Introduction
Nicotine dependence, a pervasive and challenging
addiction, continues to exact a significant toll on public health
globally (1). Despite intensive
research being made into the mechanisms underlying nicotine
addiction and its associated health consequences, effective
strategies for its prevention and interruption remain elusive
(2). In recent years, emerging
evidence has shed light on the potential interplay between nicotine
dependence and the neuroendocrine hormone, melatonin (3).
The addictive qualities of tobacco products fuel the
complex condition known as nicotine dependence, which is
characterized by compulsive cravings and physiological or
psychological dependence on nicotine. The addictive nature of
nicotine stems from its ability to activate the brain's reward
pathways, particularly the mesolimbic dopaminergic system, leading
to feelings of pleasure and reinforcement of addictive behavior
(4). Despite widespread
awareness of the health risks associated with smoking, nicotine
dependence remains a formidable public health challenge,
contributing to a myriad of acute and chronic medical conditions,
including cardiovascular disease, respiratory disorders and cancer
(5,6).
Melatonin, often referred to as the 'hormone of
darkness', is primarily synthesized nocturnally by the pineal gland
and plays a crucial role in regulating circadian rhythms (7). The suprachiasmatic nucleus (SCN) of
the hypothalamus responds to environmental light-dark cycles by
tightly controlling melatonin secretion. Traditionally recognized
for its role in promoting sleep onset and regulating the sleep-wake
cycle, the influence of melatonin extends far beyond circadian
rhythms (8). Emerging research
has highlighted the diverse physiological roles of melatonin,
including its antioxidant, immunomodulatory and neuroprotective
properties (9-12).
The potential association between melatonin and
nicotine dependence has attracted increasing attention in the
scientific community. Preclinical investigations have offered
fascinating revelations regarding the interplay of melatonin and
nicotine dependence (13,14).
Melatonin receptors are widely distributed throughout the brain,
including areas implicated in the reward pathway and
addiction-related behaviors. In animal models, evidence suggests
that melatonin may modulate the reinforcing effects of nicotine by
influencing dopaminergic neurotransmission and attenuating
nicotine-seeking behavior (15).
Additionally, the antioxidant properties of
melatonin may provide defense against the oxidative stress that
chronic nicotine exposure causes, minimizing cellular damage and
lowering the risk of nicotine-related health issues (16). Moreover, the role of melatonin in
regulating mood and stress responses may influence susceptibility
to nicotine addiction and affect smoking behavior (17).
The present comprehensive review synthesizes the
existing literature on the association between melatonin and
nicotine dependence, provides a critical analysis of the current
evidence of this association, and identifies areas for future
research. By elucidating the intricate interplay between melatonin
and nicotine, the present review aims to provide guidelines for an
improved understanding of nicotine addiction and to pave the way
for innovative approaches to smoking cessation and health
consequences.
Melatonin and its diverse physiological
roles
Melatonin, a molecule nocturnally produced and
released by the pineal gland and by numerous other cells, where its
synthesis is neither circadian nor released, has long fascinated
researchers due to its diverse physiological roles (18). Initially recognized for its
involvement in regulating circadian rhythms, the influence of
melatonin extends far beyond sleep-wake cycles and other 24-h
rhythms (19). The synthesis of
melatonin commences with the conversion of tryptophan into
serotonin, catalyzed by the enzyme, tryptophan hydroxylase.
Subsequently, serotonin undergoes two enzymatic reactions, mediated
by serotonin-N-acetyltransferase and
acetylserotonin-O-methyltransferase, to form melatonin. The
SCN located in the hypothalamus exerts precise control over the
synthesis and release of melatonin in accordance with environmental
light-dark cycles (20,21).
Melatonin plays a pivotal role in synchronizing the
circadian rhythms of the body. As darkness falls, the SCN signals
the pineal gland to increase melatonin production, promoting sleep
onset and regulating the sleep-wake cycle (22). Conversely, exposure to light
inhibits melatonin secretion, promoting chronodisruption and
wakefulness. Perturbations in the melatonin rhythm, such as those
experienced during shift work or jet lag, can lead to sleep
disturbances and impaired cognitive function (23).
Beyond its role in circadian rhythms, melatonin
exhibits potent antioxidant properties. It scavenges free radicals,
neutralizes oxidative stress and protects cells from damage. The
ability of melatonin to traverse cellular membranes and accumulate
in various organelles underscores its effectiveness as an
antioxidant. Moreover, melatonin stimulates the activity of
antioxidant enzymes, further enhancing cellular defense mechanisms
against oxidative damage (24,25).
Emerging evidence suggests that melatonin plays a
crucial role in modulating the immune system. Melatonin receptors
are present on immune cells, enabling melatonin to regulate immune
responses. Melatonin enhances the production of immune cells, such
as natural killer cells and T-lymphocytes, strengthening the
defense of the body against pathogens. Additionally, melatonin
exhibits anti-inflammatory properties, mitigating excessive immune
activation and tissue damage (26-28).
Melatonin exerts neuroprotective effects,
safeguarding the brain against various insults. It regulates
neurotransmitter release, modulates neuronal excitability and
promotes neuronal survival. The antioxidant properties of
melatonin, many of which are receptor-independent, counteract
neurotoxicity induced by oxidative stress, reducing the risk of
developing neurodegenerative disorders, such as Alzheimer's and
Parkinson's disease. Moreover, melatonin enhances neuroplasticity,
facilitating learning and memory processes (29-32).
In addition to these well-established roles,
melatonin affects reproductive function in both males and females.
In females, melatonin influences ovulation and protects the
placenta from molecular damage, while in males, it modulates
spermatogenesis and testosterone production. Melatonin receptors
are present in reproductive tissues, highlighting their direct
involvement in reproductive processes. Furthermore, the antioxidant
properties of melatonin protect the gametes of both sexes from
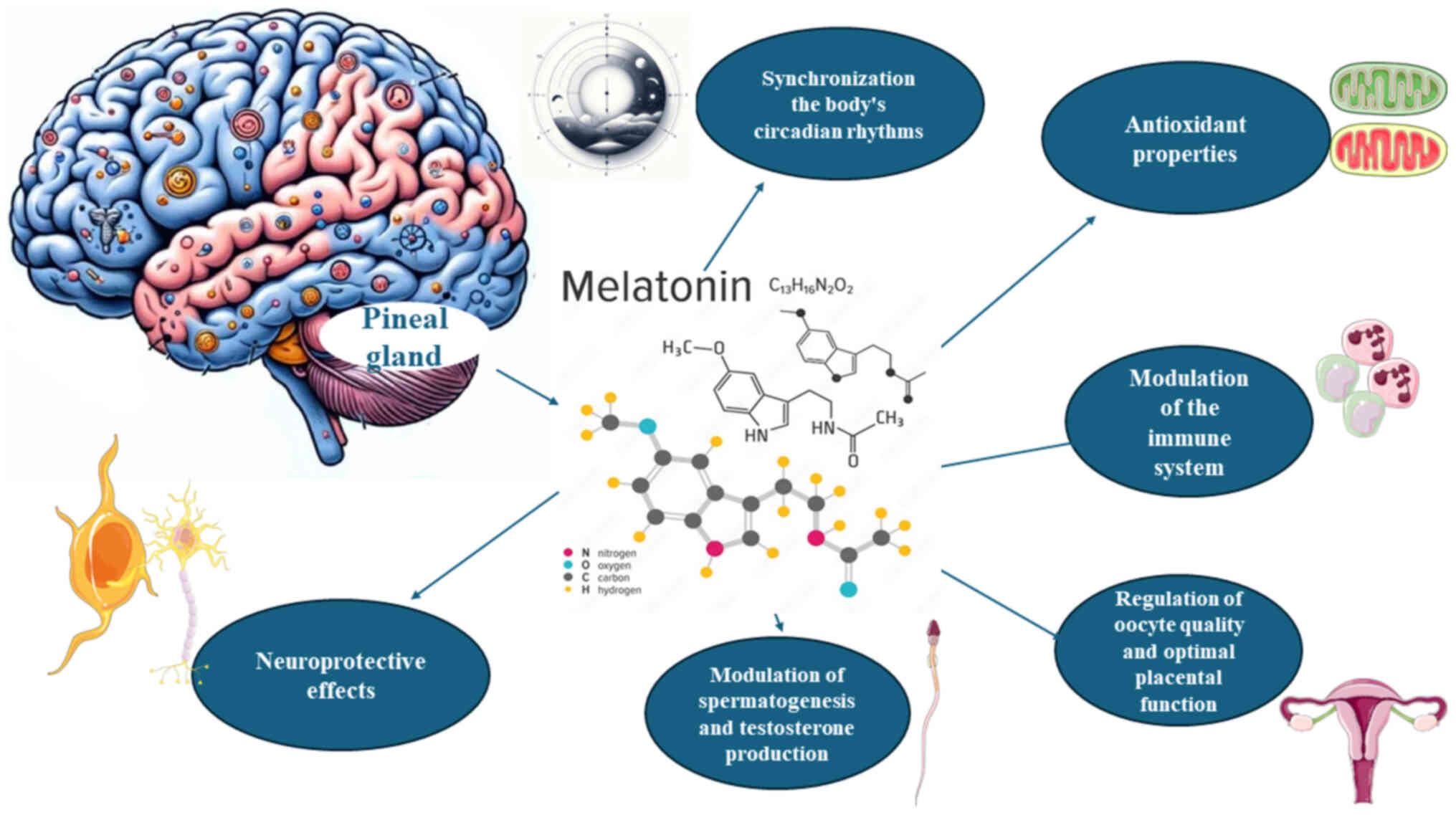
oxidative damage, ensuring reproductive success (33-35). The diverse physiological roles of
melatonin are, in part, illustrated in Fig. 1.
Overview of nicotine dependence and its
impact on health
Nicotine addiction: Mechanisms and
pathophysiology
Nicotine addiction stems from the pharmacological
effects of nicotine on the reward pathways of the brain. Upon
inhalation or ingestion, nicotine swiftly penetrates the
blood-brain barrier and attaches to nicotinic acetylcholine
receptors (nAChRs) in the mesolimbic dopaminergic system (36). This engagement prompts the
discharge of neurotransmitters, predominantly dopamine, fostering
sensations of pleasure and reinforcing addictive conduct. Chronic
nicotine exposure leads to neuroadaptations, altering the reward
circuitry of the brain and perpetuating addiction (37).
Nicotine addiction: Behavioral and
psychological factors
Beyond its pharmacological effects, a number of
behavioral and psychological factors affect nicotine dependence.
Smoking behavior often becomes intertwined with daily routines,
social interactions, and coping mechanisms, reinforcing the
habituation process. Additionally, individuals may use tobacco
products to alleviate stress, manage negative emotions, or enhance
cognitive performance, further solidifying nicotine dependence
(38). Environmental cues
associated with smoking, such as seeing cigarette advertisements or
being in social settings where smoking is prevalent, can trigger
cravings and perpetuate addiction to this dangerous molecule
(39).
Health consequences of nicotine
dependence
The inhalation of tobacco smoke exposes the body to
a toxic cocktail of chemicals, including carcinogens and harmful
gases, which damage vital organs and systems. Smoking is a leading
cause of preventable mortality worldwide, accounting for a
significant burden of disease attributable to cardiovascular
disease, respiratory disorders, and various types of cancer.
Moreover, nicotine dependence increases the risk of developing
comorbid conditions, such as hypertension, diabetes and mental
health disorders (40-51).
Cardiovascular effects
Nicotine exerts profound cardiovascular effects,
contributing to the development and progression of cardiovascular
disease. It stimulates the sympathetic nervous system, leading to
an increased heart rate, blood pressure and vasoconstriction
(40). Chronic nicotine exposure
promotes atherosclerosis, thrombosis and endothelial dysfunction,
predisposing individuals to coronary artery disease, myocardial
infarction and stroke (41).
Furthermore, the pro-inflammatory and pro-thrombotic properties of
nicotine exacerbate vascular pathology, exacerbating cardiovascular
morbidity and mortality (42).
Respiratory complications
Tobacco smoke contains numerous respiratory
irritants and carcinogens that inflict extensive damage on the
respiratory system. The inhalation of tobacco smoke damages airway
epithelial cells, impairs mucociliary clearance and induces
inflammation, predisposing individuals to chronic obstructive
pulmonary disease, emphysema and bronchitis (43). Moreover, nicotine dependence
increases the risk of developing respiratory infections and
exacerbates pre-existing respiratory conditions, leading to
respiratory failure and premature death (44).
Cancer risk
Perhaps the most well-known consequence of nicotine
dependence is its association with cancer (45). Tobacco smoke comprises >7,000
compounds; these include carcinogens, such as polycyclic aromatic
hydrocarbons and nitrosamines, which instigate and advance
oncogenic pathways (46).
Smoking stands as the primary cause behind lung cancer, responsible
for the majority of cases globally. Moreover, tobacco consumption
also escalates the likelihood of developing cancers of the pharynx,
oral cavity, larynx, bladder, esophagus, cervix and pancreas
(47). Additionally, exposure to
secondhand smoke poses a significant cancer risk to non-smokers
(48).
Mental health effects
Nicotine dependence is closely intertwined with
mental health, exacerbating symptoms of anxiety, depression and
stress. While individuals may initially use tobacco products as a
coping mechanism for stress relief or mood enhancement, chronic
nicotine exposure disrupts neurotransmitter balance and exacerbates
psychiatric symptoms. Moreover, nicotine dependence is highly
prevalent among individuals with mental health disorders,
contributing to resistance to treatment and poorer clinical
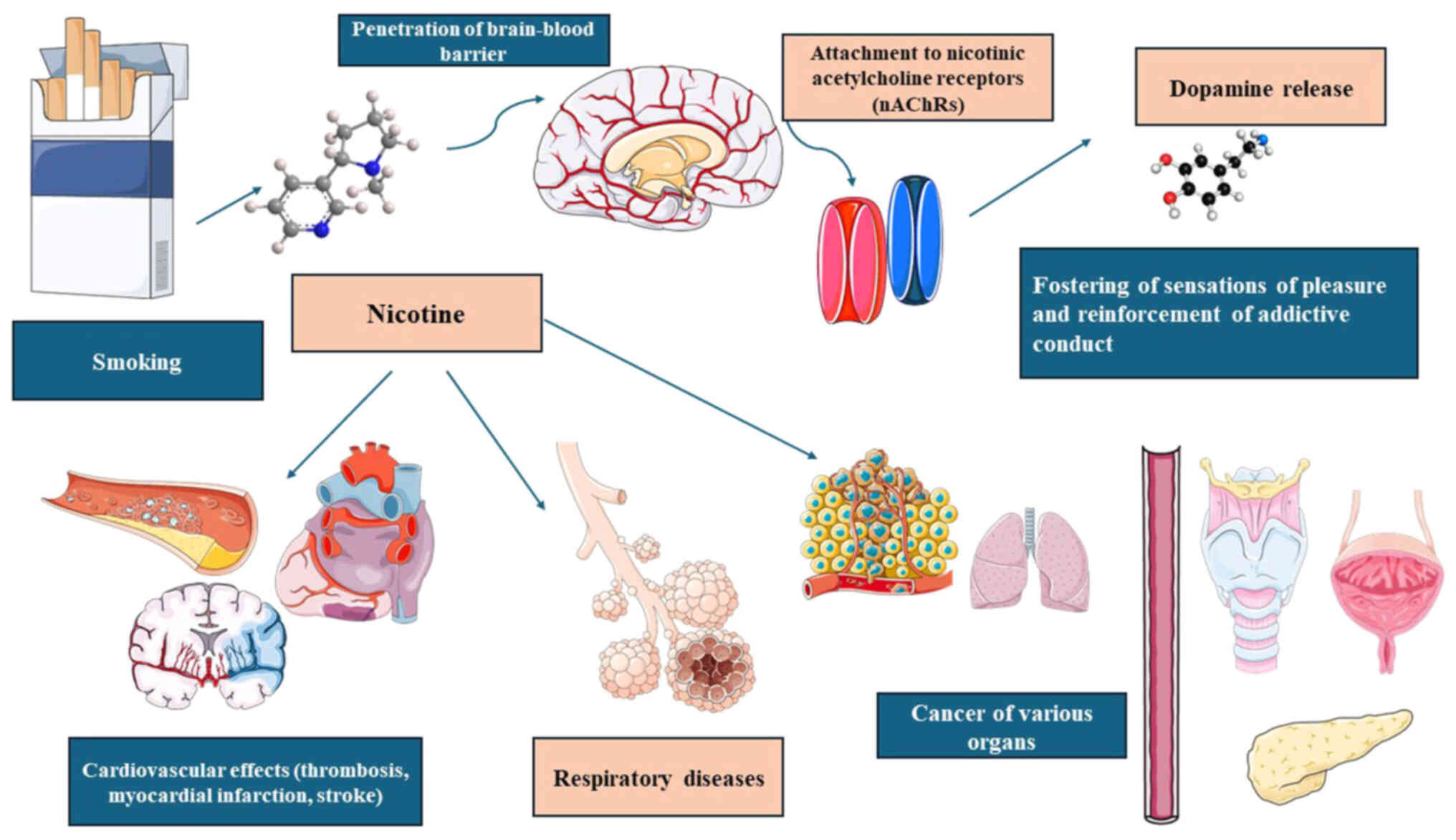
outcomes (49-51). An overview of nicotine dependence
and its impact on health is illustrated in Fig. 2.
Melatonin receptors and their distribution
in the brain
Likely central to numerous of the actions of
melatonin are its receptors, which are distributed throughout the
brain and peripheral tissues.
Types of melatonin receptors
Melatonin exerts its effects via two main types of
membrane receptors: Melatonin receptor type 1 (MT1) and melatonin
receptor type 2 (MT2), both belonging to the G protein-coupled
receptor family. MT1 receptors are predominantly coupled to
inhibitory G proteins (Gi/o), while MT2 receptors can activate both
inhibitory (Gi/o) and stimulatory (Gs) G proteins. These receptors
exhibit distinct, yet overlapping expression patterns, mediating
diverse physiological responses to melatonin (52).
Distribution in the brain
Melatonin receptors are widely distributed
throughout the brain, encompassing various regions involved in
circadian regulation, sleep-wake cycles, mood modulation and
neuroendocrine function. In the hypothalamus, MT1 and MT2 receptors
are localized in the SCN, the master circadian pacemaker, where
they regulate the timing of melatonin secretion and synchronize
biological rhythms with the light-dark cycle. Additionally,
melatonin receptors are found in the hypothalamic-pituitary axis,
where they function in the modulation of pituitary hormone
secretion related to reproductive function (53).
Circadian regulation
The SCN serves as the central hub for circadian
regulation, integrating photic and non-photic cues to entrain
circadian central and peripheral clocks. Melatonin receptors within
the SCN play a crucial role in transducing melatonin signals and
modulating neuronal activity, thereby influencing the timing of
sleep onset, body temperature and hormone secretion. Disruptions in
melatonin receptor signaling, such as those observed in circadian
rhythm disorders or shift work, can lead to sleep disturbances and
the desynchronization of biological rhythms (54-56).
Sleep-wake regulation
Beyond its role in circadian rhythms, melatonin
influences sleep-wake cycles by modulating the activity of
sleep-promoting and wake-promoting pathways in the brain. Melatonin
receptors are densely expressed in the hypothalamic nuclei, such as
the ventrolateral preoptic nucleus (VLPO) and the orexinergic
neurons in the lateral hypothalamus, which regulate sleep onset and
maintenance. The activation of MT1 receptors in the VLPO promotes
sleep by inhibiting arousal systems, while MT2 receptors modulate
the sensitivity of orexin neurons to sleep-promoting signals
(57,58).
Mood modulation
Emerging evidence suggests a role for melatonin
receptors in mood modulation and affective disorders. Melatonin
receptors are distributed in limbic structures implicated in
emotional regulation, including the hippocampus, amygdala and
prefrontal cortex (PFC) (59).
The dysregulation of melatonin receptor signaling has been
implicated in mood disorders, such as depression and bipolar
disorder. The modulation of melatonin receptors may offer a novel
therapeutic approach for mood stabilization and the management of
mood disorders (60).
Neuroprotective effects
Melatonin exerts neuroprotective effects against
oxidative stress, inflammation and neurodegeneration, mediated in
part by its receptors. Melatonin receptors are expressed in
neuronal populations vulnerable to oxidative damage, such as the
hippocampus and substantia nigra (61). The activation of melatonin
receptors enhances antioxidant defenses, reduces neuronal apoptosis
and promotes neurogenesis, thereby mitigating the progression of
neurodegenerative disorders, such as Alzheimer's, Parkinson's and
Huntington's diseases (62,63).
Therapeutic implications
The widespread distribution of melatonin receptors
in the brain as well as its receptor-independent actions underscore
the potential importance of melatonin as a therapeutic treatment
for a wide range of neurological and psychiatric disorders.
Agonists and antagonists targeting melatonin receptors have been
explored for their efficacy in sleep disorders, mood disorders and
neurodegenerative diseases. The selective modulation of melatonin
receptor subtypes may offer personalized treatment strategies with
improved efficacy and reduced side-effects (64,65).
Potential influence of melatonin on
neurotransmitter systems related to addiction
Dopaminergic system
The dopaminergic system, particularly that involved
in the mesolimbic pathway, is integral to the reinforcement of
rewarding behaviors and the development of addiction. Dopamine
release in the nucleus accumbens (NAc) mediates the pleasurable
effects of drug abuse and reinforces drug-seeking behavior
(66). Melatonin receptors,
particularly MT1 and MT2 receptors, are expressed in dopaminergic
neurons and modulate dopamine release in response to various
stimuli (67). Preclinical
studies have demonstrated that the administration of melatonin
attenuates dopamine release induced by psychostimulants, such as
cocaine and amphetamines, suggesting a potential role in modulating
reward processing and addiction vulnerability (68,69).
Glutamatergic system
The glutamatergic system plays a crucial role in
addiction, mediating synaptic plasticity and long-term potentiation
within the reward circuitry. Glutamate release in the NAc and PFC
contributes to drug-induced neuroadaptations and craving behavior
(70). Melatonin receptors are
expressed in glutamatergic neurons, where they modulate glutamate
release and synaptic transmission (71). Preclinical studies have shown
that melatonin attenuates glutamate-induced excitotoxicity and
reduces drug-seeking behavior in mouse HT22 hippocampal neurons and
in mouse retinal ganglion cells (72,73). Furthermore, melatonin may
modulate glutamate receptor expression and function, thereby
influencing synaptic plasticity and addiction-related behaviors
(74).
GABAergic system
Gamma-aminobutyric acid (GABA), the primary
inhibitory neurotransmitter in the brain, plays a critical role in
addiction by modulating the activity of dopaminergic neurons and
regulating reward-related behaviors. GABAergic interneurons in the
NAc and ventral tegmental area exert inhibitory control over the
mesolimbic dopaminergic pathway (75,76). Melatonin receptors are expressed
in GABAergic neurons, where they modulate GABA release and neuronal
excitability. Preclinical studies suggest that melatonin
administration enhances GABAergic transmission and attenuates
drug-induced hyperactivity in animal models of addiction,
highlighting its potential as a modulator of GABAergic signaling in
addiction (77).
Serotonergic system
The serotonergic system, is involved with the raphe
nuclei, modulates mood, impulsivity and reward processing,
rendering it a key target in addiction research. Serotonin
receptors are widely distributed throughout the brain, including
regions implicated in addiction such as the NAc and PFC (78). Melatonin receptors are also
expressed in serotonergic neurons, where they modulate serotonin
synthesis, release and reuptake. Preclinical studies have suggested
that melatonin may influence serotonergic neurotransmission and
mood regulation, thereby affecting addiction vulnerability and
comorbid mood disorders (79,80).
Endogenous opioid system
The endogenous opioid system, encompassing mu, delta
and kappa opioid receptors, plays a crucial role in mediating the
rewarding effects of drugs and natural reinforcers (81). Opioid receptors are densely
expressed in the mesolimbic system, where they modulate dopamine
release and reward processing (82). Melatonin receptors interact with
the endogenous opioid system to modulate opioid receptor expression
and opioid-induced analgesia. Preclinical studies have suggested
that melatonin may attenuate opioid withdrawal symptoms and reduce
opioid self-administration in animal models of addiction,
underscoring its potential as a modulator of the opioid system in
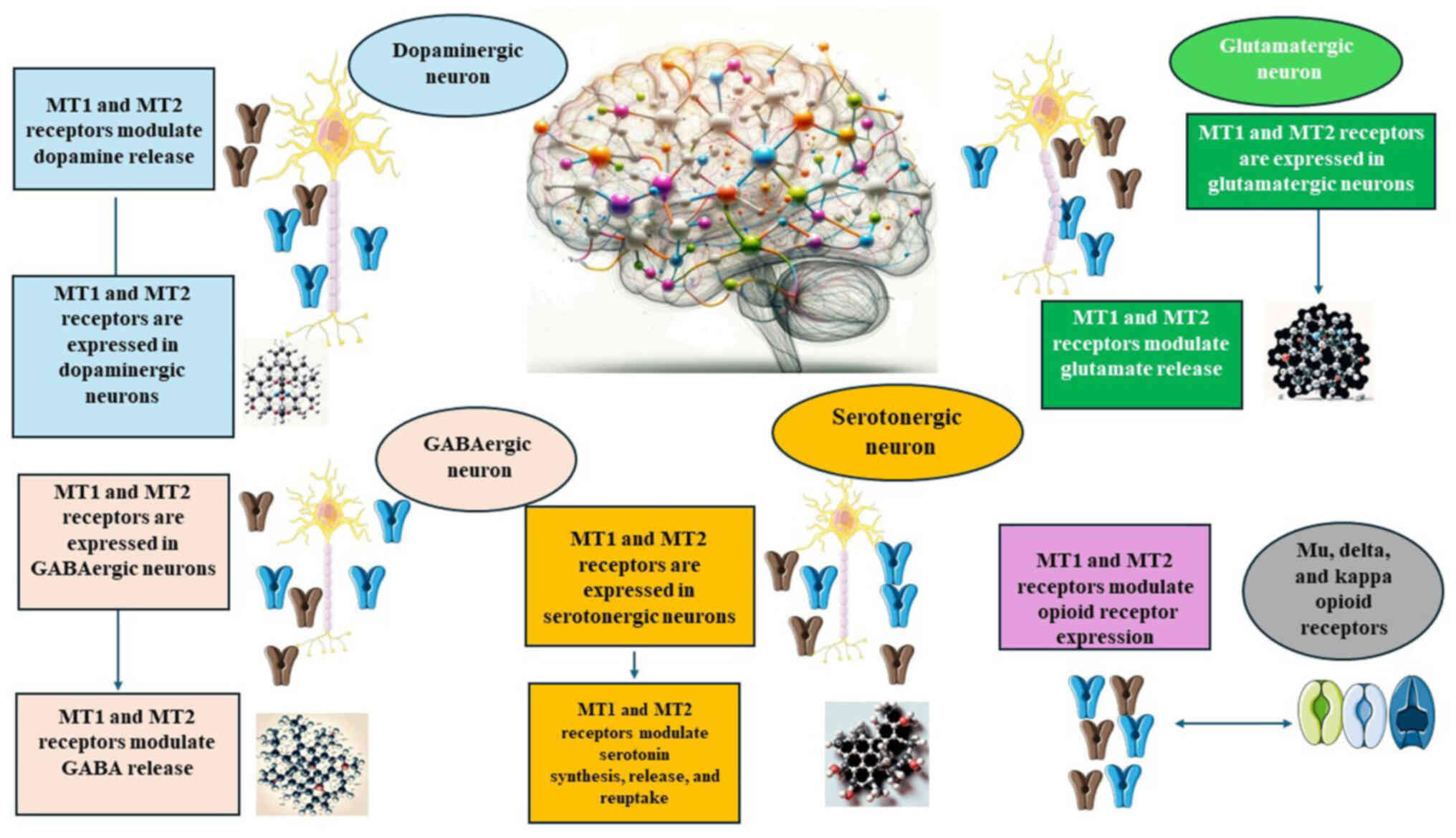
addiction (83,84). The diverse actions of melatonin
on neurotransmitter systems related to addiction are summarized in
Fig. 3.
Interplay between melatonin-regulated
circadian rhythms and nicotine cravings
Circadian regulation of nicotine
cravings
Circadian rhythms, orchestrated by melatonin in the
SCN, play a central role in regulating various physiological
processes, including sleep-wake cycles, hormone secretion and
metabolism (85). Nicotine
cravings and withdrawal symptoms often exhibit diurnal variations,
with heightened cravings observed during periods of nicotine
deprivation and reduced cravings during smoking or nicotine
administration (86). The
circadian regulation of nicotine cravings may be attributed, in
part, to the influence of melatonin on reward-related pathways and
neurotransmitter systems implicated in addiction (87).
Circadian disruptions and nicotine
cravings
Disruptions to circadian rhythms, such as those
experienced during shift work, jet lag, or chronic sleep
disturbances, can exacerbate nicotine cravings and increase
vulnerability to nicotine addiction (88). Shift workers, in particular,
often exhibit alterations in melatonin secretion and circadian
rhythms, which may contribute to heightened nicotine cravings and
impaired nicotine dependence treatment outcomes. Strategies aimed
at restoring circadian rhythmicity, such as light therapy or
melatonin supplementation, may mitigate nicotine cravings and
improve smoking cessation outcomes in individuals with circadian
disruptions (89).
Chronotherapeutic approaches to nicotine
addiction
Chronotherapeutic interventions targeting circadian
rhythms and melatonin signaling hold promise for the treatment of
nicotine addiction. Chronotherapy involves the timed administration
of medications or behavioral interventions to align treatment
efficacy with endogenous circadian rhythms (90). Melatonin supplementation or
melatonin receptor agonists may be useful if administered during
periods of heightened nicotine cravings to attenuate withdrawal
symptoms and reduce the reinforcing effects of nicotine (13). Additionally, timed exposure to
bright light or other circadian entrainment strategies may help
normalize circadian rhythms and alleviate nicotine cravings in
individuals with circadian disruptions (87).
Association between disrupted circadian
rhythms and increased vulnerability to nicotine dependence
There is mounting evidence to suggest a compelling
association between disrupted circadian rhythms and an increased
vulnerability to nicotine dependence. Studies have consistently
reported a higher prevalence of smoking among individuals with
irregular sleep patterns or those engaged in shift work, indicating
a potential interplay between circadian disruptions and nicotine
addiction (91,92).
One proposed mechanism for this potential
interaction involves the influence of circadian genes on nicotine
metabolism. Genes involved in regulating circadian rhythms also
modulate the metabolism of nicotine and other substances. Genetic
variations in these circadian genes may affect the response of an
individual to nicotine, potentially altering their susceptibility
to developing dependence (93,94).
Moreover, circadian disruptions can exert profound
effects on brain regions implicated in addiction, such as the
mesolimbic dopaminergic system. This neural circuitry underpins
reward processing and reinforcement learning which play pivotal
roles in addictive behaviors. By distrupting the normal functioning
of this system, circadian disturbances may amplify the rewarding
effects of nicotine, thereby heightening the risk of dependence
(95).
Potential role of melatonin in mitigating
withdrawal symptoms during smoking cessation
Understanding nicotine withdrawal
Nicotine, the primary psychoactive component in
tobacco, exerts its addictive effects through the activation of
nAChRs. Prolonged exposure to nicotine leads to neuroadaptations,
resulting in dependence and subsequent withdrawal symptoms upon
cessation. These symptoms can manifest as cravings, irritability,
anxiety, a depressed mood and sleep disturbances, posing
significant barriers to successful smoking cessation (96,97).
Exploring the effects of melatonin on
nicotine withdrawal
Published preclinical and clinical studies have shed
light on the potential therapeutic effects of melatonin in
mitigating nicotine withdrawal symptoms. Melatonin administration
attenuates nicotine withdrawal-induced anxiety-like behaviors and
craving-related responses, suggesting a modulatory role in the
reward circuitry of the brain (17). Furthermore, the antioxidant
properties of melatonin may mitigate the oxidative stress
associated with chronic exposure to nicotine, potentially
ameliorating neuroinflammatory processes implicated in withdrawal
symptoms (98). Additionally,
the interactions of melatonin with neurotransmitter systems,
including dopamine and GABA, could contribute to its
anti-withdrawal effects by modulating neurotransmission in key
brain regions involved in addiction (99).
Insights from studies on melatonin
supplementation in nicotine withdrawal protocols
Research on melatonin supplementation in nicotine
withdrawal protocols has yielded promising results. For example,
clinical trials on humans have also shown the potential benefits of
melatonin supplementation during smoking cessation. A randomized
controlled trial found that smokers receiving melatonin
supplementation experienced reduced withdrawal symptoms compared to
those given a placebo. Specifically, melatonin-treated individuals
reported lower levels of craving, irritability and sleep
disturbances, suggesting a therapeutic role for melatonin in
mitigating nicotine withdrawal symptoms (17).
Melatonin supplementation may also improve sleep
quality during smoking cessation, which addresses one of the most
commonly reported withdrawal symptoms (100). Sleep disturbances often
exacerbate other withdrawal symptoms and increase the risk of
relapse. By promoting improved sleep, melatonin supplementation
could enhance overall cessation outcomes and reduce the likelihood
of relapse (89).
The safety profile and tolerability of melatonin
render it an attractive adjunctive therapy for smoking cessation.
Unlike some pharmacotherapies, melatonin is a naturally occurring
molecule with few adverse effects, making it suitable for long-term
use (101,102). Additionally, melatonin
supplements are readily available over-the-counter and inexpensive,
providing convenient access to individuals seeking support during
smoking cessation (103).
Consideration of potential therapeutic
interventions targeting melatonin for nicotine dependence
Potential therapeutic interventions targeting
melatonin for nicotine dependence offer promising avenues for
enhancing smoking cessation outcomes. Several approaches could be
explored.
Melatonin supplementation can reduce nicotine
withdrawal symptoms and improve sleep quality among smokers
attempting to quit (17).
Further research is required however, to optimize dosing regimens,
treatment durations and the timing of melatonin administration to
maximize therapeutic efficacy.
Developing selective agonists targeting melatonin
receptors may offer more specific and potent therapeutic effects
compared to melatonin supplementation alone. These compounds could
modulate neural circuits implicated in nicotine addiction and
withdrawal, potentially reducing cravings and reinforcing the
effects of nicotine (104).
Combining melatonin-based interventions with
existing pharmacotherapies for smoking cessation, such as nicotine
replacement therapy (NRT) or bupropion, may produce synergistic
effects and improve treatment outcomes. These combined approaches
could target both the physiological and behavioral aspects of
nicotine dependence, providing a comprehensive approach to smoking
cessation (105).
Integrating melatonin-based interventions with
behavioral therapies, such as cognitive behavioral therapy (CBT)
may enhance the efficacy of smoking cessation interventions. CBT
addresses maladaptive behaviors and cognitive processes associated
with nicotine addiction, while melatonin supplementation could
target underlying neurobiological mechanisms contributing to
craving and withdrawal symptoms (106).
Light therapy, which usually involves exposure to
bright artificial light exposure at specific times of the day,
modulates circadian rhythms and melatonin secretion (107). Incorporating light therapy as
an adjunctive treatment for nicotine dependence may help regulate
sleep-wake cycles and improve mood, potentially reducing nicotine
cravings and withdrawal symptoms.
Promoting healthy lifestyle habits that support
optimal circadian rhythms, such as regular sleep patterns, regular
exercise and avoiding exposure to artificial light at night, may
complement melatonin-based interventions for smoking cessation.
These lifestyle modifications could enhance the efficacy of
melatonin supplementation and promote long-term smoking abstinence
(108).
Considering individual differences in melatonin
secretion patterns, circadian rhythms and genetic variations may
inform personalized treatment strategies for nicotine dependence
(87). Tailoring interventions
based on biomarkers predictive of treatment response could optimize
therapeutic outcomes and minimize adverse effects.
Developing strategies to sustain melatonin-based
interventions beyond the initial phase of smoking cessation is
crucial for preventing relapse. Long-term maintenance approaches,
such as gradual tapering of melatonin supplementation or
intermittent dosing schedules, may help maintain stable circadian
rhythms and support sustained smoking abstinence.
Identification of gaps in current
knowledge
While research on the association between melatonin
and nicotine dependence has provided valuable insight, several gaps
in current knowledge warrant further investigation. Although
preclinical studies have suggested that melatonin may modulate
nicotine-seeking behavior and withdrawal symptoms through its
interactions with neurotransmitter syst ems (68,69,72,73,77,79,80,83,84), the specific mechanisms underlying
these effects remain incompletely understood. Further research is
required to elucidate the molecular pathways and neural circuits
involved in the effects of melatonin on nicotine dependence.
Additionally, longitudinal studies tracking changes in melatonin
secretion patterns over time, particularly during nicotine
withdrawal and smoking cessation attempts, are required in order to
better understand the temporal dynamics of melatonin dysregulation
in relation to nicotine addiction.
There is a lack of research examining individual
differences in melatonin responsiveness and circadian rhythms in
relation to nicotine dependence. Investigating factors, such as
age, sex, genetic variations and comorbidities that may influence
the effects of melatonin on smoking behavior could help identify
subgroups of smokers who may benefit most from melatonin-based
interventions.
Some challenges remain in the integration of
melatonin supplementation into nicotine withdrawal protocols.
Variability in individual responses to melatonin, optimal dosing
regimens and the potential for interactions with other medications
necessitate further research. Additionally, the long-term efficacy
of melatonin supplementation in maintaining smoking abstinence
requires investigation in larger, longitudinal studies.
There is a need to identify biomarkers and
predictors of treatment response to melatonin supplementation in
nicotine dependence. Biomarkers associated with melatonin
dysregulation or circadian disruption could serve as potential
targets for personalized treatment strategies and may help stratify
individuals based on their likelihood of responding to
melatonin-based interventions.
Considering individual differences in
treatment effects
Genetic variations
Genetics play a crucial role in how individuals
metabolize and respond to melatonin. Polymorphisms in genes related
to melatonin receptors (MT1 and MT2) and circadian rhythm
regulation can affect the efficacy of melatonin in treating
nicotine dependence. For instance, variations in the genes encoding
for melatonin receptors may influence receptor sensitivity, thereby
altering the effectiveness of melatonin in modulating nicotine
cravings and withdrawal symptoms (109). Genetic testing can identify
these polymorphisms, allowing for the customization of melatonin
doses and treatment regimens to match the genetic profile of an
individual (110). Personalized
approaches based on genetic testing could enhance treatment
efficacy and reduce the risk of adverse effects, offering a more
targeted and effective intervention for nicotine dependence.
Age
Age is another critical factor influencing the
effectiveness of melatonin in treating nicotine dependence.
Melatonin production decreases with age, which may necessitate
adjustments in dosage for older individuals to achieve therapeutic
effects. Moreover, age-related changes in circadian rhythms and
sleep patterns could affect how melatonin is metabolized and
utilized by the body (111).
Younger individuals may require different dosing schedules compared
to older adults to align treatment with their specific circadian
patterns. Tailoring melatonin supplementation according to age can
optimize its effectiveness, improving treatment outcomes across
different age groups.
Sex
Sex differences can also affect how individuals
respond to melatonin treatment. Hormonal variations between males
and females can influence circadian rhythms and melatonin
metabolism. For example, fluctuations in estrogen levels during the
menstrual cycle can affect melatonin levels, and its effects on
sleep and mood (112). Research
suggests that women may experience different nicotine withdrawal
symptoms and cravings compared to men, which could influence their
response to melatonin treatment (113). By considering these
sex-specific differences, clinicians could develop more effective
treatment protocols that address the unique needs of males and
females, potentially improving adherence and outcomes.
Comorbid conditions
The presence of comorbid conditions, such as
psychiatric disorders, cardiovascular diseases, or metabolic
syndromes, can influence the effectiveness and safety of melatonin
treatment for nicotine dependence. For instance, individuals with
depression or anxiety may have altered melatonin signaling
pathways, affecting the overall treatment response (114). Tailoring melatonin
supplementation to account for these comorbid conditions involves a
comprehensive assessment of the health status of a patient and the
careful monitoring of the treatment progress.
Lifestyle factors
Lifestyle factors, including sleep patterns, diet
and exposure to light, significantly affect the effectiveness of
melatonin treatment. For instance, individuals with irregular sleep
patterns or those exposed to high levels of artificial light at
night may experience disruptions in their circadian rhythms,
affecting the therapeutic potential of melatonin (115). Lifestyle modifications, such as
improving sleep hygiene, reducing evening light exposure and
maintaining a regular sleep schedule, can enhance the effectiveness
of melatonin treatment.
Methodological challenges in studying the
melatonin-nicotine association
Studying the association between melatonin and
nicotine presents several methodological challenges, which
researchers have to consider to obtain robust and reliable
findings.
Measurement of melatonin levels
Accurately measuring melatonin levels presents a
challenge due to its episodic release, its short half-life in the
blood and its unique nocturnal elevation which requires darkness
(116,117).
The majority of studies rely on indirect measures,
such as salivary melatonin or urinary melatonin metabolite samples
to monitor its circulating concentrations, which may not capture
fluctuations accurately. Additionally, factors, such as
smoking-related changes in metabolism and excretion rates may make
measuring melatonin in smokers more challenging (118,119).
Assessment of nicotine exposure
Quantifying nicotine exposure in study participants
can be challenging, particularly in observational studies or
clinical trials where self-reported smoking status may be
unreliable. Biomarkers, such as cotinine levels in blood or urine
can provide objective measures of nicotine exposure, but may not
capture variations in nicotine intake over time accurately
(120).
Accounting for circadian rhythms
Melatonin secretion follows a circadian rhythm, with
levels peaking at night and minimal during the day (121). Studies investigating the
melatonin-nicotine association should account for these circadian
variations when assessing melatonin levels and nicotine effects.
Failure to consider circadian rhythms adequately could confound the
study results and obscure true associations between melatonin and
nicotine dependence.
Control of confounding variables
Numerous confounding factors, such as age, sex,
genetics, lifestyle factors (e.g., sleep patterns, diet) and
comorbidities (such as psychiatric disorders), may affect smoking
behavior and melatonin secretion (122,123). Failure to control for these
confounders adequately can introduce bias and limit the validity of
study findings.
Longitudinal study designs
Investigating the temporal association between
melatonin levels and nicotine dependence requires longitudinal
study designs that follow participants over time. However,
longitudinal studies are resource-intensive and may face
challenges, such as participant attrition, loss of follow-up and
changes in smoking behavior during the study, which complicates
data analysis and interpretation (124).
Experimental manipulation
Conducting experimental studies to investigate the
effects of melatonin manipulation on nicotine dependence faces
ethical and practical challenges. Ethical considerations, such as
ensuring informed consent may limit the ability to administer
melatonin or manipulate melatonin levels in human participants,
while animal models may not fully recapitulate the complex
neurobiological and behavioral aspects of nicotine addiction in
humans (125).
Generalizability of findings
Limited search examining the melatonin-nicotine
association has been conducted in controlled laboratory settings or
specific population groups, which may limit the generalizability of
findings to broader populations of smokers (17). Ensuring the diversity and
representativeness of study samples is crucial for extrapolating
findings to real-world settings (126).
Detailed discussions on clinical trial
designs and practical challenges
Clinical trial designs
Randomized controlled trials are the gold standard
for evaluating the efficacy of melatonin in reducing nicotine
dependence. Participants are randomly assigned to receive melatonin
or a placebo. Key considerations include an adequate sample size,
randomization, double-blinding and clear outcome definitions, such
as reduced nicotine cravings and withdrawal symptoms, with
long-term follow-up (127).
Crossover trials involve participants receiving both
treatment and placebo at different times, reducing variability by
serving as their own control. Key considerations are adequate
washout periods and randomized order of treatments (128). Factorial designs test multiple
interventions simultaneously, such as melatonin with CBT or NRT,
assessing interaction effects and managing increased design
complexity.
Practical challenges and solutions in
real-world applications
Ensuring participant adherence to the melatonin
regimen and study protocols is a significant challenge,
particularly during long-term follow-up. Solutions include thorough
education on adherence and study benefits, automated reminders via
text messages or phone calls, and support systems, such as peer
groups or regular check-ins.
Accurately measuring nicotine dependence and
withdrawal symptoms, along with capturing subjective outcomes such
as cravings and mood changes, presents another challenge. Solutions
involve using validated scales and questionnaires, incorporating
biomarkers such as cotinine levels, and requesting that
participants keep daily diaries.
Variability in individual responses to melatonin,
influenced by genetics, age and circadian rhythms, necessitates a
tailored approach. Effective strategies include the development of
personalized treatment plans, conducting subgroup analyses to
identify populations that benefit most, and optional genetic
testing to explore correlations between genetic markers and
treatment response.
Addressing environmental and social factors that
influence smoking behavior, such as stress and exposure to smoking
cues, is critical. Integrating melatonin with holistic
interventions, combining it with behavioral therapies such as CBT,
and engaging community resources and support networks provide a
supportive environment for smoking cessation.
Future research directions and
conclusions
Future research in the field of melatonin and
nicotine dependence is required to explore several promising
directions as outlined herein to advance our understanding and
improve treatment strategies.
Novel therapeutic targets
Identifying novel therapeutic targets within the
melatonergic system and circadian clock machinery could lead to the
development of more specific and potent interventions for nicotine
dependence. Investigating the role of melatonin receptors, clock
genes, and other components of the circadian system in nicotine
addiction may uncover new avenues for pharmacological
intervention.
Digital health interventions
Leveraging digital health technologies, such as
mobile applications, wearable devices and telemedicine platforms
could enhance the delivery and accessibility of melatonin-based
interventions for smoking cessation. Further research is required
to explore the feasibility and effectiveness of digital
interventions in supporting adherence to melatonin supplementation
and monitoring treatment outcomes remotely.
In conclusion, the interplay between melatonin and
nicotine dependence involves complex neurobiological, physiological
and behavioral mechanisms. Understanding this association could
provide insight into novel therapeutic strategies for managing
nicotine addiction and associated sleep disturbances. However,
further research is warranted in order to elucidate the precise
mechanisms underlying this interaction and to develop effective
interventions for individuals with nicotine dependence.
Availability of data and materials
Not applicable.
Authors' contributions
DAS and VEG conceptualized the study. VEG, PS, NT,
RJR and DAS made a substantial contribution to the interpretation
and analysis of the data to be included in the review, and wrote
and prepared the draft of the manuscript. RJR and DAS analyzed the
data and provided critical revisions. All authors contributed to
manuscript revision, and have read and approved the final version
of the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, the AI tool
Chat GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Widysanto A, Combest FE, Dhakal A and
Saadabadi A: Nicotine addiction (Updated 2023 Aug 8). StatPearls
[Internet]. StatPearls Publishing; Treasure Island, FL: 2024
|
|
2
|
Prochaska JJ and Benowitz NL: Current
advances in research in treatment and recovery: Nicotine addiction.
Sci Adv. 5:eaay97632019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onaolapo OJ and Onaolapo AY: Melatonin in
drug addiction and addiction management: Exploring an evolving
multidimensional relationship. World J Psychiatry. 8:64–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Picciotto MR and Kenny PJ: Mechanisms of
nicotine addiction. Cold Spring Harb Perspect Med. 11:a0396102021.
View Article : Google Scholar
|
|
5
|
Zhu J, Nelson K, Toth J and Muscat JE:
Nicotine dependence as an independent risk factor for
atherosclerosis in the national lung screening trial. BMC Public
Health. 19:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trakas N, Georgakopoulou VE, Melemeni D,
Damaskos C, Mantzouranis K, Garmpis N, Gkoufa A, Papalexis P,
Chlapoutakis S, Sklapani P, et al: Association between smoking
cessation and alterations in forced expiratory volume in one second
(FEV1). A follow-up study from a greek tobacco cessation clinic.
Addict Health. 14:87–95. 2022.PubMed/NCBI
|
|
7
|
Masters A, Pandi-Perumal SR, Seixas A,
Girardin JL and McFarlane SI: Melatonin, the hormone of darkness:
From sleep promotion to ebola treatment. Brain Disord Ther.
4:10001512014.
|
|
8
|
Hardeland R, Madrid JA, Tan DX and Reiter
RJ: Melatonin, the circadian multioscillator system and health: The
need for detailed analyses of peripheral melatonin signaling. J
Pineal Res. 52:139–166. 2012. View Article : Google Scholar
|
|
9
|
Tan DX, Manchester LC, Esteban-Zubero E,
Zhou Z and Reiter RJ: Melatonin as a potent and inducible
endogenous antioxidant: Synthesis and metabolism. Molecules.
20:18886–18906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuna-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: Inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18:8432017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bocheva G, Slominski RM, Janjetovic Z, Kim
TK, Böhm M, Steinbrink K, Reiter RJ, Kleszczyński K and Slominski
AT: Protective role of melatonin and its metabolites in skin aging.
Int J Mol Sci. 23:12382022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lempesis IG, Georgakopoulou VE, Reiter RJ
and Spandidos DA: A mid-pandemic night's dream: Melatonin, from
harbinger of anti-inflammation to mitochondrial savior in acute and
long COVID-19 (Review). Int J Mol Med. 53:282024. View Article : Google Scholar :
|
|
13
|
Horton WJ, Gissel HJ, Saboy JE, Wright KP
Jr and Stitzel JA: Melatonin administration alters nicotine
preference consumption via signaling through high-affinity
melatonin receptors. Psychopharmacology (Berl). 232:2519–2530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schiller ED, Champney TH, Reiter CK and
Dohrman DP: Melatonin inhibition of nicotine-stimulated dopamine
release in PC12 cells. Brain Res. 966:95–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markus RP, Santos JM, Zago W and Reno LA:
Melatonin nocturnal surge modulates nicotinic receptors and
nicotine-induced [3H]glutamate release in rat cerebellum slices. J
Pharmacol Exp Ther. 305:525–530. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Guo D, Wang J, Tian B, Li Y, Sun G
and Zhang H: Exogenous melatonin alleviates NO2 damage
in tobacco leaves by promoting antioxidant defense, modulating
redox homeostasis, and signal transduction. J Hazard Mater.
424:1272652022. View Article : Google Scholar
|
|
17
|
Zhdanova IV and Piotrovskaya VR: Melatonin
treatment attenuates symptoms of acute nicotine withdrawal in
humans. Pharmacol Biochem Behav. 67:131–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pandi-Perumal SR, Srinivasan V, Maestroni
GJ, Cardinali DP, Poeggeler B and Hardeland R: Melatonin: Nature's
most versatile biological signal? FEBS J. 273:2813–2838. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie X, Ding D, Bai D, Zhu Y, Sun W, Sun Y
and Zhang D: Melatonin biosynthesis pathways in nature and its
production in engineered microorganisms. Synth Syst Biotechnol.
7:544–553. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z,
Sharma R and Reiter RJ: Melatonin synthesis and function:
evolutionary history in animals and plants. Front Endocrinol
(Lausanne). 10:2492019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zisapel N: New perspectives on the role of
melatonin in human sleep, circadian rhythms and their regulation.
Br J Pharmacol. 175:3190–3199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blume C, Garbazza C and Spitschan M:
Effects of light on human circadian rhythms, sleep and mood.
Somnologie (Berl). 23:147–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chitimus DM, Popescu MR, Voiculescu SE,
Panaitescu AM, Pavel B, Zagrean L and Zagrean AM: Melatonin's
impact on antioxidative and anti-inflammatory reprogramming in
homeostasis and disease. Biomolecules. 10:12112020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kopustinskiene DM and Bernatoniene J:
Molecular mechanisms of melatonin-mediated cell protection and
signaling in health and disease. Pharmaceutics. 13:1292021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carrillo-Vico A, Reiter RJ, Lardone PJ,
Herrera JL, Fernández-Montesinos R, Guerrero JM and Pozo D: The
modulatory role of melatonin on immune responsiveness. Curr Opin
Investig Drugs. 7:423–431. 2006.PubMed/NCBI
|
|
27
|
Calvo JR, González-Yanes C and Maldonado
MD: The role of melatonin in the cells of the innate immunity: A
review. J Pineal Res. 55:103–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carrillo-Vico A, Lardone PJ,
Alvarez-Sánchez N, Rodríguez-Rodríguez A and Guerrero JM:
Melatonin: Buffering the immune system. Int J Mol Sci.
14:8638–8683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JG, Woo YS, Park SW, Seog DH, Seo MK
and Bahk WM: The neuroprotective effects of melatonin: Possible
role in the pathophysiology of neuropsychiatric disease. Brain Sci.
9:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Won E, Na KS and Kim YK: Associations
between melatonin, neuroinflammation, and brain alterations in
depression. Int J Mol Sci. 23:3052021. View Article : Google Scholar
|
|
31
|
Tozihi M, Shademan B, Yousefi H, Avci CB,
Nourazarian A and Dehghan G: Melatonin: A promising neuroprotective
agent for cerebral ischemia-reperfusion injury. Front Aging
Neurosci. 15:12275132023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Srinivasan V, Pandi-Perumal SR, Cardinali
DP, Poeggeler B and Hardeland R: Melatonin in Alzheimer's disease
and other neurodegenerative disorders. Behav Brain Funct. 2:152006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reiter RJ, Tamura H, Tan DX and Xu XY:
Melatonin and the circadian system: Contributions to successful
female reproduction. Fertil Steril. 102:321–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aversa S, Pellegrino S, Barberi I, Reiter
RJ and Gitto E: Potential utility of melatonin as an antioxidant
during pregnancy and in the perinatal period. J Matern Fetal
Neonatal Med. 25:207–221. 2012. View Article : Google Scholar
|
|
35
|
Olcese JM: Melatonin and female
reproduction: An expanding universe. Front Endocrinol (Lausanne).
11:852020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benowitz NL: Pharmacology of nicotine:
Addiction, smoking-induced disease, and therapeutics. Annu Rev
Pharmacol Toxicol. 49:57–71. 2009. View Article : Google Scholar
|
|
37
|
De Biasi M and Dani JA: Reward, addiction,
withdrawal to nicotine. Annu Rev Neurosci. 34:105–130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Chen X, Gong J and Yan Y:
Relationships between stress, negative emotions, resilience, and
smoking: Testing a moderated mediation model. Subst Use Misuse.
51:427–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stevenson JG, Oliver JA, Hallyburton MB,
Sweitzer MM, Conklin CA and McClernon FJ: Smoking environment cues
reduce ability to resist smoking as measured by a delay to smoking
task. Addict Behav. 67:49–52. 2017. View Article : Google Scholar :
|
|
40
|
Roy A, Rawal I, Jabbour S and Prabhakaran
D: Chapter 4: Tobacco and cardiovascular disease: A summary of
evidence. Cardiovascular Respiratory, and Related Disorders.
Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y and Nugent R:
3rd edition. The International Bank for Reconstruction and
Development/The World Bank; Washington, DC: 2017
|
|
41
|
Liu CC and Yeh HI: Nicotine: A
double-edged sword in atherosclerotic disease. Acta Cardiol Sin.
30:108–113. 2014.PubMed/NCBI
|
|
42
|
Benowitz NL and Burbank AD: Cardiovascular
toxicity of nicotine: Implications for electronic cigarette use.
Trends Cardiovasc Med. 26:515–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Strzelak A, Ratajczak A, Adamiec A and
Feleszko W: Tobacco smoke induces and alters immune responses in
the lung triggering inflammation, allergy, asthma and other lung
diseases: A mechanistic review. Int J Environ Res Public Health.
15:10332018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mishra A, Chaturvedi P, Datta S, Sinukumar
S, Joshi P and Garg A: Harmful effects of nicotine. Indian J Med
Paediatr Oncol. 36:24–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grando SA: Connections of nicotine to
cancer. Nat Rev Cancer. 14:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Caliri AW, Tommasi S and Besaratinia A:
Relationships among smoking, oxidative stress, inflammation,
macromolecular damage, and cancer. Mutat Res Rev Mutat Res.
787:1083652021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang CM, Corey CG, Rostron BL and
Apelberg BJ: Systematic review of cigar smoking and all cause and
smoking related mortality. BMC Public Health. 15:3902015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim AS, Ko HJ, Kwon JH and Lee JM:
Exposure to secondhand smoke and risk of cancer in never smokers: A
meta-analysis of epidemiologic studies. Int J Environ Res Public
Health. 15:19812018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
El-Sherbiny NA and Elsary AY: Smoking and
nicotine dependence in relation to depression, anxiety, and stress
in Egyptian adults: A cross-sectional study. J Family Community
Med. 29:8–16. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kutlu MG, Parikh V and Gould TJ: Nicotine
addiction and psychiatric disorders. Int Rev Neurobiol.
124:171–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Albarrak DA, Alotaibi AB, Alotaibi RF,
Alramadhan SH, Bin Muhanna AI, Aldehan AM and Bin Abdulrahman KA:
The association between nicotine dependence and mental health in
the general population of Saudi Arabia: A cross-sectional
analytical study. Int J Gen Med. 16:5801–5815. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cecon E, Oishi A and Jockers R: Melatonin
receptors: Molecular pharmacology and signalling in the context of
system bias. Br J Pharmacol. 175:3263–3280. 2018. View Article : Google Scholar :
|
|
53
|
Doghramji K: Melatonin and its receptors:
A new class of sleep-promoting agents. J Clin Sleep Med. 3(Suppl
5): S17–S23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Healy KL, Morris AR and Liu AC: Circadian
synchrony: Sleep, nutrition, and physical activity. Front Netw
Physiol. 1:7322432021. View Article : Google Scholar
|
|
55
|
Sato TK, Yamada RG, Ukai H, Baggs JE,
Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR and Hogenesch
JB: Feedback repression is required for mammalian circadian clock
function. Nat Genet. 38:312–319. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rahman SA, St Hilaire MA, Chang AM, Santhi
N, Duffy JF, Kronauer RE, Czeisler CA, Lockley SW and Klerman EB:
Circadian phase resetting by a single short-duration light
exposure. JCI Insight. 2:e894942017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schwartz MD and Kilduff TS: The
neurobiology of sleep and wakefulness. Psychiatr Clin North Am.
38:615–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wright KP, Lowry CA and Lebourgeois MK:
Circadian and wakefulness-sleep modulation of cognition in humans.
Front Mol Neurosci. 5:502012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Valdés-Tovar M, Estrada-Reyes R,
Solís-Chagoyán H, Argueta J, Dorantes-Barrón AM, Quero-Chávez D,
Cruz-Garduño R, Cercós MG, Trueta C, Oikawa-Sala J, et al:
Circadian modulation of neuroplasticity by melatonin: A target in
the treatment of depression. Br J Pharmacol. 175:3200–3208. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Boiko DI, Shkodina AD, Hasan MM, Bardhan
M, Kazmi SK, Chopra H, Bhutra P, Baig AA and Skrypnikov AM:
Melatonergic receptors (Mt1/Mt2) as a potential additional target
of novel drugs for depression. Neurochem Res. 47:2909–2924. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mehraein F, Talebi R, Jameie B, Joghataie
MT and Madjd Z: Neuroprotective effect of exogenous melatonin on
dopaminergic neurons of the substantia nigra in ovariectomized
rats. Iran Biomed J. 15:44–50. 2011.PubMed/NCBI
|
|
62
|
Shukla M, Govitrapong P, Boontem P, Reiter
RJ and Satayavivad J: Mechanisms of melatonin in alleviating
Alzheimer's disease. Curr Neuropharmacol. 15:1010–1031. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim J, Li W, Wang J, Baranov SV, Heath BE,
Jia J, Suofu Y, Baranova OV, Wang X, Larkin TM, et al: Biosynthesis
of neuroprotective melatonin is dysregulated in Huntington's
disease. J Pineal Res. 75:e129092023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Laudon M and Frydman-Marom A: Therapeutic
effects of melatonin receptor agonists on sleep and comorbid
disorders. Int J Mol Sci. 15:15924–15950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kostoglou-Athanassiou I: Therapeutic
applications of melatonin. Ther Adv Endocrinol Metab. 4:13–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Scofield MD, Heinsbroek JA, Gipson CD,
Kupchik YM, Spencer S, Smith ACW, Roberts-Wolfe D and Kalivas PW:
The nucleus accumbens: Mechanisms of addiction across drug classes
reflect the importance of glutamate homeostasis. Pharmacol Rev.
68:816–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ekmekcioglu C: Melatonin receptors in
humans: Biological role and clinical relevance. Biomed
Pharmacother. 60:97–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leeboonngam T, Pramong R, Sae-Ung K,
Govitrapong P and Phansuwan-Pujito P: Neuroprotective effects of
melatonin on amphetamine-induced dopaminergic fiber degeneration in
the hippocampus of postnatal rats. J Pineal Res. 64:e124562018.
View Article : Google Scholar
|
|
69
|
Sae-Ung K, Uéda K, Govitrapong P and
Phansuwan-Pujito P: Melatonin reduces the expression of
alpha-synuclein in the dopamine containing neuronal regions of
amphetamine-treated postnatal rats. J Pineal Res. 52:128–137. 2012.
View Article : Google Scholar
|
|
70
|
Quintero GC: Role of nucleus accumbens
glutamatergic plasticity in drug addiction. Neuropsychiatr Dis
Treat. 9:1499–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Evely KM, Hudson RL, Dubocovich ML and
Haj-Dahmane S: Melatonin receptor activation increases
glutamatergic synaptic transmission in the rat medial lateral
habenula. Synapse. 70:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang DD, Jin MF, Zhao DJ and Ni H:
Reduction of mitophagy-related oxidative stress and preservation of
mitochondria function using melatonin therapy in an HT22
hippocampal neuronal cell model of glutamate-induced
excitotoxicity. Front Endocrinol (Lausanne). 10:5502019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang C, An Y, Xia Z, Zhou X, Li H, Song S,
Ding L and Xia X: The neuroprotective effect of melatonin in
glutamate excitotoxicity of R28 cells and mouse retinal ganglion
cells. Front Endocrinol (Lausanne). 13:9861312022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jia S, Guo X, Chen Z, Li S and Liu XA: The
roles of the circadian hormone melatonin in drug addiction.
Pharmacol Res. 183:1063712022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li X and Slesinger PA: GABAB
receptors and drug addiction: Psychostimulants and other drugs of
abuse. Curr Top Behav Neurosci. 52:119–155. 2022. View Article : Google Scholar
|
|
76
|
Kalsbeek A, Garidou ML, Palm IF, Van Der
Vliet J, Simonneaux V, Pévet P and Buijs RM: Melatonin sees the
light: Blocking GABA-ergic transmission in the paraventricular
nucleus induces daytime secretion of melatonin. Eur J Neurosci.
12:3146–3154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng XP, Sun H, Ye ZY and Zhou JN:
Melatonin modulates the GABAergic response in cultured rat
hippocampal neurons. J Pharmacol Sci. 119:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sari Y, Johnson VR and Weedman JM: Role of
the serotonergic system in alcohol dependence: From animal models
to clinics. Prog Mol Biol Transl Sci. 98:401–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Míguez JM, Simonneaux V and Pévet P:
Evidence for a regulatory role of melatonin on serotonin release
and uptake in the pineal gland. J Neuroendocrinol. 7:949–956. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Micale V, Arezzi A, Rampello L and Drago
F: Melatonin affects the immobility time of rats in the forced swim
test: The role of serotonin neurotransmission. Eur
Neuropsychopharmacol. 16:538–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Befort K: Interactions of the opioid and
cannabinoid systems in reward: Insights from knockout studies.
Front Pharmacol. 6:62015.PubMed/NCBI
|
|
82
|
Fields HL and Margolis EB: Understanding
opioid reward. Trends Neurosci. 38:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dai X, Cui SG, Li SR, Chen Q and Wang R:
Melatonin attenuates the development of antinociceptive tolerance
to delta-, but not to mu-opioid receptor agonist in mice. Behav
Brain Res. 182:21–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
El-Shenawy SM, Abdel-Salam OM, Baiuomy AR,
El-Batran S and Arbid MS: Studies on the anti-inflammatory and
anti-nociceptive effects of melatonin in the rat. Pharmacol Res.
46:235–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Van Drunen R and Eckel-Mahan K: Circadian
rhythms of the hypothalamus: From function to physiology. Clocks
Sleep. 3:189–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
McLaughlin I, Dani JA and De Biasi M:
Nicotine withdrawal. Curr Top Behav Neurosci. 24:99–123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Logan RW, Williams WP III and McClung CA:
Circadian rhythms and addiction: Mechanistic insights and future
directions. Behav Neurosci. 128:387–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hasler BP, Smith LJ, Cousins JC and
Bootzin RR: Circadian rhythms, sleep, and substance abuse. Sleep
Med Rev. 16:67–81. 2012. View Article : Google Scholar
|
|
89
|
Cho YM, Kim HR, Kang MY, Myong JP and Koo
JW: Fixed night workers and failed smoking cessation. J Occup Med
Toxicol. 14:232019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cardinali DP, Brown GM and Pandi-Perumal
SR: Chronotherapy. Handb Clin Neurol. 179:357–370. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bae MJ, Song YM, Shin JY, Choi BY, Keum JH
and Lee EA: The association between shift work and health behavior:
Findings from the Korean national health and nutrition examination
survey. Korean J Fam Med. 38:86–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Short NA, Mathes BM, Gibby B, Oglesby ME,
Zvolensky MJ and Schmidt NB: Insomnia symptoms as a risk factor for
cessation failure following smoking treatment. Addict Res Theory.
25:17–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Perreau-Lenz S and Spanagel R: Clock genes
× stress × reward interactions in alcohol and substance use
disorders. Alcohol. 49:351–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Saad L, Zwiller J, Kalsbeek A and Anglard
P: Epigenetic regulation of circadian clocks and its involvement in
drug addiction. Genes (Basel). 12:12632021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lewis RG, Florio E, Punzo D and Borrelli
E: The Brain's reward system in health and disease. Adv Exp Med
Biol. 1344:57–69. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wittenberg RE, Wolfman SL, De Biasi M and
Dani JA: Nicotinic acetylcholine receptors and nicotine addiction:
A brief introduction. Neuropharmacology. 177:1082562020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jiloha RC: Biological basis of tobacco
addiction: Implications for smoking-cessation treatment. Indian J
Psychiatry. 52:301–307. 2010. View Article : Google Scholar
|
|
98
|
Reiter RJ, Mayo JC, Tan DX, Sainz RM,
Alatorre-Jimenez M and Qin L: Melatonin as an antioxidant: Under
promises but over delivers. J Pineal Res. 61:253–278. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Datta PC and King MG: Melatonin: Effects
on brain and behavior. Neurosci Biobehav Rev. 4:451–458. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fatemeh G, Sajjad M, Niloufar R, Neda S,
Leila S and Khadijeh M: Effect of melatonin supplementation on
sleep quality: A systematic review and meta-analysis of randomized
controlled trials. J Neurol. 269:205–216. 2022. View Article : Google Scholar
|
|
101
|
Foley HM and Steel AE: Adverse events
associated with oral administration of melatonin: A critical
systematic review of clinical evidence. Complement Ther Med.
42:65–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Motooka Y, Matsui T, Slaton RM, Umetsu R,
Fukuda A, Naganuma M, Hasegawa S, Sasaoka S, Hatahira H, Iguchi K
and Nakamura M: Adverse events of smoking cessation treatments
(nicotine replacement therapy and non-nicotine prescription
medication) and electronic cigarettes in the food and drug
administration adverse event reporting system, 2004-2016. SAGE Open
Med. 6:20503121187779532018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Erland LAE and Saxena PK: Melatonin
natural health products and supplements: Presence of serotonin and
significant variability of melatonin content. J Clin Sleep Med.
13:275–281. 2017. View Article : Google Scholar :
|
|
104
|
Liu J, Clough SJ, Hutchinson AJ,
Adamah-Biassi EB, Popovska-Gorevski M and Dubocovich ML: MT1 and
MT2 melatonin receptors: A therapeutic perspective. Annu Rev
Pharmacol Toxicol. 56:361–383. 2016. View Article : Google Scholar :
|
|
105
|
Giulietti F, Filipponi A, Rosettani G,
Giordano P, Iacoacci C, Spannella F and Sarzani R: Pharmacological
approach to smoking cessation: An updated review for daily clinical
practice. High Blood Press Cardiovasc Prev. 27:349–362. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Perkins KA, Conklin CA and Levine MD:
Cognitive-behavioral therapy for smoking cessation: A practical
guidebook to the most effective treatments. 1st edition. Routledge;
New York, NY: 2007
|
|
107
|
Shirani A and St Louis EK: Illuminating
rationale and uses for light therapy. J Clin Sleep Med. 5:155–163.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Patterson F, Grandner MA, Malone SK, Rizzo
A, Davey A and Edwards DG: Sleep as a target for optimized response
to smoking cessation treatment. Nicotine Tob Res. 21:139–148. 2019.
View Article : Google Scholar :
|
|
109
|
Silva ACPE, Santos MJD, Koike BDV, Moreira
MSA, Gitai DLG, de Miranda Coelho JAP and de Andrade TG: Melatonin
receptor 1B-1193T>C polymorphism is associated with diurnal
preference and sleep habits. Sleep Med. 53:106–114. 2019.
View Article : Google Scholar
|
|
110
|
Sulkava S, Ollila HM, Alasaari J, Puttonen
S, Härmä M, Viitasalo K, Lahtinen A, Lindström J, Toivola A,
Sulkava R, et al: Common genetic variation near melatonin receptor
1A gene linked to job-related exhaustion in shift workers. Sleep.
40:zsw0112017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pandi-Perumal SR, Zisapel N, Srinivasan V
and Cardinali DP: Melatonin and sleep in aging population. Exp
Gerontol. 40:911–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shechter A and Boivin DB: Sleep, hormones,
and circadian rhythms throughout the menstrual cycle in healthy
women and women with premenstrual dysphoric disorder. Int J
Endocrinol. 2010:2593452010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Leventhal AM, Waters AJ, Boyd S, Moolchan
ET, Lerman C and Pickworth WB: Gender differences in acute tobacco
withdrawal: Effects on subjective, cognitive, and physiological
measures. Exp Clin Psychopharmacol. 15:21–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Repova K, Baka T, Krajcirovicova K, Stanko
P, Aziriova S, Reiter RJ and Simko F: Melatonin as a potential
approach to anxiety treatment. Int J Mol Sci. 23:161872022.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Touitou Y, Reinberg A and Touitou D:
Association between light at night, melatonin secretion, sleep
deprivation, and the internal clock: Health impacts and mechanisms
of circadian disruption. Life Sci. 173:94–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
English J, Arendt J, Poulton A and Symons
AM: Short-term variations of circulating melatonin in the ewe. J
Pineal Res. 4:359–366. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cozzi B, Ravault JP, Ferrandi B and Reiter
RJ: Melatonin concentration in the cerebral vascular sinuses of
sheep and evidence for its episodic release. J Pineal Res.
5:535–543. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rzepka-Migut B and Paprocka J:
Melatonin-measurement methods and the factors modifying the
results. A systematic review of the literature. Int J Environ Res
Public Health. 17:19162020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Benloucif S, Burgess HJ, Klerman EB, Lewy
AJ, Middleton B, Murphy PJ, Parry BL and Revell VL: Measuring
melatonin in humans. J Clin Sleep Med. 4:66–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Florescu A, Ferrence R, Einarson T, Selby
P, Soldin O and Koren G: Methods for quantification of exposure to
cigarette smoking and environmental tobacco smoke: Focus on
developmental toxicology. Ther Drug Monit. 31:14–30. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tordjman S, Chokron S, Delorme R, Charrier
A, Bellissant E, Jaafari N and Fougerou C: Melatonin: pharmacology,
functions and therapeutic benefits. Curr Neuropharmacol.
15:434–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Valentine JA: The transformative role of
authentic partnership in the tuskegee public health ethics program.
J Healthc Sci Humanit. 8:21–29. 2018.PubMed/NCBI
|
|
123
|
Minichino A, Bersani FS, Calò WK, Spagnoli
F, Francesconi M, Vicinanza R, Delle Chiaie R and Biondi M: Smoking
behaviour and mental health disorders-mutual influences and
implications for therapy. Int J Environ Res Public Health.
10:4790–4811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Caruana EJ, Roman M, Hernández-Sánchez J
and Solli P: Longitudinal studies. J Thorac Dis. 7:E537–E540.
2015.PubMed/NCBI
|
|
125
|
Yip C, Han NR and Sng BL: Legal and
ethical issues in research. Indian J Anaesth. 60:684–688. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Faber J and Fonseca LM: How sample size
influences research outcomes. Dental Press J Orthod. 19:27–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hariton E and Locascio JJ: Randomised
controlled trials-the gold standard for effectiveness research:
Study design: Randomised controlled trials. BJOG. 125:17162018.
View Article : Google Scholar
|
|
128
|
Lim CY and In J: Considerations for
crossover design in clinical study. Korean J Anesthesiol.
74:293–299. 2021. View Article : Google Scholar : PubMed/NCBI
|