Introduction
Obesity is accompanied by chronic low-grade
inflammation that can induce knee osteoarthritis (KOA) (1,2).
In patients with KOA, the infrapatellar fat pads (IPFP) produce
numerous inflammatory cytokines and adipocytokines, which, in turn,
trigger inflammation and become a source of pain (3). With increases in the aging
population and the proportion of obese patients, the incidence of
osteoarthritis (OA) is on the rise (4). It has been demonstrated that
individuals with a higher body mass index (BMI) are at a greater
risk of developing KOA, and that being overweight can cause the
release of various cellular inflammatory factors, including
interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α, and
can also induce the production of cartilage degradation factors
(5). Moreover, metabolic
disorders caused by obesity and metabolic abnormalities may lead to
the release of adipocytokines and the occurrence of inflammation
(3,6).
Adipose tissue contains a number of different cell
types that can produce a large number of diverse cytokines. In
healthy or non-obese individuals, the levels of protein expression,
cell proliferation, differentiation and apoptosis are maintained in
a balanced and stable state (7).
When body weight increases, pro-inflammatory macrophages in the
body and immune cells such as CD8+ T cells, mast cells
and B cells enter adipose tissue, leading to a shift towards
low-grade inflammation (8). In
addition, fat cells begin to produce cytokines, known as
adipocytokines, and these changes also affect other tissues,
thereby leading to the development of multiple diseases (9). It has been demonstrated that obesity
has a marked impact on cartilage metabolism and that increased
glycosaminoglycan (GAG) release is considered as a hallmark of
cartilage destruction (10). It
has been proven that the level of free GAG produced in cartilage
tissue is positively associated with BMI (10). Moreover, it has been demonstrated
that obese patients are more likely to express IL-1β, IL-6 and
IL-18 than lean individuals (11). These factors may exert marked
effects on obesity-related diseases, including atherosclerosis and
diabetes (12-14). IL-1β is a major catabolic factor
in cartilage degradation that is highly induced in patients with OA
and is reportedly associated with BMI. Moreover, IL-18 has been
reported to induce prostaglandin E2 (PGE2), which is an important
inflammatory mediator in the synovium, and IL-6 has been reported
to be involved in bone remodeling (13).
The IPFP is an intra-articular adipose tissue that
has received much attention in recent years. From an anatomical
point of view, there are a variety of adipose tissues in the
joints, including the IPFP and a supra-fat pad (SPFP), consisting
of a quadriceps fat pad and a prefemoral fat pad, which are located
above the tibia and behind the supraorbital sac. The IPFP has
recently been described as a repository of intra-articular
cytokines and a source of inflammatory cells, such as leukocytes
and CD31+ cells (15).
The IPFP releases higher amounts of inflammatory factors than
autologous subcutaneous adipose tissue in patients with KOA.
Additionally, the size of the IPFP and the level of TNF-α are
positively associated with the BMI of patients with OA (16-18). Similarly, in high-fat diet-fed
mice, increases in weight and IPFP volume are positively correlated
with OA development (19). It has
been demonstrated that OA synovitis may be associated with
inflammatory factors released by the IPFP on the anterior surface
of the synovial membrane (20).
Therefore, the IPFP may exert paracrine functions on other joint
tissues in OA, paritcularly adjacent synovial membranes.
The nuclear factor-κB (NF-κB) signaling pathway is
one of the most significant pathways involved in the treatment and
intervention of arthritis and related inflammatory diseases
(21). A previous study found
that the NF-κB signaling pathway was activated by chondrocytes
stimulated with IL-1β (22). In
OA-affected rat cartilage tissue, NF-κB p-p65, as well as the NF-κB
signaling molecules, IKKα, IKKβ and phosphorylated IκBα, have been
shown to be upregulated (23).
However, whether the NF-κB signaling pathway is activated in
different parts of infraorbital fat pad tissue in KOA requires
further exploration. Therefore, in the present study, different
adipose tissues were collected to explore the activation of the
NF-κB pathway, and differences in the expression of inflammatory
cytokines and adipocytokines between obese and non-obese patients
with KOA. Furthermore, experiments were also conducted to
investigate the correlation between the release of related
cytokines in serum and the joint fluid of patients with BMI. The
present study aimed to focus on the IPFP and explore both the role
and the molecular mechanisms of the IPFP in obese and non-obese
patients with KOA.
Materials and methods
Human samples
At total of 32 patients with OA who under-went total
knee arthroplasty (TKA) from September, 2017 to July, 2018 at
Bayannaoer Hospital, China were recruited in the present study.
These included 22 obese patients with KOA with a BMI ≥24
(28.33±3.87, kg/m2) and 10 KOA patients with a healthy
weight (average BMI of 22.58±0.80, kg/m2). Males
accounted for 31.25% (10/32) of the patients, and females accounted
for 68.75% (22/32) of the patients. Subjects with secondary,
traumatic and/or rheumatoid arthritis, combined with severe immune
diseases were excluded. Furthermore, subcutaneous adipose tissue I,
IPFP tissue (near the synovial side II and near the patellar tendon
side IV) and suprapatellar fat body III tissues were collected from
obese and non-obese patients with KOA. The present study was
approved by the Ethics Committee of Bayannaoer Hospital and was
performed in accordance with the Helsinki Declaration. All
participants signed informed consent forms for the extraction of
knee joint fluid and the voluntary donation of IPFP specimens.
Hematoxylin and eosin (H&E)
staining
IPFP tissues collected from the patients with KOA
were dehydrated, frozen, sliced and stained with hematoxylin
(Baiaolaibo Company) and eosin (Sinopharm Group Co., Ltd.).
Subsequently, morphological differences in the tissues of obese and
lean patients with KOA were observed using an Olympus fluorescence
microscope (Olympus Corporation).
Cytokine measurement
Serum and knee joint fluid was collected and stored
at -80°C for serum cytokine analysis. The levels of
pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) were analyzed by
enzyme-linked immunosorbent assay (ELISA) kits (Santa Cruz
Biotechnology, Inc.) according to the manufacturer's
instructions.
RT-qPCR
RT-qPCR was used to examine the expression of IL-1β,
IL-6, TNF-α, leptin, adiponectin, visfatin, peroxisome
proliferator-activated receptor (PPAR)γ, IκBα and NF-κB p65 at the
mRNA level. According to the manufacturer's instructions, TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
extract total RNA. Following reverse transcription (RevertAid First
Strand cDNA Synthesis kit, #K1622, Thermo Fisher Scientific, Inc.),
SYBR-Green Master Mix (Life Technologies; Thermo Fisher Scientific,
Inc.) was used for the quantitative analysis of gene expression.
Amplification, which involved a denaturation step, and
quantification were repeated for 40 cycles (95°C for 15 sec and
60°C for 60 sec). The primer sequences are listed in Table SI. The relative gene expression
levels were calculated using the 2−ΔΔCq method (24) and are presented as the fold change
in gene transcript levels. GAPDH was used as the internal control
for RT-qPCR for normalization.
Western blot analysis
The expression of target proteins was detected by
western blot analysis. In the present study, the total protein was
extracted with RIPA lysis buffer (CW2334S, CoWin Bioscience) from
cartilage tissues, and a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology, Inc.) was used to measure the
total protein concentration of the samples. Total protein (20
µg) was loaded per well and then separated by sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE, with a 12%
separating gel and a 4% stacking gel) and transferred onto
polyvinylidene fluoride (PVDF) membranes. The membranes were
blocked with 3% non-fat milk for 1.5 h at room temperature, and
then incubated with primary anti-bodies against IL-1β (Proteintech,
16806-1-AP, 1:2,000), IL-6 (Elabscience, E-AB-30095, 1:1,000) and
TNF-α (Elabscience, E-AB-40015, 1:2,000), leptin (Abcam, ab16227,
1:4,000), adiponectin (Abcam, ab22554, 1:2,000), visfatin (Abcam,
ab236874, 1:3,000), PPARγ (Proteintech, 16643-1-AP, 1:800), IKKβ
(Abcam, ab124957, 1:1,000), IκBα (Abcam, ab32518, 1:800), p-NF-κB
p65 (Servicebio, GB11142-1, 1:500), NF-κB p65 (Servicebio, GB11997,
1:500) and β-tubulin (Proteintech, 10068-1-AP, 1:2,000) at 4°C
overnight. Following incubation with an HRP-conjugated secondary
antibody (Servicebio, GB23303, 1:3,000) for 1.5 h at room
temperature, the protein bands were visualized using an enhanced
chemiluminescence detection system (Tanon-5200, Shanghai Tanon
Science and Technology Ltd.). The results were further analyzed
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Immunofluorescence (IF)
Paraffin-embedded adipose tissue sections were
dewaxed, washed 3 times with PBS, and blocked with 5% goat serum.
The samples were incubated with a p-p65 primary antibody (1:150,
GB11142-1, Servicebio, Inc.), diluted with PBST at 4°C overnight,
washed and then incubated with secondary antibody (Alexa Fluor
594-conjugated goat anti-rabbit IgG, AS039, ABclonal) at 37°C for
60 min. After washing with PBS, 4′,6-diamidino-2-phenylindole
(DAPI, Boster, AR1176) was used for nuclear staining (37°C for 15
min). Finally, the tissues were imaged using a confocal laser
microcope (Nikon DS-U3; Nikon Corporation) after being sealed with
an antifade mounting medium (Beyotime, P0126).
Statistical analysis
All acquired data were analyzed with Microsoft Excel
and Statistical Package for Social Science software 18.0 (SPSS,
Inc.). Welch's t-test was used to assess differences in age,
clinical characteristics, and the levels of cytokines determined by
ELISA between the cases and controls. Pearson's Chi-squared test
was utilized to examine differences in sex between the cases and
controls. Correlations were analyzed by Spearman's rank correlation
test, with r values representing the correlation coefficient. A
P-value (two-tailed) <0.05 was considered to indicate a
statistically significant difference.
Results
General demographic data and indicator
levels in cases and controls
A total of 22 obese patients with KOA (mean age,
63.77±6.60 years) and 10 patients with KOA with a healthy weight
(mean age, 68.60±4.74 years) were included in the present study
(Table I). A significant
difference in age (P=0.047) was found between the cases and
controls, although no differences in sex (P=0.123) were observed
between the groups. Clinical data were collected from the patients
and significant differences were found in BMI (P<0.001), very
low-density lipoprotein (VLDL) levels (P=0.039) and erythrocyte
sedimentation rates (ESRs, P=0.043) between the obese and non-obese
patients.
 | Table IDemographics and clinical
characteristics of the cases and controls. |
Table I
Demographics and clinical
characteristics of the cases and controls.
| Variable | Cases (n=22) | Controls
(n=10) | P-value |
|---|
| Age (years) | 63.77±6.60 | 68.60±4.74 | 0.047a,c |
| Sex | | | 0.123b |
| Male | 5 | 5 | |
| Female | 17 | 5 | |
| BMI
(kg/m2) | 28.33±3.87 | 22.58±0.80 | <0.001a,c |
| HDL (mmol/l) | 1.33±0.30 | 1.29±0.46 | 0.798a |
| LDL (mmol/l) | 2.88±1.32 | 3.32±0.80 | 0.362a |
| VLDL (mmol/l) | 1.02±1.46 | 0.29±0.10 | 0.039a,c |
| CRP (mg/l) | 3.20±3.25 | 2.00±1.11 | 0.296a |
| ESR (mm/h) | 24.40±19.57 | 10.67±7.73 | 0.043a,c |
Differences in ELISA indexes were also analyzed
between the cases and controls (Table II), and it was concluded that the
expression levels of leptin in serum (P=0.033) and IL-6 in the
synovia (P=0.039) differed significantly between the obese and lean
patients with KOA.
 | Table IIDifferences in cytokine levels
determined by ELISA between the cases and controls. |
Table II
Differences in cytokine levels
determined by ELISA between the cases and controls.
| Cytokines
(pg/ml) | Cases (n=22) | Controls
(n=10) | P-value |
|---|
| Serum | | | | |
| IL-6 | 116.86±143.43 | 121.11±154.50 | 0.950 |
| IL-1β | 154.50±144.22 | 133.38±85.34 | 0.737 |
| Leptin |
92,953.26±70,742.61 |
8,538.02±2,279.42 | 0.033a |
| TNF-α | 269.64±252.16 | 155.78±96.38 | 0.101 |
| Synovia | | | | |
| IL-6 | 164.39±201.44 | 381.03±269.15 | 0.039a |
| IL-1β | 5.12±5.41 | 2.93±4.54 | 0.373 |
| Leptin |
5,826.81±3,012.33 |
3,042.15±1,540.94 | 0.007 |
| TNF-α | 19.97±24.15 | 18.71±17.01 | 0.906 |
Differences in clinical characteristics and ELISA
indexes between the male and female patients with KOA were also
analyzed (Table SII); however,
no significant differences were found; this suggested that sex was
not a factor that affected clinical data or the expression of
inflammatory cytokines and adipocytokines in obese patients with
KOA.
Correlation analysis
Correlations between ELISA indexes (IL-6, IL-1β,
leptin and TNF-α levels in serum and joint fluid) and BMI were
analyzed using SPSS software and Spearman's correlation analysis
(Table III), and the results
suggested that the expression of leptin in the synovia
significantly correlated with BMI (r=0.058, P=0.003).
 | Table IIICorrelations between ELISA indexes
and BMI in all patients with KOA. |
Table III
Correlations between ELISA indexes
and BMI in all patients with KOA.
| Location | IL-6
| IL-1β
| Leptin
| TNF-α
|
|---|
| r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| Serum | 0.258 | 0.185 | 0.113 | 0.568 | −0.084 | 0.672 | 0.279 | 0.150 |
| Synovia | −0.311 | 0.108 | 0.012 | 0.951 | 0.058a | 0.003 | 0.096 | 0.627 |
The correlations between age, clinical data and
ELISA indexes were further analyzed in all the study participants
(Table SIII). Only BMI was found
to correlate with age (r=-0.445, P=0.011). The correlations between
the patient clinical characteristics and the ELISA indexes were
then analyzed (Table IV) and it
was found that the expression of IL-1β in serum positively
correlated with the VLDL level. In serum, the expression of IL-6
significantly correlated with the levels of IL-1β, leptin and
TNF-α. In joint fluid, the expression of IL-1β positively
correlated with the level of TNF-α (P<0.05).
 | Table IVCorrelations between ELISA indexes
and clinical indexes in all patients with KOA. |
Table IV
Correlations between ELISA indexes
and clinical indexes in all patients with KOA.
| r | HDL | LDL | VLDL | CRP | ESR | IL-6
| IL-1β
| Leptin
| TNF-α
|
|---|
| Serum | Synovia | Serum | Synovia | Serum | Synovia | Serum | Synovia |
|---|
| HDL | 1.000 | −0.070 | −0.133 | 0.001 | 0.077 | −0.386 | 0.116 | −0.324 | 0.111 | −0.022 | 0.166 | −0.033 | −0.032 |
| LDL | | 1.000 | −0.020 | 0.125 | 0.100 | 0.049 | −0.169 | −0.139 | 0.087 | 0.216 | 0.155 | 0.275 | −0.058 |
| VLDL | | | 1.000 | 0.187 | 0.139 | 0.253 | 0.014 | 0.416a | 0.243 | 0.048 | −0.005 | 0.222 | 0.089 |
| CRP | | | | 1.000 | 0.087 | 0.174 | −0.380 | 0.228 | −0.083 | −0.107 | 0.108 | 0.123 | −0.132 |
| ESR | | | | | 1.000 | −0.092 | −0.182 | 0.049 | 0.274 | −0.035 | 0.193 | 0.068 | 0.116 |
| IL-6 | | | | | | | | | | | | | |
| Serum | | | | | | 1.000 | 0.002 | 0.466a | −0.222 | 0.401a | −0.167 | 0.562b | 0.009 |
| Synovia | | | | | | | 1.000 | −0.330 | 0.061 | −0.176 | −0.063 | −0.122 | 0.225 |
| IL-1β | | | | | | | | | | | | | |
| Serum | | | | | | | | 1.000 | −0.033 | 0.284 | −0.224 | 0.369 | −0.017 |
| Synovia | | | | | | | | | 1.000 | −0.209 | −0.224 | −0.015 | 0.759b |
| Leptin | | | | | | | | | | | | | |
| Serum | | | | | | | | | 1.000 | −0.099 | 0.330 | −0.175 | |
| Synovia | | | | | | | | | | | 1.000 | −0.099 | −0.186 |
| TNF-α | | | | | | | | | | | | | |
| Serum | | | | | | | | | | | | 1.000 | −0.127 |
| Synovia | | | | | | | | | | | | | 1.000 |
Observation of tissue morphological
characteristics
H&E staining was used to compare the
morphological characteristics of the sub-patellar fat pad in obese
and non-obese patients with KOA. Two samples of each group were
detected and the results revealed that there was no marked
differences in the morphology of fat cells between the non-obese
and obese group (Fig. S1).
Activation of NF-κB signaling is
increased in the IPFP of obese patients with KOA
In the present study, the activation of NF-κB in the
tissues (I, II, III and IV) of patients with KOA was examined by
RT-qPCR and western blot analysis, and the results suggested that
in obese patients with KOA, the expression of IκBα in IPFP tissues
(II and IV) was downregulated compared with that in subcutaneous
adipose tissue (P<0.05, Fig. 1A
and B). It was also found that the expression of IκBα in the
IPFP of obese patients with KOA was downregulated compared with
that in the IPFP in lean patients (P<0.05, Fig. 1C). On the contrary, the expression
of IKKβ and the phosphorylation level of p65 (p-p65/p65) were
upregulated in IPFP tissues compared to subcutaneous adipose
tissues (P<0.05; Figs. 2A, and
3A and B) in obese patients with
KOA. These expression levels in IPFP tissues were also higher in
the obese group than the lean group (P<0.05, Figs. 2B and 3C).
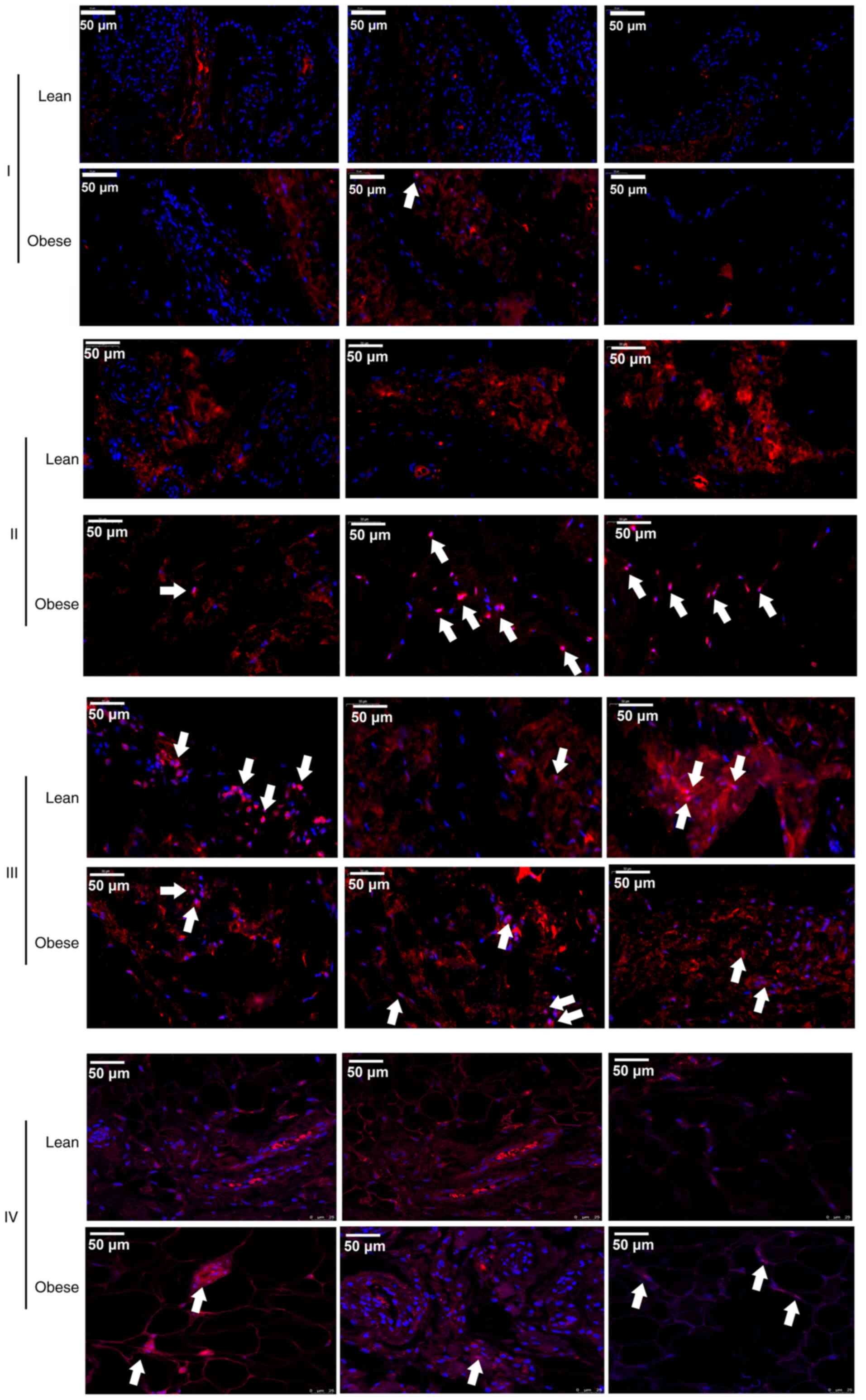
To further confirm the activation of the NF-κB
signaling pathway, IF was used to detect changes in the nuclear
localization of p-p65 in different tissues of 3 patients with KOA
of each group. The results revealed that the p-p65 (red) signals
were located in the nuclei in IPFP tissues (II and IV) and the
suprapatellar body fat (III) (Fig.
4). Moreover, it was also found that the signal intensity in
the IPFP tissues of the obese group was higher than that in IPFP
tissues of the lean controls (Fig.
4).
Expression of pro-inflammatory factors is
altered in the IPFP and other adipose tissues of obese patients
with KOA
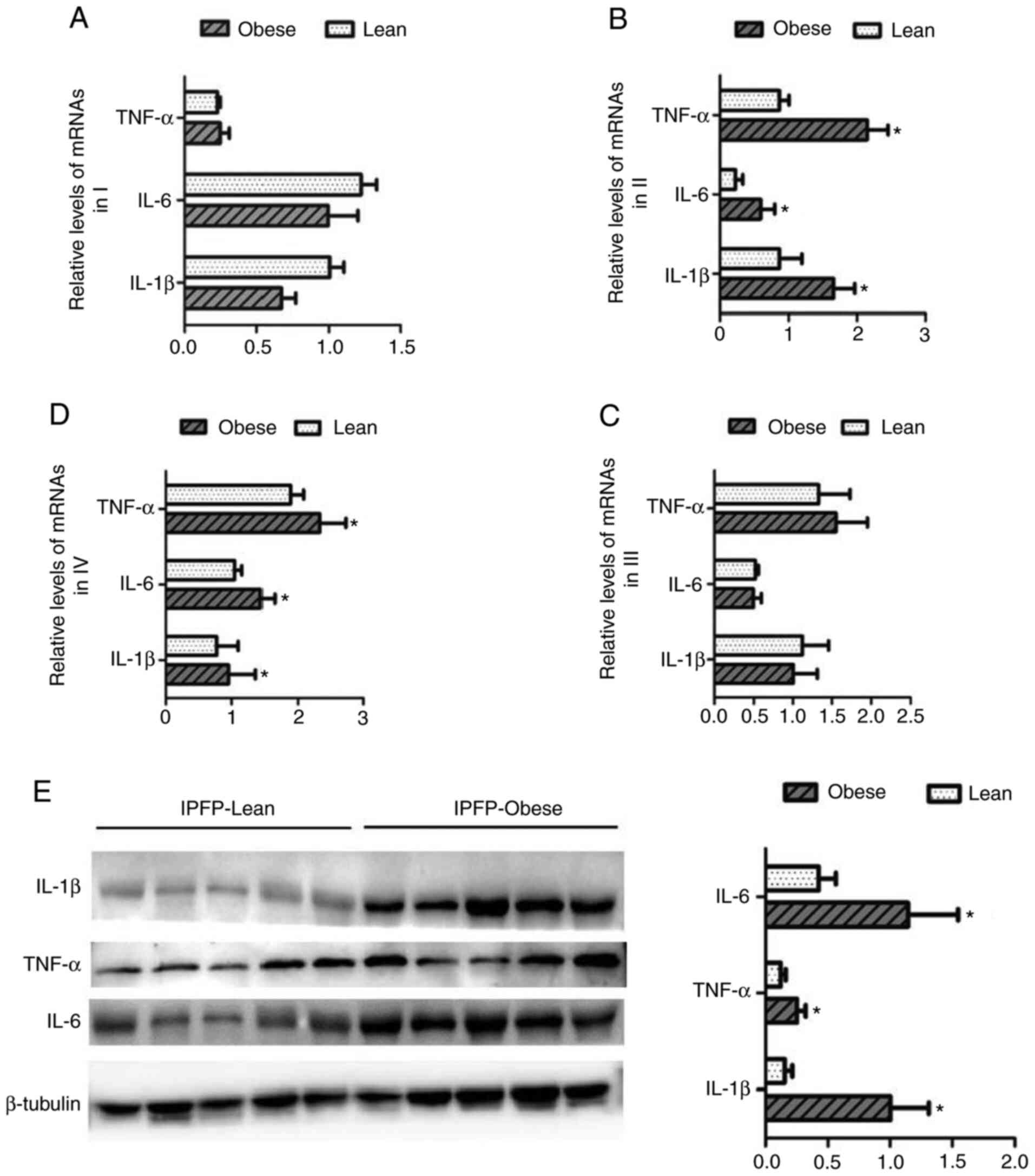
The changes in the expression levels of IL-1β, IL-6
and TNF-α in different adipose tissues from patients with KOA were
then detected. In subcutaneous adipose tissues (I) and the
suprapatellar fat body (III), the expression levels of IL-1β, IL-6
and TNF-α did not differ significantly between the lean and obese
groups (Fig. 5A and C). However,
in IPFP tissues (II and IV), the mRNA expression of levels of
related targets were markedly upregulated in the obese group than
the lean group (P<0.05, Fig. 5B
and D). The results of western blot analysis confirmed that the
expression levels of IL-1β, IL-6 and TNF-α in IPFP tissues
increased as the body weight increased (P<0.05, Fig. 5E).
Expression of adipocytokines is altered
in the IPFP and other adipose tissues of obese patients with
KOA
The changes in the expression levels of leptin,
adiponectin, visfatin and PPARγ in different adipose tissues from
patients with KOA were further verified. No significant differences
in expression levels in subcutaneous adipose tissues (I) and
suprapatellar fat body (III) were found between the obese and lean
patients with KOA (Fig. 6A and
C). However, the results also suggested that in IPFP tissues
(II and IV), the mRNA expression levels of PPARG and visfatin were
decreased, while those of leptin were increased (P<0.05,
Fig. 6B and D). Furthermore, the
protein expression levels of these targets were detected by western
blot analysis. The results revealed that in the obese group, the
expression of visfatin was significantly lower than that in the
controls, whereas that of leptin was upregulated compared to the
non-obese group (P<0.05, Fig.
6E).
 | Figure 6Differences in the expression of
leptin, adiponectin, visfatin and PPARγ in adipose tissues from the
knee joints of lean and obese patients with KOA. (A-D) The mRNA
levels of leptin, adiponectin, visfatin and PPARγ were examined by
RT-qPCR; *P<0.05 vs. I. (E) The protein levels of
leptin, adiponectin, lisfatin and PPARγ were examined by western
blot analysis; *P<0.05 vs. lean patients. KOA, knee
osteoarthritis I, subcutaneous adipose tissue; II, IPFP near the
synovial side; III, suprapatellar fat body; IV, near the patellar
tendon side. |
Discussion
OA is the most common form of arthritis and is
characterized by the loss of cartilage structure. Obesity is a
chronic inflammatory condition and is also a high risk factor for
OA (25). Obesity increases the
incidence of OA, particularly in weight-bearing joints, such as the
knee joint. It is generally believed that obesity increases the
mechanical load of articular cartilage, leading to joint
degeneration (26,27). In the present study, a total of 32
patients with KOA were recruited, including 22 obese patients and
10 patients with normal weight to investigate the molecular
mechanisms of obesity as regards the induction of KOA.
Inflammatory factors are known to participate in the
regulation of intra-articular inflammatory processes and play a key
role in the process of articular cartilage degradation, which is
crucial in the pathogenesis of OA (27). In the present study, samples of
serum and joint fluid were collected from all patients with KOA and
the expression levels of inflammatory factors, such as IL-6, IL-1β,
TNF-α, and the pro-inflammatory adipocytokine, leptin, were
detected between the obese and non-obese subjects by ELISA. The
results indicated that the leptin level in serum and the IL-6 level
in the synovia differed significantly between the cases and
controls, and that the leptin level positively correlated with BMI.
Previous studies have reported that obese individuals are more
likely to produce IL-1β, IL-6, IL-18 and TNF-α than lean
individuals (28,29). Another study demonstrated that
IL-1β was highly induced in patients with OA and was associated
with BMI (30). In addition,
leptin is also believed to play a key role in the development of
OA, and the expression level of leptin in serum has been found to
be positively associated with OA (31,32).
Recent studies have demonstrated that the IPFP, a
source of inflammatory cytokines, mediates the progression of KOA
in a paracrine manner and affects cartilage, bone and synovial
tissue inflammation (15,32,33). By examining the changes in the
expression levels of inflammatory factors in the IPFP tissues of
obese and non-obese patients, the present study found that the
expression levels of IL-6, IL-1β and TNF-α in the IPFPs of obese
patients with KOA were higher than those in the IPFPs of non-obese
patients, indicating that obese patients may be more susceptible to
the destruction of cartilage matrix in bones and joints. Studies
have demonstrated that IL-1β, TNF-α, IL-6, vascular endothelial
growth factor (VEGF), leptin, adiponectin and other factors can be
released in the IPFP (16,17)
and that these factors may exert marked effects on obesity-related
diseases, including atherosclerosis and diabetes (11,13).
The present study further explored the changes in
the expression of adipocytokines in the IPFP tissues of obese and
non-obese patients with KOA, and it was concluded that the
expression of PPARγ and visfatin was downregulated in obese
patients compared to non-obese patients, but that the expression of
leptin increased with increasing weight. PPARγ is a key
adipokinetic nuclear factor, and it has been demonstrated that
pro-inflammatory cytokines, such as IL-1β and TNF-α can
downregulate the expression of PPARγ in human chondrocytes
(34). The expression of PPARγ in
IPFP tissues has also been found to be lower than that in
subcutaneous adipose tissue (16), which is consistent with the
results of the present study. It was also found that the expression
of PPARγ in the IPFP was downregulated in obese patients compared
to non-obese patients, suggesting that PPARγ in the IPFP is related
to the dysfunction of fat metabolism. Visfatin is considered to be
involved in the pro-inflammatory process in OA, and the expression
of visfatin is enhanced by IL-1β (35,36). Moreover, studies it has been
previously found that visfatin-mediated inflammatory responses
usually activate NF-κB signaling (37). The results of the present study
revealed reduced visfatin levels in obese patients with KOA
compared with non-obese patients and suggested that visfatin plays
an important role in inflammatory reactions, and tgat visfatin is
regulated via diverse mechansims in obesity. Leptin is produced by
fat cells in the IPFP and is reduced by 40% in normal IPFP tissues
relative to subcutaneous adipose tissue (16). In patients with OA, leptin is
released into the cartilage, the IPFP, the synovium and the callus,
and normal non-OA cartilage produces less leptin than cartilage
mildly affected by OA (38).
Moreover, the findings of the present study demonstrated that
leptin was highly expressed in the IPFP tissues of obese patients
with KOA. Published results support these findings showing that the
release of inflammatory cytokines, as well as leptin and
adiponectin, is increased in the IPFPs of KOA patients cultured
in vitro (39,40). In addition, related studies have
found that leptin (alone or in combination with IL-1β) can increase
the expression of IL-6, matrix metalloproteinase (MMP)-1, MMP-3 and
MMP-13, which are involved in the regulation of the NF-κB signaling
pathway in the cartilage tissues of patients with OA (41). The present study also found a
significant positive correlation between serum leptin levels and
IL-6 levels in patients with KOA. All these findings reveal the key
roles of the IPFP in the development of KOA in obese patients.
NF-κB is an important nuclear transcription factor
that controls the expression and function of inflammatory genes.
The regulation of NF-κB-induced inflammatory factor-related changes
in target gene levels can also trigger the activation of the NF-κB
signaling pathway, which regulates the inflammatory response to a
certain extent and has a long-lasting effect. The present study
further investigated the activation of NF-κB in IPFP tissues and it
was found that IKKβ, p65 and p-p65 were highly expressed and that
their expression levels in obese patients with KOA were increased
compared with those in non-obese patients. To the best of our
knowledge, the activation of NF-κB in the IPFP tissues of obese
patients with KOA has not yet been studied; however, the
inflammatory cytokines and adipocytokines that were the focus of
the present study have been reported to participate in the NF-κB
signaling pathway (37,42,43). In addition, Hui et al
reported that leptin induces the release of collagen, the
expression of MMP-1 and MMP-13, and NF-κB signaling activation in
chondrocytes (44). These results
suggest that NF-κB is activated in the IPFP tissues of obese
patients with KOA and thus induces the expression of
inflammation-related genes and regulates cartilage
degeneration.
However, the small sample size and inevitable sample
selection bias in the present study are limitations. Furthermore,
the effect of environmental factors on KOA was not taken into
account. Future studies with larger sample sizes are required to
improve the accuracy of the assessments and to explore the
sophisticated molecular regulatory mechanisms underlying the
effects of obesity on KOA.
In conclusion, the present study suggests that the
leptin levels in the synovia positively correlate with BMI.
Moreover, obesity can cause differences in the expression of
related inflammatory cytokines and adipocytokines in patients with
KOA, and can play a role in the development of KOA by triggering
NF-κB signaling in IPFP tissues.
Supplementary Data
Acknowledgments
Not applicable.
Abbreviations:
|
KOA
|
knee osteoarthritis
|
|
IPFP
|
infrapatellar fat pad
|
|
OA
|
osteoarthritis
|
|
BMI
|
body mass index
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
PGE2
|
prostaglandin E2
|
|
SPFP
|
supra-fat pad
|
|
NF-κB
|
nuclear factor kappa B
|
|
TKA
|
total knee arthroplasty
|
|
HE
|
hematoxylineosin
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
BCA
|
bicinchoninic acid
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
PVDF
|
polyvinylidene fluoride
|
|
IF
|
immunofluorescence
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
VLDL
|
very low-density lipoprotein
|
|
ESR
|
erythrocyte sedimentation rate
|
|
VEGF
|
vascular endothelial growth factor
|
Funding
No funding was received.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author upon
reasonable request.
Authors' contributions
LD and XY conceived of and supervised the project.
Tissue samples were collected by YWu. Experiments were conducted by
LD, YM, YWa and JL. Data analyses were conducted by QW and ZT. LD
wrote the manuscript and all authors contributed to the discussion
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Bayannaoer Hospital and was performed in accordance
with the Helsinki Declaration. All participants signed informed
consent forms for the extraction of knee joint fluid and the
voluntary donation of IPFP specimens.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Oliveria SA, Felson DT, Cirillo PA, Reed
JI and Walker AM: Body weight, body mass index, and incident
symptomatic osteoarthritis of the hand, hip, and knee.
Epidemiology. 10:161–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saltiel AR and Olefsky JM: Inflammatory
mechanisms linking obesity and metabolic disease. J Clin Invest.
127:1–4. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eymard F and Chevalier X: Inflammation of
the infrapatellar fat pad. Joint Bone Spine. 83:389–393. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffin T: Biomechanical and metabolic
implications of obesity in OA. Osteoarthritis Cartilage. 26(Suppl):
S12018. View Article : Google Scholar
|
|
5
|
Francisco V, Pérez T, Pino J, López V,
Franco E, Alonso A, Gonzalez-Gay MA, Mera A, Lago F, Gómez R and
Gualillo O: Biomechanics, obesity, and osteoarthritis. The role of
adipokines: When the levee breaks. J Orthop Res. 36:595–604.
2018.
|
|
6
|
Van Beeck A, Clockaerts S, Somville J, Van
Heeswijk JH, Van Glabbeek F, Bos PK and Reijman M: Does
infrapatellar fat pad resection in total knee arthroplasty impair
clinical outcome? A systematic review. Knee. 20:226–231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimura N, Muraki S, Oka H, Tanaka S,
Kawaguchi H, Nakamura K and Akune T: Accumulation of metabolic risk
factors such as overweight, hypertension, dyslipidaemia, and
impaired glucose tolerance raises the risk of occurrence and
progression of knee osteoarthritis: A 3-year follow-up of the ROAD
study. Osteoarthritis Cartilage. 20:1217–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanneganti TD and Dixit VD: Immunological
complications of obesity. Nat Immunol. 13:707–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchholz AL, Niesen MC, Gausden EB,
Sterken DG, Hetzel SJ, Baum SZ, Squire MW and Kaplan LD: Metabolic
activity of osteoarthritic knees correlates with BMI. Knee.
17:161–166. 2010. View Article : Google Scholar
|
|
11
|
Jotanovic Z, Mihelic R, Sestan B and
Dembic Z: Role of inter-leukin-1 inhibitors in osteoarthritis: An
evidence-based review. Drugs Aging. 29:343–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guarner V and Rubio-Ruiz ME: Low-grade
systemic inflammation connects aging, metabolic syndrome and
cardiovascular disease. Interdiscip Top Gerontol. 40:99–106. 2015.
View Article : Google Scholar
|
|
13
|
Dalmas E, Venteclef N, Caer C, Poitou C,
Cremer I, Aron-Wisnewsky J, Lacroix-Desmazes S, Bayry J, Kaveri SV,
Clément K, et al: T cell-derived IL-22 amplifies IL-1β-driven
inflammation in human adipose tissue: Relevance to obesity and type
2 diabetes. Diabetes. 63:1966–1977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eymard F, Pigenet A, Citadelle D, Tordjman
J, Foucher L, Rose C, Flouzat Lachaniette CH, Rouault C, Clément K,
Berenbaum F, et al: Knee and hip intra-articular adipose tissues
(IAATs) compared with autologous subcutaneous adipose tissue: A
specific phenotype for a central player in osteoarthritis. Ann
Rheum Dis. 76:1142–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Distel E, Cadoudal T, Durant S, Poignard
A, Chevalier X and Benelli C: The infrapatellar fat pad in knee
osteoarthritis: An important source of interleukin-6 and its
soluble receptor. Arthritis Rheum. 60:3374–3377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein-Wieringa IR, Kloppenburg M,
Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H,
Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, et al:
The infrapatellar fat pad of patients with osteoarthritis has an
inflammatory phenotype. Ann Rheum Dis. 70:851–857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eymard F, Pigenet A, Citadelle D,
Flouzat-Lachaniette CH, Poignard A, Benelli C, Berenbaum F,
Chevalier X and Houard X: Induction of an inflammatory and
prodegradative phenotype in autologous fibroblast-like synoviocytes
by the infrapatellar fat pad from patients with knee
osteoarthritis. Arthritis Rheumatol. 66:2165–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwata M, Ochi H, Hara Y, Tagawa M, Koga D,
Okawa A and Asou Y: Initial responses of articular tissues in a
murine high-fat diet-induced osteoarthritis model: Pivotal role of
the IPFP as a cytokine fountain. PLoS One. 8:e607062013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bastiaansen-Jenniskens YM, Wei W, Feijt C,
Waarsing JH, Verhaar JA, Zuurmond AM, Hanemaaijer R, Stoop R and
van Osch GJ: Stimulation of fibrotic processes by the infrapatellar
fat pad in cultured synoviocytes from patients with osteoarthritis:
A possible role for prostaglandin f2α. Arthritis Rheum.
65:2070–2080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma B and Hottiger MO: Crosstalk between
Wnt/β-catenin and NF-κB signaling pathway during inflammation.
Front Immunol. 7:3782016. View Article : Google Scholar
|
|
22
|
Liao S, Zhou K, Li D, Xie X, Fang J and
Wang J: Schisantherin A suppresses interleukin-1β-induced
inflammation in human chondrocytes via inhibition of NF-κB and
MAPKs activation. Eur J Pharmacol. 780:65–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olivotto E, Otero M, Marcu KB and Goldring
MB: Pathophysiology of osteoarthritis: Canonical
NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in
cartilage degradation and chondrocyte differentiation. RMD Open.
1(Suppl 1): e0000612015. View Article : Google Scholar :
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Kulkarni K, Karssiens T, Kumar V and
Pandit H: Obesity and osteoarthritis. Maturitas. 89:22–28. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X
and Zhao Y: The relationship between body mass index and hip
osteoarthritis: A systematic review and meta-analysis. Joint Bone
Spine. 78:150–155. 2011. View Article : Google Scholar
|
|
27
|
Jiang L, Tian W, Wang Y, Rong J, Bao C,
Liu Y, Zhao Y and Wang C: Body mass index and susceptibility to
knee osteoarthritis: A systematic review and meta-analysis. Joint
Bone Spine. 79:291–297. 2012. View Article : Google Scholar
|
|
28
|
Wang X, Hunter D, Xu J and Ding C:
Metabolic triggered inflammation in osteoarthritis. Osteoarthritis
Cartilage. 23:22–30. 2015. View Article : Google Scholar
|
|
29
|
Apostolopoulos V, de Courten MP,
Stojanovska L, Blatch GL, Tangalakis K and de Courten B: The
complex immunological and inflammatory network of adipose tissue in
obesity. Mol Nutr Food Res. 60:43–57. 2016. View Article : Google Scholar
|
|
30
|
Iliopoulos D, Malizos KN, Oikonomou P and
Tsezou A: Integrative MicroRNA and proteomic approaches identify
novel osteoarthritis genes and their collaborative metabolic and
inflammatory networks. PLoS One. 3:e37402008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gandhi R, Takahashi M, Virtanen C, Syed K,
Davey JR and Mahomed NN: Microarray analysis of the infrapatellar
fat pad in knee osteoarthritis: Relationship with joint
inflammation. J Rheumatol. 38:1966–1972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lübbeke A, Finckh A, Puskas GJ, Suva D,
Lädermann A, Bas S, Fritschy D, Gabay C and Hoffmeyer P: Do
synovial leptin levels correlate with pain in end stage arthritis?
Int Orthopaedics. 37:2071–2079. 2013. View Article : Google Scholar
|
|
33
|
Francisco V, Pino J, Gonzalez-Gay MA, Mera
A, Lago F, Gómez R, Mobasheri A and Gualillo O: Adipokines and
inflammation: Is it a question of weight? Br J Pharmacol.
175:1569–1579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Afif H, Benderdour M, Mfuna-Endam L,
Martel-Pelletier J, Pelletier JP, Duval N and Fahmi H: Peroxisome
proliferator-activated receptor gamma 1 expression is diminished in
human osteoarthritis cartilage and is downregulated by
interleukin-1beta in articular chondrocytes. Arthritis Res Ther.
9:R312007. View
Article : Google Scholar
|
|
35
|
Tu C, He J, Wu B, Wang W and Li Z: An
extensive review regarding the adipokines in the pathogenesis and
progression of osteoarthritis. Cytokine. 113:1–12. 2019. View Article : Google Scholar
|
|
36
|
Springer BD, Carter JT, McLawhorn AS,
Scharf K, Roslin M, Kallies KJ, Morton JM and Kothari SN: Obesity
and the role of bariatric surgery in the surgical management of
osteoarthritis of the hip and knee: A review of the literature.
Surg Obes Relat Dis. 13:111–118. 2017. View Article : Google Scholar
|
|
37
|
Lee WJ, Wu CS, Lin H, Lee IT, Wu CM, Tseng
JJ, Chou MM and Sheu WH: Visfatin-induced expression of
inflammatory mediators in human endothelial cells through the
NF-kappaB pathway. Int J Obes (Lond). 33:465–472. 2009. View Article : Google Scholar
|
|
38
|
Simopoulou T, Malizos K, Iliopoulos D,
Stefanou N, Papatheodorou L, Ioannou M and Tsezou A: Differential
expression of leptin and leptin's receptor isoform (Ob-Rb) mRNA
between advanced and minimally affected osteoarthritic cartilage;
effect on cartilage metabolism. Osteoarthritis Cartilage.
15:872–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gross JB, Guillaume C, Gegout-Pottie P,
Reboul P, Jouzeau JY, Mainard D and Presle N: The infrapatellar fat
pad induces inflammatory and degradative effects in articular cells
but not through leptin or adiponectin. Clin Exp Rheumatol.
35:53–60. 2017.
|
|
40
|
Kontny E, Plebanczyk M, Lisowska B,
Olszewska M, Maldyk P and Maslinski W: Comparison of rheumatoid
articular adipose and synovial tissue reactivity to proinflammatory
stimuli: Contribution to adipocytokine network. Ann Rheum Dis.
71:262–267. 2012. View Article : Google Scholar
|
|
41
|
Koskinen A, Vuolteenaho K, Nieminen R,
Moilanen T and Moilanen E: Leptin enhances MMP-1, MMP-3 and MMP-13
production in human osteoarthritic cartilage and correlates with
MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp
Rheumatol. 29:57–64. 2011.PubMed/NCBI
|
|
42
|
Zhang Y, Wang S, Zhu J, Li C, Zhang T, Liu
H, Xu Q, Ye X, Zhou L and Ye L: Effect of atmospheric PM2.5 on
expression levels of NF-κB genes and inflammatory cytokines
regulated by NF-κB in human macrophage. Inflammation. 41:784–794.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li QY, Chen L, Yan MM, Shi XJ and Zhong
MK: Tectorigenin regulates adipogenic differentiation and
adipocytokines secretion via PPARγ and IKK/NF-κB signaling. Pharm
Biol. 53:1567–1575. 2015. View Article : Google Scholar
|
|
44
|
Hui W, Litherland GJ, Elias MS, Kitson GI,
Cawston TE, Rowan AD and Young DA: Leptin produced by joint white
adipose tissue induces cartilage degradation via upregulation and
activation of matrix metalloproteinases. Ann Rheum Dis. 71:455–462.
2012. View Article : Google Scholar
|