Introduction
Maxillofacial trauma is increasing on a global scale
(1), which poses challenges in
regenerating jaw tissue and fixing alveolar bone defects. However,
the potential of stem cell technology for facilitating tissue
regeneration and reconstruction has emerged (2). Notably, periodontal stem cells have
been identified as ideal seed cells for tissue regeneration, which
is attributed to their robust proliferation and versatile
differentiation capabilities (3,4).
Over the years, extensive efforts have been undertaken to unravel
the optimal conditions for osteogenic differentiation of human
periodontal ligament stem cells (hPDLSCs), and previous research
has revealed the pivotal role of the external microenvironment in
influencing stem cell differentiation towards specific cell types
(5).
Naringenin (NAR) is abundant in citrus, grape and
tomato (6), and holds notable
research and development potential. Specifically, various studies
have indicated its anti-inflammatory and antioxidant capacities
(7,8) and its role in conditions such as
tumors, cancer and hyperlipidemia (9). However, there is limited research
on the impact of NAR on bone metabolism. Kaczmarczyk-Sedlak et
al (10) and Gera et
al (11) observed improved
osteoporosis symptoms in rats treated with NAR. A previous study
also demonstrated the ability of NAR to effectively enhance the
osteogenic differentiation of hPDLSCs (12). Despite these findings, the
precise mechanism underlying the promotion of osteogenic
differentiation of hPDLSCs by NAR remains unclear.
In the past, the exploration of new drugs and the
detection of drug targets involved extensive, time-consuming and
costly experiments (13).
However, recent advancements in biomolecular technology have
offered more possibilities for modern medical research. The
development of multi-omics technology, morphology and physiology
has enabled the explanation of entire biological systems and their
activities (14).
Simultaneously, the rapid progress in network pharmacology
(15) has made the prediction of
drug action targets possible. Integrating network pharmacology with
multi-omics facilitates a comprehensive understanding of drug
effects on biological systems in a multi-dimensional, multi-level
mechanistical manner. This approach offers new perspectives on
unraveling the intricate principles of Traditional Chinese Medicine
and provides more evidence for its application (16). Consequently, the present study
aimed to explore the target and mechanism of NAR in promoting the
osteogenic differentiation of hPDLSCs using mRNA sequencing,
network pharmacology and experimental validation.
Materials and methods
Cell culture and differentiation
The present study was approved by the Ethics
Committee of The Affiliated Stomatology Hospital, Southwest Medical
University (Luzhou, China; approval no. 20221107003). Premolar
teeth were extracted for orthodontic reasons, and written consent
was provided by the guardian and patient for the use of these teeth
in the present research project. In total, 34 premolars were
collected from patients (12-14 years old) from November to
December, 2022 at The Affiliated Stomatology Hospital, Southwest
Medical University. PBS containing 5, 2 and 1%
streptomycin/penicillin were prepared and used for washing roots,
while the periodontal membrane was delicately removed from the
middle 1/3 of the roots. The periodontal ligament tissue was seeded
in a culture flask with 2 ml complete medium, which contained 89% a
MEM medium (Gibco; Thermo Fisher Scientific, Inc.), 10% fetal
bovine serum (Procell Life Science & Technology Co., Ltd.) and
1% streptomycin/penicillin (Beyotime Biotechnology Co., Ltd.). The
flask was placed inverted in an incubator at 37°C (5%
CO2). After 2-3 h, the cell culture flask was inverted.
Third-generation cells were collected for adipogenic and osteogenic
induction. The medium was changed to either osteogenic medium
[complete medium with 50 μg/ml ascorbic acid (Beyotime
Biotechnology Co., Ltd.), 100 nmol/l dexamethasone (Beijing
Solarbio Science & Technology Co., Ltd.) and 10 mmol/l
β-glycerophosphate (Beijing Solarbio Science & Technology Co.,
Ltd.)] or adipogenic induction medium [complete medium with 100
μmol/l indomethacin (Beijing Solarbio Science &
Technology Co., Ltd.), 0.5 mmol/l 3-isobutyl-1-methyl xanthine
(Beijing Solarbio Science & Technology Co., Ltd.), 10
μg/ml insulin (Beyotime Biotechnology Co., Ltd.) and 1
μmol/l dexamethasone (Beijing Solarbio Science &
Technology Co., Ltd.)] and refreshed every 3 days. Alizarin red S
staining (2%) for calcified nodules (37°C, 1 h) was performed after
28 days, while oil red O staining (0.3%) for fat droplet (37°C, 30
min) was conducted after 21 days.
Flow cytometry
Surface markers for the third-generation hPDLSCs
were identified by flow cytometry. Cells were resuspended in 1-2 ml
PBS and placed into assay tubes. Flow cytometry assay-associated
surface marker antibodies (BD Biosciences), including CD31 (cat.
no. 560984; FITC), CD44 (cat. no. 561858; PE), CD45 (cat. no.
560976; FITC), CD105 (cat. no. 560839; PE) and CD90 (cat. no.
555596; PE), were pre-diluted for use at the recommended volume per
test. These antibodies were added to the cells and incubated at 4°C
for 30 min in the dark. Subsequently, the cells were washed with
PBS thrice, resuspended in PBS and subjected to flow cytometry
using a BD FACS Calibur (BD Biosciences). Evaluation and analysis
of stem cell characteristics were conducted by detecting the
specific stem cell surface markers CD31, CD44, CD45, CD90 and
CD105. The results were analyzed by BD CellQuest Pro software 5.1
(BD Biosciences).
Cell treatment
Cells treated with NAR (10 μmol/l) were also
treated with NG-nitro-L-arginine methyl ester (L-NAME; 70
μM) to inhibit endothelial nitric oxide synthase (eNOS),
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 20 μM) to
inhibit soluble guanylate cyclase (sGC) and AKT inhibitor VIII (58
nM) to inhibit AKT, as recommended by the manufacturer
(MedChemExpress for all).
Cytotoxicity analysis
The third-generation hPDLSCs were seeded at a
density of 5×103 cells per well in 96-well plates, and
the impact of 10 μmol/l NAR on the viability of hPDLSCs was
assessed. After 7 days, the absorbance at 450 nm was measured with
a plate reader (Thermo Fisher Scientific, Inc.) following the
instructions provided by the manufacturer of Cell Counting Kit-8
(37°C, 1 h; APeXBIO Technology LLC).
Detection of protein expression by
western blotting
After 7 days of culture, cells subjected to
different treatments were washed with PBS and lysed by RIPA lysis
buffer (cat. no. PC101; Shanghai Epizyme Biotech Co., Ltd.)
containing protease (cat. no. GRF101; Shanghai Epizyme Biotech Co.,
Ltd.) and phosphatase inhibitors (cat. no. GRF102; Shanghai Epizyme
Biotech Co., Ltd.; 1:100). The BCA protein assay kit was used to
determine the concentration of proteins. After the addition of 5X
SDS-PAGE protein loading buffer, the samples underwent a 10-min
incubation in a metal water bath at 100°C. Subsequently, 20
μg protein per lane was subjected to 6, 7.5 or 10% SDS-PAGE,
then transferred onto PVDF membranes. After blocking (room
temperature, 10 min) by Protein Free Rapid Sealing Solution (cat.
no. PS108P; Shanghai Epizyme Biotech Co., Ltd.), the membranes were
incubated with 1:2,000 diluted primary antibodies (ImmunoWay
Biotechnology Company) against Runt-related transcription factor 2
(RUNX2; cat. no. YT5356), osteopontin (OPN; cat. no. YT3467), sGC
(cat. no. YN5360), protein kinase G (PKG; cat. no. YN1879),
transient receptor potential cation channel, subfamily C, member 6
(TRPC6; cat. no. YN4084), AKT (cat. no. YT0185), phosphorylated
(p)-AKT (cat. no. YP0006), eNOS (cat. no. YT3174), p-eNOS (cat. no.
YP0514) and GAPDH (cat. no. YN5585) for 24 h at 4°C, followed by a
1-h incubation with the Goat Anti-Rabbit IgG H&L antibody
(Beijing Biosynthesis Biotechnology Co., Ltd.; cat. no. YP0514;
1:3,000) at room temperature. Protein bands were developed by
Omni-ECL™ Femto Light Chemiluminescence kit (cat. no. SQ201;
Shanghai Epizyme Biotech Co., Ltd.) after immersion of the membrane
in the developing solution. GAPDH served as an internal reference
to calculate the relative expression of other target proteins by
ImageJ (version 1.52; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
After 7 days of incubation, total RNA extraction was
performed with Rapid RNA Extraction kit (Hunan Accurate Bio-Medical
Technology Co., Ltd.), and RNA was reverse transcribed using
Reverse Transcription Premix Kit according to the manufacturer's
protocol (Hunan Accurate Bio-Medical Technology Co., Ltd.). The
primer sequences for qPCR are listed in Table I. The primers (Sangon Biotech
Co., Ltd.) SYBR (Hunan Accurate Bio-Medical Technology Co., Ltd.)
and cDNA were added in octuplet tubes and qPCR was performed
following a preset program (step 1: 95°C, 30 sec, 1 cycle; step 2:
95°C, 5 sec, 40 cycles; step 3: 60°C, 30 sec, 40 cycles) (Bio-Rad
Laboratories, Inc.). Gene expression was normalized to GAPDH, which
served as an internal reference. The 2−ΔΔCq method was
used for data analysis (17).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| RUNX2 |
CCCAGTATGAGAGTAGGTGTCC |
GGGTAAGACTGGGTCATAGGACC |
| OPN |
GATGGCCTTGTATGCACCATTC |
GCAGACCTGACATCCAGTACC |
| sGC |
GACCGATTCAGATGAGGATAGGA |
GGTAAGTGGTTGGGCTGACA |
| PKG |
AACGAGCTGGGACAAGTACCG |
GATCCTTGGACTGGTGGGCTC |
| TRPC6 |
AAGACATCTTCAAGTTCATGGTC |
TCAGCGTCATCCTCAATTTCC |
Alkaline phosphatase (ALP) staining
After 7 days of incubation at 37°C, cells from
different treatment groups were washed with PBS thrice then fixed
with 4% paraformaldehyde for 30 min at room temperature. The cells
were then incubated with freshly prepared ALP staining reagent
(Sangon Biotech Co., Ltd.) at 37°C for 30 min. Subsequently, ALP
staining was observed and recorded by light microscopes and
cameras.
Alizarin red staining
Following 28 days of incubation, cells from various
treatment groups were washed with PBS thrice and subsequently fixed
with 4% paraformaldehyde for 30 min at room temperature. Next,
cells underwent 2-3 washes with 2-3 ml double-distilled water.
Finally, 1 ml alizarin red dye (Beijing Solarbio Science &
Technology Co., Ltd.) was added to each well, and the cells were
incubated for 30 min at 37°C in an incubator. The wells were then
washed thoroughly with double-distilled water, and the mineralized
nodules were observed and recorded by light microscopes and
cameras.
Molecular docking
The three-dimensional (3D) structure of the AKT
receptor protein (ID: 1UNQ) was downloaded from the Research
Collaboratory for Structural Bioinformatics Protein Data Bank (PDB)
database (https://www.rcsb.org/). Additionally,
the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was queried for NAR
(compound CID: 932), and the molecular ligand 3D structure was
retrieved and downloaded. Subsequently, both the AKT protein and
NAR molecular files were converted to a PDBQT format, which
involved the elimination of all water molecules and the addition of
polar hydrogen atoms. Molecular docking was then conducted by using
Autodock 4.2 (18) and Autodock
Vina 1.2.2 (19), and these
tools were employed to calculate the binding energy between AKT and
NAR. The visualization of the final docking results was performed
using Ligplot v.2.2.4 (EMBL-EBI) and Pymol 2.4.0 (Schrödinger,
Inc.).
Quantitative detection of differentially
expressed genes and correlation analysis
The high-throughput sequencing service and
subsequent bioinformatics analysis were provided by Novogene Co.
Ltd. (Beijing, China). Briefly, after 7 days of culture, total RNA
was extracted by TRIzol (Thermo Fisher Scientific, Inc.) from the
cells of various treatment groups. Subsequently, the gene
expression of both the control and experimental groups was
assessed, the differentially expressed mRNAs (GEO ID: GSE266150)
were identified by Novogene Co. Ltd., with the value of
|log2FoldChange|>1 and Padj <0.01. This was followed by Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, which
characterized the functional pathways associated with the
differentially expressed genes.
Protein-Protein Interaction (PPI)
network
The key genes involved in the cGMP-PKG signaling
pathway were identified using the KEGG database online tool
(https://www.genome.jp/kegg/). The NAR
target gene set was obtained through the Encyclopedia of
Traditional Chinese (20)
database (http://www.tcmip.cn/ETCM/index.php/Home/Index/index.html).
These two gene sets were entered into the STRING database
(https://cn.string-db.org/) to generate
the PPI network. The network was analyzed using Cytoscape 3.9.1
(https://cyto-scape.org/) and the genes were
ranked after calculating the score of each gene. The top ten ranked
genes were selected to construct the core sub-network.
cGMP ELISA
The cGMP ELISA was conducted using the human cyclic
guanosine monophosphate ELISA kit (cat. no. F2502-A; Beijing
Huabodeyi Biotechnology Co., Ltd.). After a 2-day incubation, the
medium from hPDLSCs from various treatment groups was collected.
The cGMP standard solutions were diluted to 0.1, 0.2, 0.4, 0.8 and
1.6 nmol/l according to the provided instructions Subsequently, the
samples and the standard solutions were added to a 96-well plate
and incubated at 37°C for 30 min. After completion of the reaction
between the samples or the standard solutions with the Enzyme
Coating Plates, the samples and the standards were discarded. Next,
the washing solution was added to the 96-well plate five times,
then 50 μl enzyme labeling reaction solution was added to
all wells except the blank wells. The reaction was then incubated
at 37°C for an additional 30 min. The enzyme labeling reaction
solution was then discarded and the 96-well plate was washed with
washing solution five times. Color rendering solution A and B were
added to the 96-well plate successively, then the 96-well plate was
incubated at 37°C for 10 min without light. Subsequently,
terminating solution was added to terminate the reaction. Finally,
the cGMP level was quantified according to the optical density (OD)
at 405 nm by comparison with the standard curves.
NO detection
NO detection was conducted using the Griess Reagent
kit (cat. no. S0021S; Beyotime Biotechnology Co., Ltd.). hPDLSCs
were seeded in 6-well plates at a density of 1.0×106
cells per well, subjected to the various aforementioned treatments
for 24 h, then the supernatant was collected. The NaNO2
standard solutions with concentrations ranging from 0 to 100
μM were prepared with complete medium. Next, 50 μl
standards and 50 μl samples were added separately to each
well of a 96-well plate. Subsequently, NO detection reagents I and
II were added (50 μl each; 37°C, 10 min). After completion
of the reaction, Finally, the resulting solutions were quantified
according to the OD at 540 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Animal treatment
A total of 12 Sprague-Dawley (SD) male rats (180-220
g, 6 weeks old) purchased from Southwest Medical University
Laboratory Animal Center (Luzhou, China), were divided equally into
the control and NAR groups. Animals were housed in a controlled
environment, including a temperature of 23±2°C, a relative humidity
of 45±5%, a 12 h light-dark cycle and access to food and water
ad libitum. Rats were examined daily for body weight, health
and behavior. Anesthesia was induced with 50 mg/kg sodium
pentobarbital by intraperitoneal injection. Hair removal was
achieved using depilatory cream and sequential incisions were made
in the left cheek tissues. Next, a 1×1×2-mm defect was abraded
underneath the molar teeth of the rats using a high-speed
handpiece. After surgery, subcutaneous injection of carprofen (5
mg/kg) was used for pain relief. The NAR group received a daily
gavage of 50 mg/kg NAR (Shanghai Macklin Biochemical Co., Ltd.)
(21,22), while the control group received
an equal volume of saline. The following conditions were set as the
humane endpoints: Initial body weight loss of >20%, inability to
eat and drink and animals without anesthesia or sedation showing
depression or low body temperatures <37°C. No SD rats exhibited
these humane endpoints. After 28 days of continuous treatment, the
SD rats were euthanized at a 50% vol/min CO2
displacement rate (according to the AVMA guidelines) (23). After determining that the rats
were not moving, not breathing and had dilated pupils, the
CO2 was switched off and the animals were observed for a
further 2 min to determine that they were dead. Next, lower jaw
samples were collected and then immersed in 4% paraformaldehyde
(room temperature, 24 h). The animal manipulation procedures were
approved by The Ethics Committee of the Laboratory Animal Center of
Southwest Medical University (approval no. 20221107-016).
Masson staining and
immunofluorescence
After 2-month decalcification using
ethylenediaminetetraacetic acid (cat. no. ST069-500 ml; Beyotime
Biotechnology Co., Ltd.), the alveolar bone tissues were embedded
in paraffin and sectioned (4-μm sections), followed by
Masson staining (room temperature, 2 h) (Beijing Solarbio Science
& Technology Co., Ltd.) and light microscopy to observe
osteogenesis at the bone defects.
For immunofluorescence staining, the tissue sections
were deparaffinized with xylene (twice for 15 min each). After
deparaffinization, the sections were rehydrated with different
concentrations of alcohol (100, 95, 80 and 70%) and purified water.
The excess water was discarded, and antigen repair was conducted by
adding an appropriate amount of antigen repair solution (cat. no.
P0088; Beyotime Biotechnology Co., Ltd.) to the tissues, then
washing with water after 10 min. Next, the sections were blocked
using 5% goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min at 37°C and then incubated overnight with
anti-RUNX2 (1:100; cat. no. YT5356) and anti-OPN (1:100; cat. no.
YT3467) antibodies (ImmunoWay Biotechnology Company) at 4°C. The
next day, the sections were incubated with a Multi-rAb
CoraLite® Plus 594-Goat Anti-Rabbit Recombinant
Secondary Antibody (1:200; cat. no. RGAR004; Proteintech Group,
Inc.) at 37°C for 1 h. Next, the sections were washed in PBS,
treated with DAPI (37°C, 10 min) and sealed with glycerol. The
sections were then observed and imaged under a fluorescence
microscope.
Micro-computed tomography (micro-CT)
A micro-CT system (SkySCAN 1176 Micro-CT; Bruker
Corporation) was employed for imaging. The imaging parameters were
as follows: Pixel size=35 mm, angular rotation step=0.8 and
voltage/current=50 kV/498 mA. The CT results were reconstructed,
and the region of the bone corresponding to the bone defect was
specifically selected for subsequent analysis. The regions of
interest were analyzed using CT-Analyser (version 1.20.3.0; Bruker
Corporation). The total volume of the tissue was calculated by the
software by setting uniform parameters. Then, the bone mineral
density (BMD), bone volume/tissue volume (BV/TV) ratio and
trabecular thickness (TbTh) were assessed.
Co-localization of NAR probes and
AKT
NAR probes labeled with fluorescein were synthesized
through an azide-alkyne cycloaddition reaction. hPDLSCs were
treated with NAR probes (10 μmol/l) and fluorescence
detection was conducted after a 2-day incubation period. The
treated cells were fixed using 4% paraformaldehyde (37°C, 30 min),
washed with PBS and blocked with 10% goat serum for 1 h (room
temperature). The blocked cells were then incubated with anti-AKT
antibody (1:100) at 4°C overnight. The next day, the samples were
incubated with a Multi-rAb CoraLite® Plus 594-Goat
Anti-Rabbit Recombinant Secondary antibody (1:200; Proteintech
Group, Inc.) at 37°C for 1 h, followed by incubation with DAPI
(37°C, 10 min). The hPDLSCs were then washed with PBS thrice,
sealed with glycerol and observed and imaged under a laser confocal
microscope.
Statistical analysis
SPSS version 21.0 (IBM Corp.) was used to analyze
the data. All data are presented as the mean ± standard deviation.
Statistical significance was evaluated using the Student's t-test
(unpaired) for independent samples to compare the differences in
the data between groups. For ≥3 groups, one-way ANOVA and Dunnett's
post hoc test were employed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Culture and characterization of
hPDLSCs
The established third-generation hPDLSCs exhibited
robust osteogenic and adipogenic differentiation capabilities
(Fig. 1C and D). Flow cytometry
analysis confirmed the identity of hPDLSCs, which revealed positive
expression of CD44 (99.84%), CD90 (99.95%) and CD105 (98.95%), and
negative expression of CD31 (0.46%) and CD45 (0.17%) (Fig. 1E). Compared with the control
group, hPDLSCs treated with 10 μM NAR for 7 days
demonstrated enhanced osteogenic potential, as indicated by ALP
staining, formation of mineralized nodules and elevated gene and
protein expression of RUNX2 and OPN (Fig. 1F, G and I-K).
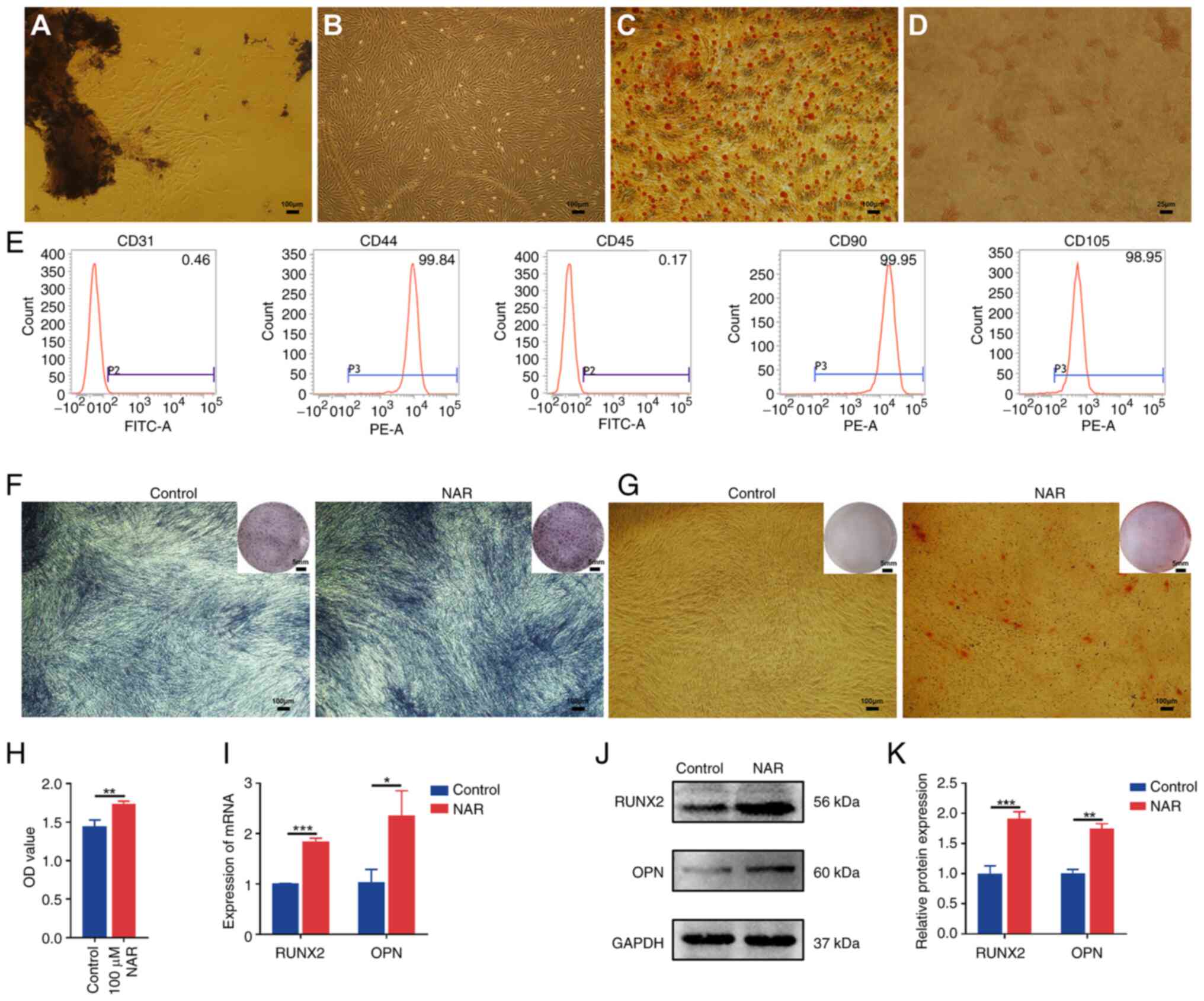 | Figure 1Isolation and identification of
hPDLSCs, and NAR-mediated promotion of hPDLSC osteogenic
differentiation. (A) The hPDLSCs around tissue blocks
(magnification, ×40). (B) The third-generation hPDLSCs
(magnification, ×40). (C) Alizarin red S staining demonstrating
osteogenic differentiation ability of hPDLSCs (magnification, ×40).
(D) Oil red O staining demonstrating adipogenic differentiation
ability of hPDLSCs (magnification, ×200). (E) Flow cytometry
results of cell surface markers. (F) Alkaline phosphatase staining
of hPDLSCs after 7 days of 10 μM NAR treatment
(magnification, ×40). (G) hPDLSCs were subjected to alizarin red
staining following incubation with NAR for 28 days (magnification,
×40). (H) Results of Cell Counting Kit 8 assay. (I) Gene and (J)
protein expression of RUNX2 and OPN in hPDLSCs after 7 days of 10
μM NAR treatment. (K) Histogram of RUNX2 and OPN protein
expression. *P<0.05, **P<0.01,
***P<0.001. hPDLSCs, human periodontal ligament stem
cells; NAR, naringenin; RUNX2, Runt-related transcription factor;
OPN, osteopontin; OD, optical density. |
Volcano plots, clustered heatmaps, KEGG
analysis and expression validation of genes
The transcriptome sequencing results revealed
differential expression of 104 genes between the control and NAR
groups (Fig. 2A and B). KEGG
analysis of these differentially expressed genes revealed the
involvement of the cGMP-PKG signaling pathway (Fig. 2C). The ELISA result revealed an
elevated cGMP protein expression in the NAR group compared with the
control group (Fig. 2D). During
the assessment of pathway factors associated with the cGMP-PKG
signaling axis, the NAR group was observed to exhibit elevated
expression levels of sGC, PKG and TRPC6 proteins and genes compared
with the control group (Fig.
2E-G).
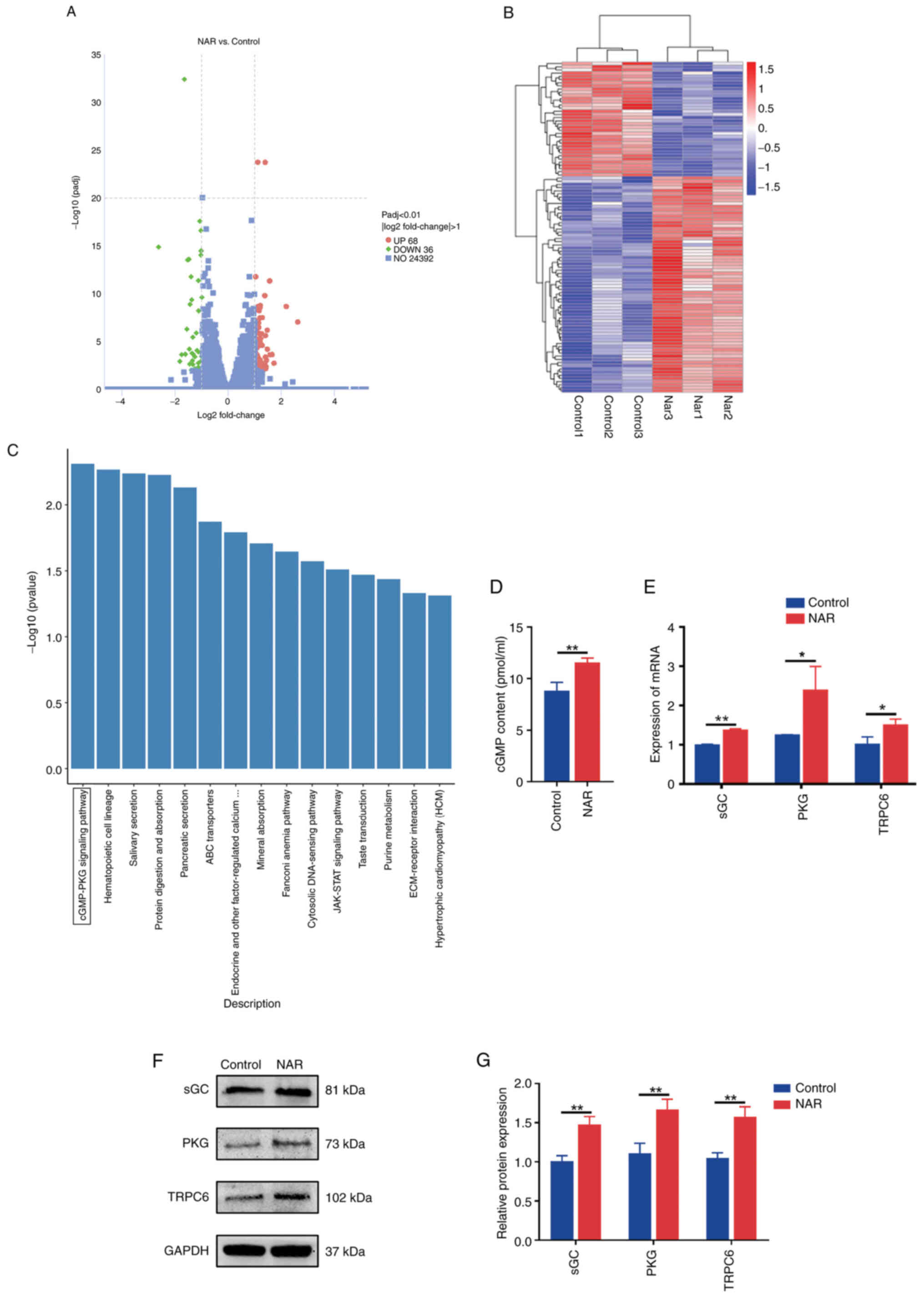 | Figure 2Volcano plot, clustered heatmap, KEGG
analysis and validation of the expression of associated factors.
(A) Volcano plot depicting the differential gene expression between
the NAR and control groups. (B) Heatmap illustrating the gene
expression differences between the NAR and control groups. (C)
Kyoto Encyclopedia of Genes and Genomes analysis of differentially
expressed genes between the NAR and control groups. (D) ELISA
detection of cGMP production. (E) Reverse
transcription-quantitative PCR-based detection of TRPC6, PKG and
sGC expression. (F and G) Protein expression analysis of PKG, TRPC6
and sGC. *P<0.05, **P<0.01. NAR,
naringenin; cGMP, cyclic guanosine monophosphate; TRPC6, transient
receptor potential cation channel, subfamily C, member 6; PKG,
protein kinase G; sGC, soluble guanylate cyclase. |
Impact of the eNOS inhibitor, L-NAME, on
NAR-induced promotion of osteogenic differentiation in hPDLSCs
Strong ALP and alizarin red staining was noted in
the NAR group compared with the control group (Fig. 3A and B), indicating that NAR
promoted the osteogenic differentiation of hPDLSCs. Additionally,
the gene and protein expression levels of the osteogenesis-related
factors, RUNX2 and OPN, were higher in the NAR group than in the
control group (Fig. 3D-F). This
elevation was accompanied by increased expression of p-eNOS, NO and
sGC (Fig. 3C-E and G). Upon the
addition of L-NAME (an eNOS inhibitor), the NAR + L-NAME group
exhibited decreased expression of p-eNOS, NO and sGC compared with
the NAR group, in addition to a decreased expression of the
osteogenic factors, RUNX2 and OPN. Furthermore, the staining
intensity of ALP and alizarin red was lower in the NAR + L-NAME
group compared with the NAR group (Fig. 3A and B).
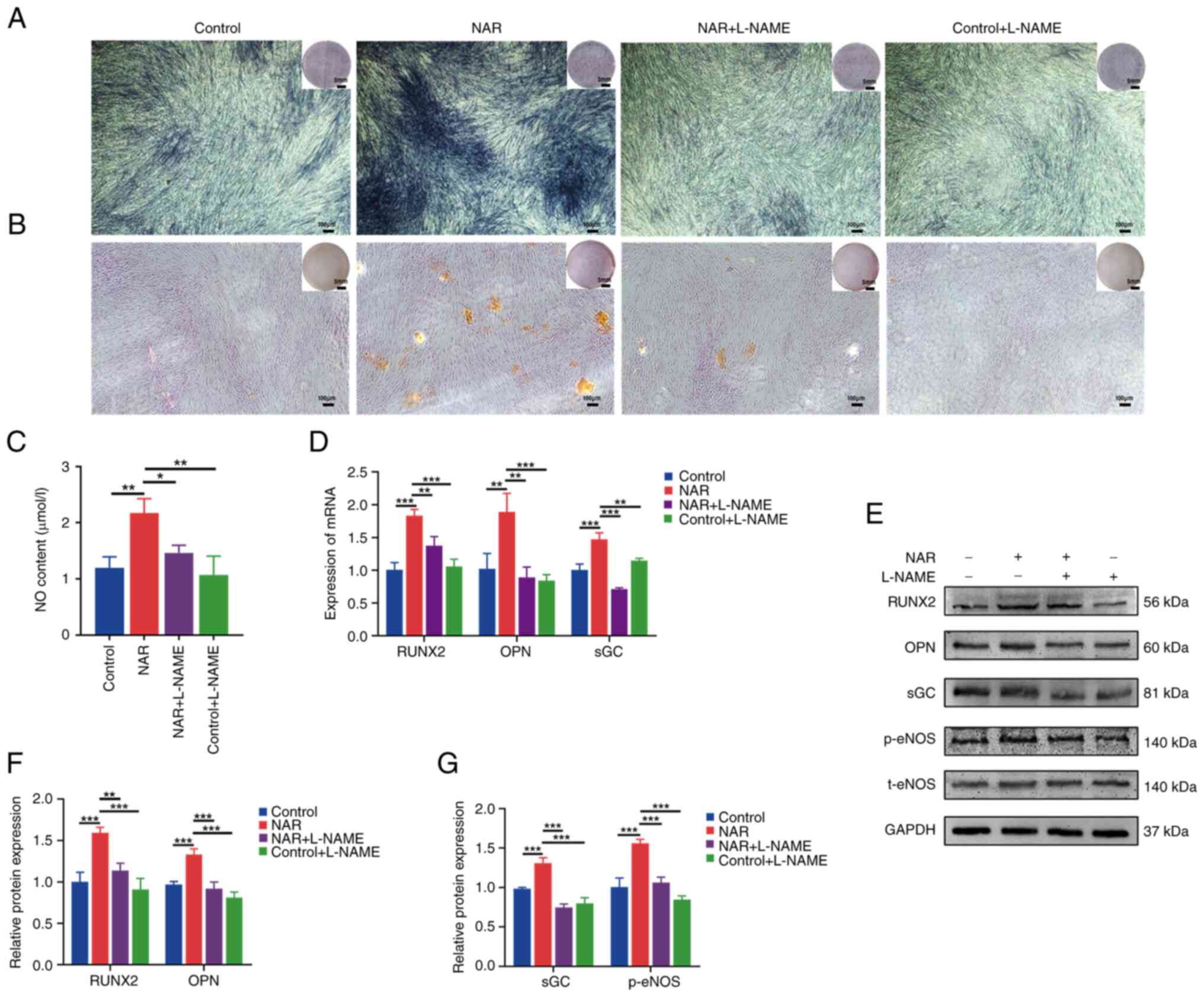 | Figure 3Ability of NAR to promote
osteogenesis in human periodontal ligament stem cells is mitigated
after L-NAME (eNOS inhibitor) treatment. (A) Alkaline phosphatase
staining in different treatment groups. (B) Alizarin red staining
in different treatment groups. (C) NO levels in different treatment
groups. (D) Gene expression levels of RUNX2, OPN and sGC in
different treatment groups. (E-G) Protein expression of RUNX2, OPN,
sGC and p-eNOS in various treatment groups. *P<0.05,
**P<0.01, ***P<0.001. NAR, naringenin;
eNOS, endothelial nitric oxide synthase; RUNX2, Runt-related
transcription factor; OPN, osteopontin; sGC, soluble guanylate
cyclase; NO, nitric oxide; p-, phosphorylated; t-, total; L-NAME,
NG-nitro-L-arginine methyl ester. |
Ability of NAR to promote the osteogenic
differentiation of hPDLSCs is decreased by the addition of ODQ, an
sGC inhibitor
Further experiments were conducted to explore the
role of the NO downstream factor, sGC. The results revealed an
increased expression of sGC, PKG, cGMP and TRPC6 in the NAR group
compared with the control group (Fig. 4C, E, F and H). Upon addition of
ODQ (an sGC inhibitor), the NAR + ODQ group exhibited decreased
expression of the osteogenesis-related factors, RUNX2 and OPN, at
both the gene and protein levels (Fig. 4D, F and G). Additionally, the
intensity of both ALP and alizarin red staining in the NAR + ODQ
group was lower compared with the NAR group (Fig. 4A and B). Furthermore, the
expression of sGC, PKG, cGMP and TRPC6 was reduced in the NAR + ODQ
group compared with the NAR group (Fig. 4C-H).
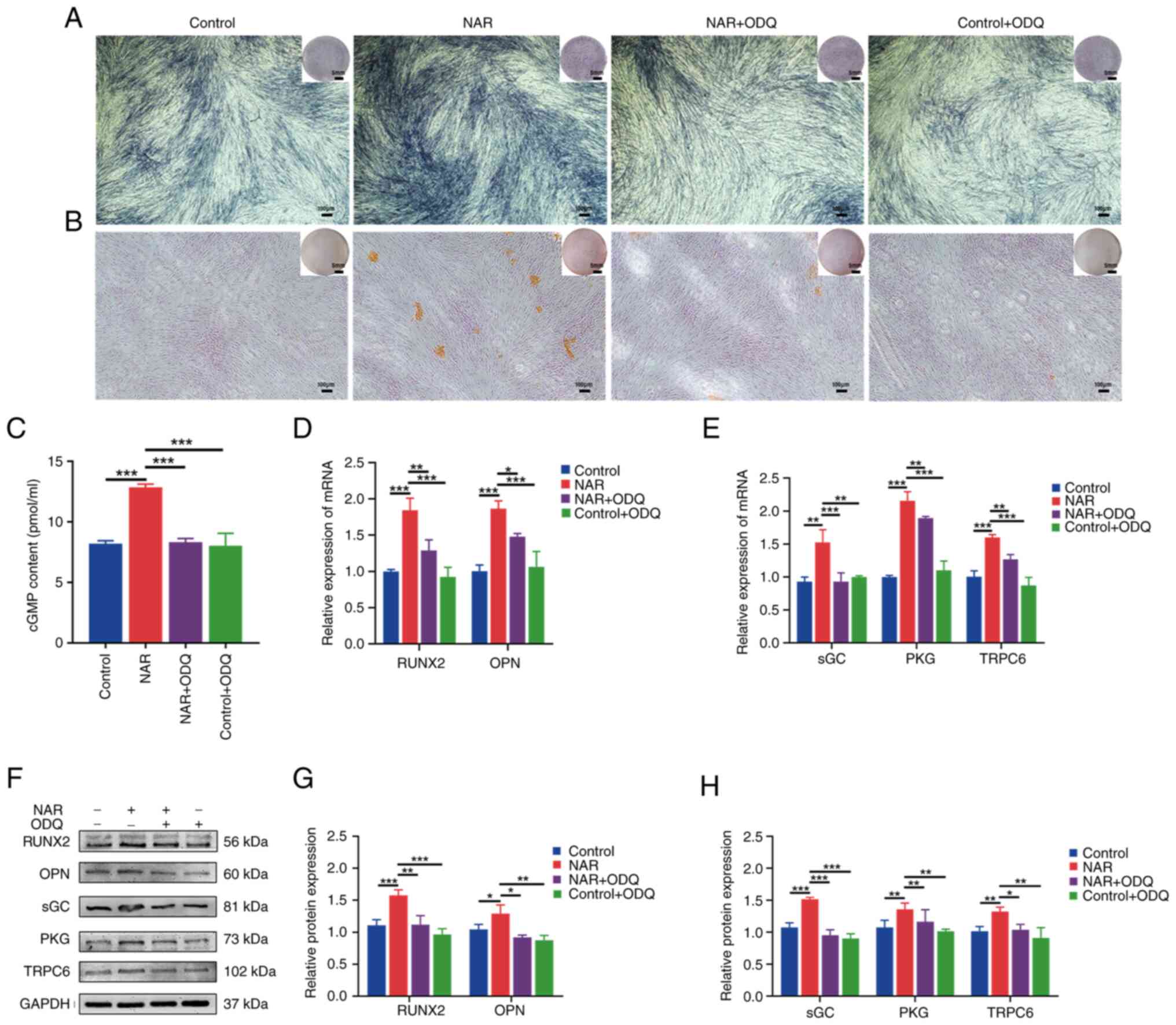 | Figure 4Ability of NAR to promote
osteogenesis in human periodontal ligament stem cells is decreased
after ODQ (an sGC inhibitor) treatment. (A) Alkaline phosphatase
staining in different treatment groups. (B) Alizarin red staining
in different treatment groups. (C) cGMP expression in different
treatment groups. (D and E) Gene expression levels of RUNX2, OPN,
sGC, PKG and TRPC6 in different treatment groups. (F-H) Protein
levels of RUNX2, OPN, sGC, PKG and TRPC6 in different treatment
groups. *P<0.05, **P<0.01,
***P<0.001. NAR, naringenin; cGMP, cyclic guanosine
monophosphate; sGC, soluble guanylate cyclase; RUNX2, Runt-related
transcription factor; OPN, osteopontin; TRPC6, transient receptor
potential cation channel, subfamily C, member 6; PKG, protein
kinase G; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. |
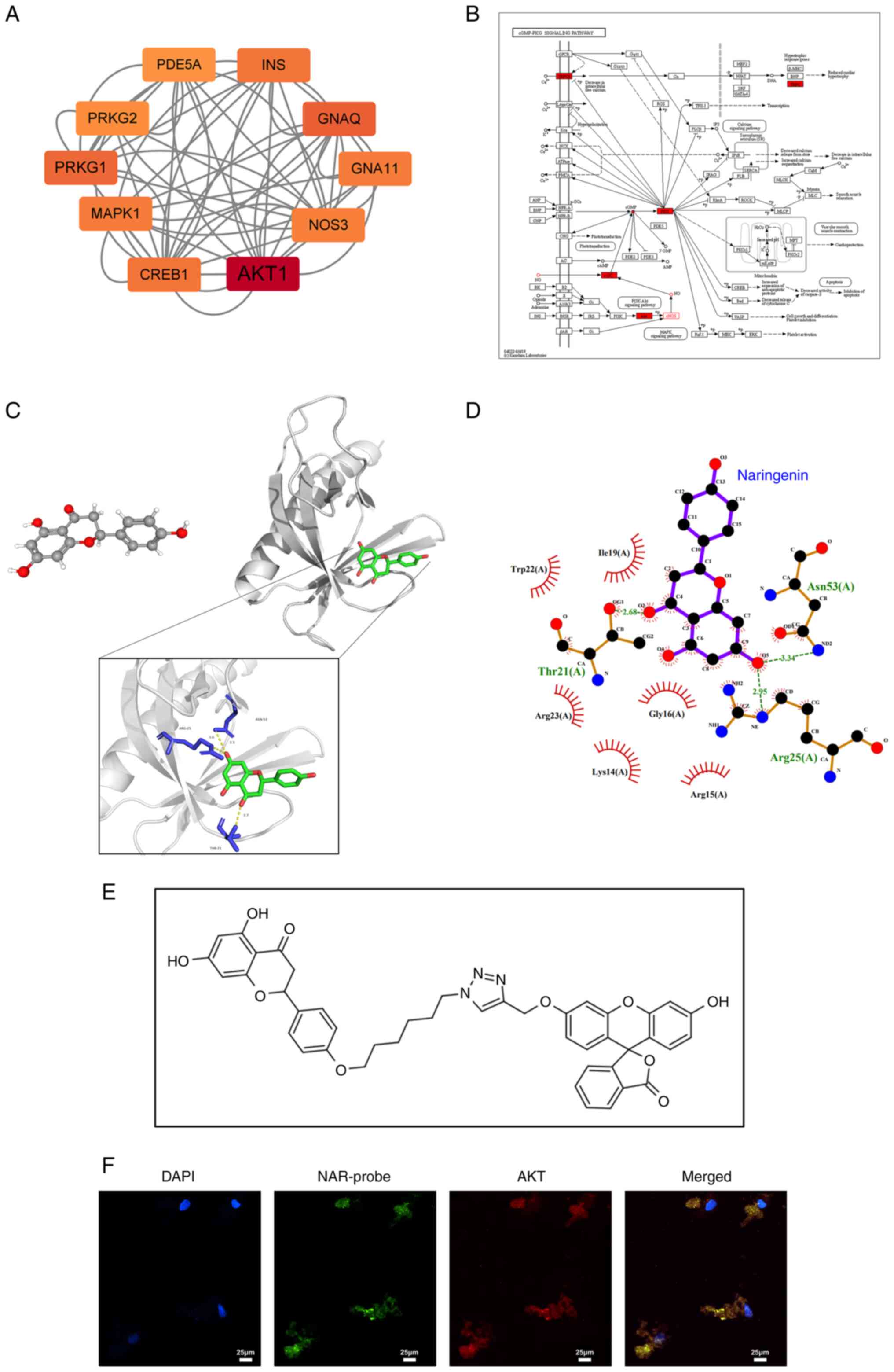
Network pharmacology screening of NAR
targeting factors and validation
NO-cGMP-PKG signaling pathway-related factors and
NAR targeting factors were input into the STRING database for
analysis. A PPI network was generated, and the top 10 genes were
used to construct the core sub-network by Cytoscape. AKT emerged as
the top-ranking gene in this network, which revealed an association
between AKT and eNOS (also termed NOS3; Fig. 5A). Subsequently, AKT, eNOS, NO,
sGC, cGMP, PKG and TRPC6 were integrated into the cGMP-PKG
signaling pathway, which revealed that AKT was situated upstream of
this pathway and could directly regulate eNOS (Fig. 5B). Fig. 5C illustrates the docking results
of NAR (structure downloaded from PubChem) with AKT molecules
(structure downloaded from the PDB), while the 2D representation is
shown in Fig. 5D. In addition, a
NAR probe labeled with fluorescein was co-cultured with hPDLSCs
(Fig. 5E), which revealed
co-localization of the fluorescence emission of the NAR probe with
that of AKT in the cytoplasm (Fig.
5F).
Ability of NAR to promote osteogenesis in
hPDLSCs is decreased by the addition of AKT inhibitor VIII
The results revealed increased expression of p-AKT
and p-eNOS in the NAR group compared with the control group
(Fig. 6D and F). Upon addition
of AKT inhibitor VIII, the NAR + AKT inhibitor VIII group exhibited
decreased expression of the osteogenesis-related factors, RUNX2 and
OPN, at both the gene and protein levels compared with the NAR
group. Additionally, ALP and alizarin red staining exhibited a
lower intensity, and the expression of p-AKT and p-eNOS was reduced
in the NAR + AKT inhibitor VIII group compared with the NAR group
(Fig. 6A-F).
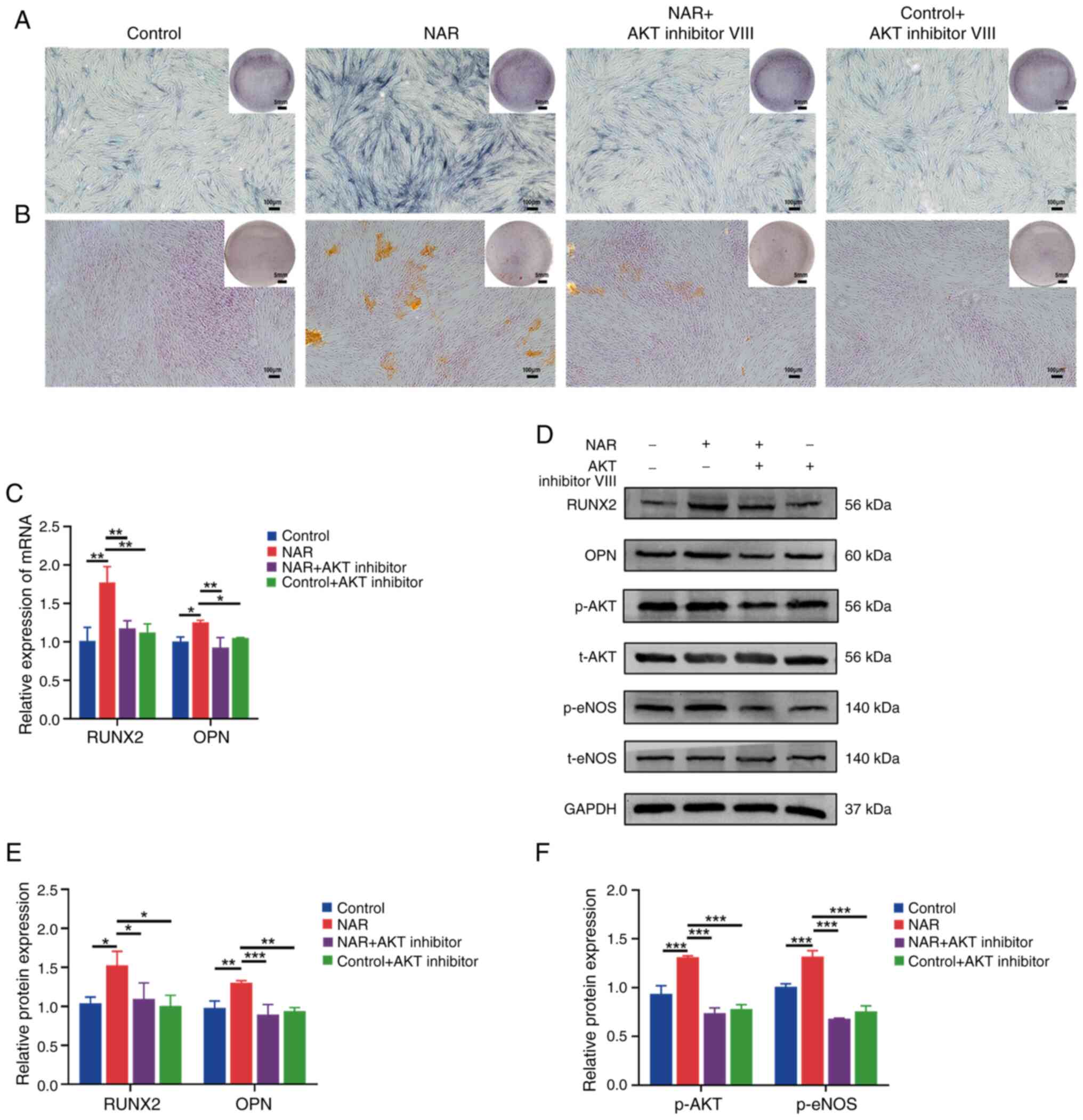 | Figure 6Impact of AKT inhibitor VIII on
NAR-mediated osteogenic promotion in human periodontal ligament
stem cells. (A) Alkaline phosphatase staining in different
treatment groups. (B) Alizarin red staining in different treatment
groups. (C) Gene expression of RUNX2 and OPN in different treatment
groups. (D-F) Protein levels of RUNX2, OPN, p-AKT and p-eNOS in
different treatment groups. *P<0.05,
**P<0.01, ***P<0.001. NAR, naringenin;
RUNX2, Runt-related transcription factor; OPN, osteopontin; eNOS,
endothelial nitric oxide synthase; p-, phosphorylated; t-,
total. |
NAR promotes the repair of alveolar bone
defects
Due to the enhanced osteogenic differentiation
observed in hPDLSCs under the influence of NAR during in
vitro experiments, it was hypothesized that NAR may promote
alveolar bone regeneration. To investigate this, an alveolar bone
defect model was established in SD rats, and a daily gavage of 50
mg/kg NAR was administered to the treatment group. Micro-CT
analysis of bone morphology revealed that, after 28 days of
treatment, new bone, BMD, BV/TV and TbTh at the bone defects were
higher in the NAR group compared with the saline-treated (control)
group (Fig. 7A-D). Masson
staining also indicated increased formation of new bone in the NAR
group (Fig. 7E).
Immunofluorescence further demonstrated higher expression of RUNX2
(Fig. 7F) and OPN (Fig. 7G) in the periodontal ligament and
new bone at the alveolar bone defects in the NAR group. These
findings suggested that NAR has the potential to promote the
healing of alveolar bone defects.
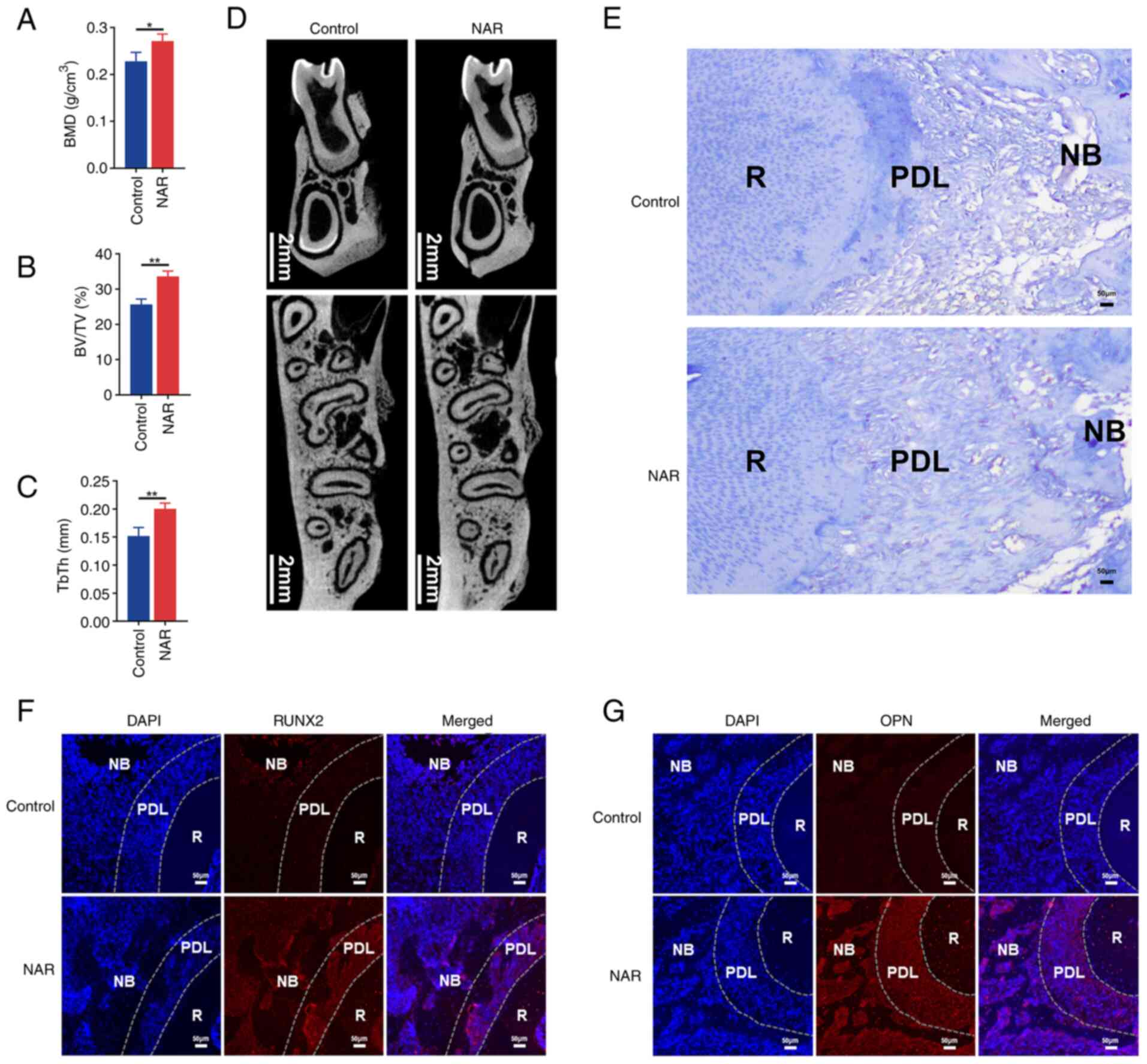 | Figure 7Ability of NAR to promote alveolar
bone repair. (A-D) Results of BMD, BV/TV ratio, TbTh and new bone
in the control and NAR groups. (E) Masson staining illustrating
tissue morphology in the control and NAR groups. (F and G)
Immunofluorescence of alveolar bone in the control and NAR groups.
*P<0.05, **P<0.01. NAR, naringenin;
RUNX2, Runt-related transcription factor; OPN, osteopontin; BMD,
bone mineral density; BT/TV, bone volume/tissue volume; TbTh,
trabecular thickness; R, root; PDL, periodontal ligament; NB, new
bone. |
Discussion
Numerous studies have employed hPDLSCs in both in
vivo and in vitro repair and regeneration experiments,
demonstrating their effectiveness in promoting osteogenesis
(24-26). However, the complexity of
material construction has hindered their widespread adoption. NAR
holds notable development and application potential as it is
readily available and easily obtainable. In the present study,
bioinformatics analysis revealed the presence of differentially
expressed factors in hPDLSCs after NAR treatment, and these factors
were associated with the NO-cGMP-PKG signaling pathway, which is
known to be involved in bone remodeling processes (27). Previous studies have suggested
the promotion of osteogenic differentiation by estradiol, fluid
shear stress and epimedium glycosides through the NO-cGMP-PKG
signaling pathway (28-30). During the cell experiments
conducted in the present study, NAR was found to enhance
osteogenesis in hPDLSCs, which was accompanied by increased
expression of NO and sGC, factors related to the NO-cGMP-PKG
pathway.
To explore the involvement of the NO-cGMP-PKG
pathway in the promotion of osteogenesis by NAR in hPDLSCs, the
present study used L-NAME (an inhibitor of eNOS phosphorylation)
and observed a decrease in both p-eNOS and NO expression, in
addition to a decreased osteogenic capacity of hPDLSCs. These
results may suggest that NO is a crucial factor in the osteogenic
promotion mediated by NAR. Additionally, as a pivotal signal in
bone remodeling (31), NO
increases cGMP concentration and promotes osteoblast proliferation
(32). The downstream factor of
NO is sGC, which has been reported to further enhance osteoblast
proliferation and differentiation and to reduce bone trabecular
loss (33). In the present
study, the addition of ODQ (an inhibitor of sGC) resulted in a
decrease in the osteogenic potential of NAR-promoted hPDLSCs, which
was coupled with reduced expression of downstream factors such as
PKG, cGMP and TRPC6. Inhibition of TRPC6 has been shown to hinder
the osteogenic capacity of periodontal ligament cells, while
activation of TRPC6 promotes it. Moreover, TRPC6-knockout mice
exhibit poorer bone regeneration in cranial defects compared with
wild-type mice, which reflects the importance of TRPC6 in bone
defect repair (34).
Beyond osteogenesis, the NO-cGMP-PKG pathway plays a
role in various biological functions such as heart failure with
preserved ejection fraction, gluconeogenesis and the circadian
clock (35-37). NAR has been shown to reduce
inflammatory pain responses induced by superoxide anion
(KO2) in mice through the NO-cGMP-PKG-ATP-sensitive
potassium channel (KATP) signaling pathway (38). In addition, NAR inhibited the
pacing activity of mouse small intestinal Cajal mesenchymal stromal
cells through a NO-cGMP-dependent pathway (39). These findings suggest that NAR
exerts diverse cellular effects by modulating the NO-cGMP-PKG
pathway.
To further investigate the regulatory mechanisms by
which NAR influences the NO-cGMP-PKG pathway, the present study
used network pharmacology. The results of computer-simulated
molecular docking revealed a strong binding affinity between NAR
and AKT, which was consistent with previous studies (40-42). As indicated by pathway mapping,
AKT was situated upstream of the NO-cGMP-PKG pathway. Thus, we
hypothesized that NAR may activate the NO-cGMP-PKG pathway by
targeting AKT. Phosphorylated AKT activates eNOS, which leads to
the release of NO and subsequent protection against high
glucose-induced apoptosis in endothelial cells (43). Similarly, the promotion of
osteogenic differentiation in bone marrow mesenchymal stem cells by
epimedium glycoside has been associated with elevated expression of
p-AKT, p-eNOS and NO (30),
which is in agreement with the increased expression of p-AKT and
p-eNOS observed following NAR treatment of hPDLSCs in the present
study. In the present study, the addition of an AKT inhibitor
resulted in decreased expression of p-AKT and p-eNOS (which
supported the aforementioned hypothesis) in NAR-treated hPDLSCs,
which was accompanied by a reduction in the ability of NARs to
promote the osteogenic differentiation of hPDLSCs.
In addition, the present study revealed that NAR
bound to the R25 site on AKT, which was consistent with the binding
site of SC79, a specific AKT activator that promotes the cytosolic
phosphorylation of AKT (44).
SC79 has been shown to contribute to the osteogenic differentiation
of human bone marrow mesenchymal stem cells (45). To explore the intracellular
binding of NAR to AKT, fluorescent NAR probes were synthesized and
employed in the present study. The observed fluorescence
co-localization of NAR with AKT confirmed that NAR may stimulate
the osteogenic differentiation of hPDLSCs by intracellularly
binding to AKT and promoting its phosphorylation. Previous studies
have reported that NAR promotes AKT phosphorylation to regulate
apoptosis and enhances the cellular cholesterol efflux process in
prostate cancer cells (46,47). Notably, NAR-promoted AKT
phosphorylation regulates various cellular biological functions
such as inducing prostate cancer apoptosis, supporting migration of
cells, improving ovulation and suppressing androgens and cystic
follicles (46,48,49). In the present study, NAR gavage
significantly promoted the repair and regeneration of alveolar bone
defects in a bone defect model. Zhou et al (50) also found that NAR significantly
reversed bone loss caused by LPS and contributed to the healing of
cranial bone defects. These findings underscore the value of NAR in
research and development for promoting bone regeneration.
However, the present study does have some
limitations. Only mRNAs from transcriptomics were analyzed for
relevant pathways, while transcriptomics also includes non-coding
RNAs. It has previously been demonstrated that non-coding RNAs have
a role in biological mechanisms (51). In the future, the functions and
mechanisms of NAR and other Chinese medicine molecules should be
further revealed from a multi-omics perspective.
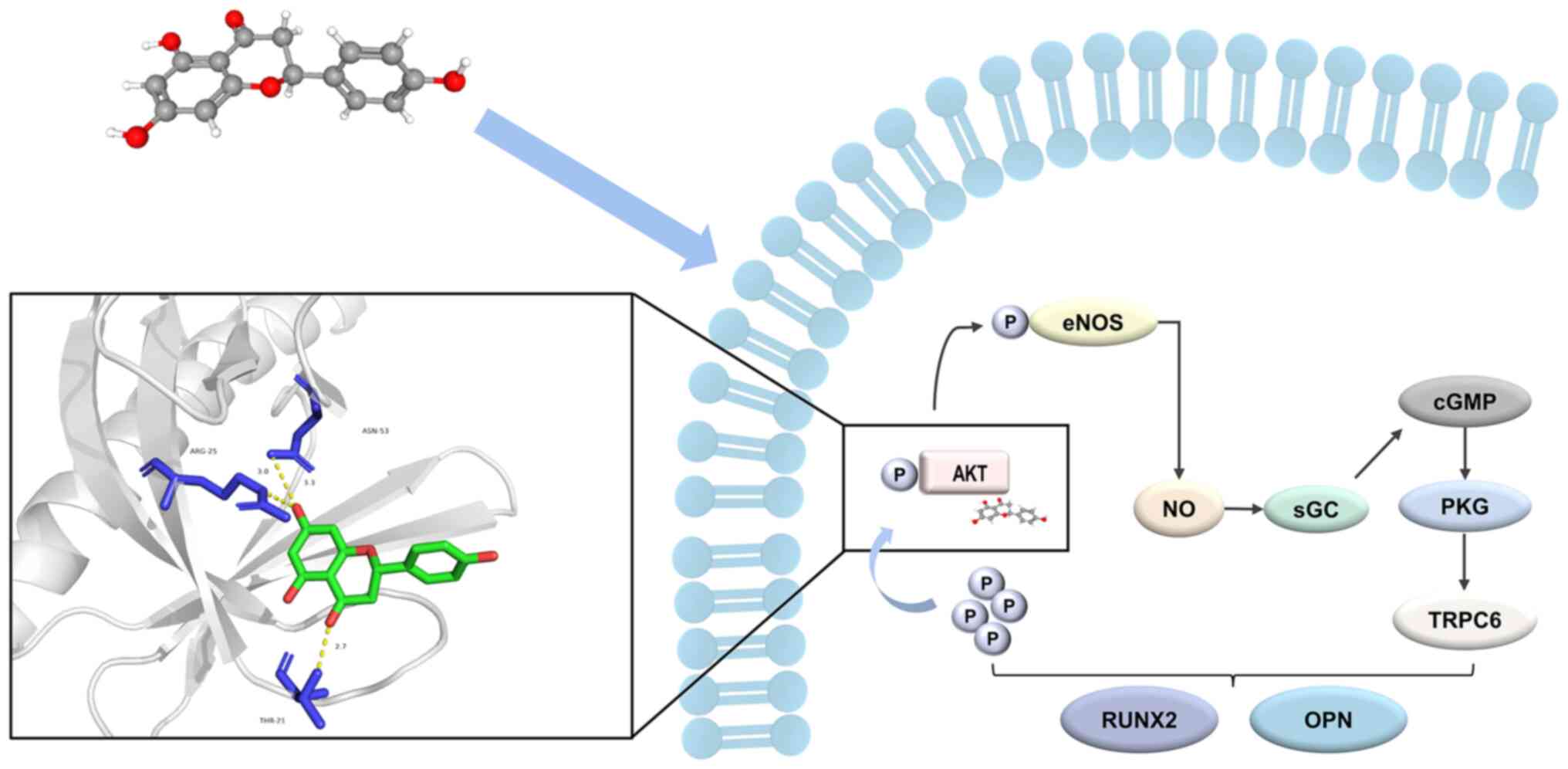
In summary, AKT has emerged as a pivotal target
through which NAR facilitates osteogenic differentiation, and the
promotion of osteogenesis in hPDLSCs by NAR is achieved by binding
to AKT to modulate the NO-cGMP-PKG signaling pathway (Fig. 8). The present findings constitute
novel insights into the underlying mechanism by which NAR promotes
the osteogenic differentiation of hPDLSCs, offering valuable
knowledge for the future development and application of NAR.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The RNA sequencing data
generated in the present study may be found in the GEO database
under accession number GSE266150 or at the following URL:
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSE266150.
Authors' contributions
SL and XX conceived and designed the experiments.
ZX, YL, QZ and LZ performed the experiments, prepared the
manuscript and analyzed the data. SL and XX confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The isolation of hPDLSCs from patient samples was
approved by the Ethics Committee of The Affiliated Stomatology
Hospital, Southwest Medical University (Luzhou, China; approval no.
20221107003). Written consent was provided by the guardians and
patients for the use of samples in the present research project.
The animal experiments followed the guidelines of the National
Institutes of Health and were approved by the Ethics Committee of
the Laboratory Animal Center of Southwest Medical University
(Luzhou, China; approval no. 20221107-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sichuan Provincial Bureau
of Traditional Chinese Medicine Project (grant no. 2023MS080),
Innovation Project of Science and Technology Department of Sichuan
Province (grant no. 2022YFS0634) and Central Guidance for Local
Scientific and Technological Development Projects (grant no.
2023ZYD0112).
References
|
1
|
Alves LS, Aragão I, Sousa MJC and Gomes E:
Pattern of maxillofacial fractures in severe multiple trauma
patients: A 7-year prospective study. Braz Dent J. 25:561–564.
2014. View Article : Google Scholar
|
|
2
|
Reddi AH: Symbiosis of biotechnology and
biomaterials: Applications in tissue engineering of bone and
cartilage. J Cell Biochem. 56:192–195. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Wang Z, Huang X, Wang Z, Chen Z,
Wang R, Chen Z, Liu W, Wu B, Fang F and Qiu W: Dynamic proteomic
profiling of human periodontal ligament stem cells during
osteogenic differentiation. Stem Cell Res Ther. 12:982021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang GTJ, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs those from
other sources: Their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang NS, Zhang C, Hwang YS and Varghese
S: Mesenchymal stem cell differentiation and roles in regenerative
medicine. Wiley Interdiscip Rev Syst Biol Med. 1:97–106. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen HJ, Inbaraj BS and Chen BH:
Determination of phenolic acids and flavonoids in Taraxacum
formosanum Kitam by liquid chromatography-tandem mass spectrometry
coupled with a post-column derivatization technique. Int J Mol Sci.
13:260–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Dai J, Liu H, Li RR, Sun PL, Du Q,
Pang LL, Chen Z and Yin KS: Naringenin inhibits allergen-induced
airway inflammation and airway responsiveness and inhibits
NF-kappaB activity in a murine model of asthma. Can J Physiol
Pharmacol. 87:729–735. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renugadevi J and Prabu SM: Naringenin
protects against cadmium-induced oxidative renal dysfunction in
rats. Toxicology. 256:128–134. 2009. View Article : Google Scholar
|
|
9
|
Uçar K and Göktaş Z: Biological activities
of naringenin: A narrative review based on in vitro and in vivo
studies. Nutr Res. 119:43–55. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaczmarczyk-Sedlak I, Wojnar W, Zych M,
Ozimina-Kamińska E and Bońka A: Effect of dietary flavonoid
naringenin on bones in rats with ovariectomy-induced osteoporosis.
Acta Pol Pharm. 73:1073–1081. 2016.PubMed/NCBI
|
|
11
|
Gera S, Sampathi S, Maddukuri S, Dodoala
S, Junnuthula V and Dyawanapelly S: Therapeutic potential of
naringenin nanosuspension: In vitro and in vivo anti-osteoporotic
studies. Pharmaceutics. 14:14492022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, He H, Zhang M, Wu Y, Xu X, Yang M
and Mei L: Assessing the effect and related mechanism of naringenin
on the proliferation, osteogenic differentiation and endothelial
differentiation of human periodontal ligament stem cells. Biochem
Biophys Res Commun. 534:337–342. 2021. View Article : Google Scholar
|
|
13
|
Wu Z, Li W, Liu G and Tang Y:
Network-based methods for prediction of drug-target interactions.
Front Pharmacol. 9:11342018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dorado G, Gálvez S, Rosales TE, Vásquez VF
and Hernández P: Analyzing modern biomolecules: The revolution of
nucleic-acid sequencing-review. Biomolecules. 11:11112021.
View Article : Google Scholar
|
|
15
|
Li S, Fan TP, Jia W, Lu A and Zhang W:
Network pharmacology in traditional chinese medicine. Evid Based
Complement Alternat Med. 2014:1384602014.PubMed/NCBI
|
|
16
|
Zhang R, Zhu X, Bai H and Ning K: Network
pharmacology databases for traditional Chinese medicine. Front
Pharmacol. 10:1232019. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morris GM, Huey R and Olson AJ: Using
AutoDock for ligand-receptor docking. Curr Protoc Bioinformatics.
Chapter 8: Unit 8.14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu HY, Zhang YQ, Liu ZM, Chen T, Lv CY,
Tang SH, Zhang XB, Zhang W, Li ZY, Zhou RR, et al: ETCM: An
encyclopaedia of traditional Chinese medicine. Nucleic Acids Res.
47(D1): D976–D982. 2019. View Article : Google Scholar :
|
|
21
|
Rahigude A, Bhutada P, Kaulaskar S, Aswar
M and Otari K: Participation of antioxidant and cholinergic system
in protective effect of naringenin against type-2 diabetes-induced
memory dysfunction in rats. Neuroscience. 226:62–72. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Renugadevi J and Prabu SM: Cadmium-induced
hepatotoxicity in rats and the protective effect of naringenin. Exp
Toxicol Pathol. 62:171–181. 2010. View Article : Google Scholar
|
|
23
|
AVMA Panel on Euthanasia. American
Veterinary Medical Association: 2000 report of the AVMA panel on
euthanasia. J Am Vet Med Assoc. 218:669–696. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren S, Zhou Y, Zheng K, Xu X, Yang J, Wang
X, Miao L, Wei H and Xu Y: Cerium oxide nanoparticles loaded
nanofibrous membranes promote bone regeneration for periodontal
tissue engineering. Bioact Mater. 7:242–253. 2021.PubMed/NCBI
|
|
25
|
Lai S, Liu C, Liu C, Fan L, Li X, Yang Y,
Zhu Y, Deng L, Xiao L and Mu Y: Lycium barbarum
polysaccharide-glycoprotein promotes osteogenesis in hPDLSCs via
ERK activation. Oral Dis. 29:3503–3513. 2023. View Article : Google Scholar
|
|
26
|
Li N, Li Z, Wang Y, Chen Y, Ge X, Lu J,
Bian M, Wu J and Yu J: CTP-CM enhances osteogenic differentiation
of hPDLSCs via NF-κB pathway. Oral Dis. 27:577–588. 2021.
View Article : Google Scholar
|
|
27
|
Kim SM, Yuen T, Iqbal J, Rubin MR and
Zaidi M: The NO-cGMP-PKG pathway in skeletal remodeling. Ann N Y
Acad Sci. 1487:21–30. 2021. View Article : Google Scholar
|
|
28
|
Joshua J, Kalyanaraman H, Marathe N and
Pilz RB: Nitric oxide as a mediator of estrogen effects in
osteocytes. Vitam Horm. 96:247–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Yuan W and Wang J: Mechanisms for
osteogenic differentiation of human mesenchymal stem cells induced
by fluid shear stress. Biomech Model Mechanobiol. 9:659–670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN,
Zhou J, Ma HP, Xian CJ and Chen KM: Icariin stimulates the
osteogenic differentiation of rat bone marrow stromal cells via
activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 66:189–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan T, Xie Y, He H, Fan W and Huang F:
Role of nitric oxide in orthodontic tooth movement (review). Int J
Mol Med. 48:1682021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalyanaraman H, Ramdani G, Joshua J,
Schall N, Boss GR, Cory E, Sah RL, Casteel DE and Pilz RB: A novel,
direct no donor regulates osteoblast and osteoclast functions and
increases bone mass in ovariectomized mice. J Bone Miner Res.
32:46–59. 2017. View Article : Google Scholar
|
|
33
|
Joshua J, Schwaerzer GK, Kalyanaraman H,
Cory E, Sah RL, Li M, Vaida F, Boss GR and Pilz RB: Soluble
guanylate cyclase as a novel treatment target for osteoporosis.
Endocrinology. 155:4720–4730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Mi J, Sun B, Yang G, Liu S, Chen
M, Yu L, Pan J and Liu Y: Role of transient receptor potential
channel 6 in the osteogenesis of periodontal ligament cells. Int
Immunopharmacol. 100:1081342021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai Z, Wu C, Xu Y, Cai J, Zhao M and Zu L:
The NO-cGMP-PKG axis in HFpEF: From pathological mechanisms to
potential therapies. Aging Dis. 14:46–62. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu M, Wang Y, Jiang Y, Zhang C, Wang H,
Sha W, Chen L, Lei T and Liu L: Berberine inhibits gluconeogenesis
in spontaneous diabetic rats by regulating the AKT/MAPK/NO/cGMP/PKG
signaling pathway. Mol Cell Biochem. 478:2013–2027. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Plano SA, Alessandro MS, Trebucq LL, Endo
S, Golombek DA and Chiesa JJ: Role of G-substrate in the
NO/cGMP/PKG signal transduction pathway for photic entrainment of
the hamster circadian clock. ASN Neuro. 13:17590914209849202021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manchope MF, Calixto-Campos C,
Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR,
Baracat MM, Casagrande R and Verri WA Jr: Naringenin inhibits
superoxide anion-induced inflammatory pain: Role of oxidative
stress cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling
pathway. PLoS One. 11:e01530152016. View Article : Google Scholar
|
|
39
|
Kim HJ and Kim BJ: Naringenin inhibits
pacemaking activity in interstitial cells of Cajal from murine
small intestine. Integr Med Res. 6:149–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin H, Wang X, Liu M, Huang M, Shen Z,
Feng J, Yang H, Li Z, Gao J and Ye X: Exploring the treatment of
COVID-19 with Yinqiao powder based on network pharmacology.
Phytother Res. 35:2651–2664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qu Y, Yang X, Li J, Zhang S, Li S, Wang M,
Zhou L, Wang Z, Lin Z, Yin Y, et al: Network pharmacology and
molecular docking study of Zhishi-Baizhu Herb Pair in the treatment
of gastric cancer. Evid Based Complement Alternat Med.
2021:23114862021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao T, Wang Q, Han S, Lu Y, Xu Y and Wang
Y: Potential molecular mechanisms of ephedra herb in the treatment
of nephrotic syndrome based on network pharmacology and molecular
docking. Biomed Res Int. 2022:92145892022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duan MX, Zhou H, Wu QQ, Liu C, Xiao Y,
Deng W and Tang QZ: Andrographolide protects against HG-induced
inflammation, apoptosis, migration, and impairment of angiogenesis
via PI3K/AKT-eNOS signalling in HUVECs. Mediators Inflamm.
2019:61683402019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jo H, Mondal S, Tan D, Nagata E, Takizawa
S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, et al:
Small molecule-induced cytosolic activation of protein kinase Akt
rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA.
109:10581–10586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu R, Chen YX, Ke QF, Gao YS and Guo YP:
SC79-loaded ZSM-5/chitosan porous scaffolds with enhanced stem cell
osteogenic differentiation and bone regeneration. J Mater Chem B.
5:5009–5018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lim W, Park S, Bazer FW and Song G:
Naringenin-induced apoptotic cell death in prostate cancer cells is
mediated via the PI3K/AKT and MAPK signaling pathways. J Cell
Biochem. 118:1118–1131. 2017. View Article : Google Scholar
|
|
47
|
Xu X, Lei T, Li W and Ou H: Enhanced
cellular cholesterol efflux by naringenin is mediated through
inhibiting endoplasmic reticulum stress-ATF6 activity in
macrophages. Biochim Biophys Acta Mol Cell Biol Lipids.
1864:1472–1482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rashid R, Tripathi R, Singh A, Sarkar S,
Kawale A, Bader GN, Gupta S, Gupta RK and Jha RK: Naringenin
improves ovarian health by reducing the serum androgen and
eliminating follicular cysts in letrozole-induced polycystic ovary
syndrome in the Sprague Dawley rats. Phytother Res. 37:4018–4041.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lim W and Song G: Naringenin-induced
migration of embrynoic trophectoderm cells is mediated via PI3K/AKT
and ERK1/2 MAPK signaling cascades. Mol Cell Endocrinol. 428:28–37.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou X, Zhang Z, Jiang W, Hu M, Meng Y, Li
W, Zhou X and Wang C: Naringenin is a potential anabolic treatment
for bone loss by modulating osteogenesis, osteoclastogenesis, and
macrophage polarization. Front Pharmacol. 13:8721882022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Adv Exp Med Biol.
937:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|