Introduction
Mechanical ventilation may cause ventilator-induced
lung injury (VILI) (1–3) that leads to acute respiratory
distress syndrome (ARDS) (4).
Mortality from ARDS has been reduced by controlling tidal volume
and ventilation pressure (5).
Increasing evidence has shown that specific target proteins have
critical function in VILI; these proteins include α-1-antitrypsin,
ephrin A2, sphingosine-1-phosphate lyase, mothers against
decapentaplegic homolog (Smad)4, p120-catenin, and SN50 (a
cell-permeable inhibitor of nuclear factor-κB) (6-8).
For example, Smad4 expression is significantly increased by
ventilation in C57BL/6 mice, and Smad4 knockdown dramatically
inhibited extracellular matrix remodeling by reducing α-smooth
muscle actin (SMA), collagen I and collagen III expression
(9). Zhao et al (10) reported that the degradation of
p120-catenin causes VILI in C57BL/6 mice, and inhibitors of protein
kinase Cα block c-Src kinase activation and p120-catenin
degradation, thus alleviating VILI. Chian et al (11) demonstrated that SN50 attenuates
inflammation and VILI. However, the pathogenesis of VILI remains
unclear.
Wnt-induced secreted protein 1 (WISP1), also known
as CCN4, is a member of the CCN family (12). Our previous studies demonstrated
that WISP1 contributes to VILI. WISP1 expression is increased in
Evans blue albumin (EBA)-sensitive A/J mice after 4 h of
ventilation, and anti-WISP1 antibodies reduce moderate tidal volume
(MTV)-induced EBA increase in A/J mice (13). Furthermore, WISP1 is reported to
act through toll-like receptor 4 (TLR4) signaling to influence VILI
(13). In addition, it was found
that WISP1 combined with αvβ3 integrin signaling increases the
TLR-induced inflammatory response in sepsis-induced lung injury
(14). Arg-Gly-Asp-Ser peptides
alleviate acute lung injury through WISP1-integrin β6 pathway
inhibition in septic mice (15).
The administration of Poly(I:C) exacerbates MTV-induced lung injury
in a WISP1- and integrin β3-dependent manner, which involves
activation of the extracellular signal-related kinase (ERK)
signaling pathway (16). The Wnt
signaling pathway includes canonical and non-canonical pathways.
The non-canonical Wnt signaling pathway includes the Wnt/planar
cell polarity and the Wnt/Ca2+ pathways,
which are stimulated by the Wnt ligands Wnt5a or Wnt11 and are
transduced through the Frizzled family and receptor tyrosine kinase
like orphan receptor (Ror)1 or Ror2 co-receptor complexes (17). Previous studies have shown that
non-canonical Wnt signaling promotes epithelial cell
differentiation, capillary development and autocrine motility
factor differentiation during lung maturation and in adult lung
fibroblasts, asthma and usual interstitial pneumonia (UIP)
(18-20). For example, Vuga et al
(21) compared the gene
expression profiles of lung fibroblasts from UIP patients to those
of normal lung fibroblasts, and revealed that Wnt5a is
significantly upregulated in UIP fibroblasts. Further analysis
revealed that WNT5a promotes proliferation, increases fibronectin
expression and inhibits H2O2-induced
apoptosis in lung fibroblasts. However, the role of non-canonical
WNT signaling in the pathogenesis of VILI is unclear.
In the present study, GEO2R was used to analyze the
differentially expressed genes (DEGs) in VILI. Gene Ontology (GO)
function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analysis of DEGs was conducted. Next, the interaction of
the identified enrichment pathways was analyzed. The Wnt signaling
pathway was associated with VILI. In addition, the non-canonical
Wnt signaling pathway may serve an important role in VILI.
Therefore, the functional role and mechanisms of the non-canonical
Wnt signaling in the pathogenesis of VILI were investigated.
Sensitive A/J mice were used to establish MTV-induced lung injury
model to test the hypothesis that non-canonical Wnt signaling has
critical function in VILI. The results indicated that modulation of
non-canonical Wnt signaling may provide novel preventive and
therapeutic strategies in VILI in the future.
Materials and methods
Bioinformatics analysis of microarray
data
Three data sets (GSE11434, GSE58169 and GSE7742)
associated with VILI in mice were collected from the GEO database
(www.ncbi.nlm.nih.gov/geo) (22–24).
Identification of differentially
expressed genes (DEGs)
GEO2R (www.ncbi.nlm.nih.gov/geo/info/geo2r.html), an
interactive web tool for comparing two or more groups of samples in
a GEO series, was used to identify DEGs across experimental
conditions. P<0.05 and log fold change >1 were chosen as the
cut-off criteria. A Venn diagram, heatmap, and cluster profiler
analysis were performed with R 3.5.1 software (cran.r-project.org/bin/windows/base/) in
the 'limma' package.
Functional enrichment analysis of
DEGs
KEGG Orthology Based Annotation System (KOBAS) 3.0
(kobas.cbi.pku.edu.cn) is a web server
for gene/protein functional annotation and for identifying the
enrichment of gene functional sets. KEGG pathway analysis was
performed using the KOBAS 3.0 online tool (25,26). P<0.05 was set as the cut-off
criterion.
VILI animal model
The animal study protocol was approved by University
of Pittsburgh institutional animal care and use committee.
Experiments were performed in accordance with the recommendations
of the National Institutes of Health Guidelines for the Use of
Laboratory Animals (27).
Sensitive A/J mice (n=36; 8-9 weeks; 1:1 male:female; 20-25 g) were
anesthetized by intraperitoneal pentobarbital injection (70 mg/kg).
The trachea was exposed, cut and intubated. To analyze the effects
of mechanical ventilation on lung injury, mice (n=6 each) were
randomized to low tidal volume (LTV; 6 ml/kg, 140 breaths/min, 0
positive end-expiratory pressure), MTV (12 ml/kg, 100 breaths/min,
0 positive end-expiratory pressure), and spontaneous breathing
groups for 6 h interventions, as described previously (28). To examine the Wnt pathway's
effects on MTV-induced lung injury, MTV-exposed mice were
randomized and intratracheally administered Y27632 [inhibitor of
Rho-associated protein kinase 1 (ROCK1); 5 mg/kg], SP600125
[inhibitor of C-Jun N-terminal kinase (JNK); 6 mg/kg] prior to MTV,
or were ventilated with MTV alone. Mice were subsequently
sacrificed and one section of the right lung was stored in liquid
nitrogen for western blotting and ELISAs; other lung sections were
fixed in 10% formaldehyde for 24 h at room temperature for
immunohistochemistry (IHC) analysis.
Measurement of alveolar-capillary
permeability
The alveolar-capillary permeability was quantified
by Evan's blue albumin (EBA) staining as previously described
(13). In brief, EBA was prepared
by adding bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to 0.5% EB to a final concentration of 4% (0.6
mM). The solution was thoroughly dissolved by gently stirring with
a magnet bar, and the EBA was then sterilely filtered through a
0.22 µm syringe filter and stored in aliquots at -80°C until
use. Each aliquot was used only once for each animal to prevent
cross-contamination. To evaluate the alveolar capillary barrier
function, EBA (20 mg/kg) was administered via the internal jugular
vein 1 h before sacrifice and tissue harvesting. At the termination
of each experiment, all animals were euthanized, and blood samples
were obtained via the right ventricle for plasma EBA measurement.
The pulmonary vasculature was then flushed with PBS to remove blood
components. The right lung was ligated at the level of the right
mainstem bronchus, excised, and weighed and stored in liquid
nitrogen for subsequent EB analysis. Once thawed, the lung tissue
was homogenized in 2 ml PBS and then incubated with 2 ml formamide
(Sigma-Aldrich; Merck KGaA) at 60°C for 18 h. Formamide extracts
were centrifuged (Beckman TLX; Beckman Coulter, Inc., Brea, CA,
USA) at 15,000×g for 30 min at 4°C, and the centrifuged
supernatants were collected to quantify lung EBA content by
dual-wavelength spectrophotometry (Beckman DU-640; Beckman Coulter)
at 620 and 740 nm (29). EBA
permeability index was calculated by dividing the corrected
pulmonary tissue EBA absorbance at 620 nm (per g of lung tissue) by
the corrected plasma EBA absorbance at 620 nm.
Histological examination
For histological examination, the lung of each
animal was fixed in 4% paraformaldehyde overnight at 4°C. Then, 4
mm thick sections were paraffin embedded and stained with
hematoxylin and eosin [(H&E) cat. no. P032IH; Auragene
Bioscience] at room temperature for 10 min. As described
previously, the histological score of the degree of lung injury was
graded on a scale from 0 to 4 (0, absent and appeared normal; 1,
light; 2, moderate; 3, strong; and 4, intense) for the following
pathological features: Alveolar congestion, neutrophil
infiltration, hemorrhage and thickness of alveolar wall membrane
formation. Five fields of view were assessed (30).
Cytokine expression in the
bronchoalveolar lavage fluid (BALF)
Mouse tumor necrosis factor (TNF)-α (cat. no.
ab100747) and interleukin (IL)-6 (cat. no. ab100713) ELISA kits
were purchased from Abcam (Cambridge, UK) and used to measure the
concentrations of TNF-α and IL-6 in BALF. All reagents were
equilibrated to room temperature and prepared prior to use
according to the manufacturer's protocol. The standards and samples
were added into wells and incubated for 2.5 h at room temperature.
The wells were washed with wash buffer four times and biotinylated
TNF-α and IL-6 were added into the wells and incubated 1 h at room
temperature with gentle shaking. Following four washes, horseradish
peroxidase (HRP)-streptavidin solution was added into the wells and
incubated for 45 min at room temperature. The wash steps were
repeated, and TMB substrate solution was added into the wells and
incubated for 30 min in the dark at room temperature. Then, stop
solution was added into the wells and a microplate reader was used
to determine the optical density of each well at 450 nm.
Total protein concentration in BALF was detected by
using bicinchoninic acid (BCA) protein assay kit (cat. no. P001B,
Auragene Bioscience) according to the manufacturer's
instructions.
Western blot analysis
Frozen lung tissues were thawed and lysed in
radioimmunoprecipitation assay buffer (cat. no. P002A; Auragene
Bioscience, Changsha, China) with protease inhibitors (cat. no.
P019A; Auragene Bioscience) and phenylmethylsulfonyl fluoride (cat.
no. P018A; Auragene Bioscience). Protein concentration was measured
with a standard BCA protein assay. Proteins were separated by 10%
SDS-PAGE and transferred to nitrocellulose membranes. Membranes
were blocked in Tris-buffered saline with 0.5% Tween-20 (TBST) and
3% BSA for 1 h at room temperature and incubated overnight with
WISP1 (cat. no. 18166-1-AP; 1:1,000; ProteinTech Group, Inc.,
Chicago, IL, USA), Ras homolog gene family, member A (RhoA; cat.
no. ab54835; 1:1,000; Abcam), phosphorylated (p-)RhoA (cat. no.
ab41435; 1:1,000; Abcam), JNK (cat. no. sc-7345; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), p-JNK (cat. no. sc-6254;
1:500; Santa Cruz Biotechnology, Inc.) or β-actin (cat. no. ab8226,
1:5,000; Abcam) antibodies. The membranes were washed with TBST
five times and incubated with HRP-conjugated goat anti-mouse (cat.
no. SA001, 1:15,000) and rabbit (cat. no. SA009; 1:15,000) IgG
secondary antibodies (Auragene Bioscience) for 40 min at room
temperature. Proteins were detected by chemiluminescence (cat. no.
P020WB; Auragene Bioscience) and quantified using ImageJ version
6.0 software (National Institutes of Health, Bethesda, MA,
USA).
Immunohistochemistry
For histological examination, a lung from each
animal was fixed in 4% paraformaldehyde overnight at 4°C. Then, the
samples were embedded in paraffin and cut into 3-µm-thick
slices. Tissue slides were heated for 2 h at 65°C and subsequently
incubated in xylene for 30 min at room temperature and in graded
alcohol (100, 95, 85 and 75% alcohol for 5 min each). The slides
were blocked in 3% H2O2 for 15 min at room
temperature to inhibit endogenous peroxidase activity and placed in
1 M sodium citrate buffer (cat. no. P019IH; Auragene Bioscience)
for antigen retrieval. The slides were incubated with the primary
antibodies against WISP1 (cat. no. 18166-1-AP; 1:100; ProteinTech
Group, Inc.), or p-Jnk1/2/3 (cat. no. YP0157; 1:200; ImmunoWay
Biotechnology Company, Plano, TX, USA) overnight at 4°C. Then, the
slides were rinsed with PBS five times and incubated with
HRP-conjugated secondary antibodies (cat. no. SA009; 1:1,000;
Auragene Bioscience) for 30 min. The slides were stained with DAB
(cat. no. P013IH; Auragene Bioscience) solution for 1 min,
counter-stained with hematoxylin (cat. no. C0107; Beyotime
Institute of Biotechnology, Haimen, China) for 15 min at room
temperature and dehydrated in a graded alcohol (85 and 95% for 5
min each) at room temperature prior to coverslipping.
Statistical analysis
Data are presented as the mean ± standard error of
the mean, and were analyzed by one-way analysis of variance
followed by Tukey's post-hoc test. Statistical analyses were
performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of differentially
expressed genes
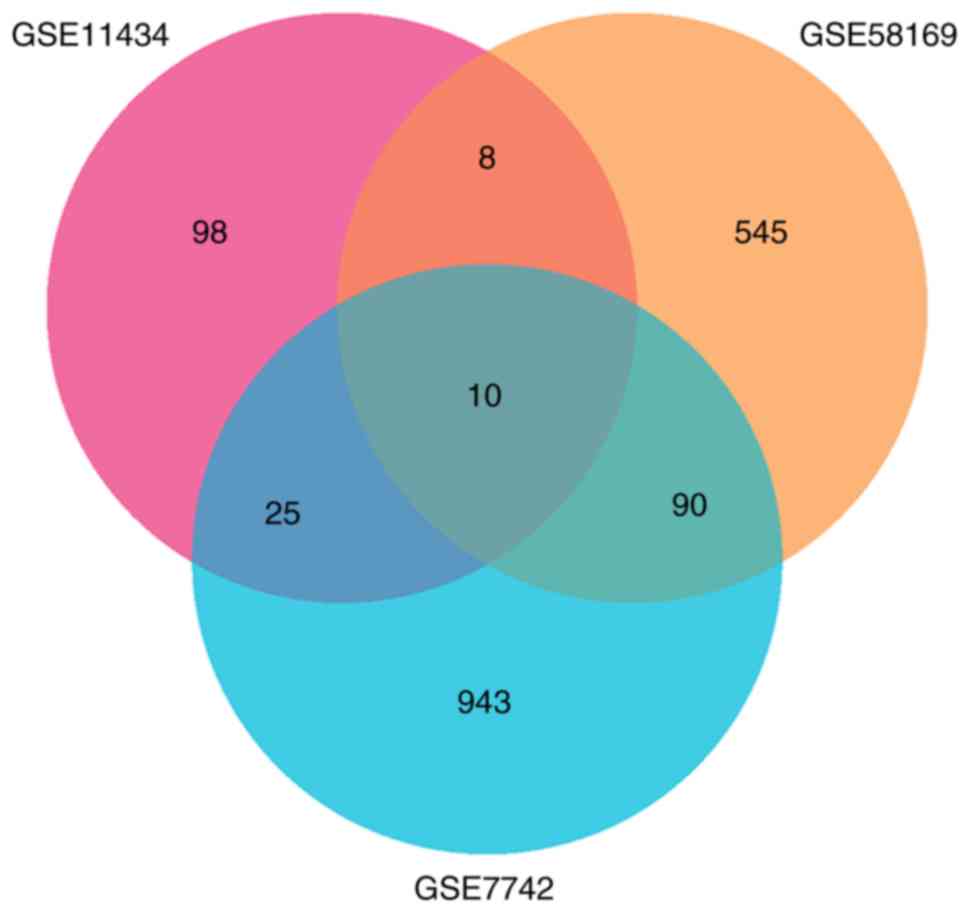
A total of 157, 757 and 1,086 DEGs were identified
from GSE11434, GSE58169, and GSE7742, respectively. There were 10
common DEGs from the three datasets that were associated with lung
injury (Fig. 1), including IL-1β,
IL-1 receptor 2, stratifin, C-type lectin domain family 4 member D,
amphiregulin, suppressor of cytokine signaling 3, S100 calcium
binding protein A1, matrix metalloproteinase 8, solute carrier
family 7 member 11 and early growth response 1.
Functional enrichment analysis of
DEGs
KOBAS 3.0 was used to analyze the pathway enrichment
of identified DEGs. KEGG analysis revealed that the upregulated
DEGs were predominantly enriched in 'HTLV-1 infection', 'Wnt
signaling pathway' and 'MAPK signaling pathway', as well as
pathways associated with VILI (31,32) (Fig.
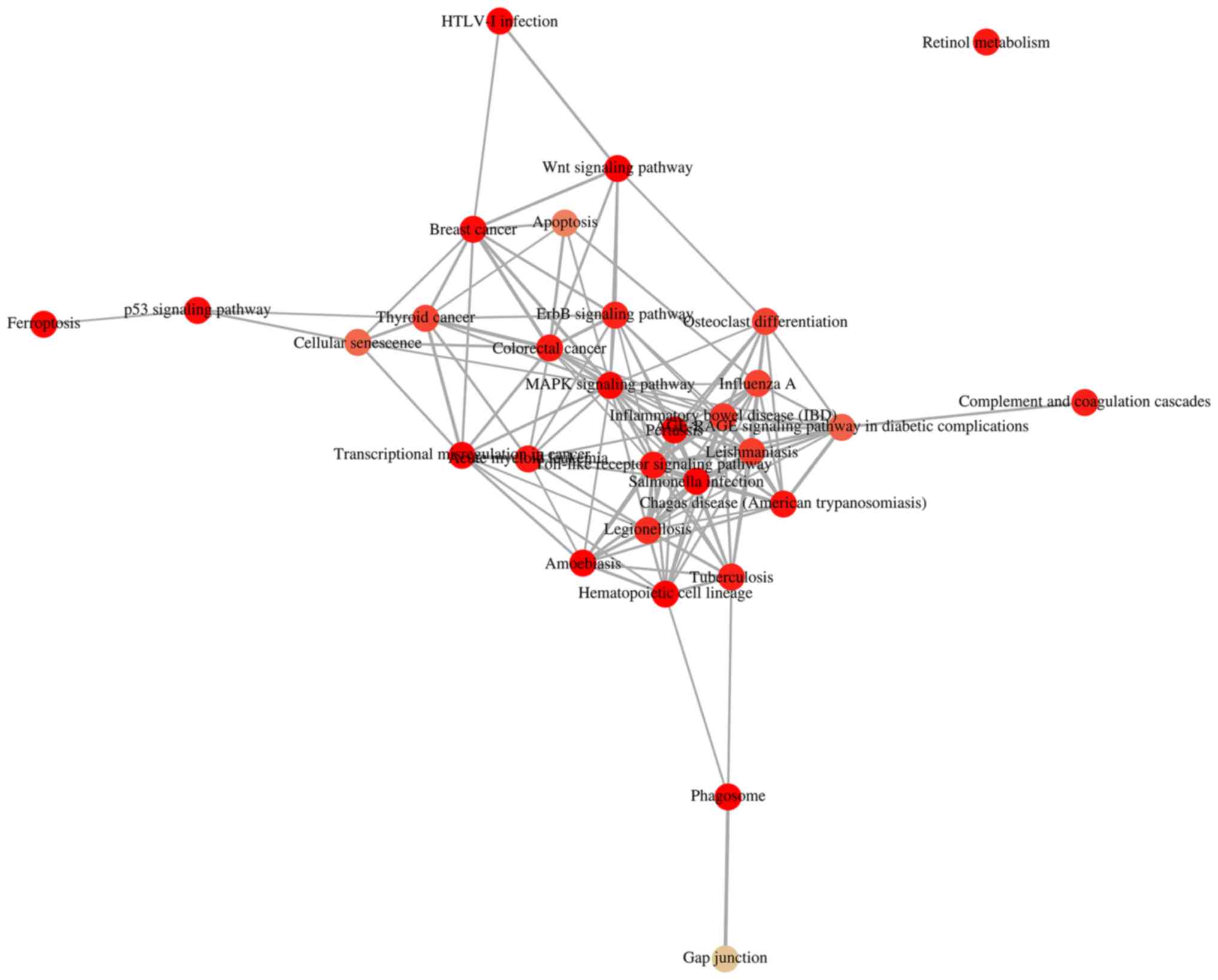
2A and B). It was demonstrated that 'HTLV-1 infection' and
'MAPK signaling pathway' were associated with 'Wnt signaling
pathway' (Fig. 3). It has been
reported that Wnt/β-catenin signaling pathway is activated in early
VILI, and inhibition of the Wnt/β-catenin signaling pathway
suppresses VILI (31,33) Thus, the Wnt pathway was selected
for further study. Heatmap analysis revealed that Wnt1 and GSK3β
were significantly increased in VILI, compared with the control
groups (Fig. 4A). In addition,
the non-canonical signaling pathway factors Wnt5a, Wnt11 and ROCK2
were activated in VILI (Fig. 4B).
The KEGG pathway enrichment demonstrated a relationship between the
identified DEGs and VILI. These analyses support the reliability of
the results in the present study.
The non-canonical Wnt signaling pathway
is activated in VILI
To investigate the role of non-canonical Wnt
signaling in VILI, animal experiments in sensitive A/J mice. To
assess VILI, alveolar-capillary permeability was measured, H&E
staining was performed, and the expression of TNF-α and IL-6, as
well as total protein, was measured. Permeability was increased in
the lungs of A/J mice ventilated with LTV and MTV compared with the
spontaneous breathing group (Fig.
5A). Histological examination illustrated that ventilation with
LTV and MTV significantly increased inflammatory cell infiltration,
pulmonary edema and alveolar septal thickening, findings which were
confirmed by histological scoring (Fig. 5B and C). Similarly, increased
TNF-α (Fig. 5D), IL-6 (Fig. 5E), and total protein (Fig. 5F) expression was detected in the
LTV and MTV groups. Collectively, these results demonstrated that
mechanical ventilation with LTV or MTV induced lung injury in A/J
mice. To investigate the role of non-canonical Wnt signaling in
VILI, the protein levels of important non-canonical Wnt signaling
pathway components were examined via western blotting and IHC. RhoA
and JNK are important proteins in non-canonical Wnt signaling
(34). Mechanical ventilation did
not alter the expression of RhoA or JNK. However, the expressions
of p-RhoA, ROCK1 and p-JNK were elevated in the LTV and MTV groups,
compared with the spontaneous breathing group (Fig. 6A and B). Furthermore, IHC revealed
that p-JNK was dramatically increased in the LTV and MTV groups,
compared with the spontaneous breathing group (Fig. 6C). Taken together, these data
indicated that non-canonical Wnt signaling pathway participated in
the pathogenesis of VILI in A/J mice.
 | Figure 5Lung injury following mechanical
ventilation with LTV or MTV in A/J sensitive mice. (A) EBA
permeability increased in mice ventilated with LTV (6 ml/kg) and
MTV (12 ml/kg) for 6 h (n=4-6). (B) Ventilation with LTV and MTV
significantly increased inflammatory cell infiltration, pulmonary
edema, alveolar septal thickening and (C) the histological score
(n=4-6). (D) TNF-α and (E) IL-6 expression, as well as (F) total
protein concentration in BALF was increased in mice ventilated with
LTV and MTV (n=4–6). *P<0.05, **P<0.01,
***P<0.001 vs. control. LTV, low tidal volume; MTV,
moderate tidal volume; BALF, bronchoalveolar lavage fluid; EBA,
Evan's blue albumin; TNF, tumor necrosis factor; IL-6, interleukin
6. |
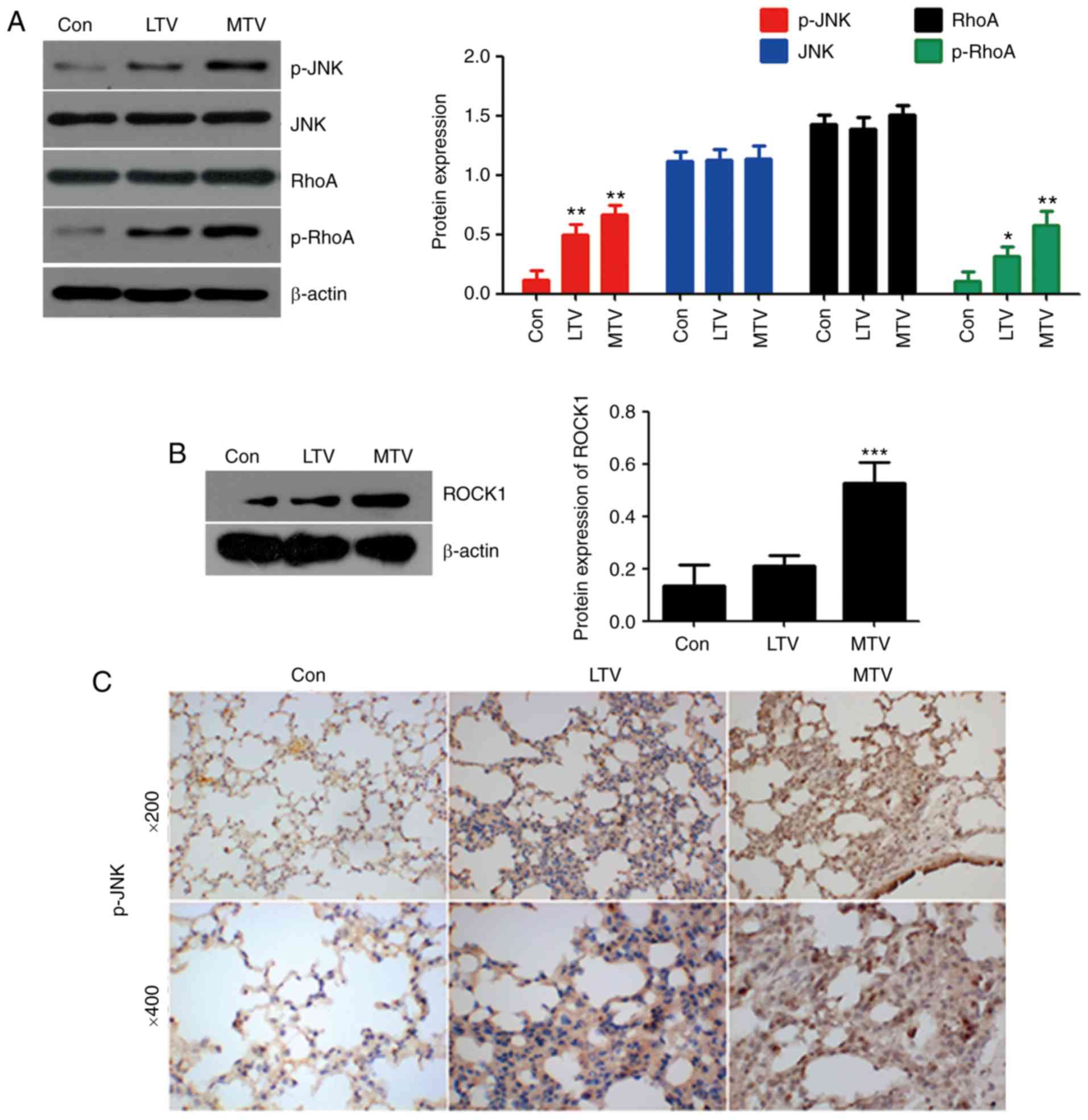 | Figure 6MTV ventilation increased
non-canonical Wnt signaling protein expression in the lungs of A/J
sensitive mice. (A) p-RhoA, p-JNK and (B) ROCK1 protein expression
was measured by western blotting. β-actin was the loading control
(n=4-6). *P<0.05, **P<0.01,
***P<0.001 vs. control. (C) Immunohistochemical
staining for p-JNK in the lungs following mechanically ventilating
with LTV and MTV for 6 h in A/J mice. LTV, low tidal volume; MTV,
moderate tidal volume; p-, phosphorylated; JNK, c-Jun N terminal
kinase; ROCK1, Rho-associated protein kinase 1; RhoA, Ras homolog
gene family, member A. |
Inhibition of non-canonical Wnt signaling
attenuated MTV-induced lung injury
To further investigate the functional role of
non-canonical Wnt signaling in the pathogenesis of VILI, inhibitors
of target proteins in the pathway were used. Sensitive A/J mice
were given Y27632 (ROCK1 inhibitor) or SP600125 (JNK inhibitor)
intratracheally before undergoing ventilation with MTV for 6 h.
Y27632 and SP600125 significantly decreased MTV-induced EBA
permeability (Fig. 7A).
Histological analysis and scoring illustrated that Y27632 and
SP600125 attenuated MTV-induced inflammatory cell infiltration,
pulmonary edema and alveolar septal thickening (Fig. 7B and C). Total protein
concentration, as well as TNF-α and IL-6 expression in BALF were
notably decreased following treatment with Y27632 or SP600125
(Fig. 7D and E). In addition, the
protein expression of JNK, p-JNK, RhoA, p-RhoA, and ROCK1 was
measured by western blotting and IHC. Western blot analysis showed
that inhibition of ROCK1 (Y27632) and JNK (SP600125) dramatically
decreased p-JNK, p-RhoA, and ROCK1 levels compared to the MTV group
(Fig. 7F and G). IHC confirmed
that p-JNK (Fig. 7H) expression
was attenuated in VILI following administration of Y27632 or
SP600125. Collectively, these data indicated that inhibition of
non-canonical and canonical Wnt signaling pathways may effectively
protect lung tissues against VILI in A/J mice.
 | Figure 7Inhibition of non-canonical Wnt
signaling attenuates MTV-induced lung injury. (A) EBA permeability
was decreased in A/J mice administered Y27632 or SP600125 prior to
MTV ventilation compared to mice ventilated with MTV alone (n=3).
(B) Administration of Y27632 or SP600125 reduced MTV-induced
inflammatory cell infiltration, pulmonary edema, alveolar septal
thickening and histological scores (n=4-6). (C) TNF-α and IL-6
expression, as well as (D) total protein concentration decreased in
BALF in A/J mice following the administration of Y27632 or SP600125
prior to MTV ventilation (n=4-6). (E) Protein quantification in
Y27632 or SP600125 treatment reduced the ventilation-induced
increase in p-RhoA, p-JNK and ROCK1. *P<0.05,
**P<0.01, ***P<0.001 vs. control. (F)
Western blotting for p-RhoA, p-JNK and ROCK1 protein expression in
A/J mice after administering Y27632 or SP600125 prior to MTV
ventilation. (G) Immunohistochemical staining for p-JNK expression
was decreased in A/J mice following the administration of Y27632 or
SP600125 prior to MTV ventilation. BALF, bronchoalveolar lavage
fluid; EBA, Evan's blue albumin; MTV, moderate tidal volume; p-,
phosphorylated; JNK, c-Jun N terminal kinase; ROCK1, Rho-associated
protein kinase 1; RhoA, Ras homolog gene family, member A; TNF,
tumor necrosis factor; IL-6, interleukin 6; Y27632, ROCK1
inhibitor; SP600125, JNK inhibitor. |
Non-canonical Wnt signaling regulates
WISP1 expression in VILI
Western blotting was performed to analyze the
regulation of WISP1 by the non-canonical Wnt pathway. The results
revealed that WISP1 expression was increased in mice ventilated
with LTV and MTV, compared with the control group (Fig. 8A). The inhibition of ROCK1 and JNK
markedly decreased MTV-induced WISP1 expression (Fig. 8B and C). These results revealed
that non-canonical Wnt signaling promoted VILI by upregulating
WISP1 expression in A/J mice.
Discussion
The present study demonstrated that non-canonical
WNT pathway proteins were overexpressed in VILI mice. To the best
of our knowledge, this is the first report to examine the
non-canonical WNT pathway in an experimental model of VILI in A/J
mice. The main findings were as follows: i) p-RhoA and JNK
expression was increased in lungs following mechanical ventilation
with LTV or MTV; ii) inhibition of the non-canonical WNT pathway by
inhibiting ROCK1 and JNK markedly decreased MTV-induced lung injury
as assessed by EBA permeability, histological scoring and
proinflammatory cytokine levels; and iii) non-canonical Wnt
signaling promoted VILI through the upregulation of WISP1
expression. These data suggested that non-canonical Wnt signaling
served significant roles in the regulation of WISP1 and the
pathogenesis of VILI in A/J mice.
Acute lung injury (ALI) has been investigated
(35,36), but the molecular mechanisms
involved in injury induced by mechanical ventilation are not well
understood. In the present study, it was found that the upregulated
DEGs in VILI were predominantly enriched in HTLV-1 infection, Wnt
signaling and MAPK signaling pathways. The correlation of these
signaling pathways was analyzed; HTLV-1 infection and the MAPK
signaling pathway were associated with Wnt signaling. It has been
previously reported that canonical WNT signaling is activated in
ALI induced by lipopolysaccharide by driving the Th17 response in
mice (37). The knockdown of
TGF-β1 and FGF-2 via the inhibition of Wnt/β-catenin signaling may
serve as a potential therapeutic strategy for bleomycin-induced
pulmonary fibrosis (38).
Additionally, Wnt signaling has critical involvement in VILI
(31). For example, MTV
exacerbates Poly(I:C)-induced lung injury in a WISP1- and integrin
β3-dependent manner, involving, at least partially, activation of
the ERK pathway (16).
Furthermore, activation of the Wnt/β-catenin signaling pathway by
mechanical ventilation is associated with ventilator-induced
pulmonary fibrosis in healthy lungs (31). miR-127 acts downstream of TLR4 to
promote proinflammatory M1 macrophage development in vitro
and in vivo, which is achieved, at least partially, through
a JNK-dependent mechanism (39).
The current in vitro study revealed that VILI
activated non-canonical Wnt signaling in sensitive A/J mice.
Initially, three GEO datasets were selected and DEGs were
identified. KEGG analysis determined that the Wnt signaling pathway
was a common node affecting other pathways. Wnt5a and Wnt11, which
are important non-canonical Wnt ligands, are activated in VILI
(40-42). Increasing evidence has shown that
the non-canonical Wnt pathway functions in cell injury. For
example, primary cilia regulate non-canonical Wnt signaling
responses in the injured kidney (43). Nakamura et al (44) reported that secreted
Frizzled-related protein 5 (SFRP5), an inhibitor of non-canonical
Wnt signaling, protects cardiac cells following
ischemia/reperfusion injury (44). Non-canonical Wnt signaling is also
associated with fibrosis (45,46). The cardiac microenvironment uses
non-canonical Wnt signaling to activate monocytes following
myocardial infarction (45).
Frizzled-7 mediates TGF-β-induced pulmonary fibrosis by mediating
non-canonical Wnt signaling (46). In the present study, it was
demonstrated that VILI induced by MTV increased p-RhoA and p-JNK
expression in A/J mice. Treatment with Y27632 and SP600125,
inhibitors of ROCK and JNK, respectively, attenuated MTV-induced
lung injury by decreasing WISP1 protein expression.
This is the first demonstration that the
non-canonical Wnt signaling pathway induced WISP1 expression and
contributed to MTV-induced lung injury in A/J mice. However, the
present study had several limitations. First, the data does not
fully confirm that the non-canonical Wnt signaling pathway was
involved in disrupting lung repair or in the early development of
fibrosis in VILI. Second, WNT5A-deficient animals were not used to
irrefutably demonstrate that this pathway contributed to the
pathogenesis of VILI. Third, the mechanism by which the
non-canonical Wnt signaling pathway regulated WISP1 remains unclear
and requires further study.
In conclusion, the present study confirmed that
mechanical ventilation induced lung injury, which was accompanied
by increasing WISP1 expression. Non-canonical Wnt signaling was
involved in mechanical VILI, and suppression of this signaling
significantly alleviated VILI. WISP1 expression was regulated by
the non-canonical Wnt signaling pathway in A/J mice. The modulation
of both WISP1 and Wnt signaling may provide novel therapeutic
strategies in VILI.
Funding
This study was supported by grants from the National
Institute of Health (grant nos. R01-GM-50441 and R01-GM-108639; to
TB and LMZ) and the Natural Science Foundation of Hunan Province of
China (grant no. 2017JJ2177; to YFX).
Availability of data and materials
Three data sets (GSE11434, GSE58169 and GSE7742)
related to ventilator-induced lung injury in mice were collected
from the GEO database (www.ncbi.nlm.nih.gov/geo).
Authors' contributions
YFX and LMZ designed the experiments and study. JC,
JFY and WO performed the experiments. BP and TB wrote the
manuscript, analyzed and interpreted the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were in accordance with the ethical standards of the
Institution Review of Hunan Cancer Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
Abbreviations:
|
WISP1
|
Wnt-induced secreted protein 1
|
|
VILI
|
ventilator-induced lung injury
|
|
MTV
|
moderate tidal volume
|
|
LTV
|
low tidal volume
|
|
p-JNK
|
phosphorylated c-Jun N-terminal
kinase
|
|
ROCK1
|
Rho-associated protein kinase 1
|
Acknowledgments
We thank Ms. Christine Heiner, Scientific Writer for
the Departments of Anesthesiology and Surgery at the University of
Pittsburgh, for her assistance with scientific editing that greatly
improved the manuscript. We would also like to show our gratitude
to Ms. Karla Woosloose, laboratory manager, for assisting with
experiments.
References
|
1
|
Hashemian SM, Mohajerani SA and Jamaati
HR: Ventilator-induced lung injury. N Engl J Med. 370:979–980.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahaman U: Mathematics of
ventilator-induced lung injury. Indian J Crit Care Med. 21:521–524.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beitler JR, Malhotra A and Thompson BT:
Ventilator-induced lung injury. Clin Chest Med. 37:633–646. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plötz FB: Ventilator-induced lung injury.
Intensive Care Med. 27:4522001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amato MB, Barbas CS, Medeiros DM, Magaldi
RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D,
Munoz C, Oliveira R, et al: Effect of a protective-ventilation
strategy on mortality in the acute respiratory distress syndrome. N
Engl J Med. 338:347–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu H, He J, Liu J, Zhang X, Yang F, Liu P
and Wang S: Alpha 1-antitrypsin ameliorates ventilator-induced lung
injury in rats by inhibiting inflammatory responses and apoptosis.
Exp Biol Med (Maywood). 243:87–95. 2018. View Article : Google Scholar
|
|
7
|
Park BH, Shin MH, Douglas IS, Chung KS,
Song JH, Kim SY, Kim EY, Jung JY, Kang YA, Chang J, et al:
Erythropoietin-producing hepatoma receptor tyrosine kinase A2
modulation associates with protective effect of prone position in
ventilator-induced lung injury. Am J Respir Cell Mol Biol.
58:519–529. 2018. View Article : Google Scholar
|
|
8
|
Suryadevara V, Fu P, Ebenezer DL,
Berdyshev E, Bronova IA, Huang LS, Harijith A and Natarajan V:
Sphingolipids in ventilator induced lung injury: Role of
sphingosine-1-phosphate. lyase Int J Mol Sci. 19:E1142018.
View Article : Google Scholar
|
|
9
|
Huang X, Zhou W and Ding S: Downregulated
Smad4 affects extracellular matrix remodeling in ventilator-induced
lung injury. Ann Clin Lab Sci. 46:451–456. 2016.PubMed/NCBI
|
|
10
|
Zhao T, Zhao H, Li G, Zheng S, Liu M, Gu C
and Wang Y: Role of the PKCα-c-Src tyrosine kinase pathway in the
mediation of p120-catenin degradation in ventilator-induced lung
injury. Respirology. 21:1404–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chian CF, Chiang CH, Chuang CH, Liu SL and
Tsai CL: SN50, a cell-permeable-inhibitor of nuclear factor-κB,
attenuates ventilator-induced lung injury in an isolated and
perfused rat lung model. Shock. 46:194–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li HH, Li Q, Liu P, Liu Y, Li J,
Wasserloos K, Chao W, You M, Oury TD, Chhinder S, et al:
WNT1-inducible signaling pathway protein 1 contributes to
ventilator-induced lung injury. Am J Respir Cell Mol Biol.
47:528–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, Ding X, Jin S, Pitt B, Zhang L,
Billiar T and Li Q: WISP1-αvβ3 integrin signaling positively
regulates TLR-triggered inflammation response in sepsis induced
lung injury. Sci Rep. 6:288412016. View Article : Google Scholar
|
|
15
|
Ding X, Wang X, Zhao X, Jin S, Tong Y, Ren
H, Chen Z and Li Q: RGD peptides protects against acute lung injury
in septic mice through Wisp1-integrin β6 pathway inhibition. Shock.
43:352–360. 2015. View Article : Google Scholar
|
|
16
|
Jin S, Chen Z, Ding X, Zhao X, Jiang X,
Tong Y, Billiar TR and Li Q: Mechanical ventilation augments
poly(I:C)induced lung injury via a WISP1-integrin β3 dependent
pathway in mice. Mol Med. 22:54–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borcherding N, Kusner D, Kolb R, Xie Q, Li
W, Yuan F, Velez G, Askeland R, Weigel RJ and Zhang W: Paracrine
WNT5A signaling inhibits expansion of tumor-initiating cells.
Cancer Res. 75:1972–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishitani T, Kishida S, Hyodo-Miura J, Ueno
N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J and
Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade
functions in the Wnt-5a/Ca(2+) pathway to antagonize
Wnt/beta-catenin signaling. Mol Cell Biol. 23:131–139. 2003.
View Article : Google Scholar :
|
|
19
|
Li C, Xiao J, Hormi K, Borok Z and Minoo
P: Wnt5a participates in distal lung morphogenesis. Dev Biol.
248:68–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Hu L, Xiao J, Chen H, Li JT,
Bellusci S, Delanghe S and Minoo P: Wnt5a regulates Shh and Fgf10
signaling during lung development. Dev Biol. 287:86–97. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vuga LJ, Ben-Yehudah A,
Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C and
Kaminski N: WNT5A is a regulator of fibroblast proliferation and
resistance to apoptosis. Am J Respir Cell Mol Biol. 41:583–589.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spassov S, Pfeifer D, Strosing K, Ryter S,
Hummel M, Faller S and Hoetzel A: Genetic targets of hydrogen
sulfide in ventilator-induced lung injury-a microarray study. PLoS
One. 9:e1024012014. View Article : Google Scholar
|
|
23
|
Dolinay T, Wu W, Kaminski N, Ifedigbo E,
Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A and Choi AM:
Mitogen-activated protein kinases regulate susceptibility to
ventilator-induced lung injury. PLoS One. 3:e16012008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wray C, Mao Y, Pan J, Chandrasena A,
Piasta F and Frank JA: Claudin-4 augments alveolar epithelial
barrier function and is induced in acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 297:L219–L227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39(Web Server issue): W316–W322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar :
|
|
27
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Chen C, Zhang Z, Zou Y, Peng M and
Wang Y: Toll-like receptor 4 knockout ameliorates neuroinflammation
due to lung-brain interaction in mechanically ventilated mice.
Brain Behav Immun. 56:42–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Su X, Yan X, Wasserloos K, Chao W,
Kaynar AM, Liu ZQ, Leikauf GD, Pitt BR and Zhang LM: Toll-like
receptor 4-myeloid differentiation factor 88 signaling contributes
to ventilator-induced lung injury in mice. Anesthesiology.
113:619–629. 2010.PubMed/NCBI
|
|
30
|
Achouiti A, van der Meer AJ, Florquin S,
Yang H, Tracey KJ, van't Veer C, de Vos AF and van der Poll T:
High-mobility group box 1 and the receptor for advanced glycation
end products contribute to lung injury during Staphylococcus aureus
pneumonia. Crit Care. 17:R2962013. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villar J, Cabrera NE, Valladares F, Casula
M, Flores C, Blanch L, Quilez ME, Santana-Rodríguez N, Kacmarek RM
and Slutsky AS: Activation of the Wnt/β-catenin signaling pathway
by mechanical ventilation is associated with ventilator-induced
pulmonary fibrosis in healthy lungs. PLoS One. 6:e239142011.
View Article : Google Scholar
|
|
32
|
Tao G, Pan L, Jing R, Lin F, Dai H and Ge
W: Study on Rac1/MAPK/ERK pathway mediated mechanism and role in
rats with ventilator induced lung injury. Zhonghua Wei Zhong Bing
Ji Jiu Yi Xue. 29:249–254. 2017.In Chinese. PubMed/NCBI
|
|
33
|
Villar J, Cabrera NE, Casula M, Valladares
F, Flores C, López-Aguilar J, Blanch L, Zhang H, Kacmarek RM and
Slutsky AS: WNT/β-catenin signaling is modulated by mechanical
ventilation in an experimental model of acute lung injury.
Intensive Care Med. 37:1201–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao J, Zhou H, Wu N and Wu L: The
non-canonical Wnt pathway negatively regulates dendritic cell
differentiation by inhibiting the expansion of Flt3+
lymphocyte-primed multipotent precursors. Cell Mol Immunol.
13:593–604. 2015. View Article : Google Scholar
|
|
35
|
Ouyang W, Zhou H, Liu C, Wang S, Han Y,
Xia J and Xu F: 25-Hydroxycholesterol protects against acute lung
injury via targeting MD-2. J Cell Mol Med. 22:5494–5503. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu F, Diao R, Liu J, Kang Y, Wang X and
Shi L: Curcumin attenuates staphylococcus aureus-induced acute lung
injury. Clin Respir J. 9:87–97. 2015. View Article : Google Scholar
|
|
37
|
Cheng L, Zhao Y, Qi D, Li W and Wang D:
Wnt/β-catenin pathway promotes acute lung injury induced by LPS
through driving the Th17 response in mice. Biochem Biophys Res
Commun. 495:1890–1895. 2018. View Article : Google Scholar
|
|
38
|
Chen X, Shi C, Meng X, Zhang K, Li X, Wang
C, Xiang Z, Hu K and Han X: Inhibition of Wnt/β-catenin signaling
suppresses bleomycin-induced pulmonary fibrosis by attenuating the
expression of TGF-β1 and FGF-2. Exp Mol Pathol. 101:22–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar
|
|
40
|
Kim J, Chang W, Jung Y, Song K and Lee I:
Wnt5a activates THP-1 monocytic cells via a β-catenin-independent
pathway involving JNK and NF-κB activation. Cytokine. 60:242–248.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koval A, Purvanov V, Egger-Adam D and
Katanaev VL: Yellow submarine of the Wnt/Frizzled signaling:
Submerging from the G protein harbor to the targets. Biochem
Pharmacol. 82:1311–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh JC, Kodjabachian L, Rebbert ML,
Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB and Nathans J:
A new secreted protein that binds to Wnt proteins and inhibits
their activities. Nature. 398:431–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saito S, Tampe B, Müller GA and Zeisberg
M: Primary cilia modulate balance of canonical and non-canonical
Wnt signaling responses in the injured kidney. Fibrogenesis Tissue
Repair. 8:62015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakamura K, Sano S, Fuster JJ, Kikuchi R,
Shimizu I, Ohshima K, Katanasaka Y, Ouchi N and Walsh K: Secreted
Frizzled-related protein 5 diminishes cardiac inflammation and
protects the heart from ischemia/reperfusion injury. J Biol Chem.
291:2566–2575. 2016. View Article : Google Scholar :
|
|
45
|
Meyer IS, Jungmann A, Dieterich C, Zhang
M, Lasitschka F, Werkmeister S, Haas J, Müller OJ, Boutros M,
Nahrendorf M, et al: The cardiac microenvironment uses
non-canonical WNT signaling to activate monocytes after myocardial
infarction. EMBO Mol Med. 9:1279–1293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guan S and Zhou J: Frizzled-7 mediates
TGF-β-induced pulmonary fibrosis by transmitting non-canonical Wnt
signaling. Exp Cell Res. 359:226–234. 2017. View Article : Google Scholar : PubMed/NCBI
|