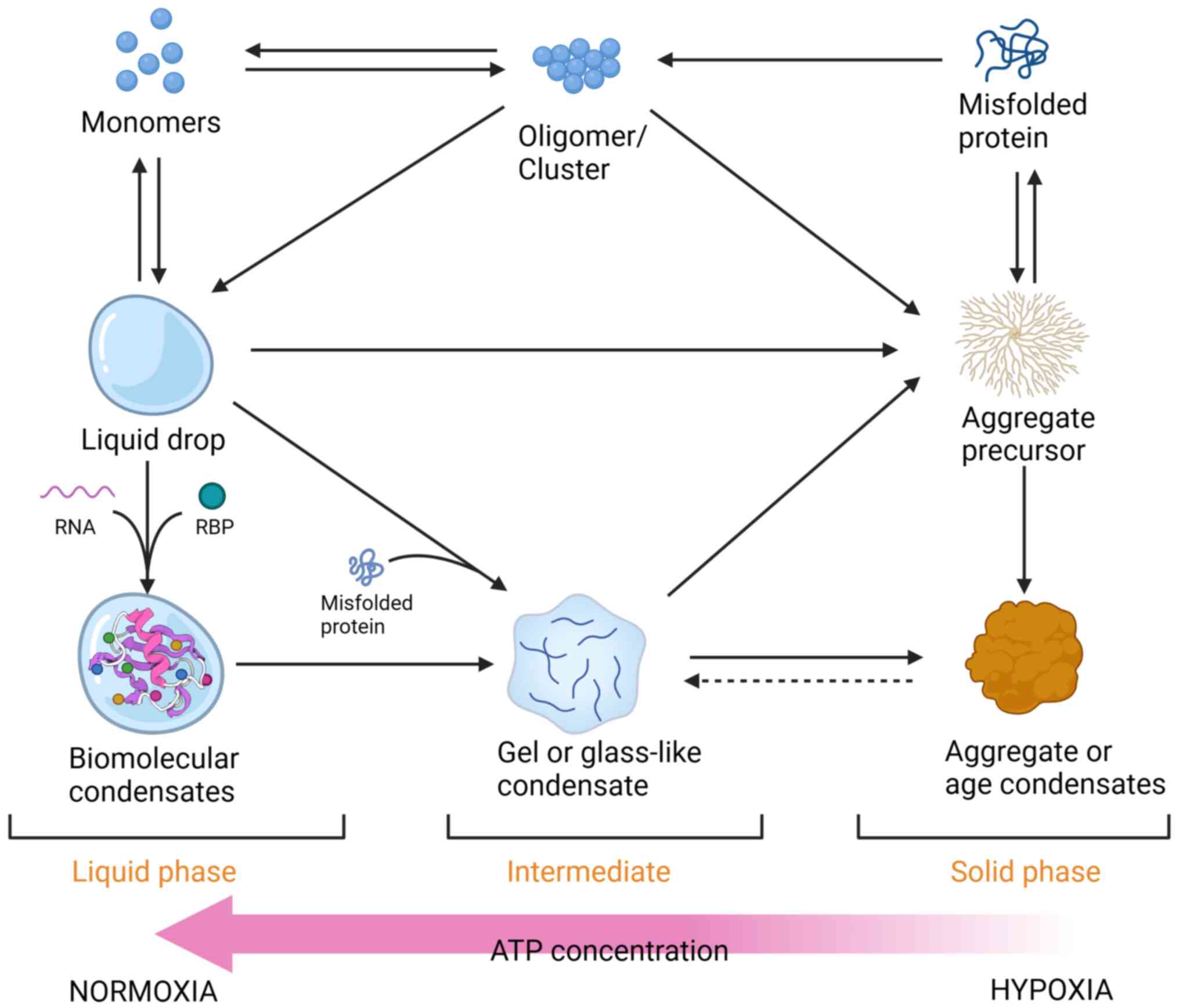
|
1
|
Kuznetsova IM, Turoverov KK and Uversky
VN: What macromolecular crowding can do to a protein. Int J Mol
Sci. 15:23090–23140. 2014.
|
|
2
|
Gaudelet T, Malod-Dognin N and Pržulj N:
Higher-order molecular organization as a source of biological
function. Bioinformatics. 34:i944–i953. 2018.
|
|
3
|
Alberti S and Hyman AA: Biomolecular
condensates at the nexus of cellular stress, protein aggregation
disease and ageing. Nat Rev Mol Cell Biol. 22:196–213. 2021.
|
|
4
|
Savastano A, Flores D, Kadavath H, Biernat
J, Mandelkow E and Zweckstetter M: Disease-associated tau
phosphorylation hinders tubulin assembly within tau condensates.
Angew Chem Int Ed Engl. 60:726–730. 2021.
|
|
5
|
Amzallag E and Hornstein E: Crosstalk
between biomolecular condensates and proteostasis. Cells.
11:24152022.
|
|
6
|
Burtscher J, Mallet RT, Burtscher M and
Millet GP: Hypoxia and brain aging: Neurodegeneration or
neuroprotection? Ageing Res Rev. 68:1013432021.
|
|
7
|
Eltzschig HK and Carmeliet P: Hypoxia and
inflammation. N Engl J Med. 364:656–665. 2011.
|
|
8
|
Schito L and Rey S: Cell-autonomous
metabolic reprogramming in hypoxia. Trends Cell Biol. 28:128–142.
2018.
|
|
9
|
Kaufman DM, Wu X, Scott BA, Itani OA, Van
Gilst MR, Bruce JE and Crowder CM: Ageing and hypoxia cause protein
aggregation in mitochondria. Cell Death Differ. 24:1730–1738.
2017.
|
|
10
|
Dasmeh P and Wagner A: Yeast Proteins may
reversibly aggregate like amphiphilic molecules. J Mol Biol.
434:1673522022.
|
|
11
|
Wilson DM III, Cookson MR, Van Den Bosch
L, Zetterberg H, Holtzman DM and Dewachter I: Hallmarks of
neurodegenerative diseases. Cell. 186:693–714. 2023.
|
|
12
|
Kohler V and Andréasson C: Reversible
protein assemblies in the proteostasis network in health and
disease. Front Mol Biosci. 10:11555212023.
|
|
13
|
Spannl S, Tereshchenko M, Mastromarco GJ,
Ihn SJ and Lee HO: Biomolecular condensates in neurodegeneration
and cancer. Traffic. 20:890–911. 2019.
|
|
14
|
Sun CL, Van Gilst M and Crowder CM:
Hypoxia-induced mitochondrial stress granules. Cell Death Dis.
14:4482023.
|
|
15
|
Jin M, Fuller GG, Han T, Yao Y, Alessi AF,
Freeberg MA, Roach NP, Moresco JJ, Karnovsky A, Baba M, et al:
Glycolytic enzymes coalesce in G bodies under hypoxic stress. Cell
Rep. 20:895–908. 2017.
|
|
16
|
Saito K, Kondo E and Matsushita M:
MicroRNA 130 family regulates the hypoxia response signal through
the P-body protein DDX6. Nucleic Acids Res. 39:6086–6099. 2011.
|
|
17
|
Lee P, Chandel NS and Simon MC: Cellular
adaptation to hypoxia through hypoxia inducible factors and beyond.
Nat Rev Mol Cell Biol. 21:268–283. 2020.
|
|
18
|
Liu C, Gao Y, Barrett J and Hu B:
Autophagy and protein aggregation after brain ischemia. J
Neurochem. 115:68–78. 2010.
|
|
19
|
Hu BR, Martone ME, Jones YZ and Liu CL:
Protein aggregation after transient cerebral ischemia. J Neurosci.
20:3191–3199. 2000.
|
|
20
|
Wouters BG and Koritzinsky M: Hypoxia
signalling through mTOR and the unfolded protein response in
cancer. Nat Rev Cancer. 8:851–864. 2008.
|
|
21
|
Koumenis C and Wouters BG: 'Translating'
tumor hypoxia: Unfolded protein response (UPR)-dependent and
UPR-independent pathways. Mol Cancer Res. 4:423–436. 2006.
|
|
22
|
Gidalevitz T, Prahlad V and Morimoto RI:
The stress of protein misfolding: From single cells to
multicellular organisms. Cold Spring Harb Perspect Biol.
3:a0097042011.
|
|
23
|
Rahman A, Saikia B, Gogoi CR and Baruah A:
Advances in the understanding of protein misfolding and aggregation
through molecular dynamics simulation. Prog Biophys Mol Biol.
175:31–48. 2022.
|
|
24
|
Chiti F and Dobson CM: Protein misfolding,
functional amyloid, and human disease. Annu Rev Biochem.
75:333–366. 2006.
|
|
25
|
Riek R: The three-dimensional structures
of amyloids. Cold Spring Harb Perspect Biol. 9:a0235722017.
|
|
26
|
Balchin D, Hayer-Hartl M and Hartl FU: In
vivo aspects of protein folding and quality control. Science.
353:aac43542016.
|
|
27
|
Korte N, Nortley R and Attwell D: Cerebral
blood flow decrease as an early pathological mechanism in
Alzheimer's disease. Acta Neuropathol. 140:793–810. 2020.
|
|
28
|
Nortley R, Korte N, Izquierdo P,
Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer
T, Khennouf L, Madry C, et al: Amyloid β oligomers constrict human
capillaries in Alzheimer's disease via signaling to pericytes.
Science. 365:eaav95182019.
|
|
29
|
Park SH, Kukushkin Y, Gupta R, Chen T,
Konagai A, Hipp MS, Hayer-Hartl M and Hartl FU: PolyQ proteins
interfere with nuclear degradation of cytosolic proteins by
sequestering the Sis1p chaperone. Cell. 154:134–145. 2013.
|
|
30
|
Heck JW, Cheung SK and Hampton RY:
Cytoplasmic protein quality control degradation mediated by
parallel actions of the E3 ubiquitin ligases Ubr1 and San1. Proc
Natl Acad Sci USA. 107:1106–1111. 2010.
|
|
31
|
Ciechanover A and Kwon YT: Degradation of
misfolded proteins in neurodegenerative diseases: Therapeutic
targets and strategies. Exp Mol Med. 47:e1472015.
|
|
32
|
Rampelt H, Kirstein-Miles J, Nillegoda NB,
Chi K, Scholz SR, Morimoto RI and Bukau B: Metazoan Hsp70 machines
use Hsp110 to power protein disaggregation. EMBO J. 31:4221–4235.
2012.
|
|
33
|
Nillegoda NB, Kirstein J, Szlachcic A,
Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A,
Aebersold R, et al: Crucial HSP70 co-chaperone complex unlocks
metazoan protein disaggregation. Nature. 524:247–251. 2015.
|
|
34
|
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum
U, Hartl FU and Behl C: Protein quality control during aging
involves recruitment of the macroautophagy pathway by BAG3. EMBO J.
28:889–901. 2009.
|
|
35
|
Quintana-Gallardo L, Martín-Benito J,
Marcilla M, Espadas G, Sabidó E and Valpuesta JM: The cochaperone
CHIP marks Hsp70- and Hsp90-bound substrates for degradation
through a very flexible mechanism. Sci Rep. 9:51022019.
|
|
36
|
Nguyen VC, Deck CA and Pamenter ME: Naked
mole-rats reduce the expression of ATP-dependent but not
ATP-independent heat shock proteins in acute hypoxia. J Exp Biol.
222:jeb2112432019.
|
|
37
|
Mitra R, Wu K, Lee C and Bardwell JCA:
ATP-independent chaperones. Annu Rev Biophys. 51:409–429. 2022.
|
|
38
|
Benjamin IJ, Kröger B and Williams RS:
Activation of the heat shock transcription factor by hypoxia in
mammalian cells. Proc Natl Acad Sci USA. 87:6263–6267. 1990.
|
|
39
|
Degrossoli A, Colhone MC, Arrais-Silva WW
and Giorgio S: Hypoxia modulates expression of the 70-kD heat shock
protein and reduces Leishmania infection in macrophages. J Biomed
Sci. 11:847–854. 2004.
|
|
40
|
Hernández R, Blanco S, Peragón J, Pedrosa
JÁ and Peinado MÁ: Hypobaric hypoxia and reoxygenation induce
proteomic profile changes in the rat brain cortex. Neuromolecular
Med. 15:82–94. 2013.
|
|
41
|
Laquatra C, Sanchez-Martin C, Dinarello A,
Cannino G, Minervini G, Moroni E, Schiavone M, Tosatto S, Argenton
F, Colombo G, et al: HIF1α-dependent induction of the mitochondrial
chaperone TRAP1 regulates bioenergetic adaptations to hypoxia. Cell
Death Dis. 12:4342021.
|
|
42
|
Zhang J, Li H, Huang Z, He Y, Zhou X,
Huang T, Dai P, Duan D, Ma X, Yin Q, et al: Hypoxia attenuates
Hsp90 inhibitor 17-DMAG-induced cyclin B1 accumulation in
hepatocellular carcinoma cells. Cell Stress Chaperones. 21:339–348.
2016.
|
|
43
|
Hogg PJ: Disulfide bonds as switches for
protein function. Trends Biochem Sci. 28:210–214. 2003.
|
|
44
|
Braakman I and Hebert DN: Protein folding
in the endoplasmic reticulum. Cold Spring Harb Perspect Biol.
5:a0132012013.
|
|
45
|
Meyer AJ, Riemer J and Rouhier N:
Oxidative protein folding: State-of-the-art and current avenues of
research in plants. New Phytol. 221:1230–1246. 2019.
|
|
46
|
Narayan M: Revisiting the formation of a
native disulfide bond: Consequences for protein regeneration and
beyond. Molecules. 25:53372020.
|
|
47
|
Koritzinsky M, Levitin F, van den Beucken
T, Rumantir RA, Harding NJ, Chu KC, Boutros PC, Braakman I and
Wouters BG: Two phases of disulfide bond formation have differing
requirements for oxygen. J Cell Biol. 203:615–627. 2013.
|
|
48
|
Bulleid NJ: Disulfide bond formation in
the mammalian endoplasmic reticulum. Cold Spring Harb Perspect
Biol. 4:a0132192012.
|
|
49
|
Braakman I and Bulleid NJ: Protein folding
and modification in the mammalian endoplasmic reticulum. Annu Rev
Biochem. 80:71–99. 2011.
|
|
50
|
Saaranen MJ and Ruddock LW: Applications
of catalyzed cytoplasmic disulfide bond formation. Biochem Soc
Trans. 47:1223–1231. 2019.
|
|
51
|
Csordás G, Weaver D and Hajnóczky G:
Endoplasmic reticulum-mitochondrial contactology: Structure and
signaling functions. Trends Cell Biol. 28:523–540. 2018.
|
|
52
|
Shin Y and Brangwynne CP: Liquid phase
condensation in cell physiology and disease. Science.
357:eaaf43822017.
|
|
53
|
Wang M and Kaufman RJ: Protein misfolding
in the endoplasmic reticulum as a conduit to human disease. Nature.
529:326–335. 2016.
|
|
54
|
Hua C, Ju WN, Jin H, Sun X and Zhao G:
Molecular chaperones and hypoxic-ischemic encephalopathy. Neural
Regen Res. 12:153–160. 2017.
|
|
55
|
Gouveia M, Xia K, Colón W, Vieira SI and
Ribeiro F: Protein aggregation, cardiovascular diseases, and
exercise training: Where do we stand? Ageing Res Rev. 40:1–10.
2017.
|
|
56
|
Okada K, Minamino T, Tsukamoto Y, Liao Y,
Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani
T, et al: Prolonged endoplasmic reticulum stress in hypertrophic
and failing heart after aortic constriction: Possible contribution
of endoplasmic reticulum stress to cardiac myocyte apoptosis.
Circulation. 110:705–712. 2004.
|
|
57
|
Tannous P, Zhu H, Nemchenko A, Berry JM,
Johnstone JL, Shelton JM, Miller FJ Jr, Rothermel BA and Hill JA:
Intracellular protein aggregation is a proximal trigger of
cardiomyocyte autophagy. Circulation. 117:3070–3078. 2008.
|
|
58
|
Pattison JS, Sanbe A, Maloyan A, Osinska
H, Klevitsky R and Robbins J: Cardiomyocyte expression of a
polyglutamine preamyloid oligomer causes heart failure.
Circulation. 117:2743–2751. 2008.
|
|
59
|
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M
and Hartl FU: Molecular chaperone functions in protein folding and
proteostasis. Annu Rev Biochem. 82:323–355. 2013.
|
|
60
|
Liang P, Zhang J and Wang B: Emerging
roles of ubiquitination in biomolecular condensates. Cells.
12:23292023.
|
|
61
|
Kaushik S and Cuervo AM: The coming of age
of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 19:365–381.
2018.
|
|
62
|
Park H, Kang JH and Lee S: Autophagy in
neurodegenerative diseases: A hunter for aggregates. Int J Mol Sci.
21:33692020.
|
|
63
|
Deng Z, Purtell K, Lachance V, Wold MS,
Chen S and Yue Z: Autophagy receptors and neurodegenerative
diseases. Trends Cell Biol. 27:491–504. 2017.
|
|
64
|
Menzies FM, Fleming A, Caricasole A, Bento
CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez
Sanchez M, Karabiyik C, et al: Autophagy and neurodegeneration:
Pathogenic mechanisms and therapeutic opportunities. Neuron.
93:1015–1034. 2017.
|
|
65
|
Frake RA, Ricketts T, Menzies FM and
Rubinsztein DC: Autophagy and neurodegeneration. J Clin Invest.
125:65–74. 2015.
|
|
66
|
Lin L, Yang P, Huang X and Zhang H, Lu Q
and Zhang H: The scaffold protein EPG-7 links cargo-receptor
complexes with the autophagic assembly machinery. J Cell Biol.
201:113–129. 2013.
|
|
67
|
Scott SV, Guan J, Hutchins MU, Kim J and
Klionsky DJ: Cvt19 is a receptor for the cytoplasm-to-vacuole
targeting pathway. Mol Cell. 7:1131–1141. 2001.
|
|
68
|
Zhang Y, Yan L, Zhou Z, Yang P, Tian E,
Zhang K, Zhao Y, Li Z, Song B, Han J, et al: SEPA-1 mediates the
specific recognition and degradation of P granule components by
autophagy in C. elegans. Cell. 136:308–321. 2009.
|
|
69
|
Ma X, Lu C, Chen Y, Li S, Ma N, Tao X, Li
Y, Wang J, Zhou M, Yan YB, et al: CCT2 is an aggrephagy receptor
for clearance of solid protein aggregates. Cell. 185:1325–1345.e22.
2022.
|
|
70
|
Cheng S, Huang Z, Jash S, Wu K, Saito S,
Nakashima A and Sharma S: Hypoxia-reoxygenation impairs
autophagy-lysosomal machinery in primary human trophoblasts
mimicking placental pathology of early-onset preeclampsia. Int J
Mol Sci. 23:56442022.
|
|
71
|
de Theije CC, Schols AMWJ, Lamers WH,
Neumann D, Köhler SE and Langen RCJ: Hypoxia impairs adaptation of
skeletal muscle protein turnover- and AMPK signaling during
fasting-induced muscle atrophy. PLoS One. 13:e02036302018.
|
|
72
|
Dao TP and Castañeda CA:
Ubiquitin-modulated phase separation of shuttle proteins: Does
condensate formation promote protein degradation? Bioessays.
42:e20000362020.
|
|
73
|
Cabe M, Rademacher DJ, Karlsson AB,
Cherukuri S and Bakowska JC: PB1 and UBA domains of p62 are
essential for aggresome-like induced structure formation. Biochem
Biophys Res Commun. 503:2306–2311. 2018.
|
|
74
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011.
|
|
75
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006.
|
|
76
|
Karagöz GE, Acosta-Alvear D and Walter P:
The unfolded protein response: detecting and responding to
fluctuations in the protein-folding capacity of the endoplasmic
reticulum. Cold Spring Harb Perspect Biol. 11:a0338862019.
|
|
77
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181. 2018.
|
|
78
|
You K, Wang L, Chou CH, Liu K, Nakata T,
Jaiswal A, Yao J, Lefkovith A, Omar A, Perrigoue JG, et al: QRICH1
dictates the outcome of ER stress through transcriptional control
of proteostasis. Science. 371:eabb68962021.
|
|
79
|
Kopp MC, Larburu N, Durairaj V, Adams CJ
and Ali MMU: UPR proteins IRE1 and PERK switch BiP from chaperone
to ER stress sensor. Nat Struct Mol Biol. 26:1053–1062. 2019.
|
|
80
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.
|
|
81
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000.
|
|
82
|
Ye J, Rawson RB, Komuro R, Chen X, Davé
UP, Prywes R, Brown MS and Goldstein JL: ER stress induces cleavage
of membrane-bound ATF6 by the same proteases that process SREBPs.
Mol Cell. 6:1355–1364. 2000.
|
|
83
|
Haze K, Yoshida H, Yanagi H, Yura T and
Mori K: Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799.
1999.
|
|
84
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
|
|
85
|
Münch C: The different axes of the
mammalian mitochondrial unfolded protein response. BMC Biol.
16:812018.
|
|
86
|
Binet F and Sapieha P: ER stress and
angiogenesis. Cell Metab. 22:560–575. 2015.
|
|
87
|
Sun LL, Chen CM, Zhang J, Wang J, Yang CZ
and Lin LZ: Glucose-regulated protein 78 signaling regulates
hypoxia-induced epithelial-mesenchymal transition in A549 cells.
Front Oncol. 9:1372019.
|
|
88
|
Raiter A, Weiss C, Bechor Z, Ben-Dor I,
Battler A, Kaplan B and Hardy B: Activation of GRP78 on endothelial
cell membranes by an ADAM15-derived peptide induces angiogenesis. J
Vasc Res. 47:399–411. 2010.
|
|
89
|
Wang Y, Alam GN, Ning Y, Visioli F, Dong
Z, Nör JE and Polverini PJ: The unfolded protein response induces
the angiogenic switch in human tumor cells through the PERK/ATF4
pathway. Cancer Res. 72:5396–5406. 2012.
|
|
90
|
Scheuner D, Song B, McEwen E, Liu C,
Laybutt R, Gillespie P, Saunders T, Bonner-Weir S and Kaufman RJ:
Translational control is required for the unfolded protein response
and in vivo glucose homeostasis. Mol Cell. 7:1165–1176. 2001.
|
|
91
|
Liu L, Cash TP, Jones RG, Keith B,
Thompson CB and Simon MC: Hypoxia-induced energy stress regulates
mRNA translation and cell growth. Mol Cell. 21:521–531. 2006.
|
|
92
|
Koumenis C, Naczki C, Koritzinsky M,
Rastani S, Diehl A, Sonenberg N, Koromilas A and Wouters BG:
Regulation of protein synthesis by hypoxia via activation of the
endoplasmic reticulum kinase PERK and phosphorylation of the
translation initiation factor eIF2alpha. Mol Cell Biol.
22:7405–7416. 2002.
|
|
93
|
Dewhirst MW, Cao Y and Moeller B: Cycling
hypoxia and free radicals regulate angiogenesis and radiotherapy
response. Nat Rev Cancer. 8:425–437. 2008.
|
|
94
|
Almendros I, Martínez-García MÁ,
Campos-Rodríguez F, Riveiro-Falkenbach E, Rodríguez-Peralto JL,
Nagore E, Martorell-Calatayud A, Hernández Blasco L, Bañuls Roca J,
Chiner Vives E, et al: Intermittent hypoxia is associated with high
hypoxia inducible factor-1α but not high vascular endothelial
growth factor cell expression in tumors of cutaneous melanoma
patients. Front Neurol. 9:2722018.
|
|
95
|
Yoon DW, So D, Min S, Kim J, Lee M,
Khalmuratova R, Cho CH, Park JW and Shin HW: Accelerated tumor
growth under intermittent hypoxia is associated with
hypoxia-inducible factor-1-dependent adaptive responses to hypoxia.
Oncotarget. 8:61592–61603. 2017.
|
|
96
|
Singleton DC and Harris AL: Targeting the
ATF4 pathway in cancer therapy. Expert Opin Ther Targets.
16:1189–1202. 2012.
|
|
97
|
Rouschop KM, van den Beucken T, Dubois L,
Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W,
Voncken JW, et al: The unfolded protein response protects human
tumor cells during hypoxia through regulation of the autophagy
genes MAP1LC3B and ATG5. J Clin Invest. 120:127–141. 2010.
|
|
98
|
Ye J, Kumanova M, Hart LS, Sloane K, Zhang
H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D and Koumenis
C: The GCN2-ATF4 pathway is critical for tumour cell survival and
proliferation in response to nutrient deprivation. EMBO J.
29:2082–2096. 2010.
|
|
99
|
Mujcic H, Nagelkerke A, Rouschop KM, Chung
S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, et
al: Hypoxic activation of the PERK/eIF2α arm of the unfolded
protein response promotes metastasis through induction of LAMP3.
Clin Cancer Res. 19:6126–6137. 2013.
|
|
100
|
Mudassar F, Shen H, O'Neill G and Hau E:
Targeting tumor hypoxia and mitochondrial metabolism with
anti-parasitic drugs to improve radiation response in high-grade
gliomas. J Exp Clin Cancer Res. 39:2082020.
|
|
101
|
Wheaton WW and Chandel NS: Hypoxia. 2.
Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol.
300:C385–C393. 2011.
|
|
102
|
Garcia-Bermudez J, Baudrier L, La K, Zhu
XG, Fidelin J, Sviderskiy VO, Papagiannakopoulos T, Molina H,
Snuderl M, Lewis CA, et al: Aspartate is a limiting metabolite for
cancer cell proliferation under hypoxia and in tumours. Nat Cell
Biol. 20:775–781. 2018.
|
|
103
|
Thomas LW, Staples O, Turmaine M and
Ashcroft M: CHCHD4 regulates intracellular oxygenation and
perinuclear distribution of mitochondria. Front Oncol.
7:712017.
|
|
104
|
Al-Mehdi AB, Pastukh VM, Swiger BM, Reed
DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF and Gillespie
MN: Perinuclear mitochondrial clustering creates an oxidant-rich
nuclear domain required for hypoxia-induced transcription. Sci
Signal. 5:ra472012.
|
|
105
|
Kim H, Scimia MC, Wilkinson D, Trelles RD,
Wood MR, Bowtell D, Dillin A, Mercola M and Ronai ZA: Fine-tuning
of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial
adaptation to hypoxia. Mol Cell. 44:532–544. 2011.
|
|
106
|
Melber A and Haynes CM: UPRmt
regulation and output: A stress response mediated by
mitochondrial-nuclear communication. Cell Res. 28:281–295.
2018.
|
|
107
|
Peter B, Waddington CL, Oláhová M,
Sommerville EW, Hopton S, Pyle A, Champion M, Ohlson M, Siibak T,
Chrzanow ska-Lightowlers ZMA, et al: Defective mitochondrial
protease LonP1 can cause classical mitochondrial disease. Hum Mol
Genet. 27:1743–1753. 2018.
|
|
108
|
Yan J, Sun CL, Shin S, Van Gilst M and
Crowder CM: Effect of the mitochondrial unfolded protein response
on hypoxic death and mitochondrial protein aggregation. Cell Death
Dis. 12:7112021.
|
|
109
|
Yoneda T, Benedetti C, Urano F, Clark SG,
Harding HP and Ron D: Compartment-specific perturbation of protein
handling activates genes encoding mitochondrial chaperones. J Cell
Sci. 117:4055–4066. 2004.
|
|
110
|
Durieux J, Wolff S and Dillin A: The
cell-non-autonomous nature of electron transport chain-mediated
longevity. Cell. 144:79–91. 2011.
|
|
111
|
Nargund AM, Pellegrino MW, Fiorese CJ,
Baker BM and Haynes CM: Mitochondrial import efficiency of ATFS-1
regulates mitochondrial UPR activation. Science. 337:587–590.
2012.
|
|
112
|
Nargund AM, Fiorese CJ, Pellegrino MW,
Deng P and Haynes CM: Mitochondrial and nuclear accumulation of the
transcription factor ATFS-1 promotes OXPHOS recovery during the
UPR(mt). Mol Cell. 58:123–133. 2015.
|
|
113
|
Fiorese CJ, Schulz AM, Lin YF, Rosin N,
Pellegrino MW and Haynes CM: The transcription factor ATF5 mediates
a mammalian mitochondrial UPR. Curr Biol. 26:2037–2043. 2016.
|
|
114
|
Quirós PM, Prado MA, Zamboni N, D'Amico D,
Williams RW, Finley D, Gygi SP and Auwerx J: Multi-omics analysis
identifies ATF4 as a key regulator of the mitochondrial stress
response in mammals. J Cell Biol. 216:2027–2045. 2017.
|
|
115
|
Michel S, Canonne M, Arnould T and Renard
P: Inhibition of mitochondrial genome expression triggers the
activation of CHOP-10 by a cell signaling dependent on the
integrated stress response but not the mitochondrial unfolded
protein response. Mitochondrion. 21:58–68. 2015.
|
|
116
|
Inigo JR and Chandra D: The mitochondrial
unfolded protein response (UPRmt): Shielding against
toxicity to mitochondria in cancer. J Hematol Oncol. 15:982022.
|
|
117
|
Sutandy FXR, Gößner I, Tascher G and Münch
C: A cytosolic surveillance mechanism activates the mitochondrial
UPR. Nature. 618:849–854. 2023.
|
|
118
|
Anderson NS and Haynes CM: Folding the
mitochondrial UPR into the integrated stress response. Trends Cell
Biol. 30:428–439. 2020.
|
|
119
|
Guo X, Aviles G, Liu Y, Tian R, Unger BA,
Lin YT, Wiita AP, Xu K, Correia MA and Kampmann M: Mitochondrial
stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway.
Nature. 579:427–432. 2020.
|
|
120
|
Alberti S, Gladfelter A and Mittag T:
Considerations and challenges in studying liquid-liquid phase
separation and biomolecular condensates. Cell. 176:419–434.
2019.
|
|
121
|
Banani SF, Lee HO, Hyman AA and Rosen MK:
Biomolecular condensates: Organizers of cellular biochemistry. Nat
Rev Mol Cell Biol. 18:285–298. 2017.
|
|
122
|
Zhang H, Ji X, Li P, Liu C, Lou J, Wang Z,
Wen W, Xiao Y, Zhang M and Zhu X: Liquid-liquid phase separation in
biology: Mechanisms, physiological functions and human diseases.
Sci China Life Sci. 63:953–985. 2020.
|
|
123
|
Hirose T, Ninomiya K, Nakagawa S and
Yamazaki T: A guide to membraneless organelles and their various
roles in gene regulation. Nat Rev Mol Cell Biol. 24:288–304.
2023.
|
|
124
|
Brangwynne CP, Eckmann CR, Courson DS,
Rybarska A, Hoege C, Gharakhani J, Jülicher F and Hyman AA:
Germline P granules are liquid droplets that localize by controlled
dissolution/condensation. Science. 324:1729–1732. 2009.
|
|
125
|
Kim J, Lee H, Lee HG and Seo PJ: Get
closer and make hotspots: Liquid-liquid phase separation in plants.
EMBO Rep. 22:e516562021.
|
|
126
|
Alberti S, Saha S, Woodruff JB, Franzmann
TM, Wang J and Hyman AA: A user's guide for phase separation assays
with purified proteins. J Mol Biol. 430:4806–4820. 2018.
|
|
127
|
Shrinivas K and Brenner MP: Phase
separation in fluids with many interacting components. Proc Natl
Acad Sci USA. 118:e21085511182021.
|
|
128
|
Galves M, Rathi R, Prag G and Ashkenazi A:
Ubiquitin signaling and degradation of aggregate-prone proteins.
Trends Biochem Sci. 44:872–884. 2019.
|
|
129
|
Snead WT and Gladfelter AS: The control
centers of biomolecular phase separation: How membrane surfaces,
PTMs, and active processes regulate condensation. Mol Cell.
76:295–305. 2019.
|
|
130
|
Sanchez-Burgos I, Espinosa JR, Joseph JA
and Collepardo-Guevara R: Valency and binding affinity variations
can regulate the multilayered organization of protein condensates
with many components. Biomolecules. 11:2782021.
|
|
131
|
Jain S, Wheeler JR, Walters RW, Agrawal A,
Barsic A and Parker R: ATPase-modulated stress granules contain a
diverse proteome and substructure. Cell. 164:487–498. 2016.
|
|
132
|
Hipp MS, Kasturi P and Hartl FU: The
proteostasis network and its decline in ageing. Nat Rev Mol Cell
Biol. 20:421–435. 2019.
|
|
133
|
Case LB, Zhang X, Ditlev JA and Rosen MK:
Stoichiometry controls activity of phase-separated clusters of
actin signaling proteins. Science. 363:1093–1097. 2019.
|
|
134
|
Franzmann TM, Jahnel M, Pozniakovsky A,
Mahamid J, Holehouse AS, Nüske E, Richter D, Baumeister W, Grill
SW, Pappu RV, et al: Phase separation of a yeast prion protein
promotes cellular fitness. Science. 359:eaao56542018.
|
|
135
|
Klosin A, Oltsch F, Harmon T, Honigmann A,
Jülicher F, Hyman AA and Zechner C: Phase separation provides a
mechanism to reduce noise in cells. Science. 367:464–468. 2020.
|
|
136
|
Riback JA, Katanski CD, Kear-Scott JL,
Pilipenko EV, Rojek AE, Sosnick TR and Drummond DA:
Stress-triggered phase separation is an adaptive, evolutionarily
tuned response. Cell. 168:1028–1040.e19. 2017.
|
|
137
|
Shin Y, Chang YC, Lee DSW, Berry J,
Sanders DW, Ronceray P, Wingreen NS, Haataja M and Brangwynne CP:
Liquid nuclear condensates mechanically sense and restructure the
genome. Cell. 175:1481–1491.e13. 2018.
|
|
138
|
Spector DL: SnapShot: Cellular bodies.
Cell. 127:10712006.
|
|
139
|
Protter DSW and Parker R: Principles and
properties of stress granules. Trends Cell Biol. 26:668–679.
2016.
|
|
140
|
Damgaard CK and Lykke-Andersen J:
Translational coregulation of 5'TOP mRNAs by TIA-1 and TIAR. Genes
Dev. 25:2057–2068. 2011.
|
|
141
|
Gwon Y, Maxwell BA, Kolaitis RM, Zhang P,
Kim HJ and Taylor JP: Ubiquitination of G3BP1 mediates stress
granule disassembly in a context-specific manner. Science.
372:eabf65482021.
|
|
142
|
Yang P, Mathieu C, Kolaitis RM, Zhang P,
Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, et al: G3BP1 is
a tunable switch that triggers phase separation to assemble stress
granules. Cell. 181:325–345.e28. 2020.
|
|
143
|
Bartoszewska S and Collawn JF: Unfolded
protein response (UPR) integrated signaling networks determine cell
fate during hypoxia. Cell Mol Biol Lett. 25:182020.
|
|
144
|
Donnelly N, Gorman AM, Gupta S and Samali
A: The eIF2α kinases: Their structures and functions. Cell Mol Life
Sci. 70:3493–3511. 2013.
|
|
145
|
Wek RC, Jiang HY and Anthony TG: Coping
with stress: eIF2 kinases and translational control. Biochem Soc
Trans. 34:7–11. 2006.
|
|
146
|
Beilsten-Edmands V, Gordiyenko Y, Kung JC,
Mohammed S, Schmidt C and Robinson CV: eIF2 interactions with
initiator tRNA and eIF2B are regulated by post-translational
modifications and conformational dynamics. Cell Discov.
1:150202015.
|
|
147
|
Kedersha N and Anderson P: Stress
granules: Sites of mRNA triage that regulate mRNA stability and
translatability. Biochem Soc Trans. 30:963–969. 2002.
|
|
148
|
Kedersha N, Chen S, Gilks N, Li W, Miller
IJ, Stahl J and Anderson P: Evidence that ternary complex
(eIF2-GTP-tRNA(i) (Met))-deficient preinitiation complexes are core
constituents of mammalian stress granules. Mol Biol Cell.
13:195–210. 2002.
|
|
149
|
Anderson P and Kedersha N: Stressful
initiations. J Cell Sci. 115:3227–3234. 2002.
|
|
150
|
Anderson P and Kedersha N: Stress
granules: The tao of RNA triage. Trends Biochem Sci. 33:141–150.
2008.
|
|
151
|
Darnell AM, Subramaniam AR and O'Shea EK:
Translational control through differential ribosome pausing during
amino acid limitation in mammalian cells. Mol Cell. 71:229–243.e11.
2018.
|
|
152
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V, Tsogka K, Sounidaki M and Stefanidis I: Differential
effects of the two amino acid sensing systems, the GCN2 kinase and
the mTOR complex 1, on primary human alloreactive CD4+
T-cells. Int J Mol Med. 37:1412–1420. 2016.
|
|
153
|
Longchamp A, Mirabella T, Arduini A,
MacArthur MR, Das A, Treviño-Villarreal JH, Hine C, Ben-Sahra I,
Knudsen NH, Brace LE, et al: Amino acid restriction triggers
angiogenesis via GCN2/ATF4 regulation of VEGF and H2S
production. Cell. 173:117–129.e14. 2018.
|
|
154
|
Liu Y, László C, Liu Y, Liu W, Chen X,
Evans SC and Wu S: Regulation of G(1) arrest and apoptosis in
hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation.
Neoplasia. 12:61–68. 2010.
|
|
155
|
Miar A, Arnaiz E, Bridges E, Beedie S,
Cribbs AP, Downes DJ, Beagrie RA, Rehwinkel J and Harris AL:
Hypoxia induces transcriptional and translational downregulation of
the type I IFN pathway in multiple cancer cell types. Cancer Res.
80:5245–5256. 2020.
|
|
156
|
Eiermann N, Haneke K, Sun Z, Stoecklin G
and Ruggieri A: Dance with the Devil: Stress granules and signaling
in antiviral responses. Viruses. 12:9842020.
|
|
157
|
Takahashi M, Higuchi M, Matsuki H, Yoshita
M, Ohsawa T, Oie M and Fujii M: Stress granules inhibit apoptosis
by reducing reactive oxygen species production. Mol Cell Biol.
33:815–829. 2013.
|
|
158
|
Lee AK, Klein J, Fon Tacer K, Lord T,
Oatley MJ, Oatley JM, Porter SN, Pruett-Miller SM, Tikhonova EB,
Karamyshev AL, et al: Translational repression of G3BP in cancer
and germ cells suppresses stress granules and enhances stress
tolerance. Mol Cell. 79:645–659.e9. 2020.
|
|
159
|
Timalsina S, Arimoto-Matsuzaki K, Kitamura
M, Xu X, Wenzhe Q, Ishigami-Yuasa M, Kagechika H and Hata Y:
Chemical compounds that suppress hypoxia-induced stress granule
formation enhance cancer drug sensitivity of human cervical cancer
HeLa cells. J Biochem. 164:381–391. 2018.
|
|
160
|
Attwood KM, Robichaud A, Westhaver LP,
Castle EL, Brandman DM, Balgi AD, Roberge M, Colp P, Croul S, Kim
I, et al: Raloxifene prevents stress granule dissolution, impairs
translational control and promotes cell death during hypoxia in
glioblastoma cells. Cell Death Dis. 11:9892020.
|
|
161
|
Liu Y, Liu Y, He Y, Zhang N, Zhang S, Li
Y, Wang X, Liang Y, Chen X, Zhao W, et al: Hypoxia-induced
FUS-circTBC1D14 stress granules promote autophagy in TNBC. Adv Sci
(Weinh). 10:e22049882023.
|
|
162
|
Li WY, Yang F, Li X, Wang LW and Wang Y:
Stress granules inhibit endoplasmic reticulum stress-mediated
apoptosis during hypoxia-induced injury in acute liver failure.
World J Gastroenterol. 29:1315–1329. 2023.
|
|
163
|
Hu L, Mao S, Lin L, Bai G, Liu B and Mao
J: Stress granules in the spinal muscular atrophy and amyotrophic
lateral sclerosis: The correlation and promising therapy. Neurobiol
Dis. 170:1057492022.
|
|
164
|
Youn JY, Dyakov BJA, Zhang J, Knight JDR,
Vernon RM, Forman-Kay JD and Gingras AC: Properties of stress
granule and P-body proteomes. Mol Cell. 76:286–294. 2019.
|
|
165
|
Kedersha N, Stoecklin G, Ayodele M, Yacono
P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE
and Anderson P: Stress granules and processing bodies are
dynamically linked sites of mRNP remodeling. J Cell Biol.
169:871–884. 2005.
|
|
166
|
Moon SL, Morisaki T, Khong A, Lyon K,
Parker R and Stasevich TJ: Multicolour single-molecule tracking of
mRNA interactions with RNP granules. Nat Cell Biol. 21:162–168.
2019.
|
|
167
|
Luo Y, Na Z and Slavoff SA: P-bodies:
Composition, properties, and functions. Biochemistry. 57:2424–2431.
2018.
|
|
168
|
Lee JI and Namkoong S: Stress granules
dynamics: Benefits in cancer. BMB Rep. 55:577–586. 2022.
|
|
169
|
Jud MC, Czerwinski MJ, Wood MP, Young RA,
Gallo CM, Bickel JS, Petty EL, Mason JM, Little BA, Padilla PA and
Schisa JA: Large P body-like RNPs form in C. elegans oocytes
in response to arrested ovulation, heat shock, osmotic stress, and
anoxia and are regulated by the major sperm protein pathway. Dev
Biol. 318:38–51. 2008.
|
|
170
|
Bett JS, Ibrahim AF, Garg AK, Kelly V,
Pedrioli P, Rocha S and Hay RT: The P-body component USP52/PAN2 is
a novel regulator of HIF1A mRNA stability. Biochem J. 451:185–194.
2013.
|
|
171
|
Carbonaro M, O'Brate A and Giannakakou P:
Microtubule disruption targets HIF-1alpha mRNA to cytoplasmic
P-bodies for translational repression. J Cell Biol. 192:83–99.
2011.
|
|
172
|
Gutierrez G: Cellular energy metabolism
during hypoxia. Crit Care Med. 19:619–626. 1991.
|
|
173
|
Hollinshead KE and Tennant DA:
Mitochondrial metabolic remodeling in response to genetic and
environmental perturbations. Wiley Interdiscip Rev Syst Biol Med.
8:272–285. 2016.
|
|
174
|
Newsholme EA and Start C: Regulation in
metabolism. John Wiley and Sons; New York and London: pp.
3491973
|
|
175
|
TeSlaa T, Bartman CR, Jankowski CSR, Zhang
Z, Xu X, Xing X, Wang L, Lu W, Hui S and Rabinowitz JD: The source
of glycolytic intermediates in mammalian tissues. Cell Metab.
33:367–378.e5. 2021.
|
|
176
|
Miura N, Shinohara M, Tatsukami Y, Sato Y,
Morisaka H, Kuroda K and Ueda M: Spatial reorganization of
Saccharomyces cerevisiae enolase to alter carbon metabolism under
hypoxia. Eukaryot Cell. 12:1106–1119. 2013.
|
|
177
|
Jang S, Nelson JC, Bend EG,
Rodríguez-Laureano L, Tueros FG, Cartagenova L, Underwood K,
Jorgensen EM and Colón-Ramos DA: Glycolytic enzymes localize to
synapses under energy stress to support synaptic function. Neuron.
90:278–291. 2016.
|
|
178
|
Webb BA, Dosey AM, Wittmann T, Kollman JM
and Barber DL: The glycolytic enzyme phosphofructokinase-1
assembles into filaments. J Cell Biol. 216:2305–2313. 2017.
|
|
179
|
Narayanaswamy R, Levy M, Tsechansky M,
Stovall GM, O'Connell JD, Mirrielees J, Ellington AD and Marcotte
EM: Widespread reorganization of metabolic enzymes into reversible
assemblies upon nutrient starvation. Proc Natl Acad Sci USA.
106:10147–10152. 2009.
|
|
180
|
Saad S, Cereghetti G, Feng Y, Picotti P,
Peter M and Dechant R: Reversible protein aggregation is a
protective mechanism to ensure cell cycle restart after stress. Nat
Cell Biol. 19:1202–1213. 2017.
|
|
181
|
Kohnhorst CL, Kyoung M, Jeon M, Schmitt
DL, Kennedy EL, Ramirez J, Bracey SM, Luu BT, Russell SJ and An S:
Identification of a multienzyme complex for glucose metabolism in
living cells. J Biol Chem. 292:9191–9203. 2017.
|
|
182
|
Fuller GG, Han T, Freeberg MA, Moresco JJ,
Ghanbari Niaki A, Roach NP, Yates JR III, Myong S and Kim JK: RNA
promotes phase separation of glycolysis enzymes into yeast G bodies
in hypoxia. Elife. 9:e484802020.
|
|
183
|
Yoshimura Y, Hirayama R, Miura N, Utsumi
R, Kuroda K, Ueda M and Kataoka M: Small-scale hypoxic cultures for
monitoring the spatial reorganization of glycolytic enzymes in
Saccharomyces cerevisiae. Cell Biol Int. 45:1776–1783. 2021.
|
|
184
|
Fuller GG and Kim JK: Compartmentalization
and metabolic regulation of glycolysis. J Cell Sci.
134:jcs2584692021.
|
|
185
|
Lu H, Gao Z, Zhao Z, Weng J and Ye J:
Transient hypoxia reprograms differentiating adipocytes for
enhanced insulin sensitivity and triglyceride accumulation. Int J
Obes (Lond). 40:121–128. 2016.
|
|
186
|
Gordon GB, Barcza MA and Bush ME: Lipid
accumulation of hypoxic tissue culture cells. Am J Pathol.
88:663–678. 1977.
|
|
187
|
Gross DA and Silver DL: Cytosolic lipid
droplets: from mechanisms of fat storage to disease. Crit Rev
Biochem Mol Biol. 49:304–326. 2014.
|
|
188
|
Lass A, Zimmermann R, Oberer M and Zechner
R: Lipolysis-a highly regulated multi-enzyme complex mediates the
catabolism of cellular fat stores. Prog Lipid Res. 50:14–27.
2011.
|
|
189
|
Farese RV Jr and Walther TC: Lipid
droplets finally get a little R-E-S-P-E-C-T. Cell. 139:855–860.
2009.
|
|
190
|
Thiam AR and Ikonen E: Lipid droplet
nucleation. Trends Cell Biol. 31:108–118. 2021.
|
|
191
|
Walther TC, Chung J and Farese RV Jr:
Lipid droplet biogenesis. Annu Rev Cell Dev Biol. 33:491–510.
2017.
|
|
192
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019.
|
|
193
|
Santinho A, Salo VT, Chorlay A, Li S, Zhou
X, Omrane M, Ikonen E and Thiam AR: Membrane curvature catalyzes
lipid droplet assembly. Curr Biol. 30:2481–2494.e6. 2020.
|
|
194
|
Zoni V, Khaddaj R, Campomanes P, Thiam AR,
Schneiter R and Vanni S: Lipid droplet biogenesis is driven by
liquid-liquid phase separation. bioRxiv. 7774662020.
|
|
195
|
Walther TC and Farese RV Jr: Lipid
droplets and cellular lipid metabolism. Annu Rev Biochem.
81:687–714. 2012.
|
|
196
|
Ward PS and Thompson CB: Signaling in
control of cell growth and metabolism. Cold Spring Harb Perspect
Biol. 4:a0067832012.
|
|
197
|
Baenke F, Peck B, Miess H and Schulze A:
Hooked on fat: The role of lipid synthesis in cancer metabolism and
tumour development. Dis Model Mech. 6:1353–1363. 2013.
|
|
198
|
Koizume S and Miyagi Y: Lipid droplets: A
key cellular organelle associated with cancer cell survival under
normoxia and hypoxia. Int J Mol Sci. 17:14302016.
|
|
199
|
Qiu B, Ackerman D, Sanchez DJ, Li B,
Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B
and Simon MC: HIF2α-dependent lipid storage promotes endoplasmic
reticulum homeostasis in clear-cell renal cell carcinoma. Cancer
Discov. 5:652–667. 2015.
|
|
200
|
Bailey AP, Koster G, Guillermier C, Hirst
EM, MacRae JI, Lechene CP, Postle AD and Gould AP: Antioxidant role
for lipid droplets in a stem cell niche of Drosophila. Cell.
163:340–353. 2015.
|
|
201
|
Rysman E, Brusselmans K, Scheys K,
Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D,
Daniëls VW, Machiels J, et al: De novo lipogenesis protects cancer
cells from free radicals and chemotherapeutics by promoting
membrane lipid saturation. Cancer Res. 70:8117–8126. 2010.
|
|
202
|
de la Rosa Rodriguez MA and Kersten S:
Regulation of lipid droplet homeostasis by hypoxia inducible lipid
droplet associated HILPDA. Biochim Biophys Acta Mol Cell Biol
Lipids. 1865:1587382020.
|
|
203
|
de la Rosa Rodriguez MA, Deng L, Gemmink
A, van Weeghel M, Aoun ML, Warnecke C, Singh R, Borst JW and
Kersten S: Hypoxia-inducible lipid droplet-associated induces DGAT1
and promotes lipid storage in hepatocytes. Mol Metab.
47:1011682021.
|
|
204
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012.
|
|
205
|
Watts ER and Walmsley SR: Inflammation and
hypoxia: HIF and PHD isoform selectivity. Trends Mol Med. 25:33–46.
2019.
|
|
206
|
Willson JA, Arienti S, Sadiku P, Reyes L,
Coelho P, Morrison T, Rinaldi G, Dockrell DH, Whyte MKB and
Walmsley SR: Neutrophil HIF-1α stabilization is augmented by
mitochondrial ROS produced via the glycerol 3-phosphate shuttle.
Blood. 139:281–286. 2022.
|
|
207
|
Chandel NS, McClintock DS, Feliciano CE,
Wood TM, Melendez JA, Rodriguez AM and Schumacker PT: Reactive
oxygen species generated at mitochondrial complex III stabilize
hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2
sensing. J Biol Chem. 275:25130–25138. 2000.
|
|
208
|
Hopfer U, Hopfer H, Jablonski K, Stahl RA
and Wolf G: The novel WD-repeat protein Morg1 acts as a molecular
scaffold for hypoxia-inducible factor prolyl hydroxylase 3 (PHD3).
J Biol Chem. 281:8645–8655. 2006.
|
|
209
|
Wong BW, Kuchnio A, Bruning U and
Carmeliet P: Emerging novel functions of the oxygen-sensing prolyl
hydroxylase domain enzymes. Trends Biochem Sci. 38:3–11. 2013.
|
|
210
|
Rantanen K, Pursiheimo J, Högel H, Himanen
V, Metzen E and Jaakkola PM: Prolyl hydroxylase PHD3 activates
oxygen-dependent protein aggregation. Mol Biol Cell. 19:2231–2240.
2008.
|
|
211
|
Theodoridis PR, Bokros M, Marijan D,
Balukoff NC, Wang D, Kirk CC, Budine TD, Goldsmith HD, Wang M,
Audas TE and Lee S: Local translation in nuclear condensate amyloid
bodies. Proc Natl Acad Sci USA. 118:e20144571182021.
|
|
212
|
Wang M, Tao X, Jacob MD, Bennett CA, Ho
JJD, Gonzalgo ML, Audas TE and Lee S: Stress-induced low complexity
RNA activates physiological amyloidogenesis. Cell Rep.
24:1713–1721.e4. 2018.
|
|
213
|
Standart N and Weil D: P-bodies: Cytosolic
droplets for coordinated mRNA storage. Trends Genet. 34:612–626.
2018.
|
|
214
|
Majerciak V, Zhou T, Kruhlak MJ and Zheng
ZM: RNA helicase DDX6 and scaffold protein GW182 in P-bodies
promote biogenesis of stress granules. Nucleic Acids Res.
51:9337–9355. 2023.
|
|
215
|
Hallacli E, Kayatekin C, Nazeen S, Wang
XH, Sheinkopf Z, Sathyakumar S, Sarkar S, Jiang X, Dong X, Di Maio
R, et al: The Parkinson's disease protein alpha-synuclein is a
modulator of processing bodies and mRNA stability. Cell.
185:2035–2056.e33. 2022.
|
|
216
|
Loll-Krippleber R and Brown GW: P-body
proteins regulate transcriptional rewiring to promote DNA
replication stress resistance. Nat Commun. 8:5582017.
|
|
217
|
Lavalée M, Curdy N, Laurent C, Fournié JJ
and Franchini DM: Cancer cell adaptability: Turning
ribonucleoprotein granules into targets. Trends Cancer. 7:902–915.
2021.
|
|
218
|
Tsai WC and Lloyd RE: Cytoplasmic RNA
granules and viral infection. Annu Rev Virol. 1:147–170. 2014.
|
|
219
|
Bargiela D, Burr SP and Chinnery PF:
Mitochondria and hypoxia: Metabolic crosstalk in cell-fate
decisions. Trends Endocrinol Metab. 29:249–259. 2018.
|
|
220
|
Taylor CT and Moncada S: Nitric oxide,
cytochrome C oxidase, and the cellular response to hypoxia.
Arterioscler Thromb Vasc Biol. 30:643–647. 2010.
|
|
221
|
Sathyanarayanan U, Musa M, Bou Dib P,
Raimundo N, Milosevic I and Krisko A: ATP hydrolysis by yeast
Hsp104 determines protein aggregate dissolution and size in vivo.
Nat Commun. 11:52262020.
|
|
222
|
Torrente MP and Shorter J: The metazoan
protein disaggregase and amyloid depolymerase system: Hsp110,
Hsp70, Hsp40, and small heat shock proteins. Prion. 7:457–463.
2013.
|
|
223
|
Jakobson CM and Jarosz DF: Metabolites
control stress granule disassembly. Nat Cell Biol. 23:1053–1055.
2021.
|
|
224
|
Grignaschi E, Cereghetti G, Grigolato F,
Kopp MRG, Caimi S, Faltova L, Saad S, Peter M and Arosio P: A
hydrophobic low-complexity region regulates aggregation of the
yeast pyruvate kinase Cdc19 into amyloid-like aggregates in vitro.
J Biol Chem. 293:11424–11432. 2018.
|
|
225
|
Cereghetti G, Wilson-Zbinden C, Kissling
VM, Diether M, Arm A, Yoo H, Piazza I, Saad S, Picotti P, Drummond
DA, et al: Reversible amyloids of pyruvate kinase couple cell
metabolism and stress granule disassembly. Nat Cell Biol.
23:1085–1094. 2021.
|
|
226
|
Haslbeck M, Miess A, Stromer T, Walter S
and Buchner J: Disassembling protein aggregates in the yeast
cytosol. The cooperation of Hsp26 with Ssa1 and Hsp104. J Biol
Chem. 280:23861–23868. 2005.
|
|
227
|
Glover JR and Lindquist S: Hsp104, Hsp70,
and Hsp40: A novel chaperone system that rescues previously
aggregated proteins. Cell. 94:73–82. 1998.
|
|
228
|
Cherkasov V, Hofmann S, Druffel-Augustin
S, Mogk A, Tyedmers J, Stoecklin G and Bukau B: Coordination of
translational control and protein homeostasis during severe heat
stress. Curr Biol. 23:2452–2462. 2013.
|
|
229
|
Kobayashi S and Welsh FA: Regional
alterations of ATP and heat-shock protein-72 mRNA following
hypoxia-ischemia in neonatal rat brain. J Cereb Blood Flow Metab.
15:1047–1056. 1995.
|
|
230
|
Oh DJ, Yu SH and Kang ET: Heat shock
protein expression in adenosine triphosphate depleted renal
epithelial cells. Korean J Intern Med. 19:149–154. 2004.
|
|
231
|
Gupta S and Knowlton AA: Cytosolic heat
shock protein 60, hypoxia, and apoptosis. Circulation.
106:2727–2733. 2002.
|
|
232
|
Eastoe J, Hatzopoulos MH and Dowding PJ:
Action of hydrotropes and alkyl-hydrotropes. Soft Matter.
7:5917–5925. 2011.
|
|
233
|
Subbarao CV, Chakravarthy IPK, Sai
Bharadwaj AVSL and Prasad KMM: Functions of hydrotropes in
solutions. Chem Eng Technol. 35:225–237. 2012.
|
|
234
|
Patel A, Malinovska L, Saha S, Wang J,
Alberti S, Krishnan Y and Hyman AA: ATP as a biological hydrotrope.
Science. 356:753–756. 2017.
|
|
235
|
Patel A, Lee HO, Jawerth L, Maharana S,
Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et
al: A liquid-to-solid phase transition of the ALS protein FUS
accelerated by disease mutation. Cell. 162:1066–1077. 2015.
|
|
236
|
Wegmann S, Eftekharzadeh B, Tepper K,
Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D,
Kamath T, Commins C, et al: Tau protein liquid-liquid phase
separation can initiate tau aggregation. EMBO J. 37:e980492018.
|
|
237
|
Ray S, Singh N, Kumar R, Patel K, Pandey
S, Datta D, Mahato J, Panigrahi R, Navalkar A, Mehra S, et al:
α-Synuclein aggregation nucleates through liquid-liquid phase
separation. Nat Chem. 12:705–716. 2020.
|
|
238
|
Hughes MP, Sawaya MR, Boyer DR,
Goldschmidt L, Rodriguez JA, Cascio D, Chong L, Gonen T and
Eisenberg DS: Atomic structures of low-complexity protein segments
reveal kinked β sheets that assemble networks. Science.
359:698–701. 2018.
|
|
239
|
Luo F, Gui X, Zhou H, Gu J, Li Y, Liu X,
Zhao M, Li D, Li X and Liu C: Atomic structures of FUS LC domain
segments reveal bases for reversible amyloid fibril formation. Nat
Struct Mol Biol. 25:341–346. 2018.
|
|
240
|
Alberti S and Hyman AA: Are aberrant phase
transitions a driver of cellular aging? Bioessays. 38:959–968.
2016.
|
|
241
|
Harmon TS, Holehouse AS, Rosen MK and
Pappu RV: Intrinsically disordered linkers determine the interplay
between phase separation and gelation in multivalent proteins.
Elife. 6:e302942017.
|
|
242
|
Nakauchi Y, Nishinami S and Shiraki K:
Glass-like protein condensate for the long-term storage of
proteins. Int J Biol Macromol. 182:162–167. 2021.
|
|
243
|
Sadati M, Nourhani A, Fredberg JJ and
Taheri Qazvini N: Glass-like dynamics in the cell and in cellular
collectives. Wiley Interdiscip Rev Syst Biol Med. 6:137–149.
2014.
|
|
244
|
Parry BR, Surovtsev IV, Cabeen MT, O'Hern
CS, Dufresne ER and Jacobs-Wagner C: The bacterial cytoplasm has
glass-like properties and is fluidized by metabolic activity. Cell.
156:183–194. 2014.
|
|
245
|
Iadanza MG, Jackson MP, Hewitt EW, Ranson
NA and Radford SE: A new era for understanding amyloid structures
and disease. Nat Rev Mol Cell Biol. 19:755–773. 2018.
|
|
246
|
Choi JM, Holehouse AS and Pappu RV:
Physical principles underlying the complex biology of intracellular
phase transitions. Annu Rev Biophys. 49:107–133. 2020.
|
|
247
|
Roberts S, Dzuricky M and Chilkoti A:
Elastin-like polypeptides as models of intrinsically disordered
proteins. FEBS Lett. 589:2477–2486. 2015.
|
|
248
|
Garaizar A, Espinosa JR, Joseph JA,
Krainer G, Shen Y, Knowles TPJ and Collepardo-Guevara R: Aging can
transform single-component protein condensates into multiphase
architectures. Proc Natl Acad Sci USA. 119:e21198001192022.
|
|
249
|
Falahati H and Wieschaus E: Independent
active and thermodynamic processes govern the nucleolus assembly in
vivo. Proc Natl Acad Sci USA. 114:1335–1340. 2017.
|
|
250
|
Eisele YS, Monteiro C, Fearns C, Encalada
SE, Wiseman RL, Powers ET and Kelly JW: Targeting protein
aggregation for the treatment of degenerative diseases. Nat Rev
Drug Discov. 14:759–780. 2015.
|
|
251
|
Wilson MR and Zoubeidi A: Clusterin as a
therapeutic target. Expert Opin Ther Targets. 21:201–213. 2017.
|
|
252
|
Sevigny J, Chiao P, Bussière T, Weinreb
PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et
al: The antibody aducanumab reduces Aβ plaques in Alzheimer's
disease. Nature. 537:50–56. 2016.
|
|
253
|
Lozupone M, Berardino G, Mollica A,
Sardone R, Dibello V, Zupo R, Lampignano L, Castellana F, Bortone
I, Stallone R, et al: ALZT-OP1: An experimental combination regimen
for the treatment of Alzheimer's disease. Expert Opin Investig
Drugs. 31:759–771. 2022.
|
|
254
|
Neumann U, Ufer M, Jacobson LH,
Rouzade-Dominguez ML, Huledal G, Kolly C, Lüönd RM, Machauer R,
Veenstra SJ, Hurth K, et al: The BACE-1 inhibitor CNP520 for
prevention trials in Alzheimer's disease. EMBO Mol Med.
10:e93162018.
|
|
255
|
Timmers M, Streffer JR, Russu A, Tominaga
Y, Shimizu H, Shiraishi A, Tatikola K, Smekens P, Börjesson-Hanson
A, Andreasen N, et al: Pharmacodynamics of atabecestat
(JNJ-54861911), an oral BACE1 inhibitor in patients with early
Alzheimer's disease: Randomized, double-blind, placebo-controlled
study. Alzheimers Res Ther. 10:852018.
|
|
256
|
Wongprayoon P and Govitrapong P: Melatonin
receptor as a drug target for neuroprotection. Curr Mol Pharmacol.
14:150–164. 2021.
|
|
257
|
Yu L, Chen Y, Wang W, Xiao Z and Hong Y:
Multi-vitamin B supplementation reverses hypoxia-induced tau
hyperphosphorylation and improves memory function in adult mice. J
Alzheimers Dis. 54:297–306. 2016.
|
|
258
|
Li S, Hafeez A, Noorulla F, Geng X, Shao
G, Ren C, Lu G, Zhao H, Ding Y and Ji X: Preconditioning in
neuroprotection: From hypoxia to ischemia. Prog Neurobiol.
157:79–91. 2017.
|
|
259
|
Zheng T, Liu H, Hong Y, Cao Y, Xia Q, Qin
C, Li M, Reiter RJ, Bai Y and Fan L: Promotion of liquid-to-solid
phase transition of cGAS by Baicalein suppresses lung
tumorigenesis. Signal Transduct Target Ther. 8:1332023.
|
|
260
|
Zhao F, Liu A, Gong X, Chen H, Wei J, Chen
B, Chen S, Yang R, Fan Y and Mao R: Hypoxia-induced RNASEH2A limits
activation of cGAS-STING signaling in HCC and predicts poor
prognosis. Tumori. 108:63–76. 2022.
|
|
261
|
Baugh EH, Ke H, Levine AJ, Bonneau RA and
Chan CS: Why are there hotspot mutations in the TP53 gene in human
cancers? Cell Death Differ. 25:154–160. 2018.
|
|
262
|
Ferretti GDS, Quarti J, Dos Santos G,
Rangel LP and Silva JL: Anticancer therapeutic strategies targeting
p53 aggregation. Int J Mol Sci. 23:110232022.
|
|
263
|
Wojtunik-Kulesza K, Rudkowska M and
Orzeł-Sajdłowska A: Aducanumab-hope or disappointment for
Alzheimer's disease. Int J Mol Sci. 24:43672023.
|
|
264
|
Salloway S, Chalkias S, Barkhof F, Burkett
P, Barakos J, Purcell D, Suhy J, Forrestal F, Tian Y, Umans K, et
al: Amyloid-related imaging abnormalities in 2 phase 3 studies
evaluating aducanumab in patients with early alzheimer disease.
JAMA Neurol. 79:13–21. 2022.
|
|
265
|
Rabinovici GD, Gatsonis C, Apgar C,
Chaudhary K, Gareen I, Hanna L, Hendrix J, Hillner BE, Olson C,
Lesman-Segev OH, et al: Association of amyloid positron emission
tomography with subsequent change in clinical management among
medicare beneficiaries with mild cognitive impairment or dementia.
JAMA. 321:1286–1294. 2019.
|