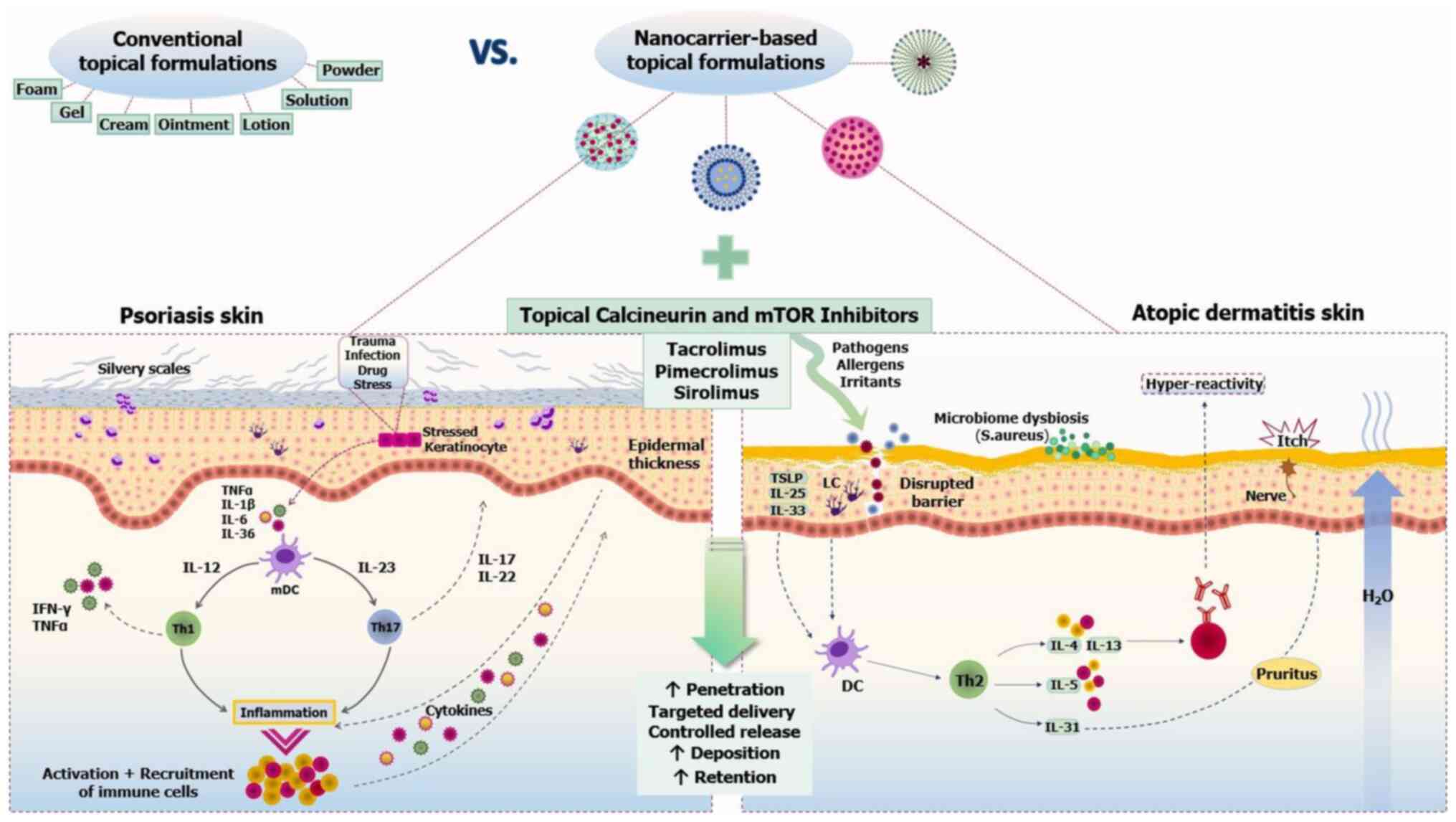
Introduction
Current therapies for inflammatory skin conditions
are increasingly directed towards non-steroidal immunomodulatory
approaches. Initially used in transplantation medicine, macrolactam
immunomodulators have broadened the treatment landscape in this
field. Lead compounds in this drug class are calcineurin
inhibitors, such as tacrolimus (TAC) and pimecrolimus (PIM), while
newer agents, such as sirolimus and its analogs, have recently
emerged as mammalian target of rapamycin (mTOR) inhibitors (mTORIs)
(1-4).
Given their efficacy in managing skin inflammation
when used systemically, macrolactam immunomodulators are currently
available or under investigation for topical application on
inflamed skin (1,2,4).
Topical calcineurin inhibitors (TCIs), known for selectively
suppressing cytokine-mediated T-cell activation and proliferation,
offer a targeted anti-inflammatory approach without compromising
skin immune homeostasis. This mode of action is particularly
beneficial in inflammatory dermatoses characterized by immune
dysregulation, such as atopic dermatitis (AD) (5-7).
Topical mTORIs have also shown inhibitory effects on epidermal and
vascular proliferation, opening new perspectives in targeting
inflammation-driven proliferative aspects in skin conditions like
psoriasis (2,4,8,9).
However, despite their potential to control skin
inflammation and hyperproliferation, the topical use of
immunomodulatory macrolactams has not always yielded satisfactory
results in both experimental and clinical settings, depending on
disease states and affected body areas (2,10-12). This therapeutic variability may
partly reflect their limited and variable skin absorption due to
unfavorable physicochemical properties, such as their hydrophobic
nature and large molecular size. Additionally, the barrier
alterations of inflamed skin further complicate their absorption
(6,10,13,14).
Despite the challenges involved in skin delivery,
topical agents for inflammatory dermatoses, such as AD or
psoriasis, should ideally overcome the stratum corneum (SC) barrier
and maintain therapeutically efficient concentrations at the site
of action in the viable epidermis and dermis without entering the
bloodstream (15,16). However, the physicochemical
features of topical calcineurin and mTOR inhibitors, while offering
a skin-selective pharmacological profile, still appear not
well-suited for intradermal delivery, especially with conventional
vehicles (14,16-18). Since the effect of drug
properties on skin permeability is crucial (19), elucidating the skin penetration
kinetics of topical macrolactam immunomodulators is therefore
important.
To optimize therapeutic outcomes, more sophisticated
nanoparticle-based approaches are being extensively explored to
improve the absorption and deposition of locally applied
therapeutics in inflamed skin. In this regard, nanotechnology has
offered a variety of promising carriers capable of delivering
macromolecules, such as TAC, in psoriatic and atopic skin,
providing an effective, safe and esthetically appealing alternative
to traditional topical vehicles (20,21).
The present review aimed to outline the
dermatokinetic profiles of topical calcineurin and mTOR inhibitors,
with special emphasis on their penetration and biodistribution in
psoriatic and atopic skin. In this context, the potential benefits
of topical sirolimus are briefly covered, as animal and human skin
studies have indicated encouraging results. Finally, it reviewed
novel preclinical approaches that utilized topically investigated
nanomaterials to explore and optimize the skin pharmacokinetics,
efficacy and safety of these 'hard-to-formulate' compounds,
focusing on comparisons between nanovectors and conventional
vehicles in psoriasis and AD skin models (Fig. 1).
The topical route
Skin delivery of bioactive agents remains a favored
approach in dermatotherapy (19). As the largest accessible organ,
the skin offers a convenient route for direct access to diseased
sites, while minimizing systemic exposure and adverse effects. In
addition, drug depots formed within the skin tissue may allow for
prolonged storage and sustained release, reducing application
frequency in favor of patient compliance (22,23).
However, when utilizing the skin for topical drug
delivery, both cutaneous biology and the physicochemical properties
of penetrants should be aligned, as the structural and functional
features of human skin may interact with penetrating compounds
(19,22,23). This section provides a brief
overview of the key factors influencing the skin absorption of
locally applied therapeutics.
Skin barriers
The skin has evolved a complex network of
interconnected barriers, i.e., the physical/mechanical,
biochemical, immune and microbiome barriers, which protect from
external insults while maintaining internal homeostasis (22,24). The outermost lipid-rich layer of
the SC, composed of tightly linked dead corneocytes, and the
underlying tight junction (TJ)-mediated paracellular sealing form a
major physical barrier against penetrating molecules, particularly
highly hydrophilic or lipophilic drugs (22,25,26).
Barrier function also involves biochemical elements,
including skin pH, natural moisturizing factors (NMFs) and skin
enzymes (22-24,27,28). The 'acid mantle' on the skin's
surface not only protects against pathogens with its antimicrobial
action but also affects drug partitioning from the vehicle into
different skin layers. Notably, pH-responsive delivery systems can
utilize local pH changes in lesional skin for targeted drug release
(27). The NMFs are essential
for maintaining skin moisture and SC integrity. Increased skin
hydration can enhance drug solubility and permeability by
rearranging the lipid matrix to create aqueous pores that
facilitate drug transport across the skin (22-24). In addition, cutaneous
bioavailability can be further altered since penetrating substances
may be trapped and biotransformed by drug-metabolizing enzymes,
mostly found in the viable epidermis (28).
Additionally, the skin harbors various
immunocompetent cells strategically located in the epidermis and
dermis (24). In the context of
drug delivery, initial views of the skin's immune surveillance have
demonstrated a dynamic immune-epithelial crosstalk. Activated
Langerhans cells (LCs) have been shown to extend their dendrites
through TJs to uptake invading molecules without compromising TJ
integrity (29,30). This interaction may provide
useful insights for translating the immune aspects of the skin
barrier into clinical implications for topical drug delivery.
Furthermore, the microbiome barrier, comprising a
diverse ecosystem of commensal microbes and their genetic material,
forms an additional barrier covering the entire skin surface
(24,31). Although the exact role of skin
flora in penetration processes remains elusive, emerging evidence
suggests that Staphylococcal strains may affect TJ functionality,
indicating an interplay between the physical and microbiome
barriers (24,25,31). In this regard, unraveling the
enzymatic pathways by which gut commensals metabolize
pharmaceuticals may partly decipher the skin microbiota-drug
interactions offering a tool for optimizing topical dermatotherapy
(31).
However, despite its complex barriers, the skin also
offers several opportunities for targeted manipulation of its
components in the context of topical drug delivery.
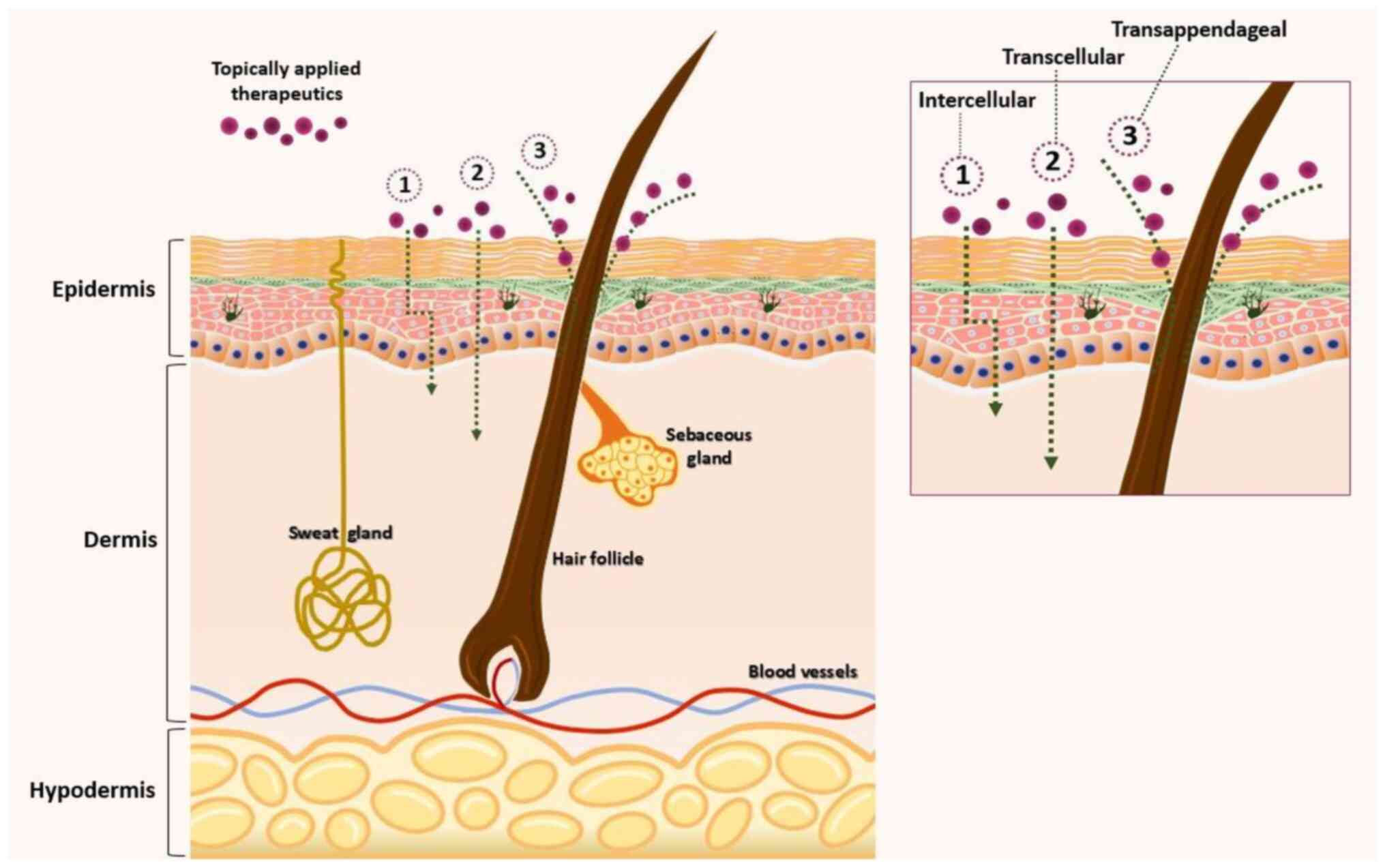
Intradermal penetration pathways
As shown in Fig.
2, drug transport across intact skin involves three main
pathways: the transcellular route (traversing corneocytes,
preferred by hydrophilic or polar compounds), the intercellular
route (across the lipid matrix, mostly used by lipophilic
molecules) and the transappendageal route (through hair follicles
and sweat glands) (22). While
the type of pathway depends on the physicochemical properties of
the drug, the intercellular path remains the preferred route for
entry into the skin. However, the importance of transfollicular
routes in skin penetration, especially for TAC, cannot be ignored
(22,32).
Physicochemical drug properties
In addition to skin barriers, it is well known that
the physicochemical properties of the permeant agent are equally
important in determining its penetration into the skin. Since drug
movement across the skin primarily occurs by diffusion via the
intercellular lipid matrix, parameters that favor topical delivery
include high pharmacological potency, low molecular weight (MW;
<500 Da) and moderate lipophilicity (octanol-water partition
coefficient-logP-between 1 and 3) (14,22,33). Thus, larger and highly
hydrophilic or lipophilic molecules, including topical calcineurin
and mTOR inhibitors, can hardly overcome the SC barrier and reach
deeper skin compartments due to their size and solubility
profiles.
Skin disease
Topical drug absorption is mainly governed by the
pathophysiological status of the skin, as specific dermatoses can
cause distinct changes in skin barrier elements and permeability
(22,30,34). For instance, in conditions such
as psoriasis and AD, impaired barrier function due to SC
disruptions, increased transepidermal water loss and alterations in
lipid composition can enhance drug diffusion in both lesional and
nonlesional skin areas (30,35,36). However, the barrier resistance in
diseased skin is not always compromised and may allow only modest
increases in penetration compared with normal skin. This suggests
that an effective barrier function can still be maintained in
certain disease stages (22,30,37). Indeed, chronic psoriatic plaques
may retain some barrier properties that limit drug absorption,
while acute lesions might exhibit enhanced permeability (38,39). This variability underscores the
need to exploit disease-specific alterations when designing drug
delivery systems tailored to particular skin conditions.
Topical calcineurin and mTOR inhibitors in
inflammatory dermatoses
Given the complexities of topical drug delivery,
special emphasis should be placed on understanding the
dermatokinetic profiles of challenging pharmaceuticals, such as
topical calcineurin and mTOR inhibitors. This will provide the
rationale for developing advanced topical formulations that can
address the limitations of conventional dosage forms and
effectively deliver these macromolecules to their target sites in
the inflamed skin.
Current challenges and limitations of
topical calcineurin inhibitors
TAC and PIM are grouped, along with cyclosporine A
(CsA), in the class of calcineurin inhibitors. The two TCIs
interact with the cytosolic protein macrophilin-12 (FK506-binding
protein; FKBP12) to form inhibitory complexes that block the
calcineurin-mediated dephosphorylation of the nuclear factor of
activated T-cells, thereby suppressing T-cell activation and
cytokine production (6,10,11). In addition, TCIs can inhibit mast
cell and neutrophil activation (6,11,40). Notably, PIM prevents mast cell
degranulation and release of pro-inflammatory mediators without
affecting LCs (6,41).
Topical preparations of TCIs are currently available
in two forms in clinical practice: TAC 0.1% or 0.03% ointment for
treating moderate to severe AD and PIM 1% cream for mild to
moderate atopic eczema (6,11). Of note, the therapeutic success
of TCIs in AD, along with their minimal risk of skin atrophy and
systemic absorption, have motivated their off-label use as
steroid-sparing agents in multiple inflammatory dermatoses,
including non-atopic dermatitis, psoriasis and vitiligo (5,11,42-45).
However, while TCIs have shown efficacy in managing
AD flares, their topical application faces a number of limitations
(46-48). Specifically, the low and variable
skin absorption of the commercial formulation of TAC ointment
cannot ensure adequate delivery to its target site in the deeper
skin layers, which can ultimately limit therapeutic outcomes
(46,47). In addition, its greasy nature and
sticky sensation, combined with application-site reactions such as
irritation, discomfort and/or pruritus, may compromise patient
compliance and satisfaction (6,48,49).
Furthermore, therapeutic outcomes with TCIs appear
to vary across different body areas and disease states. While
facial, genital and inverse psoriasis can respond well to TCIs,
refractory cases of the most common plaque-type psoriasis have
already been reported, particularly in difficult-to-treat areas
such as the scalp (2,11,12,50-52). Additionally, topically applied
TAC is often ineffective in thick psoriatic plaques unless used
under occlusion (10,53). Indeed, drug absorption in
hyperkeratotic psoriatic skin presents challenges. Although key
barrier elements, including TJ functionality and lipid composition,
are compromised, epidermal hyperplasia and excessive hyperkeratosis
can hamper penetration through the follicular routes. This may
explain the relative ineffectiveness of TCIs in treating plaque
psoriasis (12,38,39).
Considering the challenges and limitations
associated with the topical use of TAC, the skin penetration
behavior of TCIs requires special attention. The dermatokinetic
profiles of topically applied TAC and PIM are briefly covered in
the following section.
Skin pharmacokinetics: Intradermal
delivery of topical calcineurin inhibitors
This section focuses on the dermatopharmacokinetic
profiles of TCIs by summarizing the evidence from experimental and
clinical studies exploring their penetration and biodistribution in
normal and inflamed (psoriatic and atopic) skin.
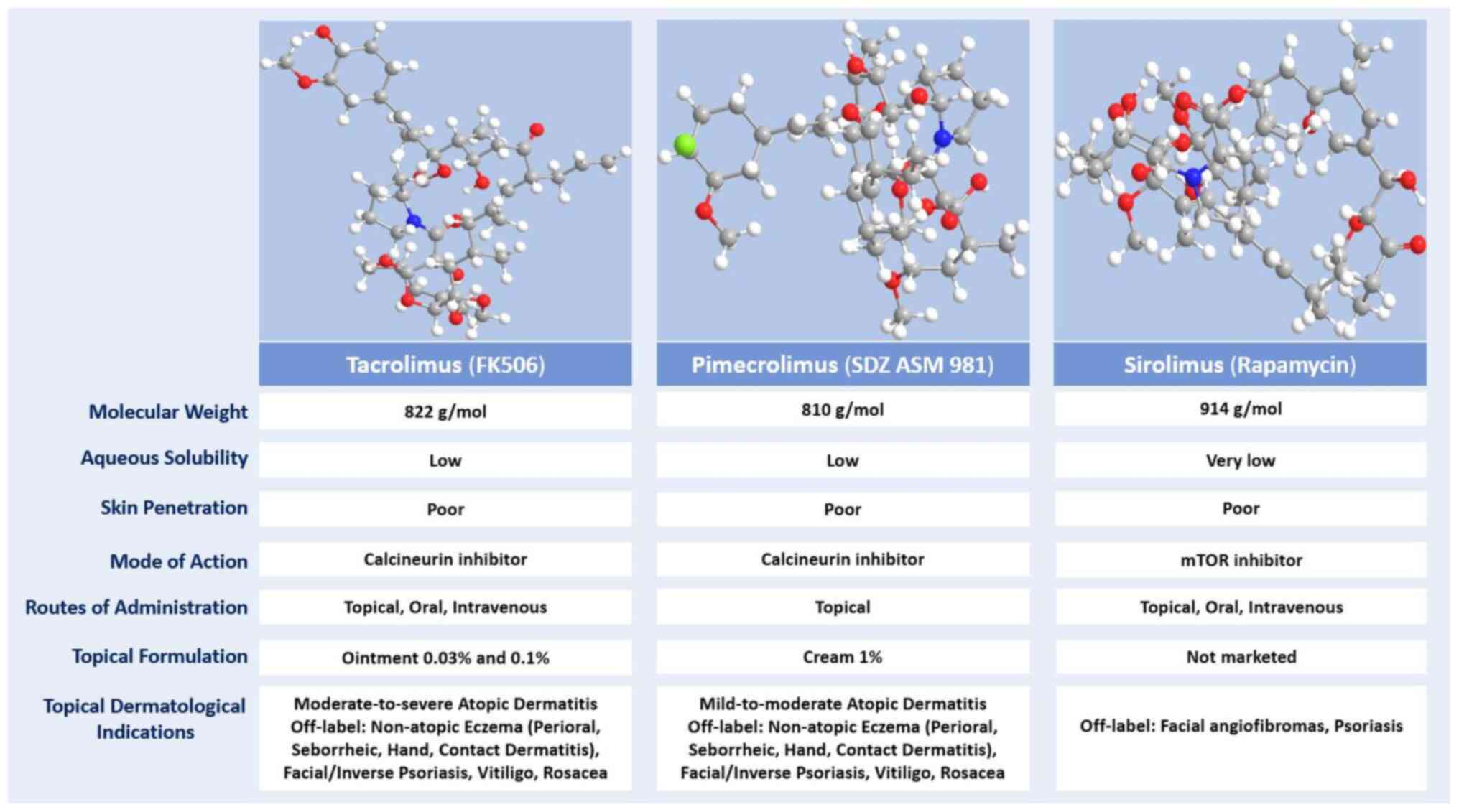
TAC (FK506), the prototype of macrolactam
immunomodulators, is a natural metabolite produced by the
fungus-like bacterium Streptomyces tsukubaensis. Classified
as a lipophilic macrolactone (logP=3.2; MW 822 Da), TAC is a
crystalline compound that is unstable in alkaline conditions and
nearly insoluble in water, but it dissolves in organic solvents
such as methanol, ethanol, acetone and chloroform (10,54,55). The newer ascomycin derivative PIM
(SDZ ASM 981) is isolated from Streptomyces hygroscopicus
var. ascomyceticus. This macrolide lactone has a molecular
weight of 810 Da and is more lipophilic than TAC (logP=6.99)
(10,56-58) (Fig. 3).
The large size and high lipophilicity of TCIs
indicate a greater affinity for the skin compartment with low
potential for percutaneous absorption into the bloodstream,
offering a more skin-selective pharmacological profile compared
with topical corticosteroids (45). However, these properties remain
not ideally suited for topical use (6,14,16,58).
In this respect, previous studies have explored the
ability of conventional topical TAC formulations to penetrate into,
and permeate through, healthy or inflamed skin using preclinical
models and human volunteers. Early ex vivo findings
demonstrated that TAC penetrates more readily in intact human skin
than topical CsA (MW 1202 Da), partly because of its higher potency
and 30% smaller molecular size (59-62). However, skin absorption remains
highly variable and usually low depending on skin barrier integrity
and TAC concentration (10).
Dermally applied TAC has been shown to reside mostly
in the SC without entering deeper layers in healthy skin (34,61,63). SC integrity represents a crucial
factor limiting TAC transport within the skin. Following a 24-h
application on ex vivo human skin, ~20-25% of TAC was found
in the SC with no drug penetrating deeper skin layers (34). Similarly, in human cadaver skin
topically treated with TAC for 24 h, only 13-23 and 0.5-1.1% of the
total dose could reach the epidermis (including the SC) and dermis,
respectively (61). In a series
of ex vivo studies, topical TAC could hardly penetrate the
intact human skin, whereas flux rates were 7-fold higher after the
SC was removed (63).
Intradermal TAC delivery enhanced as the formulation strength
increased, albeit with no evidence of depot effects (61,63). Notably, occlusion could not
affect skin absorption of TAC (60).
While barrier dysfunction in diseased skin may
theoretically enhance drug penetration, earlier ex vivo
studies demonstrated similar amounts of topically applied TAC in
the viable epidermis and dermis of both normal and inflamed porcine
skin (64). Penetration kinetics
of TAC ointment across inflamed skin were further explored in AD
patients. In barrier-disrupted human skin, TAC penetrated
efficiently into inflamed lesions to control mild to moderate AD
relapses. TAC levels beneath the SC increased with prolonged
treatment but declined with increasing skin depth. Despite drug
depot formation, only a minor proportion (3%) of TAC concentration
was sustained 7 days post-treatment, indicating short-term
retention of minimal drug amounts in upper skin layers (65).
Preclinical ex vivo and in vivo
studies on the penetration behavior of PIM in normal skin yielded
comparable results. When applied to human and porcine skin under
non- and semi-occlusive conditions, >93% of PIM remained
unabsorbed creating a depot on the skin surface, with minimal
amounts entering the epidermis. PIM levels in the SC of minipig
skin were far higher in vivo compared with the viable
epidermis and dermis (2.2 vs. 0.21 and 0.14% of the applied dose,
respectively), while dose proportions of 3.1 and 2.9% accumulated
ex vivo in the human epidermis (including the SC) and
dermis, respectively. Despite drug depot formation in the SC, skin
deposition of PIM decreased with increasing treatment duration and
was not sustained after repeated dermal application for up to 13
weeks. In fact, only 0.35% of topically applied PIM was retained in
the dermis 10 days post-treatment and it was completely eliminated
after a wash-out period of 4 weeks (66).
Despite structural and functional similarities, TCIs
display distinct differences in skin penetration dynamics. The
higher lipophilicity of PIM (logP=6.99) vs. TAC (logP=3.2) results
in a greater affinity for the skin tissue during penetration and
thus lower systemic exposure, offering a more favorable safety
profile (6,56,67). However, preclinical studies on
human and animal models showed that both TCIs diffuse similarly
across the skin (58,64), corroborating in vitro
findings (67). In all models
studied, both TAC and PIM could cross the SC to a similar degree,
achieving comparable levels in the viable epidermis and dermis
under normal and inflamed skin conditions (58,64). Notably, permeation of TAC beyond
human and animal skin was consistently higher compared with PIM,
regardless of the skin origin (animal or human) and formulation
composition. Moreover, absorption of both TCIs beyond inflamed skin
was up to 6-fold higher compared with healthy skin. Skin
inflammation seemed to enhance only the transdermal permeation of
TCIs without affecting their skin deposition (64).
Although damaged or inflamed skin may exhibit
increased permeability, potentially leading to systemic exposure,
accumulated evidence from adult and pediatric pharmacokinetic
studies has consistently shown very low or undetectable serum
concentrations of both TCIs following dermal application, far below
the levels required for systemic immunosuppression (6,45,49,58,65,68,69). Percutaneous permeation of PIM in
AD patients remains minimal, even with prolonged or extensive use
(58,68). Additionally, systemic absorption
of TAC appears to decrease with treatment duration as the skin
heals and barrier integrity is restored, indicating a favorable
long-term safety profile (45,65,69).
As reported by skin metabolism studies, no evidence
supporting the biotransformation of topical TAC and PIM has been
observed in human and porcine skin models. This suggests a low
potential for intradermal interactions that could influence the
in vivo skin bioavailability of TCIs (60,63,66).
Emerging prospects of topical mTORIs for
psoriasis and atopic dermatitis
Sirolimus (rapamycin) and its derivatives (rapalogs)
belong to a novel class of macrolactam immunomodulators. These
agents exert potent antiproliferative and immunosuppressive effects
by inhibiting mTOR, a multifunctional serine/threonine kinase of
the PI3K family. Similar to TAC, mTORIs form initial complexes with
the cytosolic FKBP12 protein but then primarily inhibit mTOR
complex 1. As a result, mTORIs induce cell cycle arrest at the
G1 to S transition, while calcineurin inhibitors block
the cell cycle at an earlier phase (G0/G1)
(1,5,10,70).
While the newer macrolides, sirolimus and
everolimus, have currently no approval for inflammatory dermatoses,
the aberrant activation of the PI3K/Akt/mTOR axis has already been
involved in the pathogenesis of psoriasis and AD (8,71-76). The upregulated PI3K/Akt/mTOR
pathway in psoriatic epidermis appears to play a key role in
disease initiation and progression by mediating Th1/Th2/Th17
imbalance, secretion of pro-inflammatory mediators, abnormal
keratinocyte proliferation and differentiation and
neovascularization (8,74-76). Although less studied in AD, the
PI3K/Akt/mTOR cascade has recently been implicated in regulating
epidermal barrier formation by influencing filaggrin processing and
lipid synthesis (71-73).
Systemic mTORIs, especially everolimus, have shown
potential in managing both psoriasis and AD (77-81). Despite limited evidence to
support the beneficial effects of topical mTORIs on inflamed skin,
cutaneous mTOR blockade appears promising in immune-mediated skin
diseases that require modulation or control of inflammation,
epidermal and vascular proliferation and keratinocyte
differentiation (4).
Indeed, topically applied sirolimus has already
demonstrated efficacy in a small clinical trial on psoriasis
patients (82). Although
preclinical data from contact dermatitis (CD) animal models were
initially not promising (83-85), subsequent studies in skin models
of AD and psoriasis indicated the broad anti-inflammatory effects
of dermally applied sirolimus both in vitro and in
vivo (82,86-92). Traditional topical formulations
of sirolimus, investigated in animal and human inflamed skin, have
shown potential for addressing several clinical, histological and
molecular aspects of cutaneous inflammation (82,86-90). Table I summarizes the key findings from
studies exploring the effects of conventional topical sirolimus
formulations on inflamed skin.
 | Table ISummary of experimental and clinical
studies on the biological effects of conventional topical
formulations of sirolimus on inflamed skin. |
Table I
Summary of experimental and clinical
studies on the biological effects of conventional topical
formulations of sirolimus on inflamed skin.
| Authors, year | Topical SIR
| Study setup
| Key findings | (Refs.) |
|---|
| Formulation type
(strength) | Skin
model/disease | Type (species) |
|---|
| Meingassner et
al, 1992 | Conventional
(0.13%, 1.2%) | DNFB-induced
CD | In vivo
(Porcine) | No effect on skin
erythema and vascular changes. | (83) |
| Meingassner et
al, 1992 | Conventional
(0.4-3.6%) |
PMA/Calcimycin-induced ICD
OXA/DNFB-induced ACD | In vivo
(Murine, Porcine) | Moderate decrease
in ICD inflammation (↓ edema by 30%).
No effect on ACD inflammation. | (84) |
| Duncan et
al, 1994 | Conventional
(0.02%, 2%) | DNFB-induced
DTH | In vitro
(HEK)
In vivo (Murine) | Stronger
keratinocyte growth inhibition than CsA in vitro.
No effect on skin erythema and T-cell infiltration in
vivo. | (85) |
| Ormerod et
al, 2005 | Solution (2.2%
followed by 8%) | Plaque Pso | In vivo
(Human subjects) | Reduced total Pso
clinical score; no effect on erythema and thickness of psoriatic
plaques.
Decreased proliferating (Ki-67) and CD4+ T-helper cells; no effect
on CD8+ cytotoxic T cells, macrophages and LCs. | (82) |
| Yang et al,
2014 | Ointment
(0.2%) | Dfb-induced AD | In vivo
(Murine) | Improved clinical
features (erythema, edema, erosion, scaling, dryness) and reduced
itch and scratching behavior.
Lesional skin: ↓ epidermal thickness; ↓ dermal inflammatory cell
infiltration (including mast and T cells); ↓ IL-4, IL-13, TSLP and
NGF; Normalization of total mTOR and p-mTOR levels.
Serum: ↓ IgE levels. | (86) |
| Jung et al,
2015 | Cream
(0.04-4%) | TNCB-induced
AD | In vivo
(Murine) | Improved clinical
signs (erythema, edema, erosion, dryness).
Lesional skin: ↓ epidermal hyperplasia; ↓ dermal edema and cell
infiltration (including eosinophils and mast cells); ↓ IL-4 and
IFN-γ.
No effect on serum IgE. | (87) |
| Bürger et
al, 2017 | Ointment (1%) | IMQ-induced
Pso | In vivo
(Murine) | Reduced clinical
erythema and scaling.
↓ Epidermal thickness and neutrophilic microabscesses; ↓ immune
cell infiltration and neovascularization.
Normalization of keratinocyte proliferation and differentiation
markers (↓ Ki-67 and KRT6; no effect on KRT10).
Partly restored skin barrier markers (↑ caspase-14, involucrin and
loricrin).
Reduced mTOR activity (↓ Rps6 and p-mTOR).
Trend for reduced immune cell migration to lymph nodes.
Serum: ↓ Total leukocytes, neutrophils and monocytes. | (88) |
| Gao et al,
2018 | Conventional (3
mg/ml) | TNF-α-stimulated
cell model
IMQ-induced Pso | In vitro
(HEK; HaCaT)
In vivo (Murine) | Restored cell
skeleton and reduced cell proliferation in vitro.
Decreased epidermal thickness in vivo.
Upregulation of TPMs in vitro and in vivo.
Reduced ERK1/2 and mTOR activity in vitro and in
vivo. | (89) |
| Kim et al,
2021 | Conventional (5
mg/ml) | IMQ + TCDD-induced
Pso | In vivo
(Murine) | Improved clinical
features (erythema, thickness, scaling).
Decreased epidermal thickness.
↓ TNF-α, IL-6, IL-17A, IL-22 and IL-23.
Decreased AHR and increased autophagy-related factors.
Normalization of oxidative stress markers (NOX-2/4,
Nrf2).
Reduced NF-κB signaling (↓ P65 protein). | (90) |
From a clinical perspective, dermally applied
sirolimus, although displaying moderate efficacy in controlling
inflammation in irritant CD models, could markedly improve the
clinical features of psoriatic and atopic skin lesions while
reducing itch and scratching behavior (82,86-88,90).
Laboratory analyses of psoriatic and atopic lesional
skin topically treated with sirolimus revealed several biological
effects. These include reduced epidermal thickness and neutrophilic
microabscesses and decreased dermal neoangiogenesis and
inflammatory cell infiltration (comprising T lymphocytes,
eosinophils and mast cells). Sirolimus also normalized markers of
keratinocyte proliferation and differentiation, such as Ki-67 and
keratins. Notably, this compound partially restored skin barrier
integrity, as indicated by the increased expression of caspase-14,
involucrin and loricrin and further contributed to regulating
cellular homeostasis, by modulating cell structure, autophagy and
oxidative stress, through the normalization of tropomyosins and
related markers (82,86-90).
Furthermore, topical sirolimus could target multiple
signaling pathways involved in skin inflammation, including mTOR,
ERK 1/2 and NF-κB. It also achieved a broad downregulation of key
inflammatory mediators, such as TNF-α, interferon-γ (IFN-γ),
interleukins (IL-4, IL-6, IL-13, IL-17A, IL-22 and IL-23) and
thymic stromal lymphopoietin (TSLP) (86-90). In addition, topical sirolimus
showed potential to impact pruritus onset and severity by reducing
nerve growth factor (NGF) levels (86).
Beyond local effects, a trend towards reduced
migration of immune cells, including lymphocytes, monocytes,
neutrophils, LCs, myeloid and plasmacytoid dendritic cells (DCs),
to draining lymph nodes has been observed (88). This suggests that sirolimus may
counteract the complex feedback loop sustaining chronic
inflammation in the atopic and psoriatic skin microenvironment
(93,94). Additionally, topical sirolimus
appeared to exert extracutaneous effects, as evidenced by decreased
serum levels of IgE, total leukocytes, neutrophils and monocytes
(86,88). This may indicate its potential to
normalize serum biomarkers involved in the so-called 'atopic or
psoriatic march', thereby mitigating systemic inflammation and
related comorbidities (94).
These findings indicate that locally applied
sirolimus may have diverse biological effects beyond its known
antiproliferative and immunosuppressive properties. Further
research is warranted to elucidate the underlying mechanisms and
the precise impact of topical sirolimus on the inflammatory and
structural components of the psoriatic and atopic skin
microenvironments.
Skin pharmacokinetics: Intradermal
delivery of topical mTORIs
Sirolimus is a lipophilic macrolactone initially
derived from the soil fungus-like bacteria Streptomyces
hygroscopicus found in Rapa Nui (Easter Island). In its natural
form, sirolimus is a white crystalline solid with a molecular
weight of 914 Da, a partition coefficient (logP) of 5.8 and low
water solubility (95,96) (Fig. 3). As a result, sirolimus is
difficult to formulate into traditional vehicles for dermal
application. Currently, topical formulations of sirolimus are not
commercially available and are typically prepared from crushed
tablets, solution, or powder forms incorporated into ointment,
cream, or gel vehicles (4,70,96,97). In addition, the physicochemical
properties of sirolimus appear suboptimal for topical use, limiting
its capacity to efficiently penetrate the epidermal barrier and
reach deeper skin compartments (17).
Thus, understanding the dermatokinetic profile of
locally applied sirolimus is important for the development and
optimization of topical formulations that ensure maximum skin
bioavailability. In this context, an early study by Ormerod et
al (82) evaluated the
penetration of topical sirolimus in ex vivo barrier-impaired
and in vivo normal human skin after a single application of
2.2 and 8% solutions. As the results showed, topical sirolimus
could diffuse into human skin with no evidence of systemic
absorption. Penetration depth seemed to enhance as the formulation
strength increased.
Several topical preparations of sirolimus have also
been explored to elucidate factors influencing intradermal
penetration, such as formulation composition and skin barrier
integrity (98-100). Tanaka et al (98) compared the skin absorption of
0.2% sirolimus formulated in gel and ointment forms using an in
vitro three-dimensional (3D) cultured human skin model. Their
findings demonstrated that the hydrogel facilitated greater skin
accumulation than the lipophilic ointment (1,360 pg/mg vs. 680
pg/mg, respectively) with lower irritation.
In an in vivo murine study, the
dermatokinetics of sirolimus were investigated utilizing different
formulations (gel, cream and lotion) as delivery vehicles. Overall,
superior skin bioavailability was observed with topical application
than oral administration of sirolimus. When locally applied,
sirolimus displayed dose-dependent skin penetration, showing
enhanced absorption and retention when applied as a gel or cream
but was less adherent to the skin when incorporated into a lotion
(99).
In a series of in vitro (on synthetic
membranes) and ex vivo (on human skin) studies, Le Guyader
et al (100) also
compared the penetration of sirolimus hydrogel, cream and ointment
formulations, demonstrating that skin flux and deposition were
higher with the hydrogel and enhanced with increased formulation
strength. This study emphasized the importance of the formulation
type and using sirolimus solubilized (rather than dispersed) at
close-to-saturation concentrations to maximize skin
bioavailability.
Penetration of topically applied sirolimus was
further evaluated in barrier-disrupted ex vivo human skin.
As expected for a hydrophobic macromolecule such as sirolimus,
absorption into the intact SC was minimal after topical application
as a solution or gel. However, in barrier-impaired skin, sirolimus
could cross the entire SC and reach deeper epidermal layers. The
penetration depth increased as the skin barrier damage enhanced
(101).
These findings suggest that the skin absorption and
deposition of topical sirolimus depend not only on dosage but also
on the administration route and the nature of the
formulation/vehicle (hydrophilic vs. lipophilic; hydrogel >
cream > ointment). The hydrogel appears to be the preferable
vehicle in terms of release and penetration profiles. Notably, the
integrity of both the SC and TJ barriers seems to have a greater
impact on sirolimus penetration into the skin than the formulation
vehicles.
Regarding safety, adverse effects of topical
sirolimus appear to be rare and mainly consist of mild
application-site reactions that are easily managed without
requiring treatment withdrawal. Systemic absorption of locally
applied sirolimus is minimal and rarely detectable, independent of
the formulation strength (4,82,97).
Novel strategies for skin inflammation:
Nanocarrier-based topical delivery of calcineurin and mTOR
inhibitors
The use of nanoproducts for encapsulating bioactive
compounds offers a novel strategy to address unfavorable
physicochemical drug properties, such as lipophilicity or
hydrophilicity, high molecular weight, stability and
bioavailability. This approach can provide enhanced skin
penetration with targeted and controlled drug release, ultimately
improving the efficacy and safety profiles of topical therapeutics
(20,21,46).
As aforementioned, due to the complexities involved
in intradermal delivery of challenging pharmaceutics, such as
calcineurin and mTOR inhibitors, more advanced nanocarrier-based
prospects are being explored to improve the penetration kinetics of
these 'hard-to-formulate' macromolecules within the skin. This
section summarized the experimental evidence on various nanovectors
investigated as prospective carriers for topical TAC, PIM and
sirolimus, focusing on research specifically conducted in psoriasis
and AD skin models.
Nanocarrier-based skin delivery of
topical calcineurin inhibitors
Several types of nanosystems have been explored to
overcome the limitations of intradermal penetration and effectively
deliver topically applied TAC to its target sites within the viable
skin.
Lipid-based nanosystems
Lipid-based nanoformulations have been widely
studied for delivering TAC in both normal and inflamed skin.
Exploring this concept, Kovačević et al (102) fabricated nanostructured lipid
carriers (NLCs) for TAC encapsulation after screening 20 selected
lipids. The prepared NLCs, composed of mixed lipid cores, displayed
favorable physicochemical properties and entrapment efficiency (EE)
of nearly 99%. The use of polyethylene glycol (PEG)-free
stabilizers ensured optimal particle stability and prevented
irritation effects (102).
TAC-loaded solid lipid nanoparticles (SLNs) and NLCs, comprising
stearic acid (SA) or beeswax as solid lipids, exhibited
satisfactory physicochemical features, with beeswax-based
nanoparticles (NPs) providing superior loading capacity (3.3 and
2.9% for NLC- and SLN-Beeswax/TAC, respectively vs. 2.7 and 2.3%
for NLC- and SLN-SA/TAC, respectively). No incompatibility between
TAC and lipid components was observed (103).
In this context, Wang et al (104) formulated TAC-loaded SLNs,
demonstrating that in vitro drug release was fast from free
TAC solution (96% at 8 h) but sustained from TAC-SLNs (55 and 86%
at 8 h and 72 h, respectively). In ex vivo studies, SLNs
achieved enhanced skin penetration and deposition of TAC in healthy
skin compared with the commercial ointment.
Kang et al (18) developed thermosensitive SLNs for
the targeted release of TAC in response to thermal variations
within the skin. The designed TAC-SLNs could cross the SC (32°C) as
intact particles and release TAC in the dermis (37°C) without
deeper diffusion. Ex vivo studies showed
temperature-dependent skin penetration; TAC-loaded SLNs failed to
traverse the skin at higher temperatures. While TAC was mainly
confined to the SC, SLNs delivered greater drug amounts into deeper
skin layers (up to 300-450 μm depth) compared with the
marketed ointment (undetectable below 150 μm). The TAC-SLN
nanoformulation was more tolerated in vivo, showing only
occasional mild erythema, whereas the commercial ointment induced
severe erythema.
Chitosan (CS) has been employed in the design of
NPs (105-108). Khan et al (105) fabricated TAC-loaded SLNs to
investigate the effects of CS coating and gel formulation on skin
delivery of TAC. In vitro drug release was faster from
uncoated SLNs and sustained from both CS-coated SLNs and SLN-gels.
Ex vivo studies showed that both SLNs and SLN-gels achieved
similar intradermal penetration, but the SLN-gel resulted in higher
retention of TAC in normal skin. Notably, the CS coating did not
markedly affect drug retention in the skin (105).
Furthermore, NLCs, considered successors to SLNs,
displayed controlled release patterns in vitro (10% of
TAC/24 h) and facilitated skin deposition of 61.7% of dermally
applied TAC ex vivo (109). Minimal toxicity to murine
fibroblasts was observed. Notably, this study also explored a novel
combination therapy using NLCs for co-delivering TAC and TNFα small
interfering RNA (siRNA) in psoriatic skin. This system exhibited
synergistic anti-psoriatic effects in vivo, preventing the
onset of erythematosquamous lesions and achieving greater TNFα
reduction (7-fold) than TAC-loaded in NLCs and commercial vehicles
(2.5- and 2-fold TNFα reduction, respectively) (109). In addition, emulsions
containing TAC-loaded NLCs outperformed the marketed ointment in
terms of TAC penetration and deposition in ex vivo skin
(NLCs: 8.6 mg/cm2; marketed ointment: 5.4
mg/cm2) (110).
CS-coated NLCs prepared for co-loading TAC and
clobetasol propionate (CP) provided an EE >90% (98% for TAC;
92.8% for CP). In vitro analysis demonstrated that
co-encapsulated NLCs released TAC more slowly than CP. While CS
coating favored TAC retention in the SC, TAC failed to diffuse
beneath the SC when loaded alone in NLCs, regardless of the
coating. Co-encapsulation of CP, especially in CS-coated NLCs,
enhanced the capacity of TAC to penetrate deeper skin layers
(106).
Vesicular nanocarriers have also gained attention
in this field (111-117). An in vivo murine study
first reported that a topical liposomal lotion enhanced cutaneous
bioavailability, resulting in 9-fold higher TAC skin levels
compared with intravenous administration. This formulation also
showed superior suppression of skin inflammation than systemic TAC
vehicles, either liposomal or traditional, as evidenced both
clinically (no erythema or edema) and pathologically (reduced cell
infiltration) (111).
Similarly, liposomal encapsulation improved the skin flux and
deposition of TAC ex vivo with respect to free TAC.
Liposomal and conventional TAC vehicles both showed similar
anti-inflammatory activity in vivo in CD skin models
(112).
In ex vivo comparison studies by Li et
al (113,114), both ethosomal and traditional
liposomal systems achieved higher epidermal accumulation of TAC
than the commercial ointment. While classic liposomes facilitated
the highest SC deposition, ethosomes delivered greater amounts of
TAC to deeper epidermal layers compared with liposomal and
conventional vehicles. The in vivo capacity to reduce
AD-like skin inflammation was in the order of ethosomes >
classic liposomes > dexamethasone cream > commercial TAC
ointment.
Using transfersomes (TFs), topical TAC was
effectively delivered into normal, atopic and psoriatic skin.
Transfersomal and liposomal carriers both exhibited improved
penetration profiles ex vivo and in vivo in terms of
TAC release, deposition and retention in the skin compared with
commercial formulations. TFs displayed enhanced delivery
properties, as evidenced by the penetration depth and amount of TAC
in both ex vivo and in vivo viable skin. Notably, TFs
were superior to classic liposomes and conventional vehicles in
vivo in restoring the clinical and pathological features of
atopic and psoriatic skin (115-117).
Liquid crystalline nanoparticles (LCNPs) have also
been used for topical TAC delivery, providing controlled drug
release and enhanced skin penetration and retention. Ex vivo
studies showed that LCNPs increased the skin concentration and
retention of TAC by 6- and ~3-fold, respectively, compared with
free TAC. When loaded into LCNPs, TAC exhibited greater
anti-psoriatic efficacy in vivo, as supported by clinical
[Psoriasis Area Severity Index (PASI) score] and pathological (skin
thickness and inflammatory infiltration) evaluations. LCNP
formulations, with or without oleic acid, were equally effective in
repairing psoriatic skin (118,119).
When comparing lipid-based nanoformulations, in
vitro TAC release at 24 h was 34, 62, 65 and 69% from LCNP,
SLN, NLC and liposomes, respectively. LCNP displayed slow, constant
release patterns, while SLN, NLC and liposomes showed an initial
burst followed by sustained release. LCNP, SLN and NLC increased
TAC skin bioavailability ex vivo by 2.5-, 2- and ~2-fold,
respectively, compared with marketed formulations. Penetration
depth decreased in the order of SLN > NLC > LCNP >
liposomes, with SLN and NLC reaching deeper skin strata than the
commercial ointment. The in vivo anti-psoriatic efficacy was
as follows: NLC=SLN > LCNP > commercial ointment >
liposomes. Unlike conventional vehicles, all nanovectors,
especially liposomes, achieved lower transepidermal water loss
values without causing skin irritation (120).
Lipid NPs (LN) and modified nanolipid carriers
(MNLC) have been exploited to deliver TAC in normal and atopic
skin. A series of in vitro and ex vivo studies showed
improved profiles in terms of skin release, penetration and
deposition of TAC with all studied nanovehicles compared with
traditional vehicles. When delivered by LN or MNLC in vivo,
TAC achieved higher levels in total skin, especially in the deeper
epidermis and dermis, without entering the bloodstream. While
nano-based and marketed formulations showed similar occlusive and
hydration effects in vitro and in vivo, the former
achieved improved skin moisture restoration ex vivo, as well
as earlier and superior AD control in vivo. Neither skin
irritation nor histological changes occurred in intact or inflamed
skin with all tested NPs, even after repeated application (48,121-124). Additionally, when TAC was
loaded into natural rhamnolipid-based NPs, no cytotoxicity was
observed in human dermal fibroblasts (125).
Microemulsions (MEs) and nanoemulsions (NEs) have
also been employed as topical vehicles for TAC (32,126-130). In vitro, MEs released
greater amounts of TAC at higher rates than the commercial ointment
(127,128). In ex vivo studies, MEs
improved the cutaneous bioavailability of TAC, as indicated by the
higher drug concentrations in animal and human skin compared with
the marketed ointment (126-129). The latter delivered 3-fold
lower TAC amounts in the deeper SC than the lecithin-based MEs
(128). Although MEs and
conventional vehicles carried similar amounts of TAC in the viable
epidermis (below the SC), MEs delivered 9-14% of TAC into the
dermis, whereas only 6.5% could reach the dermis when applied as a
traditional ointment (126).
Based on the formulation type, TAC retention in ex vivo skin
decreased in the order of conventional solution > ME-based cream
> commercial ointment (127). MEs were well tolerated in
vitro and in vivo, with no observed toxicity or skin
irritation (126-128).
ME systems have already shown therapeutic potential
for AD (127,129). As reported by Wang et al
(129), TAC-loaded MEs achieved
enhanced control of AD in vivo compared with marketed
formulations, as demonstrated by clinical and laboratory
investigations, corroborating previous findings reported by Lalan
et al (127).
Building on the benefits of MEs, a Kalonji
oil-based NE system (NE and NE-gel) was developed for the local
delivery of TAC to psoriatic plaques. In vitro TAC release
followed a slower, sustained pattern from NEs compared with free
TAC gel. With respect to the latter, NEs displayed improved ex
vivo penetration kinetics in terms of total delivery and
retention of TAC in the SC and viable epidermis. In vivo,
the Kalonji oil plus TAC-loaded NE gel showed greater
anti-psoriatic activity than the commercial ointment, as assessed
by clinical and laboratory evaluations. TAC-loaded NEs exhibited
stronger antiproliferative effects on epidermal cell lines than the
free drug (130).
In a comparative study, Savić et al
(32) fabricated NLCs and NEs to
explore their potential as topical carriers of TAC. In vitro
TAC release from NLCs was superior to that from NEs and marketed
formulations. In ex vivo studies, both NLCs and NEs achieved
greater TAC deposition in the SC than the commercial ointment, with
the highest follicular drug uptake obtained with NLCs followed by
NEs and the ointment. The latter resulted in higher transdermal
permeation of TAC compared with nanovehicles. Overall, NLCs proved
superior to NEs for dermal delivery of TAC.
Natural plant agents combined with TAC have been
used as topical antipsoriatic agents. Lipospheres co-loaded with
TAC and curcumin (CUR) displayed a slow release of both cargos
in vitro and compatibility with healthy skin in vivo.
In in vivo studies, the marketed ointment and TAC-CUR
lipospheres were equally effective but superior to betamethasone in
restoring the clinical features of psoriatic skin, such as scaling
and thickness, while the decrease in erythema followed the order of
TAC-CUR lipospheres > commercial TAC ointment > betamethasone
ointment. Among the studied formulations, only TAC-CUR lipospheres
normalized the histological psoriatic changes. Notably, TAC-CUR
lipospheres achieved the strongest suppression of TNFα and IL-22,
while IL-17 inhibition followed the order of TAC-CUR lipospheres ≈
commercial TAC ointment > TAC lipospheres > betamethasone
ointment (47).
Polymer-based nanosystems
Using micellar systems, TAC was successfully
delivered into normal and inflamed skin (131,132). While micelles remained largely
unabsorbed on the skin surface, they facilitated greater skin
deposition of TAC ex vivo compared with marketed
formulations, without permeating beyond human and porcine skin.
Improved penetration profiles were observed in porcine compared
with human skin, indicating the importance of transfollicular
pathways for micelle-mediated delivery (131).
Using X-ray microscopy, the penetration of topical
TAC incorporated into methoxy-poly(ethylene
glycol)-hexyl-substituted polylactide (mPEG-hexPLA) micelles was
investigated in an ex vivo psoriasis murine model. Slightly
increased SC deposition was observed when TAC was formulated in
micelles rather than in the commercial ointment. SC levels of
TAC-loaded micelles gradually increased until saturation and then
decreased due to deeper absorption, while amounts in the viable
epidermis remained stable. This study, although indicating
intercellular delivery routes, also suggested that micelles might
penetrate corneocytes (132).
Similar attempts have been made by Gabriel et
al (133) to design
mPEG-hexPLA-based NPs for the in vivo evaluation of TAC
penetration and anti-psoriatic effects on intact and psoriasiform
murine skin. TAC embedded into mPEG-hexPLA NPs showed enhanced
absorption in inflamed compared with normal skin, reaching ~2-fold
higher levels in psoriatic lesions than with a commercial ointment.
This nanoformulation was superior to CP in lesion clearance and
demonstrated comparable antipsoriatic efficacy to the marketed
ointment. No toxicity was observed after repeated application on
healthy skin.
In a novel approach, Zabihi et al (134) exploited polymer-based
biodegradable NPs composed of poly(lactide-co-glycerol) (PLG) to
deliver TAC into human skin. In ex vivo experiments, PLG-NPs
facilitated 80, 16 and 4% of encapsulated TAC to reach the SC,
viable epidermis and dermis, respectively, resulting in 1.74-,
1.44- and 2-fold higher drug concentrations in the SC, epidermis
and dermis, respectively, compared with the marketed ointment. The
anti-inflammatory efficacy was assessed in vitro on a 3D
reconstructed filaggrin-deficient human skin model by measuring
pro-inflammatory mediators. While the downregulation of IL-2 was
comparable between TAC-PLG-NPs and the marketed product, the NPs
were more effective in suppressing TSLP. No evidence of
cytotoxicity on primary human keratinocytes and fibroblasts was
noted.
Fereig et al (107) developed CS-based NPs for
topical application of TAC on psoriatic skin. In vitro drug
release from CS-NPs followed sustained biphasic patterns. When
delivered by CS-NPs, 82% of dermally applied TAC was accumulated in
ex vivo skin, whereas a 34% total deposition was achieved
with a conventional ointment. CS-NPs facilitated reduced flux rates
into and permeation of TAC beyond the skin compared with a
traditional ointment (24 vs. 61% permeated drug, respectively). In
in vivo experiments, CS-NPs also displayed enhanced skin
deposition of TAC (54.6 vs. 13.8% for the ointment) and achieved
faster and superior control of the clinical and pathological
psoriatic features compared with the ointment. Notably, only
TAC-loaded CS-NPs promoted hair growth in the treated areas.
CS-based NPs were further explored in combination
with nicotinamide (NIC) or hyaluronic acid (HA) to transfer TAC
into atopic skin (108,135). Under normal ex vivo and
AD-like in vivo conditions, the synergistic effect of NIC
and CS achieved greater TAC concentrations in total skin compared
with the commercial ointment. NIC-CS-NPs showed superior in
vivo anti-AD activity compared with the marketed formulation,
despite containing equal or lower TAC doses, as confirmed by
clinical, pathological and molecular analyses (108). HA coating ensured controlled
and sustained in vitro release patterns, while enhancing TAC
deposition and retention beneath the SC in ex vivo skin.
HA-covered NPs exhibited in vivo greater anti-AD efficacy
than both the uncovered CS-NPs and the commercial TAC ointment,
based on clinical and laboratory evaluations (135).
NPs prepared by HA and cholesterol (Chol)
conjugations in a NIC solution (NIC-HA-Chol NPs) synergistically
enabled deeper penetration and enhanced deposition of TAC in ex
vivo and in vivo studies on intact skin compared with
the commercial ointment. HA-Chol-NPs, with or without NIC, showed
improved drug uptake in HaCaT cells (136). Further evaluation showed that
the NIC-HA-Chol nanoformulation achieved 2.4- and 2.5-fold higher
TAC permeation and retention in ex vivo psoriatic skin,
respectively, compared with the marketed product. The in
vivo anti-psoriatic efficacy of the studied NPs, assessed via
the PASI score and epidermal thickness, was comparable to CP but
superior to the marketed ointment. The NIC-HA-Chol NPs exhibited
enhanced cellular uptake and strong anti-proliferative effects in
murine macrophage and HaCaT cells (136,137).
In this context, the dermatokinetics and
therapeutic efficacy of TAC-loaded polymeric core-multishell (CMS)
nanocarriers have been examined in vivo using an AD-like
murine model. While CMS nanoparticles delivered TAC into all skin
layers, they failed to enhance drug deposition compared with the
commercial ointment (mean TAC levels in epidermis and dermis: 36
and 77 ng/cm2 for CMS nanocarriers vs. 93 and 118
ng/cm2 for the ointment, respectively). However, both
CMS nanocarriers and the marketed ointment showed similar efficacy
in improving the clinical and pathological features of atopic skin
(138,139).
Thermoresponsive nanogels offered a new approach
for the topical delivery of TAC in ex vivo human skin.
Comparative studies showed that the commercial ointment
demonstrated superior penetration, particularly in breast vs.
abdominal skin and in barrier-impaired vs. intact skin. After
barrier disruption, nanogels, although primarily confined to the
SC, could enter deeper viable skin. TAC levels increased over time
in ointment-treated sites but remained constant in areas treated
with nanogels. The increase in IL-6 and IL-8 in damaged skin
treated with TAC was attributed to the irritative effects of TAC
and/or the vehicles used. Nanogels and the marketed ointment were
both equally effective in inhibiting T-cell proliferation (140).
As reported by Limón et al, nanostructured
hydrogels could entrap TAC both in interstitial spaces and within
gel fibers, offering a reservoir for controlled release and
protection from degradation. In vitro tests revealed
moderate rates of biphasic drug release. In ex vivo human
skin, TAC hydrogel could cross the SC and remain in the epidermis
and upper dermis with minimal percutaneous permeation. In in
vivo psoriasis models, TAC nano-hydrogel showed superior
efficacy and tolerability compared with a free TAC solution. The
latter achieved only a 9% reduction in skin thickness and failed to
prevent local adverse effects, whereas TAC nano-hydrogel achieved a
50% reduction in psoriasiform hyperplasia without causing
desquamation or hair loss (141).
Inorganic nanosystems
Recently, mesoporous silica nanoparticles (MSNs)
have been employed as carriers for topical TAC, demonstrating
potential in managing AD. TAC-loaded MSNs displayed improved in
vitro release kinetics than a free TAC gel (73 and 55% at 24 h,
respectively) without any evidence of cytotoxicity. Although both
TAC-MSNs and TAC gel showed low transdermal permeation ex
vivo (13 vs. 11%, respectively), a markedly higher amount of
TAC was deposited in the skin with MSNs than with TAC gel (75 vs.
36%, respectively). In in vivo AD-like skin, TAC-MSNs were
more effective than TAC gel in restoring both clinical and
pathological alterations (142).
Hybrid nanosystems
Hybrid nanosystems, emerging as superior
alternatives to conventional nanocarriers, have been used for
delivering TAC in psoriatic and atopic skin (143,144). Wan et al (143) developed a polymer-based ME as a
topical delivery system of TAC. Ex vivo and in vivo
studies on normal and psoriatic skin demonstrated that the studied
ME outperformed the commercial ointment in terms of delivery and
deposition of TAC across the entire skin. This system displayed
in vivo anti-psoriatic activity comparable to CP but
superior to the marketed TAC ointment, as evidenced by clinical
(PASI score) and pathological (epidermal thickness) outcomes. In
addition, this formulation showed enhanced uptake and growth
inhibition of HaCaT cells.
Shams et al (144) fabricated polymeric nanofibers
incorporating TAC-loaded ME for topical application on atopic skin.
This system displayed sustained in vitro release profiles,
with 22% of TAC released over 3 days. The tested ME-nanofibers,
applied every two days, proved equally effective as the daily use
of commercial TAC ointment in improving the AD histological
features.
Nanocarrier-based skin delivery of
topical mTORIs
Unlike TAC, only a few studies have investigated
the skin penetration and anti-inflammatory effects of topical
sirolimus nanoformulations (91,92,145,146). New generations of bioresponsive
nanocarriers, capable of exploiting key aspects of the inflamed
skin microenvironment, can release their cargos in response to
specific stimuli such as pH, temperature, or redox variations
within the skin (147). In this
regard, redox-sensitive CMS nanocarriers have been explored as
topical vehicles for sirolimus in ex vivo models of inflamed
human skin co-cultured with activated T-cells in comparison with
conventional preparations. All tested formulations delivered
sirolimus into barrier-disrupted inflamed skin, suppressing T-cell
proliferation and release of IL-2 and IL-17A. The strongest
inhibition of IL-2 release was observed with sirolimus incorporated
in CMS nanocarriers, while no effect on IL-1α, IL-6 and IL-8
pro-inflammatory cytokines was detected. Only the sirolimus-loaded
CMS nanocarriers could downregulate mTOR activity and target skin
DCs, preventing their activation and migration (91,92).
Furthermore, aqueous formulations of
sirolimus-loaded polymeric micelles have been developed for dermal
application. Micellar solution and hydrogel both increased the
bioavailability of sirolimus in total skin compared with a
conventional ointment. Greater amounts of sirolimus were deposited
in the SC, viable epidermis and dermis with micelle-based systems.
Sirolimus skin levels increased over time, indicating sustained
release kinetics. Transdermal drug permeation was undetectable for
all tested formulations (145).
Based on these results, Le Guyader et al
(146) investigated the
penetration of sirolimus-loaded polymeric micellar preparations
applied topically to ex vivo human skin. Although
micelle-based and conventional vehicles carried similar amounts of
sirolimus to the dermis (700-800 ng/cm2), a 2.5-fold
higher drug deposition in the epidermis was observed with sirolimus
formulated in micelles rather than a hydroalcoholic gel (1,900 vs.
700 ng/cm2, respectively).
Comparative nanocarrier-based skin
delivery of topical macrolactam immunomodulators
In a comparative study, Quartier et al
(54) used polymeric micelles
for the topical delivery of TAC, PIM and sirolimus. PIM exhibited a
lower linear release from the micelles in vitro, while TAC
and sirolimus showed higher releases, reaching a plateau before
further increase. In ex vivo skin, PIM was deposited in
higher amounts, especially in the epidermis, due to its greater
lipophilicity and stronger binding with skin components. TAC and
sirolimus showed lower but similar deposition levels, with greater
accumulation in the dermis. The higher water solubility of
sirolimus was associated with increased dermal levels. Transdermal
drug permeation was not observed. These findings indicate the need
for tailored micelles, even for closely related drugs, as minor
variations in drug properties, particularly aqueous solubility and
lipophilicity, can affect dermatokinetic profiles.
The wide range of nanomaterials discussed in this
section underscores their potential to overcome challenges
associated with conventional dosage forms. The key outcomes from
preclinical studies utilizing nanoparticulate systems to explore
the dermatokinetic profiles, efficacy and safety of topical TAC,
PIM and sirolimus in normal and inflamed skin models are summarized
in Table II.
 | Table IISummary of preclinical studies
utilizing nanosystems for the topical delivery of tacrolimus,
pimecrolimus and sirolimus in normal and inflamed skin models. |
Table II
Summary of preclinical studies
utilizing nanosystems for the topical delivery of tacrolimus,
pimecrolimus and sirolimus in normal and inflamed skin models.
A, Lipid-based
nanosystems
|
|---|
| Authors, year | Delivery
system | Loaded drug | Experimental set-up
| Results
|
|---|
| Skin condition | Skin model: Type
(species/method) | Release | Penetration | Therapeutic
efficacy | Safety | Key findings | (Refs.) |
|---|
| Khan et al,
2022 | SLN, CS-SLN | TAC | Normal | In vitro
(Franz-cell setup)
Ex vivo (murine) | Sustained | SLN ≈ SLN-gel | N/A | N/A | Sustained release
from CS-coated SLNs and SLN-gel. Enhanced skin retention with
SLN-gel. | (105) |
| Kang et al,
2019 | Thermo-sensitive
SLNs | TAC | Normal | In vitro
(Franz-cell setup)
Ex vivo (murine)
In vivo (rabbit) | In Dermis | ↑ | N/A | Mild erythema | Higher skin
deposition and penetration depth (300-450 μm) vs. marketed ointment
(undetectable <150 μm). | (18) |
| Wang et al,
2012 | SLN | TAC | Normal | In vitro
(dialysis membrane) Ex vivo (murine) | Sustained | ↑ | N/A | N/A | Sustained release
vs. conventional solution. Higher skin penetration and retention
vs. commercial ointment. | (104) |
| Viegas et
al, 2020 | NLC | TAC
TAC + siRNA | IQM-induced
Pso | In vitro
(dialysis membrane, murine fibroblasts)
Ex vivo (porcine)
In vivo (murine) | Sustained | ↑ | ↑ | Low
cytotoxicity | Co-delivery of TAC
and TNFα siRNA prevented psoriasis onset and achieved higher TNFα
reduction (7-fold) vs. TAC-loaded NLCs (2.5-fold) and commercial
TAC ointment (2-fold). | (109) |
| Andrade et
al, 2017 | CS-NLC | TAC + CP | Normal | In vitro
(Franz-cell setup)
Ex vivo (porcine) | Sustained | ↑ | N/A | N/A | Co-encapsulation of
CP improved TAC skin penetration ex vivo. | (106) |
| Nam et al,
2011 | NLC | TAC | Normal | Ex vivo
(murine) | N/A | ↑ | N/A | N/A | Increased skin
penetration and deposition vs. marketed product. | (110) |
| Patel et al,
2010 | LP | TAC | DNFB-induced
AD | Ex vivo
(murine)
In vivo (murine) | ↑ | ↑ | Similar to
commercial ointment | N/A | Improved skin
penetration and accumulation vs. free TAC ex vivo. | (112) |
| Erdogan et
al, 2002 | LP | TAC | OVA-induced
DTH | In vivo
(murine) | N/A | ↑ | ↑ | N/A | Superior TAC skin
deposition and anti-AD efficacy vs. systemic vehicles. | (111) |
| Li et al,
2012 | ETH, LP | TAC | DNFB-induced
AD | Ex vivo
(murine)
In vivo (murine) | N/A | ↑ | ↑ | N/A | Both vesicular
systems enhanced TAC epidermal deposition.
Anti-AD effect of ETHs > classic LPs > DXM cream >
commercial TAC ointment. | (113, 114), |
| Ren et al,
2024 | TFs | TAC | DNCB-induced
AD | In vitro
(dissolution apparatus, HaCaT, HDF)
Ex vivo (murine)
In vivo (murine) | Sustained | ↑ | ↑ | No
cytotoxicity | Increased skin
penetration depth, accumulation and retention of TAC with improved
clinical and pathological AD features vs. traditional gel.
Decreased total serum IgE. | (117) |
| Parkash et
al, 2018 | TFs, LP | TAC | DPDS-induced
Pso | Ex vivo
(murine)
In vivo (murine) | N/A | ↑ | ↑ | N/A | TFs showed deeper
and increased skin deposition and superior antipsoriatic effects
vs. classic LPs. | (116) |
| Lei et al,
2013 | TFs, LP | TAC | DNFB-induced
AD | Ex vivo
(murine)
In vivo (murine) | ↑ | ↑ | ↑ | N/A | TFs showed superior
skin penetration and AD control over classic LPs and conventional
vehicles. | (115) |
| Thapa et al,
2013, 2014 | LCNP | TAC | IQM-induced
Pso | In vitro
(dialysis membrane)
Ex vivo (murine)
In vivo (murine) | Controlled | ↑ | ↑ | N/A | Improved skin
deposition and retention and superior anti-psoriatic effects vs.
free TAC. | (118, 119) |
| Jain et al,
2019 | LCNP, SLN, NLC,
LP | TAC | Mouse-tail Pso | In vitro
(dialysis membrane)
Ex vivo (porcine)
In vivo (murine) | LCNP: 34%
SLN: 62%
NLC: 65%
LP: 69% | ↑ LCPN: 2.5x; SLN:
2x; NLC: 2x | ↑ NLC, SLN,
LCNP | No irritation; ↓
TEWL | Improved skin
bioavailability with LCPN, SLN and NLC.
Penetration to deeper skin: LP < LCNP < NLC < SLN, with
SLN and NLC penetrating deeper vs. marketed ointment. In
vivo anti-psoriatic effects of NLC = SLN > LCNP >
commercial ointment > LP. | (120) |
| Pople et al,
2013 | MNLC | TAC | DNFB-induced
AD | In vitro
(microfilters)
Ex vivo (porcine)
In vivo (murine) | N/A | ↑ | ↑ | No irritation +
histological changes; ↓ TEWL | Similar
occlusion/hydration effect and enhanced skin deposition and AD
control vs. marketed ointment. | (124) |
| Pople et al,
2011 | MNLC | TAC | Normal | In vitro
(Franz-cell setup)
Ex vivo (porcine)
In vivo (murine, rabbit) | ↑ | ↑ | N/A | No irritation | Improved skin
release, penetration and deposition vs. commercial
formulation. | (123) |
| Pople et al,
2012 | LN | TAC | DNFB-induced
AD | In vivo
(murine) | N/A | ↑ | ↑ | No histological
changes | Higher skin
penetration and retention to deeper skin vs. marketed ointment.
Faster and superior control of AD vs. marketed ointment. | (122) |
| Pople et al,
2010 | LN | TAC | Normal | In vitro
(Franz-cell setup, microfilters)
Ex vivo (porcine)
In vivo (murine, rabbit) | ↑ | ↑ | N/A | No irritation | Improved skin
release, penetration and deposition and similar occlusive and
hydrating effects vs. marketed product. | (48) |
| Wang et al,
2019 | ME | TAC | DNCB-induced
AD | In vitro
(HaCaT)
Ex vivo (murine)
In vivo (murine) | N/A | ↑ | ↑ | No cytotoxicity; ↓
TEWL | Enhanced skin
retention and anti-AD efficacy vs. commercial ointment. Decreased
serum IgE. | (129) |
| Savić et al,
2017 | ME | TAC | Normal | In vitro
(Franz-cell setup)
Ex vivo (porcine)
In vivo (human) | ↑ | ↑ | N/A | No irritation of
blank MEs in humans | Higher TAC levels
in deeper SC ex vivo vs. commercial ointment. | (128) |
| Lalan et al,
2012 | ME | TAC | TNCB-induced
AD | In vitro
(dialysis membrane)
Ex vivo (murine, human)
In vivo (murine) | ↑ | ↑ | ↑ | No irritation +
toxicity | Higher TAC skin
penetration depth and retention and superior anti-AD effects vs.
commercial ointment.
Decreased epidermal thickness and inflammatory cytokines
(IL-2/4/10) vs. commercial ointment. | (127) |
| Goebel et
al, 2011 | ME | TAC | Normal | Ex vivo
(human) | N/A | ↑ | N/A | No irritation +
vascular effects | Increased
accumulation in dermis vs. conventional TAC ointment. | (126) |
| Sahu et al,
2018 | Kalonji oil-based
NE | TAC | IQM-induced
Pso | In vitro
(dialysis membrane, A-431 epidermal cells)
Ex vivo (porcine)
In vivo (murine) | Sustained | ↑ | ↑ | N/A | Increased epidermal
retention and stronger antiproliferative effects vs. free TAC.
Greater antipsoriatic activity vs. marketed ointment.
Reduced serum TNF-α and IL-6. | (130) |
| Savić et al,
2019 | NLC, NE | TAC | Normal | In vitro
(Franz-cell setup)
Ex vivo (porcine) | ↑ NLC
> NE | ↑ | N/A | N/A | Improved SC
deposition and follicular uptake with nanovehicles. NLCs were
superior to NEs. | (32) |
| Jain et al,
2016 | Lipospheres | TAC + CUR | IQM-induced
Pso | In vitro
(dialysis membrane)
In vivo (murine) | Sustained | ↑ | ↑ | No toxicity | Improved psoriatic
histology in vivo. Higher reduction of TNFα and IL-22. | (47) |
B, Polymer-based
nanosystems
|
|---|
| Authors, year | Delivery
system | Loaded drug | Experimental set-up
| Results
|
|---|
| Skin condition | Skin model: Type
(species/method) | Release | Penetration | Therapeutic
efficacy | Safety | Key findings | (Refs.) |
|---|
| Quartier et
al, 2023 | Micelles | TAC/PIM/SIR | Normal | In vitro
(dialysis membrane)
Ex vivo (porcine) | PIM <
TAC/SIR | ↑ | N/A | N/A | Skin deposition:
PIM > TAC/SIR.
No transdermal permeation. | (54) |
| Le Guyader et
al, 2022 | Micelles | SIR | Normal | Ex vivo
(human) | N/A | ↑ | N/A | N/A | Higher epidermal
and similar dermal levels vs. conventional hydrogel. | (146) |
| Quartier et
al, 2021 | Micelles | SIR | Normal | Ex vivo
(porcine) | Sustained | ↑ | N/A | N/A | Enhanced skin
deposition without percutaneous permeation vs. traditional
ointment. | (145) |
| Lapteva et
al, 2014 | Micelles | TAC | Normal | Ex vivo
(porcine, human) | N/A | ↑ | N/A | N/A | Improved skin
accumulation without transdermal permeation. | (131) |
| Yamamoto et
al, 2019 | Micelles | TAC | IQM-induced
Pso | Ex vivo
(murine) | Sustained | ↑ | N/A | N/A | Time-dependent
higher SC deposition vs. commercial ointment with steady-state
concentrations in viable epidermis. | (132) |
| Gabriel et
al, 2016 | mPEG-hexPLA
NPs | TAC | IQM-induced
Pso | In vivo
(murine) | N/A | ↑ | ↑ | No local/systemic
toxicity | Improved
penetration in inflamed vs. normal skin. Higher accumulation in
psoriatic skin vs. marketed ointment. Antipsoriatic efficacy
superior to CP and similar to commercial ointment. | (133) |
| Zabihi et
al, 2018 | PLG NPs | TAC | 3D AD skin
model | In vitro
(PHK, PHF, 3D reconstructed filaggrin-deficient human
skin)
Ex vivo (human) | N/A | ↑ | ↑ | No
cytotoxicity | Higher skin
deposition and greater TSLP reduction vs. marketed product. | (134) |
| Fereig et
al, 2021 | CS-NPs | TAC | IQM-induced
Pso | In vitro
(dialysis membrane, Franz-cell setup)
Ex vivo (murine)
In vivo (murine) | Sustained | ↑ | ↑ | N/A | Higher skin
deposition and retention, decreased transdermal permeation and
superior antipsoriatic activity vs. commercial ointment. | (107) |
| Yu et al,
2017 | NIC-CS-NPs | TAC | DNCB-induced
AD | Ex vivo
(murine)
In vivo (murine) | N/A | ↑ | ↑ | N/A | Greater skin
accumulation and anti-AD activity vs. commercial ointment. | (108) |
| Zhuo et al
2018 | HA-CS-NPs | TAC | DNFB-induced
AD | In vitro
(Franz-cell setup)
Ex vivo (murine)
In vivo (murine) | Controlled +
Sustained | ↑ | ↑ | ↓ TEWL | HA coating enabled
controlled and sustained release, improved skin deposition and
retention and superior anti-AD effects vs. uncoated CS-NPs. | (135) |
| Wan et al,
2017 | NIC-HA-Chol
NPs | TAC | IQM-induced
Pso | In vitro
(murine macrophage cells, HaCaT)
Ex vivo (murine)
In vivo (murine) | N/A | ↑ | ↑ | N/A | Enhanced permeation
and retention in psoriatic skin vs. marketed ointment.
Antipsoriatic efficacy similar to CP and superior to commercial TAC
ointment. | (137) |
| Pan et al,
2016 | NIC-HA-Chol
NPs | TAC | Normal | In vitro
(HaCaT)
Ex vivo (murine)
In vivo (murine) | N/A | ↑ | N/A | N/A | Higher skin
penetration depth and deposition vs. marketed product. | (136) |
| Radbruch et
al, 2022 | Polymeric CMS | TAC | OXA-induced AD | In vivo
(murine) | N/A | ↓ | Similar to
commercial ointment | No systemic
toxicity | Reduced skin
deposition and transdermal permeation and similar anti-AD efficacy
vs. marketed ointment. | (138) |
| Rancan et
al, 2021, 2023 | Redox-responsive
CMS | SIR | Inflamed
skin/T-cell set-up | Ex vivo
(human skin/T-cell co-culture) | N/A | ↑ | N/A | N/A | Efficient skin
penetration.
Suppression of T-cell proliferation.
Decreased IL-2 and IL-17A; no effect on IL-1α, IL-6,
IL-8.
Targeting of DCs. Reduced mTOR activity (↓ Rps6). | (91, 92) |
| Rancan et
al, 2019 | Thermo-sensitive
nanogel | TAC | Barrier-disrupted
skin | Ex vivo
(human skin/T-cell co-culture) | Controlled | ↓ | N/A | ↑ IL-6/8 | Decreased skin
accumulation and similar antiproliferative effect on T-cells vs.
marketed formulation. | (140) |
| Limon et al,
2019 | Nano-hydrogel | TAC | TPA-induced
Pso | In vitro
(Franz-cell setup)
Ex vivo (human)
In vivo (murine) | Controlled | ↑ | ↑ | No local
desquamation or hair loss | TAC retention in
epidermis and upper dermis. Superior anti-psoriatic efficacy and
safety vs. free TAC. | (141) |
C, Inorganic
nanosystems
|
|---|
| Authors, year | Delivery
system | Loaded drug | Experimental set-up
| Results
|
|---|
| Skin condition | Skin model: Type
(species/method) | Release | Penetration | Therapeutic
efficacy | Safety | Key findings | (Refs.) |
|---|
| Parekh et
al, 2021 | MSN | TAC | DNFB-induced
AD | In vitro
(Franz-cell setup, HaCaT)
Ex vivo (murine)
In vivo (murine) | ↑ | ↑ | ↑ | No
cytotoxicity | Higher skin
deposition ex vivo and improved clinical and pathological AD
features in vivo vs. free TAC. | (142) |
D, Hybrid
nanosystems
|
|---|
| Authors, year | Delivery
system | Loaded drug | Experimental set-up
| Results
|
|---|
| Skin condition | Skin model: Type
(species/method) | Release | Penetration | Therapeutic
efficacy | Safety | Key findings | (Refs.) |
|---|
| Wan et al,
2017 | Polymer-based
ME | TAC | IQM-induced
Pso | In vitro
(HaCaT)
Ex vivo (murine)
In vivo (murine) | N/A | ↑ | ↑ | N/A | Improved skin
deposition vs. commercial ointment. Antipsoriatic efficacy similar
to CP and greater than commercial TAC ointment. | (143) |
| Shams et al,
2021 | ME-Nanofibers | TAC | DNCB-induced
AD | In vitro
(dialysis membrane) In vivo (murine) | Sustained | N/A | Similar to
commercial ointment | N/A | Every 2-day use was
equally effective as daily commercial ointment in improving AD
histology. | (144) |
However, clinical translation still needs to be
improved, as only a few studies have progressed to real-world
settings. Notably, a recent clinical trial investigating a
topically applied TAC-loaded ME on scalp psoriasis patients
reported promising results, paving the way for future clinical
applications (12).
Conclusions
Careful consideration of the administration route
is critical in treatment decision-making. While recent drug
development has primarily focused on biological and oral
small-molecule therapies for managing AD and psoriasis, systemic
use of immunosuppressants is often related to significant adverse
events. Thus, topical modalities remain the mainstay treatment for
the majority of cases with mild-to-moderate disease. Despite its
limitations, the dermal application of anti-inflammatory compounds
offers an easily accessible route that ensures localized efficacy
while reducing the risk of systemic off-target side effects.
In this regard, topical calcineurin and mTOR
inhibitors, such as TAC, PIM and sirolimus, provide more
skin-selective therapeutic options compared with topical
corticosteroids. TAC and PIM have long been associated with a
minimal risk of skin atrophy and systemic absorption into the
bloodstream, commonly seen with corticosteroid use. This offers a
safer long-term approach, especially for sensitive skin areas, such
as the facial, flexural and anogenital regions. Regarding newer
macrolides, emerging evidence suggests that topical sirolimus may
effectively target several immunological, cellular and molecular
components of the inflammatory cascades involved in AD and
psoriasis (82,86-92).
However, major challenges with conventional topical
formulations of these agents include poor skin penetration and
local irritation effects, particularly with TCIs, which can
compromise therapeutic outcomes and patient adherence. In addition,
formulating these lipophilic, poorly water-soluble and unstable
compounds into patient-friendly water-based preparations remains
difficult with traditional vehicles.
Thus, innovative strategies are needed to optimize
skin penetration and enhance the efficacy and safety of these
'hard-to-formulate' macromolecules. In this respect,
nanotherapeutics have emerged as suitable carriers to address the
limitations of traditional topical dosage forms. Nanotechnology
offers numerous advantages over conventional vehicles, such as
modified solubility, greater drug stability, protection from
degradation, encapsulation of hydrophobic payloads into hydrophilic
carriers and improved therapeutic outcomes resulting from enhanced
skin absorption, targeted delivery, controlled or sustained release
and retention at the action sites without undesirable or toxic
effects.
Based on the literature reviewed, several topically
applied nanovectors could effectively deliver TAC in both psoriatic
and atopic viable skin, facilitating controlled and/or sustained
release with minimal risk of local and off-target side effects. The
selective accumulation of specific nanovehicles in the upper
epidermis, particularly in the SC, requires special consideration
as it could serve as a reservoir from which the dermally applied
drug can be gradually released over prolonged periods, penetrating
deeper layers of viable skin. This localized retention may also
offer additional benefits for the precise targeting of psoriatic
and atopic skin, where the epidermal dysfunction appears to play a
crucial role in disease initiation and progression.
To maximize therapeutic outcomes, combined or
hybrid nanosystems that incorporate multiple therapeutically
relevant molecules or different types of nanoparticles could
provide multi-target or synergistic effects on various aspects of
the inflamed skin microenvironment. For instance, incorporating CS
films in nanoformulations offers antimicrobial properties, which
are particularly beneficial in skin disorders characterized by
microbiome dysbiosis, such as AD. In addition, the combination of
nanocarriers with gene therapy for the co-delivery of TAC and siRNA
opens new prospects for precise post-transcriptional gene
regulation and targeted silencing of disease-specific inflammatory
pathways.
While preclinical evidence on nanoparticle-based
topical delivery of sirolimus in inflamed skin is still limited,
initial findings suggest that nanocarriers could enhance its
intradermal penetration and provide foundational data for further
investigation of its potential broad effects. However, a number of
questions remain regarding the development and optimization of
topical sirolimus formulations. Whether topical mTORIs, especially
formulated in nanovehicles, could offer a viable steroid-free
alternative for AD or psoriasis patients has yet to be
determined.
Despite the potential benefits, nanomedicine-based
skin delivery of topical calcineurin and mTOR inhibitors largely
remains in its preclinical phases, mainly due to the lack of
suitable experimental models that closely mimic the in vivo
complex features of the inflamed human skin. Ex vivo studies
are often conducted on excised human or porcine skin, where barrier
properties may be altered, while in vivo studies frequently
use murine skin, which does not closely resemble human skin.
In conclusion, as the landscape of
anti-inflammatory therapies continues to evolve, ongoing research
into optimizing current modalities and developing novel
formulations remains essential. Preclinical proof-of-concept
studies are important for developing effective drug- and
disease-specific nanotherapeutics. In this context, a deeper
understanding of disease biology and in vitro-in vivo
correlations is crucial to overcome translational barriers. Further
research on ex vivo psoriatic or atopic human skin is needed
to refine preclinical models and utilize translational evidence to
advance nanomaterials designed for immunomodulatory macrolactams in
real-world settings. This may ultimately enrich and optimize the
topical management of inflammatory skin diseases in our clinical
practice.
Availability of data and materials
Not applicable.
Authors' contributions
ND and PS conceived the present study. PS performed
the literature search, designed the figures and wrote the original
draft of the manuscript. MK, MS and MP helped to revise the
manuscript. PS reviewed and edited the final version of the
manuscript under the supervision of ND. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Polytimi Sidiropoulou:
Orcid.org/0000-0002-4987-2999 Maria Papasavva:
Orcid.org/0000-0002-4221-6008 Nikolaos Drakoulis:
Orcid.org/0000-0002-7545-8089
Abbreviations:
|
3D
|
three-dimensional
|
|
AD
|
atopic dermatitis
|
|
CD
|
contact dermatitis
|
|
Chol
|
cholesterol
|
|
CMS
|
core-multishell
|
|
CP
|
clobetasol propionate
|
|
CS
|
chitosan
|
|
CsA
|
cyclosporine A
|
|
CUR
|
curcumin
|
|
DCs
|
dendritic cells
|
|
EE
|
entrapment efficiency
|
|
FKBP12
|
FK506 binding protein 12
|
|
HA
|
hyaluronic acid
|
|
HaCaT
|
immortalized human keratinocytes
|
|
IFN-γ
|
interferon-γ
|
|
IgE
|
immunoglobulin E
|
|
IL
|
interleukin
|
|
LCNPs
|
liquid crystalline nanoparticles
|
|
LCs
|
Langerhans cells
|
|
LN
|
lipid nanoparticles
|
|
ME
|
microemulsion
|
|
MNLC
|
modified nanolipid carriers
|
|
mPEG-hexPLA
|
methoxy-poly(ethylene
glycol)-hexyl-substituted polylactide
|
|
mTOR
|
mammalian target of rapamycin
|
|
mTORIs
|
mammalian target of rapamycin
inhibitors
|
|
MW
|
molecular weight
|
|
NE
|
nanoemulsion
|
|
NGF
|
nerve growth factor
|
|
NIC
|
nicotinamide
|
|
NLCs
|
nanostructured lipid carriers
|
|
NMFs
|
natural moisturizing factors
|
|
MSNs
|
mesoporous silica nanoparticles
|
|
NPs
|
nanoparticles
|
|
PASI
|
Psoriasis Area Severity Index
|
|
PEG
|
polyethylene glycol
|
|
PIM
|
pimecrolimus
|
|
SA
|
stearic acid
|
|
SC
|
stratum corneum
|
|
siRNA
|
small interfering RNA
|
|
SLNs
|
solid-lipid nanoparticles
|
|
TAC
|
tacrolimus
|
|
TCIs
|
topical calcineurin inhibitors
|
|
TFs
|
transfersomes
|
|
TJ
|
tight junction
|
|
TSLP
|
thymic stromal lymphopoietin
|
Acknowledgements
The authors would like to thank Professor Diomedes
Logothetis (Northeastern University, Boston, MA, USA) for his
insightful feedback and guidance on the present study.
Funding
No funding was received.
References
|
1
|
Rodriguez-Cerdeira C, Sanchez-Blanco E and
Molares-Vila A: Clinical application of development of
nonantibiotic macrolides that correct inflammation-driven immune
dysfunction in inflammatory skin diseases. Mediators Inflamm.
2012:5637092012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marsland AM and Griffiths CEM: Therapeutic
potential of macrolide immunosuppressants in dermatology. Expert
Opin Investig Drugs. 13:125–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kemény L: The golden ages of inflammatory
skin diseases: Skyrocketing developments in the therapy of
psoriasis and atopic dermatitis. Acad Dermatol Venereol.
35:2239–2240. 2021. View Article : Google Scholar
|
|
4
|
Leducq S, Giraudeau B, Tavernier E and
Maruani A: Topical use of mammalian target of rapamycin inhibitors
in dermatology: A systematic review with meta-analysis. J Am Acad
Dermatol. 80:735–742. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reynolds NJ and Al-Daraji WI: Calcineurin
inhibitors and sirolimus: Mechanisms of action and applications in
dermatology. Clin Exp Dermatol. 27:555–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Remitz A, De Pità O, Mota A,
Serra-Baldrich E, Vakirlis E and Kapp A: Position statement:
Topical calcineurin inhibitors in atopic dermatitis. J Eur Acad
Dermatol Venereol. 32:2074–2082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alavi A and Shear NH: New perspectives on
topical calcineurin inhibitors: Role in dermatology today and into
the future. J Cutan Med Surg. 23(4 Suppl): 3S–4S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buerger C: Epidermal mTORC1 signaling
contributes to the pathogenesis of psoriasis and could serve as a
therapeutic target. Front Immunol. 9:27862018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peramo A and Marcelo CL: Visible effects
of rapamycin (sirolimus) on human skin explants in vitro. Arch
Dermatol Res. 305:163–171. 2013. View Article : Google Scholar
|
|
10
|
Bornhövd E, Burgdorf WH and Wollenberg A:
Macrolactam immunomodulators for topical treatment of inflammatory
skin diseases. J Am Acad Dermatol. 45:736–743. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutfreund K, Bienias W, Szewczyk A and
Kaszuba A: Topical calcineurin inhibitors in dermatology. Part I:
Properties, method and effectiveness of drug use. Postepy Dermatol
Alergol. 30:165–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pinter A, Tsianakas A and Eichner A;
ScaTAC study group: Efficacy and safety of topical tacrolimus
microemulsion applied twice daily in patients with mild to moderate
scalp psoriasis. Dermatol Ther (Heidelb). 14:521–532. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirchner GI, Meier-Wiedenbach I and Manns
MP: Clinical pharmacokinetics of everolimus. Clin Pharmacokinet.
43:83–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bos JD and Meinardi MM: The 500 Dalton
rule for the skin penetration of chemical compounds and drugs. Exp
Dermatol. 9:165–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pariser D: Topical corticosteroids and
topical calcineurin inhibitors in the treatment of atopic
dermatitis: Focus on percutaneous absorption. Am J Ther.
16:264–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alomar A, Berth-Jones J, Bos JD, Giannetti
A, Reitamo S, Ruzicka T, Stalder JF and Thestrup-Pedersen K;
European Working Group on Atopic Dermatitis: The role of topical
calcineurin inhibitors in atopic dermatitis. Br J Dermatol.
151(Suppl 70): S3–S27. 2004. View Article : Google Scholar
|
|
17
|
Mao J, Wang H, Xie Y, Fu Y, Li Y, Liu P,
Du H, Zhu J, Dong L, Hussain M, et al: Transdermal delivery of
rapamycin with poor water-solubility by dissolving polymeric
microneedles for anti-angiogenesis. J Mater Chem B. 8:928–934.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang JH, Chon J, Kim YI, Lee HJ, Oh DW,
Lee HG, Han CS, Kim DW and Park CW: Preparation and evaluation of
tacrolimus-loaded thermosensitive solid lipid nanoparticles for
improved dermal distribution. Int J Nanomedicine. 14:5381–5396.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Feng X and Meng S: Site-specific
drug delivery in the skin for the localized treatment of skin
diseases. Expert Opin Drug Deliv. 16:847–867. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pandey P, Satija S, Wadhwa R, Mehta M,
Purohit D, Gupta G, Prasher P, Chellappan DK, Awasthi R, Dureja H
and Dua K: Emerging trends in nanomedicine for topical delivery in
skin disorders: Current and translational approaches. Dermatol
Ther. 33:e132922020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel-Mottaleb MM, Try C, Pellequer Y and
Lamprecht A: Nanomedicine strategies for targeting skin
inflammation. Nanomedicine (Lond). 9:1727–1743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwa C, Bauer EA and Cohen DE: Skin
biology. Dermatol Ther. 24:464–470. 2011. View Article : Google Scholar
|
|
23
|
Münch S, Wohlrab J and Neubert RHH: Dermal
and transdermal delivery of pharmaceutically relevant
macromolecules. Eur J Pharm Biopharm. 119:235–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eyerich S, Eyerich K, Traidl-Hoffmann C
and Biedermann T: Cutaneous barriers and skin immunity:
Differentiating A connected network. Trends Immunol. 39:315–327.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bäsler K, Bergmann S, Heisig M, Naegel A,
Zorn-Kruppa M and Brandner JM: The role of tight junctions in skin
barrier function and dermal absorption. J Control Release.
242:105–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andrews SN, Jeong E and Prausnitz MR:
Transdermal delivery of molecules is limited by full epidermis, not
just stratum corneum. Pharm Res. 30:1099–1109. 2013. View Article : Google Scholar :
|
|
27
|
Knudsen NØ and Pedersen GP: pH and drug
delivery. pH of the Skin: Issues and Challenges. 54. Karger
Publishers; Berlin: pp. 143–151. 2018
|
|
28
|
Pyo SM and Maibach HI: Skin metabolism:
Relevance of skin enzymes for rational drug design. Skin Pharmacol
Physiol. 32:283–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubo A, Nagao K, Yokouchi M, Sasaki H and
Amagai M: External antigen uptake by Langerhans cells with
reorganization of epidermal tight junction barriers. J Exp Med.
206:2937–2946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogt A, Wischke C, Neffe AT, Ma N, Alexiev
U and Lendlein A: Nanocarriers for drug delivery into and through
the skin-Do existing technologies match clinical challenges? J
Control Release. 242:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chavira A, Belda-Ferre P, Kosciolek T, Ali
F, Dorrestein PC and Knight R: The microbiome and its potential for
pharmacology. Concepts and Principles of Pharmacology. Handbook of
Experimental Pharmacology. Barrett JE, Page CP and Michel MC: 260.
Springer; Cham: pp. 301–326. 2019, View Article : Google Scholar
|
|
32
|
Savić V, Ilić T, Nikolić I, Marković B,
Čalija B, Cekić N and Savić S: Tacrolimus-loaded lecithin-based
nanostructured lipid carrier and nanoemulsion with propylene glycol
monocaprylate as a liquid lipid: Formulation characterization and
assessment of dermal delivery compared to referent ointment. Int J
Pharm. 569:1186242019. View Article : Google Scholar
|
|
33
|
Raphael AP, Garrastazu G, Sonvico F and
Prow TW: Formulation design for topical drug and nanoparticle
treatment of skin disease. Ther Deliv. 6:197–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Viegas J, Dias S, Carvalho AM and Sarmento
B: Characterization of a human lesioned-skin model to assess the
influence of skin integrity on drug permeability. Biomed
Pharmacother. 169:1158412023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiang A, Tudela E and Maibach HI:
Percutaneous absorption in diseased skin: An overview. J Appl
Toxicol. 32:537–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jakasa I, De Jongh CM, Verberk MM, Bos JD
and Kežić S: Percutaneous penetration of sodium lauryl sulphate is
increased in uninvolved skin of patients with atopic dermatitis
compared with control subjects. Br J Dermatol. 155:104–109. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gattu S and Maibach HI: Modest but
increased penetration through damaged skin: An overview of the in
vivo human model. Skin Pharmacol Physiol. 24:2–9. 2011. View Article : Google Scholar
|
|
38
|
Orsmond A, Bereza-Malcolm L, Lynch T,
March L and Xue M: Skin barrier dysregulation in psoriasis. Int J
Mol Sci. 22:108412021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kocsis D, Horváth S, Kemény Á,
Varga-Medveczky Z, Pongor C, Molnár R, Mihály A, Farkas D, Naszlady
BM, Fülöp A, et al: Drug delivery through the psoriatic epidermal
barrier-A 'skin-on-a-chip' permeability study and ex vivo optical
imaging. Int J Mol Sci. 23:42372022. View Article : Google Scholar
|
|
40
|
Zuberbier T, Chong SU, Grunow K, Guhl S,
Welker P, Grassberger M and Henz BM: The ascomycin macrolactam
pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of
mediator release from human dermal mast cells and peripheral blood
basophils. J Allergy Clin Immunol. 108:275–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hoetzenecker W, Meingassner JG, Ecker R,
Stingl G, Stuetz A and Elbe-Bürger A: Corticosteroids but not
pimecrolimus affect viability, maturation and immune function of
murine epidermal Langerhans cells. J Invest Dermatol. 122:673–684.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Learned C, Alsukait S and Rosmarin D:
Usage of topical calcineurin inhibitors in the medicare population
from 2013 to 2018. J Drugs Dermatol. 21:912–913. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guenther L, Lynde C and Poulin Y:
Off-label use of topical calcineurin inhibitors in dermatologic
disorders. J Cutan Med Surg. 23(4 Suppl): 27S–34S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Lu W, Yuan J, Zeng B, Li D, Zhang
F and Li J: Utility of dermoscopy for evaluating the therapeutic
efficacy of tacrolimus ointment plus 308-nm excimer laser
combination therapy in localized vitiligo patients. Exp Ther Med.
15:3981–3988. 2018.PubMed/NCBI
|
|
45
|
Bos JD: Non-steroidal topical
immunomodulators provide skin-selective, self-limiting treatment in
atopic dermatitis. Eur J Dermatol. 13:455–461. 2003.PubMed/NCBI
|
|
46
|
Kumar P, Ashawat MS, Pandit V, Singh Verma
CP, Ankalgi AD and Kumar M: Recent trends in nanocarriers for the
management of atopic dermatitis. Pharm Nanotechnol. 11:397–409.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jain A, Doppalapudi S, Domb AJ and Khan W:
Tacrolimus and curcumin co-loaded liposphere gel: Synergistic
combination towards management of psoriasis. J Control Release.
243:132–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pople PV and Singh KK: Targeting
tacrolimus to deeper layers of skin with improved safety for
treatment of atopic dermatitis. Int J Pharm. 398:165–178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hanna S, Zip C and Shear NH: What Is the
risk of harm associated with topical calcineurin inhibitors? J
Cutan Med Surg. 23(4 Suppl): 19S–26S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chat VS, Kearns DG, Uppal SK, Han G and Wu
JJ: Management of psoriasis with topicals: Applying the 2020
AAD-NPF guidelines of care to clinical practice. Cutis. 110(2
Suppl): S8–S14. 2022. View Article : Google Scholar
|
|
51
|
Malecic N and Young H: Tacrolimus for the
management of psoriasis: Clinical utility and place in therapy.
Psoriasis (Auckl). 6:153–163. 2016.PubMed/NCBI
|
|
52
|
Zonneveld IM, Rubins A, Jablonska S,
Dobozy A, Ruzicka T, Kind P, Dubertret L and Bos JD: Topical
tacrolimus is not effective in chronic plaque psoriasis. A pilot
study. Arch Dermatol. 134:1101–1102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Remitz A, Reitamo S, Erkko P, Granlund H
and Lauerma AI: Tacrolimus ointment improves psoriasis in a
microplaque assay. Br J Dermatol. 141:103–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Quartier J, Lapteva M, Boulaguiem Y,
Guerrier S and Kalia YN: Influence of molecular structure and
physicochemical properties of immunosuppressive drugs on micelle
formulation characteristics and cutaneous delivery. Pharmaceutics.
15:12782023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sehgal VN, Srivastava G and Dogra S:
Tacrolimus in dermatology-pharmacokinetics, mechanism of action,
drug interactions, dosages, and side effects: Part I. Skinmed.
7:27–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stuetz A, Grassberger M and Meingassner
JG: Pimecrolimus (Elidel, SDZ ASM 981)-preclinical pharmacologic
profile and skin selectivity. Semin Cutan Med Surg. 20:233–241.
2001. View Article : Google Scholar
|
|
57
|
Stuetz A, Baumann K, Grassberger M, Wolff
K and Meingassner JG: Discovery of topical calcineurin inhibitors
and pharmacological profile of pimecrolimus. Int Arch Allergy
Immunol. 141:199–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Billich A, Aschauer H, Aszódi A and Stuetz
A: Percutaneous absorption of drugs used in atopic eczema:
Pimecrolimus permeates less through skin than corticosteroids and
tacrolimus. Int J Pharm. 269:29–35. 2004. View Article : Google Scholar
|
|
59
|
Nghiem P, Pearson G and Langley RG:
Tacrolimus and pimecrolimus: From clever prokaryotes to inhibiting
calcineurin and treating atopic dermatitis. J Am Acad Dermatol.
46:228–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ruzicka T, Assmann T and Homey B:
Tacrolimus: The drug for the turn of the millennium? Arch Dermatol.
135:574–580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lauerma AI, Surber C and Maibach HI:
Absorption of topical tacrolimus (FK506) in vitro through human
skin: Comparison with cyclosporin A. Skin Pharmacol Physiol.
10:230–234. 1997. View Article : Google Scholar
|
|
62
|
Lauerma AI, Stein B, Lee HL, Homey B,
Bloom E and Maibach HI: Topical tacrolimus (FK506): Percutaneous
absorption and effect on allergic and irritant contact dermatitis.
J Invest Dermatol. 110:4911993.
|
|
63
|
Undre NA: Pharmacokinetics of tacrolimus
ointment: Clinical relevance. Tacrolimus Ointment. Ruzicka T and
Reitamo S: Springer; Berlin, Heidelberg: pp. 99–110. 2004,
View Article : Google Scholar
|
|
64
|
Meingassner JG, Aschauer H, Stuetz A and
Billich A: Pimecrolimus permeates less than tacrolimus through
normal, inflamed, or corticosteroid-pretreated skin. Exp Dermatol.
14:752–757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Undre NA, Moloney FJ, Ahmadi S, Stevenson
P and Murphy GM: Skin and systemic pharmacokinetics of tacrolimus
following topical application of tacrolimus ointment in adults with
moderate to severe atopic dermatitis. Br J Dermatol. 160:665–669.
2009. View Article : Google Scholar
|
|
66
|
Gschwind HP, Waldmeier F, Zollinger M,
Schweitzer A and Grassberger M: Pimecrolimus: Skin disposition
after topical administration in minipigs in vivo and in human skin
in vitro. Eur J Pharm Sci. 33:9–19. 2008. View Article : Google Scholar
|
|
67
|
Weiss HM, Fresneau M, Moenius T, Stuetz A
and Billich A: Binding of pimecrolimus and tacrolimus to skin and
plasma proteins: Implications for systemic exposure after topical
application. Drug Metab Dispos. 36:1812–1818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luger T, Boguniewicz M, Carr W, Cork M,
Deleuran M, Eichenfield L, Eigenmann P, Fölster-Holst R, Gelmetti
C, Gollnick H, et al: Pimecrolimus in atopic dermatitis: Consensus
on safety and the need to allow use in infants. Pediatr Allergy
Immunol. 26:306–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cury Martins J, Martins C, Aoki V, Gois
AF, Ishii HA and Da Silva EM: Topical tacrolimus for atopic
dermatitis. Cochrane Database Syst Rev.
2015:CD0098642015.PubMed/NCBI
|
|
70
|
Fogel AL, Hill S and Teng JMC: Advances in
the therapeutic use of mammalian target of rapamycin (mTOR)
inhibitors in dermatology. J Am Acad Dermatol. 72:879–889. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Roy T, Boateng ST, Uddin MB, Banang-Mbeumi
S, Yadav RK, Bock CR, Folahan JT, Siwe-Noundou X, Walker AL, King
JA, et al: The PI3K-Akt-mTOR and associated signaling pathways as
molecular drivers of immune-mediated inflammatory skin diseases:
Update on therapeutic strategy using natural and synthetic
compounds. Cells. 12:16712023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang J, Cui B, Chen Z and Ding X: The
regulation of skin homeostasis, repair and the pathogenesis of skin
diseases by spatiotemporal activation of epidermal mTOR signaling.
Front Cell Dev Biol. 10:9509732022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mercurio L, Albanesi C and Madonna S:
Recent updates on the involvement of PI3K/AKT/mTOR molecular
cascade in the pathogenesis of hyperproliferative skin disorders.
Front Med (Lausanne). 8:6656472021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Huang T, Lin X, Meng X and Lin M:
Phosphoinositide-3 kinase/protein kinase-B/mammalian target of
rapamycin pathway in psoriasis pathogenesis. A potential
therapeutic target? Acta Derm Venerol. 94:371–379. 2014. View Article : Google Scholar
|
|
75
|
Chamcheu JC, Chaves-Rodriquez MI, Adhami
VM, Siddiqui IA, Wood GS, Longley BJ and Mukhtar H: Upregulation of
PI3K/AKT/mTOR, FABP5 and PPARβ/δ in human psoriasis and
imiquimod-induced murine psoriasiform dermatitis model. Acta Derm
Venerol. 96:854–856. 2016.
|
|
76
|
Buerger C, Malisiewicz B, Eiser A, Hardt K
and Boehncke WH: Mammalian target of rapamycin and its downstream
signalling components are activated in psoriatic skin. Br J
Dermatol. 169:156–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wei KC and Lai PC: Combination of
everolimus and tacrolimus: A potentially effective regimen for
recalcitrant psoriasis. Dermatol Ther. 28:25–27. 2015. View Article : Google Scholar :
|
|
78
|
Frigerio E, Colombo MD, Franchi C,
Altomare A, Garutti C and Altomare GF: Severe psoriasis treated
with a new macrolide: Everolimus. Br J Dermatol. 156:372–374. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Reitamo S, Spuls P, Sassolas B, Lahfa M,
Claudy A and Griffiths CE; Sirolimus European Psoriasis Study
Group: Efficacy of sirolimus (rapamycin) administered concomitantly
with a subtherapeutic dose of cyclosporin in the treatment of
severe psoriasis: A randomized controlled trial. Br J Dermatol.
145:438–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Van Velsen SGA, Haeck IM and
Bruijnzeel-Koomen CAFM: Severe atopic dermatitis treated with
everolimus. J Dermatolog Treat. 20:365–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Feldman SR: Adherence must always be
considered: Is everolimus really ineffective as a treatment for
atopic dermatitis? J Dermatolog Treat. 20:317–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ormerod AD, Shah SAA, Copeland P, Omar G
and Winfield A: Treatment of psoriasis with topical sirolimus:
Preclinical development and a randomized, double-blind trial. Br J
Dermatol. 152:758–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Meingassner JG and Stütz A:
Immunosuppressive macrolides of the type FK 506: A novel class of
topical agents for treatment of skin diseases? J Invest Dermatol.
98:851–855. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Meingassner JG and Stütz A:
Anti-inflammatory effects of macrophilin-lnteracting drugs in
animal models of irritant and allergic contact dermatitis. Int Arch
Allergy Immunol. 99:486–489. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Duncan JI: Differential inhibition of
cutaneous T-cell-mediated reactions and epidermal cell
proliferation by cyclosporin A, FK-506, and rapamycin. J Invest
Dermatol. 102:84–88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang F, Tanaka M, Wataya-Kaneda M, Yang L,
Nakamura A, Matsumoto S, Attia M, Murota H and Katayama I: Topical
application of rapamycin ointment ameliorates Dermatophagoides
farina body extract-induced atopic dermatitis in NC/Nga mice. Exp
Dermatol. 23:568–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jung KE, Lee YJ, Ryu YH, Kim JE, Kim HS,
Kim BJ, Kang H and Park YM: Effects of topically applied rapamycin
and mycophenolic acid on TNCB-induced atopic dermatitis-like skin
lesions in NC/Nga mice. Int Immunopharmacol. 26:432–438. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bürger C, Shirsath N, Lang V, Diehl S,
Kaufmann R, Weigert A, Han YY, Ringel C and Wolf P: Blocking mTOR
signalling with rapamycin ameliorates imiquimod-induced psoriasis
in mice. Acta Derm Venerol. 97:1087–1094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao M and Si X: Rapamycin ameliorates
psoriasis by regulating the expression and methylation levels of
tropomyosin via ERK1/2 and mTOR pathways in vitro and in vivo. Exp
Dermatol. 27:1112–1119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim HR, Kim JC, Kang SY, Kim HO, Park CW
and Chung BY: Rapamycin alleviates
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced aggravated dermatitis
in mice with imiquimod-induced psoriasis-like dermatitis by
inducing autophagy. Int J Mol Sci. 22:39682021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rancan F, Guo X, Rajes K, Sidiropoulou P,
Zabihi F, Hoffmann L, Hadam S, Blume-Peytavi U, Rühl E, Haag R and
Vogt A: Topical delivery of rapamycin by means of
microenvironment-sensitive core-multi-shell nanocarriers:
Assessment of anti-inflammatory activity in an ex vivo Skin/T cell
co-culture model. Int J Nanomedicine. 16:7137–7151. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rancan F, Rajes K, Sidiropoulou P, Hadam
S, Guo X, Zabihi F, Mirastschijski U, Rühl E, Haag R, Blume-Peytavi
U and Vogt A: Efficacy of topically applied rapamycin-loaded
redox-sensitive nanocarriers in a human skin/T cell co-culture
model. Int Immunopharmacol. 117:1099032023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dong S, Li D and Shi D: Skin
barrier-inflammatory pathway is a driver of the psoriasis-atopic
dermatitis transition. Front Med (Lausanne). 11:13355512024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Furue M and Kadono T: 'Inflammatory skin
march' in atopic dermatitis and psoriasis. Inflamm Res. 66:833–842.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sehgal SN: Sirolimus: its discovery,
biological properties, and mechanism of action. Transplant Proc.
35(3 Suppl): 7S–14S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Haeri A, Osouli M, Bayat F, Alavi S and
Dadashzadeh S: Nanomedicine approaches for sirolimus delivery: A
review of pharmaceutical properties and preclinical studies. Artif
Cells Nanomed Biotechnol. 46(Suppl 1): S1–S14. 2018. View Article : Google Scholar
|
|
97
|
Balestri R, Rizzoli L, Pedrolli A, Urru
SAM, Rech G, Neri I, Girardelli CR and Magnano M: Analysis of
current data on the use of topical mTOR inhibitors in the treatment
of facial angiofibromas in tuberous sclerosis complex-an update.
Eur Acad Dermatol Venereol. 37:474–487. 2023. View Article : Google Scholar
|
|
98
|
Tanaka M, Wataya-Kaneda M, Nakamura A,
Matsumoto S and Katayama I: First left-right comparative study of
topical rapamycin vs vehicle for facial angiofibromas in patients
with tuberous sclerosis complex. Br J Dermatol. 169:1314–1318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kitayama K, Maeda S, Nakamura A, Katayama
I and Wataya-Kaneda M: Efficiency of sirolimus delivery to the skin
is dependent on administration route and formulation. J Dermatol
Sci. 94:350–353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Le Guyader G, Do B, Vieillard V, Andrieux
K and Paul M: Comparison of the in vitro and ex vivo permeation of
existing topical formulations used in the treatment of facial
angiofibroma and characterization of the variations observed.
Pharmaceutics. 12:10602020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Germer G, Ohigashi T, Yuzawa H, Kosugi N,
Flesch R, Rancan F, Vogt A and Rühl E: Improved skin permeability
after topical treatment with serine protease: Probing the
penetration of rapamycin by scanning transmission X-ray microscopy.
ACS Omega. 6:12213–12222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kovačević AB, Müller RH and Keck CM:
Formulation development of lipid nanoparticles: Improved lipid
screening and development of tacrolimus loaded nanostructured lipid
carriers (NLC). Int J Pharm. 576:1189182020. View Article : Google Scholar
|
|
103
|
Dantas IL, Bastos KTS, Machado M, Galvao
JG, Lima AD, Gonsalves JKMC, Almeida EDP, Araújo AAS, de Meneses
CT, Sarmento VHV, et al: Influence of stearic acid and beeswax as
solid lipid matrix of lipid nanoparticles containing tacrolimus. J
Therm Anal Calorim. 132:1557–1566. 2018. View Article : Google Scholar
|
|
104
|
Wang R, Li L, Wang B, Zhang T and Sun L:
FK506-loaded solid lipid nanoparticles: Preparation,
characterization and in vitro transdermal drug delivery. Afr J
Pharm Pharmacol. 6:904–913. 2012.
|
|
105
|
Khan AS, Shah KU, Mohaini MA, Alsalman AJ,
Hawaj MAA, Alhashem YN, Ghazanfar S, Khan KA, Niazi ZR and Farid A:
Tacrolimus-loaded solid lipid nanoparticle gel: Formulation
development and in vitro assessment for topical applications. Gels.
8:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Andrade LM, Silva LAD, Krawczyk-Santos AP,
Amorim ICDSM, Rocha PBRD, Lima EM, Anjos JLV, Alonso A, Marreto RN
and Taveira SF: Improved tacrolimus skin permeation by
co-encapsulation with clobetasol in lipid nanoparticles: Study of
drug effects in lipid matrix by electron paramagnetic resonance.
Eur J Pharm Biopharm. 119:142–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fereig SA, El-Zaafarany GM, Arafa MG and
Abdel-Mottaleb MMA: Tacrolimus-loaded chitosan nanoparticles for
enhanced skin deposition and management of plaque psoriasis.
Carbohydr Polym. 268:1182382021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yu K, Wang Y, Wan T, Zhai Y, Cao S, Ruan
W, Wu C and Xu Y: Tacrolimus nanoparticles based on chitosan
combined with nicotinamide: Enhancing percutaneous delivery and
treatment efficacy for atopic dermatitis and reducing dose. Int J
Nanomedicine. 13:129–142. 2017. View Article : Google Scholar
|
|
109
|
Viegas JSR, Praça FG, Caron AL, Suzuki I,
Silvestrini AVP, Medina WSG, Del Ciampo JO, Kravicz M and Bentley
MVLB: Nanostructured lipid carrier co-delivering tacrolimus and
TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv Transl
Res. 10:646–660. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nam SH, Ji XY and Park J: Investigation of
tacrolimus loaded nanostructured lipid carriers for topical drug
delivery. Bull Korean Chem Soc. 32:956–960. 2011. View Article : Google Scholar
|
|
111
|
Erdogan M, Wright JR Jr and McAlister VC:
Liposomal tacrolimus lotion as a novel topical agent for treatment
of immune-mediated skin disorders: Experimental studies in a murine
model. Br J Dermatol. 146:964–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Patel SS, Patel MS, Salampure S,
Vishwanath B and Patel NM: Development and evaluation of liposomes
for topical delivery of tacrolimus (Fk-506). J Sci Res. 2:585–596.
2010. View Article : Google Scholar
|
|
113
|
Li G, Fan C, Li X, Fan Y, Wang X, Li M and
Liu Y: Preparation and in vitro evaluation of tacrolimus-loaded
ethosomes. Sci World J. 2012:8740532012. View Article : Google Scholar
|
|
114
|
Li G, Fan Y, Fan C, Li X, Wang X, Li M and
Liu Y: Tacrolimus-loaded ethosomes: physicochemical
characterization and in vivo evaluation. Eur J Pharm Biopharm.
82:49–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lei W, Yu C, Lin H and Zhou X: Development
of tacrolimus-loaded transfersomes for deeper skin penetration
enhancement and therapeutic effect improvement in vivo. Asian J
Pharm Sci. 8:336–345. 2013. View Article : Google Scholar
|
|
116
|
Parkash V, Maan S, Chaudhary V, Jogpal V,
Mittal G and Jain V: Implementation of design of experiments in
development and optimization of transfersomal carrier system of
tacrolimus for the dermal management of psoriasis in albino wistar
rat. J Bioequiv Availab. 10:98–105. 2018. View Article : Google Scholar
|
|
117
|
Ren J, Liu T, Bi B, Sohail S and Din FU:
Development and evaluation of tacrolimus loaded nano-transferosomes
for skin targeting and dermatitis treatment. J Pharm Sci.
113:471–485. 2024. View Article : Google Scholar
|
|
118
|
Thapa RK, Baskaran R, Madheswaran T, Kim
JO, Yong CS and Yoo BK: Preparation, characterization, and release
study of tacrolimus-loaded liquid crystalline nanoparticles. J
Disper Sci Technol. 34:72–77. 2013. View Article : Google Scholar
|
|
119
|
Thapa RK and Yoo BK: Evaluation of the
effect of tacrolimus-loaded liquid crystalline nanoparticles on
psoriasis-like skin inflammation. J Dermatolog Treat. 25:22–25.
2014. View Article : Google Scholar
|
|
120
|
Jain S, Addan R, Kushwah V, Harde H and
Mahajan RR: Comparative assessment of efficacy and safety potential
of multifarious lipid based Tacrolimus loaded nanoformulations. Int
J Pharm. 562:96–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Singh KK and Pople P: Safer than safe:
Lipid nanoparticulate encapsulation of tacrolimus with enhanced
targeting and improved safety for atopic dermatitis. J Biomed
Nanotechnol. 7:40–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pople PV and Singh KK: Targeting
tacrolimus to deeper layers of skin with improved safety for
treatment of atopic dermatitis-part II: In vivo assessment of
dermatopharmacokinetics, biodistribution and efficacy. Int J Pharm.
434:70–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pople PV and Singh KK: Development and
evaluation of colloidal modified nanolipid carrier: Application to
topical delivery of tacrolimus. Eur J Pharm Biopharm. 79:82–94.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pople PV and Singh KK: Development and
evaluation of colloidal modified nanolipid carrier: Application to
topical delivery of tacrolimus, part II-In vivo assessment, drug
targeting, efficacy, and safety in treatment for atopic dermatitis.
Eur J Pharm Biopharm. 84:72–83. 2013. View Article : Google Scholar
|
|
125
|
Müller F, Hönzke S, Luthardt WO, Wong EL,
Unbehauen M, Bauer J, Haag R, Hedtrich S, Rühl E and Rademann J:
Rhamnolipids form drug-loaded nanoparticles for dermal drug
delivery. Eur J Pharm Biopharm. 116:31–37. 2017. View Article : Google Scholar
|
|
126
|
Goebel ASB, Neubert RHH and Wohlrab J:
Dermal targeting of tacrolimus using colloidal carrier systems. Int
J Pharm. 404:159–168. 2011. View Article : Google Scholar
|
|
127
|
Lalan MS, Laddha NC, Lalani J, Imran MJ,
Begum R and Misra A: Suppression of cytokine gene expression and
improved therapeutic efficacy of microemulsion-based tacrolimus
cream for atopic dermatitis. Drug Deliv Transl Res. 2:129–141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Savić V, Todosijević M, Ilić T, Lukić M,
Mitsou E, Papadimitriou V, Avramiotis S, Marković B, Cekić N and
Savić S: Tacrolimus loaded biocompatible lecithin-based
microemulsions with improved skin penetration: Structure
characterization and in vitro/in vivo performances. Int J Pharm.
529:491–505. 2017. View Article : Google Scholar
|
|
129
|
Wang Y, Cao S, Yu K, Yang F, Yu X, Zhai Y,
Wu C and Xu Y: Integrating tacrolimus into eutectic oil-based
microemulsion for atopic dermatitis: Simultaneously enhancing
percutaneous delivery and treatment efficacy with relieving side
effects. Int J Nanomedicine. 14:5849–5863. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sahu S, Katiyar SS, Kushwah V and Jain S:
Active natural oil-based nanoemulsion containing tacrolimus for
synergistic antipsoriatic efficacy. Nanomedicine (Lond).
13:1985–1998. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lapteva M, Mondon K, Möller M, Gurny R and
Kalia YN: Polymeric micelle nanocarriers for the cutaneous delivery
of tacrolimus: A targeted approach for the treatment of psoriasis.
Mol Pharm. 11:2989–3001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yamamoto K, Klossek A, Fuchs K, Watts B,
Raabe J, Flesch R, Rancan F, Pischon H, Radbruch M, Gruber AD, et
al: Soft X-ray microscopy for probing of topical tacrolimus
delivery via micelles. Eur J Pharm Biopharm. 139:68–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Gabriel D, Mugnier T, Courthion H,
Kranidioti K, Karagianni N, Denis MC, Lapteva M, Kalia Y, Möller M
and Gurny R: Improved topical delivery of tacrolimus: A novel
composite hydrogel formulation for the treatment of psoriasis. J
Control Release. 242:16–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zabihi F, Graff P, Schumacher F, Kleuser
B, Hedtrich S and Haag R: Synthesis of poly(lactide-co-glycerol) as
a biodegradable and biocompatible polymer with high loading
capacity for dermal drug delivery. Nanoscale. 10:16848–16856. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhuo F, Abourehab MAS and Hussain Z:
Hyaluronic acid decorated tacrolimus-loaded nanoparticles:
Efficient approach to maximize dermal targeting and anti-dermatitis
efficacy. Carbohydr Polym. 197:478–489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Pan W, Qin M, Zhang G, Long Y, Ruan W, Pan
J, Wu Z, Wan T, Wu C and Xu Y: Combination of hydrotropic
nicotinamide with nanoparticles for enhancing tacrolimus
percutaneous delivery. Int J Nanomedicine. 11:4037–4050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wan T, Pan W, Long Y, Yu K, Liu S, Ruan W,
Pan J, Qin M, Wu C and Xu Y: Effects of nanoparticles with
hydrotropic nicotinamide on tacrolimus: Permeability through
psoriatic skin and antipsoriatic and antiproliferative activities.
Int J Nanomedicine. 12:1485–1497. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Radbruch M, Pischon H, Du F, Haag R,
Schumacher F, Kleuser B, Mundhenk L and Gruber AD: Biodegradable
core-multishell nanocarrier: Topical tacrolimus delivery for
treatment of dermatitis. J Control Release. 349:917–928. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Unbehauen ML, Fleige E, Paulus F, Schemmer
B, Mecking S, Moré S and Haag R: Biodegradable core-multishell
nanocarriers: Influence of inner shell structure on the
encapsulation behavior of dexamethasone and tacrolimus. Polymers
(Basel). 9:3162017. View Article : Google Scholar
|
|
140
|
Rancan F, Volkmann H, Giulbudagian M,
Schumacher F, Stanko JI, Kleuser B, Blume-Peytavi U, Calderón M and
Vogt A: Dermal delivery of the high-molecular-weight drug
tacrolimus by means of polyglycerol-based nanogels. Pharmaceutics.
11:3942019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Limón D, Talló Domínguez K,
Garduño-Ramírez ML, Andrade B, Calpena AC and Pérez-García L:
Nanostructured supramolecular hydrogels: Towards the topical
treatment of Psoriasis and other skin diseases. Colloids Surf B
Biointerfaces. 181:657–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Parekh K, Hariharan K, Qu Z, Rewatkar P,
Cao Y, Moniruzzaman M, Pandey P, Popat A and Mehta T: Tacrolimus
encapsulated mesoporous silica nanoparticles embedded hydrogel for
the treatment of atopic dermatitis. Int J Pharm. 608:1210792021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wan T, Pan J, Long Y, Yu K, Wang Y, Pan W,
Ruan W, Qin M, Wu C and Xu Y: Dual roles of TPGS based
microemulsion for tacrolimus: Enhancing the percutaneous delivery
and anti-psoriatic efficacy. Int J Pharm. 528:511–523. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Shams G, Rad AN, Safdarian M, Rezaie A,
Bavarsad N and Abbaspour M: Self-microemulsification-assisted
incorporation of tacrolimus into hydrophilic nanofibers for
facilitated treatment of 2,4-dinitrochlorobenzene induced atopic
dermatitis like lesions. J Drug Deliv Sci Technol. 62:1023262021.
View Article : Google Scholar
|
|
145
|
Quartier J, Lapteva M, Boulaguiem Y,
Guerrier S and Kalia YN: Polymeric micelle formulations for the
cutaneous delivery of sirolimus: A new approach for the treatment
of facial angiofibromas in tuberous sclerosis complex. Int J Pharm.
604:1207362021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Le Guyader G, Do B, Rietveld IB, Coric P,
Bouaziz S, Guigner JM, Secretan PH, Andrieux K and Paul M: Mixed
polymeric micelles for rapamycin skin delivery. Pharmaceutics.
14:5692022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Rajes K, Walker KA, Hadam S, Zabihi F,
Rancan F, Vogt A and Haag R: Redox-responsive nanocarrier for
controlled release of drugs in inflammatory skin diseases.
Pharmaceutics. 13:372020. View Article : Google Scholar
|