Introduction
Mature chondrocytes are metabolically slow, highly
differentiated cells with a limited proliferation rate and, unlike
other tissues such as bone, a restricted ability to self-repair
cartilage degeneration (1,2).
Despite their low numbers and being the sole cell type of the
cartilage, chondrocytes serve fundamental roles in regulating
tissue properties, such as organization, remodeling, synthesis and
degradation of the surrounding matrix (3,4).
During skeletal development, chondrocytes arise from mesenchymal
progenitors to synthesize the templates or cartilage anlagen for
the developing bone (5).
Chondrogenesis occurs as a result of condensation of mesenchymal
cells. The process can be generally divided into two phases: i)
Early chondrogenesis, which is characterized by upregulated
collagen type II α1 chain (COL2A1) and SRY-box 9 (SOX9), and ii)
hypertrophy, which is characterized by upregulated collagen type X
α1 chain (COL10A1) and runt-related transcription factor 2. These
two stages are regulated by numerous factors and signaling pathways
(6), and they function to repress
each other (7-10).
The metabolic behavior of chondrocytes can be
modified by various factors, including aging, genetic makeup, and
other mechanical and chemical factors, such as joint loading and
inflammation. Proinflammatory cytokines, including tumor necrosis
factor-α (TNF-α) and interleukin-1β (IL-1β) exert catabolic and
anabolic effects, and serve important roles in the degradation and
synthesis of the extracellular matrix (ECM) (11). In osteoarthritis (OA), cartilage
deterioration is associated with disruption of the metabolic
homeostasis of chondrocytes and dysregulation of their
physiological behavior, which is reflected by the appearance of
cartilage surface fibrillation, formation of chondrocyte clusters
and alterations in the composition of ECM components (12).
SOX9 is considered the master regulator and a key
transcriptional factor in chondrocyte condensation, proliferation
and differentiation (13-16). In chondrogenesis, SOX9 acts as a
homo-dimer (17), which is
stabilized by two other SOX members of the SoxD family: L-SOX5 and
SOX6 (18). These molecules are
co-expressed with SOX9 during cell differentiation, and are
associated with the production of collagen types II and IX, as well
as aggrecan (ACAN), which is the major proteoglycan (19). SOX9 is expressed in all
prechon-drocytic and chondrocytic cells during mouse embryonic
development, whereas its expression is completely abolished in
hypertrophic chondrocytes (20-22). Mutations in the SOX9 gene lead to
campomelic dysplasia, a disease characterized by skeletal
malformation and 46,XY sex reversal (23).
Polycomb group (PcG) proteins are known to regulate
developmental genes, and abnormal expression of these proteins may
cause cancer (24,25). Scm-like with four malignant brain
tumor (MBT) domains 2 (SFMBT2) is a member of the PcG protein
family (26), which has been
reported to be involved in prostate cancer cell growth, through
repression of the homeobox B13 gene in DU145 cells (27). SFMBT2 consists of a sterile α
motif domain and four MBT domains, which are crucial for gene
regulation by recognizing and binding to methylated lysine residues
in the tails of histones H3 and H4 (28). However, to the best of our
knowledge, no study regarding its role in cartilage or OA has been
published to date.
Our previous study screened differentially expressed
genes in OA cartilage using the suppression subtractive
hybridization technique, and identified SFMBT2 as a candidate gene
from the reverse-subtracted cDNA library by sequence analysis and
similarity search with BLAST (29). Our recent study verified the
negative association of this gene with OA by analyzing the
expression of SFMBT2 in damaged cartilages from human patients with
OA compared with normal cartilages from control individuals, and
established that the down-regulation of SFMBT2 contributes to the
catabolic phenotype of chon-drocytes (30). The aim of the present study was to
examine the expression of SFMBT2 in cartilage at different stages
of its development, and during the process of chondrogenesis.
Understanding the mechanisms underlying cartilage remod-eling
during development, OA and aging, and identifying the novel factors
involved in these mechanisms may lead to the development of more
effective strategies, preventing cartilage damage and promoting
repair.
Materials and methods
Rats
Dark agouti (DA) rats, which originated from Medical
Inflammation Research, Lund University (Lund, Sweden), were bred
and maintained in polystyrene cages containing sterile wood
shavings in a specific pathogen-free animal house at the Department
of Biochemistry and Molecular Biology, Xi'an Jiaotong University
(Xi'an, China). Rats were fed standard rodent chow and water ad
libitum and were maintained in a climate-controlled environment
(temperature: 20-26°C; relative humidity: 40-70%) under a 12 h
light/dark cycle. Femoral head cartilages were surgically removed
from 12 age/weight-matched rats, four for each developmental stage
(two females, two males), after sacrificing the rats on postnatal
days 0 (D0), 21 (D21) and 42 (D42). The cartilage samples were
immediately placed in liquid nitrogen after cleaning with PBS and
were stored at -80°C for further analysis. The experiments were
approved by the Institutional Animal Ethics Committee of Xi'an
Jiaotong University (Xi'an, China).
Cell culture
The ATDC5 murine chondroblast cell line was obtained
from the European Collection of Authenticated Cell Cultures
(Salisbury, UK) and was cultured in Dulbecco's modified Eagle's
medium (DMEM)/Ham's F12 (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 5% fetal bovine serum (FBS;
Shanghai ExCell Biology, Inc., Shanghai, China). Chondrogenesis was
induced by adding ITS-supplement (insulin, 1 mg/ml; transferrin,
0.55 mg/ml; and selenium, 0.5 µg/ml) directly into the
medium. The medium was changed every 2nd or 3rd day. The cells were
harvested for RNA and protein isolation on D0, D3, D7 and D14
post-induction.
The C28/I2 human juvenile costal chondrocyte cell
line was kindly provided by Prof. Junling Cao (Institute of Endemic
Diseases, Xi'an Jiaotong University, Health Science Center, Xi'an,
China). Chondrocytes were cultured in DMEM/F12 containing 10% FBS
and 1% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Both chondrocyte lines were maintained at 37°C
in the presence of 5% CO2. Once the cells reached 90%
confluence, they were detached by treatment with 0.05% trypsin
(Shanghai ExCell Biology, Inc.) and passaged.
Transient transfection/infection with
small interfering RNAs (siRNAs)/adenovirus vectors
For the knockdown experiments, siRNAs against SFMBT2
(si-SFMBT2) and SOX9 (si-SOX9), and the scrambled negative control
siRNA (si-NC), were synthe-sized (Shanghai GenePharma Co., Ltd.,
Shanghai, China). C28/I2 cells were seeded in 12-well plates
(1x105 cells/well), after which, they were transfected
with the 80 nM si-SFMBT2, 60 nM si-SOX9 or 60 nM si-NC using
Lipofectamine® 2000 (cat. no. 11668-019; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Briefly, C28/I2 cells were incubated with
transfection complexes in serum-free medium for 4 h at 37°C in a
CO2 incubator. After 4 h, the medium was replaced with
fresh, complete medium (containing 10% FBS), and cells were
incubated at 37°C in the presence of 5% CO2. Effects of
siRNA interference were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting 12-72 h post-transfection, as indicated. The siRNA
sequences used in the present study were as follows: si-NC, sense,
UUC UCC GAA CGU GUC ACG UTT and antisense, CGU GAC ACG UUC GGA GAA
TT; si-SFMBT2, sense, 5′-GCA CUU UGU CAG CUU CCA ATT-3′ and
antisense, 5′-UUG GAA GCU GAC AAA GUG CTT-3′; and si-SOX9, sense,
5′-GCA GCG ACG UCA UCU CCA AdT dT-3′ and antisense, 5′-CGU CGC UGC
AGU AGA GGU UdT dT-3′.
The FLAG-green fluorescent protein-tagged SFMBT2
adenovirus vector (ad-SFMBT2; 1x1010 PFU/ml) and the
control adenovirus vector (ad-NC; 2x1010 PFU/ml) were
purchased from Hanbio Biotechnology Co., Ltd. (Shanghai, China).
For overexpression of SFMBT2, C28/I2 cells were seeded in 12-well
plates (1x105 cells/well) or 96-well plates
(1x104 cells/well), and were incubated with ad-NC or
ad-SFMBT2, which were added directly into the medium at the
indicated titers/multiplicities of infection (MOIs) for 4 h at 37°C
in a CO2 incubator. After 4 h, the medium containing the
viral vectors was removed and replaced with fresh medium, and the
cells were incubated at 37°C in the presence of 5% CO2.
The chondrocytes were harvested at the indicated time intervals
(0-72 h) after adenoviral infection for RNA and protein
extraction.
Chondrocyte treatment with TNF-α and
IL-1β
C28/I2 cells were serum starved for 10 h prior to
the addition of TNF-α (10 ng/ml) or IL-1β (10 ng/ml) (both from
Sino Biological Inc., Beijing, China) into the culture medium. The
cells were then incubated at 37°C in the presence of 5%
CO2 and were harvested for protein isolation 24 h
post-treatment. C28/I2 cells infected with ad-NC or ad-SFMBT2 were
treated with TNF-α 24 h post-adenoviral infection, and were
harvested for protein isolation at the indicated time points (0-48
h) following TNF-α treatment.
Total RNA extraction and RT-qPCR
Total RNA was extracted from the cultured
chondrocytes (1x105 cells/well) using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and was
reverse transcribed to cDNA using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Gene expression levels were detected by
qPCR (Agilent Stratagene Mx3005; Agilent Technologies, Inc., Santa
Clara, CA, USA) using the SYBR Green system (Roche Diagnostics,
Indianapolis, IN, USA). Thermocycling conditions were as follows:
95°C for 10 min (activation), followed by 40 cycles at 95°C for 15
sec (denaturation), 61/64°C for 30 sec (annealing) and 72°C for 30
sec (extension). Data collection was performed during each
extension phase. Relative quantification of the target genes,
normalized to the control, was calculated using the comparative Cq
(ΔΔCq) method (31). The primers
used for RT-qPCR analysis are listed in Table I.
 | Table ImRNA-specific primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
mRNA-specific primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Ta (°C) |
|---|
| ms-SFMBT2 | F:
TAACTGCTGTCCTGCCTGCT | 61 |
| R:
TGTGACATGGCCTACAGCTC | |
| ms-SOX9 | F:
TATGTGGATGTGTGCGTGTG | 61 |
| R:
CCAGCCACAGCAGTGAGTAA | |
| ms-COL2A1 | F:
AGAGCGGAGACTACTGGATTG | 61 |
| R:
CGTTAGCGGTGTTGGGAG | |
| ms-COL10A1 | F:
TGGGATGCCTCTTGTCAGTG | 61 |
| R:
GTGGGCGTGCCATTCTTAT | |
| ms-β-actin | F:
AACAGTCCGCCTAGAAGCAC | 61 |
| R:
CGTTGACATCCGTAAAGACC | |
| hsa-SFMBT2 | F:
ACGAAACAGGAGGAGGAGGAGAG | 64 |
| R:
GGAAGGGTCAGAAGCAGGAGTG | |
| hsa-SOX9 | F:
CGCACATCAAGACGGAGCAG | 61 |
| R:
TGTAGGTGAAGGTGGAGTAGAGG | |
| hsa-GAPDH | F:
CACCCACTCCTCCACCTTTG | 61 |
| R:
CCACCACCCTGTTGCTGTAG | |
Protein isolation and quantification
Total protein was isolated from the cultured
chondrocytes (1x105 cells/well) using
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing protease and phosphatase
inhibitor mixture (Bimake, Houston, TX, USA). The whole cell
lysates were incubated on ice for 30 min, centrifuged at 12,000 x g
for 15 min at 4°C to remove cellular debris, and the supernatants
were transferred to clean 1.5 ml tubes. Protein concentration in
the cleared lysates was determined by Bicinchoninic Acid Protein
Assay (Tiangen Biotech Co., Ltd., Beijing, China).
Protein sample preparation and
immunoblotting
The clear lysates were mixed with SDS loading dye
and heated at 95°C for 5 min. Subsequently, ~20 µg proteins
were separated by 10% SDS-PAGE and transferred to the
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked in 5% non-fat milk or 5% bovine
serum albumin (BSA, Amresco; VWR, Radnor, PA, USA) at room
temperature for 2 h, and then incubated at 4°C overnight with
primary antibodies. After washing three times with Tris-buffered
saline containing 0.1% Tween-20, the membrane was incubated with a
horseradish peroxidase-conjugated secondary antibody for 2 h at
room temperature. The blots were detected using the enhanced
chemiluminescence detection system (EMD Millipore). Western blots
were scanned using the GeneGnome XRQ system (Syngene, Frederick,
MD, USA), and densitometry of the bands was analyzed with GeneTools
analysis software V 4.01 (Syngene). The antibodies and their
dilutions are shown in Table
II.
 | Table IIAntibodies used in the present
study. |
Table II
Antibodies used in the present
study.
| Antibody
(origin) | Type | Species
specificity | Cat. no. | Company | Application
(dilution) |
|---|
| SFMBT2 (Rb) | Polyclonal | Human, mouse | 25256-1-AP | ProteinTech Group,
Inc. (Chicago, IL, USA) | WB (1:500) |
| SFMBT2 (Rb) | Polyclonal | Human, rat | bs-21105R | BIOSS (Beijing,
China) | IHC (1:50) |
| SOX9 (Rb) | Monoclonal | Human, mouse | ab182579 | Abcam (Cambridge,
MA, USA) | WB (1:1,000) |
| COL2A1 (Rb) | Polyclonal | Human, mouse | BA0533 | Wuhan Boster
Biological Technology, Ltd. (Wuhan, China) | WB (1:200) |
| COL10A1 (Rb) | Polyclonal | Human, mouse | BA2023 | Wuhan Boster
Biological Technology, Ltd | WB (1:500) |
| GAPDH (Rb) | Polyclonal | Human, mouse | 10494-1-AP | ProteinTech Group,
Inc. | WB (1:2,000) |
| HRP-conjugated IgG
(goat) | Polyclonal | Rabbit | 31460 | Thermo Fisher
Scientific, Inc. (Waltham, MA, USA) | WB (1:2,000) |
Immunohistochemistry
Immunohistochemical staining was performed using the
standard streptavidin peroxidase complex method. Cartilage tissues
were fixed in 4% buffered paraformaldehyde for >2 days at room
temperature, and were decalcified with buffered EDTA (12.5% EDTA,
pH 7.4) for 4 weeks at room temperature. Subsequently, the samples
were dehydrated through a graded series of alcohol, embedded in
paraffin and 5-µm sections were obtained; the tissue
sections were deparaffinized in xylene and rehydrated through a
graded series of alcohol. Following treatment with 3%
H2O2 for 10 min at room temperature and
enzymatic digestion (Antigen Retrieval Buffer, cat. no. AR0022;
Wuhan Boster Biological Technology, Ltd.) for 10 min at 37°C,
tissue sections were blocked with 5% BSA for 20 min at 4°C and were
incubated with the anti-SFMBT2 primary antibody (Table II) overnight at 4°C.
Subsequently, sections were incubated with the bioti-nylated
secondary antibody (1:1,000) for 30 min at 37°C and horseradish
peroxidase-conjugated streptavidin (1:1,000) for 30 min at 37°C,
both of which were contained within the DAB substrate kit (Wuhan
Boster Biological Technology, Ltd.). Sections were counterstained
with hematoxylin and mounted with neutral balsam. Images of the
staining were captured under a fluorescence microscope (Olympus
BX51; Olympus Corporation, Tokyo, Japan) and staining was
semi-quantified using Image-Pro® Plus (V6.0) software
(Media Cybernetics, Inc., Rockville, MD, USA).
Cell proliferation assay
Cell Counting kit-8 (CCK-8; Bimake) assay was used
to evaluate the proliferative abilities of chondrocytes. As
previously reported (32), C28/I2
cells were seeded in 96-well plates (1x104 cells/well)
in DMEM/Ham's F12 and were maintained at 37°C in the presence of 5%
CO2. Subsequently, cells were transfected/infected with
si-SFMBT2/ad-SFMBT2, alongside the respective controls
(si-NC/ad-NC). At 12, 24, 48 and 72 h post-transfection/infection,
10 µl CCK-8 solution was added directly into each well and
the cells were incubated for a further 4 h. Absorbance was measured
at 450 nm using a microplate reader (Thermo Scientific
Multiskan® Spectrum; Thermo Fisher Scientific, Inc.).
Experiments were repeated in triplicate.
Statistical analysis
All of the cell experiments were performed in
triplicate with at least three independent biological replicates.
GraphPad Prism (V5.0) software (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analyses. Data are presented as
the means ± standard error of the mean from at least three
independent values. Student's t-test or one-way analysis of
variance with Tukey's multiple comparison test were used to compare
differences between the experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
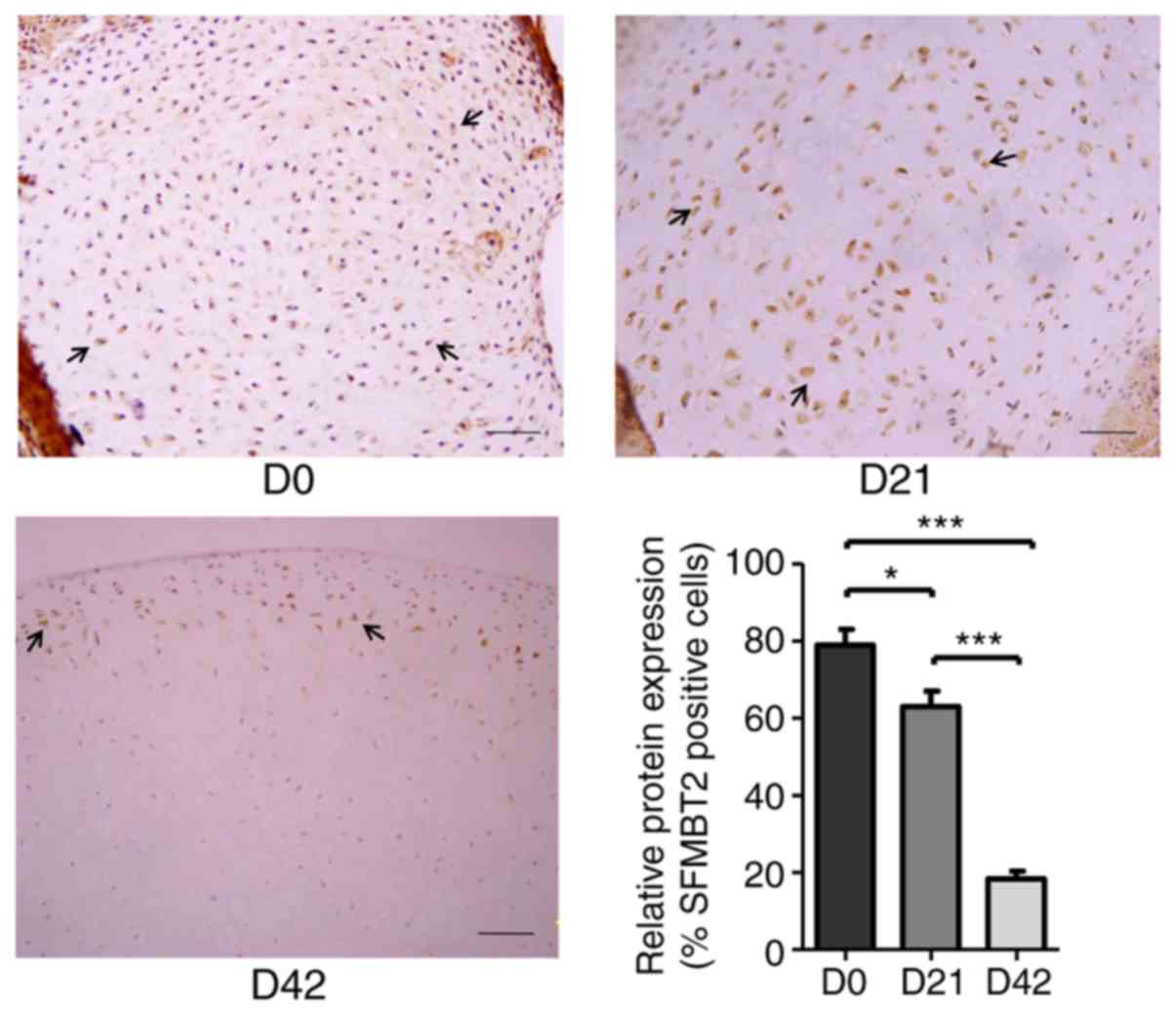
SFMBT2 is abundantly expressed during the
early stages of cartilage development
To examine the expression of SFMBT2 in articular
cartilage, femoral head cartilages were surgically isolated from DA
rats at D0, D21 and D42, which represent newborn/baby (0 years
old), ablactation/child (5 years old) and juvenile (11 years old)
developmental stages in humans, respectively (33). Immunohistochemical staining of the
cartilage samples using a specific antibody against SFMBT2 revealed
that the protein expression levels of SFMBT2 were markedly higher
at D0 (~80%) and D21 (~63%), whereas the expression was
significantly decreased at D42 (~18%) (P<0.05; Fig. 1).
SFMBT2 is upregulated during the
proliferative phase of chondrogenesis
Chondrogenesis was induced in ATDC5 cells, and
chondrocyte differentiation was verified through the expression of
key chondrogenic markers, including SOX9, COL2A1 and COL10A1. The
mRNA and protein expression levels were analyzed at specific
time-points by RT-qPCR and western blotting, respectively. As shown
in Fig. 2A, the mRNA expression
levels of SFMBT2, SOX9 and COL2A1 were markedly increased on days 3
and 7 (proliferation), but were significantly decreased at day 14
(hypertrophy) compared with at day 7 by >7-, >2- and
>16-fold, respectively (P<0.05). COL10A1 transcription
remained low during the prolifera-tive phase (days 3-7) but was
increased at day 14 by >3-fold (P<0.05), compared with D0-D7.
Western blotting results were consistent with the RT-qPCR data
(Fig. 2B). These findings
indicated that SFMBT2 may be involved in the proliferation of
chondrocytes during chondrogenesis.
 | Figure 2SFMBT2 expression during
chondrogenesis. (A and B) Relative expression levels of SFMBT2 and
chondrogenic markers (SOX9, COL2A1 and COL10A1) in ATDC5 cells
cultured in insulin-transferrin-selenium-supplemented medium, as
determined by (A) RT-qPCR and (B) western blotting. β-actin and
GAPDH were used as internal controls in RT-qPCR and western
blotting, respectively. *P<0.05,
**P<0.01, ***P<0.001 vs. the ‘D0’
group; ##P<0.01, ###P<0.001 vs. the indicated
group(s). D, day; COL10A1, collagen type X α1 chain; COL2A1,
collagen type II α1 chain; D, day; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; SFMBT2,
Scm-like with four malignant brain tumor domains 2; SOX9, SRY-box
9. |
SFMBT2 regulates SOX9 expression in human
chondrocytes
The expression levels of SFMBT2 were suppressed in
C28/I2 cells in response to si-SFMBT2. Endogenous SFMBT2 was
knocked down >70% by 80 nM si-SFMBT2 (Fig. 3A and B). Decreased levels of
SFMBT2 in C28/I2 cells led to a significant reduction in SOX9, both
at the mRNA (>1.7-fold; P<0.05) and protein levels
(>2-fold; P<0.05), as determined by RT-qPCR and western
blotting (Fig. 3A and B),
respectively. Conversely, overexpression of SFMBT2 in C28/I2 cells
was achieved by infecting the cells with ad-SFMBT2. Compared with
ad-NC, chondrocytes infected with 400 MOI ad-SFMBT2 exhibited
>18-fold higher levels of SFMBT2 (P<0.05), which resulted in
significant upregulation of SOX9 at the mRNA (>2.4-fold;
P<0.05) (Fig. 3C) and protein
levels (>7-fold; P<0.05) (Fig.
3D).
 | Figure 3SFMBT2 positively regulates SOX9.
Relative (A) mRNA and (B) protein expression levels of SFMBT2 and
SOX9 in C28/I2 cells transfected with 80 nM si-NC or si-SFMBT2, as
measured by RT-qPCR and western blotting, respectively. Relative
(C) mRNA and (D) protein expression levels of SFMBT2 and SOX9 in
C28/I2 cells infected with ad-NC or ad-SFMBT2 (400 multiplicity of
infection), as determined by RT-qPCR and western blotting,
respectively. The C28/I2 cells were harvested for RNA and protein
isolation at 24 and 48 h post-treatment with siRNA or adenoviral
vector, respectively. GAPDH was used as the internal control for
RT-qPCR and western blotting. *P<0.05;
**P<0.01; ***P<0.001. ad, adenovirus;
NC, negative control; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; SFMBT2, Scm-like with four malignant
brain tumor domains 2; si, small interfering; SOX9, SRY-box 9. |
SFMBT2 suppresses SOX9 reduction in
TNF-α-treated C28/I2 cells
Different titers (MOIs) of ad-SFMBT2 were use to
observe its effects on protein levels; as expected, ad-SFMBT2
upregulated SOX9 protein expression in a dose-dependent manner, as
determined by western blotting (Fig.
4A). To investigate whether SOX9 could regulate SFMBT2, si-SOX9
was used. C28/I2 cells transfected with 60 nM si-SOX9 exhibited a
marked decrease (>70%) in the protein expression levels of SOX9
compared with in cells transfected with si-NC. However, no change
in the expression levels of SFMBT2 was observed (Fig. 4B), thus suggesting that SFMBT2 is
located upstream of SOX9. Subsequently, SOX9 and SFMBT2 expression
was examined in C28/I2 cells treated with IL-1β (10 ng/ml) or TNF-α
(10 ng/ml). The results of a western blot analysis revealed that
SOX9 expression was decreased in response to IL-1β and TNF-α
treatment (by >2 and >3-fold, respectively). SFMBT2
expression was not significantly altered in response to IL-1β;
however, it was decreased by >1.4-fold in response to 24 h of
treatment with TNF-α (Fig. 4C).
Furthermore, C28/I2 cells were treated with TNF-α for various
durations. As shown in Fig. 4D,
SOX9 expression was decreased in TNF-α-treated chondrocytes in a
time-dependent manner. However, infecting cells with 400 MOI
ad-SFMBT2 prevented SOX9 reduction, and increased SOX9 protein
levels in TNF-α-treated chondrocytes.
 | Figure 4Exogenous SFMBT2 attenuates the
reduction in SOX9 expression in C28/I2 chondrocytes under TNF-α
treatment. (A) Left panel, dose-dependent effect of SFMBT2
overexpression on SOX9 in C28/12 cells harvested at 48 h
post-infection, as determined by western blotting (ad-SFMBT2 was
used at 200, 400 and 800 MOI, whereas ad-NC was used at 400 MOI).
Right panel, Statistical analysis of the relative protein
expression levels normalized to GAPDH. (B) Relative protein
expression levels of SOX9 and SFMBT2 in C28/I2 cells transfected
with 60 nM si-NC or si-SOX9 and harvested 48 h post-transfection,
as analyzed by western blotting. (C) Effects of IL-1β and TNF-α on
the expression levels of SOX9 and SFMBT2 in C28/I2 cells harvested
48 h post-treatment. (D) Relative protein expression levels of SOX9
in C28/I2 cells infected with ad-NC or ad-SFMBT2 (400 MOI) for 24 h
and incubated with TNF-α (10 ng/ml). The cells were harvested for
protein isolation at the indicated time points post-TNF-α treatment
and were analyzed by western blotting. GAPDH was used as the
internal control in western blot analyses. *P<0.05,
**P<0.01, ***P<0.001 vs. the Mock and
ad-NC groups; ##P<0.01, ###P<0.001 vs.
the indicated group. NS, non-significant. ad, adenovirus; IL,
interleukin; MOI, multiplicity of infection; NC, negative control;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; SFMBT2, Scm-like with four malignant brain tumor domains
2; si, small interfering; SOX9, SRY-box 9; TNF, tumor necrosis
factor. |
SFMBT2 positively regulates chondrocyte
proliferation
The proliferation of C28/I2 chondrocytes was
observed using the CCK-8. C28/12 cells were transfected/infected
with si-SFMBT2 or ad-SFMBT2, alongside their respective controls.
The results demonstrated that si-SFMBT2 transfection decreased the
proliferation of chondrocytes compared with in the si-NC group
(Fig. 5A). Conversely,
chondrocytes infected with ad-SFMBT2 exhibited increased
proliferation compared with in the ad-NC group, as measured at the
indicated time intervals (Fig.
5B).
 | Figure 5SFMBT2 positively regulates
chondrocyte proliferation. (A) Proliferation of C28/I2 cells
transfected with si-NC or si-SFMBT2, as determined 12, 24, 48 and
72 h post-transfection by CCK-8 assay. (B) Chondrocytes were
infected with ad-NC or ad-SFMBT2 for various durations (12, 24, 48
and 72 h), and cell proliferation was determined by CCK-8 assay.
*P<0.05; **P<0.01. ad, adenovirus; CCK,
cell counting kit; NC, negative control; SFMBT2, Scm-like with four
malignant brain tumor domains 2; si, small interfering. |
Discussion
A previous study reported that SFMBT2 is highly
expressed in extra-embryonic tissues, such as the placenta and yolk
sac, and is required for trophoblast maintenance and placental
development (34). The present
study analyzed the expression levels of SFMBT2 during cartilage
development. For that purpose, DA rats were raised and sacrificed
at the indicated time points. Immunohistochemical staining of
femoral head cartilage specimens collected at various stages of
cartilage development revealed that SFMBT2 was markedly expressed
during the early stages of development (D0 and D21), thus
suggesting that this gene may be involved in early cartilage
development. Notably, SFMBT2 expression was reduced in hypertrophic
chondrocytes, and at D42 its expression was confined to the
proliferating zone, thus indicating that SFMBT2 may participate in
chondrocyte proliferation. The downregulation of SFMBT2 expression
observed at D42 may indicate a lack of function of this gene in
mature articular cartilage. However, due to the complex mechanism
underlying maintenance of cartilage anabolic and catabolic
homeostasis, certain levels of molecular factors are crucial to
retain that balance. This was witnessed in our previous SFMBT2
knockdown experiments, where C28/I2 cells transfected with
si-SFMBT2 displayed altered expression levels of metabolic marker
genes, suggesting that downregulation of SFMBT2 may disturb the
homeostatic balance of chondrocytes (30).
Chondrogenesis is a process by which cartilage is
formed. It is primarily driven by the activity and expression of
the transcription factor SOX9, which stimulates and maintains the
expression of numerous cartilage-related genes, including COL2A1
and ACAN (14,35). As a prerequisite for
chondro-genesis in vivo, SOX9 activates COL2A1 transcription
by binding to the first intron enhancer through its high mobility
group DNA-binding domain (36,37). Later phases of chon-drogenesis are
marked and regulated by the sequential activation of genes,
including Indian hedgehog, runt-related transcription factor 2,
COL10A1 and matrix metalloproteinase 13 (MMP13), and the
simultaneous downregulation of SOX9 and COL2A1 (38). Following chondrogenesis,
chondrocytes remain as resting cells forming the articular
cartilage, or undergo proliferation, terminal differentiation to
chondrocyte hypertrophy and apoptosis in a process termed
endochondral ossification, whereby the hypertrophic cartilage is
replaced by bone (39).
ATDC5 cells are derived from mouse teratocarcinoma
cells (40) and are characterized
as prechondrogenic cells that remain undifferentiated if cultured
in regular medium. However, when grown in medium containing a
chondrogenic differentiation supplement, these cells can replicate
the events of chondrogenesis, from cell condensation to cartilage
nodule formation (41,42). Therefore, the ATDC5 cell line is
considered a promising in vitro cell model to study the
sequential events of cartilage formation and the factors that
influence chondrocyte behaviors during chondrogenesis. In the
present study, the expression levels of SFMBT2 were increased in
chondrogenic ATDC5 cells during the early phase of chon-drogenesis
(proliferating chondrocytes) alongside SOX9 and COL2A1, but were
decreased in the later phases (hyper-trophic differentiation) when
COL10A1 was upregulated, indicating that SFMBT2 may be involved in
the proliferation of chondrocytes. The high levels of COL10A1 at
D14 are in accordance with hypertrophic degeneration, which
physiologically precedes endochondral ossification. Under normal
physiological conditions, mature articular cartilage behaves quite
differently; SOX9 and COL2A1 levels are maintained even in the
presence of low cellular turnover. In general, in normal tissues,
cellular proliferation and differentiation are inversely related.
The chondrocytes in adult human cartilage are normally quiescent
and maintain the matrix in a low turnover state. Healthy hyaline
cartilage does not express high levels of COL10A1 and has a very
low rate of proliferation, simultaneously maintaining high SOX9 and
COL2A1 levels. Conversely, during OA development, modifications
towards hypertrophy take place (43-47).
SFMBT2 expression during the process of
chondrogenesis was almost consistent with that of SOX9. Therefore,
it was hypothesized that SFMBT2 may affect SOX9 expression in
articular chondrocyte. The present study examined the effects of
SFMBT2 knockdown and overexpression in C28/I2 cells using siRNA and
adenoviral vectors, respectively. In mature articular chondrocytes,
following SFMBT2 knockdown in vitro, SOX9 was significantly
downregulated at the mRNA and protein levels, whereas
overexpression of SFMBT2 upregulated SOX9. SOX9 is a pivotal
transcriptional regulator, which is essential for chondrocyte
phenotypic stability, differentiation (13) and proliferation (36). Downregulation of SOX9 may induce
angiogenesis, cartilage resorption, and formation of bone marrow
and endochondral bone trabeculae (22), which are associated with the
progression of OA. The present study also investigated whether SOX9
could regulate SFMBT2 expression. For that purpose, SOX9 expression
in C28/I2 cells was suppressed by targeting SOX9 mRNA using a
specific siRNA sequence; however, si-SOX9 did not affect SFMBT2
expression levels in chondrocytes, indicating that SFMBT2 is
upstream of SOX9.
Studies on chondrocyte behavior in OA suggest that
at different stages and/or locations within articular cartilage,
the processes of cartilage matrix anabolism and catabolism are
regulated through coordinated mechanisms that are not fully
understood. The degradation of articular cartilage due to
cata-bolic behavior of chondrocytes, thus leading to the
destruction of ECM components, is a characteristic of OA (48,49). In OA, the normally quiescent
chondrocytes undergo a phenotypic shift and become ‘activated’
cells, characterized by cell proliferation, cluster formation, and
increased production of matrix proteins and matrix-degrading
enzymes (49). It is generally
considered that inflammation is absent or weakly present in OA
(50); however, numerous studies
have confirmed the presence of immune cells and proinflammatory
cytokines within the synovial tissues of patients with OA (51-54). The most acknowledged background
theory is that the cartilage fragments and the degraded products
fall into the joint and come into contact with the synovium
(50). Considering them foreign
bodies, synovial cells produce inflammatory cytokines in response
to these cartilage fragments, which results in further activation
of chondrocytes, the production of matrix-degrading enzymes [i.e.
MMPs and a disintegrin and metalloproteinase with thrombospondin
motifs (ADAMTSs)] and subsequent increased cartilage destruction
(55).
Numerous studies have confirmed the upregulation of
IL-1β and TNF-α in OA joints (51-54). Nuclear factor (NF)-κB, which is a
negative regulator of SOX9 expression, is mainly regulated by TNF-α
and IL-1β (56). Activation of
NF-κB and other inflammatory pathways, such as the
mitogen-activated protein kinase signaling pathway, is required for
chondro-cytes to express MMPs, ADAMTSs and inflammatory cytokines
(44,57). In vitro studies have
suggested that NF-κB is an upstream inducer of hypoxia-inducible
factor-2α, which is another transactivator of genes expressed in
hypertrophic chondrocytes, including COL10A1, MMP13 and vascular
endothelial growth factor (58).
These findings suggest that the anabolic-catabolic balance is under
the influence of a complex network of signals that regulate
chondrocyte behavior and tissue homeostasis (59). Catabolic activity is higher in OA
(60), and due to their low
turnover rate, chon-drocytes are not able to substitute the
degraded ECM (61). However,
increased production of collagen type II and ACAN is commonly
considered an early response to cartilage degeneration in in
vivo and in vitro OA models (62,63). SOX9 is a potent transcriptional
activator of COL2A1, which participates in cartilage development
and can maintain the chondrocyte phenotype (13,20,64,65). When C28/I2 cells were incubated
with various concentrations (MOIs) of ad-SFMBT2, SOX9 protein
levels were elevated in a dose-dependent manner compared with in
the ad-NC group. Furthermore, TNF-α treatment reduced SOX9 protein
levels in C28/I2 cells. Infection with ad-SFMBT2 not only protected
the cells from a reduction in SOX9 levels, but also increased its
expression in a time-dependent manner.
The present study investigated whether alterations
in SFMBT2 levels could affect the proliferation of C28/I2 cells.
SFMBT2 was knocked down in chondrocytes, which were subjected to a
cell proliferation assay using the CCK-8. SFMBT2 knockdown
decreased cell proliferation compared with in the si-NC-transfected
cells. Conversely, the ad-SFMBT2 group displayed an increased
proliferative rate compared with in the ad-NC group. These results
suggested that SFMBT2 may function as a positive regulator of cell
proliferation in human chondrocytes.
In conclusion, the present study revealed that
SFMBT2 could target and regulate the expression of SOX9, and could
protect chondrocytes from losing SOX9 expression under TNF-α
treatment. SFMBT2 also promoted chondrocyte proliferation. Further
studies may reveal this promising molecule as a potential
therapeutic agent against OA.
Acknowledgements
The C28/I2 cell line was provided by Prof. Junling
Cao (Institute of Endemic Diseases, Xi'an Jiaotong University,
Health Science Center).
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81371986,
81772410 and 81301598), the Shaanxi Province Natural Science
Foundation (grant no. S2016YFJM1171) and the Fundamental Research
Funds for the Central Universities (grant no. xjj2015073).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
SH designed and executed the experiments, analyzed
the data and wrote the manuscript. MS and YG helped in the analyses
and interpretation of data. NM, YZ, YY, NH, EO, AS, MS, FZ and YH
helped perform the experiments. JS and SL conceived and designed
the study, critically reviewed and drafted the manuscript and take
responsibility for the integrity of the work as a whole. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal study was approved by the Institutional
Animal Ethics Committee of Xi'an Jiaotong University, Health
Science Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sandell LJ, Sugai JV and Trippel SB:
Expression of collagens I, II, X, and XI and aggrecan mRNAs by
bovine growth plate chondrocytes in situ. J Orthop Res. 12:1–14.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleland KA, James MJ, Neumann MA, Gibson
RA and Cleland LG: Differences in fatty acid composition of
immature and mature articular cartilage in humans and sheep.
Lipids. 30:949–953. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muir H: The chondrocyte, architect of
cartilage Biomechanics, structure, function and molecular biology
of cartilage matrix macromolecules. Bioessays. 17:1039–1048. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinner B, Capito RM and Spector M:
Regeneration of articular cartilage. Adv Biochem Eng Biotechnol.
94:91–123. 2005.PubMed/NCBI
|
|
5
|
Goldring MB, Tsuchimochi K and Ijiri K:
The control of chon-drogenesis. J Cell Biochem. 97:33–44. 2006.
View Article : Google Scholar
|
|
6
|
Aigner T, Zhu Y, Chansky HH, Matsen FA
III, Maloney WJ and Sandell LJ: Reexpression of type IIA
procollagen by adult articular chondrocytes in osteoarthritic
cartilage. Arthritis Rheum. 42:1443–1450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng A and Genever PG: SOX9 determines
RUNX2 trans-activity by directing intracellular degradation. J Bone
Miner Res. 25:2680–2689. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita S, Andoh M, Ueno-Kudoh H, Sato
T, Miyaki S and Asahara H: Sox9 directly promotes Bapx1 gene
expression to repress Runx2 in chondrocytes. Exp Cell Res.
315:2231–2240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Ghori-Javed FY, Rashid H, Adhami
MD, Serra R, Gutierrez SE and Javed A: Runx2 regulates endochondral
ossification through control of chondrocyte proliferation and
differentiation. J Bone Miner Res. 29:2653–2665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ulrich C, Rolauffs B, Abele H, Bonin M,
Nieselt K, Hart ML and Aicher WK: Low osteogenic differentiation
potential of placenta-derived mesenchymal stromal cells correlates
with low expression of the transcription factors Runx2 and Twist2.
Stem Cells Dev. 22:2859–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buckwalter JA and Mankin HJ: Articular
cartilage: Tissue design and chondrocyte-matrix interactions. Instr
Course Lect. 47:477–486. 1998.PubMed/NCBI
|
|
12
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bi W, Deng JM, Zhang Z, Behringer RR and
de Crombrugghe B: Sox9 is required for cartilage formation. Nat
Genet. 22:85–89. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akiyama H, Chaboissier MC, Martin JF,
Schedl A and de Crombrugghe B: The transcription factor Sox9 has
essential roles in successive steps of the chondrocyte
differentiation pathway and is required for expression of Sox5 and
Sox6. Genes Dev. 16:2813–2828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furumatsu T, Tsuda M, Taniguchi N, Tajima
Y and Asahara H: Smad3 induces chondrogenesis through the
activation of SOX9 via CREB-binding protein/p300 recruitment. J
Biol Chem. 280:8343–8350. 2005. View Article : Google Scholar
|
|
16
|
Akiyama H and Lefebvre V: Unraveling the
transcriptional regulatory machinery in chondrogenesis. J Bone
Miner Metab. 29:390–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linn FC and Sokoloff L: Movement and
Composition of interstitial fluid of cartilage. Arthritis Rheum.
8:481–494. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita S, Miyaki S, Kato Y, Yokoyama S,
Sato T, Barrionuevo F, Akiyama H, Scherer G, Takada S and Asahara
H: L-Sox5 and Sox6 proteins enhance chondrogenic miR-140 microRNA
expression by strengthening dimeric Sox9 activity. J Biol Chem.
287:22206–22215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lefebvre V, Behringer RR and de
Crombrugghe B: L-Sox5, Sox6 and Sox9 control essential steps of the
chondrocyte differentiation pathway. Osteoarthritis Cartilage.
9(Suppl A): S69–S75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ng LJ, Wheatley S, Muscat GE,
Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS
and Koopman P: SOX9 binds DNA, activates transcription, and
coexpresses with type II collagen during chondrogenesis in the
mouse. Dev Biol. 183:108–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Q, Eberspaecher H, Lefebvre V and De
Crombrugghe B: Parallel expression of Sox9 and Col2a1 in cells
undergoing chondrogenesis. Dev Dyn. 209:377–386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hattori T, Muller C, Gebhard S, Bauer E,
Pausch F, Schlund B, Bösl MR, Hess A, Surmann-Schmitt C, von der
Mark H, et al: SOX9 is a major negative regulator of cartilage
vascularization, bone marrow formation and endochondral
ossification. Development. 137:901–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Foster JW, Dominguez-Steglich MA, Guioli
S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young
ID, Goodfellow PN, et al: Campomelic dysplasia and autosomal sex
reversal caused by mutations in an SRY-related gene. Nature.
372:525–530. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bracken AP and Helin K: Polycomb group
proteins: Navigators of lineage pathways led astray in cancer. Nat
Rev Cancer. 9:773–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai HC and Baylin SB: Cancer epigenetics:
Linking basic biology to clinical medicine. Cell Res. 21:502–517.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klymenko T, Papp B, Fischle W, Köcher T,
Schelder M, Fritsch C, Wild B, Wilm M and Müller J: A Polycomb
group protein complex with sequence-specific DNA-binding and
selective methyl-lysine-binding activities. Genes Dev.
20:1110–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee K, Na W, Maeng JH, Wu H and Ju BG:
Regulation of DU145 prostate cancer cell growth by Scm-like with
four mbt domains 2. J Biosci. 38:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu S, Trievel RC and Rice JC: Human SFMBT
is a transcriptional repressor protein that selectively binds the
N-terminal tail of histone H3. FEBS Lett. 581:3289–3296. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Zhong B, Sun J, Cao J, Tian J,
Zhong N, Zhao W, Tian L, Xu P, Guo D, et al: Down-regulated HS6ST2
in osteoarthritis and Kashin-Beck disease inhibits cell viability
and influences expression of the genes relevant to aggrecan
metabolism of human chondrocytes. Rheumatology (Oxford).
50:2176–2186. 2011. View Article : Google Scholar
|
|
30
|
Hussain S, Sun M, Min Z, Guo Y, Xu J,
Mushtaq N, Heng L, Huang H, Zhao Y, Yuan Y, et al: Down-regulated
in OA cartilage, SFMBT2 contributes to NF-κB mediated ECM
degradation. J Cell Mol Med. Aug 22–2018. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Wei X, Peng G, Zheng S and Wu X:
Differentiation of umbilical cord mesenchymal stem cells into
steroidogenic cells in comparison to bone marrow mesenchymal stem
cells. Cell Prolif. 45:101–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fox JG, Cohen BJ and Loew FM: Laboratory
Animal Medicine. Academic Press Inc, Harcourt Brace Jovanovich; San
Diego, CA: 1984
|
|
34
|
Miri K, Latham K, Panning B, Zhong Z,
Andersen A and Varmuza S: The imprinted polycomb group gene Sfmbt2
is required for trophoblast maintenance and placenta development.
Development. 140:4480–4489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horton WA: Skeletal development: Insights
from targeting the mouse genome. Lancet. 362:560–569. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lefebvre V, Li P and de Crombrugghe B: A
new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in
chondro-genesis and cooperatively activate the type II collagen
gene. Embo J. 17:5718–5733. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leung KK, Ng LJ, Ho KK, Tam PP and Cheah
KS: Different cis-regulatory DNA elements mediate developmental
stage- and tissue-specific expression of the human COL2A1 gene in
transgenic mice. J Cell Biol. 141:1291–1300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuscik MJ, Hilton MJ, Zhang X, Chen D and
O'Keefe RJ: Regulation of chondrogenesis and chondrocyte
differentiation by stress. J Clin Invest. 118:429–438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lefebvre V and Smits P: Transcriptional
control of chondrocyte fate and differentiation. Birth Defects Res
C Embryo Today. 75:200–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Atsumi T, Miwa Y, Kimata K and Ikawa Y: A
chondrogenic cell line derived from a differentiating culture of
AT805 teratocarcinoma cells. Cell Differ Dev. 30:109–116. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shukunami C, Shigeno C, Atsumi T, Ishizeki
K, Suzuki F and Hiraki Y: Chondrogenic differentiation of clonal
mouse embryonic cell line ATDC5 in vitro: Differentiation-dependent
gene expression of parathyroid hormone (PTH)/PTH-related peptide
receptor. J Cell Biol. 133:457–468. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shukunami C, Ohta Y, Sakuda M and Hiraki
Y: Sequential progression of the differentiation program by bone
morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp Cell
Res. 241:1–11. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henry SP, Liang S, Akdemir KC and de
Crombrugghe B: The postnatal role of Sox9 in cartilage. J Bone
Miner Res. 27:2511–2525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Goldring MB: Chondrogenesis, chondrocyte
differentiation, and articular cartilage metabolism in health and
osteoarthritis. Ther Adv Musculoskelet Dis. 4:269–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salminen H, Vuorio E and Saamanen AM:
Expression of Sox9 and type IIA procollagen during attempted repair
of articular cartilage damage in a transgenic mouse model of
osteoarthritis. Arthritis Rheum. 44:947–955. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Binette F, McQuaid DP, Haudenschild DR,
Yaeger PC, McPherson JM and Tubo R: Expression of a stable
articular cartilage phenotype without evidence of hypertrophy by
adult human articular chondrocytes in vitro. J Orthop Res.
16:207–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hoyland JA, Thomas JT, Donn R, Marriott A,
Ayad S, Boot-Handford RP, Grant ME and Freemont AJ: Distribution of
type X collagen mRNA in normal and osteoarthritic human cartilage.
Bone Miner. 15:151–163. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goldring MB, Otero M, Tsuchimochi K, Ijiri
K and Li Y: Defining the roles of inflammatory and anabolic
cytokines in cartilage metabolism. Ann Rheum Dis. 67(Suppl 3):
iii75–iii82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goldring MB and Marcu KB: Cartilage
homeostasis in health and rheumatic diseases. Arthritis Res Ther.
11:2242009. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar
|
|
51
|
Pelletier JP and Martel-Pelletier J:
Evidence for the involvement of interleukin 1 in human
osteoarthritic cartilage degradation: Protective effect of NSAID. J
Rheumatol. 18:19–27. 1989.
|
|
52
|
Farahat MN, Yanni G, Poston R and Panayi
GS: Cytokine expression in synovial membranes of patients with
rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 52:870–875.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Muñoz-Valle JF, Oregón-Romero E,
Rangel-Villalobos H, Martínez-Bonilla GE, Castañeda-Saucedo E,
Salgado-Goytia L, Leyva-Vázquez MA, Illades-Aguiar B,
Alarcón-Romero Ldel C, Espinoza-Rojo M and Parra-Rojas I: High
expression of TNF alpha is associated with -308 and -238 TNF alpha
polymorphisms in knee osteoarthritis. Clin Exp Med. 14:61–67. 2014.
View Article : Google Scholar
|
|
55
|
Kim KI, Park YS and Im GI: Changes in the
epigenetic status of the SOX-9 promoter in human osteoarthritic
cartilage. J Bone Miner Res. 28:1050–1060. 2013. View Article : Google Scholar
|
|
56
|
Sitcheran R, Cogswell PC and Baldwin AS
Jr: NF-kappaB mediates inhibition of mesenchymal cell
differentiation through a posttranscriptional gene silencing
mechanism. Genes Dev. 17:2368–2373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schroeppel JP, Crist JD, Anderson HC and
Wang J: Molecular regulation of articular chondrocyte function and
its significance in osteoarthritis. Histol Histopathol. 26:377–394.
2011.PubMed/NCBI
|
|
58
|
Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim
BJ, Min BH and Chun JS: Hypoxia-inducible factor-2alpha is a
catabolic regulator of osteoarthritic cartilage destruction. Nat
Med. 16:687–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mueller MB and Tuan RS: Anabolic/Catabolic
balance in pathogenesis of osteoarthritis: Identifying molecular
targets. PM R. 3(Suppl 1): S3–S11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Madry H, Luyten FP and Facchini A:
Biological aspects of early osteoarthritis. Knee Surg Sports
Traumatol Arthrosc. 20:407–422. 2012. View Article : Google Scholar
|
|
61
|
Wang M, Shen J, Jin H, Im HJ, Sandy J and
Chen D: Recent progress in understanding molecular mechanisms of
cartilage degeneration during osteoarthritis. Ann N Y Acad Sci.
1240:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Matyas JR, Adams ME, Huang D and Sandell
LJ: Discoordinate gene expression of aggrecan and type II collagen
in experimental osteoarthritis. Arthritis Rheum. 38:420–425. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cs-Szabó G, Melching LI, Roughley PJ and
Glant TT: Changes in messenger RNA and protein levels of
proteoglycans and link protein in human osteoarthritic cartilage
samples. Arthritis Rheum. 40:1037–1045. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lefebvre V and de Crombrugghe B: Toward
understanding SOX9 function in chondrocyte differentiation. Matrix
Biol. 16:529–540. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lefebvre V, Huang W, Harley VR, Goodfellow
PN and de Crombrugghe B: SOX9 is a potent activator of the
chondrocyte-specific enhancer of the pro alpha1(II) collagen gene.
Mol Cell Biol. 17:2336–2346. 1997. View Article : Google Scholar : PubMed/NCBI
|