Introduction
Leukotriene B4 receptor 2 (BLT2) is a G
protein-coupled receptor for pro-inflammatory lipid mediators, such
as leukot-riene B4 (LTB4) and 12(S)-hydroxyeicosatetraenoic acid
[12(S)-HETE] (1). In recent
studies, LTB4 and its receptor, BLT2, have been demonstrated to be
closely associated with tumorigenesis. Upregulated production of
LTB4 and BLT2 has been previously noted in a number of human cancer
cells, including pancreatic, ovarian, bladder, and prostate cancer
cells, as well as lung and breast cancer cells, wherein LTB4 and
BLT2 promote cancer cell proliferation, chemoresistance, invasion
and metastasis (2-7).
Recent studies have demonstrated that following
exposure to lipopolysaccharide (LPS) in vitro and in
vivo, cancer cells become more aggressive as a result of the
stimulation of the toll-like receptor 4 (TLR4)/myeloid
differentiation primary response 88 (MyD88) pathway (8-10).
In particular, LPS-induced TLR4 overexpression in breast cancer
tissues is associated with lymph node metastasis (11,12). A previous study has demonstrated
that LPS upregulates the MyD88/5-lipoxygenase (5-LO)/BLT2 cascade
and that BLT2 depletion attenuates the ability of LPS to induce
invasiveness and interleukin-8 (IL-8) biosynthesis in aggressive
breast cancer cells. In addition, BLT2 inhibition reduces the
incidence of LPS-induced metastasis in an in vivo breast
cancer mouse model (13). On the
basis of this information, pharmaceutical or natural agents that
directly interfere with the production of BLT2, or antagonize its
signaling functions, may be effective in attenuating breast cancer
progression.
Scutellariae baicalensis Georgi is a species
of herbaceous plant in the Lamiaceae family and has several
specialized flavones, such as scutellarin, baicalein, oroxylin A
and wogonin (14). Wogonin
(5,7-dihydroxy-8-methoxyflavone), which can be found in the roots
of S. baicalensis Georgi (Scutellariae radix), is one
of the plant's active components. Wogonin is commonly used as a
traditional medicine in East Asian countries, such as China, Japan
and Korea (15), and many studies
have suggested that wogonin is widely useful due to its antiviral,
neuroprotective, anti-inflammatory and antitumor activities, which
have been demonstrated in a variety of in vitro and in
vivo models, as well as its excellent safety profile (16-19). The potent anticancer effects of
wogonin are mainly attributable to the induction of cell cycle
arrest, apoptosis, and antiangiogenesis activity in various cancer
cell lines (20-22). Although there are previous reports
about the inhibitory effect of wogonin on the proliferation and
invasion of breast cancer cells, the detailed signaling mechanisms
of the anti-invasive effects of wogonin remain unclear (20,23,24). Similarly, scutellarin, a compound
isolated from the aerial part of S. baicalensis Georgi, has
been demonstrated to exert anti-invasive effects on MCF-7 breast
cancer cells (25). Further
studies are necessary to understand the differences in the
anti-invasive signaling mechanisms of scutellarin and wogonin. In
the present study, the aggressive human breast cancer cell line
MDA-MB-231 was used to elucidate the potential mechanism of wogonin
in breast cancer cell invasiveness.
The present results demonstrated that wogonin
suppressed LPS-induced invasiveness in MDA-MB-231 cells, by
down-regulating the BLT2-mediated phosphorylation of extracellular
signal-regulated kinase (ERK) and the subsequent production of
IL-8/matrix metallopeptidase-9 (MMP-9). Additionally, treatment
with wogonin suppressed the synthesis of the BLT2 ligand LTB4, by
inhibiting 5-LO expression. Furthermore, wogonin significantly
reduced LPS-enhanced metastasis in a breast cancer mouse model.
Taken together, the present findings suggest that wogonin acts as a
novel, natural, safe and effective inhibitor of the 5-LO/BLT2
cascade, and thus attenuates invasiveness and metastasis in breast
cancer cells.
Materials and methods
Materials
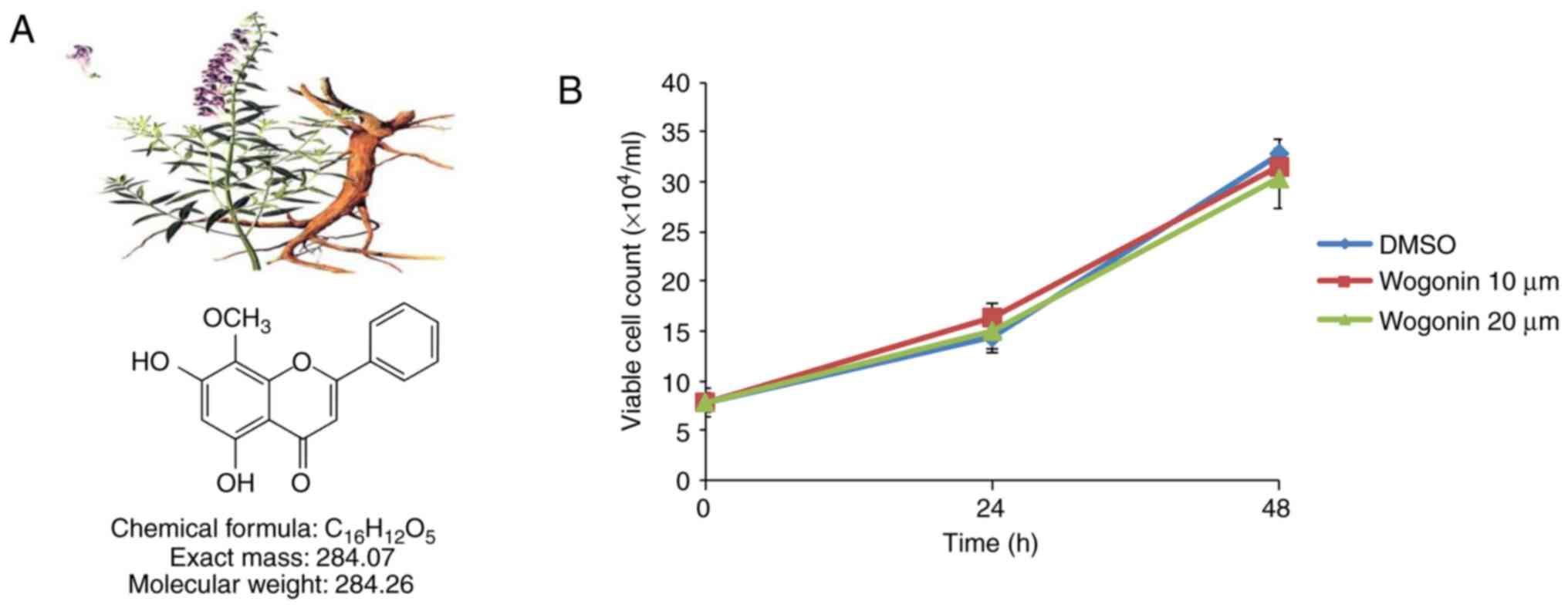
Wogonin, whose molecular formula is
C16H12O5 (Fig. 1A), was isolated from
Scutellariae radix. An air-dried material (60 g) was
extracted with hot methanol. Following concentration, the crude
extract was chromatographed on silica gel to yield a crude
crystalline fraction, which was recrystallized from methanol to
yield 5,7-dihydroxy-8-methoxyflavone (wogonin; 350 mg) (26). Wogonin was kindly provided by Dr
Ahn (Korea Research Institute of Bioscience and Biotechnology,
Daejeon, Korea). Unless otherwise indicated, samples containing
wogonin of ≥98% purity were used in all experiments. Prior to
experiments, wogonin was dissolved in dimethyl sulfoxide (DMSO) and
diluted with medium. The final DMSO concentration did not exceed
0.1% throughout the study. Fetal bovine serum (FBS) and RPMI-1640
were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA), and MK886, U75302 and LY255283 were acquired from Cayman
Chemical Co. (Ann Arbor, MI, USA). LPS (Escherichia coli
serotype O55:B5), bovine serum albumin, and DMSO were acquired from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies to 5-LO
were obtained from BD Transduction Laboratories, Inc. (BD
Biosciences, Franklin Lakes, NJ, USA), and antibodies to
phosphorylated (p-) ERK, ERK, MMP-9 and β-actin were acquired from
Cell Signaling Technology, Inc. (Danvers, MA, USA). All other
chemicals were obtained from standard sources and were of molecular
biological grade or higher.
Cell culture
The human breast cancer cell line MDA-MB-231 was
obtained from the Korean Cell Line Bank (Seoul, Korea). The
MDA-MB-231 cells were maintained in RPMI-1640 containing 10%
heat-inactivated FBS and antibiotic-antimycotic solution (Thermo
Fisher Scientific, Inc.) at 37°C in a humidified 5% CO2
atmosphere.
Cell viability
Cell viability was assessed by cell counting assays.
Briefly, 5×104 cells per well were plated in a 12-well
plate and incubated in complete culture medium prior to drug
exposure. After 24 or 48 h of treatment, aliquots were removed, and
viable cells were counted in triplicate by trypan blue
(Sigma-Aldrich; Merck KGaA) exclusion. Based on the results of the
cytotoxicity assay, a nontoxic concentration of wogonin was
selected to use on the MDA-MB-231 cells in subsequent
experiments.
Semiquantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the cells with
Easy-Blue (Intron, Sungnam, Korea), according to the manufacturer's
instructions. A portion (2 μg) of the RNA was subjected to
reverse transcription with M-MLV reverse transcriptase (Beams Bio,
Gyunggi, Korea), and then, semi-quantitative PCR analysis was
performed with a PCR PreMix kit (Intron, Sungnam, Korea) and
specific forward and reverse primers (Genotech, Daejeon, Korea),
according to the manufacturer's instructions. The sequences of the
primers used for PCR amplification are listed in Table I. The specificity of all primers
was confirmed by sequencing the PCR products. All data were
normalized to corresponding data for GAPDH. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
followed by amplification (number of cycles, temperature and
duration per step for each gene are listed in Table I), and a final extension phase at
72°C for 10 min. The reaction products were separated by
electrophoresis on a 1.5% agarose gel, stained with EtBr, and then
visualized by a ChemiDoc™ XRS+ System (Bio-Rad Laboratories,
Hercules, CA, USA).
 | Table ISequences of primers used in the
present study |
Table I
Sequences of primers used in the
present study
| Author, year | Gene | Primer | Sequence
(5′-3′) |
Denaturation/annealing/extension
Temperature (°C) | Time per step
(sec) | Total cycles | Amplicon size
(bp) | (Refs.) |
|---|
| Park and Kim,
2013 | GAPDH | Forward
Reverse |
CTGCACCACCAACTGCTTAGC
CTTCACCACCTTCTTGATGTC | 95/58/72 | 20/15/15 | 21 | 337 | (13) |
| BLT1 | Forward
Reverse |
TATGTCTGCGGAGTCAGCATGTACGC
CCTGTAGCCGACGCCCTATGT CCG | 95/67/72 | 30/30/30 | 31 | 346 | |
| BLT2 | Forward
Reverse |
AGCCTGGAGACTCTGACCGCTTTCG
GACGTAGCACCGGGTTGACGCTA | | 20/20/15 | 30 | 380 | |
| Kim et al,
2012 | IL-8 | Forward
Reverse |
ATGACTTCCAAGCTGGCCGTGGCT
TCTCAGCCCTCTTCAAAAACTTCTC | 95/61/72 | 20/20/20 | 20 | 292 | (5) |
| Kim et al,
2010 | MMP-9 | Forward
Reverse |
CACTGTCCACCCCTCAGAGC
GCCACTTGTCGGCGATAAGG | 95/62/72 | 20/20/20 | 31 | 273 | (29) |
Western blot analysis
The cells were washed with ice-cold PBS, scraped
into a lysis buffer [20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5%
Nonidet P-40; 5 mM EDTA; 1% Triton X-100; and protease inhibitors
(100 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 2
μg/ml leupeptin, and 2 μg/ml aprotinin)], maintained
at 4°C and then heated at 95°C for 5 min. Protein concentrations
were determined using the Bradford method. A total of 50 μg
protein was loaded into each lane, and then, the proteins were
separated by 8% SDS-PAGE prior to being transferred
electrophoretically to a polyvinyli-dene fluoride membrane at 100 V
for 60 min. The membrane was exposed to TBS containing 0.05%
Tween-20 and 5% dried nonfat milk for 1 h and then incubated with
primary antibodies against 5-LO (cat. no. 610695; 1:1,000; BD
Transduction Laboratories Inc.; BD Biosciences), MMP-9 (cat. no.
CST 3852), p-ERK (cat. no. 9101S), total ERK (cat. no. 9102) and
β-actin (cat. no. 4970S) (1:1,000; all from Cell Signaling
Technology, Inc.). Secondary antibodies were anti-rabbit (cat. no.
7074S; 1:2,000) or anti-mouse antibodies (cat. no. 7076S, 1:2,000)
both from Cell Signaling Technology, Inc., and then, the proteins
were visualized using enhanced chemiluminescence reagent (Amersham;
GE Healthcare, Chicago, IL, USA), according to the manufacturer's
recommendations.
RNA interference (RNAi)
BLT2-specific (5′-CCACGCAGUCAACCUUCUG-3′) (27) and pre-designed control (scrambled)
small interfering (si) RNAs (cat. no. SN-1003) were obtained from
Bioneer (Daejeon, Korea). The siRNAs were introduced into the cells
by transfection in Opti-MEM (cat. no. 31985070; Thermo Fisher
Scientific, Inc.) for the indicated times using Oligofectamine
(cat. no. 12252-011; Thermo Fisher Scientific, Inc.).
Invasion assay
The invasive potential of MDA-MB-231 cells was
assessed using BioCoat Matrigel Invasion Chambers (BD Biosciences),
as described (5,13). Cells (3.5×104) were
harvested with RPMI-1640 supplemented with 0.5% FBS and seeded in
rehydrated Matrigel inserts containing the same media. RPMI-1640
supplemented with 5% FBS, which served as a chemoattractant, was
added to the lower chamber. MDA-MB-231 cells were incubated at 37°C
for approximately 24 h, after which the cells on the upper surface
of each filter were removed, and the remaining cells were fixed in
methanol and stained with hematoxylin-eosin (H&E). The cells in
10 randomly selected high-power (×40) fields were then counted with
a CKX41 microscope (Olympus Corporation, Tokyo, Japan) equipped
with a DP71 digital camera (Olympus Corporation). Each sample was
assayed in triplicate.
IL-8 and LTB4 measurements
Conditioned media were immediately frozen and
lyophilized. ELISA kits for human IL-8 and LTB4 were obtained from
BD Biosciences (cat. no. 550799) and Enzo Life Sciences (cat. no.
ADI-900-068; Farmingdale, NY, USA), respectively. Each assay
procedure was conducted according to the manufacturer's
instructions for each kit. The concentrations of the mediators were
determined at 450 nm for IL-8 and 405 nm for LTB4, using an Epoch
microplate spectrophotometer (BioTek Instruments, Winooski, VT,
USA).
In vivo metastasis assay
This study was approved by the Ethics Committee of
Korea University, and all experimental animals used herein were
handled according to the guidelines approved by the Institutional
Animal Care and Use Committee of Korea University. All animals were
maintained under a 12-h light/dark cycle and housed at a density of
5 mice per static polycarbonate microisolator cage on disposable
bedding. Wire-lid food hoppers within the cages were filled to
capacity with rodent chow, and water was supplied by a bottle. For
the spontaneous metastasis assays, cultured MDA-MB-231 cells were
treated with LY255283 (10 μM), wogonin (20 μM) or
DMSO, prior to being treated with LPS (1 μg/ml) for 24 h, as
described previously (13). Then,
six-week-old female nude (BALB/C) mice (Daehan Biolink, Chungbuk,
Korea) were injected unilaterally in the fourth right mammary fat
pad with cultured MDA-MB-231 (3.5×106) cells in 100
μl of PBS; the cells were injected subcutaneously at the
base of the nipple. Wogonin (20 mg/kg), LY255283 (2.5 mg/kg) or
DMSO was injected intraperitoneally three times at 5-day intervals
beginning immediately following cell implantation. The animals were
sacrificed 14 weeks post-cell implantation, and the number of
metastatic nodules on the surface of the small bowel was
determined.
Statistical analysis
The data are representative of three independent
experiments, and the results are presented as the mean ± standard
deviation. Comparisons between groups were performed with one-way
analysis of variance, followed by Tukey's post-hoc test. SPSS
software (IBM SPSS Statistics for Windows, version 21.0; IBM Corp.,
Armonk, NY, USA) was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Wogonin inhibits the invasiveness of
MDA-MB-231 breast cancer cells by downregulating IL-8 and MMP-9
expression
Before studying the anti-invasive activity of
wogonin, the effect of wogonin on cell viability was assessed.
MDA-MB-231 cells were treated with wogonin at the indicated
concentrations (10 or 20 μM). The viability of MDA-MB-231
cells was not significantly affected in 24 or 48 h of treatment
with wogonin at concentrations up to 20 μM (Fig. 1B), suggesting that wogonin
exhibits no cytotoxicity at these doses in MDA-MB-231 cells. To
determine whether wogonin has any effect on the LPS-enhanced
invasive potential of MDA-MB-231 cells, Matrigel-coated Transwell
chambers were used. Similar to previous studies (13), MDA-MB-231 cell invasiveness was
increased in the LPS-treated group compared with the control group
(Fig. 2A). However, wogonin
significantly suppressed LPS-induced invasiveness in a
dose-dependent manner. Specifically, treatment with wogonin at 10
and 20 μM inhibited cell invasion by ~98% compared with the
control (Fig. 2A). Previous
studies have demonstrated that IL-8 and MMP-9 expression is
required for breast cancer cell invasiveness and metastasis
(5,13,28). To understand the signaling
mechanism by which wogonin suppressed the LPS-induced invasiveness
of the MDA-MB-231 cells, the mRNA expression levels of IL-8 and
MMP-9 were examined by RT-PCR (Fig.
2B). IL-8 and MMP-9 expression was indeed markedly increased by
LPS treatment, and 20 μM wogonin clearly suppressed
LPS-induced IL-8 and MMP-9 overexpression, in a dose-dependent
manner (Fig. 2B). This effect was
also confirmed at the protein level, by ELISA for IL-8 (Fig. 2C) and by western blotting for
MMP-9 (Fig. 2D). Taken together,
these results suggest that wogonin attenuated cell invasiveness by
downregulating the expression of IL-8 and MMP-9 in LPS-stimulated
MDA-MB-231 cells.
Wogonin inhibits BLT2 upregulation in
LPS-stimulated MDA-MB-231 cells
Next, the results demonstrated that LPS stimulation
resulted in upregulated BLT2 mRNA levels in MDA-MB-231 breast
cancer cells (Fig. 3A),
consistent with previous reports (13). Semiquantitative RT-PCR revealed
that LPS markedly increased BLT2 mRNA levels in a time-dependent
manner, but had little impact on BLT1 expression (Fig. 3A). Thus, the hypothesis that
wogonin may suppress BLT2 expression was examined. Wogonin clearly
suppressed LPS-induced BLT2 mRNA expression in a dose-dependent
manner (Fig. 3B), but did not
have an effect on BLT1 expression (Fig. 3C). Previous reports suggested that
the BLT2 cascade contributes to the production of IL-8 and MMP-9
(3,13,29). Furthermore, BLT2 depletion using
siRNA has been reported to attenuate the LPS-induced invasive
activity of MDA-MB-231 cells (13). In agreement with these results,
BLT2 depletion using siRNA, or the selective BLT2 antagonist
LY255283, clearly attenuated LPS-induced IL-8 and MMP-9 mRNA and
protein expression in MDA-MB-231 cells (Fig. 3D–F); however, the BLT1 antagonist
U75302 did not exert such effects (data not shown). These results
suggest that BLT2 may be the effector for the wogonin anti-invasion
activity.
 | Figure 3Wogonin inhibits BLT2 upregulation in
LPS-stimulated MDA-MB-231 cells. (A) Cells were treated with LPS (1
μg/ml) for the indicated times (0, 1, 3, 6, 12 and 24 h),
and then, the cell lysates were assayed for BLT1 and BLT2 mRNA
expression by semiquantitative RT-PCR. (B) Cells were incubated
with wogonin (10 or 20 μM) or LY255283 (10 μM) for 1
h, and then, they were stimulated with LPS for 24 h. The cells were
subsequently assayed for BLT2 and (C) BLT1 mRNA expression by
semiquantitative RT-PCR. (D) Cells were transfected with BLT2
(siBLT2) or control (siCont) siRNA for 24 h and then treated with
LPS for 24 h. IL-8, MMP-9 and BLT2 mRNA expression was subsequently
assayed by semiquantitative RT-PCR. (E) Cells were incubated with
LY255283 (10 μM) for 1 h and then stimulated with LPS for 24
h. The cell lysates were assayed by semiquantitative RT-PCR, or (F)
by western blot analysis. BLT, leukotriene B4 receptor; LPS,
lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain
reaction; si, small interfering; IL-8, interleukin-8; MMP-9, matrix
metallopeptidase-9. |
Wogonin suppresses the synthesis of the
BLT2 ligand in LPS-stimulated MDA-MB-231 cells
Recent studies have suggested that 5-LO expression
was increased in response to LPS stimulation in MDA-MB-231 cells
(13,30). Thus, whether wogonin affects the
expression of 5-LO and its metabolite, LTB4, was examined in
LPS-treated MDA-MB-231 cells. 5-LO expression was markedly
inhibited by wogonin treatment in LPS-stimulated MDA-MB-231 cells
(Fig. 4A). In addition, wogonin
significantly inhibited LPS-induced production of the 5-LO
metabolite LTB4 (Fig. 4B). Under
the same experimental conditions, LPS-induced IL-8 and MMP-9
expression was suppressed by pretreatment with the 5-LO-activating
protein (FLAP) inhibitor, MK886 (Fig.
4C–E). Taken together, these results suggest that wogonin
inhibits 5-LO and thus attenuates the production of its metabolite,
LTB4, and the subsequent synthesis of IL-8 and MMP-9.
 | Figure 4Wogonin suppresses the synthesis of
the BLT2 ligand in LPS-stimulated MDA-MB-231 cells. (A) Cells were
incubated with wogonin (10 or 20 μM) for 1 h and then
stimulated with LPS (1 μg/ml). After 24 h, the cell lysates
were subjected to western blot analysis with antibodies to 5-LO and
β-actin (loading control). (B) LTB4 levels in the culture
supernatants were measured by ELISA. (C) Cells were treated with
MK886 (2.5 μM) for 1 h and then stimulated with LPS for 24
h, after which IL-8 and MMP-9 mRNA expression was assayed by
semiquantitative RT-PCR. (D) IL-8 levels in the culture
supernatants were analyzed by ELISA. (E) Total cell lysates were
analyzed by western blotting to determine the protein expression of
MMP-9 and β-actin (loading control). Quantitative data are
presented as the mean ± standard deviation of three independent
experiments. ***P<0.005, with comparisons indicated
by lines. BLT, leukotriene B4 receptor; LPS, lipopolysaccharide;
5-LO, 5-lipoxygenase; LTB4, leukotriene B4; IL-8, interleukin-8;
MMP-9, matrix metallopeptidase-9; RT-PCR, reverse
transcription-polymerase chain reaction. |
Wogonin attenuates ERK phosphorylation
and thus inhibits IL-8/MMP-9 production in LPS-stimulated
MDA-MB-231 cells
Previous reports have demonstrated that ERK lies
downstream of BLT2 and upstream of MMP-9 in MDA-MB-231 cells
(24,30). Exposure to LPS increased ERK
phosphorylation in a time-dependent manner (Fig. 5A). By contrast, LPS-induced ERK
phosphorylation was markedly inhibited by wogonin in a
dose-dependent manner (Fig. 5B).
Treatment with PD98059, an ERK inhibitor, suppressed LPS-stimulated
IL-8 and MMP-9 expression at both the transcript (Fig. 5C) and protein levels (Fig. 5D and E) and inhibited
LPS-stimulated invasiveness (Fig.
5F). These data suggest that wogonin inhibits IL-8/MMP-9
production in LPS-stimulated MDA-MB-231 cells, most likely through
ERK activation.
 | Figure 5Wogonin attenuates ERK
phosphorylation in LPS-stimulated MDA-MB-231 cells. (A) Cells were
treated with LPS (1 μg/ml) for the indicated times (0, 1, 3,
6, 12 and 24 h), and then p-ERK and ERK protein levels were
measured by western blotting. (B) Cells were incubated with wogonin
(10 or 20 μM), LY255283 (10 μM), PD98059 (20
μM) or DMSO for 1 h and then stimulated with LPS for 24 h.
The cell lysates were then subjected to western blot analysis for
ERK activation. (C) Cells were incubated with PD98059 (20
μM) for 1 h, and then, they were incubated with or without
LPS (1 μg/ml) for 1 h. The cells were assayed for IL-8 and
MMP-9 mRNA expression by semiquantitative RT-PCR. (D) The levels of
secreted IL-8 in the cell supernatants were measured by ELISA. (E)
The cell lysates were analyzed by western blotting to determine
MMP-9 expression. β-actin was used as a loading control. (F) Cells
were incubated with PD98059 (20 μM) or DMSO and then treated
with or without LPS (1 μg/ml), prior to Transwell invasion
assays. Representative fields of invading cells stained with
H&E and quantification are shown. Quantitative data are
presented as the mean ± standard deviation of three independent
experiments. ***P<0.005, with comparisons indicated
by lines. ERK, extracellular signal-regulated kinase; LPS,
lipopolysaccharide; p-, phosphorylated; IL-8, interleukin-8; MMP-9,
matrix metallopeptidase-9; RT-PCR, reverse transcription-polymerase
chain reaction. |
Wogonin significantly reduces LPS-induced
metastasis in an orthotopic breast cancer model
Previously, BLT2 inhibition has been demonstrated to
significantly reduce LPS-induced metastasis in an orthotopic breast
cancer model (13). Thus, the
effect of wogonin on breast cancer metastasis induced by LPS
exposure was investigated in vivo. To study the effect of
wogonin on the LPS-driven metastasis of MDA-MB-231 cells in
vivo, the mouse orthotopic injection tumor metastasis model was
used. MDA-MB-231 cells were pretreated with wogonin (20 μM)
or DMSO, and then, they were treated with LPS (1 μg/ml) for
24 h prior to being implanted into the mammary fat pads of mice. At
14 weeks post-implantation, metastatic nodules were counted on the
small bowel of the mice. The number of nodules in the small bowel
was significantly reduced by wogonin, compared with the mice
treated with LPS alone (Fig. 6A and
B). Taken together, these results indicate that wogonin exerted
an inhibitory effect on LPS-induced metastasis in vivo.
Discussion
The present study demonstrated that wogonin
suppressed the LPS-enhanced invasiveness and metastasis of the
breast cancer cell line MDA-MB-231 in vitro and in
vivo. Additionally, the results demonstrated that wogonin
inhibited the 5-LO/BLT2/ERK/IL-8/MMP-9 cascade. These findings
suggest that this cascade may be the target through which wogonin
exerts its anticancer effects in breast cancer.
Breast cancer progression involves many steps,
including tumor growth, cancer cell invasion, and cancer cell
dissemination throughout the body (31). Cancer invasion has a central role
in metastasis and is the main cause of death in millions of breast
cancer patients (32). In
particular, MDA-MB-231 cells are highly aggressive and invasive,
and the options for the treatment of tumors comprising of such
invasive cells are limited. Thus, identifying and controlling the
molecular mechanisms that regulate the tumor cell invasion process
is key for the development of therapeutic interventions to prevent
tumor metastasis and reduce mortality in breast cancer
patients.
Wogonin has been demonstrated to inhibit the
development of malignancies and has attracted increasing attention
as a promising anticancer compound. Wogonin has been previously
reported to effectively inhibit the mobility and invasiveness of
various solid tumors, including breast cancer (24), osteo-sarcoma (33), hepatoma and melanoma (34,35). Although the anti-invasive and
antimetastatic effects of wogonin have been proven, the molecular
mechanisms of its effects are not fully understood. In addition,
its main target metabolite in cancer, especially breast cancer,
remains unclear. Previous reports have suggested that wogonin
inhibits the effects of eicosanoids generated by phospholipase A2
activation and lipoxygenase-induced fatty acid oxidation. Wogonin
significantly inhibits the release of histamine and LTB4 in rat
peritoneal exudate cells (36).
Additionally, in rat macrophages, wogonin attenuates the
biosynthesis of LTB4 and 5-hydroxyeicosatetraenoic acid (5-HETE)
(37). However, there are no
reports on the inhibitory effects of wogonin on leukotrienes
produced by lipoxygenases, such as 5-LO, in cancer cells. Thus, the
present study is the first to demonstrate that wogonin targets the
5-LO/BLT2 signaling pathway in cancer cells and thus inhibits
invasiveness. Similar to our observation, 12-LO inhibitor baicalein
has been reported to inhibit the invasion of MDA-MB-231 cells
through down-regulating various signaling pathways, including MAPK,
Wnt/β-catenin or BLT2 (13,38,39). However, under our experimental
conditions, wogonin was demonstrated to significantly inhibit the
expression levels of 5-LO, but not 12-LO (data not shown).
Therefore, it can be speculated that 5-LO, not 12-LO, may be an
important target for the action of wogonin.
5-LO controls another key pathway in arachidonic
acid metabolism; the major products of the pathway include LTB4 and
the cysteinyl leukotrienes, which are potent pro-inflammatory lipid
mediators involved in chronic inflammatory diseases and cancer
(40). 5-LO is expressed in many
cancer cells and participates in angiogenesis, invasiveness, and
cellular proliferation (41). The
present results demonstrated that 5-LO and, thus, the downstream
BLT2/ERK/IL-8/MMP-9 cascade are potential targets of wogonin. In
clinical studies, IL-8 and MMP-9 are overexpressed in breast tumor
tissues compared with normal tissues, and the expression of these
factors is correlated with high invasion potential (42,43). Consistent with these findings, we
previously reported that the expression of IL-8 promoted MDA-MB-231
cell invasiveness and metastasis through the activation of the BLT2
signaling pathway (13). Previous
reports have also revealed that wogonin suppresses IL-8 and MMP-9
expression in tumor cells. For example, wogonin attenuated IL-8
expression in LPS-induced colorectal adenocarcinoma cells (44) and inhibited breast cancer cell
invasion and metastasis in vitro, by suppressing
phorbol-12-myristate-13-acetate (PMA)-induced MMP-9 expression
(24).
The current results demonstrated that ERK lies
downstream of the BLT2 cascade in MDA-MB-231 cells (30). ERK activation is critical for IL-8
and MMP-9 expression and the promotion of the degradation of the
basement membrane and the infiltration of surrounding tissues for
the facilitation of breast tumor metastasis (45-47). In the present study, it was
demonstrated that wogonin significantly attenuated the activation
of ERK in LPS-stimulated MDA-MB-231 cells, which indicated that the
antimetastatic effect of wogonin in breast cancer may be dependent
on BLT2 expression. Additionally, wogonin remarkably suppressed
LPS-enhanced metastasis to the small bowel, suggesting that wogonin
inhibited BLT2-induced breast cancer metastasis. These findings
provide a preliminary basis for the development of wogonin-based
therapeutic herbal medicines for the treatment of metastatic breast
cancer.
In summary, the present study demonstrated that
wogonin suppressed the ability of LPS to stimulate the invasiveness
and metastasis of MDA-MB-231 cells. Furthermore, the results
revealed that the molecular mechanism responsible for the effects
of wogonin may be associated with the 5-LO/BLT2/ERK/IL-8/MMP-9
cascade (Fig. 7). Thus, the
5-LO-/BLT2 axis is a novel pathway through which wogonin may exert
its anti-invasive actions in LPS-stimulated MDA-MB-231 cells.
Acknowledgments
Not applicable.
Funding
This work was supported by the Bio & Medical
Technology Development Program (grant no. 2017M3A9D8063317), and a
Mid-Career Researcher Program (grant no. 2017R1A2B4002203),
provided by the National Research Foundation funded by the Ministry
of Science, Information and Communication Technologies and Future
Planning, Republic of Korea. This work was also supported by Basic
Science Research (grant no. 2015R1D1A1A01057757) through an NRF
funded by the Ministry of Education, Republic of Korea and the BK21
Plus Program (School of Life Sciences and Biotechnology, Korea
University).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
JHG designed the study, performed the experiments,
analyzed data, and was a major contributor in writing the
manuscript. JDW contributed to the interpretation of the results.
JIP conducted the animal study. KSA provided the wogonin and
critically revised the manuscript for intellectually important
content. JHK provided critical feedback and contributed to the
design of the present study, supervised the study and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Ethics Committee of Korea University, and performed according
to the guidelines approved by the Institutional Animal Care and Use
Committee of Korea University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Funk CD: Prostaglandins and leukotrienes:
Advances in eico-sanoid biology. Science. 294:1871–1875. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennig R, Osman T, Esposito I, Giese N,
Rao SM, Ding XZ, Tong WG, Buchler MW, Yokomizo T, Friess H and
Adrian TE: BLT2 is expressed in PanINs, IPMNs, pancreatic cancer
and stimulates tumour cell proliferation. Br J Cancer.
99:1064–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim EY, Seo JM, Kim C, Lee JE, Lee KM and
Kim JH: BLT2 promotes the invasion and metastasis of aggressive
bladder cancer cells through a reactive oxygen species-linked
pathway. Free Radic Biol Med. 49:1072–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park J, Park SY and Kim JH: Leukotriene B4
receptor 2 contributes to chemoresistance of SK-OV-3 ovarian cancer
cells through activation of signal transducer and activator of
transcription-3-linked cascade. Biochim Biophys Acta. 1863:236–243.
2016. View Article : Google Scholar
|
|
5
|
Kim H, Choi JA, Park GS and Kim JH: BLT2
up-regulates interleukin-8 production and promotes the invasiveness
of breast cancer cells. PLoS One. 7:e491862012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seo JM, Park S and Kim JH: Leukotriene B4
receptor 2 promotes invasiveness and metastasis of ovarian cancer
cells through signal transducer and activator of transcription 3
(STAT3)-dependent up-regulation of matrix metalloproteinase-2. J
Biol Chem. 287:13840–13849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JW and Kim JH: Activation of the
leukotriene B4 receptor 2-reactive oxygen species (BLT2-ROS)
cascade following detachment confers anoikis resistance in prostate
cancer cells. J Biol Chem. 288:30054–30063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harmey JH, Bucana CD, Lu W, Byrne AM,
McDonnell S, Lynch C, Bouchier-Hayes D and Dong Z:
Lipopolysaccharide-induced metastatic growth is associated with
increased angiogenesis, vascular permeability and tumor cell
invasion. Int J Cancer. 101:415–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szajnik M, Szczepanski MJ, Czystowska M,
Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M and
Whiteside TL: TLR4 signaling induced by lipopolysaccharide or
paclitaxel regulates tumor survival and chemoresistance in ovarian
cancer. Oncogene. 28:4353–4363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Zhao F, Zhang H, Zhu Y, Wu K and
Tan G: Significance of TLR4/MyD88 expression in breast cancer. Int
J Clin Exp Pathol. 8:7034–7039. 2015.PubMed/NCBI
|
|
11
|
Yang H, Wang B, Wang T, Xu L, He C, Wen H,
Yan J, Su H and Zhu X: Toll-like receptor 4 prompts human breast
cancer cells invasiveness via lipopolysaccharide stimulation and is
over-expressed in patients with lymph node metastasis. PLoS One.
9:e1099802014. View Article : Google Scholar
|
|
12
|
Ahmed A, Wang JH and Redmond HP: Silencing
of TLR4 increases tumor progression and lung metastasis in a murine
model of breast cancer. Ann Surg Oncol. 20(Suppl 3): S389–S396.
2013. View Article : Google Scholar
|
|
13
|
Park GS and Kim JH: Myeloid
differentiation primary response gene 88-leukotriene B4 receptor 2
cascade mediates lipopoly-saccharide-potentiated invasiveness of
breast cancer cells. Oncotarget. 6:5749–5759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HB and Chen F: Isolation and
purification of baicalein, wogonin and oroxylin A from the
medicinal plant Scutellaria baicalensis by high-speed
counter-current chromatography. J Chromatogr A. 1074:107–110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brekhman II, Grinevitch MA and Kyu KB:
Oriental medicine: A computerized study of complex recipes and
their components: Herbs most frequently used in traditional
Japanese and Korean medicine. Am J Chin Med. 9:134–143. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Q, Zhao L, You Q, Yang Y, Gu H, Song
G, Lu N and Xin J: Anti-hepatitis B virus activity of wogonin in
vitro and in vivo. Antiviral Res. 74:16–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan NM, Haseeb A, Ansari MY, Devarapalli
P, Haynie S and Haqqi TM: Wogonin, a plant derived small molecule,
exerts potent anti-inflammatory and chondroprotective effects
through the activation of ROS/ERK/Nrf2 signaling pathways in human
Osteoarthritis chondrocytes. Free Radic Biol Med. 106:288–301.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Yang B, Hu Y, Lu L, Lu X, Wang J, Xu
F, Yu S, Huang J and Liang X: Wogonin prevents
TLR4-NF-kappaB-medicated neuro-inflammation and improves retinal
ganglion cells survival in retina after optic nerve crush.
Oncotarget. 7:72503–72517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huynh DL, Sharma N, Kumar Singh A, Singh
Sodhi S, Zhang JJ, Mongre RK, Ghosh M, Kim N, Ho Park Y and Kee
Jeong D: Anti-tumor activity of wogonin, an extract from
Scutellaria baicalensis, through regulating different signaling
pathways. Chin J Nat Med. 15:15–40. 2017.PubMed/NCBI
|
|
20
|
Huang KF, Zhang GD, Huang YQ and Diao Y:
Wogonin induces apoptosis and down-regulates survivin in human
breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int
Immunopharmacol. 12:334–341. 2012. View Article : Google Scholar
|
|
21
|
Lin CM, Chen YH, Ong JR, Ma HP, Shyu KG
and Bai KJ: Functional role of wogonin in anti-angiogenesis. Am J
Chin Med. 40:415–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang H, Hui H, Wang Q, Li H, Zhao K, Zhou
Y, Zhu Y, Wang X, You Q, Guo Q and Lu N: Wogonin induces cell cycle
arrest and erythroid differentiation in imatinib-resistant K562
cells and primary CML cells. Oncotarget. 5:8188–8201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Xie KP, Shang F, Huo HN, Wang LM and
Xie MJ: Wogonin inhibits IGF-1-stimulated cell growth and estrogen
receptor alpha expression in breast adenocarcinoma cell and
angiogenesis of chick chorioallantoic membrane. Sheng Li Xue Bao.
64:207–212. 2012.PubMed/NCBI
|
|
24
|
Chen P, Lu N, Ling Y, Chen Y, Hui H, Lu Z,
Song X, Li Z, You Q and Guo Q: Inhibitory effects of wogonin on the
invasion of human breast carcinoma cells by downregulating the
expression and activity of matrix metalloproteinase-9. Toxicology.
282:122–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou L, Chen L and Fang L: Scutellarin
inhibits proliferation, invasion, and tumorigenicity in human
breast cancer cells by regulating HIPPO-YAP signaling pathway. Med
Sci Monit. 23:5130–5138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrison LJ, Sia GL and Sim KY:
5,7-Dihydroxy-8-methoxyflavone from Tetracera indica. Planta Med.
60:493–494. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi JA, Lee JW, Kim H, Kim EY, Seo JM, Ko
J and Kim JH: Pro-survival of estrogen receptor-negative breast
cancer cells is regulated by a BLT2-reactive oxygen species-linked
signaling pathway. Carcinogenesis. 31:543–551. 2010. View Article : Google Scholar
|
|
28
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase-9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim EY, Seo JM, Cho KJ and Kim JH:
Ras-induced invasion and metastasis are regulated by a leukotriene
B4 receptor BLT2-linked pathway. Oncogene. 29:1167–1178. 2010.
View Article : Google Scholar
|
|
30
|
Park GS and Kim JH: LPS up-regulates
ICAM-1 expression in breast cancer cells by stimulating a
MyD88-BLT2-ERK-linked cascade, which promotes adhesion to
monocytes. Mol Cells. 38:821–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Truong D, Puleo J, Llave A, Mouneimne G,
Kamm RD and Nikkhah M: Breast cancer cell invasion into a three
dimensional tumorstroma microenvironment. Sci Rep. 6:340942016.
View Article : Google Scholar
|
|
32
|
Jemal A, Ward E and Thun MJ: Recent trends
in breast cancer incidence rates by age and tumor characteristics
among U.S. women. Breast Cancer Res. 9:R282007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huynh DL, Kwon T, Zhang JJ, Sharma N, Gera
M, Ghosh M, Kim N, Kim Cho S, Lee DS, Park YH and Jeong DK: Wogonin
suppresses stem cell-like traits of CD133 positive osteosarcoma
cell via inhibiting matrix metalloproteinase-9 expression. BMC
Complement Altern Med. 17:3042017. View Article : Google Scholar
|
|
34
|
Liu X, Tian S, Liu M, Jian L and Zhao L:
Wogonin inhibits the proliferation and invasion, and induces the
apoptosis of HepG2 and Bel7402 HCC cells through NF-kappaB/Bcl-2,
EGFR and EGFR downstream ERK/AKT signaling. Int J Mol Med.
38:1250–1256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao K, Wei L, Hui H, Dai Q, You QD, Guo
QL and Lu N: Wogonin suppresses melanoma cell B16-F10 invasion and
migration by inhibiting Ras-medicated pathways. PLoS One.
9:e1064582014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim BO: Effects of wogonin, wogonoside,
and 3,5,7,2′,6′-penta hydroxyflavone on chemical mediator
production in peritoneal exduate cells and immunoglobulin E of rat
mesenteric lymph node lymphocytes. J Ethnopharmacol. 84:23–29.
2003. View Article : Google Scholar
|
|
37
|
Zeng H, Dou S, Zhao J, Fan S, Yuan X, Zhu
S, Li L, Zhang W and Liu R: The inhibitory activities of the
components of Huang-Lian-Jie-Du-Tang (HLJDT) on eicosanoid
generation via lipoxygenase pathway. J Ethnopharmacol. 135:561–568.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441. 2016.
View Article : Google Scholar :
|
|
40
|
Peters-Golden M and Henderson WR Jr:
Leukotrienes. N Engl J Med. 357:1841–1854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gautam S, Roy S, Ansari MN, Saeedan AS,
Saraf SA and Kaithwas G: DuCLOX-2/5 inhibition: A promising target
for cancer chemoprevention. Breast Cancer. 24:180–190. 2017.
View Article : Google Scholar
|
|
42
|
Green AR, Green VL, White MC and Speirs V:
Expression of cytokine messenger RNA in normal and neoplastic human
breast tissue: Identification of interleukin-8 as a potential
regulatory factor in breast tumours. Int J Cancer. 72:937–941.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Merdad A, Karim S, Schulten HJ, Dallol A,
Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM and
Al-Qahtani MH: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer: MMP-9 as a potential biomarker for
cancer invasion and metastasis. Anticancer Res. 34:1355–1366.
2014.PubMed/NCBI
|
|
44
|
Wang W, Xia T and Yu X: Wogonin suppresses
inflammatory response and maintains intestinal barrier function via
TLR4-MyD88-TAK1-mediated NF-kappaB pathway in vitro. Inflamm Res.
64:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karroum A, Mirshahi P, Benabbou N, Faussat
AM, Soria J, Therwath A, Mirshahi M and Hatmi M: Matrix
metallo-proteinase-9 is required for tubular network formation and
migration of resistant breast cancer cells MCF-7 through PKC and
ERK1/2 signalling pathways. Cancer Lett. 295:242–251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim S, Jeon M, Lee JE and Nam SJ: MEK
activity controls IL-8 expression in tamoxifen-resistant MCF-7
breast cancer cells. Oncol Rep. 35:2398–2404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jung YS and Lee SO: Apomorphine suppresses
TNF-α-induced MMP-9 expression and cell invasion through inhibition
of ERK/AP-1 signaling pathway in MCF-7 cells. Biochem Biophys Res
Commun. 487:903–909. 2017. View Article : Google Scholar : PubMed/NCBI
|