Introduction
Paraquat (PQ; 1,1-dimethyl-4,4-bipyridinium
dichloride) is an effective herbicide that is widely used in
agriculture and is highly toxic to humans (1). As of 2020, >150,000 individuals
will succumb each year to accidental or intentional exposure to PQ
(2). When PQ is absorbed into the
body it can cause damage to the lungs, liver, kidneys, heart and
other organs (3,4). The lungs have a strong dopamine
uptake system to the extent that PQ, which is chemically similar to
polyamines, accumulates mainly in the lungs (5). Therefore, when PQ is absorbed into
the bloodstream, it directly causes damage to vascular endothelial
cells. It has been demonstrated that PQ exposure induces
glutathione redox cycle dysfunction and excessive endothelial
albumin permeability in microvascular endothelial cells (6,7).
Therefore, identification of therapeutic agents that can maintain
the endothelial barrier and attenuate pulmonary microvascular
permeability may reduce mortality and improve prognosis, which is
of great significance for the clinical treatment of PQ-induced
acute lung injury (ALI), but its protective mechanism needs further
study. Endothelial cells, the basic building blocks of blood
vessels, are involved in and regulate a variety of physiological
processes, such as blood and component transport, immune responses
and vascular tone (8,9). Multiple causes of endothelial
dysfunction accelerate the progression of ALI/acute respiratory
distress syndrome (ARDS) (10).
It has been demonstrated that vascular endothelial injury can lead
to damage to the alveolar-capillary barrier, which in turn leads to
increased pulmonary microvascular permeability. The structures of
tight junctions (TJs) or adherens junctions (AJs) maintain the
integrity of the endothelial barrier (11,12). In ARDS, there is increased
permeability of the pulmonary vasculature to circulating fluids,
macromolecules and leukocytes due to a severe inflammatory response
that disrupts the endothelial barrier, leading to alveolar filling
and neutrophilic in-flow. This leads to the high mortality rate of
ARDS (13-15).
Anthrahydroquinone-2,6-disulfonate
(AH2QDS) is a reducing agent that has previously been
demonstrated to bind specifically to PQ to reduce its levels in the
body and thereby improve its survival (16-19). Additionally, it has been
demonstrated that AH2QDS has a protective effect on the
kidney in PQ poisoning (20), and
this protective mechanism may be related to cellular oxidative
stress, inflammatory injury, endoplasmic reticulum stress and
reduced apoptosis; however, its mechanism of action in ALI has not
been elucidated.
As an important signal transduction pathway in
cells, the phosphatidylinositol-3-kinase (PI3K)/protein kinase B
(AKT) signalling pathway is involved in a variety of biological
processes, such as proliferation, differentiation and
anti-inflammatory processes (21,22). It has been demonstrated that
endothelial-type nitric oxide (NO) synthase (eNOS) is a key
regulator of vascular growth and endothelial function, and can
cause relevant biological effects in endothelial cells and airway
epithelial cells, affecting the course of ALI/ARDS (23). NO can be generated by eNOS
catalysis. The excessive generation of NO can induce the generation
of oxygen free radicals, leading to tissue and organ damage, which
serves an important role in the pathophysiology of PQ-intoxicated
(24,25). To delve deeply into the critical
signaling pathway that PQ induces ALI, Sprague-Dawley (SD) rats
were utilized to establish a PQ poisoning model and these rats were
carefully divided into control, PQ poisoning and AH2QDS
treatment groups. Through detailed comparative analysis with
reference genes, enrichment analysis at the gene expression level
was conducted. The Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis revealed that the PI3K/AKT signaling
pathway exhibited a particularly significant gene enrichment
phenomenon in lung tissues after PQ poisoning and AH2QDS
treatment, indicating that it may be the primary signaling pathway
(19,26). Furthermore, according to current
understanding, the specific mechanism of the PI3K/AKT/eNOS
signaling pathway in the protective effect of AH2QDS
against PQ-induced ALI remains unclear, which precisely constitutes
the focus and core of our current study.
In the present study, PQ gavage in rats and PQ
treatment of human pulmonary microvascular endothelial cells
(HPMECs) were used as in vitro and in vivo models to
validate the aforementioned hypothesis. The results suggested that
AH2QDS could enhance the proliferation and migration of
HPMECs. In addition, AH2QDS reduced inflammatory
response and pulmonary microvascular permeability through
inactivating the PI3K/AKT/eNOS pathway, thereby attenuating
PQ-induced ALI.
Materials and methods
Establishment of the ALI model
A total of 48 male SD rats (7-8 weeks old) with an
average weight of 180-200 g were obtained from Changsha Tianqin
Biotechnology Co., Ltd., and the rats were kept at Hainan Medical
University's Experimental Animal Center using specific
pathogen-free standards, with free access to water and food. The
animal room was maintained at 22-24°C with a 12/12-h light-dark
cycle. All animal experiments are conducted in the Experimental
Animal Center of Hainan Medical University. The rats were randomly
divided into the following four groups (n=12) after 7 days of
acclimation feeding: Control group, PQ group, PQ +
AH2QDS group and AH2QDS group (20). To model the poisoning, rats were
fasted for 10 h prior to poisoning with reference to previous
experiments (19,20). The control group was given 5 ml
saline by gavage. The PQ group was administered 200 mg/kg 20% PQ
solution per rat by gavage (Henan Lane Pesticide Factory). The PQ +
AH2QDS group was given 5 ml AH2QDS by gavage
at the molar ratio of PQ:AH2QDS=1:1 (mol:mol) after 2 h
of PQ intoxication, which was used to construct the treatment
model. The AH2QDS group was administered 5 ml
AH2QDS by gavage. To adhere to the principle of humane
endpoints, the following measures were taken during the experiment
to minimize the use of animals while ensuring the scientific rigor
and humanity of the experiment. Firstly, sufficient practice was
conducted and the modelling techniques were mastered beforehand to
significantly reduce the failure rate of animal modelling, thus
ensuring the reliability and validity of the experimental results.
Secondly, during all experimental operations, it was ensured that
animals were anesthetized to minimize potential pain and discomfort
during the experiment. In addition, strict adherence to
experimental ethical norms was implemented and clear humanitarian
endpoints were formulated, tailored to the objectives of the
present study. The specific standards were as follows: i) After 72
h of PQ intervention, as the scientific goals of the research were
achieved, there was no need to continue the experiment; ii) when
animals suffered from pain that was not caused by the experiment
itself and was unexpected before the experiment starts, such as
animals with inherent defects; iii) if it was anticipated before
the experiment that the animals may suffer from pain, but the
degree of pain exceeded expectations during the actual process,
such as severe nasal and oral bleeding, severely abnormal
behaviour, and other situations; and iv) if the pain of the animals
was caused by the experiment itself, and this pain had been
anticipated before the experiment started. Once any of the
aforementioned standards were observed during the experiment, the
animals were immediately euthanized to reduce their suffering. All
animals in the present study were euthanized before the end of the
experiment once these standards were met, ensuring that no animals
would succumb due to any unnecessary reasons during the
experimental process. The animal welfare was always upheld and the
smooth progress of the experiment was ensured while maintaining the
accuracy and reliability of the experimental results. The rats were
euthanized using an overdose of sodium pentobarbital (>150
mg/kg) injected intraperitoneally following exposure to PQ for 72
h. When the cessation of the heartbeat and breathing of the rats
was confirmed, and there were no reflexes, the death of the
experimental animals was confirmed painlessly. Whole lung tissue
was excised, dried and weighed. The left lung was placed in 10%
paraformaldehyde and the right lung was placed in a
cryopreservation tube and stored in a -80°C refrigerator.
The experimental procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and approved by the Ethics Committee of the First
Affiliated Hospital of Hainan Medical University [approval no. 2020
(Research) No. (97); Haikou, China].
Haematoxylin and eosin (H&E)
staining
Rat lung tissue were paraffin-embedded in paraffin
after being fixed in 10% formaldehyde at room temperature for 24 h
and cut into 4-μm-thick sections. After successful staining
with haematoxylin and eosin, pathological changes were detected
under a light microscope. Lung tissue damage was quantified using a
score of 0 (normal) to 3 (severe) that included alveolar wall
oedema, haemorrhage, vascular congestion and polymorphonuclear
leukocyte infiltration. As previously reported, lung injuries were
categorized according to the sum of the scores (27). Lung injuries were graded on a
scale of severity from 0 to 5 (0, normal; 1, mild or very small
amount; 2, mild or small amount; 3, moderate or larger amount; 4,
severe or large amount; and 5, very severe or extremely large
amount).
Evans blue staining
To determine pulmonary vascular permeability, Evans
blue solution (2 mg/kg) was injected into the tail vein of rats,
and the rats were euthanized 1 h after the injection. Lung tissue
was perfused with PBS until the lungs turned white, resected as a
whole, dried and weighed, and the right lung was weighed in half.
The lung tissue was homogenized with formamide and incubated at
60°C in a water bath protected from light for 24 h. After
incubation, the suspension was centrifuged (4°C, 13,400 × g, 15
min) and 200 μl of the supernatant was added to a 96-well
plate, and the absorbance at 620 nm was measured using an enzyme
counter. The amount of Evans blue dye was calculated using the
standard curve method.
Immunohistochemical staining
Immunohistochemical staining was used to detect the
protein expression of vascular endothelial-cadherin (VE-cadherin),
zonula occludens-1 (ZO-1) and CD31 in paraffin sections
(4-μm-thick) of lung tissues. Paraffin sections were dewaxed
(environmental-friendly dewaxing solution; cat. no. G1128; Wuhan
Servicebio Technology Co., Ltd.), rehydrated using descending
alcohol series for antigen repair and recovered; and the sections
were placed in 3% BSA (cat. no. 36100ES25; Shanghai Yeasen
Biotechnology Co., Ltd.) for 30 min at room temperature for
blocking, and then incubated overnight at 4°C with VE-cadherin
antibody (dilution, 1:50; cat. no. XF354429; Invitrogen; Thermo
Fisher Scientific, Inc.), ZO-1 antibody (dilution, 1:1,000; cat.
no. 21773-1-AP; Proteintech Group, Inc.) and CD31 antibody
(dilution, 1:1,000; cat. no. GB113151; Wuhan Servicebio Technology
Co., Ltd.), followed by incubation with HRP-labelled goat
anti-rabbit IgG secondary antibody (dilution, 1:200; cat. no.
GB23303; Wuhan Servicebio Technology Co., Ltd.) for 1 h at room
temperature. Finally, the sections were stained with
diaminobenzidine and counterstained with haematoxylin (room
temperature, 3 min), and observed under a light microscope.
Microscopic images were evaluated using ImageJ software (Fiji
distribution of ImageJ; https://imagej.net/software/fiji/), and the area of
VE-cadherin, ZO-1 and CD31-positive cells was quantified.
Cell culture and treatment
HPMECs were purchased from Otwo Biotechnology
Development Co., Ltd. (cat. no. HTX2255) and cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (Procell Life Science & Technology Co., Ltd.) with 5%
CO2 at 37°C for proliferation. Cells were incubated with
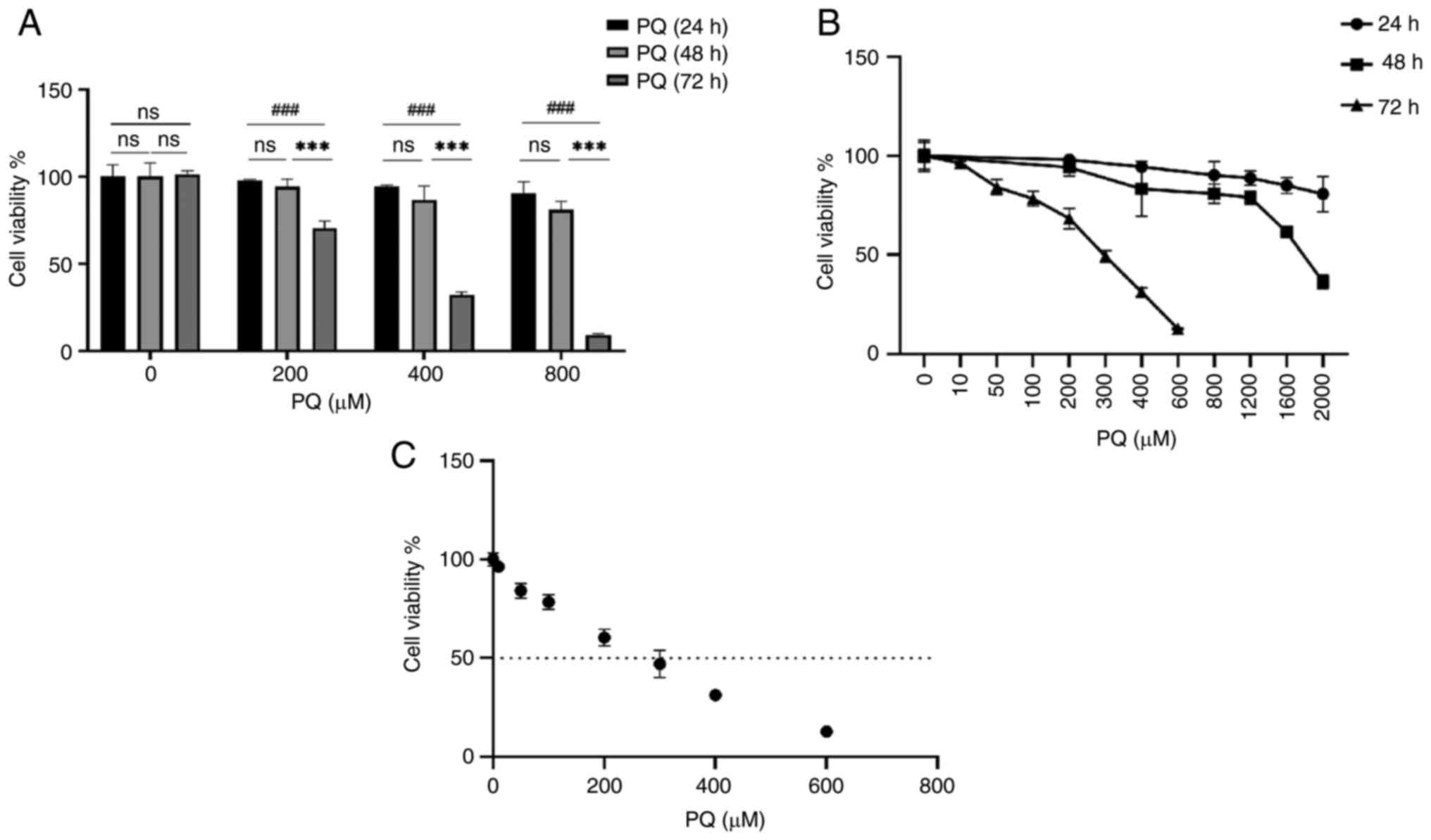
different concentrations of PQ pure (AccuStandard, Inc.) for 24, 48
and 72 h. The results are shown in Fig. S1A. Cell viability was reduced in
a dose-dependent manner, and the reduction in cell viability was
more pronounced after 72 h of treatment than at 48 and 24 h.
Although the IC50 results ranged between 200 and 300
μM (Fig. 1C), the
viability of the cells was <50% after 300 μM treatment.
Therefore, a PQ dose of 200 μM was chosen for subsequent
experiments to treat the cells for 72 h. HPMECs were then
pre-treated with different concentrations of AH2QDS for
2, 6 and 12 h, and 200 μM AH2QDS had the least
effect on cell viability (Fig. 2A and
B). Therefore, 200 μM was selected as the concentration
of AH2QDS for subsequent experiments.
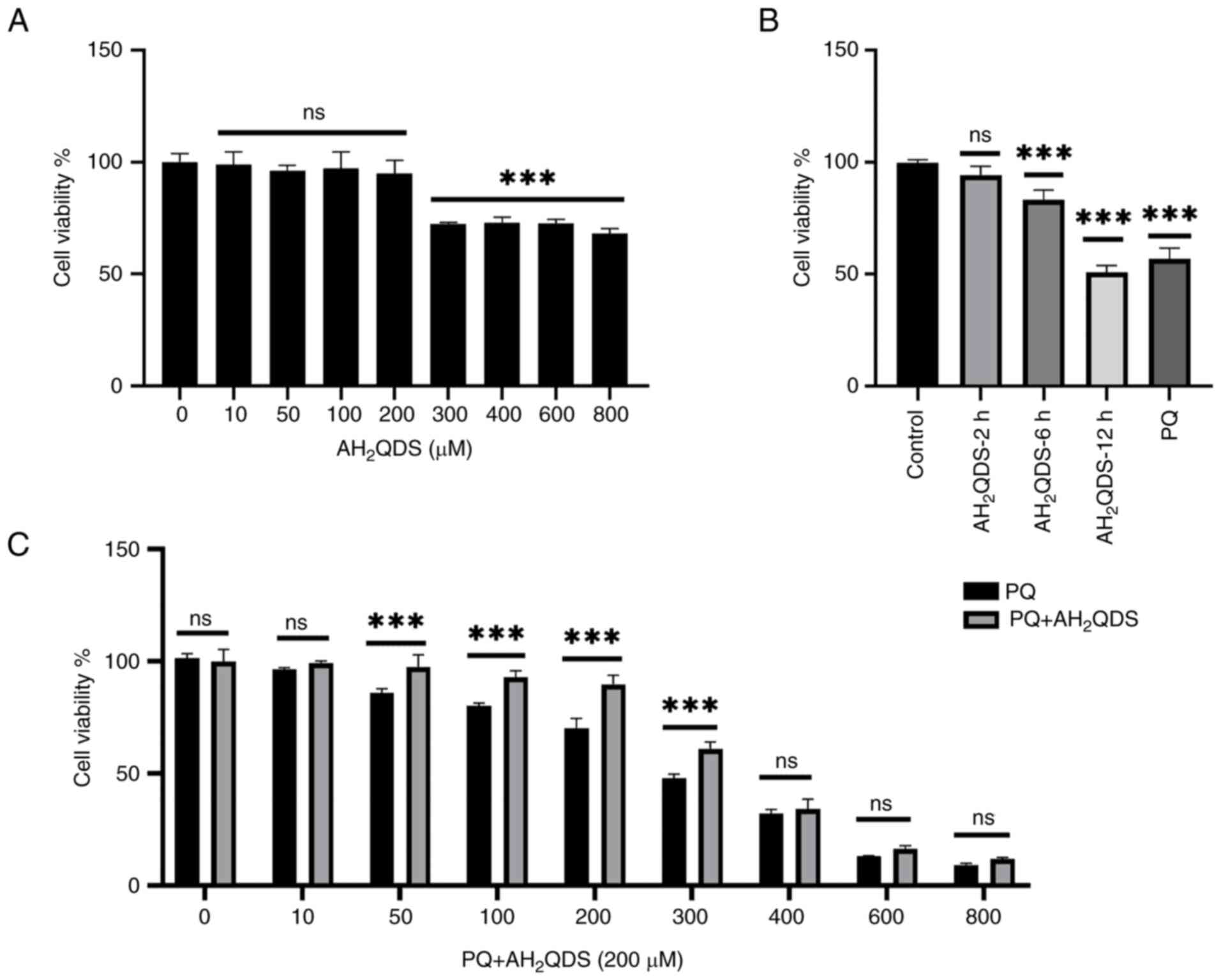 | Figure 2AH2QDS attenuates
PQ-induced cytotoxicity. (A) Cell viability was assessed after
treating cells with 0, 10, 50, 100, 200, 300, 400, 600 and 800
μM AH2QDS for 2 h. (B) Cell viability was
assessed after treating the cells with 200 μM AH2QDS for 2,
6 and 12 h. (C) Cell viability was assessed after pretreatment with
200 μM for 2 h followed by treatment of cells with 0, 10,
50, 100, 200, 300, 400, 600, and 800 μM for 72 h. Data are
expressed as the mean ± SEM of three independent experiments.
Compared with the control (0 μM PQ),
***P<0.001. AH2QDS,
anthrahydroquinone-2,6-disulfonate; PQ, paraquat; not
significant. |
The cells were divided into the following four
groups: Control group, HPMECs were treated with medium for 72 h; PQ
group, cells were stimulated with PQ (200 μM) alone for 72
h; PQ + AH2QDS group, HPMECs were treated with
AH2QDS (200 μM) for 2 h followed by stimulation
of the cells with PQ (200 μM) for 72 h; and
AH2QDS group, cells were stimulated with
AH2QDS (200 μM) alone for 72 h.
Transwell cell migration assay
Experiments were performed using Transwell plates
with a pore size of 5 μm to assess cell migration. Briefly,
3×104 HPMECs were inoculated into the upper part of the
migration chamber containing serum-free medium. The lower chamber
was filled with complete medium containing 20% fetal bovine serum
as an attractant. After 72 h of incubation, migratory cells were
stained with 0.1% crystal violet (Biosharp Life Sciences) at room
temperature for 10 min and non-migratory cells in the upper chamber
were removed with a cotton swab. Cells were then imaged and counted
under a light microscope.
Cell viability assay
To assess PQ and AH2QDS cytotoxicity,
HPMECs in the logarithmic growth phase were homogeneously
inoculated into 96-well plates at a density of 3-5×103
per cell culture (100 μl/well). After waiting for 12 h of
cell apposition, the cells were treated with different
concentrations of PQ for 24, 48 and 72 h. The cells were
pre-treated with different concentrations of AH2QDS for
2 h, and then treated with 200 μM PQ for 72 h. Cell Counting
Kit-8 (CCK-8) solution (10 μl) was added to each well
according to the manufacturer's instructions (cat. no. BS350C;
Anhui Biosharp Technology Co., Ltd.). The 96-well plates were
further incubated in a 37°C cell incubator for 1-2 h. Absorbance
was measured at 450 nm using an enzyme marker.
Measurement of intracellular NO
levels
Intracellular NO levels were measured using the
fluorescent indicator diaminofluorescein-FM diacetate (DAF-FM DA;
Shanghai Yeasen Biotechnology Co., Ltd.). Cells were inoculated at
a density of 1×104 per dish and cultured in confocal
dishes, and cells were pre-treated with AH2QDS for 2 h
and stimulated with PQ (200 μM) for 72 h. After treatment,
the medium was discarded and the cells were gently washed three
times with PBS, and then the cells were treated with 200 μl
DAF-FM DA (5 μM) for 30 min in a 37°C cell incubator. Cells
were washed three times using PBS and kept in PBS throughout the
experiment. Finally, the fluorescence intensity was measured using
a laser confocal microscope with an excitation wavelength of 488 nm
and an emission wavelength of 515 nm. The measured fluorescence
values were analysed using ImageJ software to examine the changes
in fluorescence intensity in each group.
Detection of intracellular reactive
oxygen species (ROS) levels
The level of intracellular ROS was measured using
the Reactive Oxygen Detection kit (cat. no. BL714A; Anhui Biosharp
Technology Co., Ltd.). Cells were inoculated at a density of
1×104 per dish and cultured in confocal dishes, and
cells were pre-treated with AH2QDS for 2 h and
stimulated with PQ (200 μM) for 72 h. After treatment, the
medium was discarded and the cells were gently washed three times
with PBS. Subsequently, the cells were treated with 200 μl
2',7'-dichlorodihydrofluorescein diacetate (10 μM) for 30
min at 37°C in a cell culture incubator, and washed three times
with PBS, and after that, the whole experiment was kept in
serum-free medium. Finally, the fluorescence intensity was measured
using a laser confocal microscope with an excitation wavelength of
488 nm and an emission wavelength of 515 nm. The measured
fluorescence values were analysed using ImageJ software to examine
the changes in fluorescence intensity in each group.
Detection of intracellular
malondialdehyde (MDA) and superoxide dismutase (SOD) levels
After establishing the cell model, the cells were
lysed with RIPA lysis solution and centrifuged at 13,400 g at 4°C
for 10 min, and the supernatant was the desired sample. According
to the instructions of the MDA (cat. no. BL1481A; Anhui Biosharp
Technology Co., Ltd.) and SOD (cat. no. A001-3-2; Nanjing Jiancheng
Bioengineering Institute) detection kits, the relevant reagents
were added to the reaction, and finally the absorbance values were
measured using an enzyme marker at 532 and 450 nm,
respectively.
ELISA
TNF-α, IL-1β and IL-6 levels in the supernatants of
HPMECs were detected using a human ELISA kit (Fine Biotech Co.,
Ltd.) according to the manufacturer's instructions.
Western blotting (WB)
The rat lung tissues and HPMECs were lysed with RIPA
lysis buffer (cat. no. G2002-100ML; Wuhan Servicebio Technology
Co., Ltd.) containing protease inhibitors (cat. no. BL612A; Anhui
Biosharp Technology Co., Ltd.) to prepare protein samples. After
quantification of the protein concentration using a BCA Protein
Assay Kit (cat. no. 20201ES76; Shanghai Yeasen Biotechnology Co.,
Ltd.), protein samples (40 μg) were separated by 7.5-10%
SDS-PAGE, and then transferred to PVDF transfer membranes. The
membranes were blocked with 5% skimmed milk powder for 1 h at room
temperature, and incubated with primary antibody at 4°C overnight.
The following primary antibodies were used: VE-cadherin antibody
(dilution, 1:250; cat. no. XF354429; Invitrogen; Thermo Fisher
Scientific, Inc.), ZO-1 antibody (dilution, 1:10,000; cat. no.
21773-1-AP; Proteintech Group, Inc.), IL-6 antibody (dilution,
1:1,500; cat. no. bs-0782R; BIOSS), IL-1β antibody (dilution,
1:1,000; cat. no. bs-0812R; BIOSS), TNF-α antibody (dilution,
1:1,000; cat. no. BP4903; Wuhan Boster Biological Technology,
Ltd.), PI3K antibody (dilution, 1:1,000; cat. no. 20584-1-AP;
Proteintech Group, Inc.), phosphorylated (p-)PI3K antibody
(dilution, 1:1,000; cat. no. PA5-104853; Invitrogen; Thermo Fisher
Scientific, Inc.), AKT antibody (dilution, 1:1,000; cat. no.
10176-2-AP; Proteintech Group, Inc.), p-AKT antibody (dilution,
1:1,000; cat. no. 28731-1-AP; Proteintech Group, Inc.), eNOS
antibody (dilution, 1:1,000; cat. no. 27120-1-AP; Proteintech
Group, Inc.), β-actin antibody (dilution, 1:1,000; cat. no.
GB11001-100; Wuhan Servicebio Technology Co., Ltd.) and GAPDH
antibody (dilution, 1:1,000; cat. no. GB15004-100; Wuhan Servicebio
Technology Co., Ltd.). The membranes were washed at least three
times with TBS with 1% Tween-20 (TBST) and incubated with secondary
antibody conjugated to HRP-labelled goat anti-rabbit IgG (dilution,
1:10,000; cat. no. BL003A; Anhui Biosharp Technology Co., Ltd.) at
room temperature for 2 h. After three washes with TBST, images of
the bands were captured (Super ECL Detection Reagent-ECL
Ultra-Sensitive Chemiluminescent Detection Kit; cat. no. 36208ES60;
Shanghai Yeasen Biotechnology Co., Ltd.) and analysed using a
chemical gel imager (CHAMP CHEMI TOP 610 PLUS).
Statistical analysis
All data are presented as the mean ± standard error
of the mean (SEM) of at least three independent experiments and
were analysed using GraphPad Prism8 software (Dotmatics). One-way
ANOVA followed by Tukey's multiple comparison test was used to
determine differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
PQ induces cytotoxicity in HPMECs
To study the cytotoxicity of PQ, cell viability was
examined using a CCK-8 assay. Cells were treated with pure PQ (0,
200, 400, 800, 1,200, 1,600 and 2,000 μM) for 24 and 48 h,
and cell viability was assessed at the end of culture. As shown in
Fig. S1A and B, the inhibition
of cell viability by PQ was dose- and time-dependent. After 24 h of
PQ treatment, the cell viability was decreased following 1,600
μM PQ treatment (Fig.
S1A), and after 48 h of PQ treatment, the cell viability was
decreased following 1,200 μM PQ treatment, with a cell
viability of >60% compared with the control in all cases
(Fig. S1B). However, after 72 h
of PQ treatment, the cell viability was significantly decreased
following 200 μM PQ treatment, down to 59% compared with the
control (Fig. S1C). When
calculating the IC50 of PQ, the IC50 of PQ
was 200-300 μM; however, at 300 μM, cell viability
was <50%. Therefore, 200 μM was selected as the
concentration of PQ for subsequent experiments (Fig. 1C). These results indicated that PQ
induced cytotoxicity in HPMECs in a dose- and time-dependent manner
(Fig. 1A and B).
AH2QDS attenuates PQ-induced
cytotoxicity in HPMECs
To investigate the effect of AH2QDS on
the proliferation of cells, cell viability was determined using the
CCK-8 assay. Cells were treated with 0, 10, 50, 100, 200, 300, 400,
600 and 800 μM AH2QDS for 24 h. Cell viability
was assessed at the end of the incubation. As demonstrated in
Fig. 2A, after 2 h, 0, 10, 50,
100 and 200 μM AH2QDS treatment had no
significant effect on cell viability (Fig. 2A); however, 300 μM
AH2QDS and other concentrations of AH2QDS had
an effect on cell viability. Furthermore, when treating the cells
with 200 μM AH2QDS for 2, 6 and 12 h, cell
viability was decreased after 6 h of treatment (Fig. 2B), which demonstrated the time-
and dose-dependent effects of AH2QDS on the viability of
HPMEVCs. To assess the protective effect of AH2QDS
against PQ-induced cytotoxicity, cells were pre-treated with 200
μM AH2QDS for 2 h, and then cell viability was
assessed after exposure to 200 μM PQ for 72 h. As revealed
in Fig. 2C, pretreatment with
AH2QDS significantly increased the PQ-induced decrease
in cell viability (Fig. 2C), but
this protective effect was markedly reduced by PQ at concentrations
>300 μM.
AH2QDS inhibits the PQ-induced
increase in oxidative stress and NO production in HPMECs
The intracellular oxidative stress level was
assessed by detecting ROS, NO fluorescence intensity, MDA and SOD
levels. As shown in Fig. 3A-D,
the fluorescence intensity of ROS and NO in PQ-induced HPMECs was
significantly higher compared with that in the control group,
whereas the fluorescence intensity of ROS and NO in PQ-induced
cells was decreased by pretreatment with AH2QDS,
suggesting that AH2QDS could attenuate the production of
ROS and NO in PQ-induced cells, thus reducing endothelial cell
damage. As demonstrated in Fig. 3E
and F, the levels of SOD and MDA were also examined in HPMECs.
MDA levels were significantly higher and SOD levels were lower in
the PQ group compared with the control group; while MDA levels were
decreased and SOD levels were significantly higher in the
PQ-induced cells after pretreatment with AH2QDS, which
indicated that AH2QDS could attenuate the level of
oxidative stress in the PQ-induced cells. As revealed in Fig. 3G-I, ELISA results revealed that
AH2QDS significantly inhibited the expression of TNF-α,
IL-6 and IL-1β in PQ-induced HPMECs.
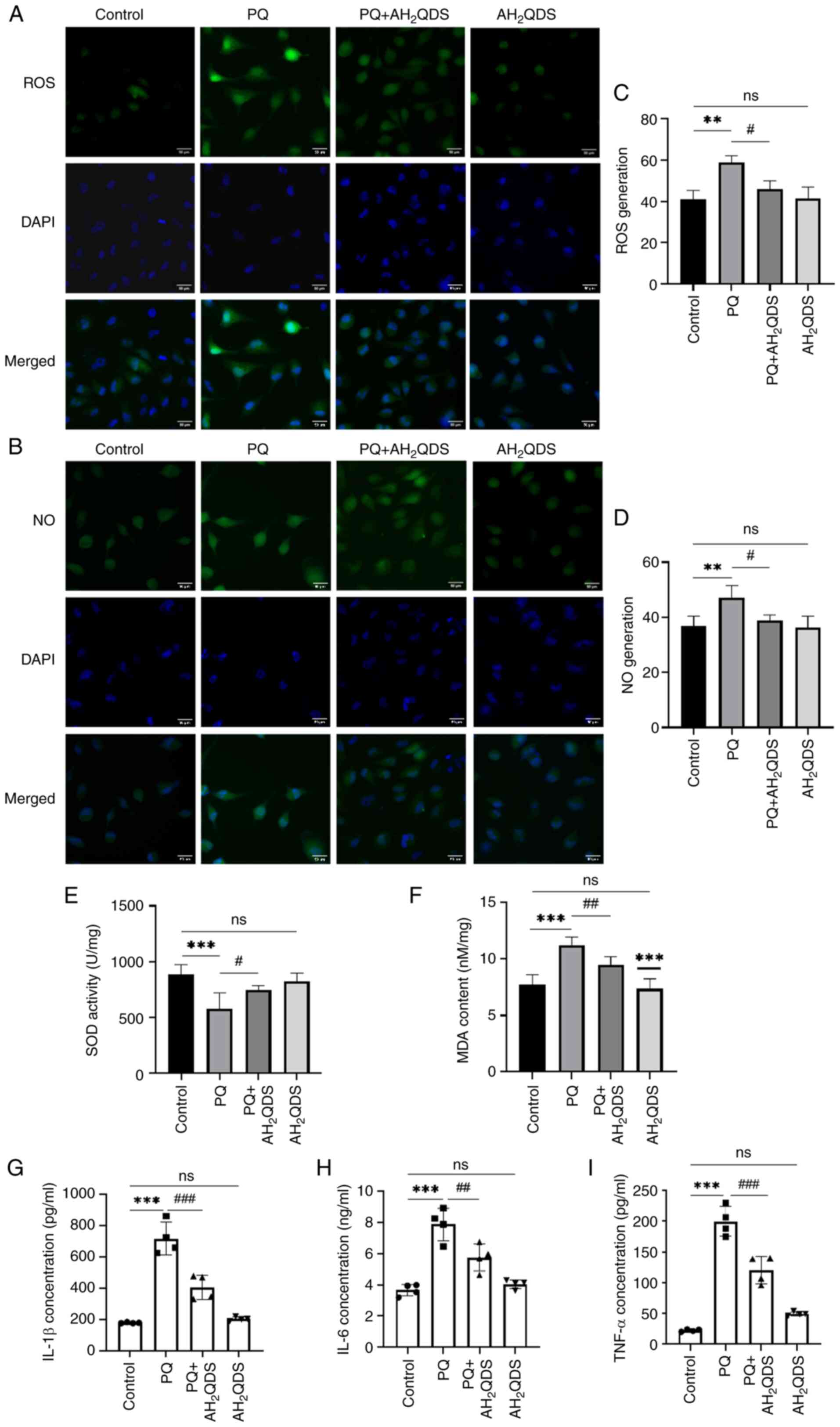 | Figure 3Effect of AH2QDS on
PQ-induced cellular oxidative stress and NO production. (A) ROS
fluorescence detection of ROS content in cells in each group. Scale
bar, 50 μm. ROS: green, DAPI: blue. (B) NO fluorescence
detection of NO content in cells in each group. Scale bar, 50
μm. NO: green, DAPI: blue. (C and D) Quantitative analysis
of ROS and NO in each group. (E and F) SOD and MDA content in each
group of human pulmonary microvascular endothelial cells. (G-I)
ELISA was performed to detect the expression levels of TNF-α, IL-6
and IL-1β in each group. Data are expressed as the mean ± SEM of
three independent experiments. Compared with the control group,
**P<0.01 and ***P<0.001. Compared with
the PQ group, #P<0.05, ##P<0.01 and
###P<0.001. AH2QDS,
anthrahydroquinone-2,6-disulfonate; PQ, paraquat; NO, nitric oxide;
ROS, reactive oxygen species; SOD, superoxide dismutase; MDA,
malondialdehyde; ns, not significant. |
AH2QDS promotes the migration
of HPMECs and maintains normal cellular connectivity
The migration of HPMECs was detected using a
Transwell assay. As displayed in Fig.
4A and B, the number of migratory cells in the PQ group was
significantly lower compared with that in the control group, while
the number was significantly higher after AH2QDS
treatment, which indicated that AH2QDS could promote the
migration of HPMECs. The intracellular protein expression levels of
VE-cadherin and ZO-1 were detected by WB, and the results are shown
in Fig. 4C-E. The protein
expression levels of VE-cadherin and ZO-1 in the PQ group were
significantly reduced, and the protein expression levels were
significantly increased after AH2QDS treatment, which
demonstrated that AH2QDS could maintain the normal
connectivity of HPMECs. The fluorescence intensity of ZO-1 protein
was detected using an immunofluorescence assay. The results in
Fig. 4F and G indicated that ZO-1
protein expression was significantly reduced in the PQ group
compared with the control group, and some cells were deficient in
protein expression. This result was reversed by AH2QDS
treatment.
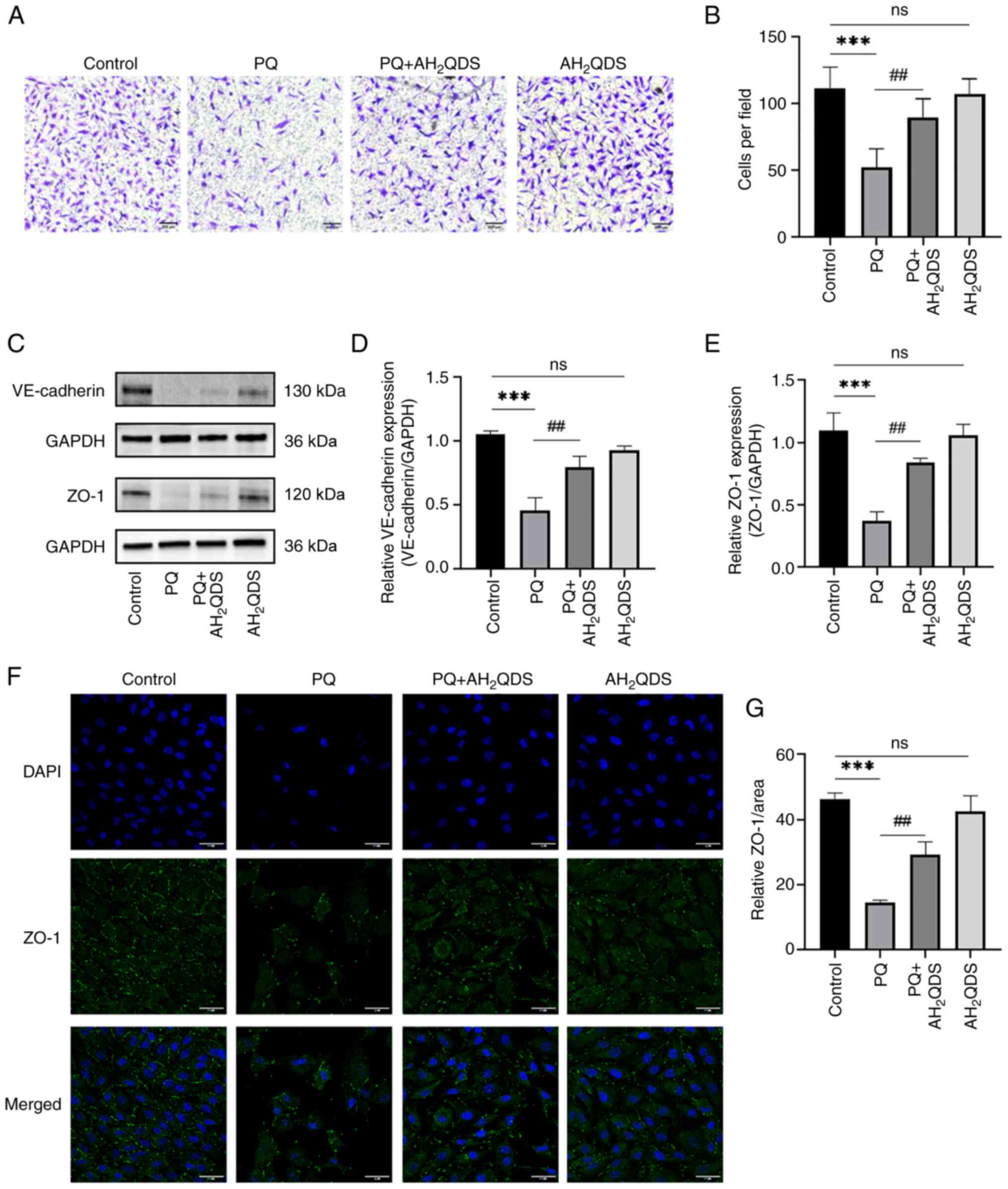 | Figure 4Effect of AH2QDS on cell
migration and connexin. (A) Transwell assay was used to detect that
AH2QDS ameliorates the PQ-induced reduction in cell migration.
Scale bar, 200 μm. (B) Number of migrating cells in the
field of view. (C-E) VE-cadherin protein and ZO-1 protein levels in
each group of HPMECs were detected by western blotting. (F)
Expression of ZO-1 protein in each group of HPMECs was measured by
confocal microscopy. Scale bar, 50 μm. ZO-1: green, DAPI:
blue. (G) ZO-1 protein-positive area in each group. Data are
expressed as the mean ± SEM of three independent experiments.
Compared with the control group, ***P<0.001. Compared
with the PQ group, ##P<0.01. AH2QDS,
anthrahydroquinone-2,6-disulfonate; PQ, paraquat; VE-cadherin,
vascular endothelial-cadherin; ZO-1, zonula occludens-1; HPMECs,
human pulmonary microvascular endothelial cells; ns, not
significant. |
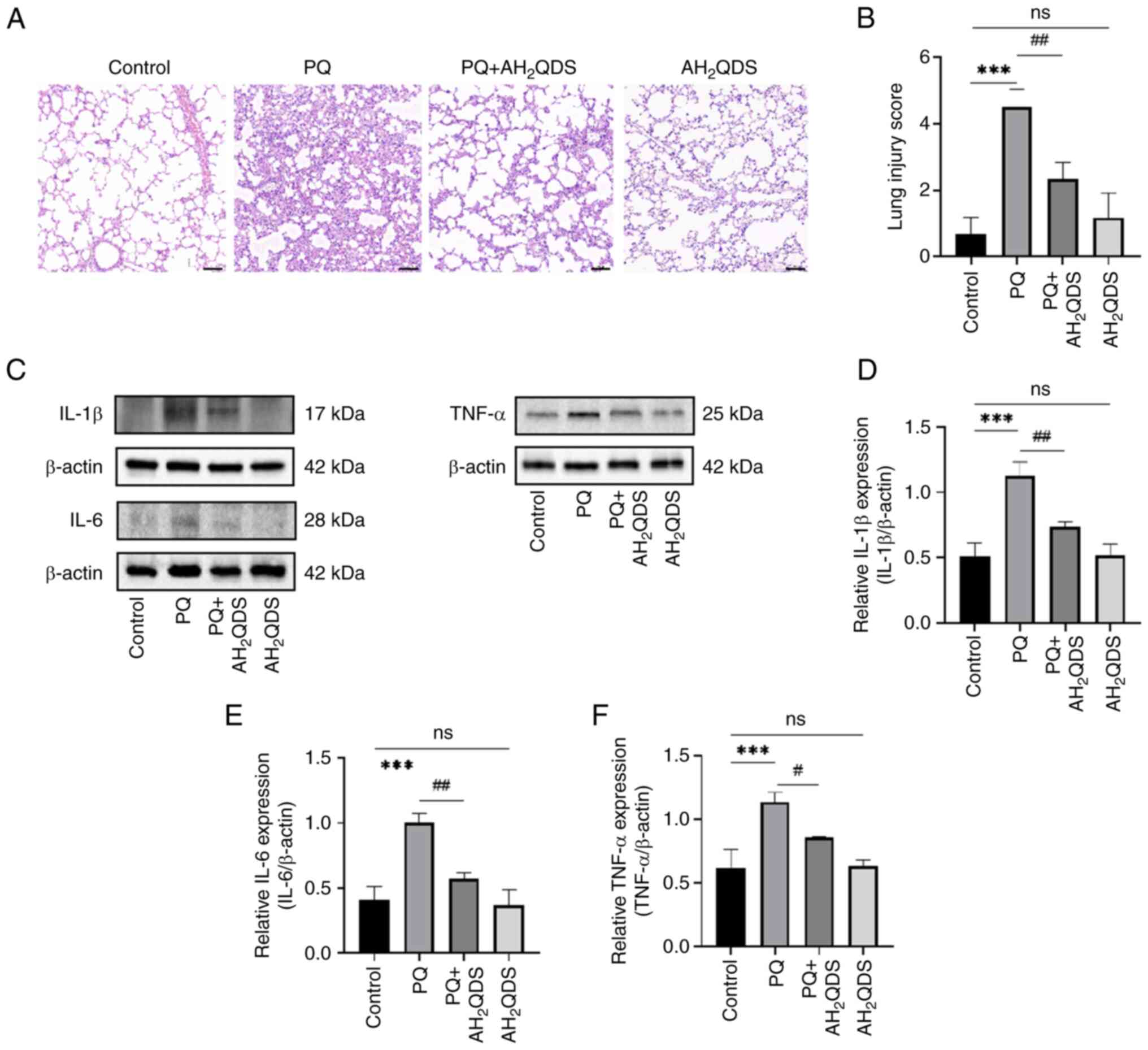
AH2QDS treatment attenuates
PQ-induced ALI in rats
To assess the effect of AH2QDS on
PQ-induced ALI in rats, a PQ-induced ALI rat model was constructed,
and pathological changes in lung tissues were detected by H&E
staining. As demonstrated in Fig.
5A, PQ treatment resulted in marked inflammatory infiltrates,
destruction of alveolar and alveolar wall capillary structures, and
pulmonary oedema in the lung tissues. These symptoms were markedly
improved by AH2QDS treatment. Additionally, as shown in
Fig. 5B, the lung injury score
was significantly higher in the PQ group compared with the control
group, whereas the lung injury score was reduced after
AH2QDS treatment. To verify that AH2QDS could
reduce inflammation in lung tissues, the protein expression levels
of IL-6, IL-1β and TNF-α in lung tissues were detected using WB. As
indicated in Fig. 5C-F, the
protein expression levels of IL-6, IL-1β and TNF-α in the PQ group
were significantly increased compared with those in the control
group; however, the protein expression levels of IL-6, IL-1β and
TNF-α were significantly decreased after AH2QDS
treatment.
AH2QDS improves PQ-induced
pulmonary vascular permeability and connexin expression in
rats
To verify that AH2QDS reduces pulmonary
oedema by decreasing pulmonary microvessel permeability and
decreasing exudation, pulmonary microvessel permeability was
examined using the Evans blue assay (Fig. 6A and B). The protein expression
levels of VE-cadherin and ZO-1 in lung tissues were detected by WB,
and the results were consistent with the results obtained in HPMECs
(Fig. 6C-E). Immunohistochemical
staining results showed that the protein expression levels of
VE-cadherin, ZO-1 and CD31 were significantly reduced in lung
tissues in rats; however, these were significantly increased in the
AH2QDS treatment group (Fig. 6F-I).
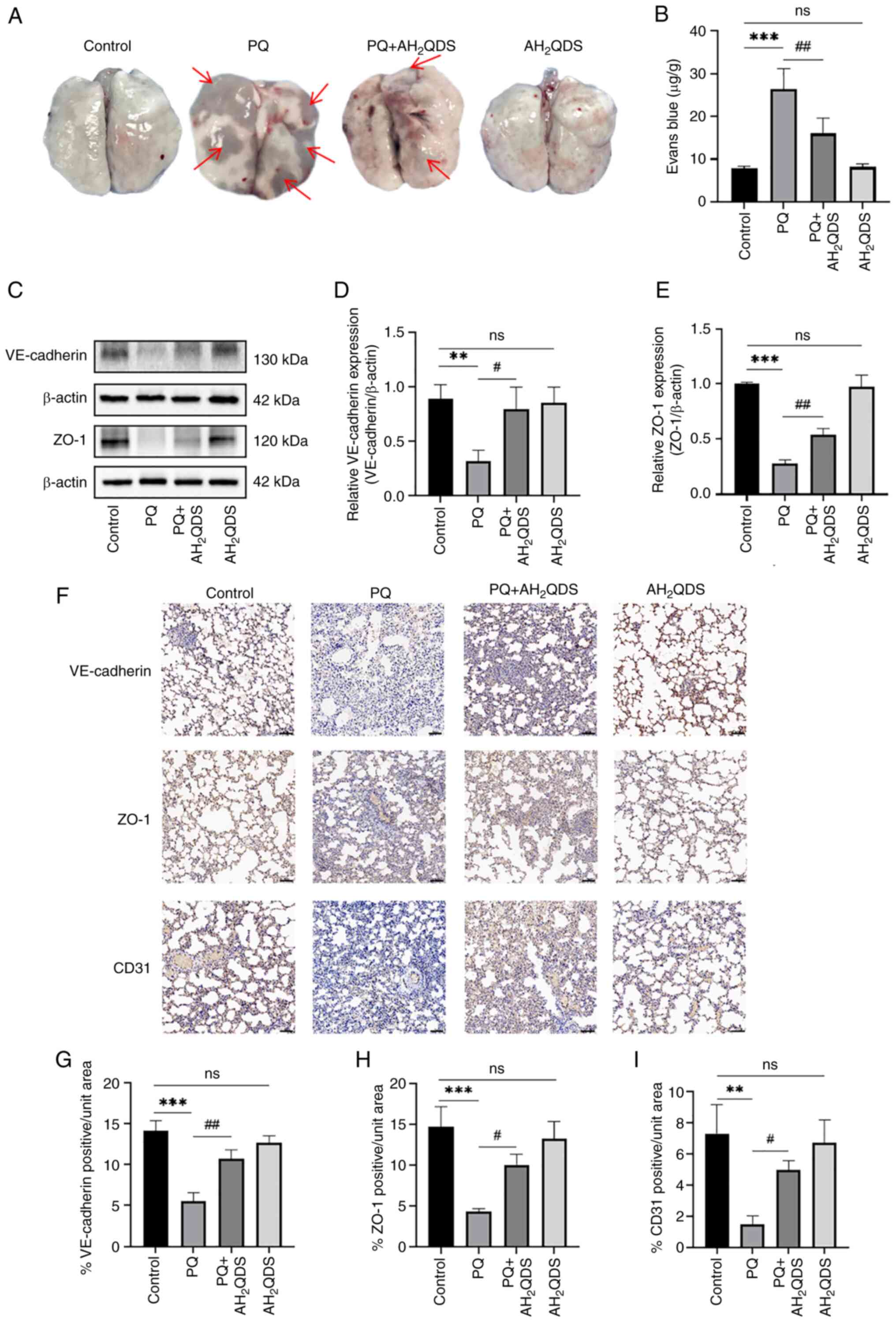 | Figure 6Effect of AH2QDS on
pulmonary microvascular permeability and connexin expression. (A
and B) EB-stained lung tissue and content of EB lung tissue for
determination of lung permeability in rats. Red arrows point to EB
exudation. (C-E) Western blotting detection of VE-cadherin and ZO-1
protein levels in lung tissues of different groups of rats. (F)
Immunohistochemical staining of VE-cadherin, ZO-1 and CD31 proteins
in different groups. Scale bar, 100 μm. (G-I)
Immunohistochemical analysis of VE-cadherin, ZO-1 and CD31 protein
expression content in different groups of rat lung tissues. Data
are expressed as the mean ± SEM of three independent experiments.
Compared with the control group, **P<0.01 and
***P<0.001. Compared with the PQ group,
#P<0.05 and ##P<0.01.
AH2QDS, anthrahydroquinone-2,6-disulfonate; EB, Evans
blue; ZO-1, zonula occludens-1; PQ, paraquat; ns, not
significant. |
AH2QDS ameliorates ALI by
modulating the PI3K/AKT/eNOS signalling pathway
To further elucidate the underlying mechanisms of
AH2QDS for the management of PQ-induced endothelial
dysfunction, the present study concentrated on the PI3K/AKT/eNOS
signalling pathway. As shown in Fig.
7A-C, in rat lung tissues, p-PI3K and p-Akt protein levels were
elevated in the PQ group compared with the control group, whereas
AH2QDS treatment reduced the protein levels of p-PI3K
and p-Akt. As demonstrated in Fig. 7D
and E, PQ increased eNOS expression in rat lung tissues, which
was reversed to a certain extent by AH2QDS treatment. As
revealed in Fig. 7F-H, the
protein levels of p-PI3K and p-Akt were elevated in HPMECs, whereas
AH2QDS treatment suppressed the protein levels of p-PI3K
and p-Akt, which was consistent with the results of the in
vitro model. The results demonstrated that the PI3K/AKT/eNOS
signalling pathway was involved in the beneficial effect of
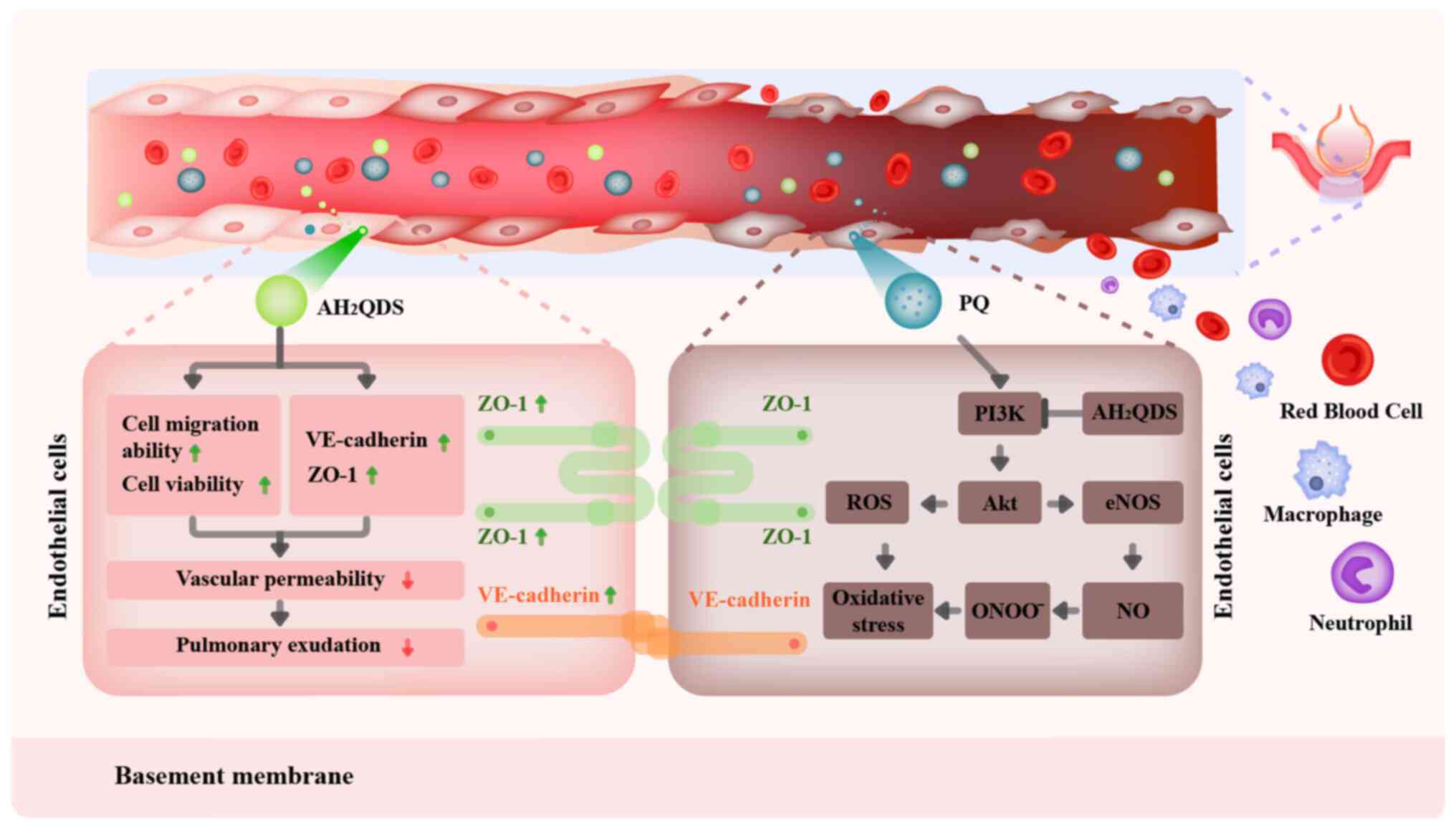
AH2QDS on PQ-induced ALI. The mechanism of
AH2QDS reducing PQ-induced pulmonary microvascular
permeability is presented in Fig.
8.
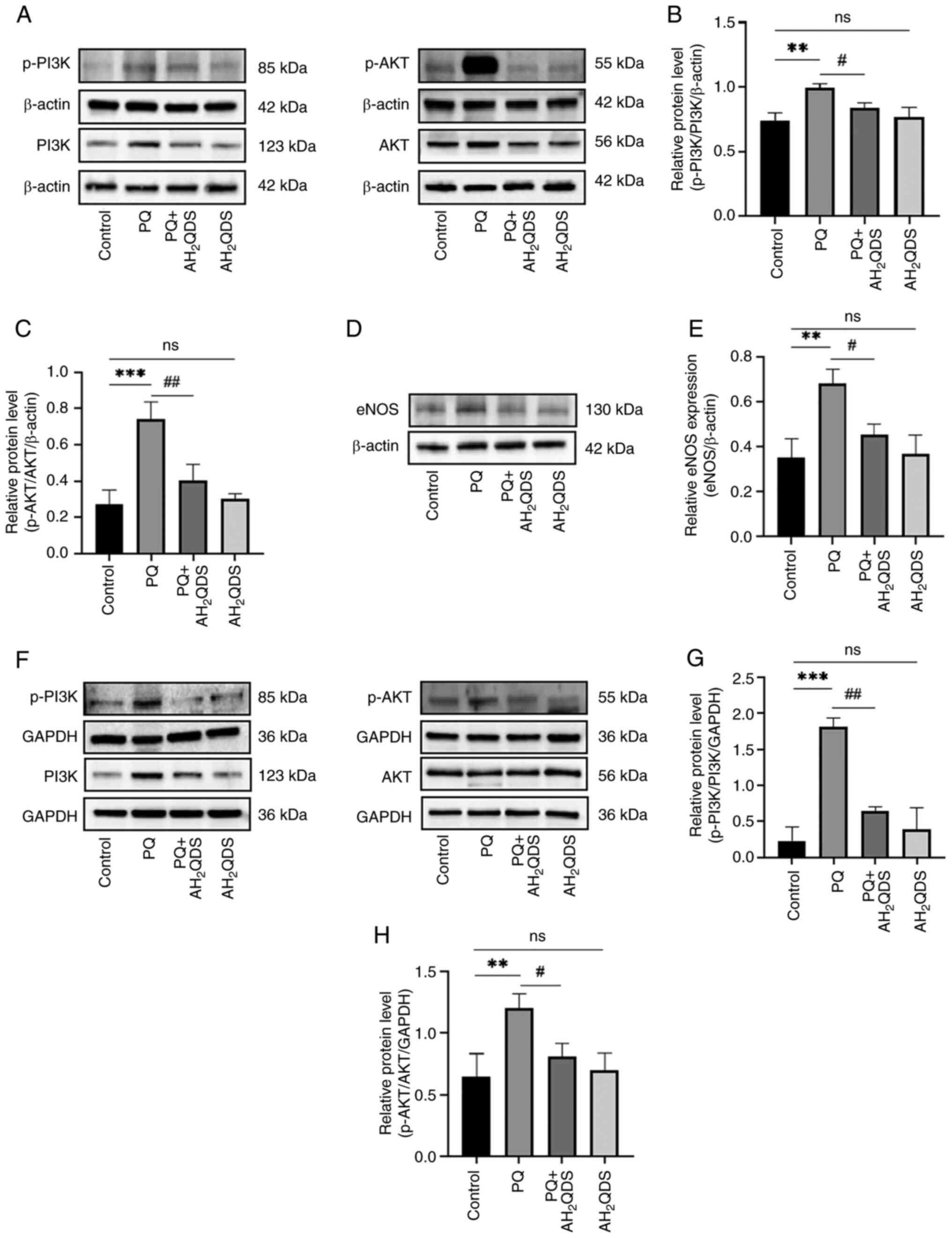 | Figure 7AH2QDS ameliorates
PQ-induced acute lung injury by modulating the PI3K/AKT/eNOS
signalling pathway. (A-C) Expression levels of p-PI3K, PI3K, p-Akt
and AKT proteins in different groups of lung tissues were detected
by WB. (D and E) Expression levels of eNOS proteins in different
groups of lung tissues detected by WB. (F-H) Expression levels of
p-PI3K, PI3K, p-Akt and AKT proteins in different groups of human
pulmonary microvascular endothelial cells were detected by WB. Data
are expressed as the mean ± SEM of three independent experiments.
Compared with the control group, **P<0.01 and
***P<0.001. Compared with the PQ group,
#P<0.05 and ##P<0.01.
AH2QDS, anthrahydroquinone-2,6-disulfonate; PQ,
paraquat; eNOS, eNOS, endothelial-type nitric oxide synthase; p-,
phosphorylated; WB, western blotting; ns, not significant. |
Discussion
PQ-induced ALI/ARDS is an acute and life-threatening
lung disease with a mortality rate of 50-70% (4). Studies have demonstrated that PQ is
absorbed into the bloodstream and first contacts endothelial cells,
leading to oxidative stress, the inflammatory response and
increased vascular permeability in pulmonary vascular endothelial
cells. This induces pulmonary oedema, intra-alveolar haemorrhage
and inflammatory cell infiltration (10,20,28,29), leading to death in severe cases
(30,31). The mechanism by which PQ leads to
endothelial cell dysfunction has not been clarified clinically and
there is a lack of specific drugs and therapeutic options.
Therefore, improving the dysfunction of the alveolar microvascular
barrier could delay the progression of ALI/ARDS, and thus, improve
patient survival (19). A recent
study by the authors demonstrated that AH2QDS could
specifically bind to PQ to reduce the PQ concentration in
vivo and improve survival in SD rats (20). AH2QDS has both potent
anti-inflammatory and antioxidant properties and is effective in
the treatment of PQ-induced acute kidney injury. By constructing an
in vitro model of PQ-induced ALI in SD rats and an in
vitro model of PQ-induced endothelial dysfunction in HPMECs,
the present study revealed that AH2QDS has potential
efficacy in treating PQ-induced endothelial cell dysfunction.
AH2QDS could attenuate PQ-induced ALI by inhibiting
oxidative stress, inflammatory responses, promoting cell migration,
enhancing cell viability and inhibiting the PI3K/AKT/eNOS
signalling pathway to reduce pulmonary microvascular
permeability.
Endothelial dysfunction is a major pathogenic
mechanism in ALI/ARDS (12,32,33). The integrity of the endothelial
barrier is disrupted after endothelial cells are damaged by
multiple pathogenic factors. TJs, AJs and migration of endothelial
cells maintain vascular permeability and reduce exudation and
inflammatory infiltration. These functions maintain endothelial
barrier function and integrity (34-36). In the present study, PQ-induced
ALI tissues were markedly damaged with increased pulmonary
microvascular permeability. This was manifested by pulmonary
oedema, haemorrhage and inflammatory cell infiltration; lung injury
assessment also verified that PQ-induced ALI induced excessive ROS
production and inflammatory responses. VE-cadherin is the major TJ
between endothelial cells. In ALI/ARDS, increased permeability
following disruption of VE-cadherin and its adhesion complexes
occurs with increased lung tissue leakage (37). ZO-1 is a key structure of AJs that
directly affects lung barrier permeability. When ZO-1 expression is
inhibited, lung permeability increases and lung barrier function is
impaired (38). CD31 is a
vascular endothelial cell-specific protein, and it has been
similarly demonstrated that maintaining vascular integrity can
effectively control the course of ALI/ARDS (39,40). The present study demonstrated that
AH2QDS attenuated the PQ-induced inflammatory response
and oxidative stress in HPMECs, upregulated the decrease in
VE-cadherin and ZO-1 protein expression due to PQ, reduced
pulmonary microvascular permeability, and ameliorated pulmonary
oedema. Elevated levels of pro-inflammatory mediators in the lungs
of patients with ALI impair endothelial cell functions, including
proliferation and migration (41,42). These toxic effects lead to an
imbalance between vasodilation and constriction, accelerating
endothelial dysfunction, disrupting vascular homeostasis, and
leading to an increase in vascular permeability, which further
exacerbates interstitial lung leakage. This is consistent with the
present experimental results, which demonstrated that
AH2QDS could markedly improve endothelial cell
migration, promote endothelial cell migration to the site of injury
and maintain the integrity of the pulmonary microvasculature. These
data suggested that AH2QDS ameliorated PQ-induced ALI by
improving endothelial dysfunction.
In the present experiments, it was found that
during the pre-treatment of HPMECs with AH2QDS,
exceeding a certain concentration range can adversely affect the
cell viability of HPMECs. Firstly, as a reducing agent, excessive
use of AH2QDS may disrupt the balance of intracellular
redox reactions, and this imbalance could potentially damage the
cell structure and function. Secondly, as a newly emerging drug,
the stability of AH2QDS and its mechanism of interaction
with cells still require further exploration. Furthermore,
different cell types may exhibit varying reactions to
AH2QDS, indicating that the impact of AH2QDS
on cell viability is a complex and multifaceted process. This
discovery not only reveals the issues that need to be addressed in
the application of AH2QDS as a reducing agent, but also
underscores its potential in scientific research. Further studies
and verifications are needed to gain a deeper understanding of the
mechanism of action of AH2QDS, thereby providing new
treatment strategies and potential drug candidates for medical
fields such as ALI related to PQ.
In a previous study by the authors, KEGG pathway
enrichment analysis revealed that the PI3K/AKT signalling pathway
was the main signalling pathway for gene enrichment in lung tissues
after PQ poisoning and AH2QDS treatment (26). The PI3K/AKT signalling pathway is
important in the regulation of cell survival as well as repairing
endothelial damage (43-47). To further investigate the
mechanism by which AH2QDS alleviated PQ-induced ALI, the
present study focused on the PI3K/AKT/eNOS signalling pathway. eNOS
is an enzyme present in large quantities that is expressed in
several cell types and promotes the production of NO. During
inflammation, eNOS induces large amounts of NO production,
generating peroxynitrite anion, which causes damage to the vascular
endothelium and contributes to the development of inflammation
(48,49). eNOS serves as a downstream target
of AKT, and the PI3K/AKT/eNOS signalling pathway is also an
important signalling pathway in cells. The present study
demonstrated that the expression levels of PI3K, AKT and eNOS were
elevated in lung tissues after PQ intoxication, whereas the
expression levels of PI3K, AKT and eNOS were significantly lower in
lung tissues treated with AH2QDS compared with the
previous ones. The NO fluorescence intensity was significantly
higher in HPMECs compared with the control group, and significantly
decreased after AH2QDS treatment compared with the PQ
group. It was demonstrated that AH2QDS could inhibit the
PI3K/AKT/eNOS signalling pathway to attenuate the inflammatory
response of endothelial cells. Therefore, the present study
revealed that the mechanism by which AH2QDS alleviated
endothelial dysfunction during PQ-induced ALI progression was
related to the PI3K/Akt/eNOS signalling pathway.
In recent years, organoid technology has become an
indispensable model system in lung research. Besides being
cultivated from adult lung stem/progenitor cells, lung organoids
can also be derived from fetal tissue or induced pluripotent stem
cells, greatly filling the technological gap in modelling lung
development in vitro (50). With continuous technological
advancements, significant progress has been made in the
characterization and refinement of organoid culture systems
(51). The strategic
implementation and continuous improvement of this technology will
provide exciting new opportunities for us to deeply understand and
explore new treatment methods, potentially leading to significant
improvements in the health conditions of patients with lung
diseases. With the continuous progress of organoid technology, its
application exploration in the field of lung diseases is
increasingly deepening. This 3D spatial structure constructed by
different types of epithelial cells, with its significant
advantages of requiring a small number of samples and short
construction time, has effectively compensated for the shortcomings
of traditional 2D cell culture and animal models. Therefore, the
organoid model has become an efficient in vitro model for
lung disease research, drug screening and disease prediction, while
also meeting strict ethical requirements for experiments. It is
involved in multiple fields such as non-specific inflammation,
infection and lung tumors. Especially during the period of the
novel coronavirus infection (COVID-19), numerous researchers have
actively attempted to use organoid technology to construct research
models for COVID-19 to explore effective treatment options, thereby
reducing respiratory symptoms, risks of multi-organ failure and
mortality (52,53). Additionally, some studies have
demonstrated the great potential of organoid technology. For
instance, Okabe et al (54) found that orthotopic fetal lung
tissue direct injection into the lungs of mice showed a preventive
effect against PQ-induced ALI. Meanwhile, Chen et al
(55) discovered that luteolin
enhances trans-epithelial sodium transport in 3D alveolar
epithelial organoid, effectively reducing respiratory symptoms.
These studies have fully proven that organoid technology has broad
application prospects in the field of personalized medicine.
In reviewing the current research, some limitations
were indeed identified. Firstly, while the present study focused on
the protective effect of AH2QDS on PQ-induced lung
microvascular endothelial dysfunction and its potential mechanism,
it has not yet compared this finding with the latest progress in
organoid research. As an emerging research tool, organoids have
shown great potential in simulating human organ functions.
Therefore, in future work, the authors will consider incorporating
the application of AH2QDS in organoid models into the
research scope to further validate its effect and mechanism.
Secondly, although the present study revealed the beneficial
effects of AH2QDS on lung tissue, it has not yet deeply
identified the key genetic targets of its action. To fully
understand the mechanism of AH2QDS, high-throughput
research such as proteomics will be conducted to find and validate
the key genes and proteins related to the function of
AH2QDS. Additionally, the present study primarily
focused on the protective effect of AH2QDS on rat lung
tissue, but it is unclear whether other organs can also benefit
from it. To broaden the application range of AH2QDS, its
protective effects will be investigated on other organs (including
the heart and brain) in subsequent studies and explore its
potential mechanisms. In terms of signaling pathway research, while
the present study paid attention to the importance of the PI3K/AKT
signaling pathway in the action of AH2QDS, it lacks
direct experimental evidence of intervention in this pathway.
Therefore, PI3K inhibitors and activators will be introduced in
subsequent studies to clarify the specific role of the PI3K/AKT
signaling pathway in the action of AH2QDS. Finally, to
more comprehensively evaluate the therapeutic effect of
AH2QDS, comparative studies with other types of
inhibitors will be conducted. Finally, to more comprehensively
evaluate the therapeutic effect of AH2QDS in treating
ALI, a series of comparative studies will be conducted.
AH2QDS shall be compared with traditional Chinese
medicines such as Yu-Ping-Feng-San and Xuanfei Baidu Formula to
explore their differences in improving ALI symptoms and reducing
inflammatory responses. Additionally, AH2QDS shall be
also compared with emerging synthetic drugs, such as inhalable
nanomaterials and extracellular vesicles, which have shown
potential value in the treatment of ALI in recent years. Through
these comparative studies, it is expected to gain a more
comprehensive understanding of the advantages and limitations of
AH2QDS in treating ALI, providing a more scientific
basis for future clinical applications. In summary, a series of
follow-up studies will be conducted to improve the mechanism of
action and therapeutic effect evaluation of AH2QDS,
aiming to provide new ideas and methods for the prevention and
treatment of related diseases.
In conclusion, AH2QDS attenuated
PQ-induced ALI by inhibiting the PI3K/Akt/eNOS signalling pathway,
suppressing inflammatory responses and oxidative stress, promoting
cell proliferation and migration, and reducing pulmonary vascular
hyperpermeability. These findings suggested that AH2QDS
may serve as an effective potential therapeutic agent for reversing
ALI/ARDS outcomes caused by PQ.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NL, YY and JC conceived and designed the study. YH,
JP and ZL provided administrative support. All authors provided
study materials. YW and JZ collected and assembled the data. CX and
HL analyzed the data. NL and YY wrote the manuscript. NL and YY
confirm the authenticity of all the raw data. JL and XL were
responsible for the critical review of the manuscript. All authors
participated in interpretation of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved [approval no. 2020
(Research) No. (97)] by the Ethics Committee of the First
Affiliated Hospital of Hainan Medical University (Haikou, China),
and was carried out in accordance with the ethical standards of
experimental animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
PQ
|
paraquat
|
|
AH2QDS
|
anthrahydroquinone-2,6-disulfonate
|
|
SD
|
Sprague-Dawley
|
|
ALI
|
acute lung injury
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
ROS
|
reactive oxygen species
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
AKT
|
protein kinase B
|
|
NO
|
nitric oxide
|
|
eNOS
|
endothelial-type NO synthase
|
|
TJs
|
tight junctions
|
|
AJs
|
adherens junctions
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
H&E
|
haematoxylin and eosin staining
|
|
WB
|
western blotting
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
PBS
|
phosphate-buffered saline
|
|
TBST
|
Tris-buffered saline with
Tween-20
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81960351) and the High-level Talent
Fund of Hainan (grant no. 822RC835).
References
|
1
|
Zhang D, Shen F, Ma S, Nan S, Ma Y, Ren L,
Li H and Yu Q: Andrographolide alleviates paraquat-induced acute
lung injury by activating the Nrf2/HO-1 pathway. Iran J Basic Med
Sci. 26:653–661. 2023.PubMed/NCBI
|
|
2
|
Amin F, Memarzia A, Roohbakhsh A, Shakeri
F and Boskabady MH: Zataria multiflora and pioglitazone affect
systemic inflammation and oxidative stress induced by inhaled
paraquat in rats. Mediators Inflamm. 2021:55750592021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar
|
|
4
|
Zhang Y, Yuan D, Li Y, Yang F, Hou L, Yu
Y, Sun C, Duan G, Meng C, Yan H, et al: Paraquat promotes acute
lung injury in rats by regulating alveolar macrophage polarization
through glycolysis. Ecotoxicol Environ Saf. 223:1125712021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Wang N, Ma Z, Wang Y, Yuan Y, Zhong
Z, Hong Y and Zhao M: Lipoxin A4 protects against paraquat-induced
acute lung injury by inhibiting the TLR4/MyD88-mediated activation
of the NF-κB and PI3K/AKT pathways. Int J Mol Med. 47:862021.
View Article : Google Scholar
|
|
6
|
Li T, Cheng S, Xu L, Lin P and Shao M:
Yue-bi-tang attenuates adriamycin-induced nephropathy edema through
decreasing renal microvascular permeability via inhibition of the
Cav-1/eNOS pathway. Front Pharmacol. 14:11389002023. View Article : Google Scholar
|
|
7
|
Huang Y and He Q: Inhibition of c-Src
protects paraquat induced microvascular endothelial injury by
modulating caveolin-1 phosphorylation and caveolae mediated
transcellular permeability. Environ Toxicol Pharmacol. 52:62–68.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu J, Chen R, An J, Wang Y, Liang M and
Huang K: Dauricine attenuates vascular endothelial inflammation
through inhibiting NF-κB pathway. Front Pharmacol. 12:7589622021.
View Article : Google Scholar
|
|
9
|
Wu B, Xu MM, Fan C, Feng CL, Lu QK, Lu HM,
Xiang CG, Bai F, Wang HY, Wu YW and Tang W: STING inhibitor
ameliorates LPS-induced ALI by preventing vascular endothelial
cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol
Sin. 43:2055–2066. 2022. View Article : Google Scholar :
|
|
10
|
Lin F, Yang Y, Wei S, Huang X, Peng Z, Ke
X, Zeng Z and Song Y: Hydrogen sulfide protects against high
glucose-induced human umbilical vein endothelial cell injury
through activating PI3K/Akt/eNOS pathway. Drug Des Devel Ther.
14:621–633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RH, Xie YX, Qiu JW and Chen JY:
Influence of LincRNA-p21 on acute lung injury in sepsis. Eur Rev
Med Pharmacol Sci. 24:5618–5626. 2020.PubMed/NCBI
|
|
12
|
Hao Y, Wang Z, Frimpong F and Chen X:
Calcium-permeable channels and endothelial dysfunction in acute
lung injury. Curr Issues Mol Biol. 44:2217–2229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan Y, Learoyd J, Meliton AY, Leff AR and
Zhu X: Inhibition of Pyk2 blocks lung inflammation and injury in a
mouse model of acute lung injury. Respir Res. 13:42012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pang L, Deng P, Liang YD, Qian JY, Wu LC,
Yang LL, Yu ZP and Zhou Z: Lipoic acid antagonizes paraquat-induced
vascular endothelial dysfunction by suppressing mitochondrial
reactive oxidative stress. Toxicol Res (Camb). 8:918–927. 2019.
View Article : Google Scholar
|
|
15
|
Cong P, Tong C, Mao S, Shi L, Shi X, Liu
Y, Jin H, Liu Y and Hou M: DDAH1 promotes lung endothelial barrier
repair by decreasing leukocyte transendothelial migration and
oxidative stress in explosion-induced lung injury. Oxid Med Cell
Longev. 2022:84076352022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu C, Li F and Zhou S: Humus respiration
and its ecological significance. Acta Ecol Sin. 29:1535–1542.
2009.
|
|
17
|
Chunyuan W, Qinfen L and Dongming W: A
rapid detoxification solution and method for glyphosate. CN Patent
201610341330.6. Filed May 23, 2016; issued September 21, 2016.
|
|
18
|
Wu C, Wu D, Liu X, Qian J and Li Q: A
specific antidote for acute paraquat poisoning. CN Patent
201910908879.2. Filed September 25, 2019; issued December 20,
2019.
|
|
19
|
Qian J, Wu CY, Wu DM, Li LH, Li Q, Deng T,
Huang QF, Xu SQ, Wang HF, Wu XX, et al:
Anthrahydroquinone-2-6-disulfonate is a novel, powerful antidote
for paraquat poisoning. Sci Rep. 11:201592021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Wang B, Lin KW, Deng T, Huang QF, Xu
SQ, Wang HF, Wu XX, Li N, Yi Y, et al:
Anthrahydroquinone-2,6-disulfonate alleviates paraquat-induced
kidney injury via the apelin-APJ pathway in rats. Asian Pac J Trop
Biomed. 12:333–342. 2022. View Article : Google Scholar
|
|
21
|
Ahmed MAE, El Morsy EM and Ahmed AAE:
Protective effects of febuxostat against paraquat-induced lung
toxicity in rats: Impact on RAGE/PI3K/Akt pathway and downstream
inflammatory cascades. Life Sci. 221:56–64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo W, Tao Y, Chen S, Luo H, Li X, Qu S,
Chen K and Zeng C: Rosmarinic acid ameliorates pulmonary
ischemia/reperfusion injury by activating the PI3K/Akt signaling
pathway. Front Pharmacol. 13:8609442022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi D, Tang X, He J, Wang D, Zhao Y, Deng
W, Deng X, Zhou G, Xia J, Zhong X and Pu S: Omentin protects
against LPS-induced ARDS through suppressing pulmonary inflammation
and promoting endothelial barrier via an Akt/eNOS-dependent
mechanism. Cell Death Dis. 7:e23602016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gopallawa I, Kuek LE, Adappa ND, Palmer JN
and Lee RJ: Small-molecule Akt-activation in airway cells induces
NO production and reduces IL-8 transcription through Nrf-2. Resp
Res. 22:2672021. View Article : Google Scholar
|
|
25
|
Liu F, Sa Y, Li Y, Liu R, Wang Z, Li S,
Zhang Y and Ma Z: Research progress on the correlation between
acute hypoxic lung injury and NO, eNOS. Med Innov China.
19:184–188. 2022.In Chinese.
|
|
26
|
Li N, Huang Y, Yi Y, Qian J, Li Q, Xu SQ,
Wang HF, Wu XX, Peng JC, Li LH, et al: Analysis of abnormal
expression of signaling pathways in PQ-induced acute lung injury in
SD rats based on RNA-seq technology. Inhal Toxicol. 36:1–12. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Li N, Zhan X, Zheng T, Huang X,
Chen Q, Song Z, Yang F, Nie H, Zhang Y, et al: Capsaicin protects
against lipopolysaccharide-induced acute lung injury through the
HMGB1/NF-κB and PI3K/AKT/mTOR pathways. J Inflamm Res.
14:5291–5304. 2021. View Article : Google Scholar :
|
|
28
|
Tatjana V, Domitille S and Jean-Charles S:
Paraquat-induced cholesterol biosynthesis proteins dysregulation in
human brain microvascular endothelial cells. Sci Rep. 11:181372021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song CY, Feng MX, Li L, Wang P, Lu X and
Lu YQ: Tripterygium wilfordii Hook.f. ameliorates paraquat-induced
lung injury by reducing oxidative stress and ferroptosis via
Nrf2/HO-1 pathway. Ecotoxicol Environ Saf. 252:1145752023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang J, Huang K, Xu S, Garcia JGN, Wang C
and Cai H: Targeting NOX4 alleviates sepsis-induced acute lung
injury via attenuation of redox-sensitive activation of
CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox
Biol. 36:1016382020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai Q, Jin Y, Jia Z and Liu Z: Paraquat
induces lung injury via miR-199-mediated SET in a mouse model.
Front Pharmacol. 13:8564412022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen K, Wang X, Wang Y, Jia Y, Zhang Y,
Wang K, Luo L, Cai W, Li J, Li S, et al: miR-125b-5p in adipose
derived stem cells exosome alleviates pulmonary microvascular
endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung
injury. Redox Biol. 62:1026552023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim Y, Bae CR, Kim D, Kim H, Lee S, Zhang
H, Noh M, Kim YM, Mochizuki N and Kwon YG: Efficacy of CU06-1004
via regulation of inflammation and endothelial permeability in
LPS-induced acute lung injury. J Inflamm (Lond). 20:132023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komarova YA, Kruse K, Mehta D and Malik
AB: Protein interactions at endothelial junctions and signaling
mechanisms regulating endothelial permeability. Circ Res.
120:179–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong W, He B, Qian H, Liu Q, Wang D, Li J,
Wei Z, Wang Z, Xu Z, Wu G, et al: RAB26-dependent autophagy
protects adherens junctional integrity in acute lung injury.
Autophagy. 14:1677–1692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng XY, Lu QY, Zhang JF, Li JF, Shi MY,
Huang SY, Yu SF, Zhao YM and Fan HJ: A novel animal model of
primary blast lung injury and its pathological changes in mice. J
Trauma Acute Care Surg. 93:530–537. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia W, Zhang H, Pan Z, Li G, Zhou Q, Hu D
and Liu Y: Inhibition of MRP4 alleviates sepsis-induced acute lung
injury in rats. Int Immunopharmacol. 72:211–217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou W, Shi G, Bai J, Ma S, Liu Q and Ma
X: Colquhounia root tablet protects rat pulmonary microvascular
endothelial cells against TNF-α-induced injury by upregulating the
expression of tight junction proteins claudin-5 and ZO-1. Evid
Based Complement Alternat Med. 2018:10246342018. View Article : Google Scholar
|
|
39
|
Birnhuber A, Fließer E, Gorkiewicz G,
Zacharias M, Seeliger B, David S, Welte T, Schmidt J, Olschewski H,
Wygrecka M and Kwapiszewska G: Between inflammation and thrombosis:
Endothelial cells in COVID-19. Eur Respir J. 58:21003772021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao Y, Jin H, Lei K, Bai LP, Pan H, Wang
C, Zhu X, Tang Y, Guo Z, Cai J and Li T: Oridonin inhibits
inflammation of epithelial cells via dual-targeting of CD31 Keap1
to ameliorate acute lung injury. Front Immunol. 14:11633972023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang N, Tian H, Zhan E, Zhai L, Jiao P,
Yao S, Lu G, Mu Q, Wang J, Zhao A, et al: Reverse-D-4F improves
endothelial progenitor cell function and attenuates LPS-induced
acute lung injury. Respir Res. 20:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen DQ, Shen MJ, Wang H, Li Y, Tang AL,
Li S, Xiong MC, Guo Y and Zhang GQ: Sirt3 maintains microvascular
endothelial adherens junction integrity to alleviate sepsis-induced
lung inflammation by modulating the interaction of VE-cadherin and
β-catenin. Oxid Med Cell Longev. 2021:89787952021. View Article : Google Scholar
|
|
43
|
Singh D, Kumar V and Singh C: IFN-γ
regulates xanthine oxidase-mediated iNOS-independent oxidative
stress in maneb- and paraquat-treated rat polymorphonuclear
leukocytes. Mol Cell Biochem. 427:133–143. 2017. View Article : Google Scholar
|
|
44
|
Li N, Sun W, Zhou X, Gong H, Chen Y, Chen
D and Xiang F: Dihydroartemisinin protects against dextran sulfate
sodium-induced colitis in mice through inhibiting the PI3K/AKT and
NF-κB signaling pathways. Biomed Res Int. 2019:14158092019.
View Article : Google Scholar
|
|
45
|
Wang L, Tang X and Li S: Propofol promotes
migration, alleviates inflammation, and apoptosis of
lipopolysaccharide-induced human pulmonary microvascular
endothelial cells by activating PI3K/AKT signaling pathway via
upregulating APOM expression. Drug Dev Res. 83:397–406. 2022.
View Article : Google Scholar
|
|
46
|
Li YH, Yuan Y, Wang YW and Zhao M: The
role of PI3K/AKT-mediated apoptosis signaling pathway in paraquat
poisoning-induced cardiac injury. Chin J Diffic Compl Cas.
20:278–282. 2021.
|
|
47
|
Zhong R, Xia T, Wang Y, Ding Z, Li W, Chen
Y, Peng M, Li C, Zhang H and Shu Z: Physalin B ameliorates
inflammatory responses in lipopolysaccharide-induced acute lung
injury mice by inhibiting NF-κB and NLRP3 via the activation of the
PI3K/Akt pathway. J Ethnopharmacol. 284:1147772022. View Article : Google Scholar
|
|
48
|
Xin W, Yanxia Z, Haixia L, Aijun L, Shuang
L, Huimin C and Zheng CW: Effect of i NOS and cell apoptosis on
renal injury in rats with acute paraquat poisoning. Mod J Integr
Traditi Chin West Med. 25:2747–2750. 2016.
|
|
49
|
Evans CE, Peng Y, Zhu MM, Dai Z, Zhang X
and Zhao YY: Rabeprazole promotes vascular repair and resolution of
sepsis-induced inflammatory lung injury through HIF-1α. Cells.
11:14252022. View Article : Google Scholar
|
|
50
|
Hughes T, Dijkstra KK, Rawlins EL and
Hynds RE: Open questions in human lung organoid research. Front
Pharmacol. 13:10830172023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liberti DC and Morrisey EE: Organoid
models: Assessing lung cell fate decisions and disease responses.
Trends Mol Med. 27:1159–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bojkova D, Klann K, Koch B, Widera M,
Krause D, Ciesek S, Cinatl J and Münch C: Proteomics of
SARS-CoV-2-infected host cells reveals therapy targets. Nature.
583:469–472. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Riva L, Yuan S, Yin X, Martin-Sancho L,
Matsunaga N, Pache L, Burgstaller-Muehlbacher S, De Jesus PD,
Teriete P, Hull MV, et al: Discovery of SARS-CoV-2 antiviral drugs
through large-scale compound repurposing. Nature. 586:113–119.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Okabe R, Chen-Yoshikawa TF, Yoshizawa A,
Hirashima T, Saito M, Date H and Takebe T: Orthotopic foetal lung
tissue direct injection into lung showed a preventive effect
against paraquat-induced acute lung injury in mice. Eur J
Cardiothorac Surg. 58:638–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen L, Yu T, Zhai Y, Nie H, Li X and Ding
Y: Luteolin enhances transepithelial sodium transport in the lung
alveolar model: Integrating network pharmacology and mechanism
study. Int J Mol Sci. 24:101222023. View Article : Google Scholar : PubMed/NCBI
|