Introduction
As a prevalent chronic inflammatory disease of the
airways, asthma is distinguished by a highly diverse etiology and a
variety of clinical features, including airway hyperresponsiveness
(AHR), underlying inflammation, fluctuating and recurring symptoms
and airflow restriction (1).
According to the difference in the type of airway inflammation
caused by the immune response, asthma may be divided into type 2
inflammation and non-type 2 inflammation. Type 2 inflammatory
asthma is mostly driven by allergens, including house dust mites
(HDMs), pollens or animal dander, and involves T helper type 2
(Th2) and innate lymphoid cell (ILC) 2 immune cells, causing the
upregulation of type 2 cytokines, such as IL-4, IL-5 and IL-13, and
leading to airway inflammation and asthma attack. However, non-type
2 asthma may be triggered by air pollutants, viral or bacterial
infection, obesity, smoking or other irritants. Immune cells
including neutrophils, Th1 cells, ILC1s, natural killer (NK) cells
and macrophages participate in non-type 2 asthma, which causes the
upregulation of non-type 2 cytokines and induces the occurrence of
the disease (2). Typical asthma
symptoms, including coughing, wheezing and shortness of breath, may
be frequently observed in type 2 inflammatory asthma (3). In addition, comorbidities such as
allergic rhinitis, atopic dermatitis, food allergies or
non-allergic disorders, such as obesity and gastroesophageal
reflux, may coexist with asthma (4). As the proportion of obese
individuals continues to increase, obesity-related asthma has
become more frequently identified, becoming a particular challenge
for asthma management due to increased medication use, decreased
steroid responsiveness, longer hospitalization, poorer quality of
life and greater severity of the disease compared with ordinary
asthma patients (5).
According to studies of asthma phenotypes and
endotypes, asthma associated with obesity has recently been
confirmed as one of the major non-type 2 driven phenotypes and a
unique respiratory metabolic phenotype (6,7),
characterized by increased levels of metabolites (8). Obesity tends to involve lymphocyte
responses of Th1 and T helper 17 (Th17) cells rather than the
classic Th2 cell response observed in asthma (9). Rastogi et al (10) reported that compared with those in
children with asthma alone, CD4+ Th1 cell levels in the peripheral
blood of obese asthmatic children were increased, and the Th2 cell
response was not enhanced (11),
implying a change in the function of lymphocytes in obese patients,
and that metabolic function may be involved in the effect of
lymphocytes (12). Furthermore,
high levels of neutrophils and fewer eosinophils were also
identified in the sputum of obese asthmatic patients (13). In the aforementioned studies, cell
function was found to be decreased, which is consistent with the
fact that most patients with obesity-related asthma have Th1 and
Th17-enhanced asthma but not Th2-enhanced asthma. However, the type
of inflammation in obesity-related asthma is still controversial.
The inflammation covered in Chapter 2 onwards mostly refers to the
more common type 2-driven inflammation, rather than non-type
2-driven inflammation, which is a special asthma phenotype. Studies
involving non-type 2 inflammation will be highlighted in the
sentences of this article.
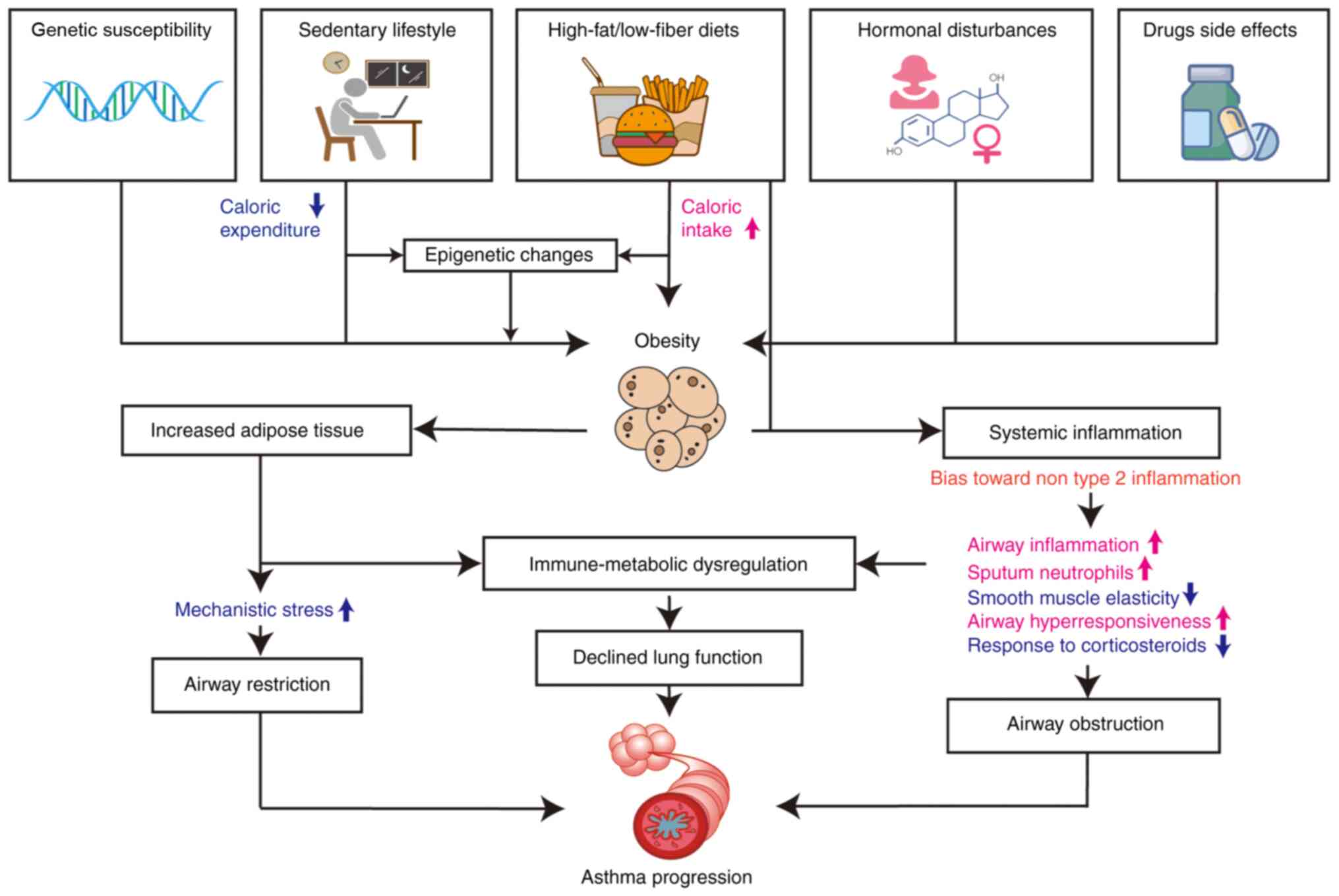
Obesity is primarily brought on by complex metabolic
dysregulation that is caused by an imbalance between calorie intake
and expenditure (14). Obese
individuals exhibit a specific non-type 2-driven chronic
subclinical inflammation, which may lead to asthma exacerbation,
aggravate airway inflammation and impair lung function (15). However, more research is required
to fully comprehend the interactions between the adipose tissue and
the lung, as well as the role served by innate and adaptive immune
cells, and the molecular basis of these intricate immuno-metabolic
interventions (Fig. 1).
The present review provides an update on the
association between obesity and asthma, paying particular attention
to current trials, studies and understanding of the probable
metabolic-inflammatory route, to provide ideas for future studies
and potential treatments.
Obesity and asthma
Association between obesity and
asthma
Obesity [body mass index (BMI) ≥30 kg/m2]
and overweight status (BMI ≥25 kg/m2) are linked to a
higher probability of developing chronic noncommunicable diseases,
a lower quality of life, exacerbation of daily symptoms and an
increased need for rescue medications (5,16).
In a multicenter retrospective cohort study of emergency department
(ED) patients aged 18 to 54 years with asthma exacerbation from 48
EDs across 23 US states during 2011 and 2012, obese adults
presenting to the ED with asthma exacerbation were determined to
have a higher risk of hospitalization compared with normal-weight
adults (17). In addition, a
k-means cluster analysis in three independent asthma populations
has validated the heterogeneity in clinical characteristics and
inflammatory biomarkers of asthma in obese individuals (18). Therefore, it is assumed that
obesity may cause persistent systemic inflammation due to
inflammatory mediators of the adipose tissue, thus elevating the
risk of airway obstruction and AHR in patients with asthma, leading
to asthma exacerbations (16).
Furthermore, the BMI, waist circumference or waist-to-height ratio
of obese populations are positively associated with current asthma,
although other metabolic syndrome components are not found to be
strongly related to current asthma (19–22). However, asthma readmissions have
also not been observed to be markedly related to obesity or
overweight status (23).
Since obesity is a major risk factor for poorly
controlled asthma, the factors contributing to the pathogenesis of
obesity-related asthma have attracted increasing attention. To a
certain extent, dietary compositions have been reported to lead to
an abnormal metabolic process, which causes obesity, as well as
airway responsiveness and inflammation in patients with asthma
(14). Obesity-causing diets are
often high in red and processed meats, fats, sugar and fried meals,
while being low in fiber. Such a dietary pattern is associated with
the immune system and asthma pathogenesis through changed
epigenetics factors, enhanced oxidative stress, abnormal expression
of adipokines and disturbed intestinal flora, causing chronic
inflammatory state, impaired lung function and respiratory symptoms
(24). An animal study revealed
that dietary fiber may influence the immune response through short
chain fatty acids (SCFAs) as early as during in utero
development (25). In addition, a
high-fat diet (HFD) may lead to airway disease even if it does not
result in obesity, as illustrated by studies in both mouse models
and humans (26,27).
Epidemiology of obesity-related
asthma
According to official documentation from the
American Thoracic Society published in 2010, obesity is a risk
factor for adult asthma and the concept is widely accepted due to
its in-depth study (28).
Depending on the age of onset, obesity has been demonstrated to be
associated with different asthma phenotypes and endotypes (29). Inadequate lung function, obesity
and metabolic syndrome were found to be associated in a US study of
teenagers (30). Obese patients
with early-onset asthma have a greater AHR, more severe airway
obstruction and an increased exacerbation risk compared with obese
patients with late-onset asthma, with similar levels of IgE and
eosinophilic inflammation (29).
According to a study from Brazil, severe asthma in adolescents was
markedly associated with certain aspects of metabolic syndrome,
particularly insulin resistance, but not with the BMI (31). This suggests that the BMI may not
be a reliable parameter for severely obese children and adolescents
due to being an artificially defined indicator (32). While it has been demonstrated in a
number of studies that obese children have an increased risk of
developing asthma, a different study reported the opposite, finding
that a history of asthma and medication had an impact on childhood
and adolescent obesity (33).
These specific features in asthmatic children imply that the
phenotype of children should be distinguished from late-onset
obesity-related asthma in adults. The aforementioned results
suggest that obesity is more likely to be an asthma comorbidity for
early-onset asthma, which is less severe but less responsive to
treatment, whereas obesity has a causative influence on late-onset
asthma, which is generally severe. Furthermore, it cannot be ruled
out that a sizeable portion of adult patients with obesity-related
asthma were children who originally had type 2-driven asthma, with
obesity developing later due to other factors (34). Therefore, further research is
still needed to determine the underlying causes of obesity and
asthma in various age groups.
The notable characteristics of the non-type 2-driven
phenotype of obesity-related asthma are more frequently observed in
women, and female sex is considered to be a key component of this
asthma phenotype (35). Obesity
may increase the chance of developing asthma in women, but not in
men, according to a Canadian study (36). In addition, it has been revealed
that the BMI is substantially associated with asthma severity only
in women and not in men, suggesting that hormonal state,
particularly during the early menarche, may serve a role in asthma
pathogenesis (37). Overweight
and obesity among girls before pubertal onset can advance the age
of onset of puberty and the age of menarche, providing evidence for
the association between obesity and hormonal state (38). Only among women, central adiposity
has been reported to exhibit a strong association with asthma
(39). In obese women but not in
obese men, obesity has also been revealed to be associated with
moderate AHR (40). In brief, the
relationship between obesity and asthma is particularly notable in
women and the development of asthma may be influenced by hormone
levels and the activity of estrogens (37). However, no consensus has been
reached on whether female sex should be recognized as a risk factor
in obesity-related asthma (22).
Although sex has been described as having no influence on
obesity-related asthma in children (39), according to a meta-analysis,
maternal obesity during pregnancy may increase the risk of asthma
in children; however, since the inclusion criteria were not
carefully adhered to, the findings of the study may require more
substantiating evidence (41).
As a result, there are currently numerous
unrecognized epidemiological patterns of obesity-related asthma,
particularly in children and adolescents. It is still unclear what
distinguishes the various endotypes of asthma in obese individuals
and this needs to be further clarified (Fig. 1).
Possible pathogenesis of asthma in obese
individuals
Numerous epidemiological studies have demonstrated
the link between obesity and asthma; however, the underlying
mechanisms are still unclear and further investigation through
various clinical and animal experiments is ongoing. Metabolic
disorders impair airway function in obese individuals through
various mechanisms such as HFD, disordered L-arginine/nitric oxide
pathways, mitochondrial dysfunction, insulin resistance and
abnormal lipid metabolism. Macrophages, lymphocytes, intestinal
floras and certain adipokines produced by adipocytes in adipose
tissue are important in regulating metabolic balance, with a strong
connection to the immunological response in patients with
obesity-related asthma (42).
Oxidative stress, mitochondrial dysfunction, genetics and
epigenetics factors, which are involved in the abnormal immune
responses in asthma, may also be associated with metabolic
dysfunction, indicating their crucial roles in the pathogenic
mechanisms of obesity-related asthma (43) (Fig.
2).
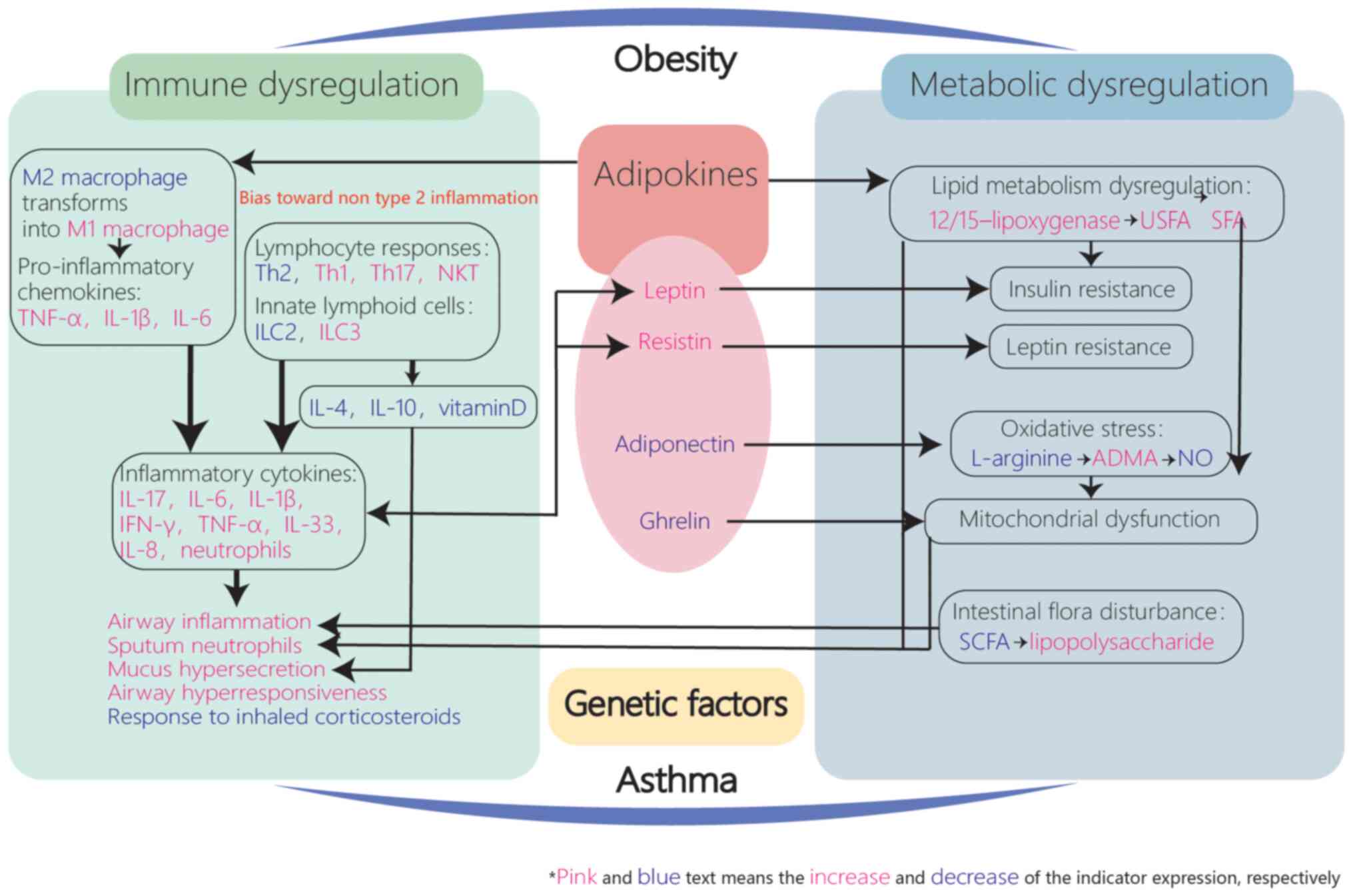 | Figure 2.Immuno-inflammatory mechanisms of
obesity-related asthma. Adipokines including leptin, resistin,
adiponectin and ghrelin may contribute to metabolic and immune
dysregulation in patients with asthma via complex interactions and
lead to inflammation which bias toward non-type 2, implying the
important role of increased adipose tissue in asthma progression.
TNF-α, tumor necrosis factor-α; Th, T helper type; IL, innate
lymphoid; ILC, innate lymphoid cell; NKT, natural killer T; IFN,
interferon; USFA, unsaturated fatty acids; SFA, saturated fatty
acids; ADMA, asymmetric dimethylarginine; NO, nitric oxide; SCFA,
short chain fatty acid. |
Inflammation in obesity-related
asthma
Cells
Macrophages, neutrophils, ILCs, NKT cells, Th1 cells
and Th17 cells secrete a variety of cytokines and chemokines under
stimulation, acting on bronchial smooth muscle, eventually leading
to a series of asthma symptoms such as tracheal constriction,
airway remodeling, increased mucus secretion, AHR and impaired lung
function (44). However, obese
patients with asthma have immune responses that differ from those
of normal-weight patients with asthma. Besides studies focusing on
innate immunity in inflammation of patients with obesity-related
asthma, studies focusing on the adaptive immune system have also
attracted attention, helping to explore the potential mechanisms of
the specific immune responses of obese patients with asthma
(9) (Table I).
 | Table I.Cells and adipokines involved in
obesity-related asthma pathogenesis. |
Table I.
Cells and adipokines involved in
obesity-related asthma pathogenesis.
| A, Cells |
|---|
|
|---|
| Name | Effect | Expression in
obesity-related asthma and possible mechanism | (Refs.) |
|---|
| M1 type of
macrophages |
Pro-inflammatory | Increased | (50) |
|
|
| Increased secretion
of TNF-α, IL-1β, |
|
|
|
| IL-6 and MCP-1 |
|
| M2 type of
macrophages |
Anti-inflammatory | Decreased | (53) |
|
|
| Decreased secretion
of TGF-β and prostaglandin E2 |
|
| Th17 cells |
Pro-inflammatory | Increased | (58) |
|
|
| IL-17 generated by
Th17 cells secretes |
|
|
|
| increased IL-6,
TNF-α and IL-8 |
|
| Th1 cells |
Pro-inflammatory | Increased | (13) |
|
|
| Increased secretion
of IL-2, IL-3 and TNF-α |
|
| Th2 cells |
Pro-inflammatory | Elusive |
|
| Eosinophils |
Pro-inflammatory | Elusive |
|
| Neutrophils |
Pro-inflammatory | Increased | (70) |
| NKT cells |
Pro-inflammatory | Increased | (65) |
|
| B,
Adipokines |
|
| Name | Effect | Expression in
obesity-related asthma and possible mechanism | (Refs.) |
|
| Leptin | Regulation of
energy | Increased | (81) |
|
| balance and
appetite | Increased secretion
of IL-6, TNF-α |
|
|
| Pro-inflammatory
effect | and interferon-γ,
activation of mast |
|
|
|
| cells and NF-κB,
and imbalance of |
|
|
|
| the regulation of
Th1/Th2 cells |
|
| Resistin | Reduces the
sensitivity | Increased | (80) |
|
| of the body to
insulin | Boosts the
activation of NF-κB and |
|
|
| Pro-inflammatory
effect | secretion of TNF-α,
IL-6 and IL-1 |
|
| Adiponectin | Regulates
various | Decreased | (74) |
|
| metabolic
processes | Weakens effects
that inhibit IL-6 |
|
|
| and energy
balance | and TNF-α |
|
|
| Anti-inflammatory
effect |
|
|
| Ghrelin | Regulates energy
balance | Decreased | (92) |
|
| and appetite | Weakens effects
that inhibit IL-1, IL-6 |
|
|
| Anti-inflammatory
effect | and TNF-α |
|
i) Macrophages
Normal lung tissues have M1 and M2 types of
macrophages to maintain the balance of pulmonary function and
status (45). Immune cells in
normal adipose tissue typically comprise M2 macrophages, CD4+T
cells and regulatory T cells, which may collectively adjust heat
production, the immune response and fat metabolism. Long-term
overnutrition may lead to hypertrophy, vascularization, hypoxia and
necrosis of adipose tissue, leading to macrophage infiltration in
adipose tissue and surrounding necrotic tissue, increasing the
levels of pro-inflammatory cytokines such as IL-6 and tumor
necrosis factor-α (TNF-α). Along with the over-expressed
pro-inflammatory cells (M1 macrophages), integrins (CD11b and
CD11c) and other metabolic endotoxins and adipokines,
anti-inflammatory M2 macrophages transform into pro-inflammatory M1
macrophages (46). When
inflammatory factors released by adipose tissue reach the lungs
through the circulatory system, airway inflammation and AHR may
occur (47,48). The abnormal activity and
metabolism of macrophages may be an important mechanism in the
pathological process of obesity-related asthma.
As cells with high inflammatory activity, M1
macrophages may secrete various pro-inflammatory adipokines and
chemokines, which lead to a state of subclinical inflammation in
adipose tissues (49), and these
adipokines and chemokines include TNF-α, IL-1β, IL-6 and monocyte
chemoattractant protein-1 (50),
the elevated expression of which is observed in obesity-related
asthma even without antigen activation (51), implying a role of M1 macrophages
in asthma pathogenesis. TNF-α secreted by M1 macrophages may bind
to receptors on airway smooth muscle, contract the muscle and
induce intracellular signal transduction and inflammation (11), which are positively related to the
BMI of patients with asthma (52). Obese mice lacking TNF-α receptor 2
were not observed to have enhanced airway reactivity, indicating
the importance of TNF-α activation in asthma pathogenesis (51).
M2 macrophages inhibit inflammation through
secretion of efferocytosis-related molecules and anti-inflammatory
mediators such as transforming growth factor-β and prostaglandin E2
(53). In a previous study,
efferocytosis and biomarkers reflecting the function of M2
macrophages were decreased in the sputum of obese asthmatic
patients, while the levels of M1 macrophages were higher than those
of patients with asthma with a normal body weight (54), implying that the effect of
suppressing inflammation was weakened because of the excessive
polarization of M2 macrophages (55).
ii) Lymphocytes
Obesity tends to represent the lymphocyte response
of Th1 and Th17 cells, associated with the increased portion of Th1
and Th17 cells, which is associated with the altered portion of Th2
and regulatory T cells (43).
IL-17 can directly induce airway hyperreactivity
(56). Increased IL-17 (secreted
by ILC3s) and increased activity of the NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) inflammasome in lung tissues
of HFD-induced mice (57)
suggested that IL-17 may promote inflammation in obese individuals.
Furthermore, IL-17 generated by Th17 cells may stimulate the
production of inflammatory molecules such as IL-6, TNF-α and IL-8
by activating the mitogen-activated protein kinase (MAPK) or
nuclear factor-κb (NF-κB) signaling pathway, thus promoting airway
inflammation (58). In addition,
airway neutrophilic leukocytosis is a feature of obesity-related
asthma, while lung neutrophilic aggregation is closely associated
with the excessive secretion of IL-17, IL-6 and TNF-α from Th17
cells, which may aggravate inflammatory manifestations in airways
(59,60).
Compared to individuals with a normal BMI, increased
Th1 cell levels in adipose tissue of obese individuals cause the
immune system to enter a pro-inflammatory state. Furthermore,
cytokines IL-2, IL-3, TNF-α and interferon-γ produced by adipocytes
and immune cells to maintain tissue integrity may further promote
inflammation of the airways (13). Reduced regulatory T cells in
adipose tissue may also be associated with obesity, causing
inflammation and insulin resistance (61).
ILC2s and ILC3s, produced by common helper innate
lymphoid progenitors, may be associated with the exacerbation of
allergic airway inflammation in activating gene 12/2 and
leptin-deficient HFD-HDM mice, indicating the involvement of ILCs
(34,57). Furthermore, NLRP3 may act as a key
regulator of the innate immune response of the host (62). NLRP3 and IL-17 (secreted by ILC3)
have also been found to be important mediators for the acceleration
of AHR in mouse models. After knockout of the genes related to
adaptive immunity in HFD-fed mice, AHR remained measurable,
demonstrating the independent role of innate immunity in the
mechanism (57).
The effect of Th2 cells on obesity-related asthma
remains disputed. IL-33, produced by epithelial cells, helps
maintain the integrity and metabolism of adipose tissue. IL-33
induces ILCs to produce IL-5 and IL-13, which accelerate the
activation of eosinophils. Eosinophils emit IL-4, which contribute
to polarizing M2 macrophages and diverging beige adipocytes
(63,64). Therefore, anti-IL-33 biologics may
be a potential therapeutic in certain cases. The association
between obesity and Th2 type CD4+ cell differentiation remains
controversial. Certain studies have reported AHR and increased
secretion of Th2 cytokine and eosinophilia in HFD-induced obesity
(34,65). Furthermore, in in vitro
research, Th2 cells differentiated from human CD4+ cells were
associated with melanin hormone production, which was associated
with increased appetite and obesity (66), revealing a comparable pathogenic
pathway for the Th2 response and obesity.
The important role of lymphocytes in asthma has been
demonstrated, while the varied phenotypes and endotypes of asthma
related to distinct immune responses, particularly for obese
patients with asthma, remain unclear. There is still more research
to be done on the various lymphocyte subtypes involved in
immunological processes.
iii) Eosinophils
Although advances have been achieved in type
2-driven asthma, non-type 2-driven asthma is poorly understood.
Studies have found that obese female patients often present with
typical non-type 2-driven asthma, which is clearly associated with
metabolic imbalance (35).
As an important biomarker of type 2-driven and
allergic asthma, the role of eosinophils in obesity-related asthma
remains contentious. In animal studies, different mouse models that
imitate human obesity-related asthma have varying eosinophil
levels. HFD-ovalbumin (OVA)-induced mice exhibited reduced
eosinophils, increased IL-10 and upregulated inflammatory cytokines
IL-5 and TNF-α in their bronchoalveolar lavage fluid (BALF), while
the eosinophil levels gradually increased while being transported
from bone marrow to lung tissues (67). In addition, increased levels of
eosinophils and AHR have also been reported in BALF of an
HFD-HDM-induced mouse model (34). Furthermore, in another study,
HFD-OVA-induced obese mice exhibited lower susceptibility to
allergic sensitization, which was positively associated with
increased airway eosinophilia, anti-OVA IgE antibodies and IL-6
(68). Considering that AKR mice
are more likely to reflect the obesity and AHR phenotypes, the
various usages of sensitizing and stimulating substances and the
diverse sampling and detection modes in mouse models, the
aforementioned results may need further verification. On the other
hand, in a clinical investigation (69), asthma was reported to be
positively associated with obesity with or without increased
eosinophil inflammation in overweight asthma patients. In addition,
eosinophils and IgE levels were identical in obese and non-obese
asthmatic children.
Despite the fact that obesity-related asthma is
regarded as the primary subtype of non-type 2-driven asthma, no
direct evidence of reduced eosinophils has been found in animal and
clinical studies. The clinical co-existence of asthma and obesity
may not only be due to non-type 2-driven immunity due to
differences in individual behavior, suggesting that further studies
on the critical factors that may trigger the complex interactions
are required.
iv) Neutrophils
Previous studies suggest that neutrophils may serve
a role in the etiology of obesity-related asthma. A previous study
revealed higher neutrophil levels in the blood and sputum of obese
asthmatic patients, particularly in women (70). However, neutrophils were
considerably reduced in obese female asthmatic patients after
losing weight and in male patients after reducing saturated fatty
acid (SFA) intake (71). Even in
asthmatic patients without obesity, an HFD was able to markedly
increase neutrophils and impair the effect of bronchodilators
(27).
This evidence suggests that neutrophils may be
involved in the pathogenesis of obesity-related asthma,
particularly in women, as a direct result of obesity or an HFD.
v) Others
NKT cell levels were found to be decreased in common
obesity with a slightly increased degree of activation, while the
expression and activation of NKT cells were more noticeably
increased in obese patients with asthma (65), suggesting that NKT cell
differentiation is inhibited in obesity and more active in
obesity-related asthma, implying a positive association with airway
inflammation.
Various cellular molecules have been observed to be
related to immunologic pathways, and thus, potentially affect the
progression of airway inflammation. CD38, as an important metabolic
enzyme located on the surface of plasma cells, T cells, NK cells,
dendritic cells and other cells, serves a role in T-cell priming,
as well as dendritic and neutrophil cell migration (72). Highly expressed CD38 in lungs of
HFD-OVA obese mice was found to induce AHR, potentially by
mediating the upregulation of TNF-α (73).
In conclusion, abundant studies in humans and
animals have provided evidence for how immune responses impact the
pathogenesis of asthma in obese individuals. However, it is still
unclear how immunity and metabolism interact in detail.
Obesity-related asthma has been observed to be closely associated
with innate immune responses, while the potential role of adaptive
immune responses still requires further research.
Adipokines
In obesity, adipose tissue usually exhibits the
states of hypertrophy and hyperplasia, giving rise to increased
levels of pro-inflammatory mediators and higher secretion of
inflammatory cytokines, including TNF-α, IFN-γ, IL-6 and IL-17,
leading to dysregulated fat metabolism and airway inflammation
(28). Furthermore, adipokines
are peptide substances produced by adipose tissue, which may act as
critical mediators in both metabolism and the immunological
response through their endocrine activity that reveal the
functional state of adipose tissue (74) (Fig.
2 and Table I).
i) Leptin
The adipokine leptin, an important mediator secreted
by adipose tissue, binds to and activates the leptin receptor
(LEP-R) in the brain and activates JAK-STAT3, suppressing
neuropeptide Y and agouti-related protein, which increase food
intake, and increasing proopiomelanocortin and
corticotrophin-releasing hormone, which decrease food intake, and
thus, regulates the energy balance and appetite through a negative
feedback mechanism between adipose tissue and the hypothalamus
(75). When fat cells increase,
leptin levels also increase and leptin binds to LEP-R to send
signals to increase satiety, thus controlling food intake, as well
as to promote energy expenditure (76). Congenital leptin deficiency, high
but ineffective leptin and leptin resistance may lead to obesity
(77).
Clinical research indicated that leptin was
upregulated in obese individuals, which was positively associated
with AHR, and may be normalized after weight loss surgery (48). In another clinical study, patients
with obesity-related asthma demonstrated elevated levels of leptin,
which may trigger asthmatic inflammation in the obese phenotype of
asthma (78). Furthermore, leptin
resistance may be developed in obese individuals, which may further
exacerbate obesity by causing a greater sense of hunger (79), which is an adverse factor in
asthmatic complications. However, in a clinical study, leptin
levels were not found to be associated with asthma (80), indicating the possibility that
leptin is involved in asthma as a regulator rather than an
etiologic factor in asthma development. Obese mouse models with
elevated leptin levels also exhibit an exacerbation of allergic
asthma and increased AHR (79,81). Studies found that, besides
regulating appetite, leptin may also induce the pro-inflammatory
state by increasing IL-6, TNF-α and interferon-γ, activating mast
cells and NF-κB, and unbalancing the regulation of Th1/Th2 cells
(81). Furthermore, the pathway
for leptin to affect allergic responses was investigated in mice,
indicating that rapamycin complex 1, MAPK and STAT3 in Th2 cells
may be involved in enhancing the production of Th2 cytokines to
produce more IL-4, IL-13 and IL-5, which cause airway symptoms
(82).
Although leptin is assumed to be a pro-inflammatory
factor, there is no precise in vitro or in vivo
evidence to support this hypothesis. Interrelations of leptin with
allergic reactions still need further proof and investigation.
ii) Resistin
Resistin is another hormone released by adipose
tissue that may reduce the sensitivity of the body to insulin,
leading to insulin resistance (83). Resistin has been observed to be
elevated in obesity, which may boost the activation of NF-κB and
secretion of inflammatory cytokines, such as TNF-α, IL-6 and IL-1,
which may in turn stimulate resistin production, contributing to
severe exacerbations of asthma in obese patients (78). Furthermore, overweight patients
with asthma have higher resistin and leptin levels than
normal-weight patients with asthma (78), and high resistin levels have been
demonstrated to be negatively associated with lung function
(84), suggesting a potential
role for resistin in asthmatic processes. However, a study reported
no difference in resistin levels between obese and non-obese
patients with asthma, indicating that resistin may be a predictor
of asthma risk and resistin/adiponectin may act as a forced
expiratory volume in 1 sec predictor in asthma (85). At present, the role of resistin in
obesity-related asthma is still controversial and requires further
exploration.
iii) Adiponectin
Adiponectin is a peptide hormone and the most
abundant adipokine in adipose tissue, and is involved in the
regulation of various metabolic processes and energy balance
(86), affecting
anti-inflammation pathways and cellular metabolism (87).
In contrast to leptin, resistin and TNF-α,
adiponectin levels may decrease with obesity, with higher
neutrophil and eosinophil levels, and pulmonary vascular
remodeling, demonstrating a diminished anti-inflammatory impact
linked to asthma attacks (87,88). Adiponectin can inhibit airway
inflammatory and oxidative stress (87), potentially by inhibiting IL-6 and
TNF-α, which are important pro-inflammation factors in asthma
mechanisms (74). HFD-OVA mouse
models have indicated its regulatory effect on cellular metabolism
via adenosine monophosphate-activated protein kinase signaling
pathways (87), demonstrating
that adipokines may act not only on immunity but also metabolism,
thus serving an important role in asthma associated with obesity.
However, contradictory results have been obtained in clinical
studies, which found no statistically significant difference in
adiponectin levels between obese and non-obese adults with asthma
(85). Furthermore, adiponectin
levels may differ in female and male adults due to the inhibitory
effect of testosterone on adiponectin (89), which requires further
exploration.
iv) Ghrelin
Besides leptin, ghrelin is another appetite hormone
regulating metabolism and energy balance (90), causing increased food intake and
decreased fat utilization (91).
The expression of pro-inflammatory cytokines such as IL-1, IL-6 and
TNF has been demonstrated to be inhibited by ghrelin (92). Furthermore, obese individuals
exhibit less ghrelin expression and an impaired inhibitory effect
of ghrelin on inflammation, which is considered to be a
physiological protective mechanism to regulate the energy balance
(93).
In brief, adipokines appear to be possible mediators
that affect the immune responses in obese patients with asthma,
although the detailed mechanisms still require to be
determined.
Oxidative stress
The metabolic imbalance in obese patients with
asthma may be closely associated with oxidative stress. Reduced
levels of L-arginine with high levels of asymmetric
dimethylarginine (ADMA) may promote the uncoupling of nitric oxide
synthase (NOS) and inhibit the production of NO, resulting in
decreased production of NO and excessive production of reactive
oxygen species (ROS) (94), which
may in turn impair the airway physiological function of NO
bioavailability and bronchiectasis (95). ROS, performing as oxidants in the
human body, are normally produced in small amounts by mitochondria
(96), while altered metabolism
in mitochondria caused by an obesity-related chronic inflammatory
state may lead to the overexpression of ROS, which can in turn
trigger mitochondrial dysfunction (97). Excessive ROS may disturb cellular
homeostasis and lead to an over-oxidation state in cells, reported
as a key element in oxidative stress, which has been linked to
asthma etiology and an increase in airway inflammation (98) (Fig.
2).
Obesity may cause mitochondrial dysfunction by
overwhelming the Krebs cycle and the mitochondrial respiratory
chain, leading to increased ROS formation (99,100). HFD mouse models exhibited higher
levels of oxidative stress indicators, including increased
uncoupled inducible NOS and lower NO, but without enhanced airway
inflammation (100,101). Elevated levels of ROS resulting
from infiltrating immune cells and consequential oxidative stress
are implicated in the pathological processes underlying asthma.
When combined with obesity, oxidative stress is exacerbated,
leading to tissue damage and promoting airway inflammation and
hyperresponsiveness (102).
However, there is no certain evidence that obesity-induced
overexpressed ROS can potentiate type 2 or non-type 2 inflammation
in asthma. The plasma L-arginine/ADMA ratio was found to be
decreased in patients with late-onset asthma, with more evident
airway symptoms, worse lung function and reduced fractional exhaled
NO (103), which is negatively
associated with the BMI (104,105). Obese patients with asthma may
have higher oxidative stress biomarkers levels and NO deficiency,
causing airway dysfunction, increased comorbidities and impaired
glucocorticoid sensitivity (106,107).
In summary, oxidative stress manifesting with
altered L-arginine and NO metabolism may act as a potential pathway
in obese patients with asthma. Furthermore, key inflammatory
cytokines in asthma, including IL-4, are markedly associated with
obesity and mitochondrial dysfunction (105). IL-4, as an allergic inflammatory
mediator, may induce ADMA aggregation in airway epithelial cells,
leading to increased ROS and mitochondrial dysfunction, which are
also associated with the immune responses in asthma (105), resulting in airway damage and
remodeling (106).
Fatty acids
Previous research has indicated that disturbances in
lipid metabolism may be linked to the mechanism of obesity-induced
asthma and may be one of the possible causes of the failure of
traditional therapies.
During the metabolism of unsaturated fatty acids
(USFA), 12/15-lipoxygenase, which acts as a catalyzer in the
hydrogenation and oxidation of USFA, may be involved in insulin
resistance in obese individuals (108). In addition, 12/15-lipoxygenase
is involved in mitochondrial dysfunction. The disruption of calcium
homeostasis and subsequent mitochondrial dysfunction in airway
epithelial cells, caused by overload of mitochondrial calcium, may
lead to the manifestation of asthma symptoms (109). IL-4, an important inflammatory
agent, may also induce mitochondrial dysfunction (110), causing increased neutrophil
aggregation and more severe airway damage (111) (Fig. 2).
On the other hand, SFA metabolism has also been
reported to be associated with immunological responses. HFD-induced
obesity may be associated with higher levels of SFA and nutritional
factors, which may trigger immune-metabolic disorders (112), leading to AHR and neutrophilic
airway inflammation (113–115). When palmitic acid, the primary
SFA component in HFD, was added directly to a HDM mouse model,
similar effects on the airways were also observed (116).
Intestinal flora
Intestinal floras act as major biological barriers
and immunomodulatory function regulators to the human body. Obesity
may cause intestinal flora disorders, leading to increased
intestinal mucosal permeability and decreased SCFA, involving the
immunomodulatory dysfunction in asthma (117).
Studies have revealed increased lipopolysaccharides
in the plasma of obese asthmatic patients and HFD-induced
lipopolysaccharides may enter the blood circulation through the
intestinal mucosa with increased permeability, activate the NF-κB
signaling pathway and produce pro-inflammatory molecules such as
TNF-α and IL-6, eventually resulting in AHR and asthma exacerbation
(118). On the other hand, an
HFD or low-soluble-fiber diet may alter the gut microbiome and is
associated with lower levels of SCFAs that enter the circulation,
thereby lowering the ability of SCFAs to inhibit dendritic cells to
stimulate allergic inflammation, causing augmented allergic airway
inflammation (117).
Furthermore, a high-fiber diet may alter the composition of the
intestinal and lung microbiota of mice. Intestinal microbiota
metabolize fiber, increase circulating SCFA levels and further
regulate the differentiation and activation of regulatory T cells,
improving the immune environment of the lungs, thus inhibiting the
occurrence of allergic inflammation and weight gain (117).
Although several studies have implied the role of
intestinal floras in asthma mechanisms, studies in this field are
still lacking and further exploration is required.
Genetics
As a more precise predictor of asthma features than
family history, the high-risk genotype of asthma commonly overlaps
with that of obesity (119),
implying similarities in gene expression rooted in lipid metabolism
and allergic mechanisms (120).
Numerous susceptibility loci for asthma and obesity have been
identified, including adrenoceptor β2 gene (ADRB2), nuclear
receptor subfamily 3 group C member 1 (NR3C1) and TNF-α gene
(121,122) (Table II). ADRB2 affects the sympathetic
nervous system, thus exerting an influence on the respiration and
metabolism systems (123). The
glucocorticoid receptors encoded by NR3C1 may regulate airway
inflammations, while TNF-α induces immune-inflammatory responses
(123). Furthermore, an HFD
upregulates the expression levels of chitinase-3-like protein 1
gene, whose expressed products are associated with obesity and
asthma (124). In addition, the
protein kinase Cα, leptin and ADRB3 genes have been found to be
related to asthma and BMI (122,125,126). Furthermore, several loci,
including ADRB2, TNF, leukotriene A4 hydrolase,
glucosamine-6-phosphate deaminase 2 and roundabout guidance
receptor 1, have been implicated in the pathogenesis of obese
asthma in children (121,127).
 | Table II.Genes and loci relevant to
obesity-related asthma. |
Table II.
Genes and loci relevant to
obesity-related asthma.
| Introduction | Gene and loci | Mechanism of
action | (Refs.) |
|---|
| Susceptibility loci
for asthma and obesity | ADRB2 | Affects the
sympathetic nervous system, thus influencing respiration and
metabolism | (118–120) |
|
| NR3C1 | The glucocorticoid
receptors encoded by NR3C1 regulate airway inflammation |
|
|
| TNF-α gene | TNF-α induces
immune-inflammatory responses. |
|
| HFD-upregulated
gene | CH13L1 gene | The expressed
products are related to obesity and asthma | (121) |
| Genes related to
asthma and BMI | PRKCA, LEP, ADRB3
genes | Unknown | (119,122,123) |
| Loci involved in
obese asthmatic children | ADRB2, TNF, LTA4H,
GNPDA2 and ROBO1 | Unknown | (119,124) |
Epigenetic mechanisms lead to heritable changes in
the function of genes without altering the DNA sequence, which may
be caused by environmental changes and dietary intake (128,129). Of note, prenatal and early-life
environmental exposures have been reported to affect the risk of
asthma and obesity in the adult stage epigenetically (128,130).
Histone modification, as one of the epigenetic
mechanisms, is coordinated by histone acetyltransferases and
histone deacetylases (HDACs), which comprise 11 classic subtypes,
HDAC1-HDAC11. Inflammation of the airways and metabolic disorders
linked to obesity may both be accompanied by HDAC9 expression.
Studies have demonstrated that increased HDAC9 levels lead to
impaired adipocyte differentiation induced by an HFD, and
inhibiting the HDAC9 gene improves adipocyte differentiation and
systemic metabolism, bringing about weight loss and increased
sensitivity to glucose and insulin (131) with suppressed airway
inflammation (25). Furthermore,
SCFAs acts as an HDAC inhibitor, which may suppress inflammation by
inhibiting the NF-κB signaling pathway (132). Patients with asthma who have
reduced HDAC2 levels are less responsive to glucocorticoid therapy
(133).
DNA methylation, as one of the epigenetic
mechanisms, may inhibit the promoter of genes encoding molecules in
obese asthmatic children, including C-C motif chemokine ligand 5,
IL-2 receptor α and T-box 21. Increased methylation is also
observed at the low-affinity receptor for IgE (Fc fragment of IgE
receptor II) and the transforming growth factor β1 gene (134).
In terms of genetic aspects, although the knowledge
regarding the connection between obesity and asthma remains
insufficient, genetic factors may have a potential effect on the
morbidities of both asthma and obesity.
Potential treatments
The investigation of inflammatory mechanisms in
obesity-related asthma is of significant importance for the
advancement of clinical treatments. A comprehensive understanding
of specific pathways involved in inflammation and targeting
critical factors such as adipokines, gut microbiota and genes that
contribute to immunological inflammatory response may facilitate
clinical trials aimed at optimizing asthma therapies and
prevention. Efficient therapies available for obese patients with
asthma are currently under investigation. The effectiveness of
weight loss has been demonstrated, while certain medications
targeting the immune response in obese patients with asthma are
also being further explored.
Weight loss
Weight loss accomplished through dietary
adjustments, lifestyle interventions, medication applications, or
bariatric surgery may markedly improve asthma outcomes (135–142). For obese individuals,
improvements in asthma control, lung function and overall quality
of life have been found after losing weight, with a trial revealing
that obese individuals who lose at least 10% of their body weight
may experience a meaningful improvement of clinical symptoms
(137). Except for the
mechanical effect of weight loss on improving lung function, its
effects on the immune-metabolic route of asthma remain unclear.
Diet-induced weight loss may reduce airway
inflammation with suppressed AHR (135). The crucial role of TNF-α as a
mediator connecting asthma and obesity was also detected in mouse
models (138). However, the
heterogeneous results of human studies suggested that the role of
weight loss was more complex. Although marked clinical improvements
in asthma control were observed, the levels of inflammation were
not altered in obese asthmatic children after dietary-induced
weight loss (139). A
weight-loss program combining dietary and weight-loss medicine
(sibutramine or orlistat) intake also revealed marked clinical
improvements but without improvements in airway inflammation or
bronchial reactivity (140).
However, markedly improved clinical symptoms and life quality were
found in adults who lost weight through comprehensive intervention
of dietary restriction and exercise, while decreased airway
inflammation was also observed with improved neutrophilic,
eosinophilic and vitamin D levels, which differed from the
unaltered inflammation in the aforementioned studies (71,141). Bariatric surgical treatments
have been demonstrated to improve asthma outcomes as well,
explained by improved small airway function and decreased systemic
inflammation (136), as well as
reduced metabolic inflammation, improved AHR and adjusted T-cell
differentiation, which is involved in asthma pathogenesis (142). In obese mice, bariatric surgery
and dietary-induced weight loss had equivalent effects in reducing
the elevated AHR (135). These
findings suggest that weight loss may be a potential therapeutic
and daily management strategy for obese asthma patients, although
its anti-inflammatory effect remains uncertain and requires further
validation in human studies.
Medications
Medications aimed at regulating the abnormal immune
responses in obese patients with asthma are currently at the
exploratory stage, with a series of medications being potential
treatments.
Celastrol is a natural bioactive molecule and has
been found to have an anti-inflammatory effect to reduce AHR in
allergic asthma, with a reduction in Th17 mRNA expression in mouse
models (143,144). Since the Notch signaling pathway
is involved in allergic airway inflammation and metabolism
(145,146), γ-secretase inhibitors that can
block the signaling pathway have been found to decrease the Th17
response and AHR in mice (147).
C-X-C motif chemokine receptor 2 antagonist (SCH527123) is a
small-molecule drug for non-type 2 asthma that has been revealed to
markedly reduce neutrophil levels and improve asthma symptoms
(148). However, it does not
improve the clinical outcome of patients with refractory asthma
(148,149) and its effect in obese patients
with asthma remains unclarified. Certain medications at off-label
dosages, including macrolides, statins and low-dose theophylline,
may have a potential effect on non-type 2 asthma. Azithromycin can
reduce asthma attacks and airway inflammation in patients with
non-eosinophilic asthma (150,151). Statins, which have an
immunomodulatory effect (152),
may reduce hospitalization and restore the glucocorticoid
sensitivity of patients with asthma (153), while their effectiveness in
obese patients with asthma still requires further verification.
Glucocorticoids may inhibit the expression of
pro-inflammatory genes, partly through their negative regulation of
MAPK signaling pathways (154).
Proinflammatory environments characterized by cytokines such as
IL-1, IL-6 and TNF-α are observed in obese individuals and these
are regulated by p38 MAPK or are its potential regulators, and
thus, may alter glucocorticoid responses in obese patients with
asthma (154). TNF-α expression
has been reported to increase as the BMI increases in patients with
asthma, implying its potential role in interfering with the process
of the glucocorticoid response in obese or overweight individuals
with asthma (52). Monoclonal
antibodies (mabs) against IL-17 or TNF-α may act on neutrophilic
inflammation and restore glucocorticoid sensitivity in mouse models
(155). However, clinical trials
on brodalumab have provided disappointing results (156), and whether TNF-α mab improves
glucocorticoid sensitivity in obese asthmatic patients remains
unclear.
Insulin resistance may lead to metabolic syndrome
and type 2 diabetes (157). As
an important comorbidity of obesity-related asthma, insulin
resistance is closely related to asthma development and
exacerbation (21,158). Therefore, treatments targeting
the metabolic process are critical for asthma control. Certain
therapies to improve insulin resistance, such as metformin and
sulfonylureas, have been found to improve asthma symptoms (159,160). In addition, glucagon-like
peptide 1 receptor (GLP-1R) agonists, as diabetes medications, may
raise insulin and inhibit glucagon secretion, as well as promote
weight loss by delaying gastric emptying and increasing satiety
(161), markedly inhibit airway
inflammation and reduce airway eosinophilia, mucus production and
AHR (162,163), suggesting their potential
therapeutic implications for obesity-related asthma. Applications
of GLP-1R agonists in patients with asthma combined with type 2
diabetes reduced the frequency of asthma exacerbations (164), implying their potential role in
treating obesity-related asthma associated with metabolic
dysfunction.
The aforementioned studies revealed potential
methods for obesity-related asthma treatment, which still need to
be substantiated by further studies. In addition, through analysis
of gene expression, asthma phenotypes and endotypes associated with
obesity may be further differentiated at the molecular level
(6,165) and more corresponding phenotypic
biomarkers may be found, thus contributing to finding possible
methods for the effective treatment of obesity-related asthma.
Conclusions
Patients with obesity-related asthma exhibit
inflammatory dysregulation, which differs from ordinary patients
with asthma, and this may partly explain the clinical
characteristics of obesity-related asthma in epidemiological
studies, including severe asthma symptoms, difficulty in
controlling symptoms and obvious resistance to conventional drugs
such as glucocorticoids. However, numerous epidemiological aspects
in this field remain unclear, particularly the correlation between
asthma related to obesity and factors such as age, gender and
BMI.
Metabolic disorders may hinder airway function in
obese individuals through various mechanisms. Based on the present
review, various factors such as oxidative stress, mitochondrial
dysfunction, genetics and epigenetics related to the obese state
may have impacts on asthma inflammation. In patients with
obesity-related asthma, macrophages, lymphocytes, intestinal flora
and certain adipokines produced by adipose tissue are also strongly
linked to the immunological response through inhibiting the
anti-inflammatory effect, activating multiple inflammatory
signaling pathways and secreting various pro-inflammatory mediators
and chemokines. In addition, genetic factors may potentially affect
both asthma and obesity morbidity, including the genes ADRB2, NR3C1
and TNF-α.
Based on these findings and hypotheses, directions
for future exploration are provided. Aiming at the special non-type
2 inflammatory types and inflammatory factors that are different
between obesity-related asthma and ordinary asthma, the
pathological process of inflammation and the mechanism of drug
resistance will be further clarified by future studies, providing
insightful targets and ideas for the management of obesity-related
asthma. Furthermore, the role of various adipokines and genetic
factors in the inflammatory process of asthma has not been fully
elucidated, which may be expected to provide potential targets for
the treatment of asthma in obese individuals through further
research.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Province Graduate
Education Quality Improvement Program Project of China (Teaching
Case Base Construction Project of Professional Degree Postgraduates
in Shandong Province) (grant no. SDYAL21057).
Availability of data and materials
Not applicable.
Authors' contributions
ZQ, CH and YC conceived and designed the review.
ZQ, CH, HY, JL, DL and YW wrote the manuscript and prepared the
figures. ZQ, HY, DL and YC performed the literature search. JL, DL
and YW critically revised the manuscript for important intellectual
content. All authors have read and approved the final manuscript.
All authors are responsible for all aspects of the work and approve
the submission in its current form. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
National Asthma Education and Prevention
Program, . Expert panel report 3 (EPR-3): Guidelines for the
diagnosis and management of asthma-summary report 2007. J Allergy
Clin Immunol. 120 (5 Suppl):S94–S138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fahy JV: Type 2 inflammation in
asthma-present in most, absent in many. Nat Rev Immunol. 15:57–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Asthma-hope for the
future? Lancet. 386:10142015. View Article : Google Scholar
|
|
4
|
Lemanske RF Jr and Busse WW: Asthma. JAMA.
278:1855–1873. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shashaty MGS and Stapleton RD:
Physiological and management implications of obesity in critical
illness. Ann Am Thorac Soc. 11:1286–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koczulla AR, Vogelmeier CF, Garn H and
Renz H: New concepts in asthma: Clinical phenotypes and
pathophysiological mechanisms. Drug Discov Today. 22:388–396. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lötvall J, Akdis CA, Bacharier LB, Bjermer
L, Casale TB, Custovic A, Lemanske RF Jr, Wardlaw AJ, Wenzel SE and
Greenberger PA: Asthma endotypes: A new approach to classification
of disease entities within the asthma syndrome. J Allergy Clin
Immunol. 127:355–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maniscalco M, Paris D, Melck DJ, D'Amato
M, Zedda A, Sofia M, Stellato C and Motta A: Coexistence of obesity
and asthma determines a distinct respiratory metabolic phenotype. J
Allergy Clin Immunol. 139:1536–1547.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLaughlin T, Liu LF, Lamendola C, Shen L,
Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, et al:
T-cell profile in adipose tissue is associated with insulin
resistance and systemic inflammation in humans. Arterioscler Thromb
Vasc Biol. 34:2637–2643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rastogi D, Fraser S, Oh J, Huber AM,
Schulman Y, Bhagtani RH, Khan ZS, Tesfa L, Hall CB and Macian F:
Inflammation, metabolic dysregulation, and pulmonary function among
obese urban adolescents with asthma. Am J Respir Crit Care Med.
191:149–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ather JL, Poynter ME and Dixon AE:
Immunological characteristics and management considerations in
obese patients with asthma. Expert Rev Clin Immunol. 11:793–803.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fracchia KM and Walsh CM: Metabolic
mysteries of the inflammatory response: T cell polarization and
plasticity. Int Rev Immunol. 34:3–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umetsu DT: Mechanisms by which obesity
impacts upon asthma. Thorax. 72:174–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peters U, Suratt BT, Bates JHT and Dixon
AE: Beyond BMI: Obesity and lung disease. Chest. 153:702–709. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon AE and Poynter ME: Mechanisms of
asthma in obesity. Pleiotropic aspects of obesity produce distinct
asthma phenotypes. Am J Respir Cell Mol Biol. 54:601–608. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasegawa K, Tsugawa Y, Lopez BL, Smithline
HA, Sullivan AF and Camargo CA Jr: Body mass index and risk of
hospitalization among adults presenting with asthma exacerbation to
the emergency department. Ann Am Thorac Soc. 11:1439–1444. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haldar P, Pavord ID, Shaw DE, Berry MA,
Thomas M, Brightling CE, Wardlaw AJ and Green RH: Cluster analysis
and clinical asthma phenotypes. Am J Respir Crit Care Med.
178:218–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assad N, Qualls C, Smith LJ, Arynchyn A,
Thyagarajan B, Schuyler M, Jacobs DR Jr and Sood A: Body mass index
is a stronger predictor than the metabolic syndrome for future
asthma in women. The longitudinal CARDIA study. Am J Respir Crit
Care Med. 188:319–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brumpton BM, Camargo CA Jr, Romundstad PR,
Langhammer A, Chen Y and Mai XM: Metabolic syndrome and incidence
of asthma in adults: The HUNT study. Eur Respir J. 42:1495–1502.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardet JC, Ash S, Kusa T, Camargo CA Jr
and Israel E: Insulin resistance modifies the association between
obesity and current asthma in adults. Eur Respir J. 48:403–410.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beuther DA and Sutherland ER: Overweight,
obesity, and incident asthma: A meta-analysis of prospective
epidemiologic studies. Am J Respir Crit Care Med. 175:661–666.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonzalez-Barcala FJ, Nieto-Fontarigo JJ,
Lourido-Cebreiro T, Rodríguez-García C, San-Jose ME, Carreira JM,
Calvo-Alvarez U, Cruz MJ, Facal D, Garcia-Sanz MT, et al: Obesity
does not increase the risk of asthma readmissions. J Clin Med.
9:2212020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han YY, Forno E, Shivappa N, Wirth MD,
Hébert JR and Celedón JC: The dietary inflammatory index and
current wheeze among children and adults in the United States. J
Allergy Clin Immunol Pract. 6:834–841.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thorburn AN, McKenzie CI, Shen S, Stanley
D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, et al:
Evidence that asthma is a developmental origin disease influenced
by maternal diet and bacterial metabolites. Nat Commun. 6:73202015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fricke K, Vieira M, Younas H, Shin MK,
Bevans-Fonti S, Berger S, Lee R, D'Alessio FR, Zhong Q, Nelson A,
et al: High fat diet induces airway hyperresponsiveness in mice.
Sci Rep. 8:64042018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wood LG, Garg ML and Gibson PG: A high-fat
challenge increases airway inflammation and impairs bronchodilator
recovery in asthma. J Allergy Clin Immunol. 127:1133–1140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suratt BT, Ubags NDJ, Rastogi D, Tantisira
KG, Marsland BJ, Petrache I, Allen JB, Bates JHT, Holguin F,
McCormack MC, et al: An official American thoracic society workshop
report: Obesity and metabolism. An emerging frontier in lung health
and disease. Ann Am Thorac Soc. 14:1050–1059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holguin F, Bleecker ER, Busse WW, Calhoun
WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E,
Jarjour NN, et al: Obesity and asthma: An association modified by
age of asthma onset. J Allergy Clin Immunol. 127:1486–1493.e2.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Forno E, Han YY, Muzumdar RH and Celedón
JC: Insulin resistance, metabolic syndrome, and lung function in US
adolescents with and without asthma. J Allergy Clin Immunol.
136:304–311.e8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuschnir FC, Felix MMR, Caetano Kuschnir
MC, Bloch KV, Azevedo de Oliveira Costa Jordão E, Solé D, Ledo
Alves da Cunha AJ and Szklo M: Severe asthma is associated with
metabolic syndrome in Brazilian adolescents. J Allergy Clin
Immunol. 141:1947–1949.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freedman DS, Butte NF, Taveras EM, Lundeen
EA, Blanck HM, Goodman AB and Ogden CL: BMI z-scores are a poor
indicator of adiposity among 2- to 19-year-olds with very high
BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring).
25:739–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Salam MT, Alderete TL, Habre R,
Bastain TM, Berhane K and Gilliland FD: Effects of childhood asthma
on the development of obesity among school-aged children. Am J
Respir Crit Care Med. 195:1181–1188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Everaere L, Ait-Yahia S, Molendi-Coste O,
Vorng H, Quemener S, LeVu P, Fleury S, Bouchaert E, Fan Y, Duez C,
et al: Innate lymphoid cells contribute to allergic airway disease
exacerbation by obesity. J Allergy Clin Immunol. 138:1309–1318.e11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bel EH: Obesity and asthma control: Do
gender, age, and ethnicity really matter? Am J Respir Crit Care
Med. 187:667–668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Dales R, Tang M and Krewski D:
Obesity may increase the incidence of asthma in women but not in
men: Longitudinal observations from the Canadian national
population health surveys. Am J Epidemiol. 155:191–19. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Varraso R, Siroux V, Maccario J, Pin I and
Kauffmann F; Epidemiological Study on the Genetics and Environment
of Asthma, : Asthma severity is associated with body mass index and
early menarche in women. Am J Respir Crit Care Med. 171:334–339.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xi X, Wu D, Wu W, Zhou Y, Zhang Q, Wang Y,
Wang H and Liu Q: The influence of the trajectory of obesity
indicators on the age of pubertal onset and pubertal tempo in
girls: A longitudinal study in Chongqing, China. Front Public
Health. 11:10257782023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Forno E, Han YY, Libman IM, Muzumdar RH
and Celedón JC: Adiposity and asthma in a nationwide study of
children and adults in the United States. Ann Am Thorac Soc.
15:322–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sposato B, Scalese M, Scichilone N,
Pammolli A, Balducci MT, Migliorini MG and Scala R: BMI can
influence adult males' and females' airway hyperresponsiveness
differently. Multidiscip Respir Med. 7:452012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Forno E, Young OM, Kumar R, Simhan H and
Celedón JC: Maternal obesity in pregnancy, gestational weight gain,
and risk of childhood asthma. Pediatrics. 134:e535–e546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dixon AE and Rincón M: Metabolic
dysfunction: Mediator of the link between obesity and asthma?
Lancet Respir Med. 4:533–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miethe S, Guarino M, Alhamdan F, Simon HU,
Renz H, Dufour JF, Potaczek DP and Garn H: Effects of obesity on
asthma: Immunometabolic links. Pol Arch Intern Med. 128:469–477.
2018.PubMed/NCBI
|
|
44
|
DeKruyff RH, Yu S, Kim HY and Umetsu DT:
Innate immunity in the lung regulates the development of asthma.
Immunol Rev. 260:235–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hussell T and Bell TJ: Alveolar
macrophages: Plasticity in a tissue-specific context. Nat Rev
Immunol. 14:81–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Crewe C, An YA and Scherer PE: The ominous
triad of adipose tissue dysfunction: Inflammation, fibrosis, and
impaired angiogenesis. J Clin Invest. 127:74–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lumeng CN, Deyoung SM, Bodzin JL and
Saltiel AR: Increased inflammatory properties of adipose tissue
macrophages recruited during diet-induced obesity. Diabetes.
56:16–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sideleva O, Suratt BT, Black KE, Tharp WG,
Pratley RE, Forgione P, Dienz O, Irvin CG and Dixon AE: Obesity and
asthma: An inflammatory disease of adipose tissue not the airway.
Am J Respir Crit Care Med. 186:598–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang C, Wang B, Kaliannan K, Wang X, Lang
H, Hui S, Huang L, Zhang Y, Zhou M, Chen M and Mi M: Gut microbiota
mediates the protective effects of dietary capsaicin against
chronic low-grade inflammation and associated obesity induced by
high-fat diet. mBio. 8:e00470–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oliveira AG, Araujo TG, Carvalho BM,
Guadagnini D, Rocha GZ, Bagarolli RA, Carvalheira JB and Saad MJ:
Acute exercise induces a phenotypic switch in adipose tissue
macrophage polarization in diet-induced obese rats. Obesity (Silver
Spring). 21:2545–2556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Williams AS, Chen L, Kasahara DI, Si H,
Wurmbrand AP and Shore SA: Obesity and airway responsiveness: Role
of TNFR2. Pulm Pharmacol Ther. 26:444–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sutherland ER, Goleva E, Strand M, Beuther
DA and Leung DY: Body mass and glucocorticoid response in asthma.
Am J Respir Crit Care Med. 178:682–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McCubbrey AL and Curtis JL: Efferocytosis
and lung disease. Chest. 143:1750–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fernandez-Boyanapalli R, Goleva E,
Kolakowski C, Min E, Day B, Leung DY, Riches DW, Bratton DL and
Sutherland ER: Obesity impairs apoptotic cell clearance in asthma.
J Allergy Clin Immunol. 131:1041–1047. 1047.e1–e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kudo M, Melton AC, Chen C, Engler MB,
Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, et al:
IL-17A produced by αβ T cells drives airway hyper-responsiveness in
mice and enhances mouse and human airway smooth muscle contraction.
Nat Med. 18:547–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim HY, Lee HJ, Chang YJ, Pichavant M,
Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et
al: Interleukin-17-producing innate lymphoid cells and the NLRP3
inflammasome facilitate obesity-associated airway hyperreactivity.
Nat Med. 20:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chehimi M, Vidal H and Eljaafari A:
Pathogenic role of IL-17-producing immune cells in obesity, and
related inflammatory diseases. J Clin Med. 6:682017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Apostolopoulos V, de Courten MP,
Stojanovska L, Blatch GL, Tangalakis K and de Courten B: The
complex immunological and inflammatory network of adipose tissue in
obesity. Mol Nutr Food Res. 60:43–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nguyen DV, Linderholm A, Haczku A and
Kenyon N: Glucagon-like peptide 1: A potential anti-inflammatory
pathway in obesity-related asthma. Pharmacol Ther. 180:139–143.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Feuerer M, Herrero L, Cipolletta D, Naaz
A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S and
Mathis D: Lean, but not obese, fat is enriched for a unique
population of regulatory T cells that affect metabolic parameters.
Nat Med. 15:930–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin Y, Lv X, Sun C, Sun Y, Yang M, Ma D,
Jing W, Zhao Y, Cheng Y, Xuan H and Han L: TRIM50 promotes NLRP3
inflammasome activation by directly inducing NLRP3 oligomerization.
EMBO Rep. 23:e545692022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee MW, Odegaard JI, Mukundan L, Qiu Y,
Molofsky AB, Nussbaum JC, Yun K, Locksley RM and Chawla A:
Activated type 2 innate lymphoid cells regulate beige fat
biogenesis. Cell. 160:74–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Molofsky AB, Nussbaum JC, Liang HE, Van
Dyken SJ, Cheng LE, Mohapatra A, Chawla A and Locksley RM: Innate
lymphoid type 2 cells sustain visceral adipose tissue eosinophils
and alternatively activated macrophages. J Exp Med. 210:535–549.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen YP, Zhang JH, Li CQ, Sun QX and Jiang
XH: Obesity enhances Th2 inflammatory response via natural killer T
cells in a murine model of allergic asthma. Int J Clin Exp Med.
8:15403–15412. 2015.PubMed/NCBI
|
|
66
|
Sandig H, McDonald J, Gilmour J, Arno M,
Lee TH and Cousins DJ: Human Th2 cells selectively express the
orexigenic peptide, pro-melanin-concentrating hormone. Proc Natl
Acad Sci USA. 104:12440–12444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Calixto MC, Lintomen L, Schenka A, Saad
MJ, Zanesco A and Antunes E: Obesity enhances eosinophilic
inflammation in a murine model of allergic asthma. Br J Pharmacol.
159:617–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dietze J, Böcking C, Heverhagen JT,
Voelker MN and Renz H: Obesity lowers the threshold of allergic
sensitization and augments airway eosinophilia in a mouse model of
asthma. Allergy. 67:1519–1529. 2012.PubMed/NCBI
|
|
69
|
Cvejoska-Cholakovska V, Kocova M,
Velikj-Stefanovska V and Vlashki E: The association between asthma
and obesity in children-inflammatory and mechanical factors. Open
Access Maced J Med Sci. 7:1314–1319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Telenga ED, Tideman SW, Kerstjens HA,
Hacken NH, Timens W, Postma DS and van den Berge M: Obesity in
asthma: More neutrophilic inflammation as a possible explanation
for a reduced treatment response. Allergy. 67:1060–1068. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Scott HA, Gibson PG, Garg ML, Pretto JJ,
Morgan PJ, Callister R and Wood LG: Dietary restriction and
exercise improve airway inflammation and clinical outcomes in
overweight and obese asthma: A randomized trial. Clin Exp Allergy.
43:36–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guedes AGP, Jude JA, Paulin J, Kita H,
Lund FE and Kannan MS: Role of CD38 in TNF-alpha-induced airway
hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol.
294:L290–L299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chong L, Zhang W, Yu G, Zhang H, Zhu L, Li
H, Shao Y and Li C: High-fat-diet induces airway
hyperresponsiveness partly through activating CD38 signaling
pathway. Int Immunopharmacol. 56:197–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sood A and Shore SA: Adiponectin, leptin,
and resistin in asthma: Basic mechanisms through population
studies. J Allergy (Cairo). 2013:7858352013.PubMed/NCBI
|
|
75
|
Ahima RS: Revisiting leptin's role in
obesity and weight loss. J Clin Invest. 118:2380–2383.
2008.PubMed/NCBI
|
|
76
|
Park HK and Ahima RS: Physiology of
leptin: Energy homeostasis, neuroendocrine function and metabolism.
Metabolism. 64:24–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dubern B and Clement K: Leptin and leptin
receptor-related monogenic obesity. Biochimie. 94:2111–2115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Muc M, Todo-Bom A, Mota-Pinto A,
Vale-Pereira S and Loureiro C: Leptin and resistin in overweight
patients with and without asthma. Allergol Immunopathol (Madr).
42:415–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shore SA, Schwartzman IN, Mellema MS,
Flynt L, Imrich A and Johnston RA: Effect of leptin on allergic
airway responses in mice. J Allergy Clin Immunol. 115:103–109.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jartti T, Saarikoski L, Jartti L, Lisinen
I, Jula A, Huupponen R, Viikari J and Raitakari OT: Obesity,
adipokines and asthma. Allergy. 64:770–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zheng H, Wu D, Wu X, Zhang X, Zhou Q, Luo
Y, Yang X, Chock CJ, Liu M and Yang XO: Leptin promotes allergic
airway inflammation through targeting the unfolded protein response
pathway. Sci Rep. 8:89052018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mai XM, Chen Y and Krewski D: Does leptin
play a role in obesity-asthma relationship? Pediatr Allergy
Immunol. 20:207–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Huang F, Del-Río-Navarro BE,
Torres-Alcántara S, Pérez-Ontiveros JA, Ruiz-Bedolla E,
Saucedo-Ramírez OJ, Villafaña S, Sánchez Muñoz F, Bravo G and Hong
E: Adipokines, asymmetrical dimethylarginine, and pulmonary
function in adolescents with asthma and obesity. J Asthma.
54:153–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ballantyne D, Scott H, MacDonald-Wicks L,
Gibson PG and Wood LG: Resistin is a predictor of asthma risk and
resistin:adiponectin ratio is a negative predictor of lung function
in asthma. Clin Exp Allergy. 46:1056–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gelsinger C, Tschoner A, Kaser S and
Ebenbichler CF: Adipokine update-new molecules, new functions. Wien
Med Wochenschr. 160:377–390. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhu L, Chen X, Chong L, Kong L, Wen S,
Zhang H, Zhang W and Li C: Adiponectin alleviates exacerbation of
airway inflammation and oxidative stress in obesity-related asthma
mice partly through AMPK signaling pathway. Int Immunopharmacol.
67:396–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
de Lima Azambuja R, da Costa Santos
Azambuja LS, Costa C and Rufino R: Adiponectin in asthma and
obesity: Protective agent or risk factor for more severe disease?
Lung. 193:749–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Frederiksen L, Højlund K, Hougaard DM,
Mosbech TH, Larsen R, Flyvbjerg A, Frystyk J, Brixen K and Andersen
M: Testosterone therapy decreases subcutaneous fat and adiponectin
in aging men. Eur J Endocrinol. 166:469–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ma Y, Zhang H, Guo W and Yu L: Potential
role of ghrelin in the regulation of inflammation. FASEB J.
36:e225082022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wiedmer P, Nogueiras R, Broglio F,
D'Alessio D and Tschöp MH: Ghrelin, obesity and diabetes. Nat Clin
Pract Endocrinol Metab. 3:705–712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin- and activation-induced proinflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tschöp M, Weyer C, Tataranni PA,
Devanarayan V, Ravussin E and Heiman ML: Circulating ghrelin levels
are decreased in human obesity. Diabetes. 50:707–709. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lu Y, Van Bever HP, Lim TK, Kuan WS, Goh
DY, Mahadevan M, Sim TB, Ho R, Larbi A and Ng TP: Obesity, asthma
prevalence and IL-4: Roles of inflammatory cytokines, adiponectin
and neuropeptide Y. Pediatr Allergy Immunol. 26:530–536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ito T, Kubo M, Nagaoka K, Funakubo N,
Setiawan H, Takemoto K, Eguchi E, Fujikura Y and Ogino K: Early
obesity leads to increases in hepatic arginase I and related
systemic changes in nitric oxide and L-arginine metabolism in mice.
J Physiol Biochem. 74:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kim HR, Ingram JL and Que LG: Effects of
oxidative stress on airway epithelium permeability in asthma and
potential implications for patients with comorbid obesity. J Asthma
Allergy. 16:481–499. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wiegman CH, Michaeloudes C, Haji G, Narang
P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J,
et al: Oxidative stress-induced mitochondrial dysfunction drives
inflammation and airway smooth muscle remodeling in patients with
chronic obstructive pulmonary disease. J Allergy Clin Immunol.
136:769–780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
de Mello AH, Costa AB, Engel JDG and Rezin
GT: Mitochondrial dysfunction in obesity. Life Sci. 192:26–32.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Singh VP, Aggarwal R, Singh S, Banik A,
Ahmad T, Patnaik BR, Nappanveettil G, Singh KP, Aggarwal ML, Ghosh
B and Agrawal A: Metabolic syndrome is associated with increased
oxo-nitrative stress and asthma-like changes in lungs. PLoS One.
10:e01298502015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Winnica D, Que LG, Baffi C, Grasemann H,
Fiedler K, Yang Z, Etling E, Wasil K, Wenzel SE, Freeman B and
Holguin F: l-Citrulline prevents asymmetric
dimethylarginine-mediated reductions in nitric oxide and
nitrosative stress in primary human airway epithelial cells. Clin
Exp Allergy. 47:190–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Michaeloudes C, Abubakar-Waziri H, Lakhdar
R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK and Chung KF:
Molecular mechanisms of oxidative stress in asthma. Mol Aspects
Med. 85:1010262022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Holguin F, Comhair SA, Hazen SL, Powers
RW, Khatri SS, Bleecker ER, Busse WW, Calhoun WJ, Castro M,
Fitzpatrick AM, et al: An association between L-arginine/asymmetric
dimethyl arginine balance, obesity, and the age of asthma onset
phenotype. Am J Respir Crit Care Med. 187:153–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mishra V, Banga J and Silveyra P:
Oxidative stress and cellular pathways of asthma and inflammation:
Therapeutic strategies and pharmacological targets. Pharmacol Ther.
181:169–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pattnaik B, Bodas M, Bhatraju NK, Ahmad T,
Pant R, Guleria R, Ghosh B and Agrawal A: IL-4 promotes asymmetric
dimethylarginine accumulation, oxo-nitrative stress, and hypoxic
response-induced mitochondrial loss in airway epithelial cells. J
Allergy Clin Immunol. 138:130–141.e9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Grasemann H and Holguin F: Oxidative
stress and obesity-related asthma. Paediatr Respir Rev. 37:18–21.
2021.PubMed/NCBI
|
|
107
|
To M, Kono Y, Ogura N, Mikami S, Honda N,
Hitani A, Kano I, Haruki K and To Y: Obesity-related systemic
oxidative stress: An important factor of poor asthma control.
Allergol Int. 67:147–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nunemaker CS, Chen M, Pei H, Kimble SD,
Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, et al:
12-Lipoxygenase-knockout mice are resistant to inflammatory effects
of obesity induced by Western diet. Am J Physiol Endocrinol Metab.
295:E1065–E1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mabalirajan U, Rehman R, Ahmad T, Kumar S,
Leishangthem GD, Singh S, Dinda AK, Biswal S, Agrawal A and Ghosh
B: 12/15-Lipoxygenase expressed in non-epithelial cells causes
airway epithelial injury in asthma. Sci Rep. 3:15402013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Panda L, Gheware A, Rehman R, Yadav MK,
Jayaraj BS, Madhunapantula SV, Mahesh PA, Ghosh B, Agrawal A and
Mabalirajan U: Linoleic acid metabolite leads to steroid resistant
asthma features partially through NF-κB. Sci Rep. 7:95652017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mabalirajan U, Rehman R, Ahmad T, Kumar S,
Singh S, Leishangthem GD, Aich J, Kumar M, Khanna K, Singh VP, et
al: Linoleic acid metabolite drives severe asthma by causing airway
epithelial injury. Sci Rep. 3:13492013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
da Silva-Santi LG, Antunes MM,
Caparroz-Assef SM, Carbonera F, Masi LN, Curi R, Visentainer JV and
Bazotte RB: Liver fatty acid composition and inflammation in mice
fed with high-carbohydrate diet or high-fat diet. Nutrients.
8:6822016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Deopurkar R, Ghanim H, Friedman J,
Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A and
Dandona P: Differential effects of cream, glucose, and orange juice
on inflammation, endotoxin, and the expression of Toll-like
receptor-4 and suppressor of cytokine signaling-3. Diabetes Care.
33:991–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kien CL, Bunn JY, Fukagawa NK, Anathy V,
Matthews DE, Crain KI, Ebenstein DB, Tarleton EK, Pratley RE and
Poynter ME: Lipidomic evidence that lowering the typical dietary
palmitate to oleate ratio in humans decreases the leukocyte
production of proinflammatory cytokines and muscle expression of
redox-sensitive genes. J Nutr Biochem. 26:1599–1606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ralston JC, Lyons CL, Kennedy EB, Kirwan
AM and Roche HM: Fatty acids and NLRP3 inflammasome-mediated
inflammation in metabolic tissues. Annu Rev Nutr. 37:77–102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tashiro H, Takahashi K, Sadamatsu H, Kato
G, Kurata K, Kimura S and Sueoka-Aragane N: Saturated fatty acid
increases lung macrophages and augments house dust mite-induced
airway inflammation in mice fed with high-fat diet. Inflammation.
40:1072–1086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Trompette A, Gollwitzer ES, Yadava K,
Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod
LP, Harris NL and Marsland BJ: Gut microbiota metabolism of dietary
fiber influences allergic airway disease and hematopoiesis. Nat
Med. 20:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tuomi K and Logomarsino JV: Bacterial
lipopolysaccharide, lipopolysaccharide-binding protein, and other
inflammatory markers in obesity and after bariatric surgery. Metab
Syndr Relat Disord. 14:279–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Belsky DW, Sears MR, Hancox RJ, Harrington
H, Houts R, Moffitt TE, Sugden K, Williams B, Poulton R and Caspi
A: Polygenic risk and the development and course of asthma: an
analysis of data from a four-decade longitudinal study. Lancet
Respir Med. 1:453–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hallstrand TS, Fischer ME, Wurfel MM,
Afari N, Buchwald D and Goldberg J: Genetic pleiotropy between
asthma and obesity in a community-based sample of twins. J Allergy
Clin Immunol. 116:1235–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Danielewicz H: What the genetic background
of individuals with asthma and obesity can reveal: Is β2-adrenergic
receptor gene polymorphism important? Pediatr Allergy Immunol
Pulmonol. 27:104–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Melén E, Himes BE, Brehm JM, Boutaoui N,
Klanderman BJ, Sylvia JS and Lasky-Su J: Analyses of shared genetic
factors between asthma and obesity in children. J Allergy Clin
Immunol. 126:631–637.e1-e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Weiss ST: Obesity: Insight into the
origins of asthma. Nat Immunol. 6:537–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ahangari F, Sood A, Ma B, Takyar S,
Schuyler M, Qualls C, Dela Cruz CS, Chupp GL, Lee CG and Elias JA:
Chitinase 3-like-1 regulates both visceral fat accumulation and
asthma-like Th2 inflammation. Am J Respir Crit Care Med.
191:746–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Murphy A, Tantisira KG, Soto-Quirós ME,
Avila L, Klanderman BJ, Lake S, Weiss ST and Celedón JC: PRKCA: A
positional candidate gene for body mass index and asthma. Am J Hum
Genet. 85:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kuo NW, Tung KY, Tsai CH, Chen YC and Lee
YL: β3-Adrenergic receptor gene modifies the association between
childhood obesity and asthma. J Allergy Clin Immunol.
134:731–733.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Vijayakanthi N, Greally JM and Rastogi D:
Pediatric obesity-related asthma: The role of metabolic
dysregulation. Pediatrics. 137:e201508122016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Potaczek DP, Harb H, Michel S, Alhamwe BA,
Renz H and Tost J: Epigenetics and allergy: From basic mechanisms
to clinical applications. Epigenomics. 9:539–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Rohde K, Keller M, la Cour Poulsen L,
Blüher M, Kovacs P and Böttcher Y: Genetics and epigenetics in
obesity. Metabolism. 92:37–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Palmer DJ, Huang RC, Craig JM and Prescott
SL: Nutritional influences on epigenetic programming: Asthma,
allergy, and obesity. Immunol Allergy Clin North Am. 34:825–837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chatterjee TK, Basford JE, Knoll E, Tong
WS, Blanco V, Blomkalns AL, Rudich S, Lentsch AB, Hui DY and
Weintraub NL: HDAC9 knockout mice are protected from adipose tissue
dysfunction and systemic metabolic disease during high-fat feeding.
Diabetes. 63:176–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Waldecker M, Kautenburger T, Daumann H,
Busch C and Schrenk D: Inhibition of histone-deacetylase activity
by short-chain fatty acids and some polyphenol metabolites formed
in the colon. J Nutr Biochem. 19:587–593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bhavsar P, Ahmad T and Adcock IM: The role
of histone deacetylases in asthma and allergic diseases. J Allergy
Clin Immunol. 121:580–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Rastogi D, Suzuki M and Greally JM:
Differential epigenome-wide DNA methylation patterns in childhood
obesity-associated asthma. Sci Rep. 3:21642013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ather JL, Chung M, Hoyt LR, Randall MJ,
Georgsdottir A, Daphtary NA, Aliyeva MI, Suratt BT, Bates JH, Irvin
CG, et al: Weight loss decreases inherent and allergic methacholine
hyperresponsiveness in mouse models of diet-induced obese asthma.
Am J Respir Cell Mol Biol. 55:176–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
van Huisstede A, Rudolphus A, Castro
Cabezas M, Biter LU, van de Geijn GJ, Taube C, Hiemstra PS and
Braunstahl GJ: Effect of bariatric surgery on asthma control, lung
function and bronchial and systemic inflammation in morbidly obese
subjects with asthma. Thorax. 70:659–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ma J, Strub P, Xiao L, Lavori PW, Camargo
CA Jr, Wilson SR, Gardner CD, Buist AS, Haskell WL and Lv N:
Behavioral weight loss and physical activity intervention in obese
adults with asthma. A randomized trial. Ann Am Thorac Soc. 12:1–11.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim JY, Sohn JH, Lee JH and Park JW:
Obesity increases airway hyperresponsiveness via the TNF-α pathway
and treating obesity induces recovery. PLoS One. 10:e01165402015.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Jensen ME, Gibson PG, Collins CE, Hilton
JM and Wood LG: Diet-induced weight loss in obese children with
asthma: A randomized controlled trial. Clin Exp Allergy.
43:775–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Dias-Júnior SA, Reis M, de Carvalho-Pinto
RM, Stelmach R, Halpern A and Cukier A: Effects of weight loss on
asthma control in obese patients with severe asthma. Eur Respir J.
43:1368–1377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Freitas PD, Ferreira PG, Silva AG,
Stelmach R, Carvalho-Pinto RM, Fernandes FL, Mancini MC, Sato MN,
Martins MA and Carvalho CR: The role of exercise in a weight-loss
program on clinical control in obese adults with asthma. A
randomized controlled trial. Am J Respir Crit Care Med. 195:32–42.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Dixon AE, Pratley RE, Forgione PM,
Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J,
Raymond D, Poynter ME, Bunn JY and Irvin CG: Effects of obesity and
bariatric surgery on airway hyperresponsiveness, asthma control,
and inflammation. J Allergy Clin Immunol. 128:508–515.e1-e2. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kim DY, Park JW, Jeoung D and Ro JY:
Celastrol suppresses allergen-induced airway inflammation in a
mouse allergic asthma model. Eur J Pharmacol. 612:98–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zeng Z, Lin X, Zheng R, Zhang H and Zhang
W: Celastrol alleviates airway hyperresponsiveness and inhibits
Th17 responses in obese asthmatic mice. Front Pharmacol. 9:492018.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Bi P and Kuang S: Notch signaling as a
novel regulator of metabolism. Trends Endocrinol Metab. 26:248–255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang MT, Chiu CJ and Chiang BL:
Multi-faceted notch in allergic airway inflammation. Int J Mol Sci.
20:35082019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zeng Z, Wang L, Ma W, Zheng R, Zhang H,
Zeng X, Zhang H and Zhang W: Inhibiting the Notch signaling pathway
suppresses Th17-associated airway hyperresponsiveness in obese
asthmatic mice. Lab Invest. 99:1784–1794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Nair P, Gaga M, Zervas E, Alagha K,
Hargreave FE, O'Byrne PM, Stryszak P, Gann L, Sadeh J and Chanez P;
Study Investigators, : Safety and efficacy of a CXCR2 antagonist in
patients with severe asthma and sputum neutrophils: A randomized,
placebo-controlled clinical trial. Clin Exp Allergy. 42:1097–1103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
O'Byrne PM, Metev H, Puu M, Richter K,
Keen C, Uddin M, Larsson B, Cullberg M and Nair P: Efficacy and
safety of a CXCR2 antagonist, AZD5069, in patients with
uncontrolled persistent asthma: A randomised, double-blind,
placebo-controlled trial. Lancet Respir Med. 4:797–806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Brusselle GG, Vanderstichele C, Jordens P,
Deman R, Slabbynck H, Ringoet V, Verleden G, Demedts IK, Verhamme
K, Delporte A, et al: Azithromycin for prevention of exacerbations
in severe asthma (AZISAST): A multicentre randomised double-blind
placebo-controlled trial. Thorax. 68:322–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gibson PG, Yang IA, Upham JW, Reynolds PN,
Hodge S, James AL, Jenkins C, Peters MJ, Marks GB, Baraket M, et
al: Effect of azithromycin on asthma exacerbations and quality of
life in adults with persistent uncontrolled asthma (AMAZES): A
randomised, double-blind, placebo-controlled trial. Lancet.
390:659–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hothersall E, McSharry C and Thomson NC:
Potential therapeutic role for statins in respiratory disease.
Thorax. 61:729–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Huang CC, Chan WL, Chen YC, Chen TJ, Chou
KT, Lin SJ, Chen JW and Leu HB: Statin use in patients with asthma:
A nationwide population-based study. Eur J Clin Invest. 41:507–512.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Clark AR: MAP kinase phosphatase 1: A
novel mediator of biological effects of glucocorticoids? J
Endocrinol. 178:5–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dejager L, Dendoncker K, Eggermont M,
Souffriau J, Van Hauwermeiren F, Willart M, Van Wonterghem E,
Naessens T, Ballegeer M, Vandevyver S, et al: Neutralizing TNFα
restores glucocorticoid sensitivity in a mouse model of
neutrophilic airway inflammation. Mucosal Immunol. 8:1212–1225.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Busse WW, Holgate S, Kerwin E, Chon Y,
Feng J, Lin J and Lin SL: Randomized, double-blind,
placebo-controlled study of brodalumab, a human anti-IL-17 receptor
monoclonal antibody, in moderate to severe asthma. Am J Respir Crit
Care Med. 188:1294–1302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wu TD, Brigham EP, Keet CA, Brown TT,
Hansel NN and McCormack MC: Association between
prediabetes/diabetes and asthma exacerbations in a claims-based
obese asthma cohort. J Allergy Clin Immunol Pract. 7:1868–1873.e5.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Peters MC, Mauger D, Ross KR, Phillips B,
Gaston B, Cardet JC, Israel E, Levy BD, Phipatanakul W, Jarjour NN,
et al: Evidence for exacerbation-prone asthma and predictive
biomarkers of exacerbation frequency. Am J Respir Crit Care Med.
202:973–982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wu TD, Keet CA, Fawzy A, Segal JB, Brigham
EP and McCormack MC: Association of metformin initiation and risk
of asthma exacerbation. A claims-based cohort study. Ann Am Thorac
Soc. 16:1527–1533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Szczeklik A, Pietoń R and Sieradzki J:
Alternation in both insulin release and its hypoglycemic effects in
atopic bronchial asthma. J Allergy Clin Immunol. 66:424–427. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Campbell JE and Drucker DJ: Pharmacology,
physiology, and mechanisms of incretin hormone action. Cell Metab.
17:819–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Bloodworth MH, Rusznak M, Pfister CC,
Zhang J, Bastarache L, Calvillo SA, Chappell JD, Boyd KL, Toki S,
Newcomb DC, et al: Glucagon-like peptide 1 receptor signaling
attenuates respiratory syncytial virus-induced type 2 responses and
immunopathology. J Allergy Clin Immunol. 142:683–687.e12. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Toki S, Goleniewska K, Reiss S, Zhang J,
Bloodworth MH, Stier MT, Zhou W, Newcomb DC, Ware LB, Stanwood GD,
et al: Glucagon-like peptide 1 signaling inhibits allergen-induced
lung IL-33 release and reduces group 2 innate lymphoid cell
cytokine production in vivo. J Allergy Clin Immunol.
142:1515–1528.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Foer D, Beeler PE, Cui J, Karlson EW,
Bates DW and Cahill KN: Asthma exacerbations in patients with type
2 diabetes and asthma on glucagon-like peptide-1 receptor agonists.
Am J Respir Crit Care Med. 203:831–840. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Pepper AN, Renz H, Casale TB and Garn H:
Biologic therapy and novel molecular targets of severe asthma. J
Allergy Clin Immunol Pract. 5:909–916. 2017. View Article : Google Scholar : PubMed/NCBI
|