Introduction
Bone cancer pain (BCP) is a significant and
life-altering pain, and is the most common symptom of bone
metastasis, which occur in almost all types of cancer. Among all
the cancer types, the relative incidence of breast, prostate and
thyroid cancer is 75, 75 and 40%, respectively (1). It was estimated that there were 19.3
million new cancer cases in 2020, according to the GLOBOCAN 2020
database (2). Moreover, the
5-year relative survival rate of cancer patients increases to 68%,
depending on the advanced treatment and improved diagnoses.
However, 60-84% of patients with advanced-stage cancer experience
varying degrees of bone pain (3).
The mainly clinical symptoms of BCP are stable background pain,
occasional breakthrough pain and ongoing pain (4). BCP is described by patients as
annoying, gnawing, aching and nagging, and is exacerbated by weight
bearing or movement; it markedly impairs the functional capacity
and daily functions of patients (5). The pathogenesis of BCP involves a
complex mixture of tumor cells, bone cells, inflammatory
microenvironment and the neuronal tissue (6). Therefore, research on the mechanisms
of BCP may be useful for pain management.
Neuroinflammation in the spinal cord plays a pivotal
role in the pathogenesis and maintenance of chronic pain, and is
characterized by the infiltration of leukocytes, the activation of
glial cells, and the production/secretion of pro-inflammatory
cytokines and chemokines (7,8).
Increased spinal inflammation has been reported in rodent models of
several chronic pain, including inflammatory pain (9), neuropathic pain (10), chemotherapy-induced pain (11) and BCP (12,13). Research has provided evidence of
neuroinflammation in the spinal cord of patients with chronic pain.
Patients suffering from lumbar radiculopathy exhibit elevated
levels of neuroinflammation marker translocator proteins in the
spinal cord (14). A previous
study demonstrated that the autopsy of spinal cord tissue from a
patient with longstanding complex regional pain syndrome revealed
the activation of astrocytic and microglial cells (15). It has been demonstrated that in
patients positive for human immunodeficiency virus who suffer from
chronic pain, the levels of the pro-inflammatory cytokines, tumor
necrosis factor α (TNF-α) and interleukin (IL)-1β, and the
astrocytic markers, glial fibrillary acidic protein (GFAP) and
S100β, are markedly increased in the spinal dorsal horn (16). The pharmacological inhibition of
glial activation and pro-inflammatory cytokine release has been
shown to exert an anti-nociceptive effect in animals (17). Of note, it has been shown that
patients with spinal cord injury on an anti-inflammatory diet
exhibit a decreased expression of interleukin (IL)-1β and IL-6, and
an attenuation of sensory neuropathic pain (18). Electroacupuncture has also been
found to exert a therapeutic effect on inflammatory pain by
suppressing the expression of IL-1β and TNF-α (19). Oxidative stress in the spinal cord
is a key contributor to neuroinflammation and neuropathic pain. A
previous study demonstrated that the intrathecal injection of the
reactive oxygen species (ROS) donor, tert-butyl hydroperoxide, led
to transient hyperalgesia. However, systemic or intrathecal
post-treatment with ROS scavengers significantly reduced
hyperalgesia in a mouse model of capsaicin-induced pain (20). Glutathione also exerts an
analgesic effect in models of neuropathic pain by reducing the ROS
content and increasing the expression of antioxidant enzymes
(21). In a a previous study, in
rats with cancer-induced bone pain, the inhibition of the oxidative
stress response and the elevation of the activity of the
antioxidant enzyme, superoxide dismutase (SOD), reduced the pain
sensitivity of rats (22).
Increased levels of oxidative stress activate a variety of
transcription factors in inflammatory pathways and trigger the
inflammatory response (23).
Thus, targeting spinal oxidative stress and inflammation may be a
potential therapeutic strategy for neuropathic pain.
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (Akt) pathway participates in inflammatory cellular
responses, mediates neuropathic pain and functions as a novel
therapeutic target for inflammation-related diseases (24). LY294002, an inhibitor of PI3K, has
been shown to alleviate endometriosis-associated sciatic nerve pain
by inhibiting the PI3K/Akt/mammalian target of rapamycin signaling
pathway (25). In a rat model of
chronic constriction injury (CCI), the intrathecal injection of
LY294002 was found to significantly block CCI-increased Akt
phosphorylation and attenuate mechanical allodynia (26). In another study, in a rat model of
BCP-morphine tolerance, the levels of phosphorylated (p-)PI3K, Akt
and JNK1/2 were upregulated in the L4-6 spinal dorsal horn;
however, the intrathecal injection of LY294002 decreased the
phosphorylation levels of these proteins and attenuated the
development of morphine tolerance (27).
In the present study, to investigate the effects of
LY294002 on BCP and explore the underlying mechanisms, rats with
BCP were intrathecally injected with LY294002 and the pain
behaviors, motor ability, spinal inflammation and mitochondrial
function were detected. The present study provides experimental
data which may prove to be useful in alleviating BCP.
Materials and methods
Animals
A total of 36 male Sprague-Dawley (SD) rats,
weighing 180-200 g (6-8 weeks old), were purchased from the Hubei
Province Experimental Animal Center (Wuhan, China). All animals
were housed in a temperature-controlled environment (22±1°C) and
55±5% humidity with a 12-h light/dark cycle with ad libitum
access to food and water. All procedures were conducted in
accordance with the Chinese National Guidelines for the ethical
review of animal welfare (GB/T 35892-2018) and approved by the
Ethics Committee of Hubei University of Science and Technology
(approval number 2020-01-900). The rats were allowed to acclimatize
to their environment for 5 days prior to the start of the
experiments. A total of 36 rats were randomly divided into four
groups as follows: The sham-operated (sham), LY294002, BCP and BCP
+ LY294002 group, with 9 rats in each group.
Cells and cell culture
MRMT-1 rat breast cancer cells (cat. no. RCB2860;
Jennio Biotech Co., Ltd.) were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 50 U/ml penicillin and 50
µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% carbon dioxide. Rat glioma cells (C6) (cat. no.
CBP60888; Jennio Biotech Co., Ltd.) were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum, 50
U/ml penicillin and 50 µg/ml streptomycin at 37°C with 5%
carbon dioxide.
Establishment of rat model of BCP and
drug administration
To establish the rat model of BCP, MRMT-1 cells were
collected and resuspended in Hank's balance salt solution (HBSS)
(cat. no. 14170146; Gibco; Thermo Fisher Scientific, Inc.) and kept
on ice until use. The rats were anaesthetized with pentobarbital
sodium (50 mg/kg, intraperitoneal injection). The left hind limb
was disinfected with 75% ethanol and 5.0×105 MRMT-1
cells in HBSS were slowly injected into the intramedullary space of
bones of the rats. The rats in the sham group were injected with an
equivalent volume of the vehicle (HBSS). After 14 days of modeling,
an intrathecal injection was administered using a 25-µl
microsyringe, which was inserted into the intervertebral space of
the rats between L5 and L6 vertebrae. The PI3K inhibitor, LY294002
(Selleck Chemicals), was dissolved in DMSO, and diluted with 0.9%
NaCl (v/v 1:1) prior to use. The rats in the LY294002 and BCP +
LY294002 groups were intrathecally injected with 10 µl
LY294002 (1.5 mg/kg). The rats in the sham and BCP groups received
an intrathecal injection of the same volume of the vehicle.
Bone X-ray and histological analysis
A roentgenograph of the left tibia was performed on
the 14th day following the inoculation of the MRMT-1 cells. On day
14, 3 rats in each group were euthanized with an overdose of
pentobarbital sodium (150 mg/kg) by intraperitoneal injection, the
left tibiae were collected and fixed in 4% paraformaldehyde for 24
h, decalcified in 10% EDTA (Gibco; Thermo Fisher Scientific, Inc.)
solution. The bone tissue was dehydrated and embedded in paraffin,
and cut into 4-µm-thick sections using a microtome (Thermo
Fisher Scientific, Inc.). The bone sections were then dewaxed and
stained with hematoxylin and eosin (H&E) (Beyotime Institute of
Biotechnology). Briefly, the sections were dyed with hematoxylin
solution for 3 min and washed with tap water for 10 sec. The
sections were stained with eosin for 3 min and washed with tap
water for 10 sec. The dehydration and transparent treatment were
conducted by putting the slices into 80% ethanol (5 min), 90%
ethanol (5 min), 95% ethanol (5 min), 100% ethanol (5 min, two
times), xylene (5 min, times). The temperature used for all these
procedures was 25°C. Images were obtained using a microscope
(Olympus IX73; Olympus Corporation).
Paw withdrawal threshold (PWT) test
The rats were placed in a 30×30×30 cm plexiglass
chamber and were allowed to acclimatize to their environment for at
least 30 min prior to the start of the behavioral experiments. von
Frey filaments (Stoelting Co.; ranging from 0.4 to 26 g) were used
by stimulating the left hind paws of the rats. Briefly, the
filaments were pressed vertically against the plantar surfaces
until the filaments were bent and held for 6-8 sec. Under this
condition, a brisk withdrawal and paw flinching was considered a
positive response. Once a positive response occurred, the von Frey
filament with the next lower force was applied, and whenever a
negative response occurred, the filament with the next higher force
was applied. The pattern of positive and negative withdrawal
response was used to determine the PWT.
Flinching number analysis
The rats were placed in a 30×30×30 cm plexiglass
chamber and were allowed to acclimatize to their environment for at
least 30 min prior to the start of the experiment. The number of
flinches was recorded and counted for 5 min and repeated three
times (28).
Rotarod test
An accelerating rotarod (ZS-RDM, Beijing Zhongshi
Dichuang Science and Technology Development Co., Ltd.) was used to
assess the motor coordination and balance of the animals. At 3 days
prior to the start of the experiment, the animals received
acclimatization training at a fixed speed of 4 r/min for 10 min
repeating three times with 10-min intervals. At the beginning of
experiment, the rotation speed was fixed at 10 r/min for 10 sec and
then accelerated for 10 sec. Subsequently, the rod was working at a
speed of 20 r/min for 30 sec and then accelerated for 10 sec. The
movement was continuously carried out for 10 min, repeating three
times with an interval of 10 min. The latency of the rats to fall
was recorded.
Western blot analysis
Following 12 h of LY294002 and vehicle
administration, another 3 rats from each group were euthanized by
an overdose of pentobarbital sodium (150 mg/kg) by intraperitoneal
injection and then subjected to decapitation. The spinal cord
(L4-L6) was collected into ice-cold RIPA lysis buffer (Beyotime
Institute of Biotechnology) containing a cocktail of protease
inhibitors, homogenized on ice, and centrifugated at 12,000 × g,
4°C for 15 min. The supernatant was collected and the protein
concentration was measured using a BCA kit (Beyotime Institute of
Biotechnology). Equal amounts of protein samples (35 µg)
were separated by SDS-polyacrylamide gel (8-12%) electrophoresis,
and electrically transferred onto PVDF membranes (MilliporeSigma).
The membranes were then blocked with QuickBlock™ blocking buffer
(Beyotime Institute of Biotechnology) at 25°C for 1.5 h and
incubated with the proper primary antibodies overnight at 4°C. The
following antibodies were used: IL-1β (1:1,000; cat. no. AF5103;
Affinity Biosciences), TNF-α (1:1,000; cat. no. AF7014; Affinity
Biosciences), GFAP (1:1,000; cat. no. BF0345; Affinity
Biosciences), p-Akt (1:500; cat. no. AP1259; ABclonal Biotech Co.,
Ltd.), Akt (1:1,000; cat. no. A20799; ABclonal Biotech Co., Ltd.),
nuclear factor (NF)-κB (1:1,000; cat. no. BF8005; Affinity
Biosciences), BAX (1:1,000; cat. no. A20227; ABclonal Biotech Co.,
Ltd.), NADH:ubiquinone oxidoreductase subunit B11 (NDUFB11;
1:1,000; cat. no. A15617; ABclonal Biotech Co., Ltd.),
dihydroorotate dehydrogenase (DHODH; 1:1,000; cat. no. A6899;
ABclonal Biotech Co., Ltd.) and β-actin (1:50,000; cat. no. AC026;
ABclonal Biotech Co., Ltd.). Following incubation (25°C, 1 h) with
goat anti-rabbit IgG (H + L)-HRP (1:10,000; cat. no. AS014;
ABclonal Biotech Co., Ltd.) or goat anti-mouse IgG (H + L)-HRP
(1:10,000; cat. no. AS003; ABclonal Biotech Co., Ltd.) secondary
anti-bodies, the membranes were visualized with iBright 1500
(Invitrogen; Thermo Fisher Scientific, Inc.). The bands of target
protein were analyzed using ImageJ 1.48v software (National
Institutes of Health).
Immunofluorescence assay
Following treatment with LY294002 for 12 h, another
3 rats from each group were deeply anesthetized and myocardial
perfused with 0.9% NaCl containing heparin, and subsequently
switched perfusate to 4% polyformaldehyde. Following perfusion, the
spinal cord was collected and fixed in 4% paraformaldehyde for 24
h, dehydrated, embedded in paraffin, and cut into 4-µm-thick
sections using a microtome (Thermo Fisher Scientific, Inc.). The
spinal cord sections were dewaxed, boiled at 95°C in antigen repair
solution (Beyotime Institute of Biotechnology) for 15 min, cooled
naturally to normal temperature, incubated with 3%
H2O2 solution (Guangdong Hengjian
Pharmaceutical Co., Ltd.) for 10 min, blocked with
immunofluorescence blocking solution (Beyotime Institute of
Biotechnology) at 25°C for 1 h and subsequently incubated with
primary antibodies at 4°C for 24 h. Including IL-1β (1:100; cat.
no. AF5103; Affinity Biosciences), GFAP (1:100; cat. no. BF0345;
Affinity Biosciences), PI3K (1:100; cat. no. AF6241; Affinity
Biosciences), p-Akt (1:100; cat. no. AP1259; ABclonal Biotech Co.,
Ltd.), NDUFB11(1:100; cat. no. A15617; ABclonal Biotech Co., Ltd.)
and BAX (1:100; cat. no. A20227; ABclonal Biotech Co., Ltd.), and
goat-rabbit IgG H&L (FITC) (1:500; cat. no. ab6717; Abcam) at
25°C for 1 h. They were then mounted with antifade mounting medium
(Beyotime Institute of Biotechnology). Images were acquired using a
fluorescence microscope (Olympus Corporation). The intensity was
analyzed using ImageJ 1.48v software (National Institutes of
Health).
Transmission electron microscopy (TEM)
assay
The morphology of the mitochondria in the spinal
cord were confirmed using electron microscopy with negative
staining. Briefly, the spinal cord tissues from another 3 rats from
each group were isolated, cut into ~1 mm3 cubes, fixed
in 2.5% glutaraldehyde (Sigma-Aldrich Shanghai Trading Co., Ltd.)
at 25°C for 4 h and post-fixed with 1% osmium tetroxide (Electron
Microscopy China) at 25°C for 2 h. Ultrathin sections were
post-stained with uranyl acetate (Electron Microscopy China) for 30
min and lead citrate (Electron Microscopy China) for 15 min and
then examined using an HC-1 transmission electron microscope
(Hitachi Corporation) operating at 120 kV.
Detection of manganese SOD (Mn-SOD)
activity
To detect the enzyme activity of Mn-SOD, the
CuZn/Mn-SOD assay kit with WST-8 (cat. no. S0103; Beyotime
Institute of Biotechnology) were used. Briefly, the spinal cords
were homogenized in ice-cold phosphate-buffered saline (PBS) and
centrifuged at 12,000 × g, 4°C for 15 min. The supernatant was
collected and incubated with Cu/Zn-SOD inhibitors for 1 h at 37°C.
After mixing with WST-8 enzyme working solution for 20 min at 37°C,
the absorbance value at OD450 nm of each pore was
measured. The Mn-SOD activity was expressed as units per milligram
of total protein (U/mg protein).
Malondialdehyde (MDA) assay
The levels of MDA were measured using the MDA Assay
kit (cat. no. S0131S; Beyotime Institute of Biotechnology).
Briefly, the spinal cord tissues were lysis using ice-cold RIPA
lysis buffer (Beyotime Institute of Biotechnology) and centrifuged
at 12,000 × g, 4°C for 15 min. The supernatant acquired was mixed
with MDA working solution, heated at 95°C for 15 min, and
centrifugated at 1,000 × g, 25°C for 10 min after cooling naturally
to room temperature. The supernatant was collected and the
absorbance was measured at 532 nm. The MDA levels were calculated
according to the standard curve and expressed as µmol/mg
protein.
Measurement of mitochondrial membrane
potential (MMP)
MMP was measured using a fluorescent probe JC-1
(Beyotime Institute of Biotechnology). The C6 cells were inoculated
in the 24-well plate with sterilized coverslips, stimulated with
IL-1β (5 ng/ml; Beyotime Institute of Biotechnology) for 30 min and
incubated with 1 µM LY294002 at 37°C for 24 h. The cells
were then cultured with 200 µl JC-1 for 20 min at 37°C in
the dark, and the fluorescence intensity was detected using a
fluorescence microscope (Olympus Corporation). Mitochondrial
uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 10
µM) treatment was used as positive control, since it
disrupts mitochondrial integrity and induced the loss of MMP
(29).
Measurement of mitochondrial ROS
generation
MitoSOX Red mitochondrial superoxide indicator
(Thermo Fisher Scientific, Inc.), a novel fluorogenic dye, was used
to detect mitochondrial superoxide in C6 cells. Briefly, the cells
were inoculated in the 24-well plate with sterilized coverslips,
stimulated with IL-1β, incubated with LY294002, subsequently
incubated with 5 µM MitoSOX Red (Molecular Probes, Thermo
Fisher Scientific, Inc.) in the dark at 37°C for 10 min and
detected under a fluorescence microscope (Olympus Corporation).
Statistical analysis
All statistical analyses were performed using the
SPSS 26 software package (IBM Corp.). One-way analysis of variance
(one-way ANOVA) was used to evaluate the differences between the
experimental groups followed by Bonferroni post hoc tests. The
results of the behavioral tests are presented as the mean ± SEM.
Other experiment data are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Intratibial inoculation of tumor cells
induces bone destruction, hyperalgesia and motor impairment in
rats
After 5 days of acclimatization, the rats were
inoculated with MRMT-1 cells to establish the model of BCP. To
verify the establishment of the BCP model, X-rays, H&E staining
and behavioral tests were performed (Fig. 1A). Radiographs of the rat tibiae
were obtained on day 14 post-surgery and revealed notable bone
destruction, particularly at the proximal epiphysis. Fewer
radiolucent lesions in the tibiae were observed in the rats in the
sham group (Fig. 1B). Moreover,
the histological analysis of the tibiae from rats with BCP revealed
that there was an increased cell infiltration in the bone marrow
spaces and a loss of normal bone structure, whereas bone loss was
not evident in the sham group (Fig.
1C). Pain behavioral testing revealed that there were no
significant differences in mechanical withdrawal, the number of
spontaneous flinches and latency to fall between the rats in the
sham and BCP groups prior to cancer cell inoculation (Fig. 1D-F). Following the intratibial
inoculation of MRMT-1 cells, mechanical allodynia, spontaneous
flinching behavior and the impairment of motor functions developed
on day 4 and persisted for at least 14 days. By contrast, no marked
changes were observed in the PWT value, the number of spontaneous
flinches and the latency to fall in the rats in the sham group
following surgery. Taken together, these results suggested that the
inoculation of MRMT-1 cells induced bone destruction, pain
hypersensitivity and motor impairment in the rats.
 | Figure 1Establishment of the rat model of BCP
and the assessment of bone morphology, pain and locomotor behaviors
in rats. (A) Schematic diagram of the study design: Inoculation of
MRMT-1 cells to establish BCP in rats following acclimation. The
behavioral tests were performed on days 0, 4, 7, 11 and 14
post-surgery. Tissue samples were then collected for morphological
and protein expression analyses. (B) Radiographs of the tibiae from
the rats in the sham and BCP groups on day 14 after the MRMT-1 cell
inoculation (n=5). The red arrow indicates the structural
destruction in rats with BCP. (C) Hematoxylin and eosin staining of
tibial sections revealed bone loss and bone destruction on day 14
following MRMT-1 cell inoculation (n=5). The blue arrows indicate
the discontinuous trabecular structure. Scale bars, 100 µm.
(D) The PWT of rats from the sham and BCP group in response to von
Frey filaments at the indicated time points (days 0, 4, 7, 11 and
14 following inoculation). (E) The number of spontaneous flinches
in a 5-min period of the sham and BCP group rats. (F) The latency
to fall of the sham and BCP group rats in the rotarod test. The
data of behavioral tests are presented as the mean ± SEM from at
least 9 rats in each group. *P<0.05 vs. the sham
group. BCP, bone cancer pain; PWT, paw withdrawal threshold. |
LY294002 treatment alleviates pain
hypersensitivity and motor impairment
To confirm the effects of the PI3K/Akt pathway on
pain behaviors, the expression and localization of PI3K and Akt in
the spinal cords of the rats were analyzed, and the pain behavior
and motor performance were further assessed following treatment
with the PI3K inhibitor, LY294002. Immunofluorescence analysis
revealed the enhanced fluorescence signals of PI3K and p-Akt in the
spinal dorsal horn of rats with BCP (Fig. 2A-C). Western blot analysis also
revealed an increase in the level of p-Akt in the rats with BCP
(Fig. 2D and E). Upon treatment
with LY294002, the levels of PI3K and p-Akt were significantly
decreased in the spinal cords of rats with BCP, as detected using
immunofluorescence and western blot analysis. However, LY294002 had
no effect on the PI3K/Akt pathway in the spinal cords of the rats
in the sham group (Fig. 2A-E).
Subsequently, behavioral tests were performed following treatment
with LY294002. The results indicated that the intrathecal injection
of LY294002 significantly increased the PWT values (Fig. 2F), decreased the numbers of
spontaneous flinches (Fig. 2G)
and increased the latency to fall (Fig. 2H) in the rats with BCP. However,
there were no significant changes between the rats in the sham and
LY294002 groups (Fig. 2F-H).
These results indicated that LY294002 exerted an inhibitory effect
on mechanical pain and spontaneous pain, and a beneficial effect on
the motor impairment of rats with BCP.
 | Figure 2Effects of LY294002 on the PI3K/Akt
signaling pathway and behaviors of rats with BCP. (A)
Immunofluorescence staining of PI3K and p-Akt in the spinal cord
sections from the rats in the sham, LY294002-treated sham, BCP and
LY294002-treated BCP groups. Scale bars, 20 µm. (B and C)
Quantitative analysis of the fluorescence intensity of PI3K and
p-Akt. Data are expressed as the mean ± SD (n=3). (D) The protein
levels of p-Akt (Ser473) and total Akt in lumbar spinal cord were
detected using western blot analysis. (E) Quantification of Akt
phosphorylation. Data are expressed as the ratio of p-Akt/total Akt
and each bar represents the mean ± SD (n=3). Comparison of (F) PWT
value, (G) numbers of flinches, and (H) latency to fall in the
sham, LY294002, BCP and BCP + LY294002 groups. Data are expressed
as the mean ± SEM (n=9). *P<0.05 vs. sham group,
#P<0.05 vs. BCP group. BCP, bone cancer pain; PWT,
paw withdrawal threshold. |
LY294002 treatment inhibits astrocytic
activation and the inflammatory response in rats with BCP
The robust astrocyte activation and the release of
IL-1β from activated astrocytes are closely related to chronic pain
(30). To identify the activation
of astrocytes and spinal inflammation, immunofluorescence staining
was performed for GFAP, a marker of abnormal activation and the
proliferation of astrocytes (an astrocytic marker) (31) and IL-1β. The results revealed that
compared with the sham group, the fluorescence intensities of GFAP
and IL-1β in the spinal dorsal horns of rats with BCP were
significantly enhanced (Fig.
3A-C). Western blot analysis revealed the increased expression
levels of GFAP and IL-1β in rats with BCP. In addition to IL-1β,
upregulated TNF-α protein levels were also detected in the rats
with BCP (Fig. 3D and E).
Treatment with LY294002 significantly reduced the fluorescence
intensities of GFAP and IL-1β, and decreased the protein levels of
GFAP, IL-1β and TNF-α in the rats with BCP, and exerted inhibitory
effects on both astrocyte activation and pro-inflammatory cytokine
expression. However, no significant changes were detected in the
levels of GFAP, IL-1β and TNF-α in the rats in the sham and
LY294002 groups (Fig. 3). Since
the nuclear transcription factor NF-κB mediated the expression of
pro-inflammatory cytokines, the expression of NF-κB was further
detected. The results revealed that LY294002 treatment also reduced
the expression of NF-κB in the rats with BCP (Fig. 3D and E). These data indicated that
LY294002 treatment inhibited the spinal astrocytic activation and
inflammatory response in rats with BCP.
 | Figure 3Effects of LY294002 on spinal GFAP,
IL-1β, TNF-α and NF-κB protein levels. (A) Immunofluorescence
staining of GFAP and IL-1β on spinal cord sections from the sham,
LY294002-treated sham, BCP and LY294002-treated BCP group rats.
Scale bars, 20 µm. (B and C) Quantitative analysis of GFAP
and IL-1β fluorescence intensity. Data are expressed as the mean ±
SD (n=3). (D) Western blot analysis of GFAP, IL-1β, TNF-α and NF-κB
protein expression in the lumbar spinal cord. (E) Quantification of
the protein levels by relative greyscale analysis. Data are
expressed as mean ± SD (n=3). *P<0.05 vs. sham group,
#P<0.05 vs. BCP group. BCP, bone cancer pain; GFAP,
glial fibrillary acidic protein; IL, interleukin; TNF-α, tumor
necrosis factor α. |
LY294002 treatment blocks mitochondrial
dysfunction in rats with BCP
The PI3K/Akt pathway regulates mitochondrial
function and dynamics by inhibiting BAX translocation from the
cytoplasm to the mitochondria (32); therefore, the BAX expression level
was analyzed in the present study. In contrast to the sham group,
the fluorescence intensity and protein level of BAX were
significantly upregulated in the BCP group, while LY294002
treatment reduced both the BAX fluorescence intensity and the
protein level (Fig. 4A and B).
Second, the mitochondrial morphology was observed with TEM. The TEM
images revealed that the mitochondria in the sham group contained a
whole inner membrane, outer membrane and cristae in an oval shape.
However, in the BCP group, mitochondrial cristae were lost, and the
mitochondrial size and perimeter were decreased (Fig. 4C). Subsequently, the changes in
mitochondrial function were determined by determining the activity
of the Mn-SOD enzyme and the levels of mitochondrial proteins. As
shown in Fig. 4D, Mn-SOD
activity, the primary antioxidant enzyme in the mitochondria,
markedly decreased in the spinal cords of rats with BCP. LY294002
treatment remarkably increased the Mn-SOD activity of BCP rats
(Fig. 4D). Apart from this
antioxidant, the levels of the marker of oxidative stress, MDA,
were analyzed; an increase in the MDA level was observed in the
spinal cords of rats in the BCP group. LY294002 treatment increased
Mn-SOD activity and reduced the MDA level in the rats with BCP
(Fig. 4D and E). Subsequently,
the expression levels of the mitochondrial protein, NDUFB11 (a
component of mitochondrial complex I) and DHODH (an antioxidant
which inhibits lipid peroxidation in the mitochondria) were
examined using immunofluorescence and western blot analysis. The
fluorescence intensity of NDUFB11 in the spinal dorsal horn was
markedly decreased in the BCP group, and the intensity was
increased following treatment with LY294002 (Fig. 4F and G). Western blot analysis
revealed that the expression level of spinal NDUFB11 was decreased
in the rats with BCP and LY294002 treatment upregulated the NDUFB11
expression level (Fig. 4H and I).
By contrast, the relative level of DHODH was increased in the BCP
group and LY294002 treatment decreased the DHODH protein level
(Fig. 4H and I). These results
indicated that LY294002 treatment reduced oxidative stress and
restored mitochondrial function in rats with BCP.
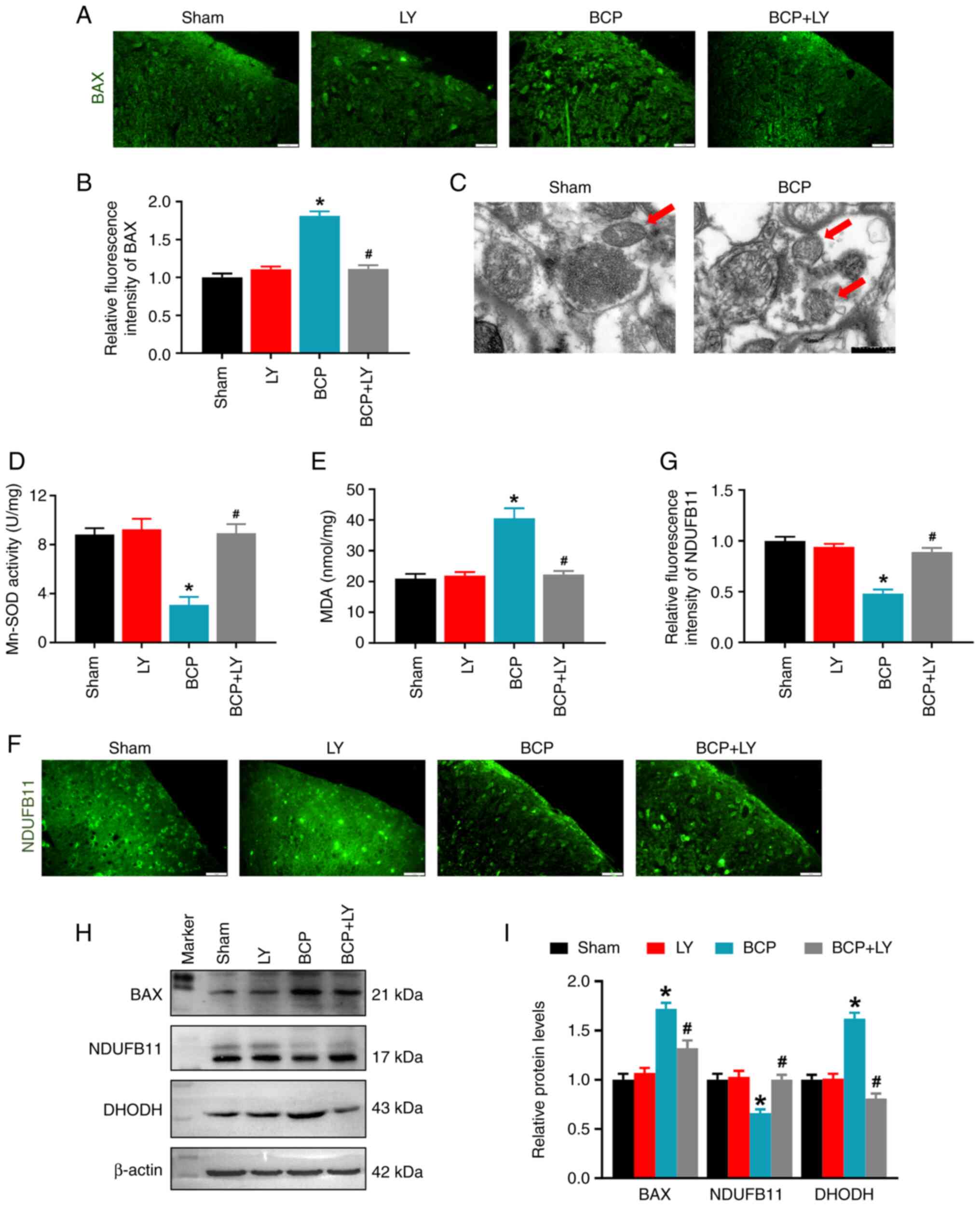 | Figure 4Effects of LY294002 on the spinal BAX
level and mitochondrial morphology and function. (A)
Immunofluorescence staining of BAX on spinal cord sections. Scale
bars, 20 µm. (B) Quantitative analysis of BAX fluorescence
intensity. Data are expressed as the mean ± SD (n=3). (C)
Representative transmission microscopy images of mitochondria in
the spinal cord of rats in the sham and BCP groups. Scale bars, 0.5
µm. The red arrows indicate the mitochondria. (D) Changes in
Mn-SOD activity detected using the Mn-SOD Assay kit. Data are
expressed as the mean ± SD (n=3). (E) Changes in the MDA level of
the spinal cord. Data are expressed as the mean ± SD (n=3). (F)
Immunofluorescence staining of NDUFB11 on spinal cord sections.
Scale bars, 20 µm. (G) Quantitative analysis of NDUFB11
fluorescence intensity. Data are expressed as the mean ± SD (n=3).
(H) The protein levels of BAX, NDUFB11 and DHODH in lumbar spinal
cord were detected using western blot analysis. (I) Quantification
of the protein level by relative greyscale analysis. Data are
expressed as the mean ± SD (n=3). *P<0.05 vs. sham
group, #P<0.05 vs. BCP group. BCP, bone cancer pain;
Mn-SOD, manganese superoxide dismutase; MDA, malondialdehyde;
NDUFB11, NADH:ubiquinone oxidoreductase subunit B11; DHODH,
dihydroorotate dehydrogenase. |
LY294002 treatment improves mitochondrial
function in C6 cells
To verify the effects of LY294002 on mitochondrial
function, changes in MMP and mitochondrial ROS were detected using
JC-1 and the MitoSOX red indicator. For JC-1 analysis, the
monomeric green form indicates a lower MMP, while red aggregates
indicate a higher MMP. MitoSOX red indicators specifically bind
with mitochondrial superoxide. As shown in Fig. 5, IL-1β stimulation in C6 cells
induced a lower MMP. LY294002 treatment restored the MMP in the
IL-1β + LY group (Fig. 5A and C).
IL-1β stimulation enhanced Mito-ROS intensity, and LY294002
treatment markedly reduced the mitochondrial ROS level in the IL-1β
+ LY294002 group (Fig. 5B and D).
However, LY294002 had no effect on the control cells.
Discussion
Cancer metastases is the cause of 90% of
cancer-related deaths, and bones are the third most frequent target
sites of metastases after the lungs and liver (33). Breast cancer, as the most common
type of cancer, tends to spread to the bones more often than other
parts of the body. Once cancer has spread to the bones, tumor cells
growing in bone marrow induce osteolysis and bone destruction,
injure the peripheral nerves and stimulate the inflammatory process
(34). These reactions in the
bone cause peripheral sensitization presenting as the activated
pain signaling pathway and a lowered pain threshold (35). In the present study, MRMT-1 rat
breast carcinoma cells were inoculated into the intramedullary
space of rat tibiae and induced bone damage, pain hypersensitive
and motor disability. These symptoms indicated that the rat model
of BCP was successfully established and suitable for research.
Peripheral noxious stimuli are conveyed to the
spinal cord and are finally passed to numerous parts of the brain
(36). During these processes,
the spinal cord undergoes central sensitization and functions on
pain maintenance (37).
Therefore, spinal cord stimulation has been used to treat a variety
of pain conditions and improve the quality of life of patients
(38). Astrocytes in the spinal
dorsal horn exhibit variable morphological and functional
alterations, and stimulate pain hypersensitivity following
peripheral nerve injury conditions (39). In the present study, astrocytes
were activated and pro-inflammatory cytokine expression was
increased in the spinal cords of rats with BCP, which were
suppressed by treatment with the PI3K inhibitor, LY294002.
PI3Ks play a key role in the inflammatory response.
The selective inhibition of PI3Ks offers a therapeutic opportunity
for inflammatory pathologies (40). In the present study, LY294002 was
used to treat rats with BCP and its inhibitory effects on
inflammatory signal activation were observed. Several signal
pathways are involved in the effects of PI3K/Akt signaling on
spinal inflammation and pain. The inhibition of PI3Kα/δ/β plays a
key role in the inflammatory response to damage and infection
(41,42). PI3K/Akt inhibition by compound 8C
and PIK-75 has been shown to markedly decrease the production and
biological activity of pro-inflammatory cytokines, such as TNF-α
and IL-6 in a NF-κB-dependent manner (43,44), which is considered the central
mediator of inflammatory process by inducing the expression of
various pro-inflammatory genes (45,46). In the present study, it was found
that LY294002 treatment inhibited the activation of the PI3K/Akt
signal pathway, and decreased the upregulated expression of NF-κB,
TNF-α and IL-1β in the spinal cords of rats with BCP. Accordingly,
LY294002 suppressed spinal inflammation via the PI3K/Akt/NF-κB
pathway.
Mitochondrial cristae membranes contain a number of
enzymes for electron transport chain (47) and oxidative phosphorylation
(48). NDUFB11 is a relatively
small integral membrane protein and is absolutely essential for the
assembly of an active mitochondrial complex I of respiratory chain
(49). The mitochondrial enzyme
DHODH couples the respiratory chain to the de novo pyrimidine
biosynthesis pathway (50),
associates with the function of respiratory complexes II and III
(51), and mediates ROS
generation (52). Mitochondrial
structure, distribution and function are dependent on energy
demands. High energy demands and high rates of ATP production and
consumption in the spinal dorsal horn drive mitochondrial
destruction and dysfunction (53). The present study demonstrated
that, in the spinal cords of rats with BCP, mitochondrial structure
and function were damaged, exhibiting disappeared cristae, a
decreased Mn-SOD activity, a reduced NDUFB11 expression, and an
increased DHODH expression. Moreover, cell research has indicated
that inflammation induced MMP deficiency and elevated mitochondrial
ROS levels. The increased production of mitochondrial ROS triggers
the activation of the NF-κB inflammatory pathway and promotes the
synthesis of pro-inflammatory cytokines (54). BAX localizes largely in the
cytoplasm, redistributes to the mitochondria in response to
stimuli, triggers the release of cytochrome c, and
consequently induces mitochondrial dysfunction (55). It has been reported that LY294002
treatment inhibits the PI3K/Akt pathway, suppresses BAX
translocation to the mitochondria and reduces the release of
mitochondrial ROS (56). The
knockdown of DHODH has been shown to increase BAX expression
(57). Herein, LY294002 treatment
suppressed BAX and DHODH expression, increased NDUFB11 expression,
elevated Mn-SOD activity, and reduced the mitochondrial and
cellular ROS levels. Taken together, the inhibition of PI3K/Akt
signaling by LY294002 restored mitochondrial function in rats with
BCP.
In conclusion, the present study demonstrates that,
in the process of BCP, the PI3K/Akt pathway is activated,
mitochondrial dysfunction is induced, and the spinal inflammatory
response is triggered. The inhibition of the PI3K/Akt pathway by
LY294002 treatment alleviates BCP by suppressing BAX and DHODH
activity, reducing the mitochondrial ROS level and consequently
decreasing the NF-κB-mediated inflammatory signal in the spinal
cord (Fig. 6).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
All authors made a significant contribution to the
study. HZ and MX conceived and designed the study, and drafted and
edited the manuscript. JZ, YY, SZ, LY and LL performed the
experiments, JD and QT collected and analyzed the data. HZ, MX, JD
and QT critically revised the manuscript. All authors have read and
approved the final manuscript. JZ and YY confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All procedures involving animals were conducted in
accordance with the Chinese National Guidelines for ethical review
of animal welfare (GB/T 35892-2018) and approved by the Ethics
Committee of Hubei University of Science and Technology (approval
no. 2020-01-900).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81901149, 81971066 and 32100823),
and the Hubei University of Science and Technology Program (grant
nos. 2020TD02 and BK202116).
References
|
1
|
Zajączkowska R, Kocot-Kępska M, Leppert W
and Wordliczek J: Bone pain in cancer patients: Mechanisms and
current treatment. Int J Mol Sci. 20:60472019. View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mercadante S: Malignant bone pain:
Pathophysiology and treatment. Pain. 69:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallaway MS, Townsend JS, Shelby D and
Puckett MC: Pain among cancer survivors. Prev Chronic Dis.
17:E542020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kane CM, Hoskin P and Bennett MI: Cancer
induced bone pain. BMJ. 350:h3152015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapoor R, Saxena AK, Vasudev P, Sundriyal
D and Kumar A: Cancer induced bone pain: Current management and
future perspectives. Med Oncol. 38:1342021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vergne-Salle P and Bertin P: Chronic pain
and neuroinflammation. Joint Bone Spine. 88:1052222021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji RR, Xu ZZ and Gao YJ: Emerging targets
in neuroinflammation-driven chronic pain. Nat Rev Drug Discov.
13:533–548. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Li Y and Tu Y: Lidocaine
attenuates CFA-induced inflammatory pain in rats by regulating the
MAPK/ERK/NF-κB signaling pathway. Exp Ther Med. 21:2112021.
View Article : Google Scholar
|
|
10
|
Sommer C, Leinders M and Üçeyler N:
Inflammation in the pathophysiology of neuropathic pain. Pain.
159:595–602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wahlman C, Doyle TM, Little JW, Luongo L,
Janes K, Chen Z, Esposito E, Tosh DK, Cuzzocrea S, Jacobson KA and
Salvemini D: Chemotherapy-induced pain is promoted by enhanced
spinal adenosine kinase levels through astrocyte-dependent
mechanisms. Pain. 159:1025–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng XQ, Wu YH, Huang JF and Wu AM:
Neurophysiological mechanisms of cancer-induced bone pain. J Adv
Res. 35:117–127. 2021. View Article : Google Scholar
|
|
13
|
Li MY, Ding JQ, Tang Q, Hao MM, Wang BH,
Wu J, Yu LZ, Jiao M, Luo BH, Xie M and Zhu HL: SIRT1 activation by
SRT1720 attenuates bone cancer pain via preventing Drp1-mediated
mitochondrial fission. Biochim Biophys Acta Mol Basis Dis.
1865:587–598. 2019. View Article : Google Scholar
|
|
14
|
Albrecht DS, Ahmed SU, Kettner NW, Borra
RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV,
et al: Neuroinflammation of the spinal cord and nerve roots in
chronic radicular pain patients. Pain. 159:968–977. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del VL, Schwartzman RJ and Alexander G:
Spinal cord histopathological alterations in a patient with
longstanding complex regional pain syndrome. Brain Behav Immun.
23:85–91. 2009. View Article : Google Scholar
|
|
16
|
Shi Y, Gelman BB, Lisinicchia JG and Tang
SJ: Chronic-pain-associated astrocytic reaction in the spinal cord
dorsal horn of human immunodeficiency virus-infected patients. J
Neurosci. 32:10833–10840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Luo M, Meng D, Pan H, Shen H, Shen
J, Yao M and Xu L: Tetrahydropalmatine exerts analgesic effects by
promoting apoptosis and inhibiting the activation of glial cells in
rats with inflammatory pain. Mol Pain. 17:174480692110421172021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allison DJ, Thomas A, Beaudry K and Ditor
DS: Targeting inflammation as a treatment modality for neuropathic
pain in spinal cord injury: A randomized clinical trial. J
Neuroinflammation. 13:1522016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu ML, Wei RD, Zhang T, Wang JM, Cheng Y,
Qin FF, Fu SP, Lu ZG and Lu SF: Electroacupuncture relieves pain
and attenuates inflammation progression through inducing IL-10
Production in CFA-Induced mice. Inflammation. 43:1233–1245. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwartz ES, Lee I, Chung K and Mo Chung
J: Oxidative stress in the spinal cord is an important contributor
in capsaicin-induced mechanical secondary hyperalgesia in mice.
Pain. 138:514–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroth A, Santos MDCQ, Borella da Silva TC,
Santos Silveira EM and Partata WA: Aqueous leaf extract from Luehea
divaricata Mart. Modulates oxidative stress markers in the spinal
cord of rats with neuropathic pain. J Ethnopharmacol.
268:1136742021. View Article : Google Scholar
|
|
22
|
Long H, Zheng H, Ai L, Osman K and Liu Z:
Down-Regulation of NOX4 expression in dorsal horn of spinal cord
could alleviate cancer-induced bone pain in rats by reducing
oxidative stress response. Cancer Manag Res. 12:10929–10938. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hussain T, Tan B, Yin Y, Blachier F,
Tossou MC and Rahu N: Oxidative stress and inflammation:What
polyphenols can do for us? Oxid Med Cell Longev. 2016:74327972016.
View Article : Google Scholar
|
|
24
|
Duarte A, Silveira GG, Soave DF, Costa JPO
and Silva AR: The Role of the LY294002-A non-selective inhibitor of
phosphatidylinositol 3-Kinase (PI3K) pathway-in cell survival and
proliferation in cell line SCC-25. Asian Pac J Cancer Prev.
20:3377–3383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Qin X, Lu X and Jiang J: Effects of
inhibiting the PI3K/Akt/mTOR signaling pathway on the pain of
sciatic endometriosis in a rat model. Can J Physiol Pharmacol.
97:963–970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu W, Lv Y and Ren F: PI3K/Akt pathway is
required for spinal central sensitization in neuropathic pain. Cell
Mol Neurobiol. 38:747–755. 2018. View Article : Google Scholar
|
|
27
|
Jiang B, Zhong X, Fang J, Zhang A, WangD
W, Liang Y, Fang J, Chen F and Du J: Electroacupuncture attenuates
morphine tolerance in rats with bone cancer pain by inhibiting
PI3K/Akt/JNK1/2 signaling pathway in the spinal dorsal horn. Integr
Cancer Ther. 20:15347354219952372021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mao Y, Wang C, Tian X, Huang Y, Zhang Y,
Wu H, Yang S, Xu K, Liu Y, Zhang W, et al: Endoplasmic reticulum
stress contributes to nociception via neuroinflammation in a murine
bone cancer pain model. Anesthesiology. 132:357–372. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao C, Xu D, Liu X, Fang Y, Yi J, Li X
and Guo B: Iridium (III) complex-loaded liposomes as a drug
delivery system for lung cancer through mitochondrial dysfunction.
Int J Nanomedicine. 13:4417–4431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Donnelly CR, Andriessen AS, Chen G, Wang
K, Jiang C and Maixner W: Central nervous system targets: Glial
cell mechanisms in chronic pain. Neurotherapeutics. 17:846–860.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee P, Pedrini S, Stoops E, Goozee
K, Villemagne VL, Asih PR, Verberk IMW, Dave P, Taddei K, Sohrabi
HR, et al: Plasma glial fibrillary acidic protein is elevated in
cognitively normal older adults at risk of Alzheimer's disease.
Transl Psychiatry. 11:272021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsuruta F, Masuyama N and Gotoh Y: The
phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax
translocation to mitochondria. J Biol Chem. 277:14040–14047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sindhi V and Erdek M: Interventional
treatments for metastatic bone cancer pain. Pain Manag. 9:307–315.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawton AJ, Lee KA, Cheville AL, Ferrone
ML, Rades D, Balboni TA and Abrahm JL: Assessment and management of
patients with metastatic spinal cord compression: A
multidisciplinary review. J Clin Oncol. 37:61–71. 2019. View Article : Google Scholar
|
|
35
|
Laakso H, Lehto LJ, Paasonen J, Salo R,
Canna A, Lavrov I, Michaeli S, Gröhn O and Mangia S: Spinal cord
fMRI with MB-SWIFT for assessing epidural spinal cord stimulation
in rats. Magn Reson Med. 86:2137–2145. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paolini F, Ferini G, Bonosi L, Costanzo R,
Brunasso L, Benigno UE, Porzio M, Gerardi RM, Giammalva GR, Umana
GE, et al: Spinal cord stimulationto treat unresponsive cancer
pain: A possible solution in palliative oncological therapy. Life
(Basel). 12:5542022.
|
|
37
|
Bosma RL, Mojarad EA, Leung L, Pukall C,
Staud R and Stroman PW: FMRI of spinal and supra-spinal correlates
of temporal pain summation in fibromyalgia patients. Hum Brain
Mapp. 37:1349–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang R, Lao L, Ren K and Berman BM:
Mechanisms of acupuncture-electro acupuncture on persistent pain.
Anesthesiology. 120:482–503. 2014. View Article : Google Scholar
|
|
39
|
Ji RR, Donnelly CR and Nedergaard M:
Astrocytes in chronic pain and itch. Nat Rev Neurosci. 20:667–685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Kuramitsu Y, Baron B, Kitagawa T,
Tokuda K, Akada J, Maehara SI, Maehara Y and Nakamura K: PI3K
inhibitor LY294002, as opposed to wortmannin, enhances AKT
phosphorylation in gemcitabine-resistant pancreatic cancer cells.
Int J Oncol. 50:606–612. 2017. View Article : Google Scholar
|
|
41
|
Jo H, Lo PK, Li Y, Loison F, Green S, Wang
J, Silberstein LE, Ye K, Chen H and Luo HR: Deactivationof Akt by a
small molecule inhibitor targeting pleckstrin homology domain and
facilitating Akt ubiquitination. Proc Natl Acad Sci USA.
108:6486–6491. 2011. View Article : Google Scholar
|
|
42
|
Yu W, Sun H, Zha W, Cui W, Xu L, Min Q and
Wu J: Apigenin attenuates adriamycin-induced cardiomyocyte
apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement
Alternat Med. 2017:25906762017. View Article : Google Scholar
|
|
43
|
Sala V, Della Sala A, Ghigo A and Hirsch
E: Roles of phosphatidyl inositol 3 kinase gamma (PI3Kγ) in
respiratory diseases. Cell Stress. 5:40–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dagia NM, Agarwal G, Kamath DV,
Chetrapal-Kunwar A, Gupte RD, Jadhav MG, Dadarkar SS, Trivedi J,
Kulkarni-Almeida AA, Kharas F, et al: A preferential
p110alpha/gamma PI3K inhibitor attenuates experimental inflammation
by suppressing the production of proinflammatory mediators in a
NF-kappaB-dependent manner. Am J Physiol Cell Physiol.
298:C929–C941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hellenbrand DJ, Quinn CM, Piper ZJ,
Morehouse CN, Fixel JA and Hanna AS: Inflammation after spinal cord
injury: A review of the critical timeline of signaling cues and
cellular infiltration. J Neuroinflammation. 18:2842021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khutornenko AA, Dalina AA, Chernyak BV,
Chumakov PM and Evstafieva AG: The role of dihydroorotate
dehydrogenase in apoptosis induction in response to inhibition of
the mitochondrial respiratory chain complex III. Acta Naturae.
6:69–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boukalova S, Hubackova S, Milosevic M,
Ezrova Z, Neuzil J and Rohlena J: Dihydroorotate dehydrogenase in
oxidative phosphorylation and cancer. Biochim Biophys Acta Mol
Basis Dis. 1866:1657592020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Torraco A, Bianchi M, Verrigni D, Gelmetti
V, Riley L, Niceta M, Martinelli D, Montanari A, Guo Y, Rizza T, et
al: A novel mutation in NDUFB11 unveils a new clinical phenotype
associated with lactic acidosis and sideroblastic anemia. Clin
Genet. 91:441–447. 2017. View Article : Google Scholar
|
|
50
|
Qian Y, Liang X, Kong P, Cheng Y, Cui H,
Yan T, Wang J, Zhang L, Liu Y, Guo S, et al: Elevated DHODH
expression promotes cell proliferation via stabilizing β-catenin in
esophageal squamous cell carcinoma. Cell Death Dis. 11:8622020.
View Article : Google Scholar
|
|
51
|
Hatinguais R, Pradhan A, Brown GD, Brown
AJP, Warris A and Shekhova E: Mitochondrial reactive oxygen species
regulate immune responses of macrophages to aspergillus fumigatus.
Front Immunol. 12:6414952021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kirkland RA and Franklin JL: Bax, reactive
oxygen, and cytochrome c release in neuronal apoptosis. Antioxid
Redox Signal. 5:589–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rabchevsky AG, Michael FM and Patel SP:
Mitochondria Focused neurotherapeutics for spinal cord injury. Exp
Neurol. 330:1133322020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhong Z, Umemura A, Sanchez-Lopez E, Liang
S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, et al:
NF-κB restricts inflammasome activation via elimination of damaged
mitochondria. Cell. 164:896–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cogliati S, Frezza C, Soriano ME, Varanita
T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A,
Gomes LC, et al: Mitochondrial cristae shape determines respiratory
chain supercomplexes assembly and respiratory efficiency. Cell.
155:160–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tian H, Li S and Yu K: DJ-1 alleviates
high glucose-induced endothelial cells injury via PI3K/Akt-eNOS
signaling pathway. Mol Med Rep. 17:1205–1211. 2018.
|
|
57
|
Liu L, Dong Z, Lei Q, Yang J, Hu H, Li Q,
Ji Y, Guo L, Zhang Y, Liu Y and Cui H: Inactivation/deficiency of
DHODH induces cell cycle arrest and programed cell death in
melanoma. Oncotarget. 8:112354–112370. 2017. View Article : Google Scholar
|




















