Introduction
Esophageal squamous cell carcinoma (ESCC), a major
histological type of esophageal cancer, is one of the most lethal
malignant cancers. In 2018, there were an estimated 455,800 new
cases and 400,200 deaths related to ESCC worldwide (1). The 5-year survival rate of patients
with ESCC is <10% in China, and <20% in the United States. In
China, ESCC is the main type of esophageal cancer, and its
incidence has gradually increased over the past decade (2). ESCC can be caused by a variety of
factors, including heavy smoking, alcohol consumption, foods rich
in nitrosamines or contaminated with mycotoxins, Barrett's
esophagus and esophageal reflux disease (3). Traditional treatments for ESCC
include surgical resection, chemotherapy and radiotherapy. However,
the majority of patients still suffer from tumor recurrence and
metastasis following complete ESCC resection (4). Chemotherapy is a widely used
alternative in ESCC treatment; however, drug resistance is
widespread (5). Therefore, it is
imperative to explore the pathogenesis of ESCC and develop novel
therapeutic targets for ESCC treatment and drug resistance.
Malignant tumors result from the instability of the
cell genome, which can occur when processes including DNA repair,
DNA replication and chromosome segregation are altered. Genetic
mutation is the most common cause of DNA damage. Xeroderma
pigmentosum complementation group D (XPD) can regulate the
transcription initiation and cleavage repair of damaged nucleotide
sequences and maintain the biological process in a normal and
orderly fashion (6). XPD plays an
important role in the repair of damage caused by oxidative stress
(7). A previous study found that
XPD gene polymorphism increases the risk of lung cancer in
residents of coal mines (8). XPD
polymorphisms are associated with the development of pre oral
cancer as well as oral cancer and its clinical course (9). It has been found that XPD can
inhibit the proliferation and migration of hepatocellular carcinoma
cells (10). However, the
biological roles of XPD in ESCC remain unclear.
In the present study, the mechanisms through which
XPD participates in the tumorigenesis and progression of ESCC were
investigated. An XPD gene-encoding plasmid was trans-fected into
ESCC cell lines (EC9706 and EC109 cells), and changes in the
molecular biological behavior of EC9706 or EC109 cells were
observed. In addition, the molecular mechanisms underlying the
XPD-mediated regulation of ESCC cell growth and invasion were
investigated.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco; Thermo Fisher
Scientific, Inc. The vacant vector plasmid pEGFP-N2 and the
recombinant plasmid pEGFP-N2/XPD were generously provided by
Jiangxi Provincial Key Laboratory of Molecular Medicine and these
plasmids have been described in previous studies (10,11). Lipofectamine™ 2000 and TRIzol
reagent were purchased from Invitrogen; Thermo Fisher Scientific,
Inc. PCR primers were synthesized by Sangon Biotech. The total
protein extraction kit was purchased from AmyJet Scientific Inc.
The reverse transcription kit was purchased from Fermentas; Thermo
Fisher Scientific, Inc. The Annexin V-FITC/PI kit was purchased
from Vazyme Biotech. The Cell Counting kit-8 was purchased from
Solarbio Science Technology. Transwell chambers were purchased from
BD Biosciences. Anti-XPD (ab54676) primary antibody was purchased
from Abcam. Primary antibodies against phosphoinositide 3-kinase
(PI3K; #4249), AKT (#4685), p-AKT (Ser473) (#4060), Bcl-2 (#15071),
p21 (#2947), p-p65 (Ser536) (#3033), p65 (#8242), p-signal
transducer and activator of transcription 3 (STAT3; Tyr705)
(#9145), STAT3 (#12640), p-p38 mitogen-activated protein kinase
(MAPK; Thr180/Tyr182) (#4511), p38 MAPK (#8690) and β-actin (#4970)
were purchased from Cell Signaling Technology, Inc. Horseradish
peroxidase-conjugated secondary antibodies (ZB-2305 and ZB-2306)
were purchased from Beijing Zhongshan Golden Bridge Biotechnology
Co. Ltd. Cisplatin, fluorouracil and LY294002 were purchased from
MedChemExpress (MCE).
Clinical specimens
A total of 20 ESCC tissue samples and adjacent
normal esophageal tissue samples (>5 cm away from the tumor)
were collected from patients who underwent gastroscopy, endoscopic
biopsy and pathological diagnosis at the Department of
Gastroenterology of Third Affiliated Hospital of Nanchang
University (Nanchang, China) between September, 2018 and December,
2018. The 20 ESCC cases were obtained from 14 males and 6 females
aged 41-77 years. No patient had received radiotherapy or
chemotherapy prior to the endoscopic biopsy. The present study was
approved by the Human Ethics Committee of Third Affiliated Hospital
of Nanchang University and prior written consent was obtained from
all patients.
Cells and cell culture
The human ESCC cell lines, EC9706 and EC109, were
obtained from the American Type Culture Collection (ATCC). All
cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin and incubated in a
humidified incubator at 37°C, 5% CO2 and 95% air.
Cell transfection
The EC9706 or EC109 Cells were divided into 3 groups
as follows: i) The untransfected control group (Ctrl); ii) pEGFP-N2
empty plasmid transfection group (pEGFP-N2); and iii) the
pEGFP-N2/XPD plasmid transfection group (pEGFP-N2/XPD). The
detailed procedures for transfection were described in a previous
study by the authors (12). At 48
h following the transfection of the pEGFP-N2 or pEGFP-N2/XPD
plasmids, green fluorescence was observed under a fluorescence
microscope (Olympus Corp.). Western blot analysis and RT-qPCR were
used to detect the protein and mRNA expression levels of XPD.
Cell proliferation assay
At 0, 24, 48, 72 or 96 h following transfection with
XPD plasmid, the EC9706 or EC109 cells were seeded into 96-well
culture plates at a density of 4×103 cells/well. In
addition, for the pEGFP-N2/XPD + LY294002 group, the EC9706 or
EC109 cells were treated with 10 µmol/l of LY294002 for 0,
24, 48, 72 or 96 h following transfection with XPD plasmid. The
cells in each group were washed with PBS and incubated with 100
µl CCK-8 solution for 1 h at 37°C. The absorbance at 450 nm
was measured using a microplate reader (Thermo Fisher Scientific,
Inc.). Each independent experiment was performed 3 times. Data were
calculated as the means ± SD.
Cell apoptosis assay
The EC9706 or EC109 cells in each group were
trypsinized and collected by centrifugation at 37°C for 5 min at a
speed of 1,000 × g. A total of 1×105 cells were then
resuspended in 500 µl of buffer and incubated with Annexin
V-FITC/PI kit for 15 min at room temperature. The apoptotic rate of
EC9706 or EC109 cells was detected using a flow cytometer
(FACSCalibur, BD Biosciences). Each experiment was performed in
triplicate independently.
Cell Transwell migration and invasion
assays
Cell migration and invasion assays were performed
using Transwell chambers without or with Matrigel according to the
manufacturer's instructions and as previously described (12). A total of 2×105 EC9706
or EC109 cells were seeded into the upper chamber of the insert in
serum-free DMEM. The lower chamber of the insert contained DMEM
supplemented with 10% FBS as a chemoattractant. Following
incubation in a humidified incubator at 37°C 5% CO2 and
95% air for 48 h, EC9706 or EC109 cells remaining on the insert's
top layer were wiped off with a cotton swab. Cells that migrated or
invaded to the lower surface of the membrane were stained with
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) for 20 min at room temperature and imaged under an inverted
light microscope (x50 magnification). Cells in 5 fields were
counted to calculate the cell migration or invasion. Each
experiment was performed in triplicate.
Chemosensitivity assay
At 24 h following transfection with the XPD plasmid,
EC9706 or EC109 cells were seeded into 96-well plates at a density
of 4×103 cells/well. The medium was then discarded and
the cells were incubated at 37°C in the presence of 0, 20, 40, 80
and 100 µg/ml cisplatin or fluorouracil for 72 h. EC9706 or
EC109 cell sensitivity to cisplatin or fluorouracil in each group
was detected by CCK-8 assay as described above. Cell viability (%)
was calculated by using the following formula: OD value (0, 20, 40,
80, or 100 µg/ml)/OD value (0 µg/ml) ×100%.
RT-qPCR
Total RNA was isolated from all tissue samples,
EC9706, or EC109 cells according to the standard TRIzol method. A
total of 1 µg RNA was used as a template for cDNA synthesis
using a reverse transcription kit at 37°C for 15 min and 85°C for
30 sec. XPD, PI3K, AKT, Bcl-2, c-Myc, p21, Cyclin D1, vascular
endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-9
and β-actin primers were designed using Primer Premier 5.0
software. The primer sequences are presented in Table I. Quantitative PCR was performed
using TB Green Premix Ex Taq (Takara Bio, Inc.) under the following
thermal cycling conditions: Initial denaturation at 95°C for 5 min;
subsequent 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The
relative expression mRNA levels of XPD, PI3K, AKT, Bcl-2, c-Myc,
p21, Cyclin D1, VEGF and MMP-9 were calculated using the
2-ΔΔCq (13) method.
β-actin was used as an endogenous control. All reactions were
repeated 3 times.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Primer
sequences |
|---|
| XPD | F:
5′-TCTGCCTCTGCCCTATGAT-3′ |
| R:
5′-CGATTCCCTCGGACACTTT-3′ |
| PI3K | F:
5′-TGGCCTTAGCTCTTAGCCAAACAC-3′ |
| R:
5′-ATTGGAACACGGCCTTTGACA-3′ |
| AKT | F:
5′-CTGTGCCTATGCTGCCCAT-3′ |
| R:
5′-CAGTGCGATGTCGTGGAGG-3′ |
| Bcl-2 | F:
5′-GGATAACGGAGGCTGGGATGC-3′ |
| R:
5′-GACTTCACTTGTGGCCCAGAT-3′ |
| c-Myc | F:
5′-TGTGTTACGGTCGCGTCTTT-3′ |
| R:
5′-AACAGCTCGGTCACCATCTC-3′ |
| p21 | F:
5′-GACCTGTCACTGTCTTGTAC-3′ |
| R:
5′-CTCTCATTCAACCGCCTAG-3′ |
| Cyclin D1 | F:
5′-CCAGACCCACGTTTCTTTGC-3′ |
| R:
5′-ATCCCTAGAAACACCACGGC-3′ |
| VEGF | F: 5′-
ACCCACTCACTGGCTGTTT-3′ |
| R:
5′-CGGGCTCTGAGATGTTCAG-3′ |
| MMP-9 | F: 5′-
TAGACGCTGCTCCCCTCA-3′ |
| R:
5′-GCGGGTGTAACCATAGCG-3′ |
| β-actin | F:
5′-AAGGTGACAGCAGTCGGTT-3′ |
| R:
5′-TGTGTGGACTTGGGAGAGG-3′ |
Western blot analysis
Total protein of all tissue samples, EC9706 or EC109
cells was extracted using a total protein extraction kit. The
protein concentration was measured using the BCA assay kit
according to the manufacturer's instructions. Protein samples were
separated by 10% SDS polyacrylamide gel electrophoresis and
transferred to nitrocellulose membranes. The membranes were blocked
with 5% non-fat milk in Tris buffered saline containing Tween-20.
The membranes were then incubated with specific primary antibodies
against XPD (1:1,000), PI3K (1:1,000), AKT (1:1,000), p-AKT
(1:2,000), Bcl-2 (1:1,000), p21 (1:2,000), p65 (1:1,000), p-p65
(1:1,000), STAT3 (1:1,000), p-STAT3 (1:2,000), p38 MAPK (1:1,000),
p-p38 MAPK (1:1,000) and β-actin (1:3,000) at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated
secondary antibodies (1:800) for 1 h at room temperature. Finally,
the signal was visualized using ECL reagent. ImageJ 1.8.0 software
was used to determine the density of the protein bands.
Anti-β-actin antibody was used as an endogenous control.
Statistical analysis
All experiments were performed at least 3 times and
all final data were expressed as the mean ± SD. Graphpad prism 7.0
software was used to evaluate the experimental data through one-way
analysis of variance (ANOVA) among groups and the LSD post hoc
test. The t-test was used to determine differences between 2
groups. Differences in the expression levels of XPD in ESCC tissue
samples were evaluated using a Fisher's exact test or Pearson's
χ2 test. Differences at P<0.05 were considered
statistically significant.
Results
XPD expression is downregulated in human
ESCC tissue samples
To examine the expression level of XPD in ESCC, 20
pairs of human ESCC tissue samples and adjacent normal esophageal
tissue samples were collected to detect the mRNA and protein
expression levels of XPD. The mRNA and protein expression levels of
XPD were markedly decreased in human ESCC tissue samples compared
to adjacent normal esophageal tissue samples (Figs. 1 and S1). The association between XPD
expression and various clinicopathological characteristics is shown
in Table II. XPD expression was
associated with histological grade (P=0.011) and clinical TNM stage
(P=0.032), indicating that XPD may be associated with the
progression of ESCC.
 | Table IIAssociation between XPD expression
and clinico-pathological factors of ESCC patients. |
Table II
Association between XPD expression
and clinico-pathological factors of ESCC patients.
| Factors | No. of
patients | XPD expression
| P-value |
|---|
| Low | High |
|---|
| Sex | | | | 0.354 |
| Male | 14 | 8 | 6 | |
| Female | 6 | 5 | 1 | |
| Age (years) | | | | 0.174 |
| <55 | 7 | 3 | 4 | |
| ≥55 | 13 | 10 | 3 | |
| Histological
(differentiation) grade | | | | 0.011 |
| Well | 1 | 0 | 1 | |
| Moderately | 5 | 1 | 4 | |
| Poorly | 14 | 12 | 2 | |
| TNM stage | | | | 0.032 |
| I | 2 | 0 | 2 | |
| II | 7 | 3 | 4 | |
| III | 6 | 5 | 1 | |
| IV | 5 | 5 | 0 | |
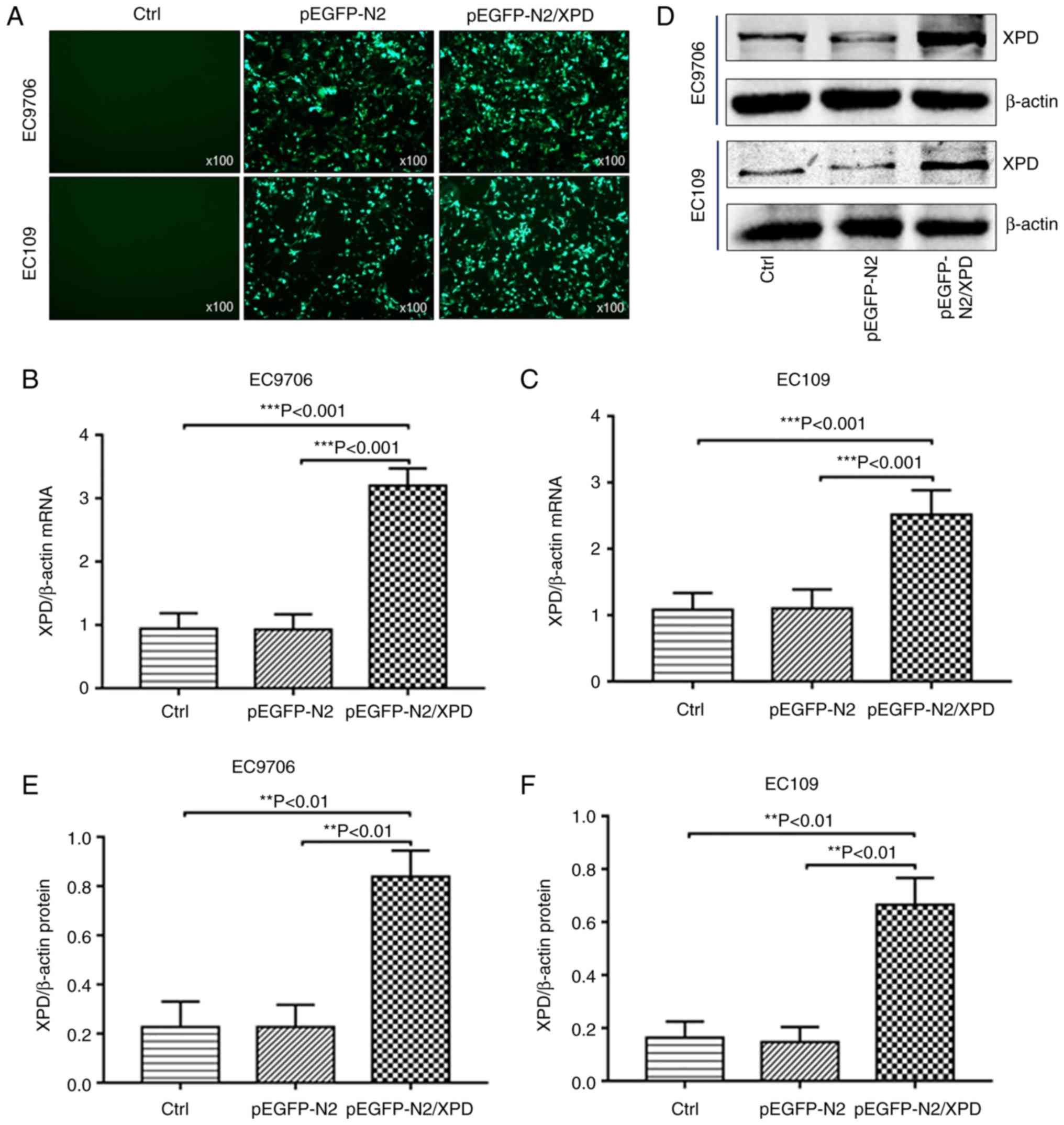
Successful transfection of XPD plasmid
and upregulation of XPD
The pEGFP-N2/XPD plasmid or pEGFP-N2 plasmid were
transiently transfected into EC9706 cells and EC109 cells. At 48 h
following transfection, the cells were observed under a
fluorescence microscope. As shown in Fig. 2A, cells successfully transfected
with pEGFP-N2/XPD or pEGFP-N2 plasmid exhibited green fluorescence.
No green fluorescence was observed in the control (Ctrl) group. The
results of western blot analysis and RT-qPCR revealed that the mRNA
(Fig. 2B and C) and protein
(Fig. 2D-F) expression levels of
XPD in EC9706 cells or EC109 cells of the pEGFP-N2/XPD group were
markedly upregulated.
XPD overexpression suppresses the
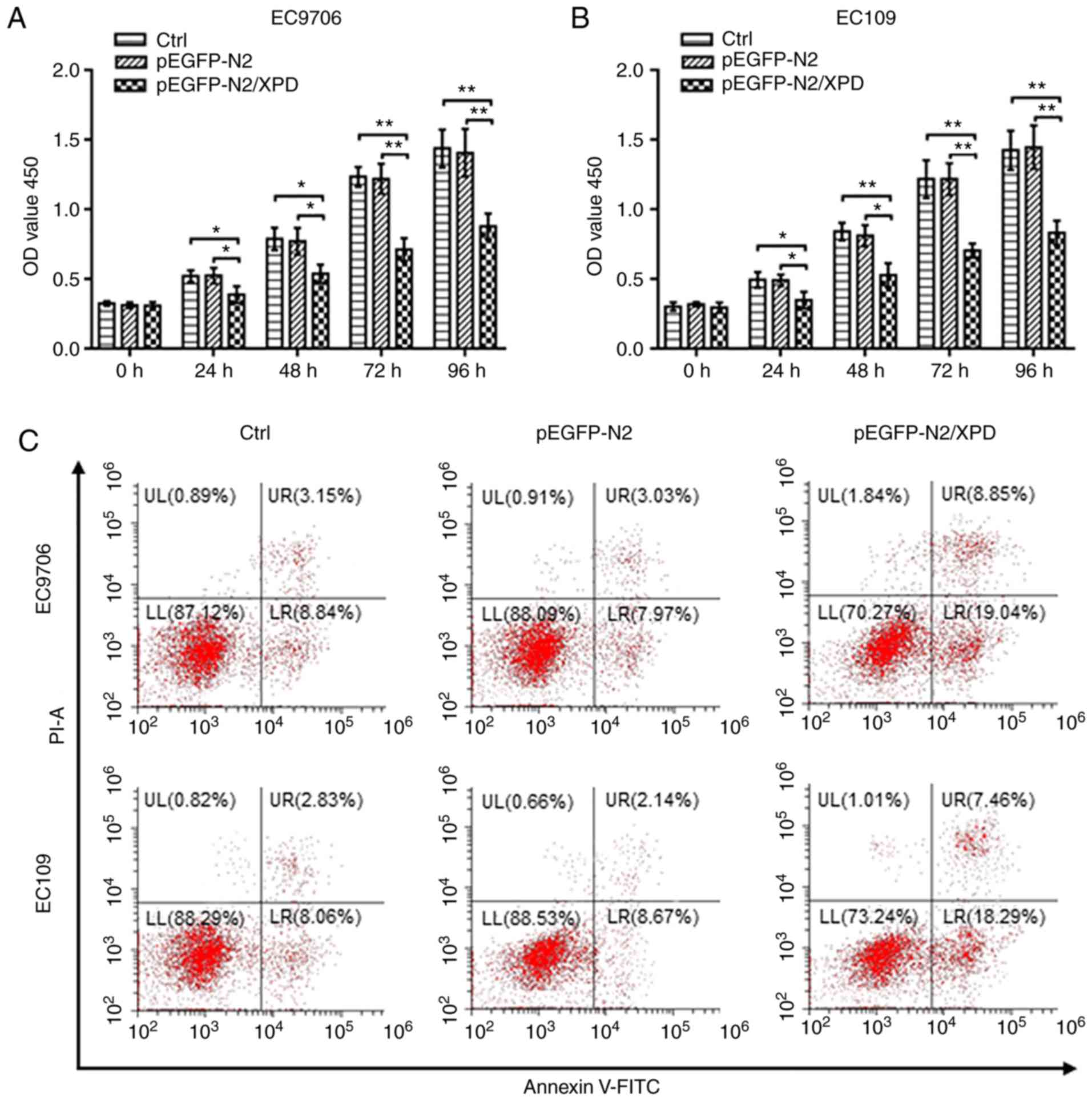
proliferation of EC9706 cells and EC109 cells
Cell viability was used to investigate whether XPD
overexpression affects the proliferation of EC9706 or EC109 cells.
At 24, 48, 72 or 96 h following transfection with XPD plasmid, the
optical density (OD) value of the pEGFP-N2/XPD group was
significantly reduced compared to the that of the control (Ctrl)
group and pEGFP-N2 group (P<0.05 or P<0.01; Fig. 3A and B). There was no significant
difference in the OD value between the Ctrl group and pEGFP-N2
group (P>0.05; Fig. 3A and B).
XPD overexpression can thus inhibit the proliferation of EC9706 and
EC109 cells.
Upregulation of XPD increases the
apoptosis of EC9706 and EC109 cells
As shown in Fig.
3C-E, compared with the Ctrl group and the pEGFP-N2 group, the
apoptotic rate of the EC9706 or EC109 cells in the pEGFP-N2/XPD
group was significantly higher (P<0.001). There was no marked
difference in the cell apoptotic rate between the Ctrl group and
the pEGFP-N2 group (P>0.05). XPD upregulation can thus promote
the apoptosis of EC9706 and EC109 cells.
XPD overexpression inhibits the migration
and invasion of EC9706 and EC109 cells
Transwell migration and invasion assays were used to
examine the effects of XPD overexpression on the migration and
invasion of EC9706 or EC109 cells. As shown in Fig. 3F-H, compared to the Ctrl group and
pEGFP-N2 group, the migratory capacity of the EC9706 or EC109 cells
in the pEGFP-N2/XPD group was markedly decreased (P<0.01).
Compared to the Ctrl group and the pEGFP-N2 group, the upregulation
of XPD in the EC9706 cells or EC109 cells significantly reduced the
invasive ability (P<0.001; Fig.
3I-K). There was no significant difference in the migratory and
invasive capacities between the pEGFP-N2 group and Ctrl group
(P>0.05; Fig. 3F-K). XPD
upregulation can thus inhibit the migration and invasion of EC9706
or EC109 cells.
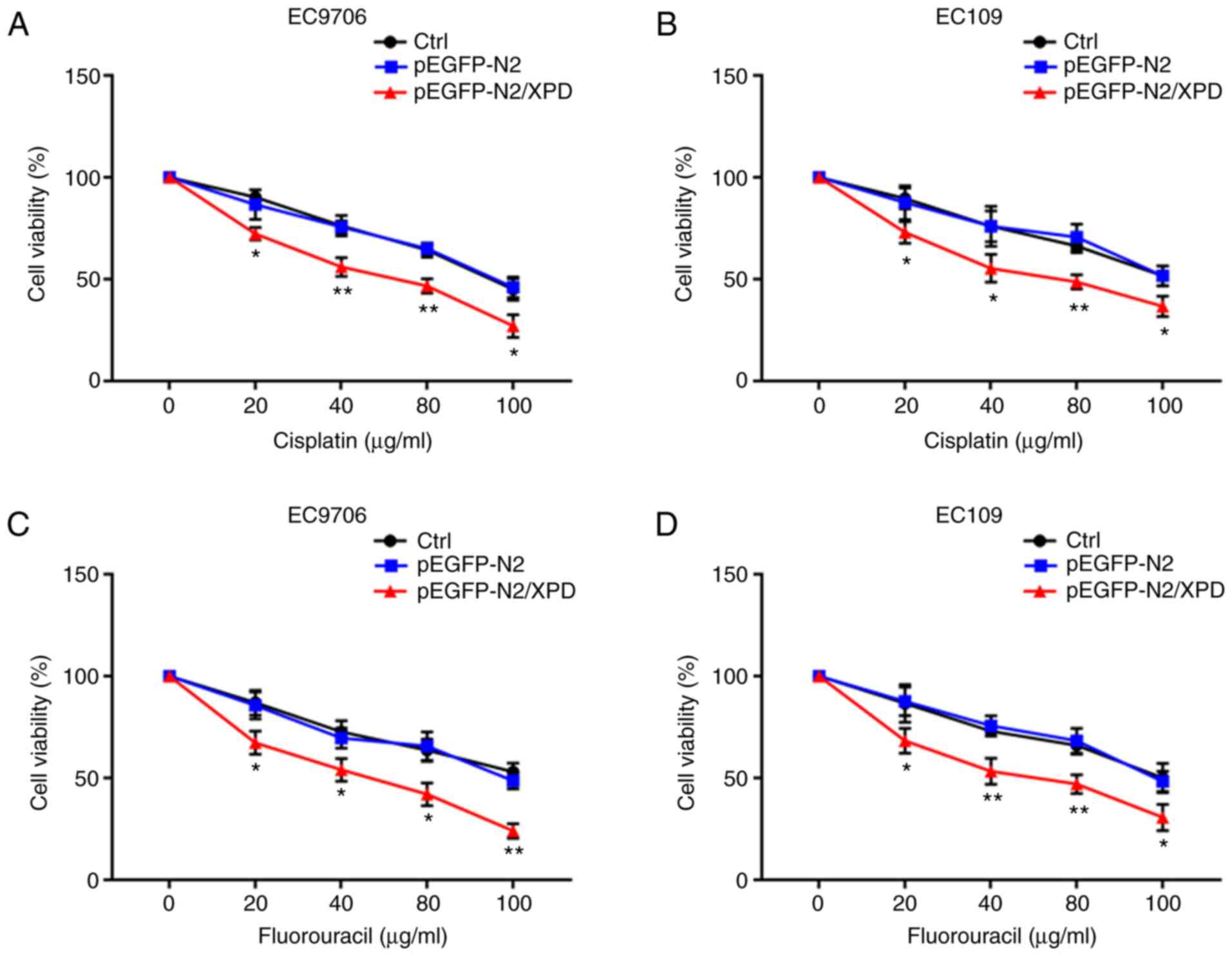
Overexpression of XPD enhances the
chemosensitivity of EC9706 and EC109 cells to cisplatin or
fluorouracil
At 24 h following transfection with XPD plasmid, the
EC9706 or EC109 cells were incubated in the presence of 0, 20, 40,
80 and 100 µg/ml cisplatin or fluorouracil for 72 h. As
shown in Fig. 4, the results of
CCK-8 assay revealed that compared with the Ctrl group and the
pEGFP-N2 group, the chemosensitivity of the EC9706 and EC109 cells
to cisplatin or fluorouracil in the pEGFP-N2/XPD group was markedly
enhanced (P<0.05 or P<0.01). XPD overexpression can thus
increase the chemosensitivity of EC9706 and EC109 cells to
cisplatin or fluorouracil.
Upregulation of XPD inhibits the PI3K/AKT
signaling pathway
Previous studies have found that the tumorigenesis
and progression of ESCC are associated with the PI3K/AKT signaling
pathway (14,15). In the present study, RT-qPCR and
western blot analysis were used to investigate the potential
effects of XPD overexpression on the expression levels of PI3K/AKT
signaling pathway-related factors in the EC9706 or EC109 cells. As
shown in Fig. 5A and B, compared
with the pEGFP-N2 group and the Ctrl group, the mRNA expression
levels of PI3K, Bcl-2, c-Myc, Cyclin D1, VEGF and MMP-9 in the
EC9706 or EC109 cells of pEGFP-N2/XPD group were markedly decreased
(P<0.05 or P<0.01), whereas the mRNA expression of p21 was
markedly increased (P<0.01). There was no significant difference
in mRNA expression level of AKT among the pEGFP-N2/XPD, pEGFP-N2
and Ctrl groups (P>0.05). As shown in Fig. 5C-F, compared to the pEGFP-N2 group
and the Ctrl group, the protein expression levels of PI3K, P-AKT
and Bcl-2 in the pEGFP-N2/XPD group were markedly decreased
(P<0.05 or P<0.01), whereas the protein expression level of
p21 in the pEGFP-N2/XPD group was significantly upregulated
(P<0.05 or P<0.01). There was no significant difference in
the protein expression level of AKT among the pEGFP-N2/XPD,
pEGFP-N2 and Ctrl groups (P>0.05). In addition, there was no
significant difference in the expression levels of PI3K/AKT
signaling pathway-associated factors between the pEGFP-N2 group and
the Ctrl group (P>0.05). To further investigate the involvement
of the PI3K/AKT pathway in XPD-inhibited ESCC progression, the
PI3K/AKT pathway inhibitor, LY294002 (10 µM) was used.
Following transfection with XPD plasmid, the EC9706 or EC109 cells
in the pEGFP-N2/XPD + LY294002 group were treated with 10
µmol/l of LY294002 for 0, 24, 48, 72 or 96 h. The
proliferative abilities of the EC9706 or EC109 cells were then
detected by CCK-8 assay. The results revealed that XPD
overexpression markedly inhibited the proliferative capabilities of
the EC9706 or EC109 cells, and LY294002 significantly enhanced the
suppressive effects (Fig. 5G and
H, P<0.05 or P<0.01). To determine the association
between XPD and other signaling pathways in ESCC, such as the
NF-κB, JAK2/STAT3 and MAPK signaling pathways (16-18), the protein expression levels of
p65, p-p65, STAT3, p-STAT3, p38 MAPK and p-p38 MAPK were detected
in the EC9706 cells following the overexpression of XPD. As shown
in Fig. 5I and J, there was no
significant difference in the protein expression levels of NF-κB
p65, JAK2/STAT3, and p38 MAPK signaling pathways among the
pEGFP-N2/XPD, pEGFP-N2 and Ctrl groups (P>0.05). These results
indicated that the upregulation of XPD can inhibit the PI3K/AKT
signaling pathway in ESCC.
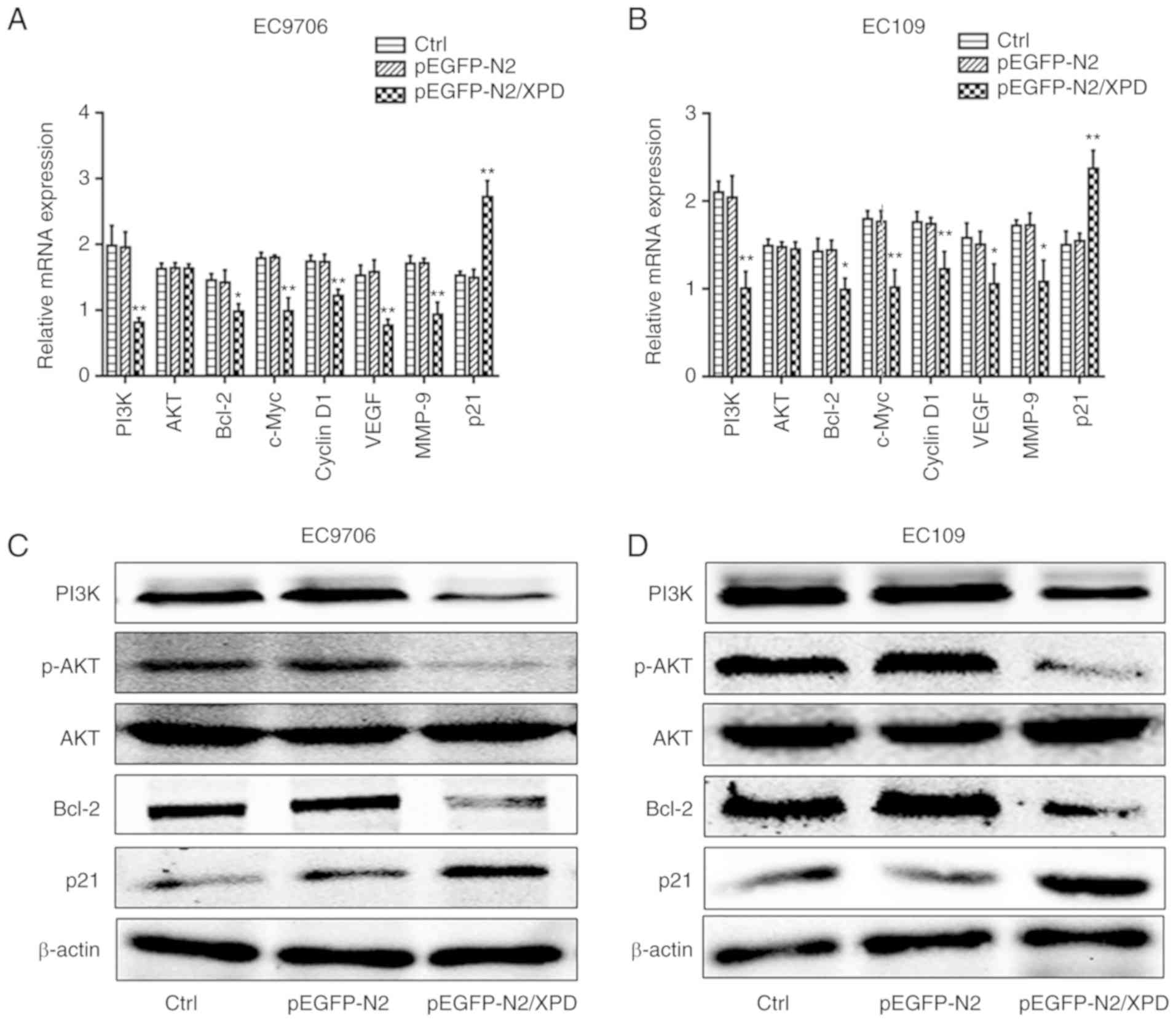 | Figure 5Effects of XPD overexpression on
multiple signaling pathways in EC9706 cells and EC109 cells. (A and
B) The mRNA expression levels of PI3K, AKT, Bcl-2, c-Myc, Cyclin
D1, VEGF, MMP-9 and p21 in EC9706 or EC109 cells were detected by
RT-qPCR. *P<0.05 or **P<0.01, vs. Ctrl
group or pEGFP-N2 group (n=3). (C-F) The protein expression levels
of PI3K, p-AKT, AKT, Bcl-2 and p21 in EC9706 or EC109 cells were
detected by western blot analysis. (G and H) Following treatment
with or without LY294002 (10 µM), cell proliferation was
examined by CCK-8 assay. *P<0.05 or
**P<0.01, vs. pEGFP-N2/XPD group (n=3). (I and J) The
protein expression levels of p-p65, p65, p-STAT3, STAT3, p-p38, and
p38 in EC9706 cells were detected by western blot analysis. Data
represent the means ± standard deviation. pEGFP-N2/XPD,
pEGFP-N2/XPD plasmid was transfected into EC9706 or EC109 cells;
pEGFP-N2, pEGFP-N2 plasmid was transfected into EC9706 or EC109
cells; Ctrl, no transfection; pEGFP-N2/XPD + LY294002, pEGFP-N2/XPD
plasmid was transfected into EC9706 or EC109 cells and cells were
then treated with LY294002 (10 µM); XPD, xeroderma
pigmentosum complementation group D. |
Discussion
ESCC is one of the most common malignancies and the
most commonly diagnosed type of esophageal cancer (19). ESCC ranks as the 6th most lethal
cancer worldwide due to its high mortality (19,20). Esophageal cancer is often
diagnosed at an advanced stage and the patient survival rate is
very low due to the lack of specific symptoms and effective early
diagnostic methods (21).
Although multiple treatments, such as surgical resection,
chemotherapy, and radiotherapy have been applied for ESCC
treatment, the prognosis of ESCC remains poor (22-24). Therefore, it is of utmost
importance to better understand the molecular mechanisms
responsible for the development of ESCC and to explore novel
therapies with which to improve the survival of patients with
ESCC.
XPD is located on 19q13.2-q13.3 and encodes an
ATP-dependent DNA helicase (25).
XPD751 polymorphism has been shown to be associated with the
occurrence and development of a wide range of malignancies, such as
esophageal cancer, gastric cancer, and colorectal cancer (26-28). Previous studies have demonstrated
that XPD expression serves as a tumor suppressor in HCC (10,11,29). In a present study by the authors,
it was found that the mRNA and protein expression levels of XPD in
ESCC tissue samples were significantly lower than those in the
adjacent normal esophageal tissue samples. To determine the role of
XPD in ESCC, the in vitro cellular effects of XPD
overexpression in ESCC were investigated through XPD transfection
into EC9706 and EC109 cells. In the present study, it was
demonstrated that XPD gene overexpression significantly reduced the
proliferation and inhibited the migration and invasion of EC9706 or
EC109 cells, whilst increasing cell apoptosis. Additionally, the
upregulation of XPD gene enhanced the chemosensitivity of EC9706
and EC109 cells to cisplatin or fluorouracil.
Previous studies have indicated that the PI3K/AKT
signaling pathway plays an important role in the occurrence,
development and invasion of malignant tumors, such as esophageal
cancer, colon cancer and gastric cancer (30-32). A previous study by the authors
demonstrated that the inhibition of the activation of AKT and the
promotion of the expression of p21 inhibited cell proliferation and
promotes cell apoptosis in hepatocellular carcinoma (12). In the present study, XPD was shown
to be involved in the phosphorylation of AKT. Following XPD
upregulation, the protein expression level of p-AKT was
significantly decreased, indicating that XPD overexpression may
inhibit the activation of AKT and suppress PI3K/AKT signal
transduction. p21 has been demonstrated to be involved in cell
cycle progression and apoptosis, as well as in behaviors essential
for tumorigenesis and tumor progression (33). The present study also demonstrated
that the mRNA and protein expression levels of p21 were
significantly upregulated following the overexpression of XPD.
Previous studies have demonstrated that c-Myc, Cyclin D1 and Bcl-2
play crucial roles in regulating tumorigenesis and are
significantly upregulated during tumor progression (34-36). The present study also revealed
that the expression levels of c-Myc, Cyclin D1 and Bcl-2 were
significantly downregulated following the over-expression of XPD.
Previous studies have demonstrated that the functions of VEGF and
MMP-9 are essential for tumor invasion (37,38). The present study also demonstrated
that the mRNA expression levels of VEGF and MMP-9 were both
significantly decreased following the overexpression of XPD. As
shown by the results of the present study, the expression level of
XPD was low in ESCC tissues, EC9706 cells, or EC109 cells; thus,
XPD overexpression experiments were conducted and this is the
reason that XPD knockdown experiments were not conducted in the
EC9706 or EC109 cells. As such, the fact that there no XPD
knockdown experiments were performed is a limitation of the present
study.
In conclusion, the findings of the present study
demonstrate that the upregulation of XPD inhibits the
proliferation, abrogates the migration and invasion, and promotes
the apoptosis of EC9706 and EC109 cells by inhibiting the PI3K/AKT
signaling pathway. XPD overexpression also enhanced the
chemosensitivity of EC9706 and EC109 cells to cisplatin or
fluorouracil. Based on the results of the present study, XPD may
thus become a potential target for ESCC treatment and drug
resistance in the future.
Supplementary Data
Funding
The present study was supported by the National
Natural Science Foundation of China (grant. no. 81660408) and the
Health and Family Planning Commission Science and Technology Plan
of Jiangxi Province (grant. no. 20184002).
Availability of data and materials
The datasets used in the present study can be
obtained from the corresponding author upon reasonable request.
Authors' contributions
JJ, SL and NF designed the experiments. JJ, SL, LZL,
LZ, LY, LHG and YQH performed the experiments. SL contributed to
the data analysis. JJ wrote the manuscript and conducted the
revision of the manuscript. NF was responsible for the final
modification of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee of Third Affiliated Hospital of Nanchang University and
prior written consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Bin Li, Dr
Jian-Bin Qin and Dr Feng Deng (Department of Gastroenterology,
Third Affiliated Hospital of Nanchang University) for providing
their valuable assistance with this research.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yutong H, Xiaoli X, Shumei L, Shan S, Di L
and Baoen S: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with esophageal cancer in a high
incidence area in China. Arch Med Res. 46:557–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S,
Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al:
Alcohol drinking, cigarette smoking, and the development of
squamous cell carcinoma of the esophagus: Molecular mechanisms of
carcinogenesis. Int J Clin Oncol. 15:135–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon DH, Jeon JH, Yang HC, Kim YI, Lee JY,
Kim MS, Lee JM and Lee GK: Intramural metastasis as a risk factor
for recurrence in esophageal squamous cell carcinoma. Ann Thorac
Surg. 106:249–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, Chen S, Yu J, Li Y, Zhang XY, Yang
L, Zhang H, Hou Q, Jiang M, Brunicardi FC, et al: Single-cell
transcriptome analyses reveal molecular signals to intrinsic and
acquired paclitaxel resistance in esophageal squamous cancer cells.
Cancer Lett. 420:156–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oksenych V and Coin F: The long unwinding
road: XPB and XPD helicases in damaged DNA opening. Cell Cycle.
9:90–96. 2010. View Article : Google Scholar
|
|
7
|
Lerner LK, Moreno NC, Rocha CRR, Munford
V, Santos V, Soltys DT, Garcia CCM, Sarasin A and Menck CFM:
XPD/ERCC2 mutations interfere in cellular responses to oxida-tive
stress. Mutagenesis. 34:341–354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minina VI, Bakanova ML, Soboleva OA,
Ryzhkova AV, Titov RA, Savchenko YA, Sinitsky MY, Voronina EN,
Titov VA and Glushkov AN: Polymorphisms in DNA repair genes in lung
cancer patients living in a coal-mining region. Eur J Cancer Prev.
28:522–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nigam K, Yadav SK, Samadi FM, Bhatt ML,
Gupta S and Sanyal S: Risk modulation of oral pre cancer and cancer
with polymorphisms in XPD and XPG genes in North Indian population.
Asian Pac J Cancer Prev. 20:2397–2403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Z, Wang Y and Ding H: XPD suppresses
cell proliferation and migration via miR-29a-3p-Mdm2/PDGF-B axis in
HCC. Cell Biosci. 9:62019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding H, Xu JJ, Huang Y, Du FT and Zhang
JX: XPD could suppress growth of HepG2.2.15 and down-regulate the
expression of hepatitis B virus x protein through P53 pathway.
Biochem Biophys Res Commun. 419:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang DH, Jian J, Li S, Zhang Y and Liu
LZ: TPX2 silencing exerts anti-tumor effects on hepatocellular
carcinoma by regulating the PI3K/AKT signaling pathway. Int J Mol
Med. 44:2113–2122. 2019.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Wang L, Zhang Z, Yu X, Li Q, Wang Q, Chang
A, Huang X, Han X, Song Y, Hu J, et al: SOX9/miR-203a axis drives
PI3K/AKT signaling to promote esophageal cancer progression. Cancer
Lett. 468:14–26. 2020. View Article : Google Scholar
|
|
15
|
Zheng TL, Li DP, He ZF and Zhao S: MiR-145
sensitizes esophageal squamous cell carcinoma to cisplatin through
directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int.
19:2502019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Zheng S, Liu T, Liu Q, Chen Y, Tan
D, Ma R and Lu X: MCP2 activates NF-κB signaling pathway promoting
the migration and invasion of ESCC cells. Cell Biol Int.
42:365–372. 2018. View Article : Google Scholar
|
|
17
|
Lu Z, Lu C, Li C, Jiao Y, Li Y and Zhang
G: Dracorhodin perchlorate induces apoptosis and G2/M cell cycle
arrest in human esophageal squamous cell carcinoma through
inhibition of the JAK2/STAT3 and AKT/FOXO3a pathways. Mol Med Rep.
20:2091–2100. 2019.PubMed/NCBI
|
|
18
|
Wang WW, Zhao ZH, Wang L, Li P, Chen KS,
Zhang JY, Li WC, Jiang GZ and Li XN: MicroRNA-134 prevents the
progression of esophageal squamous cell carcinoma via the
PLXNA1-mediated MAPK signalling pathway. EBioMedicine. 46:66–78.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
20
|
Wiedmann MW and Mössner J: New and
emerging combination therapies for esophageal cancer. Cancer Manag
Res. 5:133–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohashi S, Miyamoto S, Kikuchi O, Goto T,
Amanuma Y and Muto M: Recent advances from basic and clinical
studies of esophageal squamous cell carcinoma. Gastroenterology.
149:1700–1715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong H, Jie H, Liyu R, Zerui C, Borong S
and Hongwei L: Prognostic significance of middle paraesophageal
lymph node metastasis in resectable esophageal squamous cell
carcinoma: A STROBE-compliant retrospective study. Medicine
(Baltimore). 98:e175312019. View Article : Google Scholar
|
|
23
|
Zhao Y, Han L, Zhang W, Shan L, Wang Y,
Song P, Peng C and Zhao X: Preoperative chemotherapy compared to
postoperative adjuvant chemotherapy for squamous cell carcinoma of
the thoracic oesophagus with the detection of circulation tumour
cells randomized controlled trial. Int J Surg. 73:1–8. 2020.
View Article : Google Scholar
|
|
24
|
Zhang Z, Xu L, Di X, Zhang C, Ge X and Sun
X: A retrospective study of postoperative radiotherapy for locally
advanced esophageal squamous cell carcinoma. Ann Palliat Med.
8:708–716. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan Q, Chen Z, Chen Q and Zhi X: XRCC1
and XPD polymorphisms and their relation to the clinical course in
hepa-tocarcinoma patients. Oncol Lett. 14:2783–2788. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon HH, Catalano PJ, Murphy KM, Skaar TC,
Philips S, Powell M, Montgomery EA, Hafez MJ, Offer SM, Liu G, et
al: Genetic variation in DNA-repair pathways and response to
radio-chemotherapy in esophageal adenocarcinoma: A retrospective
cohort study of the eastern cooperative oncology group. BMC Cancer.
11:1762011. View Article : Google Scholar
|
|
27
|
Engin AB, Karahalil B, Engin A and
Karakaya AE: DNA repair enzyme polymorphisms and oxidative stress
in a Turkish population with gastric carcinoma. Mol Biol Rep.
38:5379–5386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang MY, Wang JY, Huang ML, Chang HJ and
Lin SR: Polymorphisms in XPD and ERCC1 associated with colorectal
cancer outcome. Int J Mol Sci. 14:4121–4134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng JF, Li LL, Lu J, Yan K, Guo WH and
Zhang JX: XPD functions as a tumor suppressor and dysregulates
autophagy in cultures HepG2 cells. Med Sci Monit. 21:1562–1568.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Javadinia SA, Shahidsales S, Fanipakdel A,
Mostafapour A, Joudi-Mashhad M, Ferns GA and Avan A: The esophageal
cancer and the PI3K/AKT/mTOR signaling regulatory microRNAs: A
novel marker for prognosis, and a possible target for
immuno-therapy. Curr Pharm Des. 24:4646–4651. 2018. View Article : Google Scholar
|
|
31
|
Han B, Jiang P, Li Z, Yu Y, Huang T, Ye X
and Li X: Coptisine-induced apoptosis in human colon cancer cells
(HCT-116) is mediated by PI3K/Akt and mitochondrial-associated
apoptotic pathway. Phytomedicine. 48:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Y, Zhang J, Hou L, Wang G, Liu H,
Zhang R, Chen X and Zhu J: LncRNA AK023391 promotes tumorigenesis
and invasion of gastric cancer through activation of the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 36:1942017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parveen A, Akash MS, Rehman K and Kyunn
WW: Dual role of p21 in the progression of cancer and its
treatment. Crit Rev Eukaryot Gene Expr. 26:49–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang D, Qi J, Liu R, Dai B, Ma W, Zhan Y
and Zhang Y: c-Myc plays a key role in TADs-induced apoptosis and
cell cycle arrest in human hepatocellular carcinoma cells. Am J
Cancer Res. 5:1076–1088. 2015.PubMed/NCBI
|
|
35
|
Cheng G, Zhang L, Lv W, Dong C, Wang Y and
Zhang J: Overexpression of cyclin D1 in meningioma is associated
with malignancy grade and causes abnormalities in apoptosis,
invasion and cell cycle progression. Med Oncol. 32:4392015.
View Article : Google Scholar
|
|
36
|
Alam M, Kashyap T, Pramanik KK, Singh AK,
Nagini S and Mishra R: The elevated activation of NFκB and AP-1 is
correlated with differential regulation of Bcl-2 and associated
with oral squamous cell carcinoma progression and resistance. Clin
Oral Investig. 21:2721–2731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su F, Geng J, Li X, Qiao C, Luo L, Feng J,
Dong X and Lv M: SP1 promotes tumor angiogenesis and invasion by
activating VEGF expression in an acquired trastuzumab-resistant
ovarian cancer model. Oncol Rep. 38:2677–2684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao G, Zhang H, Huang Z, Lv L and Yan F:
Cortactin and Exo70 mediated invasion of hepatoma carcinoma cells
by MMP-9 secretion. Mol Biol Rep. 43:407–414. 2016. View Article : Google Scholar : PubMed/NCBI
|