Introduction
Human dental pulp stem cells (hDPSCs) are
self-renewing, highly proliferative, multi-potent stem cells that
are derived from enzymatically disaggregated adult human dental
pulp (1). More than 90% of dental
pulp cells (DPCs) display positive expression of the endothelial
cell markers CD29, CD44 and CD146, while >20% of DPCs express
STRO-1, a mesenchymal stromal progenitor marker. Furthermore, DPCs
have a negative expression of hematopoietic markers, including
CD34, CD45 and CD133, and the endothelial marker, CD106 (2). Previous studies have demonstrated
that hDPSCs exhibit a higher proliferative rate compared with human
bone marrow stromal cells (hBMSCs), and are able to differentiate
into odontoblasts/osteoblasts, chondrocytes, adipocytes and neural
cells in vitro (1,3,4).
However, unlike BMSCs, DPSCs exhibit an odontogenic capability to
form specific crystalline structures in mineralized nodules,
similar to physiological dentin but distinct from bone structures
(5). A previous study revealed
that DPSCs, similar to mesenchymal stem cells (MSCs), possess
immunomodulatory properties, and that Fas ligand governs the
immunoregulatory properties of DPSCs in the induction of T-cell
apoptosis (6). Taken together,
hDPSCs may represent good sources of stem cells for experimental
and clinical studies.
Aspirin, also known as acetylsalicylic acid (ASA),
is the most widely used antipyretic, analgesic and non-steroidal
anti-inflammatory drug (7). It
affects multiple biological pathways by inhibiting cyclooxygenase
and decreasing the production of prostaglandins (7). Previous studies have suggested that
aspirin has the potential to promote bone regeneration (8–11).
Aspirin increased the osteogenic capacity of bone marrow MSCs
(BMMSCs) by targeting the telomerase activity, and inhibited
osteoclast activity in mice (8).
In addition, aspirin promoted BMMSC-based bone regeneration via
inhibiting tumor necrosis factor-α and interferon-γ production in
skull defect models (9).
Administration of aspirin was capable of improving BMSC-mediated
calvarial bone regeneration in a porcine model (10), as well as osteogenic
differentiation and immunomodulation mediated by stem cells derived
from exfoliated deciduous teeth (11). Aspirin has also been demonstrated
to enhance the function of periodontal ligament stem cells and may
have regenerative dentistry applications (12). Combined use of aspirin and
adipose-derived stem cells has been reported to partially reverse
bone loss caused by castration in rats (13). In addition, regular administration
of aspirin may have a moderate beneficial effect on bone mineral
density in human patients (14).
However, to the best of our knowledge, no previous studies have
assessed the effect of aspirin on the osteogenic capacity of
hDPSCs.
In the present study, the impact of aspirin on bone
repair on hDPSC-seeded anorganic bovine bone (Bio-Oss), a
tissue-engineered construct, was assessed in a rat calvarial defect
model.
Materials and methods
Isolation and culture of hDPSCs
The present study was approved by the Ethical Board
of the Second Affiliated Hospital of Harbin Medical University
(Harbin, China). Written informed consent was obtained from the
parents of the healthy donors (age, 14–18 years; 5 males and 2
females) undergoing orthodontic treatments. Briefly, cells were
isolated from dental pulp tissue of extracted permanent teeth as
previously described (1,3,4).
The cells were cultured in Human Mesenchymal Stem Cell Growth
Medium (Cyagen Biosciences, Inc., Guangzhou, China) supplemented
with 10% fetal bovine serum (FBS), 10 mM glutamine and 100 U/ml
penicillin-streptomycin at 37°C with 5% CO2 in
humidified incubator. At 3–4 days later, non-adherent cells were
removed, and the medium was changed every 2 days thereafter. After
~14 days, colony formation unit-fibroblasts were formed (1), and various methodologies were
performed.
A mixed colony culture was performed similarly to a
multi-colony derived cell culture, as reported previously (15). Briefly, primary cells were
passaged when colonies began to merge on days 12–14 with 0.25%
trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), following which all colonies and other cells were combined.
For a picked colony culture, after 14–16 days, individual round
colonies (>10 per flask) were selected and pooled together in a
new flask. Upon reaching 80–90% confluency, cells were collected
with 0.25% trypsin-EDTA and passaged. At passage 3 (P3), adherent
cells were collected, characterized and used in further
experiments.
Flow cytometric analysis for cell
characterization
P3 cells at confluence in 25-cm2 flasks
were collected and stained at 4°C for 30 min with antibodies
against human CD29 (1:100; cat. no. 559882), CD44 (1:100; cat. no.
550989), CD133 (1:100; cat. no. 566593) and CD146 (1:100; cat. no.
550315; BD Biosciences, San Jose, CA, USA), as well as anti-STRO-1
antibody (1:50; cat. no. FAB1038G; R&D Systems, Minneapolis,
MN, USA). Subsequently, cells were analyzed using a FACS Calibur
flow cytometer and Cell Quest software (BD Biosciences).
Multilineage differentiation of
hDPSCs
P3 cells were seeded at 15×104 cells/well
in 6-well plates (Corning Incorporated, Corning, NY, USA) and
cultured in Human Mesenchymal Stem Cell Growth Medium (Cyagen
Biosciences, Inc., Guangzhou, China) supplemented with 10% FBS, 10
mM glutamine and 100 U/ml penicillin-streptomycin at 37°C with 5%
CO2 in a humidified incubator. Cells were then treated
with osteogenic, chondrogenic and adipogenic induction medium for
2–3 weeks as previously reported (16). Trilineage differentiation of
hDPSCs was evaluated by alizarin red, alcian blue and Oil Red O
stains using a Human Mesenchymal Stem Cell Differentiation kit
(Thermo Fisher Scientific, Inc.), in accordance with the
manufacturer's protocol.
Aspirin cytotoxicity assay
The effect of aspirin on hDPSC viability was
assessed using Cell Counting Kit-8 (Beyotime Institute of
Biotechnology, Nantong, China), in accordance with the protocol
provided by the manufacturer. Briefly, hDPSCs were seeded at a
density of 4,000 cells/well in a 96-well flat-bottom plate (Corning
Incorporated) in triplicate. Cells were maintained in 100 μl
standard medium containing 0, 25, 50, 100, 200 or 400 μg/ml
aspirin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 24, 48
or 72 h.
Alkaline phosphatase (ALP) staining and
Alizarin red staining (ARS)
Cells were cultured in Human Mesenchymal Stem Cell
Osteogenic Differentiation Medium (Cyagen Biosciences, Inc.)
containing 10% FBS, 100 U/ml penicillin-streptomycin, 0.2 mM
ascorbate, 10 mM β-glycerophosphate and 10−7 M
dexamethasone. After 24 h, aspirin (0, 25, 50 and 100 μg/ml)
was added to hDPSCs that were seeded at 15×104
cells/well in 6-well plates (Corning Incorporated).
ALP staining was conducted at day 14 using a
BCIP/NBT staining kit (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer's protocol. NBT
formazan was collected using 100 mM cetylpyridinium chloride
monohydrate (CPC; cat. no. C9002-25G; Sigma-Aldrich, Merck KGaA),
and absorbance was read at 560 nm with a microplate reader (iMARK
Microplate Absorbance Reader; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
For the ARS assay, hDPSCs were cultured for 21 days
and then fixed with 75% ethanol, followed by staining with 2%
alizarin red (Beijing Solarbio Bioscience & Technology Co.,
Ltd., Beijing, China; pH 4.2). Unbound and nonspe-cifically bound
stain was removed by rinsing with distilled water. Calcium-bound
stain was collected with 100 mM CPC, and absorbance was read at 560
nm with a microplate reader (iMARK Microplate Absorbance Reader;
Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were homogenized for RNA extraction using the
RNeasy mini kit (Qiagen, Hilden, Germany). The RNA concentrations
were measured by a Nanovue spectrophotometer (GE Healthcare Life
Sciences, Marlborough, MA, USA), and the total RNA was then
reverse-transcribed to cDNA using the Prime Script First Strand
cDNA Synthesis kit (Takara Bio, Inc., Kusatsu, Japan) on the
MxPro-Mx3000P Real-Time PCR System (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). PCR was then performed
to determine the expression levels of target genes, and gene
expression was normalized to that of β-actin. The relative
differences in the PCR results were calculated by using the
2−ΔΔCq method (17).
The thermo-cycling conditions were as follows: 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The
primers used in this analysis are listed in Table I (Invitrogen; Thermo Fisher
Scientific, Inc.).
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Reverse
(5′-3′) | Forward
(5'-3') |
|---|
| RUNX2 |
CAGATGGGACTGTGGTTACTG |
GAGGATTTGTGAAGACGGTTA |
| Col-I |
AAGACGAAGACATCCCACCAA C |
AGATCACGTCATCGCACAAC |
| OCN |
AGGGCAGCGAGGTAGTGAAGA |
AGAGGAGCAGAACTGGGGTTG |
| β-actin |
GGGCCGGACTCGTCATAC |
CCTGGCACCCAGCACAAT |
Western blotting
The cells were harvested and proteins were extracted
with RIPA lysis buffer (Beyotime, Shanghai, China). The protein
concentration was determined using the BCA protein Assay (Beyotime,
Shanghai, China). Equal aliquots of 40 μg per sample were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (10–12%) and transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore, Bedford, MA, USA).
Following blocking in 5% nonfat dry milk (dissolved in TBST, TBS
plus 0.1% Tween-20) for 1 h at room temperature, the proteins of
interest were probed with primary antibodies overnight at 4°C:
osteocalcin (1:1,000; cat. no. ab13418; Abcam, Cambridge, UK),
collagen I (1:1,000; cat. no. ab6308; Abcam), runt-related
transcription factor 2 (RUNX2;1:1,000; cat. no. 12556; Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-actin (1:5,000;
cat. no. 3700; Cell Signaling Technology, Inc.). Subsequently,
membranes were incubated with IRDye 800CW-labeled goat anti-rabbit
IgG (H+L; 1:10,000; cat. no. 926-32211; LI-COR Biosciences,
Lincoln, NE, USA) and goat anti-mouse IgG (1:10,000; H+L; cat. no.
926-32210; LI-COR Biosciences) for 1 h at room temperature. The
blots were then visualized using an Infrared Imaging System (LI-COR
Biosciences, Lincoln, NE, USA). The band density was quantified
using Odyssey software version 3.0 (LI-COR Biosciences) and
normalized to β-actin.
Generation of rat calvarial bone defects
and transplantation
The animal studies were approved by the Ethical
Board of the Second Affiliated Hospital of Harbin Medical
University. The 40 adult male Sprague-Dawley rats aged 9–10 weeks
(200–250 g) used in this study were supplied by the Animal Center
of the Second Affiliated Hospital of Harbin Medical University
(Harbin, China; No. SYXK, 2013-002). The rats were housed in an
animal facility with 20–23°C, 40–60% humidity and a 12-h light/dark
cycle. Standard laboratory chow and water ad libitum were
supplied. hDPSCs were cultured in Human Mesenchymal Stem Cell
Osteogenic Differentiation Medium prior to implantation into the
cranial defects. Briefly, Sprague-Dawley rats were anesthetized
with intraperitoneal injection of 300 mg/kg chloral hydrate (10%;
cat. no. C8383; Sigma-Aldrich; Merck KGaA), and then bicortical
defects of 5-mm diameter were created with a stainless-steel
trephine (18). The rats were
randomly assigned to four different groups (n=10 per group) with
the following: i) Untreated group, unfilled defects; ii) BO group,
0.02 g Bio-Oss (Geistlich Pharma AG, Wolhusen, Switzerland) only;
iii) DPSC/BO group, hDPSCs (8×106 cells) + Bio-Oss; and
iv) DPSC/BO/ASA group, hDPSCs (8×106 cells) treated with
100 μg/ml aspirin for 3 days using Bio-Oss as a carrier.
Following the placement of the materials, the surgical site was
covered with a native collagen membrane (Biogide®;
Geistlich Pharma AG), and the soft tissues were closed with
sutures. All the animals received a single dose of ampicillin (100
mg/kg; A6920, Beijing Solarbio Bioscience & Technology Co.,
Ltd.) 12 h post-surgery. The rats were sacrificed with an overdose
of pentobarbital sodium (100 mg/kg; intravenous) at 8 or 12 weeks
postoperatively, and the calvaria were immediately excised and
fixed in 4% neutral-buffered formaldehyde.
Electron microscopy
To observe cells adhesion on scaffolds in
vitro, hDPSCs were seeded on Bio-Oss at a density of
8×106 cells per 0.02 g Bio-Oss and cultured for 24 h.
The samples were fixed in 2.5% glutaraldehyde and evaluated by
scanning electron microscopy (SEM; S-3400; Hitachi, Ltd., Tokyo,
Japan).
Radiography and micro-computed tomography
(CT) scanning
Explanted calvaria samples were radiographed by
X-ray (Faxitron Bioptics LLC, Tucson, AZ, USA) and scanned by a
micro-CT scanner (μCT35; Scanco Medical AG, Bassersdorf,
Switzerland) to examine the new bone within the defect region.
Histology
The specimens were fixed, decalcified and
paraffin-embedded. Sections (4-μm) were prepared and then
stained with hematoxylin and eosin (H&E) or with Masson's
trichrome (MTS). New bone formation within the defect was measured
histomorphometrically using an image analysis software (Image Pro
Plus, version 7.0; Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis
The results are reported as the mean ± standard
deviation of three independent experiments. The data were analyzed
using GraphPad Prism software, version 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA). Multiple comparisons were performed by
one-way analysis of variance followed by Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
hDPSCs express MSC markers and have
multilineage differentiation potential in vitro
hDPSCs were characterized at P3 by flow cytometry,
and the majority of cells were found to express STRO-1, CD146, CD44
and CD29. By contrast, CD133 expression was not evident (Fig. 1A). Furthermore, hDPSCs were
cultured by picking established colonies at passage 0, which
increased the percentage of STRO-1+ cells at P3 compared
with the mixed colony culture method (44.9 vs. 3.25%; P<0.05).
STRO-1 was expressed by ≤70% of cells (mean value, 44.9%; Fig. 1B). The cells proliferated rapidly
following the subculture and homogeneously exhibited a
fibroblast-like spindle shape (Fig.
1C). Cells were positive for ARS, alcian blue staining and Oil
red O staining in response to osteogenic, chondrogenic and
adipogenic induction, respectively (Fig. 1D–F). Taken together, these results
indicated that hDPSCs possess MSC properties, and that the picked
colony culture method is suitable for multipotent hDPSC culture and
augments the STRO-1+ subpopulation.
Aspirin enhances osteogenic
differentiation of hDPSCs in vitro
The present study first assessed the toxicity of
aspirin on hDPSCs in vitro. Aspirin at concentrations of
≤100 μg/ml had no significant effect on the viability of
hDPSCs within 72 h, while 200 and 400 μg/ml aspirin caused a
significant decrease in cell viability at 48 and 72 h (Fig. 2A). Therefore, ≤100 μg/ml
aspirin was used to treat hDPSCs in the following experiments.
Next, it was demonstrated that aspirin increased ALP activity,
whose expression by functional osteoblasts precedes mineralization
(19), in a dose-dependent manner
(Fig. 2B and E). The
hDPSC-osteoinductive function of aspirin was then assessed by ARS,
and the results revealed that 21-day aspirin treatment at doses of
50 and 100 μg/ml significantly augmented mineralized nodule
formation by ~2–3 fold (Fig. 2C, D
and F).
 | Figure 2Aspirin increases the mineralization
of hDPSCs in vitro. (A) Aspirin was non-toxic to hDPSCs at a
concentration of ≤100 μg/ml, as shown by CCK-8 assay. (B)
hDPSCs treated with different doses of aspirin (0, 25, 50 and 100
μg/ml), exhibiting increased ALP activity at 14 days and (C)
capability of forming min-eralized nodules at 21 days in a
dose-dependent manner. (D) A magnified view of the mineralized
matrix by alizarin red staining. Scale bar, 200 μm.
Significantly increased (E) ALP activity and (F) calcium deposition
were observed in cells treated with 100 μg/ml aspirin,
compared with the untreated group. *P<0.05,
**P<0.01 and ****P<0.0001. hDPSCs,
human dental pulp stem cells; CCK-8, Cell Counting Kit-8; ALP,
alkaline phosphatase; OD, optical density; ns, non-significant. |
The study further examined the effects of aspirin on
the expression levels of osteogenesis-associated markers, including
RUNX2, collagen I and osteocalcin, in hDPSCs (Fig. 3). The results demonstrated that
high doses of ASA (100 μg/ml) significantly improved the
osteogenic differentiation of hDPSCs compared with the untreated
groups in vitro. RUNX2 upregulation was observed on days 5
and 7 (Fig. 3A and D), while
collagen I (Fig. 3B and E) and
osteocalcin (Fig. 3C and F) were
significantly upregu-lated on day 14, at the protein and mRNA
levels (Fig. 3). Cumulatively,
these data suggested that aspirin enhanced the osteogenic
differentiation potential of hDPSCs in the ex vivo
culture.
Aspirin improves hDPSC-based bone
formation in vivo
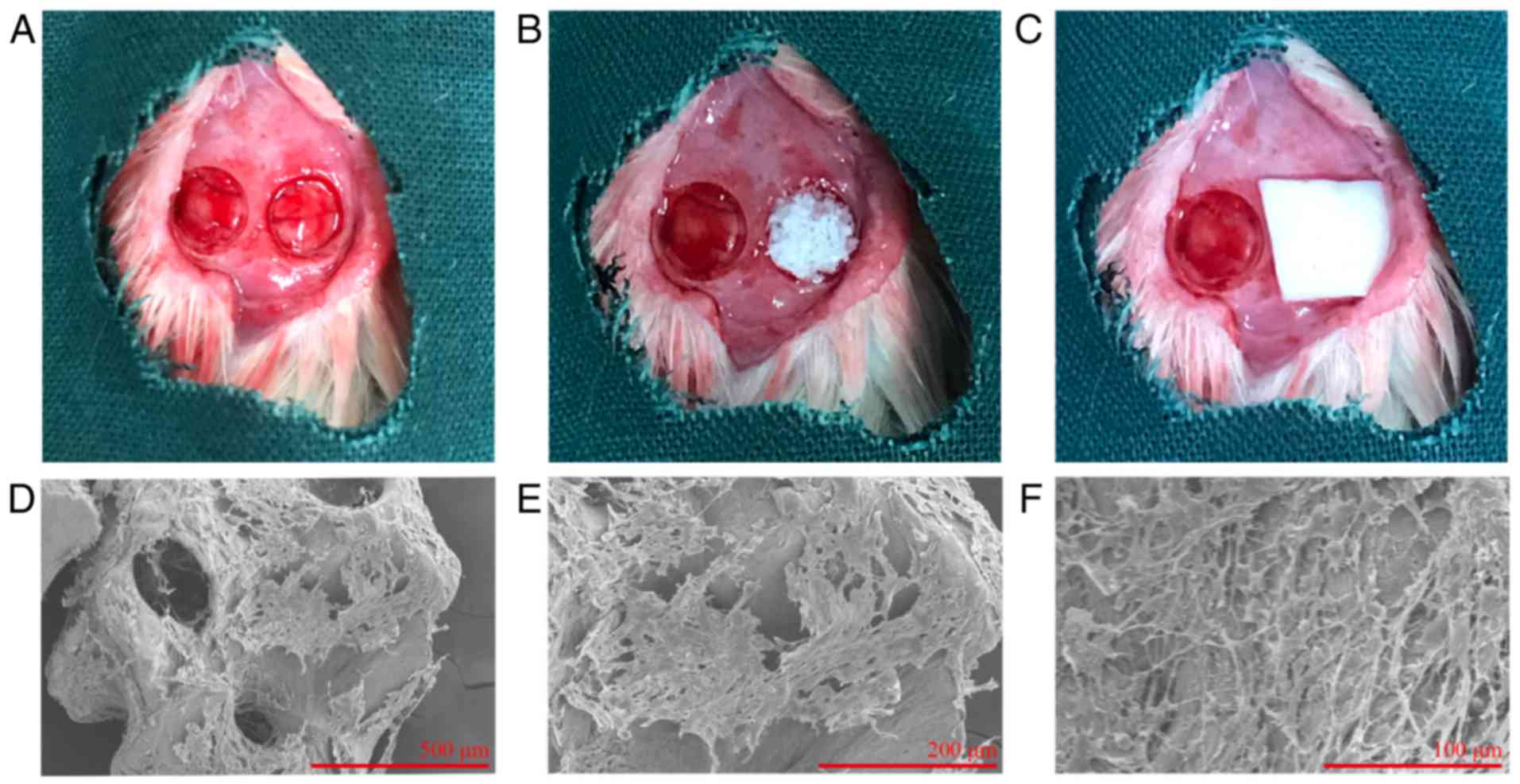
Using a rat calvarial defect model (Fig. 4A–C), it was confirmed that aspirin
enhanced the bone-forming capacity of hDPSCs in vivo. hDPSCs
were seeded on Bio-Oss and incubated for 24 h prior to SEM
examination. It was observed that hDPSCs dispersed as a monolayer
and covered parts of the Bio-Oss surface (Fig. 4D–F). In addition, radiographs
revealed incomplete healing after 8 or 12 weeks in untreated rats
(Fig. 5A and B). By contrast,
Bio-Oss significantly increased bone healing in the cranial defect
model, and hDPSCs seeded on Bio-Oss further augmented the
high-density area.
To assess whether aspirin improved new bone
formation, the calvarial bone specimens were histologically
analyzed, and aspirin was found to enhance the hDPSC-mediated bone
formation in vivo (Fig.
6A–D). H&E and MTS staining revealed minimal new bone
formation around the margins of the native bone in the untreated
groups, where soft fibrous tissue filled the center of the defect
area. Limited new bone formation was evident in the BO group, while
a moderate amount of bone formation was noted in defects treated
with DPSC/BO. Aspirin treatment resulted in abundant mineralized
tissue formation at 8 and 12 weeks post-surgery (Fig. 6A and C). According to the H&E
staining, more new bone was evident at the bottom of the defect
during the earlier period of bone formation, and the untreated
group exhibited significantly less new bone density in comparison
with the other three groups at 8 weeks. Aspirin-treated hDPSCs
exhibited significantly increased new bone formation, with
woven/lamellar features in the defect area, as compared with the
other groups at 12 weeks (Fig. 6B and
D), indicating a pro-osteogenic effect exerted by aspirin
(P<0.05). These findings suggested that aspirin improved bone
formation when hDPSCs were seeded Bio-Oss in a rat cranial defect
model.
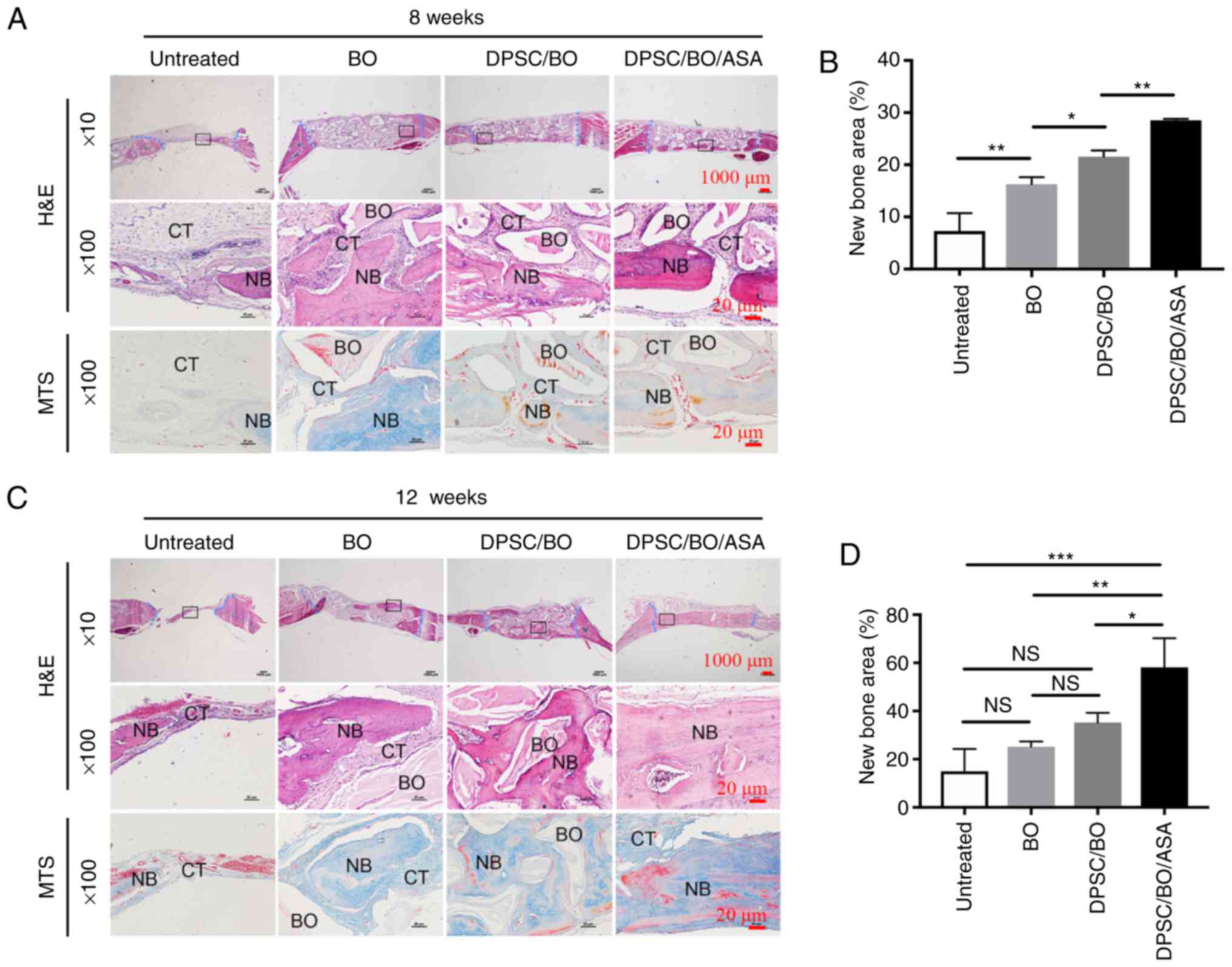 | Figure 6Aspirin treatment significantly
improves hDPSC-based bone formation in a rat calvavial defect
model. (A) Calvarial bone specimens that were untreated, or treated
with BO, hDPSCs+BO or hDPSCs+BO+ASA were retrieved at 8 weeks
post-surgery, and (B) quantitative analyses, based on H&E
staining, of the percentage of new bone formation between different
groups was performed. (C) Calvarial bone specimens collected at 12
weeks post-surgery in the different groups, and (D) H&E-based
quantitative analysis of new bone formation. Sections were stained
with H&E and MTS, and images were captured at low (×10; scale
bar, 1,000 μm) and high magnification (×100; scale bar, 20
μm). The edge of the defects was shown in blue in the low
magnification images. H&E staining results were analyzed using
Image-Pro Plus 6.0 software. *P<0.05,
**P<0.01 and ***P<0.001. hDPSC, human
dental pulp stem cell; BO, Bio-Oss; ASA, acetylsalicylic acid
(aspirin); NB, new bone; CT, connective tissue; H&E,
hematoxylin and eosin; MTS, Masson's trichrome. |
Discussion
hDPSCs are easily isolated from the teeth of healthy
donors undergoing orthodontic treatments. In addition, hDPSCs
proliferate faster than hBMSCs and can differentiate into multiple
cell types, particularly osteogenic cells (20). Previous studies have demonstrated
that hDPSCs possess the ability to differentiate into neural,
odontogenic and osteogenic cells, with the latter two cell types
being able to form mineral-ized nodules in vitro (21,22). It has also been demonstrated that
multi-colony-derived DPSCs have a potential capacity to proliferate
in vitro and to regenerate dentin in vivo, as
compared with single-colony-derived strains (3). In the present study, hDPSCs were
cultured using mixed colony culture and picked colony culture
methods at passage 0, the two of which initiated successful cell
expansion.
DPSCs have been reported to express CD10, CD29,
CD44, CD59, CD73, CD90, CD105, CD150 and CD166, but not
hematopoietic cell surface markers, such as CD45, CD14, CD18, CD24,
CD34 or CD133 (6,23). A previous study revealed that
STRO-1 and CD146 (also known as melanoma cell adhesion molecule or
MUC18) function as markers for pre-osteogenic stem cells and
high-purity BMMSCs, respectively (24,25). The STRO-1+ fraction
represented ~6% of the total pulp cells, which have higher capacity
for colony formation and osteoblast differentiation (24,26). In the present study, it was
demonstrated that STRO-1 was expressed by ≤70% of hDPSCs (mean
value, 44.9%). This finding is consistent with previous studies
reporting that STRO-1 is a marker of pre-osteogenic populations,
the expression of which is lost upon cell proliferation and
differentiation into mature osteoblasts.
Although DPSCs have similar characteristics to
BMMSCs, DPSCs exhibit reduced osteogenic and adipogenic potentials
compared with BMMSCs (1,6). The current study focused on
investigating various potent regulators, such as cytokines or
drugs, to regulate the differentiation of DPSCs. It has been
reported that aspirin has an anti-proliferative effect on BMMSCs at
high concentrations, but not at low ones (50–200 μg/ml)
(27). This is consistent with
the results of the present study, which revealed that aspirin
exerted little effect on the number of hDPSCs at low concentrations
(<100 μg/ml). That is likely due to different resources
and protocols used for MSC culture that may result in different
responses to aspirin.
DPSCs and BMSCs exhibited a similar expression
pattern of bone markers, including ALP, collagen I, osteocalcin and
osteopontin (1). RUNX2 is the
early osteoblastic transcription factor (28). Collagen I, the dominant fibrous
protein in hard tissues, such as bone and dentin, is secreted by
mature osteoblasts. Osteoblasts produce a collagen extracellular
matrix that becomes mineralized through various signaling
molecules, particularly bone sialoprotein, osteopontin and
osteocalcin (29). Osteocalcin is
considered to be the latest-functioning expression marker in mature
osteoblasts (28). It has been
demonstrated that aspirin can affect the expression levels of those
markers. For instance, Liu et al (11) reported that low doses of aspirin
(10 and 50 μg/ml) treatment, but not a high dose (200
μg/ml), upregulated RUNX2 and ALP expression levels. This
was consistent with the results of the present study, which
demonstrated that the expression levels of RUNX2, collagen I and
osteocalcin were elevated when the cells were treated with aspirin.
This suggests that aspirin enhanced the osteogenic capacity of
hDPSCs in vitro.
Bone engineering in craniomaxillofacial surgery
requires scaffold/supporting materials, adequate target cells and
osteo-genesis-inducing factors (30). Several in vivo experiments
have indicated that DPSCs have potential applications in bone
engineering (31), while aspirin
promotes the bone-forming ability of BMSCs (9) and stem cells from human exfoliated
deciduous teeth (11). The
present study used Bio-Oss as a carrier to transplant hDPSCs into
rat cranial defects with or without aspirin treatment. It was
identified that aspirin treatment promoted hDPSC-induced bone
regeneration. Bio-Oss is a commercially available bone substitute
with osteoconductive properties that supports new bone formation
for implant dentistry and maxillofacial surgery (32,33). However, a consensus regarding the
biodegradation of Bio-Oss is yet to be reached. A number of
investigators have observed that the bovine bone mineral is
replaced by the newly formed bone (34,35), while others have indicated that
the resorption process of Bio-Oss was markedly slow (36,37). Histological and
histomorphometrical analyses in the current study indicated that
the majority of the Bio-Oss particles were surrounded by new bone
formation, indicating that Bio-Oss was biocompatible and
osteoconductive. In addition, according to the H&E staining
results, new bone was located adjacent to the dura mater during the
early period of bone formation. Similarly, it has previously been
reported that new bone formation localized to the area of the
scaffold adjacent to the dura mater potentially indicates strong
paracrine signaling between the underlying dura mater and the cells
within the defect (38).
In conclusion, the present study demonstrated that
hDPSCs exhibit stem cell properties, including expression of MSC
markers and a capacity to differentiate into multiple cell lineages
in vitro. This suggests that hDPSCs may be a feasible source
of MSCs. Furthermore, aspirin improved the potential of osteogenic
differentiation of hDPSCs in vitro and in vivo. Thus,
the present study provides a promising basis for an hDPSC-based
bone regeneration therapy.
Acknowledgments
The authors would like to thank Dr Quan Liu (Harbin
Medical University, Harbin, China for the critical revision of this
article.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81570951 and 81500816), the
Special Foundation for Sino-Russian Translational Medicine Research
Center of Harbin Medical University (grant no. CR201412 and
CR201504), the Natural Science Foundation of Heilongjiang Province
of China (grant no. H2015103), the Research Innovation Fund of
Harbin Medical University (grant no. 2016LCZX19), the Science
Foundation of the Second Affiliated Hospital of Harbin Medical
University (grant no. CX2016-20), and the Research Grant of Health
and Family Planning Commission of Heilongjiang Province (grant no.
2016-060).
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
BZ and YL conceived and designed the experiments.
MY, YZ, NM and XX performed the experiments. MY, WH and HJ analyzed
the data. MY wrote the paper.
Ethics approval and consent to
participate
The experimental protocols of the present study were
approved by the Ethical Board of the Second Affiliated Hospital of
Harbin Medical University (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei X, Ling J, Wu L, Liu L and Xiao Y:
Expression of mineralization markers in dental pulp cells. J Endod.
33:703–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gronthos S, Brahim J, Li W, Fisher LW,
Cherman N, Boyde A, DenBesten P, Robey PG and Shi S: Stem cell
properties of human dental pulp stem cells. J Dent Res. 81:531–535.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Gronthos S and Shi S: Dental pulp
stem cells. Methods Enzymol. 419:99–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
About I, Bottero MJ, de Denato P, Camps J,
Franquin JC and Mitsiadis TA: Human dentin production in vitro. Exp
Cell Res. 258:33–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Wang L, Jin Y and Shi S: Fas
ligand regulates the immu-nomodulatory properties of dental pulp
stem cells. J Dent Res. 91:948–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith JB and Willis AL: Aspirin
selectively inhibits pros-taglandin production in human platelets.
Nat New Biol. 231:235–237. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K,
Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One. 3:e26152008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen
C, Xu X, Yang R, Chen W, Wang S and Shi S: Mesenchymal stem
cell-based tissue regeneration is governed by recipient T
lymphocytes via IFN-γ and TNF-α. Nat Med. 17. pp. 1594–1601. 2011,
View Article : Google Scholar
|
|
10
|
Cao Y, Xiong J, Mei S, Wang F, Zhao Z,
Wang S and Liu Y: Aspirin promotes bone marrow mesenchymal stem
cell-based calvarial bone regeneration in mini swine. Stem Cell Res
Ther. 6:2102015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Chen C, Liu S, Liu D, Xu X, Chen X
and Shi S: Acetylsalicylic acid treatment improves differentiation
and immunomodulation of SHED. J Dent Res. 94:209–218. 2015.
View Article : Google Scholar :
|
|
12
|
Abd Rahman F, Mohd Ali J, Abdullah M, Abu
Kasim NH and Musa S: Aspirin enhances osteogenic potential of
periodontal ligament stem cells (PDLSCs) and modulates the
expression profile of growth factor-associated genes in PDLSCs. J
Periodontol. 87:837–847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Li W, Liu Y, Zhang X and Zhou Y:
Co-administration of aspirin and allogeneic adipose-derived stromal
cells attenuates bone loss in ovariectomized rats through the
anti-inflammatory and chemotactic abilities of aspirin. Stem Cell
Res Ther. 6:2002015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane
NE, Hochberg MC, Stone K and Nevitt MC: Aspirin and NSAID use in
older women: Effect on bone mineral density and fracture risk. J
Bone Miner Res. 11:29–35. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuznetsov SA, Krebsbach PH, Satomura K,
Kerr J, Riminucci M, Benayahu D and Robey PG: Single-colony derived
strains of human marrow stromal fibroblasts form bone after
transplantation in vivo. J Bone Miner Res. 12:1335–1347. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei M, Li K, Li B, Gao LN, Chen FM and Jin
Y: Mesenchymal stem cell characteristics of dental pulp and
periodontal ligament stem cells after in vivo transplantation.
Biomaterials. 35:6332–6343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
18
|
Spicer PP, Kretlow JD, Young S, Jansen JA,
Kasper FK and Mikos AG: Evaluation of bone regeneration using the
rat critical size calvarial defect. Nat Protoc. 7:1918–1929. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi S, Gronthos S, Chen S, Reddi A,
Counter CM, Robey PG and Wang CY: Bone formation by human postnatal
bone marrow stromal stem cells is enhanced by telomerase
expression. Nat Biotechnol. 20:587–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nuti N, Corallo C, Chan BM, Ferrari M and
Gerami-Naini B: Multipotent differentiation of human dental pulp
stem cells: A literature review. Stem Cell Rev. 12:511–523. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu M, Sun Y, Liu Y, Yuan M, Zhang Z and
Hu W: Modulation of the differentiation of dental pulp stem cells
by different concentrations of β-glycerophosphate. Molecules.
17:1219–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Yao J, Yuan M, Zhang Z and Hu W:
Osteoblasts can induce dental pulp stem cells to undergo osteogenic
differentiation. Cytotechnology. 65:223–231. 2013. View Article : Google Scholar :
|
|
23
|
Ferro F, Spelat R, Beltrami AP, Cesselli D
and Curcio F: Isolation and characterization of human dental pulp
derived stem cells by using media containing low human serum
percentage as clinical grade substitutes for bovine serum. PLoS
One. 7:e489452012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesen-chymal stem cells in human bone marrow and
dental pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gronthos S and Zannettino AC: A method to
isolate and purify human bone marrow stromal stem cells. Methods
Mol Biol. 449:45–57. 2008.PubMed/NCBI
|
|
26
|
Yu J, He H, Tang C, Zhang G, Li Y, Wang R,
Shi J and Jin Y: Differentiation potential of STRO-1+
dental pulp stem cells changes during cell passaging. BMC Cell
Biol. 11:322010. View Article : Google Scholar
|
|
27
|
Tang J, Xiong J, Wu T, Tang Z, Ding G,
Zhang C, Wang S and Liu Y: Aspirin treatment improved mesenchymal
stem cell immunomodulatory properties via the
15d-PGJ2/PPARγ/TGF-β1 pathway. Stem Cells Dev.
23:2093–2103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun L, Blair HC, Peng Y, Zaidi N, Adebanjo
OA, Wu XB, Wu XY, Iqbal J, Epstein S, Abe E, et al: Calcineurin
regulates bone formation by the osteoblast. Proc Natl Acad Sci USA.
102:17130–17135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wiesmann HP, Meyer U, Plate U and Höhling
HJ: Aspects of collagen mineralization in hard tissue formation.
Int Rev Cytol. 242:121–156. 2005. View Article : Google Scholar
|
|
30
|
Graziano A, D'Aquino R, Laino G and
Papaccio G: Dental pulp stem cells: A promising tool for bone
regeneration. Stem Cell Rev. 4:21–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morad G, Kheiri L and Khojasteh A: Dental
pulp stem cells for in vivo bone regeneration: A systematic review
of literature. Arch Oral Biol. 58:1818–1827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sculean A, Chiantella GC, Windisch P, Gera
I and Reich E: Clinical evaluation of an enamel matrix protein
derivative (Emdogain) combined with a bovine-derived xenograft
(Bio-Oss) for the treatment of intrabony periodontal defects in
humans. Int J Periodontics Restorative Dent. 22:259–267.
2002.PubMed/NCBI
|
|
33
|
Valentini P and Abensur DJ: Maxillary
sinus grafting with anorganic bovine bone: A clinical report of
long-term results. Int J Oral Maxillofac Implants. 18:556–560.
2003.PubMed/NCBI
|
|
34
|
Klinge B, Alberius P, Isaksson S and
Jönsson J: Osseous reponse to implant natural bone mineral and
synthetic hydroxylapatite ceramic in the repair of experimental
skull bone defects. J Oral Maxillofac Surg. 50:241–249. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jensen SS, Aaboe M, Pinholt EM,
Hjørting-Hansen E, Melsen F and Ruyter IE: Tissue reaction and
material characteristics of four bone substitutes. Int J Oral
Maxillofac Implants. 11:55–66. 1996.PubMed/NCBI
|
|
36
|
Berglundh T and Lindhe J: Healing around
implants placed in bone defects treated with Bio-Oss. An
experimental study in the dog. Clin Oral Implants Res. 8:117–124.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Piattelli M, Favero GA, Scarano A, Orsini
G and Piattelli A: Bone reactions to anorganic bovine bone
(Bio-Oss) used in sinus augmentation procedures: A histologic
long-term report of 20 cases in humans. Int J Oral Maxillofac
Implants. 14:835–840. 1999.PubMed/NCBI
|
|
38
|
Cowan CM, Shi YY, Aalami OO, Chou YF, Mari
C, Thomas R, Quarto N, Contag CH, Wu B and Longaker MT:
Adipose-derived adult stromal cells heal critical-size mouse
calvarial defects. Nat Biotechnol. 22:560–567. 2004. View Article : Google Scholar : PubMed/NCBI
|