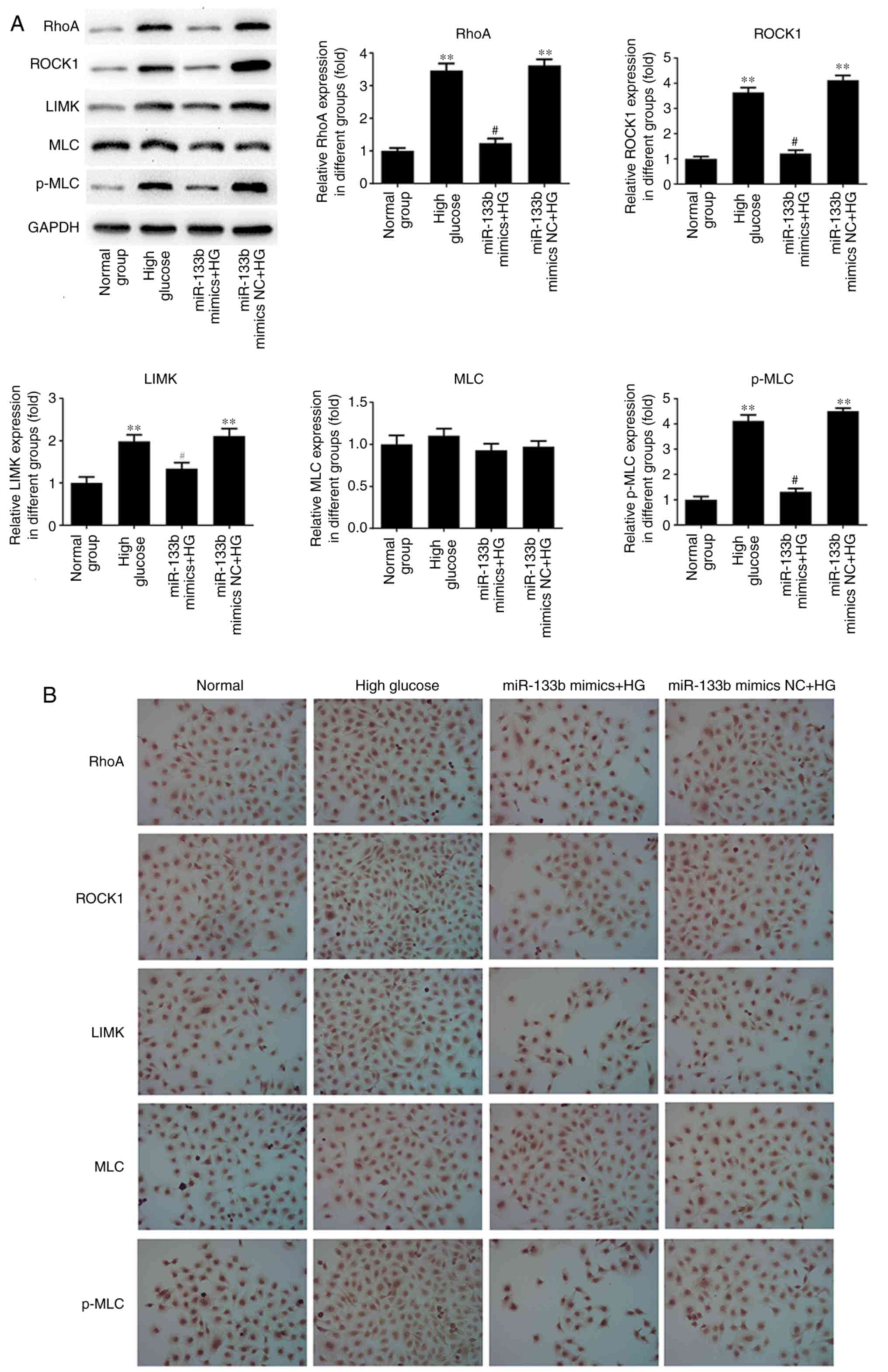 | Figure 9miR-133b overexpression downregulates
RhoA, ROCK1 and LIMK protein expression levels. (A) Western blot
bands and quantitative analysis of RhoA, ROCK1, LIMK, MLC and p-MLC
protein levels. (B) Immunocytochemical analysis was performed to
assess the expression of RhoA, ROCK1, LIMK, MLC and p-MLC proteins.
n=3. *P<0.05 and **P<0.01 vs. normal
group. #P<0.05 and ##P<0.01 vs. high
glucose group. hRECs, human retinal endothelial cells; miR,
microRNA; RhoA, ras homolog family member A; ROCK1, Rho-associated
protein kinase 1; LIMK, LIM domain kinase 1; MLC, myosin light
chain; p-MLC, phosphorylated-MLC; NC, negative control; HG, high
glucose. |
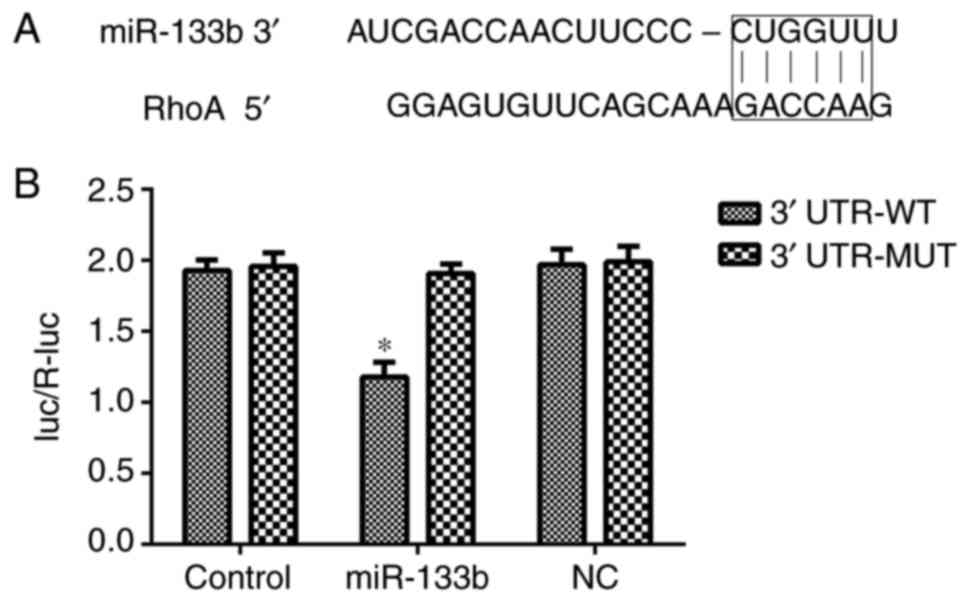
RhoA is a direct target of miR-133b in
hRECs
To elucidate the molecular mechanisms underlying the
effect of miR-133b in hRECs, TargetScan was used to predict the
potential targets of miR-133b. As shown in Fig. 10, the 3′-UTR sequence of RhoA
mRNA was found to match the sequence of miR-133b. To confirm that
RhoA is a direct target of miR-133b, its wild-type 3′-UTR sequence
(3′-UTR-WT) and a mutant 3′-UTR sequence (3′-UTR-MT) were cloned
into a lucif-erase reporter vector. The luciferase activity of the
RhoA 3′-UTR-WT following cell transfection with miR-133b mimic was
significantly inhibited. In addition, miR-133b inhibitors increased
the expression of RhoA in hRECs (Figs. 5 and 6), while miR-133b mimics decreased the
expression of RhoA in high-glucose-induced hRECs (Figs. 8 and 9). These data indicated that RhoA is a
direct target of miR-133b in hRECs.
Discussion
DR is a common microvascular complication of DM. A
series of endocrine and metabolic alterations occur in
hyperglycemia-induced hRECs, causing disorders of organ structure
and function (16,17). In addition, DR is the leading
cause of blindness worldwide. Hyperglycemia, hypertension and
dyslipidemia constitute three major risk factors of DR (18).
miRNAs are involved in a variety of physiological
processes, including developmental timing, cell proliferation,
apoptosis, hematopoiesis and neural patterning (12). miR-133b has been reported to
function as a tumor suppressor in non-small-cell lung cancer
(19), as well as to inhibit cell
proliferation, migration and invasion in gastric cancer (20) and hepatocellular carcinoma
(21). In the present study, the
role of miR-133b in high-glucose-induced hRECs was investigated.
hRECs were cultured in normal- or high-glucose medium for 1, 2 and
3 days, and then an Annexin V-APC/7-AAD staining assay was
performed to assess cell apoptosis. The results revealed that high
glucose significantly attenuated the apop-totic rate of hRECs in a
time-dependent manner. Furthermore, miR-133b inhibitors promoted
proliferation and repressed apoptosis in hRECs, whereas miR-133b
mimics repressed proliferation and promoted apoptosis in
high-glucose-induced hRECs. These data demonstrated that abnormal
proliferation of high-glucose-induced hRECs may be inhibited by
transfection with miR-133b mimic.
RhoA is mainly associated with the regulation of the
cytoskeleton, particularly regarding actin stress fiber formation
and actomyosin contraction (22).
RhoA activates ROCK, which regulates LIMK, which then stimulates
cofilin to effectively reorganize the actin cytoskeleton of cells
(23). It has been demonstrated
that the RhoA/ROCK1/MLC signaling pathway was associated with actin
stress fiber formation in the retinal pigment epithelium (24), and with ethanol-induced apoptosis
by anoikis in astrocytes (25).
The RhoA/ROCK1 signaling pathway has also been found to modulate
microvascular endothelial cell dysfunction (10). In the present study, the results
of RT-qPCR, western blotting and immunocytochemical assay
demonstrated that the mRNA and protein expression levels of RhoA,
ROCK1 and LIMK, as well as the p-MLK protein level, were
significantly increased in a time-dependent manner by high glucose
concentration. These results suggested that RhoA/ROCK1 may be a
novel target for the treatment of DR.
Bioinformatics analysis performed in the present
study also predicted that RhoA was a direct target of miR-133b.
miR-133b has previously been observed to regulate neurite outgrowth
via the ERK1/2 and PI3K/Akt signaling pathways by targeting RhoA
expression (26). In the present
study, miR-133b inhibitors promoted the mRNA and protein expression
levels of RhoA, ROCK1 and LIMK, as well as p-MLK protein, in hRECs.
By contrast, miR-133b mimics repressed the mRNA and protein
expression levels of RhoA, ROCK1 and LIMK, as well as p-MLK protein
expression, in high-glucose-induced hRECs. These results suggested
that miR-133b may be involved in DR via the RhoA/ROCK1 signaling
pathway.
In conclusion, high-glucose treatment in hRECs was
observed to promote the proliferation and inhibit the apop-tosis of
these cells via the RhoA/ROCK signaling pathway. Furthermore,
overexpression of miR-133b was observed to inhibit proliferation
and promote apoptosis in a DR cell model by downregulating the
RhoA/ROCK signaling pathway.
Acknowledgments
Not applicable.
Funding
The present study was supported by the 13th
high-level talents training project of 'Six Talent Peaks' of
Jiangsu Province (grant no. WSN-242), the Science and Technology
Project of Jiangsu Province TCM (grant no. LZ11125), the Health
Science and Technology Project of Wuxi Health Bureau (grant no.
MD201211) and the Wuxi Science and Technology Project (grant no.
CSZ00N1225).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY wrote the manuscript and interpreted the data. YY
and KW analyzed the data and revised the manuscript. QZ and YT
searched the literature and collected the data. JW designed the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boussageon R, Bejan-Angoulvant T,
Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger
S, Wright JM, Gueyffier F and Cornu C: Effect of intensive glucose
lowering treatment on all cause mortality, cardiovascular death,
and micro-vascular events in type 2 diabetes: Meta-analysis of
randomised controlled trials. BMJ. 343:d41692011. View Article : Google Scholar
|
|
2
|
Hartnett ME, Baehr W and Le YZ: Diabetic
retinopathy, an overview. Vision Res. 139:1–6. 2017. View Article : Google Scholar
|
|
3
|
García de la Torre N, Fernández-Durango R,
Gómez R, Fuentes M, Roldán-Pallarés M, Donate J, Barabash A, Alonso
B, Runkle I, Durán A, et al: Expression of angiogenic microRNAs in
endothelial progenitor cells from type 1 diabetic patients with and
without diabetic retinopathy. Invest Ophthalmol Vis Sci.
56:4090–4098. 2015. View Article : Google Scholar
|
|
4
|
Loukovaara S, Gucciardo E, Repo P, Vihinen
H, Lohi J, Jokitalo E, Salven P and Lehti K: Indications of
lymphatic endothelial differentiation and endothelial progenitor
cell activation in the pathology of proliferative diabetic
retinopathy. Acta Ophthalmol. 93:512–523. 2015. View Article : Google Scholar
|
|
5
|
García-Ramírez M, Turch M, Simó-Servat O,
Hernández C and Simó R: Silymarin prevents diabetes-induced
hyperpermeability in human retinal endothelial cells. Endocrinol
Diabetes Nutr. 65:200–205. 2018.In English, Spanish. View Article : Google Scholar
|
|
6
|
Choi SH, Chung M, Park SW, Jeon L, Kim JH
and Yu YS: Relationship between pericytes and endothelial cells in
retinal neovascularization: A histological and immunofluorescent
study of retinal angiogenesis. Korean J Ophthalmol. 32:70–76. 2018.
View Article : Google Scholar
|
|
7
|
Bifulco M: Role of the isoprenoid pathway
in ras transforming activity, cytoskeleton organization, cell
proliferation and apoptosis. Life Sci. 77:1740–1749. 2005.
View Article : Google Scholar
|
|
8
|
Crick DC, Andres DA and Waechter CJ: Novel
salvage pathway utilizing farnesol and geranylgeraniol for protein
isoprenylation. Biochem Biophys Res Commun. 237:483–487. 1997.
View Article : Google Scholar
|
|
9
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
10
|
Lu QY, Chen W, Lu L, Zheng Z and Xu X:
Involvement of RhoA/ROCK1 signaling pathway in
hyperglycemia-induced microvascular endothelial dysfunction in
diabetic retinopathy. Int J Clin Exp Pathol. 7:7268–7277. 2014.
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar
|
|
13
|
McArthur K, Feng B, Wu Y, Chen S and
Chakrabarti S: MicroRNA-200b regulates vascular endothelial growth
factor-mediated alterations in diabetic retinopathy. Diabetes.
60:1314–1323. 2011. View Article : Google Scholar :
|
|
14
|
Feng B, Chen S, George B, Feng Q and
Chakrabarti S: miR133a regulates cardiomyocyte hypertrophy in
diabetes. Diabetes Metab Res Rev. 26:40–49. 2010. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
16
|
Á Castilho, Madsen E, Ambrósio AF, Veruki
ML and Hartveit E: Diabetic hyperglycemia reduces Ca2+
permeability of extrasynaptic AMPA receptors in AII amacrine cells.
J Neurophysiol. 114:1545–1553. 2015. View Article : Google Scholar
|
|
17
|
Baptista FI, ÁF Castilho, Gaspar JM,
Liberal JT, Aveleira CA and Ambrósio AF: Long-term exposure to high
glucose increases the content of several exocytotic proteins and of
vesicular GABA transporter in cultured retinal neural cells.
Neurosci Lett. 602:56–61. 2015. View Article : Google Scholar
|
|
18
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012. View Article : Google Scholar
|
|
19
|
Zhen Y, Liu J, Huang Y, Wang Y, Li W and
Wu J: miR-133b inhibits cell growth, migration, and invasion by
targeting MMP9 in non-small cell lung cancer. Oncol Res.
25:1109–1116. 2017. View Article : Google Scholar
|
|
20
|
Cheng Y, Jia B, Wang Y and Wan S: miR-133b
acts as a tumor suppressor and negatively regulates ATP citrate
lyase via PPARgamma in gastric cancer. Oncol Rep. 38:3220–3226.
2017. View Article : Google Scholar
|
|
21
|
Li H, Xiang Z, Liu Y, Xu B and Tang J:
MicroRNA-133b inhibits proliferation, cellular migration, and
invasion via targeting LASP1 in hepatocarcinoma cells. Oncol Res.
25:1269–1282. 2017. View Article : Google Scholar
|
|
22
|
Zilberman Y, Alieva NO, Miserey-Lenkei S,
Lichtenstein A, Kam Z, Sabanay H and Bershadsky A: Involvement of
the Rho-mDia1 pathway in the regulation of Golgi complex
architecture and dynamics. Mol Biol Cell. 22:2900–2911. 2011.
View Article : Google Scholar :
|
|
23
|
Kiss C, Li J, Szeles A, Gizatullin RZ,
Kashuba VI, Lushnikova T, Protopopov AI, Kelve M, Kiss H,
Kholodnyuk ID, et al: Assignment of the ARHA and GPX1 genes to
human chromosome bands 3p213 by in situ hybridization and with
somatic cell hybrids. Cytogenet Cell Genet. 79:228–230. 1997.
View Article : Google Scholar
|
|
24
|
Ruiz-Loredo AY, López E and López-Colomé
AM: Thrombin promotes actin stress fiber formation in RPE through
Rho/ROCK-mediated MLC phosphorylation. J Cell Physiol. 226:414–423.
2011. View Article : Google Scholar
|
|
25
|
Miñambres R, Guasch RM, Perez-Aragó A and
Guerri C: The RhoA/ROCK-I/MLC pathway is involved in the
ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci.
119:271–282. 2006. View Article : Google Scholar
|
|
26
|
Lu XC, Zheng JY, Tang LJ, Huang BS, Li K,
Tao Y, Yu W, Zhu RL, Li S and Li LX: MiR-133b Promotes neurite
outgrowth by targeting RhoA expression. Cell Physiol Biochem.
35:246–258. 2015. View Article : Google Scholar
|