Introduction
Myocardial fibrosis, which is a morphological
feature of ventricular remodeling, develops as a result of several
different heart diseases, including myocardial infarction,
hypertension, atherosclerosis and myocarditis (1,2).
Excessive extracellular matrix (ECM) production often disrupts the
tissues' physiological architecture (3). In the heart, the primary components
of the ECM network structure are predominantly collagens I and III.
Massive cardiac collagen deposition can lead to ventricular
stiffness and diastolic and systolic dysfunction (4,5).
These adverse changes can result in heart failure and even sudden
cardiac death (4,6,7).
Treatment strategies for myocardial fibrosis are still a focus of
clinical attention.
Transforming growth factor β1 (TGF-β1) has been
confirmed as a primary regulator of myocardial fibrosis (8,9).
TGF-β1 exerts its biological functions by binding to the active
TGF-β receptors type I and II (TβRI and II), which causes
phosphorylation of TβRI and II (9). The activated receptors subsequently
phosphorylate receptor-regulated mothers against decapentaplegic
homolog (Smad) proteins and increase the common-regulated Smad
protein expression. These Smad proteins are the primary downstream
signaling molecules of TGF-β1 (10). It is well known that the
upregulated TGF-β expression resulting from tissue injury plays a
role in the tissue repair process and scar formation (11). In terms of post-infarct
remodeling, TGF-β1 is involved in myocardial fibrosis via ECM
synthesis upregulation by fibroblasts; as a consequence,
TGF-β1/Smads is considered to be the primary signaling pathway that
contributes to myocardial fibrosis (12). Therefore, the inhibition of TGF-β
signaling is a promising approach for inhibiting myocardial
fibrosis.
Gentianella acuta (G. acuta), which
belongs to the Gentianaceae, is one of the most commonly used herbs
in Mongolian medicine to cure hepatitis, jaundice, fever and
headache. In China, this herb is widely distributed in Hebei, Inner
Mongolia, Shanxi and Shandon. The whole grass of G. acuta
has been used to treat angina by the local herdsmen in Inner
Mongolia for a long time. In these regions, G. acuta was
called 'guixincao'. Ewenki people had a habit of drinking
'guixincao' tea and used it to treat angina by chewing or water
decoction (13). Certain
experimental results have indicated that G. acuta exerts
anti-inflammatory, antioxidant, antibacterial and antiarrhythmic
effects, thereby preventing cardiovascular and cerebrovascular
diseases (14-16). Numerous studies have shown that
G. acuta and its active components have a protective effect
on the heart (16,17). In the authors' previous study, it
was found that G. acuta significantly improved cardiac
function and inhibited myocardial fibrosis in a rat model (18). However, the detailed mechanism
underlying G. acuta's anti-myocardial fibrotic
effects is not clear. The aim of the present study was to
investigate whether the mechanism underlying G. acuta's
anti-myocardial fibrosis effects is based on the TGF-β1/Smads
signaling pathway, which will provide evidence for clinical
application.
Materials and methods
Primary drugs and reagents
Isoproterenol (ISO) was purchased from Tokyo
Chemical Industry, Ltd. Bellidifolin, swertianolin and
demethylbellidifolin were acquired from Chengdu Alfa Biotechnology,
Co., Ltd. Masson trichrome and wheat germ agglutinin (WGA) staining
kit were purchased from Wuhan Servicebio Technology, Ltd.
Antibodies for α-smooth actin (α-SMA; cat. no. ab32575), TGF-β1
(cat. no. ab92486), Smad4 (cat. no. ab40759), phosphorylated (P)-
and total Smads2 (cat. nos. ab53100 and ab40855) and 3 (cat. nos.
ab52903 and ab40854) were purchased from Abcam. The antibody for
TβRI (cat. no. GB11271) was purchased from Wuhan Servicebio
Technology, Ltd. The antibody for TβRII (cat. no. A11788) was
purchased from ABclonal Biotech Co., Ltd. Antibodies for collagens
I (cat. no. AF7001), III (cat. no. AF0136), phospho-TβRI (cat. no.
AF8080) and II (cat. no. AF8191) were purchased from Affinity
Biosciences. Antibody for GAPDH (cat. no. 60004-1-Ig) was obtained
from Wuhan Sanying Biotechnology. Horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (cat. no. ZDR-5306) and
HRP-conjugated anti-mouse IgG (cat. no. ZDR-5307) antibodies were
obtained from Beijing Zhongshan Golden Bridge Biotechnology, Co.,
Ltd.; OriGene Technologies, Inc. The SP9000 immunohistochemical
staining kit was purchased from Beijing Zhongshan Golden Bridge
Biotechnology, Co., Ltd.; OriGene Technologies, Inc.
Preparation of plant material
The whole plant of G. acuta was purchased
from the market in Inner Mongolia and authenticated by Professor
Zheng Yu-Guang (Department of Pharmacognosy, School of Pharmacy,
Hebei University of Chinese Medicine). According to body surface
area conversion equations (human: Rat=1: 6.3) for translating
dosages from human to animal (19,20), the clinical dosage of G.
acuta (6 g/d) in adults is ~ equal to the daily dosage of 0.6
g/kg in rat. The dosages of G. acuta adopted in the
experiments are 0.3, 0.6 and 1.2 g/kg, respectively. G.
acuta's dried whole plant was ground and soaked in a 20-fold
volume of distilled water for 20 min. The mixture, which was
decocted twice at 100°C for 30 min, was then filtered and
concentrated at 80°C. The dark brown aqueous extracts were stored
in a refrigerator at 4°C until use.
Animal protocol
A total of 50 male Sprague-Dawley rats (5 weeks old)
were obtained from the Experimental Animal Centre of Hebei Medical
University (Shijiazhuang, China) and maintained in an animal
facility (22-23°C; 55-60% humidity) on a 12-h light/dark cycle with
free access to food and water. All experiments were conducted in
accordance with the P.R. China legislation for the use and care of
laboratory animals. The animal care and study protocols were
approved by the Institutional Animal Care and Use Committee of
Hebei University of Chinese Medicine (no. 1703440).
The myocardial fibrosis model was established as
described previously (21,22).
Briefly, ISO (5 mg/kg/day) was injected subcutaneously into rats
for seven days. A total of 50 rats were equally divided into the
following five groups at random: i) Control group (Control); ii)
model group (ISO); iii) low-dose drug intervention group (ISO+G.
acuta 0.3 g/kg); iv) middle-dose drug intervention group
(ISO+G. acuta 0.6 g/kg); and v) high-dose drug intervention
group (ISO+G. acuta 1.2 g/kg). All drugs were administered
via oral gavage and the treatment period lasted 21 days beginning
from the day after the first ISO injection. On the 22nd day, the
rats were anaesthetized and then sacrificed. All the samples were
cut from the same location of heart, the mid left ventricular (2.5
mm above the apex). The tissues were then fixed for 24 h at room
temperature with 4% paraformaldehyde for histopathological analyses
and immunohistochemical measurements, and the fresh myocardial
tissues were stored at -80°C for western blotting analysis.
Morphological detection of heart
The heart tissues were fixed in 4% paraformaldehyde,
embedded in paraffin wax and cut into 5-µm sections.
Hematoxylin and eosin (H&E), Masson trichrome and WGA staining
were performed in accordance with the manufacturer's protocol in
order to observe histopathological changes and collagen deposition
in the heart tissues. Sections were dehydrated, made transparent
and sealed. Staining was visualized and images were captured using
light microscopy and fluorescence microscope (Leica Microsystems
GmbH).
Immunohistochemical staining
Immunohistochemical staining was performed for
detection of collagens I and III, α-smooth muscle actin (α-SMA),
TGF-β1, Smads2, 3 and 4 expression according to the manufacturer's
protocol of the SP9000 immunohistochemical staining kit. Briefly,
heart samples were fixed in 4% paraformaldehyde, embedded in
paraffin and cut into 5-µm sections. The sections were then
de-paraffinized, incubated with 3% hydrogen peroxide for 10 min at
room temperature in order to block endogenous peroxidase activity
and then incubated for 30 min at room temperature with 3% goat
serum (OriGene Technologies, Inc.) to block nonspecific binding.
Next, the sections were incubated with a primary antibody (collagen
I, 1:100; collagen III, 1:100; α-SMA, 1:500; TGF-β1, 1:50; Smad2,
1:50; Smad3, 1:1,000; and Smad4, 1:100) at 4°C overnight. After
washing with phosphate-buffered saline (PBS), the sections were
incubated with secondary antibodies from the SP9000 kit for 15 min
each at room temperature. Finally, the sections were incubated with
3, 3'-diaminobenzedine for 2 min at room temperature and observed
under a light microscope (Leica Microsystems GmbH).
Western blotting
The heart tissues were washed twice with ice-cold
PBS and lysed in radioimmunoprecipitation assay buffer containing a
protease inhibitor cocktail, phenylmethyl sulfonyl fluoride, and
phosphatase inhibitors. The protein concentration was measured
using a bicinchoninic acid Protein Assay kit (Wuhan Servicebio
Technology, Ltd.). An equal amount of protein (20 µg/lane)
was separated using 10% SDS-PAGE and then transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
5% skim milk for 90 min at room temperature and then individually
incubated with the appropriate primary antibody (collagen I,
1:1,000; collagen III, 1:1,000; α-SMA, 1:5,000; TGF-β1, 1:1,000;
TβRI, 1:1,000; P-TβRI, 1:3,000; TβRII, 1:1,000; P-TβRII, 1:3,000;
Smad2, 1:2,000; P-Smad2, 1:1,000; Smad3, 1:10,000; P-Smad3,
1:2,000; and Smad4, 1:5,000) at 4°C overnight. Subsequently, the
membranes were incubated with the appropriate HRP-conjugated
secondary antibody (HRP-conjugated anti-rabbit IgG and
HRP-conjugated anti-mouse IgG, both 1:20,000) at room temperature
for 60 min. The immunoreactive bands were detected using the
enhanced luminol-based ECL reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and the Fusion FX5 Spectra multifunction
laser-scanning system. The expression of each protein of interest
was normalized to that of GAPDH and was analyzed using Image J 3.0
software (National Institute of Health).
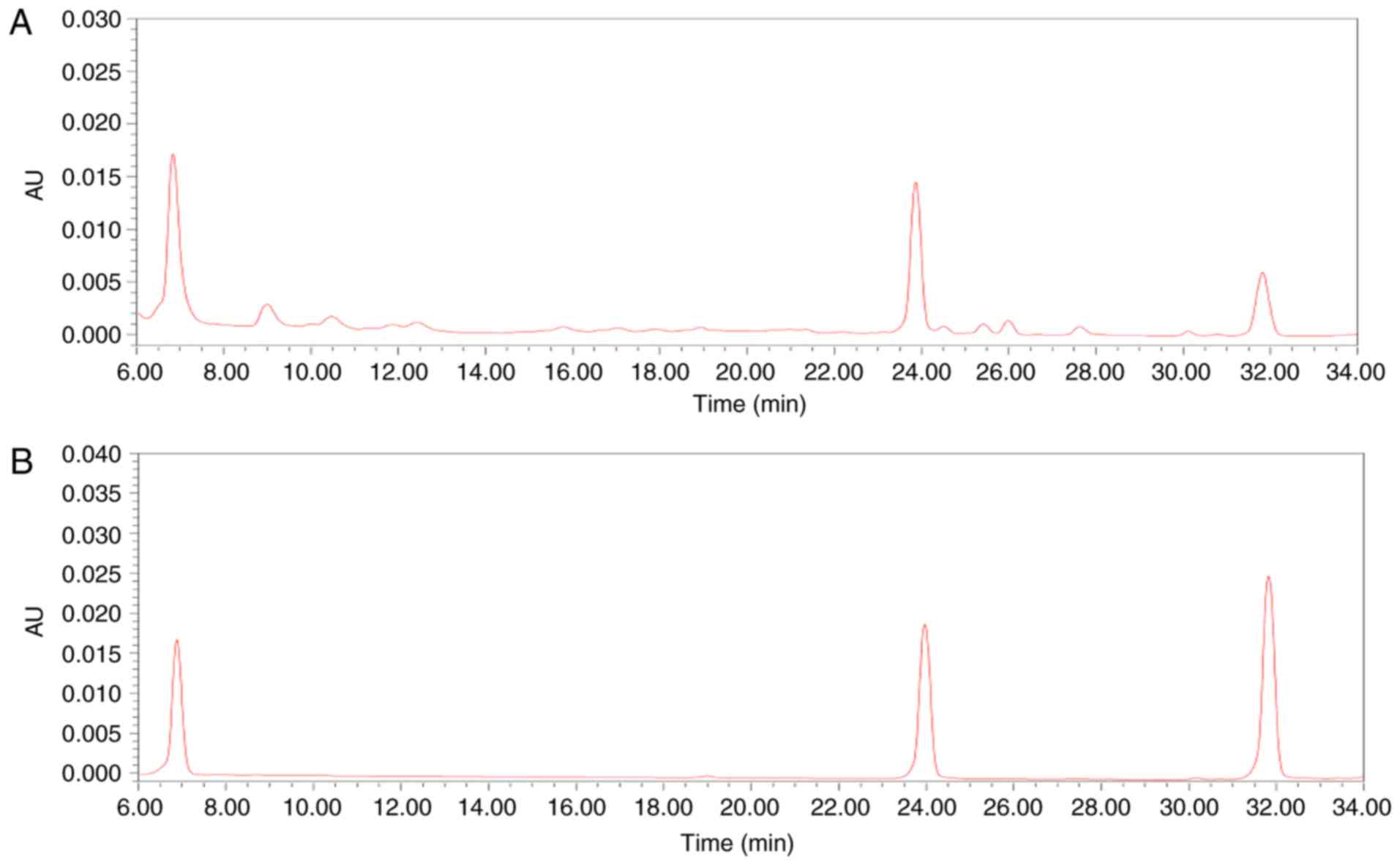
High performance liquid chromatography
(HPLC) analysis
The sample solutions were prepared by dissolving 0.2
g of G. acuta in 50 ml water. The aqueous extracts of G.
acuta were evaluated by HPLC analysis. The standard solutions
of i) swertianolin, ii) demethylbellidifolin, and iii) bellidifolin
were prepared to concentrations of 0.02 mg/ml. HPLC analysis was
performed on a XBridge C18 (4.6×250 mm, 5 µm, Waters
Corporation) maintained at 35°C. Mobile phases A and B were
acetonitrile and 0.1% acetic acid, respectively. The flow rate was
set at 1.0 ml/min. The detection wavelength was set at 254 nm to
identify xanthones from G. acuta (Fig. 1).
Statistical analysis
Each experiment was repeated three times. Data are
expressed as the mean ± standard error of the mean or mean ±
standard deviation. All statistical analyses were performed with
SPSS 21.0 statistical software (IBM, Corp.). Differences were
analyzed using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
G. acuta ameliorates ISO-induced
myocardial fibrosis and cardiac remodeling in rats
Morphological examination of heart tissue sections
stained with H&E revealed that an ISO-induced myocardial
fibrosis rat model had been successfully established. Compared with
the control group, heart tissues from ISO-treated rats exhibited
widespread myocardial structural disorder and necrotic
degeneration. Compared with the ISO group, G. acuta
treatment produced a marked improvement in ISO-induced myocardial
necrosis and structural disorder (Fig. 2A and B). Masson trichrome staining
revealed that the collagen deposition was significantly increased
in the ISO-treated rat hearts. However, G. acuta treatment
caused a significant reduction in collagen deposition compared with
the ISO group (P<0.05; Fig. 2A and
C). WGA staining indicated that ISO could significantly induce
cardiomyocytes injury and hypertrophy (P<0.01), and treatment
with G. acuta could inhibit myocardial hypertrophy (Fig. 2A and D). These results indicate
that G. acuta treatment caused an inhibition of collagen
deposition and ameliorated ISO-induced myocardial fibrosis and left
ventricle remodeling.
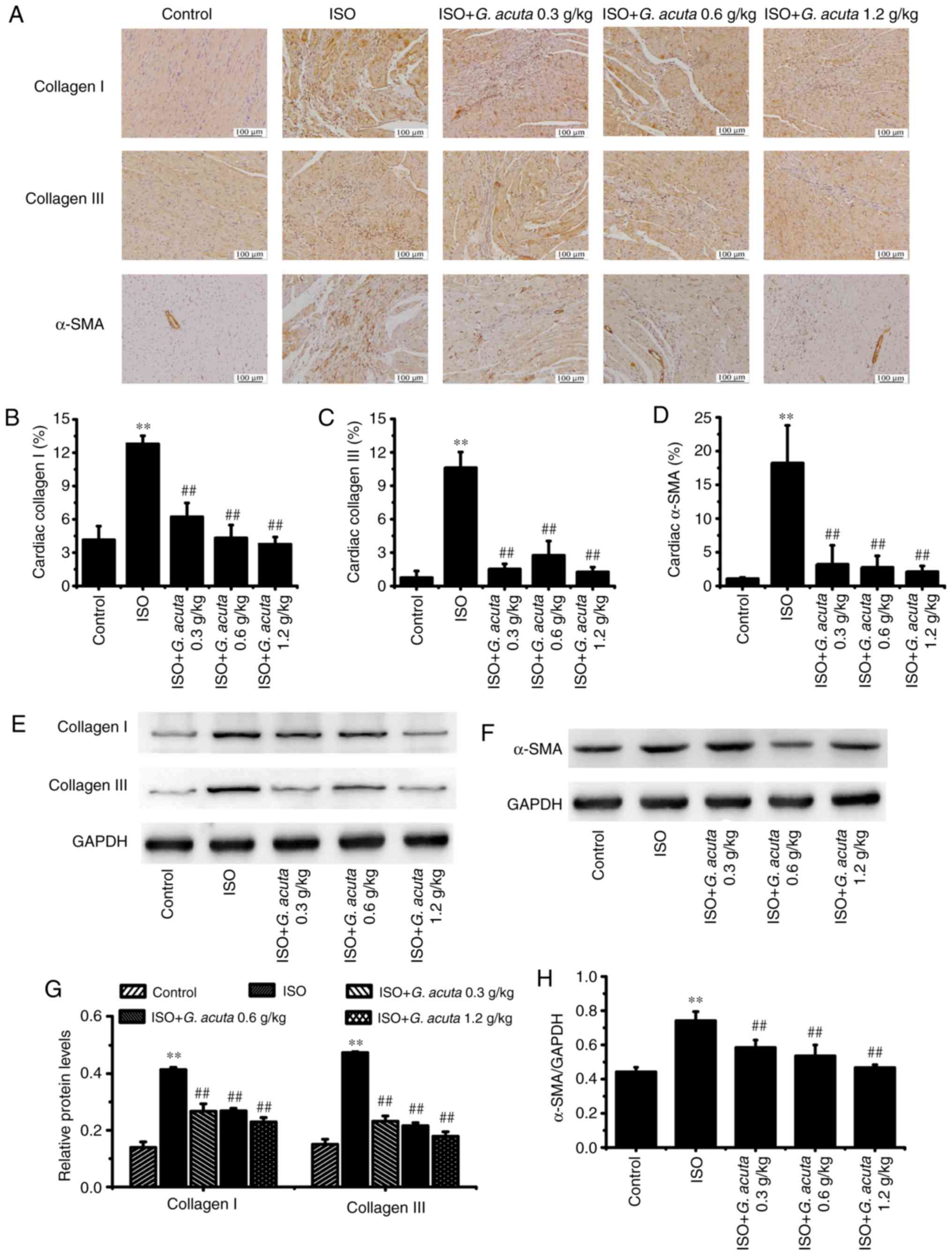
G. acuta inhibits the expression of
collagens I, III and α-SMA induced by ISO
In order to further confirm G. acuta's
inhibitory effects on myocardial fibrosis, the content and
distribution of collagen types I and III, the two major components
of the ECM, were quantified using immunohistochemical staining and
western blotting. Immunohistochemical staining revealed that the
expression of myocardial collagens I and III were markedly enhanced
in the ISO group vs. the control group. G. acuta treatment
decreased ISO-induced collagens I and III expression enhancement
(Fig. 3A-C). Western blotting
results indicated that G. acuta treatment inhibited the
collagens I and III expression (Fig.
3E and G). These findings demonstrate that G. acuta
treatment caused a decrease in collagens I and III expression in
the process of ISO-induced myocardial fibrosis.
A crucial event in myocardial fibrosis is the
conversion of cardiac fibroblasts (CFs) to myofibroblasts (MFs).
MFs express contractile proteins, including α-SMA. Therefore, the
expression of α-SMA, which is a typical marker of MFs
differentiation, was analyzed using immunohistochemical staining
and western blotting. In the control group, α-SMA-positive staining
was observed in the blood vessels. In comparison with the control
group (without ISO), an abundance of ISO-induced brown particles
was present in the fibrotic areas, while G. acuta treatment
ameliorated the ISO-induced positive brown particles (Fig. 3A and D). Western blotting results
indicated that the expression level of α-SMA was significantly
downregulated by G. acuta (P<0.01; Fig. 3F and H).
These findings indicate that G. acuta
treatment caused inhibition of ISO-induced myocardial fibrosis by
reducing collagens I, III and α-SMA expression in the heart
tissue.
G. acuta treatment inhibits ISO-induced
TGF-β1 expression and the phosphorylation of TβRI and II
TGF-β1 is the major regulator of the fibrogenic
process. In order to identify whether TGF-β1 is involved in G.
acuta-mediated attenuation of myocardial fibrosis, TGF-β1
levels were examined in the heart tissue. Immunohistochemical
analysis revealed that the expression levels of TGF-β1 were
increased in the ISO group compared with the control group.
However, compared with the ISO group, G. acuta treatment
caused a significant decrease in the ISO-induced TGF-β1 expression
levels (P<0.01; Fig. 4A and
B). Western blotting results showed that TGF-β1 expression was
significantly decreased in G. acuta treatment group
(P<0.01; Fig. 4C). These
results indicate that G. acuta treatment inhibited
ISO-induced TGF-β1 expression in heart tissues.
Phosphorylation of the TGF-β receptor is responsible
for the initiation of multiple TGF-β signaling pathways. In order
to confirm whether G. acuta affects TGF-β receptors, the
expression and phosphorylated levels of TβRI and II were examined
by western blotting. The results indicated that TβRI expression and
levels of TβRI and II phosphorylation were significantly
upregulated in the ISO group compared with the control group
(P<0.01). Moreover, G. acuta treatment abolished
upregulation of these ISO-induced proteins expression and
phosphorylation levels (Fig. 5).
These results demonstrate that G. acuta attenuated TβRI
expression and inhibited the phosphorylation and activation of TβRI
and II.
 | Figure 5G. acuta causes a reduction in
ISO-induced TβRI levels and the phosphorylation of TβRI and II. (A)
Western blotting analysis of TβRII, phosphory-lated (P)-TβRII,
TβRI, P-TβRI. (B) Bar graphs show fold-changes of the TβRII,
P-TβRII, TβRI, P-TβRI expression, as analyzed by western blotting.
GAPDH was used as a loading control (n=3). (C) Bar graphs show
fold-changes of P-TβRII/TβRII and (D) P-TβRI/TβRI. Data are shown
as mean ± standard error of the mean. **P<0.01 vs.
the Control and ##P<0.01 vs. ISO. ISO, isoproterenol;
G. acuta, Gentianella acuta; P-TβR, phosphorylated-
TGF-β receptor. |
G. acuta inhibits ISO-activated
TGF-β1/smads signaling
The TGF-β1/Smads signaling pathway plays a critical
role in myocardial fibrosis. Smad proteins are the primary
downstream molecules of TβRI. In order to investigate whether G.
acuta caused a reduction in the ISO-induced expression and
activation of Smads, immunohistochemical staining and western
blotting were performed. In comparison with the control group, the
immunoreactivities of Smads2, 3 and 4 were significantly increased
in the ISO group. Moreover, G. acuta treatment reduced the
increase in ISO-induced Smads2, 3 and 4 expression (Fig. 6A). Western blot analysis showed
that G. acuta treatment caused a decrease in the Smads2 and
4 expression. In addition, compared with the control group, Smads2
and 3 phosphorylation levels were significantly increased in the
ISO group (P<0.01). G. acuta treatment caused a
significant reduction in Smads2 and 3 phosphorylation levels,
compared with the ISO group (P<0.05; Fig. 6B-E). These results demonstrate
that G. acuta treatment attenuated Smads2 and 4 expression
and inhibited activation of ISO-induced Smads2 and 3 via
phosphorylation. In conclusion, the present data suggested that
G. acuta caused an inhibition of myocardial fibrosis through
the regulation of the TGF-β1/Smads signaling pathway (Fig. 7).
 | Figure 6G. acuta causes inhibition of
ISO-activated TGF-β1/Smads signaling. (A) Immunohistochemistry
staining to detect Smads2, 3 and 4; scale bars=100 µm. (B)
Western blotting analysis of Smad2, P-Smad2, Smad3, P-Smad3 and
Smad4 expression. (C) Bar graphs show fold-changes of Smad2,
P-Smad2, Smad3, P-Smad3 and Smad4 expression as analyzed by western
blotting. GAPDH was used as a loading control (n=3). (D) Bar graphs
show fold-changes of P-Smad2/Smad2 and (E) P-Smad3/Smad3. Data are
shown as mean ± standard error of the mean. **P<0.01
vs. control, #P<0.05 and ##P<0.01 vs.
ISO. P-Smad, phosphorylated-mothers against decapentaplegic
homolog; ISO, isoproterenol; G. acuta, Gentianella
acuta. |
Discussion
In mammals, the ability to regenerate the heart
after birth is limited and the adult mammalian heart rarely
exhibits the capacity for self-renewal. Myocardial fibrosis is a
morphological feature of ventricular remodeling in a variety of
heart diseases involved in myocardial infarction (23). Accompanying the fibrosis, normal
structural components of heart tissue can be disrupted. Employment
of ISO has been popular for the induction of myocardial fibrosis in
rats. A previous study showed that the pathophysiological change of
left ventricular induced by ISO could persist up to 21 days after a
single subcutaneous injection (24). In the authors' previous study, it
was confirmed that G. acuta could decrease ISO-increased
heart rate, improve cardiac function and ameliorate myocardial
fibrosis (18). However, the
detailed mechanism of G. acuta on myocardial fibrosis was
not clear. In this study, H&E and Masson trichrome staining
demonstrated that G. acuta alleviated cardiac structural
disorder and myocardial fibrosis induced by ISO, which were
consistent with the authors' previous study (18). In addition, WGA staining
determined that G. acuta also inhibited ISO-induced
myocardial hypertrophy. These results determined that G.
acuta treatment ameliorated ISO-induced myocardial fibrosis and
ventricular remodeling.
ECM is a crucial element and regulator in numerous
biological tissues (25). In the
heart, the collagen matrix plays an important role in supporting
muscle cells and blood vessels via connecting cells and muscle to
govern the architecture and maintain an important diastolic and
systolic myocardial stiffness (26,27). Adverse cardiac remodeling and
myocardial fibrosis lead to the alteration of ECM in heart tissue.
Fibrillar collagen replaces dead cardiomyocytes and scar tissue
formation occurs in the infarct zone along with the excessive
deposition of collagen in the interstitium remote to the infarction
site (28). One of the key events
in myocardial fibrosis is the transformation of myocardial CFs into
MFs. MFs are the primary cells involved in the repair of
pathological tissue, which produce collagen during the fibrotic
process (29,30). High α-SMA expression is a key MFs
feature (31). In this study, it
was determined that ISO induced the excessive deposition of
collagen and G. acuta decreased the collagen deposition by
immunohistochemical staining, that results were in consistent with
the authors' previous study (18). It was also verified that G.
acuta downregulated collagen expression by western blotting.
Furthermore, G. acuta treatment ameliorated the expression
of α-SMA in the context of ISO-induced myocardial fibrosis. These
data suggest that G. acuta inhibited the conversion of CFs
to MFs, reduced collagen synthesis and improved myocardial
fibrosis.
TGF-β family members consist of three isoforms
(TGF-β1, TGF-β2 and TGF-β3), which are involved in numerous
cellular processes, including cell differentiation, organ
development, wound healing and immune regulation (10). However, the distinct biological
functions of different isoforms in vivo are still under
debate. Accumulating evidence has revealed that TGF-β1 plays a
vital role in fibrogenesis and myocardial remodeling (32). TGF-β1 mediates the process of ECM
production, which can induce CF differentiation and promote
collagen synthesis (33). In this
study, the present results showed that TGF-β1 expression was
upregulated by ISO in the heart, which was attenuated by G.
acuta treatment. The results were consistent with the authors'
previous study (18). Therefore,
anti-fibrotic effects of G. acuta may be mediated by
inhibiting the TGF-β1 signaling at least partly.
TGF-β1 signaling regulates tissue fibrosis and
tissue scarring by activating specific downstream serine/threonine
kinase receptors. TβRII is the specific downstream serine/threonine
kinase receptor of TGF-β1. TGF-β1 combines with TβRII, which
results in TβRII autophosphorylation (34). It is essential for its kinase
activity and regulation of downstream signaling to regulate tissue
fibrosis and tissue scarring. As a result, the activated TβRII
further binds and phosphorylates TβRI (32). Activation of this receptor complex
leads to downstream signaling molecule activation and then signal
transmission into the cells. In the present study, G. acuta
treatment alleviated the ISO-induced TβRI and II phosphorylation.
These results indicated that G. acuta could block the TGF-β
signaling pathway activation.
TGF-β signaling includes canonical and non-canonical
(Smad and non-Smad-based, respectively) signaling pathways
(12). Numerous studies have
demonstrated that the Smad-dependent pathway contributes to
myocardial fibrosis in the TGF-β-induced signaling pathway
(10,12,35,36). Smad proteins are the core
downstream signaling molecules of TGF-β1 and they are mediated by
the TGF-β receptor. Smads2 and 3 can be phosphorylated and
activated. The latter becomes a transcription complex by further
combining with Smad4. After this event, the complex moves into the
nucleus to complete the process of intracellular signaling
transduction via mediation of the target genes such as collagen and
α-SMA, leading to myocardial fibrosis (37). In the present study, it was
demonstrated that G. acuta caused a decrease in Smads2 and 3
phosphorylation levels and inhibited Smads2 and 4 expression in
ISO-induced myocardial fibrosis. It was confirmed that G.
acuta inhibited myocardial fibrosis via TGF-β1/Smads signaling
pathway suppression.
An increasing number of studies have demonstrated
that G. acuta exerts a protective effect on the heart
(15,16). In the authors' previous study, it
was found that G. acuta caused inhibition of myocardial
fibrosis (18). In the present
study, the mechanism underlying the anti-fibrotic effect was
further clarified to be based upon the TGF-β1/Smads signaling
pathway. Phytochemical analysis showed that G. acuta
contains numerous kinds of constituents, such as xanthones,
flavonoids, monoterpenes, lignans and phenolic acids, and xanthones
were elucidated to be main constituents in the plant (14-16,38,39). Recently, a few studies have
explored the effects of the primary active components of this plant
on anti-myocardial injury (14-16) and xanthones from G. acuta
can protect cardiomyocytes from injury (16,40). The present study first revealed
that the aqueous extracts of G. acuta, a form of clinical
application, contains three xanthones components by HPLC (13). The above results indicated that
xanthones were a kind of major bioactivity substances in G.
acuta. However, except for xanthones, it has been reported that
phenolics, flavonoids and lignan glycosides also have a protective
effect on the heart (17,41). Therefore, the effect of G.
acuta on myocardial fibrosis may be the potential synergistic
effect of various compounds. In addition, because of that, it is
difficult to find a suitable plant drug or single compound as a
positive control that was the main limitation of the present study.
Based upon the present study, it was hypothesized that the
inhibitor of TβR may be a potential positive control, such as
Galunisertib or LY2109761, which exhibited preclinical
anti-fibrotic effects (42,43).
For the first time, the present study demonstrated
that G. acuta treatment causes a reduction in TGF-β1 and
TβRI expression and TβRI and II phosphorylation levels, thereby
suppressing the Smads2 and 4 expression and Smads2 and 3
activation. This suppression further inhibits the ISO-induced α-SMA
and collagens I and III expression in rats. These findings
demonstrate that G. acuta has potential anti-fibrotic
function via inhibition of the TGF-β1/Smads signaling transduction
pathway. It will be necessary to confirm the specific active
ingredients that are responsible for the therapeutic effects of
G. acuta.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81573698
and 31271466), the Science Foundation for Youth of the Higher
Education Institutions of Hebei Province (grant no. 2016137), the
Projects of Hebei Provincial Administration of Traditional Chinese
Medicine (grant nos. 2016003 and 2012080) and the Graduate
Innovation Funding Projects of Hebei Province (grant no.
CXZZBS2018155).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
As the corresponding author, AL contributed to the
conception and design of the study. HY contributed to the study's
design, performed the experiments, analyzed the data and prepared
the manuscript for writing. GX performed western blot analysis. JHS
established the experimental animal model. CZ contributed
significantly to HPLC analysis and identification of the effective
constituent xanthones. YZ, JNS, YFL and YL were involved in
analyzing the data.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee of Hebei University of Chinese
Medicine (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Masci PG, Schuurman R, Andrea B, Ripoli A,
Coceani M, Chiappino S, Todiere G, Srebot V, Passino C, Aquaro GD,
et al: Myocardial fibrosis as a key determinant of left ventricular
remodeling in idiopathic dilated cardiomyopathy: A
contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc
Imaging. 6:790–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixon JA and Spinale FG: Pathophysiology
of myocardial injury and remodeling: Implications for molecular
imaging. J Nucl Med. 51(Suppl3r 1): 102S–106S. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Zhao Q and Kong W: Extracellular
matrix remodeling and cardiac fibrosis. Matrix Biol. 68-69:490–506.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frangogiannis NG: Cardiac fibrosis: Cell
biological mechanisms, molecular pathways and therapeutic
opportunities. Mol Aspects Med. 65:70–99. 2019. View Article : Google Scholar
|
|
5
|
Czubryt MP: Common threads in cardiac
fibrosis, infarct scar formation, and wound healing. Fibrogenesis
Tissue Repair. 5:192012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyers TA and Townsend D: Early right
ventricular fibrosis and reduction in biventricular cardiac reserve
in the dystrophin-deficient mdx heart. Am J Physiol Heart Circ
Physiol. 308:H303–H315. 2015. View Article : Google Scholar :
|
|
7
|
Raman B, Ariga R, Spartera M,
Sivalokanathan S, Chan K, Dass S, Petersen SE, Daniels MJ, Francis
J, Smillie R, et al: Progression of myocardial fibrosis in
hypertrophic cardiomyopathy: Mechanisms and clinical implications.
Eur Heart J Cardiovasc Imaging. 20:157–167. 2019. View Article : Google Scholar :
|
|
8
|
Goumans MJ and Ten Dijke P: TGF-β
signaling in control of cardiovascular function. Cold Spring Harb
Perspect Biol. 10:a0222102018. View Article : Google Scholar
|
|
9
|
Yue Y, Meng K, Pu Y and Zhang X:
Transforming growth factor beta (TGF-β) mediates cardiac fibrosis
and induces diabetic cardiomyopathy. Diabetes Res Clin Pract.
133:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leask A: Potential therapeutic targets for
cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF,
partners in fibroblast activation. Circ Res. 106:1675–1680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gyorfi AH, Matei AE and Distler JHW:
Targeting TGF-β signaling for the treatment of fibrosis. Matrix
Biol. 68-69:8–27. 2018. View Article : Google Scholar
|
|
13
|
Wunir, Chunliang and Khasbagan: Ewenki
folk medicinal plants and its comparison with Mongolian medicine.
Chin J Ethnomed Ethnopharm. 18:156–158. 2009.In Chinese.
|
|
14
|
Liu Y, Ni Y, Ruan J, Qu L, Yu H, Han L,
Zhang Y and Wang T: Bioactive gentixanthone and gentichromone from
the whole plants of Gentianella acuta (Michx.) Hulten. Fitoterapia.
113:164–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Wu G, Liu H, Xing N, Sun Y, Zhai
Y, Yang B, Kong AT, Kuang H and Wang Q: Cardioprotective effect of
the xanthones from Gentianella acuta against myocardial
ischemia/reperfusion injury in isolated rat heart. Biomed
Pharmacother. 93:626–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Wu G, Yu Y, Liu H, Yang B, Kuang H
and Wang Q: Xanthones isolated from Gentianella acuta and their
protective effects against H2O2-induced
myocardial cell injury. Nat Prod Res. 32:2171–2177. 2018.
View Article : Google Scholar
|
|
17
|
Yu Y, Wang ZB, Zhai YD, Song PY, Wang QH,
Yang BY and Kuang H: Lignan glycosides from Gentianella acuta
(Michx.) Hulten and their protective effects against
H2O2-induced apop-tosis in H9c2
cardiomyoblast. Rec Nat Prod. 8:234–241. 2014.
|
|
18
|
Li AY, Wang JJ, Yang SC, Zhao YS, Li JR,
Liu Y, Sun JH, An LP, Guan P and Ji ES: Protective role of
Gentianella acuta on isoprenaline induced myocardial fibrosis in
rats via inhibition of NF-κB pathway. Biomed Pharmacother.
110:733–741. 2019. View Article : Google Scholar
|
|
19
|
Xu SY, Bian RL and Chen X: Experimental
methodology of pharmacology. People's Medical Publishing House;
Beijing: 1982
|
|
20
|
Blanchard OL and Smoliga JM: Translating
dosages from animal models to human clinical trials-revisiting body
surface area scaling. FASEB J. 29:1629–1634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Chen X, Chen L, Zhou X, Zheng G,
Zhang H, Huang W and Cai J: Anti-fibrosis effect of scutellarin via
inhibition of endothelial-mesenchymal transition on
isoprenaline-induced myocardial fibrosis in rats. Molecules.
19:15611–15623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan Y, Xu L, Wang Y, Tuerdi N, Ye M and Qi
R: Preventive effects of astragaloside IV and its active sapogenin
cycloas-tragenol on cardiac fibrosis of mice by inhibiting the
NLRP3 inflammasome. Eur J Pharmacol. 833:545–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Zhan Y, Wang Y, Han D, Tao B, Luo
Z, Ma S, Wang Q, Li X, Fan L, et al: Chitosan/silk fibroin modified
nanofibrous patches with mesenchymal stem cells prevent heart
remodeling post-myocardial infarction in rats. Acta Biomater.
80:154–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koga M, Kuramochi M, Karim MR, Izawa T,
Kuwamura M and Yamate J: Immunohistochemical characterization of
myofibro-blasts appearing in isoproterenol-induced rat myocardial
fibrosis. J Vet Med Sci. 81:127–133. 2019. View Article : Google Scholar
|
|
25
|
Daley WP and Yamada KM: ECM-modulated
cellular dynamics as a driving force for tissue morphogenesis. Curr
Opin Genet Dev. 23:408–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gyöngyösi M, Winkler J, Ramos I, Do QT,
Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, et al:
Myocardial fibrosis: Biomedical research from bench to bedside. Eur
J Heart Fail. 19:177–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voorhees AP and Han HC: Biomechanics of
cardiac function. Compr Physiol. 5:1623–1644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nielsen SH, Mouton AJ, DeLeon-Pennell KY,
Genovese F, Karsdal M and Lindsey ML: Understanding cardiac
extracellular matrix remodeling to develop biomarkers of myocardial
infarction outcomes. Matrix Biol. 75-76:43–57. 2019. View Article : Google Scholar
|
|
29
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: The role of cardiac fibroblasts in post-myocardial heart tissue
repair. Exp Mol Pathol. 101:231–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Putten S, Shafieyan Y and Hinz B:
Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol.
93:133–142. 2016. View Article : Google Scholar
|
|
31
|
Gabbiani G: The myofibroblast in wound
healing and fibrocontractive diseases. J Pathol. 200:500–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dobaczewski M, Chen W and Frangogiannis
NG: Transforming growth factor (TGF)-β signaling in cardiac
remodeling. J Mol Cell Cardiol. 51:600–606. 2011. View Article : Google Scholar
|
|
33
|
Shinde AV, Humeres C and Frangogiannis NG:
The role of α-smooth muscle actin in fibroblast-mediated matrix
contraction and remodeling. Biochim Biophys Acta Mol Basis Dis.
1863:298–309. 2017. View Article : Google Scholar
|
|
34
|
Luo K and Lodish HF: Positive and negative
regulation of type II TGF-beta receptor signal transduction by
autophosphorylation on multiple serine residues. EMBO J.
16:1970–1981. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li PF, He RH, Shi SB, Li R, Wang QT, Rao
GT and Yang B: Modulation of miR-10a-mediated TGF-β1/Smads
signaling affects atrial fibrillation-induced cardiac fibrosis and
cardiac fibroblast proliferation. Biosci Rep. 39:BSR201819312019.
View Article : Google Scholar
|
|
36
|
Khalil H, Kanisicak O, Prasad V, Correll
RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, et al:
Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac
fibrosis. J Clin Invest. 127:3770–3783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Chu J, Yi P, Dong W, Saultz J,
Wang Y, Wang H, Scoville S, Zhang J, Wu LC, et al: SMAD4 promotes
TGF-β-independent NK cell homeostasis and maturation and antitumor
immunity. J Clin Invest. 128:5123–5136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding Z, Liu Y, Ruan J, Yang S, Yu H, Chen
M, Zhang Y and Wang T: Bioactive constituents from the whole plants
of Gentianella acuta (Michx.) Hulten. Molecules. 22:E13092017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng CY, Wu Q, Yin DD, Li B, Li SS, Tang
ZQ, Xu YJ and Wang LS: Determination of xanthones and flavonoids of
methanol extracts obtained from different parts of the plants of
three Gentianaceae species. J Pharm Biomed Anal. 161:455–463. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren K, Su H, Lv LJ, Yi LT, Gong X, Dang
LS, Zhang RF and Li MH: Effects of four compounds from Gentianella
acuta (Michx.) Hulten on hydrogen peroxide-induced injury in H9c2
cells. Biomed Res Int. 2019:26929702019. View Article : Google Scholar :
|
|
41
|
Tungmunnithum D, Thongboonyou A, Pholboon
A and Yangsabai A: Flavonoids and other phenolic compounds from
medicinal plants for pharmaceutical and medical aspects: An
overview. Medicines (Basel). 5:E932018. View Article : Google Scholar
|
|
42
|
Hammad S, Cavalcanti E, Werle J, Caruso
ML, Dropmann A, Ignazzi A, Ebert MP, Dooley S and Giannelli G:
Galunisertib modifies the liver fibrotic composition in the Abcb4Ko
mouse model. Arch Toxicol. 92:2297–2309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luangmonkong T, Suriguga S, Adhyatmika A,
Adlia A, Oosterhuis D, Suthisisang C, de Jong KP, Mutsaers HAM and
Olinga P: In vitro and ex vivo anti-fibrotic effects of LY2109761,
a small molecule inhibitor against TGF-β. Toxicol Appl Pharmacol.
355:127–137. 2018. View Article : Google Scholar : PubMed/NCBI
|