Osteoporosis is closely linked to imbalances in bone
homeostasis. The maintenance of bone homeostasis mainly depends on
the balance between osteoclasts and osteoblasts; osteoclasts
mediate bone resorption and osteoblasts mediate bone formation. In
organisms, bone tissue constantly undergoes remodeling process
through the synergistic action of bone marrow mesenchymal cells
(BMSCs), osteoblasts, osteocytes and osteoclasts (6). BMSCs can multi-directionally
differentiate into osteogenesis, adipogenesis and cartilage. BMSCs
also promote calcium deposition and bone formation (7). Osteoblasts are derived from BMSCs
and play a key role in the process of bone formation. They are
mainly distributed on the surface of bone and synthesize bone
matrix by secreting collagen and bone matrix proteins. Osteoblasts
can synthesize and secrete the main components of bone, including
collagen fibers, chondroitin sulfate, phosphoric acid and calcium.
These secretions are important components of the bone matrix and
play an important role in maintaining the structure and function of
bone. Osteoclasts, originating from the hematopoietic
monocyte-macrophage system, are a special type of terminally
differentiated cells. They are involved in the process of bone
remodeling through the uptake of bone matrix and minerals, as well
as the secretion of organic acids and protein hydrolyzing enzymes,
thus maintaining the normal structure and function of the skeleton
(8). As osteoblast-derived
cells, osteocytes maintain mature bone metabolism by mediating
cytokine sensing, transduction and secretion to modulate bone
resorption and formation (9).
Under pathological conditions, osteoclast activity increases, while
osteogenesis is either weakened or impaired, consequently
destroying bone homeostasis. Over time, osteoporosis develops
further.
During bone homeostasis and repair, Wnt/β-catenin,
Hedgehog, bone morphogenetic protein (BMP-2)/Smad and
phosphatidylinositol-3-kinase/protein kinase B and others are the
major regulatory signaling pathways during skeletal growth and
development. The Wnt/β-catenin signaling pathway plays a key
regulatory role in bone development and formation by affecting the
proliferation and differentiation of osteoblasts and osteoclast
generation. Wnt is composed of 19 secreted glycoproteins and has
the function of regulating cell growth, differentiation and
apoptosis (10,11). The receptor activator of nuclear
factor-κB ligand (RANK-L)/RANK/MAPK and the NF-κB axes are the
major regulatory signaling pathways in osteoclast formation and
function. RANKL integrates with the osteoclast surface receptor
RANK, which in turn recruits tumor necrosis factor
receptor-associated factor (TRAF) 6 and further activates multiple
downstream signaling pathways, such as three mitogen-activated
protein kinases (MAPKs) including p38 kinase (p38MAPK),
extracellular signal-regulated kinase (ERK) and C-Jun N-terminal
kinase (12,13).
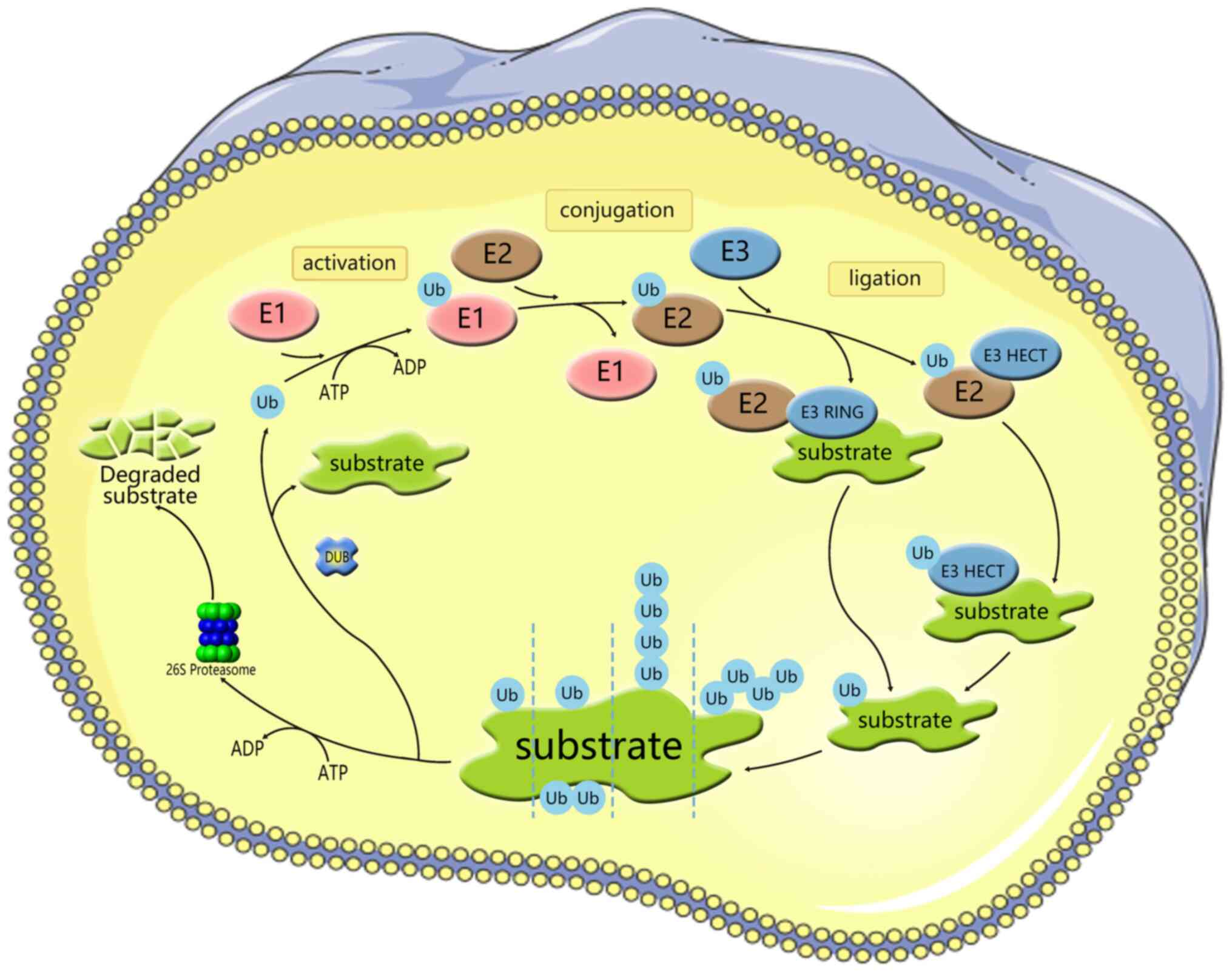
Ubiquitination is a conserved, widespread and
dynamic post-translational modification involved in regulating the
activity and stability of thousands of proteins, thereby affecting
most cellular functions and responses (14). Ubiquitylation is a process
mediated by the covalent attachment of ubiquitin (Ub) to the target
protein, catalyzed by a series of enzymatic cascades, including
E1-mediating activation, E2-mediating coupling and E3-mediating
connection step (Fig. 1)
(15). E3 then targets the
protein by promoting the formation of an isopeptide bond between
the C-terminal carboxyl group of Ub and one of the seven lysine
residues of Ub (K6/11/27/29/33/48/63) or the N-terminal methionine
of Ub (M1) (16-18). Subsequently, modified substrate
proteins are degraded by 26S protein complexes or exhibit cellular
function (19,20). E3 provides the reaction rate and
substrate specificity for this cascade reaction. Accumulating
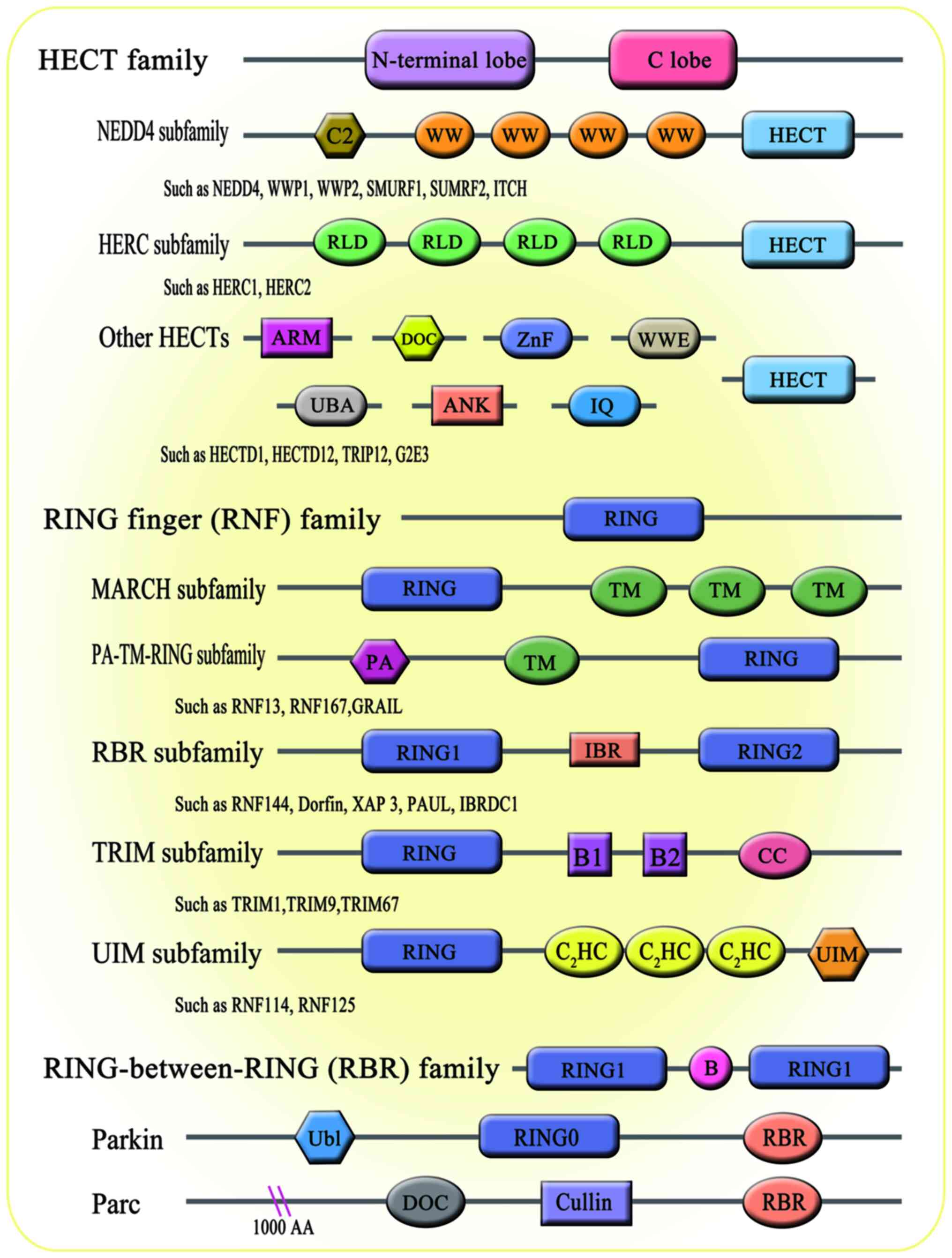
evidence has shown that E3 ligases are associated with a number of
biological processes, including bone homeostasis (21). There are 600-1,000 E3 ligases
encoded by the human genome. According to the domain structure and
ubiquitination mechanism through which E3 ligases interact with
target proteins, E3 members are broadly divided into three major
subfamilies: i) Homology to E6AP C-Terminus (HECT) family; ii)
really interesting new gene figure (RING) family; and iii)
RING-between-RING (RBR; Fig. 2).
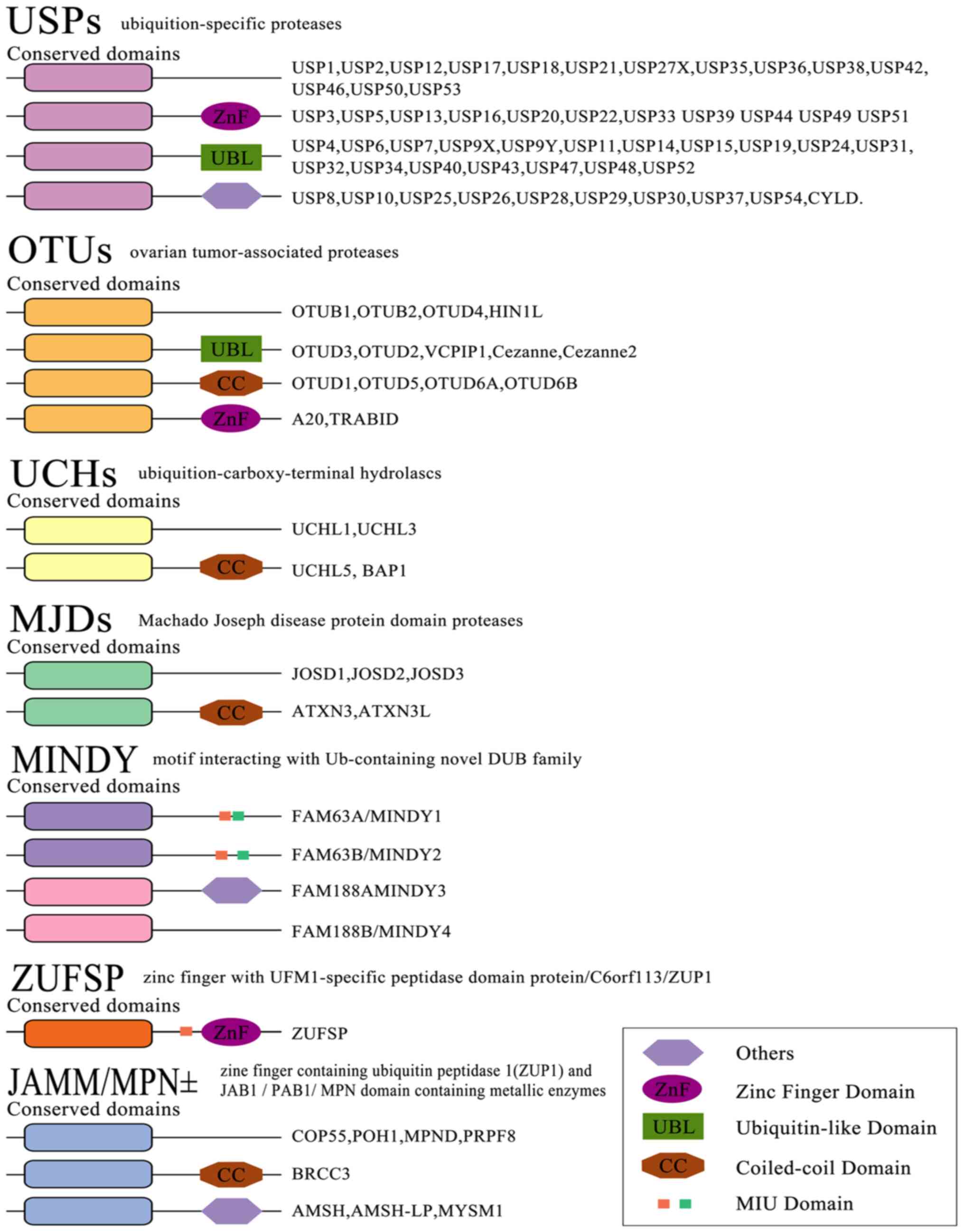
The reversibility of ubiquitination modification is achieved by
deubiquitinating enzymes (DUBs) (22). At present, >100 DUBs are
encoded in the human genome based on the catalytic region. They can
be classified into two categories or six subfamilies (Fig. 3) (23-28).
In recent years, a growing body of evidence suggests
that ubiquitination plays an important regulatory role in the
maintenance of bone homeostasis. Osteoporosis and other related
bone diseases are accompanied by abnormal expression and
dysfunction of ubiquitination and de-ubiquitination enzymes. As a
potential research hotspot for bone metabolism-related diseases and
a new target for drug development, ubiquitination has a broad
prospect in the field of osteoporosis prevention and treatment. The
current review discusses the specific mechanism and role of
ubiquitination in bone remodeling to provide a more theoretical
basis and practical guidance for the treatment of related diseases
(Fig. 4; Table I).
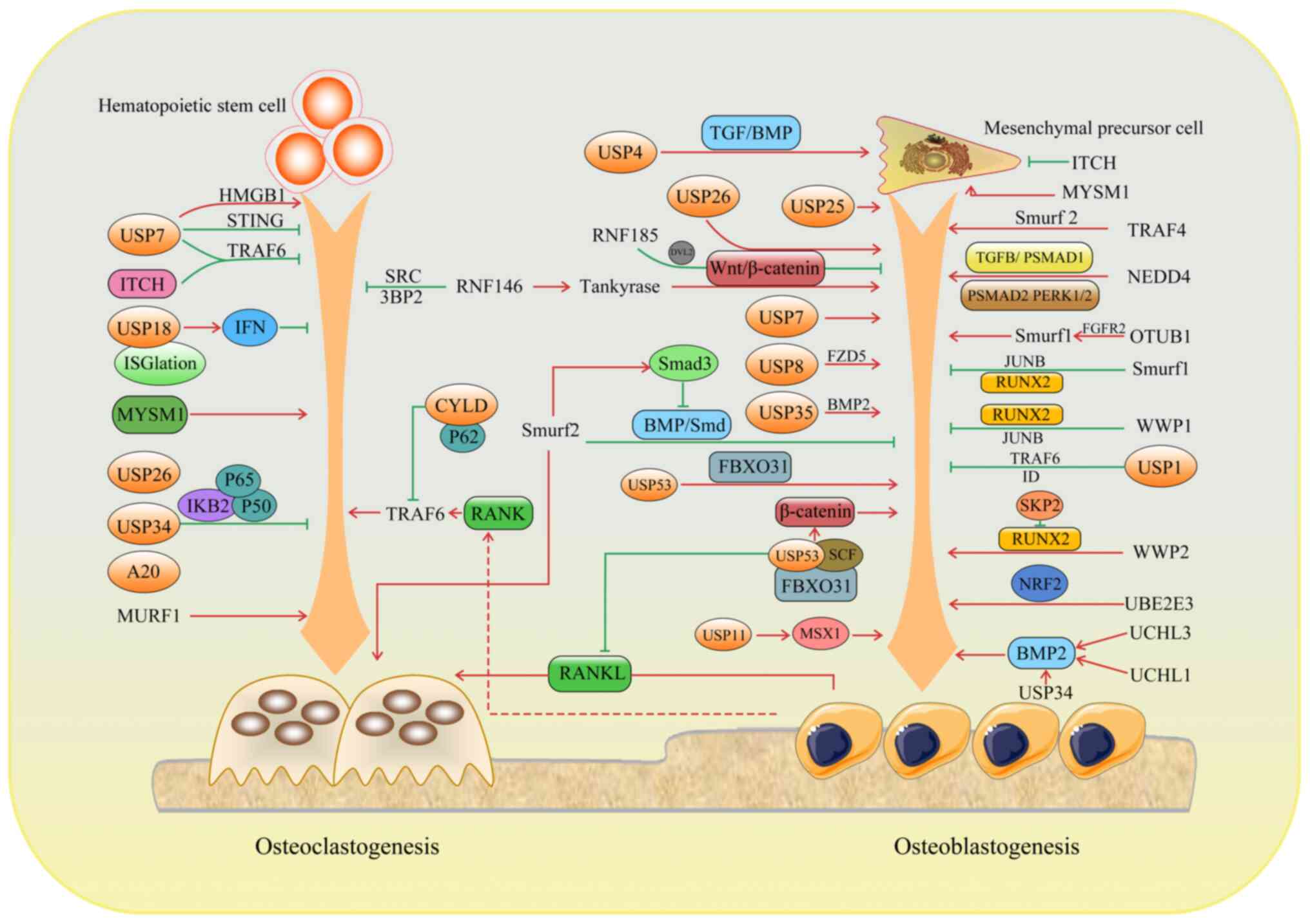
In a growing number of studies, bone remodeling has
been linked to ubiquitination. E3 Ub ligases are mainly involved in
osteoblast differentiation and bone formation. In the present
review, the function and regulation of E3 ligases in bone
homeostasis is discussed.
Belonging to the C2-WW-HECT region Ub ligases,
Smurf1 downregulates the level of alkaline phosphatase and
osteocalcin and suppresses the osteogenic activity of osteoblasts,
resulting in osteoporosis and fragility fractures. Al-Rawi et
al (29) found that the
micro duplication of the Smurf1 gene led to the first case of
childhood osteoporosis. Mechanically, Smurf1 affects the process of
osteogenic differentiation through various mechanisms. Runt-related
transcription factor 2 (Runx2) is a core transcription factor for
directed differentiation of the osteoblast cell lineage and plays a
crucial role in promoting osteoblast differentiation. It is
involved in the Ub-proteasome pathway, which regulates osteoblast
differentiation and bone formation by interacting with and
ubiquitinating Runx2 (30). The
BMP signaling pathway plays a critical biological role in bone
development and post-natal bone formation, mainly by regulating the
differentiation and function of mesenchymal stem cells (MSCs) and
osteoblasts. Smurf1 degrades BMP downstream signaling molecules
also in an Ub-dependent manner and when Smurf1 is inhibited, bone
formation is enhanced in osteoporotic mice with markedly
age-related osteoporosis (31).
Jun B proto-oncogene (JunB) is an ubiquitinated substrate of Smurf1
and a stimulator of MSC proliferation and differentiation into
osteoblasts. Smurf1 is able to bind to and ubiquitinate JunB, which
is subsequently degraded by the proteasome. This degradation
process reduces the protein level of JunB, which inhibits its
transcriptional activity and further affects the proliferation and
differentiation of MSCs, leading to the regulation of bone
metabolism (32). Additionally,
the P53/microRNA (miR/miRNA)-17/Smurf1 pathway inhibits
osteogenesis through suppressing MSC function in age-related
models, leading to osteoporosis (33). However, the underlying mechanism
remains elusive.
WWP1 is a 922-amino acid long HECT type E3 Ub
protein ligase that interacts with the proline-rich polypeptide
motif of target proteins to regulate protein transport,
degradation, signaling and transcription. The role of WWP1 in bone
homeostasis was first discovered in 2006 by Shu et al
(39). WWP1 can polyubiquitinate
Runx2 and decrease the differentiation and migration of
osteoblasts. The role of the intracellular adaptor protein
Schnurri-3 (Shn3) in osteoblastic bone formation is well
established. Shn3 is able to regulate Runx2 protein levels through
a delicate mechanism. Specifically, Shn-3 is able to interact with
WWP1 and recruit it to Runx2. Once WWP1 binds to Runx2, it is able
to promote the ubiquitination of Runx2, which in turn triggers the
process of Runx2 degradation. In this way, Shn3 effectively
controls the intracellular levels of the Runx2 protein, indirectly
affecting the process of bone tissue formation and mineralization
(40). Additionally, during
inflammation-mediated osteoporosis models, WWP1 targets JunB for
ubiquitination and degradation in MSCs to inhibit MSC
differentiation into osteoblasts (41). As with WWP1, WWP2 belongs to the
HECT type of E3 Ub protein ligases. WWP2 mediates
monoubiquitination of Runx2 leading to its transactivation and
resulting in increased osteogenic activity and promotion of
osteogenic differentiation (42).
The RING finger (RNF) 146, a RING domain and
PAR-acylation dependent cytoplasmic E3 ligase, is indispensable in
bone formation. RNF146 can improve MSC self-renewal and
osteogenesis/adipogenesis, leading to increased bone mass (43). In osteoclast differentiation,
RNF146 can promote the ubiquitination and degradation of AXIN,
thereby regulating the Wnt signaling pathway and activating
β-catenin to promote osteoclast differentiation. In addition, BP2
activity is indispensable for SRC tyrosine kinase in osteoclast
differentiation. RNF146 deubiquitinates 3BP2, thereby inhibiting
SRC activity and reducing osteoclast differentiation ability.
Furthermore, RNF146 expression can be suppressed by RANKL through
the NF-κB pathway, by regulating the stability of AXIN and 3BP2,
thereby intervening in osteoclast differentiation (44).
RNF185, also known as FLJ38628, is a RING domain E3
Ub ligase. In a high-throughput screening (45) of synthetic silencing RNA
libraries targeting 5,000 human genes, RNF185 was identified as an
endogenous inhibitor of osteoblastic norms. Deficiency of RNF185
can initiate human MSCs to differentiate into osteoblasts. An
additional study further revealed the underlying mechanism through
which RNF185 negatively regulates osteogenesis by degrading
dishevelled (Dvl) 2 and downregulating the Wnt signaling pathway
(46).
In bone metabolism, ITCH negatively regulates the
differentiation of mesenchymal progenitor cells into osteoblasts by
mediating the proteasomal degradation of the JunB protein, a
positive osteoblast regulator (47). In addition, in animal
experiments, high expression of ITCH was detected in mouse fracture
callus. Several positive regulatory factors for osteoblast
differentiation, including Runx2, increase in the callus of
Itch−/− mice fractures and thus the differentiation of
osteoblasts is also markedly enhanced (48). In osteoclast formation, ITCH
inhibits TRAF6 de-ubiquitination by binding the deubiquitinating
enzyme cylindromatosis to inhibit RANKL-induced osteoclast. ITCH
transcription is directly regulated by RANKL during osteoclast
differentiation via the NF-κB (49).
S-phase kinase-associated protein (SKP) 2 regulates
a wide range of cell cycle proteins by targeting the
SKP1-Cullin1-F-box for ubiquitination and proteasomal degradation
(50). SKP2 negatively targets
Runx2 for Ub-mediated degradation to inhibit osteogenic
differentiation (51).
Muscle-specific RING finger E3 ligase 1 (MURF1), a RING-type E3 Ub
ligase, exhibits a critical role in inducing skeletal muscle
atrophy. It has been reported that prolonged bed rest for various
reasons, such as fractures, spinal cord injury, brain disease and
exposure to microgravity, causes loss of muscle mass and strength,
which further leads to bone loss. Mechanistically, MURF1 is
upregulated and increases protein degradation and muscle wasting in
numerous muscle atrophy models (52). The suppression of MURF1 can
reduce osteoclastogenesis (53).
Ubiquitin-conjugating enzyme E2 E3 (UBE2E3) is negatively and
positively associated with age and osteogenic genes, respectively.
The lack of UBE2E3 was further shown to promote osteoporosis
development and overexpression could inhibit cell aging and
increase osteogenic differentiation of old BMSCs (54). TRAF4 is markedly reduced in bone
sections of ovariectomized rats with local osteoporosis and
patients with osteoporosis (55). Further mechanisms indicate that
the TRAF4 degrades Smurf2 to enhance osteoblastic differentiation
of MSCs. Neural precursor cell expressed developmentally
downregulated protein 4 (NEDD4), a member of the HECT domain
family, participates in cell proliferation, apoptosis, autophagy
and other biological processes to regulate mammalian growth and
development (56,57). NEDD4 enhances osteoblast
proliferation and bone mass accumulation by inducing the
degradation of TGF-1β-activated pSMAD1 and improving the activation
of the pSMAD2 and pERK1/2 pathways (58).
DUBs are involved in DNA repair, gene transcription,
apoptosis, autophagy, DNA repair and immune responses (19). DUBs act as critical regulators
for bone remodeling by regulating basic multicellular unit
differentiation and/or function.
Human adipose-derived stem cells (hASCs) can be
extracted and isolated from human adipose tissue and have the
potential for self-renewal and multidirectional differentiation.
hASCs can be induced to differentiate in a variety of directions,
such as adipogenic, osteogenic and chondrogenic. During osteogenic
differentiation of hASCs, the expression level of USP7 changes and
is positively associated with the degree of osteogenic
differentiation. This implies that USP7 may play an important role
in the osteogenic differentiation of hASCs (59). USP1 controls the NF-κB signaling
pathway by inhibiting TRAF6 ubiquitination, thereby inhibiting
osteoblast pyroptosis (60).
USP1 deletion results in osteogenic impairment in mice and marked
osteopenia. Overexpression of USP1 in MSCs inhibits osteogenic
differentiation and promotes the preservation of MSC features. The
mechanism is that USP1 deubiquitinated inhibitors of DNA-binding
proteins to promote the preservation of MSC signatures (61). USP8 in bone progenitor cells
prevents the degradation of the Frizzy-5 ubiquitinated Wnt receptor
to ensure Wnt-induced osteogenesis, thus promoting bone formation
(62). USP11 can stabilize the
Msh homeobox 1 (MSX1) protein to upregulate the expression of
osteogenic differentiation-related markers in human MSCs,
consequently enhancing osteogenic differentiation (63). The USP34 protein is involved in
bone formation by modulating BMP2 signaling in MSCs. When BMSCs or
pre-osteoblasts are conditionally knocked out of USP34, mice have
low bone mass. Mechanically, USP34 stabilizes both Smad1 and RUNX2
for osteogenic differentiation and bone formation (64). Ubiquitin C-terminal hydrolase-L3
(UCHL3) regulates BMP2-induced Smad1 polyubiquitination to promote
osteoblast differentiation (65).
USP4 affects osteogenic differentiation through
multiple mechanisms. The ubiquitination of the Dvl protein can
activate the Wnt/β-catenin signaling pathway to affect osteoblast
differentiation (66). By
removing the lysine-63-linked polyUb chain from Dvl, USP4 strongly
inhibits Wnt/β-catenin signal transduction to weaken osteogenic
differentiation (67).
Regulatory pathways associated with TGF/BMP are also important in
bone growth (68). USP4 can
target the TGF-β receptor for deubiquitination and enhance the
activation of the TGF-β/BMP signaling pathway to promote the
proliferation and differentiation of MSCs (69).
USP53 enhances osteogenic function and
differentiation in human BMSCs by stabilizing key proteins
associated with osteogenic differentiation and regulating the BMP
signaling pathway. Moreover, USP53-overexpressing human BMSCs
markedly promote bone regeneration in murine calvaria bone defects.
Mechanistically, the interaction of USP53 with F-box protein 31
revealed a new role for USP53 in cellular signaling and protein
degradation. This interaction specifically involves the degradation
of β-catenin by the Skp1/Cul1/F-box protein complex, a key process
that is essential for the regulation of the Wnt signaling pathway
(70). In addition, USP53 acting
on the vitamin D-receptor-SMAD3 pathway increases RANKL-dependent
osteoclastogenesis through osteoblasts (71).
UCHL1 was originally identified in neurons. CXCL7, a
chemokine with a C-X-C motif, is associated with the posterior
longitudinal ligament ossification of the spine (OPLL) phenotype,
manifested by dyskinesia, ectopic ossification of posterior
ligament tissue and osteoporosis. UCHL1 is overexpressed in the
serum of mice with a CXCL7 gene deletion and in patients with OPLL
(72). In patients with
periodontitis, UCHL1 leads to periodontal ligament stem cell
osteogenesis and alveolar bone loss by regulating the BMP2/Smad
signaling pathway. However, it is unclear which molecule is
deubiquitinated by UCHL1. By inhibiting the high expression of
UCHL1, periodontal bone loss is reduced and inflammation is
alleviated (73).
CYLD negatively regulates osteoclastogenesis.
Consistently, the number and activity of osteoclasts in CYLD
knockout mice increases and the mice exhibit severe osteoporosis
(74). With the use of
proteasome inhibitors, CYLD accumulation increases, leading to
impairment of osteoclast production and function (75). Mechanically, CYLD, through
deubiquitination, is able to remove the Ub chain on TRAF6 and
inactivate it, thereby inhibiting the activation of the RANK
signaling pathway (76).
A20 has been shown to mainly regulate osteoclast
formation. A20 overexpression has anti-inflammatory effects on
human periodontal ligament cells (HPDLCs) and blocks osteoclast
differentiation (77). A20
knockout mice have increased osteoclasts, thinner bone trabeculae
and are prone to develop severe osteoporosis (78). A20 induction by intravenous gamma
globulin attenuates RANKL inducing NF-κB signaling to reduce
osteoclastogenesis in vivo as well as inhibits bone
resorption, thus preserving bone mass (79). Mechanistically, the A20
anti-osteoclast function is mainly dependent on inhibiting NF-κB
activation by altering IκBα ubiquitination-mediated degradation,
which physically interacts with NF-κB to inhibit its activation
(80). TRAF6-dependent autophagy
in HPDLCs is inhibited for the reduced expression of A20 with 2%
hypoxia treatment, resulting in decreased osteoclast
differentiation and formation. The underlying mechanism involves
A20 inhibiting K63-linked, while it enhances K48-based
ubiquitination of TRAF6 to suppress TRAF6 activation and promote
TRAF6 proteasomal degradation, respectively, thus inhibiting
autophagy under hypoxic conditions (81).
Myb-like, Swi3p, Rsc8p and Moira (SWIRM) and MPN
domain containing 1 (MYSM1) is a metalloprotease composed of three
domains, MPN, SWI2/SNF2 ISWI-like SANT (SANT) and SWIRM (82). MYSM1 is not only an important
regulator of hematopoiesis and immunity (83), but also essential for maintenance
of bone homeostasis. Mysm1−/− mice showed enhanced
autonomic differentiation of MSCs and accelerated adipogenesis, as
evidenced by reduced bone mass in long bones and cranial bones
(84). A study demonstrated that
osteoclast progenitor cells in Mysm1−/− mice showed
decreased proliferative ability and markedly decreased osteoclast
number and absorption activity (85). However, the underlying mechanism
of MYSM1 in osteoclast differentiation and function remains
enigmatic.
The main goal of osteoporosis medications is to
reduce the risk of osteoporotic fractures and improve the quality
of life of patients by relieving pain, increasing bone density and
preventing fractures (94).
According to the different mechanisms of action, anti-osteoporosis
drugs are generally placed in three categories: i) Inhibitors of
bone resorption; ii) promoters of bone formation; and iii)
dual-acting drugs. Bone formation promoters are mainly parathyroid
hormone receptor agonists, including teriparatide and appalatide.
Sclerostatin monoclonal is a double-acting drug (95). There are five main categories of
bone resorption inhibitors: i) Bisphosphonates (BPs); ii) RANKL
inhibitors; iii) estrogen; iv) selective estrogen receptor
modulators; and iv) calcitonin (Table II).
BPs are widely used in the treatment of
osteoporosis, where they are effective in inhibiting bone
resorption and increasing bone mass at vertebral, non-vertebral and
hip sites, thereby reducing the risk of fracture (96). However, oral BPs can increase
upper gastrointestinal symptoms in patients and ~33% of patients
receiving first intravenous zoledronic acid develop flu-like
symptoms. In addition, prolonged use of BP medications may result
in reduced blood supply to the jaws, thereby increasing the risk of
osteonecrosis of the jaws as well as inhibition of bone turnover,
leading to impaired bone remodeling, thereby increasing the risk of
atypical fractures (97).
Denosumab is also the most common osteoporosis drug in the clinic.
It can be combined with RANKL to markedly reduce the degree of
osteoporosis in patients. If Denosumab was stopped without other
osteoporosis drugs, bone mineral density showed a steep decline
(98). It is noteworthy that
rashes, cellulitis, femoral shaft or subtrochanteric fractures and
jaw necrosis may occur during use. The application of estrogen in
women aged <60 years or 10 years after menopause can effectively
prevent bone loss caused by menopause. Estrogen acts by indirectly
regulating calcitonin, parathyroid hormone and 1,25-(OH)2D3.
However, Estrogen replacement therapy has been limited clinically
due to potential adverse effects, including venous thromboembolism,
cerebrovascular disease, stroke and breast cancer (99). Menopausal hormone therapy (MHT)
has its unique advantages in the treatment of postmenopausal
osteoporosis (100,101). In the mid-1990s and early
2000s, in most European and North American countries, MHT is widely
used to prevent the risk of postmenopausal osteoporosis and related
bone fractures in women. Following the publication of the
randomized Women's Health Initiative (WHI) report (102), which found that MHT increased
the risk of coronary heart disease, stroke and breast cancer, there
was a dramatic decline in MHT prescribing worldwide. The current
trend is to recommend lower doses of estrogen for effective relief
of vasomotor symptoms and prevent vaginal atrophy with an improved
bleeding profile than with higher estrogen dosages within 10 years
of menopause than was recommended a few years ago. For the
prevention of endometrial cancer caused by MHT, it is recommended
to add progesterone (103). In
addition, compared with oral estrogen therapy, transdermal estrogen
patch can reduce the risk of venous thrombosis and atherosclerotic
vascular disease and also protect the heart (104). Therefore, low-dose non-oral MHT
may be considered for women with cardiovascular risk factors, older
women and those who choose to use MHT for extended periods to
protect bone (105,106). In conclusion, individualized
treatment is advocated for hormone replacement therapy and
different formulations, dosages and MHT regimens are considered at
the same time. The artificial synthesis of selective estrogen
receptor modulators can selectively act on different tissue
estrogen receptors to reduce the incidence of breast cancer in
women (107). However, some
side effects, including vasomotor symptoms, muscle spasm and venous
thrombosis, remain. The Use of Raloxifene in Heart Disease (RUTH)
study showed that Raloxifene increased fatal stroke (0.7‰ increase
in absolute risk) and the risk of the occurrence of venous
thromboembolic disease (absolute risk increase by 1.3‰) (108).
Parathyroid hormone is one of the important hormones
that regulate calcium and phosphorus metabolism, as well as bone
conversion. It has been shown that parathyroid hormone promotes
bone anabolic metabolism that is dependent on low-dose and
intermittent administration. The duration and dosage of
teriparatide use are closely associated with rat bone tumors
(109), but post-marketing
surveillance did not reveal a higher than expected risk of
osteosarcoma (110). Calcitonin
reduces blood calcium and phosphorus mainly by inhibiting
osteoclasts. However, long-term use of calcitonin leads to clinical
resistance. In addition, there may be an increased risk of
cardiovascular complications. This may be associated with the
effect of calcitonin on the cardiovascular system; it may affect
the systolic and diastolic function of the heart, or the relaxation
and contraction of blood vessels (111). Monoclonal sclerostin
romosozumab promotes the formation of bone mass, while it inhibits
the resorption of bone, rapidly forming bone on trabecular and
cortical bone surfaces to increase bone density and strength
(112). The most common adverse
reactions include arthralgia, nasopharyngitis, back pain and
injection site reactions, rare mandibular osteonecrosis and
atypical fractures. The black box warning on the potential
cardiovascular risks of romosozumab, including serious adverse
events such as non-fatal myocardial infarction, non-fatal stroke
and cardiovascular death, may indeed place some limitations on its
clinical use (95). Osteoporosis
guidelines emphasize vitamin D and calcium supplementation as basic
measures. Importantly, it has been shown that when patients with
osteoporosis are treated with anti-osteoporosis drugs, the
effectiveness of these drugs is markedly reduced without adequate
vitamin D and calcium supplementation (113).
UPS is critical for bone cell formation and
differentiation. Based on these mechanisms of action, drugs
targeting these Ub ligases and deubiquitinating enzymes for the
treatment of osteoporosis and other related bone diseases continue
to be developed. For example, by inhibiting the activity of certain
Ub ligases, it may be possible to promote bone formation or inhibit
bone resorption for the treatment of osteoporosis. By contrast, by
activating the activity of certain deubiquitinating enzymes, it may
also be possible to have a positive impact on the treatment of
skeletal diseases.
Catalpol, a biologically active cyclic enol ether
terpene glucoside extracted from the traditional Chinese Medicine
Rehmannia glutinosa, inhibits the ubiquitination and
degradation of phosphatase and tensin homolog to increase
phosphatase activity and then inhibits downstream AKT and NF-κB
associated with RANKL signal pathways, consequently suppressing
osteoclast formation and bone absorption (114). In mice with LPS and
ovariectomized-induced bone loss, catalpol improves bone loss by
attenuating osteoclast activity (114). Melatonin is a biologically
active amine hormone secreted by the pineal gland of the brain and
is a signaling molecule with a circadian rhythm, participating in
various physiological processes including bone metabolism (109). Melatonin inhibits RANKL
receptor activators to inhibit osteolysis and prevent bone loss and
also promotes osteoblast differentiation and activity by melatonin
receptor 2 (115). Melatonin
reduces the ubiquitination of the Smad1 protein preventing its
degradation by inhibiting Smurf1 activity, thereby stabilizing BMP
Smad1 signaling activity and restoring inflammatory factor-induced
osteogenesis (116). Carnosic
acid (CA), an amino acid substance with strong antioxidant and
anti-aging effects, is widely used in weight loss foods,
nutritional supplements and other fields. CA binds to the
ligand-binding region of cholesterol and estrogen
receptor-associated receptor α, promoting its ubiquitination and
proteasomal degradation, thereby inhibiting RANKL-associated
osteoclast formation and ovariectomy-induced bone loss (117).
Beraprost can improve postmenopausal osteoporosis by
downregulating the NEDD4-mediated ubiquitination-proteasome
degradation of Runx2 (118).
The selective complexes B06 and B75 block binding of Smurf1 to Ub,
thereby stabilizing the level of the BMP signaling component
Smad1/5 protein and enhancing osteoblast activity. This also offers
promising new clinical prospects for the therapy of osteoporosis
(119). miR-195-5p can target
and inhibit the activation of BMP-2/SMAD/Akt/Runx2 pathway
signaling by Smurf1, to promote osteogenic differentiation in
ovariectomized mouse models (120). Chalcone derivatives enhance
local bone mass after lumbar spine fusion surgery in normal BMP-2
mice with high expression of Smurf1 (31). Bortezomib, is a 26S proteasome
inhibitor which inhibits the degradation of polyUb protein by
suppressing the hydrolysis activity of the proteasome. Bortezomib
regulates proteasomal degradation of Runx2 via the Wnt/β-catenin
signaling pathway (121),
inducing osteogenic differentiation and promoting osteogenesis
(122). Furthermore, a recent
study suggested that bortezomib may treat post-menopausal
osteoporosis by inhibiting the Smurf-mediated
ubiquitination-proteasome process (123). Withaferin A, also a proteasome
inhibitor, promotes osteoblast proliferation and differentiation as
well as regulates bone anabolism to promote osteoporotic fracture
healing (124).
miRNAs participate in the progress in osteoporosis
through a complex signaling pathway network (125). miR-21-5p inhibits osteoclast
differentiation by targeting SKP2. In an ovariectomy (OVX) mouse
model injected with miR-21-5p, the number of osteoclasts was
reduced and the degree of osteoporosis was improved (126). Neat1, the mechanoreceptor long
non-coding RNA (lncRNA), under mechanical stimulation, regulates
osteogenic function by nuclear retention of the
paraplaque-dependent E3 Ub ligase Smurf1 mRNA. In Neat1-knockout
hindlimb unloading mice, no bone damage or bone loss are found.
This provides a treatment reference for bone loss caused by
long-term space flight or inactivity and osteoporosis associated
with aging (127). In addition,
SNHG1 lncRNA inhibits osteogenic differentiation through
NEDD4-mediated ubiquitination by suppressing the activation of the
p38-MAPK signaling pathway, a key trigger factor in bone formation
(128). In recent years, a new
drug delivery system, nanoparticles, has been used to treat
osteoporosis. Ferumoxytol and ferucarbotran, as clinical
nanoparticles, exert anti-osteoporosis effects by inhibiting
osteoclastogenesis and participating in bone metabolism.
Nanoparticles trigger the upregulation of p62, resulting in the
recruitment of CYLD to enhance deubiquitination and inactivation of
TRAF6, the main controller of the RANKL and NF-κB signal (129). When downstream activation of
the NF-κB signaling pathway and the MAPK signaling pathway is
attenuated, the proliferation and differentiation processes of
osteoclast precursors may be impeded, leading to a reduction in
osteoclast formation. Intravenous injection can improve bone
quality in OVX mice and it is anticipated to be an alternative drug
for the treatment of osteoporosis (129).
In addition, several clinically used drugs have been
discovered to be involved in bone homeostasis via the UPS system,
such as thalidomide (130),
lansoprazole (131), vitisin A,
clomimidazole and zoledronic acid (132), all of which inhibit or induce
ubiquitination of related substrates.
The present review introduced and summarized the
current literature on ubiquitination and deubiquitination in
osteoporosis and bone homeostasis, highlighting the critical
importance of Ub-dependent proteolytic systems in skeletal cell
function. The E3 connecting enzymes are mainly regulated by
transcription factors and they have notable protein participation
in bone formation in the signaling pathway of BMSC and osteoblast
proliferation and differentiation. Only a few studies have shown
that RNF146 (44) and Smurf2
(35) regulate osteoclasts. The
E3 protease system can influence the bone remodeling process by
regulating different target proteins. They recognize and degrade
proteins that are involved in bone formation, thereby regulating
the rate and extent of bone formation. At the same time, they also
recognize and degrade proteins involved in bone resorption, thereby
controlling the process of bone resorption. This precise regulatory
mechanism is essential for maintaining bone homeostasis.
Research on the regulation of deubiquitinating
enzymes on osteoblasts and osteoclasts is limited and the mechanism
and molecules of action have not been fully elucidated. To date,
the relationship between MJDs and MINDY families with skeletal cell
differentiation and function has not been reported. Elucidating the
intrinsic mechanisms by which DUB gene alterations regulate
osteoblast and osteoclast functions is important for the
development of novel and more effective therapeutic strategies for
osteoporosis. Designing drugs targeting specific genetic
alterations in the DUB gene to avoid its dysregulation is one of
the important directions for developing novel osteoporosis
treatment strategies. Through in-depth study of the function and
regulatory mechanisms of the DUB gene, potential drug targets can
be identified and targeted drugs can be designed to regulate the
function of osteoblasts and osteoclasts. These drugs can correct
the imbalance of bone metabolism by altering the activity or
expression level of the DUB gene, thus achieving the goal of
treating bone diseases such as osteoporosis. In the future, through
in-depth study of the function and regulatory mechanism of the DUB
gene, as well as the design of drugs targeting its specific genetic
alterations, it is anticipated to provide new ideas and methods for
the treatment of skeletal diseases such as osteoporosis.
Meanwhile, several anti-osteoporosis drugs commonly
used in the clinic were discussed in the present review as well as
their disadvantages and the current clinical studies on
ubiquitination-related drugs for osteoporosis. At present,
anti-osteoporosis drugs have shown some efficacy in clinical
research and their benefits outweigh the risk of side effects, but
patients lack compliance due to the aforementioned side effects,
high costs and sequential treatment. Although designing drugs to
target specific genetic alterations in the DUB gene is challenging
and the adverse effects and the interactions with other drugs are
not yet clear, the future research direction may be to treat
osteoporosis by mediating ubiquitination.
Furthermore, the internal mechanisms by which
ubiquitination regulates skeletal cell function have not been
thoroughly investigated and the specific sites of ubiquitination
modification and the interactions of different molecules acting at
the same site have not been clarified. Decoding the pathways
between ubiquitination and other modification mechanisms has also
not been described in depth. These practical mechanisms should be
further investigated in the future.
Not applicable.
CC conceived the study. XF wrote the first draft. RZ
drew the figures. GX, WL, PF, CC and RG revised the manuscript and
figures. All authors read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was supported by grants from the Qinghai
Provincial Department of Science and Technology (grant no.
2022-ZJ-951Q).
|
1
|
The Lancet Diabetes Endocrinology:
Osteoporosis: Overlooked in men for too long. Lancet Diabetes
Endocrinol. 9:12021. View Article : Google Scholar
|
|
2
|
Shen Y, Huang X, Wu J, Lin X, Zhou X, Zhu
Z, Pan X, Xu J, Qiao J, Zhang T, et al: The global burden of
osteoporosis, low bone mass, and its related fracture in 204
countries and territories, 1990-2019. Front Endocrinol (Lausanne).
13:8822412022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Huang Z, Wang Y, Xu W, Chen H, Xu
J, Luo S, Zhang Y, Zhao D and Hu J: The efficacy and safety of
denosumab in postmenopausal women with osteoporosis previously
treated with bisphosphonates: A review. J Orthop Translat. 22:7–13.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu XM, Li N, Li K, Li XY, Zhang P, Xuan YJ
and Cheng XG: Discordance in diagnosis of osteoporosis by
quantitative computed tomography and dual-energy X-ray
absorptiometry in Chinese elderly men. J Orthop Translat. 18:59–64.
2018. View Article : Google Scholar
|
|
5
|
Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP,
Zhang M, Bai LY, Chen XX, Zhang C, Shi L and Rui YF: The regulative
effect and repercussion of probiotics and prebiotics on
osteoporosis: Involvement of brain-gut-bone axis. Crit Rev Food Sci
Nutr. 63:7510–7528. 2023. View Article : Google Scholar
|
|
6
|
Intemann J, De Gorter DJJ, Naylor AJ,
Dankbar B and Wehmeyer C: Importance of osteocyte-mediated
regulation of bone remodelling in inflammatory bone disease. Swiss
Med Wkly. 150:w201872020.PubMed/NCBI
|
|
7
|
Amarasekara DS, Kim S and Rho J:
Regulation of osteoblast differentiation by cytokine networks. Int
J Mol Sci. 22:28512021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edwards JR and Mundy GR: Advances in
osteoclast biology: Old findings and new insights from mouse
models. Nat Rev Rheumatol. 7:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar
|
|
10
|
Han L, Wu J, Wang M, Zhang Z, Hua D, Lei S
and Mo X: RNA modification-related genetic variants in genomic loci
associated with bone mineral density and fracture. Genes (Basel).
13:18922022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Chen Q and Xu H: Wnt/β-catenin
signal transduction pathway in prostate cancer and associated drug
resistance. Discov Oncol. 12:402021. View Article : Google Scholar
|
|
12
|
Hu R, Chen L, Chen X, Xie Z, Xia C and
Chen Y: Aloperine improves osteoporosis in ovariectomized mice by
inhibiting RANKL-induced NF-κB, ERK and JNK approaches. Int
Immunopharmacol. 97:1077202021. View Article : Google Scholar
|
|
13
|
Hou H, Peng Q, Wang S, Zhang Y, Cao J,
Deng Y, Wang Y, Sun WC and Wang HB: Anemonin attenuates
RANKL-induced osteoclastogenesis and ameliorates LPS-induced
inflammatory bone loss in mice via modulation of NFATc1. Front
Pharmacol. 10:16962020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng L, Meng T, Chen L, Wei W and Wang P:
The role of ubiquitination in tumorigenesis and targeted drug
discovery. Signal Transduct Target Ther. 5:112020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai J, Culley MK, Zhao Y and Zhao J: The
role of ubiquitination and deubiquitination in the regulation of
cell junctions. Protein Cell. 9:754–69. 2018. View Article : Google Scholar :
|
|
16
|
van Huizen M and Kikkert M: The role of
atypical ubiquitin chains in the regulation of the antiviral innate
immune response. Front Cell Dev Biol. 7:3922020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akutsu M, Dikic I and Bremm A: Ubiquitin
chain diversity at a glance. J Cell Sci. 129:875–880. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gundogdu M and Walden H: Structural basis
of generic versus specific E2-RING E3 interactions in protein
ubiquitination. Protein Sci. 28:1758–1770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mennerich D, Kubaichuk K and Kietzmann T:
DUBs, hypoxia, and cancer. Trends Cancer. 5:632–653. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Komander D and Rape M: The ubiquitin code.
Annu Rev Biochem. 81:203–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng N and Shabek N: Ubiquitin ligases:
Structure, function, and regulation. Annu Rev Biochem. 86:129–157.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao J, Guo J, Wang Y, Ma Q, Shi Y, Cheng
F, Lu Q, Fu W, Ouyang G, Zhang J, et al: Research progress of DUB
enzyme in hepatocellular carcinoma. Front Oncol. 12:9202872022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clague MJ, Urbé S and Komander D: Breaking
the chains: Deubiquitylating enzyme specificity begets function.
Nat Rev Mol Cell Biol. 20:338–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bello AI, Goswami R, Brown SL, Costanzo K,
Shores T, Allan S, Odah R and Mohan RD: Deubiquitinases in
neurodegeneration. Cells. 11:5562022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu
W, Cohen RE and Shi Y: Crystal structure of a UBP-family
deubiquitinating enzyme in isolation and in complex with ubiquitin
aldehyde. Cell. 111:1041–1054. 2002. View Article : Google Scholar
|
|
26
|
Johnston SC, Larsen CN, Cook WJ, Wilkinson
KD and Hill CP: Crystal structure of a deubiquitinating enzyme
(human UCH-L3) at 1.8 A resolution. EMBO J. 16:3787–3796. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y and Reverter D: Molecular mechanisms
of DUBs regulation in signaling and disease. Int J Mol Sci.
22:9862021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komander D, Clague MJ and Urbé S: Breaking
the chains: Structure and function of the deubiquitinases. Nat Rev
Mol Cell Biol. 10:550–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Rawi R, Al-Beshri A, Mikhail FM and
McCormick K: Fragile bones secondary to SMURF1 gene duplication.
Calcif Tissue Int. 106:567–573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Glimcher LH, Jones DC and Wein MN: Control
of postnatal bone mass by the zinc finger adapter protein
Schnurri-3. Ann N Y Acad Sci. 1116:174–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang C, Peng S, Li J, Lu J, Guan D, Jiang
F, Lu C, Li F, He X, Zhu H, et al: Inhibition of osteoblastic
Smurf1 promotes bone formation in mouse models of distinctive
age-related osteoporosis. Nat Commun. 9:34282018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao L, Huang J, Guo R, Wang Y, Chen D and
Xing L: Smurf1 inhibits mesenchymal stem cell proliferation and
differentiation into osteoblasts through JunB degradation. J Bone
Miner Res. 25:1246–1256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu W, Qi M, Konermann A, Zhang L, Jin F
and Jin Y: The p53/miR-17/Smurf1 pathway mediates skeletal
deformities in an age-related model via inhibiting the function of
mesenchymal stem cells. Aging (Albany NY). 7:205–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koganti P, Levy-Cohen G and Blank M:
smurfs in protein homeostasis, signaling, and cancer. Front Oncol.
8:2952018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Z, Greenblatt MB, Yan G, Feng H, Sun J,
Lotinun S, Brady N, Baron R, Glimcher LH and Zou W: SMURF2
regulates bone homeostasis by disrupting SMAD3 interaction with
vitamin D receptor in osteoblasts. Nat Commun. 8:145702017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonewald LF and Mundy GR: Role of
transforming growth factor-beta in bone remodeling. Clin Orthop
Relat Res. 261–276. 1990.PubMed/NCBI
|
|
37
|
Bai Y and Ying Y: The post-translational
modifications of Smurf2 in TGF-β signaling. Front Mol Biosci.
7:1282020. View Article : Google Scholar
|
|
38
|
Kushioka J, Kaito T, Okada R, Ishiguro H,
Bal Z, Kodama J, Chijimatsu R, Pye M, Narimatsu M, Wrana JL, et al:
A novel negative regulatory mechanism of Smurf2 in BMP/Smad
signaling in bone. Bone Res. 8:412020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shu L, Zhang H, Boyce BF and Xing L:
Ubiquitin E3 ligase Wwp1 negatively regulates osteoblast function
by inhibiting osteoblast differentiation and migration. J Bone
Miner Res. 28:1925–1935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jones DC, Wein MN, Oukka M, Hofstaetter
JG, Glimcher MJ and Glimcher LH: Regulation of adult bone mass by
the zinc finger adapter protein Schnurri-3. Science. 312:1223–1227.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L, Huang J, Zhang H, Wang Y, Matesic
LE, Takahata M, Awad H, Chen D and Xing L: Tumor necrosis factor
inhibits mesenchymal stem cell differentiation into osteoblasts via
the ubiquitin E3 ligase Wwp1. Stem Cells. 29:1601–1610. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu W, He X, Hua Y, Li Q, Wang J and Gan
X: The E3 ubiquitin ligase WWP2 facilitates RUNX2 protein
transactivation in a mono-ubiquitination manner during osteogenic
differentiation. J Biol Chem. 292:11178–11188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W,
Yu T, Yang R, Wang R, Zhou Y and Shi S: Circulating apoptotic
bodies maintain mesenchymal stem cell homeostasis and ameliorate
osteopenia via transferring multiple cellular factors. Cell Res.
28:918–933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsumoto Y, Larose J, Kent OA, Lim M,
Changoor A, Zhang L, Storozhuk Y, Mao X, Grynpas MD, Cong F and
Rottapel R: RANKL coordinates multiple osteoclastogenic pathways by
regulating expression of ubiquitin ligase RNF146. J Clin Invest.
127:1303–1315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Y and Ding S: A high-throughput siRNA
library screen identifies osteogenic suppressors in human
mesenchymal stem cells. Proc Natl Acad Sci USA. 104:9673–9678.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou Y, Shang H, Zhang C, Liu Y, Zhao Y,
Shuang F, Zhong H, Tang J and Hou S: The E3 ligase RNF185
negatively regulates osteogenic differentiation by targeting Dvl2
for degradation. Biochem Biophys Res Commun. 447:431–436. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Li X, Zhang H, Gu R, Wang Z, Gao Z
and Xing L: Ubiquitin E3 ligase Itch negatively regulates
osteoblast function by promoting proteasome degradation of
osteogenic proteins. Bone Joint Res. 6:154–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H and Xing L: Ubiquitin e3 ligase
itch negatively regulates osteoblast differentiation from
mesenchymal progenitor cells. Stem Cells. 31:1574–1583. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Wu C, Matesic LE, Li X, Wang Z,
Boyce BF and Xing L: Ubiquitin E3 ligase Itch negatively regulates
osteoclast formation by promoting deubiquitination of tumor
necrosis factor (TNF) receptor-associated factor 6. J Biol Chem.
288:22359–22368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang F, Zhao Y and Sun Y: USP2 is an SKP2
deubiquitylase that stabilizes both SKP2 and its substrates. J Biol
Chem. 297:1011092021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thacker G, Kumar Y, Khan MP, Shukla N,
Kapoor I, Kanaujiya JK, Lochab S, Ahmed S, Sanyal S, Chattopadhyay
N and Trivedi AK: Skp2 inhibits osteogenesis by promoting
ubiquitin-proteasome degradation of Runx2. Biochim Biophys Acta.
1863:510–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eddins MJ, Marblestone JG, Suresh Kumar
KG, Leach CA, Sterner DE, Mattern MR and Nicholson B: Targeting the
ubiquitin E3 ligase MuRF1 to inhibit muscle atrophy. Cell Biochem
Biophys. 60:113–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bettis T, Kim BJ and Hamrick MW: Impact of
muscle atrophy on bone metabolism and bone strength: Implications
for muscle-bone crosstalk with aging and disuse. Osteoporos Int.
29:1713–1720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Cai G, Chen P, Jiang T and Xia Z:
UBE2E3 regulates cellular senescence and osteogenic differentiation
of BMSCs during aging. PeerJ. 9:e122532021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Wang P, Xie Z, Wang S, Cen S, Li M,
Liu W, Tang S, Ye G, Zheng G, et al: TRAF4 positively regulates the
osteogenic differentiation of mesenchymal stem cells by acting as
an E3 ubiquitin ligase to degrade Smurf2. Cell Death Differ.
26:2652–2666. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
An H, Krist DT and Statsyuk AV: Crosstalk
between kinases and Nedd4 family ubiquitin ligases. Mol Biosyst.
10:1643–1657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wiszniak S, Harvey N and Schwarz Q: Cell
autonomous roles of Nedd4 in craniofacial bone formation. Dev Biol.
410:98–107. 2016. View Article : Google Scholar
|
|
58
|
Jeon SA, Lee JH, Kim DW and Cho JY:
E3-ubiquitin ligase NEDD4 enhances bone formation by removing
TGFβ1-induced pSMAD1 in immature osteoblast. Bone. 116:248–258.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang Y, Lv L, Li W, Zhang X, Jiang Y, Ge W
and Zhou Y: Protein deubiquitinase USP7 is required for osteogenic
differentiation of human adipose-derived stem cells. Stem Cell Res
Ther. 8:1862017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun D, Peng Y, Ge S and Fu Q: USP1
inhibits NF-κB/NLRP3 induced pyroptosis through TRAF6 in
osteoblastic MC3T3-E1 cells. J Musculoskelet Neuronal Interact.
22:536–545. 2022.PubMed/NCBI
|
|
61
|
Williams SA, Maecker HL, French DM, Liu J,
Gregg A, Silverstein LB, Cao TC, Carano RA and Dixit VM: USP1
deubiquitinates ID proteins to preserve a mesenchymal stem cell
program in osteosarcoma. Cell. 146:918–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chaugule S, Kim JM, Yang YS, Knobeloch KP,
He X and Shim JH: Deubiquitinating enzyme USP8 is essential for
skeletogenesis by regulating Wnt signaling. Int J Mol Sci.
22:102892021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kaushal K, Tyagi A, Karapurkar JK, Kim EJ,
Tanguturi P, Kim KS, Jung HS and Ramakrishna S: Genome-wide
CRISPR/Cas9-Based screening for deubiquitinase subfamily identifies
ubiquitin-specific protease 11 as a novel regulator of osteogenic
differentiation. Int J Mol Sci. 23:8562022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Guo YC, Wang MY, Zhang SW, Wu YS, Zhou CC,
Zheng RX, Shao B, Wang Y, Xie L, Liu WQ, et al: Ubiquitin-specific
protease USP34 controls osteogenic differentiation and bone
formation by regulating BMP2 signaling. EMBO J. 37:e993982018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim JY, Lee JM and Cho JY: Ubiquitin
C-terminal hydrolase-L3 regulates Smad1 ubiquitination and
osteoblast differentiation. FEBS Lett. 585:1121–1126. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang P, Yan R, Zhang X, Wang L, Ke X and
Qu Y: Activating Wnt/β-catenin signaling pathway for disease
therapy: Challenges and opportunities. Pharmacol Ther. 196:79–90.
2019. View Article : Google Scholar
|
|
67
|
Zhou F, Li F, Fang P, Dai T, Yang B, van
Dam H, Jia J, Zheng M and Zhang L: Ubiquitin-specific protease 4
antagonizes osteoblast differentiation through dishevelled. J Bone
Miner Res. 31:1888–8198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guasto A and Cormier-Daire V: Signaling
pathways in bone development and their related skeletal dysplasia.
Int J Mol Sci. 22:43212021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Herhaus L and Sapkota GP: The emerging
roles of deubiquitylating enzymes (DUBs) in the TGFβ and BMP
pathways. Cell Signal. 26:2186–2192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Baek D, Park KH, Lee KM, Jung S, Joung S,
Kim J and Lee JW: Ubiquitin-specific protease 53 promotes
osteogenic differentiation of human bone marrow-derived mesenchymal
stem cells. Cell Death Dis. 12:2382021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hariri H, Kose O, Bezdjian A, Daniel SJ
and St-Arnaud R: USP53 regulates bone homeostasis by controlling
rankl expression in osteoblasts and bone marrow adipocytes. J Bone
Miner Res. 38:578–596. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tsuru M, Ono A, Umeyama H, Takeuchi M and
Nagata K: Ubiquitin-dependent proteolysis of CXCL7 leads to
posterior longitudinal ligament ossification. PLoS One.
13:e01962042018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lin L, Li S, Hu S, Yu W, Jiang B, Mao C,
Li G, Yang R, Miao X, Jin M, et al: UCHL1 impairs periodontal
ligament stem cell osteogenesis in periodontitis. J Dent Res.
102:61–71. 2023. View Article : Google Scholar
|
|
74
|
Cao Y, Zhang X, Hu M, Yang S, Li X, Han R,
Zhou J, Li D and Liu D: CYLD inhibits osteoclastogenesis to
ameliorate alveolar bone loss in mice with periodontitis. J Cell
Physiol. 238:1036–1045. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ang E, Pavlos NJ, Rea SL, Qi M, Chai T,
Walsh JP, Ratajczak T, Zheng MH and Xu J: Proteasome inhibitors
impair RANKL-induced NF-kappaB activity in osteoclast-like cells
via disruption of p62, TRAF6, CYLD, and IkappaBalpha signaling
cascades. J Cell Physiol. 220:450–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jin W, Chang M, Paul EM, Babu G, Lee AJ,
Reiley W, Wright A, Zhang M, You J and Sun SC: Deubiquitinating
enzyme CYLD negatively regulates RANK signaling and
osteoclastogenesis in mice. J Clin Invest. 118:1858–1866. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hong JY, Bae WJ, Yi JK, Kim GT and Kim EC:
Anti-inflammatory and anti-osteoclastogenic effects of zinc finger
protein A20 overexpression in human periodontal ligament cells. J
Periodontal Res. 51:529–539. 2016. View Article : Google Scholar
|
|
78
|
Lee EG, Boone DL, Chai S, Libby SL, Chien
M, Lodolce JP and Ma A: Failure to regulate TNF-induced NF-kappaB
and cell death responses in A20-deficient mice. Science.
289:2350–2354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee MJ, Lim E, Mun S, Bae S, Murata K,
Ivashkiv LB and Park-Min KH: Intravenous immunoglobulin (IVIG)
attenuates TNF-induced pathologic bone resorption and suppresses
osteoclastogenesis by inducing A20 expression. J Cell Physiol.
231:449–458. 2016. View Article : Google Scholar :
|
|
80
|
Martens A and van Loo G: A20 at the
crossroads of cell death, inflammation, and autoimmunity. Cold
Spring Harb Perspect Biol. 12:a0364182020. View Article : Google Scholar
|
|
81
|
Yan K, Wu C, Ye Y, Li L, Wang X, He W, Ren
S and Xu Y: A20 inhibits osteoclastogenesis via TRAF6-dependent
autophagy in human periodontal ligament cells under hypoxia. Cell
Prolif. 53:e127782020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Birol M and Echalier A: Structure and
function of MPN (Mpr1/Pad1 N-terminal) domain-containing proteins.
Curr Protein Pept Sci. 15:504–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fiore A, Liang Y, Lin YH, Tung J, Wang H,
Langlais D and Nijnik A: Deubiquitinase MYSM1 in the hematopoietic
system and beyond: A current review. Int J Mol Sci. 21:30072020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li P, Yang YM, Sanchez S, Cui DC, Dang RJ,
Wang XY, Lin QX, Wang Y, Wang C, Chen DF, et al: Deubiquitinase
MYSM1 Is essential for normal bone formation and mesenchymal stem
cell differentiation. Sci Rep. 6:222112016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Haffner-Luntzer M, Kovtun A, Fischer V,
Prystaz K, Hainzl A, Kroeger CM, Krikki I, Brinker TJ, Ignatius A
and Gatzka M: Loss of p53 compensates osteopenia in murine Mysm1
deficiency. FASEB J. 32:1957–1968. 2018. View Article : Google Scholar
|
|
86
|
Lin YC, Zheng G, Liu HT, Wang P, Yuan WQ,
Zhang YH, Peng XS, Li GJ, Wu YF and Shen HY: USP7 promotes the
osteoclast differentiation of CD14+ human peripheral blood
monocytes in osteoporosis via HMGB1 deubiquitination. J Orthop
Translat. 40:80–91. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xie Z, Wu Y, Shen Y, Guo J, Yuan P, Ma Q,
Wang S, Jie Z, Zhou H, Fan S and Chen S: USP7 inhibits
osteoclastogenesis via dual effects of attenuating TRAF6/TAK1 axis
and stimulating STING signaling. Aging Dis. 14:2267–2283. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mustachio LM, Lu Y, Kawakami M, Roszik J,
Freemantle SJ, Liu X and Dmitrovsky E: Evidence for the
ISG15-specific deubiquitinase USP18 as an antineoplastic target.
Cancer Res. 78:587–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu X, Lu Y, Chen Z, Liu X, Hu W, Zheng L,
Chen Y, Kurie JM, Shi M, Mustachio LM, et al: The
ubiquitin-specific peptidase USP18 promotes lipolysis, fatty acid
oxidation, and lung cancer growth. Mol Cancer Res. 19:667–677.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yim HY, Park C, Lee YD, Arimoto K, Jeon R,
Baek SH, Zhang DE, Kim HH and Kim KI: Elevated response to type I
IFN enhances RANKL-mediated osteoclastogenesis in Usp18-knockout
mice. J Immunol. 196:3887–3895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Q, Wang M, Xue H, Liu W, Guo Y, Xu R,
Shao B and Yuan Q: Ubiquitin-specific protease 34 inhibits
osteoclast differentiation by regulating NF-κB signaling. J Bone
Miner Res. 35:1597–1608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li C, Qiu M, Chang L, Qi J, Zhang L,
Ryffel B and Deng L: The osteoprotective role of USP26 in
coordinating bone formation and resorption. Cell Death Differ.
29:1123–1136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhu Q, Fu Y, Cui CP, Ding Y, Deng Z, Ning
C, Hu F, Qiu C, Yu B, Zhou X, et al: OTUB1 promotes osteoblastic
bone formation through stabilizing FGFR2. Signal Transduct Target
Ther. 8:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
LeBoff MS, Greenspan SL, Insogna KL,
Lewiecki EM, Saag KG, Singer AJ and Siris ES: The clinician's guide
to prevention and treatment of osteoporosis. Osteoporos Int.
33:2049–2102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Eastell R, Rosen CJ, Black DM, Cheung AM,
Murad MH and Shoback D: Pharmacological management of osteoporosis
in postmenopausal women: An endocrine society* clinical
practice guideline. J Clin Endocrinol Metab. 104:1595–1622. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
McClung M, Harris ST, Miller PD, Bauer DC,
Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK and Lewiecki EM:
Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug
holiday. Am J Med. 126:13–20. 2013. View Article : Google Scholar
|
|
97
|
Shane E, Burr D, Abrahamsen B, Adler RA,
Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, et
al: Atypical subtrochanteric and diaphyseal femoral fractures:
Second report of a task force of the American society for bone and
mineral research. J Bone Miner Res. 29:1–23. 2014. View Article : Google Scholar
|
|
98
|
Deeks ED: Denosumab: A review in
postmenopausal osteoporosis. Drugs Aging. 35:163–173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Morin SN, Feldman S, Funnell L,
Giangregorio L, Kim S, McDonald-Blumer H, Santesso N, Ridout R,
Ward W, Ashe MC, et al: Clinical practice guideline for management
of osteoporosis and fracture prevention in Canada: 2023 Update.
CMAJ. 195:E1333–E1348. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lobo RA, Pickar JH, Stevenson JC, Mack WJ
and Hodis HN: Back to the future: Hormone replacement therapy as
part of a prevention strategy for women at the onset of menopause.
Atherosclerosis. 254:282–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gosset A, Pouillès JM and Trémollieres F:
Menopausal hormone therapy for the management of osteoporosis. Best
Pract Res Clin Endocrinol Metab. 35:1015512021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results from
the women's health initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Prior JC, Seifert-Klauss VR, Giustini D,
Adachi JD, Kalyan S and Goshtasebi A: Estrogen-progestin therapy
causes a greater increase in spinal bone mineral density than
estrogen therapy-a systematic review and meta-analysis of
controlled trials with direct randomization. J Musculoskelet
Neuronal Interact. 17:146–154. 2017.PubMed/NCBI
|
|
104
|
de Villiers TJ, Hall JE, Pinkerton JV,
Pérez SC, Rees M, Yang C and Pierroz DD: Revised global consensus
statement on menopausal hormone therapy. Maturitas. 91:153–155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Levin VA, Jiang X and Kagan R: Estrogen
therapy for osteoporosis in the modern era. Osteoporos Int.
29:1049–1055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Abdi F, Mobedi H, Bayat F, Mosaffa N,
Dolatian M and Ramezani Tehrani F: The effects of transdermal
estrogen delivery on bone mineral density in postmenopausal women:
A meta-analysis. Iran J Pharm Res. 16:380–389. 2017.PubMed/NCBI
|
|
107
|
Mosca L: Rationale and overview of the
raloxifene use for the heart (RUTH) trial. Ann N Y Acad Sci.
949:181–185. 2001. View Article : Google Scholar
|
|
108
|
Ensrud K, Genazzani AR, Geiger MJ, McNabb
M, Dowsett SA, Cox DA and Barrett-Connor E: Effect of raloxifene on
cardiovascular adverse events in postmenopausal women with
osteoporosis. Am J Cardiol. 97:520–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Vahle JL, Long GG, Sandusky G, Westmore M,
Ma YL and Sato M: Bone neoplasms in F344 rats given teriparatide
[rhPTH(1-34)] are dependent on duration of treatment and dose.
Toxicol Pathol. 32:426–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Andrews EB, Gilsenan AW, Midkiff K,
Sherrill B, Wu Y, Mann BH and Masica D: The US postmarketing
surveillance study of adult osteosarcoma and teriparatide: Study
design and findings from the first 7 years. J Bone Miner Res.
27:2429–2437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xie J, Guo J, Kanwal Z, Wu M, Lv X,
Ibrahim NA, Li P, Buabeid MA, Arafa EA and Sun Q: Calcitonin and
bone physiology: In vitro, in vivo, and clinical investigations.
Int J Endocrinol. 2020:32368282020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Holdsworth G, Roberts SJ and Ke HZ: Novel
actions of sclerostin on bone. J Mol Endocrinol. 62:R167–R185.
2019. View Article : Google Scholar
|
|
113
|
Weaver CM, Alexander DD, Boushey CJ,
Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC
and Wang DD: Calcium plus vitamin D supplementation and risk of
fractures: An updated meta-analysis from the national osteoporosis
foundation. Osteoporos Int. 27:367–376. 2016. View Article : Google Scholar :
|
|
114
|
Meng J, Zhang W, Wang C, Zhang W, Zhou C,
Jiang G, Hong J, Yan S and Yan W: Catalpol suppresses
osteoclastogenesis and attenuates osteoclast-derived bone
resorption by modulating PTEN activity. Biochem Pharmacol.
171:1137152020. View Article : Google Scholar
|
|
115
|
Ferlazzo N, Andolina G, Cannata A,
Costanzo MG, Rizzo V, Currò M, Ientile R and Caccamo D: Is
melatonin the cornucopia of the 21st century? Antioxidants (Basel).
9:10882020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lian C, Wu Z, Gao B, Peng Y, Liang A, Xu
C, Liu L, Qiu X, Huang J, Zhou H, et al: Melatonin reversed tumor
necrosis factor-alpha-inhibited osteogenesis of human mesenchymal
stem cells by stabilizing SMAD1 protein. J Pineal Res. 61:317–327.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zheng ZG, Cheng HM, Zhou YP, Zhu ST, Thu
PM, Li HJ, Li P and Xu X: Dual targeting of SREBP2 and ERRα by
carnosic acid suppresses RANKL-mediated osteoclastogenesis and
prevents ovariectomy-induced bone loss. Cell Death Differ.
27:2048–2065. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zheng HL, Xu WN, Zhou WS, Yang RZ, Chen
PB, Liu T, Jiang LS and Jiang SD: Beraprost ameliorates
postmenopausal osteoporosis by regulating Nedd4-induced Runx2
ubiquitination. Cell Death Dis. 12:4972021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang Y, Wang C, Cao Y, Gu Y and Zhang L:
Selective compounds enhance osteoblastic activity by targeting HECT
domain of ubiquitin ligase Smurf1. Oncotarget. 8:50521–50533. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ye LC, Qian LF, Liang L, Jiang LJ, Che ZY
and Guo YH: Overexpression of miR-195-5p reduces osteoporosis
through activating BMP-2/SMAD/Akt/RUNX2 pathway via targeting
SMURF1. J Biol Regul Homeost Agents. 35:1201–1216. 2021.
|
|
121
|
Oyajobi BO, Garrett IR, Gupta A, Flores A,
Esparza J, Muñoz S, Zhao M and Mundy GR: Stimulation of new bone
formation by the proteasome inhibitor, bortezomib: implications for
myeloma bone disease. Br J Haematol. 139:434–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Uyama M, Sato MM, Kawanami M and Tamura M:
Regulation of osteoblastic differentiation by the proteasome
inhibitor bortezomib. Genes Cells. 17:548–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Fang Y, Liu Y, Zhao Z, Lu Y, Shen X, Zhu
T, Hou M, He F, Yang H, Zhang Y, et al: Bortezomib rescues
ovariectomy-induced bone loss via SMURF-mediated ubiquitination
pathway. Oxid Med Cell Longev. 2021:96612002021. View Article : Google Scholar
|
|
124
|
Khedgikar V, Kushwaha P, Gautam J, Verma
A, Changkija B, Kumar A, Sharma S, Nagar GK, Singh D, Trivedi PK,
et al: Withaferin A: A proteasomal inhibitor promotes healing after
injury and exerts anabolic effect on osteoporotic bone. Cell Death
Dis. 4:e7782013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kabekkodu SP, Shukla V, Varghese VK, D'
Souza J, Chakrabarty S and Satyamoorthy K: Clustered miRNAs and
their role in biological functions and diseases. Biol Rev Camb
Philos Soc. 93:1955–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Huang Y, Yang Y, Wang J, Yao S, Yao T, Xu
Y, Chen Z, Yuan P, Gao J, Shen S and Ma J: miR-21-5p targets SKP2
to reduce osteoclastogenesis in a mouse model of osteoporosis. J
Biol Chem. 296:1006172021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu C, Gao X, Li Y, Sun W, Xu Y, Tan Y, Du
R, Zhong G, Zhao D, Liu Z, et al: The mechanosensitive lncRNA Neat1
promotes osteoblast function through paraspeckle-dependent Smurf1
mRNA retention. Bone Res. 10:182022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jiang Y, Wu W, Jiao G, Chen Y and Liu H:
LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus
inhibits osteogenic differentiation of bone marrow mesenchymal stem
cells. Life Sci. 228:208–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu L, Jin R, Duan J, Yang L, Cai Z, Zhu
W, Nie Y, He J, Xia C, Gong Q, et al: Bioactive iron oxide
nanoparticles suppress osteoclastogenesis and ovariectomy-induced
bone loss through regulating the TRAF6-p62-CYLD signaling complex.
Acta Biomater. 103:281–292. 2020. View Article : Google Scholar
|
|
130
|
Liu Y, Huang X, He X, Zhou Y, Jiang X,
Chen-Kiang S, Jaffrey SR and Xu G: A novel effect of thalidomide
and its analogs: Suppression of cereblon ubiquitination enhances
ubiquitin ligase function. FASEB J. 29:4829–4839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Mishima K, Kitoh H, Ohkawara B, Okuno T,
Ito M, Masuda A, Ishiguro N and Ohno K: Lansoprazole upregulates
polyubiquitination of the TNF receptor-associated factor 6 and
facilitates Runx2-mediated osteoblastogenesis. EBioMedicine.
2:2046–2061. 2015. View Article : Google Scholar
|
|
132
|
Li X, Sun W, Li J, Wang M, Zhang H, Pei L,
Boyce BF, Wang Z and Xing L: Clomipramine causes osteoporosis by
promoting osteoclastogenesis via E3 ligase Itch, which is prevented
by zoledronic acid. Sci Rep. 7:413582017. View Article : Google Scholar : PubMed/NCBI
|