Introduction
Prolactinomas are the most common type of
hormone-secreting pituitary tumors, which cause hyperprolactinemia
(1). Patients with prolactinomas
usually exhibit symptoms of sexual dysfunction, menstrual
disturbance and infertility, and prolactinomas account for ~50% of
all patients with pituitary adenomas which are brought to medical
attention (2,3). Bromocriptine is generally considered
to be the first-choice treatment for patients with prolactinoma
(4). However, at least 30% of
patients do not respond to bromocriptine (5). Thus, improved systemic therapeutic
approaches are urgently required. Recent studies have suggested a
rational combination strategy consisting of a chemosensitizing
agent and bromocriptine to fill this therapeutic void (5).
In our clinical practice, increased prolactin (PRL)
levels were observed in patients with bromocriptine-resistant
prolactinoma (5). Several studies
have also revealed that PRL serves important roles in cell
dissemination, survival and drug resistance of prolactinomas
(6,7). PRL binds to the prolactin receptor
(PRLR) forming a complex which further activates the classical
prolactin (PRL)/Janus kinase-2 (JAK2)/signal transducer and
activator of transcription 5A (STAT5) pathway (8). Thus, the JAK2/STAT5 pathway may
contribute to drug resistance in prolactinomas. Furthermore, basal
activation of PRL/JAK2/STAT5 signaling has been observed in
chemoresistant tumors (9). Based
on this background, it is hypothesized that blocking this signaling
pathway may reverse bromocriptine resistance. Among the possible
inhibitors of the PRL/JAK2/STAT5 signaling cascade components, the
STAT5 inhibitor, pimozide, an orally active antipsychotic drug
approved by the Food and Drug Administration (FDA) in September
2011 was investigated in the present study (10,11). Pimozide has long been used to
treat motor tics and schizophrenia (10,11). It is widely used in our clinical
practice due its high clinical safety, low risk of side effects and
reasonable price. Pimozide acts on neurons in the CNS by blocking
dopaminergic, serotonergic and other central nervous system
receptors (12). Recent studies
have also suggested that pimozide inhibits cancer growth in
neuro-blastoma, lymphoblastoma, breast cancer and non-small cell
lung cancer (13-17). As pimozide can cross the
blood-brain barrier (12), it is
hypothesized that it may reduce/eliminate bromocriptine resistance
in prolactinoma cells.
The aim of the present study was to evaluate the
activation status of the JAK2/STAT5 signaling pathway in
bromocriptine-resistant prolactinoma tissues and to assess the
potential chemotherapeutic sensitizing effect of pimozide on the
bromocriptine-resistant prolactinoma cells. As tumor stem cells
serve a vital role in tumor recurrence, metastasis and drug
resistance (16,18,19), the effect of pimozide on
prolactinoma cell stemness was also assessed. Collectively, these
experiments provide a rationale for further clinical studies on the
use of pimozide as a chemosensitizer in bromocriptine-resistant
prolactinomas.
Materials and methods
Reagent and antibodies
CD133 (product code ab16518), nestin (product codes
ab105389 and ab6142), SRY-box transcription factor 2 (Sox2)
(product code ab93689), B-cell lymphoma extra-large (Bcl-xL)
(product code ab32370) and Ki67 (product code ab16667) antibodies
were obtained from Abcam. PRLR (product no. 13552), Stat5 (product
no. 94205), p-Stat5 (Tyr694; product no. 9359), Akt (product no.
4691), p-Akt (Ser473; product no. 4060) and p-JAK2 (Tyr1007/1008;
product no. 3776) primary antibodies were purchased from Cell
Signaling Technology, Inc. Erk2 (sc-1647), p-ERK (cat. no sc-7383),
JAK2 (cat. no. sc-390539), cyclin D1 (cat. no. sc-8396), and
β-actin (cat. no. sc-81178) primary antibodies were purchased from
Santa Cruz Biotechnology, Inc. Horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (cat. no. G-21040) and goat
anti-rabbit IgG (cat. no. G-21234) secondary antibodies were
purchased from Thermo Fisher Scientific, Inc.
Prolactinoma specimens
Currently, bromocriptine is used as an initial
therapeutic agent in China, while cabergoline has not been approved
for clinical application yet (20). Thus, in the present study,
bromocriptine resistance was defined as the failure to normalize
PRL levels or to reduce tumor size by ≥50%, after a 3-month course
of bromocriptine (≥15 mg/day) (3,5).
All patients (9 men, 7 women; age range 16-63; mean age 43.50,
interquartile range, 38-51 years) received surgery between July
2013 and July 2019 at the Department of Neurosurgery, The First
Affiliated Hospital of Henan University of Science and Technology
(HUST). Eight patients with prolactinoma who had received
bromocriptine first-line treatment following relapse, and eight
control patients who responded positively to bromocriptine therapy,
but who also received surgery for large tumors or poor medical
compliance, were included in the present study. The diagnoses of
clinical prolactinoma were according to clinical and hormonal
evaluation, histological assessment and PRL exclusive
immunoreactivity. The PRL blood levels of the patients ranged from
154 to 232 µg/l before receiving medication. For each
sample, the tissue was bisected; one half was frozen for protein
and RNA extraction, and the other half was fixed and embedded in
paraffin. Tissue sections were fixed with 10% neutral buffered
formalin (cat. no. DF0111; Beijing Leagene Biotechnology Co., Ltd.)
overnight at room temperature (RT) followed by an embedding in
paraffin wax. Sections, 4-µm thick, were deparaffinized with
xylene and then rehydrated with distilled water through an ethanol
series. Informed consent was obtained from all patients recruited,
and the study protocol was approved by the Research Ethics
Committee of the First Affiliated Hospital of HUST Institutional
Review Board (approval no. 2013-08).
Cell culture
Rat prolactinoma MMQ cells were purchased from the
China Infrastructure of Cell Line Resources, Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences. The
bromocriptine-resistant MMQ/BRO cells were established by exposing
MMQ cells to increasing concentrations of bromocriptine (5-100
µM; CAS 25614-03-3; Santa Cruz Biotechnology, Inc.) for 6
months, and the resultant cell line was termed MMQ/BRO. MMQ/BRO
cells were continuously cultured with BRO (15 µM) to
preserve their resistance. All cells were maintained in DMEM/F12
culture medium (Wuhan Boster Biotechnology Co., Ltd.) supplemented
with 5% fetal bovine serum (FBS; Wuhan Boster Biotechnology Co.,
Ltd.), 10% horse serum (Wuhan Boster Biotechnology Co., Ltd.),
penicillin and streptomycin (100 µg/ml each; Genom Biotech
Pvt., Ltd.) in a humidified incubator at 37°C (5%
CO2).
Primary culture of pituitary adenoma
tissue
The pituitary adenoma tissue was cultured as
described in our previous study (21). Following surgery, the tissue was
enzymatically digested using a Human Tumor Dissociation kit (cat.
no. 130-095-929; Miltenyi Biotec, Inc.). After dissociation, the
tissue was filtered using anti-human Fibroblast MicroBeads (cat.
no. 130050601; Miltenyi Biotec GmbH). Subsequently,
1×106 cells/well were cultured in complete DMEM/F12,
supplemented with penicillin 100 units, streptomycin 100 g/ml, 15
mM HEPES, 3,151 mg/l glucose, 55 mg/l sodium pyruvate, 365 mg/l
L-glutamine, 1,200 mg/l sodium bicarbonate, pH 7.0-7.4 (cat. no.
PYG0085; Wuhan Boster Biotechnology Co., Ltd.).
Isolation of CD133+
nestin+ MMQ cells
As described in our previous study (21), MMQ cells were isolated using CD133
(cat. no. 130097049; Miltenyi Biotec, Inc.) and Anti-Nestin
Magnetic Beads (cat. no. MB106970-T46; Sino Biological, Inc.). The
cells were cultured in DMEM/F12 medium supplied with N2-supplement
(5 ml; cat. no. 17502048; Invitrogen; Thermo Fisher Scientific,
Inc.), penicillin 100 units, streptomycin 100 g/ml, 15 mm HEPES,
3151 mg/l glucose, 55 mg/l sodium pyruvate, 365 mg/l L-glutamine,
1,200 mg/l sodium bicarbonate, pH 7.0-7.4. N-acetylcysteine (60
HEPES, 3,151 mg/l, glucose, 55 mg/l sodium pyruvate, 365 mg/l
L-Alanine; cat. no. CC-4323; Lonza Bioscience, Inc.), epidermal
growth factor (20 ng/ml; cat. no. 11376454001; Roche Diagnostics),
human basic fibroblast growth factor (20 ng/ml; product no. 61977;
Cell Signaling Technology, Inc.), leukemic inhibitory factor (10
ng/ml; cat. no. 123-07; ScienCell Research Laboratories, Inc.).
Cell transfection
The STAT5 small interfering (si)RNA
(SignalSilence® Stat5 siRNA I; cat. no. 6275) system was
obtained from Cell Signaling Biotechnology, Inc. A total of
2×105 cells/well primary human pituitary adenoma (HPA)
cells were seeded into 6-well culture plates in DMEM/F12 medium.
After cells were ~70% confluent, the primary
bromocrip-tine-resistant human prolactinoma cells were transfected
with STAT5 siRNA. Then the cells were treated with 20 µM
bromocriptine in the presence or absence of 10 µM pimozide.
The STAT5 siRNA was transfected at a concentration of 100 nM using
Lipofectamine® 3000 (cat. no. L3000001; Thermo Fisher
Scientific, Inc.) transfection reagent per the manufacturer's
protocol. Briefly, 25 µl of Opti-MEM™ I medium was mixed
with 1.5 µl Lipofectamine 3000 reagent in tube 1. Next, 25
µl Opti-MEM I medium, 250 ng DNA (2.5 µg/µl)
and 0.5 µl P3000™ reagent were added and mixed in tube 2.
Tube 2 solution was then added to tube 1 and mixed well. Then, the
mixture was incubated at room temperature for 15 min and 50
µl of complex was added to the cells. The plate was gently
swirled to ensure homogeneous distribution of the complex to the
entire well. Subsequently, 48 h after transfection, the cells were
evaluated for further experiments. After 48 h, the medium was
replaced with fresh medium.
Cell proliferation assay
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8; cat. no. AR1199; Wuhan Boster Biotechnology Co.,
Ltd.) assay. The cytotoxicity of pimozide and/or bromocriptine
towards primary human bromocriptine-resistant prolactinoma cells
(HPA-BRO) were screened using a CCK-8 assay. The cells were seeded
(1×103 cells/well) in 96-well plates overnight at 37°C
and then treated with pimozide (5, 10 and 20 µM) in the
presence or absence of bromocriptine (5, 10 and 20 µM) for 2
days. Following stimulation, 10 µl CCK-8 reagent was added
to the medium, followed by a 4-h incubation at 37°C. The absorption
was measured using a microplate reader at 450 nm. The cell
viability of each group was calculated relative to the controls,
which were normalized to 100% survival. All analyses were performed
in triplicate.
Sphere formation assay
A total of 1 ml cell suspension (1×103
cells) was plated per well in triplicate in a 24-well Corning
ultra-low attachment plate in DMEM/F12 medium, supplemented with 10
µg/ml insulin (Sigma-Aldrich; Merck KGaA), 1 µg/ml
hydrocortisone (Sigma-Aldrich; Merck KGaA), 1X B27 (Thermo Fisher
Scientific, Inc.), 20 ng/ml EGF, 20 ng/ml bFGF and 4 µg/ml
heparin (all from Stemcell Technologies, Inc.). After 24 h, the
cells were treated with indicated doses of 10 µM pimozide,
20 µM bromocriptine or combined treatment for 7 days to form
primary spheres. Cells were nourished every 3 days through the
addition of 50-100 µl medium per well. Sphere formation was
determined by dividing the total number of spheres by the number of
cells plated. For secondary sphere formation assays, primary
spheres were dissociated into single cells then replated into new
ultra-low attachment 24-well plates for another 7 days. Primary and
secondary sphere assays were performed in at least triplicates.
Cell cycle, apoptosis and cell marker
analysis
Cells were seeded (1×104 cells/plate in
complete medium) for 24 h. Subsequently, the cells were treated
with 10 µM pimozide, 20 µM bromocriptine or combined
treatment for 24 h. The collected cells were fixed in 70% ethanol
overnight at −20°C. Then fixed cells were washed, and incubated in
PBS with FxCycle™ PI/RNase Staining Solution (cat. no. F10797
Invitrogen; Thermo Fisher Scientific, Inc.) with RNase A (0.5
mg/ml) and propidium iodide (PI; 50 µg/ml) for 30 min at
37°C. The percentage of cells in each phase of the cell cycle was
quantified using a Guava® easyCyte™ 8 flow cytometer
(EMD Millipore) with ModFit software 5.0 (Verity Software House).
For cell marker analysis, cells were incubated with anti-CD133,
anti-nestin or anti-Sox2 antibodies conjugated to fluorochromes for
30 min at 37°C and analyzed using a flow cytometric system
(FACSCalibur; BD Biosciences). Gates were set on the basis of
staining with isotype controls (product code ab125938; Abcam) so
that no more than 0.1% of cells were detected with control
antibodies. The cells in each group were quantified based on three
independent experiments.
Western blot analysis
Cells were collected for lysate preparation. The
RIPA Lysis and Extraction Buffer (cat. no. 89901; Thermo Fisher
Scientific, Inc.) was used for protein extraction following the
manufacturer's protocol. Briefly, cells were washed twice with cold
PBS. Cold RIPA Buffer was added to the cells. Then, 1 ml of buffer
was used per 75-cm2 flask containing cells. This was
maintained on ice for 5 min and the plate was swirled occasionally
for uniform spreading. The lysate was gathered to one side using a
cell scraper, and then the lysate was collected and transfered to a
microcentrifuge tube. The samples were entrifuged at ~14,000 × g
for 15 min to collect the cell debris. The Pierce™ Rapid Gold BCA
Protein Assay Kit (cat. no. A53225; Thermo fisher scientific) was
used for protein determination. Briefly, 20 µl of each
standard sample replicate was pipetted into a microplate well.
Then, 200 µl of the wash buffer was added to each well and
the plate was thoroughly mixed on a plate shaker for 30 sec and
subsequently incubated at room temperature for 5 min. The
absorbance at or near 480 nm was measured on a plate reader.
Subtracted the average 480 nm absorbance measurement of the blank
standard replicates from the 480 nm measurements of all other
individual standard and unknown sample replicates. A standard curve
was prepared by plotting the average blank-corrected 480 nm
measurement for each BSA standard vs. its concentration in
µg/ml. The standard curve was used to determine the protein
concentration of each sample. Equal quantities of protein (50
µg) were loaded onto a 10% SDS gel, resolved using 10%
SDS-PAGE, and transferred to a nitrocellulose membrane for western
blot analysis. After blocking the membrane in 5% milk in TBST for 1
h at room temperature, the membranes were incubated in diluted
primary antibodies (1:1,000) overnight at 4°C. Subsequently, the
membranes were washed and incubated in HRP-labeled secondary
antibodies (1:5,000) for 1.5 h at room temperature. SuperSignal
West Pico ECL solution (Thermo Fisher Scientific, Inc.) was added
to the membranes to enhance the chemiluminescent signal. Protein
bands were imaged using a FluorChemE imager (Alpha Innotech). The
ImageJ analysis of the western-blot strip gray-scale value (band
density) was used for relative quantification. Average pixel
intensity was obtained after image inversion from black to white
pixels using the freeware ImageJ from the National Institutes of
Health.
Animals and treatments
For the tumor grafting experiments, primary cells
obtained from human prolactinoma tissues were cultured in complete
DMEM/F12 and harvested during the logarithmic phase of growth.
After adjusting the cell number based on viability,
1×106 viable cells were subcutaneously injected into the
flank of nude mice. A total number of 16 (8 male, 8 female) nude
mice were used in our study. Upon arrival, at 6 weeks of age,
animals were weighed (18-20 grams), ear tagged, and split into 4
different groups (n=4) following a stratified randomization scheme
in order for all groups to have a similar body weight distribution
at the beginning. All mice were housed in same-sex groups of 5, in
type II polycarbonate cages in individually ventilated caging
systems with bedding and water and food ad libitum with a
standardized NIH-31 diet (cat. no. LAD-NIH-31 diet; TROPHIC Animal
Feed High-tech Co., Ltd.). Cages were cleaned every week. On those
occasions, mice were also weighed and examined to evaluate their
health. The animal room had a controlled 12-h light/dark cycle
(lights on at 6:00 AM), temperature (22±2°C), and relative humidity
(45-65%). Food intake was measured by weighing uneaten pellets.
Pimozide (200 µg/mouse), bromocriptine (20 mg/kg) or
combined treatment was administered via an intraperitoneal
injection into mice in the treatment group for 24 days. Injections
were performed from the 6th day after the initial intraperitoneal
injection until the 30th day. Tumor volumes were measured every
other day. The tumor volume was calculated as follows: Tumor
volume=longest diameter × shortest diameter2 × 0.5.
Tumor grafts were removed and processed for further analysis. All
procedures involving mice were approved by the HUST Institutional
Animal Care and Use Committee and performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals, 8th edition, 2011.
Immunohistochemical staining
Tumor specimens were derived from formalin-fixed,
paraffin-embedded tissue samples. Following antigen retrieval and
blocking, tumor tissues were incubated with Ki67 (diluted 1:100)
and p-STAT5 (diluted 1:50) antibodies overnight at 4°C. The slides
were then incubated with biotinylated anti-rabbit IgG secondary
antibody (cat. no. PK-7200; 1:1,000 dilution;Vectastain Elite
ABC-HRP Kit; Vector Laboratories, Inc.) for 30 min at room
temperature, followed by incubation in Vectastain Elite ABC Reagent
for 30 min at room temperature. For all slides, a diaminobenzidine
detection kit (Vector Laboratories, Inc.) was used according to the
manufacturer's protocol. The sections were then counterstained with
Meyer's hematoxylin for 8 min at 37°C, dehydrated, and mounted.
Slides were observed and imaged using a light Nikon ECLIPSE E100
microscope at a magnification of ×40 or ×100.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc.). A Duncan's test was used to
assess differences between multiple groups using one-way ANOVA. A
unpaired t-test was used to compare the means of two groups. Data
are presented as the mean ± SD. P<0.05 was considered to
indicate a statistically significant difference.
Results
Basal activation of PRL/JAK2/STAT5
signaling in bromocriptine-resistant tumor cells
The role of PRL/JAK2/STAT5 signaling in
bromocriptine-resistant prolactinoma has not been explored, to the
best of our knowledge. To address this issue, PRL/JAK2/STAT5
signaling was assessed using immunohistochemistry in 8
bromocriptine-resistant and 8 bromocriptine-sensitive prolactinoma
specimens. Although not all the differences were statistically
significant, drug-resistant samples exhibited higher basal levels
of p-JAK2, p-STAT5 and PRLR protein expression (Fig. 1). For further investigation, MMQ
cells were used to establish bromocriptine-resistant cells
(MMQ/BRO). As described in a previous study (5), the bromocriptine-resistant cells
were treated for 6 months with increasing concentrations of
bromocriptine. In line with this previous study (5), the MMQ/BRO cells expressed higher
basal levels of p-JAK2, p-STAT5 and PRLR in comparison with their
parental MMQ cells (Fig. 1).
Collectively, these data indicated that PRL/JAK2/STAT5 signaling
was activated in bromocriptine-resistant tumor cells, which may
contribute to bromocriptine resistance in prolactinomas.
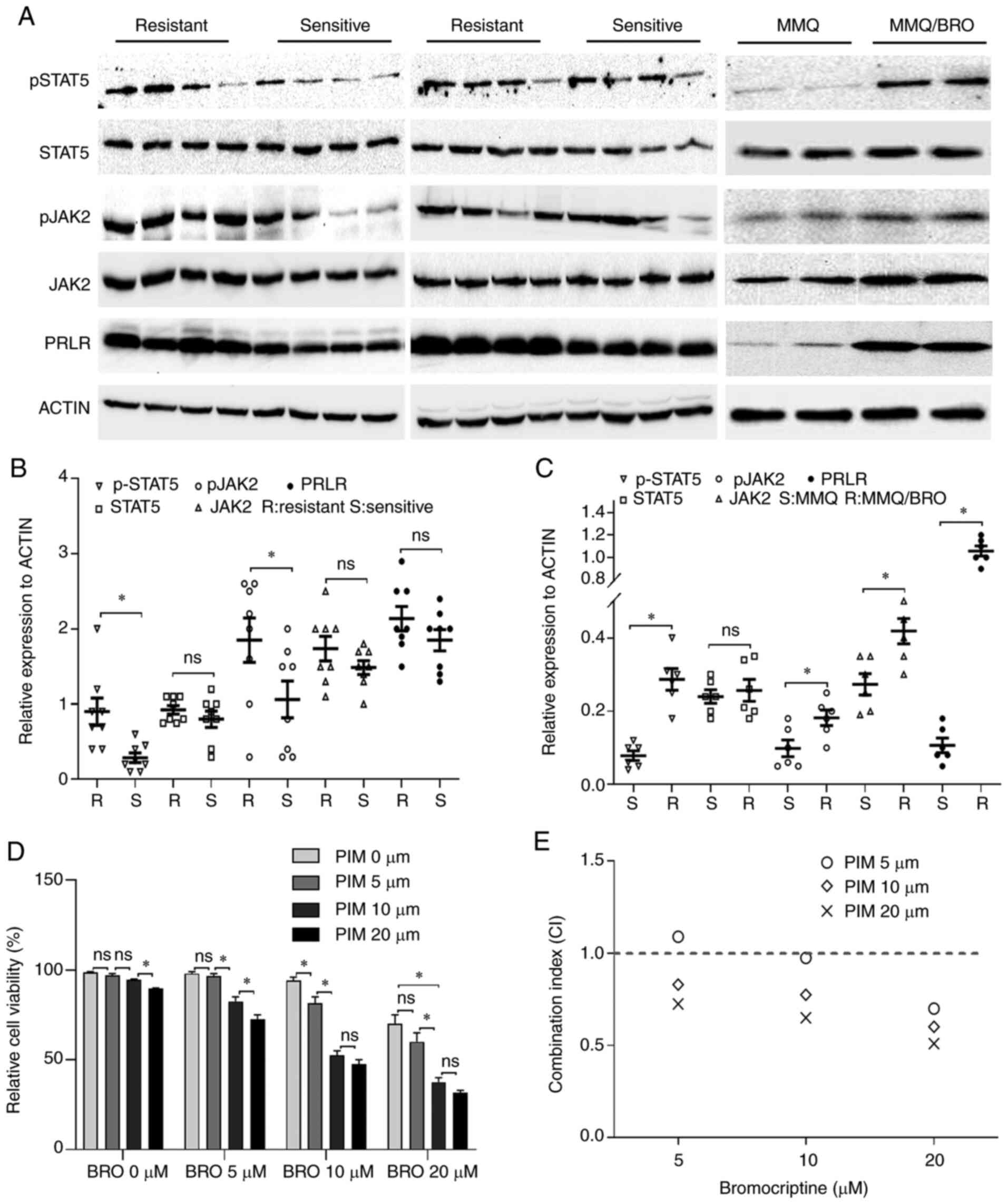 | Figure 1Increased p-STAT, p-JAK2 and PRLR
expression in bromocriptine-resistant prolactinoma tissues and MMQ
cells. (A) Western blots of p-STAT5, STAT5, p-JAK2, JAK2 and PRLR
protein levels in bromocriptine-resistant and -sensitive
prolactinoma tissues and MMQ cells. (B) Scatter plots of p-STAT5,
STAT5, p-JAK2, JAK2 and PRLR protein levels in 8
bromocriptine-sensitive (Sensitive) and 8 bromocriptine-resistant
(Resistant) prolactinoma tissues. Data are presented as the mean ±
SD (Student's t-test; *P<0.05). (C) Scatter plots of
p-STAT5, STAT5, p-JAK2, JAK2 and PRLR protein levels in
bromocriptine-sensitive (S) and bromocriptine-resistant (R) MMQ
cells. Data are presented as the mean ± SD (Student's t-test;
*P<0.05). (D) Pimozide enhanced inhibition of MMQ/BRO
cell proliferation mediated by bromocriptine. MMQ/BRO cells were
treated with bromocriptine (5, 10 and 20 µM) in the presence
of pimozide (5, 10 and 20 µM) for three days. Cell viability
was measured using a CCK-8 assay. Results are presented as the mean
± standard error of the mean; *P<0.05. (E) CI of
bromocriptine and pimozide based on the CCK-8 assays. Pimozide
synergistically enhanced bromocriptine-induced growth inhibition,
as indicated by a CI <1. p-, phospho; PRLR, prolactin receptor;
CI, combination index; CCK-8, Cell Counting Kit-8; CON, control;
BRO, bromocriptine; PIM, pimozide; ns, not significant; |
Pimozide enhances bromocriptine-induced
cytotoxicity in prolactinoma cells
Given that pimozide acts as an inhibitor of STAT5
(22), a CCK-8 assay was used to
determine whether pimozide sensitized prolactinoma cells to
bromocriptine chemotherapy. The dose was initially optimized for
combined treatment. CCK-8 assay revealed that 5 µM pimozide
or 5 µM bromocriptine treatment for 48 h did not result in
any significant cytotoxic effects in HPA-BRO cells. A combined
treatment of 10 µM pimozide and 20 µM bromocriptine
significantly reduced cell viability to 52.81±4.13% compared with 5
µM pimozide plus 20 µM bromocriptine treatment
(Fig. 1D and E). Collectively,
these data indicated a strong synergism between the toxicity of
pimozide and bromocriptine treatment in primary human
bromocriptine-resistant prolactinoma cells.
Pimozide enhances bromocriptine-induced
anti-stemness efficiency in prolactinoma cells
Given that tumor stem cells have been suggested to
be responsible for drug resistance (5), whether pimozide and bromocriptine
combined treatment affected prolactinoma cell stemness was
determined. As revealed in our previous study (20), the CD133+
nestin+ stem-like MMQ cells were isolated using CD133
and nestin magnetic micro beads (Fig.
2A and B). Since Sox2 is known as a marker for pituitary
adenoma stem cells (23), after
isolation, the purity of CD133-, nestin- and Sox2-positive cells
was also examined by flow cytometry. The proportion of CD133-,
nestin- and Sox2-positive cells was 69.31, 61.58 and 30.64%,
respectively (Fig. 2C). Although
there were only 30.64% Sox2-positive cells, the proportion was
significantly higher than 1.71% of parental MMQ cells (data not
shown). As revealed in Fig. 2A,
the isolated cells also displayed sphere-forming capacity in the
present study. All these data supported to some extent the
existence of stemness in the isolated CD133+
nestin+ stem-like MMQ cells. Since the sphere formation
assay is the gold standard assay to assess the stemness of cells
(24), the effect of the pimozide
and bromocriptine combined treatment on the stemness of
CD133+ nestin+ pituitary adenoma stem-like
cells was assessed using the sphere formation assay. Because in the
aforementioned CCK-8 assay it was revealed that the concentration
of pimozide (10 µM) and bromocriptine (20 µM) was the
minimum dose required to achieve the maximum synthetic lethal
effect in vitro, we employed pimozide (10 µM) and
bromocriptine (20 µM) for the experiment. The results
revealed that 7 days of pimozide and bromocriptine combined
treatment significantly reduced tumor sphere formation in the
CD133+ nestin+ pituitary adenoma stem-like
cells compared with either drug alone (Fig. 2A and E). Furthermore, the combined
treatment reduced the expression of CD133 levels in the tumor
spheres of CD133+ nestin+ pituitary adenoma
stem-like cells compared to the pimozide or bromocriptine
monotherapy (Fig. 2D and F).
These data indicated that pimozide sensitized prolactinoma cells to
bromocriptine chemotherapy through inhibition of cell growth and
stemness.
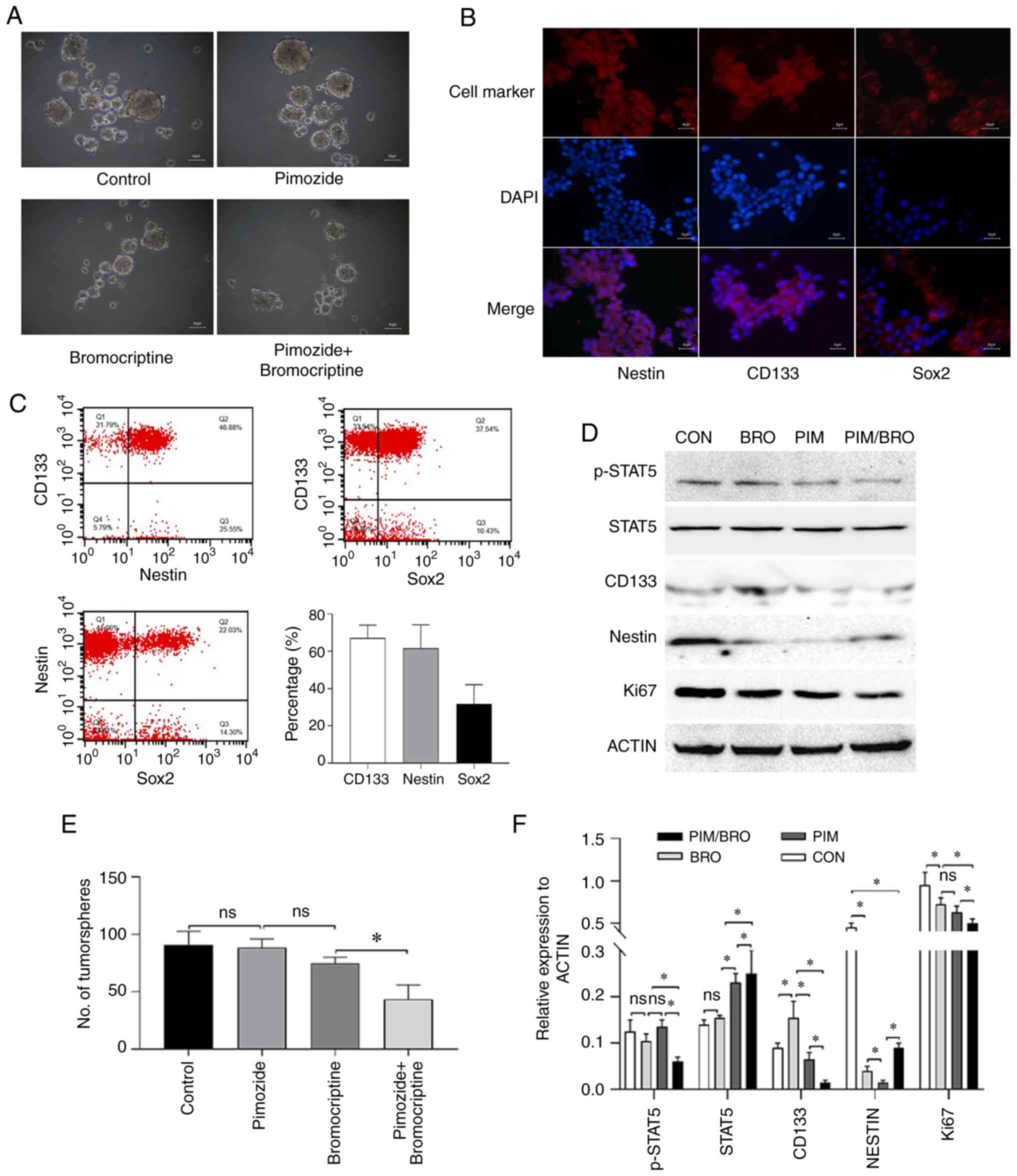 | Figure 2(A) Representative images of the
tumorsphere formation assay of CD133+ nestin+
stem-like MMQ cells. Scale bar represents 50 µm. (B)
Immunofluorescence analysis of CD133, nestin or Sox2 (red) and DAPI
(blue) in CD133+ nestin+ stem-like MMQ cells
(magnification, ×10). Scale bar represents 50 µm. (C) The
representative cytometric dot-plots of nestin, CD133 and Sox2
expression in CD133+ nestin+ stem-like MMQ
cells. (D) Representative western blots of p-STAT5, STAT5, CD133,
nestin and Ki67 protein expression in CD133+
nestin+ stem-like MMQ cells treated with vehicle
control, 10 µM pimozide, 20 µM bromocriptine or both
drugs combined. Pimozide in combination with bromocriptine
suppressed the expression of the tumor stem cell marker proteins
CD133 and nestin, compared with either drug alone. Results are
presented as the mean ± standard error of the mean. (E)
Quantitative results of the CD133+ nestin+
stem-like MMQ cell tumorsphere formation assay treated with vehicle
control, 10 µM Pimozide, 20 µM bromocriptine or both
drugs combined for 7 days. The combination treatment suppressed the
formation of tumorspheres and growth of the CD133+
nestin+ stem-like MMQ cells, compared with bromocriptine
alone. Results are presented as the mean ± standard error of the
mean. *P<0.05. (F) Quantification of p-STAT5, STAT5,
CD133, nestin and Ki67 protein levels in CD133+
nestin+ stem-like MMQ cells treated with vehicle
control, 10 µM pimozide, 20 µM bromocriptine or both
drugs combined. Data are presented as the mean ± SD (Student's
t-test; *P<0.05). p-, phospho-; CON, control; BRO,
bromocriptine; PIM, pimozide; STAT5, signal transducer and
activator of transcription 5A; Sox2, SRY-box transcription factor
2; ns, not significant. |
Pimozide enhances bromocriptine-induced
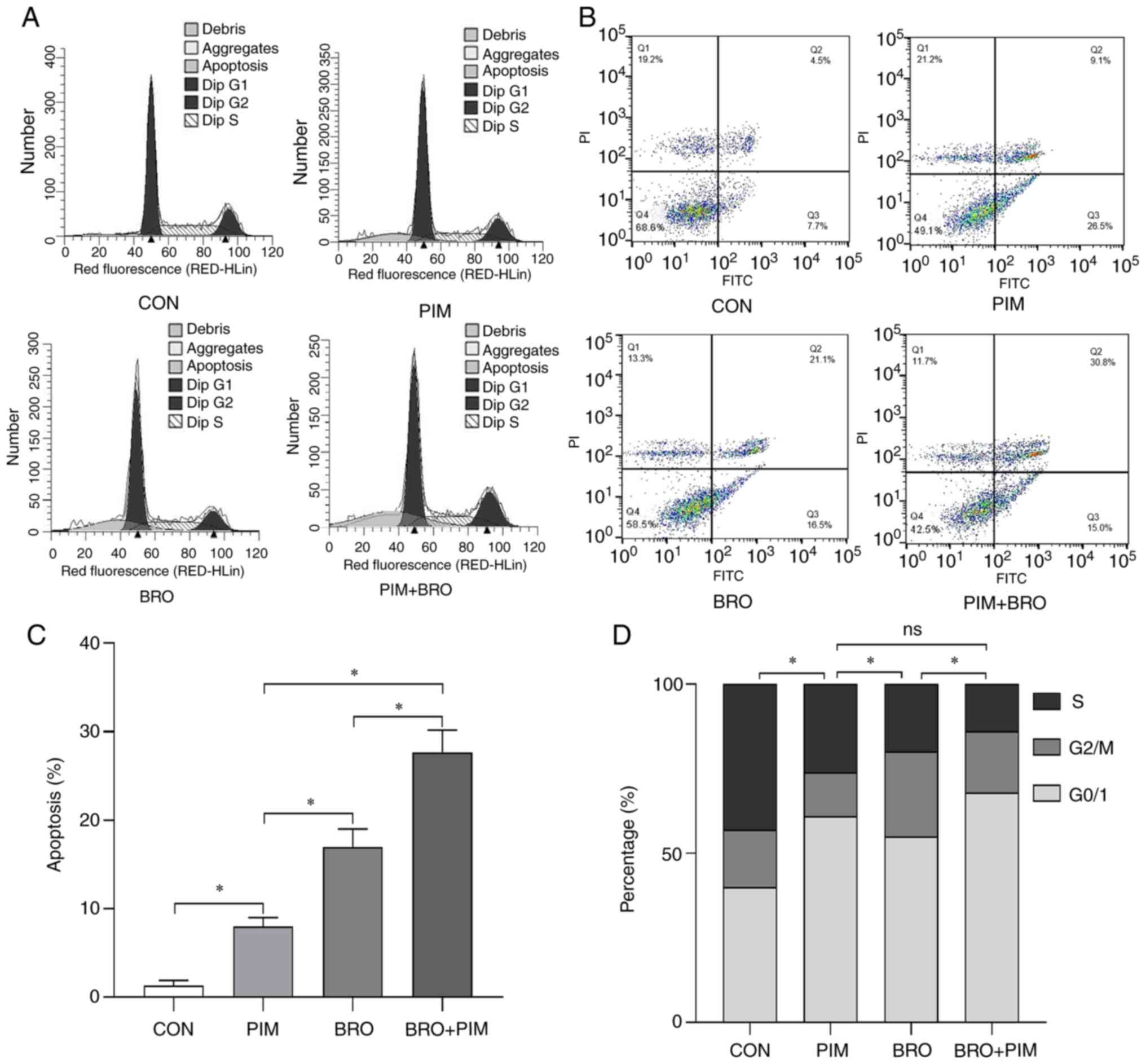
cell cycle arrest and apoptosis in MMQ-BRO cells
To identify the cellular mechanisms underlying the
chemosensitizing effects of pimozide on bromocriptine treatment,
the apoptosis rates and cell cycle distribution were analyzed in
prolactinoma cells. As there is always a small proportion of
fibroblast cells mixed with HPA cells after long term culture, the
heterogeneity of the primary cultured HPA cells may undermine the
reliability of proliferation and signaling research (25). In the present study, MMQ/BRO cells
were used for cell cycle and apoptosis analysis (Fig. 3A and B). Although the ratio was
relatively low, flow cytometric analysis revealed increased
apoptosis in MMQ-BRO+PIM (28.34±2.83%) cells following 24 h of the
combined treatment compared with either pimozide (8.84±2.15%) or
bromocriptine alone (17.81±1.10%) (Fig. 3C). In terms of cell cycle
distribution, 24 h of the combined treatment resulted in cell cycle
arrest at the G0/1 stage (68.82±4.15%), compared with either
pimozide (60.87±3.16%) or bromocriptine alone (51.92±5.12%)
(Fig. 3D). These results
indicated that pimozide sensitized prolactinoma cells to
bromocriptine chemotherapy by inducing cell cycle arrest and
apoptosis.
Pimozide augments the cytotoxicity of
bromocriptine via inhibition of STAT5/Bcl-xL and STAT5/cyclin D1
signaling
The molecular mechanisms underlying the
pimozide-induced augmented antitumor effects to bromocriptine
treatment were assessed. As the heterogeneous nature of the primary
HPA cells may have undermined the reliability of the molecular
mechanism research, the effect of pimozide on the expression of
apoptosis and cell cycle signaling-related regulators, including
anti-apoptotic proteins Bcl-xL and cell cycle regu-lator (cyclin
D1) expression in MMQ/BRO cells was assessed. Treatment with 10
µM pimozide for 24 h decreased p-STAT5, Bcl-xL and cyclin D1
expression levels compared to the control. Bromocriptine at 20
µM for 24 h resulted in higher p-STAT5 and Bcl-xL but lower
cyclin D1 expression levels compared to the control. Furthermore,
the combined treatment with pimozide abrogated the
bromocriptine-induced p-STAT5 and Bcl-xL upregulation (Fig. 4A and B).
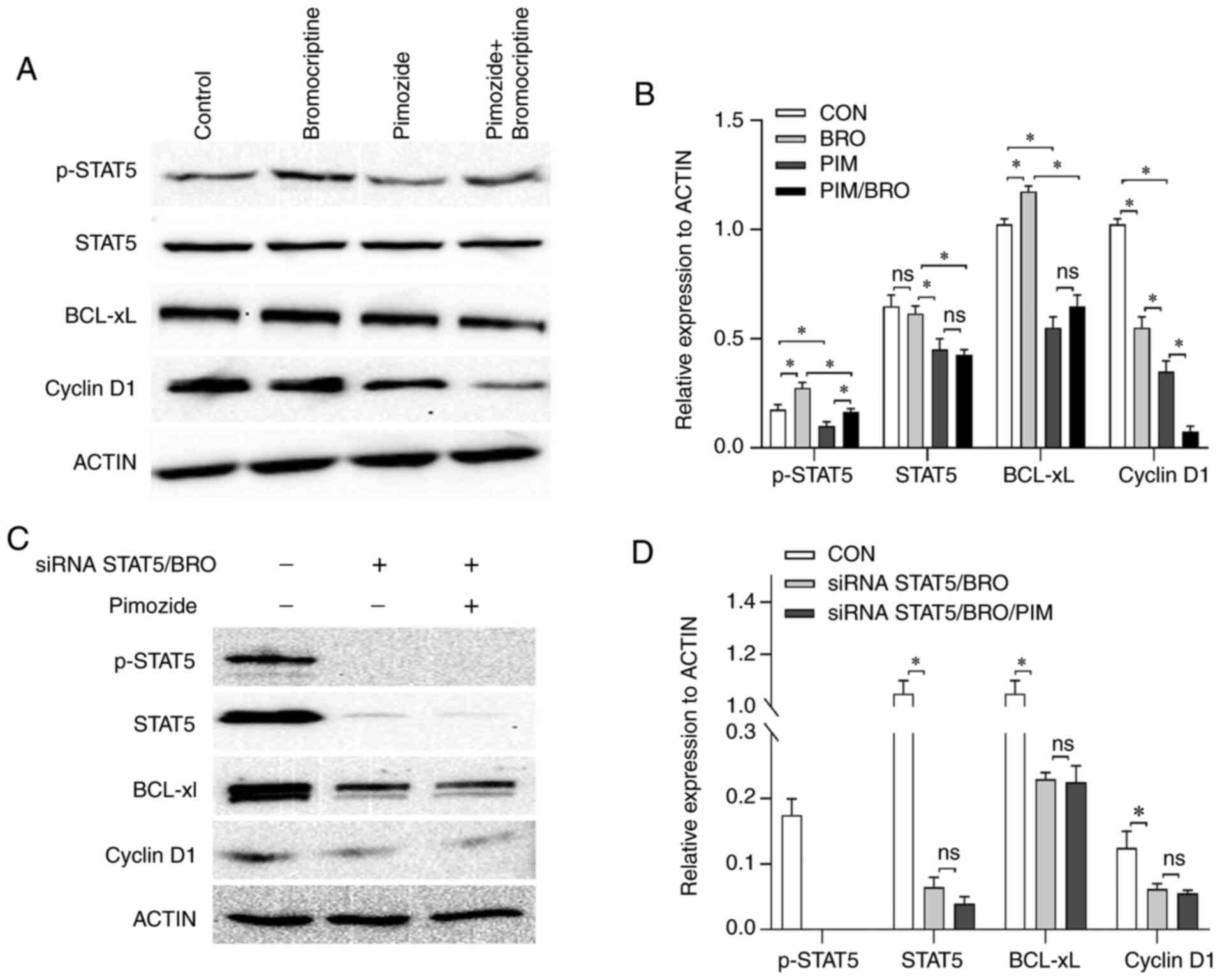 | Figure 4(A) Representative western blot
images and (B) quantification of p-STAT5, STAT5, Bcl-xL and cyclin
D1 protein expression levels in MMQ/BRO cells treated with vehicle
control, 10 µM pimozide, 20 µM bromocriptine or both
drugs combined for 24 h. Pimozide abrogated the
bromocriptine-induced p-STAT5 and Bcl-xL upregulation for 24 h. (C)
Representative western blots and (D) quantification of p-STAT5,
STAT5, Bcl-xL and cyclin D1 protein expres-sion levels in
STAT5-knockdown primary bromocriptine-resistant human prolactinoma
cells treated with 20 µM bromocriptine in the presence or
absence of 10 µM pimozide for 24 h. Data are presented as
the mean ± SD (Student's t-test; *P<0.05). Compared
with bromocriptine treatment alone, the combination of pimozide
with bromocriptine did not exert any notable additional
antiproliferative effects in siRNA STAT5/BRO cells. p-, phospho-;
STAT5, signal transducer and activator of transcription 5A; Bcl-xL,
B-cell lymphoma extra-large; si, small interfering; CON, control;
BRO, bromocriptine; PIM, pimozide. |
As pimozide is an inhibitor of STAT5 (22), the role of STAT5 on cell
proliferation signaling in primary culture bromocriptine-resistant
prolactinoma cells was assessed. A STAT5 siRNA was used to
knockdown STAT5. STAT5 knockdown decreased cyclin D1 and Bcl-xL
expression compared to the control (Fig. 4C and D). Additionally, STAT5
knockdown significantly increased bromocriptine cytotoxicity in
primary culture prolactinoma cells. Furthermore, compared with
bromocriptine treatment alone, pimozide in combination with
bromocriptine did not exert any notable additional
antiproliferative effects in STAT5-knockdown cells (data not
shown). These results indicated that STAT5/cyclin D1 and
STAT5/Bcl-xL contributed to the acquired antitumor effects induced
by pimozide in combination with bromocriptine.
Pimozide synergizes with bromocriptine in
suppressing tumor growth, STAT5 signaling and stem cell marker
expression in human prolactinoma xenograft tissues
To investigate the in vivo therapeutic
effects of pimozide-bromocriptine combination, the effect of the
combined treatment on subcutaneous growth of human
bromocriptine-resistant prolactinoma tissue xenografts was
assessed. As described in our previous study (21), human prolactinoma tissue was
subcutaneously injected into nude mice. After palpable tumors had
formed, the mice were randomized into four groups receiving
control, pimozide and bromocriptine alone, or a combined treatment.
The tumorigenesis assay demonstrated that the antitumor effects of
the drug combination were significantly greater than either agent
alone (Fig. 5D and E). H&E
images of xenografts displayed monomorphic giant cells with
granular cytoplasm, round nuclei with finely dispersed chromatin
and multiple distinct nucleoli. KI67 nuclear and CD133 cytoplasmic
staining was also confirmed (Fig.
5A). Moreover, toxicity of drugs was evaluated based on the
food intake and weight of the mice (Fig. 5F and G). The body weight gain and
total food intake in all groups was similar at the end of the
experiment. These data indicated that drug administration had no
severe effects.
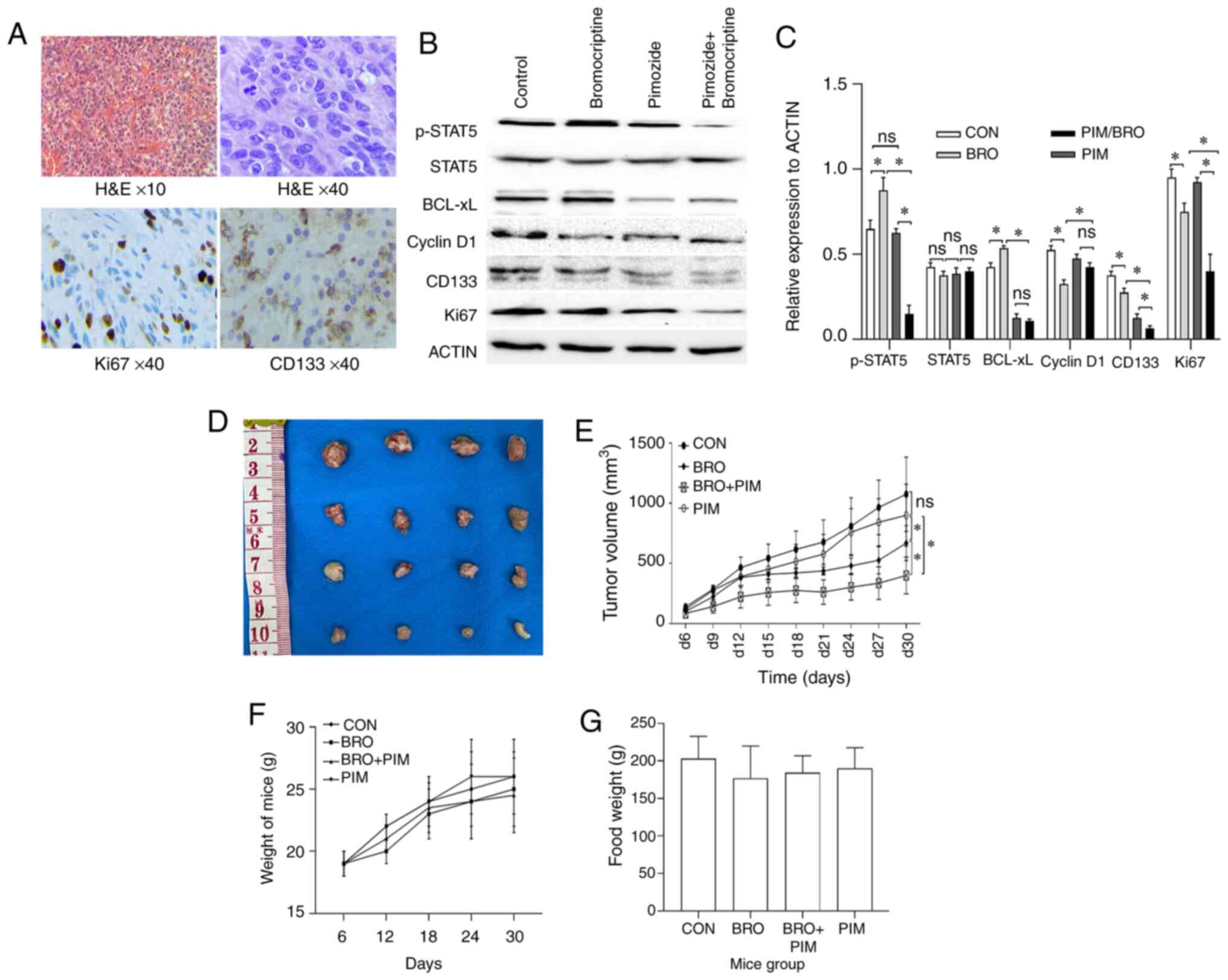 | Figure 5(A) Representative images of H&E
and immunohistochemistry staining of CD133 and Ki67 in human
prolactinoma tissue xenograft tumors (magnification, ×40).
Decreased CD133 and Ki67 protein expression levels were observed in
the bromocriptine/pimozide group tumors. (B) Representative western
blot images and (C) quantification of p-STAT5, STAT5, Bcl-xL,
cyclin D1, CD133 and Ki67 protein expression levels in human
prolactinoma tissue xeno-grafts. Pimozide in combination with
bromocriptine suppressed the expression of tumor stem cell marker
proteins CD133, compared with either drug alone. (D) and (E)
Synergistic inhibitory effect of bromocriptine and pimozide on
tumor growth in nude mice. Human prolactinoma tissue xenograft mice
were treated daily with 20 mg/kg bromocriptine in the absence or
presence of 200 µg/mouse pimozide every three days (n=3 per
group). Pimozide combined with bromocriptine significantly
suppressed xenograft growth. Tumor volumes were measured using
calipers every other day. The tumor volume was calculated as
follows: 0.5 × length × width2. Results are presented as
the mean ± standard error of the mean. *P<0.05. (F)
Body weight change and (G) food intake in mice during the
experimental period. Data are presented as the mean ± SD.
Statistical significance was calculated using t-test. H&E,
hematoxylin and eosin; p-, phospho-; STAT5, signal transducer and
activator of transcription 5A; Bcl-xL, B-cell lymphoma extra-large;
si, small interfering; CON, control; BRO, bromocriptine; PIM,
pimozide; ns, not significant. |
The effect of pimozide on the expression of
STAT5/cyclin D1 and STAT5/Bcl-xL signaling in vivo was also
assessed. Western blotting revealed that pimozide treatment in
xenograft mice significantly reduced p-STAT5, CD133 and Ki67
protein expression levels in tumors receiving the combined
treatment compared with the pimozide monotherapy controls.
Unexpectedly, cyclin D1 and Bcl-xL expression slightly decreased,
but the difference was not statistically significant (Fig. 5B and C). Collectively, these data
indicated that pimozide may have the potential to serve as a
chemosensitizer to bromocriptine in bromocriptine-resistant cells
in vivo.
Discussion
In the present study, increased expression of p-JAK2
and p-STAT5 was observed in bromocriptine-resistant prolactinoma
tissues and cells. In our clinical practice, patients with
bromocriptine-resistant prolactinoma usually suffer from
hyperprolactinemia (26-28). Increased serum levels of PRL tend
to bind PRLR forming a complex, and further activate the classical
PRL/JAK2/STAT5 pathways (8,29,30). Previous studies have also revealed
that STAT5 has several target genes, among which, cell cycle
regulator cyclin D1 is a STAT5 target gene. STAT5 can bind at -481
bp of the cyclin D1 promoter and promote cell cycle progression
(31-34). In addition, STAT5-deficient mice
were revealed to exhibit decreased Bcl-xL expression which resulted
in the suppression of cell growth (35,36). As hyperactivated STAT5 leads to
the aberrant expression of its target genes including
antiapoptotic, proliferative and pro-inflammatory genes, which
promote tumorigenesis (33,37), it was hypothesized that activated
STAT5/cyclin D1 and STAT5/Bcl-xL signaling may contribute to the
bromocriptine resistance in prolactinomas.
In the present study, 10 µM pimozide alone
did not exhibit any notable antitumor efficacy in
bromocriptine-resistant cells. However, a supra-additive effect on
growth inhibition was observed when pimozide was combined with
bromocriptine. Pimozide combined with bromocriptine treatment
induced apoptosis and G0/G1 cycle arrest in
bromocriptine-resistant cells. Furthermore, selective
siRNA-mediated STAT5 inhibition reduced the viability of cells and
downregulated the expression of Bcl-xL and cyclin D1. Pimozide
combined with bromocriptine did not exert any notable additional
antipro-liferative effects in STAT5-siRNA primary culture human
prolactinoma cells. Mechanistically, pimozide was effective in
inhibiting STAT5 and its downstream effectors, cyclin D1 and
Bcl-xL. Since cyclin D1 is a modulator of the cell cycle and Bcl-xL
affects apoptosis (9,38,39), it was proposed that STAT5/cyclin
D1 and STAT5/Bcl-xL may contribute to the antitumor effects of
pimozide.
Bromocriptine regulates several signaling pathways
in tumorigenesis and progression, including the PI3K/AKT and the
MAPK/JNK/ERK signaling pathways (4,40).
Previous studies have also revealed that STAT proteins are
important downstream targets of MAPK and JAK signaling (19,41-43). Although the exact mechanism is not
completely understood, it has been hypothesized that there may be
crosstalk between the STAT5 and JNK/MAPK pathways. In the present
study, bromocriptine alone resulted in upregulation of p-STAT5 and
Bcl-xL expression, and downregulation of cyclin D1 expression
compared to the control. Pimozide treatment decreased Bcl-xL
expression, but had no obvious effect on p-STAT5 expresion compared
to the control. When pimozide was combined with bromocriptine, the
expression of p-STAT5 and Bcl-xL was further decreased compared to
bromocriptine monotherapy. This opposing p-STAT5 regulation may be
due to the several roles of JNK in STAT5 modulation and the
crosstalk between JNK/STAT5 and JAK2/STAT5 in different types and
statuses of studied cells. In the present study, no statistical
difference was observed in the baseline STAT5 expression between
the bromocriptine-resistant and sensitive human prolactinoma
tissues. The are two possible reasons for this unexpected result.
The first is that the total amount of STAT5 may be affected by a
variety of pathophysiological factors in vivo, thus there
may be no statistical difference in the total amount. Second, the
overall sample size of this study was relatively small, and more
patient samples are required for further study. Nevertheless,
understanding the crosstalk between JNK/MAPK, JAK2 and STAT5 will
be helpful to illustrate the heterogeneity that exists between
different types of cancer.
The combined therapy may have possibly increased
toxicity. Both JNK/MAPK and JAK signaling pathways exert important
roles in human cell physiology (37,44). Undesirable consequences may be
encountered upon simultaneous inhibition of both pathways. In the
present study, no noticeable side effects were observed in the
mice. The combination of bromocriptine and pimozide was found to
yield improved antitumor activity compared with either drug alone.
These data suggest that the dual inhibition strategy was effective
in patients with bromocriptine resistance. Both bromocriptine and
pimozide have been approved by the FDA for several years, and no
obvious side effects and pharmaceutical incompatibility of the two
drugs has been reported (10).
The results of the present study indicated that concurrent blockade
of both pathways may be efficacious, but at the expense of possible
toxicity, which requires further investigation. It is known that
pimozide may cause signs of an allergic reaction: Urticaria,
swelling of your face, lips, tongue (45). Bromocriptine may cause some
unwanted effects including dizziness and nausea (46). Patients should be informed of
these possible side effects, and get emergency medical help if they
have relevant symptoms.
The results of the present study support the use of
pimozide as a potential chemosensitizer to bromocriptine in
prolactinoma-resistant patients. The results revealed that
bromocriptine induced STAT5 activation, and cyclin D1 inhibition.
Additionally, pimozide in combination with bromocriptine decreased
p-STAT5 and Bcl-xL expression levels compared to bromocriptine
monotherapy. This enhanced inhibition further reversed
bromocriptine resistance in prolactinoma cells. The schematic model
that summarizes the proposed mechanisms of pimozide in prolactinoma
bromocriptine resistance is presented in Fig. 6. Based on these data, pimozide in
combination with the current standard, bromocriptine, may be of
interest as a potential therapeutic for the treatment of
bromocriptine-resistant prolactinomas. Additional studies are
required to further explore pimozide as a chemosensitizer
clinically for the treatment of patients with
bromocriptine-resistant prolactinomas.
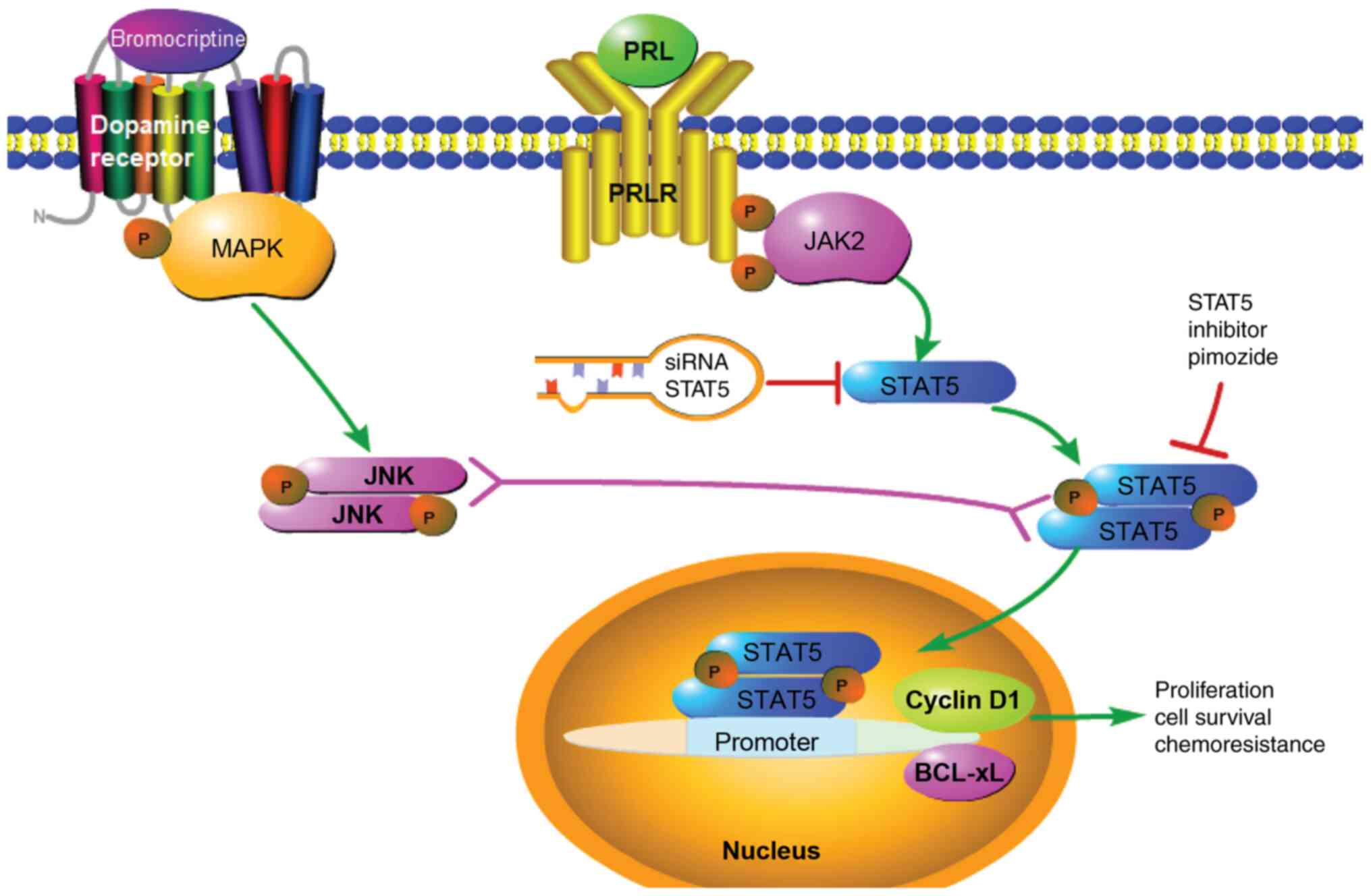 | Figure 6Proposed mechanism of pimozide in
prolactinoma bromocriptine resistance. Pimozide inhibits the
expression of STAT5, which suppresses cyclin D1 and Bcl-xL,
resulting in reversal of bromocriptine resistance. PRL contributes
to drug resistance through activation of the JAK2/STAT5 signaling
pathway. Bromocriptine treatment alone increased STAT5 and Bcl-xL
activation, and reduced cyclin D1 activity in prolactinoma cells.
When combined with bromocriptine, pimozide suppressed activation of
STAT5, which further decreased cyclin D1 and Bcl-xL expression.
Further mechanistic studies are required for detailed
characterization. The solid arrow represents a promoting effect,
the red T symbol represents an inhibitory effect and the double
headed arrow represents possible crosstalk between signaling. PRL,
prolactin; PRLR, prolactin receptor; STAT5, signal transducer and
activator of transcription 5A; Bcl-xL, B-cell lymphoma
extra-large. |
Funding
The present study was supported by funding from the
National Natural Science Foundation of China (grant no.
U1404822).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZX designed the study and wrote the manuscript. ZX,
KZ, XW and ZLi supervised the study, contributed to data
acquisition and revised the manuscript. JL, QD, CS, ZLiu, XY, ZLi
and ZS collected and analyzed the data, and performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the First
Affiliated Hospital of Henan University of Science and Technology
(HUST) Institutional Review Board (approval no. 2013-08). Written
informed consent was obtained from each donor and patient. All
procedures involving mice were approved by the HUST Institutional
Animal Care and Use Committee and performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals, 8th edition, 2011.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Junqiang Yan at
the Neurotumor Laboratory of the First Affiliated Hospital of Henan
University of Science and Technology (Luoyang, China) for technical
support.
References
|
1
|
Mete O, Cintosun A, Pressman I and Asa SL:
Epidemiology and biomarker profile of pituitary adenohypophysial
tumors. Mod Pathol. 31:900–909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, He Y, Zhang X, Yan X and Huang Y:
Clinicopathological analysis of 250 cases of pituitary adenoma
under the new WHO classification. Oncol Lett. 19:1890–1898.
2020.PubMed/NCBI
|
|
3
|
Lim CT and Korbonits M: Update on the
clinicopathology of pituitary adenomas. Endocr Pract. 24:473–488.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang C, Sun R, Wen G, Zhong C, Yang J, Zhu
J, Cong Z, Luo X and Ma C: Bromocriptine and cabergoline induce
cell death in prolactinoma cells via the ERK/EGR1 and AKT/mTOR
pathway respectively. Cell Death Dis. 10:3352019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molitch ME: Pharmacologic resistance in
prolactinoma patients. Pituitary. 8:43–52. 2005. View Article : Google Scholar
|
|
6
|
Kavarthapu R and Dufau ML: Essential role
of endogenous prolactin and CDK7 in estrogen-induced upregulation
of the prolactin receptor in breast cancer cells. Oncotarget.
8:27353–27363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clevenger CV, Gadd SL and Zheng J: New
mechanisms for PRLr action in breast cancer. Trends Endocrinol
Metab. 20:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorvin CM, Newey PJ, Rogers A, Stokes V,
Neville MJ, Lines KE, Ntali G, Lees P, Morrison PJ, Singhellakis
PN, et al: Association of prolactin receptor (PRLR) variants with
prolactinomas. Hum Mol Genet. 28:1023–1037. 2019. View Article : Google Scholar :
|
|
9
|
Kwinta BM, Grzywna E, Krzyżewski RM and
Adamek D: The quantitative evaluation of the immunohistochemical
expression of the pituitary adenomas. Folia Med Cracov. 57:83–96.
2017.
|
|
10
|
Naguy A: Pimozide: An old wine in a new
bottle. Indian J Psychol Med. 39:382–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Preskorn SH: Changes in the product label
for pimozide illustrate both the promises and the challenges of
personalized medicine. J Clin Psychiatry. 73:1191–1193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedman JI, Lindenmayer JP, Alcantara F,
Bowler S, Parak M, White L, Iskander A, Parrella M, Adler DN,
Tsopelas ND, et al: Pimozide augmentation of clozapine inpatients
with schizophrenia and schizoaffective disorder unresponsive to
clozapine monotherapy. Neuropsychopharmacology. 36:1289–1295. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rondanin R, Simoni D, Maccesi M, Romagnoli
R, Grimaudo S, Pipitone RM, Meli M, Cascio A and Tolomeo M: Effects
of pimo-zide derivatives on pSTAT5 in K562 cells. ChemMedChem.
12:1183–1190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rondanin R, Simoni D, Romagnoli R,
Baruchello R, Marchetti P, Costantini C, Fochi S, Padroni G,
Grimaudo S, Pipitone RM, et al: Inhibition of activated STAT5 in
Bcr/Abl expressing leukemia cells with new pimozide derivatives.
Bioorg Med Chem Lett. 24:4568–4574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Zheng H, Sun Y, Yu Q and Li L:
Signal transducer and activator of transcription 5a inhibited by
pimozide may regulate survival of goat mammary gland epithelial
cells by regulating parathyroid hormone-related protein. Gene.
551:279–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JJ, Cai N, Chen GZ, Jia CC, Qiu DB,
Du C, Liu W, Yang Y, Long ZJ and Zhang Q: The neuroleptic drug
pimozide inhibits stem-like cell maintenance and tumorigenicity in
hepatocellular carcinoma. Oncotarget. 8:17593–17609. 2017.
View Article : Google Scholar :
|
|
17
|
Zhou W, Chen MK, Yu HT, Zhong ZH, Cai N,
Chen GZ, Zhang P and Chen JJ: The antipsychotic drug pimozide
inhibits cell growth in prostate cancer through suppression of
STAT3 activation. Int J Oncol. 48:322–328. 2016. View Article : Google Scholar
|
|
18
|
Hadzijusufovic E, Keller A, Berger D,
Greiner G, Wingelhofer B, Witzeneder N, Ivanov D, Pecnard E,
Nivarthi H, Schur F, et al: STAT5 is expressed in
CD34+/CD38-stem cells and serves as a potential
molecular target in ph-negative myeloproliferative neoplasms.
Cancers (Basel). 12:10212020. View Article : Google Scholar
|
|
19
|
Subramaniam D, Angulo P, Ponnurangam S,
Dandawate P, Ramamoorthy P, Srinivasan P, Iwakuma T, Weir SJ,
Chastain K and Anant S: Suppressing STAT5 signaling affects
osteosarcoma growth and stemness. Cell Death Dis. 11:1492020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Jin D, Lian W, Xing B, Feng M, Liu
X and Wang R: Clinical characteristics and surgical outcome of
prolactinoma in patients under 14 years old. Medicine (Baltimore).
98:e143802019. View Article : Google Scholar
|
|
21
|
Zhao Y, Xiao Z, Chen W, Yang J, Li T and
Fan B: Disulfiram sensitizes pituitary adenoma cells to
temozolomide by regulating O6-methylguanine-DNA methyltransferase
expression. Mol Med Rep. 12:2313–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turhan AG: STAT5 as a CML target: STATinib
therapies. Blood. 117:3252–3253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Florio T: Adult pituitary stem cells: From
pituitary plasticity to adenoma development. Neuroendocrinology.
94:265–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Würth R, Barbieri F, Pattarozzi A,
Gaudenzi G, Gatto F, Fiaschi P, Ravetti JL, Zona G, Daga A, Persani
L, et al: Phenotypical and pharmacological characterization of
stem-like cells in human pituitary adenomas. Mol Neurobiol.
54:4879–4895. 2017. View Article : Google Scholar
|
|
26
|
Santos-Pinheiro F, Penas-Prado M,
Kamiya-Matsuoka C, Waguespack SG, Mahajan A, Brown PD, Shah KB,
Fuller GN and McCutcheon IE: Treatment and long-term outcomes in
pituitary carcinoma: A cohort study. Eur J Endocrinol. 181:397–407.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akinduro OO, Lu VM, Izzo A, De Biase G,
Vilanilam G, Van Gompel JJ, Bernet V, Donaldson A, Olomu O, Meyer
FB, et al: Radiographic and hormonal regression in prolactinomas:
An analysis of treatment failure. World Neurosurg. 129:e686–e694.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HY, Lin SJ, Zhao WG and Wu ZB:
Cabergoline versus bromocriptine for the treatment of giant
prolactinomas: A quantitative and systematic review. Metab Brain
Dis. 33:969–976. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hachim IY, Shams A, Lebrun JJ and Ali S: A
favorable role of prolactin in human breast cancer reveals novel
pathway-based gene signatures indicative of tumor differentiation
and favorable patient outcome. Hum Pathol. 53:142–152. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wen Y, Zand B, Ozpolat B, Szczepanski MJ,
Lu C, Yuca E, Carroll AR, Alpay N, Bartholomeusz C, Tekedereli I,
et al: Antagonism of tumoral prolactin receptor promotes
autophagy-related cell death. Cell Rep. 7:488–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalita A, Gupta S, Singh P, Surolia A and
Banerjee K: IGF-1 stimulated upregulation of cyclin D1 is mediated
via STAT5 signaling pathway in neuronal cells. IUBMB Life.
65:462–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao Y, Li Z, Lou C and Zhang Y: Expression
of phosphorylated Stat5 predicts expression of cyclin D1 and
correlates with poor prognosis of colonic adenocarcinoma. Int J
Colorectal Dis. 26:29–35. 2011. View Article : Google Scholar
|
|
33
|
Brockman JL and Schuler LA: Prolactin
signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol
Cell Endocrinol. 239:45–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Magné S, Caron S, Charon M, Rouyez MC and
Dusanter-Fourt I: STAT5 and Oct-1 form a stable complex that
modulates cyclin D1 expression. Mol Cell Biol. 23:8934–8945. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian YH, Xiao Q, Chen H and Xu J:
Dexamethasone inhibits camptothecin-induced apoptosis in C6-glioma
via activation of Stat5/Bcl-xL pathway. Biochim Biophys Acta.
1793:764–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujinaka Y, Takane K, Yamashita H and
Vasavada RC: Lactogens promote beta cell survival through
JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem.
282:30707–30717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanie T, Abe A, Matsuda T, Kuno Y,
Towatari M, Yamamoto T, Saito H, Emi N and Naoe T: TEL-Syk fusion
constitutively activates PI3-K/Akt, MAPK and JAK2-independent STAT5
signal pathways. Leukemia. 18:548–555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moldovan IM, Şuşman S, Pîrlog R, Jianu EM,
Leucuţa DC, Melincovici CS, Crişan D and Florian IŞ: Molecular
markers in the diagnosis of invasive pituitary adenomas-an
immunohisto-chemistry study. Rom J Morphol Embryol. 58:1357–1364.
2017.
|
|
39
|
Bălinişteanu B, Cîmpean AM, Ceauşu AR,
Corlan AS, Melnic E and Raica M: High Ki-67 expression is
associated with prolactin secreting pituitary adenomas. Bosn J
Basic Med Sci. 17:104–108. 2017.
|
|
40
|
Oshige T, Nakamura Y, Sasaki Y, Kawano S,
Ohki T, Tsuruta M, Tokubuchi I, Nakayama H, Yamada K, Ashida K, et
al: Bromocriptine as a potential glucose-lowering agent for the
treatment of prolactinoma with type 2 diabetes. Intern Med.
58:3125–3128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gschwantler-Kaulich D, Grunt TW, Muhr D,
Wagner R, Kölbl H and Singer CF: HER Specific TKIs exert their
antineoplastic effects on breast cancer cell lines through the
involvement of STAT5 and JNK. PLoS One. 11:e01463112016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hazzan T, Eberle J, Worm M and Babina M:
Thymic stromal lymphopoietin interferes with the apoptosis of human
skin mast cells by a dual strategy involving STAT5/Mcl-1 and
JNK/Bcl-xL. Cells. 8:8292019. View Article : Google Scholar :
|
|
43
|
Kim U, Kim CY, Lee JM, Ryu B, Kim J, Shin
C and Park JH: Pimozide inhibits the human prostate cancer cells
through the generation of reactive oxygen species. Front Pharmacol.
10:15172020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nyga R, Pecquet C, Harir N, Gu H,
Dhennin-Duthille I, Régnier A, Gouilleux-Gruart V, Lassoued K and
Gouilleux F: Activated STAT5 proteins induce activation of the PI
3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding
adapter. Biochem J. 390:359–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gupta MA, Vujcic B, Pur DR and Gupta AK:
Use of antipsychotic drugs in dermatology. Clin Dermatol.
36:765–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Biller BM: Hyperprolactinemia. Int J
Fertil Womens Med. 44:74–77. 1999.PubMed/NCBI
|