Introduction
Diabetes mellitus (DM) is a serious illness
associated with an increased risk of cardiovascular complications,
including dyslipidemia, coronary artery disease, hypertension and
myocardial infarction (1,2). In addition to cardiovascular injury,
two other affected vital organs are the kidney [diabetic
nephropathy (DN)] and eyes (3),
with DN being one of the most common complications for patients
with diabetic (4,5). The pathogenesis of DN involves
mesangial expansion, basement membrane thickening, glomerular
hypertrophy and renal fibrosis (6-8),
among which progressive renal fibrosis is the important
pathological characteristic of DN (9). The epithelial-mesenchymal transition
(EMT) of renal tubular epithelial cells is one of the underlying
mechanisms of renal fibrosis and encompasses a range of events
whereby epithelial cells no longer exhibit certain epithelial
traits, such as E-cadherin expression, and instead acquire typical
characteristics of mesenchymal cells, such as α-smooth muscle actin
(α-SMA) expression (10). A
number of studies have reported that the process is connected to
the production of interstitial myofibro-blasts during nephropathy
(11-13). Constant downregulation of
E-cadherin is reported to occur via transcriptional repression,
mediated by transcription factors including snail, slug, twist and
zinc finger E-box-binding homeobox 1 (ZEB-1) (14-16), which control E-cadherin
transcription by cross interaction with the E-box binding sites in
the E-cadherin promoter (17,18). However, the cellular molecular
mechanisms underlying EMT are not completely understood (19). Therefore, it is vital to clarify
the pathogenic mechanisms of EMT to formulate appropriate
interventions.
Mammalian target of rapamycin (mTOR) is a
serine/threonine protein kinase that regulates a series of
growth-associated cellular processes. mTOR includes two complexes
termed mTOR complex 1 (mTORC1) and complex 2 (mTORC2) (20). However, only mTORC1 is sensitive
to rapamycin inhibition, and controls cell proliferation and growth
via the phosphorylation of certain downstream targets, such as
ribosomal protein S6 kinase β-1 (p70S6K) (20). Aberrant activation of the
mTORC1/p70S6K pathway has been reported to be involved in the
pathogenesis of DN (21).
Furthermore, mTORC1/p70S6K signaling may mediate renal tubular EMT
during DN (19).
Astragaloside IV (AST) is a small molecular saponin
that is one of the main active ingredients extracted from
Astragalus membranaceus (22,23). Growing evidence has confirmed that
AST has a wide spectrum of pharmacological effects (24-28). However, to the best of our
knowledge, the effect of AST on the EMT of renal tubular cells
during DN has not yet been reported. Therefore, the present study
was performed to investigate whether AST has an effect on EMT in
renal tubular cells and to clarify the potential mechanisms
involved.
Materials and methods
Cell culture and intervention
HK-2, the human proximal tubular epithelial cell
line, was obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 5.56 mmol/l D-glucose [normal
glucose (NG)]. For induction of EMT, the HK-2 cells (at ~60%
confluence) were cultivated with high glucose (HG) medium including
60 mmol/l D-glucose for 72 h (19). Mannitol medium including 5.56
mmol/l glucose and 54.44 mmol/l mannitol, was used as an osmotic
control (MA). AST, purchased from Shanghai YuanYe Biotechnology
Co., Ltd. (Shanghai, China), was added when the cell culture medium
was changed from NG to HG medium at a concentration of 50
µg/ml [AST low (ASTL)], 100 µg/ml [AST medium (ASTM)]
or 200 µg/ml [AST high (ASTH)]. For the rapamycin
administration group (Rap), rapamycin (Cell Signaling Technology,
Inc., Danvers, MA, USA) was added at a concentration of 20 nmol/l
(19).
Western blot analysis
Protein was isolated from HK-2 cells using lysis
buffer including 1% NP-40, 1 mmol/l Na3VO4, 1
mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l EDTA, 20 mmol/l NaF,
50 mmol/l Tris (pH 7.6), and 150 mmol/l NaCl. The concentration of
proteins was measured with a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China) following the
manufacturer’s protocol. For immunoblotting, equivalent quantities
of protein (80 µg) from the different groups were separated
by SDS-PAGE on 8% gels and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA), which
were blocked in TBS-Tween containing 3% bovine serum albumin (BSA)
at room temperature for 1 h, and then incubated with primary
antibodies at 4°C overnight. The primary antibodies against mTOR
(cat. no. 2983T; 1:1,000), phospho-mTOR (Ser2448; cat. no. 5536T;
1:1,000), p70S6K (cat. no. 2708T; 1:1,000), phospho-p70S6K (Thr389;
cat. no. 9234T; 1:1,000), snail (cat. no. 3879T; 1:1,000), slug
(cat. no. 9585T; 1:1,000), twist (cat. no. 46702S; 1:1,000), ZEB-1
(cat. no. A1500; 1:1,000) and E-cadherin (cat. no. 3195T; 1:1,000)
were purchased from Cell Signaling Technology, Inc., and against
α-SMA (cat. no. ab32575; 1:1,000) and GAPDH (cat. no. ab181602;
1:1,000) from Abcam (Cambridge, UK). Secondary antibody (cat. no.
C40721-02; 1:1,000) was obtained from LI-COR Biosciences, (Lincoln,
NE, USA) and incubation was at 4°C overnight. Quantification was
performed by measuring the signal intensity of the protein bands
with ImageJ software v1.46 (National Institutes of Health,
Bethesda, MD, USA).
Immunofluorescence
HK-2 cells (3×104/ml) were seeded into
12-well plates containing glass coverslips. Following treatment,
they were washed three times with cold PBS and then fixed with 100%
cold methanol for 20 min at -20°C. Following three more washes with
PBS, the HK-2 cells were blocked in 5% BSA at room temperature for
1 h, and then labeled with E-cadherin (1:200; cat. no. 3195T; Cell
Signaling Technology, Inc.) and α-SMA (1:500; cat. no. ab32575;
Abcam) antibodies at room temperature for 2 h. The slips were then
incubated with DyLight 594 donkey anti-rabbit IgG (1:200; cat. no.
E032421-01; EarthOx Life Sciences, Millbrae, CA, USA) for 1 h at
room temperature. Subsequently, the nuclei were counter-stained
with DAPI for 2 min, and then the cells were washed with PBS three
times prior to mounting with fluorescence mounting medium. Images
were captured with an Olympus BX43F fluorescence microscope
(Olympus Corporation, Tokyo, Japan).
ELISA
The protein levels of fibronectin (FN) and collagen
type IV (Col IV) were measured via enzyme-linked immunosorbent
assays (cat. nos. 30357H and 30588H; Shanghai Boyun Biotech Co.,
Ltd., Shanghai, China;) according to the manufacturer’s
instructions.
Statistical analysis
Data are expressed as means ± standard error.
Statistical analyses were performed using one-way analysis of
variance for multiple data comparisons, followed by the
Neuman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
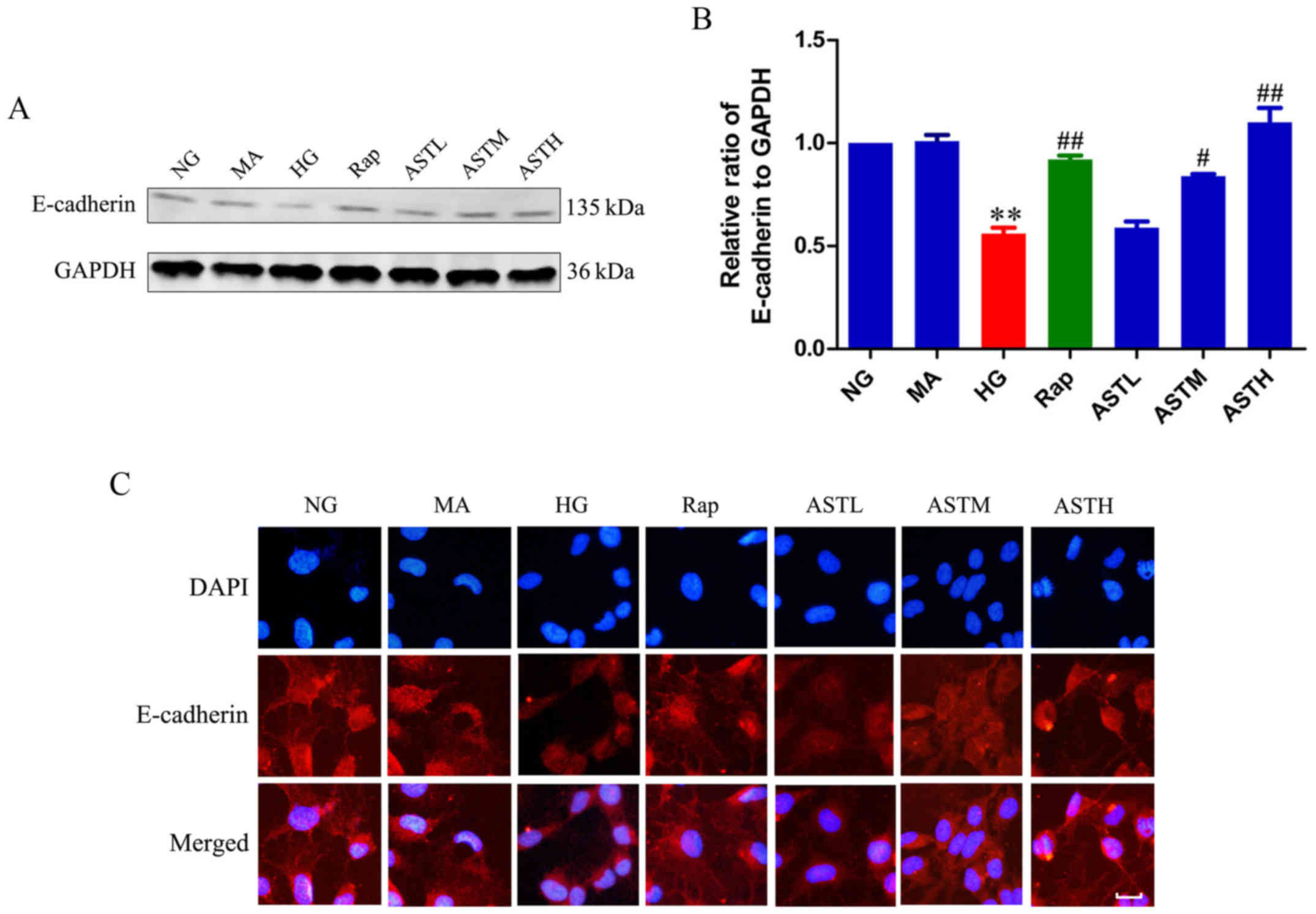
Effects of AST on E-cadherin
expression
As demonstrated in Fig. 1A and B, the HG group exhibited
significantly lower E-cadherin expression compared with the NG
group (P<0.01). Additionally, the expression level of E-cadherin
in the MA group did not differ significantly to that in the NG
group, indicating that there were no obvious effects generated by
the osmotic pressure. Rapamycin administration increased the
expression of E-cadherin compared with HG treatment (P<0.01).
Notably, the ASTM- and ASTH-treated groups also exhibited markedly
higher E-cadherin expression compared with the HG group (P<0.05
and P<0.01, respectively). These results revealed E-cadherin
expression was decreased in HK-2 cells induced by high glucose,
detected by western blotting and immunofluorescence, while ASTM-
and ASTH treatment could increase E-cadherin expression compared
with HG (Fig. 1C).
Effects of AST on α-SMA expression
Expression of α-SMA was upregulated in the HG group
compared with the NG group (P<0.01; Fig. 2A and B). Additionally, there was
no significant difference between the NG and MA groups, indicating
no obvious effects generated by the osmotic pressure. In the
rapamycin group, the mTOR inhibitor downregulated α-SMA expression
compared with HG treatment alone (P<0.01), and administration of
AST could also significantly decrease the expression of α-SMA
compared with HG alone (P<0.01). The results were validated by
immunofluorescence (Fig. 2C).
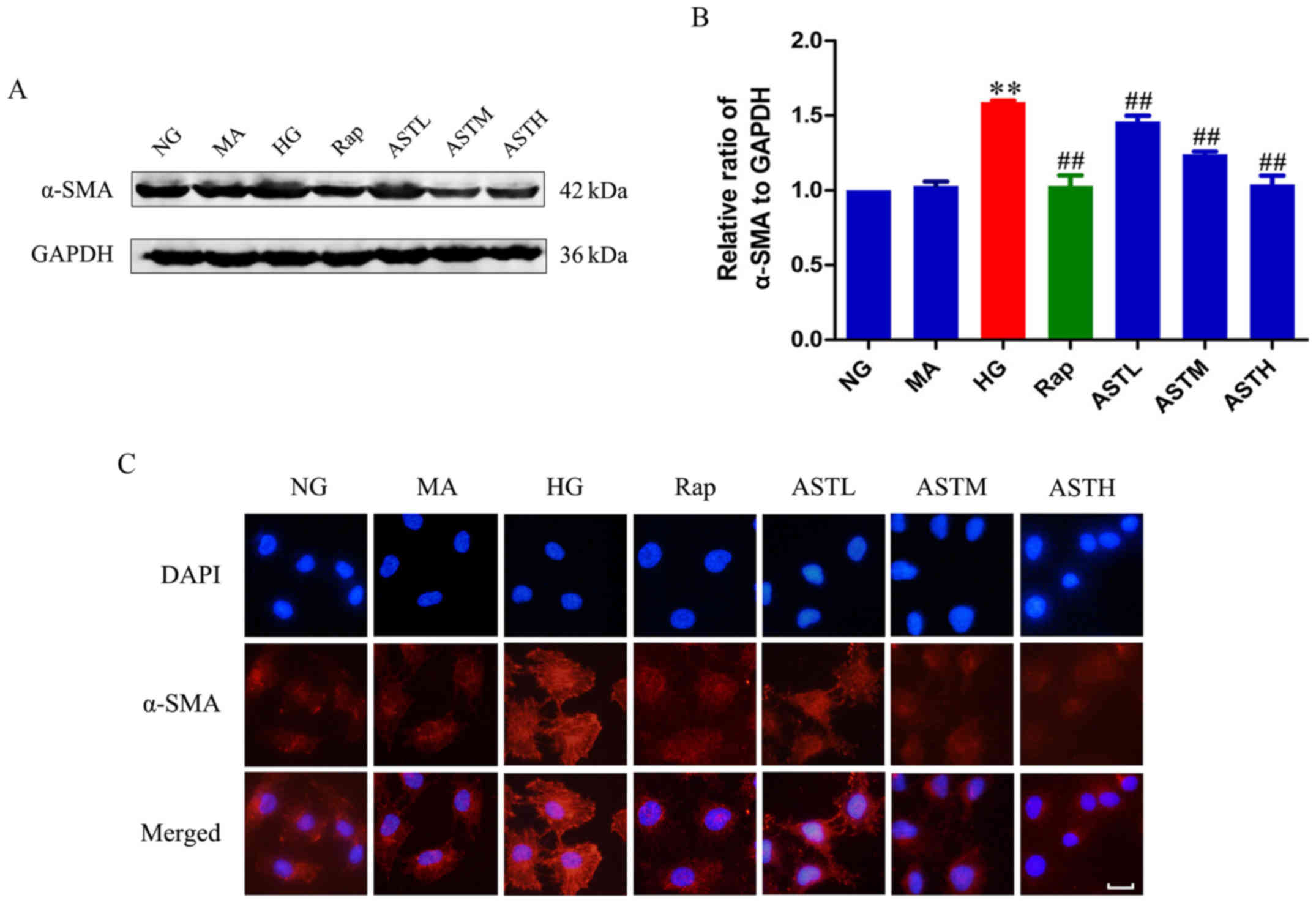 | Figure 2Effects of AST on α-SMA expression in
HK-2 cells. (A) Western blotting bands of α-SMA. (B) Densitometry
analysis of α-SMA. (C) Expression of α-SMA via immunofluorescence.
Data are expressed as the mean ± standard error. n=3. Scale bar, 20
µm.**P<0.01 vs. NG; ##P<0.01 vs.
HG group. AST, astragaloside IV; NG, normal glucose 5.56 mmol/l;
MA, normal glucose 5.56 mmol/l + mannitol 54.44 mmol/l; HG, high
glucose 60 mmol/l; Rap, high glucose + 20 nmol/l rapamycin; ASTL,
high glucose + 50 µg/ml AST; ASTM, high glucose + 100
µg/ml AST; ASTH, high glucose + 200 µg/ml AST; α-SMA,
α-smooth muscle actin. |
Effects of AST on mTORC1/p70S6K
signaling
As demonstrated in Fig. 3A and B, the HG group exhibited
markedly higher levels of phosphorylated mTOR compared with the NG
group (P<0.01). Additionally, the phosphorylation of p70S6K, a
main downstream target of mTOR, was also significantly increased in
the HG group compared with in the NG group (P<0.01; Fig. 3C and D). These results revealed
that HG activated mTORC1/p70S6K signaling. Furthermore, rapamycin
reversed the abnormal activation induced by HG (P<0.01). There
was no significant difference between the NG and MA groups,
indicating no obvious effects generated by the osmotic pressure
alone. Notably, the AST downregulated the phosphorylation of mTOR
and its main downstream target, p70S6K, indicating that AST
inhibits mTORC1/p70S6K signaling.
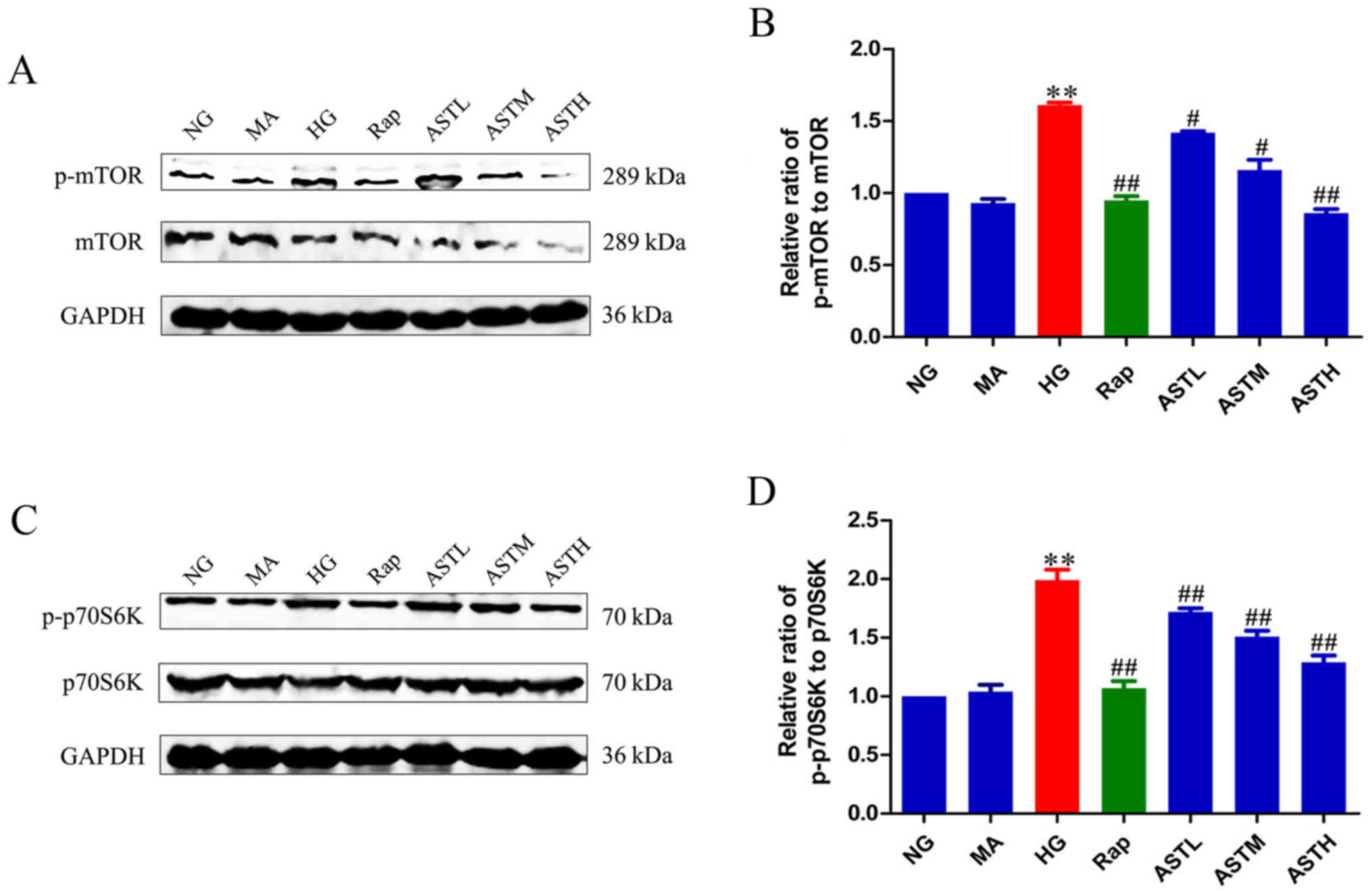 | Figure 3Effects of AST on mTORC1/p70S6K
signaling in HK-2 cells. (A) Western blot analysis of p-mTOR. (B)
Densitometry analysis of p-mTOR. (C) Western blot analysis of
p-p70S6K. (D) Densitometry analysis of p-p70S6K. Data are expressed
as mean ± standard error. n=3. **P<0.01 vs. NG group;
#P<0.05 vs. HG group; ##P<0.01 vs. HG
group. AST, astragaloside IV; NG, normal glucose 5.56 mmol/l; MA,
normal glucose 5.56 mmol/l + mannitol 54.44 mmol/l; HG, high
glucose 60 mmol/l; Rap, high glucose + 20 nmol/l rapamycin; ASTL,
high glucose + 50 µg/ml AST; ASTM, high glucose + 100
µg/ml AST; ASTH, high glucose + 200 µg/ml AST; p-
phospho-; mTOR, mammalian target of rapamycin; p70S6K, ribosomal
protein S6 kinase β-1. |
Effects of AST on transcription factors
(snail, slug, twist and ZEB-1)
E-cadherin is a marker protein of epithelial layers
and its expression is downregulated when EMT occurs (14-16,19). The zinc finger transcription
factors (snail, slug, twist and ZEB-1) suppress E-cadherin
expression (14-16,19). To verify whether these
transcription factors have a role in EMT of HK-2 cells induced by
high glucose, the protein expression of snail, slug, twist and
ZEB-1 was determined. The expression of snail and twist was
significantly enhanced in the HG group compared with in the NG
group (P<0.01), while slug and ZEB-1 expression exhibited no
significant changes (Fig. 4).
Meanwhile, rapamycin treatment could decrease the expression of
snail and twist when compared with HG treatment (P<0.01).
Interestingly, the AST administrations could also down-regulate the
protein levels of snail and twist (P<0.05, P<0.01). In
addition, there was no significant difference between the NG and MA
groups, indicating no obvious effects generated by the osmotic
pressure alone.
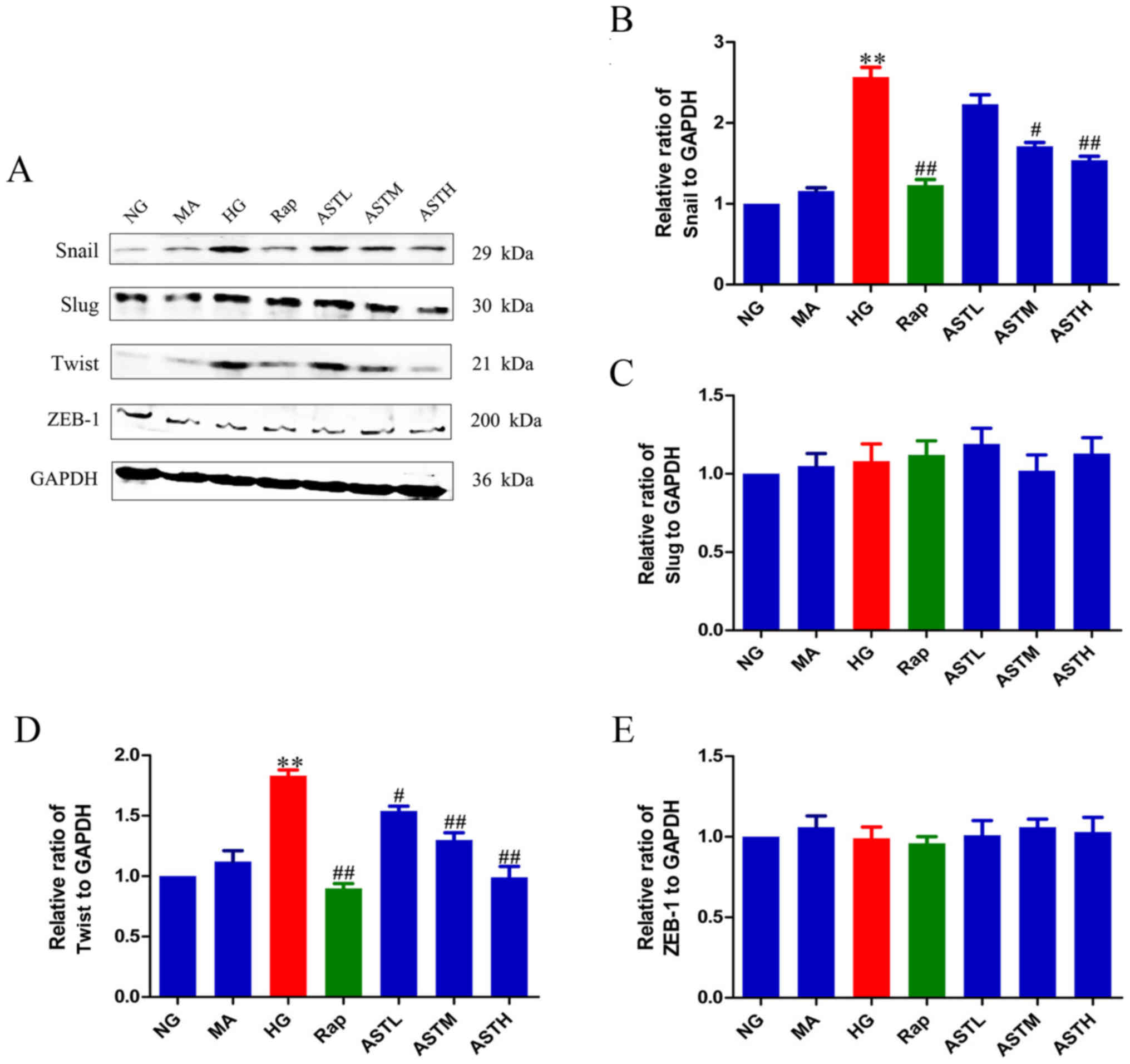 | Figure 4Effects of AST on transcriptional
factors, snail, slug, twist and ZEB-1. (A) Western blot analysis of
snail, slug, twist and ZEB-1. Relative abundance of (B) snail, (C)
slug, (D) twist and (E) ZEB-1. Data are expressed as mean ±
standard error. n=3. **P<0.01 vs. NG group;
#P<0.05 vs. HG group; ##P<0.01 vs. HG
group. AST, astragaloside IV; NG, normal glucose 5.56 mmol/l; MA,
normal glucose 5.56 mmol/l + mannitol 54.44 mmol/l; HG, high
glucose 60 mmol/l; Rap, high glucose + 20 nmol/l rapamycin; ASTL,
high glucose + 50 µg/ml AST; ASTM, high glucose + 100
µg/ml AST; ASTH, high glucose + 200 µg/ml AST; ZEB-1,
zinc finger E-box-binding homeobox 1. |
Effects of AST on FN and Col IV
The major extracellular matrix (ECM) proteins FN and
Col IV are regarded as markers of fibrogenesis and can cause renal
fibrosis when accumulated in DN. As evident in Fig. 5, the protein levels of FN and Col
IV were significantly increased in the HG group compared with in
the NG group (P<0.01); and no significant differences were
observed between the MA and NG groups, indicating no marked effects
generated by the osmotic pressure alone. In turn, treatment with
rapamycin or AST downregulated the elevated FN and Col IV levels
compared with HG treatment (P<0.01).
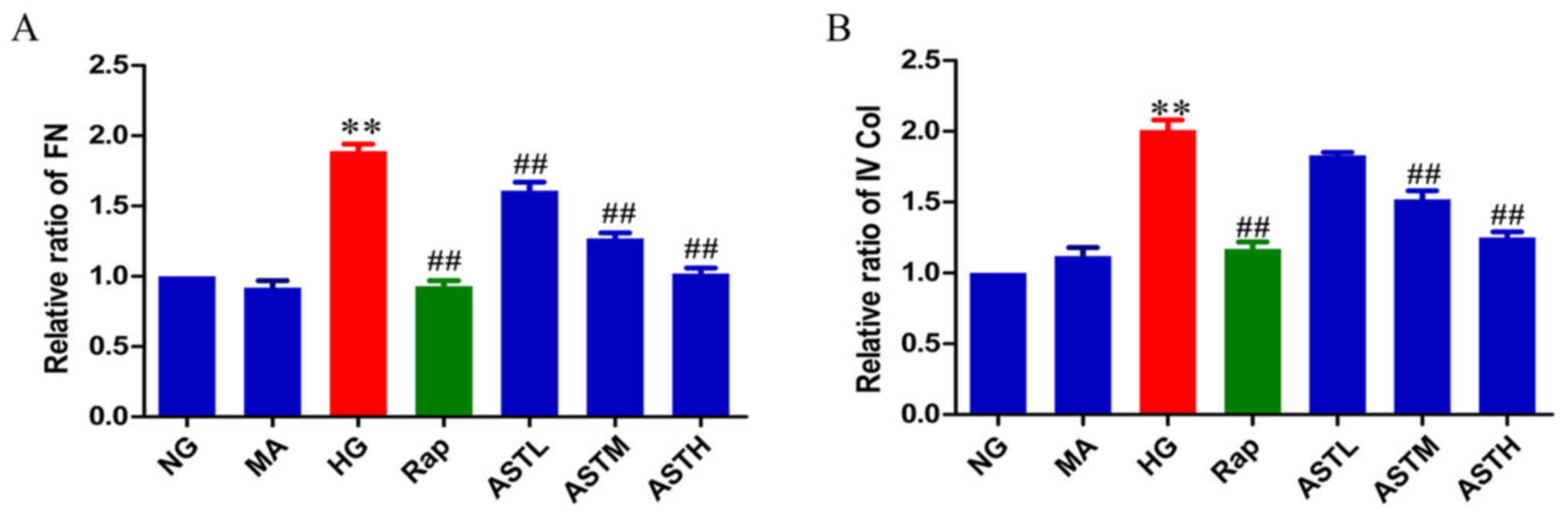 | Figure 5Effects of AST on FN and IV Col. (A)
Relative abundance of FN. (B) Relative abundance of IV Col. Data
are expressed as the mean ± standard error. n=3.
**P<0.01 vs. NG group; ##P<0.01 vs. HG
group. FN, fibronectin; AST, astragaloside IV; NG, normal glucose
5.56 mmol/l; MA, normal glucose 5.56 mmol/l + mannitol 54.44
mmol/l; HG, high glucose 60 mmol/l; Rap, high glucose + 20 nmol/l
rapamycin; ASTL, high glucose + 50 µg/ml AST; ASTM, high
glucose + 100 µg/ml AST; ASTH, high glucose + 200
µg/ml AST; IV Col, collagen type IV. |
Discussion
The incidence of DM has been increasing worldwide
and as a secondary complication, DN is one of the most significant
causes of end-stage renal disease (29,30). In China (31) and the United States (32), ~16 and 26% of patients with DN
develop end-stage renal disease, respectively; thus, effective and
safe treatment strategies to delay the progression of DN are
urgently required in the clinic (21). However, little progress has been
made in the treatment of patients with DN (33). Although certain novel drugs,
including dipeptidyl peptidase 4 inhibitors (34-36), sodium glucose cotransporter 2
inhibitors (37,38) and glucagon-like peptide 1 agonists
(39), can help patients
presenting with early-stage DN by tightly controlling blood glucose
level, it remains largely unknown whether these drugs can
ameliorate EMT of renal tubular cells, which also occurs in DN.
Astragalus membranaceus, a traditional Chinese
herbal medicine, is widely used as a remedy in the treatment of a
variety of clinical diseases, including seasonal allergic rhinitis
(40), ischemic heart disease
(41), leucopenia (42) and DN (43). AST is one of the main active
ingredients of Astragalus membranaceus, the potential
pharmaceutical properties of which may include anti-inflammatory
(25), anti-oxidative injury
(44), anti-cancer (28), anti-hepatitis (27), anti-chronic heart failure
(26) and anti-diabetes effects
(24,45). The current study aimed to
determine whether AST altered EMT in renal tubular cells and to
verify the potential mechanisms involved. To the best of our
knowledge, the current study is the first to explore the effect and
mechanism of AST on the EMT of renal tubular epithelial cells and
whether the mTORC1/p70S6K signaling pathway in involved in this
effect.
EMT is a physiological process required for wound
healing, tissue remodeling and embryogenesis (19); however, EMT is also implicated in
pathological processes of various diseases (13,46). For example, during DN, renal
tubular epithelial cells undergo a trans-differentiation process
and become myofibroblasts, which are the primary source of ECM in
renal cells. Major ECM proteins, including FN and Col IV, are often
regarded as markers of fibrogenesis (47-49), and their accumulation in patients
with DN can lead to renal fibrosis (11,50).
In the present study, the detection of EMT marker
proteins demonstrated that high glucose significantly downregulated
E-cadherin expression and upregulated the generation of α-SMA.
Furthermore, levels of FN and Col IV were increased by high
glucose. In turn, treatment with AST could ameliorate the changes
stimulated by high glucose. Previous studies have indicated that
the mTORC1/p70S6K pathway has an important role in DN, with
blockade of this pathway reported to slow DN development (19,21).
It was previously reported that quercetin
effectively ameliorated the high glucose-induced EMT of HK-2 and
NRK-52E cells and inhibited the activation of mTORC1/p70S6K
(19). In vivo, diabetic
rats exhibited a significant decline in renal function and severe
renal fibrosis at 14 weeks after STZ injection, and mTORC1/p70S6K
was activated in the renal cortex of the diabetic rats. Treatment
with quercetin alleviated the decline in renal function, and the
progression of renal fibrosis and inhibited mTORC1/p70S6K
activation in the diabetic renal cortex (19). A HK-2 cell model was validated in
the previous study, and based on this, HK-2 cells treated with high
glucose was selected as the cell model to verify the effects of AST
on EMT in the present study. The results of the current study
confirmed previous findings, revealing that the phosphorylation of
mTOR and its downstream target, p70S6K, was markedly increased in
HK-2 cells induced by high glucose. Treatment with rapamycin
inhibited the activation of the mTORC1/p70S6K pathway.
Additionally, the association of mTORC1/p70S6K signaling with the
EMT of renal tubular epithelial cells was confirmed. Notably, a
study by Lu et al (19)
indicated that the mTORC1/p70S6K pathway is also involved in the
regulation of transcription factor expression, including snail and
twist. In the present study, inhibiting mTORC1/p70S6K signaling via
rapamycin downregulated the increased protein expression of snail
and twist induced by high glucose. These results indicated that
activation of mTORC1/p70S6K signaling promoted the progression of
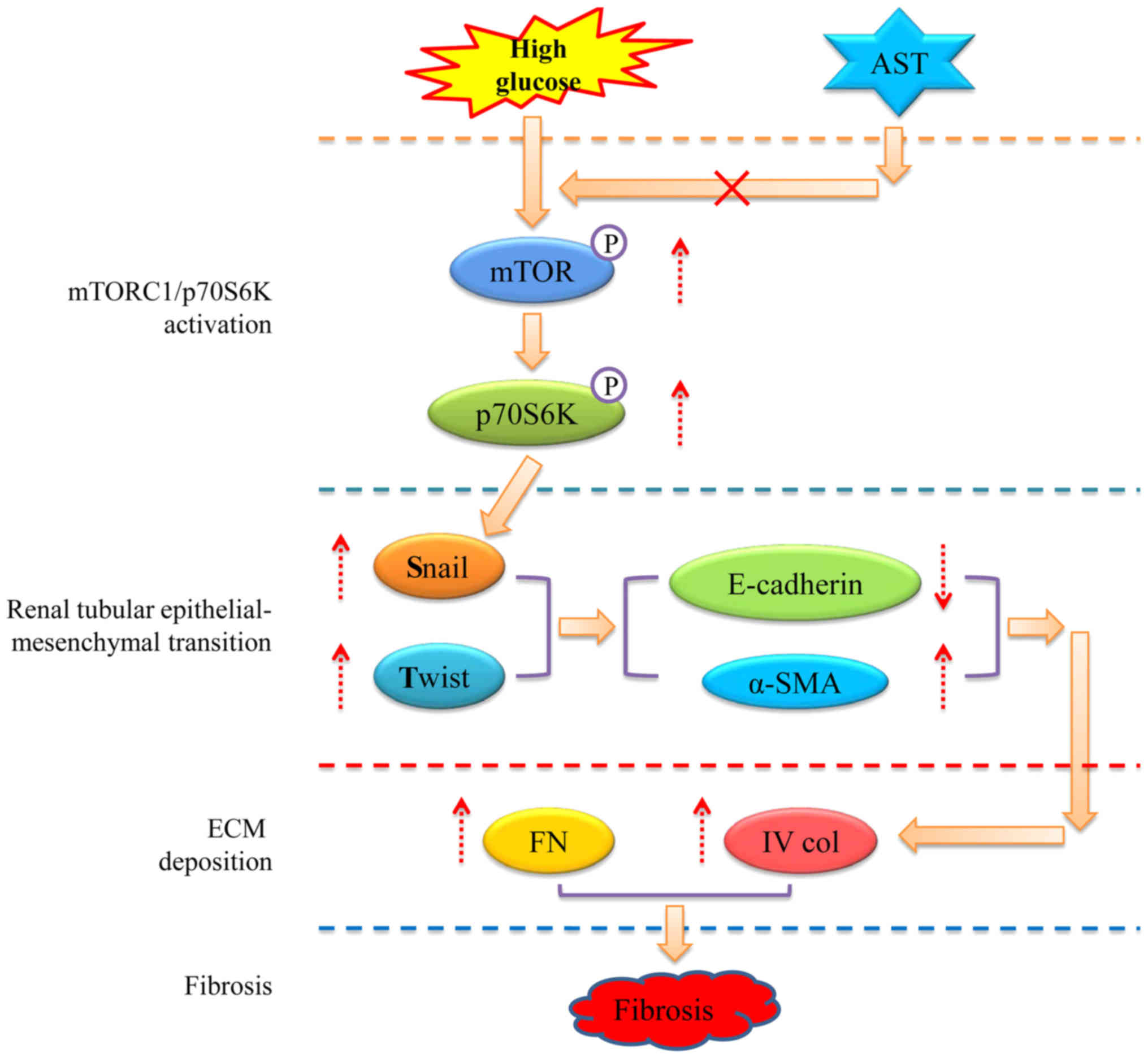
EMT by regulating snail and twist expression. Furthermore, AST
administration inhibited the mTORC1/p70S6K pathway and reduced the
expression of snail and twist; the potential mechanism is
illustrated in Fig. 6. However,
not studying more markers was a limitation of the present study.
The findings of the present study require further validation in the
future.
In conclusion, to the best of our knowledge, the
present study is the first research to determine the effects of AST
on EMT in HK-2 cells via the mTORC1/p70S6K signaling pathway. The
present findings provided confirmation that AST reduces EMT in
renal tubular cells stimulated by high glucose via mTORC1/p70S6K
signaling, and subsequent downregulation of the expression of the
transcription factors snail and twist in HK-2 cells. The
conclusions will be validated using in vivo models in future
studies.
Funding
The present study was supported by the Young Medical
Talents of Wuxi (grant no. QNRC020), Young Project of Wuxi Health
and Family Planning Research (grant no. Q201706), and Wuxi Science
and Technology Development Guidance Plan (Medical and Health Care;
grant no. CSZON1744).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
DW and ZC conceived and designed the study. DW, XC,
YY and CL performed the experiments. DW and XC wrote the paper. YY
and CL critically reviewed and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Dixit P, Ghaskadbi S, Mohan H and
Devasagayam TP: Antioxidant properties of germinated fenugreek
seeds. Phytother Res. 19:977–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts KT: The potential of fenugreek
(Trigonella foenum-graecum) as a functional food and nutraceutical
and its effects on glycemia and lipidemia. J Med Food.
14:1485–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins AJ, Foley RN, Herzog C, Chavers B,
Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, et al:
US Renal Data System 2010 Annual Data Report. Am J Kidney Dis.
57(Suppl 1): A8e1–e526. 2011. View Article : Google Scholar
|
|
5
|
Reutens AT and Atkins RC: Epidemiology of
diabetic nephropathy. Contrib Nephrol. 170:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosius FC, Khoury CC, Buller CL and Chen
S: Abnormalities in signaling pathways in diabetic nephropathy.
Expert Rev Endocrinol Metab. 5:51–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steffes MW, Osterby R, Chavers B and Mauer
SM: Mesangial expansion as a central mechanism for loss of kidney
function in diabetic patients. Diabetes. 38:1077–1081. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ziyadeh FN: The extracellular matrix in
diabetic nephropathy. Am J Kidney Dis. 22:736–744. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
tran-scriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badid C, Desmouliere A, Babici D,
Hadj-Aissa A, McGregor B, Lefrancois N, Touraine JL and Laville M:
Interstitial expression of alpha-SMA: An early marker of chronic
renal allograft dysfunction. Nephrol Dial Transplant. 17:1993–1998.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hills CE and Squires PE: The role of TGF-β
and epithelial-to mesenchymal transition in diabetic nephropathy.
Cytokine Growth Factor Rev. 22:131–139. 2011.PubMed/NCBI
|
|
12
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype. Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
19
|
Lu Q, Ji XJ, Zhou YX, Yao XQ, Liu YQ,
Zhang F and Yin XX: Quercetin inhibits the mTORC1/p70S6K
signaling-mediated renal tubular epithelial-mesenchymal transition
and renal fibrosis in diabetic nephropathy. Pharmacol Res.
99:237–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Hu W, Han WB, Liu YL, Tu Y, Yang HM,
Fang QJ, Zhou MY, Wan ZY, Tang RM, et al: Inhibition of
Akt/mTOR/p70S6K Signaling Activity With Huangkui Capsule Alleviates
the Early Glomerular Pathological Changes in Diabetic Nephropathy.
Front Pharmacol. 9:4432018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei
XY, Gao L and Gao GD: Astragaloside IV protects against focal
cerebral ischemia/reperfusion injury correlating to suppression of
neutrophils adhesion-related molecules. Neurochem Int. 60:458–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du Q, Zhang S, Li A, Mohammad IS, Liu B
and Li Y: Astragaloside IV: Astragaloside IV Inhibits Adipose
Lipolysis and Reduces Hepatic Glucose Production via Akt Dependent
PDE3B Expression in HFD-Fed Mice. Front Physiol. 9:152018.
View Article : Google Scholar
|
|
25
|
Li C, Yang F, Liu F, Li D and Yang T:
NRF2/HO-1 activation via ERK pathway involved in the
anti-neuroinflammatory effect of Astragaloside IV in LPS induced
microglial cells. Neurosci Lett. 666:104–110. 2018. View Article : Google Scholar
|
|
26
|
Tang B, Zhang JG, Tan HY and Wei XQ:
Astragaloside IV inhibits ventricular remodeling and improves fatty
acid utili-zation in rats with chronic heart failure. Biosci Rep.
38:382018. View Article : Google Scholar
|
|
27
|
Wang S, Li J, Huang H, Gao W, Zhuang C, Li
B, Zhou P and Kong D: Anti-hepatitis B virus activities of
astragaloside IV isolated from radix Astragali. Biol Pharm Bull.
32:132–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010–2030. Diabetes Res
Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
31
|
Liu ZH: Nephrology in china. Nat Rev
Nephrol. 9:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Afkarian M, Zelnick LR, Hall YN, Heagerty
PJ, Tuttle K, Weiss NS and de Boer IH: Clinical Manifestations of
Kidney Disease Among US Adults With Diabetes, 1988–2014. JAMA.
316:602–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Boer IH: A new chapter for diabetic
kidney disease. N Engl J Med. 377:885–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Penno G, Garofolo M and Del Prato S:
Dipeptidyl peptidase-4 inhibition in chronic kidney disease and
potential for protection against diabetes-related renal injury.
Nutr Metab Cardiovasc Dis. 26:361–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang D, Zhang G, Chen X, Wei T, Liu C,
Chen C, Gong Y and Wei Q: Sitagliptin ameliorates diabetic
nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol
Med. 41:2784–2792. 2018.PubMed/NCBI
|
|
36
|
Zhang GY, Wang DD, Cao Z, Wei T, Liu CX
and Wei QL: Sitagliptin ameliorates high glucose-induced cell
proliferation and expression of the extracellular matrix in
glomerular mesangial cells. Exp Ther Med. 14:3862–3867. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wanner C, Inzucchi SE, Lachin JM, Fitchett
D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC
and Zinman B: Empagliflozin and progression of kidney disease in
type 2 diabetes. N Engl J Med. 375:323–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al: Empagliflozin, cardiovascular outcomes, and mortality in
type 2 diabetes. N Engl J Med. 373:2117–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muskiet MHA, Tonneijck L, Smits MM, van
Baar MJB, Kramer MHH, Hoorn EJ, Joles JA and van Raalte DH: GLP-1
and the kidney: From physiology to pharmacology and outcomes in
diabetes. Nat Rev Nephrol. 13:605–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matkovic Z, Zivkovic V, Korica M, Plavec
D, Pecanic S and Tudoric N: Efficacy and safety of Astragalus
membranaceus in the treatment of patients with seasonal allergic
rhinitis. Phytother Res. 24:175–181. 2010.
|
|
41
|
Li SQ, Yuan RX and Gao H: Clinical
observation on the treatment of ischemic heart disease with
Astragalus membranaceus. Zhongguo Zhong Xi Yi Jie He Za Zhi.
15:77–80. 1995.In Chinese. PubMed/NCBI
|
|
42
|
Zhang C, Zhu C, Ling Y, Zhou X, Dong C,
Luo J and Liu Y: The clinical value of Huangqi injection in the
treatment of leucopenia: A meta-analysis of clinical controlled
trials. PLoS One. 8:e831232013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li M, Wang W, Xue J, Gu Y and Lin S:
Meta-analysis of the clinical value of Astragalus membranaceus in
diabetic nephropathy. J Ethnopharmacol. 133:412–419. 2011.
View Article : Google Scholar
|
|
44
|
Qiao Y, Fan CL and Tang MK: Astragaloside
IV protects rat retinal capillary endothelial cells against high
glucose-induced oxidative injury. Drug Des Devel Ther.
11:3567–3577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Wang DD, Wei T, He SM, Zhang GY
and Wei QL: Effects of astragalosides from Radix Astragali on high
glucose-induced proliferation and extracellular matrix accumulation
in glomerular mesangial cells. Exp Ther Med. 11:2561–2566. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nieto MA: Epithelial-Mesenchymal
Transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gonzalez J, Klein J, Chauhan SD, Neau E,
Calise D, Nevoit C, Chaaya R, Miravete M, Delage C, Bascands JL, et
al: Delayed treatment with plasminogen activator inhibitor-1 decoys
reduces tubulointerstitial fibrosis. Exp Biol Med (Maywood).
234:1511–1518. 2009. View Article : Google Scholar
|
|
48
|
Wang JY, Yin XX, Wu YM, Tang DQ, Gao YY,
Wan MR, Hou XY and Zhang B: Ginkgo biloba extract suppresses
hypertrophy and extracellular matrix accumulation in rat mesangial
cells. Acta Pharmacol Sin. 27:1222–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Kang YS, Dai C, Kiss LP, Wen X and
Liu Y: Epithelial-to-mesenchymal transition is a potential pathway
leading to podocyte dysfunction and proteinuria. Am J Pathol.
172:299–308. 2008. View Article : Google Scholar : PubMed/NCBI
|