Intervertebral disc degeneration (IVDD) is a disease
characterized by degeneration of the spinal disc, accompanied by
the gradual loss of proteoglycans and water in the nucleus pulposus
(NP) of the IVD (1). IVDD can
lead to changes in the structure of the spine, which can adversely
affect the normal physiological function of the spine (2). In addition, IVDD is the
prerequisite and pathological basis for degenerative disease of the
lumbar spine, including lumbar disc herniation and stenosis
(3). Epidemiological studies
have shown that IVDD is the primary factor of lower back pain
(LBP), which is a common clinical symptom (4). In 2019, the global adult incidence
of LBP was 7.34% (5,6). As the leading factor in the global
disability burden, LBP-related disability cases increased by 47%
from 1990 to 2019 (5). The
lifetime instances of LBP in Americans amounts to ~80% (7). A notable burden is imposed on
society when people struggle with recurrent LBP that causes harm to
their physical and mental health (8,9).
However, the exact pathogenesis of IVDD is not clear and there is a
lack of effective treatment options for IVDD and secondary LBP.
Oral metformin is a prescription medication most
frequently used for treating diabetes, including type 2 diabetes
mellitus (T2DM). Patients with T2DM who use metformin have
excellent safety profiles, along with satisfactory blood glucose
control. This drug rarely causes adverse reactions such as
hypoglycaemia (10-12). The primary cause of fasting
hyperglycaemia in patients with T2DM is increased liver glucose
production. Various studies have noted that metformin controls
hyperglycaemia by inhibiting gluconeogenesis in the liver (13-16). Metformin is efficient at
alleviating a wide range of conditions, such as obesity (17), eating disorders (18), tumours (19), cardiovascular disease (20,21), polycystic ovary syndrome
(22), kidney disease (23), cell senescence (24), osteoporosis (OP) (25) and osteoarthritis (OA) (26). DM is significantly associated
with IVDD (27). DM can promote
IVDD by abnormal blood glucose regulation and damaging IVD
metabolism, which makes IVDD a complication of diabetes (28). Due to the association between
IVDD and DM, metformin has been demonstrated to alleviate IVDD
(29-34). Recent research has revealed that
metformin may boost its curative effect on IVDD by encouraging the
release of mesenchymal stem cell (MSC)-derived extracellular
nanovesicles (30).
The hypoglycaemic effect of metformin is primarily
mediated by inhibiting the mitochondrial respiratory chain in the
liver and decreasing the production of ATP, which leads to the
activation of AMP-activated kinase (AMPK) and increases the ratio
of AMP/ATP, thereby inhibiting liver glycogenolysis and glucose
production (35,36). Improvement of IVDD is associated
with the AMPK signalling pathway (29,37). Autophagy is an internal recycling
mechanism that has been preserved throughout evolution to degrade
damaged or old cytoplasm in order to preserve cells in a state of
equilibrium (38). Impaired
autophagy in IVD cells is a primary contributor to IVDD.
Appropriate induction of autophagy can slow progression of IVDD,
but excessive autophagy can lead to IVD cell death (3,39). Hypoxia can decrease excessive
autophagy by limiting reactive oxygen species (ROS) production and
inactivating the AMPK/mTOR signalling pathway, thereby contributing
to the survival of IVD cells (37). Metformin induces autophagy in NP
cells of IVD in an AMPK-dependent manner, which suggests the
importance of the AMPK pathway for the treatment of IVDD with
metformin (29). The present
review summarizes research advances on metformin and IVDD and
potential mechanisms of metformin in controlling the onset and
progression of IVDD. These results may provide fresh perspectives
on how metformin is clinically used to prevent and treat IVDD and
facilitate further research into its mechanism of action.
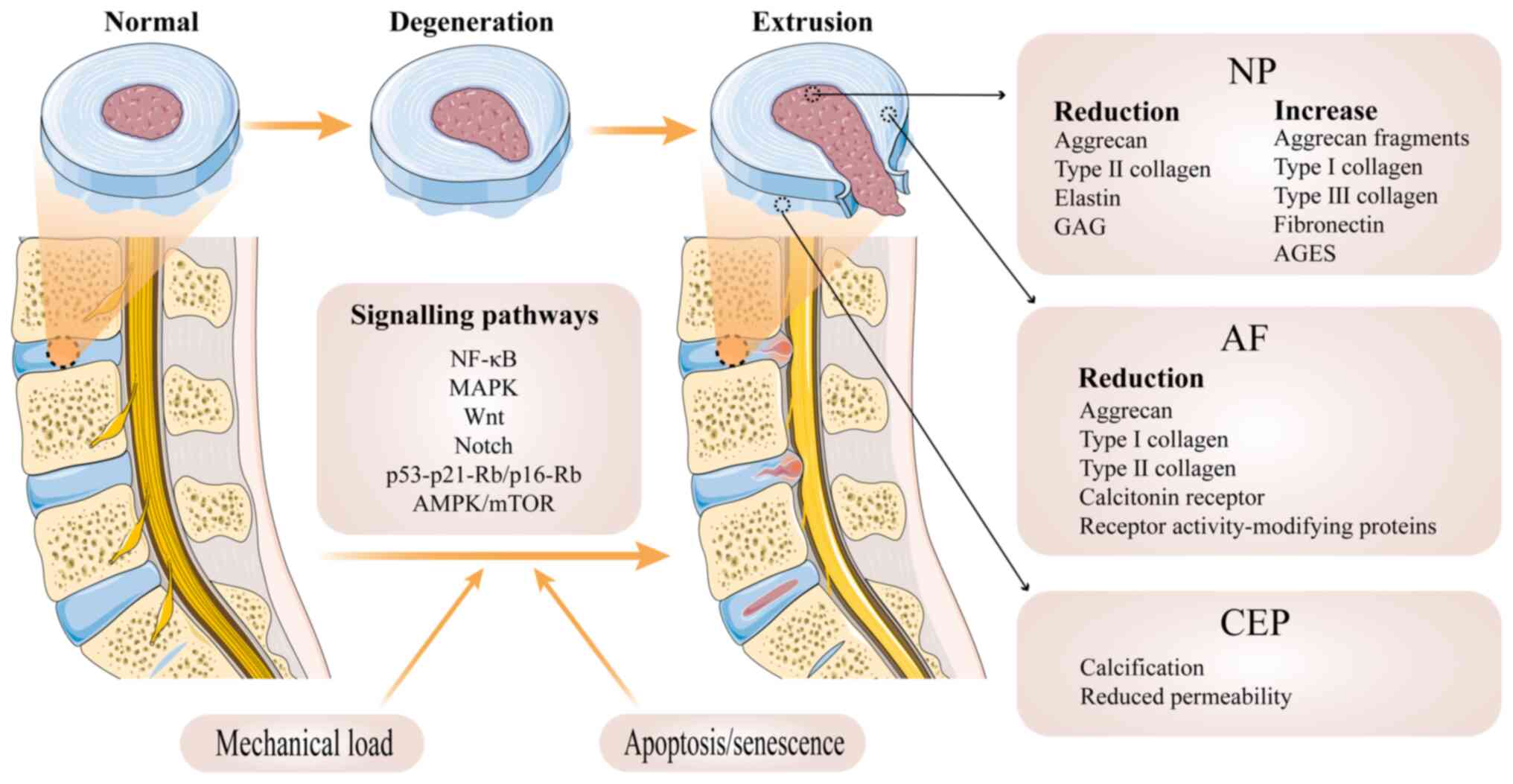
The IVD is a key structure in the human spinal
joint, connecting the upper and lower cones to perform movements
and carry the load of the spine. The IVD is made up of three
components: NP, anulus fibrosus (AF) and the cartilage endplate
(CEP) and cone endplate on the upper and lower surfaces (40) (Fig. 1). The NP is composed of
chondrocyte-like cells and has a large number of randomly
distributed collagen fibres and radially arranged elastic fibers,
the most important component of which is aggregated proteoglycan
(aggrecan) (41,42), which provides the osmotic
properties needed to resist compression and maintain the height of
the IVD (43). The AF is
composed primarily of water, collagen, aggregated and
non-aggregated proteoglycan and non-collagenous protein (44). The AF is structurally composed of
15-25 concentric rings or lamellae composed of type I collagen
fibres that are reversed in their alignment between consecutive
cones (45). The AF is used to
accommodate the NP and maintain NP pressurization under compressive
loading (43,46). The CEP mostly is composed of type
II collagen, glycosaminoglycan (GAG), and water (47) in similar proportions to those
found in articular cartilage (48). The CEP exists in a highly
hydrated proteoglycan matrix reinforced by collagen fibres, which
fibers may contribute to the connection between the CEP and AF
(49), while elastic fibers are
key for the connection between the NP and adjacent CEP (50). CEP can separate the IVD from the
adjacent cone while accommodating the NP (51). CEP can act as a semipermeable
membrane that facilitates material exchange between cone layers
(52), which provides nutrients
to the cone and eliminates metabolites. CEP can act as a cushion
and evenly distribute the load (53).
Aberrant cell-mediated response to increasing
collapse of structure is a prevalent route of IVDD (Fig. 1). Degenerative IVD is
characterized by structural failure accompanied by accelerated
cellular senescence (45).
Although mechanical overload is hypothesized to be the direct cause
of IVDD, it is only an aggravating factor. The premature weakening
of IVD is one of the key factors leading to disc-related issues.
This degeneration can be caused by various factors, including
genetic predisposition, age, insufficient transport of metabolites,
and loading history (45).
A key stage in the development of the IVDD is the
degradation of the extracellular matrix (ECM) by NP and AF cells.
In NP cells, the matrix exhibits a decrease in GAG, aggrecan and
elastin, an alteration in collagen and an increase in fragmented
aggrecan, fibronectin and advanced glycosylation end products
(AGES) (54). The decrease in
type II collagen expression in NP cells is accompanied by an
increase in type I and III collagen, ensuring that the absolute
amount of collagen remains constant. However, there is an
association between changes in type X collagen and calcification of
the CEP. High expression of type X collagen may stimulate
preosteoblasts to undergo osteogenesis in chondrocytes, leading to
calcification of CEP (55,56). In addition, fragments of aggrecan
are filtered out of the IVD (57), and the decrease in aggrecan is
hypothesized to be closely related to a decrease in the hydration
capacity and ability to withstand load of NP cells (58). Additionally, the formation of
AGES can increase stiffness and fragility of collagen fibres
(54). This may lead to a
decrease in the elasticity of the IVD, making it more susceptible
to injury and degeneration, which in turn affects the stability and
function of the spine (54). In
addition, syndecan-4, a transmembrane acetyl heparan sulfate
proteoglycan, may mediate degradation of ECM and aggregated
proteins via thrombospondin motif 5 and MMPs (59,60). The small leucine-rich
proteoglycans are also impaired by degradation (61). This may lead to a loss of
elasticity and stability of the ECM, affecting cell-matrix
interactions and, consequently, the physiological function and
structural stability of the tissue (62). Decrease expression of islet
amyloid polypeptide, calcitonin receptor and receptor
activity-modifying proteins in AF cells disrupts ECM homeostasis,
thereby promoting IVDD (61).
Furthermore, the reduction of type I and type II collagen and
lectins in AF cells leads to a transition of their load-bearing
form to an inner annular elastic cushioning. Prolonged transition
may result in compressed pain and AF damage (51). Alterations in the activity of
certain enzymes, such as histoproteinases, matrix proteases and
polymerase, may indirectly trigger degeneration of the disc
(55). For example, MMPs may
cause apoptosis in NP cells and calcification of the CEP (63,64).
Numerous signalling pathways are thought to be
closely associated with IVDD, such as the NF-κB and MAPK pathways,
which advance the progression of IVDD by regulating proinflammatory
mediators (65) such as TNF-α,
IL-1β and IL-6. In addition, initiation and development of disc
disease may be dependent on the Wnt and the Notch pathways.
Activation of the Wnt pathway accelerates NP cell senescence
(66), while the
hypoxia-activated Notch pathway can influence the course of IVDD
via proinflammatory factors (66). DNA damage also plays a role in
IVDD, leading to the loss of proteoglycans, decreased disc height
and increased cellular senescence (67,68). DNA damage and telomere shortening
induce replicative senescence in IVD cells primarily through
activation of the p53/p21-retinoblastoma (Rb)/p16-Rb signalling
pathway (69,70). In addition, oxidative stress
serves an important role in IVDD and ROS damage the structure and
function of IVD by damaging lipids, DNA and protein (71). Hydrogen peroxide promotes
senescence by activating the senescence pathway, which stops NP
cells at the G0/G1 phase of the cell cycle (72). The effects of oxidative stress
also indicate activation of the p53/p21-Rb/p16-Rb signalling
pathway (73). Inflammation
promotes senescence in IVD cells and the inflammatory factors TNF-α
and IL-1α, -1β, -6 and -17 enhance catabolism of the ECM (73).
Sirtuin (SIRT)1 can protect against IVD by
inhibiting the p53-dependent and p16-dependent cellular senescence
pathways and it can activate the Akt signalling pathway to protect
NP cells from apoptosis (73,74). Moderate autophagy can mitigate
apoptosis and delay IVDD in response to oxidative stress (75). Most autophagy is activated via
AMPK/mTOR-dependent pathways (76). Overactivated autophagy may
adversely affect IVD and hypoxic conditions can inactivate the
AMPK/mTOR signalling pathway by limiting ROS production and
decreasing activation of autophagy in NP cells through a pathway
involving hypoxia-inducible factor 1α (75). In addition, autophagy safeguards
CEP cells against calcification brought on by the intermittent,
cyclic mechanical strain (77).
Apoptosis is associated with IVDD in NP cells, which
are associated with activation of caspase-3 activity, oxidative
stress and SIRT1 (37).
Extrinsically, TNF-α regulates Fas/Fas ligand levels and thus
activates caspase-3/9 activity (66), and may cause a significant
increase in a disintegrin and metalloproteinase with thrombospondin
motifs (ADAMTS) family of disintegrins and ADAMTS-7 in degenerating
NP cells (78). In addition, in
degenerating IVD, microRNAs (miRs) are differentially expressed,
including downregulation of miR-155, production of miR-10b and
miR-27a, and elevation of miR-21, and downregulation of miR-155
also activates caspase-3 activity to promote apoptosis (37). Additionally, p38 MAPK, JNK1/2 and
ERK1/2 in the MAPK family may damage the IVD (78). It has been shown that the MAPK
family exerts proapoptotic effects on senescence via direct or
indirect activation of the p53/p21-Rb/p16-Rb pathway (73). In addition, apoptosis in AF and
CEP cells is associated with the presence of the MAPK family and
caspase-3, -8 and -9 (37).
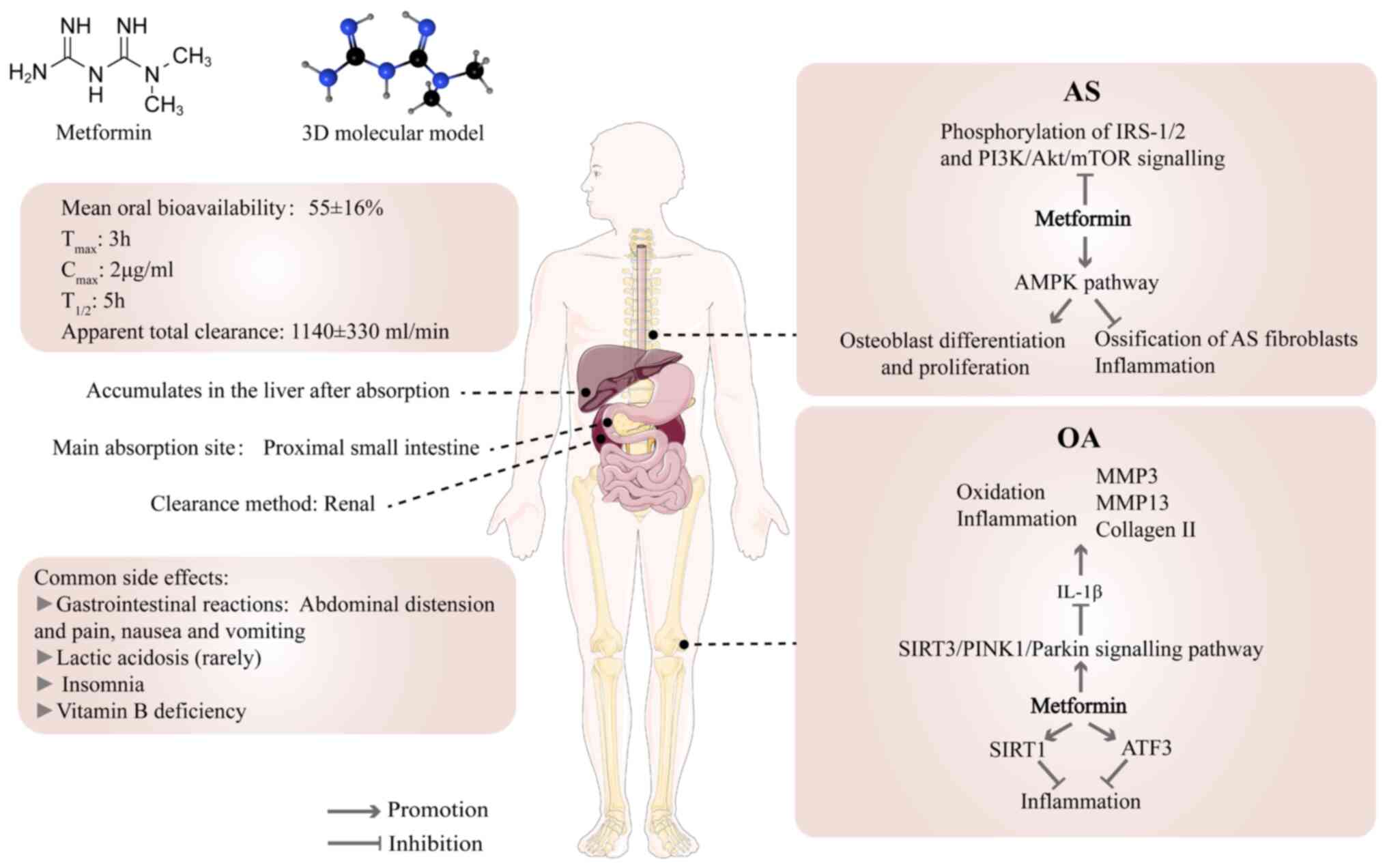
Following oral administration, metformin is
dispersed in the gastrointestinal tract, liver and kidney (85). The mean oral bioavailability of
metformin is 55±16%. Absorption is mainly limited to the proximal
small intestine, and it accumulates in the liver after absorption
(83). The peak plasma
concentration is reached ~3 h after oral administration, and the
peak in the liver appears within 1 h of administration (83,86). The plasma concentrations of
metformin are 1-4 mg/l in patients who received 1-2 g/day metformin
oral treatment (12). At 4 h
after drug intake, the liver absorbs 80% of metformin and the body
eliminates 98% within 24 h (87). Metformin is primarily excreted
through the kidneys. Elimination half-life and apparent total
clearance in individuals with normal renal function are 5 h and
1,140±330 ml/min, respectively (83). The absorption, liver utilization
and renal excretion of metformin are primarily mediated by organic
cation transporters (OCTs). Studies have shown that proton
inhibitors decrease metformin transport by inhibiting OCTs
(24,83).
To lessen gastrointestinal adverse reactions such as
diarrhoea, malignant vomiting and so on, metformin should be taken
with meals. Eating can affect drug absorption, but the decreased
rate of absorption is not clinically significant for most patients
(24,83,88). The faecal recovery of metformin
is 20-30% of the oral dose, and metformin is not readily detectable
in faeces after intravenous administration, suggesting that faecal
metformin is not absorbed or utilized (83).
Degenerative skeletal diseases include OP, OA and
IVDD, as well as diseases such as ankylosing spondylitis (AS) and
periodontitis (24) (Fig. 2). The use of metformin in the
treatment of degenerative bone diseases has been verified to be
beneficial (24,89). The incidence of OP is lower among
patients who have diabetes with carcinoma in situ who are
treated with metformin than those not receiving metformin (25). Metformin improves the long-term
outcome for people who are significantly overweight and have knee
OA (90). To the best of our
knowledge however, there have been no clinical studies showing the
effectiveness of metformin in the treatment of IVDD. Since the NP
is similar to articular cartilage, which consists of water,
collagen and proteoglycans, (91-93) and NP and bone tissue have common
components and origins (94,95), the mechanisms by which metformin
affects them are similar.
Many studies have shown that activation of AMPK has
a notable effect on musculoskeletal disorder (96-98). AMPK activity is significantly
downregulated in human and mouse chondrocytes with OA and
significantly elevated following metformin administration (82). Metformin can lead to osteoblast
differentiation in vitro by activating AMPK, promoting bone
matrix synthesis and osteoblast proliferation (99). Metformin also stimulates the
regenerative process in bone lesions in diabetic and non-diabetic
rats (99). Metformin serves an
effective role in inhibiting ossification and inflammation in AS
fibroblasts by activating the AMPK pathway (100). The aforementioned studies
suggest an important role of the AMPK signalling pathway in
treatment of degenerative skeletal diseases with metformin.
However, the direct targets of metformin that act via the AMPK
pathway in these diseases remain unclear. Recent studies have shown
that metformin binds presenilin Enhancer 2 (PEN2) and initiates a
signalling pathway that intersects with the AMPK/activated
lysosomal glucose sensing pathway via ATP6AP1 (101-103). Whether metformin acts through
this signalling pathway in skeletal degenerative disease remains
unclear.
Phosphoinositide 3-kinase (PI3K)/Akt is one of the
key pathways that regulates cell behaviour (including apoptosis,
proliferation and differentiation); previous studies have shown
that this pathway is a key regulator of osteoblasts and is
associated with fracture healing (104-107). Metformin ability to regulate
the PI3K/Akt pathway could contribute to its efficacy in the
treatment of AS (100).
Metformin reduces insulin and insulin-like growth factor-1 (IGF-1)
levels, thereby inhibiting phosphorylation of insulin receptor
substrate 1/2 (IRS-1/2) and PI3K/Akt/mTOR signalling (24), which is key for the control of
skeletal degenerative lesions.
Metformin is an efficient and typically
well-tolerated drug with notable hypoglycaemic effects but there
are some adverse events (Fig.
2); the most prevalent negative reactions include
gastrointestinal discomfort, including abdominal distension and
pain, nausea and vomiting. These symptoms are typically mild, but
medication may need to be suspended and the dose adjusted (24,88,116,117). In rare cases, metformin is
related to lactic acidosis. Studies have shown that cardiovascular
disease and liver and kidney damage following treatment are likely
to be the main causes of lactic acidosis and there is no causal
relationship with metformin (24,118,119). It has been reported that 1.4%
of patients treated with metformin have insomnia, which may be
associated with changes in blood glucose levels (120). DM with depression often suffer
from insomnia (121,122). In addition, long-term metformin
treatment may lead to vitamin B12 deficiency and require regular
monitoring to decrease the incidence of side effects (123-125). Researchers determined the
milk-to-plasma ratio of metformin in lactating patients and showed
that metformin was safe during lactation and did not cause side
effects (10,24). However, maternal and infant risks
and individual differences should be considered and metformin is
not recommended for pregnant patients (24).
As a highly evolutionarily conserved
serine/threonine protein kinase, AMPK is commonly expressed in
eukaryotic cells (126). The
role of AMPK in the development of IVDD is becoming more obvious
(Table I). Activation of the
AMPK signalling pathway has been observed in human IVDD (127). AMPK phosphorylation is
increased in rat IVDD. IVDD is a complex process involving both
apoptosis and senescence (29).
In degenerating rat NP cells, AMPK activation further
dephosphorylates mTOR and inhibition of AMPK-mediated mTOR
facilitates apoptosis and senescence while suppressing cell
proliferation and cell cycle progression (34,128). Meanwhile, human AF cell
apoptosis is influenced by AMPK (129).
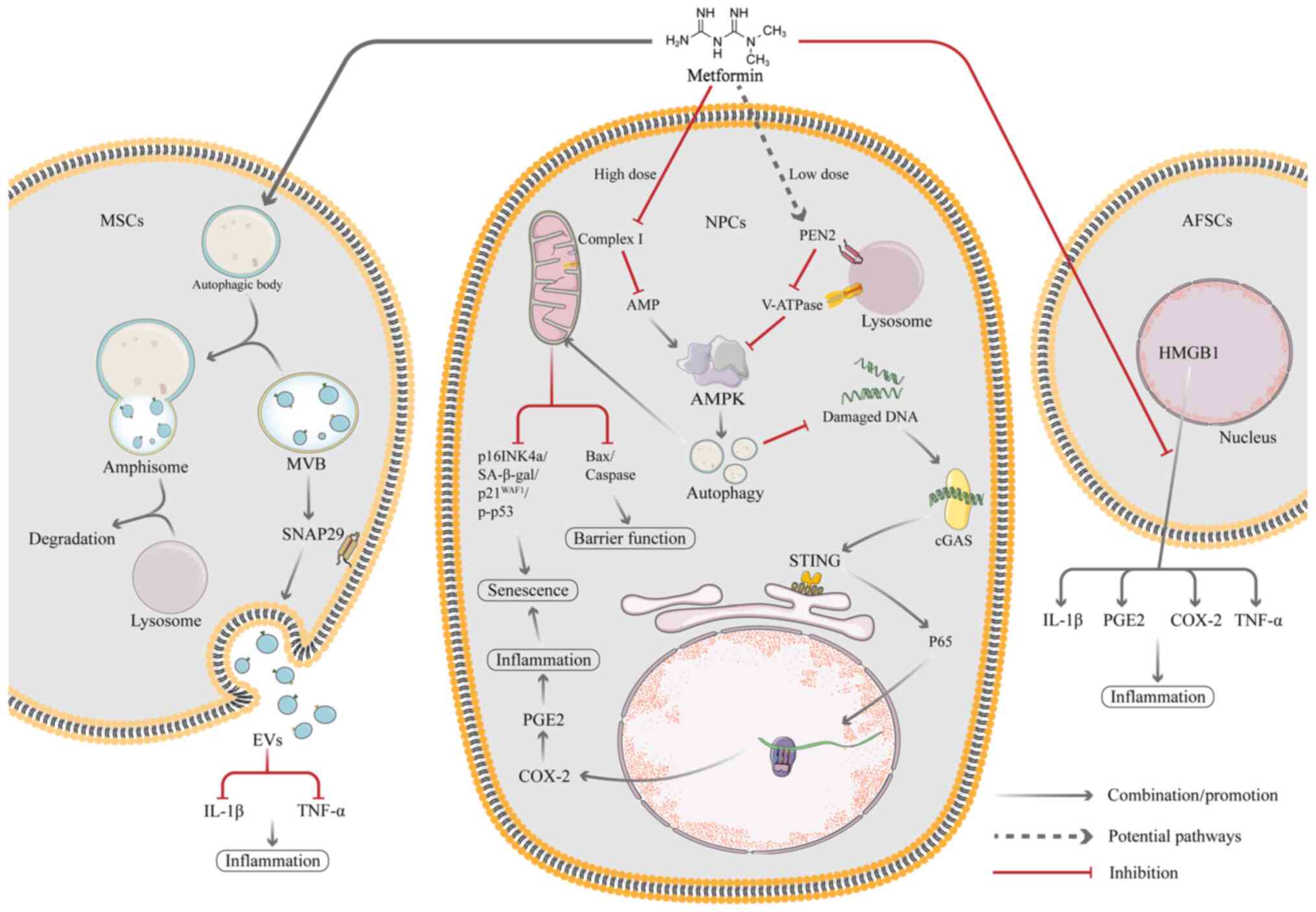
EVs are vesicular secretions produced by MSCs that
mediate the biological effects of MSCs (134-136). Compared with MSC-based cell
treatment, EV therapy provides a number of potential benefits,
including decreased manufacturing costs, greater stability and ease
of sterilization, storage and infusion therapy (134). EV therapy has been considered a
promising alternative strategy for treatment with MSCs. Autophagic
activity is associated with EV biogenesis and secretion and the
regulation of autophagy may influence EV production (137,138). Metformin, which is an AMPK
activator, is associated with autophagy activation (139). In a recent study, Liao et
al (30) showed that
metformin treatment effectively promotes the secretion of EVs in
vitro and that this process is associated with autophagy
activation (30). Metformin
activates autophagy in MSCs primarily via AMPK signalling and
promotes formation of multivesicular bodies (MVBs) or amphipathic
bodies, which is followed by membrane fusion and EV release. In
addition, metformin treatment induces phosphorylation of
synaptosome associated protein (SNAP29), a key SNARE protein that
mediates fusion of MVBs with the plasma membrane, thereby
facilitating the release of EVs (30). Liao et al (30) also hypothesized that metformin
facilitates release of EVs by altering the expression of
inter-α-trypsin inhibitor heavy chain 4, which serves a key role in
the regulation of cell proliferation and inflammatory responses.
The secretome of MSCs (including EVs) exerts antioxidant and
anti-inflammatory effects to rescue resident senescent cells in the
IVD; EV treatment reduces the rate of senescent NP cells and delays
the progression of IVDD (140).
One of the key indicators of the innate immune
response is cyclic GMP-AMP synthase (cGAS)/stimulator of interferon
genes (STING) signalling and its primary function is to recognize
and respond to cytoplasmic DNA. After cGAS recognizes and binds DNA
in the cytoplasm, STING initiates downstream responses (31). Senescent cells secrete a variety
of cytokines called the senescence-associated secretory phenotype
(SASP). SASP factors affect the intracellular environment and
induce local or systemic dysfunction. However, it has been
demonstrated that cGAS/STING signalling can stimulate production of
inflammatory factors that lead to SASP factor secretion by
senescent cells and accelerate ageing (141). Ren et al (31) demonstrated that metformin
inhibits the activation of the cGAS/STING pathway by activating
autophagy, thereby inhibiting the inflammatory response and NP cell
ageing and ultimately delaying IVDD (31). During IVDD, release of
inflammatory factors is the primary cause of excessive senescence
in myeloid cells (31). Several
studies have proven that oxidative stress is present in
degenerative discs and ROS production disrupts intracellular
homeostasis and leads to inflammation and accelerated senescence in
NP cells (142-144). The cGAS/STING signalling axis
consists of the second messenger loop cGAS of the interferon gene
and circulating GMP-AMP receptor-stimulating factor. The
inflammatory response and cellular senescence both rely on this
pathway, which is part of the innate immune system. cGAS sequesters
damaged DNA fragments in the cytoplasm in senescent myeloid cells,
which then recruits downstream STING to activate the NF-κB
signalling pathway, promoting the release of inflammatory cytokines
(145). In addition, the
cGAS/STING signalling pathway is a pathway that is responsive to
cytoplasmic DNA. Under normal conditions, intracellular DNA is
stable and present in the nucleus and mitochondria (146-148). When DNA is damaged, histone
γ-H2AX (DNA damage marker) is phosphorylated and damaged DNA
fragments enter the cytoplasm and are recognized by cGAS, thus
activating STING and its downstream molecules (149). NF-κB has been identified as a
downstream molecule of cGAS-STING. Damaged DNA fragments induce
cGAS/STING activation, which phosphorylates P65 and promotes its
translocation to the nucleus (150,151). This promotes the production of
inflammatory cytokines, which then induce oxidative stress, leading
to degradation and destruction of ECM and accelerated cellular
senescence (152). The study
has also shown that silencing STING gene inhibits senescence and
that overexpression of STING promotes senescence in myeloid cells
(152), which suggests that
cGAS/STING signalling may serve as a novel therapeutic target to
inhibit senescence and mitigate the progression of IVDD (Fig. 3).
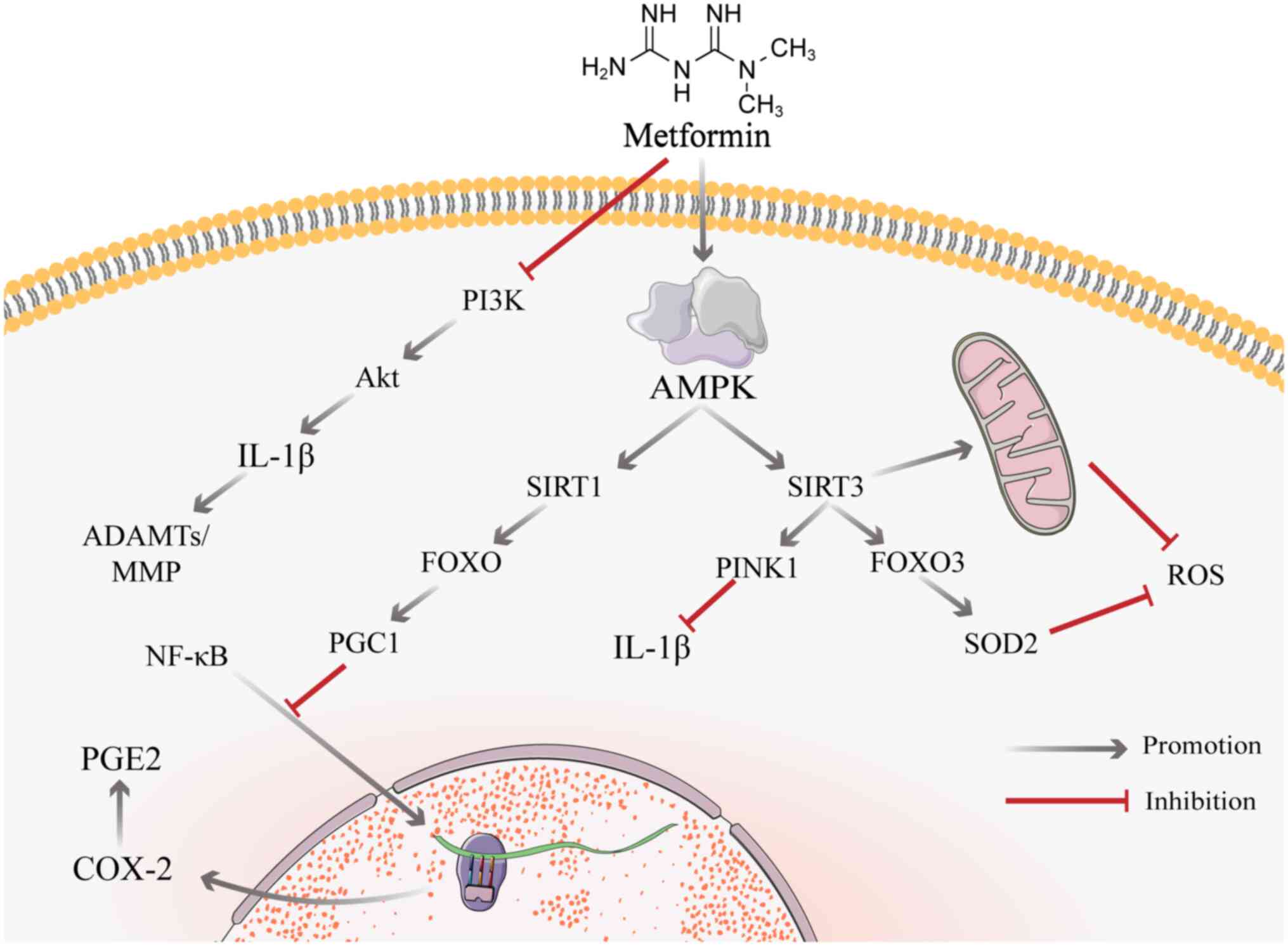
NF-κB is a key mediator of cellular responses to
inflammation; the downstream factor prostaglandin E2 (PGE2)
disrupts IVD cell matrix homeostasis, thereby destroying the disc
(153,154). There is substantial evidence
that inhibiting NF-κB inhibits the inflammatory response and the
production of catabolic factors in IVD cells (153,155), thus providing a potential
strategy for inhibiting disc degeneration. Unlike conventional
non-steroidal anti-inflammatory drugs, metformin does not inhibit
COX-2 (PGE2 synthase) through an enzymatic response but rather
regulates COX-2 expression via NF-κB (33). Metformin results in a large
decrease in nuclear ectopic NF-κB and a significant decrease in
PGE2 levels following dual stimulation of mechanical and
inflammatory stress (33). The
effect of metformin on proinflammatory gene expression is likely
due to blockade of NF-κB translocation to the nucleus via the
SIRT1/FOXO/Peroxisome proliferator-activated receptor gamma
coactivator 1 (PGC1) pathway induced by activated AMPK (33,156).
High mobility group box 1 (HMGB1) is a nuclear
protein that binds to DNA and is a cofactor for gene transcription
(157). Under normal
conditions, HMGB1 is in the nucleus and serves a role in regulating
DNA stability and transcription, but it is released into ECM in
response to certain stimulatory conditions (such as LPS) (158), which promotes expression of
PGE2 and some inflammatory factors (TNF-α, IL-1β and IL-6)
(159,160). Metformin exerts antiaging and
anti-inflammatory effects by inhibiting HMGB1 translocation and
catabolic products of AFSCs. HMGB1 also significantly decreases the
levels of SA-β-gal activity, inflammatory factors (TNF-, IL-1 and
IL-6), and certain catabolic enzymes (MMP3 and 13) in AFSCs
(32) (Fig. 4).
SIRT3 is a key factor for IVDD. A key mechanism of
AGE-induced oxidative stress and secondary human NP apoptosis is
impairment of SIRT3 activity and the mitochondrial antioxidant
network (181). Nicotinamide
mononucleotide (NMN) activates SIRT3 via the AMPK/PGC-1α pathways
(181,182). By preventing
compression-induced mitochondrial division, SIRT3 expression
improves mitochondrial function, decreases the formation of ROS
(183) and activates the
SIRT3/FOXO3/SOD2 signalling pathway; SIRT3 activates transcription
factor FOXO3a, which in turn promotes superoxide dismutase 2 (SOD2)
synthesis and ROS catabolism (184), which reduces NP cell stress and
the degradation of ECM. The activating effect of metformin on SIRT3
is evident, and metformin significantly increases the expression of
Histone H3 Lysine 79 trimethylation (H3K79me3) in the SIRT3
promoter region by stimulating AMPK production, thereby increasing
levels of SIRT3 (185). In
addition, metformin inhibits the inflammatory response in myeloid
cells via the SIRT3/PINK1/Parkin signalling pathway (110). When NAD is used to inhibit the
effect of SIRT3, a significant decrease in the effect of metformin
also occurs (186). This
finding suggests that metformin may act via SIRT3 to improve
IVDD.
In senescent NP cells, expression of SIRT6 is
decreased, and SIRT6 decreases levels of the replicative senescence
marker SA-β-gal, which inhibits IL-1β-induced apoptosis and
activates autophagy in NP cells by inhibiting phosphorylation of
mTOR; activation of autophagy inhibits stress-induced apoptosis in
NP cells and regulates the expression and activity of certain
mechanistic catabolic enzymes (187). For example, MMP-3 (Matrix
Metalloproteinases) and MMP-13 are enzymes that are frequently
regulated during autophagy, and they play important roles in
extracellular matrix degradation and interstitial cell remodelling
(188). ADAMTS-4 (A Disintegrin
and Metalloproteinase with Thrombospondin Motifs) and ADAMTS-5 are
typical examples of autophagy-regulated enzymes, and their activity
and expression change significantly during articular cartilage and
intervertebral disc degeneration (189). Autophagy may affect cell
metabolism and survival by regulating the number and function of
lysosomes to influence the activity of endogenous mechanolytic
enzymes (190). Metformin
augments the expression of SIRT6 in vitro (191). It has also been shown that
SIRT2 can decrease the expression of P21 and P53 and thus inhibit
ageing and oxidative stress in NP cells (192), and there is a relationship
between metformin and SIRT2 (193). However, whether metformin
ameliorates IVDD directly by acting on SIRT-related signalling
pathways requires further study.
To the best of our knowledge, the use of metformin
in the treatment of IVDD has not been validated by clinical data.
However, in clinically relevant conditions, such as obesity, aging
and diabetes, that may be a direct or indirect inducer of IVDD,
metformin has been used in clinical treatment or trials (24,194,195). Patients experiencing insulin
sensitivity and resistance who are overweight exhibit increased
weight loss with metformin (196). To the best of our knowledge,
few clinical trials directly examine the effects of metformin on
human aging (197,198). A randomized placebo-controlled
clinical trial of metformin is underway to prevent frailty in
elderly patients with poor glucose tolerance and examine the
effects of metformin on several markers of aging (199). However, multiple preclinical
experiments have established that metformin can be beneficial in
treating IVDD (29-34). By promoting autophagy or
controlling release of EVs from bone marrow MSCs, metformin
prevents apoptosis and senescence in NP cells (29,30). In addition, a randomized,
double-blind, placebo-controlled, crossover trial showed that
metformin delayed senescence in the elderly population via
metabolic and non-metabolic effects (200), suggesting that metformin plays
a role in ageing-related diseases. The Targeting Aging with
Metformin (TAME) trial was approved by the Food and Drug
Administration (FDA, America) in 2015 (199,200). The TAME trial may make
metformin the first approved anti-aging drug; as it is not testing
metformin against a single disease, but rather a collection of
age-related diseases, it establishes aging as a medical condition
that can be intervened in or treated, rather than an irreversible
process outside of human control. This shift in the concept of
aging may make it easier to conduct future anti-aging clinical
trials and the increase in the number of clinical trials associated
with cellular senescence as a major contributor to IVDD may
facilitate the clinical scope of investigating metformin for IVDD
in future (201,202). Metformin is the first-line drug
for T2DM, especially for obesity-associated and gestational
diabetes (203). Metformin
positively regulates obesity, aging and diabetes through AMPK
pathway signaling, PI3K/AKT/mTOR pathway phosphorylation, oxidative
stress, autophagy and other pathways to alleviate and improve
disease progression (194,204). Ramanathan et al
(33) confirmed that the
inflammatory response in IVD treated with metformin is
significantly inhibited. In addition, studies have shown that
metformin promotes angiogenesis in the injured spinal cord by
activating enzyme pathways, thereby improving neurological function
after spinal cord injury in aged mice and promoting recovery of
motor function following injury (205,206). Although metformin has not yet
been used for clinical trials of IVDD, the aforementioned
preclinical and clinical trials of diseases closely related to IVDD
suggest potential mechanisms.
In clinical practice, metformin is often used as an
adjunctive therapeutic agent. For example, metformin has
anti-inflammatory properties (165). When combined with drugs that
also have certain anti-inflammatory effects, metformin produces
significant synergistic anti-inflammatory effects. Metformin and
pioglitazone have different mechanisms of action on oxidative
stress; combination of these two drugs is more effective in
improving oxidative stress (161). Sildenafil + metformin + leucine
and metformin + atorvastatin combination showed higher efficacy in
lowering oxidative stress and decreasing inflammatory markers
compared with each drug alone (162,163). IVDD often occurs in conjunction
with DM, aging and inflammation (207). Especially in elderly patients
with DM, chronic inflammation and IVDD, metformin can be used to
treat DM as well as to slow down the further progression of IVDD.
There have been numerous clinical studies of metformin in the
treatment of OA (ICTRP, https://trialsearch.who.int/) (Table II), and there are many
similarities between IVDD and OA, both in terms of the mechanism of
occurrence and at the preventive and therapeutic levels (7,208-210). Specifically, for example, both
IVDD and OA involve chronic inflammation and apoptotic processes,
and elevated inflammatory markers such as IL-1β, IL-6, and TNF-α,
to name a few, are present in patients with both IVDD and OA
(211,212). Metformin may have potential for
the treatment of IVDD. In the future, whether metformin can be used
independently to delay IVDD or as an adjunctive drug to delay IVDD
while assisting in the treatment of other diseases, and in what
ways its benefits can be increased, need to be corroborated by
basic research studies and clinical trials.
Although metformin affects IVDD in numerous
molecular processes, there are still notable difficulties in its
therapeutic use. The first is that the mechanism by which metformin
acts on IVDD has not been fully elucidated and confirmed. Metformin
has been shown to induce autophagy via AMPK, control MSC EV release
and activate the cGAS/STING pathway to prevent apoptosis and
senescence in NP cells (29-31). More research is required to
understand additional distinct anti-inflammatory and
autophagy-activating mechanisms of metformin in IVD cells. In
addition, existing studies are cell culture and preclinical
studies; to the best of our knowledge, there is a lack large-scale,
randomized, double-blind, placebo-controlled clinical trials
(29-33). Designing controls is challenging
due to the number of potential confounding variables, including
sample size, age, presence of other common conditions (particularly
diabetes), adherence to treatment and follow-up accuracy. The drug
concentration of oral metformin in the target area, feasibility of
the local route of administration, the half-life of the drug and
effect of the combined drug on efficacy are also factors that need
to be explored in clinical experiments.
There are many studies showing that metformin does
not lead to lower blood glucose when treating other conditions in
non-diabetic patients because metformin does not stimulate insulin
secretion (213-215). In recent years, metformin has
been studied for the treatment of other clinical conditions,
including cancer, heart disease and neurodegenerative disease such
as IVDD (24). However, when
metformin is used in non-diabetic patients, doctors may closely
monitor their blood glucose levels to ensure they are in the normal
range as other medications or health conditions may interact with
metformin to affect blood glucose (216). Therefore, structure-activity
relationship (SAR) or quantitative SAR studies could be considered
to enhance the effect of metformin by modifying its structure to
some extent and ensuring that it does not lower blood glucose
levels.
Following oral administration, bioavailability of
metformin is relatively low as it is primarily absorbed from the
upper part of the small intestine (217). Incomplete absorption may be
ameliorated by use of convenient drug delivery systems, such as
bioadhesion and gastric retention drug delivery systems (218). In addition, due to the short
biological half-life of metformin, repeated high doses of metformin
are required, which usually results in decreased patient compliance
and an increased incidence of side effects, such as diarrhoea,
nausea and weight loss. Metformin also causes lactic acidosis,
which is sometimes fatal (217). It is also necessary to develop
new formulation strategies for metformin to improve its
bioavailability, reduce the frequency of administration and
minimize gastrointestinal side effects and toxicity. Drug delivery
systems (such as particles, nanoparticles, liposomes and lipoid)
are useful systems to overcome difficulties associated with
conventional dosage forms. These systems offer advantages over
conventional dosage forms, such as effective protection of the drug
from enzymatic degradation, decreased drug side effects,
improvement of patient comfort and compliance, control or site of
drug release and improvement of relative bioavailability of the
drug (219). In particulate
dosage form, nanoparticle drug delivery systems are unique and may
enhance targeted delivery of metformin to specific sites of action,
which has been used as a promising approach for the treatment of
cancer (220). However, whether
metformin in the form of particles and nanoparticles as carriers is
potentially important for its goal of delaying IVDD needs to be
further investigated. Additionally, adverse medication reactions,
particularly gastrointestinal symptoms, need to be detected and
assessed further before metformin is used in the clinical treatment
of IVDD. Besides, the combination of metformin with other drugs
also needs to be considered in terms of drug interactions and
adverse effects, as well as whether metformin can be used
clinically for the treatment of IVDD. Specific experiments need to
be designed to validate this on a case-by-case basis, as well as
more basic research.
Globally, IVDD has notable health implications. For
example, IVDD can lead to low back or neck pain (212), IVDD may lead to reduced disc
height and spinal deformity, which can affect a patient's motor
function (221), and IVDD may
also lead to nerve compression, causing neurological symptoms such
as sciatica and myelopathy (222). Metformin, as a first-line drug
for the treatment of T2DM, may also contribute to the treatment of
IVDD. The present review summarizes potential mechanisms of
metformin for the treatment of IVDD. Metformin induces autophagy
via the AMPK pathway and inhibits senescence and apoptosis of NP
cells (29). Metformin promotes
the production of EVs through autophagy and inactivates the
cGAS/STING signalling pathway, improving the senescence of myeloid
cells (30,31). Moreover, it has been shown that
metformin inhibits senescence and apoptosis of medullary cells by
suppressing inflammatory responses (32,33). Finally, metformin may act on IVDD
by activating the SIRT signalling pathway, providing a theoretical
basis for more basic and clinical studies of metformin in IVDD and
associated disease. For elderly patients with both diabetes and
IVDD-related diseases, metformin may serve as an adjunctive
medication for the treatment of IVDD. The present review provides
direction and for future preclinical and clinical studies of
metformin in IVDD.
Not applicable.
HT, JL and WY conceived the study. WY, YY, YW and
ZG wrote and edited the manuscript. ZG and WY constructed figures
and tables. JZ, WG and YC performed the literature review. YL, HW,
LZ and YW revised the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82072492) and the Excellent Youth
Scientific Research Projects in Anhui Universities (grant no.
2022AH030117).
|
1
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis Cartilage.
24:398–408. 2016. View Article : Google Scholar
|
|
2
|
Cornaz F, Widmer J, Farshad-Amacker NA,
Spirig JM, Snedeker JG and Farshad M: Biomechanical contributions
of spinal structures with different degrees of disc degeneration.
Spine (Phila Pa 1976). 46:E869–E877. 2021. View Article : Google Scholar
|
|
3
|
Lan T, Shiyu H, Shen Z, Yan B and Chen J:
New insights into the interplay between miRNAs and autophagy in the
aging of intervertebral discs. Ageing Res Rev. 65:1012272021.
View Article : Google Scholar
|
|
4
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar
|
|
5
|
GBD 2019 Diseases and Injuries
Collaborators: Global burden of 369 diseases and injuries in 204
countries and territories, 1990-2019: A systematic analysis for the
global burden of disease study 2019. Lancet. 396:1204–1222. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Lai X, Li C, Yang Y, Gu S, Hou W,
Zhai L and Zhu Y: Focus on the impact of social factors and
lifestyle on the disease burden of low back pain: Findings from the
global burden of disease study 2019. BMC Musculoskelet Disord.
24:6792023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goode AP, Carey TS and Jordan JM: Low back
pain and lumbar spine osteoarthritis: How are they related? Curr
Rheumatol Rep. 15:3052013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maher C, Underwood M and Buchbinder R:
Non-specific low back pain. Lancet. 389:736–747. 2017. View Article : Google Scholar
|
|
9
|
Chou R: Low back pain. Ann Intern Med.
174:ITC113–ITC128. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apostolova N, Iannantuoni F, Gruevska A,
Muntane J, Rocha M and Victor VM: Mechanisms of action of metformin
in type 2 diabetes: Effects on mitochondria and
leukocyte-endothelium interactions. Redox Biol. 34:1015172020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez-Rangel E and Inzucchi SE:
Metformin: Clinical use in type 2 diabetes. Diabetologia.
60:1586–1593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LaMoia TE and Shulman GI: Cellular and
molecular mechanisms of metformin action. Endocr Rev. 42:77–96.
2021. View Article : Google Scholar :
|
|
13
|
An H and He L: Current understanding of
metformin effect on the control of hyperglycemia in diabetes. J
Endocrinol. 228:R97–R106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foretz M, Hébrard S, Leclerc J,
Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F and
Viollet B: Metformin inhibits hepatic gluconeogenesis in mice
independently of the LKB1/AMPK pathway via a decrease in hepatic
energy state. J Clin Invest. 120:2355–2369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caton PW, Nayuni NK, Kieswich J, Khan NQ,
Yaqoob MM and Corder R: Metformin suppresses hepatic
gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol.
205:97–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YD, Park KG, Lee YS, Park YY, Kim DK,
Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, et al: Metformin
inhibits hepatic gluconeogenesis through AMP-activated protein
kinase-dependent regulation of the orphan nuclear receptor SHP.
Diabetes. 57:306–314. 2008. View Article : Google Scholar
|
|
17
|
Masarwa R, Brunetti VC, Aloe S, Henderson
M, Platt RW and Filion KB: Efficacy and safety of metformin for
obesity: A systematic review. Pediatrics. 147:e202016102021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Day EA, Ford RJ, Smith BK,
Mohammadi-Shemirani P, Morrow MR, Gutgesell RM, Lu R, Raphenya AR,
Kabiri M, McArthur AG, et al: Metformin-induced increases in GDF15
are important for suppressing appetite and promoting weight loss.
Nat Metab. 1:1202–1208. 2019. View Article : Google Scholar
|
|
19
|
Kamarudin MNA, Sarker MMR, Zhou JR and
Parhar I: Metformin in colorectal cancer: Molecular mechanism,
preclinical and clinical aspects. J Exp Clin Cancer Res.
38:4912019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo F, Das A, Chen J, Wu P, Li X and Fang
Z: Metformin in patients with and without diabetes: A paradigm
shift in cardiovascular disease management. Cardiovasc Diabetol.
18:542019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Griffin SJ, Leaver JK and Irving GJ:
Impact of metformin on cardiovascular disease: A meta-analysis of
randomised trials among people with type 2 diabetes. Diabetologia.
60:1620–1629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diamanti-Kandarakis E, Economou F,
Palimeri S and Christakou C: Metformin in polycystic ovary
syndrome. Ann N Y Acad Sci. 1205:192–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song A, Zhang C and Meng X: Mechanism and
application of metformin in kidney diseases: An update. Biomed
Pharmacother. 138:1114542021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Gan D, Lin S, Zhong Y, Chen M, Zou
X, Shao Z and Xiao G: Metformin in aging and aging-related
diseases: Clinical applications and relevant mechanisms.
Theranostics. 12:2722–2740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu CH, Chung CH, Kuo FC, Chen KC, Chang
CH, Kuo CC, Lee CH, Su SC, Liu JS, Lin FH, et al: Metformin
attenuates osteoporosis in diabetic patients with carcinoma in
situ: A nationwide, retrospective, matched-cohort study in Taiwan.
J Clin Med. 9:28392020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhang B, Liu WX, Lu K, Pan H, Wang
T, Oh CD, Yi D, Huang J, Zhao L, et al: Metformin limits
osteoarthritis development and progression through activation of
AMPK signalling. Ann Rheum Dis. 79:635–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Liu X, Wang Y, Cao F, Chen Z, Hu Z,
Yu B, Feng H, Ba Z, Liu T, et al: Intervertebral disc degeneration
in mice with type II diabetes induced by leptin receptor
deficiency. BMC Musculoskelet Disord. 21:772020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cannata F, Vadalà G, Ambrosio L, Fallucca
S, Napoli N, Papalia R, Pozzilli P and Denaro V: Intervertebral
disc degeneration: A focus on obesity and type 2 diabetes. Diabetes
Metab Res Rev. 36:e32242020. View Article : Google Scholar
|
|
29
|
Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y,
Cai N, Tang Q, Wang C, Yan M, et al: Metformin protects against
apoptosis and senescence in nucleus pulposus cells and ameliorates
disc degeneration in vivo. Cell Death Dis. 7:e24412016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liao Z, Li S, Lu S, Liu H, Li G, Ma L, Luo
R, Ke W, Wang B, Xiang Q, et al: Metformin facilitates mesenchymal
stem cell-derived extracellular nanovesicles release and optimizes
therapeutic efficacy in intervertebral disc degeneration.
Biomaterials. 274:1208502021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren C, Jin C, Li C, Xiang J, Wu Y, Zhou Y,
Sun L, Zhang X and Tian N: Metformin inactivates the cGAS-STING
pathway through autophagy and suppresses senescence in nucleus
pulposus cells. J Cell Sci. 135:jcs2597382022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Y, Yuan F, Deng C, He F, Zhang Y, Shen
H, Chen Z and Qian L: Metformin decreases LPS-induced inflammatory
response in rabbit annulus fibrosus stem/progenitor cells by
blocking HMGB1 release. Aging (Albany NY). 11:1252–10265. 2019.
|
|
33
|
Ramanathan R, Firdous A, Dong Q, Wang D,
Lee J, Vo N and Sowa G: Investigation into the anti-inflammatory
properties of metformin in intervertebral disc cells. JOR Spine.
5:e11972022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaya YE, Karaarslan N, Yilmaz I, Sirin DY,
Akalan H and Ozbek H: A study of the effects of metformin, a
biguanide derivative, on annulus fibrosus and nucleus pulposus
cells. Turk Neurosurg. 30:434–441. 2020.PubMed/NCBI
|
|
35
|
Stephenne X, Foretz M, Taleux N, van der
Zon GC, Sokal E, Hue L, Viollet B and Guigas B: Metformin activates
AMP-activated protein kinase in primary human hepatocytes by
decreasing cellular energy status. Diabetologia. 54:3101–3110.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maruthur NM, Tseng E, Hutfless S, Wilson
LM, Suarez-Cuervo C, Berger Z, Yue Chu Y, Iyoha E, Segal JB and
Bolen S: Diabetes medications as monotherapy or metformin-based
combination therapy for type 2 diabetes: A systematic review and
meta-analysis. Ann Intern Med. 164:740–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Zhao X, Shen H and Zhang C:
Molecular mechanisms of cell death in intervertebral disc
degeneration (Review). Int J Mol Med. 37:1439–1448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong CY and Zhang HH: Autophagy as a
potential therapeutic target in intervertebral disc degeneration.
Life Sci. 273:1192662021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Wang C, Xue L, Zhang F and Liu J:
Melatonin suppresses apoptosis of nucleus pulposus cells through
inhibiting autophagy via the PI3K/Akt pathway in a high-glucose
culture. Biomed Res Int. 2021:46042582021.PubMed/NCBI
|
|
40
|
Humzah MD and Soames RW: Human
intervertebral disc: Structure and function. Anat Rec. 220:337–356.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bach FC, de Vries SAH, Krouwels A,
Creemers LB, Ito K, Meij BP and Tryfonidou MA: The species-specific
regenerative effects of notochordal cell-conditioned medium on
chondrocyte-like cells derived from degenerated human
intervertebral discs. Eur Cell Mater. 30:132–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Urban JPG and Roberts S: Degeneration of
the intervertebral disc. Arthritis Res Ther. 5:120–130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dowdell J, Erwin M, Choma T, Vaccaro A,
Iatridis J and Cho SK: Intervertebral disk degeneration and repair.
Neurosurgery. 80(3S): S46–S54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oegema TR Jr: Biochemistry of the
intervertebral disc. Clin Sports Med. 12:419–439. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bruehlmann SB, Rattner JB, Matyas JR and
Duncan NA: Regional variations in the cellular matrix of the
annulus fibrosus of the intervertebral disc. J Anat. 201:159–171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roberts S, Menage J, Duance V, Wotton S
and Ayad S: 1991 Volvo Award in basic sciences. Collagen types
around the cells of the intervertebral disc and cartilage end
plate: An immunolocalization study. Spine (Phila Pa 1976).
16:1030–1038. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mwale F, Roughley P and Antoniou J:
Distinction between the extracellular matrix of the nucleus
pulposus and hyaline cartilage: A requisite for tissue engineering
of intervertebral disc. Eur Cell Mater. 8:58–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maroudas A, Stockwell RA, Nachemson A and
Urban J: Factors involved in the nutrition of the human lumbar
intervertebral disc: Cellularity and diffusion of glucose in vitro.
J Anat. 120:113–130. 1975.PubMed/NCBI
|
|
50
|
Yu J, Winlove PC, Roberts S and Urban JPG:
Elastic fibre organization in the intervertebral discs of the
bovine tail. J Anat. 201:465–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pattappa G, Li Z, Peroglio M, Wismer N,
Alini M and Grad S: Diversity of intervertebral disc cells:
Phenotype and function. J Anat. 221:480–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moon SM, Yoder JH, Wright AC, Smith LJ,
Vresilovic EJ and Elliott DM: Evaluation of intervertebral disc
cartilaginous endplate structure using magnetic resonance imaging.
Eur Spine J. 22:1820–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Broberg KB: On the mechanical behaviour of
intervertebral discs. Spine (Phila Pa 1976). 8:151–165. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rider SM, Mizuno S and Kang JD: Molecular
mechanisms of intervertebral disc degeneration. Spine Surg Relat
Res. 3:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Raj PP: Intervertebral disc:
Anatomy-physiology-pathophysiology-treatment. Pain Pract. 8:18–44.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Colombini A, Lombardi G, Corsi MM and
Banfi G: Pathophysiology of the human intervertebral disc. Int J
Biochem Cell Biol. 40:837–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lyons G, Eisenstein SM and Sweet MB:
Biochemical changes in intervertebral disc degeneration. Biochim
Biophys Acta. 673:443–453. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Roughley PJ, Melching LI, Heathfield TF,
Pearce RH and Mort JS: The structure and degradation of aggrecan in
human intervertebral disc. Eur Spine J. 15(Suppl 3): S326–S332.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Binch ALA, Shapiro IM and Risbud MV:
Syndecan-4 in intervertebral disc and cartilage: Saint or synner?
Matrix Biol. 52-54:355–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu K, Wang X, Zhang Q, Liang A, Zhu H,
Huang D, Li C and Ye W: Sp1 downregulates proinflammatory
cytokine-induced catabolic gene expression in nucleus pulposus
cells. Mol Med Rep. 14:3961–3968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Roughley PJ: Biology of intervertebral
disc aging and degeneration: Involvement of the extracellular
matrix. Spine (Phila Pa 1976). 29:2691–2699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sjöberg AP, Manderson GA, Mörgelin M, Day
AJ, Heinegård D and Blom AM: Short leucine-rich glycoproteins of
the extracellular matrix display diverse patterns of complement
interaction and activation. Mol Immunol. 46:830–839. 2009.
View Article : Google Scholar :
|
|
63
|
Huang YC, Urban JP and Luk KD:
Intervertebral disc regeneration: Do nutrients lead the way? Nat
Rev Rheumatol. 10:561–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang H, Zhu T, Zhang L and Wu Q: Stromal
cell-derived factor-1 induces matrix metalloproteinase expression
in human endplate chondrocytes, cartilage endplate degradation in
explant culture, and the amelioration of nucleus pulposus
degeneration in vivo. Int J Mol Med. 41:969–976. 2018.
|
|
65
|
Hiyama A, Sakai D and Mochida J: Cell
signaling pathways related to pain receptors in the degenerated
disk. Global Spine J. 3:165–174. 2013. View Article : Google Scholar :
|
|
66
|
Kondo N, Yuasa T, Shimono K, Tung W, Okabe
T, Yasuhara R, Pacifici M, Zhang Y, Iwamoto M and Enomoto-Iwamoto
M: Intervertebral disc development is regulated by Wnt/β-catenin
signaling. Spine (Phila Pa 1976). 36:E513–E518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lotz JC and Ulrich JA: Innervation,
inflammation, and hypermobility may characterize pathologic disc
degeneration: review of animal model data. J Bone Joint Surg Am.
88(Suppl 2): S76–S82. 2006.
|
|
68
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Olovnikov AM: A theory of marginotomy. The
incomplete copying of template margin in enzymic synthesis of
polynucleotides and biological significance of the phenomenon. J
Theor Biol. 41:181–190. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Stein GH, Beeson M and Gordon L: Failure
to phosphorylate the retinoblastoma gene product in senescent human
fibroblasts. Science. 249:666–669. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Feng C, Yang M, Lan M, Liu C, Zhang Y,
Huang B, Liu H and Zhou Y: ROS: Crucial intermediators in the
pathogenesis of intervertebral disc degeneration. Oxid Med Cell
Longev. 2017:56015932017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X,
Zhang X, Shen J, Fang J and Jiang W: SIRT1 inhibits apoptosis of
degenerative human disc nucleus pulposus cells through activation
of Akt pathway. Age (Dordr). 35:1741–1753. 2013. View Article : Google Scholar
|
|
75
|
Chen JW, Ni BB, Zheng XF, Li B, Jiang SD
and Jiang LS: Hypoxia facilitates the survival of nucleus pulposus
cells in serum deprivation by down-regulating excessive autophagy
through restricting ROS generation. Int J Biochem Cell Biol.
59:1–10. 2015. View Article : Google Scholar
|
|
76
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu HG, Yu YF, Zheng Q, Zhang W, Wang CD,
Zhao XY, Tong WX, Wang H, Liu P and Zhang XL: Autophagy protects
end plate chondrocytes from intermittent cyclic mechanical tension
induced calcification. Bone. 66:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Deosarkar SD, Arsule AD and Kalyankar TM:
Effect of antidiabetic metformin hydrochloride on physicochemical
properties of cationic surfactant cetyltrimethylammonium bromide in
aqueous solutions. Colloids Surf A: Physicochem Eng Asp.
613:1260522021. View Article : Google Scholar
|
|
80
|
Dołowy M, Jampilek J and Bober-Majnusz K:
A comparative study of the lipophilicity of metformin and
phenformin. Molecules. 26:66132021. View Article : Google Scholar :
|
|
81
|
Ismail AH, Al-Garawi ZS, Al-Shamari K and
Salman AT: Metformin compounds: A review on the importance and the
possible applications. J Phys Conf Ser. 1853:0120602021. View Article : Google Scholar
|
|
82
|
He M, Lu B, Opoku M, Zhang L, Xie W, Jin
H, Chen S, Li Y and Deng Z: Metformin prevents or delays the
development and progression of osteoarthritis: New insight and
mechanism of action. Cells. 11:30122022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Graham GG, Punt J, Arora M, Day RO, Doogue
MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick
CM, et al: Clinical pharmacokinetics of metformin. Clin
Pharmacokinet. 50:81–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bailey CJ: Metformin: Historical overview.
Diabetologia. 60:1566–1576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
He L: Metformin and systemic metabolism.
Trends Pharmacol Sci. 41:868–881. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tucker GT, Casey C, Phillips PJ, Connor H,
Ward JD and Woods HF: Metformin kinetics in healthy subjects and in
patients with diabetes mellitus. Br J Clin Pharmacol. 12:235–246.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wilcock C and Bailey CJ: Accumulation of
metformin by tissues of the normal and diabetic mouse. Xenobiotica.
24:49–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sambol NC, Brookes LG, Chiang J, Goodman
AM, Lin ET, Liu CY and Benet LZ: Food intake and dosage level, but
not tablet vs solution dosage form, affect the absorption of
metformin HCl in man. Br J Clin Pharmacol. 42:510–512. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Song Y, Wu Z and Zhao P: The function of
metformin in aging-related musculoskeletal disorders. Front
Pharmacol. 13:8655242022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen S, Ruan G, Zeng M, Chen T, Cao P,
Zhang Y, Li J, Wang X, Li S, Tang S, et al: Association between
metformin use and risk of total knee arthroplasty and degree of
knee pain in knee osteoarthritis patients with diabetes and/or
obesity: A retrospective study. J Clin Med. 11:47962022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
El-Arabey AA, Abdalla M and Ali Eltayb W:
Metformin: Ongoing journey with superdrug revolution. Adv Pharm
Bull. 9:1–4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mazor M, Best TM, Cesaro A, Lespessailles
E and Toumi H: Osteoarthritis biomarker responses and cartilage
adaptation to exercise: A review of animal and human models. Scand
J Med Sci Sports. 29:1072–1082. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Vinatier C, Merceron C and Guicheux J:
Osteoarthritis: From pathogenic mechanisms and recent clinical
developments to novel prospective therapeutic options. Drug Discov
Today. 21:1932–1937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Williams S, Alkhatib B and Serra R:
Development of the axial skeleton and intervertebral disc. Curr Top
Dev Biol. 133:49–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li X, Lee JP, Balian G and Greg Anderson
D: Modulation of chondrocytic properties of fat-derived mesenchymal
cells in co-cultures with nucleus pulposus. Connect Tissue Res.
46:75–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jørgensen SB, Richter EA and Wojtaszewski
JF: Role of AMPK in skeletal muscle metabolic regulation and
adaptation in relation to exercise. J Physiol. 574:17–31. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ,
Oh KS, Koh EH, Won JC, Kim MS, Oh GT, et al: AMPK activation
increases fatty acid oxidation in skeletal muscle by activating
PPARalpha and PGC-1. Biochem Biophys Res Commun. 340:291–295. 2006.
View Article : Google Scholar
|
|
98
|
Lee-Young RS, Canny BJ, Myers DE and
McConell GK: AMPK activation is fiber type specific in human
skeletal muscle: effects of exercise and short-term exercise
training. J Appl Physiol (1985). 107:283–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bahrambeigi S, Yousefi B, Rahimi M and
Shafiei-Irannejad V: Metformin; an old antidiabetic drug with new
potentials in bone disorders. Biomed Pharmacother. 109:1593–1601.
2019. View Article : Google Scholar
|
|
100
|
Qin X, Jiang T, Liu S, Tan J, Wu H, Zheng
L and Zhao J: Effect of metformin on ossification and inflammation
of fibroblasts in ankylosing spondylitis: An in vitro study. J Cell
Biochem. 119:1074–1082. 2018. View Article : Google Scholar
|
|
101
|
Ma T, Tian X, Zhang B, Li M, Wang Y, Yang
C, Wu J, Wei X, Qu Q, Yu Y, et al: Low-dose metformin targets the
lysosomal AMPK pathway through PEN2. Nature. 603:159–165. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sakamoto K and Jessen N: PEN2: Metformin's
new partner at lysosome. Cell Res. 32:507–508. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Liu C, Zhang S, Xue J, Zhang H and Yin J:
Evaluation of PEN2-ATP6AP1 axis as an antiparasitic target for
metformin based on phylogeny analysis and molecular docking. Mol
Biochem Parasitol. 255:1115802023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dong J, Xu X, Zhang Q, Yuan Z and Tan B:
The PI3K/AKT pathway promotes fracture healing through its
crosstalk with Wnt/β-catenin. Exp Cell Res. 394:1121372020.
View Article : Google Scholar
|
|
105
|
Watabe H, Furuhama T, Tani-Ishii N and
Mikuni-Takagaki Y: Mechanotransduction activates α5β1 integrin and
PI3K/Akt signaling pathways in mandibular osteoblasts. Exp Cell
Res. 317:2642–2649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang Z, Ma F, Wang J, Zhou Z, Liu B, He X,
Fu L, He W and Cooper PR: Extracellular signal-regulated kinase
mitogen-activated protein kinase and phosphatidylinositol
3-kinase/Akt signaling are required for lipopolysaccharide-mediated
mineralization in murine odontoblast-like cells. J Endod.
41:871–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tang Y, Mo Y, Xin D, Xiong Z, Zeng L, Luo
G and Cao Y: Regulation of osteoblast autophagy based on
PI3K/AKT/mTOR signaling pathway study on the effect of
β-ecdysterone on fracture healing. J Orthop Surg Res. 16:7192021.
View Article : Google Scholar
|
|
108
|
Lee SH, Lee JH, Lee HY and Min KJ: Sirtuin
signaling in cellular senescence and aging. BMB Rep. 52:24–34.
2019. View Article : Google Scholar :
|
|
109
|
Hu X, Feng G, Meng Z, Ma L and Jin Q: The
protective mechanism of SIRT1 on cartilage through regulation of
LEF-1. BMC Musculoskelet Disord. 22:6422021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang C, Yang Y, Zhang Y, Liu J, Yao Z and
Zhang C: Protective effects of metformin against osteoarthritis
through upregulation of SIRT3-mediated PINK1/Parkin-dependent
mitophagy in primary chondrocytes. Biosci Trends. 12:605–612. 2019.
View Article : Google Scholar
|
|
111
|
Chen Y, Wu YY, Si HB, Lu YR and Shen B:
Mechanistic insights into AMPK-SIRT3 positive feedback
loop-mediated chondrocyte mitochondrial quality control in
osteoarthritis pathogenesis. Pharmacol Res. 166:1054972021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lin S, Guo H, You X, Zhang Z and Ye H:
SND1 aggravates mitochondrial damage, apoptosis and extracellular
matrix degradation in IL-1β-stimulated chondrocytes via PINK1/BECN1
pathway. Eur J Med Res. 28:3712023. View Article : Google Scholar
|
|
113
|
Hu D, Xie F, Xiao Y, Lu C, Zhong J, Huang
D, Chen J, Wei J, Jiang Y and Zhong T: Metformin: A potential
candidate for targeting aging mechanisms. Aging Dis. 12:480–493.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xing H, Liang C, Wang C, Xu X, Hu Y and
Qiu B: Metformin mitigates cholesterol accumulation via the
AMPK/SIRT1 pathway to protect osteoarthritis chondrocytes. Biochem
Biophys Res Commun. 632:113–121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kang W, Wang T, Hu Z, Liu F, Sun Y and Ge
S: Metformin inhibits porphyromonas gingivalis
lipopolysaccharide-influenced inflammatory response in human
gingival fibroblasts via regulating activating transcription
factor-3 expression. J Periodontol. 88:e169–e178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Saluja M, Pareek KK and Swami YK: Study of
diversity of metformin related gastrointestinal side effects. J
Assoc Physicians India. 68:36–38. 2020.PubMed/NCBI
|
|
117
|
Lamos EM, Stein SA and Davis SN:
Combination of glibenclamide-metformin HCl for the treatment of
type 2 diabetes mellitus. Expert Opin Pharmacother. 13:2545–2554.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Stades AME, Heikens JT, Erkelens DW,
Holleman F and Hoekstra JBL: Metformin and lactic acidosis: Cause
or coincidence? A review of case reports. J Intern Med.
255:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Eppenga WL, Lalmohamed A, Geerts AF,
Derijks HJ, Wensing M, Egberts A, De Smet PA and de Vries F: Risk
of lactic acidosis or elevated lactate concentrations in metformin
users with renal impairment: A population-based cohort study.
Diabetes Care. 37:2218–2224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
O CK, Siu BW, Leung VW, Lin YY, Ding CZ,
Lau ES, Luk AO, Chow EY, Ma RC, Chan JC, et al: Association of
insomnia with incident chronic cognitive impairment in older adults
with type 2 diabetes mellitus: A prospective study of the Hong Kong
diabetes register. J Diabetes Complications. 37:1085982023.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wiwanitkit S and Wiwanitkit V: Metformin
and sleep disorders. Indian J Endocrinol Metab. 16(Suppl 1):
S63–S64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rosekind MR and Gregory KB: Insomnia risks
and costs: health, safety, and quality of life. Am J Manag Care.
16:617–626. 2010.PubMed/NCBI
|
|
123
|
Wu H, Esteve E, Tremaroli V, Khan MT,
Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M,
Planas-Fèlix M, et al: Metformin alters the gut microbiome of
individuals with treatment-naive type 2 diabetes, contributing to
the therapeutic effects of the drug. Nat Med. 23:850–858. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Salvatore T, Pafundi PC, Marfella R, Sardu
C, Rinaldi L, Monaco L, Ricozzi C, Imbriani S, Nevola R, Adinolfi
LE and Sasso FC: Metformin lactic acidosis: Should we still be
afraid? Diabetes Res Clin Pract. 157:1078792019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Rahman F and Tuba S: Lactic acidosis
associated with metformin in patients with diabetic kidney disease.
Med Arch. 76:297–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Du J, Xu M, Kong F, Zhu P, Mao Y, Liu Y,
Zhou H, Dong Z, Yu Z, Du T, et al: CB2R attenuates intervertebral
disc degeneration by delaying nucleus pulposus cell senescence
through AMPK/GSK3β pathway. Aging Dis. 13:552–267. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang Z, Wu J, Teng C, Wang J, Yu J, Jin
C, Wang L, Wu L and Lin Z, Yu Z and Lin Z: Orientin downregulating
oxidative stress-mediated endoplasmic reticulum stress and
mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus
pulposus cells in vitro and attenuated intervertebral disc
degeneration in vivo. Apoptosis. 27:1031–1048. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Harada M, Tadevosyan A, Qi X, Xiao J, Liu
T, Voigt N, Karck M, Kamler M, Kodama I, Murohara T, et al: Atrial
fibrillation activates AMP-dependent protein kinase and its
regulation of cellular calcium handling: Potential role in
metabolic adaptation and prevention of progression. J Am Coll
Cardiol. 66:47–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ,
He XG, Gao YC and Kang XW: Sirtuins and intervertebral disc
degeneration: Roles in inflammation, oxidative stress, and
mitochondrial function. Clin Chim Acta. 508:33–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu Y, Lin J, Wu X, Guo X, Sun H, Yu B,
Shen J, Bai J, Chen Z, Yang H, et al: Aspirin-mediated attenuation
of intervertebral disc degeneration by ameliorating reactive oxygen
species in vivo and in vitro. Oxid Med Cell Longev.
2019:71898542019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dai X, Chen Y, Yu Z, Liao C, Liu Z, Chen J
and Wu Q: Advanced oxidation protein products induce annulus
fibrosus cell senescence through a NOX4-dependent, MAPK-mediated
pathway and accelerate intervertebral disc degeneration. PeerJ.
10:e138262022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: Implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Brennan MÁ, Layrolle P and Mooney DJ:
Biomaterials functionalized with MSC secreted extracellular
vesicles and soluble factors for tissue regeneration. Adv Funct
Mater. 30:19091252020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Varderidou-Minasian S and Lorenowicz MJ:
Mesenchymal stromal/stem cell-derived extracellular vesicles in
tissue repair: Challenges and opportunities. Theranostics.
10:5979–5997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rani S, Ryan AE, Griffin MD and Ritter T:
Mesenchymal stem cell-derived extracellular vesicles: Toward
cell-free therapeutic applications. Mol Ther. 23:812–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Latifkar A, Ling L, Hingorani A, Johansen
E, Clement A, Zhang X, Hartman J, Fischbach C, Lin H, Cerione RA
and Antonyak MA: Loss of sirtuin 1 alters the secretome of breast
cancer cells by impairing lysosomal integrity. Dev Cell.
49:393–408.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Minakaki G, Menges S, Kittel A,
Emmanouilidou E, Schaeffner I, Barkovits K, Bergmann A, Rockenstein
E, Adame A, Marxreiter F, et al: Autophagy inhibition promotes
SNCA/alpha-synuclein release and transfer via extracellular
vesicles with a hybrid autophagosome-exosome-like phenotype.
Autophagy. 14:98–119. 2018. View Article : Google Scholar :
|
|
139
|
Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J
and Yan H: Metformin induces autophagy and G0/G1 phase cell cycle
arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways.
J Exp Clin Cancer Res. 37:632018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shen M, Yu H, Jin Y, Mo J, Sui J, Qian X
and Chen T: Metformin facilitates osteoblastic differentiation and
M2 macrophage polarization by PI3K/AKT/mTOR pathway in human
umbilical cord mesenchymal stem cells. Stem Cells Int.
2022:94988762022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Han X, Chen H, Gong H, Tang X, Huang N, Xu
W, Tai H, Zhang G, Zhao T, Gong C, et al: Autolysosomal degradation
of cytosolic chromatin fragments antagonizes oxidative
stress-induced senescence. J Biol Chem. 295:4451–4463. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tang Z, Hu B, Zang F, Wang J, Zhang X and
Chen H: Nrf2 drives oxidative stress-induced autophagy in nucleus
pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect
intervertebral disc from degeneration. Cell Death Dis. 10:5102019.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wang J, Huang C, Lin Z, Pan X, Chen J,
Zheng G, Tian N, Yan Y, Zhang Z, Hu J, et al: Polydatin suppresses
nucleus pulposus cell senescence, promotes matrix homeostasis and
attenuates intervertebral disc degeneration in rats. J Cell Mol
Med. 22:5720–5731. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kang L, Zhang H, Jia C, Zhang R and Shen
C: Targeting oxidative stress and inflammation in intervertebral
disc degeneration: Therapeutic perspectives of phytochemicals.
Front Pharmacol. 13:9563552022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Glück S, Guey B, Gulen MF, Wolter K, Kang
TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L and Ablasser A:
Innate immune sensing of cytosolic chromatin fragments through cGAS
promotes senescence. Nat Cell Biol. 19:1061–1070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Cheng Z, Dai T, He X, Zhang Z, Xie F, Wang
S, Zhang L and Zhou F: The interactions between cGAS-STING pathway
and pathogens. Signal Transduct Target Ther. 5:912020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chen Q, Sun L and Chen ZJ: Regulation and
function of the cGAS-STING pathway of cytosolic DNA sensing. Nat
Immunol. 17:1142–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hu MM and Shu HB: Innate immune response
to cytoplasmic DNA: Mechanisms and DIseases. Annu Rev Immunol.
38:79–98. 2020. View Article : Google Scholar
|
|
149
|
Motwani M, Pesiridis S and Fitzgerald KA:
DNA sensing by the cGAS-STING pathway in health and disease. Nat
Rev Genet. 20:657–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kim J, Kim HS and Chung JH: Molecular
mechanisms of mitochondrial DNA release and activation of the
cGAS-STING pathway. Exp Mol Med. 55:510–519. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Guo Y, Gu R, Gan D, Hu F, Li G and Xu G:
Mitochondrial DNA drives noncanonical inflammation activation via
cGAS-STING signaling pathway in retinal microvascular endothelial
cells. Cell Commun Signal. 18:1722020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ritchie C, Carozza JA and Li L:
Biochemistry, cell biology, and pathophysiology of the innate
immune cGAS-cGAMP-STING pathway. Annu Rev Biochem. 91:599–628.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
154
|
Vo NV, Sowa GA, Kang JD, Seidel C and
Studer RK: Prostaglandin E2 and prostaglandin F2α differentially
modulate matrix metabolism of human nucleus pulposus cells. J
Orthop Res. 28:1259–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tisherman R, Coelho P, Phillibert D, Wang
D, Dong Q, Vo N, Kang J and Sowa G: NF-κB signaling pathway in
controlling intervertebral disk cell response to inflammatory and
mechanical stressors. Phys Ther. 96:704–711. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ren H, Shao Y, Wu C, Ma X, Lv C and Wang
Q: Metformin alleviates oxidative stress and enhances autophagy in
diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell
Endocrinol. 500:1106282020. View Article : Google Scholar
|
|
157
|
Chen Q, Guan X, Zuo X, Wang J and Yin W:
The role of high mobility group box 1 (HMGB1) in the pathogenesis
of kidney diseases. Acta Pharm Sin B. 6:183–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Tang D, Kang R, Van Houten B, Zeh HJ,
Billiar TR and Lotze MT: High mobility group box 1 (HMGB1)
phenotypic role revealed with stress. Mol Med. 20:359–362. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Leclerc P, Wähämaa H, Idborg H, Jakobsson
PJ, Harris HE and Korotkova M: IL-1β/HMGB1 complexes promote The
PGE2 biosynthesis pathway in synovial fibroblasts. Scand J Immunol.
77:350–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Appavoo E, Hajam IA, Muneeswaran NS,
Kondabattula G, Bhanuprakash V and Kishore S: Synergistic effect of
high-mobility group box-1 and lipopolysaccharide on cytokine
induction in bovine peripheral blood mononuclear cells. Microbiol
Immunol. 60:196–202. 2016. View Article : Google Scholar
|
|
161
|
Wang Y, Che M, Xin J, Zheng Z, Li J and
Zhang S: The role of IL-1β and TNF-α in intervertebral disc
degeneration. Biomed Pharmacother. 131:1106602020. View Article : Google Scholar
|
|
162
|
Song Y, Wang Y, Zhang Y, Geng W, Liu W,
Gao Y, Li S, Wang K, Wu X, Kang L and Yang C: Advanced glycation
end products regulate anabolic and catabolic activities via
NLRP3-inflammasome activation in human nucleus pulposus cells. J
Cell Mol Med. 21:1373–1387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Dong HC, Li PN, Chen CJ, Xu X, Zhang H,
Liu G, Zheng LJ and Li P: Sinomenine attenuates cartilage
degeneration by regulating miR-223-3p/NLRP3 inflammasome signaling.
Inflammation. 42:1265–1275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Xu H, Mei Q, Xu B, Liu G and Zhao J:
Expression of matrix metalloproteinases is positively related to
the severity of disc degeneration and growing age in the East Asian
lumbar disc herniation patients. Cell Biochem Biophys.
70:1219–1225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Vo NV, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Liu Q, Jin L, Shen FH, Balian G and Li XJ:
Fullerol nanoparticles suppress inflammatory response and
adipogenesis of vertebral bone marrow stromal cells-a potential
novel treatment for intervertebral disc degeneration. Spine J.
13:1571–1580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Xian H, Liu Y, Rundberg Nilsson A,
Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench
GR, Lewis G, Chen W, et al: Metformin inhibition of mitochondrial
ATP and DNA synthesis abrogates NLRP3 inflammasome activation and
pulmonary inflammation. Immunity. 54:1463–1477.e11. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Jiang Y, Xie Z, Yu J and Fu L: Resveratrol
inhibits IL-1β-mediated nucleus pulposus cell apoptosis through
regulating the PI3K/Akt pathway. Biosci Rep. 39:BSR201900432019.
View Article : Google Scholar
|
|
169
|
Isoda K, Young JL, Zirlik A, MacFarlane
LA, Tsuboi N, Gerdes N, Schönbeck U and Libby P: Metformin inhibits
proinflammatory responses and nuclear factor-kappaB in human
vascular wall cells. Arterioscler Thromb Vasc Biol. 26:611–617.
2006. View Article : Google Scholar
|
|
170
|
Carafa V, Rotili D, Forgione M, Cuomo F,
Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A and
Altucci L: Sirtuin functions and modulation: From chemistry to the
clinic. Clin Epigenetics. 8:612016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Dai H, Sinclair DA, Ellis JL and Steegborn
C: Sirtuin activators and inhibitors: Promises, achievements, and
challenges. Pharmacol Ther. 188:140–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ohguchi K, Itoh T, Akao Y, Inoue H, Nozawa
Y and Ito M: SIRT1 modulates expression of matrix
metalloproteinases in human dermal fibroblasts. Br J Dermatol.
163:689–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Dvir-Ginzberg M, Gagarina V, Lee EJ and
Hall DJ: Regulation of cartilage-specific gene expression in human
chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J
Biol Chem. 283:36300–36310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Qu L, Yu Y, Qiu L, Yang D, Yan L, Guo J
and Jahan R: Sirtuin 1 regulates matrix metalloproteinase-13
expression induced by Porphyromonas endodontalis lipopolysaccharide
via targeting nuclear factor-κB in osteoblasts. J Oral Microbiol.
9:13175782017. View Article : Google Scholar
|
|
175
|
Abdelmawgoud H and El Awady RR: Effect of
Sirtuin 1 inhibition on matrix metalloproteinase 2 and Forkhead box
O3a expression in breast cancer cells. Genes Dis. 4:240–246. 2017.
View Article : Google Scholar
|
|
176
|
Gagarina V, Gabay O, Dvir-Ginzberg M, Lee
EJ, Brady JK, Quon MJ and Hall DJ: SirT1 enhances survival of human
osteoarthritic chondrocytes by repressing protein tyrosine
phosphatase 1B and activating the insulin-like growth factor
receptor pathway. Arthritis Rheum. 62:1383–1392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Song YM, Lee YH, Kim JW, Ham DS, Kang ES,
Cha BS, Lee HC and Lee BW: Metformin alleviates hepatosteatosis by
restoring SIRT1-mediated autophagy induction via an AMP-activated
protein kinase-independent pathway. Autophagy. 11:46–59. 2015.
View Article : Google Scholar :
|
|
178
|
Rogacka D, Audzeyenka I, Rychłowski M,
Rachubik P, Szrejder M, Angielski S and Piwkowska A: Metformin
overcomes high glucose-induced insulin resistance of podocytes by
pleiotropic effects on SIRT1 and AMPK. Biochim Biophys Acta Mol
Basis Dis. 1864:115–125. 2018. View Article : Google Scholar
|
|
179
|
Shen J, Lan Y, Ji Z and Liu H: Sirtuins in
intervertebral disc degeneration: Current understanding. Mol Med.
30:442024. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Xu J, Shao T, Lou J, Zhang J and Xia C:
Aging, cell senescence, the pathogenesis and targeted therapies of
intervertebral disc degeneration. Front Pharmacol. 14:11729202023.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Song Y, Li S, Geng W, Luo R, Liu W, Tu J,
Wang K, Kang L, Yin H, Wu X, et al: Sirtuin 3-dependent
mitochondrial redox homeostasis protects against AGEs-induced
intervertebral disc degeneration. Redox Biol. 19:339–353. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lin J, Du J, Wu X, Xu C, Liu J, Jiang L,
Cheng X, Ge G, Chen L, Pang Q, et al: SIRT3 mitigates
intervertebral disc degeneration by delaying oxidative
stress-induced senescence of nucleus pulposus cells. J Cell
Physiol. 236:6441–6456. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Hu B, Wang P, Zhang S, Liu W, Lv X, Shi D,
Zhao L, Liu H, Wang B, Chen S and Shao Z: HSP70 attenuates
compression-induced apoptosis of nucleus pulposus cells by
suppressing mitochondrial fission via upregulating the expression
of SIRT3. Exp Mol Med. 54:309–323. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhou TY, Wu YG, Zhang YZ, Bao YW and Zhao
Y: SIRT3 retards intervertebral disc degeneration by anti-oxidative
stress by activating the SIRT3/FOXO3/SOD2 signaling pathway. Eur
Rev Med Pharmacol Sci. 23:9180–9188. 2019.PubMed/NCBI
|
|
185
|
Karnewar S, Neeli PK, Panuganti D,
Kotagiri S, Mallappa S, Jain N, Jerald MK and Kotamraju S:
Metformin regulates mitochondrial biogenesis and senescence through
AMPK mediated H3K79 methylation: Relevance in age-associated
vascular dysfunction. Biochim Biophys Acta Mol Basis Dis.
1864:1115–1128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Du Y and Zhang J, Fang F, Wei X, Zhang H,
Tan H and Zhang J: Metformin ameliorates
hypoxia/reoxygenation-induced cardio-myocyte apoptosis based on the
SIRT3 signaling pathway. Gene. 626:182–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Chen J, Xie JJ, Jin MY, Gu YT, Wu CC, Guo
WJ, Yan YZ, Zhang ZJ, Wang JL, Zhang XL, et al: Sirt6
overexpression suppresses senescence and apoptosis of nucleus
pulposus cells by inducing autophagy in a model of intervertebral
disc degeneration. Cell Death Dis. 9:562018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Shan L, Wang F, Zhai D, Meng X, Liu J and
Lv X: Matrix metalloproteinases induce extracellular matrix
degradation through various pathways to alleviate hepatic fibrosis.
Biomed Pharmacother. 161:1144722023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Valenti MT, Dalle Carbonare L, Zipeto D
and Mottes M: Control of the autophagy pathway in osteoarthritis:
Key regulators, therapeutic targets and therapeutic strategies. Int
J Mol Sci. 22:27002021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Ryter SW, Cloonan SM and Choi AM:
Autophagy: A critical regulator of cellular metabolism and
homeostasis. Mol Cells. 36:7–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Mu W and Wang Z, Ma C, Jiang Y, Zhang N,
Hu K, Li L and Wang Z: Metformin promotes the proliferation and
differentiation of murine preosteoblast by regulating the
expression of sirt6 and oct4. Pharmacol Res. 129:462–474. 2018.
View Article : Google Scholar
|
|
192
|
Yang M, Peng Y, Liu W, Zhou M, Meng Q and
Yuan C: Sirtuin 2 expression suppresses oxidative stress and
senescence of nucleus pulposus cells through inhibition of the
p53/p21 pathway. Biochem Biophys Res Commun. 513:616–622. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Tang X, Chen XF, Wang NY, Wang XM, Liang
ST, Zheng W, Lu YB, Zhao X, Hao DL, Zhang ZQ, et al: SIRT2 acts as
a cardioprotective deacetylase in pathological cardiac hypertrophy.
Circulation. 136:2051–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Dutta S, Shah RB, Singhal S, Dutta SB,
Bansal S, Sinha S and Haque M: Metformin: A review of potential
mechanism and therapeutic utility beyond diabetes. Drug Des Devel
Ther. 17:1907–1932. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yanovski JA, Krakoff J, Salaita CG,
McDuffie JR, Kozlosky M, Sebring NG, Reynolds JC, Brady SM and
Calis KA: Effects of metformin on body weight and body composition
in obese insulin-resistant children: A randomized clinical trial.
Diabetes. 60:477–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Seifarth C, Schehler B and Schneider HJ:
Effectiveness of metformin on weight loss in non-diabetic
individuals with obesity. Exp Clin Endocrinol Diabetes. 121:27–31.
2013.
|
|
197
|
Piskovatska V, Storey KB, Vaiserman AM and
Lushchak O: The use of metformin to increase the human healthspan.
Adv Exp Med Biol. 1260:319–332. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Valencia WM, Palacio A, Tamariz L and
Florez H: Metformin and ageing: Improving ageing outcomes beyond
glycaemic control. Diabetologia. 60:1630–1638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Espinoza SE, Musi N, Wang CP, Michalek J,
Orsak B, Romo T, Powers B, Conde A, Moris M, Bair-Kelps D, et al:
Rationale and study design of a randomized clinical trial of
metformin to prevent frailty in older adults with prediabetes. J
Gerontol A Biol Sci Med Sci. 75:102–109. 2020. View Article : Google Scholar :
|
|
200
|
Kulkarni AS, Brutsaert EF, Anghel V, Zhang
K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP and
Barzilai N: Metformin regulates metabolic and nonmetabolic pathways
in skeletal muscle and subcutaneous adipose tissues of older
adults. Aging Cell. 17:e127232018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wang C, Chen B, Feng Q, Nie C and Li T:
Clinical perspectives and concerns of metformin as an anti-aging
drug. Aging Med (Milton). 3:266–275. 2020. View Article : Google Scholar
|
|
202
|
World's first anti-aging TAME trial gets
green-light-Longevity. Technology. https://www.longevity.technology/worlds-first-anti-aging-trial-gets-green-light/.
Accessed July 1, 2020
|
|
203
|
American Diabetes Association: Erratum:
Standards of Care in diabetes-2023 abridged for primary care
providers. Clin diabetes. 2023.41:4–31. View Article : Google Scholar
Clin Diabetes. 41:3282023. View Article : Google Scholar
|
|
204
|
Sun R, Feng J and Wang J: Underlying
mechanisms and treatment of cellular senescence-induced biological
barrier interruption and related diseases. Aging Dis. 15:612–639.
2024. View Article : Google Scholar :
|
|
205
|
Zhao JY, Sheng XL, Li CJ, Qin T, He RD,
Dai GY, Cao Y, Lu HB, Duan CY and Hu JZ: Metformin promotes
angiogenesis and functional recovery in aged mice after spinal cord
injury by adenosine monophosphate-activated protein
kinase/endothelial nitric oxide synthase pathway. Neural Regen Res.
18:1553–1562. 2023. View Article : Google Scholar :
|
|
206
|
Chen Q, Xie D, Yao Q and Yang L: Effect of
metformin on locomotor function recovery in rat spinal cord injury
model: A meta-analysis. Oxid Med Cell Longev. 2021:19480032021.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Chao-Yang G, Peng C and Hai-Hong Z: Roles
of NLRP3 inflammasome in intervertebral disc degeneration.
Osteoarthritis Cartilage. 29:793–801. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: a
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chen WK, Yu XH, Yang W, Wang C, He WS, Yan
YG, Zhang J and Wang WJ: lncRNAs: Novel players in intervertebral
disc degeneration and osteoarthritis. Cell Prolif. 50:e123132017.
View Article : Google Scholar
|
|
210
|
Fujiwara A, Lim TH, An HS, Tanaka N, Jeon
CH, Andersson GB and Haughton VM: The effect of disc degeneration
and facet joint osteoarthritis on the segmental flexibility of the
lumbar spine. Spine (Phila Pa 1976). 25:3036–3044. 2000. View Article : Google Scholar
|
|
211
|
Fine N, Lively S, Séguin CA, Perruccio AV,
Kapoor M and Rampersaud R: Intervertebral disc degeneration and
osteoarthritis: A common molecular disease spectrum. Nat Rev
Rheumatol. 19:136–152. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X,
Iatridis JC and Zheng Z: Painful intervertebral disc degeneration
and inflammation: from laboratory evidence to clinical
interventions. Bone Res. 9:72021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Mojtahedzadeh M, Jafarieh A, Najafi A,
Khajavi MR and Khalili N: Comparison of metformin and insulin in
the control of hyperglycaemia in non-diabetic critically ill
patients. Endokrynol Pol. 63:206–211. 2012.PubMed/NCBI
|
|
214
|
Farkhondeh T, Amirabadizadeh A, Aramjoo H,
Llorens S, Roshanravan B, Saeedi F, Talebi M, Shakibaei M and
Samarghandian S: Impact of metformin on cancer biomarkers in
non-diabetic cancer patients: A systematic review and meta-analysis
of clinical trials. Curr Oncol. 28:1412–1423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Ko KP, Ma SH, Yang JJ, Hwang Y, Ahn C, Cho
YM, Noh DY, Park BJ, Han W and Park SK: Metformin intervention in
obese non-diabetic patients with breast cancer: Phase II
randomized, double-blind, placebo-controlled trial. Breast Cancer
Res Treat. 153:361–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Lalau JD, Arnouts P, Sharif A and De Broe
ME: Metformin and other antidiabetic agents in renal failure
patients. Kidney Int. 87:308–322. 2015. View Article : Google Scholar
|
|
217
|
Cetin M and Sahin S: Microparticulate and
nanoparticulate drug delivery systems for metformin hydrochloride.
Drug Deliv. 23:2796–2805. 2016. View Article : Google Scholar
|
|
218
|
Adikwu MU, Yoshikawa Y and Takada K:
Bioadhesive delivery of metformin using prosopis gum with
antidiabetic potential. Biol Pharm Bull. 26:662–666. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Varde NK and Pack DW: Microspheres for
controlled release drug delivery. Expert Opin Biol Ther. 4:35–51.
2004. View Article : Google Scholar
|
|
220
|
Alexis F, Pridgen EM, Langer R and
Farokhzad OC: Nanoparticle technologies for cancer therapy. Handb
Exp Pharmacol. 55–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Feng Y, Egan B and Wang J: Genetic factors
in intervertebral disc degeneration. Genes Dis. 3:178–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Cosamalón-Gan I, Cosamalón-Gan T,
Mattos-Piaggio G, Villar-Suárez V, García-Cosamalón J and
Vega-Álvarez JA: Inflammation in the intervertebral disc
herniation. Neurocirugia (Astur : Engl Ed). 32:21–35. 2021.In
English, Spanish. View Article : Google Scholar
|