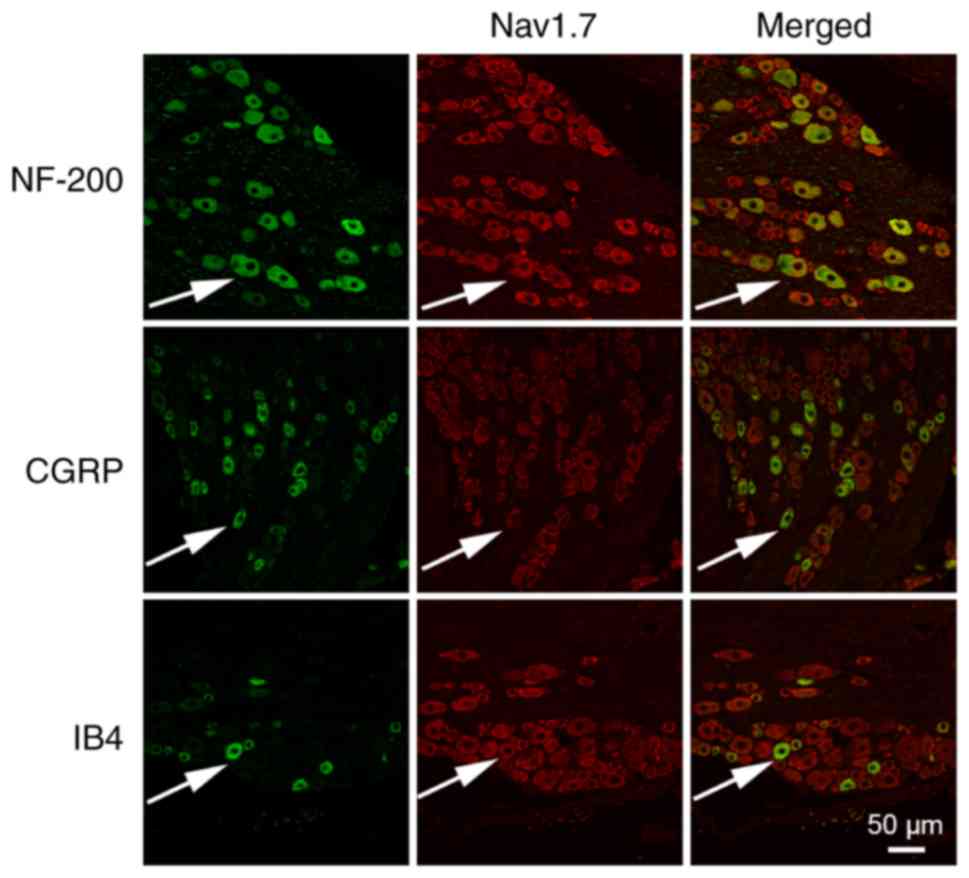
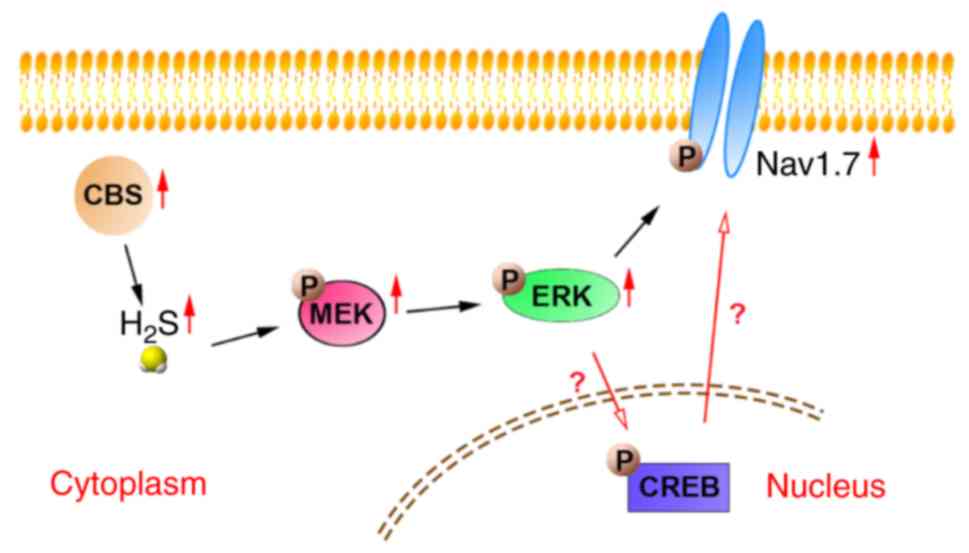
|
1
|
Jensen TS, Baron R, Haanpää M, Kalso E,
Loeser JD, Rice AS and Treede RD: A new definition of neuropathic
pain. Pain. 152:2204–2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouhassira D, Lantéri-Minet M, Attal N,
Laurent B and Touboul C: Prevalence of chronic pain with
neuropathic characteristics in the general population. Pain.
136:380–387. 2008. View Article : Google Scholar
|
|
3
|
Zhang Y, Wang K, Lin M, Li Q and Hong Y:
Inhibition of morphine tolerance by MrgC receptor via modulation of
interleukin-1β and matrix metalloproteinase 9 in dorsal root
ganglia in rats. Eur J Pharmacol. 815:10–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Li N, Duan G, Liu Y, Guo S, Wang C,
Zhu C and Zhang X: Increased Na1.7 expression in the dorsal root
ganglion contributes to pain hypersensitivity after plantar
incision in rats. Mol Pain. 14:17448069187823232018. View Article : Google Scholar
|
|
5
|
Kawai H, Asaoka N, Miyake T, Nagayasu K,
Nakagawa T, Shirakawa H and Kaneko S: Neurotropin inhibits neuronal
activity through potentiation of sustained K currents in primary
cultured DRG neurons. J Pharmacol Sci. 137:313–316. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pachuau J and Martin-Caraballo M:
Expression pattern of T-type Ca (2+) channels in embryonic chick
nodose ganglion neurons. Dev Neurobiol. 67:1901–1914. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitamura N, Nagami E, Matsushita Y, Kayano
T and Shibuya I: Constitutive activity of transient receptor
potential vanilloid type 1 triggers spontaneous firing in nerve
growth factor-treated dorsal root ganglion neurons of rats. IBRO
Rep. 5:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang W, Berta T, Kim YH, Lee S, Lee SY
and Ji RR: Expression and role of voltage-gated sodium channels in
human dorsal root ganglion neurons with special focus on
Nav1.7, species differences, and regulation by
paclitaxel. Neurosci Bulletin. 34:4–12. 2018. View Article : Google Scholar
|
|
9
|
Koenig J, Werdehausen R, Linley JE, Habib
AM, Vernon J, Lolignier S, Eijkelkamp N, Zhao J, Okorokov AL, Woods
CG, et al: Regulation of Nav1.7: A conserved SCN9A
natural anti-sense transcript expressed in dorsal root ganglia.
PLoS One. 10:e01288302015. View Article : Google Scholar
|
|
10
|
Kim DT, Rossignol E, Najem K and Ospina
LH: Bilateral congenital corneal anesthesia in a patient with SCN9A
mutation, confirmed primary erythromelalgia, and paroxysmal extreme
pain disorder. J AAPOS. 19:478–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faber CG, Hoeijmakers JG, Ahn HS, Cheng X,
Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, et
al: Gain of function Naν1.7 mutations in idiopathic small fiber
neuropathy. Ann Neurol. 71:26–39. 2012. View Article : Google Scholar
|
|
12
|
Wang Y, Qu R, Hu S, Xiao Y, Jiang X and Xu
GY: Upregulation of cystathionine β-synthetase expression
contributes to visceral hyperalgesia induced by heterotypic
intermittent stress in rats. PLoS One. 7:e531652012. View Article : Google Scholar
|
|
13
|
Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X
and Xu GY: Neonatal maternal deprivation sensitizes voltage-gated
sodium channel currents in colon-specific dorsal root ganglion
neurons in rats. Am J Physiol Gastrointest Liver Physiol.
304:G311–G321. 2013. View Article : Google Scholar
|
|
14
|
Yan J, Hu S, Zou K, Xu M, Wang Q, Miao X,
Yu SP and Xu GY: Inhibition of cystathionine β-synthetase
suppresses sodium channel activities of dorsal root ganglion
neurons of rats with lumbar disc herniation. Sci Rep. 6:381882016.
View Article : Google Scholar
|
|
15
|
Ji RR and Strichartz G: Cell signaling and
the genesis of neuropathic pain. Sci STKE.
2004:reE142004.PubMed/NCBI
|
|
16
|
Stamboulian S, Choi JS, Ahn HS, Chang YW,
Tyrrell L, Black JA, Waxman SG and Dib-Hajj SD: ERK1/2
mitogen-activated protein kinase phosphorylates sodium channel
Na(v)1.7 and alters its gating properties. J Neurosci.
30:1637–1647. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Casals-Díaz L, Casas C and Navarro X:
Changes of voltage-gated sodium channels in sensory nerve
regeneration and neuropathic pain models. Restor Neurol Neurosci.
33:321–334. 2015.PubMed/NCBI
|
|
18
|
Zhang M, Gao CX, Wang YP, Ma KT, Li L, Yin
JW, Dai ZG, Wang S and Si JQ: The association between the
expression of PAR2 and TMEM16A and neuropathic pain. Mol Med Rep.
17:3744–3750. 2018.
|
|
19
|
Norcini M, Sideris A, Martin Hernandez LA,
Zhang J, Blanck TJ and Recio-Pinto E: An approach to identify
microRNAs involved in neuropathic pain following a peripheral nerve
injury. Front Neurosci. 8:2662014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu D, Wu X, Grabauskas G and Owyang C:
Butyrate-induced colonic hypersensitivity is mediated by
mitogen-activated protein kinase activation in rat dorsal root
ganglia. Gut. 62:1466–1474. 2013. View Article : Google Scholar
|
|
21
|
Deuis JR, Wingerd JS, Winter Z, Durek T,
Dekan Z, Sousa SR, Zimmermann K, Hoffmann T, Weidner C, Nassar MA,
et al: Analgesic effects of GpTx-1, PF-04856264 and CNV1014802 in a
mouse model of Nav17-mediated pain. Toxins (Basel). 8. pp. pii:
E782016, View Article : Google Scholar
|
|
22
|
Zhang H, Reichert E and Cohen AE: Optical
electrophysiology for probing function and pharmacology of
voltage-gated ion channels. ELife. 5:pii: e152022016. View Article : Google Scholar
|
|
23
|
Choi JS, Boralevi F, Brissaud O,
Sánchez-Martín J, Te Morsche RH, Dib-Hajj SD, Drenth JP and Waxman
SG: Paroxysmal extreme pain disorder: A molecular lesion of
peripheral neurons. Nat Rev Neurol. 7:51–55. 2011. View Article : Google Scholar
|
|
24
|
Cox JJ, Reimann F, Nicholas AK, Thornton
G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y,
et al: An SCN9A channelopathy causes congenital inability to
experience pain. Nature. 444:894–898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He W, Young GT, Zhang B, Cox PJ, Cho LT,
John S, Paciga SA, Wood LS, Danziger N, Scollen S and Vangjeli C:
Functional confirmation that the R1488* variant in SCN9A results in
complete loss-of-function of Na1.7. BMC Med Genet. 19:1242018.
View Article : Google Scholar
|
|
26
|
Cummins TR, Dib-Hajj SD and Waxman SG:
Electrophysiological properties of mutant Nav1.7 sodium
channels in a painful inherited neuropathy. J Neurosci.
24:8232–8236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geha P, Yang Y, Estacion M, Schulman BR,
Tokuno H, Apkarian AV, Dib-Hajj DS and Waxman SG: Pharmacotherapy
for pain in a family with inherited erythromelalgia guided by
genomic analysis and functional profiling. JAMA Neurol. 73:659–667.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang X, Djouhri L, McMullan S, Berry C,
Waxman SG, Okuse K and Lawson SN: Intense isolectin-B4 binding in
rat dorsal root ganglion neurons distinguishes C-fiber nociceptors
with broad action potentials and high Nav1.9 expression.
J Neurosci. 26:7281–7292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Cavanaugh DJ, Nemenov MI and
Basbaum AI: The modality-specific contribution of peptidergic and
non-peptidergic nociceptors is manifest at the level of dorsal horn
nociresponsive neurons. J Physiol. 591:1097–1110. 2013. View Article : Google Scholar :
|
|
30
|
Tan CY, Ma KT, Si JQ, Zhou Y, Qu ZW, Zhang
M, Chen QY, Tian JJ, Xu ZZ and Deng SY: Type of excitability of DRG
neurons in rats with neuropathic pain. Chin J Mod Med. 29:1–7.
2019.
|
|
31
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimura H: Hydrogen sulfide as a
neuromodulator. Mol Neurobiol. 26:13–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore PK, Bhatia M and Moochhala S:
Hydrogen sulfide: From the smell of the past to the mediator of the
future? Trends Pharmacol Sci. 24:609–611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R: Two's company, three's a crowd:
Can H2S be the third endogenous gaseous transmitter?
FASEB J. 16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kombian SB, Reiffenstein RJ and Colmers
WF: The actions of hydrogen sulfide on dorsal raphe serotonergic
neurons in vitro. J Neurophysiol. 70:81–96. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dello Russo C, Tringali G, Ragazzoni E,
Maggiano N, Menini E, Vairano M, Preziosi P and Navarra P: Evidence
that hydrogen sulphide can modulate hypothalamopituitary-adrenal
axis function: In vitro and in vivo studies in the rat. J
Neuroendocrinol. 12:225–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eto K and Kimura H: A novel enhancing
mechanism for hydrogen sulfide-producing activity of cystathionine
beta-synthase. J Biol Chem. 277:42680–42685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Julian D, Statile JL, Wohlgemuth SE and
Arp AJ: Enzymatic hydrogen sulfide production in marine
invertebrate tissues. Comp Biochem Physiol A Part, Mol Integr
Physiol. 133:105–115. 2002. View Article : Google Scholar
|
|
39
|
Chen X, Jhee KH and Kruger WD: Production
of the neuromodulator H2S by cystathionine beta-synthase
via the condensation of cysteine and homocysteine. J Biol Chem.
279:52082–52086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yusuf M, Kwong Huat BT, Hsu A, Whiteman M,
Bhatia M and Moore PK: Streptozotocin-induced diabetes in the rat
is associated with enhanced tissue hydrogen sulfide biosynthesis.
Biochem Biophys Res Commun. 333:1146–1152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Levonen AL, Lapatto R, Saksela M and
Raivio KO: Human cystathionine gamma-lyase: Developmental and in
vitro expression of two isoforms. Biochem J. 347:291–295. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith HS: Hydrogen sulfide's involvement
in modulating nociception. Pain Physician. 12:901–910.
2009.PubMed/NCBI
|
|
43
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boehning D and Snyder SH: Novel neural
modulators. Ann Rev Neurosci. 26:105–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Awata S, Nakayama K, Suzuki I, Sugahara K
and Kodama H: Changes in cystathionine gamma-lyase in various
regions of rat brain during development. Biochem Mol Biol Int.
35:1331–1338. 1995.PubMed/NCBI
|
|
46
|
Xu GY, Winston JH, Shenoy M, Zhou S, Chen
JD and Pasricha PJ: The endogenous hydrogen sulfide producing
enzyme cystathionine-beta synthase contributes to visceral
hypersensitivity in a rat model of irritable bowel syndrome. Mol
Pain. 5:442009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hatakeyama Y, Takahashi K, Tominaga M,
Kimura H and Ohta T: Polysulfide evokes acute pain through the
activation of nociceptive TRPA1 in mouse sensory neurons. Mol Pain.
11:242015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maeda Y, Aoki Y, Sekiguchi F, Matsunami M,
Takahashi T, Nishikawa H and Kawabata A: Hyperalgesia induced by
spinal and peripheral hydrogen sulfide: Evidence for involvement of
Cav3.2 T-type calcium channels. Pain. 142:127–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kawabata AT, Ishiki K, Nagasawa K, Yoshida
S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S and
Nishikawa H: Hydrogen sulfide as a novel nociceptive messenger.
Pain. 132:74–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schicho R, Krueger D, Zeller F, Von
Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B
and Schemann M: Hydrogen sulfide is a novel prosecretory
neuromodulator in the Guinea-pig and human colon. Gastroenterology.
131:1542–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Krueger D, Foerster M, Mueller K, Zeller
F, Slotta-Huspenina J, Donovan J, Grundy D and Schemann M:
Signaling mechanisms involved in the intestinal pro-secretory
actions of hydrogen sulfide. Neurogastroenterol Motil.
22:1224–1231. e319–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao S, Liu FF, Wu YM, Jiang YQ, Guo YX
and Wang XL: Upregulation of spinal NMDA receptors mediates
hydrogen sulfide-induced hyperalgesia. J Neurol Sci. 363:176–181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu S, Xu W, Miao X, Gao Y, Zhu L, Zhou Y,
Xiao Y and Xu GY: Sensitization of sodium channels by cystathionine
β-synthetase activation in colon sensory neurons in adult rats with
neonatal maternal deprivation. Exp Neurol. 248:275–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qu R, Tao J, Wang Y, Zhou Y, Wu G, Xiao Y,
Hu CY, Jiang X and Xu GY: Neonatal colonic inflammation sensitizes
voltage-gated Na (+) channels via upregulation of cystathionine
β-synthetase expression in rat primary sensory neurons. Am J
Physiol Gastrointest Liver Physiol. 304:G763–G772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang HJ, Xu X, Xie RH, Rui YY, Zhang PA,
Zhu XJ and Xu GY: Prenatal maternal stress induces visceral
hypersensitivity of adult rat offspring through activation of
cystathionine- β-synthase signaling in primary sensory neurons. Mol
Pain. 14:17448069187774062018. View Article : Google Scholar
|
|
56
|
Lan A, Liao X, Mo L, Yang C, Yang Z, Wang
X, Hu F, Chen P, Feng J, Zheng D and Xiao L: Hydrogen sulfide
protects against chemical hypoxia-induced injury by inhibiting
ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.
PLoS One. 6:e259212011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gobbi G, Ricci F, Malinverno C, Carubbi C,
Pambianco M, Panfilis GD, Vitale M and Mirandola P: Hydrogen
sulfide impairs keratinocyte cell growth and adhesion inhibiting
mitogen-activated protein kinase signaling. Lab Invest.
89:994–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang G, Sun X and Wang R: Hydrogen
sulfide-induced apoptosis of human aorta smooth muscle cells via
the activation of mitogen-activated protein kinases and caspase-3.
FASEB J. 18:1782–1784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang F, Sun W, Yang Y, Wang Y, Li CL, Fu
H, Wang XL, Yang F, He T and Chen J: SDF1-CXCR4 signaling
contributes to persistent pain and hypersensitivity via regulating
excitability of primary nociceptive neurons: Involvement of
ERK-dependent Nav1.8 up-regulation. J Neuroinflammation.
12:2192015. View Article : Google Scholar
|
|
60
|
Chen Y, Zhang Y, Huo Y, Wang D and Hong Y:
Adrenomedullin mediates tumor necrosis factor-α-induced responses
in dorsal root ganglia in rats. Brain Res. 1644:183–191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma W, Zheng WH, Powell K, Jhamandas K and
Quirion R: Chronic morphine exposure increases the phosphorylation
of MAP kinases and the transcription factor CREB in dorsal root
ganglion neurons: An in vitro and in vivo study. Eur J Neurosci.
14:1091–1104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang WX, Wu Q, Liang SS, Zhang XK, Hu Q,
Chen QH, Huang HJ, Xu L and Lou FQ: Dexmedetomidine promotes the
recovery of neurogenesis in aged mouse with postoperative cognitive
dysfunction. Neurosci Lett. 677:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sabbir MG and Fernyhough P: Muscarinic
receptor antagonists activate ERK-CREB signaling to augment neurite
outgrowth of adult sensory neurons. Neuropharmacology. 143:268–81.
2018. View Article : Google Scholar : PubMed/NCBI
|