Introduction
Pulmonary hypertension (PH) is a cardiopulmonary
vascular disease characterized by continued increases in pulmonary
arterial pressure and pulmonary vascular resistance (1). Current treatment strategies mainly
focus on reducing pulmonary vascular resistance and increasing
blood flow. However, these approaches are not effective for a long
period of time. Therefore, the identification of a precise
therapeutic target for PH is critical. The hallmark feature of PH
is medial pulmonary artery hyperplasia, which is mainly caused by
the abnormal proliferation and aggregation of pulmonary artery
smooth muscle cells (PASMCs) (2,3).
Therefore, the proliferation, migration, apoptosis and
extracellular matrix deposition of PASMCs are critical targets for
studying PH.
A number of growth factors are related to PH and
vascular remodeling, including platelet-derived growth factor
(PDGF)-BB and transforming growth factor (TGF)-β (4). The inhibition of the PDGF receptor
has been shown to increase the survival rate of rats with
monocrotaline (MCT)-induced PH (5,6).
The TGF-β superfamily contains a large number of cytokine growth
factors that control numerous cellular functions, including
proliferation, migration, differentiation, and extracellular matrix
secretion and deposition (4). It
has been reported that PDGF-BB activates the TGF-β1/SMAD2/3
signaling pathway, and induces the growth and proliferation of rat
PASMCs (7). The TGF-β1/SMAD
pathway is activated in animals with MCT and hypoxia-induced PH
(8-10) and in patients with PH (11). The activation of the
TGF-β1/SMAD2/3 signaling pathway is one of the factors contributing
to the occurrence and development of PH.
Inwardly rectifying K+ channel (KIR)2.1
encoded by the KCNJ2 gene is a member of the classical
inwardly rectifying K+ channel family (KIR2 subfamily).
In previous research, KIR2.1 was considered the main component of
the inward rectifying potassium current of the heart and an
essential component of the stable resting membrane potential of
cardiomyocytes (12). However,
KIR2.1 is related to cell proliferation and migration (13-17) and vascular remodeling (18). According to previous studies,
KIR2.1 is expressed in isolated rat basilar artery, coronary
artery, mesenteric artery smooth muscle cells (19), renal arteriole smooth muscle cells
(20) and PASMCs (21). KIR2.1 gene knockout significantly
inhibits the proliferation and migration of rat vascular smooth
muscle cells (VSMCs) stimulated by PDGF-BB (22). However, researchers have not yet
determined whether KIR2.1 participates in the proliferation and
migration of PASMCs.
Therefore, the present study aimed to examine the
effects of KIR2.1 inhibition on the proliferation and migration of
PASMCs induced by PDGF-BB. In addition, the present study examined
whether KIR2.1 regulates the TGF-β1/Smad2/3 signaling pathway by
maintaining the depolarized membrane potential of the cell
membrane, and whether it regulates the proliferation and migration
of PASMCs.
Materials and methods
Animal model and treatment strategy
A total of 12 Sprague-Dawley (SD) rats (8-10 weeks
old, weighing 200-250 g) were used in the experiments. The rats
were purchased from Beijing Vital River Experimental Animal Co.,
Ltd. (license no. SCXK Beijing 2016-0006). The animals were
maintained in environmentally controlled conditions (adequate cage
size; free access to food and water; temperature, 22±2°C; humidity,
50-55%) on a 12-h light/12-h dark cycle. All procedures involving
animals were performed in accordance with ethical standards and
approved by the Institutional Animal Care and Use Committee of the
Affiliated Hospital of Shihezi University School of Medicine
(approval no. A 2020-165-01). Applicable guidelines were followed
in accordance with the 'Guide for the Care and Use' published by
the American Physiological Society (23).
A total of 12 rats were randomly and equally divided
into two groups as follows: The control (CON) group and MCT group
[60 mg/kg MCT (24-26) administered by intraperitoneal
(i.p.) injection on the first day; Sigma-Aldrich; Merck KGaA].
Doppler echocardiography
measurements
The Doppler echo parameter, pulmonary artery
acceleration time (PAAT) is considered an echocardiographic
indicator of PH (27). PAAT is
the time interval between the onset of systolic pulmonary arterial
flow and peak flow velocity. Previous studies have demonstrated
that PAAT is inversely proportional to pulmonary vascular
resistance (28-30). If the pulmonary vascular
resistance increases, the pulmonary artery pressure also increases.
Doppler echocardiography was used to assess PH on the 28th day
following model establishment in the SD rats. A transthoracic
closed-chest echocardiography was performed using a Vivid E9
ultrasound system equipped with a 12-MHz transducer (GE Healthcare;
Cytiva). The rats were anesthetized by administering an i.p.
injection of 3% sodium pentobarbital (40 mg/kg). PAAT was measured
near the pulmonary valve on the left side of the chest. EchoPAC™
BT11 software (v.6.5; GE Healthcare; Cytiva) was used to analyze
the data.
Right ventricular hypertrophy
measurement
For the measurement of right ventricular
hypertrophy, the rats were euthanized by an i.p. injection of 3%
sodium pentobarbital (100 mg/kg) combined with CO2
anesthesia at a displacement of 70% vol/min. The heart tissue was
collected and weighed to evaluate the right ventricular hypertrophy
index (RVHI). The atrium and external blood vessels were separated
from the isolated heart in 0.9% normal saline. The weights of both
the right ventricle (RV) and left ventricle (LV) plus septum (S)
were recorded. The RVHI was calculated using the formula: [RV/(LV +
S)].
Histopathological examination of lung
tissue
Lung tissues were harvested, fixed in 4%
paraformaldehyde (Boster Co., Ltd.), dehydrated, cleared, waxed,
embedded, sectioned, patched and cut into slices
(4-µm-thick). Hematoxylin and eosin (H&E) staining or
Masson's trichome staining (Beijing Solarbio Biotechnology Co.,
Ltd.) were used to evaluate the pulmonary artery morphology. Lung
tissues were observed and photographed using a digital camera
(BX51; Olympus Corporation). Image-Pro Plus v.6.0 software (Media
Cybernetics, Inc.) was used for the quantitative analysis.
Pulmonary vascular remodeling (PVR) was evaluated by calculating
the percentage of the thickness of the vessel wall (WT%) and the
percentage of the vessel wall area (WA%): WT%=[2× (blood vessel
outer diameter-blood vessel inner diameter)]/(blood vessel outer
diameter) ×100%; WA%=(total area of blood vessel-blood vessel
internal area)/total area of blood vessel ×100% (31). Two professional pathologists
randomly selected 20 different non-overlapping fields from each
section and analyzed PVR and lung fibrosis with Image-Pro Plus
v.6.0 software (Media Cybernetics, Inc.). The pulmonary fibrosis
index was analyzed by calculating the ratio of the total area of
collagen to the total area of connective tissue in each field of
view, as previously described (32).
Cell culture and treatment
Human PASMCs (HPASMCs) (Shanghai iCell Bioscience
Inc.) were cultured in high-glucose DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS)
and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). The cells were incubated at 37°C for 24 h in a humidified 5%
CO2 atmosphere.
For the cell treatments, the cells were first
pre-treated with the KIR2.1 pathway blocker, ML133 (20 µM,
ApexBio) (14,17,33), or the TGF-β1/SMAD signaling
pathway blocker, SB431542 (10 µM, ApexBio), for 24 h and
were then treated with PDGF-BB (25 ng/ml, PeproTech, Inc.)
(34-36) for 24 h. Cells in the control group
were not treated. The experiment was repeated six times (n=6).
Cell scratch assay
HPASMCs in the logarithmic growth phase were plated
as monolayers on six-well plates and cultured at 37°C with 5%
CO2 until the cell density reached 80%. A cell-free band
was uniformly generated at the center of each well using a 1-ml
pipet tip. The cells were washed twice with PBS and then incubated
with 2 ml 2% DMEM/F12 low-serum medium and pretreated with 0
µM ML133 for 24 h, then treated with 25 ng/ml PDGF-BB for 24
h. Images were recorded and assessed at 0 and 24 h using an Olympus
inverted microscope (Olympus Corporation). The migration distance
was estimated using ImageJ v1.8.0 software (National Institutes of
Health). The cell migration rate (%)=[(0 h average scratch area-24
h average scratch area)/0 h average scratch area] ×100% (37-39).
Transwell assay
The Transwell™ chamber (Corning, Inc.) was placed in
a 24-well culture plate. Subsequently, 200 µl of the cell
suspension (cell density of 104 cells/ml) were
inoculated into the upper chamber. Complete DMEM/F12 containing 10%
FBS (600 µl) was then added to the lower chamber. 20
µM ML133 was added for 24 h after the cells adhered to the
well, then treated with 25 ng/ml PDGF-BB for 24 h. Following the
intervention, the cells were cultured with fresh medium at 37°C in
a humidified atmosphere containing 5% CO2 for 24 h.
Subsequently, the cells below the membrane were fixed with 4%
paraformaldehyde for 10 min at room temperature and stained with
0.1% crystal violet for 30 min at room temperature. A cotton swab
was used to gently remove the cells from the upper surface of the
chamber. Following observation with a light microscope and imaging
with a digital camera (BX51, Olympus Corporation), five different
fields of view were randomly selected; the cells that invaded the
submembrane surface were counted, and the number of invaded cells
reflected the strength of the invasive ability of the HPASMCs
(37-39).
Immunofluorescence staining
The paraffin-embedded tissue sections were dewaxed,
and antigen retrieval was performed. The cells were seeded on
six-well glass slides for fixation. Triton X (0.3%) was used to
permeabilize the membrane. The cells were then incubated overnight
at 4°C with the following antibodies: Anti-KIR2.1 (1:100 dilution,
cat. no. ab109750), anti-osteopontin (OPN; 1:500 dilution, cat. no.
ab8448), anti-proliferating cell nuclear antigen (PCNA; 1:200
dilution, cat. no. ab29) and anti-α-smooth muscle actin (α-SMA; 1:
500; cat. no. ab124964) (all from Abcam). The following day, the
cells were incubated with FITC-labeled goat anti-rabbit IgG (1:100;
cat. no. ZF-0311) or TRITC-labeled anti-mouse IgG (1:100; ZF-0313)
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) antibodies in a
dark box at 37°C for 2 h. The nuclei were stained with DAPI
(1:1,000, D9542; Sigma-Aldrich; Merck KGaA) at 37°C in a dark box
for 20 min. The cells were observed and photographed under a
fluorescence inverted microscope (LSM710; Carl Zeiss AG). Proteins
were semi-quantitatively analyzed using Image-Pro Plus 6.0 software
(National Institutes of Health).
Western blot analysis
Total protein was extracted from HPASMC suspensions
or pulmonary vascular tissue homogenates from SD rats with RIPA
Lysis Buffer (Thermo Fisher Scientific, Inc.) in western blot
analysis. The BCA protein determination method was used to
determine the protein concentration. Proteins aliquots (40
µg/lane) were separated using standard sodium lauryl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, (8-10% gel))
and transferred to 0.45 µm nitrocellulose membranes
(Invitrogen; Thermo Fisher Scientific, Inc.). The membranes were
incubated overnight at 4°C with the following primary antibodies:
Anti-GAPDH (1:10,000 dilution, cat. no. ab8245), anti-KIR2.1
(1:1,000 dilution, cat. no. ab109750), anti-TGF-β1 (1:1,000
dilution, cat. no. ab92486), anti-SMAD2 (1:1,000 dilution, cat. no.
ab40855), anti-SMAD3 (1:1,000 dilution, cat. no. ab40854),
anti-phosphorylated (p-)SMAD2 (1:1,000 dilution, cat. no.
ab188334), anti-p-SMAD3 (1:1,000 dilution, cat. no. ab52903),
anti-OPN (1:1,000 dilution, cat. no. ab8448) and anti-PCNA (1:1,000
dilution, cat. no. ab29) (all from Abcam). Subsequently, the
membranes were incubated with HRP-labeled anti-mouse IgG (1:20,000
dilution; cat. no. ZB-2305) or anti-rabbit IgG (1:10,000 dilution;
cat. no. ZB-5301; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) at room temperature for 2 h. Luminescence reagents were
obtained from the SuperSignal™ West Pico luminescence substrate kit
(Thermo Fisher Scientific, Inc.) and incubated with the membranes.
Protein bands were quantified using ImageJ v1.8.0 software
(National Institutes of Health).
Statistical analysis
The statistical software packages SPSS 21.0 and
GraphPad Prism 8.0 were used to analyze the experimental results.
All data are presented as the mean ± standard deviations (means ±
SD). The Kolmogorov-Smirnov test was used for each set of data and
the data were found to be normally distributed. Differences between
two groups were analyzed using unpaired t-tests. Differences in
data from more than two groups were analyzed using one-way analysis
of variance (ANOVA), followed by Tukey's multiple comparisons test.
A value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Right ventricular remodeling and PVR in
rats with PH
The echocardiogram of the blood flow in the
pulmonary artery was detected using the Doppler ultrasonic
diagnostic instrument. Compared with the CON group (Fig. 1A), the MCT group exhibited a
midsystolic notch, with the peak shifting forward, and the PAAT
value was decreased (P<0.01, n=6; Fig. 1B). The RVHI% of the rats in the
MCT group was significantly higher than that in the CON group
(P<0.01, n=6; Fig. 1C).
 | Figure 1Right ventricular remodeling and
pulmonary artery remodeling in pulmonary hypertension. (A) The
pulmonary hemodynamic spectrum. (B) Comparison of PAAT. (C)
Comparison of RVHI%. (D) H&E staining of the small pulmonary
artery. (E) H&E staining of the middle pulmonary artery; scale
bars, 50 µm (top panels) and 25 µm (bottom panels).
The red arrows indicate the pulmonary artery. (F) Statistical
analysis of the WT% and WA% of pulmonary arteries; scale bar, 50 or
25 µm. (G) Masson's trichrome staining of lung tissue. (H)
Analysis of the fibrotic index of pulmonary vascular tissue; scale
bar=50 or 25 µm. WT% (S), WT% of the small pulmonary artery;
WA% (S), WA% of the small pulmonary artery; WT% (M), WT% of the
middle pulmonary artery; WA% (m), WA% of the middle pulmonary
artery. **P<0.01, MCT vs. CON (n=6, data were
analyzed using a t-test). PAAT, pulmonary artery acceleration time;
RVHI%, right ventricular hypertrophy index percentage; WT%,
percentage of the thickness of the vessel wall; WA%, percentage of
the vessel wall area; MCT, monocrotaline; CON, control. |
H&E staining was performed to observe the
structural differences between the small pulmonary arteries
(diameter, 15-50 µm) (Fig.
1D) and the middle pulmonary arteries (diameter, 50-150
µm) (Fig. 1E). The
pulmonary artery cells in the CON group were evenly distributed,
continuous and structurally intact, while the pulmonary artery
cells in the MCT group exhibited a disordered arrangement, the area
of the lumen was reduced, and the thickness of the tube wall was
significantly increased. The pulmonary artery WT% and WA% of the
MCT group were significantly higher than that of the CON group
(P<0.01, n=6; Fig. 1F).
Masson's trichrome staining was used to observe
collagen deposition and lung fibrosis (Fig. 1G). The analysis revealed evident
collagen deposition in the pulmonary blood vessels and lung tissues
of the MCT group of rats (P<0.01, Fig. 1H). The lung tissue was evidently
fibrotic.
Expression of KIR2.1, OPN, PCNA and
TGF-β1/SMAD2/3 signaling pathway proteins in pulmonary blood
vessels and lung tissues of rats with PH
Immunohistochemistry and immunofluorescence staining
were performed to detect changes in the expression of KIR2.1
(Fig. 2A and B), the
migration-related protein, OPN (Fig.
2A and C), and the proliferation-related protein, PCNA
(Fig. 2A and D), in rat lung
tissue. The KIR2.1, OPN and PCNA proteins were widely expressed in
lung tissues and pulmonary blood vessels. The semi-quantitative
analysis of the fluorescence intensity revealed that KIR2.1, OPN,
and PCNA expression was significantly increased in the MCT group
(P<0.01, n=6) compared with that in the CON group (Fig. 2E).
 | Figure 2Expression of KIR2.1, OPN, PCNA and
TGF-β1/SMAD2/3 signaling pathway proteins in pulmonary vessels and
lung tissues of rats with pulmonary hypertension. (A)
Immunohistochemical staining for KIR2.1, OPN and PCNA; scale bar,
25 µm. (B) Immunofluorescence staining for α-SMA and KIR2.1;
(C) Immunofluorescence staining for α-SMA and OPN. (D)
Immunofluorescence staining for α-SMA and PCNA. Scale bars, 50
µm. The white arrows indicate the pulmonary blood vessels.
(E) Analysis of the relative expression of the KIR2.1, OPN and PCNA
proteins using immunofluorescence staining. (F) Representative
western blots illustrating KIR2.1, OPN, PCNA, TGF-β1, SMAD2, SMAD3,
p-SMAD2, p-SMAD3 and GAPDH levels. (G) Analysis of the protein
levels of KIR2.1, OPN, PCNA, TGF-β1, SMAD2, p-SMAD2, SMAD3 and
p-SMAD3. *P<0.05 and **P<0.01, MCT vs.
CON (n=6, data were analyzed using a t-test). KIR2.1, inwardly
rectifying K+ channel 2.1; OPN, osteopontin; PCNA,
proliferating cell nuclear antigen; α-SMA, α-smooth muscle actin;
MCT, monocrotaline; CON, control. |
Western blot analysis was conducted to further
detect KIR2.1, OPN and PCNA protein levels in the tissue homogenate
of rat pulmonary blood vessels (Fig.
2F). The results were consistent with those of the
semi-quantitative analysis of immunofluorescence staining.
Significantly higher levels of the KIR2.1, OPN and PCNA proteins
were detected in the MCT group compared with the CON group
(P<0.01 or P<0.05, n=6; Fig.
2G). Furthermore, western blot analysis was used to assess the
expression of proteins in the TGF-β1/SMAD2/3 signaling pathway in
tissue homogenates of pulmonary blood vessels (Fig. 2F). Following treatment with MCT,
the levels of the TGF-β1 and p-SMAD2/3 proteins were significantly
increased compared with those in the CON group (P<0.01 or
P<0.05, n=6; Fig. 2G).
PDGF-BB upregulates KIR2.1 protein
expression, and promotes the proliferation and migration of
HPASMCs, which are inhibited by ML133
HPASMCs were treated with PDGF-BB and cell
proliferation, migration and changes in KIR2.1 protein expression
were observed to further investigate the role of KIR2.1 in PVR.
Cell proliferation and migration were analyzed using scratch and
Transwell assays (Fig. 3A and C).
Following stimulation with PDGF-BB, the scratch healing ability of
the HPASMCs was enhanced, and the number of cells that migrated to
the lower surface of the Transwell™ chamber increased (P<0.01,
n=6). Moreover, cell scratch healing and the number of cells
migrating through the Transwell™ were reduced following
pre-treatment with the KIR2.1 protein inhibitor, ML133 (P<0.01,
n=6; Fig. 3B and D). The
expression of the migration-related protein, OPN, and the
proliferation-related protein, PCNA, in HPASMCs was detected using
western blot analysis (Fig. 4A).
The results were consistent with the phenotype. Following PDGF-BB
intervention, the protein levels of OPN and PCNA in the HPASMCs
were significantly increased (P<0.01, n=6), and OPN and the OPN
and PCNA protein levels were significantly decreased in cells
pre-treated with ML133 (P<0.01, n=6; Fig. 4C and D).
 | Figure 4ML133 alters the expression of
related proteins in human pulmonary artery smooth muscle cells
treated with PDGF-BB. (A) Representative western blots illustrating
the levels of KIR2.1, OPN, PCNA, TGF-β1, SMAD2, SMAD3, p-SMAD2,
p-SMAD3 and GAPDH. (B) Analysis of KIR2.1 expression. (C) Analysis
of OPN expression. (D) Analysis of PCNA expression. (E) Analysis of
TGF-β1 expression. (F) Analysis of p-SMAD2 and SMAD2 levels. (G)
Analysis of p-SMAD3 and SMAD3 levels. *P<0.05 and
**P<0.01, PDGF-BB vs. CON; #P<0.05 and
##P<0.01, PDGF-BB + ML133 vs. PDGF-BB (n=6, data were
analyzed using one-way ANOVA). PDGF-BB, platelet-derived growth
factor-BB; KIR2.1, inwardly rectifying K+ channel 2.1;
OPN, osteopontin; PCNA, proliferating cell nuclear antigen; CON,
control. |
Immunofluorescence staining and western blot
analysis were also performed to determine KIR2.1 protein expression
in the HPASMCs (Figs. 3E and
4A). The results of
immunofluorescence staining revealed that KIR2.1 was mainly located
in the cell membrane and cytoplasm. The semi-quantitative analysis
of the fluorescence intensity revealed a significantly higher
protein expression of KIR2.1 in the HPASMCs stimulated with PDGF-BB
than that in the CON group (P<0.01, n=6; Fig. 3E). However, KIR2.1 protein
expression was decreased in the PDGF-BB + ML133 group (P<0.01,
n=6; Fig. 3F). The results of
western blot analysis were consistent with those from the
semi-quantitative analysis of immunofluorescence staining.
Following stimulation with PDGF-BB, KIR2.1 protein expression in
the HPASMCs was significantly increased (P<0.01, n=6), whereas
it was significantly decreased following pre-treatment with ML133
(P<0.01, n=6; Fig. 4B).
ML133 blocks the activation of the
TGF-β1/SMAD2/3 signaling pathway induced by PDGF-BB
The levels of TGF-β1/SMAD2/3 signaling pathway
proteins in HPASMCs treated with PDGF-BB were examined using
western blot analysis to investigate the underlying molecular
mechanisms (Fig. 4A). PDGF-BB
increased the levels of TGF-β1, p-SMAD2 and p-SMAD3 in HPASMCs
(P<0.01 or P<0.05, n=6) and activated the TGF-β1/SMAD2/3
signaling pathway. Following pre-treatment with ML133, the levels
of TGF-β1 and p-SMAD2/3 were significantly decreased (P<0.01 or
P<0.05, n=6), and the TGF-β1/SMAD2/3 signaling pathway was
inhibited (Fig. 4E-G).
The TGF-β1/SMAD2/3 blocker, SB431542,
inhibits the proliferation and migration of HPASMCs, but does not
affect KIR2.1 expression
The HPASMCs were pre-treated with the TGF-β1/SMAD2/3
inhibitor, SB431542, and the changes in cell proliferation were
observed following the PDGF-BB intervention to further elucidate
the role of the TGF-β1/SMAD2/3 signaling pathway in HPASMC
proliferation and migration. Compared with the PDGF-BB group, the
cell scratch healing level was reduced in the PDGF-BB + SB431542
group (Fig. 5A and C), and the
number of cells migrating through the Transwell™ was decreased
(P<0.01 or P<0.05, n=6; Fig. 5B
and D), indicating that cell proliferation and migration were
decreased. The expression of the OPN and PCNA proteins was examined
using western blot analysis (Fig.
6A), and the results were consistent with the phenotype.
Following pre-treatment with SB431542, OPN and PCNA protein
expression was significantly decreased (P<0.01 or P<0.05,
n=6; Fig. 6C and D).
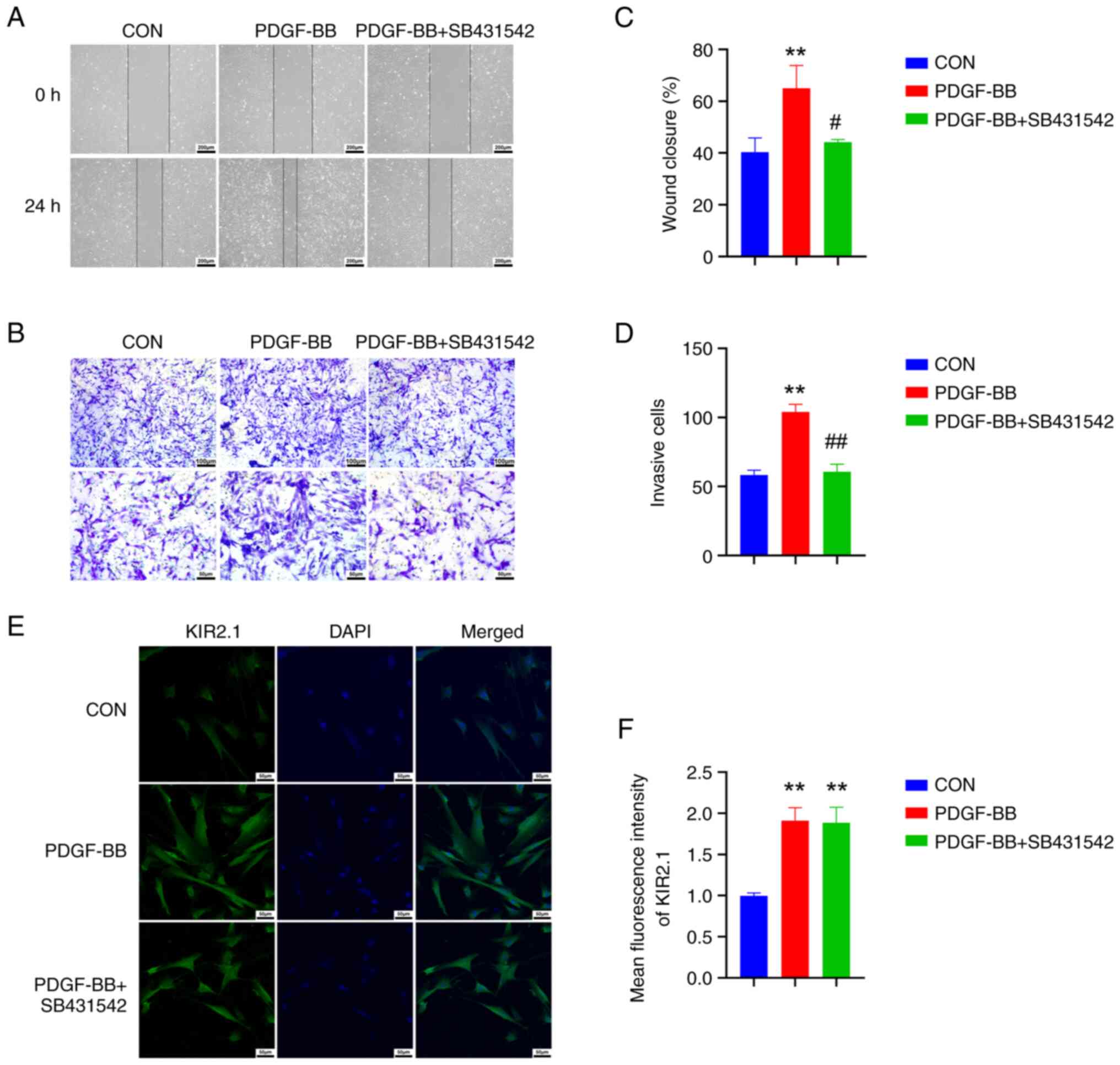 | Figure 5SB431542 reverses the proliferation
and migration of human pulmonary artery smooth muscle cells induced
by PDGF-BB. (A) Cell scratch images; scale bar, 200 µm. (B)
Crystal violet staining results from the Transwell assay; scale
bars, 100 µm (top panel) and 50 µm (bottom panel).
(C) Quantitative analysis of the cell scratch healing rate. (D)
Quantitative analysis of cell invasion. (E) Immunofluorescence
staining for KIR2.1; scale bar, 50 µm. (F) Analysis of the
relative expression of the KIR2.1 protein using immunofluorescence
staining. **P<0.01, PDGF-BB vs. CON;
#P<0.05 and ##P<0.01, PDGF-BB +
SB431542 vs. PDGF-BB (n=6, data were analyzed using one-way ANOVA).
PDGF-BB, platelet-derived growth factor-BB; KIR2.1, inwardly
rectifying K+ channel 2.1; CON, control. PDGF-BB,
platelet-derived growth factor-BB; KIR2.1, inwardly rectifying
K+ channel 2.1; CON, control. |
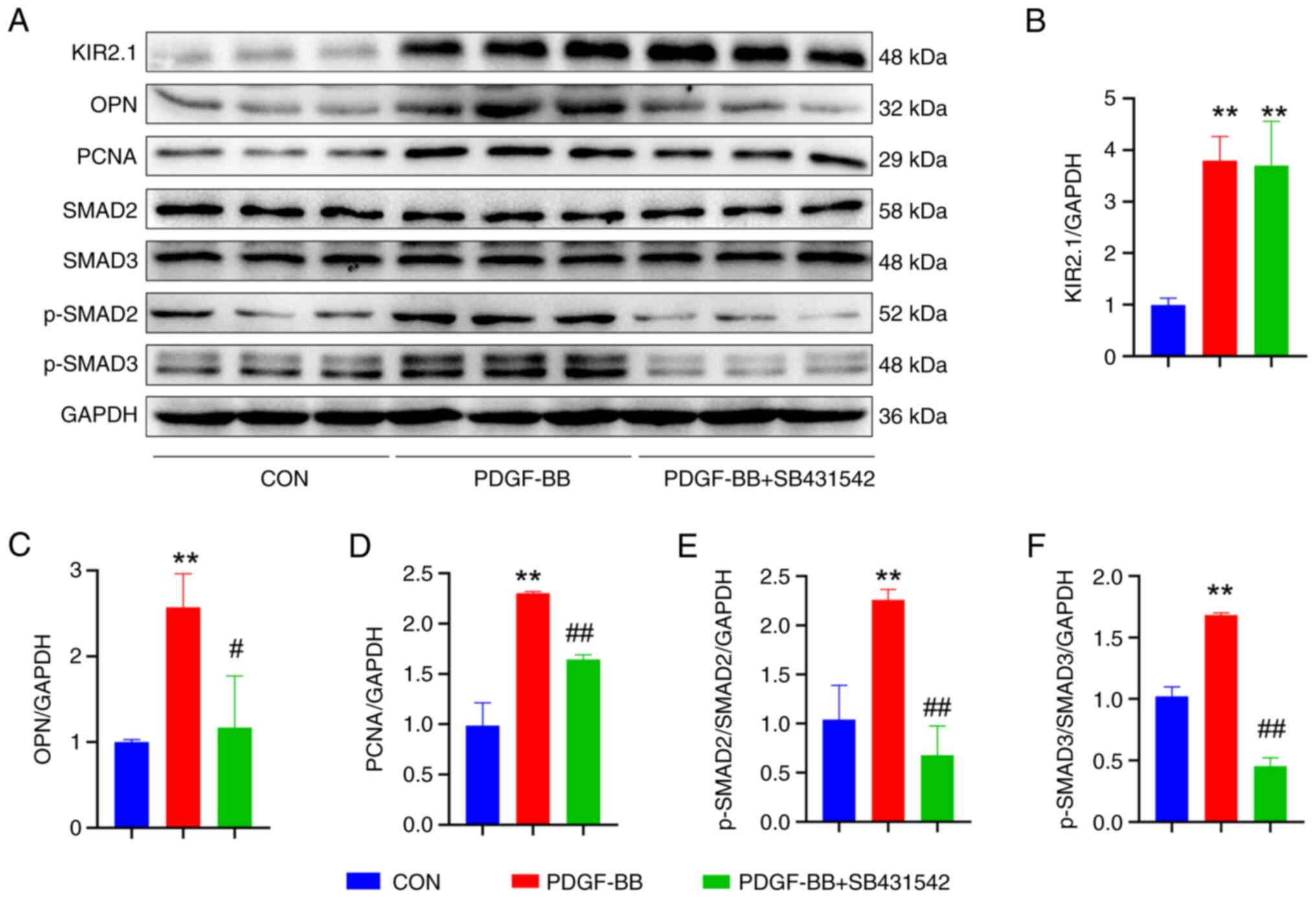 | Figure 6SB431542 alters the expression of
related proteins in human pulmonary artery smooth muscle cells
treated with PDGF-BB. (A) Representative western blots illustrating
the levels of KIR2.1, OPN, PCNA, TGF-β1, SMAD2, SMAD3, p-SMAD2,
p-SMAD3 and GAPDH. (B) Analysis of KIR2.1 levels. (C) Analysis of
OPN levels. (D) Analysis of PCNA levels. (E) Analysis of p-SMAD2
and SMAD2 levels. (F) Analysis of p-SMAD3 and SMAD3 levels.
**P<0.01, PDGF-BB vs. CON; #P<0.05 and
##P<0.01, PDGF-BB + SB431542 vs. PDGF-BB (n=6, data
were analyzed using one-way ANOVA). PDGF-BB, platelet-derived
growth factor-BB; KIR2.1, inwardly rectifying K+ channel
2.1; OPN, osteopontin; PCNA, proliferating cell nuclear antigen;
CON, control. |
Based on the aforementioned results, it was found
that PDGF-BB upregulates KIR2.1 protein expression in HPASMCs and
activates the TGF-β1/SMAD2/3 signaling pathway. The present study
then further investigated the upstream and downstream association
between KIR2.1 and the TGF-β1/SMAD2/3 signaling pathway by
detecting changes in KIR2.1 levels following pre-treatment with
SB431542. The results of immunofluorescence staining and western
blot analysis (Figs. 5E and
6A) revealed that the SMAD2/3
signaling pathway was inhibited following pre-treatment with
SB431542 (P<0.01 or P<0.05, n=6; Fig. 6E and F); however, no significant
differences in KIR2.1 protein expression were observed between the
PDGF-BB + SB431542 group and the PDGF-BB group (P>0.05, n=6;
Figs. 5F and 6B).
On the whole, PDGF-BB activated the TGF-β1/SMAD2/3
signaling pathway and upregulated the expression of OPN and PCNA to
promote cell proliferation and migration. ML133 inhibited KIR2.1
channel activation and modulated the downstream TGF-β1/SMAD2/3
signaling pathway. The activation of the TGF-β1/SMAD2/3 signaling
pathway was blocked by SB431542; however, the expression of KIR2.1
was not affected, indicating that KIR2.1 is located upstream of the
TGF-β1/SMAD2/3 signaling pathway (Fig. 7).
Discussion
The main findings of the present study were the
following: Significant right ventricular remodeling and PVR were
observed in the rats with PH, and obvious PVR and pulmonary
fibrosis were detected. The expression of the KIR2.1, OPN and PCNA
proteins in pulmonary vessels and lung tissues increased, and the
TGF-β1/SMAD2/3 signaling pathway was activated. PDGF-BB upregulated
the expression of the KIR2.1 protein, activated the TGF-β1/SMAD2/3
signaling pathway, and promoted the proliferation and migration of
smooth muscle cells. The KIR2.1 protein inhibitor, ML133, blocked
the activation of the TGF-β1/SMAD2/3 signaling pathway by PDGF-BB,
and inhibited the proliferation and migration of smooth muscle
cells. SB431542, an inhibitor of TGF-β1/SMAD2/3 signaling, reduced
the proliferation and migration of HPASMCs induced by PDGF-BB, but
did not affect KIR2.1 expression.
The main characteristics of PH are PVR and right
ventricular hypertrophy (1). An
intraperitoneal injection of MCT is one of the most classic methods
used to establish PH models. In the present study, the MCT group
exhibited a decrease in PAAT and an increase in RVHI%. In addition,
the expression of the migration-related protein, OPN, and the
proliferation-related protein, PCNA, was detected in pulmonary
blood vessels and lung tissues. The results revealed significantly
higher levels of the OPN and PCNA proteins in rats with PH than in
the control group. The regulatory role of PDGF-BB in VSMC
proliferation, migration and phenotype has been extensively studied
(40,41). In the present study, in
vitro, it was found that PDGF-BB stimulation promoted HPASMC
proliferation and migration, and upregulated OPN and PCNA protein
expression in the cells.
K+ channels are closely related to the
proliferation and apoptosis of PASMCs (42). To date, four different types of
K+ channels have been identified in smooth muscle cells:
Voltage-gated K+ (Kv) channels (43,44), ATP-sensitive potassium
(KATP) channels (45),
large conductance Ca2+ -activated K+ (BK Ca)
channels (44,46,47) and KIR channels (22,41,48). The contribution of KIR2.1 to
proliferation and migration is relatively controversial and may
also be dependent on the cell type. In human heart
c-kit+ progenitor cells, the cell membrane potential is
depolarized by silencing the KIR2.1 channel, and cell proliferation
is not affected, although the cell migration rate is increased
(16). In microglia, the
inhibition of KIR2.1 has been found to increase cell proliferation,
but to reduce cell migration (17). In rat VSMC, the knockdown of
KIR2.1 expression has been shown to inhibit PDGF-BB-induced cell
proliferation, migration, phenotype and intimal hyperplasia
following balloon injury (22).
In the human thoracic aorta, KIR2.1 has been found to be related to
VSMC proliferation and vascular remodeling (18). In the present study, the protein
expression of KIR2.1 was detected in pulmonary blood vessels and
lung tissues, and a significantly higher protein expression of
KIR2.1 was observed in pulmonary arteries and lung tissues of rats
with PH than in the control group. KIR2.1 is closely related to PH
development and PVR. In vitro, PDGF-BB promoted cell
proliferation and migration, while upregulating the protein
expression of KIR2.1 in cells. Following treatment with the KIR2.1
inhibitor, ML133, the proliferation and migration induced by
PDGF-BB were inhibited, while the protein expression levels of OPN
and PCNA were decreased. Therefore, the present study demonstrated
that the KIR2.1 channel participates in PASMC proliferation and
migration, as well as in PVR. The present study aimed to verify the
current changes in KIR2.1 function using the whole-cell patch clamp
technique. However, due to the limited HPASMC volume and small
KIR2.1 current, the experimental process and results were not
ideal. Therefore, the whole-cell patch clamp technique could not be
used for validation experiments in the present study.
For cell proliferation, cell viability (49), apoptosis [expression of
apoptosis-related genes (Bcl-2, cleaved caspase-3 and Bax)]
(50) and the cell cycle
(51) can further influence cell
proliferation. The inhibition of KIR2.1 channel-induced
depolarization has been shown to promote the biological activity
and differentiation of late endothelial progenitor cells (52). Another study found that the
inhibition of KIR2.1 channels increased cell mobility without
affecting cell cycling progression in human cardiac
c-kit+ progenitor cells (16). A previous study on BV2 microglial
cells demonstrated that hypoxia induced the apoptosis of cells by
upregulating KIR2.1 protein to activate mitochondria-related
apoptotic pathways (50).
However, such experiments have not been previously reported using
PASMCs, at least to the best of our knowledge. Therefore, whether
KIR2.1 channels can affect the proliferation and migration of
PASMCs by regulating cell proliferation, cell viability and
apoptosis warrants further investigation.
The TGF-β signaling pathway regulates various
cardiovascular diseases (53). In
the MCT-induced PH model, the expression of the TGF-β1 protein and
the levels of p-SMAD2/3 have been shown to be significantly
increased, promoting the proliferation and migration of smooth
muscle cells, PVR and increasing pulmonary vascular resistance,
ultimately accelerating the occurrence and development of PH
(54). In the present study,
in vivo, the levels of TGF-β1 and p-SMAD2/3 proteins in rats
with PH were higher than those in the control group, and the
TGF-β1/SMAD2/3 signaling pathway was activated, consistent with the
findings of previous research (54). In vitro, PDGF-BB increased
the TGF-β1 and p-SMAD2/3 protein levels in HPASMCs, and activated
the TGF-β1/SMAD2/3 signaling pathway. At the same time, TGF-β1 is
also an indicator of fibrosis (55). In vivo, the present study
found that the pulmonary blood vessels and lung tissues of the rats
with PH induced by MCT exhibited obvious fibrosis. TGF-β1 is
closely related to a depolarized membrane potential (56). When applied for 24 to 48 h, TGF-β1
causes substantial membrane depolarization concomitant with a
several-fold increase of transmembrane currents (56). In the in vitro experiments
in the present study, following the inhibition of KIR2.1, the
protein expression levels of TGF-β1 and p-SMAD2/3 were decreased,
and the TGF-β1/SMAD2/3 signaling pathway was inhibited. The
activation of the KIR2.1 channel promotes K+ ion influx
and cell membrane depolarization and may regulate the
TGF-β1/SMAD2/3 signaling pathway to alter the proliferation and
migration of HPASMCs.
In the present study, he TGF-β1/SMAD2/3 signaling
pathway inhibitor, SB431542, was used to further verify the
upstream and downstream relationship between KIR2.1 and the
TGF-β1/SMAD2/3 signaling pathway. The blockade of the
TGF-β1/SMAD2/3 signaling pathway reversed PDGF-BB-induced cell
proliferation and migration, and reduced the OPN and PCNA protein
levels. However, blocking the TGF-β1/SMAD2/3 signaling pathway did
not affect the protein expression of KIR2.1. Therefore, KIR2.1 is
located upstream of the TGF-β1/SMAD2/3 signaling pathway. PDGF-BB
upregulates KIR2.1 protein expression, activates the KIR2.1
channel, promotes K+ ion influx and cell membrane
depolarization, and then activates the TGF-β1/SMAD2/3 signaling
pathway to regulate PASMC proliferation and migration (Fig. 7).
In conclusion, the present study demonstrates that
KIR2.1 is involved in PVR and the generation of PH, potentially as
the KIR2.1 channel regulates the activity of the TGF-β1/SMAD2/3
signaling pathway by regulating cell membrane depolarization,
thereby modulating the proliferation and migration of PASMCs and
participating in PVR. Therefore, KIR2.1 may serve as a potential
therapeutic target for the prevention or treatment of PH.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NC, LL and JQS conceived and designed the
experiments. NC, WYS and NA conducted the experiments. RJG, WJQ,
LJYK, AMZ and JRZ assisted with the experiments. NC, KTM, LL and
XCT analyzed the data. NC and JQS wrote the manuscript. NC, JQS and
LL revised and reviewed the manuscript. All authors discussed and
commented on the manuscript and all authors have read and approved
the final manuscript. NC and JQS confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
All procedures involving animals were performed in
accordance with ethical standards and approved by the Institutional
Animal Care and Use Committee of the Affiliated Hospital of Shihezi
University School of Medicine (approval no. A 2020-165-01).
Applicable guidelines were followed in accordance with the 'Guide
for the Care and Use' published by the American Physiological
Society (23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The present study was performed at the Key
Laboratory of Xinjiang Endemic and Ethnic Diseases of Xinjiang
Provincial Department of Physiology, Shihezi University School of
Medicine.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81560081 and 81960188), the
Financial science and technology plan project of Xinjiang
production and Construction Corps (grant no. 2020AB019) and the
Research Project of Shihezi University (grant no. ZZZC201954A).
References
|
1
|
Crosby A, Jones F, Kolosionek E, Southwood
M, Purvis I, Soon E, Butrous G, Dunne DE and Morrell NW:
Praziquantel reverses pulmonary hypertension and vascular
remodeling in murine schistosomiasis. Am J Respir Crit Care Med.
184:467–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pabani S and Mousa S: Current and future
treatment of pulmonary hypertension. Drugs Today (Barc).
48:133–147. 2012. View Article : Google Scholar
|
|
3
|
Kim G, Ryan J, Marsboom G and Archer SL:
Epigenetic mechanisms of pulmonary hypertension. Pulm Circ.
1:347–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schermuly RT, Dony E, Ghofrani HA,
Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N,
Seeger W and Grimminger F: Reversal of experimental pulmonary
hypertension by PDGF inhibition. J Clin Invest. 115:2811–2821.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klein M, Schermuly RT, Ellinghaus P,
Milting H, Riedl B, Nikolova S, Pullamsetti SS, Weissmann N, Dony
E, Savai R, et al: Combined tyrosine and serine/threonine kinase
inhibition by sorafenib prevents progression of experimental
pulmonary hypertension and myocardial remodeling. Circulation.
118:2081–2090. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue Y, Li YQ, Fu S, Wu YT, Zhu L, Hua L,
Lv JY, Li YL and Yang DL: Osthole inhibits cell proliferation by
regulating the TGF-β1/Smad/p38 signaling pathways in pulmonary
arterial smooth muscle cells. Biomed Pharmacother. 121:1096402020.
View Article : Google Scholar
|
|
8
|
Wang XB, Wang W, Zhu XC, Ye WJ, Cai H, Wu
PL, Huang XY and Wang LX: The potential of asiaticoside for
TGF-β1/Smad signaling inhibition in prevention and progression of
hypoxia-induced pulmonary hypertension. Life Sci. 137:56–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu W, Liu D, Liang C, Ochs T, Chen S, Chen
S, Du S, Tang C, Huang Y, Du J and Jin H: Sulfur dioxide protects
against collagen accumulation in pulmonary artery in association
with downregulation of the transforming growth factor β1/smad
pathway in pulmonary hypertensive rats. J Am Heart Assoc.
5:e0039102016. View Article : Google Scholar
|
|
10
|
Ma W, Han W, Greer PA, Tuder RM, Toque HA,
Wang KKW, Caldwell RW and Su Y: Calpain mediates pulmonary vascular
remodeling in rodent models of pulmonary hypertension, and its
inhibition attenuates pathologic features of disease. J Clin
Invest. 121:4548–4566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas M, Docx C, Holmes AM, Beach S,
Duggan N, England K, Leblanc C, Lebret C, Schindler F, Raza F, et
al: Activin-like kinase 5 (ALK5) mediates abnormal proliferation of
vascular smooth muscle cells from patients with familial pulmonary
arterial hypertension and is involved in the progression of
experimental pulmonary arterial hypertension induced by
monocrotaline. Am J Pathol. 174:380–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hibino H, Inanobe A, Furutani K, Murakami
S, Findlay I and Kurachi Y: Inwardly rectifying potassium channels:
Their structure, function, and physiological roles. Physiol Rev.
90:291–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu L, Wang Q, Gu J, Zhang H and Gu Y:
Modulation of actin filament dynamics by inward rectifying of
potassium channel Kir2.1. Int J Mol Sci. 21:74792020. View Article : Google Scholar :
|
|
14
|
Ji CD, Wang YX, Xiang DF, Liu Q, Zhou ZH,
Qian F, Yang L, Ren Y, Cui W, Xu SL, et al: Kir2.1 interaction with
Stk38 promotes invasion and metastasis of human gastric cancer by
enhancing MEKK2MEK1/2ERK1/2 signaling. Cancer Res. 78:3041–3053.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anton R, Ghenghea M, Ristoiu V, Gattlen C,
Suter MR, Cojocaru PA, Popa-Wagner A, Catalin B and Deftu AF:
Potassium channels Kv1.3 and Kir2.1 but not Kv1.5 contribute to BV2
cell line and primary microglial migration. Int J Mol Sci.
22:20812021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Li G, Che H, Sun HY, Xiao GS,
Wang Y and Li GR: Effects of BKCa and Kir2.1 channels on cell
cycling progression and migration in human cardiac c-kit+
progenitor cells. PLoS One. 10:e01385812015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lam D and Schlichter L: Expression and
contributions of the Kir2.1 inward-rectifier K(+) channel to
proliferation, migration and chemotaxis of microglia in
unstimulated and anti-inflammatory states. Front Cell Neurosci.
9:1852015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karkanis T, Li S, Pickering JG and Sims
SM: Plasticity of KIR channels in human smooth muscle cells from
internal thoracic artery. Am J Physiol Heart Circ Physiol.
284:H2325–H2334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradley K, Jaggar J, Bonev A, Heppner TJ,
Flynn ER, Nelson MT and Horowitz B: Kir2.1 encodes the inward
rectifier potassium channel in rat arterial smooth muscle cells. J
Physio. 515(Pt 3): pp. 639–651. 1999, View Article : Google Scholar
|
|
20
|
Chilton L, Loutzenhiser K, Morales E,
Breaks J, Kargacin G and Loutzenhiser R: Inward rectifier K(+)
currents and Kir2.1 expression in renal afferent and efferent
arterioles. J Am Soc Nephrol. 19:69–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tennant B, Cui Y, Tinker A and Clapp L:
Functional expression of inward rectifier potassium channels in
cultured human pulmonary smooth muscle cells: Evidence for a major
role of Kir2.4 subunits. J Membr Biol. 213:19–29. 2006. View Article : Google Scholar
|
|
22
|
Qiao Y, Tang C, Wang Q, Wang D, Yan G and
Zhu B: Kir2.1 regulates rat smooth muscle cell proliferation,
migration, and post-injury carotid neointimal formation. Biochem
Biophys Res Commun. 477:774–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological society
Physiologist. 39:208–111. 1996.
|
|
24
|
Barman S, Li X, Haigh S, Kondrikov D,
Mahboubi K, Bordan Z, Stepp DW, Zhou J, Wang Y, Weintraub DS, et
al: Galectin-3 is expressed in vascular smooth muscle cells and
promotes pulmonary hypertension through changes in proliferation,
apoptosis, and fibrosis. Am J Physiol Lung Cell Mol Physiol.
316:L784–L797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian L, Wu D, Dasgupta A, Chen KH, Mewburn
J, Potus F, Lima PDA, Hong Z, Zhao YY, Hindmarch CCT, et al:
Epigenetic metabolic reprogramming of right ventricular fibroblasts
in pulmonary arterial hypertension: A pyruvate dehydrogenase
kinase-dependent shift in mitochondrial metabolism promotes right
ventricular fibrosis. Circ Res. 126:1723–1745. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Fan Z, Wang L, Liu LQ, Li XZ, Li
L, Si JQ and Ma KT: Carbenoxolone decreases monocrotaline-induced
pulmonary inflammation and pulmonary arteriolar remodeling in rats
by decreasing the expression of connexins in T lymphocytes. Int J
Mol Med. 45:81–92. 2020.
|
|
27
|
Flues K, Moraes-Silva I, Mostarda C, Souza
PRM, Diniz GP, Moreira ED, Piratello AC, Chaves MLB, Angelis KD,
Salemi VMC, et al: Cardiac and pulmonary arterial remodeling after
sinoaortic denervation in normotensive rats. Auton Neurosci.
166:47–53. 2012. View Article : Google Scholar
|
|
28
|
Yared K, Noseworthy P, Weyman AE, McCabe
E, Picard MH and Baggish AL: Pulmonary artery acceleration time
provides an accurate estimate of systolic pulmonary arterial
pressure during transthoracic echocardiography. J Am Soc
Echocardiogr. 24:687–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Zhang F, Li J, Liu Z, Kou Y, Song
Y, Xu H, Wang H and Wang Y: Using pulmonary artery acceleration
time to evaluate pulmonary hemodynamic changes on preterm infants
with respiratory distress syndrome. Transl Pediatr. 10:2287–2297.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaky A, Zafar I, Masjoan-Juncos JX, Husain
M, Mariappan N, Morgan CJ, Hamid T, Frölich MA, Ahmad S and Ahmad
A: Echocardiographic, biochemical, and electrocardiographic
correlates associated with progressive pulmonary arterial
hypertension. Front Cardiovasc Med. 8:7056662021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Zhou R, Zhang M, Tan HR and Yu JQ:
Betaine attenuates monocrotaline-induced pulmonary arterial
hypertension in rats via inhibiting inflammatory response.
Molecules. 23:12742018. View Article : Google Scholar :
|
|
32
|
Zhang LZ, Fan ZR, Wang L, Liu LQ, Li XZ,
Li L, Si JQ and Ma KT: Carbenoxolone decreases
monocrotaline-induced pulmonary inflammation and pulmonary
arteriolar remodeling in rats by decreasing the expression of
connexins in T lymphocytes. Int J Mol Med. 45:81–92. 2020.
|
|
33
|
Baldwin SN, Sandow SL, Mondéjar-Parreño G,
Stott JB and Greenwood IA: K(V)7 channel expression and function
within rat mesenteric endothelial cells. Front Physiol.
11:5987792020. View Article : Google Scholar
|
|
34
|
Ji Z, Li J and Wang J: Jujuboside b
inhibits neointimal hyperplasia and prevents vascular smooth muscle
cell dedifferentiation, proliferation, and migration via activation
of AMPK/PPAR-γ signaling. Front Pharmacol. 12:6721502021.
View Article : Google Scholar
|
|
35
|
Zuo W, Liu N, Zeng Y, Xiao Z, Wu K, Yang
F, Li B, Song Q, Xiao Y and Liu Q: Luteolin ameliorates
experimental pulmonary arterial hypertension via suppressing
hippo-YAP/PI3K/AKT signaling pathway. Front Pharmacol. 12:663551.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu W, Lim TC, Alshihri A, Rajappa R, Wang
L, Kurisawa M and Spector M: Platelet-derived growth factor
stimulated migration of bone marrow mesenchymal stem cells into an
injectable gelatin-hydroxyphenyl propionic acid matrix.
Biomedicines. 9:2032021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An Z, Liu Y, Song ZS, Tang H, Yuan Y and
Xu ZY: Mechanisms of aortic dissection smooth muscle cell phenotype
switch. J Thorac Cardiovasc Surg. 154:1511–1521.e6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song W, Li L, Jia Q, Cao N, Li L, Ma K and
Si J: Monocrotaline pyrrole induces A549 cells and activates
TGF-β1/SMAD2/SMAD3 pathway to promote proliferation and migration
of human pulmonary artery smooth muscle cells. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 36:527–534. 2020.In Chinese. PubMed/NCBI
|
|
39
|
Jia Q, Li L, Song W, Cao N, Li L, Ma K and
Si J: Up-regulation of connexin 43 (Cx43) by angiotensin II
promotes the proliferation and migration of human pulmonary artery
smooth muscle cells. Xi Bao Yu Fen Zi Mian Yi Xue Za. 36:616–621.
2020.In Chinese.
|
|
40
|
Raines EW: PDGF and cardiovascular
disease. Cytokine Growth Factor Rev. 15:237–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang C, Wang D, Luo E, Yan G, Liu B and
Hou J: Activation of inward rectifier K(+) channel 2.1 by PDGF-BB
in rat vascular smooth muscle cells through protein kinase a.
Biomed Res Int. 1:43708322020.
|
|
42
|
Burg ED, Remillard CV and Yuan JXJ:
Potassium channels in the regulation of pulmonary artery smooth
muscle cell proliferation and apoptosis: Pharmacotherapeutic
implications. Br J Pharmacol. 153:S99–S111. 2008. View Article : Google Scholar
|
|
43
|
Mondejar-Parreño G, Perez-Vizcaino F and
Cogolludo A: Kv7 channels in lung diseases. Front Physiol.
11:6342020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iqbal H, Verma AK, Yadav P, Alam S, Shafiq
M, Mishra D, Khan F, Hanif K, Negi AS and Chanda D:
Antihypertensive effect of a novel angiotensin II receptor blocker
fluorophenyl benzimidazole: Contribution of cGMP, voltage-dependent
calcium channels, and BK channels to vasorelaxant mechanisms. Front
Pharmacol. 12:6111092021. View Article : Google Scholar
|
|
45
|
Jin X, Wu Y, Cui N, Jiang C and Li SS:
Methylglyoxal-induced miR-223 suppresses rat vascular K channel
activity by downregulating Kir6.1 mRNA in carbonyl stress. Vascula
Pharmacol. 128-129:1066662020. View Article : Google Scholar
|
|
46
|
Shen X, Zhang L, Jiang L, Xiong W, Tang Y,
Lin L and Yu T: Alteration of sphingosine-1-phosphate with aging
induces contractile dysfunction of colonic smooth muscle cells via
Ca2+ -activated K channel (BKCa)
upregulation. Neurogastroenterol Motil. 33:e140522021. View Article : Google Scholar
|
|
47
|
Li Y, Bai J, Yang YH, Hoshi N and Chen DB:
Hydrogen sulfide relaxes human uterine artery via activating smooth
muscle BKCa channels. Antioxidants (Basel). 9:11272020. View Article : Google Scholar
|
|
48
|
Yuan XJ: Voltage-gated K+ currents
regulate resting membrane potential and [Ca2+]i in pulmonary
arterial myocytes. Circ Res. 77:370–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Zhang W, Gao Q, Cao X, Li Y, Li X,
Min Z, Yu Y, Guo Y and Shuai L: Extractive from hypericum ascyron L
promotes serotonergic neuronal differentiation in vitro. Stem Cell
Res. 31:42–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie YF, Wang Y, Rong Y, He W, Yan M, Li X,
Si J, Li L, Zhang Y and Ma K: Hypoxia induces apoptosis of
microglia BV2 by upregulating Kir2.1 to activate
mitochondrial-related apoptotic pathways. Dis Markers.
17:58558892022.
|
|
51
|
Gao Q, Zhang W, Zhao Y, Tian Y, Wang Y,
Zhang J, Geng M, Xu M, Yao C, Wang H, et al: High-throughput
screening in postimplantation haploid epiblast stem cells reveals
Hs3st3b1 as a modulator for reprogramming. Stem Cells Transl Med.
10:743–755. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang X, Cui X, Li X, Yan H, Li H, Guan X,
Wang Y, Liu S, Qin X and Cheng M: Inhibition of Kir2.1
channel-induced depolarization promotes cell biological activity
and differentiation by modulating autophagy in late endothelial
progenitor cells. J Mol Cell Cardiol. 127:57–66. 2019. View Article : Google Scholar
|
|
53
|
Goumans M and Dijke PT: TGF-β signaling in
control of cardiovascular function. Cold Spring Harbor Perspect
Biol. 10:a0222102018. View Article : Google Scholar
|
|
54
|
Cao N, Tang X, Gao R, Kong L, Zhang J, Qin
W, Hu N, Zhang A, Ma K, Li L and Si JQ: Galectin-3 participates in
PASMC migration and proliferation by interacting with TGF-β1. Life
Sci. 1274:1193472021. View Article : Google Scholar
|
|
55
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Salvarani N, Maguy A, De Simone S,
Miragoli M, Jousset F and Rohr S: TGF-β1 (Transforming
Growth Factor-β1) plays a pivotal role in cardiac
myofibroblast arrhythmogenicity. Cir Arrhythm Electrophysiol.
10:e0045672017.
|





















