Introduction
Periodontitis is a chronic inflammatory disease that
can cause irreversible damage to the tissues that support the
teeth, such as the periodontal ligament, cementum and alveolar
bone, thereby leading to the loss of the affected tooth (1). Due to the importance of the
periodontium, periodontal therapy is aimed at suppressing disease
progression and promoting the regeneration of the affected
periodontal tissue or the structures that support the tooth.
Studies have reported the significance of stem cell-based tissue
engineering technology in repairing and regenerating damaged
tissues and maintaining a highly orderly internal environment
(2-4). Human periodontal ligament stem
cells (hPDLSCs) are mesenchymal stem cells (MSCs) obtained from
human periodontal ligament tissue in a normal or periodontitis
environment (5). In vivo
and in vitro experiments have verified that hPDLSCs have
good proliferative, self-renewal and multidirectional
differentiation potentials (6,7).
The osteogenic differentiation ability of hPDLSCs is superior to
that of other odontogenic stem cells, such as dental pulp cells,
gingival mesenchymal stem cells and dental follicle stem cells
(8,9). However, the biological behavior of
stem cells is closely associated with the tissue microenvironment.
For instance, an inflammatory microenvironment may alter the
differentiation ability of stem cells, and weaken their osteogenic
differentiation and tissue regeneration ability (5,10). Therefore, an effective strategy
is required to modulate the differentiation potential of hPDLSCs
during inflammation.
Tumor necrosis factor-α (TNF-α) is the main
regulator of several pro-inflammatory cytokines, including
interleukin (IL)-1β and IL-6 that are released by monocytes and
macrophages, as well as by endothelial cells in periodontitis
(11). It is associated with
osteoclastogenesis, the absorption of alveolar bone and the
inhibition of osteogenesis.
Nuclear factor (NF)-κB is an important nuclear
transcription factor that regulates cell apoptosis, inflammatory
responses and osteoblast differentiation. Elevated TNF-α levels
negatively affect the osteogenic differentiation of mesenchymal
stem cells via the activation of the NF-κB signaling pathway when
it binds its receptor (12,13). Moreover, NF-κB is a priming
signal for the activation of the NOD-like receptor family pyrin
domain-containing protein 3 (NLRP3) inflammasome that is involved
in the expression of NLRP3 protein (14). The NLRP3 inflammasome is a
protein complex composed of a receptor protein (NLRP3), adaptor
protein (ASC) and effector protein (procaspase-1). It plays a vital
role in the innate immunity of infections, inflammatory and chronic
diseases, such as Alzheimer's disease, type 2 diabetes and
osteoporosis (15). The assembly
of the NLRP3 inflammasome, followed by the activation of caspase-1,
and the maturation of IL-1β and IL-18, trigger an inflammatory
cascade response (16). Advanced
glycation end products significantly upregulate the expression of
NLRP3 in hPDLSCs through the NF-κB signaling pathway (17). Studies have demonstrated that
NLRP3 activation increases adipogenesis and inhibits the
osteogenesis of human umbilical cord stem cells, while the
silencing of NLRP3 alleviates estrogen depletion-induced
osteoporosis in mice (18,19). Most importantly, previous
clinical studies have reported that gene polymorphisms of NLRP3 are
associated with susceptibility to periodontitis and the expression
levels of NLRP3 inflammasome-associated proteins are particularly
enhanced in periodontitis (20,21). However, whether NLRP3 associates
with NF-κB to inhibit the osteogenic differentiation of hPDLSCs
needs to be elucidated in a model of TNF-α-induced
periodontitis.
Quercetin (Quer; 3,3′,4′,5,7-pentahydroxyflavone), a
flavonoid derived from common vegetables and fruits, such as
apples, onions and blueberries, is known to exhibit anti- tumor,
anti-inflammatory and antioxidant properties (22). Quer has been reported to reduce
periodontitis damage in rats and to improve the osteogenic
differentiation abilities of bone marrow-derived MSCs (BMMCs)
during inflammation to alleviate osteoporosis symptoms (23,24). Furthermore, it has been
demonstrated that Quer can reverse lipopolysaccharide (LPS)-induced
osteoblast apoptosis and restore the impaired osteogenic
differentiation ability of MC3T3-E1 cells (25). However, it has not yet been
established whether Quer reduces osteogenic damage to hPDLSCs
during TNF-α-induced inflammation. It has also been shown that Quer
decreases osteoclast formation by inhibiting IL-17-induced receptor
activator of nuclear factor κB ligand (RANKL) expression and
inhibits bone resorption in rheumatoid arthritis via the
suppression of NF-κB (26).
Recent studies based on rat models in vivo, have
demonstrated that Quer treatment reduces NLRP3 inflammasome-related
protein expression and inflammatory cytokine levels (27,28). In addition, Quer has been shown
to exert protective effects against endoplasmic reticulum
stress-related endothelial cell damage and isoniazid-induced L02
cell apoptosis by suppressing NLRP3 inflammasome activation
(29,30).
Based on these findings, it was hypothesized that
Quer could attenuate the suppression of the osteogenic
differentiation of hPDLSCs in TNF-α-induced inflammation and that
the underlying mechanisms may be associated with the NF-κB/NLRP3
inflammasome pathway.
Materials and methods
Cells and cell culture
The approval for the present study was provided by
the Committee on Ethics of the Stomatology Hospital of Shandong
University (protocol no. GR201806). From April, 2019 to September,
2019, freshly extracted teeth were collected from orthodontic
volunteers aged 16-22 years at the Stomatology Hospital of Shandong
University. All participants provided signed informed consent in
accordance with the Helsinki Declaration. The method of hPDLSC
isolation from teeth was similar to a previously reported one
(31). Briefly, the extracted
teeth were placed in a 15 ml centrifuge tube with α-MEM (Biological
Industries) amended with 5% antibiotics (100 U/ml penicillin, 10
mg/ml streptomycin) on ice. They were transferred to an ultra-clean
workbench as soon as possible for use in further experiments. Each
tooth was washed thrice in phosphate-buffered saline (PBS;
Biological Industries) containing 5% antibiotics. Periodontal
ligament tissue from the middle third of the root surface was
gently scraped with a sterile surgical blade and cut into tiny
fragments, which were then seeded into culture dishes (25 cm).
hPDLSCs were grown at 37°C in medium supplemented with α-MEM, 20%
fetal bovine serum (FBS; Biological Industries) and 1% antibiotics
in a 5% CO2 incubator. Following inoculation 3-4 h, the
culture dish (25 cm) was turned over to ensure that the hPDLSCs
touched the culture medium. The medium was refreshed after every 3
days until the cells grew out from the tissue sections. The cells
were passaged at 80-90% confluence and the cells at passages 3 to 5
were used for further experiments.
Colony-forming assay
The hPDLSCs were cultured in a 10-cm diameter
culture plate (1,000 cells per plate) in α-MEM supplemented with
10% FBS [common medium (CM)]. After 7 days, the hPDLSCs were rinsed
thrice using PBS, then fixed in 4% polyformaldehyde, after which
they were stained with 0.1% crystal violet (Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 15 min.
The cell clones were observed under a microscope (Olympus Corp.),
and aggregates of >3 mm were considered as clones.
Flow cytometric analysis of hPDLSC
surface marker phenotypes
Passage 3 cells were digested using trypsin,
followed by washing using PBS. A total of 100 μl of prepared
cell suspension was then incubated with antibodies (FITC mouse
anti-human CD90, 5 μl, PE mouse anti-human CD44, 5
μl, PerCP-Cy™5.5 mouse anti-human CD105, 5 μl, APC
mouse anti-human CD73, 5 μl, PE hMSC isotype control
negative cocktail, 20 μl, PE hMSC negative cocktail, 20
μl, hMSC isotype control positive cocktail, 20 μl,
hMSC positive cocktail, 20 μl, #562245, BD Biosciences)
conjugated with a monoclonal fluorescent dye in the dark for 20 min
at 4°C. Cells were detected using flow cytometry, as per the
manufacturer's protocol (#660519, BD Biosciences). The
MSC-associated positive cocktail, including CD105-PerCP-Cy,
CD73-APC, CD44-PE, CD90-FITC and MSC-associated negative cocktail
that included CD34 PE, CD45 PE, CD19 PE, HLA-DR PE and CD11b PE
(#562245, BD Biosciences) were used. Respective negative and
positive isotype control cocktails were used as systemic controls.
Final database analysis was carried out using FlowJo software
(Beckman coulter, Inc.).
Multi-lineage differentiation assays of
hPDLSCs
The hPDLSCs were incubated in 6-well dishes at
1×105 cells/well with CM. At 80-90% density, the
corresponding culture medium was replaced to examined osteogenesis
and adipogenesis.
For osteogenesis, cells were exposed to an
osteogenic induction medium (OIM) containing α-MEM with 10% FBS,
β-glycerophosphate (10 mM), dexamethasone (10 nM) and ascorbic acid
(50 mg/l; Beijing Solarbio Science & Technology Co., Ltd.) for
21 days. The cells were then incubated with Alizarin Red solution
(Sigma-Aldrich; Merck KGaA) at 22°C for 10 min to observe the
mineralization.
For adipogenesis, cells were treated in an
adipogenic induction medium containing α-MEM with 10% FBS, insulin
(10 mg/l), dexamethasone (1 μM), indomethacin (0.2 mM) and
isobutyl-methylxanthine (0.5 mM; Beijing Solarbio Science &
Technology Co., Ltd.) for 28 days. Subsequently, the hPDLSCs were
incubated with Oil Red O solution at 22°C for 20 min to observe
lipid droplets.
Cell proliferation assay
According to the manufacturer′s instructions, the
cell counting kit-8 (CCK-8; MedChemexpress Co., Ltd.) kit was
utilized to detect the proliferative ability of hPDLSCs. A total of
4,000 cells/well were plated in a 96-well plate and cultured at
37°C for 24 h with CM. The hPDLSCs were then subjected to various
concentrations (0, 0.01, 0.1, 1, 5, 10, 50 and 100 μM) of
Quer (Beijing Solarbio Science & Technology Co., Ltd.) for 3
days. Cell viability was examined at 24, 48 and 72 h. Moreover, the
hPDLSCs were stimulated with various concentrations (0, 1, 10, 20,
30, 50 ng/ml) of TNF-α (cat. no. 300-01A Peprotech Inc.) for 4
days. CCK-8 reagent (10 μl) was mixed with 90 μl
complete culture medium per well and incubated at 37°C with the
cells 1 h later. The absorbance at 450 nm was measured with the
SPECTROstar plate reader (BMG Labtech Inc.).
Alkaline phosphatase (ALP) activity and
ALP staining assay
Following 7 days of osteogenesis, the ALP activity
of the cells was assessed using the ALP activity kit (Nanjing
Jiancheng Bioengineering Institute) as per the producer's
protocols. For ALP staining, the hPDLSCs were fixed in
paraformaldehyde (4%), then stained with an ALP staining kit
(Beyotime Institute of Biotechnology) at room temperature for 10
min after 7 days. Cells were observed and examined using an
inverted microscope (Olympus Corp.).
Alizarin Red staining (ARS) assay
Following 21 days of osteogenic induction, the cells
were rinsed 3 times then fixed in paraformaldehyde (4%), after
which they were stained using an Alizarin Red solution (Beijing
Solarbio Science & Technology Co., Ltd.) for 10 min at
22°C.
Gene expression analysis
TRIzol reagent (Qingdao Haosail Science &
Technology Co., Ltd.) was utilized to isolate total RNA from cells
as per producer's suggestions. Subsequently, 1 μg RNA was
reverse transcribed as the template strand using the
HiScript® III Reverse Transcriptase kit (#R323-01,
Nanjing Vazyme Biotech Co., Ltd.) to obtain cDNA. RT-qPCR was
performed by The Roche Light Cycler® 480II in a 10
μl reaction volume with the SYBR qPCR Master Mix (Nanjing
Vazyme Biotech Co., Ltd.). The PCR cycling conditions were as
follows: Initial denaturation at 95°C for 30 sec followed by 45
cycles of denaturation at 95°C for 5 sec and annealing 65°C for 30
sec. Changes in target gene expression were calculated using the
2−ΔΔCq method (32).
The primers used in this assay were as follows: GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGAALP-3′; collagen I (COL1) forward,
5′-GCTGATGATGCCAATGTGGTT-3′ and reverse,
5′-CCAGTCAGAGTGGCACATCTTG-3′; ALP forward,
5′-GTGAACCGCAACTGGTACTC-3′ and reverse, 5′-GAGCTGCGTAGCGATGTCC-3′;
runt-related transcription factor 2 (RUNX2) forward,
5′-GTTTCACCTTGACCATAACCGT-3′ and reverse,
5′-GGGACACCTACTCTCATACTGG-3′; NLRP3 forward,
5′-ACGACTGCGTCTCATCAAGG-3′ and reverse, 5′-CATCGGGGTCAAACAGCAAC-3′;
caspase-1 forward, 5′-GTGCAGGACAACCCAGCTAT-3′ and reverse,
5′-TGCGGCTTGACTTGTCCATT-3′; IL-1β forward,
5′-GTACCTGTCCTGCGTGTTGA-3′ and reverse, 5′-GGGAACTGGGCAGACTCAAA-3′;
IL-6 forward, 5′-CCTTCGGTCCAGTTGCCTTCT-3′ and reverse,
5′-CAGTGCCTCTTTGCTGCTTTC-3′.
Western blot analysis
Proteins were extracted from the cells as previously
described (7). Briefly, the
hPDLSCs were rinsed in PBS, then lysed on ice in RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.) comprising 1%
phosphatase inhibitor (Boster Biological Technology Co., Ltd.) plus
1% PMSF (Beijing Solarbio Science & Technology Co., Ltd.).
Following ultrasonic cracking and centrifugation (12,000 × g; 20
min; 4°C), the concentration of proteins was determine using the
BCA assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.). Subsequently, the proteins from different groups were
resolved on 10% SDS-PAGE and transferred onto a PVDF membrane. A
total of 20 μg proteins from different groups were loaded on
10% SDS-PAGE and separated through electrophoresis. Subsequently,
the separated proteins were eletro-blotted onto a PVDF membrane.
Blocking was performed using 5% non-fat dry milk at 22°C for 1 h,
then probed with rabbit anti-human GAPDH polyclonal (1:20,000, cat.
no. 10494-1-AP; ProteinTech Group, Inc.), rabbit anti-human RUNX2
monoclonal (1:1,000, cat. no. ab23981; Abcam), rabbit anti
human-COL1 mono- clonal (1:1,000, cat. no. #84336; Cell Signaling
Technology, Inc.), rabbit anti human-ALP monoclonal (1:5,000, cat.
no. ab108337; Abcam), rabbit anti human-NLRP3 polyclonal (1:1,000,
cat. no. WL02635; Wanlei Biotech Co., Ltd.), rabbit anti
human-procaspase-1 polyclonal (1:500, cat. no. WL02996; Wanlei
Biotech Co., Ltd., China), rabbit anti human-caspase-1 polyclonal
(1:500, cat. no. WL03450; Wanlei Biotech Co., Ltd.), rabbit anti
human-phosphorylated (p-)p65 polyclonal (1:500, cat. no. WL02169;
Wanlei Biotech Co., Ltd.), rabbit anti human-p-IκBα polyclonal
(1:500, cat. no. WL02495; Wanlei Biotech Co., Ltd.), rabbit anti
human-IκBα polyclonal (1:500, cat. no. WL01936; Wanlei Biotech Co.,
Ltd.), rabbit anti human-p65 monoclonal (1:1,000, cat. no. #59674;
Cell Signaling Technology, Inc.) at 4°C for 24 h. This was followed
by incubation with a horseradish peroxidase-labeled goat
anti-rabbit IgG secondary antibody (1:20,000, cat. no. 7074S; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h. Protein
detection was performed using a chemiluminescent HRP (EMD
Millipore) and the expression levels of target proteins were
analyzed using ImageJ 1.47V software (National Institutes of
Health) and normalized to GAPDH expression.
NLRP3 interfering
The hPDLSCs were seeded into a 6-well plate with CM
and transfected with siRNA targeting NLRP3 (si-NLRP3 forward,
5′-CCUCGGUACUCAGCACUAATT-3′ and reverse, 5′-UUAGUGCUGAGUACCGAG-3′;
20 μM) or negative control (si-NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; 20 μM) (Shanghai GenePharma
Technology Co., Ltd.) with Micropoly-transfecter™ cell reagent
(#MT115; Nantong Micropoly Biotech Co., Ltd.) when the cells were
at a density of 70-80%. Following 24 h of treatment, the proteins
were extracted to examine the interference efficiency.
Statistical analysis
Data were analyzed using GraphPad Prism, v. 8.0.
One-way analysis of variance (ANOVA) was employed to compare data
between multiple groups. All post hoc analyses were performed using
Tukey's test. The t-test was used to compare data between 2 groups.
All tests were carried out in 3 replicates, and data are reported
as the means ± SD. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Cell culture and characterization of
hPDLSCs
hPDLSCs were obtained from periodontal ligament
tissues by tissue block culture. They were mainly long, fusiform
and fibroblast-like cells under an inverted microscope (Fig. 1A). Flow cytometry analysis
revealed that passage 3 hPDLSCs positively expressed mesenchymal
surface markers (CD90, CD105, CD73 and CD44), and negatively
expressed hematopoietic or endothelial-specific markers (CD11b,
CD34, CD19, HLA-DR and CD45) (Fig.
1E). Furthermore, the hPDLSCs exhibited their colony formation
properties (Fig. 1B). In
multi-lineage differentiation potential assays, osteogenic
differentiation detected by ARS exhibited mineralized nodules
(Fig. 1C), and adipogenic
differentiation revealed lipid droplets following staining with Oil
Red O solution (Fig. 1D).
 | Figure 1Cell culture and characterization of
hPDLSCs. (A) hPDLSCs (P3) displaying spindle fibroblast-like
morphology under a phase-contrast microscope (scale bar, 200
μm). (B) Detection of single clones of hPDLSCs (scale bar,
200 μm). (C) Osteogenic differentiation ability of hPDLSCs
assayed by ARS (scale bar, 100 μm). (D) Adipogenic
differentiation ability of hPDLSCs assayed by Oil Red O staining
(scale bar, 50 μm). (E) Negative expression of CD11b, CD34,
CD19, CD45 and HLA-DR, and positive expression of CD90, CD105,
CD44, and CD73 in hPDLSCs assayed by flow cytometric analysis.
hPDLSCs, human periodontal ligament stem cells; P3, passage 3; ARS,
Alizarin Red staining. |
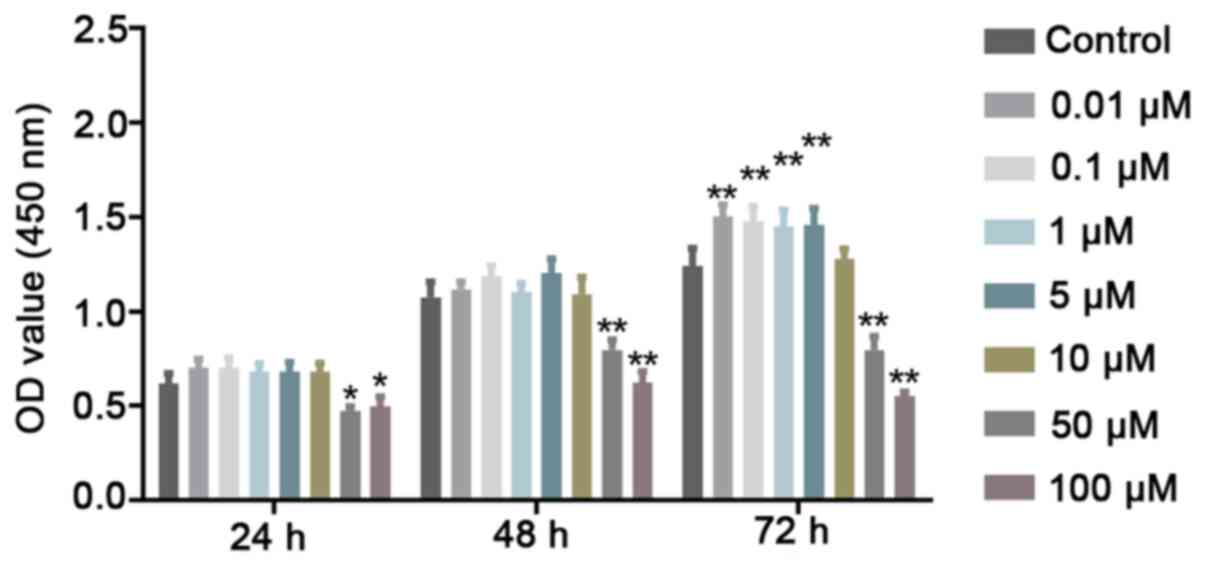
Low concentrations of Quer exert a
non-toxic effect on hPDLSCs
To evaluate cell viability, CCK-8 assay was
performed to determine whether Quer exerts potential cytotoxic
effects on the hPDLSCs. It was revealed that Quer did not affect
hPDLSC viability at concentrations up to 10 μM at 24 and 48
h. However, at high concentrations, such as 50 and 100 μM,
Quer significantly exhibited cellular toxicity against the hPDLSCs
at the 3 time points examined (Fig.
2). Therefore, these 2 higher concentrations were excluded from
further experiments.
Optimum concentration of Quer exerts a
protective effect against hPDLSCs osteogenic damage induced by
TNF-α
Relative to the control group, various
concentrations of TNF-α did not exert an obvious effect on hPDLSC
proliferation, as revealed by CCK-8 assay (Fig. 3A). Following osteogenic induction
for 7 days, ALP activity revealed that TNF-α concentrations >20
ng/ml significantly decreased the hPDLSC osteogenic differentiation
ability (Fig. 3B and C). When
the hPDLSCs were cultured in OIM, Quer concentrations >0.01
μM, particularly those between 1-10 μM, reversed the
TNF-α-induced inhibition of ALP activity (Fig. 3D). Therefore, the 1 μM
concentration of Quer was used in the subsequent experiments.
 | Figure 3The optimum concentration of Quer
exerts a protective effect against TNF-α-induced hPDLSC osteogenic
damage. (A) hPDLSCs were cultured with various concentrations (1,
10, 20, 30 and 50 ng/ml) of TNF-α for 4 days, and cell
proliferation was examined on days 1-3 and 4 using the CCK-8 assay
kit. (B) hPDLSCs were exposed in OIM with 0, 1, 10, 20, 30 and 50
ng/ml TNF-α, and ALP activity was detected after 7 days.
**P<0.01 vs. the control, NS (no significant
difference) vs. control. (C) Illustrative images of ALP staining in
OIM with 0, 1, 10, 20, 30 and 50 ng/ml TNF-α for 7 days (scale bar,
100 μm). (D) hPDLSCs were exposed in OIM containing 20 ng/ml
TNF-α with various concentrations of Quer (0, 0.01, 0.1, 1, 5, and
10 μM), and ALP activity was detected after 7 days.
*P<0.05 vs. TNF-α group (without Quer);
**P<0.01 vs. TNF-α group (without Quer). hPDLSCs,
human periodontal ligament stem cells; Quer, quercetin; OIM,
osteogenic induction medium; TNF-α, tumor necrosis factor-α; ALP,
alkaline phosphatase. |
Quer inhibits the induction of IL-1β and
IL-6 induced by TNF-α
To investigate the mimicking of the inflammatory
environment stimulated by TNF-α, the hPDLSCs were stimulated with
Quer (1 μM), TNF-α (20 ng/ml), or their combination in CM.
RT-qPCR analysis revealed that Quer + TNF-α significantly
downregulated the mRNA expression levels of IL-1β and IL-6 in the
hPDLSCs relative to the group stimulated with TNF-α alone. However,
Quer alone did not affect the IL-1β and IL-6 gene expression levels
(Fig. 4). Therefore, the in
vitro inflammatory environment was successfully established,
and 1 μM Quer had no significant pro-inflammatory
effect.
Quer reverses the inhibitory effects of
TNF-α on the osteogenic differentiation of hPDLSCs
Osteogenic differentiation was observed in order to
ascertain the effects of Quer on osteogenesis under normal
conditions or in a TNF-α-induced inflammatory microenvironment.
Following 7 days of osteogenic induction, western blot analysis
revealed that the protein levels of COL1, ALP and RUNX2 were
downregulated in the TNF-α group, while the levels of these
osteogenic differentiation-related proteins were evidently
upregulated following the addition of Quer (Fig. 5A and B). RT-qPCR revealed that
the gene expression levels of COL1 and RUNX2 were markedly
suppressed in the TNF-α group relative to the control group.
However, the suppression of these osteogenesis-associated genes was
significantly reversed in the Quer + TNF-α group (Fig. 5D). Following 7 days of osteogenic
induction, ALP activity and ALP staining assay revealed a decreased
and improved osteogenic ability of the hPDLSCs in TNF-α group and
in the Quer + TNF-α group, respectively (Fig. 5C and E). After 21 days of
osteogenic induction, ARS detected more mineralized nodules in the
Quer + TNF-α group than in the TNF-α group (Fig. 5F). These findings suggested that
Quer reversed the inhibitory effect of TNF-α on the osteogenesis of
hPDLSCs.
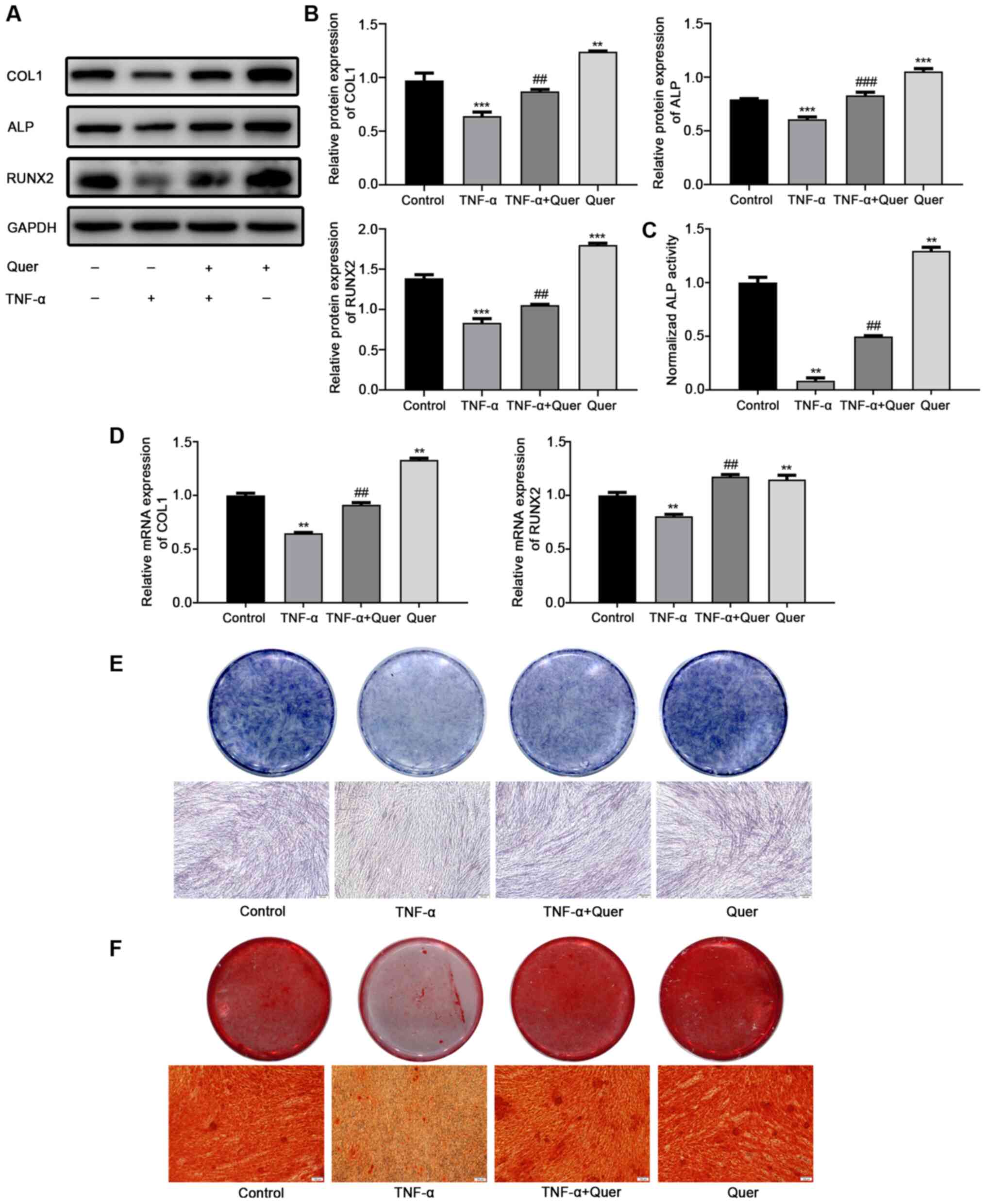 | Figure 5Reversing effect of Quer on
TNF-α-induced suppression of osteogenesis. hPDLSCs were treated
with Quer, TNF-α, or their combination for 7 days and/or 21 days in
an OIM. (A) Protein levels of COL1, ALP and RUNX2 were measured by
western blot analysis on day 7. (B) Band intensities were
quantified using ImageJ software. **P<0.01,
***P<0.001 vs. control; ##P<0.01,
###P<0.001 vs. TNF-α. (C) ALP activity detection
following 7 days of osteogenic induction. **P<0.01
vs. control; ##P<0.01 vs. TNF-α. (D) mRNA expression
levels of COL1 and RUNX2 were measured by RT-qPCR day 7.
**P<0.01 vs. control; ##P<0.01 vs.
TNF-α. (E) ALP staining detection following osteogenic induction
for 7 days (scale bar, 100 μm). (F) Alizarin Red staining
detection following 21 days of osteogenic induction (scale bar, 100
μm). hPDLSCs, human periodontal ligament stem cells; Quer,
quercetin; OIM, osteogenic induction medium; TNF-α, tumor necrosis
factor-α; ALP, alkaline phosphatase. |
Quer disrupts the TNF-α-induced
activation of the NF-κB/NLRP3 inflammasome pathway in hPDLSCs
To clarify the possible mechanisms through which
Quer attenuated the TNF-α-induced suppression of the osteogenesis
of hPDLSCs, the levels of NF-κB pathway-associated proteins and its
downstream molecule, NLRP3, were examined by RT-qPCR and western
blot analysis. As shown in Fig. 6A
and B, the levels of p-p65 and p-IκBα were markedly increased
in the TNF-α group compared to the control group. By contrast, the
levels of p-p65 and p-IκBα were significantly decreased (Fig. 6A and B) following treatment with
Quer. These results revealed that Quer disrupted the NF-κB pathway
in the model of TNF-α-induced periodontitis. At the same time,
western blot analysis was performed to observe the expression
levels of NLRP3, procaspase-1 and caspase-1, which were found to be
significantly elevated in the TNF-α group. In the Quer + TNF-α
group, the levels of these proteins were found to be downregulated
when compared to the TNF-α group (Fig. 6C and D). Additionally, NLRP3
inflammasome-associated gene expression was consistent with the
results obtained for protein expression (Fig. 6E). These findings indicated that
Quer suppressed the activation of NLRP3 stimulated by TNF-α in
hPDLSCs.
 | Figure 6Effect of Quer on the activation of
the NF-κB/NLRP3 pathway in TNF-α-induced hPDLSCs. hPDLSCs were
treated with 1 μM Quer, 20 ng/ml TNF-α, or their combination
for 24 h in a CM. (A) Protein expression levels of p-p65, p65,
p-IκBα and IκBα. (B) Band intensities were quantified using ImageJ
software. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. TNF-α. (C)
Protein expression levels of NLRP3, procaspase-1 and caspase-1. (D)
Band intensities were quantified using the ImageJ software.
**P<0.01 vs. control; #P<0.05,
##P<0.01 vs. TNF-α. (E) mRNA expression levels of
NLRP3 and caspase-1. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. TNF-α. hPDLSCs,
human periodontal ligament stem cells; Quer, quercetin; TNF-α,
tumor necrosis factor-α; NLRP3, NOD-like receptor family pyrin
domain-containing protein 3. |
Silencing of of NLRP3 reverses
TNF-α-induced osteogenic damage to hPDLSCs
To explore the effects of NLRP3 protein on
TNF-α-induced osteogenic damage to hPDLSCs, si-NLRP3 was used to
decrease NLRP3 expression. The NLRP3 protein level was markedly
decreased in the si-NLRP3, but not in the si-NC group (Fig. 7A). Additionally, the changes in
the levels of NF-κB signaling pathway-associated proteins were
examined following the silencing of NLRP3 in the TNF-α-induced
inflammatory microenvironment. Western blot analysis revealed that
the expression levels of p-p65 and p-IκBα were markedly upregulated
in the TNF-α + si-NC group and the TNF-α + si-NLRP3 group. No
significant differences were observed between the 2 groups
(Fig. 7B and C). That is, the
TNF-α-induced activation of the NF-κB signaling pathway was not
inhibited by the use of NLRP3 siRNA. hPDLSCs osteogenesis was then
induced and the effects of NLRP3 silencing on TNF-α-induced
osteogenic damage were investigated. After 7 days of osteogenic
differentiation, western blot analysis revealed that the levels of
osteogenic differentiation-associated proteins, including COL1, ALP
and RUNX2 were downregulated in the TNF-α + si-NC group. However,
the silencing of of NLRP3 reversed the TNF-α-induced inhibition of
osteogenic differentiation ability. The effect was consistent with
the Quer + TNF-α + si-NC group (Fig.
7D and E). Moreover, RT-qPCR, ALP staining and ALP activity
assay revealed a notably upregulated osteogenic ability in the Quer
+ TNF-α + si-NC or the TNF-α + si-NLRP3 groups, compared to the
TNF-α + si-NC group (Fig. 7F-H).
Taken together, these results demonstrated that the silencing of
NLRP3 protected against TNF-α-induced osteogenic damage to hPDLSCs,
consistent with the effects of Quer.
Discussion
Periodontitis is a severe oral disease that results
in the defection of periodontal supporting tissue, long-term
inflammatory cytokines stimulation, and can cause teeth loss
(33). Unlike traditional
treatment methods, such as scaling and root planning for
periodontitis, tissue regenerative technology, which utilizes
combination treatment with drugs and stem cells, has more potential
therapeutic properties (34,35). The selected drug should inhibit
inflammatory factor secretion, while stem cells have high
proliferation and multiple differentiation potentials to restore
the balance of bone formation and resorption for periodontitis
therapy. Studies have documented that hPDLSCs have more
undifferentiated MSC characteristics and are suitable seed cells
for bone tissue regeneration (6,7).
In the present study, hPDLSCs were successfully isolated from
volunteers with excellent stem cell properties.
The expression of TNF-α, as a key pro-inflammatory
cytokine, is elevated during periodontal disease progression
(36). The overexpression of
TNF-α elevates osteoclast activity, resulting in bone destruction
and a decreased osteoblast bone formation ability (37,38). As previously reported, various
concentrations of TNF-α do not exert an obvious effect on hPDLSCs
proliferation, while elevated concentrations of TNF-α exhibit a
negative regulatory effect on osteogenesis (39). The present study found that a
TNF-α concentration of 20 ng/ml was required to mimic an
inflammatory micro- environment in vitro, which was
demonstrated by decreased ALP staining and ALP activity.
To investigate a novel strategy for alleviating
TNF-α-induced periodontal supportive tissue destruction, Quer was
used. Quer is a natural flavonoid compound that exhibits
anti-inflammatory, antioxidant and cardiovascular protective
properties at an optimum concentration (40). Quer is a potential candidate for
preventing various pathological diseases due to its extensive
pharmacological properties. It has been shown that Quer can inhibit
the IL-17-induced RANKL production and decrease IL-17-stimulated
osteoclastogenesis in the bone destructive process (26). A previous study revealed that
Quer decreased LPS-induced osteoclast formation, ligature-promoted
periodontal inflammation and bone destruction with experimental
periodontitis in rats (23). The
present study found that Quer at concentrations of <10 μM
did not exhibit any cytotoxicity, but significantly prevented
TNF-α-induced osteogenic damage, particularly at the 1-10 μM
concentrations.
In periodontal disease, the upregulation of IL-1β
and IL-6 is closely associated with the pathophysiology of
periodontitis and can accelerate the degeneration of inflammatory
periodontal tissues (41,42).
The present study used RT-qPCR to examine the mRNA expression
levels of IL-1β and IL-6. Elevated IL-1β and IL-6 expres- sion
levels indicated that the model of inflammation was successfully
mimicked by TNF-α stimulation in vitro. Quer treatment
decreased the TNF-α-induced production of IL-1β and IL-6, and did
not exert pro-inflammatory effects on hPDLSCs without TNF-α.
Moreover, the gene level of IL-1β following the different
treatments suggested that the NLRP3 inflammasome may be activated
in the process. Subsequently, the levels of the osteogenic
differentiation representative genes and proteins, COL1, ALP and
RUNX2, were determined by RT-qPCR and western blot analysis,
respectively. The results demonstrated that Quer (1 μM)
significantly restored the osteogenic ability of the hPDLSCs which
had been impaired by TNF-α. Furthermore, ALP staining and the
results of ARS were in accordance with the obtained mRNA and
protein expression levels. It has been previously demonstrated that
Quer can promote mouse BMMC proliferation and osteogenic ability
(43). Similarly, the present
study found that Quer improved the osteogenic ability of hPDLSCs
when compared to the control group without TNF-α. These findings
revealed that Quer antagonized the TNF-α-induced inhibition of
hPDLSC osteogenesis, which may be due to the suppression of
inflammation and the pro-osteogenic effects on hPDLSCs.
The NF-κB signaling pathway, as a significant
downstream pathway of TNF-α, is activated when TNF-α binds TNF
receptor 1 (TNFR1) to inhibit osteogenesis-associated gene
transcription and regulate osteogenic differentiation (12,44,45). Studies have documented that
various flavonoids, including Quer, can suppress LPS- or
TNF-α-induced key protein expression of the NF-κB signaling pathway
(46,47). These studies prompted us to
determine whether the NF-κB pathway is a potential responsive
mechanism through which Quer reverses the inhibitory osteogenic
differentiation of TNF-α induced hPDLSCs. Consistent with previous
studies (46,47), the results of the present study
demonstrated that the 1 μM Quer concentration significantly
inhibited the TNF-α-induced phosphorylation of p65 and IκBα in the
hPDLSCs. Additionally, the NF-κB signaling pathway is a priming
signal for activating the NLRP3 inflammasome (14). It was also found that the
activation of the NF-κB signaling pathway was not significantly
influenced by the silencing of of NLRP3 in the TNF-α-induced
inflammatory microenvironment. Studies have revealed that Quer can
attenuate diabetic encephalopathy and protect against
isoniazid-induced hepatotoxicity by suppressing the NLRP3 pathway
(30,48). Likewise, the present study
determined that Quer downregulated the expression of NLRP3
inflammasome-associated proteins and genes treated in the hPDLSCs
stimulated with TNF-α. Recent studies have revealed that NLRP3
inflammasome activation can suppress the osteogenesis of MSCs and
can contribute to the estrogen deficiency-induced suppression of
osteogenesis in ovariectomized mice (18,19). However, few studies have
demonstrated the effect of NLRP3 on the osteogenic differentiation
of hPDLSCs stimulated with TNF-α. The present study indicated that
NLRP3 silencing reversed TNF-α-induced osteogenic damage to
hPDLSCs, similar to Quer treatment. Therefore, Quer treatment
reversed the inhibition of osteogenic differentiation induced by
TNF-α, which may be associated with the inhibition of the
NF-κB/NLRP3 inflammasome pathway.
In conclusion, the present study demonstrated that
Quer reversed the suppression of the osteogenesis of hPDLSCs in an
in vitro model of TNF-α-induced periodontitis by inhibiting
the relative targets involved in the NF-κB/NLRP3 inflammasome
pathway. These findings provide a basis for the use of the drug and
stem cell combinations as an effective treatment agent for bone
regeneration under inflammatory conditions.
Funding
The present study was supported by the Construction
Engineering Special Fund of Taishan Scholars (grant no.
ts201511106) and the National Natural Science Foundation of China
(grant no. 82071148).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XX designed the study. WZ, LJ and BZ performed the
experiments. JL, YX and YNW analyzed the experimental data. WZ
wrote the article. All authors listed have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was authorized by the Medical
Ethical committee of School of Stomatology, Shandong University
(protocol no. GR201806). Signed informed consent was provided from
every participant according to the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the Director of
Shandong Provincial Key Laboratory of Oral Tissue Regeneration for
providing technical support with the study.
References
|
1
|
Bartold PM and Van Dyke TE: Periodontitis:
A host-mediated disruption of microbial homeostasis. Unlearning
learned concepts. Periodontol 2000. 62:203–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L,
Ji HL, Tse HF, Fu QL and Lian Q: Mesenchymal stem cells and
immunomodulation: Current status and future prospects. Cell Death
Dis. 7:e20622016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moradi SL, Golchin A, Hajishafieeha Z,
Khani MM and Ardeshirylajimi A: Bone tissue engineering: Adult stem
cells in combination with electrospun nanofibrous scaffolds. J Cell
Physiol. 233:6509–6522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouchi T and Nakagawa T: Mesenchymal stem
cell-based tissue regeneration therapies for periodontitis. Regen
Ther. 14:72–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia Y, Tang HN, Wu RX, Yu Y, Gao LN and
Chen FM: Cell responses to conditioned media produced by
patient-matched stem cells derived from healthy and inflamed
periodontal ligament tissues. J Periodontol. 87:e53–e63. 2016.
View Article : Google Scholar
|
|
6
|
Bright R, Hynes K, Gronthos S and Bartold
PM: Periodontal ligament-derived cells for periodontal regeneration
in animal models: A systematic review. J Periodontal Res.
50:160–172. 2015. View Article : Google Scholar
|
|
7
|
Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen
Y and Xu X: An in vitro comparative study of multisource derived
human mesenchymal stem cells for bone tissue engineering. Stem
Cells Dev. 27:1634–1645. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trubiani O, Pizzicannella J, Caputi S,
Marchisio M, Mazzon E, Paganelli R, Paganelli A and Diomede F:
Periodontal ligament stem cells: Current knowledge and future
perspectives. Stem Cells Dev. 28:995–1003. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winning L, El Karim IA and Lundy FT: A
Comparative analysis of the osteogenic potential of dental
mesenchymal stem cells. Stem Cells Dev. 28:1050–1058. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XY, He XT, Wang J, Li X, Xia Y, Tan YZ
and Chen FM: Role of the P2X7 receptor in inflammation-mediated
changes in the osteogenesis of periodontal ligament stem cells.
Cell Death Dis. 10:202019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartold PM, Cantley MD and Haynes DR:
Mechanisms and control of pathologic bone loss in periodontitis.
Periodontol 2000. 53:55–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbas S, Zhang YH, Clohisy JC and Abu-Amer
Y: Tumor necrosis factor-alpha inhibits pre-osteoblast
differentiation through its type-1 receptor. Cytokine. 22:33–41.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J
and Yang P: Dose-specific effects of tumor necrosis factor alpha on
osteogenic differentiation of mesenchymal stem cells. Cell Prolif.
44:420–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo H, Callaway JB and Ting JP:
Inflammasomes: Mechanism of action, role in disease, and
therapeutics. Nat Med. 21:677–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Zhang L, Lu W, Tan X, Yue J, Wang P,
Xu W, Ye L and Huang D: The effect of NLRP inflammasome on the
regulation of AGEs-induced inflammatory response in human
periodontal ligament cells. J Periodontal Res. 54:681–689. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Chen K, Wan X, Wang F, Guo Z and
Mo Z: NLRP3 inflammasome activation in mesenchymal stem cells
inhibits osteogenic differentiation and enhances adipogenic
differentiation. Biochem Biophys Res Commun. 484:871–877. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu L, Zhang L, Wang Z, Li C, Li S, Li L,
Fan Q and Zheng L: Melatonin suppresses estrogen deficiency-induced
osteoporosis and promotes osteoblastogenesis by inactivating the
NLRP3 inflammasome. Calcif Tissue Int. 103:400–410. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Alencar JB, Zacarias JMV, Tsuneto PY,
Souza VH, Silva COE, Visentainer JEL and Sell AM: Influence of
inflammasome NLRP3, and IL1B and IL2 gene polymorphisms in
periodontitis susceptibility. PLoS One. 15:e02279052020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Isaza-Guzmán DM, Medina-Piedrahíta VM,
Gutiérrez-Henao C and Tobón-Arroyave SI: Salivary levels of NLRP3
inflammasome-related proteins as potential biomarkers of
periodontal clinical status. J Periodontol. 88:1329–1338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, inflammation and immunity.
Nutrients. 8:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng WC, Huang RY, Chiang CY, Chen JK,
Liu CH, Chu CL and Fu E: Ameliorative effect of quercetin on the
destruction caused by experimental periodontitis in rats. J
Periodontal Res. 45:788–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan Z, Min J, Zhao Y, Cheng Q, Wang K,
Lin S, Luo J and Liu H: Quercetin rescued TNF-alpha-induced
impairments in bone marrow-derived mesenchymal stem cell
osteogenesis and improved osteoporosis in rats. Am J Transl Res.
10:4313–4321. 2018.
|
|
25
|
Guo C, Yang RJ, Jang K, Zhou XL and Liu
YZ: Protective effects of pretreatment with quercetin against
lipopolysaccharide-induced apoptosis and the inhibition of
osteoblast differentiation via the MAPK and Wnt/β-Catenin pathways
in MC3T3-E1 cells. Cell Physiol Biochem. 43:1547–1561. 2017.
View Article : Google Scholar
|
|
26
|
Kim HR, Kim BM, Won JY, Lee KA, Ko HM,
Kang YS, Lee SH and Kim KW: Quercetin, a plant polyphenol, has
potential for the prevention of bone destruction in rheumatoid
arthritis. J Med Food. 22:152–161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zendedel A, Johann S, Mehrabi S, Joghataei
MT, Hassanzadeh G, Kipp M and Beyer C: Activation and regulation of
NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal
cord injury model. Mol Neurobiol. 53:3063–3075. 2016. View Article : Google Scholar
|
|
28
|
Jiang W, Huang Y, Han N, He F, Li M, Bian
Z, Liu J, Sun T and Zhu L: Quercetin suppresses NLRP3 inflammasome
activation and attenuates histopathology in a rat model of spinal
cord injury. Spinal Cord. 54:592–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Xu X, Li Y, Kou J, Huang F, Liu B
and Liu K: Quercetin, luteolin and epigallocatechin gallate
alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with
regulation of AMPK in endothelial cells. Eur J Pharmacol.
745:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Qu X, Gao H, Zhai J, Tao L, Sun
J, Song Y and Zhang J: Quercetin attenuates NLRP3 inflammasome
activation and apoptosis to protect INH-induced liver injury via
regulating SIRT1 pathway. Int Immunopharmacol. 85:1066342020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao B, Zhang Y, Xiong Y and Xu X: Rutin
promotes the formation and osteogenic differentiation of human
periodontal ligament stem cell sheets in vitro. Int J Mol Med.
44:2289–2297. 2019.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Bosshardt DD: The periodontal pocket:
Pathogenesis, histopathology and consequences. Periodontol 2000.
76:43–50. 2018. View Article : Google Scholar
|
|
34
|
Huang J, Liu L, Jin S, Zhang Y, Zhang L,
Li S, Song A and Yang P: Proanthocyanidins promote osteogenic
differentiation of human periodontal ligament fibroblasts in
inflammatory environment via suppressing NF-κB signal pathway.
Inflammation. 43:892–902. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yue H, Xu X, Liu Q, Li X, Xiao Y and Hu B:
Effects of non-surgical periodontal therapy on systemic
inflammation and metabolic markers in patients undergoing
haemodialysis and/or peritoneal dialysis: A systematic review and
meta-analysis. BMC Oral Health. 20:182020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Romero-Castro NS, Vázquez-Villamar M,
Muñoz-Valle JF, Reyes-Fernández S, Serna-Radilla VO,
García-Arellano S and Castro-Alarcón N: Relationship between TNF-α,
MMP-8, and MMP-9 levels in gingival crevicular fluid and the
subgingival microbiota in periodontal disease. Odontology.
108:25–33. 2020. View Article : Google Scholar
|
|
37
|
Ohori F, Kitaura H, Ogawa S, Shen WR, Qi
J, Noguchi T, Marahleh A, Nara Y, Pramusita A and Mizoguchi I:
IL-33 inhibits TNF-α-induced osteoclastogenesis and bone
resorption. Int J Mol Sci. 21:11302020. View Article : Google Scholar
|
|
38
|
Tan J, Zhou L, Xue P, An Y, Luo L, Zhang
R, Wu G, Wang Y, Zhu H and Wang Q: Tumor necrosis factor-α
attenuates the osteogenic differentiation capacity of periodontal
ligament stem cells by activating PERK signaling. J Periodontol.
87:e159–e171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qin Z, Fang Z, Zhao L, Chen J, Li Y and
Liu G: High dose of TNF-α suppressed osteogenic differentiation of
human dental pulp stem cells by activating the Wnt/β-catenin
signaling. J Mol Histol. 46:409–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Casado-Diaz A, Anter J, Dorado G and
Quesada-Gómez JM: Effects of quercetin, a natural phenolic
compound, in the differentiation of human mesenchymal stem cells
(MSC) into adipocytes and osteoblasts. J Nutr Biochem. 32:151–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noh MK, Jung M, Kim SH, Lee SR, Park KH,
Kim DH, Kim HH and Park YG: Assessment of IL-6, IL-8 and TNF-α
levels in the gingival tissue of patients with periodontitis. Exp
Ther Med. 6:847–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marchesan JT: Inflammasomes as
contributors to periodontal disease. J Periodontol. 91(Suppl 1):
S6–S11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pang XG, Cong Y, Bao NR, Li YG and Zhao
JN: Quercetin stimulates bone marrow mesenchymal stem cell
differentiation through an estrogen receptor-mediated pathway.
Biomed Res Int. 2018:41780212018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Hu C, Wang G, Li L, Kong X, Ding Y
and Jin Y: Nuclear factor-κB modulates osteogenesis of periodontal
ligament stem cells through competition with β-catenin signaling in
inflammatory microenvironments. Cell Death Dis. 4:e5102013.
View Article : Google Scholar
|
|
45
|
Wang LM, Zhao N, Zhang J, Sun QF, Yang CZ
and Yang PS: Tumor necrosis factor-alpha inhibits osteogenic
differentiation of pre-osteoblasts by downregulation of EphB4
signaling via activated nuclear factor-kappaB signaling pathway. J
Periodontal Res. 53:66–72. 2018. View Article : Google Scholar
|
|
46
|
Li T, Li F, Liu X, Liu J and Li D:
Synergistic anti-inflammatory effects of quercetin and catechin via
inhibiting activation of TLR4-MyD88-mediated NF-κB and MAPK
signaling pathways. Phytother Res. 33:756–767. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Yu C, Ji K, Wang X, Li X, Xie H,
Wang Y, Huang Y, Qi D and Fan H: Quercetin reduces TNF-α-induced
mesangial cell proliferation and inhibits PTX3 production:
Involvement of NF-κB signaling pathway. Phytother Res.
33:2401–2408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu T, Lu XY, Shi JJ, Liu XQ, Chen QB, Wang
Q, Chen YB and Zhang SJ: Quercetin protects against diabetic
encephalopathy via SIRT1/NLRP3 pathway in db/db mice. J Cell Mol
Med. 24:3449–3459. 2020. View Article : Google Scholar : PubMed/NCBI
|