Compared with the increase in osteoclast activity,
decreased osteogenesis is the most important factor in the
occurrence and development of osteoporosis. On the one hand, bone
resorption by osteoclasts contributes to the metabolism of bone
tissue (8), while on the other
hand, inhibiting osteoclastogenesis relieves further loss of bone
mass, but does not improve bone mass, and patients are still in an
osteoporotic state (9).
Therefore, the role of osteoblasts is key to exploring the
regulation of multiple types of osteoporosis. Osteoblasts are
differentiated from bone marrow mesenchymal stem cells (BM-MSCs)
(10). BM-MSCs are pluripotent
stem cells with multidirectional differentiation ability (11). Adipogenic differentiation is
another main differentiation direction that is balanced with
osteogenesis (12). Increasing
the adipogenesis of BM-MSCs is an important factor in the
development of osteoporosis, as it decreases osteogenesis (13). Therefore, determining the role of
fat formation will contribute to unifying the mechanisms of the
pathogenesis of osteoporosis.
Postmenopausal women are at the highest risk of
osteoporosis. A previous study indicated that more than one-half of
postmenopausal women suffer from metabolic syndrome, and nearly 60%
of them have dyslipidemia (14).
Furthermore, estrogen deficiency can induce hyperlipidemia in
animals (15,16). With aging, the activity of lipid
metabolic enzymes undergoes obvious changes (17). Lipid peroxidation also accelerates
the aging process (18).
Additionally, lipid metabolism dysfunction and type 2 diabetes are
inextricably linked (19).
Obesity is not only an important risk factor for type 2 diabetes,
but hyperinsulinemia in diabetic patients also affects the
synthesis and degradation of lipids (20-22). Lipid metabolism disorders are
common in patients with several typical types of osteoporosis.
Therefore, the present review aims to systematically discuss the
role of lipid metabolism in the occurrence and development of
osteoporosis.
Obesity is a high risk factor for osteoporosis. The
view that the accumulation of fat increases the protection of bones
is doubted and challenged (23).
Based on the balance of osteogenesis and adipogenesis, the
expansion of bone marrow adipose tissue is common in populations at
a high risk for osteoporosis, leading to decreased bone formation
(24). Bone mineral density
decreases significantly with increasing fat levels in bone marrow
and blood, and obesity increases the risk of fracture by
approximately six-fold (25,26). There is a significant negative
correlation between visceral adipose tissue and bone mineral
density (27). Additionally, a
population-based study indicated that the weight-adjusted waist
circumference index was positively correlated with hip and spine
fractures (28). Redistribution
of adipose tissue and the infiltration of muscle are important in
the pathogenesis of fractures (29). The extra weight in obese
individuals leads to the occurrence of osteoporosis due to the
considerable load on the joints and bones. Calcium deficiency and
poor calcium deposition are the main pathogeneses of
obesity-induced osteoporosis. Obese individuals have difficulty
absorbing vitamin B12 and vitamin D, which is not conducive to bone
tissue remodeling (30). In a
previous study, 86.2% of obese women were reported to be deficient
in vitamin D and had difficulty absorbing calcium (31). Vitamin D deficiency can alter
adipogenesis, lipogenesis and lipolysis, and exacerbate obesity
(32). The vicious cycle of
obesity and vitamin D accelerates bone loss. Hypovitaminosis D also
occurs during the weight loss process (33). Aging also reduces the absorption
of vitamin D, which increases the risk of bone loss and
osteoporosis (34). Therefore, an
appropriate intake of vitamin D and calcium contributes to
improving the adverse effects of obesity and weight loss on bone
remodeling.
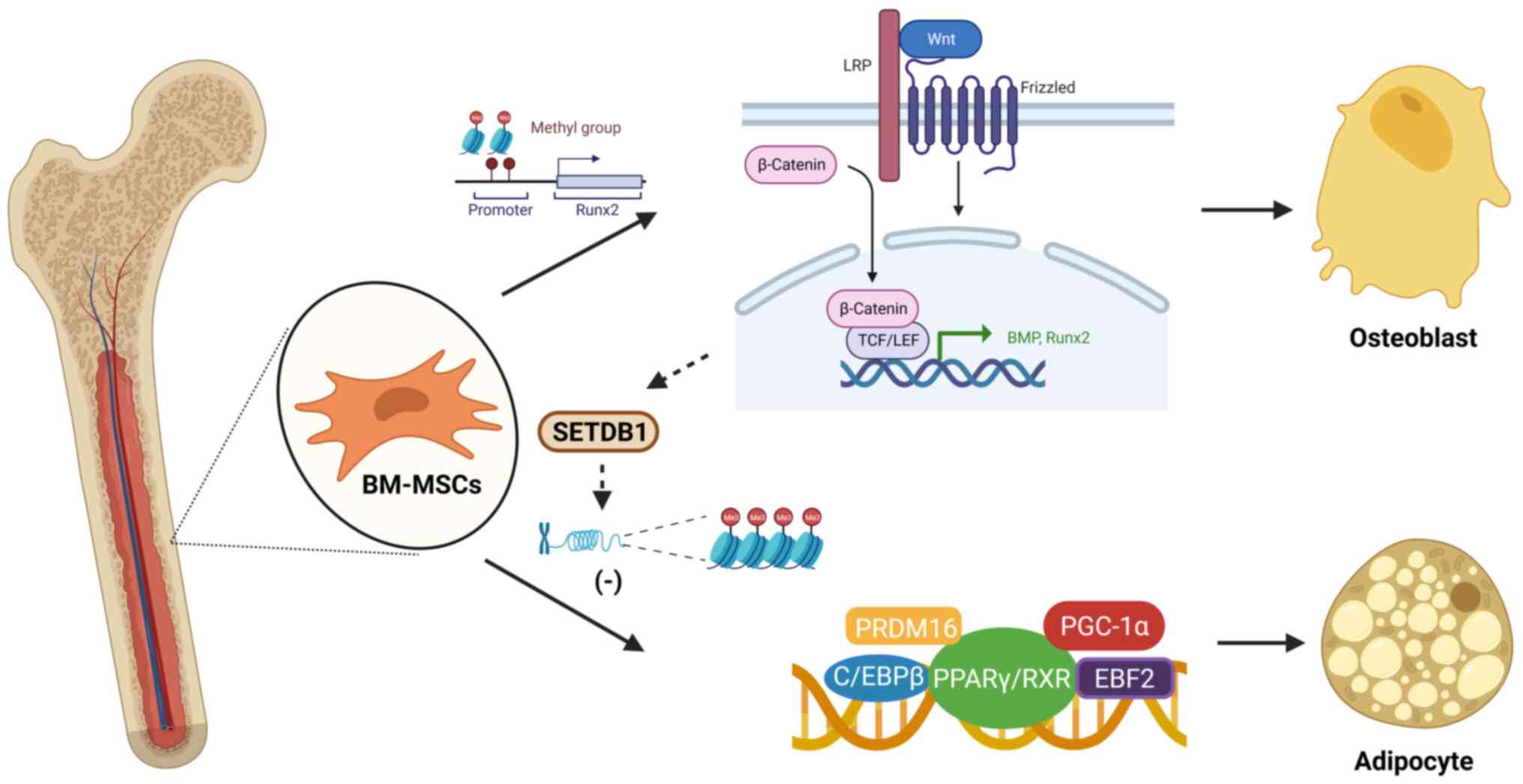
BM-MSCs are pluripotent stem cells with self-renewal
and multidirectional differentiation abilities that are the
precursor cells of osteoblasts and adipocytes (35). There is a mutual balance and
modulation between these two differentiation trends (36). Scientists have discovered that
peroxisome proliferator-activated receptor γ (PPARγ) and Wnt
signaling are factors that mediate the balance between osteogenesis
and adipogenesis (37).
Activation of Wnt/β-catenin signaling promotes the expression of
bone morphogenetic proteins (38,39). PPARγ inhibits the osteogenic
effect of the Wnt/β-catenin signaling pathway by activating the Wnt
inhibitor Dickkopf and directly acting on the β-catenin nuclear
transcription factor complex (40). DNA methylation plays an important
role in BM-MSC differentiation. Methylation of histone H3 lysine 9
dimethylation (H3K9me2) at the runt-related transcription factor 2
(Runx2) promoter modulates the osteogenic differentiation and
mineralization of BM-MSCs (41).
In one study, a DNA methylation profile revealed that zinc-finger E
homeobox-binding transcription factors participated in the
osteogenic and adipogenic differentiation of BM-MSCs, and were
correlated with body mass index and PPARγ expression (42). The non-canonical Wnt pathway
participates in the inhibition of PPARγ by activating
histone-lysine N-methyltransferase SETDB1 to induce histone H3K9
methylation of target genes (43,44) (Fig.
1).
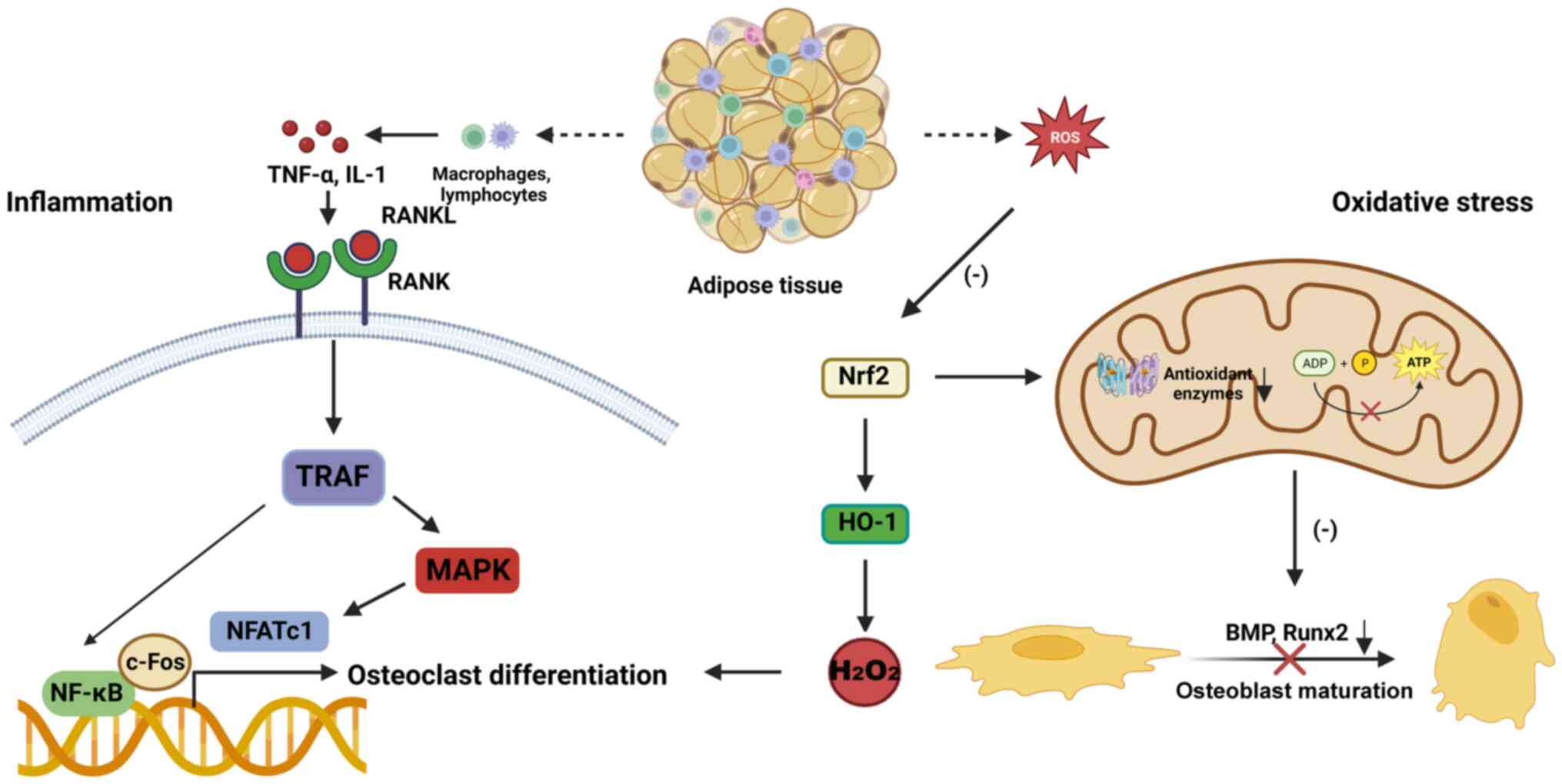
Inflammation and oxidative stress are the main
pathological changes in the development of osteoporosis (Fig. 2). Inflammatory status is a common
element for pathological change in obese individuals. The
accumulation of adipose tissue can induce chronic inflammation and
lead to an imbalance in the release of hormones and adipokines
(45). Adipocytes can directly
release inflammatory factors, including TNF-α, IL-6, C-reactive
protein and adiponectin (46).
The metabolic activity of adipocytes is increased in obese
individuals, who require a large amount of protein synthesis.
Endoplasmic reticulum stress occurs when the endoplasmic reticulum
cannot meet protein synthesis needs, thus activating the
inflammatory response (47).
Macrophages and lymphocytes are also activated to release
inflammatory factors in adipose tissue (48). Additionally, the abundance of
fatty acid-producing bacteria increases in the intestines of obese
individuals, leading to intestinal mucosa injury and an
inflammatory response to promote systemic chronic inflammation
(49). Inflammation is regarded
as an important mediator of obesity-induced osteoporosis. In mice
fed a high-fat diet (HFD), serum lipid levels increase, bone
mineral density decreases, and serum inflammatory factors,
including IL-1 and TNF-α, increase (50,51). IL-1 activates the NF-κB and MAPK
pathways by stimulating TNF receptor-associated factor 6 (TRAF6) to
promote osteoclastogenesis with the assistance of receptor
activator of nuclear factor κB ligand (RANKL) (52). TNF-α slows the differentiation of
osteoblasts and enhances the activity of osteoclasts by recruiting
TRAF and activating the NF-κB/c-Fos/nuclear factor of activated
T-cells cytoplasmic 1 (NFATc1) pathway, which is independent of the
RANKL/RANK system (53,54). Additionally, a HFD induces many
CD11c+ macrophages to aggregate and express IL-18 and
IL-1β (55). Macrophages
participate in the pathogenesis of osteonecrosis, which is the main
mechanism by which immune cells affect bone metabolism (56). Macrophages are also progenitors of
osteoclasts that contribute to bone absorption (57). In conclusion, limiting the
activity of macrophages and the release of inflammatory factors
helps alleviate the damage to bone balance caused by
hyperlipidemia.
Oxidative stress is another important pathological
state induced by obesity that accelerates bone metabolism disorders
(58). In one study, the levels
of serum markers of oxidative stress, including hydrogen peroxide
and malondialdehyde, in obese individuals almost doubled compared
with those in individuals of normal weight (mean age, 71.0±5.7)
(59). Oxidized low-density
lipoprotein is an oxidative stress biomarker that is involved in
the negative effects of obesity (60). Mitochondrial dysfunction is the
main cause of obesity-induced oxidative stress (61). Hyperlipidemia destroys the
structure of the mitochondria, changes the membrane potential and
affects ATP synthesis (62).
Obese individuals have difficulties clearing reactive oxygen
species (ROS) based on decreased antioxidant enzyme activity,
leading to ROS accumulation and the aggravation of oxidative stress
(63). In HFD-fed mice, serum
total antioxidant capacity and levels of superoxide dismutase,
which is associated with bone biomechanical strength and
microarchitecture, are decreased (64). Hyperlipidemia also decreases the
expression of nuclear factor erythroid 2-related factor 2 (Nrf2)
and antioxidant enzymes in bone tissue (50). HFD consumption induces the
overexpression of ROS to inhibit the Wnt/β-catenin pathway
(65). Oxidative injury decreases
the expression of BMP2 and Runx2 in osteoblasts (66). Oxidized lipids contribute to
PPARγ-induced adipogenesis and inhibit β-catenin-induced
osteogenesis in osteoporosis (67,68). HFD consumption reduces the
glutathione/oxidized glutathione ratio to not only inhibit bone
formation, but also to increase the expression of bone resorption
markers such as cross-linked N-telopeptides of bone type І collagen
(69). HFD intake promotes
osteoclast activity and differentiation by inhibiting the Nrf2/heme
oxygenase-1/catalase signaling pathway (70).
Triglycerides are an important form of fat; they are
the main energy source in the body, and have the greatest storage
and production capacity. Triglyceride levels were positively
associated with an increased risk of osteoporosis in a study of
serum fat markers in 481 individuals (71). The levels of triglycerides were
obviously different among the normal, osteopenia and osteoporosis
groups (71), which indicated
that variations in triglycerides were strongly related to the
occurrence and development of osteoporosis (72). Some drugs for the treatment of
osteoporosis, such as bisphosphonates and calcium, have also been
found to cause abnormal triglyceride metabolism in adipose tissue
while promoting bone growth, showing that interfering with fat
metabolism is beneficial for improving bone mass (73,74). At the cellular level, the
adipogenic differentiation of BM-MSCs leads to the accumulation of
triglycerides, which are a risk factor for osteogenesis (75). Triglycerides decrease the
expression of the bone growth factor FGF2 and increase the
expression of the inflammatory mediator TNF-α, which inhibits the
proliferation of osteoblasts (76). Notably, appropriate modification
of triglycerides and adjustment of their concentration can improve
bone mineral density by promoting the transdifferentiation of
chondrocytes to osteoblasts in postmenopausal mice (77).
Cholesterol is a substance involved in the
structural arrangement of the body and the regulation of cell
function. As an important synthetic substance consisting of
estrogen and vitamin D, cholesterol is involved in the regulation
of bone metabolism (78).
Previous studies have indicated that serum total cholesterol (TC)
levels are negatively correlated with bone mineral density
(71,79). A high-cholesterol diet inhibits
the differentiation and proliferation of osteoblasts, and reduces
bone formation (66,80). Osteoclast synthesis also requires
exogenous cholesterol (81).
Cholesterol is classified as high-density lipoprotein cholesterol
(HDL-C) and low-density lipoprotein cholesterol (LDL-C). A number
of studies have demonstrated the fact that HDL-C is positively
associated with bone mineral density (82-84). Dysfunctional HDL-C increases the
expression of PPARγ and decreases the expression of osteogenic
markers (85). When HDL-C
inhibits the activity of inflammatory factors, these factors
suppress osteogenic formation via the Wnt/β-catenin axis (86). In contrast to HDL-C, LDL-C is a
negative regulator of bone homeostasis. On the one hand, LDL-C
inhibits alkaline phosphatase activity and cell mineralization to
interfere with osteogenesis (87), while on the other hand, LDL-C
activates RANKL and promotes cell fusion during osteoclastogenesis
(88,89). Based on this evidence, decreasing
TC levels and increasing the proportion of HDL-C are beneficial for
attenuating the development of osteoporosis.
Phospholipids are the main components of biological
membrane structures. Phospholipids interfere with bone homeostasis
mainly in their oxidized form (90). The accumulation of oxidized
phospholipids leads to a systemic inflammatory state by influencing
immunocytes, which causes inflammatory bone loss (91). Various phospholipids exhibit
toxicity to osteoblasts after oxidation (92). Oxidized phospholipids reduce the
expression of osteogenic markers and attenuate parathyroid hormone
signaling (93). Bioactive
oxidized phospholipids also decrease the response of BM-MSCs to
osteogenic factors to inhibit osteogenesis by binding to receptors
on the cell surface (94).
Additionally, a previous study indicated that oxidized
phospholipids could enhance the production of RANKL by T
lymphocytes to promote osteoclastogenesis (95). These phospholipids also induce
osteoblasts to secrete cell cytokines such as IL-6 and TNF-α, both
of which contribute to osteoclast differentiation (96). Neutralization of oxidized
phospholipids is beneficial for improving bone mass (97,98). The oxidation-specific epitopes of
oxidized phospholipids are potential targets for osteoporosis
treatment (99).
Glycolipids are a class of lipid compounds involved
in the biological structure of cell membranes and are closely
related to the development of osteoporosis (100). Glycolipid-induced toxicity is an
important factor in diabetic patients with osteoporosis (101). Menopause-related hormone therapy
for osteoporosis is also relevant to glycolipid metabolism
(102). Leucine-rich
repeat-containing G-protein coupled receptor 4, which is related to
glycolipids, has been shown to have an osteogenic effect by
upregulating the expression of components of the Wnt/β-catenin
signaling pathway (103). Some
studies have indicated that glycolipids conjugated to receptors on
natural killer (NK) T cells protect against osteolytic pathogenesis
(104,105). However, invariant NK T cells
increase the expression of RANKL to promote osteoclastogenesis
(106). The effect of
glycolipids on NK cells might be a key factor in
osteoimmunology.
Bile acid is the main route of cholesterol
conversion, and it is also the main component of bile and is
involved in fat metabolism. Serum metabolomic analysis of
ovariectomized mice revealed that serum bile acid levels were
closely related to the development of postmenopausal osteoporosis
(107). Serum bile acid level is
positively correlated with bone mineral density (108). However, different types of bile
acids have different effects on bone metabolism. Osteoporosis is a
common complication of biliary cholangitis (109). The use of ursodeoxycholic acid
to treat cholestatic liver disease plays a positive role in the
treatment of osteoporosis (110). Ursodeoxycholic acid also
promotes the differentiation of osteoblasts by increasing the
expression of Runx2 and inhibiting osteoblast apoptosis induced by
bilirubin (109,111). Targeted stimulation of bile acid
receptors contributes to preventing osteoporosis in postmenopausal
mice (112,113). In contrast to ursodeoxycholic
acid, lithocholic acid plays a negative role in bone balance. In
human osteoblasts, lithocholic acid decreased the expression of
osteogenic proteins, dampened the effect of vitamin D and increased
the expression of apoptosis markers in osteoblasts (114,115). Additionally, lithocholic acid
enhances osteoclast activity by upregulating RANKL expression
(109). Overall, increasing the
level of deoxycholic acid might prevent the occurrence of
osteoporosis.
Triglyceride metabolism results in the production of
glycerol and large amounts of fatty acids, both of which affect
bone homeostasis. Glycerol is widely used in drug modification and
the design of bone scaffolds via tissue engineering technology due
to its satisfactory permeability and membrane fusion properties
(116-118). Fatty acids are classified as
saturated and unsaturated fatty acids. Unsaturated fatty acids are
generally considered beneficial to the human body (119). However, the positive effect
depends on the ratio of n-3 fatty acids to n-6 fatty acids. With an
increase in the ratio of n-3/n-6 fatty acids, the bone mineral
density increases and the fracture ratio decreases (120). n-3 fatty acids can reverse the
effects of aging and promote the proliferation and differentiation
of osteoblasts (121). Fatty
acids are catabolized in the liver to produce ketone bodies, which
are involved in bone metabolism. Acetoacetate can promote
osteoblast differentiation and generate far fewer free radicals
than the equivalent amount of glucose under the same conditions,
thereby reducing oxidative damage to osteoblast precursor cells
(122,123). However, β-hydroxybutyrate plays
a negative role in osteogenic differentiation (122). As aforementioned, high
triglyceride levels are detrimental to bones, and the effects of
their ketogenic metabolites are multifaceted. Decreasing
triglyceride levels and adjusting the ratio of their ketogenic
metabolites will increase bone mass in patients with osteoporosis.
Genes associated with fatty acid biosynthesis and degradation
participate in the regulation of bone metabolism. Acyl-CoA
synthetase long-chain family members (ACSLs) play a key role in
fatty acid metabolism by converting free long-chain fatty acids
into fatty acyl-CoA esters. ACSL1 is a potential biomarker of
osteoporosis, as it modulates the activity of microRNAs during
adipogenesis (124). ACSL1 is
also involved in the inflammatory response in osteoporosis
(125). Previous studies found
that ACSL3 is significantly correlated with total hip bone mineral
density (126), while ACSL5 is
associated with sarcopenia during hip fractures (127). Differential gene analysis via
the Gene Expression Omnibus database revealed that ACSL5 is a
potential target for osteoporosis treatment (128). Malonyl-CoA-acyl carrier protein
transacylase (MCAT) is a component of the fatty acid synthase
complex in mitochondria and is the specific substrate of the zinc
finger DHHC-type palmitoyltransferase 13 (ZDHHC13) enzyme. ZDHHC13
deficiency leads to the accumulation of MCAT proteins and induces
mitochondrial damage, causing osteoporosis (129) (Table I).
The incidence of obesity in postmenopausal women is
increasing. With increasing age, the metabolism of body fat slows.
According to past dietary habits, obesity will inevitably occur.
Estrogen is an important endogenous hormone that regulates lipid
metabolism. On the one hand, estrogen affects the distribution of
fat in the body. As estrogen levels decrease, fat is redistributed
and accumulates from the limbs and trunk to the abdomen and viscera
(130). The levels of fatty acid
metabolites are increased in visceral adipose tissue (131). On the other hand, estrogen
regulates lipid synthesis and decomposition. Estrogen regulates
hypothalamic neurons and transmits signals to control adipose
tissue catabolism and thermogenesis (132). Estrogen receptor α mediates the
activation of thermogenic uncoupling protein-1 to promote fat
consumption (133). Estrogen
receptor α also promotes histone modification and regulates the DNA
methylation of genes associated with lipid metabolism to inhibit
adipogenesis (134). In an
estrogen-deficient state, β-oxidation of free fatty acids to
provide energy does not occur, leading to fat accumulation
(135). Postmenopausal obesity
is a high risk factor for the development of osteoporosis. Obesity
accelerates bone loss and increases bone fragility in
postmenopausal women (136).
Obesity is positively correlated with the occurrence of all-cause
fractures but protects against pelvic fractures in postmenopausal
women (137). A meta-analysis
indicated that serum adipokines were potential predictors of bone
mineral density and fracture risk in postmenopausal women (138). Selective inhibition of
adipogenesis could prevent the development of osteoporosis in
ovariectomized (OVX) mice (139). High levels of β-crosslap and low
levels of procollagen type 1 N-terminal propeptide indicate an
imbalance in bone formation and resorption in postmenopausal obese
women (140). The decrease in
plasma calcium and phosphorus levels also indicated weak
osteogenesis in obese OVX mice. The levels of obesity-associated
proteins, which colocalize with tartrate-resistant acid phosphatase
(TRAP) and upregulate NFATc1 and c-FOS expression to promote
RANKL-mediated osteoclast differentiation, are increased in
postmenopausal obese mice (141). Increasing calcium intake could
help reduce postmenopausal weight and increase serum leptin levels,
which helps alleviate bone loss (142).
Obesity is related to and impacts diabetes. Patients
with diabetes are prone to abnormal blood lipid levels, as fat
synthesis is reduced, degradation is accelerated and disorders of
lipid metabolism cause an increase in blood lipids (143). In diabetes, large amounts of
fatty acids and glycerol enter the liver due to accelerated fat
degradation with a decrease in the insulin/glucagon ratio.
Excessive fatty acids are re-esterified into triglycerides and
released into the bloodstream in the form of very-low-density
lipoproteins (VLDLs) (144).
Additionally, the activity of lipoprotein lipase decreases, making
it difficult for VLDLs and chylomicrons to be cleared from the
plasma (145). Abnormal hormone
secretion in diabetes promotes the activity of β-hydroxy
β-methylglutaryl-CoA reductase to increase cholesterol synthesis
(146). The synthesis of
triglycerides also increases in diabetic patients (147). In addition, adipose tissue
secretes a variety of inflammatory factors, such as leptin and
adiponectin, which reduce insulin sensitivity and aggravate
diabetes (148). Abnormal lipid
metabolism is a driving factor of the development of osteoporosis
in diabetic patients (149). TC,
triglyceride and LDL-C levels are negatively correlated with bone
mineral density in diabetic patients. Hyperglycemia inhibits
osteogenesis and promotes adipogenic differentiation (150). High glucose-induced lipid
peroxidation leads to ferroptosis in osteoblasts (151,152). In a previous study, diabetic
mice with excess fat showed obviously elevated TRAP levels, which
indicated enhanced bone resorption (153).
Osteoblasts are differentiated from mesenchymal stem
cells in the bone marrow. BM-MSCs have multiple differentiation
abilities, and an improvement in one differentiation ability will
affect the abilities other types of differentiation. Among these
differentiation trends, osteogenesis and adipogenesis are
considered relevant groups with a clear negative correlation
(158). The present review
discusses the osteogenic and adipogenic differentiation of BM-MSCs
and the mutual regulatory effects mediated by the PPARγ and
Wnt/β-catenin signaling pathways. The review also examines the role
of other lipid substances in the occurrence and development of
osteoporosis. The results indicate that most of these substances
play a dual role in bone metabolism. Excessive accumulation of
lipids inhibits osteogenesis, while proper stimulation increases
bone mass. The total body fat content is clearly negatively
correlated with bone mineral density (159). Moreover, lipid metabolism
disorders induce specific pathologies, including inflammation and
oxidative stress, to alter the bone microenvironment. Numerous
secreted inflammatory factors, but mainly IL-1 and TNF-α, promote
the differentiation of osteoclasts. Oxidative damage also inhibits
osteogenesis and reduces bone strength. Additionally, lipid
metabolism disorders are common in populations that are at high
risk for osteoporosis, including postmenopausal women, diabetic
patients and obese individuals. Lipidomic profiling contributes to
the diagnosis, prevention and treatment of osteoporosis (160).
The present review highlights the mutual regulation
of the osteogenic and adipogenic differentiation of BM-MSCs, and
the role of various lipids in the development of osteoporosis, and
discusses the mechanism by which lipids affect the skeletal system.
The bidirectional effect of lipids on bone metabolism suggests that
a reasonable adjustment of the content and proportion of lipids
will increase bone mass. In addition, relieving inflammatory storms
and oxidative damage induced by lipid imbalances is key to
preventing bone loss. This review contributes to unifying theories
on the pathogenesis of osteoporosis and optimizing treatments for
osteoporosis.
Not applicable.
XW was responsible for data curation and writing the
original draft. CZ performed data curation and helped in writing of
the original draft. GZ performed data curation and writing of the
original draft. KY was responsible for funding acquisition,, and
reviewing and editing the manuscript. LT was responsible for
funding acquisition, project administration, resources, and
reviewing and editing the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by grants from the National
Science Fund for Distinguished Young Scholars (no. 32200943), the
Shenyang Young and Middle-aged Innovative Talents Project (no.
RC210171) and the China Postdoctoral Science Foundation (no.
2022M723520).
|
1
|
Ebeling PR, Nguyen HH, Aleksova J, Vincent
AJ, Wong P and Milat F: Secondary osteoporosis. Endocr Rev.
43:240–313. 2022. View Article : Google Scholar
|
|
2
|
Flores LE, Nelson S, Waltman N, Kupzyk K,
Lappe J, Mack L and Bilek LD: Examining effects of habitual
physical activity and body composition on bone structure in early
post-menopausal women: A pQCT analysis. Osteoporos Int. 33:425–433.
2022. View Article : Google Scholar
|
|
3
|
Compston J, Cooper A, Cooper C, Francis R,
Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P and Wilkins M;
National Osteoporosis Guideline Group (NOGG): Guidelines for the
diagnosis and management of osteoporosis in postmenopausal women
and men from the age of 50 years in the UK. Maturitas. 62:105–108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirza F and Canalis E: Management of
endocrine disease: Secondary osteoporosis: Pathophysiology and
management. Eur J Endocrinol. 173:R131–R151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY
and Yu SN: A network meta-analysis on the short-term efficacy and
adverse events of different anti-osteoporosis drugs for the
treatment of postmenopausal osteoporosis. J Cell Biochem.
119:4469–4481. 2018. View Article : Google Scholar
|
|
6
|
Ying S, Sifan W, Yujiao W, Rongyi C,
Qingrong H, Lili M, Huiyong C and Lindi J: The roles of miRNA,
lncRNA and circRNA in the development of osteoporosis. Biol Res.
53:402020. View Article : Google Scholar
|
|
7
|
Feng K, Yu M, Lou X, Wang D, Wang L and
Ren W: Multi-omics analysis of bone marrow mesenchymal stem cell
differentiation differences in osteoporosis. Genomics.
115:1106682023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gritsaenko T, Pierrefite-Carle V, Creff G,
Simoneau B, Hagège A, Farlay D, Pagnotta S, Orange F, Jaurand X,
Auwer CD, et al: Low doses of uranium and osteoclastic bone
resorption: Key reciprocal effects evidenced using new in vitro
biomimetic models of bone matrix. Arch Toxicol. 95:1023–1037. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung HJ, Cho L, Shin JS, Lee J, Ha IH,
Park HJ and Lee SK: Effects of JSOG-6 on protection against bone
loss in ovariectomized mice through regulation of osteoblast
differentiation and osteoclast formation. BMC Complement Altern
Med. 14:1842014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dey D, Jingar P, Agrawal S, Shrivastava V,
Bhattacharya A, Manhas J, Garg B, Ansari MT, Mridha AR, Sreenivas
V, et al: Symphytum officinale augments osteogenesis in human bone
marrow-derived mesenchymal stem cells in vitro as they
differentiate into osteoblasts. J Ethnopharmacol. 248:1123292020.
View Article : Google Scholar
|
|
11
|
Zhang L, Yuan Y, Wu W, Sun Z, Lei L, Fan
J, Gao B and Zou J: Medium-intensity treadmill exercise exerts
beneficial effects on bone modeling through bone marrow mesenchymal
stromal cells. Front Cell Dev Biol. 8:6006392020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zuo R, Liu M, Wang Y, Li J, Wang W, Wu J,
Sun C, Li B, Wang Z, Lan W, et al: BM-MSC-derived exosomes
alleviate radiation-induced bone loss by restoring the function of
recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell
Res Ther. 10:302019. View Article : Google Scholar
|
|
13
|
Cheng H, Qiu L, Ma J, Zhang H, Cheng M, Li
W, Zhao X and Liu K: Replicative senescence of human bone marrow
and umbilical cord derived mesenchymal stem cells and their
differentiation to adipocytes and osteoblasts. Mol Biol Rep.
38:5161–5168. 2011. View Article : Google Scholar
|
|
14
|
Chedraui P, Miguel GS, Vintimilla-Sigüenza
I, Villacreses D, Romero-Huete L, Domínguez A, Jaramillo W, Escobar
GS, Pérez-López FR, Genazzani AR, et al: The metabolic syndrome and
its components in postmenopausal women. Gynecol Endocrinol.
29:563–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hur HJ, Jeong YH, Lee SH and Sung MJ:
Quercitrin ameliorates hyperlipidemia and hepatic steatosis in
ovariectomized mice. Life (Basel). 10:2432020.PubMed/NCBI
|
|
16
|
Chen L, Liu Y, Tang Z, Shi X, Song Z, Cao
F, Wei P, Li M, Li X, Jiang D, et al: Improvements in estrogen
deficiency-induced hypercholesterolemia by Hypericum perforatum L.
extract are associated with gut microbiota and related metabolites
in ovariectomized (OVX) rats. Biomed Pharmacother. 135:1111312021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mutlu AS, Duffy J and Wang MC: Lipid
metabolism and lipid signals in aging and longevity. Dev Cell.
56:1394–1407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miró O, Casademont J, Casals E, Perea M,
Urbano-Márquez A, Rustin P and Cardellach F: Aging is associated
with increased lipid peroxidation in human hearts, but not with
mitochondrial respiratory chain enzyme defects. Cardiovasc Res.
47:624–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van de Wiel A: Diabetes mellitus and
alcohol. Diabetes Metab Res Rev. 20:263–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maggio CA and Pi-Sunyer FX: Obesity and
type 2 diabetes. Endocrinol Metab Clin North Am. 32:805–822. 2003.
View Article : Google Scholar
|
|
21
|
Comuzzie AG, Tejero ME, Funahashi T,
Martin LJ, Kissebah A, Takahashi M, Kihara S, Tanaka S, Rainwater
DL, Matsuzawa Y, et al: The genes influencing adiponectin levels
also influence risk factors for metabolic syndrome and type 2
diabetes. Hum Biol. 79:191–200. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Z, Huo J, Ding X, Yang M, Li L, Dai J,
Hosoe K, Kubo H, Mori M, Higuchi K and Sawashita J: Coenzyme Q10
improves lipid metabolism and ameliorates obesity by regulating
CaMKII-Mediated PDE4 inhibition. Sci Rep. 7:82532017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fassio A, Idolazzi L, Rossini M, Gatti D,
Adami G, Giollo A and Viapiana O: The obesity paradox and
osteoporosis. Eat Weight Disord. 23:293–302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ali D, Tencerova M, Figeac F, Kassem M and
Jafari A: The pathophysiology of osteoporosis in obesity and type 2
diabetes in aging women and men: The mechanisms and roles of
increased bone marrow adiposity. Front Endocrinol (Lausanne).
13:9814872022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lopes KG, Rodrigues EL, da Silva Lopes MR,
do Nascimento VA, Pott A, Guimarães RCA, Pegolo GE and Freitas KC:
Adiposity metabolic consequences for adolescent bone health.
Nutrients. 14:32602022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salzmann SN, Ortiz Miller C, Carrino JA,
Yang J, Shue J, Sama AA, Cammisa FP, Girardi FP and Hughes AP: BMI
and gender increase risk of sacral fractures after multilevel
instrumented spinal fusion compared with bone mineral density and
pelvic parameters. Spine J. 19:238–245. 2019. View Article : Google Scholar
|
|
27
|
Perna S, Gasparri C, Allehdan S, Riva A,
Petrangolini G, Ferraris C, Guido D, Alalwan TA and Rondanelli M:
Discovering the Physio-pathological mechanisms of interaction
between bone mineral density, muscle mass, and visceral adipose
tissue in female older adults through structural equation modeling.
J Clin Med. 12:22692023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao J, Zhang Y, Tan C and Tan W:
Associations between weight-adjusted waist index and fractures: A
population-based study. J Orthop Surg Res. 18:2902023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piñar-Gutierrez A, García-Fontana C,
García-Fontana B and Muñoz-Torres M: Obesity and bone health: A
complex relationship. Int J Mol Sci. 23:83032022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aaseth JO and Alexander J: Postoperative
osteoporosis in subjects with morbid obesity undergoing bariatric
surgery with gastric bypass or sleeve gastrectomy. Nutrients.
15:13022023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albaik M, Khan JA, Sindi I, Akesson KE and
McGuigan FEA: Bone mass in Saudi women aged 20-40 years: The
association with obesity and vitamin D deficiency. Arch Osteoporos.
17:1232022. View Article : Google Scholar
|
|
32
|
Di Filippo L, De Lorenzo R, Giustina A,
Rovere-Querini P and Conte C: Vitamin d in osteosarcopenic obesity.
Nutrients. 14:18162022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bassatne A, Chakhtoura M, Saad R and
Fuleihan GE: Vitamin D supplementation in obesity and during weight
loss: A review of randomized controlled trials. Metabolism.
92:193–205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bosetti M, Sabbatini M, Calarco A, Borrone
A, Peluso G and Cannas M: Effect of retinoic acid and vitamin D3 on
osteoblast differentiation and activity in aging. J Bone Miner
Metab. 34:65–78. 2016. View Article : Google Scholar
|
|
35
|
Wang C, Tian W, Hu SY, Di CX, He CY, Cao
QL, Hao RH, Dong SS, Liu CC, Rong Y, et al: Lineage-selective super
enhancers mediate core regulatory circuitry during adipogenic and
osteogenic differentiation of human mesenchymal stem cells. Cell
Death Dis. 13:8662022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao RH, Guo Y, Wang C, Chen F, Di CX, Dong
SS, Cao QL, Guo J, Rong Y, Yao S, et al: Lineage-specific
rearrangement of chromatin loops and epigenomic features during
adipocytes and osteoblasts commitment. Cell Death Differ.
29:2503–2518. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang
B, Zhang D, Rao P and Xiao J: PPARγ and wnt signaling in adipogenic
and osteogenic differentiation of mesenchymal stem cells. Curr Stem
Cell Res Ther. 11:216–225. 2016. View Article : Google Scholar
|
|
38
|
Yao XT, Li PP, Liu J, Yang YY, Luo ZL,
Jiang HT, He WG, Luo HH, Deng YX and He BC: Wnt/β-Catenin promotes
the osteoblastic potential of BMP9 through Down-Regulating Cyp26b1
in mesenchymal stem cells. Tissue Eng Regen Med. 20:705–723. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Zhao Y, Xie Z, Li M, Liu Y and Tu
X: Activating Wnt/β-Catenin signaling in osteocytes promotes
osteogenic differentiation of BMSCs through BMP-7. Int J Mol Sci.
23:160452022. View Article : Google Scholar
|
|
40
|
Vallée A and Lecarpentier Y: Crosstalk
between peroxisome Proliferator-Activated receptor gamma and the
canonical WNT/β-Catenin pathway in chronic inflammation and
oxidative stress during carcinogenesis. Front Immunol. 9:7452018.
View Article : Google Scholar
|
|
41
|
Kang P, Wu Z, Huang Y, Luo Z, Huo S and
Chen Q: Histone H3K9 demethylase JMJD2B/KDM4B promotes osteogenic
differentiation of bone marrow-derived mesenchymal stem cells by
regulating H3K9me2 on RUNX2. PeerJ. 10:e138622022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gómez R, Barter MJ, Alonso-Pérez A,
Skelton AJ, Proctor C, Herrero-Beaumont G and Young DA: DNA
methylation analysis identifies key transcription factors involved
in mesenchymal stem cell osteogenic differentiation. Biol Res.
56:92023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takada I, Kouzmenko AP and Kato S: Wnt and
PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev
Rheumatol. 5:442–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takada I, Suzawa M, Matsumoto K and Kato
S: Suppression of PPAR transactivation switches cell fate of bone
marrow stem cells from adipocytes into osteoblasts. Ann N Y Acad
Sci. 1116:182–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barakat B and Almeida MEF: Biochemical and
immunological changes in obesity. Arch Biochem Biophys.
708:1089512021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ellulu MS, Khaza'ai H, Abed Y, Rahmat A,
Ismail P and Ranneh Y: Role of fish oil in human health and
possible mechanism to reduce the inflammation.
Inflammopharmacology. 23:79–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ajoolabady A, Lebeaupin C, Wu NN, Kaufman
RJ and Ren J: ER stress and inflammation crosstalk in obesity. Med
Res Rev. 43:5–30. 2023. View Article : Google Scholar
|
|
48
|
Sardi C, Martini E, Mello T, Camelliti S,
Sfondrini L, Marcucci F, Kallikourdis M, Sommariva M and Rumio C:
Effect of acetylsalicylic acid on inflamed adipose tissue. Insulin
resistance and hepatic steatosis in a mouse model of diet-induced
obesity. Life Sci. 264:1186182021. View Article : Google Scholar
|
|
49
|
Zhou X, Pak S, Li D, Dong L, Chen F, Hu X
and Ma L: Bamboo shoots modulate gut microbiota, eliminate obesity
in high-fat-diet-fed mice and improve lipid metabolism. Foods.
12:13802023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He H, Zhang Y, Sun Y, Zhang Y, Xu J, Yang
Y and Chen J: Folic acid attenuates high-fat diet-induced
osteoporosis through the AMPK signaling pathway. Front Cell Dev
Biol. 9:7918802021. View Article : Google Scholar
|
|
51
|
Kang YS, Kim JC, Kim JS and Kim SH:
Effects of swimming exercise on serum irisin and bone FNDC5 in rat
models of High-Fat Diet-Induced osteoporosis. J Sports Sci Med.
18:596–603. 2019.PubMed/NCBI
|
|
52
|
Walsh MC, Kim GK, Maurizio PL, Molnar EE
and Choi Y: TRAF6 autoubiquitination-independent activation of the
NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS One.
3:e40642008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu WJ, Xia CL, Ou SJ, Yang Y, Ma YF, Hou
YL, Yang QP, Zhang J, Li JW, Qi Y and Xu CP: Novel elongator
protein 2 inhibitors mitigating tumor necrosis Factor-α induced
osteogenic differentiation inhibition. Biomed Res Int.
2021:36645642021. View Article : Google Scholar
|
|
54
|
Yao Z, Getting SJ and Locke IC: Regulation
of TNF-induced osteoclast differentiation. Cells. 11:1322021.
View Article : Google Scholar
|
|
55
|
Zhang Y, Li Q, Rao E, Sun Y, Grossmann ME,
Morris RJ, Cleary MP and Li B: Epidermal Fatty Acid binding protein
promotes skin inflammation induced by high-fat diet. Immunity.
42:953–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gkouveris I, Soundia A, Gouveris P, Zouki
D, Hadaya D and Tetradis S: Macrophage involvement in
Medication-related osteonecrosis of the jaw (MRONJ): A
comprehensive, short review. Cancers (Basel). 14:3302022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Russo R, Zito F and Lampiasi N: MiRNAs
expression profiling in Raw264.7 macrophages after Nfatc1-Knockdown
elucidates potential pathways involved in osteoclasts
differentiation. Biology (Basel). 10:10802021.PubMed/NCBI
|
|
58
|
Korkmaz HA and Özkan B: Impact of obesity
on bone metabolism in Children. J Pediatr Endocrinol Metab.
35:557–565. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Leanza G, Conte C, Cannata F, Isgrò C,
Piccoli A, Strollo R, Quattrocchi CC, Papalia R, Denaro V,
Maccarrone M, et al: Oxidative stress in postmenopausal women with
or without obesity. Cells. 12:11372023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gutiérrez-Solis AL, Garrido-Dzib AG,
Rochel-Pérez A, Magallón-Zertuche V, Chávez-Loría G, Medina-Vera I
and Avila-Nava A: Oxidative stress biomarkers in mexican subjects
with overweight and obesity: A systematic review. Metab Syndr Relat
Disord. 21:188–196. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cojocaru K, Cojocaru KA, Luchian I, Ursu
RG, Butnaru O and Foia L: Mitochondrial dysfunction, oxidative
stress, and therapeutic strategies in diabetes, obesity, and
cardiovascular disease. Antioxidants (Basel). 12:6582023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jing J, Peng Y, Fan W, Han S, Peng Q, Xue
C, Qin X, Liu Y and Ding Z: Obesity-induced oxidative stress and
mitochondrial dysfunction negatively affect sperm quality. FEBS
Open Bio. 13:763–778. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lubkowska A, Dudzińska W and Pluta W:
Antioxidant enzyme activity and serum HSP70 concentrations in
relation to insulin resistance and lipid profile in lean and
overweight young men. Antioxidants (Basel). 12:6552023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xia B, Zhu R, Zhang H, Chen B, Liu Y, Dai
X, Ye Z, Zhao D, Mo F, Gao S, et al: Lycopene improves bone quality
and regulates AGE/RAGE/NF-кB signaling pathway in high-fat
diet-induced obese mice. Oxid Med Cell Longev. 2022:36970672022.
View Article : Google Scholar
|
|
65
|
Wang YN, Jia TT, Feng Y, Liu SY, Zhang WJ,
Zhang DJ and Xu X: Hyperlipidemia impairs osseointegration via the
ROS/Wnt/β-Catenin pathway. J Dent Res. 100:658–665. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
You L, Sheng ZY, Tang CL, Chen L, Pan L
and Chen JY: High cholesterol diet increases osteoporosis risk via
inhibiting bone formation in rats. Acta Pharmacol Sin.
32:1498–1504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Almeida M, Ambrogini E, Han L, Manolagas
SC and Jilka RL: Increased lipid oxidation causes oxidative stress,
increased peroxisome proliferator-activated receptor-gamma
expression, and diminished pro-osteogenic Wnt signaling in the
skeleton. J Biol Chem. 284:27438–27448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ronis MJ, Mercer K and Chen JR: Effects of
nutrition and alcohol consumption on bone loss. Curr Osteoporos
Rep. 9:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xiao Y, Cui J, Li YX, Shi YH, Wang B, Le
GW and Wang ZP: Dyslipidemic high-fat diet affects adversely bone
metabolism in mice associated with impaired antioxidant capacity.
Nutrition. 27:214–220. 2011. View Article : Google Scholar
|
|
70
|
Li G, Park JN, Park HJ, Suh H and Choi HS:
High Cholesterol-Induced bone loss is attenuated by arctiin via an
action in osteoclasts. Nutrients. 14:44832022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kan B, Zhao Q, Wang L, Xue S, Cai H and
Yang S: Association between lipid biomarkers and osteoporosis: A
cross-sectional study. BMC Musculoskelet Disord. 22:7592021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lu CW, Wang CH, Hsu BG and Tsai JP: Serum
osteoprotegerin level is negatively associated with bone mineral
density in patients undergoing maintenance hemodialysis. Medicina
(Kaunas). 57:7622021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Martini C, Sosa FN, Malvicini R, Pacienza
N, Yannarelli G, Del C and Vila M: Alendronate inhibits
triglyceride accumulation and oxidative stress in adipocytes and
the inflammatory response of macrophages which are associated with
adipose tissue dysfunction. J Physiol Biochem. 77:601–611. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mao H, Wang W, Shi L, Chen C, Han C, Zhao
J, Zhuo Q, Shen S, Li Y and Huo J: Metabolomics and physiological
analysis of the effect of calcium supplements on reducing bone loss
in ovariectomized rats by increasing estradiol levels. Nutr Metab
(Lond). 18:762021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sutjarit N, Thongon N, Weerachayaphorn J,
Piyachaturawat P, Suksamrarn A, Suksen K, Papachristou DJ and Blair
HC: Inhibition of adipogenic differentiation of human bone
Marrow-Derived mesenchymal stem cells by a phytoestrogen
diarylheptanoid from curcuma comosa. J Agric Food Chem.
68:9993–10002. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang H, Luo L and Liu Z, Li Y, Tong Z and
Liu Z: Role of TNF-α and FGF-2 in the fracture healing disorder of
type 2 diabetes model induced by high fat diet followed by
streptozotocin. Diabetes Metab Syndr Obes. 13:2279–2288. 2020.
View Article : Google Scholar :
|
|
77
|
Zhang T, Tian Y, Wang Q, Fu M, Xue C and
Wang J: Comparative study of DHA with different molecular forms for
ameliorating osteoporosis by promoting Chondrocyte-to-Osteoblast
transdifferentiation in the growth plate of ovariectomized mice. J
Agric Food Chem. 69:10562–10571. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Antonenko A, Leahy A, Babenko M and Lyons
D: Low dose hydrophilic statins are the preferred agents for
females at risk of osteoporosis. Bone Rep. 16:1011522022.
View Article : Google Scholar
|
|
79
|
Liu J, Deng X, Liang X and Li L: The
phytoestrogen glabrene prevents osteoporosis in ovariectomized rats
through upregulation of the canonical Wnt/β-catenin signaling
pathway. J Biochem Mol Toxicol. 35:e226532021. View Article : Google Scholar
|
|
80
|
Zhou Y, Deng T, Zhang H, Guan Q, Zhao H,
Yu C, Shao S, Zhao M and Xu J: Hypercholesterolaemia increases the
risk of high-turnover osteoporosis in men. Mol Med Rep.
19:4603–4612. 2019.PubMed/NCBI
|
|
81
|
Luegmayr E, Glantschnig H, Wesolowski GA,
Gentile MA, Fisher JE, Rodan GA and Reszka AA: Osteoclast
formation, survival and morphology are highly dependent on
exogenous cholesterol/lipoproteins. Cell Death Differ. 11(Suppl 1):
S108–S118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dumitru N, Carsote M, Cocolos A, Petrova
E, Olaru M, Dumitrache C and Ghemigian A: The link between bone
osteocalcin and energy metabolism in a group of postmenopausal
women. Curr Health Sci J. 45:47–51. 2019.PubMed/NCBI
|
|
83
|
Papachristou NI, Blair HC, Kypreos KE and
Papachristou DJ: High-density lipoprotein (HDL) metabolism and bone
mass. J Endocrinol. 233:R95–R107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tang Y, Wang S, Yi Q, Xia Y and Geng B:
High-density lipoprotein cholesterol is negatively correlated with
bone mineral density and has potential predictive value for bone
loss. Lipids Health Dis. 20:752021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Barsh GS and Schwartz MW: Genetic
approaches to studying energy balance: Perception and integration.
Nat Rev Genet. 3:589–600. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
86
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dawodu D, Patecki M, Dumler I, Haller H
and Kiyan Y: oxLDL inhibits differentiation of mesenchymal stem
cells into osteoblasts via the CD36 mediated suppression of Wnt
signaling pathway. Mol Biol Rep. 46:3487–3496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Okayasu M, Nakayachi M, Hayashida C, Ito
J, Kaneda T, Masuhara M, Suda N, Sato T and Hakeda Y: Low-density
lipoprotein receptor deficiency causes impaired osteoclastogenesis
and increased bone mass in mice because of defect in osteoclastic
cell-cell fusion. J Biol Chem. 287:19229–19241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hada N, Okayasu M, Ito J, Nakayachi M,
Hayashida C, Kaneda T, Uchida N, Muramatsu T, Koike C, Masuhara M,
et al: Receptor activator of NF-κB ligand-dependent expression of
caveolin-1 in osteoclast precursors, and high dependency of
osteoclastogenesis on exogenous lipoprotein. Bone. 50:226–236.
2012. View Article : Google Scholar
|
|
90
|
Lee KG, Lee GB, Yang JS and Moon MH:
Perturbations of lipids and oxidized phospholipids in lipoproteins
of patients with postmenopausal osteoporosis evaluated by
asymmetrical flow field-flow fractionation and nanoflow
UHPLC-ESI-MS/MS. Antioxidants (Basel). 9:462020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Leitinger N: The role of phospholipid
oxidation products in inflammatory and autoimmune diseases:
Evidence from animal models and in humans. Subcell Biochem.
49:325–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fallah A, Pierre R, Abed E and Moreau R:
Lysophosphatidylcholine-induced cytotoxicity in osteoblast-like
MG-63 cells: Involvement of transient receptor potential vanilloid
2 (TRPV2) channels. Mol Membr Biol. 30:315–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huang MS, Morony S, Lu J, Zhang Z,
Bezouglaia O, Tseng W, Tetradis S, Demer LL and Tintut Y:
Atherogenic phospholipids attenuate osteogenic signaling by BMP-2
and parathyroid hormone in osteoblasts. J Biol Chem.
282:21237–21243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang L, Chai Y, Li C, Liu H, Su W, Liu X,
Yu B, Lei W, Yu B, Crane JL, et al: Oxidized phospholipids are
ligands for LRP6. Bone Res. 6:222018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Graham LS, Parhami F, Tintut Y, Kitchen
CM, Demer LL and Effros RB: Oxidized lipids enhance RANKL
production by T lymphocytes: Implications for lipid-induced bone
loss. Clin Immunol. 133:265–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tseng W, Lu J, Bishop GA, Watson AD, Sage
AP, Demer L and Tintut Y: Regulation of interleukin-6 expression in
osteoblasts by oxidized phospholipids. J Lipid Res. 51:1010–1016.
2010. View Article : Google Scholar :
|
|
97
|
Palmieri M, Almeida M, Nookaew I,
Gomez-Acevedo H, Joseph TE, Que X, Tsimikas S, Sun X, Manolagas SC,
Witztum JL and Ambrogini E: Neutralization of oxidized
phospholipids attenuates age-associated bone loss in mice. Aging
Cell. 20:e134422021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Palmieri M, Kim HN, Gomez-Acevedo H, Que
X, Tsimikas S, Jilka RL, Manolagas SC, Witztum JL and Ambrogini E:
A neutralizing antibody targeting oxidized phospholipids promotes
bone anabolism in chow-fed young adult mice. J Bone Miner Res.
36:170–185. 2021. View Article : Google Scholar
|
|
99
|
Ambrogini E, Que X, Wang S, Yamaguchi F,
Weinstein RS, Tsimikas S, Manolagas SC, Witztum JL and Jilka RL:
Oxidation-specific epitopes restrain bone formation. Nat Commun.
9:21932018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Inagaki M: Structure and biological
activity of glycosphingolipids from starfish and feather stars.
Yakugaku Zasshi. 128:1187–1194. 2008.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liang B, Shen X, Lan C, Lin Y, Li C, Zhong
S and Yan S: Glycolipid toxicity induces osteogenic dysfunction via
the TLR4/S100B pathway. Int Immunopharmacol. 97:1077922021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ran SY, Yu Q, Chen Y and Lin SQ:
Prevention of postmenopausal osteoporosis in Chinese women: A
5-year, double-blind, randomized, parallel placebo-controlled
study. Climacteric. 20:391–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yu WJ, Zhang Z, Fu WZ, He JW, Wang C and
Zhang ZL: Association between LGR4 polymorphisms and peak bone
mineral density and body composition. J Bone Miner Metab.
38:658–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Melgar-Rodríguez S, Cafferata EA, Díaz NI,
Peña MA, González-Osuna L, Rojas C, Sierra-Cristancho A, Cárdenas
AM, Díaz-Zúñiga J and Vernal R: Natural Killer T (NKT) cells and
periodontitis: Potential regulatory role of NKT10 cells. Mediators
Inflamm. 2021:55739372021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Naruo M, Negishi Y, Okuda T, Katsuyama M,
Okazaki K and Morita R: Alcohol consumption induces murine
osteoporosis by downregulation of natural killer T-like cell
activity. Immun Inflamm Dis. 9:1370–1382. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tilkeridis K, Kiziridis G, Ververidis A,
Papoutselis M, Kotsianidis I, Kitsikidou G, Tousiaki NE, Drosos G,
Kapetanou A, Rechova KV, et al: Immunoporosis: A new role for
invariant natural Killer T (NKT) cells through overexpression of
nuclear Factor-κB ligand (RANKL). Med Sci Monit. 25:2151–2158.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wen K, Tao L, Tao Z, Meng Y, Zhou S, Chen
J, Yang K, Da W and Zhu Y: Fecal and serum metabolomic signatures
and microbial community profiling of postmenopausal osteoporosis
mice model. Front Cell Infect Microbiol. 10:5353102020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhao YX, Song YW, Zhang L, Zheng FJ, Wang
XM, Zhuang XH, Wu F and Liu J: Association between bile acid
metabolism and bone mineral density in postmenopausal women.
Clinics (Sao Paulo). 75:e14862020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ruiz-Gaspà S, Guañabens N, Jurado S,
Combalia A, Peris P, Monegal A and Parés A: Bilirubin and bile
acids in osteocytes and bone tissue. Potential role in the
cholestatic-induced osteoporosis. Liver Int. 40:2767–2775. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ahn TK, Kim KT, Joshi HP, Park KH, Kyung
JW, Choi UY, Sohn S, Sheen SH, Shin DE, Lee SH and Han IB:
Therapeutic potential of tauroursodeoxycholic acid for the
treatment of osteoporosis. Int J Mol Sci. 21:42742020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ruiz-Gaspà S, Dubreuil M, Guañabens N,
Combalia A, Peris P, Monegal A and Parés A: Ursodeoxycholic acid
decreases bilirubin-induced osteoblast apoptosis. Eur J Clin
Invest. 44:1206–1214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li Z, Huang J, Wang F, Li W, Wu X, Zhao C,
Zhao J, Wei H, Wu Z, Qian M, et al: Dual targeting of bile acid
Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) prevents
estrogen-dependent bone loss in mice. J Bone Miner Res. 34:765–776.
2019. View Article : Google Scholar
|
|
113
|
Wang Q, Wang G, Wang B and Yang H:
Activation of TGR5 promotes osteoblastic cell differentiation and
mineralization. Biomed Pharmacother. 108:1797–1803. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ruiz-Gaspà S, Guañabens N, Jurado S,
Dubreuil M, Combalia A, Peris P, Monegal A and Parés A: Bile acids
and bilirubin effects on osteoblastic gene profile. Implications in
the pathogenesis of osteoporosis in liver diseases. Gene.
725:1441672020. View Article : Google Scholar
|
|
115
|
Ruiz-Gaspà S, Guañabens N, Enjuanes A,
Peris P, Martinez-Ferrer A, de Osaba MJ, Gonzalez B, Alvarez L,
Monegal A, Combalia A and Parés A: Lithocholic acid downregulates
vitamin D effects in human osteoblasts. Eur J Clin Invest.
40:25–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Medrano-David D, Lopera AM, Londoño ME and
Araque-Marín P: Formulation and characterization of a new
injectable bone substitute composed PVA/Borax/CaCO3 and
demineralized bone matrix. J Funct Biomater. 12:462021. View Article : Google Scholar
|
|
117
|
Deng D, Pan C, Wu Z, Sun Y, Liu C, Xiang
H, Yin P and Shang D: An integrated metabolomic study of
osteoporosis: Discovery and quantification of hyocholic acids as
candidate markers. Front Pharmacol. 12:7253412021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Naito C, Katsumi H, Yoneto K, Omura M,
Nishidono M, Kamei S, Mizoguchi A, Tamba A, Tanaka A, Morishita M
and Yamamoto A: Development of a phosphoric Acid-Mediated
hyaluronic acid gel sheet for efficient transdermal delivery of
alendronate for Anti-osteoporotic therapy. Pharmaceutics.
11:6422019. View Article : Google Scholar
|
|
119
|
Asefy Z, Tanomand A, Hoseinnejhad S,
Ceferov Z, Oshaghi EA and Rashidi M: Unsaturated fatty acids as a
co-therapeutic agents in cancer treatment. Mol Biol Rep.
48:2909–2916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xiao WJ, Ke YH, He JW, Zhang H, Yu JB, Hu
WW, Gu JM, Gao G, Yue H, Wang C, et al: Polymorphisms in the human
ALOX12 and ALOX15 genes are associated with peak bone mineral
density in Chinese nuclear families. Osteoporos Int. 23:1889–1897.
2012. View Article : Google Scholar
|
|
121
|
Wu Y, Zhang M, Chen X, Zhou Y and Chen Z:
Metabolomic analysis to elucidate the change of the n-3
polyunsaturated fatty acids in senescent osteoblasts. Biosci
Biotechnol Biochem. 85:611–620. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Saito A, Yoshimura K, Miyamoto Y, Kaneko
K, Chikazu D, Yamamoto M and Kamijo R: Enhanced and suppressed
mineralization by acetoacetate and β-hydroxybutyrate in osteoblast
cultures. Biochem Biophys Res Commun. 473:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Board M, Lopez C, van den Bos C, Callaghan
R, Clarke K and Carr C: Acetoacetate is a more efficient
energy-yielding substrate for human mesenchymal stem cells than
glucose and generates fewer reactive oxygen species. Int J Biochem
Cell Biol. 88:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yi X, Liu J, Wu P, Gong Y, Xu X and Li W:
The key microRNA on lipid droplet formation during adipogenesis
from human mesenchymal stem cells. J Cell Physiol. 235:328–338.
2020. View Article : Google Scholar
|
|
125
|
Li L, Wang XQ, Liu XT, Guo R and Zhang RD:
Integrative analysis reveals key mRNAs and lncRNAs in monocytes of
osteoporotic patients. Math Biosci Eng. 16:5947–5971. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Reppe S, Refvem H, Gautvik VT, Olstad OK,
Høvring PI, Reinholt FP, Holden M, Frigessi A, Jemtland R and
Gautvik KM: Eight genes are highly associated with BMD variation in
postmenopausal Caucasian women. Bone. 46:604–612. 2010. View Article : Google Scholar
|
|
127
|
Kang YJ, Yoo JI and Baek KW: Differential
gene expression profile by RNA sequencing study of elderly
osteoporotic hip fracture patients with sarcopenia. J Orthop
Translat. 29:10–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang X, Chen K, Chen X, Kourkoumelis N,
Li G, Wang B and Zhu C: Integrative analysis of genomics and
transcriptome data to identify regulation networks in female
osteoporosis. Front Genet. 11:6000972020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shen LF, Chen YJ, Liu KM, Haddad ANS, Song
IW, Roan HY, Chen LY, Yen JJY, Chen YJ, Wu JY and Chen YT: Role of
S-Palmitoylation by ZDHHC13 in mitochondrial function and
metabolism in liver. Sci Rep. 7:21822017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Andersson T, Söderström I, Simonyté K and
Olsson T: Estrogen reduces 11beta-hydroxysteroid dehydrogenase type
1 in liver and visceral, but not subcutaneous, adipose tissue in
rats. Obesity (Silver Spring). 18:470–475. 2010. View Article : Google Scholar
|
|
131
|
Yamatani H, Takahashi K, Yoshida T, Soga T
and Kurachi H: Differences in the fatty acid metabolism of visceral
adipose tissue in postmenopausal women. Menopause. 21:170–176.
2014. View Article : Google Scholar
|
|
132
|
Mahboobifard F, Pourgholami MH, Jorjani M,
Dargahi L, Amiri M, Sadeghi S and Tehrani FR: Estrogen as a key
regulator of energy homeostasis and metabolic health. Biomed
Pharmacother. 156:1138082022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yepuru M, Eswaraka J, Kearbey JD, Barrett
CM, Raghow S, Veverka KA, Miller DD, Dalton JT and Narayanan R:
Estrogen receptor-{beta}-selective ligands alleviate high-fat diet-
and ovariectomy-induced obesity in mice. J Biol Chem.
285:31292–31303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bjune JI, Strømland PP, Jersin R, Mellgren
G and Dankel SN: Metabolic and epigenetic regulation by estrogen in
adipocytes. Front Endocrinol (Lausanne). 13:8287802022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ko SH and Jung Y: Energy metabolism
changes and dysregulated lipid metabolism in postmenopausal women.
Nutrients. 13:45562021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ali D, Figeac F, Caci A, Ditzel N, Schmal
C, Kerckhofs G, Havelund J, Faergeman N, Rauch A, Tencerova M and
Kassem M: High-fat diet-induced obesity augments the deleterious
effects of estrogen deficiency on bone: Evidence from
ovariectomized mice. Aging Cell. 21:e137262022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu HF, Meng DF, Yu P, De JC and Li HY:
Obesity and risk of fracture in postmenopausal women: A
meta-analysis of cohort studies. Ann Med. 55:22035152023.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lee S, Kim JH, Jeon YK, Lee JS, Kim K,
Hwang SK, Kim JH, Goh TS and Kim YH: Effect of adipokine and
ghrelin levels on BMD and fracture risk: An updated systematic
review and meta-analysis. Front Endocrinol (Lausanne).
14:10440392023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sardar A, Gautam S, Sinha S, Rai D,
Tripathi AK, Dhaniya G, Mishra PR and Trivedi R: Nanoparticles of
naturally occurring PPAR-γ inhibitor betulinic acid ameliorates
bone marrow adiposity and pathological bone loss in ovariectomized
rats via Wnt/β-catenin pathway. Life Sci. 309:1210202022.
View Article : Google Scholar
|
|
140
|
López-Gómez JJ, Pérez-Castrillón JL,
García de Santos I, Pérez-Alonso M, Izaola-Jauregui O, Primo-Martín
D and De Luis-Román DA: Influence of obesity on bone turnover
markers and fracture risk in postmenopausal women. Nutrients.
14:16172022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhuang J, Ning H, Wang M, Zhao W, Jing Y,
Liu X, Zu J, Kong P, Wang X, Sun C and Yan J: Downregulated fat
mass and obesity-associated protein inhibits bone resorption and
osteoclastogenesis by nuclear factor-kappa B inactivation. Cell
Signal. 87:1101372021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wawrzyniak N, Suliburska J, Kulczyński B,
Kołodziejski P, Kurzawa P and Gramza-Michałowska A:
Calcium-Enriched pumpkin affects serum leptin levels and fat
content in a rat model of postmenopausal osteoporosis. Nutrients.
13:23342021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Fu Q, Zhang Z, Hu W and Yang Y: The
correlation of triglyceride/high-density lipoprotein cholesterol
ratio with muscle mass in type 2 diabetes patients. BMC Endocr
Disord. 23:932023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kalhan SC, Bugianesi E, McCullough AJ,
Hanson RW and Kelley DE: Estimates of hepatic glyceroneogenesis in
type 2 diabetes mellitus in humans. Metabolism. 57:305–312. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Niu YG and Evans RD: Myocardial metabolism
of triacylglycerol-rich lipoproteins in type 2 diabetes. J Physiol.
587:3301–3315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ishikawa M, Iwasaki Y, Yatoh S, Kato T,
Kumadaki S, Inoue N, Yamamoto T, Matsuzaka T, Nakagawa Y, Yahagi N,
et al: Cholesterol accumulation and diabetes in pancreatic
beta-cell-specific SREBP-2 transgenic mice: A new model for
lipotoxicity. J Lipid Res. 49:2524–2534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yang Q, Xu H, Zhang H, Li Y, Chen S, He D,
Yang G, Ban B, Zhang M and Liu F: Serum triglyceride glucose index
is a valuable predictor for visceral obesity in patients with type
2 diabetes: A cross-sectional study. Cardiovasc Diabetol.
22:982023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gavin KM, Sullivan TM, Maltzahn JK,
Jackman MR, Libby AE, MacLean PS, Kohrt WM, Majka SM and Klemm DJ:
Hematopoietic stem Cell-Derived adipocytes modulate adipose tissue
cellularity, leptin production and insulin responsiveness in female
mice. Front Endocrinol (Lausanne). 13:8448772022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chen Z, Zhao GH, Zhang YK, Shen GS, Xu YJ
and Xu NW: Research on the correlation of diabetes mellitus
complicated with osteoporosis with lipid metabolism, adipokines and
inflammatory factors and its regression analysis. Eur Rev Med
Pharmacol Sci. 21:3900–3905. 2017.PubMed/NCBI
|
|
150
|
Figeac F, Tencerova M, Ali D, Andersen TL,
Appadoo DRC, Kerckhofs G, Ditzel N, Kowal JM, Rauch A and Kassem M:
Impaired bone fracture healing in type 2 diabetes is caused by
defective functions of skeletal progenitor cells. Stem Cells.
40:149–164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Jin C, Tan K, Yao Z, Lin BH, Zhang DP,
Chen WK, Mao SM, Zhang W, Chen L, Lin Z, et al: A novel
Anti-Osteoporosis mechanism of VK2: Interfering with ferroptosis
via AMPK/SIRT1 pathway in type 2 diabetic osteoporosis. J Agric
Food Chem. 71:2745–2761. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yang Y, Lin Y, Wang M, Yuan K, Wang Q, Mu
P, Du J, Yu Z, Yang S, Huang K, et al: Targeting ferroptosis
suppresses osteocyte glucolipotoxicity and alleviates diabetic
osteoporosis. Bone Res. 10:262022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kanda J, Furukawa M, Izumo N, Shimakura T,
Yamamoto N, Takahashi HE and Wakabayashi H: Effects of the
linagliptin, dipeptidyl peptidase-4 inhibitor, on bone fragility
induced by type 2 diabetes mellitus in obese mice. Drug Discov
Ther. 14:218–225. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Peyman H, Elizabeth E, Dominik O and
Robert B: Bone evaluation study-2: Update on the epidemiology of
osteoporosis in Germany. Arch Osteoporos. 19:262024. View Article : Google Scholar
|
|
155
|
Matsunaga T, Miyagi M, Nakazawa T, Murata
K, Kawakubo A, Fujimaki H, Koyama T, Kuroda A, Yokozeki Y, Mimura
Y, et al: Prevalence and characteristics of spinal sagittal
malalignment in patients with osteoporosis. J Clin Med.
10:28272021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Louwers YV and Visser JA: Shared genetics
between age at menopause, early menopause, POI and other traits.
Front Genet. 12:6765462021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Farooqui KJ, Mithal A, Kerwen AK and
Chandran M: Type 2 diabetes and bone fragility-An under-recognized
association. Diabetes Metab Syndr. 15:927–935. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Russell AL, Lefavor R, Durand N, Glover L
and Zubair AC: Modifiers of mesenchymal stem cell quantity and
quality. Transfusion. 58:1434–1440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Giudici KV, de França NAG, Peters BSE,
Fisberg RM and Martini LA: Associations between markers of glucose
metabolism and bone measures among diabetic and non-diabetic
adults. J Diabetes Metab Disord. 20:1247–1255. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Aleidi SM, Al-Ansari MM, Alnehmi EA,
Malkawi AK, Alodaib A, Alshaker M, Benabdelkamel H and Abdel Rahman
AM: Lipidomics profiling of patients with low bone mineral density
(LBMD). Int J Mol Sci. 23:120172022. View Article : Google Scholar : PubMed/NCBI
|