Introduction
Nasal polyps (NPs) are pink-colored, tear-drop
shaped outgrowths, which form either in the nose or in the
paranasal sinuses. NPs are characterized by tissue remodeling,
consisting of stromal and epithelial cell proliferation,
inflammatory cell infiltration, goblet cell hyperplasia, pseudocyst
formation, focal fibrosis, edema, and basement membrane thickening,
with a high recurrence rate in the nose and paranasal sinuses
(1,2). The majority of individuals with NPs
exhibit morbidities, including nasal congestion, rhinorrhea,
decreased taste, anosmia and headaches, which reduce the
individual’s quality of life (3).
NPs typically embody a chronic infiltration of inflammatory cells.
The recurrence of NPs, following surgical procedure, is common;
therefore, many patients require additional procedures. The
etiology and pathophysiology of NPs formation remain to be fully
elucidated. However, according to reports by other research groups,
a remodeling process is considered to be involved; damage to the
mucosal epithelium is accompanied by extracellular matrix (ECM)
protein accumulation and inflammatory cell infiltration (4).
Several types of cells, including epithelial cells,
T cells, mast cells and fibroblasts, are involved in the
pathogenesis of NPs (5). Among
these, fibroblasts are the major structural components of NP
architecture and are actively involved in NP formation (6). Fibroblasts are found in the stroma
and are the cellular source of ECM components, including collagen
and fibronectin, the excessive deposition of which is
characteristic of the majority of fibrotic responses (7).
Marine algae have been traditionally used in folk
medicine and as ingredients in food in Asian countries. Marine
algae are rich sources of vitamins, minerals, dietary fibers,
proteins, polyunsaturated fatty acids, essential amino acids and
bioactive substances (8), which
have antioxidant, anti-inflammatory, antiviral, anticoagulant,
anticancer, immunomodulatory and antibacterial activities (9,10).
Marine algae also form a potential resource for bioactive secondary
metabolites, which may provide useful leads for the development of
pharmaceuticals (11,12). Phlorotannins (PTNs), a group of
hydrophilic phenolic compounds from marine algae, consist of
polymers of phloroglucinol units with a wide range of molecular
sizes (126–650 kDa) (13). PTNs
have diverse bioactivities, including antioxidant,
anti-inflammatory, antiviral, antitumor, antidiabetic and
anticancer properties (14,15). However, neither the antifibrotic
activity nor the regulatory mechanism of PTNs in nasal
polyp-derived fibroblasts (NPDFs) have been reported
previously.
The present study investigated the effect of PTNs on
TGF-β-induced myofibroblast differentiation and expression of
profibrotic proteins, including collagen type-1 (Col-1) and
fibronectin, in NPDFs, which mimic the conditions of NP formation.
The potential regulatory mechanism of PTNs on the expression of
profibrotic factors was also examined.
Materials and methods
Reagents
The PTNs were provided by Professor W. K. Jung
(Pukyong National University, Busan, Korea). TGF-β was purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). The Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Antibodies against α-smooth
muscle actin (α-SMA; cat. no. ab5694) and Col-1 (cat. no. ab88147)
were purchased from Abcam (Cambridge, MA, USA). Antibodies against
actin (cat. no. 612656) and fibronectin (cat. no. 610077) were
purchased from BD Biosciences (San Jose, CA, USA). Antibodies
against goat anti-mouse IgG-horseradish peroxidase (HRP) conjugate
(cat. no. LF-SA8001) and GAPDH (cat. no. LF-PA0018) were purchased
from Young In Frontier Co., Ltd. (Seoul, Korea). Antibodies against
phosphorylated (p-) small mothers against decapentaplegic (Smad)2
(cat. no. 3101) and p-Smad3 (cat. no. 9520) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Smad2/3-specific small interfering (si)RNAs (cat. no. sc-37238)
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rat tail type-collagen was purchased from BD Biosciences.
NP-derived fibroblast culture
Patients with NPs were recruited and NPDFs were
cultured as in our previous report (16). Individuals were diagnosed with NPs
based on the minimal criteria for chronic rhinosinusitis with NPs.
A total of 15 subjects (male:female, 9:6; median age, 43) with NPs
and 15 subjects with deviated nasal septa were recruited from the
Department of Otorhinolaryngology, Inje University Pusan Paik
Hospital (Pusan, Korea) between July 2017 and September 2017.
Written informed consent was obtained from each patient and the
study was approved by the Ethics Committees of Inje University
Pusan Paik Hospital (Pusan, Korea). A NP was defined as the
presence of endoscopically visible bilateral polyps growing from
the middle nasal meatus into the nasal cavities, and affecting the
ethmoid and maxillary sinuses on computed tomography (CT) of the
paranasal sinus. NPs were obtained from the region of the middle
meatus at the beginning of the surgical procedure. As a control,
nasal mucosal tissue was also obtained from the inferior turbinate
(IT) of patients who underwent a septoturbinoplasty. The subjects
had no history of nasal allergy, asthma, or aspirin sensitivity.
The diagnosis of allergy was based on both a history of allergy and
the results of ImmunoCAP or skin prick tests. No patient had
received steroids (systemic or topical), nonsteroidal
anti-inflammatory drugs, antihistamines, or macrolide antibiotics
during the 4 weeks before the biopsy. NPDFs were isolated from
surgical tissues by enzymatic digestion with collagenase (500 U/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), hyaluronidase (30
U/ml; Sigma-Aldrich; Merck KGaA), and DNase (10 U/ml;
Sigma-Aldrich; Merck KGaA). Cells were cultured in Dulbecco’s
Modified Eagle Medium (DMEM) containing 10% (v/v) heat-inactivated
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 1,000 U/ml penicillin, and 1,000 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
in an atmosphere containing 5% CO2. The purity of the
NPDFs was confirmed by flow cytometry and characteristic
spindle-shaped cell morphology. Experimental cells were used in the
fourth to six cell passages.
Immunohistochemistry
To detect Col-1 and fibronectin,
immunohistochemistry was performed as previously reported (16). Briefly, 5-µm-thick NP
sections were prepared from formalin-fixed paraffin-embedded
tissues. The sections were incubated overnight with Col-1 (1:100)
and fibronectin (1:300) antibodies at 4°C overnight. The slides
were then incubated with anti-mouse IgG-HRP at a 1:2,000 dilution
for 1 h at room temperature in the dark. DAB was used as a
chromogen, and Mayer’s hematoxylin was used for counter-staining.
The expression levels of Col-1 and fibronectin were evaluated under
a digital slide scanner (NanoZoomer 2.0-RS; Hamamatsu, Shizuoka,
Japan).
Cell viability assay
Cellular viability was assessed using the CCK-8
(Dojindo Molecular Technologies, Inc.). In a 96-well microplate,
NPDFs (1×105 cells/well) were treated with PTNs (5, 10
and 30 µM). Following incubation for 24 h at 37°C in an
atmosphere of 5% CO2, the cells were washed twice with
PBS. CCK-8 solution was then added to each well and incubated at
37°C for 1 h, followed by an absorbance analysis at 450 nm using a
microplate reader (SpectraMax M2e; Molecular Devices LLC,
Sunnyvale, CA, USA). All assays were performed in triplicate.
Western blot analysis
The cells were lysed with lysis buffer (Mammalian
Cell-PE LB; G-Biosciences, St. Louis, MO, USA). Protein
concentration was quantified using the Bradford method (Bio-Rad
protein assay dye reagent concentration; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Equal quantities of protein (20 mg) were
separated on 10% SDS-polyacrylamide mini-gels and transferred onto
nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont,
UK). Membranes were blocked in 5% non-fat milk diluted in
Tris-buffered saline/0.1% Tween-20 (TBST) at room temperature for 1
h. Following incubation with the appropriate primary antibody
(a-SMA, Col-1, fibronectin, p-Smad2 and p-Smad3) at a dilution of
1:1,000 overnight at 4°C, the membranes were incubated for 1 h at
room temperature with secondary antibody conjugated to HRP (goat
anti-mouse IgG; 1:1,000). Following three washes with TBST, the
immunoreactive bands were visualized using an ECL detection system
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and band
intensities were evaluated quantitatively using the Multi gauge
version 2.2 software (Fuji Film, Tokyo, Japan).
Silencing of Smad2/3 by synthetic
siRNAs
At 16 h following plating, the cells were
transfected with Smad2/3-siRNAs (40, 80 and 100 nM) using the siRNA
transfection reagent (Santa Cruz Biotechnology, Inc.), in
accordance with the manufacturer’s protocol. Following 6 h of
incubation, an equal volume of fibroblast growth medium 2 (cat. no.
C-23020; PromoCell, Heidelberg, Germany) was added. The cells were
then used for estimating the expression of α-SMA, fibronectin and
Col-1 at 16 h post-transfection. The transfection efficiency was
evaluated by western blot analysis of the protein expression of
Smad2/3.
Rat tail type-1 collagen gel contraction
assay
The rat tail type-1 collagen gel contraction assay
was performed as previously described (16). Briefly, type-1 collagen from the
rat tail was diluted with fibroblast basal medium (CC-3131; Lonza
Group, Ltd., Basel, Switzerland) to a concentration of 1 mg/ml and
mixed with NPDFs to reach a final concentration of 1×105
cells/ml. Following the addition of 1 N NaOH as per the
manufacturer’s protocol, 500 µl of the cell-collagen mixture
was added into each well of a 24-well cell culture plate. The plate
was incubated at 37°C for 30 min. The cells were then incubated in
fibroblast growth medium 2 overnight, and treated with PTNs and
TGF-β1, as indicated. The gel sizes were measured using ImageJ
software (version 1.51j8; National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All statistical analyses were performed with GraphPad
Prism software 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Comparisons between groups were performed by Dunnett’s multiple
range tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of Col-1 and fibronectin in NP
tissues
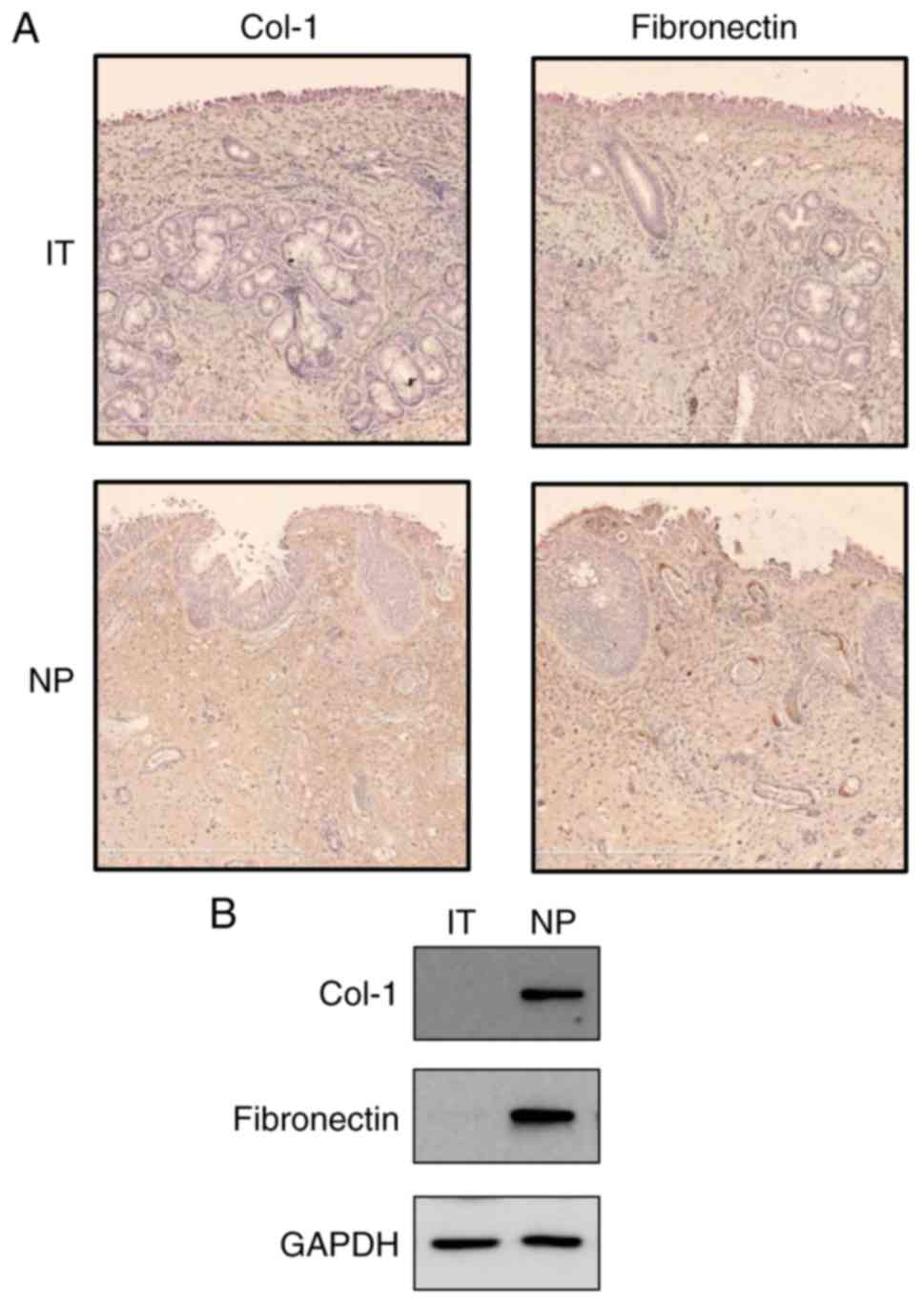
To examine whether Col-1 and fibronectin were
expressed in NP tissues, immunohistochemistry was performed in IT
tissues and NP tissues. In the NP tissues, Col-1 and fibronectin
immunoreactivity was detected in lesions in which overall stroma
was observed (Fig. 1A). In
addition, Col-1 and fibronectin proteins were found to be expressed
in the NP tissue lysates via western blot analysis (Fig. 1B). However, minimal expression
signals of the Col-1 and fibronectin proteins were detected in the
IT tissues.
Effects of PTNs on the viability of
NPDFs
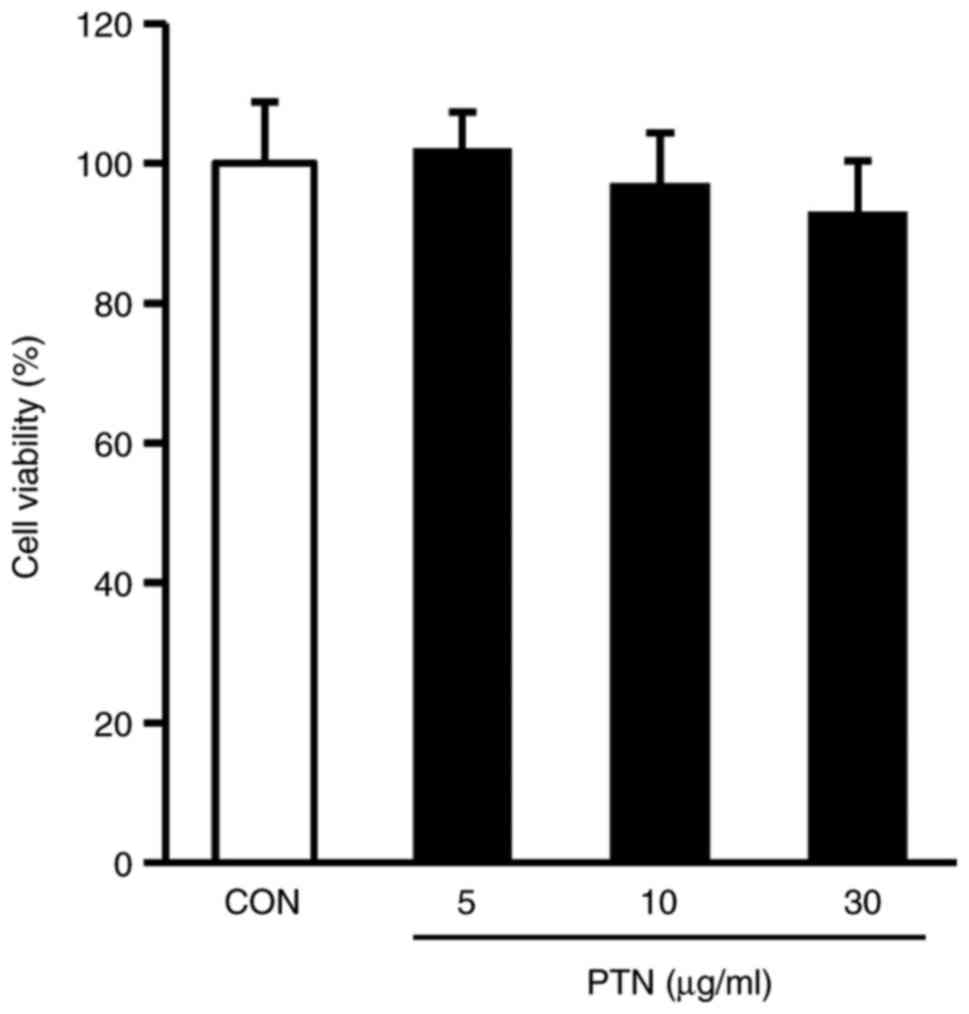
The viability of the NPDFs treated with PTNs was
examined using the CCK-8 assay. There was no cytotoxicity towards
NPDFs at PTN doses up to 30 µg/ml (Fig. 2). On the basis of these results, a
PTN concentration range of 5–30 µg/ml was selected for the
subsequent experiments.
Effect of PTNs on protein expression
levels of α-SMA, Col-1, and fibronectin in TGF-β1-induced
NPDFs
To determine whether PTNs attenuated the
TGF-β1-induced expression of α-SMA, Col-1 and fibronectin in
TGF-β1-stimulated NPDFs, the cells were treated with various
concentrations of PTNs (5–30 µg/ml) for 30 min, prior to
TGF-β1 stimulation for 24 h. It was found that the expression
levels of α-SMA, Col-1 and fibronectin were significantly
attenuated in a PTN concentration-dependent manner (Fig. 3).
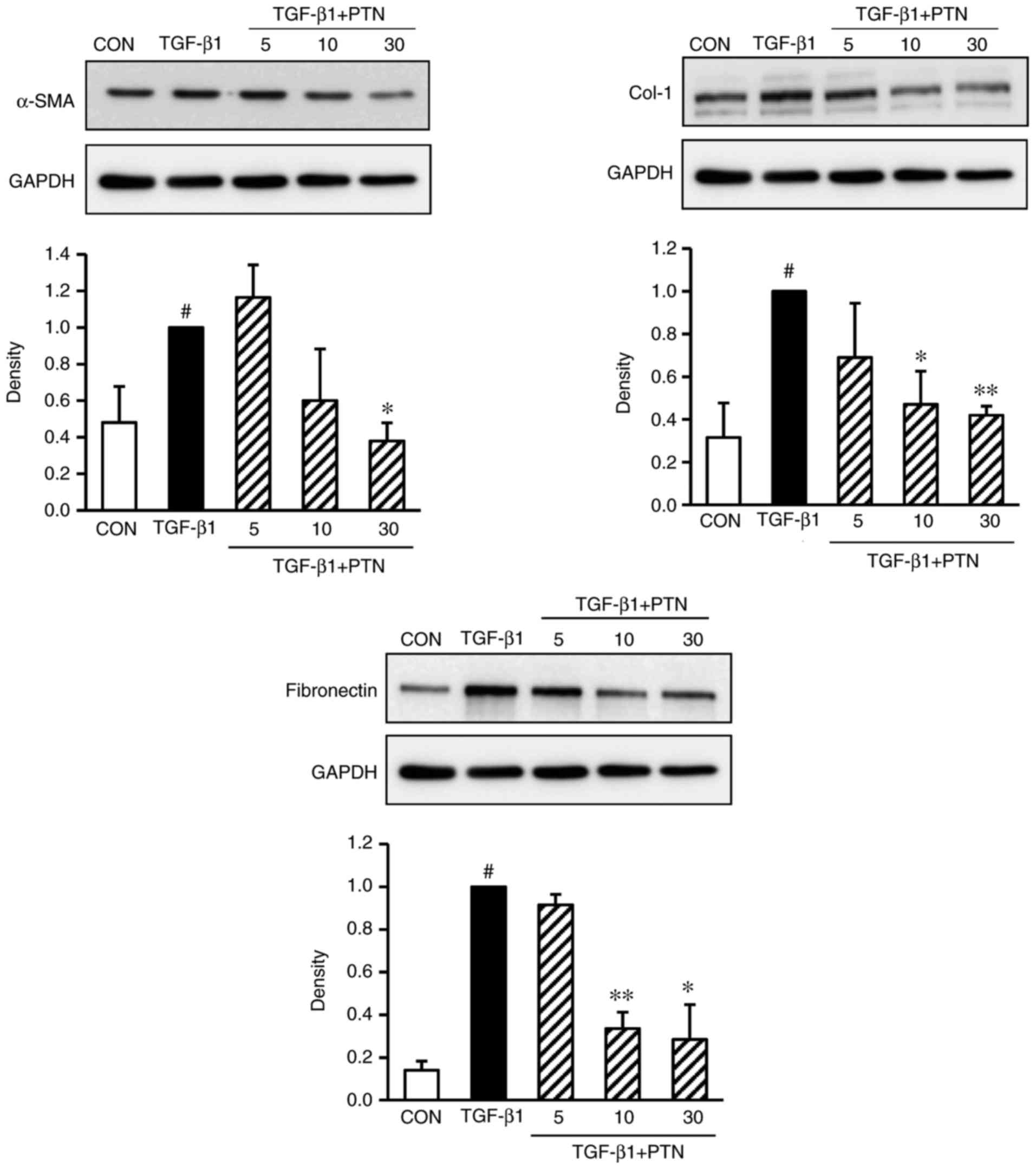 | Figure 3Effect of PTNs on protein expression
levels of α-SMA, Col-1 and fibronectin in TGF-β1-stimulated nasal
polyp-derived fibroblasts. The cells were seeded at
2×105 cells/ml and incubated with various concentrations
(5, 10, and 30 µM) of PTNs for 1 h prior to TGF-β1
stimulation (1 ng/ml). Following stimulation with TGF-β1 for 3 h,
and the protein expression of α-SMA, Col-1 and fibronectin was
determined by western blot analysis. GAPDH was used as an internal
control. Each value indicates the mean ± standard error of the
mean, and is representative of results obtained from three
independent experiments. #P<0.05, vs. CON group (no treatment);
*P<0.05 and **P<0.01 vs. TGF-β1 group.
PTN, phlorotannin; TGF-β1, transforming growth factor-β1; α-SMA,
α-smooth muscle actin; Col-1, collagen type-1; CON, control. |
PTNs inhibits TGF-β1-stimulated Smad2/3
signaling pathways
The phosphorylation of Smad2 and Smad3 in NPDFs was
markedly enhanced by TGF-β1 induction (Fig. 4A). However, when the cells were
pretreated for 30 min with PTNs, particularly at 30 µg/ml,
prior to TGF-β1 stimulation for 24 h, the phosphorylation of Smad2
and Smad3 was significantly reduced. Therefore, the antifibrotic
effects of PTNs may be mediated by the inhibition of TGF-β1-induced
Smad2/3 signaling pathways.
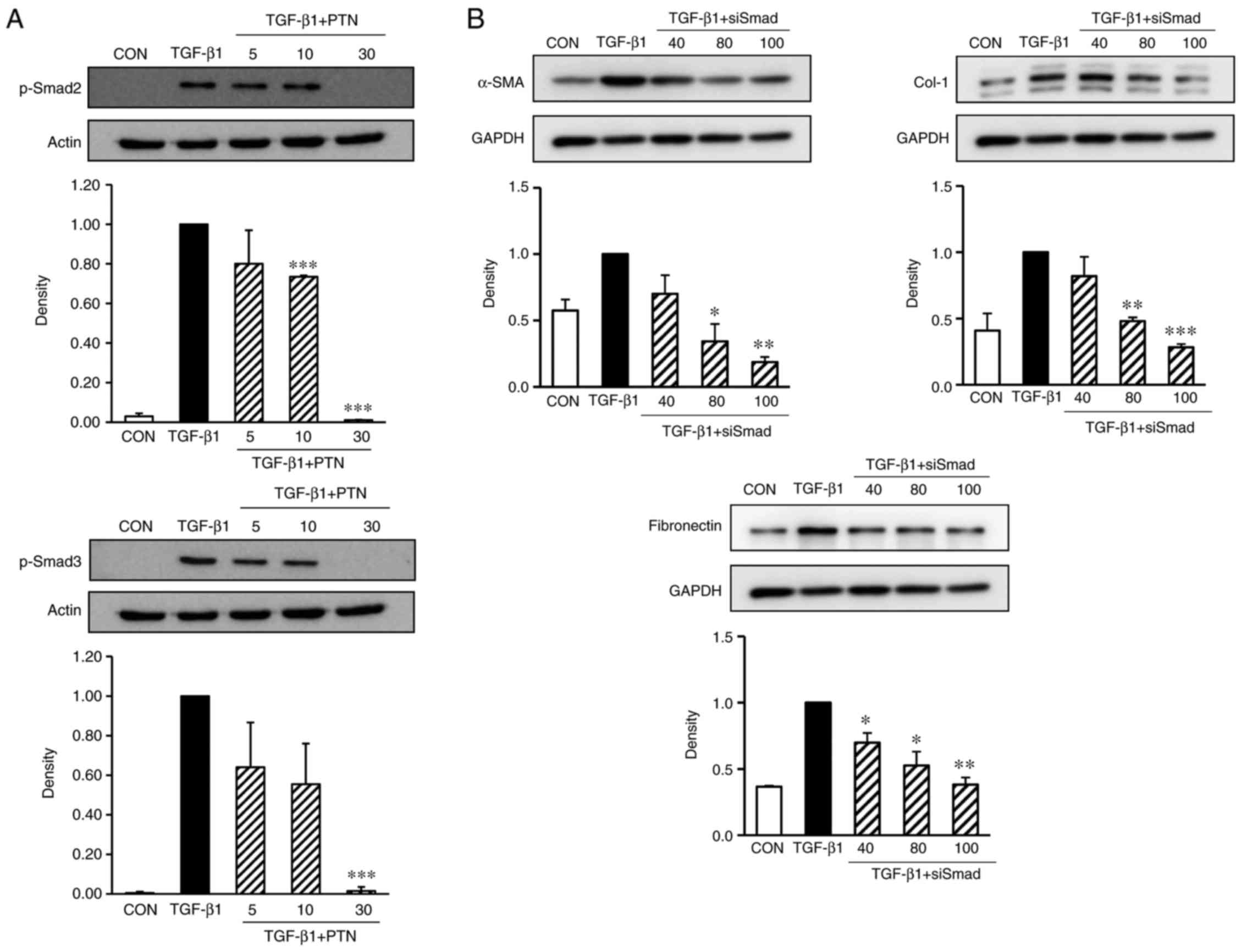 | Figure 4Effect of PTNs on Smad2/3 activation
in TGF-β1-stimulated NPDFs. (A) NPDFs were treated with either the
vehicle or the indicated concentrations of PTNs (5–30 µM)
for 30 min, prior to stimulation with TGF-β1 (1 ng/ml) for 30 min.
Nuclear protein extracts were then prepared and subjected to
western blot analysis with antibodies specific for p-Smad2 and
p-Smad3. The results presented are representative of three
independent experiments. (B) Smad2/3-silencing inhibited the
protein expression of α-SMA, Col-1 and fibronectin in
TGF-β1-stimulated NPDFs. The NPDFs were transfected with the
indicated concentrations of siSmad2/3 (40, 80, and 100 nM) for 24
h, prior to stimulation with TGF-β1 (1 ng/ml) for 24 h. Each bar
represents the mean ± standard error of the mean from three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. TGF-β1
group. NAPDFs, nasal polyp-derived fibroblasts; PTN, phlorotannin;
TGF-β1, transforming growth factor-β1; Smad, small mothers against
decapentaplegic; p-Smad, phosphorylated Smad; α-SMA, α-smooth
muscle actin; Col-1, collagen type-1; siSmad: Smad2/3 small
interfering RNA; CON, control. |
Silencing of Smad2/3 inhibits the
TGF-β1-induced expression of α-SMA, Col-1 and fibronectin in
NPDFs
To confirm whether Smad2/3 are critical to the
TGF-β1-induced expression of α-SMA, Col-1 and fibronectin in
TGF-β1-stimulated NPDFs, siRNAs were used to knock down the Smad2/3
genes in the NPDFs, and the expression levels of α-SMA, Col-1 and
fibronectin were examined. As expected, the siRNA-mediated
silencing of Smad2/3 resulted in significantly reduced the
expression levels of α-SMA, Col-1 and fibronectin (Fig. 4B).
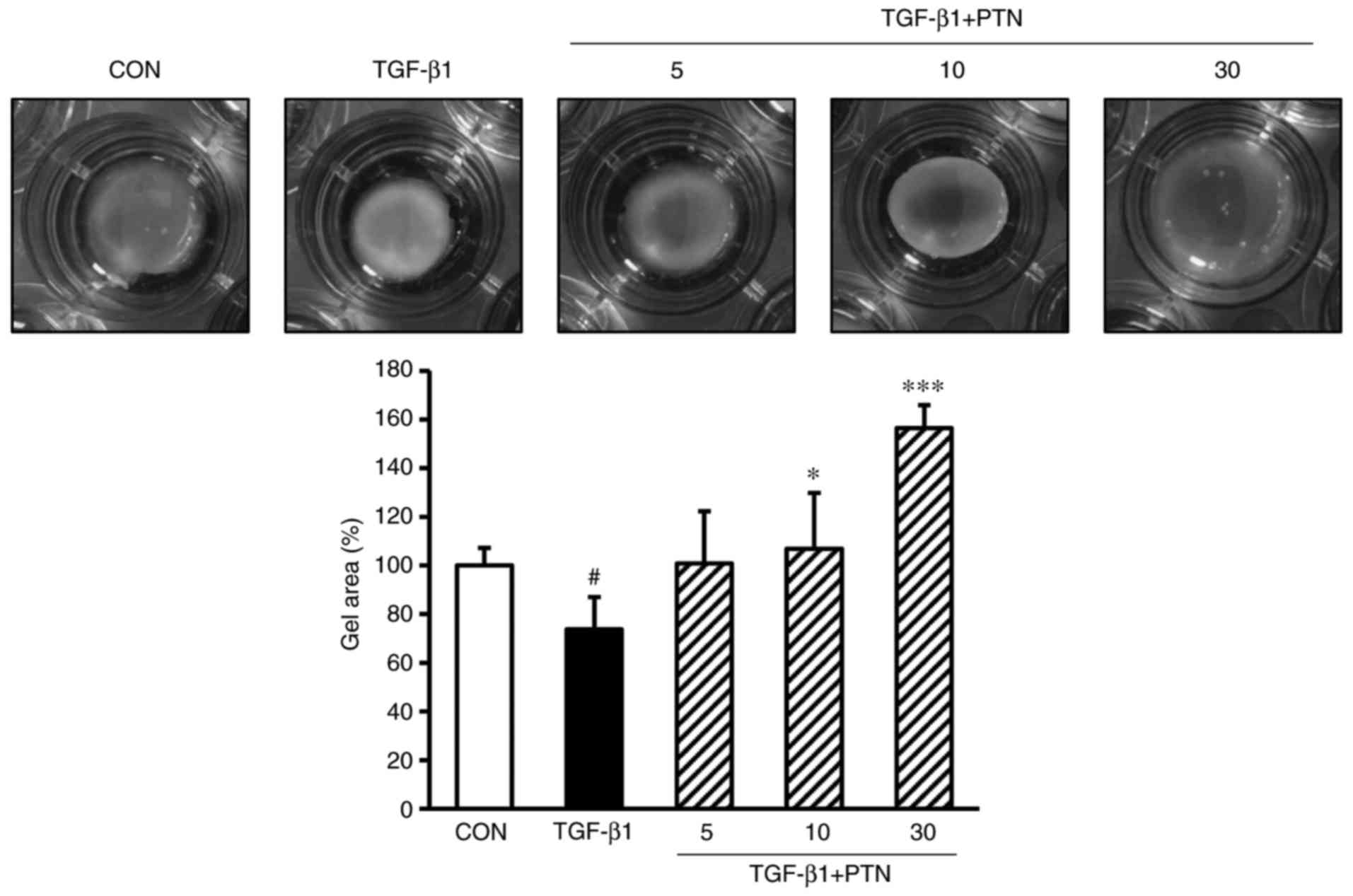
PTNs inhibits TGF-β1-induced fibroblast
contractile activity
The cells were cultured in type-1 collagen gel, as
described above. The cells were then pretreated with PTNs (5, 10 or
30 µg/ml) for 30 min, followed by TGF-β1 (1 ng/ml)
stimulation for 24 h. Stimulation with TGF-β1 resulted in a
decrease in the size of the collagen gel (73.71% vs.
TGF-β1-untreated group; P<0.05), whereas pretreatment with the
PTNs was observed to inhibit this contraction effect at PTN
concentrations of 5, 10 and 30 µg/ml (100.86, 106.90 and
156.46% vs. TGF-β1-untreated group, respectively), as shown in
Fig. 5 (P<0.05 and
P<0.001).
Discussion
The present study investigated the antifibrotic
effect and signaling mechanisms involved in the regulation by PTNs,
which are well known anti-inflammatory agents. Accumulating
evidence suggests that PTNs have a protective effect against
inflammatory diseases (17,18). NPs are associated with chronic
inflammation and are characterized by structural abnormalities,
including stromal fibrosis in the sinus, that cause them grow.
Therefore, inhibition of the inflammatory process and attenuation
of the fibrotic process is considered to be a promising strategy
for the therapy of NPs.
The present study investigated the association
between the morbidity and expression levels of ECM proteins in the
NPs, using immunohistochemical and western blot assays. As shown in
Fig. 1, the expression levels of
Col-1 and fibronectin were higher in the NP tissues and lysates
than those in the IT tissues used as a control. Therefore, the high
expression levels of Col-1 and fibronectin were correlated with the
morbidity of NPs. On the basis of this result, the antifibrogenic
effect and inhibitory signaling mechanism in vitro were
investigated using PTNs in TGF-β1-induced NPDFs.
Although diverse factors have been implicated in the
development and progression of fibrosis, TGF-β, one of the most
potent fibrogenic factors, is considered to be crucial in the
pathogenesis of fibrosis. TGF-β is a secreted homodimeric protein
that regulates multiple biological processes, including cell
proliferation, differentiation, migration, extracellular matrix
production, angiogenesis and apoptosis (19,20). The excessive elevation of TGF-β
correlates with diverse fibrotic disorders, including pulmonary
fibrosis, cardiac fibrosis, cirrhosis, glomerulosclerosis, diabetic
nephropathy, Crohn’s disease, rheumatoid arthritis,
radiation-induced fibrosis and myocarditis in various human organs
(21). Enhanced TGF-β levels have
been observed in NPs, suggesting that TGF-β is also involved in the
pathogenesis of NPs (22,23) It is well known that TGF-β induces
fibroblast activation, proliferation and differentiation.
Fibroblasts are found in the stroma of NPs and are considered to be
important in development of fibrosis. Previous investigations on
the fibroblasts of NPs showed that exposure of TGF-β1 stimulated
myofibroblast differentiation, induced collagen production and
increased α-SMA (24,25). To elucidate the antifibrotic
activity of PTNs, the present study investigated myofibroblast
differentiation and profibrotic protein expression, in addition to
the mechanism underlying the effect of PTNs in TGF-β1-stimulated
NPDFs.
Fibroblasts can be activated by various chemical
signals, which promote their proliferation and differentiation into
myofibroblasts (7).
Myofibroblasts are characterized by their morphology, functional
properties and gene expression. They are the principal effector
cells, which synthesize profibrotic proteins, including α-SMA, and
high quantities of ECM proteins, particularly Col-1 and
fibronectin. Myofibroblasts are important in ECM remodeling in
several pathological conditions of the human airway, including
asthma, chronic rhinosinusitis and NPs (26). In NPs, myofibroblasts are
considered to originate via the differentiation of resident NP
fibroblasts. The expression of α-SMA is the hallmark of
myofibroblast differentiation and is critical for its function.
Fibronectin, a multifunctional glycoprotein involved in tissue
remodeling, is known to be a chemoattractant for fibroblasts and
can be released in increased quantities by the fibroblasts in
response to various cytokines (27). Compared with that in the normal
control IT tissues, Col-1 was found to be increased in all NPs.
Collagen deposition was most abundant in the sub-mucosal connective
tissue and in the basement membrane zone (28). TGF-β1 induces
fibroblast-to-myofibroblast differentiation, and increases the
expression of α-SMA, Col-1 and fibronectin. Therefore, the
approaches to reduce the conversion of fibroblasts to
myofibroblasts and ECM proteins may be beneficial therapeutic
strategies for NPs. In the present study, it was found that the
expression levels of α-SMA, fibronectin and Col-1 were
significantly induced in TGF-β1-stimulated NPDFs. However, the
results showed that PTNs inhibited the expression of α-SMA,
fibronectin and Col-1 in response to TGF-β1 in the absence of
cytotoxic concentrations. These results suggested that PTNs
suppressed TGF-β1-induced myofibroblast differentiation and ECM
protein accumulation in NPDFs.
The present study also investigated the signal
pathways underlying the inhibition of α-SMA and ECM levels by PTN
treatment. TGF-β is recognized by two heterodimeric membrane
receptors, type-1 and type-II TGF-β receptors, which are
transmembrane serine/threonine kinases (29). The Smad-dependent signal
transduction system is necessary for TGF-β signaling, and the
TGF-β/Smad signaling pathway is one of the most common pathways in
fibrosis. When TGF-β1 binds to its receptor, Smad2/3 is
phosphorylated and binds with Smad4, consequently translocating to
the nucleus, where these complexes activate the transcription of
profibrotic genes and induce fibrogenesis (30). In the present study, PTN treatment
was observed to attenuate TGF-β1-induced Smad2 and Smad3
phosphorylation in the nucleus (Fig.
4A). To further confirm the role of Smad2/3 in the inhibitory
effect of PTNs, siRNAs were used to knock down the Smad2/3 genes
prior to TGF-β1 treatment in NPDFs; the levels of α-SMA, Col-1 and
fibronectin were then measured. As expected, the siRNA-mediated
silencing of Smad2/3 resulted in significant inhibition of the
production of TGF-β1-induced α-SMA and ECM proteins (Fig. 4B). These data demonstrated that
PTNs inhibited myofibroblast differentiation and ECM protein
accumulation by inhibiting the phosphorylation of Smad2/3 pathways
in TGF-β1-stimulated NPDFs.
Finally, the present study assessed the effect of
PTN treatment on type-1 collagen gel contraction mediated by
TGF-β1-stimulated NPDFs. Myofibroblasts have increased contractile
activity owing to their elevated expression levels of α-SMA with
increasing mechanical load (31).
Simulation with TGF-β1 resulted in a decrease in the size of the
collagen gel, indicating an increase in contractility, whereas
pretreatment with PTNs was observed to inhibit the collagen gel
contraction (Fig. 5).
Collectively, these results confirmed that PTNs suppressed the
TGF-β1-mediated fibrotic process in vitro.
In conclusion, the results of the present study
demonstrated that PTNs effectively suppressed TGF-β1-augmented
myofibroblast differentiation, ECM protein accumulation, and
collagen gel contraction in vitro, by inhibiting the
phosphorylation of Smad2/3 signaling pathways in NPDFs. These
results suggested that PTNs may be potential therapeutic agents for
treating NP formation. Furthermore, this possibility has important
implications in the development of novel therapeutic approaches for
managing any fibrotic disorder in the future.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Marine
Biodiversity Institute of Korea Research program (grant no.
2018M00700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JP, GC, DL and IC conceived and designed the project
and prepared the manuscript. MY, JL, JY, WP and TK performed the
experiments. SeP and SS performed statistical analysis and data
interpretation. SaP, DL and IC analyzed the data. DL and IC wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Local Ethics Committee
of Busan Paik Hospital, Inje University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cho JS, Kang JH, Shin JM, Park IH and Lee
HM: Inhibitory effect of delphinidin on extracellular matrix
production via the MAPK/NF-κB pathway in nasal polyp-derived
fibroblasts. Allergy Asthma Immunol Res. 7:276–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pawankar R: Nasal polyposis: An update:
Editorial review. Curr Opin Allergy Clin Immunol. 3:1–6. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung JW, Park IH, Cho JS and Lee HM:
Naringenin inhibits extracellular matrix production via
extracellular signal-regulated kinase pathways in nasal
polyp-derived fibroblasts. Phytother Res. 27:463–467. 2013.
View Article : Google Scholar
|
|
4
|
Cho JS, Moon YM, Um JY, Moon JH, Park IH
and Lee HM: Inhibitory effect of ginsenoside Rg1 on extracellular
matrix production via extracellular signal-regulated protein
kinase/activator protein 1 pathway in nasal polyp-derived
fibroblasts. Exp Biol Med (Maywood). 237:663–669. 2012. View Article : Google Scholar
|
|
5
|
Kondo S, Kagami S, Urushihara M, Kitamura
A, Shimizu M, Strutz F, Müller GA and Kuroda Y: Transforming growth
factor-beta1 stimulates collagen matrix remodeling through
increased adhesive and contractive potential by human renal
fibroblasts. Biochim Biophys Acta. 1693:91–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakagawa T, Yamane H, Nakai Y, Shigeta T,
Takashima T and Takeda Z: Comparative assessment of cell
proliferation and accumulation of extracellular matrix in nasal
polyps. Acta Otolaryngol Suppl. 538:205–208. 1998. View Article : Google Scholar
|
|
7
|
Kendall RT and Feghali-Bostwick CA:
Fibroblasts in fibrosis: Novel roles and mediators. Front
Pharmacol. 5:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suleria HA, Osborne S, Masci P and Gobe G:
Marine-based nutraceuticals: An innovative trend in the food and
supplement industries. Mar Drugs. 13:6336–6351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JC, Hou MF, Huang HW, Chang FR, Yeh
CC, Tang JY and Chang HW: Marine algal natural products with
anti-oxidative, anti-inflammatory, and anti-cancer properties.
Cancer Cell Int. 13:552013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wijesinghe WA and Jeon YJ: Exploiting
biological activities of brown seaweed Ecklonia cava for potential
industrial applications: A review. Int J Food Sci Nutr. 63:225–235.
2012. View Article : Google Scholar
|
|
11
|
Sekar D and Kolanjinathan K: Antibacterial
activity of marine macroalgae Padina gymnospora and Turbinaria
conoides collected from Mandapam Coast of Tamilnadu, India. Int J
Adv Res Biol Sci. 2:146–152. 2015.
|
|
12
|
Kolanjinathan K, Ganesh P and Saranraj P:
Pharmacological importance of seaweeds: A Review. World J Fish and
Marine Sci. 6:1–15. 2014.
|
|
13
|
Wijesekara I and Kim SK:
Angiotensin-I-converting enzyme (ACE) inhibitors from marine
resources: Prospects in the pharmaceutical industry. Mar Drugs.
8:1080–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eom SH, Kim YM and Kim SK: Antimicrobial
effect of phlorotannins from marine brown algae. Food Chem Toxicol.
50:3251–3255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vo TS, Ngo DH and Kim SK: Marine algae as
a potential pharmaceutical source for anti-allergic therapeutics.
Process Biochem. 47:386–394. 2012. View Article : Google Scholar
|
|
16
|
Lee DS, Lee CM, Park SK, Yim MJ, Lee JM,
Choi G, Yoo JS, Jung WK, Park S, Seo SK, et al: Anti-inhibitory
potential of an ethanolic extract of Distromium decumbens on
pro-inflammatory cytokine production in Pseudomonas aeruginosa
lipopolysaccharide-stimulated nasal polyp-derived fibroblasts. Int
J Mol Med. 40:1950–1956. 2017.PubMed/NCBI
|
|
17
|
Jung HA, Jin SE, Ahn BR, Lee CM and Choi
JS: Anti-inflammatory activity of edible brown alga Eisenia
bicyclis and its constituents fucosterol and phlorotannins in
LPS-stimulated RAW264.7 macrophages. Food Chem Toxicol. 59:199–206.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang YI, Woo JH, Seo YJ, Lee KT, Lim Y and
Choi JH: Protective effect of brown alga phlorotannins against
hyper-inflammatory responses in lipopolysaccharide-induced sepsis
models. J Agric Food Chem. 64:570–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Gu X and Yi S: The regulatory
effects of transforming growth factor-β on nerve regeneration. Cell
Transplant. 26:381–394. 2017. View Article : Google Scholar :
|
|
21
|
Pohlers D, Brenmoehl J, Löffler I, Müller
CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW and Wolf G:
TGF-beta and fibrosis in different organs - molecular pathway
imprints. Biochim Biophys Acta. 1792:746–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CH, Chai CY, Ho KY, Kuo WR, Tai CF,
Lin CS, Tsai SM, Wu SC and Juan KH: Expression of transforming
growth factor-beta 1 and alpha-smooth muscle actin of myofibroblast
in the pathogenesis of nasal polyps. Kaohsiung J Med Sci.
17:133–138. 2001.PubMed/NCBI
|
|
23
|
Coste A, Lefaucheur JP, Wang QP, Lesprit
E, Poron F, Peynegre R and Escudier E: Expression of the
transforming growth factor beta isoforms in inflammatory cells of
nasal polyps. Arch Otolaryngol Head Neck Surg. 124:1361–1366. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin JM, Park JH, Park IH and Lee HM:
Pirfenidone inhibits transforming growth factor β1-induced
extracellular matrix production in nasal polyp-derived fibroblasts.
Am J Rhinol Allergy. 29:408–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin JM, Park JH, Park IH and Lee HM:
Doxycycline inhibits TGF-β1-induced extracellular matrix production
in nasal polyp-derived fibroblasts. Int Forum Allergy Rhinol.
6:256–263. 2016. View Article : Google Scholar
|
|
26
|
Park SK, Jin YD, Park YK, Yeon SH, Xu J,
Han RN, Rha KS and Kim YM: IL-25-induced activation of nasal
fibroblast and its association with the remodeling of chronic
rhinosinusitis with nasal polyposis. PLoS One. 12:e01818062017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugiura H, Ichikawa T, Liu X, Kobayashi T,
Wang XQ, Kawasaki S, Togo S, Kamio K, Mao L, Ann Y, et al:
N-acetyl-L-cysteine inhibits TGF-β1-induced profibrotic responses
in fibroblasts. Pulm Pharmacol Ther. 22:487–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Molet SM, Hamid QA and Hamilos DL: IL-11
and IL-17 expression in nasal polyps: Relationship to collagen
deposition and suppression by intranasal fluticasone propionate.
Laryngoscope. 113:1803–1812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn JY, Kim MH, Lim MJ, Park S, Lee SL,
Yun YS and Song JY: The inhibitory effect of ginsan on TGF-β
mediated fibrotic process. J Cell Physiol. 226:1241–1247. 2011.
View Article : Google Scholar
|
|
30
|
Zhou L, Dong X, Wang L, Shan L, Li T, Xu
W, Ding Y, Lai M, Lin X, Dai M, et al: Casticin attenuates liver
fibrosis and hepatic stellate cell activation by blocking
TGF-β/Smad signaling pathway. Oncotarget. 8:56267–56280.
2017.PubMed/NCBI
|
|
31
|
Wipff PJ, Rifkin DB, Meister JJ and Hinz
B: Myofibroblast contraction activates latent TGF-beta1 from the
extracellular matrix. J Cell Biol. 179:1311–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|