Introduction
Rheumatoid arthritis (RA) is among the most common
autoimmune disorders and is characterized by painful, swollen
joints, the formation of an invasive pannus, chronic articular
inflammation, and degradation of cartilage and bone (1-3).
Fibroblast-like synoviocytes (FLS) are the most abundant cell type
composing the intimal lining of the synovium. In normal conditions,
the synovial membrane facilitates smooth articulation of the joint,
while in RA, FLS take on an invasive pathological phenotype. RA-FLS
secrete proinflammatory cytokines and degradative enzymes that
promote chronic inflammation of the joint, as well as cartilage and
bone destruction (4). It is
well-established that secretion of interleukins (ILs) including
IL-6 and IL-8 by FLS contributes to inflammation and progression of
RA (5-7). Monocyte chemoattractant protein
(MCP)-1 is an important chemokine that recruits macrophages to
infiltrate the synovium (8).
Matrix metalloproteinases (MMPs) are essential to cartilage
remodeling, but increased expression of MMPs, including MMP-1,
MMP-3 and MMP-13, is observed in RA. Overexpression of these
enzymes drives excessive cartilage degradation and destruction of
the joint by cleaving type II collagen and aggrecan (9,10).
Together, these processes create the ideal conditions for the
formation of an invasive pannus. In normal physiology, the
deleterious effects of cytokines, chemokines and degradative
enzymes are tightly regulated by complementary anti-inflammatory
cytokines, such as IL-4. Previous studies have demonstrated that
IL-4 is lacking in the RA synovium and treatment with IL-4/IL-10
exerts significant protective effects against cartilage destruction
(11,12). Expression of transforming growth
factor (TGF)-1β has also been demonstrated to exert a protective
effect in RA by inhibiting expression of proinflammatory cytokines
(12,13).
Tumor necrosis factor (TNF)-α is another major
cytokine involved in RA. TNF-α is upregulated in the synovium and
synovial fluid of patients with RA and is recognized as serving a
pivotal role in the pathogenesis of RA by inducing oxidative
stress, production of proinflammatory cytokines and chemokines, and
expression of degradative enzymes by FLS, thereby promoting pannus
invasion and sustained inflammation (14-16). Anti-TNF-α and anti-IL-6 therapies
have become a cornerstone of RA treatment. However, not all
patients respond to these strategies (17). Recently, treatment methods that
modulate multiple cytokines by targeting signaling pathways through
specific receptors have been receiving attention as a novel
treatment approach for RA (17).
Bexarotene is a specific retinoid X receptor (RXR) subfamily
agonist that was approved by the Food and Drug Administration (FDA)
for the treatment of T-cell lymphoma in 1999. Bexarotene has been
noted to have a low occurrence of retinoid side effects (18,19). RXRs regulate cell apoptosis and
exert antiangiogenic effects, thereby serving as a novel treatment
target for various types of cancers (19). In the present study, the effects
of bexarotene treatment were investigated in primary human FLS
exposed to TNF-α. The results demonstrated that specific agonism of
RXR by bexarotene significantly suppressed TNF-α-induced release of
IL-6, IL-8 and MCP-1. In addition, bexarotene treatment resulted in
a downregulation of expression of MMPs, increased levels of the
anti-inflammatory cytokines IL-4 and TGF-β1, and attenuation of
oxidative stress. Notably, the present study indicated that these
effects of RXR agonism were mediated through the p38
mitogen-activated protein kinase (MAPK)/nuclear factor (NF)-κB
signaling pathway.
Materials and methods
Cell culture and treatment
Human subject studies were designed in accordance
with the World Medical Association Declaration of Helsinki Ethical
Principles for Medical Research Involving Human Subjects.
Experimental procedures were approved by the Institutional Ethics
Committee at the First Affiliated Hospital of Henan University of
Science and Technology. Written informed consent was obtained from
all of the participants. Primary human FLS were isolated as
previously described (20). The
tissues were collected and cut into small pieces in a cell culture
hood before being digested with 0.2% collagenase (Sigma-Aldrich;
Merck KGaA) overnight in a 37°C and 5% CO2 humidified
incubator. The isolated cells were then seeded into 10 cm Eppendorf
cell culture dishes in DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (penicillin 0.1 mg/ml, gentamycin
0.05 mg/ml, and amphotericin B 50 ng/ml). The medium was changed
every 3 days, passaged twice, and 2x105 cells were seeded into each
well of a 6-well dish for subsequent experiments. FLS were treated
with 10 ng/ml TNF-α in the presence or absence of 150 and 300 nM
bexarotene for 24 h.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR) analysis
After the indicated treatments, cells were washed
twice with cold Dulbecco's phosphate-buffered saline (DPBS) and
treated with 0.5 ml TRIzol (cat. no. 15596026; Thermo Fisher
Scientific, Inc.). Total RNA was isolated following the protocol
from the manufacturer. A DNase (Thermo Fisher Scientific, Inc.)
treatment was included in the end, following the supplier's
protocol. The RNA concentration was measured using a NanoDrop 2000
spectrophotometer. Two μg total RNA was adjusted to the same
concentration before being reverse-transcribed into cDNA using a
Verso 1-Step RT-PCR kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. The qPCR SYBR green mix and cycling conditions were
used as previously described (21). qPCR experiments were performed
using a Roche LightCycler 480 (Roche Applied Science) with a
384-multiwell format. The qPCR was performed with the following
conditions: A 5 min denaturation step at 94°C, 40 cycles of a 30
sec denaturation step at 94°C, a 30 sec annealing step at 56°C, and
a 45 sec extension step at 72°C. Relative fold changes of target
gene expression were then calculated using the 2−ΔΔCq
method (21). The following
primers were used in the present study: RXR, forward 5′-CAT GTT TGA
CTG TAT GGA TG-3′ and reverse 5′-AGC CCT TAC ATC CCT CAC AG-3′;
IL-6, forward 5′-GGT ACA TCC TCG ACG GCA TCT-3′ and reverse 5′-GTG
CCT CTT TGC TGC TTT CAC-3′; IL-8, forward 5′-TTT CTG TTA AAT CTG
GCA ACC CTA GT-3′ and reverse 5′-ATA AAG GAG AAA CCA AGG CAC
AGT-3′; MCP-1, forward 5′-ATG CAA TCA ATG CCC CAG TC-3′ and reverse
5′-TGC AGA TTC TTG GGT TGT GG-3′; MMP-1, forward 5′-CCT AGT CTA TTC
ATA GCT AAT CAA GAG GAT GT-3′ and reverse 5′-AGT GGA GGA AAG CTG
TGC ATA C-3′; MMP-3, forward 5′-CCT CTA TGG ACC TCC CAC AGA ATC-3′
and reverse 5′-GGT GCT GAC TGC ATC GAA GGA CAA A-3′; MMP-13,
forward 5′-CTG GCC TGC TGG CTC ATG CTT-3′ and reverse 5′-CCT CAG
AAA GAG CAG CAT CGA TAT G-3′; IL-4, forward 5′-GCC ACC ATG AGA AGG
ACA CT-3′ and reverse 5′-ACT CTG GTT GGC TTC CTT CA-3′; TGF-β1,
forward 5′-CCC TGG ACA CCA ACT ATT GC-3′ and reverse 5′-TGC GGA AGT
CAA TGT ACA GC-3′; and GAPDH, forward 5′-ACC CCT TCA TTG ACC TCA
AC-3′ and reverse 5′-CTT GAC GGT GCC ATG GAA TT-3′.
Western blot analysis
Total protein was isolated from FLS with a Total
Protein Extraction kit (Thermo Fisher Scientific, Inc.). Protein
concentration was determined using the bicinchoninic acid method
(Sigma-Aldrich; Merck KGaA). Equal amounts of protein (20
μg) from cells were loaded onto a 10% SDS-PAGE and
transferred to a polyvinylidene fluoride membrane (EMD Millipore)
(22). Membranes were blocked
with 5% BSA in TBS for 2 h at room temperature and then incubated
with primary antibodies at 4°C overnight. After three washes with
TBST, membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody at room temperature (RT) for 2
h. Blots were detected using the Immobilon Western Chemiluminescent
HRP Substrate system (Merck KGaA). The following primary antibodies
were used in this study: RXR (cat. no. sc-553; 1:2,000; Santa Cruz
Biotechnology, Inc.), tubulin (cat. no. NB600-936; 1:5,000; Novus
Biologicals, Ltd.), p38 (cat. no. 8690; 1:3,000; Cell Signaling
Technology, Inc.), phosphorylated (p-) p38 (cat. no. 4511; 1:1,000;
Cell Signaling Technology, Inc.), inhibitor α of κB (IκBα; cat. no.
4814; 1:1,000; Cell Signaling Technology, Inc.), p-IκBα (cat. no.
2859; 1:1,000; Cell Signaling Technology, Inc.), NF-κB (cat. no.
6956; 1:3,000; Cell Signaling Technology, Inc.) and lamin B1 (cat.
no. 13435; 1:5,000; Cell Signaling Technology, Inc.). The following
secondary antibodies were used: anti-rabbit HRP-linked secondary
antibody (cat. no. 7074; 1:3,000; Cell Signaling Technology, Inc.)
and anti-mouse HRP-linked secondary antibody (cat. no. 7076;
1:3,000; Cell Signaling Technology, Inc.).
Measurement of intracellular reactive
oxygen species (ROS)
The patterns of oxidative stress in FLS were
assessed by measuring intracellular ROS. FLS were treated with 10
ng/ml TNF-α in the presence or absence of 150 and 300 nM bexarotene
for 24 h. After the indicated treatments, cells were incubated with
10 μM 2',7'-dichlorodihydrofluo-rescein diacetate (DCFH-DA)
for 30 min in the dark. With this assay, the reduced DCFH-DA gets
oxidized and converted into fluorescent dichlorofluorescein by
intracellular ROS (23). The
fluorescent signals were visualized using a fluorescence microscope
(Zeiss GmbH). Fluorescence intensity of ROS images was quantified
using the software ImageJ version 1.51 (National Institutes of
Health). Regions of interest (ROI) were defined and the average
number of cells present in the ROI was counted. Then ROS activity
was calculated as follows: Average ROS = the integrated density
value/average cell number.
ELISA
FLS were treated with 10 ng/ml TNF-α in the presence
or absence of 150 and 300 nM bexarotene for 24 h. Supernatants were
collected to examine the secretion of IL-6, IL-8, MCP-1, IL-4 and
TGF-β1. Cells were lysed and lysates were used to determine the
levels of MMP-1, MMP-3 and MMP-13. Commercial ELISA kits (R&D
Systems, Inc.) were used for all the measurements (human IL-6, cat.
no. D6050; human IL-8, cat. no. D8000C; human MCP-1, cat. no.
DCP00; human TGF-β1, cat. no. DB100B; human MMP-1, cat. no. DY901B;
human MMP-3, cat. no. DMP300; and human MMP-13, cat. no. DY511), in
accordance with the manufacturer's instructions.
Luciferase activity determination
FLS were seeded into 24-well plates. Twenty-four h
later, NF-κB reporter plasmids (Beyotime Institute of
Biotechnology) and a Renilla luciferase plasmid (Promega
Corporation) were transfected into cells using Lipofectamine 2000
(thermo Fisher Scientific, Inc.), in accordance with the
manufacturer's instructions. At 24 h post-transfection, cells were
treated with 10 ng/ml TNF-α in the presence or absence of 150 and
300 nM bexarotene for 24 h. Cells were then lysed, and the
luciferase activity was measured using a Dual-Luciferase Reporter
Assay System (Promega Corporation). The relative promoter activity
was calculated as firefly luminescence/Renilla
luminescence.
Nuclear protein extraction
The nuclear extracts of FLS were obtained using the
NE-PER™ Nuclear and Cytoplasmic Extraction Reagent (cat. no. 78833;
Thermo Fisher Scientific, Inc.), in accordance with the
manufacturer's instructions. The nuclear protein lamin B1 was used
as a control for the nuclear fraction.
Statistical analysis
All values are expressed as means ± standard
deviation. Statistical analysis was performed using SPSS version
18.0 software (SPSS, Inc.). Differences among the groups were
detected using the one-way analysis of variance, followed by
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
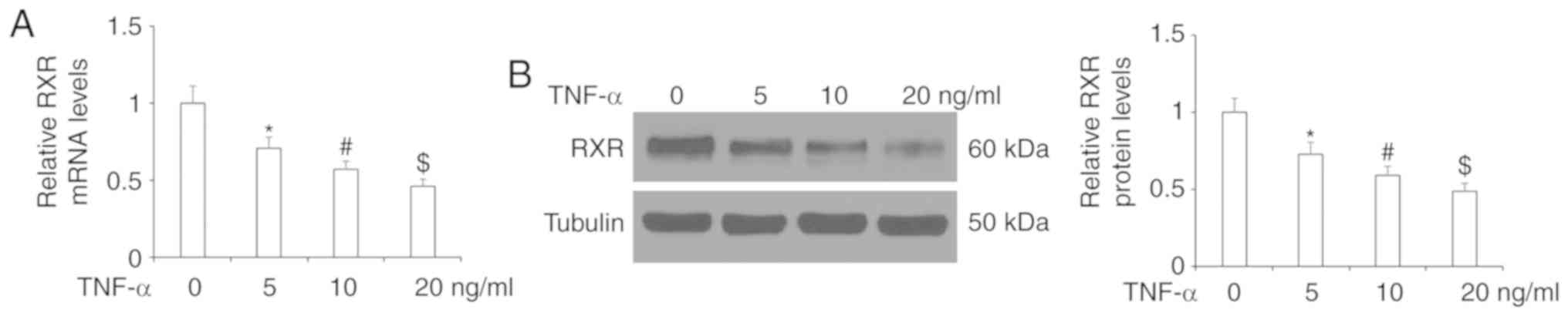
First, we determined whether RXR is expressed on
human FLS. As shown by the results of RT-qPCR and western blot
analyses in Fig. 1, RXR was
expressed in FLS and its expression was significantly reduced at
both the mRNA and protein levels upon exposure to TNF-α. The
inhibitory effect of TNF-α on RXR expression was dose-dependent.
Next, the effects of RXR agonism on various factors of RA was
investigated using bexarotene. Human FLS were treated with TNF-α in
the presence or absence of bexarotene. As shown by the results of
RT-qPCR in Fig. 2A, TNF-α
stimulation increased the mRNA expression levels of IL-6, IL-8 and
MCP-1 by 5.2-, 4.6- and 7.6-fold, respectively. Western blot
analysis confirmed that TNF-α stimulation increased the protein
expression levels of IL-6, IL-8 and MCP-1 by 4.5-, 4.3- and
7.1-fold, respectively (Fig. 2B).
However, the expression of all three of these cytokines was reduced
to roughly 2-fold at the mRNA level (Fig. 2A) and 1.5- to 2-fold at the
protein level (Fig. 2B) by
treatment with bexarotene, compared with TNF-α stimulation alone.
RT-qPCR and ELISA analyses were employed to determine the effects
of RXR agonism on TNF-α-induced expression of MMPs by FLS. Briefly,
cells were exposed to TNF-α in the presence or absence of
bexarotene. As shown in Fig. 3,
TNF-α stimulation increased expression of MMP-1, MMP-3 and MMP-13
by 4.2-, 5.5- and 6.5-fold, respectively, at the mRNA levels and
3.8-, 4.5-, and 5.4-fold at the protein level. Notably, treatment
with bexarotene reduced this TNF-α-induced overexpression of these
enzymes to <2-fold at both the mRNA and protein levels (Fig. 3).
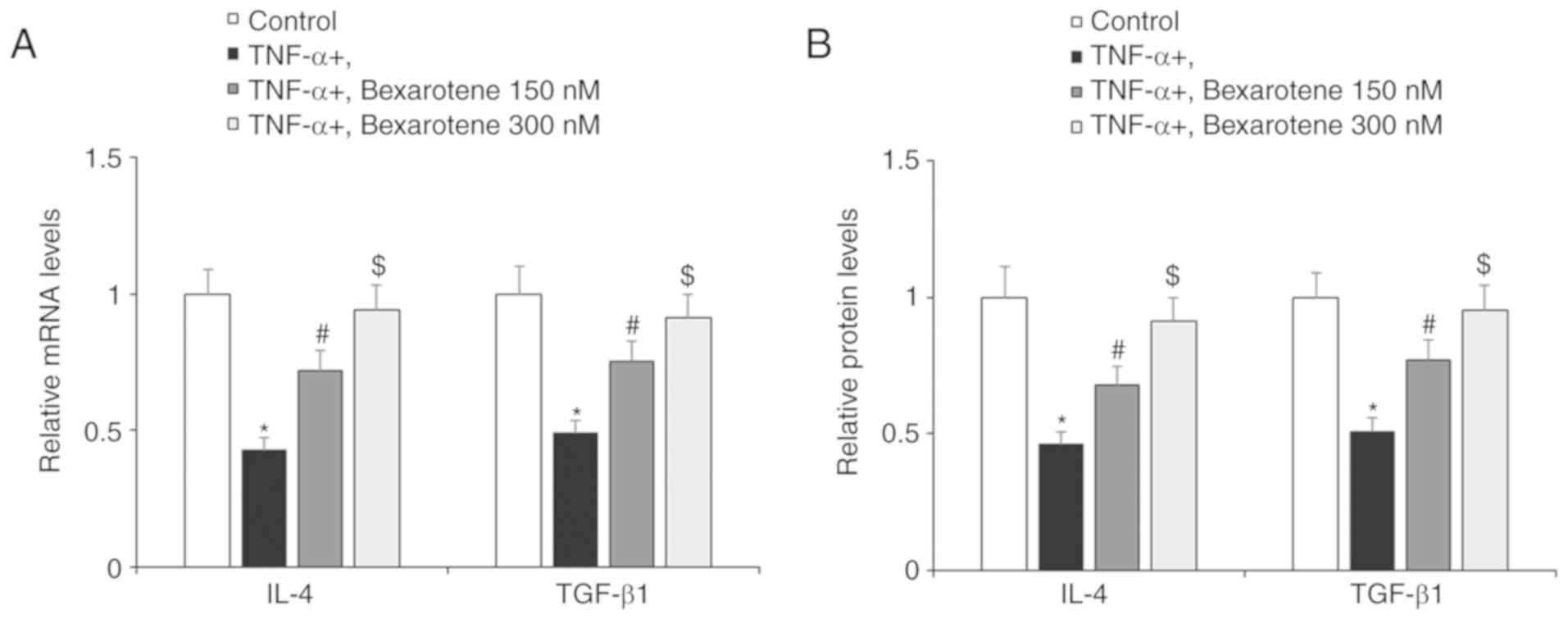
Next, the present study investigated the effect of
RXR agonism by bexarotene on the anti-inflammatory and antioxidant
factors IL-4 and TGF-β1. As shown in Fig. 4, exposure to TNF-α reduced
expression of IL-4 and TGF-β1 by roughly half at both the mRNA and
protein levels. However, RXR agonism by bexarotene significantly
restored the expression of these protective factors, almost to the
levels of the control cells (Fig.
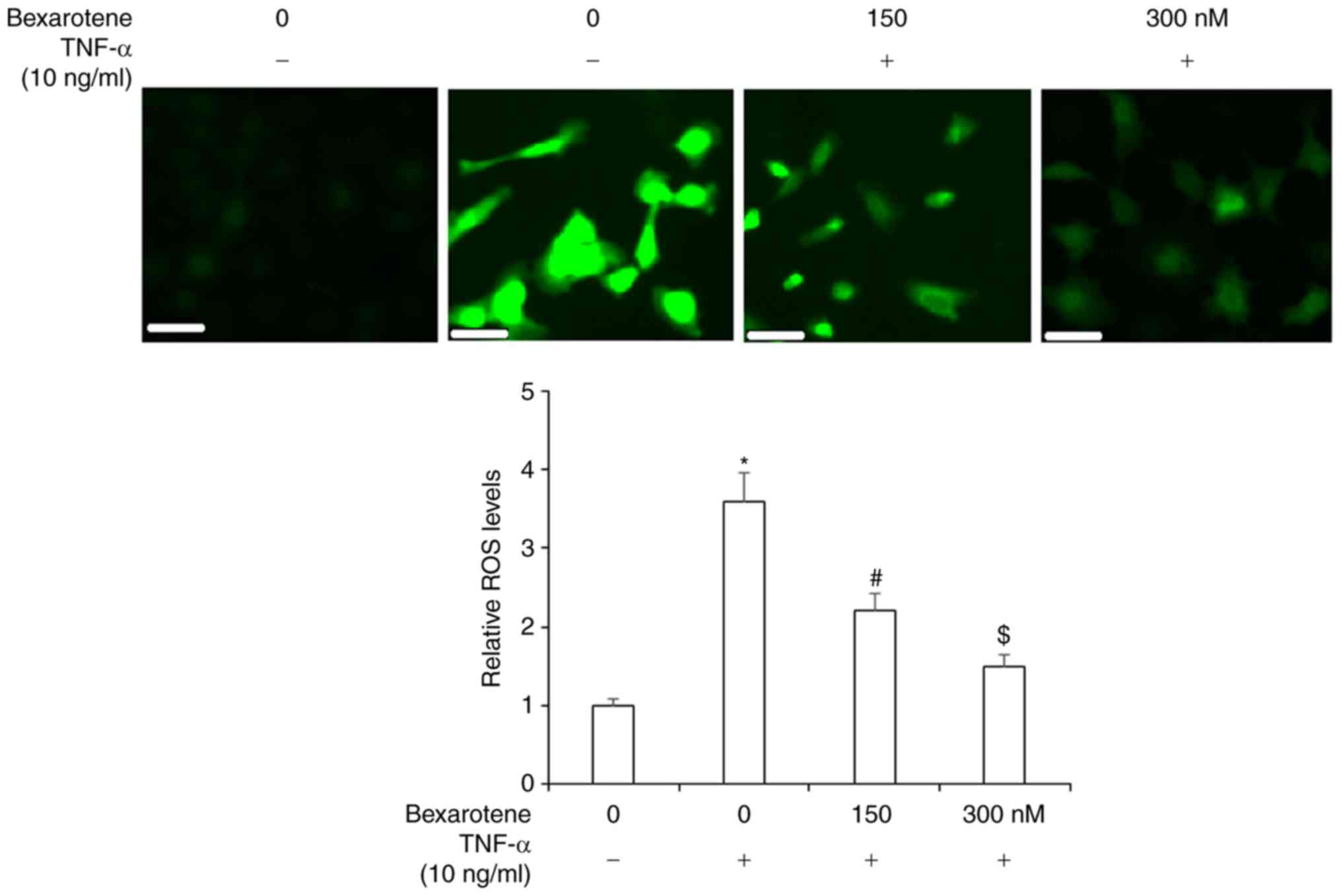
4). To investigate the effects of bexarotene on oxidative
stress, ROS production was determined using the DCFH-DA assay. As
shown in Fig. 5, ROS production
was increased to ~3.5-fold upon exposure to TNF-α, but treatment
with bexaro-tene significantly ameliorated this effect.
Finally, the cellular signaling pathways involved in
mediating the effects of RXR agonism were explored. The p38 MAPK
pathway is widely studied as a treatment target in RA due to its
crucial role in chronic inflammation (24). As shown in Fig. 6, TNF-α stimulation increased the
levels of p-p38 protein to ~4-fold compared with the control
unstimulated cells, while treatment with bexarotene significantly
reduced the levels of p-p38 to ~1.5-fold. The NF-κB pathway is
regarded as perhaps the most important pathway involved in the
inflammatory response (25). To
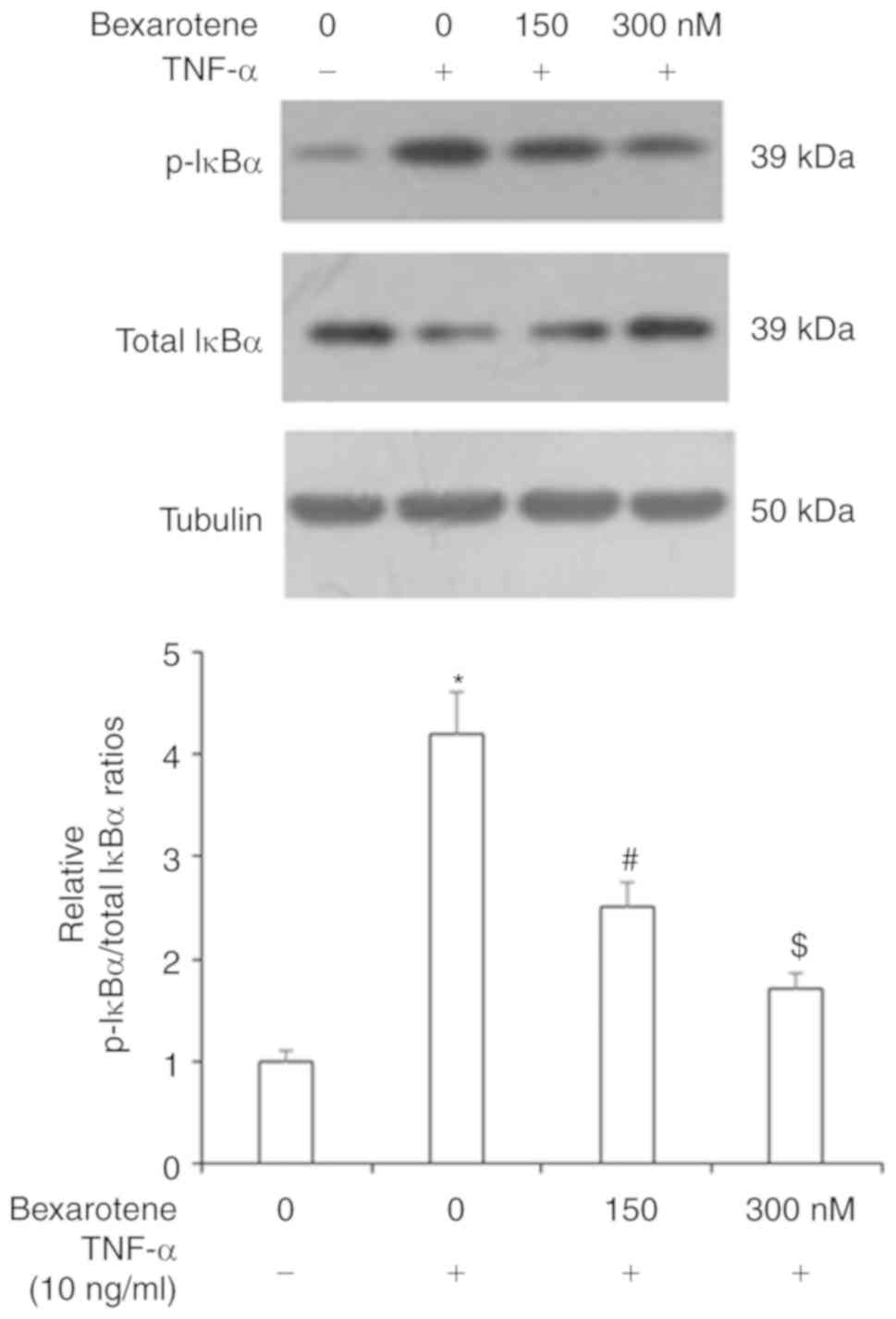
determine the effects of RXR agonism on NF-κB signaling activation,
first, the effect of bexarotene on its inhibitor, IκBα, was
investigated. As shown in Fig. 7,
exposure to TNF-α increased the levels of p-IκBα to >4-fold
compared with the control unstimulated cells, but treatment with
bexaro-tene reduced the levels of p-IκBα to <2-fold. Similarly,
the results of western blot analysis revealed that TNF-α
stimulation increased the levels of nuclear p-65 protein to
~4-fold, and this effect was reduced to <2-fold following
bexarotene treatment (Fig. 8A).
Luciferase reporter assay revealed that TNF-α stimulation increased
NF-κB activity by 85-fold, while treatment with bexarotene
significantly reduced the luciferase activity of NF-κB in a
dose-dependent manner (Fig. 8B).
These findings indicated that bexarotene exerted dose-dependent
protective effects against insult from TNF-α and that these effects
were mediated via the p38 MAPK/IκBα/NF-κB pathway.
Discussion
RA is a painful chronic inflammatory disease for
which, despite its high prevalence, a reliable therapy is still not
available. Recent studies have focused on targeted
agonism/antagonism of specific receptors as a means to modulate the
expression of cytokines, chemokines, and degradative enzymes
involved in the pathogenesis of RA (26). RXR is a member of the superfamily
of nuclear receptors and functions via homodimerization with itself
or heterodimerization with peroxisome proliferator-activated
receptors (PPARs) (27,28). Both retinoic acid receptors (RARs)
and retinoid X receptors (RXRs) have α, β and γ subtypes and
interact with retinoids. Retinoids refer to natural or synthetic
vitamers of all-trans-retinol (vitamin A), and have long
been recognized as having the ability to exert pleiotropic effects,
including inhibition of synthesis of MMPs and regulation of the
cell cycle, differentiation and apoptosis. While TNF-α is known to
induce cell death in many cell types, RA FLS exhibit increased
proliferation and resistance to apoptosis in response to TNF-α,
thereby contributing to their invasive nature (29,30). Bexarotene (Targetrin) is currently
the only synthetic rexinoid approved by the FDA for clinical use,
but this class of drugs is currently expanding, with new selective
RXR ligands with higher specificity and lower side effects under
development. Bexarotene is approved for the treatment of T-cell
lymphoma and is also being investigated as a potential treatment
for breast and lung cancers due to its antiproliferative effects
(31,32). To the best of our knowledge, the
present study is the first to have investigated the role of RXR
agonism by bexarotene in human FLS. First, it was determined that
expression of RXR was downregulated in human FLS upon exposure to
TNF-α, thereby demonstrating a potential treatment target against
TNF-α-mediated RA progression. Then, the results demonstrated that
bexarotene exerted beneficial effects against TNF-α-induced markers
of RA, by downregulating expression of proinflammatory cytokines,
chemokines and collagenases, and by upregulating the expression of
protective cytokines. Notably, the present findings revealed that
the effects of bexarotene in TNF-α-stimulated FLS were mediated
through the p38 MAPK/NF-κB pathway.
Previous studies with bexarotene have demonstrated
its anti-inflammatory effects. A recent study reported that
bexarotene reduced the expression of inflammatory cytokines in a
controlled cortical impact mouse model (33). Another recent study found that the
formation of PPARα/β/γ-RXRα heterodimers and Cytochrome p450 4F6
expression reduced inflammation-associated hypotension in a septic
shock rat model (34). The
present results provided further evidence of the anti-inflammatory
capacity of bexarotene by demonstrating that bexarotene
significantly inhibited TNF-α-induced expression of IL-6, IL-8 and
HMGB1 in FLS. While agonism of RXRs using bexarotene combination
therapy has been shown to decrease the expression of MCP-1 in rat
mesangial cells and human umbilical arterial endothelial cells
(35,36), the present study is the first to
demonstrate the ability of bexarotene to exert this effect in FLS.
Previous research using a photoaged skin mouse model did not find
any significant effect of RXR agonism on ultraviolet-induced
expression of MMP-3 and MMP-13. However, the present findings
demonstrated that RXR agonism significantly reduced expression of
MMP-1, MMP-3 and MMP-13 induced by TNF-α in FLS (37). The p38 MAPK/NF-κB pathway is
widely studied as a treatment target against chronic inflammatory
diseases, including RA (38). The
involvement of the p38 MAPK/NF-κB pathway in the anti-inflammatory
effects of RXR was demonstrated in an earlier study using a
combination of PPARγ and RXR ligands (39). RXR has also been shown to modulate
NF-κB activation (40). The
present results demonstrated that RXR agonism with bexarotene
suppressed activation of the p38 MAPK/NF-κB pathway by preventing
phosphorylation of p38 and IκBα, as well as reducing p65 protein
levels in the nucleus. These findings suggested that RXR agonism
using bexarotene may exert novel protective effects against the
development and progression of RA induced by TNF-α.
Funding
This study was funded by the First Affiliated
Hospital of Henan University of Science and Technology.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL contributed to the conception and design of the
study. YL, QX, YW, LZ, PZ, XH and JW contributed to data
acquisition, data analysis and interpretation. YL drafted and
critically revised the article. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Experimental procedures were approved by the
Institutional Ethics Committee at the First Affiliated Hospital of
Henan University of Science and Technology. Written informed
consent was obtained from all of the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Sweeney SE and Firestein GS: Rheumatoid
arthritis: Regulation of synovial inflammation. Int J Biochem Cell
Biol. 36:372–378. 2004. View Article : Google Scholar
|
|
2
|
Pap T and Korb-Pap A: Cartilage damage in
osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat
Rev Rheumatol. 11:606–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bresnihan B: Pathogenesis of joint damage
in rheumatoid arthritis. J Rheumatol. 26:717–719. 1999.PubMed/NCBI
|
|
4
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nanki T, Nagasaka K, Hayashida K, Saita Y
and Miyasaka N: Chemokines regulate IL-6 and IL-8 production by
fibroblast-like synoviocytes from patients with rheumatoid
arthritis. J Immunol. 167:5381–5385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Georganas C, Liu H, Perlman H, Hoffmann A,
Thimmapaya B and Pope RM: Regulation of IL-6 and IL-8 expression in
rheumatoid arthritis synovial fibroblasts: The dominant role for
NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 165:7199–7206.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokota K, Miyazaki T, Hirano M, Akiyama Y
and Mimura T: Simvastatin inhibits production of interleukin 6
(IL-6) and IL-8 and cell proliferation induced by tumor necrosis
factor-alpha in fibroblast-like synoviocytes from patients with
rheumatoid arthritis. J Rheumatol. 33:463–471. 2006.PubMed/NCBI
|
|
8
|
Harigai M, Hara M, Yoshimura T, Leonard
EJ, Inoue K and Kashiwazaki S: Monocyte chemoattractant protein-1
(MCP-1) in inflammatory joint diseases and its involvement in the
cytokine network of rheumatoid synovium. Clin Immunol Immunopathol.
69:83–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green MJ, Gough AK, Devlin J, Smith J,
Astin P, Taylor D and Emery P: Serum MMP-3 and MMP-1 and
progression of joint damage in early rheumatoid arthritis.
Rheumatology (Oxford). 42:83–88. 2003. View Article : Google Scholar
|
|
10
|
Agere SA, Akhtar N, Watson JM and Ahmed S:
RANTES/CCL5 induces collagen degradation by activating MMP-1 and
MMP-13 expression in human rheumatoid arthritis synovial
fibroblasts. Front Immunol. 8:13412017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lubberts E, Joosten LA, Chabaud M, van den
Bersselaar L, Oppers B, Coenen-de Roo CJ, Richards CD, Miossec P
and van den Berg WB: IL-4 gene therapy for collagen arthritis
suppresses synovial IL-17 and osteoprotegerin ligand and prevents
bone erosion. J Clin Invest. 105:1697–1710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chemel M, Brion R, Segaliny A, Lamora A,
Charrier C, Brulin B, Maugars Y, Le Goff B, Heymann D and
Verrecchia F: Bone morphogenetic protein 2 and transforming growth
factor-β1 inhibit the expression of the pro-inflammatory cytokine
IL-34 in rheumatoid arthritis synovial fibroblasts. Am J Pathol.
187:156–162. 2017. View Article : Google Scholar
|
|
13
|
Sugiura Y, Niimi T, Sato S, Yoshinouchi T,
Banno S, Naniwa T, Maeda H, Shimizu S and Ueda R: Transforming
growth factor β1 gene polymorphism in rheumatoid arthritis. Ann
Rheum Dis. 61:826–828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mateen S, Zafar A, Moin S, Khan AQ and
Zubair S: Understanding the role of cytokines in the pathogenesis
of rheumatoid arthritis. Clin Chim Acta. 455:161–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kageyama Y, Takahashi M, Ichikawa T,
Torikai E and Nagano A: Reduction of oxidative stress marker levels
by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid
arthritis. Clin Exp Rheumatol. 26:73–80. 2008.PubMed/NCBI
|
|
16
|
Charles P, Elliott MJ, Davis D, Potter A,
Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, deWoody K,
et al: Regulation of cytokines, cytokine inhibitors, and
acute-phase proteins following anti-TNF-alpha therapy in rheumatoid
arthritis. J Immunol. 163:1521–1528. 1999.PubMed/NCBI
|
|
17
|
Gottenberg JE, Brocq O, Perdriger A,
Lassoued S, Berthelot JM, Wendling D, Euller-Ziegler L, Soubrier M,
Richez C, Fautrel B, et al: Non-TNF-targeted biologic vs. a second
anti-TNF drug to treat rheumatoid arthritis in patients with
insufficient response to a first anti-TNF drug: A randomized
clinical trial. JAMA. 316:1172–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Liu Y, Wang J, Chen L, Zhang W and
Yan X: Preparation, in vitro and in vivo evaluation of bexarotene
nanocrystals with surface modification by folate-chitosan
conjugates. Drug Deliv. 23:79–87. 2016. View Article : Google Scholar
|
|
19
|
Yen WC, Prudente RY, Corpuz MR,
Negro-Vilar A and Lamph WW: A selective retinoid X receptor agonist
bexarotene (LGD1069, targretin) inhibits angiogenesis and
metastasis in solid tumours. Br J Cancer. 94:654–660. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamanishi Y, Boyle DL, Green DR, Keystone
EC, Connor A, Zollman S and Firestein GS: P53 tumor suppressor gene
mutations in fibroblast-like synoviocytes from erosion synovium and
non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther.
7:R12–R18. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Terraneo L, Bianciardi P, Malavalli A,
Mkrtchyan G, Spann SN, Lohman J, Samaja M and Vandegriff KD:
Hemoglobin extravasation in the brain of rats exchange-transfused
with hemoglobin-based oxygen carriers. Artif Cells Nanomed
Biotechnol. 45:710–716. 2017. View Article : Google Scholar
|
|
23
|
Ma S, Bai Z, Wu H and Wang W: The DPP-4
inhibitor saxagliptin ameliorates ox-LDL-induced endothelial
dysfunction by regulating AP-1 and NF-κB. Eur J Pharmacol.
851:186–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schett G, Zwerina J and Firestein G: The
p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid
arthritis. Ann Rheum Dis. 67:909–916. 2008. View Article : Google Scholar
|
|
25
|
Mitchell JP and Carmody RJ: NF-κB and the
transcriptional control of inflammation. Int Rev Cell Mol Biol.
335:41–84. 2018. View Article : Google Scholar
|
|
26
|
Noack M and Miossec P: Selected cytokine
pathways in rheumatoid arthritis. Semin Immunopathol. 39:365–383.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Emdad L, Das SK, Hu B, Kegelman T, Kang
DC, Lee SG, Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC: A promiscuous
protein partner critical in cancer, obesity, and CNS diseases. Adv
Cancer Res. 131:97–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiazza F and Collino M: Peroxisome
proliferator-activated receptors (PPARs) in glucose control. Mol
Nutri Diabetes. 105–114. 2016. View Article : Google Scholar
|
|
29
|
Vincenti MP, Clark IM and Brinckerhoff CE:
Using inhibitors of metalloproteinases to treat arthritis. Easier
said than done? Arthritis Rheum. 37:1115–1126. 1994.
|
|
30
|
Mosquera N, Rodriguez-Trillo A,
Mera-Varela A, Gonzalez A and Conde C: Uncovering Cellular retinoic
acid-binding protein 2 as a potential target for rheumatoid
arthritis synovial hyper-plasia. Sci Rep. 8:87312018. View Article : Google Scholar
|
|
31
|
Uray IP and Brown PH: Chemoprevention of
hormone receptor-negative breast cancer: New approaches needed.
Recent Results Cancer Res. 188:147–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang D, Leal AS, Carapellucci S, Zydeck
K, Chaaban N, Sporn MB, Wagner CE and Liby KT: Comparison of the
rexinoids Bexarotene and Pyrimidine-Bexarotene in a preclinical
model of lung carcinogenesis. FASEB J. 31(Suppl 1): S671–S616.
2017.
|
|
33
|
Zhong J, Cheng C, Liu H, Huang Z, Wu Y,
Teng Z, He J, Zhang H, Wu J, Cao F, et al: Bexarotene protects
against traumatic brain injury in mice partially through
apolipoprotein E. Neuroscience. 343:434–448. 2017. View Article : Google Scholar
|
|
34
|
Tunctan B, Kucukkavruk SP, Temiz-Resitoglu
M, Guden DS, Sari AN and Sahan-Firat S: Bexarotene, a selective
RXRα agonist, reverses hypotension associated with inflammation and
tissue injury in a rat model of septic shock. Inflammation.
41:337–355. 2018. View Article : Google Scholar
|
|
35
|
Kim DH, Lee GC, Kim CH, Oh SW, Han KH and
Han SY: Anti-inflammatory effect of combination therapy with
rosiglitazone and all-trans retinoic acid on high glucose-induced
MCP-1 response in rat mesangial cells. Biomed Res. 28:463–467.
2017.
|
|
36
|
Escudero P, Martinez de Marañón A, Collado
A, Gonzalez- Navarro H, Hermenegildo C, Peiró C, Piqueras L and
Sanz MJ: Combined sub-optimal doses of rosuvastatin and bexarotene
impair angiotensin II-induced arterial mononuclear cell adhesion
through inhibition of Nox5 signaling pathways and increased
RXR/PPARα and RXR/PPARγ interactions. Antioxid Redox Signal.
22:901–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Niu X, Xiao S and Ma H: Retinoic
acid ameliorates photo-aged skin through RAR-mediated pathway in
mice. Mol Med Rep. 16:6240–6247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Westra J and Limburg PC: p38
mitogen-activated protein kinase (MAPK) in rheumatoid arthritis.
Mini Rev Med Chem. 6:867–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Desreumaux P, Dubuquoy L, Nutten S,
Peuchmaur M, Englaro W, Schoonjans K, Derijard B, Desvergne B,
Wahli W, Chambon P, et al: Attenuation of colon inflammation
through activators of the retinoid X receptor (RXR)/peroxisome
proliferator-activated receptor gamma (PPARgamma) heterodimer: A
basis for new therapeutic strategies. J Exp Med. 193:827–838. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan Y, Wang J, Wei L, He B, Wang C and
Wang B: Iron deficiency activates pro-inflammatory signaling in
macrophages and foam cells via the p38 MAPK-NF-κB pathway. Int J
Cardiol. 152:49–55. 2011. View Article : Google Scholar
|